New records of leaf galls and arthropod oviposition scars in Permian–Triassic Gondwanan gymnosperms
Stephen McLoughlinDepartment of Paleobotany, Swedish Museum of Natural History, Box 50007, SE-104 05, Stockholm, Sweden. Email: steve.mcloughlin@nrm.se
Australian Journal of Botany 59(2) 156-169 https://doi.org/10.1071/BT10297
Submitted: 8 November 2010 Accepted: 24 January 2011 Published: 28 March 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Single, midrib-positioned galls and midrib-flanking oviposition scars are described from four species of Permian glossopterid foliage from Australia and South Africa. Several of these traces have been mistaken previously for glossopterid reproductive organs or fructification detachment scars. A single Early Triassic corystosperm leaf from Australia is reported bearing multiple disc-like galls on both the midrib and pinnules. A Middle Triassic taeniopterid gymnosperm leaf from Australia is described hosting oviposition scars between consecutive secondary veins flanking the midrib. These fossils attest to a much richer record of plant–arthropod interactions in the late Palaeozoic and early Mesozoic of high-latitude Gondwana than previously reported, and indicate that herbivory and reproductive strategies involving galling and foliar ovipositioning were re-established relatively soon after the end-Permian mass extinction event that saw major turnovers in both the flora and insect fauna.
Introduction
Gall-formation (cecidogeny) represents one of nine major categories of arthropod damage to, or physical interaction with, plants (the Functional Feeding Groups of Labandeira et al. 2007); the others are external foliage feeding, piercing-and-sucking, leaf-mining, seed predation, boring, palynivory, nectarivory and ovipositioning. Although the last of these categories does not strictly involve feeding, it commonly produces diagnostic markings and acid-resistant structures on leaves that can be detected in the fossil record (Pott et al. 2008). Supplementing these categories is a range of additional fossil evidence (e.g. mouthpart structure, coprolites, detached scale-insect shields) that helps elucidate arthropod life-strategies intimately associated with certain plant organs through time (Labandeira 1997; Tosolini and Pole 2010).
There has been no consensus on a rigorous definition of the term ‘gall’. The expression is generally used for a biochemically induced growth response (especially thickening and reorientation) of plant tissue around a single or multiple infecting agent(s), but it excludes simple tissue deformation (pseudogalls) caused by piercing and sucking insects (Wool 1984; Krassilov et al. 2008). Although most modern galls can be related to their inducing species, few fossil galls preserve their perpetrators. Several attempts to categorise and formally name galls using morphological criteria (e.g. Ludwig 1857; Mani 1964; van Amerom 1973; Vjalov 1975; Meyer 1987; Krassilov et al. 2008) have met with little acceptance, because a single parasite may induce galls of multiple forms depending on the host organ and species. A universally accepted classification scheme for galls is currently lacking. In the most extensive overview of fossil plant–arthropod interactions, Labandeira et al. (2007) provided no hierarchical classification but discriminated 34 categories of ancient galls on the basis of size, morphology, anatomy and position on the host organ. Each was given a simple numerical designation within their list of plant ‘Damage types’.
Modern galls are produced by a wide range of organisms including insects (at least seven orders and over 13 000 species), mites, nematodes, bacteria, viruses and fungi (Mani 1992; Shorthouse et al. 2005). The gall-forming habit arose independently in many families within these groups (Meyer 1987; Roskam 1992). Each gall-forming agent generally has a preferred host species and organ. The aberrant plant tissue forming the gall wall typically provides physical protection, but also constitutes a food source, for a single invasive parasite species, although tri-specific (e.g. fungus–midge–angiosperm) associations are known to occur (Raman et al. 2005; Rohfritsch 2008). Large, elaborate and morphologically consistent galls of subaerial plant parts, such as those described below, are typically produced by insects and mites.
Records of fossil plant galls are sparse but many examples may have been overlooked in the past studies. A review of fossil galls by Larew (1992) documented only 26 pre-Pliocene examples, most of which were from the Cenozoic and Cretaceous. Scott et al. (1994) expanded the record with 25 new gall types from the Cretaceous and Paleogene; however, they noted only a single putative example (on the medullosan seed-fern Odontopteris) from the Palaeozoic (Permian of central Germany) (Potonié 1893), and no convincing records of pre-Cretaceous Mesozoic galls were known at that time. Since the mid-1990s, increased interest in the evolution of arthropod–plant–fungus interactions has led to the description of many additional gall types (Labandeira et al. 2007), although records older than mid-Cretaceous remain scarce. The mid-Cretaceous spike in galling activity appears to correlate with the radiation of angiosperms and a concomitant pulse in insect diversity. The oldest recorded galls are elongate growths produced by an unknown holometabolan insect on the petioles of a medullosan seed-fern (Stipitopteris) from the latest Carboniferous (c. 302 million years ago) of North America (Labandeira and Phillips 1996, 2002). A few Permian galls have been reported (e.g. Potonié 1893; Adami-Rodrigues et al. 2004; Labandeira 2006; Prevec et al. 2009); however, the cecidogenic character of most is equivocal. Following the demise of several key plant groups and major reorganisation of insect biotas during the end-Permian mass extinction event, evidence of galling is again sparse until the Late Triassic, from which time several examples have been documented in both hemispheres.
Diverse arthropods deposit their eggs within (endophytic ovipositioning) or on (ectophytic ovipositioning) plant organs without producing true galls (i.e. where feeding on the host is not involved). The oldest records of ovipositioning are from the latest Carboniferous and Permian, and most commonly involve palaeodictyopteroid and odonatopteroid insects depositing eggs on sphenophytes or a narrow range of gymnosperms (Béthoux et al. 2004; Labandeira 2006; Prevec et al. 2009). Oviposition scars are typically linear to lenticular with orientations and distributions closely related to the venation pattern of the host plant (Labandeira et al. 2007). Evidence of ovipositioning and the range of host plants notably increase in the Mesozoic (Labandeira 2006).
The record of fossil arthropod–plant interactions is markedly greater from the northern hemisphere, with a few local exceptions (e.g. from the Late Triassic Molteno Formation, South Africa) (Scott et al. 2004), essentially corresponding to the intensity of palaeobotanical sampling (Labandeira 2006). Here, I document some of the earliest records of galls and ovipositioning scars on southern hemisphere plants. The significance of the plant–arthropod interactions is explored with respect to the dramatic changes in floras and insect faunas at the close of the Palaeozoic.
Materials and methods
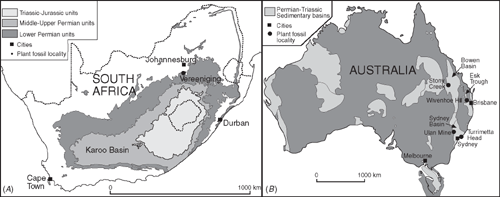
The present study is based on 13 fossil specimens derived from various stratigraphic intervals within four sedimentary basins (Fig. 1A, B) and stored in the collections of the Swedish Museum of Natural History, Stockholm, Sweden (prefixed NRMS), the University of Queensland’s Department of Earth Sciences collections held at the Queensland Museum, Brisbane, Australia (UQF), The Geological Survey of New South Wales, Sydney, Australia (MMF), The Australian Museum, Sydney, Australia (AMF), Vaal Teknorama Museum, Vereeniging, South Africa (VM) and the Bernard Price Institute for Palaeontology, Johannesburg, South Africa (BP). Registration numbers of the studied specimens, together with details of their preservation, host stratigraphic unit and age, are listed before the descriptions of individual damage types below. The fossils were photographed with a Canon EOS 40D digital camera under strong unilateral light from various angles to obtain optimum expression of surface relief for what are, in many examples, subtle features. In some cases, a polarising filter was employed to enhance the image contrast. The Permian and Triassic galls were compared with the full suite of published fossil examples and a range of modern forms collected by the author and examples held in the herbarium of the Swedish Museum of Natural History.
|
Results
Galls on Palaeovittaria kurtzii(Glossopteridales: Early Permian)

|
Locality. Quarries owned by Vereeniging Refractories (ex. Vereeniging Brick and Tile Co), situated on the northern bank of the Vaal River, 6 km south of Vereeniging, South Africa (26°42′S, 27°55′E; Le Roux and Anderson 1977; Fig. 1A).
