Agent-based modelling study of antimicrobial-resistant Neisseria gonorrhoeae transmission in men who have sex with men: towards individualised diagnosis and treatment
Adam K. Zienkiewicz A B * , Nicolás Verschueren van Rees A B * , Martin Homer A , Jason J. Ong C D E , Hannah Christensen E , Darryl Hill F , Katharine J. Looker E , Paddy Horner E , Gwenda Hughes G H and Katy M. E. Turner B E I
G H and Katy M. E. Turner B E I
A Department of Engineering Mathematics, University of Bristol, Bristol BS8 1UB, UK.
B School of Veterinary Sciences, University of Bristol, Langford House, Langford, Bristol BS40 5DU, UK.
C Clinical Research and Development, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK.
D Central Clinical School, Monash University, Clayton, Vic. 3800, Australia.
E Population Health Sciences, University of Bristol, Oakfield House, Oakfield Grove, Bristol BS8 2BN, UK.
F School of Cellular and Molecular Medicine, University of Bristol, Biomedical Sciences Building, University Walk, Bristol BS8 1TD, UK.
G Instituto de Medicina Tropical, Universidade de São Paulo, Avenuenida Dr Enéas Carvalho de Aguiar, 470, CEP 05403-000, São Paulo, Brasil.
H Blood Safety, Hepatitis, STI & HIV Division, National Infection Service, Public Health England, NW9 5EQ, UK.
I Corresponding author. Email: katy.turner@bristol.ac.uk
Sexual Health 16(5) 514-522 https://doi.org/10.1071/SH18235
Submitted: 15 December 2018 Accepted: 29 July 2019 Published: 3 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: Antimicrobial-resistant (AMR) gonorrhoea is a global public health threat. Discriminatory point-of-care tests (POCT) to detect drug sensitivity are under development, enabling individualised resistance-guided therapy. Methods: An individual-based dynamic transmission model of gonorrhoea infection in MSM living in London has been developed, incorporating ciprofloxacin-sensitive and resistant strains. The time-dependent sexual contact network is captured by periodically restructuring active connections to reflect the transience of contacts. Different strategies to improve treatment selection were explored, including discriminatory POCT and selecting partner treatment based on either the index case or partner susceptibility. Outcomes included population prevalence of gonorrhoea and drug dose counts. Results: It is shown that using POCT to detect ciprofloxacin-sensitive infections could result in a large decrease in ceftriaxone doses (by 70% compared with the reference case in the simulations of this study). It also suggests that ceftriaxone use can be reduced with existing technologies, albeit to a lesser degree; either using index case sensitivity profiles to direct treatment of partners, or testing notified partners with strain discriminatory laboratory tests before treatment, reduced ceftriaxone use in our model (by 27% and 47% respectively). Conclusions: POCT to detect ciprofloxacin-sensitive gonorrhoea are likely to dramatically reduce reliance on ceftriaxone, but requires the implementation of new technology. In the meantime, the proportion of unnecessary ceftriaxone treatment by testing partners before treatment could be reduced significantly. Alternatively, index case sensitivity profiles could be used to select effective treatments for partners.
Additional keywords: antimicrobial resistance, diagnostics, gonorrhoea, point-of-care test, resistance-guided therapy.
Introduction
Gonorrhoea is caused by infection with the Gram-negative bacterium, Neisseria gonorrhoeae. Urethral infection in men commonly causes discharge (>80%) and dysuria (>50%), with an onset period of 2–5 days after exposure.1 Complications arising from gonorrhoea include epididymo-orchitis, prostatitis, conjunctivitis, arthritis, pelvic inflammatory disease, chronic pelvic pain, ectopic pregnancy and tubal factor infertility.1,2 Diagnoses of gonorrhoea in England increased from 14 985 in 2008, to 44 676 cases in 2017, of which nearly half were reported in men who have sex with men (MSM).3 MSM bear a disproportionate burden of sexually transmissible infections (STIs).4,5 Gonorrhoea is on the World Health Organization (WHO) high-priority list, requiring immediate action due to concern about the spread of antimicrobial-resistant (AMR) strains and the prospect of untreatable, multi-drug-resistant gonorrhoea.6,7 Currently, N. gonorrhoeae has developed resistance to all major classes of antibiotics.8
AMR gonorrhoea strains have been reported since the early 1940s; by the end of the decade, over 90% of treatments with sulfonamides failed in vitro.8–10 In England, the Gonococcal Resistance to Antimicrobials Surveillance Program (GRASP) has reported on microbiological resistance in gonorrhoea since 2000. In 2004, GRASP reported increased N. gonorrhoeae resistance to ciprofloxacin (14.1%),11 resulting in a change in UK guidelines, to extended spectrum cephalosporins (cefixime).12 Subsequently, increasing cefixime resistance led to another guideline change in 2011, to combination therapy; injectable ceftriaxone with oral azithromycin.2 Most recently in 2019, due to concerns about azithromycin resistance, the guidelines have been further updated to recommend 1 g ceftriaxone or 500 mg ciprofloxacin, if susceptibility is known (https://www.bashhguidelines.org/current-guidelines/urethritis-and-cervicitis/gonorrhoea-2019/). Despite the changes to first-line treatment recommendations, resistance to previously used drugs has remained high and stable; for example, ciprofloxacin (36.4%) and tetracycline (43.7%), although resistance to penicillin has slowly decreased from a peak of 17.1% in 2014 to 10.8% in 2017.13 Interestingly, cefixime resistance increased and then decreased, which may suggest a fitness cost of resistance.13,14
The current situation is therefore that our first-line antibiotic therapy (ceftriaxone) is also the last line. In 2014, the world’s first documented treatment failure to dual ceftriaxone–azithromycin therapy was reported in England,15 with increasing reports of cases in 2018.6,16–18 There is an urgent need, therefore, to develop new diagnostics and treatment strategies in order to preserve ceftriaxone efficacy and to slow or reduce the spread of antibiotic-resistant strains.19 Given the available susceptibility data, it is clear that many infections could be treated with previous antibiotics; ~90% with penicillin alone.13 However, it is essential that alternative treatments are accurately targeted to prevent treatment failure, preserve efficacy of last-line antibiotics and reduce the selection pressure for resistance.
