Understanding variability in heat yields of wet sclerophyll forest fuels
Wey Yao Wong A * , Jane Cawson
A * , Jane Cawson  B , Thomas Duff
B , Thomas Duff  C , Patrick Lane A and Gary Sheridan
C , Patrick Lane A and Gary Sheridan  A
A
A
B
C
Abstract
Fireline intensity is important for understanding fire behaviour. Heat yield – the amount of energy released by fuels, calculated by subtracting energy lost by vaporising moisture from a fuel’s calorific value – is considered the least variable component of fireline intensity. Recent work suggests it may be more variable than assumed, though how it varies between fuels and seasons remains unclear.
This study aims to determine how heat yields vary between fuels and seasons in terms of calorific values, hydrogen content and fuel moisture.
We sampled common wet sclerophyll forest fuels over a year, measuring their moisture content. We determined their calorific value with bomb calorimetry, and hydrogen content with elemental analysis.
Fuel heat yields varied substantially between species and seasons, with some species having large seasonal variations. The heat yields of live fuels were significantly lower than dead fuels.
Heat yields are highly variable between fuels. Accounting for species composition and seasonal variation may be important for accurately estimating heat yield at the forest-stand scale.
Heat yields are more variable than previously assumed and have been overestimated in some models. This could have implications for fireline intensity.
Keywords: calorimetry, energy release, fireline intensity, fuel, fuel moisture, heat of combustion, mountain ash, wildfire.
Introduction
Fireline intensity – or the heat released per unit length of flame front – is an important metric in many operational fire behaviour models (Keeley 2009). It relates to the extent of damage caused by a fire (McArthur and Cheney 2015; Volkova et al. 2019), as well as its suppressibility (Alexander 2000; Penney et al. 2019). The potential energy that could be released by a burning fuel – its heat yield – is needed for calculating fireline intensity (Byram 1959).
There are numerous ways of estimating heat yield, but a common approach is to subtract from the gross calorific value of a fuel, the latent heat of vaporisation of the water of reaction and a further 24 kJ kg−1 for each percent of moisture in the fuel (Van Wagner 1972). Originally, Byram’s (1959) approach also subtracted the heat required to separate water from its physically bound state, as well as heat losses from radiation and incomplete combustion of fuels. However, the separation of bound water is a relatively small heat loss and is therefore often not included in the estimation of heat yields, such as in Alexander (1982). On the other hand, emitted radiation may contribute a large proportion of the heat of combustion, but is not subtracted from heat yield due to its significant contribution to fire behaviour and difficulty in its measurement (Van Wagner 1972). The completeness of fuel consumption is also difficult to estimate, and thus often not subtracted from heat yield (Van Wagner 1972).
Byram’s (1959) paper on fireline intensity states that heat yield does not vary widely between different types of fuels. However, the values provided were only for wood and litter, not fresh foliage. Van Wagner (1972) subsequently estimated that the effect of fluctuations in the calorific value of western conifer foliage were likely to be offset by coinciding fluctuations in moisture content. Many fire behaviour models have therefore used a fixed value for heat yield for all fuel types with the assumption that its variation is insignificant (Van Wagner et al. 1992; Cheney et al. 2012). However, the heat yield of other fuel types (e.g. fresh foliage, bark) and other species have yet to be measured in context with their moisture contents, so it is unclear if Van Wagner’s (1972) results are generalisable.
There could be seasonal variation in heat yield due to differences in plant physiology and phenology, which could differ by plant growth forms (trees, shrubs, herbs). Grouping fuels by their condition (live or dead), or growth form could be a useful way of incorporating a wider diversity of heat yields into fireline intensity calculations. Live- and dead fuels have significantly different fuel moisture contents (FMCs) (Nelson 2001) and dynamics in calorific values (Hughes 1971), hence their heat yields are likely to be most different. Additionally, the heat yields of live fuels may form distinct groups based on growth form. Differences in calorific values of plant growth forms (trees, shrubs, herbs) (Rivera et al. 2012; Yan et al. 2018) compounded with seasonal variation in FMC of live and dead fuel (Brown et al. 2022), may result in significant differences in heat yields between fuel groups.
In this study, we sought to better understand how the heat yields of forest fuels vary between fuel types and seasons, using a case study in wet sclerophyll forest of south-eastern Australia. Specifically, we asked:
Methods
We quantified the heat yields of common live and dead fuels in wet sclerophyll forest by measuring their FMC, gross calorific value and hydrogen content over the course of a year. We focussed on species comprising understorey live fuels, as the flammability of this strata is considered most important from a fuel hazard perspective in Australian forests (Gould et al. 2011).
Site description
Samples were collected from an area of wet sclerophyll forest, located approximately 70 km east of Melbourne, Australia. The climate in this region consists of mild winters and warm summers, classified as climate type Cfb (Peel et al. 2007). The region experiences a mean maximum temperature of 25.4°C in January and a minimum temperature of 2.8°C in July. Mean annual rainfall is 1446 mm for the nearest permanent weather station (Powelltown, station number 086282) (BoM 2025). The region has deep soils with rich organic matter topped with abundant litter (Ashton 1975), which is frequently too wet to burn throughout the year (Burton et al. 2019).