Stratigraphy and age. From the fluvio-lacustrine Vryheid Formation, Middle Ecca Group, Karoo Supergroup, northern Karoo Basin (Le Roux and Anderson 1977; Keyser 1997). Palynological correlation of these deposits with Australian successions indicates a Sterlitamakian to early Baigendzhinian (mid-Early Permian, c. 290–280 million years ago) age (Millsteed 1994).
Palaeolatitude. From ~70°S, on the basis of Scotese’s (1997) palaeogeographic reconstructions.
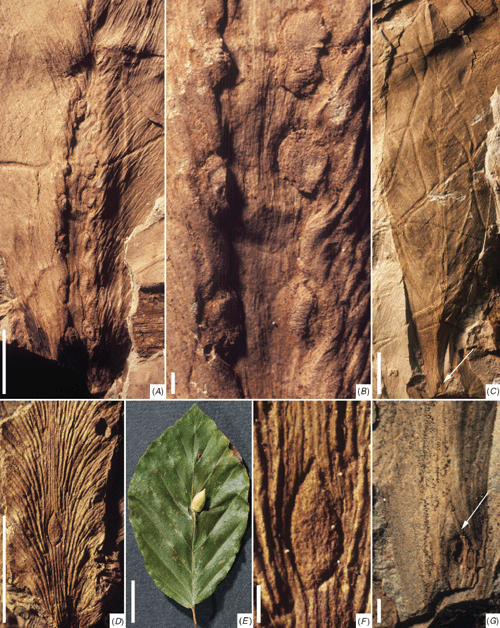
Description. Several incomplete leaf impressions (fully described by Plumstead (1958) and Anderson and Anderson (1985)) with a maximum preserved length of 92 mm and maximum width of 42 mm host single elongate elliptical to wedge-shaped indentations centred on the midrib (Fig. 2A–E). The lateral veins depart the midrib at a very acute angle, arch minimally across the lamina, bifurcate sparsely and intersect the margin at 10–20°. The secondary venation of some specimens is distorted slightly around the elliptical indentation (Fig. 2A). The indentations measure 17–26 mm long, 7–12 mm wide, and are essentially featureless apart from irregular longitudinal striations and an ill-defined narrow depression near the rim (Fig. 2F–H). The position of these anomalous, midrib-aligned structures varies among specimens from the petiole–lamina transition (Fig. 2B, C) to about mid-way along the leaf (Fig. 2A).
Remarks. These leaves are referable to Palaeovittaria kurtzii Feistm., a relatively uncommon glossopterid species distributed mainly through central Gondwana (India and southern Africa) and ranging throughout most of the Permian but more common in the early part of the period (Srivastava 1956; Anderson and Anderson 1985). The anomalous indentations along the midribs of the studied leaf impressions, corresponding to raised structures on the original leaf, were interpreted by Plumstead (1958, 1969) and Anderson and Anderson (1985) as ovuliferous reproductive structures. However, the lack of diagnostic features such as seed scars and a discrete marginal wing, together with evidence of distortion of the secondary venation, suggests that they represent the positions of galls similar to that of the Late Permian Australian specimen described below. The variable position of these features on the midrib, from near the petiole to about middle of the leaf also argues against these being reproductive organs. Large galls, although differing in shape and host species, are common on the midribs and major secondary veins of both fossil and modern leaves (Dreger-Jauffret and Shorthouse 1992; Labandeira et al. 2007).
Gall on Glossopteris acutifolia (Glossopteridales: Late Permian)

|
Locality. Ulan Coal Mine (32°14′30″S, 149°44′15″E; Fig. 1B), north-western Sydney Basin, 225 km north-west of Sydney, central eastern New South Wales, Australia.
Stratigraphy and age. The fossil likely derives from shales above the ‘Ulan Seam’ – the major coal seam exploited at Ulan Mine. Most workers regard this seam as laterally equivalent to the Lithgow and Bayswater coal seams or upper Lidsdale Seam, all of which belong to the fluvial–paludal Cullen Bullen Subgroup of the Illawarra Coal Measures (Tadros 1995; Yoo et al. 1995). These units host a Permian Upper Stage 5a–b palynoflora (of Price 1983), corresponding to a Wordian–Capitanian (middle–late Middle Permian: 268–260 million years ago) age (Ogg et al. 2008).
Palaeolatitude. Located at a ~60°S, on the basis of Scotese’s (1997) palaeogeographic maps.
Description. This specimen represents the proximal half of a Glossopteris leaf, with part of the petiole preserved (gross preserved dimensions: 64 mm long, 30 mm wide). Undisturbed lateral venation typically departs the robust midrib at ~20°, and arches gently across the lamina to intersect the margin at ~40–50°, with a marginal density of 15–20 per cm. The leaf bears a single large gall centred on the midrib near the transition from the petiole to the main lamina (this transition being gradational in glossopterids; Fig. 3A). Secondary venation and the lamina margin are slightly arched around the gall. The elliptical gall is ~12 mm long and 8.5 mm wide, with the long axis aligned along the midrib (Fig. 3B). Strong compression and loss of organic matter from the centre of the gall prevent assessment of its original thickness. However, the significant distortion of the lamina suggests that this gall was robust. On the basis of its sedimentary impression, the gall was smooth apart from weak concentric wrinkling near the base, and formed a slightly raised mound set in a shallow depression on the lamina.
Remarks. The leaf is here assigned to Glossopteris acutifolium McLoughlin, a moderately common species in Middle–Upper Permian strata of eastern Australia (McLoughlin 1994a), on the basis of its size, relatively low-angle lateral veins and moderate vein density. The gall is located proximally on the midrib, in a position typically occupied by ovuliferous reproductive structures in glossopterids (see e.g. Anderson and Anderson 1985; McLoughlin 1990a). However, the studied specimen lacks the diagnostic pitted or tuberculate morphology of glossopterid ovuliferous organs. Furthermore, the latter do not distort the secondary venation or lamina margin even when tightly appressed to the leaf (see numerous examples illustrated by Anderson and Anderson 1985). The large size and greater distortion of venation than typically expressed by arthropod oviposition scars (see e.g. Labandeira et al. 2007) strongly favour the elliptical feature on AMF119492 being a gall.
Gall on Glossopteris xiphophylla (Glossopteridales: Late Permian)
|
Locality. Tributary of Stony Creek (24°41′25″S, 148°16′15″E; Fig. 1B), south-western Bowen Basin, ~19 km west-south-west of Consuelo Station, east–central Queensland, Australia.
Stratigraphy and age. From the predominantly lacustrine upper Black Alley Shale, which contains an Upper Stage 5c palynoflora (McLoughlin 1988) and overlies the highest occurrence of the brachiopod index Echinalosia ovalis (Maxwell) (Briggs 1998). On this basis, it is ascribed a Wuchiapingian (early Late Permian: ~260–254 million years ago) age (Shi et al. 2010).