Utilising point-of-care tests (POCT) was identified as a key priority for controlling curable STIs in the review by O’Neill.17,19,20 POCT is any method that can be used to provide accurate and rapid diagnostics, informing treatment within a single clinical visit. A previous modelling paper demonstrated that POCT with lower sensitivity and specificity could be cost-effective in controlling STIs if they could be produced at low enough cost, which may be especially relevant in low-income settings to support shifting away from current syndromic management approaches.21 A systematic review of current developments in POCT for STIs has recently been conducted,22 and it is likely that POCT will become available that can detect and differentiate resistant gonorrhoea strains and/or susceptibility to alternative antibiotic treatments.
Mathematical modelling allows for the testing of possible interventions in public health systems before they are applied in reality, and given a sufficiently detailed and well-calibrated model, enables comparison of the benefits of different possible intervention strategies.23 We have previously developed a static model to evaluate the cost-effectiveness of POCT and POCT AMR tests for gonorrhoea,20,24,25 but a key limitation was the lack of infection dynamics to evaluate the effect of interventions at the population level and to predict gonorrhoea incidence over time. Published models of gonorrhoea include ordinary differential equation approaches, which are fast, require only a small number of parameters and are easy to analyse. However, they assume a well-mixed population, and fail to model the true structure of sexual partnership networks.26,27 Alternative approaches include individual-based models that are typically computationally intensive, require large numbers of parameters and repeated simulations.28–30 The key advantage compared with static or ordinary equation models is that individual-level effects such as contact tracing, partner notification or testing of targeted index-linked treatment choices or interventions can be straightforwardly included, and the model can be extended without altering the underlying contact structures and dynamics. Individual-based models can be used as an additional tool for evaluation of longer-term dynamic effects of both POCT, vaccination or novel drug treatments,31–34 and this paper adds another tool.
In this study, we report the development of a novel individual-based model, with multiple strain transmission dynamics across a hybrid static-dynamic sexual contact network. The model reproduces key epidemiological details of MSM gonorrhoea infection, such as low prevalence and reinfection, while retaining observed features of the contact network topology over extended time periods. It allows a flexible choice of complex (individual-based) treatment, testing and tracing options, including strain-sensitive diagnostic tests with variable time delays, which we use to evaluate the effect of a range of novel control strategies.
Methods
Model overview
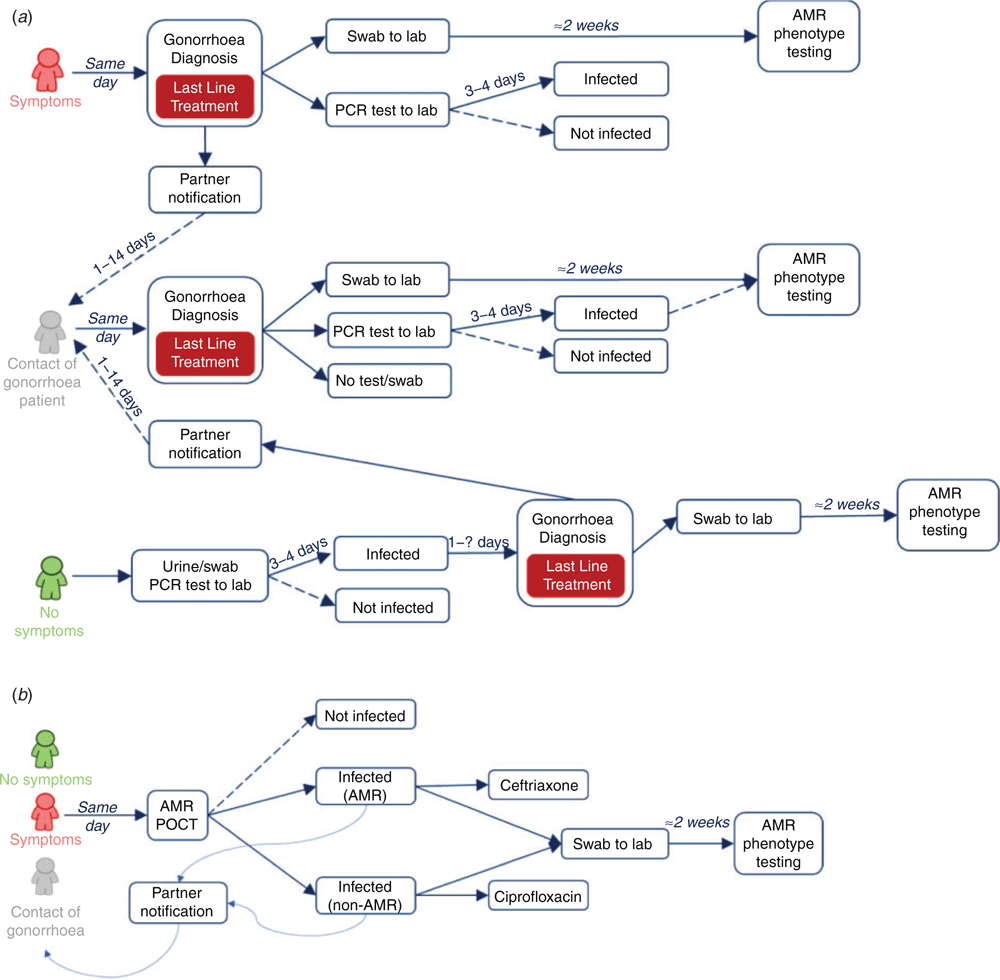