Wet sclerophyll forest in Victoria is often dominated by Mountain ash (Eucalyptus regnans F.Muell.), forming a tall open canopy. The understorey beneath the E. regnans is structurally diverse, consisting of subdominant ferns, shrubs and grasses. The sampled species, along with their growth form and common names, are listed in Table 1, and dominant species at each site are listed in Table 2. E. regnans is an obligate seeder, regenerating in single aged stands from seeds released during infrequent but intense fires (Ashton 1981).
| Growth form | Species name | Common name | |
|---|---|---|---|
| Tree | Acacia dealbata Link | Silver wattle | |
| Eucalyptus regnans F.Muell. | Mountain ash | ||
| Persoonia arborea F.Muell. | Tree geebung | ||
| Shrub | Correa lawrenceana Hook. | Mountain correa | |
| Hedycarya angustifolia A.Cunn. | Austral mulberry | ||
| Olearia argophylla (Labill.) Benth. | Musk daisy-bush | ||
| Pomaderris aspera Sieber ex DC. | Hazel pomaderris | ||
| Prostanthera melissifolia F.Muell. | Balm mint-bush | ||
| Fern | Blechnum wattsii Tindale | Hard water-fern | |
| Dicksonia antarctica Labill. | Soft tree-fern | ||
| Polystichum proliferum (R.Br.) C.Presl | Mother shield-fern | ||
| Graminoids | Gahnia sieberiana Kunth | Red-fruit saw-sedge | |
| Tetrarrhena juncea R.Br. | Forest wiregrass |
| Site | Last burnt | Coordinates | Elevation (m) | Slope | Aspect | Canopy species | Dominant understorey species sampled at each site | |
|---|---|---|---|---|---|---|---|---|
| Young ash | 2017 | −37°54′1.749″ | 735.14 | 4.87 | 158.4 | Eucalyptus regnans | Tetrarrhena juncea | |
| 145°43′56.301″ | Acacia dealbata | Pomaderris aspera | ||||||
| Prostanthera melissifolia | ||||||||
| Gahnia sieberiana | ||||||||
| Mature ash | 1939 | −37°54′11.729″ | 724.76 | 13.15 | 159.7 | Eucalyptus regnans | Blechnum wattsii | |
| 145°45′18.756″ | Dicksonia antarctica | |||||||
| Polystichum proliferum | ||||||||
| Scrub | 1939 | −37°54′24.393″ | 635.06 | 15.07 | 166.7 | Acacia dealbata | Blechnum wattsii | |
| 145°44′30.861″ | Acacia mearnsii | Correa lawrenceana | ||||||
| Persoonia arborea | Dicksonia antarctica | |||||||
| Pomaderris aspera | ||||||||
| Olearia argophylla | ||||||||
| Prostanthera melissifolia | ||||||||
| Tetrarrhena juncea |
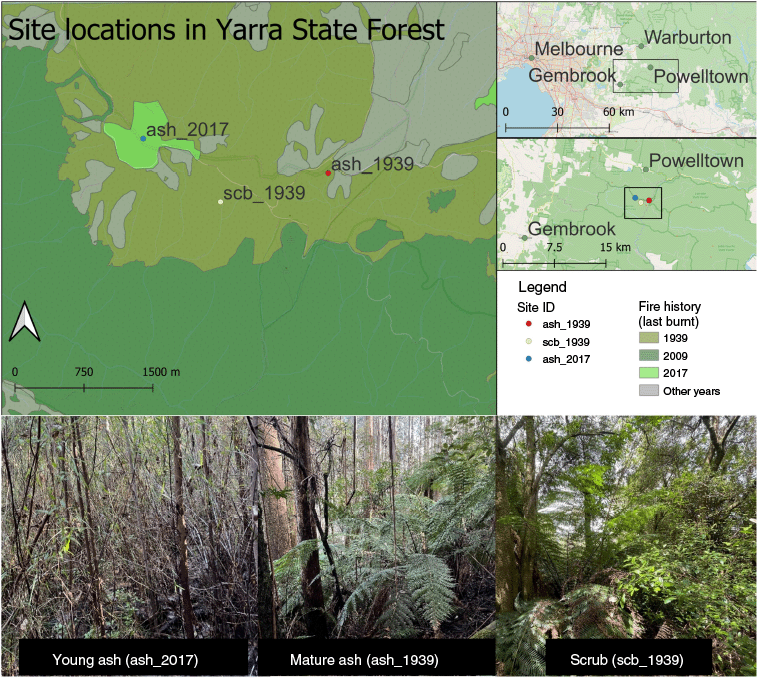
Study site characteristics and dominant canopy and understorey species at each site that was collected for this study.
Samples were collected from three forest sites that we called: ‘Young ash’, ‘Mature ash’ and ‘Scrub’ (collapsed ash, ash = E. regnans) (Fig. 1). The contrasting forest structures and compositions at these sites, representing different forest states, reflect the variable fire history across the landscape (Burton et al. 2019) (Fig. 1). The young ash site was burnt in 2017 after being logged. Both the mature ash and scrub sites were burnt in 1939, with the scrub site also being burnt in 1926 and 1932, leading to localised extirpation (or collapse) of the E. regnans there.
Field sampling
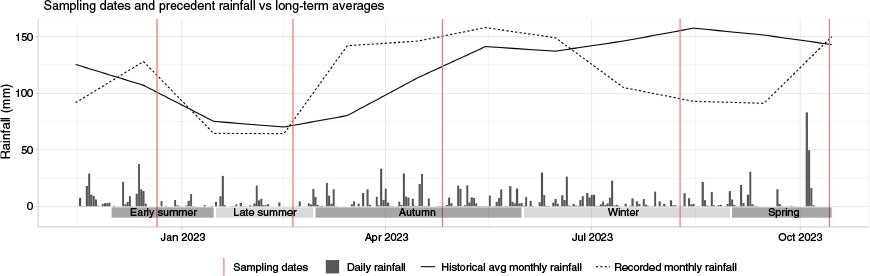
Fuel samples were collected in field campaigns from the three sites from December 2022 to September 2023: in early summer (21 December 2022), late summer (19 February 2023), autumn (26 April 2023), winter (9 August 2023) and spring (12 September 2023). All samples were collected between 12 pm and 3 pm on a clear day to minimise dew or precipitation on the fuel surface. Dead fuels collected in winter had free water on their surface, so their results were omitted as free water can cause large errors in calculated FMC (Pollet and Brown 2007). All reported FMC values were from fuels that were free of surface water during collection. Daily rainfall at the sites over the sampling period is shown in Fig. 2. All collected fuel samples were weighed in the field and placed into double-bagged Ziploc bags for transport.
Sample collection dates and antecedent rainfall, including recorded monthly averages at the site and comparison with Bureau of Meteorology (BoM) historical average monthly rainfall. Sampling dates were: early summer (21 December 2022), late summer (19 February 2023), autumn (26 April 2023), winter (9 August 2023) and spring (12 September 2023).
