Prenatal establishment of the foal gut microbiota: a critique of the in utero colonisation hypothesis
Kirsty L. Mols A C , Gry B. Boe-Hansen
A C , Gry B. Boe-Hansen  B , Deirdre Mikkelsen A , Wayne L. Bryden A and A. Judy Cawdell-Smith A
B , Deirdre Mikkelsen A , Wayne L. Bryden A and A. Judy Cawdell-Smith A
A Equine Research Unit, School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
B School of Veterinary Science, The University of Queensland, Gatton, Qld 4343, Australia.
C Corresponding author. Email: k.mols@uq.edu.au
Animal Production Science 60(18) 2080-2092 https://doi.org/10.1071/AN20010
Submitted: 7 January 2020 Accepted: 12 June 2020 Published: 23 November 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Bacteria colonisation of the foal’s gastrointestinal tract (GIT) is a critical developmental stage, effecting subsequent immunological and health outcomes. It has long been thought that the equine fetus develops in a sterile intrauterine environment and GIT colonisation commences at birth. Research now suggests that bacteria isolated from amniotic fluid are the initial colonisers of the fetal GIT, and exposure to the dam’s microbiota and the external environment during birth provide supplementary colonisation. This in utero colonisation hypothesis has only recently been examined in the horse and microbiota were detected in the amniotic fluid and meconium of healthy equine pregnancies. This review highlights the possible colonisation routes of these bacteria into the fetal compartments and examines their likely origins from the existing maternal microbiome. However, the current data describing the amniotic microbiota of the horse are limited and there is a need for research to fill this gap. Understanding the significance of intrauterine microbes for foal GIT colonisation may provide strategies to improve neonatal health.
Keywords: amniotic fluid, gastrointestinal tract, meconium, sterile womb.
Introduction
Microbiota colonisation of the intestinal tract, during the early stages of neonatal life, plays a critical role in the immunological and physiological development of animals and humans (Schoster et al. 2013). In the horse, disrupted, imbalanced or incomplete colonisation has a major impact on foal health and can result in infectious gastrointestinal tract (GIT) diseases, which cause significant costs to the equine industry (Wohlfender et al. 2009). This typically results in neonatal losses, treatment expenses and a decrease of potential future earnings in the sale ring, on racetracks or in the breeding industry (Hong et al. 1993). The economic repercussions involved in attempting to improve the health of foals from GIT diseases is also considerable (Rose et al. 2018). Infections can result from prenatal exposure in the dam, or postnatally due to pathogenic organisms in the environment (Foote et al. 2012). The outcome of pathogen exposure is highly dependent on the immunological status of the neonate and juvenile animal (Torrazza and Neu 2011). Therefore, it is important to understand how and when these initial resident microbes commence colonisation of the GIT. This will allow the development of strategies to promote adequate immunological development. Moreover, it will permit delineation of the role of the microbiota in equine behaviour and temperament, an area of significant interest in human health (Borre et al. 2014; Aatsinki et al. 2019; Zhuang et al. 2019), with important implications for farm animal behaviour and welfare (Kraimi et al. 2019).
The longstanding ‘sterile womb paradigm’ describes neonates from normal pregnancies being born devoid of microbiota, having developed in a sterile intrauterine environment including the placenta (Costa et al. 2016). The colonisation of the foal and the GIT is thought to commence at birth from exposure to the mother and the external environment (Aagaard et al. 2014). Research is now challenging this view, with the detection of microbial communities in the human placenta, amniotic fluid and neonatal meconium of apparently normal pregnancies (Cao et al. 2014; Wassenaar and Panigrahi 2014; Panelli et al. 2018). Traditionally, the presence of bacteria in the placenta was thought to be pathological, indicating an intrauterine infection; however, bacteria have also been discovered in humans in the absence of histological infection over the past few decades (Prince et al. 2015). Thus, there is increasing support for the hypothesis that the initial microbial colonisation commences in utero. The role that placental microbes play in establishing the neonate intestinal microbiota has not yet been established, and the timing and route of this initial colonisation remain unclear. However, it is well established that intestinal microbes play an important role in the development of postnatal immunity, and this fundamental knowledge gap could be vital in further understanding the developmental origin of health and disease (Rehbinder et al. 2018). Moreover, it is likely that the bacteria in the amniotic fluid modulate the fetal immune system, thus preparing the neonate for the external environment outside the uterus (Hemberg et al. 2015). The focus of this review is to challenge traditional thinking on microbial colonisation of the neonatal GIT of the foal, by examining the in utero colonisation hypothesis, especially how the amniotic microbiota are established and their likely origins from the microbiota of the mare.
The traditional view of foal GIT colonisation
Foals, like all mammalian species, were thought to develop in a sterile intrauterine environment. This sterile womb paradigm was first suggested in 1885 by Theodor Escherich who declared human meconium to be free of viable bacteria. Further independent studies in 1927 and 1934 found the majority (62%, n = 150) of human meconium samples from healthy pregnancies to be free of both aerobic and anaerobic bacteria, apparently confirming the sterile placenta hypothesis (Burrage 1927; Hall and O’Toole 1934; Perez-Muñoz et al. 2017).
According to this traditional view, the foal’s GIT is first colonised during birth, through exposure to the dam’s vaginal, faecal and skin microbiota, as well as the immediate surrounding environment. Colonisation of the GIT is then further evolved by nursing and behaviours such as coprophagy (Charbonneau et al. 2016; Costa et al. 2016; Alipour et al. 2018; Costa and Weese 2018).
Studies that established the sterile womb paradigm employed traditional culture-based methods and microscopy (Burrage 1927; Hall and O’Toole 1934; Perez-Muñoz et al. 2017). After these early studies, microbiological research on human meconium stopped for over 30 years, until the sterile womb was challenged by Jiménez et al. (2008), who reported 100% (n = 21) of human meconium samples positive for bacterial growth. This sparked additional research, and recent studies have now employed modern sequencing technologies to further develop the idea that neither the placenta nor the fetal environment are sterile (Perez-Muñoz et al. 2017). The relevance and accuracy of both the culture-based and modern sequencing techniques are currently vigorously debated. The argument is advanced that positive samples from healthy pregnancies are the result of sampling contamination. However, later in this review, it will be established that microbiota detected in amniotic fluid and meconium do not appear to originate from the mother’s reproductive tract or external environment, which would be the expected origin of contamination. Instead the evidence suggests an internal mother–fetus microbiological interaction. This human microbiome research implies that in utero colonisation of the fetal GIT is likely to occur in all mammalian species, including the horse.
The challenging hypothesis: in utero colonisation of the foal
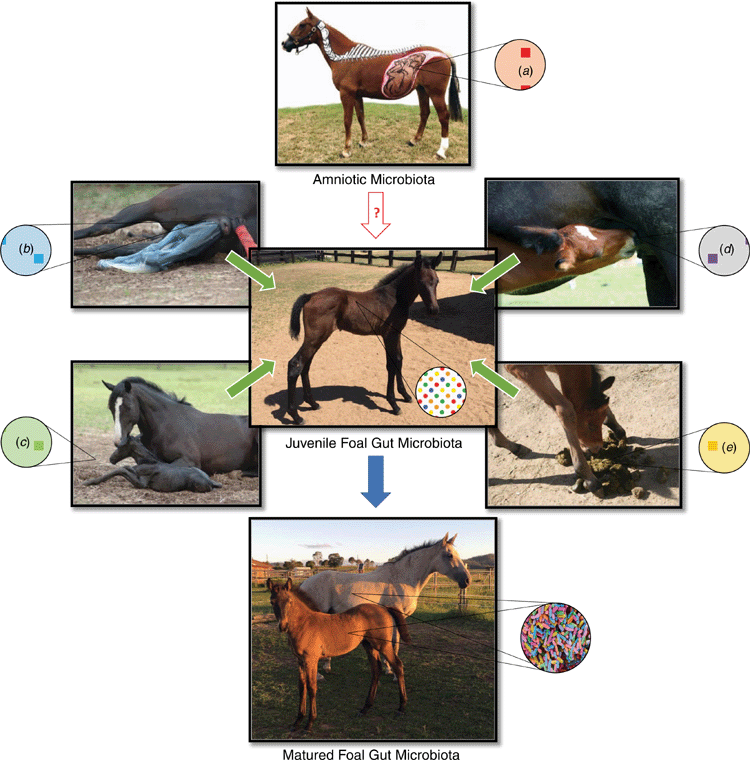
Bacteria have now been detected in foal first pass meconium, suggesting that the GIT colonisation process may commence in utero, and is then supplemented by, instead of originating from, the exposure to further microbes during birth and later life (Jacquay et al. 2018; Quercia et al. 2019), as shown in Fig. 1. This hypothesis has been strongly debated, with very few studies questioning this theory in horses, although in utero colonisation is now gradually being accepted in human microbiome research (Aagaard et al. 2014; Collado et al. 2016; Chu et al. 2017). Fetal colonisation of the GIT plays a critical role in health development and disease prevention in young foals (Costa et al. 2016). Developing a mechanistic understanding of this process by establishing whether bacterial colonisation commences in utero, could fundamentally change our understanding of foal health and, subsequently, influence our management practises of both pregnant mares and newborn foals.