We developed a stochastic, discrete-time Markov model, which describes individual-level gonorrhoea transmission, treatment and recovery (illustrated schematically in Fig. 1), within a time-varying sexual partnership network. Two independent strains are considered, which can be treated with two different drug therapies, ciprofloxacin (Cipr) or ceftriaxone (Ceft). The strains differ only in their response to treatment: one (non-AMR) strain is susceptible to both drug therapies, while the other (AMR) strain is susceptible to Ceft but resistant to Cipr. Recovery from either infection may be spontaneous (natural recovery) or through treatment. As individuals show negligible immunity to re-infection, and remain susceptible to further infections, the model of each individual’s infection status is of susceptible-infected-susceptible (SIS) type.26 Treatment may be sought by individuals with symptomatic infections, prescribed through partner notification (tracing), patient recall following routine (asymptomatic) screening or misdiagnosis revealed by laboratory testing. The model includes flexible combinations of diagnosis and treatment scenarios, summarised in Table 1 and is described in the outline below. Three patient groups are considered, as outlined in Figure 1, defined as: Symptomatic patients (diagnosed based on clinical presentation); Asymptomatic infections (diagnosed based on laboratory tests); and Traced contacts (presumptive treatment).
Full details of the model, its implementation and calibration using data on gonorrhoea in MSM living in London, and the resulting values of epidemiological parameters, are presented in the Supplementary Material. The simulation code is available online: https://data.bris.ac.uk/data/dataset/3erdo698eboli2ptxi324rsuhg.
Scenario analysis
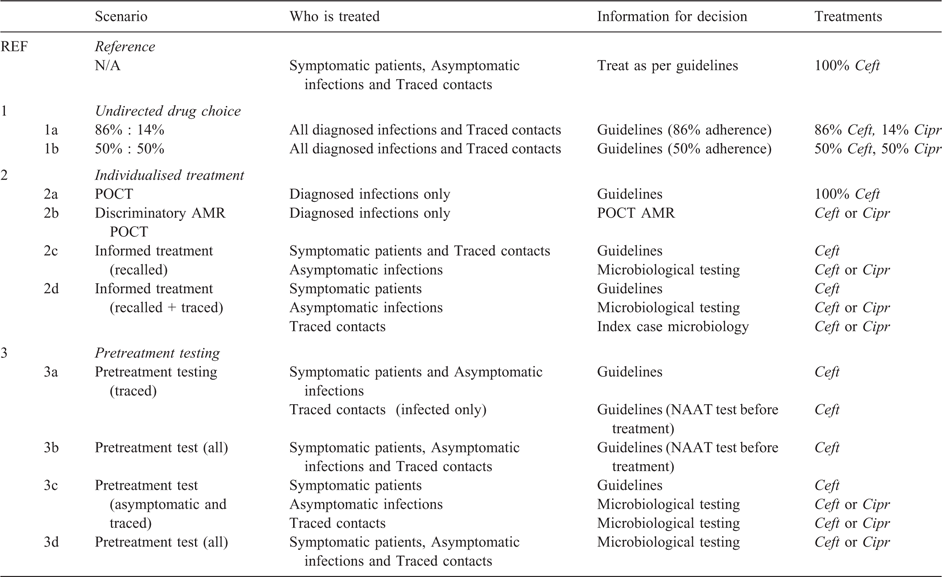
The diagnosis and treatment scenarios we consider are designed to assess the effect (on infection prevalence and the volume and efficacy of drug choice) of POCT and other informed treatment strategies. We compare a baseline reference scenario with additional scenarios divided into three categories: (1) Undirected drug choice; (2) Individualised treatment; and (3) Pretreatment testing.
Undirected drug choice – treatment not always according to guidelines, but not based on any additional diagnostic information (probabilistic adherence to guidelines).
Individualised treatment – treatment decision is based on diagnostic test results, either from a discriminatory POCT (same day) or using current laboratory microbiological susceptibility test results (~2 weeks).
Pretreatment testing – treatment is only given once a diagnostic test has been performed (either POCT or laboratory nucleic acid amplification test (NAAT)). For symptomatic and asymptomatic patients, treatment may then be based on current guidelines or individualised based on results of susceptibility testing of an individual’s infection (laboratory or POCT). For traced contacts, the treatment decision can be based on guidelines or on the microbiological test results of the index case.
Baseline situation
The reference scenario (REF) is based on current gonorrhoea treatment guidelines, in which there is 100% adherence to Ceft as the first-line treatment. Treatment is given to all individuals, who either present with a symptomatic infection, attend a clinic after recall following testing or are traced through a treated partner.