One aggregated sample was collected for each live fuel species at a site. The sample consisted of shoots collected from at least three separate individuals of the same species, with each shoot harvested being <3 mm including stems and live foliage (protocol 19 in Yebra et al. 2024). Dead foliage was removed from all live fuel samples. Samples from ferns consisted of mature, non-reproductive fronds, and samples of graminoids consisted of non-flowering stems and attached leaves. Shoots were collected from the top sections of shrubs, and the lower sections of tree canopies due to access constraints. Canopy (overstorey) fuels – E. regnans, Acacia dealbata Link – were only collected during late summer and winter as overstorey FMC is likely to be less responsive to seasonal variations in soil moisture compared to understorey live fuels due to deeper roots (Agee et al. 2002). Furthermore, their calorific values are available in the literature (Dickinson and Kirkpatrick 1985; Frederick et al. 1985).
The sampling protocol for dead fuel moisture followed (Slijepcevic et al. 2018). Collected litter consisted of fallen leaves and twigs <6 mm in diameter, with surface litter being collected from the top 1 cm of litter, and subsurface litter being collected from below the top 1 cm of litter down to mineral soil. Surface and subsurface litter samples were aggregated from three random points within 10 m of the centre of the site, each time from points that had not been previously sampled. Elevated eucalyptus bark ribbons were collected from a height of 1.5 m from at least three points within 10 m of the centre of the site.
Lab measurements
Fresh samples were weighed in the field, then dried at 105°C for a week (Matthews 2010), or until the mass of the samples stopped reducing. The FMC was calculated as the difference between the fresh and dry mass divided by the dry mass of the fuel (Eqn 1):
Oven dry samples were ground using a Retsch Ultra Centrifugal Mill ZM200 with a 1.0 mm filter. Each ground sample consisted of all leaves and twigs collected for fuel moisture sampling. The dried powdered samples were stored in airtight aluminium tins while awaiting calorimetry. Gross calorific value was determined with oxygen bomb calorimetry according to the ISO 1716 standard using the CAL3K Bomb Calorimeter. Approximately 0.3 g of the powdered sample was loaded into gelatine capsules to prevent loose powder from dispersing during ignition inside the bomb calorimeter. The resulting gross calorific values were adjusted for the mass and calorific value of the gelatine capsule (Atwater and Snell 1903). Elemental composition (including hydrogen content) was determined using the Thermo Scientific FlashSmart CHNS analyser.
Calculation of heat yield
Heat yields for each fuel were calculated by subtracting water-related heat losses from the gross calorific value (Eqn 2), following (Byram 1959; Van Wagner 1972). Heat yield is calculated in MJ kg−1, GCV (MJ kg−1) is the gross calorific value (also called gross heat of combustion, higher heat content or high heat of combustion), FMC is the fuel moisture content of a fuel as a percent of its dry weight, and Hd is the percent hydrogen composition of a fuel. Radiation losses, losses from the separation of bound water, and incomplete combustion are not included in the estimation of heat yields, which is typical for the estimation of heat yield for use in fireline intensity (Alexander 1982).
Calorific values were not determined for E. regnans and A. dealbata, as they have been previously reported (Dickinson and Kirkpatrick 1985; Frederick et al. 1985). For these species, we assumed that the hydrogen content was 5.747%, which corresponds to 1.263 MJ kg−1 of latent heat absorbed for the vaporisation of the water of reaction for woody fuels (Byram 1959; Alexander 1982).
Data analysis
For calculating heat yields of a fuel for a given season, replicate values for FMC, calorific value and hydrogen content of species common across multiple sites were averaged (mean). There were no statistically significant differences in the heat yield between sampling sites (P > 0.05), so values were averaged if a species was found in multiple sites.
An analysis of variance (ANOVA) was performed to determine whether the differences in heat yield between the fuel types were statistically significant. Fuels were grouped by growth forms (shrub, fern, graminoids) as well as the fuel strata (surface, near-surface, elevated). The analysis was conducted using R statistical software (version 4.3.3) (R Core Team 2021), and all graphs were produced using the ggplot package (Wickham 2016).
The net contribution of seasonal change attributed to FMC and GCV was calculated for each fuel as the absolute value of the difference in GCV between two seasons (ΔGCV), minus the absolute value of the difference in FMC (ΔFMC). For example, if ΔGCV and ΔFMC contributed equally to heat yield, their difference would be 0, while if ΔGCV was 1 kJ kg−1 while ΔFMC was 2 kJ kg−1, the net contribution would be 1 kJ kg−1 from ΔFMC.
Results
Variations in heat yield between fuel types and seasons
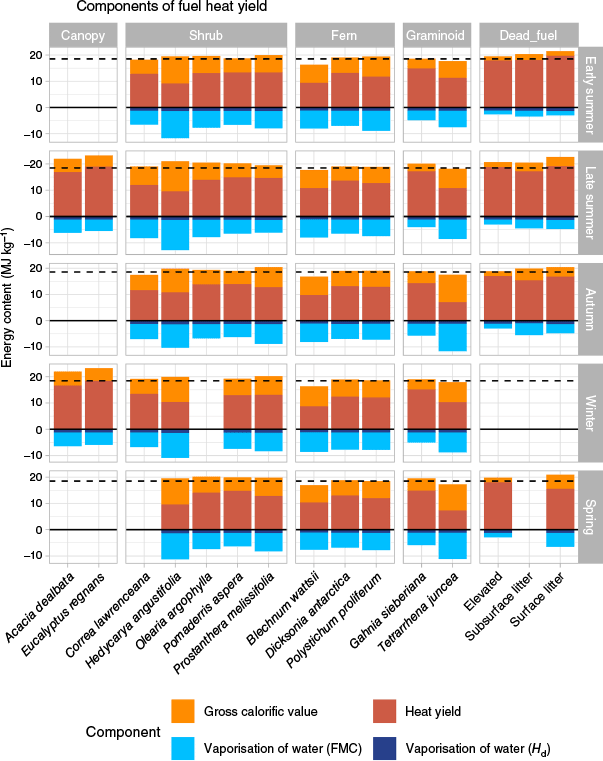
There was substantial variation in the heat yields of the fuels measured in this study. On average, bark ribbons had the highest heat yield across seasons (19.53 MJ kg−1 in early summer), while forest wiregrass (Tetrarrhena juncea R.Br.) had the lowest heat yield across seasons (6.18 MJ kg−1 in autumn). Heat yields peaked in late summer, with a second smaller peak in winter/spring across most fuels (Fig. 3).
