Extending the use period of hormonal long-acting reversible contraceptive devices in Australia: exploring patterns of use in a cohort of users before and after COVID-19 guidance
Tahlee B. Stevenson A , Alice R. Rumbold B , Kelly Hall A , Jenni Ilomaki C , Deborah Bateson
A , Alice R. Rumbold B , Kelly Hall A , Jenni Ilomaki C , Deborah Bateson  D , Danielle Mazza E and Luke E. Grzeskowiak B F *
D , Danielle Mazza E and Luke E. Grzeskowiak B F *
A
B
C
D
E
F
Abstract
As the accessibility of health services fluctuated throughout the COVID-19 pandemic, recommendations supporting off-label extended use of hormonal long-acting reversible contraceptive (LARC) devices began to appear around the globe. Supported by emerging evidence, these recommendations were intended to encourage consumers to postpone device replacement and reduce the need for face-to-face care interactions.
In this population-based cohort study, data from the nationally representative Australian Pharmaceutical Benefits Scheme 10% sample were analysed. Specifically, logistic regression analysis was undertaken for females aged 15–49 years who had a hormonal LARC dispensed to them within the COVID-19 pandemic period (between February 2017 and November 2021) to assess timing of replacement, compared with timing for pre-COVID counterparts.
Extended use periods were observed in less than 10% of hormonal implant or intrauterine device users in both the pre- and post-COVID cohorts, with 40% replacing their device on time, and around 50% with no record of replacement. No statistically significant changes occurred after the onset of COVID-19.
Despite recommendations for extending LARC device use periods, typical usage patterns were maintained in Australia throughout the COVID-19 pandemic. Given these extended use periods have since been made permanent in Australian clinical guidelines, targeted education for consumers and providers is likely to be required to ensure understanding, uptake and consistent implementation.
Keywords: contraceptive devices, COVID-19, female, hormonal contraception, intrauterine devices, long-acting reversible contraception.
Introduction
Long-acting reversible contraceptive (LARC) devices are widely recognised as the most effective method of reversible contraception.1 In the Australian setting, there are two types of hormonal LARC devices, the subdermal hormonal implant (Implanon NXT®) and levonorgestrel intrauterine devices (IUDs; the Mirena® and Kyleena®). At the time these data were collected, the Implanon NXT® was licensed for contraceptive use for a period of 3 years and the Mirena® and Kyleena® for 5 years.2 All of these hormonal LARC devices are able to be accessed at a subsidised cost through the Australian Government’s Pharmaceutical Benefits Scheme (PBS). Despite this, the proportion of Australian consumers who opt to use these methods is relatively low, likely as a result of the low number of providers that possess the knowledge and capability to counsel consumers about and insert these devices, and the lack of timely and affordable appointments with these providers.3,4
It was speculated that the impact of these barriers might be amplified throughout the COVID-19 pandemic as a result of increased pressure on health systems and staff, supply chain disruptions and lockdown restrictions.5 In line with emerging clinical evidence, recommendations in support of ‘off-label’ extended use periods for LARC devices were developed locally and internationally by March of 2020.6,7 These recommendations were endorsed and shared by many reproductive health peak bodies and suggested using LARC devices for longer periods than those they were licensed to provide contraceptive coverage for. The hope was that by extending these use periods, the need for and frequency of face-to-face care interactions could be reduced while also ensuring that consumer’s contraceptive needs were met.6,8 Specifically, within Australia the recommended use period was extended by 1 year for both the Implanon NXT® and the Mirena®, resulting in a 4 year and 6 year total period of use, respectively. Guidance recommended advising consumers to defer device replacement and removal to align with these timeframes regardless of their intentions to replace their device or not.6
Currently, the extent to which these extended use recommendations were incorporated into clinical practice in Australia is unknown. Therefore, our aim was to explore PBS prescribing data for hormonal LARCs to determine whether the timing and spacing of device dispensing changed after the onset of COVID-19 and indicated uptake of extended periods.