Foal development in utero
For in utero colonisation to be initiated, the amniotic fluid microbiota must interact with the developing fetus. Two stages of development must be established to determine the timing of initial colonisation. These include, first, the commencement and development of the fetal GIT and, second, the point in which amniotic fluid first enters the fetal GIT.
Fetal GIT development
The development of the foal’s GIT commences in utero during gastrulation, beginning as early as Day 12 after fertilisation (Gaivão et al. 2014). Gastrulation is the first major morphogenetic change the embryo undergoes and is the stage at which simple cells of the conceptus are transitioned into more complex layers consisting of the major tissue endoderm, mesoderm and ectoderm. This process gives rise to the primitive intestine and it is during organogenesis that the inner endoderm develops into the complete fetal digestive tract and its associated organs, including the pancreas and liver. The primitive intestine of the fetus is well defined by Day 25 of gestation, and completion of organogenesis occurs after Day 40, but the GIT is still immature at this stage (Platt 1984; Franciolli et al. 2011). Although the primitive fetal GIT is developed, in utero colonisation is more likely to commence when the physiological process of fetal swallowing begins.
Fetal swallowing
Fetal swallowing is a key component of further GIT maturation and allows the passage of nutrient-rich amniotic fluid into the lumen of the primitive GIT. The commencement of fetal swallowing is not well defined in the horse; extrapolations can be made from human development to horses. First, for fetal swallowing to begin, organogenesis must be complete, which occurs after Day 40 of gestation (Platt 1984; Franciolli et al. 2011). Second, fetal swallowing of amniotic fluid contributes to the accumulation of meconium, and, in humans, it is known that meconium begins to form as early as the 12th week, coinciding with the end of the first trimester (Stinson et al. 2018; Woźniak et al. 2018). In horses, the transition from first to second trimester is ~14 weeks of gestation; therefore, it can be assumed that fetal swallowing commences between Day 40 and Day 100 of gestation. Amniotic fluid volume increases in the mare from Week 8 of gestation (Whitwell and Jeffcott 1975), further indicating that it is likely that fetal swallowing and in utero GIT colonisation commence during this time frame.
The process of fetal swallowing plays a significant role in the growth and development of the fetus. It has been demonstrated in pigs, sheep and rabbits that preventing amniotic fluid swallowing by surgical oesophageal ligation leads to inhibition of gastric development and acid secretion (Mulvihill et al. 1986; Trahair and Sangild 2000; Sangild et al. 2002; Cellini et al. 2006). However, infusion of amniotic fluid at the same rate returns gastric weight, gastric acid secretion, and serum gastrin concentrations back to normal (Mulvihill et al. 1986). Although this has yet to be demonstrated in horses, the same relationships are likely to occur in the equine fetus. If amniotic microbiota are initially introduced to the fetal GIT at the commencement of fetal swallowing, it is possible that they influence the growth and development of the fetus.
As mentioned previously, fetal swallowing of amniotic fluid is considered a major contributor to the formation and accumulation of meconium in the fetal GIT throughout pregnancy (Costa et al. 2016). The in utero colonisation hypothesis has been developed from the recent detection of bacteria in foal meconium and their suggested amniotic fluid origin (Jacquay et al. 2018; Quercia et al. 2019), further supporting the idea that fetal swallowing commences in utero GIT colonisation.
Amniotic microbiota
The presence of bacteria in the amniotic fluid and placenta were thought to be associated only with infection and pathology. The investigation into the presence of amniotic microbiota in healthy pregnancies was prompted after the detection of non-pathogenic bacteria in the meconium of newborn foals that appeared to originate from the amniotic fluid (Hong et al. 1993; Foote et al. 2012; Wassenaar and Panigrahi 2014; Hemberg et al. 2015; Quercia et al. 2019). This novel area of research in equine science has now detected bacteria and other microbes in amniotic fluid from healthy equine pregnancies, indicating that microorganisms may indeed exist in the amniotic fluid, thereby negating the concept of a sterile womb (Hemberg et al. 2015; Quercia et al. 2019).
Hemberg et al. (2015) examined 46 amniotic fluid samples from foaling Standardbred mares on one stud farm in Sweden. Bacterial growth was found in amniotic fluid samples collected at parturition from 26 pregnancies, resulting in both healthy and unhealthy foals, as defined by the foal’s ability to stand unaided within 2 h (normal) or requiring more than 2 h respectively. The group requiring more than 2 h to stand also had more complications during foaling and postpartum than did the healthy group. Pure cultures were isolated from 33% of the amniotic fluid samples, mixed growth of no more than two different bacteria were isolated from 24% of the samples, and 43% of the samples were negative for bacterial growth. Of the positive cultures, coagulase-negative staphylococci were the bacteria most frequently isolated, occurring in 54% of all positive samples. Other bacteria isolated from the amniotic fluid included haemolytic streptococci, and isolates identified as belonging to Pantoea spp., Micrococcus spp., Escherichia coli and Acinetobacter spp. However, the main limitation of these findings was the use of culture-dependent analysis, as this approach would have highlighted the dominant bacteria capable of growing under the selective culture conditions, thus biasing and limiting in-depth analysis of the whole microbiota present. Interestingly, there were no significant differences between the types of bacterial species detected in the amniotic fluid samples collected from the healthy and unhealthy foal groups (Hemberg et al. 2015).
Recently, Quercia et al. (2019) utilised next generation sequencing-based techniques to characterise the microbiota present in equine amniotic fluid samples, and in foal meconium samples collected from 13 Standardbred mare–foal pairs. Subsequent bioinformatics showed that bacterial sequences assigned to 32 unique operational taxonomic units (OTUs), were detected in both the amniotic fluid and meconium samples, indicating a strong microbial similarity between the two sample types (Quercia et al. 2019). It agrees with the similarity found in humans by Ardissone et al. (2014), with over 50% of bacteria identified in meconium also being detected in amniotic fluid. The source of the microbiota in the horse is yet to be elucidated; however, the OTUs identified in both the amniotic fluid and foal meconium samples were related to well known opportunistic bacteria, as well as other mare microbiota that are resident to the skin, oral cavity and the gut. For example, Quercia et al. (2019) reported OTUs from bacterial species belonging to the genera Pseudomonas, Sphingomonas, Aerococcus and Streptococcus, all of which are regarded as opportunistic bacteria. OTUs were also related to Staphylococcus and Acinetobacter, which are genera that have been identified as part of the mare’s skin microbiota, and some OTUs were identified to Delftia, a genus that has been detected as a component of the mare’s oral microbial community. Microbes belonging to the phylum Bacteroidetes, and the genera Akkermansia and Faecalibacterium, were identified from the mare’s distal GIT (Quercia et al. 2019).