Seasonal trends of heat yields for each fuel, grouped by growth form. Error bars are standard deviations, and the black line is the heat yield average for the growth form or fuel group.
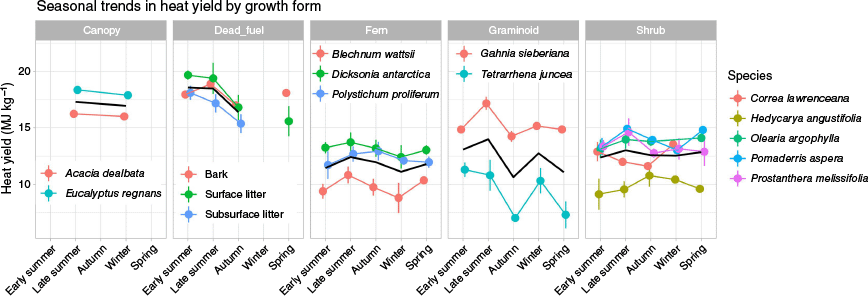
Averaged across all fuel types, heat yields increased slightly from early summer to late summer by 0.58 MJ kg−1. This was followed by a decrease of 1.31 MJ kg−1 from late summer to autumn, which was in part due to the simultaneous increase in moisture content of around 20.21% for each fuel on average, and decrease in gross calorific value of 0.83 MJ kg−1 (Supplementary Table S1). Heat yields increased slightly (0.31 MJ kg−1) from autumn to winter, and were relatively stable from winter to spring. Assuming the spring heat yields correspond to the spring preceding the early summer results, heat yields increased by around 1.45 MJ kg−1 from spring to early summer, which correspond to decreases in FMC of around 7.51% (as 7.51 units of FMC), and increases in calorific values of 1.27 MJ kg−1.
However, there were significant differences in the heat yield dynamics between fuel types. The fuel with the greatest fluctuation in heat yield was Tetrarrhena juncea (Fig. 3). Its heat yield decreased by 4.03 MJ kg−1 (40%) from late summer to autumn, then rose by 3.52 MJ kg−1 (57%) from autumn to winter, then decreased again by 3.21 MJ kg−1 (33%) from winter to spring. The other graminoid, Gahnia sieberiana, also had similarly large fluctuations in heat yield and similar seasonal trends as T. juncea, with the largest variation being a decrease of 3.05 MJ kg−1 (18%) from late summer to autumn. On the other hand, the fuel with the least dynamic heat yield was the soft treefern (Dicksonia antarctica Labill.), whose heat yield did not vary by more than 1.0 MJ kg−1 (4%) from season to season. However, this was not the case for all ferns. Heat yields of the fern Blechnum wattsii fluctuated between seasons, with the largest variation being an increase in 1.63 MJ kg−1 (20%) from winter to spring. Despite differences in the magnitude of the seasonal variations, the overall seasonal trends in heat yields were similar for the canopy, dead fuel, ferns and graminoids.
There were some differences in the seasonal trend of shrubs. From early summer to autumn, the heat yield of Correa lawrenceana decreased by 1.33 MJ kg−1 (12% decrease), yet over the same time period, Hedycarya angustifolia had an increase of 1.76 MJ kg−1 (17% increase) (Fig. 3). Autumn was also a seasonal minimum for C. lawrenceana and Prostanthera melissifolia, a seasonal maximum for H. angustifolia, and a seasonal midpoint for Olearia argophylla and Pomaderris aspera.
Variation in heat yield between fuel groups and growth forms
There was significant difference in heat yield between live and dead fuels (P < 0.001) across all seasons, as well as between fuels grouped by strata (i.e. litter bed, live understorey, bark and canopy) (Table 3). A post hoc Tukey test showed that the heat yields of understorey live fuels were significantly different from canopy fuels, litter and bark.
| Heat yield (MJ kg−1) | Range (min–max) | ||
|---|---|---|---|
| Live vs dead fuels | |||
| Dead fuels | 17.41 ± 1.50a | 7.02–18.99 | |
| Live fuels | 12.20 ± 2.66b | 15.37–19.67 | |
| Finer classes of fuels | |||
| Bark | 17.84 ± 0.78a | 16.99–18.89 | |
| Litter | 17.18 ± 1.81a | 15.37–19.67 | |
| Canopy | 17.36 ± 1.21a | 16.64–18.99 | |
| Live understorey | 11.77 ± 2.26b | 7.02–17.17 | |
| Growth forms within live understorey | |||
| Shrubs | 12.10 ± 1.84a | 9.13–14.91 | |
| Graminoids | 11.80 ± 3.68a | 7.02–17.17 | |
| Ferns | 11.21 ± 1.59a | 8.08–13.72 | |
Means and standard deviations of heat yields for each of the fuel classes averaged over all seasons. Different superscript letters (a,b) indicate statistically significant differences between each class (P < 0.05) within the larger group.
However, heat yields of understorey fuels grouped by growth form were not significantly different. This is due to the large variation in the heat yields of individual species within each growth form. Differences in heat yield between species within the same growth form were especially large for graminoids, with the largest difference being 7.98 MJ kg−1 in spring between Tetrarrhena juncea and Gahnia sieberiana, or around 68% of the mean value for the graminoid group (Fig. 3). The in-group differences between the ferns were smaller, with a relatively consistent difference between the lowest heat yield (Blechnum wattsii) and the highest (Dicksonia antarctica) of around 3.93 MJ kg−1, or 35% of the mean value of the fern group. The heat yields of some shrub species were similar, with an average of 4.60 MJ kg−1 difference between the lowest (Hedycarya angustifolia) and highest members (various sp.), or approximately 38% of the mean heat yield. Within the canopy group, heat yields differed by 2.05 MJ kg−1 or about 15% of the group mean, which were similar to the magnitude of difference among dead fuels (2.17 MJ kg−1 or 12% of group mean).