Methods
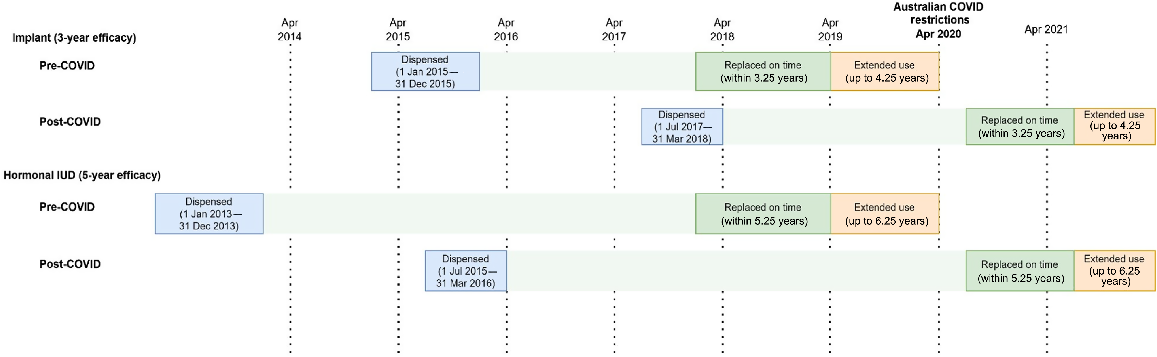
We used the PBS 10% sample dataset; this is a nationally representative, individual-level extract including all PBS dispensing data for a random 10% sample of Australians eligible for subsidised medicines.9 This dataset has previously been used to evaluate patterns and changes in hormonal LARC dispensing in Australia within the COVID-19 pandemic period more generally.10 We analysed data for females aged 15–49 years (inclusive), defining the study population as those who had a hormonal LARC dispensed to them between February 2017 and November 2021 (PBS Item codes: 8633J and 8487Q). The study population was split into two cohorts: pre-COVID, where LARC replacement was due prior to the onset of pandemic-related restrictions, and post-COVID, where LARC replacement was due after the onset of the pandemic-related restrictions (Fig. 1). The pre-COVID cohort included those who had an IUD dispensed between 1 January 2013 and 31 December 2013 or an implant dispensed between 1 January 2015 and 31 December 2015. The post-COVID cohort included those who had an IUD dispensed between 1 July 2015 and 31 March 2016 or an implant dispensed between 1 July 2017 and 31 March 2018. We allowed a 3-month buffer when examining repeat dispensing date to allow for a time lag between dispensing and insertion. Therefore, expected replacement timing was within 5.25 years for an IUD and 3.25 years for the implant. Repeat dispensing that occurred within the 5.25–6.25 years of initial dispensing of a hormonal IUD, or 3.25–4.25 years after initial dispensing of an implant, was classified as extended use. Users that swapped from one hormonal LARC method to another (e.g. implant to IUD) were still considered to have replaced their device. Those with no repeat dispensing occurring within these designated time periods were classified as having no replacement.
Overview of the process used to define study cohorts on the basis of hormonal long-acting reversible contraceptive type and whether replacement was due before or after the onset of COVID-19 restrictions in Australia in April 2020.
We undertook logistic regression analysis to separately compare the likelihood of extended use (compared with no replacement or on-time replacement) and no replacement (compared with extended use or on-time replacement), adjusting for potential confounders including age, state/territory, concessional status and whether it was the first dispensing of that LARC type or not. Differences in proportions between pre-COVID and post-COVID cohorts were compared using a Chi-squared test. Statistical significance was defined as a two-sided P value of <0.05. Statistical analyses were undertaken using Stata MP 17 (Stata, College Station, TX, USA).
Results
A total of 31,371 females were included in the study cohort, 17,764 of whom were dispensed a hormonal implant and 13,607 a hormonal IUD. Characteristics of individuals are presented in Table 1. Among hormonal implant users, compared with those in the pre-COVID cohort, those in the post-COVID cohort were more likely to be first-time users of the same LARC type, and less likely to be concession card holders and have changed their LARC type (all P < 0.001). Among hormonal IUD users, those in the post-COVID cohort were less likely to be first-time users of the same LARC type or concession holders (P < 0.001), but no differences were evident with respect to other characteristics.