Quercia et al. (2019) also compared the bacteria of amniotic fluid and meconium with those of the mare’s distal GIT. Among all three environments, six OTUs were shared, namely, Escherichia coli, Enterococcus faecalis, Streptococcus equinus, Agathobacter ruminis, Paraclostridium bifermentas and Acinetobacter iwoffii. No OTUs were exclusively shared between amniotic fluid and mare faeces, whereas 75 OTUs were shared between meconium and the mare’s distal GIT samples, while 32 OTUs were common between meconium and amniotic fluid samples. These results suggest internal transmission routes for the microbes, where the mare’s microbiota are vertically transmitted to the fetus. The shared OTUs of the meconium with both the amniotic fluid and mare’s distal GIT further suggest that each of these sites provides a specific contribution to the overall meconium microbial community (Quercia et al. 2019). These colonisation routes have also been demonstrated in humans (Charbonneau et al. 2016; Pelzer et al. 2017).
One limitation of the study by Quercia et al. (2019) is the inability to differentiate between live and dead bacteria due to the sole use of next generation sequencing, with no culture for viability assessment. However, the culture of live bacteria by Hemberg et al. (2015) confirmed that at least some bacteria present in amniotic fluid are viable. Whether all bacterial DNA detected in amniotic fluid and meconium comes from live cells remains to be determined. Moreover, the source of these bacteria can be questioned. Research in humans and mice has observed distinct similarities between the amniotic microbiota and the mother’s oral microbiota (Fardini et al. 2010; Ardissone et al. 2014). Although Quercia et al. (2019) demonstrated similarities among the amniotic, meconium and the mare’s faecal microbiota, other locations of the dam, such as the oral cavity, were not examined. Since the dam’s oral microbiota would be contributing to the GIT, and, subsequently, the faecal microbiomes, it could also be influencing the amniotic microbiota. However, this hypothesis has not yet been explored in the horse.
These early studies have provided evidence of non-pathological prenatal exposure of the equine fetus to microbes; however, there are some limitations. These findings are limited in scope due to the small number of samples and the alternate culture-dependent and culture-independent analytical methods used. Furthermore, as meconium composition is considered as a record of changes occurring throughout pregnancy (Woźniak et al. 2018), it can be questioned whether sampling just one section of the meconium would provide an accurate analysis of the entire microbiota and the potential GIT colonisation process. Variations between distal versus proximal samples of the meconium could indicate the origin of bacteria in the foal’s GIT. It could potentially demonstrate whether the bacteria enter from swallowed amniotic fluid and colonise the GIT, or from the external environment starting at birth. Nonetheless, the presence of live bacteria and components of bacterial DNA in the amniotic fluid and the meconium casts serious doubt on the application of the sterile womb paradigm to equine pregnancy. This is an area that needs additional research to further explore the relationship between amniotic and meconium microbiota, and to determine how microbes first enter the fetal compartments and, in turn, influence the development of the foal from prenatal into neonatal life.
Proposed routes of colonisation
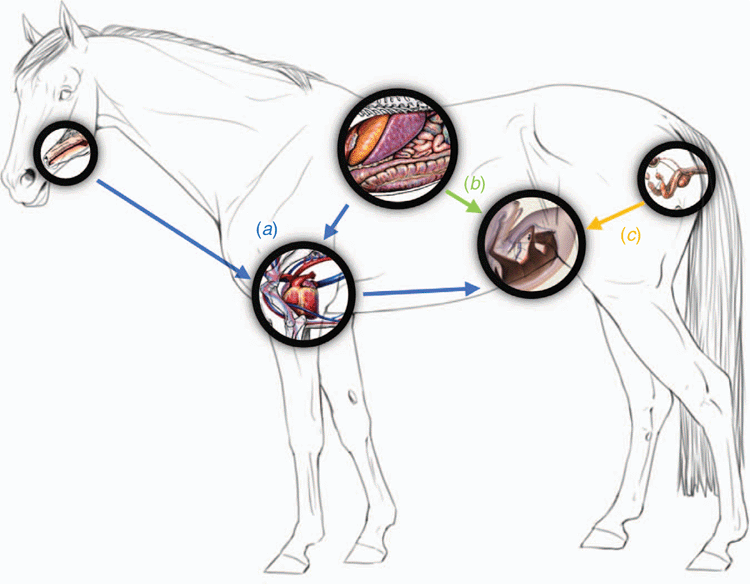
How microbes enter the fetal compartments is not entirely clear. To our knowledge, no research has focused on the colonisation routes in horses, but parallels can be drawn from studies examining humans and other animal models. Microbial taxa that have been found in the placenta, both pathogenic and non-pathogenic, most frequently originate from the mother, and three different colonisation routes have been proposed (Charbonneau et al. 2016; Pelzer et al. 2017), as demonstrated for the horse in Fig. 2. First, the ascension of microorganisms from the vagina and cervix to the uterus. Second, the haematogenous spread from the oral cavity to the placenta, and, third, maternal dendritic cells sampling bacteria from the intestinal lumen, which are internalised, and then transported to the placenta (Pararas et al. 2006; Moreno and Franasiak 2017; Pelzer et al. 2017). Introduction of bacteria can also occur iatrogenically during invasive procedures, such as amniocentesis or percutaneous fetal blood sampling (Pararas et al. 2006). This section will focus on the natural ascension and transfer from mother to offspring of such microorganisms, rather than those introduced via iatrogenic routes, which would have minimal relevance in the horse, since amniocentesis are very rarely being performed in the mare (Schmidt et al. 1991).
The first proposed route, for colonisation of normal amniotic fluid, is ascension of bacteria from the genital tract through the cervix and into the placenta. This route has been well defined for the invasion of pathogens causing intrauterine infections, namely placentitis. The most common pathogens implicated include Streptococcus equi subspecies zooepiddemicus, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa (Hong et al. 1993; Foote et al. 2012). The bacteria then infect various locations including the fetal membranes, umbilical cord, placenta, amniotic fluid and, finally, the fetus (Hitti et al. 2001; Fardini et al. 2010). It is currently unknown whether, in the absence of infection, non-pathogenic bacteria can ascend from the genital tract of the mare into the fetal compartments.
The second proposed route of colonisation is the haematogenous transfer of microbiota from distal locations in the dam, including the oral cavity and the GIT, to the placenta. This idea originated when an increasing number of human studies reported intrauterine infections caused by bacterial species that were not found in the lower genital tract, but instead are commensal species originating from the oral cavity, suggestive of an haematogenous transfer mechanism (Fardini et al. 2010; Han et al. 2010). Some of these species include Fusobacterium nucleatum and Bergeyella, Eikenella, Capnocytophaga spp., and Porphyromonas gingivalis (Fardini et al. 2010; Mesa et al. 2013). Fusobacterium nucleatum and Bergeyella spp. were identified to be associated with preterm birth, with the same clonal species being found in the subgingival plaque of the mother, but not in the supragingival plaque, or vaginal, or rectal, microbiota (Fardini et al. 2010; Han et al. 2010). Other epidemiologic studies have also established a link among oral microbiota, periodontal disease and preterm birth, with Aagaard et al. (2015) demonstrating that the placental microbiota are more homologous with those of the oral cavity, as opposed to the vagina. Mice have been used as human models to confirm that a diverse group of oral bacteria can translocate to the placenta and the meconium of the newborn (Jiménez et al. 2008; Fardini et al. 2010). Further studies have suggested that haematogenous transfer routes are involved with bacterial transmission during pregnancy (Fardini et al. 2010; Mesa et al. 2013; Cao et al. 2014; Aagaard et al. 2015). For example, Gram-negative periodontal pathogens, including their products such as lipopolysaccharides, have been shown to enter the circulatory system and produce low-grade bacteraemia, that could then translocate to the placenta or uterus to influence birth outcomes (Mesa et al. 2013). Another study was able to isolate microorganisms belonging to the genera Enterococcus, Streptococcus, Staphylococcus and Propionibacterium from umbilical cord blood, which also supports a haematogenous transfer of bacteria from distal maternal locations to the fetus (Cao et al. 2014). Similar results have been reported for cattle, with their calves’ umbilical blood harbouring key uterine pathogens such as Bacteroides spp., Fusobacterium spp. and Prophyromonas spp. Presence of uterine pathogens in faeces and blood indicates the feasibility of spread of haematogenous bacteria from the gut to the uterus. Ascending contamination of the uterus cannot be discarded as an important route, as the vagina also harbours the main uterine pathogens. However, other uterine pathogens such as Prevotella spp., Helcococcus spp., Filifactor spp., Campylobacter spp. and Arcanobacterium spp. were not found as part of the cow’s core vaginal microbiome, which may also indicate a haematogenous transfer route (Jeon et al. 2017). It has been suggested that the mechanism by which microbiota move through the circulatory system could be facilitated by other bacteria such as Fusobacterium nucleatum. These have the ability to bind to vascular endothelium and alter permeability, functioning as an ‘enabler’ for other bacteria, such as Escherichia coli, to move through the circulatory system (Wassenaar and Panigrahi 2014). Studies in mice and cattle have also shown that bacteria can be transported to extra-intestinal sites by mononuclear cells (Jeon et al. 2017). Detailed mechanisms of haematogenous transfer of non-pathogenic microbiota from distal locations of the mare to the placenta and fetal compartments have not been described in the horse.