Palaeolatitude. Located at ~55–60°S in the Late Permian (Scotese 1997).
Description. The impression of the proximal portion of this glossopterid leaf bears a single small narrowly ovate body centred on, and aligned along, the midrib (Fig. 4D). The preserved portion of the leaf is 42 mm long and 12 mm in maximum width. Lateral veins arise at 10–50° from the midrib and arch gently across the lamina to intersect the margin at 45–60°, with a density of ~25 per cm. Secondary veins dichotomise and rejoin 2–4 times across the lamina to produce linear–crescentic meshes. The ovate body is 3.5 mm long, 1.5 mm wide, and lacks any consistent ornamentation (Fig. 4F). Veins flanking this body are slightly diverted around its periphery.
Remarks. This leaf is referable to Glossopteris xiphophylla, a common and widespread species in the Upper Permian of the Bowen Basin (McLoughlin 1994b). McLoughlin (1990b) originally interpreted this specimen as a juvenile or aborted glossopterid ovuliferous fructification. However, the lack of diagnostic ovule-attachment scars or a marginal protective wing, together with distortion of the adjacent lamina veins, suggests that this is not a reproductive organ. Prevec et al. (2009) reinterpreted this specimen as a solitary oviposition scar. The size and position of the damaged area along the leaf midrib are certainly similar to some other late Palaeozoic oviposition scars (see e.g. Labandeira and Allen 2007: figs 6.6–7), but the distinctly pointed–ovate shape of the structure differs from the typically rounded–elliptical or spindle-shaped features of confidently identified oviposition scars (Labandeira et al. 2007). Although definitive attribution is not possible in the absence of organic preservation, an alternative interpretation is that this structure represents a small collapsed or infilled gall because its margins are sunken with respect to the level of the lamina impression (indicating a raised rim on the original organ). In shape and position on the major vein, this body superficially resembles galls produced by the gall midge Mikiola fagi (Hartig) (Diptera: Cecidomyiidae) on modern Fagus sylvatica L. leaves (Fig. 4E), although attachment of the putative Permian gall only at the base cannot be determined from the impression. Nevertheless, the morphological similarity of these features suggests that plant–arthropod interactions involving the adoption of architectural traits parallel to modern forms have been operational since the late Palaeozoic.
Biseriate arthropod oviposition scars on Glossopteris bucklandensis (Glossopteridales: Late Permian)
Locality. Tributary of Stony Creek (24°42′38″S, 148°15′42″E; Fig. 1B), Bowen Basin, ~21 km west-south-west of Consuelo Station, east–central Queensland, Australia (McLoughlin 1990a).
Stratigraphy and age. Upper Black Alley Shale of Wuchiapingian (early Late Permian: ~260–254 million years ago) age (McLoughlin 1988; Shi et al. 2010).
Palaeolatitude. Positioned at ~55–60°S (Scotese 1997).
Description. See McLoughlin (1990a) for a full description of the host leaf – a paratype of Glossopteris bucklandensis McLoughlin. This leaf impression bears a 48-mm-long, ~10-mm-wide, raised area along its mid-line, which incorporates at least seven distinct opposite pairs of circular to elliptical scars flanking the midvein (Fig. 4A). The scars range in size from 2 × 3 to 3 × 4 mm and are separated longitudinally by 1–4 mm. The scars are generally raised above the surface of the impression on UQF76185a (Fig. 4B), indicating that these structures were sunken into the original leaf. The counterpart, UQF76185b, shows scars that are neither strongly raised nor sunken, but are smoother than the surrounding lamina. The scars are locally wrinkled and surrounded by a depressed rim, but are otherwise featureless.
Remarks. The scars flanking the midrib of UQF76185a,b were originally interpreted to represent imprints on the lamina produced by seeds attached to a Senotheca-like fructification (McLoughlin 1990a). Senotheca is a linear organ aligned along a glossopterid midrib and bears paired seeds (Banerjee 1969). However, Senotheca generally has over 35 pairs of very small seeds (or seed scars) that are typically <1 mm apart (McLoughlin 1990a), in contrast to the widely spaced robust scars evident on G. bucklandensis. Prevec et al. (2009: pl. 11, figs 3–5, 9, 10) illustrated several Glossopteris leaves from the Late Permian Normandien Formation of the Karoo Basin, South Africa, that bear regimented elliptical indendations and mounds flanking the midrib that were interpreted to represent insect oviposition scars. These are remarkably similar in size and arrangement to the scars on UQF76185a,b, although the former are more regularly elliptical and less deeply indented. Both forms are here interpreted to represent arthropod oviposition scars, although there is a weak possibility that they represent galls on the basis of their strong surface relief and slight distortion of the secondary venation. Prevec et al. (2009) suggested that members of the Palaeodictyoptera (an extinct Palaeozoic insect group) and Odonatoptera (including the modern damselflies and dragonflies but also many extinct groups) were the likely producers of the oviposition scars on the South African leaves, and these are the probable generators of the scars on UQF76185a,b.
Solitary arthropod oviposition scar on Glossopteris bucklandensis (Glossopteridales: Late Permian)
Locality, stratigraphy, age and palaeolatitude. As for UQF76185a,b described above.
Description. This leaf is the holotype of Glossopteris bucklandensis (Fig. 4C) and was fully described by McLoughlin (1990a). The leaf bears a distinct spindle-shaped scar (4 mm long and 1 mm wide) slightly off-centred along the base of the midrib (Fig. 4G). The central part of this feature is embedded moderately into the rock matrix, indicating that it was formed by an originally raised (positive) structure. Apart from a slightly raised and thickened rim (on the impression), the structure is otherwise featureless.
Remarks. The damage to the base of UQF76186a was originally interpreted to represent the likely detachment point of a Senotheca-like fructification (McLoughlin 1990a). However, its slightly off-centred position on the midrib argues against this. It is similar in shape to a range of arthropod oviposition scars developed on the lamina and midrib of several Glossopteris morphospecies from the Late Permian of South Africa (Prevec et al. 2009), and was likely formed by the same process. However, in the absence of organic preservation, interpretation as a gall-attachment site cannot be excluded. This scar is notably different from the oviposition features on the conspecific leaf UQF76185a,b, described above, in its shape and solitary aspect. White (1961: figs 8, 9) illustrated two additional Glossopteris leaves from the Early Permian Agate Creek Volcanic Group, northern Queensland, with similar solitary scars or thickenings on their midribs that are likely to have derived from the same style of arthropod interaction.
Galls on Dicroidium odontopteroides (Corystospermales: Early Triassic)

|
Locality. Turrimetta Head (33°41′46″S, 151°18′51″E; Fig. 1B), Sydney Basin, 20 km north-north-east of Sydney city centre, New South Wales, Australia.
Stratigraphy and age. Newport Formation – correlated by Retallack (1977) to the Aratrisporites tenuispinosus Palynozone of late Olenekian to Anisian (late Early to early Middle Triassic: ~247–237 million years ago) age (Helby et al. 1987).
Palaeolatitude. Located at ~55–60°S (Scotese 1997).