The reference scenario is calibrated (see Supplementary Material) to equilibrate on endemic prevalence and incidence appropriate for an MSM population similar to that of London; prevalence of ~4% (equally divided between AMR and non-AMR strains), and approximately five positive diagnoses per day per 10 000 individuals. A recent analysis of MSM in London found the self-reported gonorrhoea diagnosis in the last year to be 5.2% (Gay Mens Survey) and national Public Health England (PHE) data report high rates of gonorrhoea diagnoses in MSM in London.33,34
Comparison scenarios
Scenario 1, undirected drug choice, attempts to reflect the reality of current UK prescribing, where less than 100% Ceft usage has been reported.13
We consider two options as counterfactuals to evaluate the effect of lower than 100% adherence to recommended guidelines such that some infections (with an AMR strain) are not cured. Here, we assume that drug treatment is assigned in an undirected manner, with Ceft or Cipr treatment given in the absence of knowledge of strain phenotype, with fixed probability. Two fixed ratios are considered: (1a) 86% Ceft and 14% Cipr (in accordance with findings of the GRASP 2014 report); or (1b) equal likelihood of Ceft and Cipr.
Scenario 2, individualised treatment, simulates the effect of two different possible methods to incorporate strain phenotyping into drug choice. (2a) An idealised (100% accurate) POCT that identifies, on the day of clinic attendance, whether an individual is infected with gonorrhoea, in a non-discriminatory manner. (2b) Ideal scenario of AMR POCT, where the strain is also identified. The prescription decision follows accordingly: Ceft is prescribed only to infected individuals (2a) or the strain-appropriate choice of Ceft or Cipr (2b). The second approach is to use an ‘informed’ prescription decision based on strain phenotypic data obtained through laboratory testing, either for individuals recalled for treatment as a result of prior testing (2c), or additionally for those traced through partner notification (2d), using the phenotype of the index patient to inform the treatment choice of their traced partners.
Scenario 3 adds ‘pretreatment testing’ to previous scenarios: individuals attending for treatment are tested for infection before any prescription decision is made. The pathway by which individuals receive treatment becomes the same as those attending via standard asymptomatic testing, thus accruing additional delay due to laboratory test and patient recall delays. The aim is to address treatment wastage due to, for example, treating traced contacts who are assumed to be infected regardless of whether they show symptoms; (3a) pretreatment testing is applied to traced partners of index cases; (3b) pretreatment testing of all individuals, whether traced or treatment-seeking due to symptoms. In reality, it would be highly unusual for clinicians to await test results before treating a patient with obvious symptoms; however, this scenario provides a comparison that can highlight potential sources of treatment wastage. As per the reference scenario, treatments for (3a) and (3b) are exclusively treated with Ceft.
Finally, in scenarios (3c) and (3d), informed discriminatory treatment is included as per scenarios (2c) and (2d), with additional pretreatment testing according to (3a) and (3b) above. In these scenarios, we therefore allow treatment with Cipr as an alternative to Ceft, depending on strain discriminatory testing results.
Experimental procedure and outputs
Simulations for each treatment scenario include an equilibration period (10 000 days) in which transmission dynamics and treatment protocols are fixed according to the reference scenario. This ensures that strain prevalence and incidence settle on stable target values, and are sufficiently transmitted to and distributed within the contact network (10 000 individuals). After the equilibration period, a new scenario is activated, modifying the relevant model treatment pathways as described above.
We collect outputs after one (simulated) year, to make comparisons between different scenarios. Specifically, we analyse disease prevalence and incidence – both by strain, and overall – and drug dosages – both total prescription counts, and sub-divided by efficacy. There are four efficacy categories: optimal treatment (Ceft when an individual was infected with the AMR strain, or Cipr if infected only with the non-AMR strain), over-treatment (Ceft when infected only with the non-AMR strain), under-treatment (Cipr when infected with the AMR strain) and wasted treatment (either drug prescribed in the absence of infection).
To obtain robust statistics from the inherently stochastic transmission processes, 500 realisations of each scenario were simulated, each with different partnership network dynamics and initial infection states.
Results
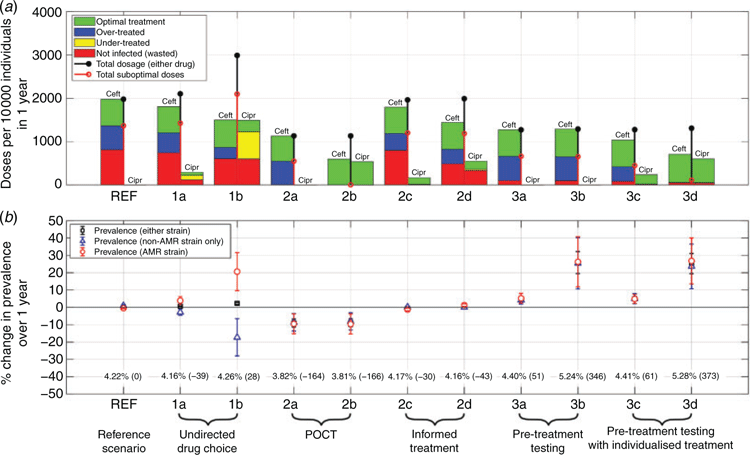
The data shown in Fig. 2 summarise the key outcomes of simulations given the treatment scenarios described above (summarised in Table 1), 1 year after having switched from the reference scenario (REF). Figure 2a provides the treatment breakdown in terms of the number of doses of each drug prescribed and their efficacy depending on the different infection states of individuals. In Figure 2b we report the change in strain prevalence over the course of the same year.
|
Reference scenario
Simulating the model as per the REF scenario with 100% exclusive Ceft treatment for an additional year after the nominal equilibration period yields the following annual statistics, providing a baseline for comparison with alternative strategies: 4.2% overall prevalence (AMR strain: 2.18%, non-AMR only: 2.04%, co-infected: 0.16%) and 1977 per 10 000 individual doses of Ceft. Of these doses, 31.0% were optimally prescribed, while the remaining 69.0% comprised 27.8% over-treatments (individuals with no AMR infection component) and 41.2% ‘wasted’ (individuals treated but not infected with either strain).