Dendritic cell sampling is the third proposed route through which bacterial translocation from the GIT to the placenta can occur. Transfer across the GIT epithelium is restricted by a well established barrier; however, dendritic cells can penetrate this barrier and enter the intestinal lumen via their intraepithelial dendrite extensions. This allows the dendritic cells to internalise bacteria from the GIT and shuttle them across the epithelial barrier to the periphery (Foti and Ricciardi-Castagnoli 2005; Nicoletti et al. 2009). In vitro and in vivo studies in mice have examined the ability of dendritic cells to sample both non-pathogenic Escherichia coli and pathogenic Salmonella (Rescigno et al. 2001; Wallace et al. 2016). It was determined that, in both cases, dendritic cells take up the bacteria directly, although, in the presence of pathogens, their numbers doubled. It was concluded that dendritic cells have the ability to penetrate and sample the GIT lumen, and to facilitate transfer of both pathogenic and non-pathogenic bacteria to peripheral sites through the lymphatic system (Rescigno et al. 2001; Wallace et al. 2016). Once pathogenic bacteria are in the lymphatic system, an efficient immune response is initiated. Dendritic cells play a key role in immunological regulation but are a new concept in the in utero colonisation hypothesis; therefore, their ability to transfer non-pathogenic bacteria to the equine placenta has not yet been explored.
These three proposed routes have not been defined in the horse as mechanisms for non-pathogenic bacteria to transfer into the placenta and fetal compartments. Preliminary studies on the equine amniotic microbiome have shown similarities to the dam’s GIT microbiota (Quercia et al. 2019). Therefore, it is possible that haematogenous transfer and dendritic cell sampling also occur in the horse and transfer maternal bacteria to the placenta and fetal fluids. More studies are required to define the mechanisms underpinning vertical transmission of bacteria to the fetal gut of the foal in utero.
The microbiota of the dam
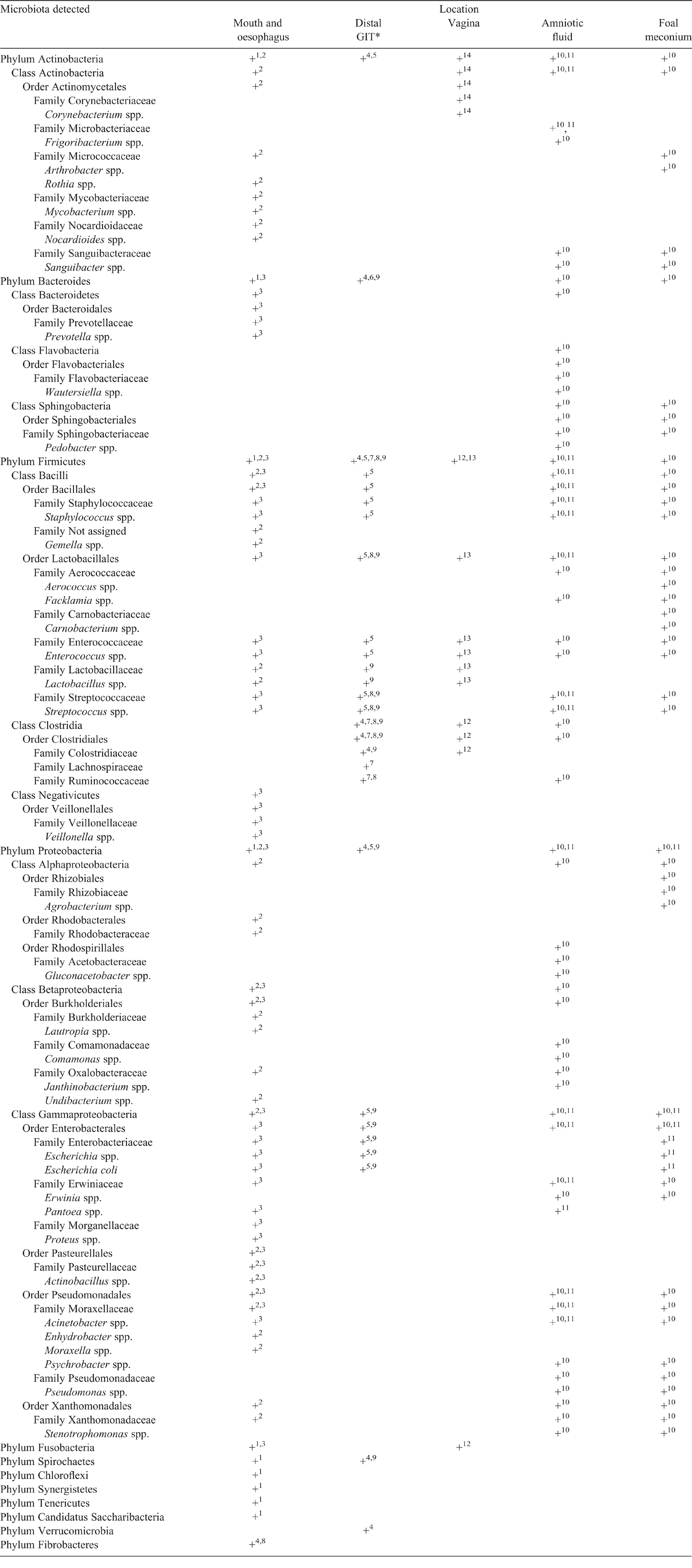
There is no doubt that during birth, the foal is exposed to a considerable number of bacteria. However, as the in utero colonisation hypothesis implies that bacteria are also being relocated internally from a variety of the dam’s microbial sites to create an amniotic microbiota during pregnancy, it is important to understand what microbiota occur normally in the mare. Some similarities between the bacteria in amniotic fluid and the dam’s microbiomes have been identified (Table 1); however, the current amniotic data are too limited to justify any statistical analysis and infer relevance. It is important to note that the normal composition of the equine microbiome can vary among regions, ages and management systems, and there are inter-horse variations (Costa and Weese 2018), which would likely have an impact on the comparisons to the amniotic microbiome. Here, we focus on the oral, distal GIT and reproductive tract microbiota of the mare, as these are the origin of the proposed routes of colonisation.
|
Oral microbiota
There are limited data on the oral microbiome of the healthy horse, with most studies having focused on disease such as periodontitis. Kennedy et al. (2016) identified 179 microbial family groups in the oral cavity of the horse; however, only 7% of these were associated with the healthy oral microbiome. The most discriminative bacteria identified from the healthy oral microbiota belonged to the phyla Proteobacteria and Firmicutes (Table 1; Kennedy et al. 2016). These results were supported by Gao et al. (2016), who identified additional phyla in the healthy equine subgingival microbiome, including Actinobacteria, Bacteroidetes, Chloroflexi, Fusobacteria, Spirochaetes, Synergistetes, Tenericutes and Saccharibacteria. These same phyla have been identified in the human oral microbiome (Gao et al. 2016), which is noteworthy, because human oral bacteria, such as Bergeyella spp. from the phylum Bacteroidetes, and Fusobacterium nucleatum, have been identified in placental infections causing adverse pregnancy outcomes, indicating the possible haematogenous oral-placenta route of colonisation proposed earlier (Fardini et al. 2010; Pelzer et al. 2017).