Description. This incomplete pinnate leaf is 76 mm long and ~49 mm wide (Fig. 5A). Several pinnules have detached or are concealed. Pinnules are inserted on the rachis at 50–60° in opposite to alternate arrangement and reach 25 mm long and 8 mm wide. Pinnules have full basal attachment to the rachis, with a straight acroscopic margin and slightly decurrent basiscopic margin; the remainder of the pinnule margin is slightly undulate and forms a broadly rounded apex. Each pinnule has a weakly defined mid-vein, giving off sparsely dichotomous subsidiary veins at 40–50°. The leaf bears a minimum of 23 circular to slightly elliptical scars, ~13 of which are positioned on the rachis or on pinnule bases abutting the rachis, the remainder being distributed along the pinnules (mostly on, or close to, the mid-veins). The scars are typically ~3 mm in diameter on the pinnules but tend to be larger on the rachis where they reach 5.5 mm in maximum dimensions (Fig. 5B). The scars are generally represented by a flat disc set in a lamina depression. The central part of the disc is raised to form a 0.5-mm-diameter low tubercle (Fig. 5C–E). The best-preserved examples show weak striae radiating from the central tubercle to the disc margin (Fig. 5E). About half the scars apparently have their main expression on the concealed side of the leaf, and in these cases the corresponding position on the exposed surface is represented by a relatively featureless low mound surrounded by a depressed rim (Fig. 5B).
Remarks. Retallack (1977) referred conspecific leaves from this formation to Dicroidium lancifolium lancifolium (Morris) Gothan, and these were later reassigned to Dicroidium odontopteroides (Morris) Gothan forma odontopteroides by Anderson and Anderson (1985). Although similar-sized elliptical scars are commonly produced by scale insects (see e.g. piercing and sucking Damage Types 53, 77, 86 and 128 of Labandeira et al. 2007), such scars typically differ from those described above in having regular concentric ornament corresponding to shield-growth increments (Wappler and Ben-Dov 2008). Furthermore, published records of the Coccoidea (scale insects) are rare from the early Mesozoic, the group having undergone its major radiation in the Cretaceous and Cenozoic, in tandem with angiosperms (Wappler and Ben-Dov 2008; Tosolini and Pole 2010).
Although many galls are robustly thickened structures, flat, discoid galls similar to those on MMF13070 are also manifest in leaves of modern plants. For example, galls produced by Neuroterus quercusbaccarum L. (Cynipidae: Hymenoptera) on modern Quercus robur L. (Fig. 5F) are of similar shape and size with weak radial ornament but differ in bearing distinct scales or hairs. Modern Neuroterus galls are also shortly pedicellate and typically detach from the leaf before senescence, whereas the fossil galls were evidently retained on the leaf. Because only the very oldest Hymenoptera fossils are recorded from the Triassic, it is likely that the galls produced by modern Neuroterus are convergent in morphology, with the presumably single-chambered forms found on the Dicroidium leaf studied here. MMF13070 shows a remarkably heavy infestation of galls, given that galling has been reported only rarely on Permian–Triassic Gondwanan gymosperms (Pant and Srivastava 1995; Banerjee and Bera 1998; Adami-Rodrigues et al. 2004; Scott et al. 2004; Prevec et al. 2009). Significantly, the only galling structures identified within the intensively studied Molteno Formation flora (early Late Triassic) of South Africa were also on Dicroidium leaves (D. crassinervis: Scott et al. 2004).
Arthropod oviposition scars on Taeniopteris parvilocus (gymnosperm: Middle Triassic)

|
Locality. Road-cutting on the western side of Wivenhoe Hill (27°22′36″S, 152°35′21″E; Fig. 1B), Esk Trough, ~45 km west-north-west of Brisbane, Queensland, Australia.
Stratigraphy and age. Esk Formation; dated as Anisian–Ladinian (Middle Triassic: 246–229 milliion years) on the basis of palynostratigraphy (de Jersey 1975) and a radiometric date of 236–242 ± 5 million years from lavas in the overlying and partly interdigitating Neara Volcanics (Webb 1982a).
Palaeolatitude. Located at ~50°S during the Middle Triassic (Scotese 1997).
Description. This specimen is an incomplete leaf impression (lacking counterpart, Fig. 6A), measuring 45 mm long and 21 mm in maximum width (length of the complete leaf is estimated to have been ~110 mm). The margins are entire but neither apex nor base is preserved. The leaf has a stout (1 mm wide) midvein that gives off slender lateral veins at >65°, with a density of 12 per cm. These rarely bifurcate, arch only slightly and run parallel (0.5–0.7 mm apart) across the lamina to the margin. Along both sides of the midrib and situated between the bases of the majority of the lateral veins are circular to elliptical scars with a maximum diameter of 0.5–1 mm. The scars are essentially featureless apart from a slightly raised and discoloured (mineral-stained) rim (Fig. 6E, F). Although a scar is evident between each pair of lateral veins in the proximal half of the leaf fragment (Fig. 6B), the scars are more sporadic in the distal half and disappear well before the leaf apex (Fig. 6A, E). The leaf also shows a small area of rimmed discoloration in mid-lamina (Fig. 6D) and a zone of irregular marginal damage towards the apex (Fig. 6C).
Remarks. The leaf’s morphology is consistent with Taeniopteris parvilocus Anderson & Anderson 1989, reported widely across Gondwana from the Middle to early Late Triassic (Anderson and Anderson 1989). The damage features flanking the midrib of this leaf are interpreted to represent insect oviposition scars on the basis of their shape and arrangement similar to scars on UQF76185a,b, described above, and with examples illustrated by Labandeira et al. (2007: Damage Type 76) and Prevec et al. (2009: pl. 11, figs 3–5, 9, 10). Similar midrib-flanking arrangements of eggs are produced by a range of modern insects, e.g. Galerucella spp. (Chrysomelidae: Coleoptera) and Austroasca spp. (Cucadellidae: Hemiptera). Oviposition scars with broadly similar arrangement from the Paleogene and attributed to the ichnogenus Paleoovoidus (Sarzetti et al. 2009: fig. 2.3) were probably produced by members of the Zygoptera (Odonata). Webb (1982b: fig. 4B) reported insect oviposition scars on Dictyophyllum bremerense Shirley (Dipteridaceae) from the same rock unit as NRMS089026. However, the scars on that fern are slightly smaller and occur in elliptical clusters rather than in regular files. The region of mid-lamina discoloration bounded by a stained rim and located between two sets of veins (Fig. 6A, D) may represent arthropod hole-feeding damage similar to Damage Type 02 of Labandeira et al. (2007) or a small area of surface feeding similar to Damage Type 28 illustrated by the same authors. Two areas of damage near the apex of the leaf (Fig. 6C) are broadly similar to deeply incised margin feeding (Damage Type 15) of Labandeira et al. (2007).
Discussion
Palaeoecological interactions
The fossil galls and oviposition scars described here add to a growing battery of evidence for probable arthropod interactions with Gondwanan late Palaeozoic and early Mesozoic plants (see summaries by Adami-Rodrigues et al. 2004; Scott et al. 2004; Labandeira 2006; Prevec et al. 2009). The Gondwanan Permian now yields evidence of all arthropod functional feeding groups, except seed predation, leaf mining and nectarivory, the last of which is difficult to detect in the fossil record (Labandeira 2006). Moreover, mine-like traces have recently been detected on South African glossopterid leaves (Prevec et al. 2009: pl. 13, figs 1–4). Unambiguous examples of all functional feeding groups are evident in the Gondwanan fossil record by the Triassic. Records of Gondwanan plant–arthropod interactions are overwhelmingly associated with the dominant gymnosperms of the time, namely glossopterids (in the Permian) and corystosperms (in the Triassic); only a few examples are known from other plant groups (Webb 1982b; Rozefelds and Sobbe 1987; Scott et al. 2004; Beattie 2007). External foliage-feeding traces are by far the most common evidence of arthropod interactions in these floras so the new records of galling and oviposition add breadth to the known range of feeding and reproductive strategies of arthropods in Permian–Triassic forest communities.