Scenario 1: undirected drug choice
Undirected drug choice leads to an increase in the overall dosing by 6.3% (scenario 1a) and 51.2% (1b) above the reference value. While there is a reduction in the number of Ceft doses in both scenarios, of 8.6% (1a) and 24.3% (1b), the number of suboptimal doses were both greater than in the reference scenario (1424 doses (1a) and 2096 doses (1b) per 10 000 individuals).
Undirected drug choice leads to new optimal and suboptimal treatments compared with the reference scenario; in the former case, individuals treated with Cipr who were infected only with the susceptible strain, and in the latter, treatment failures for individuals treated with Cipr who were infected with the resistant strain. Under-treatment comprised 4.7% of doses in scenario (1a) and 21.2% in scenario (1b).
Undirected treatment also affects strain prevalence. While the overall prevalence (infections with either strain) increased only by a small margin – 0.6% (1a) and 2.3% (1b) – individual strain prevalence diverged significantly. In particular, the AMR strain prevalence increased by 3.8% (1a) and 20.6% (1b), with corresponding decreases in the susceptible strain prevalence of 2.8% (1a) and 17.3% (1b).
Scenario 2: individualised treatment
POCT (scenarios 2a, 2b) represents an ideal regime of individualised treatment, and has significant benefits in all measures that we consider.
Non-discriminatory POCT (2a) leads to a 42.7% decrease in the overall number of doses with respect to the reference scenario (both Ceft prescription only). Optimal doses accounted for 51.6% of the total, with the remaining doses all over-treatments, as expected. Instant positivity testing reduces the proportion of wasted doses to zero. The overall infection prevalence also decreased, by 9.1%, with a 9.5% reduction in the AMR strain prevalence, and 8.6% reduction in individuals with susceptible-only infections.
Discriminatory POCT (2b) leads to the same total number of doses as the non-discriminatory test, but the ability to prescribe Cipr leads to a large decrease (of 69.8%) in the number of Ceft doses. As each detected strain is treated with the correct drug, a 100% optimal dose ratio is achieved, with no under/over-treatments or wasted doses; strain discrimination shifts over-treatments in scenario (2a) to optimal treatments (with Cipr) in scenario (2b). The overall infection prevalences are broadly similar to those for scenario (2a).
In contrast to POCT, informed treatment choice (scenarios 2c, 2d) seeks to individualise treatment as currently achievable, via (strain discriminatory) laboratory testing for screened individuals (2c) and additionally for those identified via contact tracing (2d), using the phenotype of the index patient. Both improve drug usage, with respect to the reference scenario. When applied to screened patients (2c), there is an 0.8% decrease in total doses, of which 91.4% were Ceft, representing a 9.3% decrease with respect to the reference scenario, but there remained a significant proportion of over-treated (19.9%) and wasted doses (41.3%). Extending informed choice to secondary cases identified via partner notification (2d), the total number of doses is similar to the reference scenario, but there is a significant reduction in the number of Ceft doses (by 27.2%), an increase in optimal doses, fewer over-treatments and similar wastage. The overall prevalence, both overall and per strain, are very similar to the reference scenario in both informed choice scenarios.
Overall, both informed choice scenarios (2c, 2d) represent an improvement in drug usage, compared with the reference scenario, with a higher proportion of optimal treatments and many fewer under-treatments.
Scenario 3: pretreatment testing
Pretreatment testing seeks to address treatment wastage by testing groups of treatment-seeking individuals before making a prescription decision. Two scenarios (3a and 3b) apply this to Ceft-only prescribing by testing traced partners before treatment (3a), or testing all individuals before treatment (3b), while a further two scenarios (3c and 3d) extend the informed choice scenarios (2c and 2d) with additional pretreatment testing, as in scenarios 3a and 3b respectively. The results of all four scenarios show a significant improvement in drug usage, approaching the level of POCT, but at the cost of increased prevalence (due to the delays in treatment leading to increased duration of infection).
In the Ceft-only pretreatment testing scenarios, the total number of doses was reduced by ~35% (both 3a and 3d) compared with the reference scenario, accompanied by increases of 55% (3a) and 60% (3b) in the proportion of optimal treatments. While a significant proportion of prescriptions were over-treatments (3a: 44.1%, 3b: 43.3%), there was a substantial reduction in wasted treatments, to ~8.1% (3a) and 7.4% (3b) compared with over 41% at reference. The negligible difference between these reductions suggests that wastage in the reference scenario is primarily due to treating uninfected traced partners. However, the additional delays due to pretreatment screening lead to increases in prevalence, of 4.7% (3a) and 25.8% (3b).