Dead fuels had the greatest seasonal fluctuation in heat yield. As a group, their mean heat yields increased by 1.97 MJ kg−1 from spring to early summer and decreased by 2.00 MJ kg−1 from late summer to autumn (Fig. 3). By comparison, the mean heat yield of live fuels only had a slight increase (0.79 MJ kg−1) from early summer to late summer, and a decrease (−1.02 MJ kg−1) from late summer to autumn.
However, among all the fuel groups, the most dynamic were the graminoids, with the largest change in heat yields being a decrease of 3.35 MJ kg−1 from late summer to autumn. Both graminoids species had similar seasonal dynamics, with large declines in heat yields from late summer to autumn, followed by a small peak in winter before declining in spring (Fig. 3).
Heat yield components driving seasonal dynamics
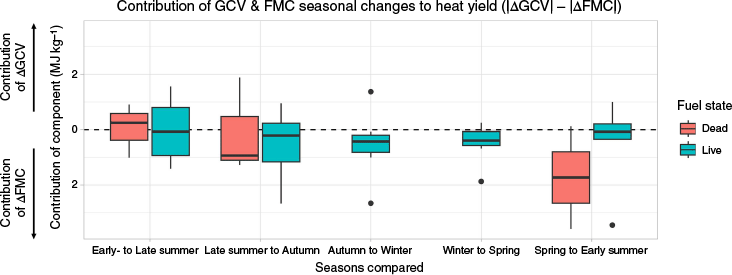
Dynamics in FMC had the greatest effect on heat yield across seasons, followed by GCV (Fig. 4). Hydrogen composition was the least dynamic variable, changing by less than 1% across all seasons for all fuels (Fig. 5). FMC dynamics was the primary driver of heat yield variation for dead fuels which are highly responsive to atmospheric conditions, while the contribution of FMC to heat yields of live fuels remained relatively constant throughout the season (Fig. 5). However, Tetrarrhena juncea is a notable exception, showing substantial variation in FMC throughout the year. Despite the strong influence of FMC on fluctuations in heat yield, heat yields of even the wettest fuels were still strongly positive. Average GCV had a slightly greater contribution to heat yield than FMC in dead fuels from early summer to late summer, outweighing FMC by about 0.05 MJ kg−1 (Fig. 4).
The contribution towards heat yield of seasonal changes in gross calorific value (ΔGCV) and fuel moisture content (ΔFMC) for live and dead fuels, calculated as the difference between the absolute values of ΔGCV and ΔFMC. The relative contribution of FMC and GCV is a magnitude, hence the non-negative axis.
Components of fuel heat yield: Gross calorific value (orange), Heat yield (red), Vaporisation of water (FMC) = heat of vaporisation for FMC (cyan) and Vaporisation of water (Hd) = heat of vaporisation for the water of reaction from fuel hydrogen content (dark blue). Dotted line is 18.6 MJ kg−1, the currently used heat yield value in the Australian Fire Danger Rating System.
Discussion
Heat yields vary between species and seasons
The heat yields of different co-occurring species were highly variable. There were substantial differences in heat yields between species, as well as between seasons. The graminoids Tetrarrhena juncea and Gahnia sieberiana had the largest seasonal fluctuations in heat yield, while the treefern Dicksonia antarctica had the smallest seasonal variation.
The heat yield values we measured were higher than similar studies in Australian forests, with the heat yield of A. dealbata being more than double of what was reported in Cawson et al. (2023) and significantly higher than those reported by Añón et al. (1995) for understorey vegetation in Spain. This was due to the measurement of heats of combustion of fully hydrated fuels in Cawson et al. (2023), and high moisture contents as well as higher hydrogen content values in Añón et al. (1995). Though the species sampled were from taxonomically diverse groups, the heat yields of ferns appear to be consistently the lowest of all fuels in these studies.
Our results suggest that variations in species composition in forest stands, or the seasonal timing of a fire, could contribute to differences in fireline intensity. Wet sclerophyll forests can vary substantially in species composition – dominated by grasses, ferns, or an assemblage of shrubs over a ridge or further downslope (Ashton 1976). Forests with successional states in response to different fire histories and time since fires may have drastically different species composition with implications for the heat yield of a given forest stand. Similarly, seasonal differences in heat yield between a late summer and autumn for Tetrarrhena juncea could contribute to differences in fireline intensity for forests dominated by this species. The differing trends in heat yields between shrub species also suggests there may be a need for species-specific inputs for predicting fireline intensities when a particular species is highly dominant. Depending on the relative abundance of live fuels and on season, there may be a substantial difference between the default heat yield value currently used for certain ecosystems and the actual heat yields of fuels.
Assuming the live- and dead fuel loads are equal in a wet forest scrub, the averaged heat yield would be around 14.8 MJ kg−1 (Table 3). The default value for forest is 18.6 MJ kg−1, and the difference of 3.8 MJ kg−1 is comparable to a 20.4% decrease in the fireline intensity, fuel load consumed, or rate of spread terms. For comparison, a similar percentage reduction in the Australian Fire Danger Rating System’s (AFDRS's) fuel availability term corresponds to a reduction in drought factor (DF) from 10 to 6.3, given Keetch Byram Drought Index (KBDI) = 203.2 and Wind Reduction Factor (WRF) = 5. Alternatively, given a more reasonable KBDI = 125 and WRF = 5, DF reduces from 10 to 8.9. With an increased focus on spatially mapping potential fireline intensities in Australia (Leonard et al. 2023), more accurate heat yield values may be important to consider.
Understory live fuels have lower and more variable heat yields than dead fuels
Heat yields for live fuels were significantly lower and more variable than for dead fuels. Furthermore, the understorey live fuel heat yields were substantially lower than default values used in some fire behaviour models (Van Wagner et al. 1992; Cheney et al. 2012). In contrast, the difference between the live canopy and dead fuel heat yields and the default value were less substantial. The variability in heat yields for understorey live fuels can be attributed to significant differences in FMC between species, and to a smaller extent differences in their gross calorific values.