| Implant cohorts | P value | Hormonal IUD cohorts | P value | ||||
|---|---|---|---|---|---|---|---|
| Pre-COVID | Post-COVID | Pre-COVID | Post-COVID | ||||
| n = 10 689 | n = 7 075 | n = 7 652 | n = 5 955 | ||||
| User type, n (%) | <0.001 | <0.001 | |||||
| Previously used | 4139 (38.7%) | 2533 (35.8%) | 2498 (32.6%) | 2331 (39.1%) | |||
| First use | 6550 (61.3%) | 4542 (64.2%) | 5154 (67.4%) | 3624 (60.9%) | |||
| Age, n (%) | <0.001 | 0.059 | |||||
| 15–19 years | 2373 (22.2%) | 1626 (23.0%) | 177 (2.3%) | 158 (2.7%) | |||
| 20–24 years | 2859 (26.7%) | 1734 (24.5%) | 755 (9.9%) | 648 (10.9%) | |||
| 25–29 years | 2050 (19.2%) | 1344 (19.0%) | 1136 (14.8%) | 951 (16.0%) | |||
| 30–34 years | 1495 (14.0%) | 1110 (15.7%) | 1542 (20.2%) | 1148 (19.3%) | |||
| 35–39 years | 1100 (10.3%) | 757 (10.7%) | 1864 (24.4%) | 1409 (23.7%) | |||
| 40–44 years | 812 (7.6%) | 504 (7.1%) | 2178 (28.5%) | 1641 (27.6%) | |||
| State/territory, n (%) | 0.246 | 0.138 | |||||
| New South Wales | 2581 (24.1%) | 1784 (25.2%) | 2126 (27.8%) | 1669 (28.0%) | |||
| Victoria | 2658 (24.9%) | 1716 (24.3%) | 1706 (22.3%) | 1403 (23.6%) | |||
| Other states/territories | 5450 (51.0%) | 3575 (50.5%) | 3820 (49.9%) | 2883 (48.4%) | |||
| Concession status, n (%) | <0.001 | <0.001 | |||||
| No concession | 6772 (63.5%) | 4680 (66.3%) | 5537 (72.4%) | 4459 (75.0%) | |||
| Concession holder | 3898 (36.5%) | 2382 (33.7%) | 2108 (27.6%) | 1488 (25.0%) | |||
| LARC replacement, n (%) | 0.821 | 0.400 | |||||
| No replacement | 5578 (52.2%) | 3724 (52.6%) | 4030 (52.7%) | 3168 (53.2%) | |||
| Replaced on time | 4225 (39.5%) | 2764 (39.1%) | 2972 (38.8%) | 2319 (38.9%) | |||
| Extended use | 886 (8.3%) | 587 (8.3%) | 650 (8.5%) | 468 (7.9%) | |||
| Changed LARC method,An (%) | 1320 (12.3%) | 764 (10.8%) | 0.002 | 357 (4.7%) | 252 (4.2%) | 0.225 | |
Extended use periods were observed in around 8% of hormonal implant or IUD users in both the pre- and post-COVID cohorts. In both cohorts, close to 40% replaced their devices on time, and around 50% had no record of replacement. When compared with the pre-COVID cohort, for the post-COVID cohort that used implants there was no significant difference in the likelihood of extended use (adjusted Odds Ratio (aOR) 1.01; 0.91–1.13) or no replacement (aOR 1.00; 0.94–1.06). Similarly, among hormonal IUD users in the post-COVID cohort there were also no significant differences evident in the likelihood of extended use (aOR 0.90; 0.79–1.02) or no replacement (aOR 1.06; 0.99–1.14).
Discussion
This study is the first to examine nationally representative prescribing data to explore LARC device usage periods within Australia before and during the COVID-19 pandemic. Despite concerns about reduced access to contraceptive care, we observed no difference in the proportion of users extending the use period of their LARC devices. Additionally, there was no difference observed in the proportion of users that did not replace their LARC devices. These findings demonstrate that despite the recommendations, original usage periods (3 years for a hormonal implant and 5 years for hormonal IUDs) and typical replacement patterns were maintained. This invites further exploration of consumer and provider awareness of extended use recommendations, experiences when requesting or advising extended use, and perspectives regarding the acceptability of extended use periods.
It is unclear whether extended use recommendations were not implemented more widely because they were not understood or preferred, or simply because they were not required. Within Australia there were considerable efforts made to ensure that contraceptive care remained reasonably accessible throughout the pandemic period.11 This sustained commitment to maintaining access may have meant that there was little need for users to extend their LARC usage period. It is also possible there was confusion surrounding whether extended use recommendations were to be implemented at all times or only in times when services were struggling to meet the demand for appointments.
There is a growing body of evidence demonstrating that some hormonal LARC devices can be used safely and provide effective conception for periods considerably longer than the period for which they were initially licensed for use. Specifically, the Mirena® is effective for up to 8 years12,13 and the Implanon NXT® for up to 5 years.14,15 Despite this, the Australian Mirena® product information and licensing was not updated to reflect this until mid-2024,16 and to date the Implanon® information and licensing remains unchanged.17 Additionally, at the time in question there were no Australian clinical guidelines for contraception provision, and the international guidelines that were most commonly referred to were not updated to reflect extended use periods until 2024.18,19 This lag between evidence being generated and then reflected in regulatory and guidance documents may have contributed to confusion and uncertainty for providers.