Scenarios (3c) and (3d) reduce total drug usage by similar levels to scenarios (3a) and (3b), but with improved dose optimality because Cipr can be prescribed. In scenario (3c), 65.0% of prescriptions are optimal, with Ceft usage reduced by 47.5% with respect to the reference scenario. Over-treatments (26.6%) still occur, as symptomatic treatment-seekers do not receive individualised treatments, but under-treatment with Cipr (0.2%) is rare – representing those individuals who are infected with an AMR strain discordantly with their partner. In scenario (3d), individualised treatment is available to all individuals, leading to a very high dose optimality (91.5%), almost complete elimination of under-treatment and large reduction in the use of Ceft (by 64.3%) compared with the reference scenario; a similar dosing pattern to the discriminatory POCT. The majority of suboptimal drug use is wasted treatment, caused by natural recovery during the test and recall delay. While the laboratory testing delay has benefits in reducing drug use, it also leads to increased gonorrhoea prevalence, to ~4.4% in scenarios 3a and 3c, and to ~5.3% in scenarios 3b and 3c, rises of 4% and 25% with respect to the reference scenario.
Discussion
We have considered the potential effect of current and near future diagnostic test technologies (laboratory and POCT) on the management and control of gonorrhoea in an exemplar high-risk population in a UK city (MSM in London). Point-of-care tests, which do not identify AMR, could still have an important role in reducing unnecessary antibiotic prescriptions (i.e. of uninfected contacts), but POCT AMR would be the ideal situation that could not only reduce prescription of ceftriaxone, but also potentially reduce prevalence due to reductions in treatment delays (mainly for asymptomatic patients). Some improvements could be made using current data by informing treatment of contacts based on the index susceptibility profile. The technical and informatics challenges of this may outweigh potential gains, given that same-day testing is already available in some clinics (e.g. Dean St Express clinic), and that POCT tests are available and POCT AMR tests are likely to be available within a few years. Conversely, given the current financial climate and cuts to sexual health services, it may be prudent to try to optimise the use of current susceptibility test data, as large scale investment in new testing technology and service reorganisation or modernisation may not be financially viable.
This study makes available a flexible, individual-based stochastic network simulation model, calibrated to reproduce the transmission dynamics of gonorrhoea in a population of MSM in London. Notably, we developed an efficient algorithm to reproduce heterogeneous patterns of breaking and forming partnerships between individuals while retaining the longer-term cumulative degree distribution. We then used this model framework to explore potential interventions such as POCT or phenotypic resistance profiling, which could enable individually tailored antibiotic usage and control antibiotic resistant gonorrhoea. As for all mathematical models, results are dependent on both modelling assumptions and on parametrisation; while we have used epidemiologically plausible parameter values, the conclusions below use quantitative results from the model to identify qualitative differences between model scenarios.
We found that, compared with the reference scenario (assuming that 50% of infections are resistant to ciprofloxacin), discriminatory POCT, which detect genetic resistance markers could both reduce the use of ceftriaxone (by ∼70%) and reduce overall gonorrhoea prevalence by reducing current delays in treatment of asymptomatic infections. Antibiotic-resistant gonorrhoea is a serious public health threat6–8 and notably the review by O’Neill called for rapid roll-out of rapid diagnostic tests to detect resistance and direct treatment.19 Most highly resistant organisms occur in the context of hospital settings and clinically unwell patients; however, gonorrhoea is mostly diagnosed in outpatient, community and online testing settings; often patients are asymptomatic and infections are non-lethal and largely curable. These considerations make it less likely that expensive diagnostics will be considered cost-effective for STI clinics, which are already being asked to make significant cost-savings, and even less likely in low-income settings.19,30 However, the use of POCT to reduce clinic attendances and reduce time-to-treatment could be cost-effective even without consideration of AMR.20
We also considered the potential effect of treating individuals, attending clinics as a result of partner notification, with antibiotics, as determined by the resistance profile of the index case (directed treatment). This strategy was found to reduce prescriptions of ceftriaxone by 27%. The effectiveness of this strategy depends on the delays assumed between index and partner treatment, but might be feasible in at least a proportion of cases. Crucially, neither AMR POCT nor index-directed partner treatment resulted in a relative increase in resistant strains of AMR gonorrhoea. We also estimated that ~40% of treatments are given to individuals who are not actually infected. This is in line with previous static analyses,19,20,25 which suggested that POCT would reduce over-treatment, although its relevance to preventing emergence and spread of resistance is not fully understood.
Antibiotic stewardship encourages the use of appropriate antibiotics by using the right antibiotic, at the right dose, for the right duration and at the right time (https://www.cdc.gov/antibiotic-use/stewardship-report/improving-antibiotic-use.html). We identified potential antibiotic wastage due to treating traced partners who are uninfected. Pre-testing symptomatic patients to determine antibiotic susceptibility before treatment is not advised due to introducing unnecessary treatment delays and consequent increase in prevalence. It may be possible, however, with current technology to introduce individualised treatment for asymptomatic patients or traced contacts (microbiological susceptibility testing of index cases) if suitable information systems can be put in place to link patient records to the results of susceptibility testing (either their own or the index case), together with appropriate follow up of cases.