Galls can form in all parts of a plant; however, ~80% of extant examples occur on leaves, although this proportion may not have been the case in the ancient past (Labandeira 2002). The evidence thus far from Gondwanan glossopterids is consistent with this, because galls have not been found on organs other than leaves in this well-studied plant group. Galls form on almost all modern groups of higher plants and can be found in most terrestrial ecosystems on evergreen and deciduous, and annual and perennial taxa, although a preference for long-lived hosts appears to be a typical phenomenon (Crespi et al. 1997). Leaf galls may be initiated at any stage of foliar development, from within the bud to mature leaves. Because mid- to high-palaeolatitude glossopterids and corystosperms are interpreted to have been winter-deciduous (Retallack 1980), the Gondwanan gall-inducing organisms are likely to have had an infection–maturation–reproduction cycle tuned to the seasonal production of leaves in the host plants.
The role of galls in whole-plant and community ecology may be complex. Although galls cause tissue damage and intuitively reduce the ‘fitness’ of the host plant (Silva et al. 1996; Cuevas-Reyes et al. 2006), they also make leaves less palatable to a range of herbivores (Karban and Myers 1989). Although galls increase leaf mortality in some cases (Williams and Whitham 1986), even intense gall development rarely leads to the death of the host plant. Given the scarcity of galls thus far reported from the Permian, gall-inducing arthropods probably played a relatively minor role in influencing the fitness of plants in Gondwanan glossopterid forest mires.
The location of large galls in a proximal position on the midrib of glossopterids may be significant in explaining the solitary nature of galls on this plant group. Whitham (1978) indicated that populations of the extant aphid, Pemphigus betae Doane, actively compete for optimal position (proximal midvein region) on a leaf – the galling site strongly affecting the parasite’s maturation and reproductive success. This commonly results in the development of only a single gall per leaf. Preferential ovipositioning on the proximal midrib of glossopterids by an adult arthropod may have permitted monopolisation of resources by the gall-forming larva, and avoidance of the infected leaf by other ovipositing adults.
Few studies have attempted quantification of arthropod damage on Gondwanan fossil-leaf assemblages. Of these, Adami-Rodrigues et al. (2004) reported that 8.24% of leaves (predominantly Glossopteris and a few Noeggerathiopsis) in an Early Permian assemblage from Brazil showed evidence of arthropod damage. A single Late Permian fossil assemblage from the Karoo Basin, South Africa, recently yielded evidence of 22 distinctive damage types on 137 of the total of 9772 plant organs scored (=1.4%), which were almost exclusively glossopterids (Prevec et al. 2009). Analysis of several assemblages from the early Late Triassic Molteno Formation, South Africa, revealed levels of arthropod damage on leaves ranging from 3 to 25%. The majority of damage was recorded on corystosperms, ginkgoaleans and voltzialean conifers; however, a range of subsidiary gymnosperms was also affected (Scott et al. 2004).
Given the small sample size, quantitative surveys were not attempted in the present study. Nevertheless, the new records suggest that organisms producing galls and oviposition scars on leaves were more widespread and common in the late Palaeozoic and early Mesozoic than previously suspected (cf. Larew 1992; Scott et al. 2004; Labandeira 2006). The Permian examples described here are among the earliest Gondwanan gall types; the oldest of these being only ~12 million years younger than the most ancient gall types yet reported (Labandeira and Phillips 2002).
Additional putative galls and oviposition scars
The scarcity of reported galls on glossopterids may, in part, stem from these features being readily confused with poorly preserved or immature reproductive organs. Reproductive structures and galls both apparently attach to the midribs of Glossopteris leaves and develop similar shapes (Plumstead 1958; Anderson and Anderson 1985; McLoughlin 1990b). Consequently, several other records of ill-defined leaf-borne structures interpreted by previous authors to represent ovuliferous organs deserve reinvestigation. Among these, a 14 × 7-mm elliptical organ attached to the midrib of a late Early Permian Glossopteris leaf from India (Sen 1963) is potentially a gall, on the basis of its lack of clearly defined seed scars or a marginal wing characteristic of glossopterid fructifications. Similarly, solitary ill-defined elliptical structures, <10 mm long, centred on the midribs of several Glossopteris leaves from the late Early Permian of India and assigned to Bokarospermum maheshwari (Singh 2002) probably represent oviposition scars or galls on the basis of their size, shape and distribution. Smaller circular markings found scattered across the lamina of some other late Early Permian Glossopteris leaves from India (Sen 1955) may represent fungal damage or diagenetic mineral staining.
Lanceolatus bonairensis Menendez (1962) from the Early Permian of Argentina is represented by the impression of a partially enrolled Glossopteris leaf with a cluster of small circular indentations flanking the midrib. The irregular disposition of the indentations and their position adjacent to the midrib, together with the lack of any associated imprint signalling the outline of a fructification, suggest that these features represent arthropod oviposition scars. Bordy and Prevec (2008: plates 3E, F) illustrated South African Glossopteris leaves with dense oviposition scars in the proximal half that Plumstead (1970) had similarly misidentified as fructifications.
An unusual multi-chambered globular structure, 30–40 mm in diameter, from the Early Permian of South Africa, assigned to Breytenia plumsteadiae by Melville (1983a), has been interpreted as an angiosperm-like glossopterid reproductive structure enclosing seeds in locule-like chambers (Plumstead 1962, 1963; Melville 1983b). However, this structure lacks the lamina-like architecture of typical glossopterid fructifications (Gould and Delevoryas 1977); hence, other researchers have found Melville’s interpretation unconvincing and the fossil has generally been excluded from subsequent reviews of glossopterid reproductive organs. Nevertheless, the biological affinity of this complex structure remains unresolved and it deserves re-appraisal to assess whether it may represent an early example of a large multi-chambered gall.
End-Palaeozoic community turnover
Arthropod interactions with the Permian glossopterid-dominated flora and the Triassic corystosperm-dominated flora in Gondwana represent part of Labandeira’s (2006) Herbivore Expansion Phases 3 and 4, respectively. The transition between these pulses essentially equates to the global restructuring of plant and insect communities during the end-Permian mass extinction, when insects suffered the loss of about eight orders and a reduction in family-level diversity of >50%, together with dramatic changes in relative diversity (Anderson et al. 1999; Labandeira 2005; Erwin 2006). The new records of Early Triassic galls and Middle Triassic ovipositioning scars from the Sydney Basin and Esk Trough represent the earliest records of such structures in Gondwana subsequent to the end-Permian biotic crisis (Labandeira 2006). If Palaeozoic Gondwanan gall-inducing arthropods disappeared with their host plants (glossopterids) during the end-Permian extinction event, then this specialised feeding and reproductive strategy was clearly already re-established in the Gondwanan floras by the Middle Triassic (6 million years later).
Given the rich fossil floras represented in Gondwanan Permian and Triassic strata, a systematic survey of all adpression assemblages is likely to reveal a wealth of additional evidence for arthropod–plant interactions. Such surveys, combined with studies of permineralised peats (Weaver et al. 1997; Kellogg and Taylor 2004), coprolite-content analysis (Chin 2007; Vijaya et al. 2009), mesofossil studies (Tosolini and Pole 2010) and vertebrate dental microwear investigations (Teaford 1991), offer multiple future avenues for reconstructing the food webs and ecology of the unique austral polar forests of the late Palaeozoic and earliest Mesozoic.
