A threatened ecological community: research advances and priorities for Banksia woodlands
Alison L. Ritchie A B K , Lauren N. Svejcar
A B K , Lauren N. Svejcar  B C , Bronwyn M. Ayre
B C , Bronwyn M. Ayre  A B , Julian Bolleter
A B , Julian Bolleter  D , Aaron Brace E , Michael D. Craig
D , Aaron Brace E , Michael D. Craig  A C , Belinda Davis B , Robert A. Davis
A C , Belinda Davis B , Robert A. Davis  A E , Eddie J. B. van Etten
A E , Eddie J. B. van Etten  E , Joseph B. Fontaine
E , Joseph B. Fontaine  C , William M. Fowler
C , William M. Fowler  C I , Ray H. Froend
C I , Ray H. Froend  E , Christine Groom
E , Christine Groom  A , Giles E. S. J. Hardy
A , Giles E. S. J. Hardy  C F , Paula Hooper
C F , Paula Hooper  D , Anna J. M. Hopkins
D , Anna J. M. Hopkins  E , Michael Hughes
E , Michael Hughes  C , Siegfried L. Krauss
C , Siegfried L. Krauss  A B , Matthias Leopold
A B , Matthias Leopold  G , Ben P. Miller
G , Ben P. Miller  A B H , Russell G. Miller
A B H , Russell G. Miller  B C H , Cristina E. Ramalho
B C H , Cristina E. Ramalho  A , Katinka X. Ruthrof
A , Katinka X. Ruthrof  C H , Christopher Shaw F , Jason C. Stevens
C H , Christopher Shaw F , Jason C. Stevens  A B , Ryan Tangney
A B , Ryan Tangney  B J , Leonie E. Valentine
B J , Leonie E. Valentine  A , Erik J. Veneklaas
A , Erik J. Veneklaas  A G and Richard J. Hobbs
A G and Richard J. Hobbs  A
A
A School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Kings Park Science, Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, 2 Kattidj Close, Kings Park, WA 6005, Australia.
C Environmental and Conservation Sciences, College of Science, Health, Engineering and Education, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
D Australia Urban Design Research Centre, School of Design, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6005, Australia.
E Centre for Ecosystem Management, School of Science, Edith Cowan University, 270 Joondalup Drive, Joondalup, WA 6027, Australia.
F Centre for Phytophthora Science and Management, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
G UWA School of Agriculture and Environment, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6005, Australia.
H Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, 17 Dick Perry Avenue, Kensington, WA 6151, Australia.
I Parks and Wildlife Service, Department of Biodiversity, Conservation and Attractions, PO Box 1266, Mandurah, WA 6210, Australia.
J Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
K Corresponding author. Email: alison.ritchie@uwa.edu.au
Australian Journal of Botany 69(2) 53-84 https://doi.org/10.1071/BT20089
Submitted: 27 July 2020 Accepted: 22 January 2021 Published: 10 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
The rapid expansion of urban areas worldwide is leading to native habitat loss and ecosystem fragmentation and degradation. Although the study of urbanisation’s impact on biodiversity is gaining increasing interest globally, there is still a disconnect between research recommendations and urbanisation strategies. Expansion of the Perth metropolitan area on the Swan Coastal Plain in south-western Australia, one of the world’s thirty-six biodiversity hotspots, continues to affect the Banksia Woodlands (BWs) ecosystem, a federally listed Threatened Ecological Community (TEC). Here, we utilise the framework of a 1989 review of the state of knowledge of BWs ecology and conservation to examine scientific advances made in understanding the composition, processes and functions of BWs and BWs’ species over the last 30 years. We highlight key advances in our understanding of the ecological function and role of mechanisms in BWs that are critical to the management of this ecosystem. The most encouraging change since 1989 is the integration of research between historically disparate ecological disciplines. We outline remaining ecological knowledge gaps and identify key research priorities to improve conservation efforts for this TEC. We promote a holistic consideration of BWs with our review providing a comprehensive document that researchers, planners and managers may reference. To effectively conserve ecosystems threatened by urban expansion, a range of stakeholders must be involved in the development and implementation of best practices to conserve and maintain both biodiversity and human wellbeing.
Keywords: Swan Coastal Plain, biodiversity hotspot, Mediterranean Climate Ecosystem, synthesis, urbanisation.
Introduction
Urban land cover is projected to increase by 1.5 million square kilometres worldwide, and urban populations rise to 5 billion people by 2030 (Seto et al. 2011). This growth is forecast to have high impacts on native species and ecosystems (Hahs et al. 2009; Seto et al. 2011), making the conservation of remnant ecosystems within urban matrices critical (McCarthy et al. 2006; Lawson et al. 2008; Wintle et al. 2019), and the utilisation of scientific knowledge for informing urbanisation strategies imperative. In Australia, cities are generally recognised as hotspots for threatened species (Ives et al. 2016). An example of an urban area undergoing the challenge of balancing increased urbanisation pressures and conservation of biodiversity is the metropolitan region of Perth, Western Australia. Perth is representative of many cities sitting within global biodiversity hotspots where rapid urban development has resulted in extensive loss of native ecosystems.