Comparing these bacteria identified in the oral cavity (Meyer et al. 2010; Gao et al. 2016; Kennedy et al. 2016) with those detected in amniotic fluid and meconium by Quercia et al. (2019; as outlined in Table 1), only one family was shared between the oral cavity and meconium, namely, Xanthomonadaceae. Although this result would indicate no relationship between oral and amniotic microbiomes, the data were collected from one study that has not been replicated. That study also included only bacteria with the highest abundance from each site; therefore, it is possible that oral bacteria may appear in the placental fluids at a lower abundance than the other sites. Alternatively, oral bacteria may play a role only in adverse pregnancy outcomes and not be present in the normal amniotic microbiota. This could be supported by the presence of Actinobacillus spp. in the equine oral cavity, which has previously been described as a pathogen causing abortion in the mare, but has not been isolated from normal pregnancies in recent studies (Layman et al. 2014; Quercia et al. 2019). At this stage in equine amniotic-microbiota research, it is unclear whether oral bacteria form part of the normal inhabitants in the fetal compartments.
Distal GIT microbiome
The GIT harbours a complex microbial community consisting of archaea, bacteria, fungi, parasites and viruses (Costa et al. 2016; Costa and Weese 2018). The intestinal microbiota are recognised for their essential role in maintaining host health, by providing protection against overgrowth of pathogenic organisms, contributing to development of the intestinal epithelium, breaking down complex feed particles, and modulating the local immune system and metabolic functions (Costa and Weese 2012, 2018; Schoster et al. 2013; Wallace et al. 2016). A properly functioning GIT, along with a balanced intestinal microbiota, is fundamental for maintenance of host health and performance, and the ecological system must respond to changes so as to maintain gut homeostasis (Martin et al. 2017). Disruption of the normal microbial community is associated with problems including colitis, laminitis and diarrhoea in young foals. These GIT diseases are leading causes of morbidity and mortality (Costa and Weese 2012; Costa et al. 2016). The resident equine GIT microbiota have been predominantly evaluated using faecal samples, due to ease of non-invasive collection. However, faeces are representative only of the distal part of the GIT tract, and while having shown microbial similarities to that of the caecum, faecal microbes are a poor indicator of the composition of the microbiota present in the small intestine (Schoster et al. 2013; Costa and Weese 2018). Therefore, the following discussion will focus on the distal GIT or hindgut microbiota.
Consistent with the majority of mammalian species, the equine distal gut consists predominantly of Firmicutes (Table 1; Willing et al. 2009; Kuhl et al. 2011; Schoster et al. 2013; Costa and Weese 2018). Bacteroidetes and Verrucomicrobia are the second-most abundant phyla, and- with the development of next generation sequencing, bacteria of the class Clostridia, which includes several Clostridium species, have also been recognised as dominant species present in the distal intestinal tract of the adult horse (Costa and Weese 2018). The amniotic and meconium microbiota also include Firmicutes, with distinct similarities to the distal GIT of the adult horse (Table 1). Comparing the results from Quercia et al. (2019) with previously published literature, Escherichia coli and the family Ruminococcaceae are present both in the distal GIT and the amniotic fluid. In addition, the genera Enterococcus, Streptococcus and Staphylococcus are present in the amniotic fluid and meconium as well as in the distal GIT (Willing et al. 2009; Kuhl et al. 2011; Schoster et al. 2013).
These similarities suggest that microbes from the distal GIT of the dam could be a significant source of the amniotic fluid microbiota. Moreover, the proximal GIT microbiota could also be a source of microbes, but its composition is unknown. In addition, the bacteria detected in the distal GIT are essentially a snapshot in time, and many factors can affect the microbiota present, including age, diet, environmental conditions, disease and the use of supplements such as probiotics (Willing et al. 2009; Schoster 2018; De La Torre et al. 2019). Therefore, comparisons among studies should be considered with caution. Future studies that examine the origin of the equine amniotic microbiome could minimise these variables by comparing amniotic fluid and the dam’s distal GIT from samples collected at the same time point. Furthermore, using such an approach throughout a mare’s pregnancy would be beneficial for determining whether changes in the GIT microbiome are reflected in the amniotic or meconium microbiota at birth.
Reproductive tract microbiota
Bacteria in the equine uterus have previously been associated with inflammation and endometritis. It is now thought that the mare may establish and support a normal moderately diverse uterine microbiome sourced from vaginal microbiota during puberty and mating (Heil et al. 2018; Pugh 2018). The composition of the normal uterine microbiome appears similar to populations found on the external cervical os, which can be explained by the open cervix, and communication between the uterine lumen and cranial vagina during oestrus. Heil et al. (2018) reported on the beta diversity, and although over 5400 OTUs were detected, the taxonomical identification of these sequences remains unclear. It has been documented that the normal vaginal microbiota of the horse consist of the genera Lactobacillus, Enterococcus, Coryneobacterium and Fusobacterium, as well as the family Clostridiaceae, suggesting that these may also form part of the normal uterine microbiota (Table 1) (Fraga et al. 2008; Hoyles et al. 2013; Pugh 2018).
In contrast, the pathogenic bacteria causing endometritis in horses have been well defined. Microorganisms that have been associated with this disease include Streptococcus zooepidemicus, α-haemolytic Streptococcus spp., Pseudomonas aeruginosa, Pseudomonas spp., Corynebacterium spp., Staphylococcus spp., Escherichia coli, Klebsiella spp., and some yeasts and fungi (Diel de Amorim et al. 2016). Interestingly, some of these bacteria that have previously been defined as pathogens causing endometritis, have now been detected in the amniotic fluid from healthy pregnancies of mares, including Streptococcus spp., Staphylococcus spp., Pseudomonas spp. and E. coli (Quercia et al. 2019). Such findings suggest that these microorganisms may, instead, be opportunistic pathogens that can cause pathology under altered conditions, but are usually part of the normal uterine microbiome. The fact that under some conditions they do cause inflammation and pathology suggests that it may be the stability of the microbial community that contributes to a healthy pregnancy, whereas disruption of the resident microbiota allows for organisms to colonise and proliferate rapidly, causing dysbiosis (Pugh 2018). This suggests that an imbalance in the microbial community is the cause of complications, rather than is the presence of individual microorganisms.
Supporting this idea, Clostridium spp. has been observed in the upper and lower reproductive tract of the mare, and a reduction in its population appears to result in the overgrowth of other organisms such as Fusobacterium spp. It has been suggested that some organisms belonging to the class Clostridia exert a protective effect in the uterus during pregnancy, although this has not been explored in detail (Pugh 2018). Since the resident uterine microbiota in the mare are not well defined, no clear comparisons of their role in establishing the amniotic microbiota can be made at this time. It appears that a stable community can help maintain or support a healthy pregnancy, but it is unclear whether these microbes can infiltrate a healthy pregnancy and contribute to the amniotic microbiota.
Conclusions
The long-standing paradigm that a healthy pregnancy is dependent on a sterile placental environment is currently vigorously debated. Few anatomical sites can be described as naturally sterile; therefore, the observation that the placenta harbours a unique microbiota is not surprising, especially since it is the first organ to nourish and maintain the fetus. The presence of microbes in the healthy placenta, umbilical cord and meconium of humans has sparked interest that fetal microbial contact in utero is a normal phenomenon occurring across all mammalian species, including the horse.
Bacteria have now been detected in the amniotic fluid and meconium from healthy equine pregnancies and it is our contention that these bacteria commence colonisation of the GIT in utero. This, in turn, will have an impact on the fetal immune system, preparing the foal for the environment outside the uterus. However, this is a new area of research and it is not well defined what bacteria are present in the normal amniotic microbiome of the horse and what role they play during pregnancy and fetal development.