As demonstrated in recent research from the US, there is a need for widespread education for both providers and consumers to accompany changes such as this, to ensure clarity, understanding of supporting evidence and consistent implementation.20 The importance of this has been reinforced in subsequent studies that have suggested that the number and frequency of updates to LARC information and guidance in recent years has created confusion and contributed to misinformation being passed on to consumers querying extended use options.21,22 Although these findings emerged from the US, given that a lack of provider knowledge and misconceptions regarding LARC use and suitability are established barriers to uptake in the Australian context,4 it is likely that these changes may also be contributing to confusion here. To overcome this and support consistent implementation, targeted educational campaigns and updates sharing the information and evidence in support of extended LARC usage periods will be paramount. The option to use LARC devices for a longer time period may also contribute to increased uptake, as consumers may perceive them to be increasingly cost effective and acceptable if the frequency of invasive and uncomfortable insertion procedures is reduced.
Strengths of this study include use of the nationally representative and high-quality PBS 10% sample database, which contains individual-level longitudinal data for a large cohort of LARC users. Limitations include restriction of analysis to hormonal LARC devices (copper IUD use periods were not explored as these devices are not covered by the PBS) and the assumption that the date of dispensing corresponded closely with the date of device insertion. Additionally, from dispensing data alone we are unable to elicit the indication for device use; specifically, whether it was for contraceptive purposes alone or had other intended therapeutic benefit such as reduced pelvic pain or menstrual bleeding (where an extended use period may not have been fit for purpose). Also, within the no replacement cohort we are unable to tell when or why these consumers removed their device.
Conclusion
Despite specific recommendations encouraging extended use periods for LARC devices, PBS dispensing data indicate that typical useage patterns were maintained within the Australian setting. As we move beyond the disruptions of the COVID-19 pandemic, the priority must be ensuring that high-quality, evidence-based and equitable contraceptive care is provided consistently throughout Australia. Information sharing and education will need to be fundamental components of initiatives that aim to increase awareness and understanding of extended LARC device usage periods and LARC uptake more generally. Care providers and clinical support staff must be aware of updated guidelines and regulatory documents if they are to implement them and must understand the evidence that underpins them if they are to recommend that consumers follow them.
Data availability
The study data cannot be publicly shared for reasons of ethics and privacy. Access to aggregate data, however, may be granted upon request to corresponding author if deemed appropriate. All authors had full access to all study data (including statistical reports and tables).
Conflicts of interest
Authors Deborah Bateson and Danielle Mazza have received honoraria, and research and travel funding from pharmaceutical companies marketing LARC products including Bayer and Organon. The authors have no further conflicts of interest to declare.
References
1 Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs 2011; 71: 969-980.
| Crossref | Google Scholar |
2 Bahamondes L, Fernandes A, Monteiro I, Bahamondes MV. Long-acting reversible contraceptive (LARCs) methods. Best Pract Res Clin Obstet Gynaecol 2020; 66: 28-40.
| Crossref | Google Scholar | PubMed |
3 Grzeskowiak LE, Calabretto H, Amos N, Mazza D, Ilomaki J. Changes in use of hormonal long-acting reversible contraceptive methods in Australia between 2006 and 2018: a population-based study. Aust N Z J Obstet Gynaecol 2021; 61: 128-134.
| Crossref | Google Scholar | PubMed |
4 Mazza D, Bateson D, Frearson M, Goldstone P, Kovacs G, Baber R. Current barriers and potential strategies to increase the use of long-acting reversible contraception (LARC) to reduce the rate of unintended pregnancies in Australia: an expert roundtable discussion. Aust N Z J Obstet Gynaecol 2017; 57: 206-212.
| Crossref | Google Scholar | PubMed |
5 Riley T, Sully E, Ahmed Z, Biddlecom A. Estimates of the potential impact of the COVID-19 pandemic on sexual and reproductive health in low- and middle-income countries. Int Perspect Sex Reprod Health 2020; 46: 73-76.