The key strengths of this study are that we used a hybrid static-dynamic network model, which has the flexibility to be extended in several ways without significantly increasing model run time, including: alternative network structures; increasing number of strains; increased complexity of infection process within individuals, taking into account HIV status; vaccination status or multiple anatomical sites of infection. There are limitations to our analysis; we have assumed in the first instance that strains are at equilibrium (50/50) before the introduction of an intervention and are identical except in response to antibiotic treatment. For simplicity, we also assume that all strains remain 100% susceptible to the last-line drug, ceftriaxone. This is reasonable to explore the relative effects of different interventions, but could form the subject of further investigation. We do not consider any explicit de novo evolution of new resistance or decreasing susceptibility to antibiotic treatment, although there is potential for individual-level variation in treatment response due to the model structure chosen. Further, we have not accounted for variability in epidemiological parameters such as transmissibility or duration either due to biological variability in strains or behavioural differences due to condom use or treatment-seeking behaviours. We have included asymptomatic and symptomatic infections with corresponding differences in treatment-seeking behaviour and hence duration of infection.
In summary, POCT to detect ciprofloxacin-sensitive gonorrhoea infection could contribute to reduced reliance on ceftriaxone. We could significantly reduce the proportion of unnecessary ceftriaxone treatments by testing partners before treatment, and use existing phenotype data more informatively. For example, if laboratory turnaround times are fast enough, index case sensitivity profiles could be used to select effective treatments for partners. Future work should prioritise further modelling, attempting to recapture time series and explicit models of resistance evolution. There is also a need to estimate the costs and timeframe of emergence of new resistant strains of gonorrhoea.
Conflicts of interest
AK Zienkiewicz, KME Turner and M Homer gratefully acknowledge funding by BristolBridge (grant number EP/M027546/1) under the EPSRC Bridging the Gaps between the Engineering and Physical Sciences and Antimicrobial Resistance cross-council AMR initiative. KME Turner, H Christensen and NV van Rees acknowledge support from the NIHR Health Protection Research Unit in Evaluation of Interventions at University of Bristol. JJ Ong acknowledges support from the Australian National Health and Medical Research Council Sidney Sax Postdoctoral Research Fellowship. KME Turner has received consultancy fees from Aquarius Population for previous work on gonorrhoea/chlamydia point-of-care tests (2016) and travel expenses from Hologic (2017) for unrelated work. The other authors declare no conflicts of interest.
Acknowledgements
This work was carried out using the computational facilities of the Advanced Computing Research Centre, University of Bristol – http://www.bris.ac.uk/acrc/. We also acknowledge Helen Fifer at Public Health England for her assistance in providing data. The study was partially funded by the National Institute of Health Research (NIHR) Health Protection Research Unit in Evaluation of Interventions at University of Bristol, in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.
References
[1] Holmes KK. Sexually transmitted diseases, 4th edn. New York: McGraw-Hill Education; 2008.[2] Bignell C, Fitzgerald M, Guideline Development Group British Association for Sexual Health and HIV UK UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 2011; 22 541–7.
| UK national guideline for the management of gonorrhoea in adults, 2011.Crossref | GoogleScholarGoogle Scholar | 21998172PubMed |
[3] Public Health England. Sexually transmitted infections: annual data tables 2008–2017. London: Public Health England; 2018. Available online at: https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables [verified 13 August 2019].
[4] Town K, Obi C, Quaye N, Chisholm S, Hughes G, Group GC. Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales. Sex Transm Infect 2017; 93 39–45.
| Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales.Crossref | GoogleScholarGoogle Scholar | 27382010PubMed |
[5] World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: WHO; 2016.
[6] Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27 587–613.
| Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future.Crossref | GoogleScholarGoogle Scholar | 24982323PubMed |
[7] Kampmeier RH. Introduction of sulfonamide therapy for gonorrhea. Sex Transm Dis 1983; 10 81–4.
| Introduction of sulfonamide therapy for gonorrhea.Crossref | GoogleScholarGoogle Scholar | 6362039PubMed |
[8] Rosenblatt-Farrell N. The landscape of antibiotic resistance. Environ Health Perspect 2009; 117 A244–50.
| The landscape of antibiotic resistance.Crossref | GoogleScholarGoogle Scholar | 19590668PubMed |
[9] Fenton KA, Ison C, Johnson AP, Rudd E, Soltani M, Martin I, et al Ciprofloxacin resistance in Neisseria gonorrhoeae in England and Wales in 2002. Lancet 2003; 361 1867–9.
| Ciprofloxacin resistance in Neisseria gonorrhoeae in England and Wales in 2002.Crossref | GoogleScholarGoogle Scholar | 12788575PubMed |
[10] Bignell CJ. BASHH guideline for gonorrhoea. Sex Transm Infect 2004; 80 330–1.
| BASHH guideline for gonorrhoea.Crossref | GoogleScholarGoogle Scholar | 15459396PubMed |
[11] Public Health England. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) – data to May 2018. London: Public Health England; 2018. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/746261/GRASP_2017_report.pdf [verified 13 August 2019].
[12] Whittles LK, White PJ, Didelot X. Estimating the fitness cost and benefit of cefixime resistance in Neisseria gonorrhoeae to inform prescription policy: a modelling study. PLoS Med 2017; 14 e1002416
| Estimating the fitness cost and benefit of cefixime resistance in Neisseria gonorrhoeae to inform prescription policy: a modelling study.Crossref | GoogleScholarGoogle Scholar | 29088226PubMed |
[13] Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016; 374 2504–6.
| Failure of dual antimicrobial therapy in treatment of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 27332921PubMed |
[14] Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, et al Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 2018; 23 pii=1800323
| Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018.Crossref | GoogleScholarGoogle Scholar | 29991383PubMed |
[15] Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18 717–8.
| Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29976521PubMed |
[16] Katz AR. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis 2018; 24 608
| Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017.Crossref | GoogleScholarGoogle Scholar |
[17] O’Neill LJ. Tackling drug-resistant infections globally: final report and recommendations – the Review on Antimicrobial Resistance chaired by Jim O’Neill. 2016. Available online at: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf [verified 13 August 2019].