Acknowledgements
I thank Zhen Yong-yi (Australian Museum), Ian Percival (Geological Survey of New South Wales) and Alex Cook and Kristin Spring (Queensland Museum) for access to specimens in their care. Marion Bamford is thanked for providing images of fossils from the Bernard Price Institute of Palaeontology, Johannesburg. Yvonne Arremo (Swedish Museum of Natural History) photographed modern gall types. I thank Torsten Wappler (University of Bonn) for his opinions on the identity of the Triassic galls. The comments of two anonymous reviewers helped improve the manuscript. Funding is acknowledged from the Swedish Research Council and an Australian Research Council Linkage Project (LP100100339).
References
Adami-Rodrigues K, Iannuzzi R, Pinto ID (2004) Permian plant–insect interactions from a Gondwana flora of southern Brazil. Fossils and Strata 51, 106–125.Anderson JM, Anderson HM (1985) ‘Palaeoflora of southern Africa. Prodromus of South African megafloras Devonian to Lower Cretaceous.’ (A.A. Balkema: Rotterdam, The Netherlands)
Anderson JM, Anderson HM (1989) ‘Palaeoflora of southern Africa, Molteno Formation (Triassic) 2. Gymnosperms (excluding Dicroidium).’ (A.A. Balkema: Rotterdam, The Netherlands)
Anderson JM, Anderson HM, Archangelsky S, Bamford M, Chandra S, Dettmann ME, Hill RS, McLoughlin S, Rösler O (1999) Patterns of Gondwana plant colonisation and diversification. African Journal of Earth Sciences 28, 145–167.
| Patterns of Gondwana plant colonisation and diversification.Crossref | GoogleScholarGoogle Scholar |
Banerjee M (1969) Senotheca murulidihensis a new glossopteridean fructification from India associated with Glossopteris taeniopteroides Feistm. In ‘J. Sen memorial volume’. (Eds H Santapau, AK Ghosh, SK Roy, S Chanda, SK Choudhuri) pp. 359–368. (Botanical Society of Bengal: Calcutta, India)
Banerjee M, Bera S (1998) Record of zoocecidia on leaves of Glossopteris browniana Brongn. from Mohuda Basin, Upper Permian, Indian Lower Gondwana. Indian Biologist 30, 58–61.
Beattie R (2007) The geological setting and palaeoenvironmental and palaeoecological reconstructions of the Upper Permian insect beds at Belmont, New South Wales, Australia. African Invertebrates 48, 41–57.
Béthoux O, Galtier J, Nel A (2004) Earliest evidence of insect endophytic oviposition. Palaios 19, 408–413.
| Earliest evidence of insect endophytic oviposition.Crossref | GoogleScholarGoogle Scholar |
Bordy EM, Prevec R (2008) Sedimentology, palaeontology and palaeo-environments of the Middle (?) to Upper Permian Emakwenzini Formation (Karoo Supergroup, South Africa). South African Journal of Geology 111, 429–458.
| Sedimentology, palaeontology and palaeo-environments of the Middle (?) to Upper Permian Emakwenzini Formation (Karoo Supergroup, South Africa).Crossref | GoogleScholarGoogle Scholar |
Briggs DJC (1998) Permian Productidina and Strophalosiidina from the Sydney–Bowen Basin and New England Orogen: systematics and biostratigraphic significance. Memoir of the Association of Australasian Palaeontologists 19, 1–258.
Chin K (2007) The paleobiological implications of herbivorous dinosaur coprolites from the Upper Cretaceous Two Medicine Formation of Montana: why eat wood? Palaios 22, 554–566.
| The paleobiological implications of herbivorous dinosaur coprolites from the Upper Cretaceous Two Medicine Formation of Montana: why eat wood?Crossref | GoogleScholarGoogle Scholar |
Cuevas-Reyes P, Quesada M, Oyama K (2006) Abundance and leaf damage caused by gall-inducing insects in a Mexican tropical dry forest. Biotropica 38, 107–115.
Crespi BJ, Carmean DA, Chapman TW (1997) Ecology and evolution of galling thrips and their allies. Annual Review of Entomology 42, 51–71.
| Ecology and evolution of galling thrips and their allies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjvFSgsw%3D%3D&md5=7ba5a7f9ab88a6ded5c364d084ff4918CAS | 15012307PubMed |
de Jersey NJ (1975) Miospore zones in the lower Mesozoic of southeastern Queensland. In ‘Gondwana geology: papers from the 3rd Gondwana symposium, Canberra, 1973’. (Ed. KSW Campbell) pp. 159–172. (ANU Press: Canberra)
Dreger-Jauffret F, Shorthouse JD (1992) Diversity of gall-inducing insects and their galls. In ‘Biology of insect-induced galls’. (Eds JD Shorthouse, O Rohfritsch) pp. 8–33. (Oxford University Press: Oxford, UK)
Erwin DH (2006) ‘Extinction. How life on Earth nearly ended 250 million years ago.’ (Princeton University Press: Princeton, NJ)
Gould RE, Delevoryas T (1977) The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs. Alcheringa 1, 387–399.
| The biology of Glossopteris: evidence from petrified seed-bearing and pollen-bearing organs.Crossref | GoogleScholarGoogle Scholar |
Helby R, Morgan R, Partridge AD (1987) A palynological zonation of the Australian Mesozoic. Memoir of the Association of Australasian Palaeontologists 4, 1–94.
Karban R, Myers JH (1989) Induced plant responses to herbivory. Annual Review of Ecology and Systematics 20, 331–348.
| Induced plant responses to herbivory.Crossref | GoogleScholarGoogle Scholar |
Kellogg DW, Taylor EL (2004) Evidence of oribatid mite detritivory in Antarctica during the late Paleozoic and Mesozoic. Journal of Paleontology 78, 1146–1153.
| Evidence of oribatid mite detritivory in Antarctica during the late Paleozoic and Mesozoic.Crossref | GoogleScholarGoogle Scholar |
Keyser N (Compiler) (1997) ‘Geological map of the Republic of South Africa.’ (Council for Geoscience: Pretoria, South Africa)
Krassilov V, Silantieva N, Lewy Z (2008) Part I. Traumas on fossil leaves from the Cretaceous of Israel. In ‘Plant–arthropod interactions in the early angiosperm history. Evidence from the Cretaceous of Israel’. (Eds V Krassilov, A Rasnitsyn) pp. 1–187. (Pensoft Publishers: Sofia, Bulgaria)
Labandeira CC (1997) Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annual Review of Ecology and Systematics 28, 153–193.
| Insect mouthparts: ascertaining the paleobiology of insect feeding strategies.Crossref | GoogleScholarGoogle Scholar |
Labandeira CC (2002) The history of associations between plants and animals. In ‘Plant–animal interactions: an evolutionary approach’. (Eds CM Herrera, O Pellmyr) pp. 26–74, 248–261. (Blackwell: London)
Labandeira CC (2005) The fossil record of insect extinction: new approaches and future directions. American Entomologist 51, 14–29.
Labandeira CC (2006) The four phases of plant-arthropod associations in deep time. Geologica Acta 4, 409–438.
Labandeira CC, Allen EG (2007) Minimal insect herbivory for the Lower Permian Coprolite Bone Bed site of north-central Texas, USA, and comparison to other late Paleozoic floras. Palaeogeography, Palaeoclimatology, Palaeoecology 247, 197–219.
| Minimal insect herbivory for the Lower Permian Coprolite Bone Bed site of north-central Texas, USA, and comparison to other late Paleozoic floras.Crossref | GoogleScholarGoogle Scholar |
Labandeira CC, Phillips TL (1996) A Carboniferous insect gall: insight into early ecologic history of the Holometabola. Proceedings of the National Academy of Sciences, USA 93, 8470–8474.
| A Carboniferous insect gall: insight into early ecologic history of the Holometabola.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkvFyksb0%3D&md5=90ca22be4f02f1a3c81ac1d759d5a964CAS |
Labandeira CC, Phillips TL (2002) Stem borings and petiole galls from Pennsylvanian tree ferns of Illinois, USA: implications for the origin of the borer and galling functional-feeding-groups and holometabolous insects. Palaeontographica 264, 1–84.