The equine placenta and fetal fluids have vital roles in growth and development of the fetus. Further, the amniotic fluid that surrounds the fetus is the first fluid to enter the fetal GIT and significantly contributes to the development and accumulation of meconium. Meconium in the foal documents the changes occurring within the amniotic fluid throughout pregnancy, and since amniotic fluid can be used as an indicator of fetal health, the meconium is also regarded as useful for evaluating the neonatal foal. It is currently unknown whether microbial changes occurring throughout gestation can also be detected in the meconium of the foal.
It is not known when and how bacteria enter the amniotic fluid during pregnancy, but the species detected show similarities to maternal microbiomes, suggesting that the amniotic microbiome is established from internal sites within the mare. Determining what bacteria compose the amniotic microbiome, when they enter the fetal compartments and colonise the amniotic fluid and their potential origins will provide crucial information as to how they influence the development of the foal from fetal to neonatal life. The significance of intrauterine microbes for foal GIT colonisation remains to be elucidated but could be key in improving neonatal health and development.
Conflicts of interest
The authors declare no conflicts of interest. WLB and GB are members of the Editorial Board of Animal Production Science but had no role in the peer review and acceptance of this paper.
Acknowledgements
This research did not receive any specific funding.
References
Aagaard K, Ma J, Antony K, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Science Translational Medicine 6, 1–11.| The placenta harbors a unique microbiome.Crossref | GoogleScholarGoogle Scholar |
Aagaard K, Ma J, Prince A, Chu D, Showalter L, Antony K, Versalovic J (2015) Association of preterm birth with alterations in the microbiome and linkage to host mtDNA variants. American Journal of Obstetrics and Gynecology 212, S10–S10.
| Association of preterm birth with alterations in the microbiome and linkage to host mtDNA variants.Crossref | GoogleScholarGoogle Scholar |
Aatsinki A-K, Lahti L, Uusitupa H-M, Munukka E, Keskitalo A, Nolvi S, O’Mahony S, Pietilä S, Elo LL, Eerola E, Karlsson H, Karlsson L (2019) Gut microbiota composition is associated with temperament traits in infants. Brain, Behavior, and Immunity 80, 849–858.
| Gut microbiota composition is associated with temperament traits in infants.Crossref | GoogleScholarGoogle Scholar | 31132457PubMed |
Alipour MJ, Jalanka J, Pessa-Morikawa T, Kokkonen T, Satokari R, Hynönen U, Iivanainen A, Niku M (2018) The composition of the perinatal intestinal microbiota in cattle. Scientific Reports 8, 1–14.
| The composition of the perinatal intestinal microbiota in cattle.Crossref | GoogleScholarGoogle Scholar |
Ardissone AN, de la Cruz AG, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, Murgas-Torrazza R, Sharma R, Hudak ML, Triplett EW, Neu J (2014) Meconium microbiome analysis indentifies bacteria correlated with premature birth. PLoS One 9, 1–8.
| Meconium microbiome analysis indentifies bacteria correlated with premature birth.Crossref | GoogleScholarGoogle Scholar |
Borre YE, O’keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in Molecular Medicine 20, 509–518.
| Microbiota and neurodevelopmental windows: implications for brain disorders.Crossref | GoogleScholarGoogle Scholar | 24956966PubMed |
Burrage S (1927) Bacteria in the supposedly sterile meconium. Journal of Bacteriology 13, 47–48.
Cao B, Stout MJ, Lee I, Mysorekar IU (2014) Placental microbiome and its role in preterm birth. NeoReviews 15, e537–e545.
| Placental microbiome and its role in preterm birth.Crossref | GoogleScholarGoogle Scholar | 25635174PubMed |
Cellini C, Xu J, Buchmiller TL (2006) Effect of esophageal ligation on small intestinal development in normal and growth-retarded fetal rabbits. Journal of Pediatric Gastroenterology and Nutrition 43, 291–298.
| Effect of esophageal ligation on small intestinal development in normal and growth-retarded fetal rabbits.Crossref | GoogleScholarGoogle Scholar | 16954949PubMed |
Charbonneau MR, Blanton LV, DiGiulio DB, Relham DA, Lebrilla CB, Mills DA, Gordon JI (2016) A microbial perspective of human developmental biology. Nature 535, 48–55.
| A microbial perspective of human developmental biology.Crossref | GoogleScholarGoogle Scholar | 27383979PubMed |
Chu D, Stewart C, Seferovic M, Suter M, Cox J, Vidaeff A, Aagard K (2017) Profiling of microbiota in second trimester amniotic fluid reveals a distinctive community present in the mid trimester and predictive of the placental microbiome at parturition. American Journal of Obstetrics and Gynecology 216, S18–S19.
| Profiling of microbiota in second trimester amniotic fluid reveals a distinctive community present in the mid trimester and predictive of the placental microbiome at parturition.Crossref | GoogleScholarGoogle Scholar |
Collado MC, Rautava S, Isolari E, Salminen S (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports 6, 1–13.
| Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid.Crossref | GoogleScholarGoogle Scholar |
Costa MC, Weese JS (2012) The equine intestinal microbiome. Animal Health Research Reviews 13, 121–128.
| The equine intestinal microbiome.Crossref | GoogleScholarGoogle Scholar | 22626511PubMed |
Costa MC, Weese JS (2018) Understanding the intestinal microbiome in health and disease. The Veterinary Clinics of North America. Equine Practice 34, 1–12.
| Understanding the intestinal microbiome in health and disease.Crossref | GoogleScholarGoogle Scholar | 29402480PubMed |
Costa MC, Stampfli HR, Allen-Vercoe E, Weese JS (2016) Development of the faecal microbiota in foals. Equine Veterinary Journal 48, 681–688.
| Development of the faecal microbiota in foals.Crossref | GoogleScholarGoogle Scholar | 26518456PubMed |
De La Torre U, Henderson JD, Furtado KL, Pedroja M, Elenamarie O, Mora A, Pechanec MY, Maga EA, Mienaltowski IMJ (2019) Utilizing the fecal microbiota to understand foal gut transitions from birth to weaning. PLoS One 14, 1–18.
| Utilizing the fecal microbiota to understand foal gut transitions from birth to weaning.Crossref | GoogleScholarGoogle Scholar |
Diel de Amorim M, Gartley CJ, Foster RA, Hill A, Scholtz EL, Hayes A, Chenier TS (2016) Comparison of clinical signs, endometrial culture, endometrial cytology, uterine low-volume lavage, and uterine biopsy and combinations in the diagnosis of equine endometritis. Journal of Equine Veterinary Science 44, 54–61.
| Comparison of clinical signs, endometrial culture, endometrial cytology, uterine low-volume lavage, and uterine biopsy and combinations in the diagnosis of equine endometritis.Crossref | GoogleScholarGoogle Scholar |
Fardini Y, Chung P, Dumm R, Joshi N, Han YW (2010) Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infection and Immunity 78, 1789–1796.
| Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection.Crossref | GoogleScholarGoogle Scholar | 20123706PubMed |
Foote AK, Ricketts SW, Whitwell KE (2012) A racing start in life? The hurdles of equine feto-placental pathology. Equine Veterinary Journal 44, 120–129.
| A racing start in life? The hurdles of equine feto-placental pathology.Crossref | GoogleScholarGoogle Scholar |
Foti M, Ricciardi-Castagnoli P (2005) Antigen sampling by mucosal dendritic cells. Trends in Molecular Medicine 11, 394–396.
| Antigen sampling by mucosal dendritic cells.Crossref | GoogleScholarGoogle Scholar | 16087403PubMed |
Fraga M, Perelmuter K, Delucchi L, Cidade E, Zunino P (2008) Vaginal lactic acid bacteria in the mare: evaluation of the probiotic potential of native Lactobacillus spp. and Enterococcus spp. strains. Antonie van Leeuwenhoek 93, 71–78.