[18] Turner KM, Christensen H, Adams EJ, McAdams D, Fifer H, McDonnell A, et al Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: a modelling study. BMJ Open 2017; 7 e015447
| Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: a modelling study.Crossref | GoogleScholarGoogle Scholar | 28864707PubMed |
[19] Vickerman P, Watts C, Alary M, Mabey D, Peeling RW. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect 2003; 79 363–7.
| Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women.Crossref | GoogleScholarGoogle Scholar | 14573829PubMed |
[20] Herbst de Cortina S, Bristow CC, Joseph Davey D, Klausner JD. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect Dis Obstet Gynecol 2016; 2016 4386127
| A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.Crossref | GoogleScholarGoogle Scholar | 27313440PubMed |
[21] Keeling MJ, Danon L. Mathematical modelling of infectious diseases. Br Med Bull 2009; 92 33–42.
| Mathematical modelling of infectious diseases.Crossref | GoogleScholarGoogle Scholar | 19855103PubMed |
[22] Adams EJ, Ehrlich A, Turner KM, Shah K, Macleod J, Goldenberg S, et al Mapping patient pathways and estimating resource use for point of care versus standard testing and treatment of chlamydia and gonorrhoea in genitourinary medicine clinics in the UK. BMJ Open 2014; 4 e005322
| Mapping patient pathways and estimating resource use for point of care versus standard testing and treatment of chlamydia and gonorrhoea in genitourinary medicine clinics in the UK.Crossref | GoogleScholarGoogle Scholar | 25056977PubMed |
[23] Turner KM, Round J, Horner P, Macleod J, Goldenberg S, Deol A, et al An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England. Sex Transm Infect 2014; 90 104–11.
| An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England.Crossref | GoogleScholarGoogle Scholar | 24273127PubMed |
[24] Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis 1978; 5 51–6.
| Dynamics and control of the transmission of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 10328031PubMed |
[25] Turner KM, Garnett GP. The impact of the phase of an epidemic of sexually transmitted infection on the evolution of the organism. Sex Transm Infect 2002; 78 i20–30.
| The impact of the phase of an epidemic of sexually transmitted infection on the evolution of the organism.Crossref | GoogleScholarGoogle Scholar | 12083443PubMed |
[26] Ghani AC, Ison CA, Ward H, Garnett GP, Bell G, Kinghorn GR, et al Sexual partner networks in the transmission of sexually transmitted diseases: an analysis of gonorrhea cases in Sheffield, UK. Sex Transm Dis 1996; 23 498–503.
| Sexual partner networks in the transmission of sexually transmitted diseases: an analysis of gonorrhea cases in Sheffield, UK.Crossref | GoogleScholarGoogle Scholar | 8946636PubMed |
[27] Chen MI, Ghani AC, Edmunds WJ. A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations. J R Soc Interface 2009; 6 775–91.
| A metapopulation modelling framework for gonorrhoea and other sexually transmitted infections in heterosexual populations.Crossref | GoogleScholarGoogle Scholar | 18986961PubMed |
[28] Chen MI, Ghani AC. Republished paper: populations and partnerships: insights from metapopulation and pair models into the epidemiology of gonorrhoea and other sexually transmitted infections. Sex Transm Infect 2010; 86 iii63–9.
| 21098058PubMed |
[29] Whittles LK, White PJ, Didelot X. A dynamic power-law sexual network model of gonorrhoea outbreaks. PLOS Comput Biol 2019; 15 e1006748
| A dynamic power-law sexual network model of gonorrhoea outbreaks.Crossref | GoogleScholarGoogle Scholar | 30849080PubMed |
[30] Hui BB, Ryder N, Su JY, Ward J, Chen MY, Donovan B, et al Exploring the benefits of molecular testing for gonorrhoea antibiotic resistance surveillance in remote settings. PLoS One 2015; 10 e0133202
| Exploring the benefits of molecular testing for gonorrhoea antibiotic resistance surveillance in remote settings.Crossref | GoogleScholarGoogle Scholar | 26181042PubMed |
[31] Craig AP, Gray RT, Edwards JL, Apicella MA, Jennings MP, Wilson DP, et al The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 2015; 33 4520–5.
| The potential impact of vaccination on the prevalence of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 26192351PubMed |
[32] Kenyon CR, Schwartz IS. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg Infect Dis 2018; 24 1195–203.
| Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 29912682PubMed |
[33] Ruf M, Delpech V, Osuagwu U, Brown AE, Robinson E, Chadborn T. Men who have sex with men: estimating the size of at-risk populations in London primary care trusts. Int J STD AIDS 2011; 22 25–9.
| Men who have sex with men: estimating the size of at-risk populations in London primary care trusts.Crossref | GoogleScholarGoogle Scholar | 21364063PubMed |
[34] Prah P, Hickson F, Bonell C, McDaid LM, Johnson AM, Wayal S, et al Men who have sex with men in Great Britain: comparing methods and estimates from probability and convenience sample surveys. Sex Transm Infect 2016; 92 455–63.
| Men who have sex with men in Great Britain: comparing methods and estimates from probability and convenience sample surveys.Crossref | GoogleScholarGoogle Scholar | 26965869PubMed |
* Joint first authors.