Labandeira CC, Wilf P, Johnson KR, Marsh F (2007) ‘Guide to insect (and other) damage types on compressed plant fossils. Version 3.0.’ (Smithsonian Institution: Washington, DC)
Larew HG (1992) Fossil galls. In ‘Biology of insect-induced galls’. (Eds JD Shorthouse, O Rohfritsch) pp. 50–59. (Oxford University Press: New York)
Le Roux SF, Anderson HM (1977) A review of the localities and flora of the lowest Permian Karoo strata at Vereeniging, South Africa. Palaeontologia africana 20, 27–42.
Ludwig R (1857) Fossile Pflanzen aus der altestem Abtheilung der rheinisch-wetterauer Tertiär-Formation. Palaeontographica 8, 81–109.
Mani MS (1964) ‘Ecology of plant galls.’ (W. Junk: The Hague, The Netherlands)
Mani MS (1992) Introduction to Cecidology. In ‘Biology of insect-induced galls’. (Eds JD Shorthouse, O Rohfritsch) pp. 1–7. (Oxford University Press: Oxford, UK)
McLoughlin S (1988) Geology of the Inglis Dome, Denison Trough, central Queensland. Papers of the Department of Geology, University of Queensland 12, 229–263.
McLoughlin S (1990a) Some Permian glossopterid fructifications and leaves from the Bowen Basin, Queensland, Australia. Review of Palaeobotany and Palynology 62, 11–40.
| Some Permian glossopterid fructifications and leaves from the Bowen Basin, Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
McLoughlin S (1990b) Late Permian glossopterid fructifications from the Bowen and Sydney Basins, eastern Australia. Geobios 23, 283–297.
| Late Permian glossopterid fructifications from the Bowen and Sydney Basins, eastern Australia.Crossref | GoogleScholarGoogle Scholar |
McLoughlin S (1994a) Late Permian plant megafossils from the Bowen Basin, Queensland, Australia: Part 2. Palaeontographica (B) 231, 1–29.
McLoughlin S (1994b) Late Permian plant megafossils from the Bowen Basin, Queensland, Australia: Part 3. Palaeontographica (B) 231, 31–62.
Melville R (1983a) Two new genera of Glossopteridae. Botanical Journal of the Linnean Society 86, 275–277.
| Two new genera of Glossopteridae.Crossref | GoogleScholarGoogle Scholar |
Melville R (1983b) Glossopteridae, Angiospermidae and the evidence for angiosperm origins. Botanical Journal of the Linnean Society 86, 279–323.
| Glossopteridae, Angiospermidae and the evidence for angiosperm origins.Crossref | GoogleScholarGoogle Scholar |
Menendez CA (1962) Hallazgo de una fructificación en la Flora de Glossopteris de la provincia de Buenos Aires (Lanceolatus bonariensis sp. nov.). Con consideraciones sobre la nomenclatura de fructificaciones de Glossopteris. Ameghiniana 2, 175–182.
Meyer J (1987) ‘Plant galls and gall inducers.’ (Gebrüder Borntraeger: Berlin)
Millsteed BD (1994) Palynological evidence for the age of the Permian Karoo coal deposits near Vereeniging, northern Orange Free State, South Africa. South African Journal of Geology 97, 15–20.
Ogg JG, Ogg G, Gradstein FM (2008) ‘The concise geologic time scale.’ (Cambridge University Press: Cambridge, UK)
Pant DD, Srivastava PC (1995) Lower Gondwana insect remains and evidences of insect–plant interaction. In ‘Proceedings of the first international conference on global environment and diversification through geological time’. (Eds DD Pant, DD Nautiyal, AN Bhatnagar, KR Surange, MD Bose, PK Khare) pp. 317–326. (Society of Indian Plant Taxonomists: Allahabad, India)
Plumstead EP (1958) Further fructifications of the Glossopteridae and a provisional classification based on them. Transactions – Geological Society of South Africa 61, 1–58.
Plumstead EP (1962) Possible angiosperms from Lower Permian coal of the Transvaal. Nature 194, 594–595.
| Possible angiosperms from Lower Permian coal of the Transvaal.Crossref | GoogleScholarGoogle Scholar |
Plumstead EP (1963) The influence of plants and environment on the developing animal life of Karoo times. South African Journal of Science 59, 147–152.
Plumstead EP (1969) ‘Three thousand million years of plant life in Africa.’ Alex L Du Toit Memorial Lectures 11. (Geological Society of South Africa: Pretoria, South Africa)
Plumstead EP (1970) Recent progress and the future of palaeobotanical correlation in Gondwanaland. In ‘Proceedings 2nd IUGS symposium on Gondwana stratigraphy and palaeontology’. (Ed. SH Haughton) pp. 139–144. (Council for Scientific and Industrial Research, South Africa: Pretoria, South Africa)
Potonié H (1893) Die Flora des Rothliegenden von Thüringen. Abhandlungen der Koniglich Preussischen Geologischen Landesandstalt 9, 1–298.
Pott C, Labandeira CC, Krings M, Kerp H (2008) Fossil insect eggs and ovipositional damage on bennettitalean leaf cuticles from the Carnian (Upper Triassic) of Austria. Journal of Paleontology 82, 778–789.
| Fossil insect eggs and ovipositional damage on bennettitalean leaf cuticles from the Carnian (Upper Triassic) of Austria.Crossref | GoogleScholarGoogle Scholar |
Prevec R, Labandeira CC, Neveling J, Gastaldo RA, Looy CV, Bamford M (2009) Portrait of a Gondwanan ecosystem: a new late Permian fossil locality from KwaZulu-Natal, South Africa. Review of Palaeobotany and Palynology 156, 454–493.
| Portrait of a Gondwanan ecosystem: a new late Permian fossil locality from KwaZulu-Natal, South Africa.Crossref | GoogleScholarGoogle Scholar |
Price PL (1983) A Permian palynostratigraphy for Queensland. In ‘Proceedings of the symposium on the Permian geology of Queensland’. (Ed. Anonymous) pp. 155–212. (Geological Society of Australia, Queensland Division: Brisbane)
Raman A, Schaefer CW, Withers TM (2005) Galls and gall-inducing arthropods: an overview of their biology, ecology, and evolution. In ‘Biology, ecology, and evolution of gall-inducing arthropods’. (Eds A Raman, CW Schaefer, TM Withers) pp. 1–33. (Science Publishers Inc.: Enfield, NH)
Retallack GJ (1977) Reconstructing Triassic vegetation of eastern Australasia: a new approach for the biostratigraphy of Gondwanaland. Alcheringa 1, 247–278.
| Reconstructing Triassic vegetation of eastern Australasia: a new approach for the biostratigraphy of Gondwanaland.Crossref | GoogleScholarGoogle Scholar |
Retallack GJ (1980) Late Carboniferous to Middle Triassic megafossil floras from the Sydney Basin. In ‘A guide to the Sydney Basin’. (Eds C Herbert, RJ Helby) pp. 384–430. (Geological Survey of NSW Bulletin 26: Sydney)
Rohfritsch O (2008) Plants, gall midges, and fungi: a three-component system. Entomologia Experimentalis et Applicata 128, 208–216.