| Crossref | Google Scholar | PubMed |
6 Family Planning Alliance Australia. Extended use of an ongoing access to LARCs during the COVID-19 pandemic – Family Planning Alliance Australia Statement 2020; 2020. Available at https://familyplanningallianceaustralia.org.au/wp-content/uploads/2023/06/LARCs-during-the-COVID-19-pandemic-FPAA-APR-2020.pdf [accessed March 2023]
7 The Faculty of Sexual and Reproductive Healthcare. Essential services in sexual and reproductive healthcare; 2020. Available at https://bsacp.org.uk/wp-content/uploads/2020/03/fsrh-position-essential-srh-services-during-covid19-24-march-2020.pdf [accessed March 2023]
8 Mazza D. Women’s Sexual and Reproductive Health COVID-19 Coalition Bulletin #3, 10th June 2020. SPHERE NHMRC Centre of Research Excellence in Sexual and Reproductive Health for Women in Primary Care; 2020. Available at https://mailchi.mp/3121d6d142d1/srh-covid-19-bulletin-5032793?e=6321eec329
9 Mellish L, Karanges EA, Litchfield MJ, Schaffer AL, Blanch B, Daniels BJ, Segrave A, Pearson SA. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634.
| Crossref | Google Scholar | PubMed |
10 Stevenson TB, Rumbold AR, Moore V, Hall K, Ilomaki J, Mazza D, Bateson D, Grzeskowiak LE. Longitudinal trends in uptake of hormonal long-acting reversible contraception devices throughout the COVID-19 pandemic: an Australian population-based study. BMJ Sex Reprod Health 2024; 50: 262-269.
| Crossref | Google Scholar | PubMed |
11 Newman CE, Fraser D, Ong JJ, Bourne C, Grulich AE, Bavinton BR. Sustaining sexual and reproductive health through COVID-19 pandemic restrictions: qualitative interviews with Australian clinicians. Sex Health 2022; 19: 525-532.
| Crossref | Google Scholar | PubMed |
12 Jensen JT, Lukkari-Lax E, Schulze A, Wahdan Y, Serrani M, Kroll R. Contraceptive efficacy and safety of the 52-mg levonorgestrel intrauterine system for up to 8 years: findings from the Mirena extension trial. Am J Obstet Gynecol 2022; 227: 873.E1-873.E12.
| Crossref | Google Scholar |
13 Harrison CV, Igwe-Kalu C, Eide L. An integrative review of extended use of intrauterine devices. Nurs Womens Health 2023; 27: 427-434.
| Crossref | Google Scholar |
14 McNichols C, Swor E, Wan L, Peipert JF. Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device – two years beyond FDA-approved duration. Am J Obstet Gynecol 2017; 216: 586.e1-586.e6.
| Crossref | Google Scholar |
15 Ali M, Akin A, Bahamondes L, Brache V, Habib N, Landoulsi S, Hubacher D, WHO study group on subdermal contraceptive implants for women. Extended use up to 5 years of the etonogestrel-releasing subdermal contraceptive implant: comparison to levonorgestrel-releasing subdermal implant. Hum Reprod 2016; 31: 2491-2498.
| Crossref | Google Scholar | PubMed |
16 Therapeutic Goods Administration. Australian Product Information MIRENA® (levonorgestrel) intrauterine drug delivery system; 2024. Available at https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2009-PI-01235-3
17 Therapeutic Goods Administration. Australian Product Information: Implanon NXT® Subdermal Implant; 2023. Available at https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2011-PI-02693-3
18 The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). C-Gyn 3 Contraception – Category: Clinical Guideline (Signposting); 2024. Available at https://ranzcog.edu.au/wp-content/uploads/Contraception-Clinical-Guideline.pdf
19 The Faculty of Sexual and Reproductive Healthcare. FSRH Guideline – Intrauterine Contraception; 2025. Available at https://www.fsrh.org/Common/Uploaded%20files/documents/fsrh-clinical-guideline-intrauterine-contraception-mar-23-amended.pdf
20 Rigler N, Kully G, Hildebrand MC, Averbach S, Mody SK. Offering extended use of the contraceptive implant via an implementation science framework: a qualitative study of clinicians’ perceived barriers and facilitators. BMC Health Serv Res 2024; 24: 679.
| Crossref | Google Scholar |
21 de Oliveira BR, Alsamman S, Pai M, Mody SK. “My IUD is expiring”: A national U.S. mystery client study regarding use of the LNG 52-mg IUD for pregnancy prevention beyond 5 years after multiple FDA extensions. Contraception 2024; 137: 110483.
| Crossref | Google Scholar | PubMed |
22 Pai M, Rigler N, de Oliveira BR, Mody SK. “My implant is expiring”: a national US mystery shopper study regarding extended use of the contraceptive implant. Contraception 2023; 123: 110038.
| Crossref | Google Scholar | PubMed |