| Vaginal lactic acid bacteria in the mare: evaluation of the probiotic potential of native Lactobacillus spp. and Enterococcus spp. strains.Crossref | GoogleScholarGoogle Scholar | 17588124PubMed |
Franciolli ALR, Cordeiro BM, da Fonseca ET, Rodrigues MN, Sarmento CAP, Ambrosio CE, de Carvalho AF, Miglino MA, Silva LA (2011) Characteristics of the equine embryo and fetus from days 15 to 107 of pregnancy. Theriogenology 76, 819–832.
| Characteristics of the equine embryo and fetus from days 15 to 107 of pregnancy.Crossref | GoogleScholarGoogle Scholar |
Gaivão MMF, Rambags BPB, Stout TAE (2014) Gastrulation and the establishment of the three germ layers in the early horse conceptus. Theriogenology 82, 354–365.
| Gastrulation and the establishment of the three germ layers in the early horse conceptus.Crossref | GoogleScholarGoogle Scholar |
Gao W, Chan Y, You M, Lacap-Bugler DC, Leung WK, Watt RM (2016) In-depth snapshot of the equine subgingival microbiome. Microbial Pathogenesis 94, 76–89.
| In-depth snapshot of the equine subgingival microbiome.Crossref | GoogleScholarGoogle Scholar | 26550763PubMed |
Hall IC, O’Toole E (1934) Bacterial flora of first specimens of meconium passed by fifty new-born infants. American Journal of Diseases of Children 47, 1279–1285.
Han YW, Fardini Y, Chen C, Iacampo KH, Peraino VA, Shamonki JM, Redline RW (2010) Term stillbirth caused by oral Fusobacterium nucleatum. Obstetrics and Gynecology 115, 442–445.
| Term stillbirth caused by oral Fusobacterium nucleatum.Crossref | GoogleScholarGoogle Scholar | 20093874PubMed |
Heil BA, Thompson SK, Davolli GM, King G, Sones JL (2018) Metagenetic characterization of the resident equine uterine microbiome using multiple techniques. Journal of Equine Veterinary Science 66, 111
| Metagenetic characterization of the resident equine uterine microbiome using multiple techniques.Crossref | GoogleScholarGoogle Scholar |
Hemberg E, Einarsson S, Kútvölgyi G, Lundeheim N, Bagge E, Båverud V, Jones B, Morrell JM (2015) Occurrence of bacteria and polymorphonuclear leukocytes in fetal compartments at parturition; relationships with foal and mare health in the peripartum period. Theriogenology 84, 163–169.
| Occurrence of bacteria and polymorphonuclear leukocytes in fetal compartments at parturition; relationships with foal and mare health in the peripartum period.Crossref | GoogleScholarGoogle Scholar | 25850610PubMed |
Hitti J, Hillier SL, Agnew KJ, Krohn MA, Reisner DP, Eschenbach DA (2001) Vaginal indicators of amniotic fluid infection in preterm labor. Obstetrics and Gynecology 97, 211–219.
Hong CB, Donahue JM, Giles RCJ, Petrites-Murphy MB, Poonacha KB, Roberts AW, Smith BJ, Tramontin RR, Tuttle PA, Swerczek TW (1993) Equine abortion and stillbirth in central Kentucky during 1988 and 1989 foaling seasons. Journal of Veterinary Diagnostic Investigation 5, 560–566.
| Equine abortion and stillbirth in central Kentucky during 1988 and 1989 foaling seasons.Crossref | GoogleScholarGoogle Scholar | 8286455PubMed |
Hoyles L, Ortman K, Cardew S, Foster G, Rogerson F, Falsen E (2013) Corynebacterium uterequi sp. nov., a non-lipophilic bacterium isolated from urogenital samples from horses. Veterinary Microbiology 165, 469–474.
| Corynebacterium uterequi sp. nov., a non-lipophilic bacterium isolated from urogenital samples from horses.Crossref | GoogleScholarGoogle Scholar | 23618836PubMed |
Jacquay E, Zeglin L, Lillich J, Jones E, Kouba J (2018) Characterization of foal fecal microbiome from birth to weaning and the relationship to mare milk and mare feces. Journal of Animal Science 96, 33
| Characterization of foal fecal microbiome from birth to weaning and the relationship to mare milk and mare feces.Crossref | GoogleScholarGoogle Scholar |
Jeon SJ, Cunha F, Vieira-Neto A, Bicalho RC, Lima S, Bicalho ML, Galvão KN (2017) Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 5, 1–13.
| Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows.Crossref | GoogleScholarGoogle Scholar |
Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM (2008) Is meconium from health newborns actually sterile? Research in Microbiology 159, 187–193.
| Is meconium from health newborns actually sterile?Crossref | GoogleScholarGoogle Scholar | 18281199PubMed |
Kennedy R, Lappin DF, Dixon PM, Buijs MJ, Zaura WC, O’Donnell L, Bennett D, Brandt BW, Riggio MP (2016) The microbiome associated with equine periodontitis and oral health. Veterinary Research 47, 1–9.
| The microbiome associated with equine periodontitis and oral health.Crossref | GoogleScholarGoogle Scholar |
Kraimi N, Dawkins M, Gebhardt-Henrich SG, Velge P, Rychlik I, Volf J, Creach P, Smith A, Colles F, Leterrier C (2019) Influence of the microbiota–gut–brain axis on behavior and welfare in farm animals: a review. Physiology and Behavior 210, 1–12.
| Influence of the microbiota–gut–brain axis on behavior and welfare in farm animals: a review.Crossref | GoogleScholarGoogle Scholar |
Kuhl J, Winterhoff N, Wulf M, Schweigert FJ, Schwendenwein I, Bruckmaier RM, Aurich JE, Kutzer P, Aurich C (2011) Changes in faecal bacteria and metabolic parameters in foals during the first six weeks of life. Veterinary Microbiology 151, 321–328.
| Changes in faecal bacteria and metabolic parameters in foals during the first six weeks of life.Crossref | GoogleScholarGoogle Scholar | 21511405PubMed |
Layman QD, Rezabek GB, Ramachandran A, Love BC, Confer AW (2014) A retrospective study of equine actinobacillosis cases: 1999–2011. Journal of Veterinary Diagnostic Investigation 26, 365–375.
| A retrospective study of equine actinobacillosis cases: 1999–2011.Crossref | GoogleScholarGoogle Scholar | 24742921PubMed |
Martin EJ, Reed KJ, Kunz IGZ, Coleman RJ, Coleman SJ (2017) Normal variation and changes over time in the equine intestinal microbiome. Journal of Equine Veterinary Science 52, 60
| Normal variation and changes over time in the equine intestinal microbiome.Crossref | GoogleScholarGoogle Scholar |
Mesa F, Pozo E, Blanc V, Puertas A, Bravo M, O’Valle F (2013) Are periodontal bacterial profiles and placental inflammatory infiltrate in pregnancy related to birth outcomes? Journal of Periodontology 84, 1327–1336.
| Are periodontal bacterial profiles and placental inflammatory infiltrate in pregnancy related to birth outcomes?Crossref | GoogleScholarGoogle Scholar | 23121458PubMed |
Meyer W, Kacza J, Schnapper A, Verspohl J, Hornickel I, Seeger J (2010) A first report on the microbial colonisation of the equine oesophagus. Annals of Anatomy 192, 42–51.
| A first report on the microbial colonisation of the equine oesophagus.Crossref | GoogleScholarGoogle Scholar | 19942420PubMed |
Moreno I, Franasiak JM (2017) Endometrial microbiota: new player in town. Fertility and Sterility 108, 32–39.
| Endometrial microbiota: new player in town.Crossref | GoogleScholarGoogle Scholar | 28602480PubMed |
Mulvihill SJ, Stone MM, Fonkalsrud EW, Debas HT (1986) Trophic effect of amniotic fluid on fetal gastrointestinal development. The Journal of Surgical Research 40, 291–296.