| Plants, gall midges, and fungi: a three-component system.Crossref | GoogleScholarGoogle Scholar |
Roskam JC (1992) Evolution of the gall-inducing guild. In ‘Biology of insect-induced galls’. (Eds JD Shorthouse, O Rohfritsch) pp. 34–49. (Oxford University Press: Oxford, UK)
Rozefelds AC, Sobbe I (1987) Problematic insect leaf mines from the Upper Triassic Ipswich Coal Measures of southeastern Queensland, Australia. Alcheringa 11, 51–57.
| Problematic insect leaf mines from the Upper Triassic Ipswich Coal Measures of southeastern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Sarzetti LC, Labandeira CC, Muzón J, Wilf P, Cúneo NR, Johnson KR, Genise JF (2009) Odonatan endophytic oviposition from the Eocene of Patagonia: the ichnogenus Paleoovoidus and implications for behavioral stasis. Journal of Paleontology 83, 431–447.
| Odonatan endophytic oviposition from the Eocene of Patagonia: the ichnogenus Paleoovoidus and implications for behavioral stasis.Crossref | GoogleScholarGoogle Scholar |
Scotese CR (1997) ‘Paleogeographic atlas. PALEOMAP progress report 90-0497.’ (Department of Geology, University of Texas: Arlington, TX)
Scott AC, Stephenson J, Collinson ME (1994) The fossil record of plant galls. In ‘Plant galls: organisms, interactions, populations’. (Ed. MAJ Williams) pp. 447–470. (Systematics Association Special Volume 49, Clarendon Press: Oxford, UK)
Scott AC, Anderson JM, Anderson HM (2004) Evidence of plant–insect interactions in the Upper Triassic Molteno Formation of South Africa. Journal of the Geological Society 161, 401–410.
Sen J (1955) On some fructifications borne on Glossopteris leaves. Botaniska Notiser 108, 245–251.
Sen J (1963) A glossopteridean fructification from India. Nature 200, 1124
| A glossopteridean fructification from India.Crossref | GoogleScholarGoogle Scholar | 14098462PubMed |
Shi GR, Waterhouse JB, McLoughlin S (2010) The Lopingian of Australasia: a review of biostratigraphy, correlations, palaeogeography and palaeobiogeography. Geological Journal 45, 230–263.
| The Lopingian of Australasia: a review of biostratigraphy, correlations, palaeogeography and palaeobiogeography.Crossref | GoogleScholarGoogle Scholar |
Shorthouse JD, Wool D, Raman A (2005) Gall-inducing insects – Nature’s most sophisticated herbivores. Basic and Applied Ecology 6, 407–411.
| Gall-inducing insects – Nature’s most sophisticated herbivores.Crossref | GoogleScholarGoogle Scholar |
Silva IM, Andrade GI, Fernandes GW, Lemos Filho JP (1996) Parasitic relationships between a gall-forming insect Tomoplagia rudolphi (Diptera: Tephritidae) and its host plant (Vernonia polyanthes, Asteraceae). Annals of Botany 78, 45–48.
| Parasitic relationships between a gall-forming insect Tomoplagia rudolphi (Diptera: Tephritidae) and its host plant (Vernonia polyanthes, Asteraceae).Crossref | GoogleScholarGoogle Scholar |
Singh SM (2002) Seeds, fructifications, bracts and calamitalean axes from the Karanpura and Bokaro group of coalfields. The Palaeobotanist 51, 73–79.
Srivastava PN (1956) Studies in the Glossopteris flora of India – 4. Glossopteris, Gangamopteris and Palaeovittaria from the Raniganj Coalfield. The Palaeobotanist 5, 1–45.
Tadros NZ (1995) Sydney–Gunnedah Basin overview. In ‘Geology of Australian coal basins’. (Eds CR Ward, HJ Harrington, CW Mallett, JW Beeston) pp. 163–175. (Geological Society of Australia, Coal Geology Group, Special Publication 1: Sydney)
Teaford MF (1991) Dental microwear: what can it tell us about diet and dental function? In ‘Advances in dental anthropology’. (Eds MA Larsen, CS Kelley) pp. 341–356. (Alan R. Liss: New York)
Tosolini A-MP, Pole M (2010) Insect and clitellate annelid traces in mesofossil assemblages from the Cretaceous of Australasia. Alcheringa 34, 397–419.
| Insect and clitellate annelid traces in mesofossil assemblages from the Cretaceous of Australasia.Crossref | GoogleScholarGoogle Scholar |
van Amerom HWJ (1973) Gibt es Cecidien im Karbon bei Calamiten und Asterophylliten? In ‘Compte rendu septième congrès international de stratigraphie et de géologie du carbonifère’. (Ed. K-H Josten) pp. 63–83. (Van Acken: Krefeld, Germany)
Vijaya , Prasad GVR, Singh K (2009) Late Triassic palynoflora from the Pranhita–Godavari Valley, India: evidence from vertebrate coprolites. Alcheringa 33, 91–111.
| Late Triassic palynoflora from the Pranhita–Godavari Valley, India: evidence from vertebrate coprolites.Crossref | GoogleScholarGoogle Scholar |
Vjalov OS (1975) Fossil remains of insect feeding. Paleontologicheskiy Sbornik 1–2, 147–155.
Wappler T, Ben-Dov Y (2008) Preservation of armoured scale insects on angiosperm leaves from the Eocene of Germany. Acta Palaeontologica Polonica 53, 627–634.
| Preservation of armoured scale insects on angiosperm leaves from the Eocene of Germany.Crossref | GoogleScholarGoogle Scholar |
Weaver L, McLoughlin S, Drinnan AN (1997) Fossil woods from the Upper Permian Bainmedart Coal Measures, northern Prince Charles Mountains, East Antarctica. AGSO Journal of Australian Geology & Geophysics 16, 655–676.
Webb JA (1982a) Triassic radiometric dates from eastern Australia. In ‘Numerical dating in stratigraphy’. (Ed. GS Odin) pp. 515–521. (John Wiley: New York)
Webb JA (1982b) Triassic species of Dictyophyllum from eastern Australia. AIcheringa 6, 79–91.
| Triassic species of Dictyophyllum from eastern Australia.Crossref | GoogleScholarGoogle Scholar |
White ME (1961) Permian plant fossils from the Agate Creek Volcanics, north Queensland. Bureau of Mineral Resources, Geology and Geophysics, Australia Record 1961(20), 1–10. [unpublished; available from Geoscience Australia: http://www.ga.gov.au/].
Whitham TG (1978) Habitat selection by Pemphigus aphids in response to resource limitation and competition. Ecology 59, 1164–1176.
| Habitat selection by Pemphigus aphids in response to resource limitation and competition.Crossref | GoogleScholarGoogle Scholar |
Williams AG, Whitham TG (1986) Premature leaf abscission: an induced plant defense against gall aphids. Ecology 67, 1619–1627.
| Premature leaf abscission: an induced plant defense against gall aphids.Crossref | GoogleScholarGoogle Scholar |
Wool D (1984) Gall-forming aphids. In ‘The biology of gall insects’. (Ed. TN Ananthakrishnan) pp. 11–58. (Edward Arnold: New Delhi, India)
Yoo EK, Norman A, McDonald I (1995) Sydney Basin – Western Coalfied. In ‘Geology of Australian coal basins’. (Eds CR Ward, HJ Harrington, CW Mallett, JW Beeston) pp. 231–245. Coal Geology Group, Special Publication 1. (Geological Society of Australia: Sydney)