Due to the significant difference in the heat yields of live understorey and dead fuels, it may be necessary to separately account for the live understorey heat yields in forests where they are abundant. A possible approach is to scale heat yields based on fuel loads, where the heat yield of individual components contributes to a part of the heat yield of a forest stand. A similar approach through partitioning reaction intensity has been explored in the National Fire Danger Rating System (Fujioka et al. 2021). Reaction intensity (heat released per unit area of flame front) calculated using the reaction velocity, multiplied by fuel load, low heat content (net calorific value), and mineral- and moisture damping coefficients (Rothermel 1972). Reaction velocity is dependent on fuel characteristics (e.g. surface area, packing ratio), which may vary considerably between fuel types. Therefore, to account for the different properties of fuels, Fujioka et al. (2021) used a weighted sum of the energy release of each fuel component to calculate reaction intensity of the forest as a whole. Although they did not account for differences in calorific values in the partitioning of reaction intensity, its inclusion may be warranted given the variability in calorific values.
The significant difference between the heat yields of understorey and overstorey live fuels highlights the importance of accounting for the vertical distribution of fuels and their heat yields. In fire behaviour models where fuel strata are progressively included in a fire based on flame height (Cheney et al. 2012), it may be useful to account for the average heat yield of each fuel strata. For example, an understorey comprising abundant live fuel is likely to have a lower heat yield than if it was primarily made up of dead fuels. The higher heat yield of canopy fuels compared to live understorey fuels may be a factor contributing to the very high intensity of a crown fires.
Seasonal variation in heat yield was primarily driven by FMC dynamics
Fuel moisture dynamics were the primary driver of seasonal variations in heat yields, but fluctuations in fuel moisture were not the only driver. Peaks and troughs in heat yield were driven by fluctuations in both fuel moisture and calorific value, which at times reinforced each other and at other times counteracted one another. This contrasts with Philpot et al. (1971) who found fluctuations in moisture and calorific value offset each other during the summer season. We found large differences between the moisture- and calorific- dynamics between species, meaning there was no consistent trend overall.
Although the results show a relatively small contribution of FMC to the seasonal variability of heat yields for live fuels, its effect is likely to be greater during drought. FMC of the live fuels are likely to drop significantly in response to drought (Nolan et al. 2022), leading to greater availability of the calorific value for release as heat during combustion. The calorific value of live fuels are also likely to change in response to drought stress, variation in photosynthetic rates and changes in the composition of plant organs (Hnilička et al. 2015). Therefore, the dynamics of FMC and GCV on heat yield in response to drought stress is an important direction for further research on this topic.
Incomplete realisation of heat yield during forest fire
The use of calorific values as a measure of potential heat release is not representative of field conditions. Byram’s (1959) heat yield takes into account other sources of heat losses, including the separation of bound water and radiative losses. Not all of the available heat in a fuel is released during flaming combustion, with smouldering combustion releasing significantly less heat at the fire front than flaming combustion (Rein 2016). Factors such as fuel particle size, composition, arrangement, oxygen concentration and heat flux contribute to heat release during combustion (Shafizadeh and Bradbury 1979; Yang et al. 2024). These factors mean that species like Tetrarrhena juncea with the lowest heat yield can still be highly flammable due to its structure (Fogarty 1993).
Fuel moisture is another important factor for combustion and realisation of the heat yield. Some heat is used to separate bound water from wood and evaporate water, which can substantially reduce the heat yield of fuels depending on their moisture content (Byram 1959). This heat requirement may be significant enough to reduce or even suppress flame-front energy release (May et al. 2019). Furthermore, when moisture in fuels is vaporised during combustion, the resulting water vapour dilutes the pyrolysates, making it more difficult to generate a flammable mixture (Janssens 2005; McAllister et al. 2012). Additionally, water uptake into foliage continues during burning (Hoffmann et al. 2021), which may further increase the amount of water vapour released, reducing realised heat yield.
Fire behaviour models have attempted to account for the reductions in heat yield in various ways. Rothermel’s (1972) reaction intensity approach uses lower heat content (net calorific value), further reduced by moisture and mineral content to calculate heat yield in terms of energy release per area. This better accounts for the effect of moisture, as fuels above their ‘moisture of extinction’ are unlikely to burn. Likewise, the AFDRS applies a ‘fuel availability’ term (Duff et al. 2018), that reduces the proportion of fuel load available to burn, but does not adjust the heat yield.
Instead of accounting for theoretical heat losses in fuel heat release during combustion, some have used more empirical approaches. The heat of combustion as measured with oxygen bomb calorimeters may not accurately represent burning under field conditions, where oxygen may be a factor limiting the extent of combustion. Cone calorimeters burn fuels in air and have long been considered more accurate measures of the heat release of non-forest fuels (Janssens 2002), and these methods have been adopted for vegetative fuels (Weise et al. 2005). Studies using cone calorimeters have been able to compare the ‘effective heat of combustion’ – the amount of heat produced per unit mass lost – against gross calorific value (Madrigal et al. 2011), as well as the contribution of forest fuels to the heat release of an advancing flame front (Melnik et al. 2022). These measures of fuel heat release reported from cone calorimeter experiments were found to be consistently lower than gross calorific values measured from bomb calorimetry (Mišić et al. 2024). Methods employing cone calorimetry are able to capture the completeness of combustion, as well as the effect of fuel structure (Madrigal et al. 2009), both of which reduce fuel heat release. On the other hand, these factors are not accounted for in typical measurements of gross calorific values as measured with bomb calorimetry. Further research into these new methods is important for better representation of heat release during fire, and these dynamic heat yield estimates should gradually be adopted to better reflect fuel flammability.
Conclusions
Heat yields of common fuels in wet sclerophyll forest, and their seasonal variations were determined. There were significant differences in the heat yields of live and dead fuels, as well as between understorey live fuels and overstorey live fuels. However, high variability in heat yield between species meant, the heat yields of growth forms (shrubs, ferns, graminoids) were not significantly different. There were substantial differences between the heat yields of live fuels and large seasonal variations for some fuels, with FMC being the primary driver of seasonal change.