| Trophic effect of amniotic fluid on fetal gastrointestinal development.Crossref | GoogleScholarGoogle Scholar | 3702386PubMed |
Nicoletti C, Regoli M, Bertelli E (2009) Dendritic cells in the gut: to sample and to exclude? Mucosal Immunology 2, 462
| Dendritic cells in the gut: to sample and to exclude?Crossref | GoogleScholarGoogle Scholar | 19687786PubMed |
Panelli S, Schneider L, Bandi C, Zuccotti GV, D’Auria E (2018) Is there life in the meconium? A challenging, burning question. Pharmacological Research 137, 148–149.
| Is there life in the meconium? A challenging, burning question.Crossref | GoogleScholarGoogle Scholar | 30296570PubMed |
Pararas MV, Skevaki CL, Kafetzis DA (2006) Preterm birth due to maternal infection: causative pathogens and modes of prevention. European Journal of Clinical Microbiology & Infectious Diseases 25, 562–569.
| Preterm birth due to maternal infection: causative pathogens and modes of prevention.Crossref | GoogleScholarGoogle Scholar |
Pelzer E, Gomez-Arango LF, Barrett HL, Nitert MD (2017) Review: maternal health and the placental microbiome. Placenta 54, 30–37.
| Review: maternal health and the placental microbiome.Crossref | GoogleScholarGoogle Scholar | 28034467PubMed |
Perez-Muñoz ME, Arrieta M, Ramer-Tait AE, Walter J (2017) A critical assessment of the ‘sterile womb’ and ‘in utero colonization’ hypotheses: implications for research on the pioneer infant microbiome Microbiome 5, 1–19.
| A critical assessment of the ‘sterile womb’ and ‘in utero colonization’ hypotheses: implications for research on the pioneer infant microbiomeCrossref | GoogleScholarGoogle Scholar |
Platt H (1984) Growth of the equine foetus. Equine Veterinary Journal 16, 247–252.
| Growth of the equine foetus.Crossref | GoogleScholarGoogle Scholar | 6383808PubMed |
Prince A, Chu D, Seferovic M, Antony K, Ma J, Aagaard K (2015) The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harbor Perspectives in Medicine 5, 1–24.
| The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome.Crossref | GoogleScholarGoogle Scholar |
Pugh G (2018) ‘The role of microbial diversity in female reproductive health: an analysis of the microbiome’s influence on pregnancy outcomes in postparturient mares.’ (ProQuest Dissertations Publishing, Michigan, USA)
Quercia S, Freccero F, Castagnetti C, Soverini M, Turroni S, Biagi E, Rampelli S, Lanci A, Mariella J, Chinellato E, Brigidi P, Candela M (2019) Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals. Equine Veterinary Journal 51, 231–237.
| Early colonisation and temporal dynamics of the gut microbial ecosystem in Standardbred foals.Crossref | GoogleScholarGoogle Scholar | 29931762PubMed |
Rehbinder EM, Lodrup Carlsen KC, Staff AC, Angel IL, Landro L, Hilde K, Gaustad P, Rudi K (2018) Is amniotic fluid of women with uncomplicted term pregnancices free of bacteria? American Journal of Obstetrics and Gynecology 219, 289.e1–289.e12.
| Is amniotic fluid of women with uncomplicted term pregnancices free of bacteria?Crossref | GoogleScholarGoogle Scholar |
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J, Ricciardi-Castagnoli P (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunology 2, 361–367.
| Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria.Crossref | GoogleScholarGoogle Scholar | 11276208PubMed |
Rose BV, Firth M, Morris B, Roach JM, Wathes DC, Verheyen KLP, de Mestre AM (2018) Descriptive study of current therapeutic practises, clinical reproductive findings and incidence of pregnancy loss in intensively managed thoroughbred mares. Animal Reproduction Science 188, 74–84.
| Descriptive study of current therapeutic practises, clinical reproductive findings and incidence of pregnancy loss in intensively managed thoroughbred mares.Crossref | GoogleScholarGoogle Scholar | 29146097PubMed |
Sangild PT, Schmidt M, Elnif J, Björnvad CR, Weström BR, Buddington RK (2002) Prenatal develoment of gastrointestinal function in the pig and the effects of fetal esophageal obstruction. Pediatric Research 52, 416–424.
| Prenatal develoment of gastrointestinal function in the pig and the effects of fetal esophageal obstruction.Crossref | GoogleScholarGoogle Scholar | 12193678PubMed |
Schmidt AR, Williams MA, Carleton CL, Darien BJ, Derksen FJ (1991) Evaluation of transabdominal ultrasound-guided amniocentesis in the late gestational mare. Equine Veterinary Journal 23, 261–265.
| Evaluation of transabdominal ultrasound-guided amniocentesis in the late gestational mare.Crossref | GoogleScholarGoogle Scholar | 1915224PubMed |
Schoster A (2018) Probiotic use in equine gastrointestinal disease. The Veterinary Clinics of North America. Equine Practice 34, 13–24.
| Probiotic use in equine gastrointestinal disease.Crossref | GoogleScholarGoogle Scholar | 29402478PubMed |
Schoster A, Arroyo LG, Staempfli HR, Weese JS (2013) Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism. BMC Research Notes 6, 1–9.
| Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism.Crossref | GoogleScholarGoogle Scholar |
Stinson LF, Keelan JA, Payne MS (2018) Comparison of meconium DNA extraction methods for use in microbiome studies. Frontiers in Microbiology 9, 1–14.
| Comparison of meconium DNA extraction methods for use in microbiome studies.Crossref | GoogleScholarGoogle Scholar |
Torrazza RM, Neu J (2011) The developing intestinal microbiome and its relationship to health and disease in the neonate. Journal of Perinatology 31, S29–S34.
| The developing intestinal microbiome and its relationship to health and disease in the neonate.Crossref | GoogleScholarGoogle Scholar |
Trahair JF, Sangild PT (2000) Fetal organ growth in response to oesophageal infusion of amniotic fluid, colostrum, milk or gastrin-releasing peptide: a study in fetal sheep. Reproduction, Fertility and Development 12, 87–95.
| Fetal organ growth in response to oesophageal infusion of amniotic fluid, colostrum, milk or gastrin-releasing peptide: a study in fetal sheep.Crossref | GoogleScholarGoogle Scholar |
Wallace JG, Gohir W, Sloboda DM (2016) The impact of early life gut colonisation on metabolic and obesogenic outcomes: what have animal models shown us? Journal of Developmental Origins of Health and Disease 7, 15–24.
| The impact of early life gut colonisation on metabolic and obesogenic outcomes: what have animal models shown us?Crossref | GoogleScholarGoogle Scholar | 26399435PubMed |
Wassenaar TM, Panigrahi P (2014) Is a foetus developing in a sterile environment? Letters in Applied Microbiology 59, 572–579.
| Is a foetus developing in a sterile environment?Crossref | GoogleScholarGoogle Scholar | 25273890PubMed |
Whitwell KE, Jeffcott LB (1975) Morphological studies on the fetal membranes of the normal singleton foal at term. Research in Veterinary Science 19, 44–55.
| Morphological studies on the fetal membranes of the normal singleton foal at term.Crossref | GoogleScholarGoogle Scholar | 1153897PubMed |
Willing B, Vörös A, Roos S, Jones C, Jansson A, Lindberg JE (2009) Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training. Equine Veterinary Journal 41, 908–914.
| Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training.Crossref | GoogleScholarGoogle Scholar | 20383990PubMed |
Wohlfender FD, Barrelet FE, Doherr MG, Straub R, Meier HP (2009) Diseases in neonatal foals. Part 1: the 30-day incidence of disease and the effect of prophylactic antimicrobial drug treatment during the first three days post partum Equine Veterinary Journal 41, 179–185.
Woźniak MK, Jaszczak E, Wiergowski M, Polkowska Ż, Namieśnik J, Biziuk M (2018) Meconium analysis as a promising diagnostic tool for monitoring fetal exposure to toxic substances: recent trends and perspectives. Trends in Analytical Chemistry 109, 124–141.
Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z (2019) Intestinal microbiota in early life and its implications on childhood health. Genomics, Proteomics and Bioinformatics 17, 13–25.
| Intestinal microbiota in early life and its implications on childhood health.Crossref | GoogleScholarGoogle Scholar | 30986482PubMed |