Observed inter-species differences in heat yield and seasonal dynamics for understory live fuel suggest that heat yields are more variable than previously assumed. Therefore, using a variable value for heat yield for forests with substantially different compositions could improve predictions of fireline intensity across the landscape. Significant differences between understorey live fuels and canopy fuels also suggest it may be beneficial to vary the heat yield input by strata in fire models that include the vertical distributions of fuels. As live fuels are the primary store of moisture, which is the main component reducing heat yield, changes in their moisture content under drought is likely to significantly increase heat yields.
There are other factors that may further reduce actual heat yields due to incomplete realisation during forest fires, often referred to as incomplete combustion. Common values for heat yields used in fire behaviour models may be overestimating fireline intensity due to incomplete realisation of heat yields, but this does not suggest that fuels themselves are any less flammable. Both theoretical and empirical approaches have been used to account for these further reductions, but more research is needed to resolve the uncertainty around heat yields in Australian forests.
Data availability
The data collected in this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Gary Sheridan is an Associate Editor of International Journal of Wildland Fire but was not involved in the peer review or any decision-making process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
This research was funded by an Australian Government Research Training Program (RTP) scholarship, as well as the Integrated Forest Ecosystem Research (IFER) program, which is a joint research agreement between the University of Melbourne and the Victorian Department of Energy, Environment and Climate Action.
Acknowledgements
This research was conducted on Wurundjeri land, and the authors pay our respect to their Elders past and present. We thank Joe Eliades at the Monash Metabolic Phenotyping Platform and Anthony De Girolamo at the Monash Department of Chemical and Biological Engineering for their calorimetry and elemental analysis services respectively. We also thank Dr Alex Filkov and Julio Najera Umana at the University of Melbourne Creswick for their assistance with calorimetry. Finally, we also thank the three anonymous reviewers for their detailed comments and suggestions for the manuscript.
References
Agee JK, Wright CS, Williamson N, Huff MH (2002) Foliar moisture content of Pacific Northwest vegetation and its relation to wildland fire behavior. Forest Ecology and Management 167, 57-66.
| Crossref | Google Scholar |
Alexander ME (1982) Calculating and interpreting forest fire intensities. Canadian Journal of Botany 60(4), 349-357.
| Crossref | Google Scholar |
Alexander ME (2000) Fire behaviour as a factor in forest and rural fire suppression. Forest Research Bulletin (No. 197, 5). iv + 28 pp. Available at https://www.ruralfireresearch.co.nz/__data/assets/pdf_file/0009/63945/31037-FireBehaviourSuppression.pdf
Añón JAR, López FF, Castiñeiras JP, Ledo JP, Regueira LN (1995) Calorific values and flammability for forest wastes during the seasons of the year. Bioresource Technology 52(3), 269-274.
| Crossref | Google Scholar |
Ashton DH (1975) Studies of litter in Eucalyptus regnans forests. Australian Journal of Botany 23(3), 413-433.
| Crossref | Google Scholar |
Ashton DH (1976) The development of even-aged stands of Eucalyptus regnans F. Muell. in Central Victoria. Australian Journal of Botany 24, 397-414.
| Crossref | Google Scholar |
Atwater WO, Snell JF (1903) Description of a bomb-calorimeter and method of its use. Journal of the American Chemical Society 25(7), 659-699.
| Crossref | Google Scholar |
BoM (2025) Bureau of Meteorology Climate Data Online. Available at http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=&p_stn_num=086094
Brown TP, Hoylman ZH, Conrad E, Holden Z, Jencso K, Jolly WM (2022) Decoupling between soil moisture and biomass drives seasonal variations in live fuel moisture across co-occurring plant functional types. Fire Ecology 18(1), 14.
| Crossref | Google Scholar |
Burton JE, Cawson J, Noske P, Sheridan G (2019) Shifting states, altered fates: divergent fuel moisture responses after high frequency wildfire in an obligate seeder Eucalypt forest. Forests 10(5), 436.
| Crossref | Google Scholar |
Cawson JG, Burton JE, Pickering BJ, Demetriou V, Filkov AI (2023) Quantifying the flammability of living plants at the branch scale: which metrics to use? International Journal of Wildland Fire 32, 1404-1421.
| Crossref | Google Scholar |
Cheney NP, Gould JS, Lachlan Mccaw W, Anderson WR (2012) Predicting fire behaviour in dry eucalypt forest in southern Australia. Forest Ecology and Management 280, 120-131.
| Crossref | Google Scholar |
Dickinson KJM, Kirkpatrick JB (1985) The flammability and energy content of some important plant species and fuel components in the forests of southeastern Tasmania. Journal of Biogeography 12(2), 121-134.
| Crossref | Google Scholar |
Duff TJ, Cawson JG, Cirulis B, Nyman P, Sheridan GJ, Tolhurst KG (2018) Conditional performance evaluation: using wildfire observations for systematic fire simulator development. Forests 9(4), 189.
| Crossref | Google Scholar |
Frederick DJ, Madgwick HAI, Jurgensen MF, Oliver GR (1985) Dry matter content and nutrient distribution in an age series of Eucalyptus regnans plantations in New Zealand. New Zealand Journal of Forestry Science 15(2), 158-179.
| Google Scholar |
Fujioka FM, Weise DR, Chen SC, Kim SH, Kafatos MC (2021) Reaction intensity partitioning: a new perspective of the National Fire Danger Rating System Energy Release Component. International Journal of Wildland Fire 30(5), 351-364.
| Crossref | Google Scholar |
Gould JS, Lachlan McCaw W, Phillip Cheney N (2011) Quantifying fine fuel dynamics and structure in dry eucalypt forest (Eucalyptus marginata) in Western Australia for fire management. Forest Ecology and Management 262(3), 531-546.
| Crossref | Google Scholar |
Hnilička F, Hniličková H, Hejnák V (2015) Use of combustion methods for calorimetry in the applied physiology of plants. Journal of Thermal Analysis and Calorimetry 120(1), 411-417.
| Crossref | Google Scholar |
Hoffmann WA, Rodrigues AC, Uncles N, Rossi L (2021) Hydraulic segmentation does not protect stems from acute water loss during fire. Tree Physiology 41(10), 1785-1793.
| Crossref | Google Scholar | PubMed |
Hughes MK (1971) Seasonal calorific values from a deciduous woodland in England. Ecology 52(5), 923-926.
| Crossref | Google Scholar |
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. International Journal of Wildland Fire 18(1), 116-126.
| Crossref | Google Scholar |
Leonard J, Arena A, Opie K, Shrestha DL, Song Y, Swedosh W, Leighton B, Tang H, Yu J, Sarker C, Robertson D, Gomes Da Cruz M, Newnham G (2023) ‘National Bushfire Intelligence Capability (NBIC) Stage 1 Collection.’ (CSIRO) 10.25919/AVJ5-SS75
Madrigal J, Hernando C, Guijarro M, Díez C, Marino E, De Castro AJ (2009) Evaluation of forest fuel flammability and combustion properties with an adapted mass loss calorimeter device. Journal of Fire Sciences 27(4), 323-342.
| Crossref | Google Scholar |
Madrigal J, Guijarro M, Hernando C, Díez C, Marino E (2011) Effective heat of combustion for flaming combustion of Mediterranean forest fuels. Fire Technology 47(2), 461-474.
| Crossref | Google Scholar |
Matthews S (2010) Effect of drying temperature on fuel moisture content measurements. International Journal of Wildland Fire 19(6), 800-802.
| Crossref | Google Scholar |
May N, Ellicott E, Gollner M (2019) An examination of fuel moisture, energy release and emissions during laboratory burning of live wildland fuels. International Journal of Wildland Fire 28(3), 187-197.
| Crossref | Google Scholar |
McAllister S, Grenfell I, Hadlow A, Jolly WM, Finney M, Cohen J (2012) Piloted ignition of live forest fuels. Fire Safety Journal 51, 133-142.
| Crossref | Google Scholar |
McArthur AG, Cheney NP (2015) The characterization of fires in relation to ecological studies, with an Introduction by Neil Burrows. Fire Ecology 11(1), 3-9.
| Crossref | Google Scholar |
Melnik OM, Paskaluk SA, Ackerman MY, Melnik KO, Thompson DK, McAllister SS, Flannigan MD (2022) New in-flame flammability testing method applied to monitor seasonal changes in live fuel. Fire 5(1), 1.
| Crossref | Google Scholar |
Mišić N, Protić M, Raos M, Vukadinović A (2024) A literature review of key findings in fundamental forest fire research. Facta Universitatis, Series: Working and Living Environmental Protection 037. 10.22190/FUWLEP240213003M
Nolan RH, Foster B, Griebel A, Choat B, Medlyn BE, Yebra M, Younes N, Boer MM (2022) Drought-related leaf functional traits control spatial and temporal dynamics of live fuel moisture content. Agricultural and Forest Meteorology 319(March), 108941.
| Crossref | Google Scholar |
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11(5), 1633-1644.
| Crossref | Google Scholar |
Penney G, Habibi D, Cattani M (2019) Firefighter tenability and its influence on wildfire suppression. Fire Safety Journal 106, 38-51.
| Crossref | Google Scholar |
Rivera JdD, Davies GM, Jahn W (2012) Flammability and the heat of combustion of natural fuels: a review. Combustion Science and Technology 184(2), 224-242.
| Crossref | Google Scholar |
Shafizadeh F, Bradbury AGW (1979) Smoldering combustion of cellulosic materials. Journal of Thermal Insulation 2(3), 141-152.
| Crossref | Google Scholar |
Slijepcevic A, Anderson WR, Matthews S, Anderson DH (2018) An analysis of the effect of aspect and vegetation type on fine fuel moisture content in eucalypt forest. International Journal of Wildland Fire 27(3), 190-202.
| Crossref | Google Scholar |
Volkova L, Weiss Aparicio AG, Weston CJ (2019) Fire intensity effects on post-fire fuel recovery in Eucalyptus open forests of south-eastern Australia. Science of The Total Environment 670, 328-336.
| Crossref | Google Scholar | PubMed |
Weise DR, White RH, Beall FC, Etlinger M (2005) Use of the cone calorimeter to detect seasonal differences in selected combustion characteristics of ornamental vegetation. International Journal of Wildland Fire 14(3), 321-338.
| Crossref | Google Scholar |
Yan P, Xu L, He N (2018) Variation in the calorific values of different plants organs in China. PLoS One 13(6), e0199762.
| Crossref | Google Scholar | PubMed |
Yang J, Wang H, Wang R, Xu J, Huang W, Hu Y (2024) Experimental and theoretical study on the smoldering combustion of size-fractioned forest duff particles. International Journal of Heat and Mass Transfer 231, 125883.
| Crossref | Google Scholar |
Yebra M, Scortechini G, Adeline K, Aktepe N, Almoustafa T, Bar-Massada A, Beget ME, Boer M, Bradstock R, Brown T, Castro FX, Chen R, Chuvieco E, Danson M, Değirmenci CÜ, Delgado-Dávila R, Dennison P, Di Bella C, Domenech O, Féret J-B, Forsyth G, Gabriel E, Gagkas Z, Gharbi F, Granda E, Griebel A, He B, Jolly M, Kotzur I, Kraaij T, Kristina A, Kütküt P, Limousin J-M, Martín MP, Monteiro AT, Morais M, Moreira B, Mouillot F, Msweli S, Nolan RH, Pellizzaro G, Qi Y, Quan X, Resco de Dios V, Roberts D, Tavşanoğlu Ç, Taylor AFS, Taylor J, Tüfekcioğlu İ, Ventura A, Younes Cardenas N (2024) Globe-LFMC 2.0, an enhanced and updated dataset for live fuel moisture content research. Scientific Data 11(1), 332.
| Crossref | Google Scholar | PubMed |