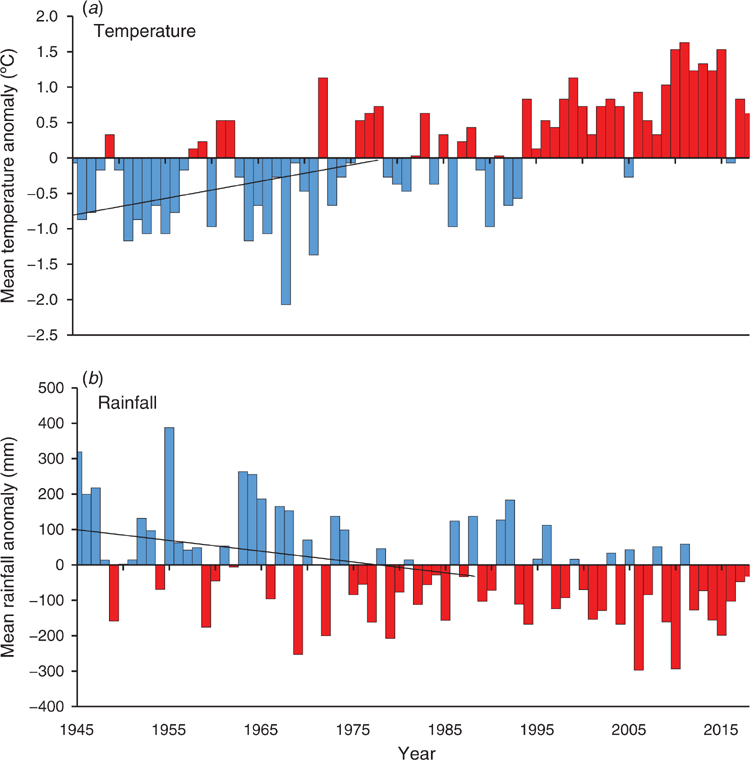
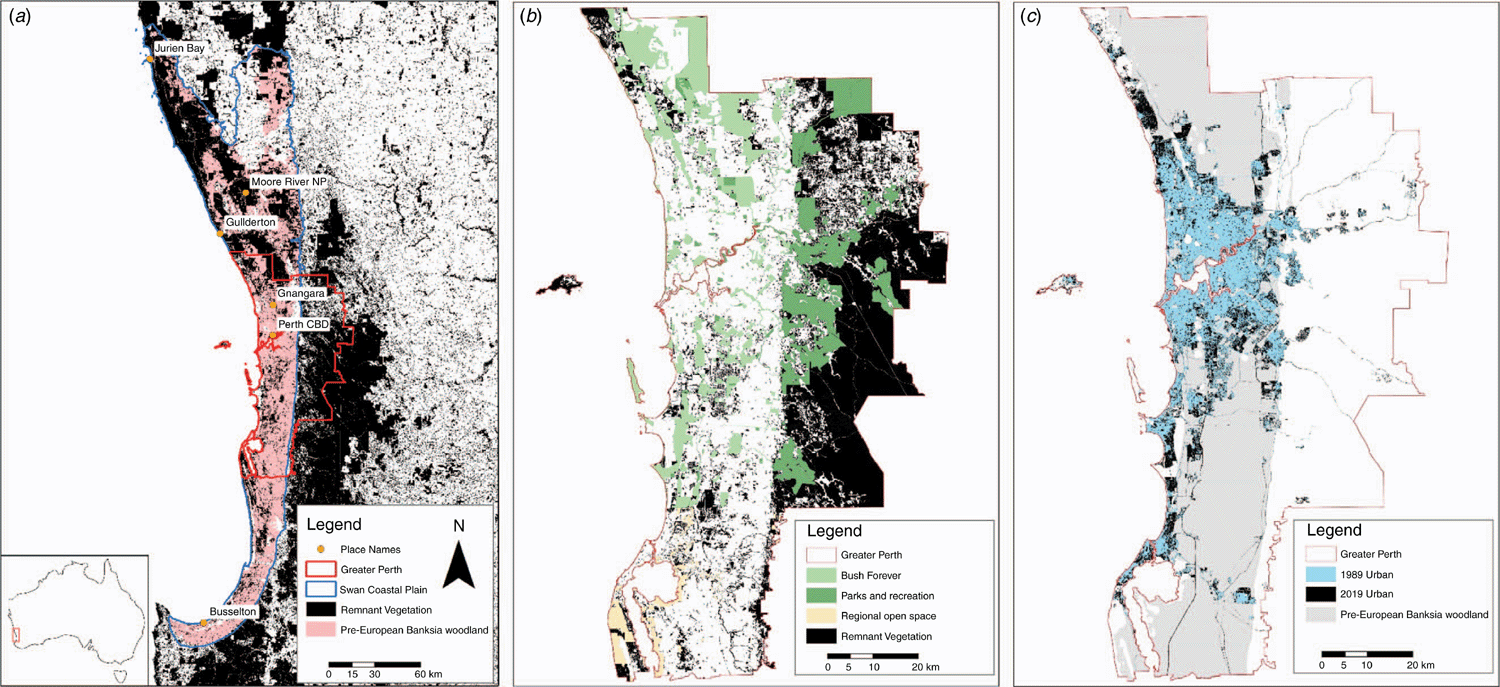
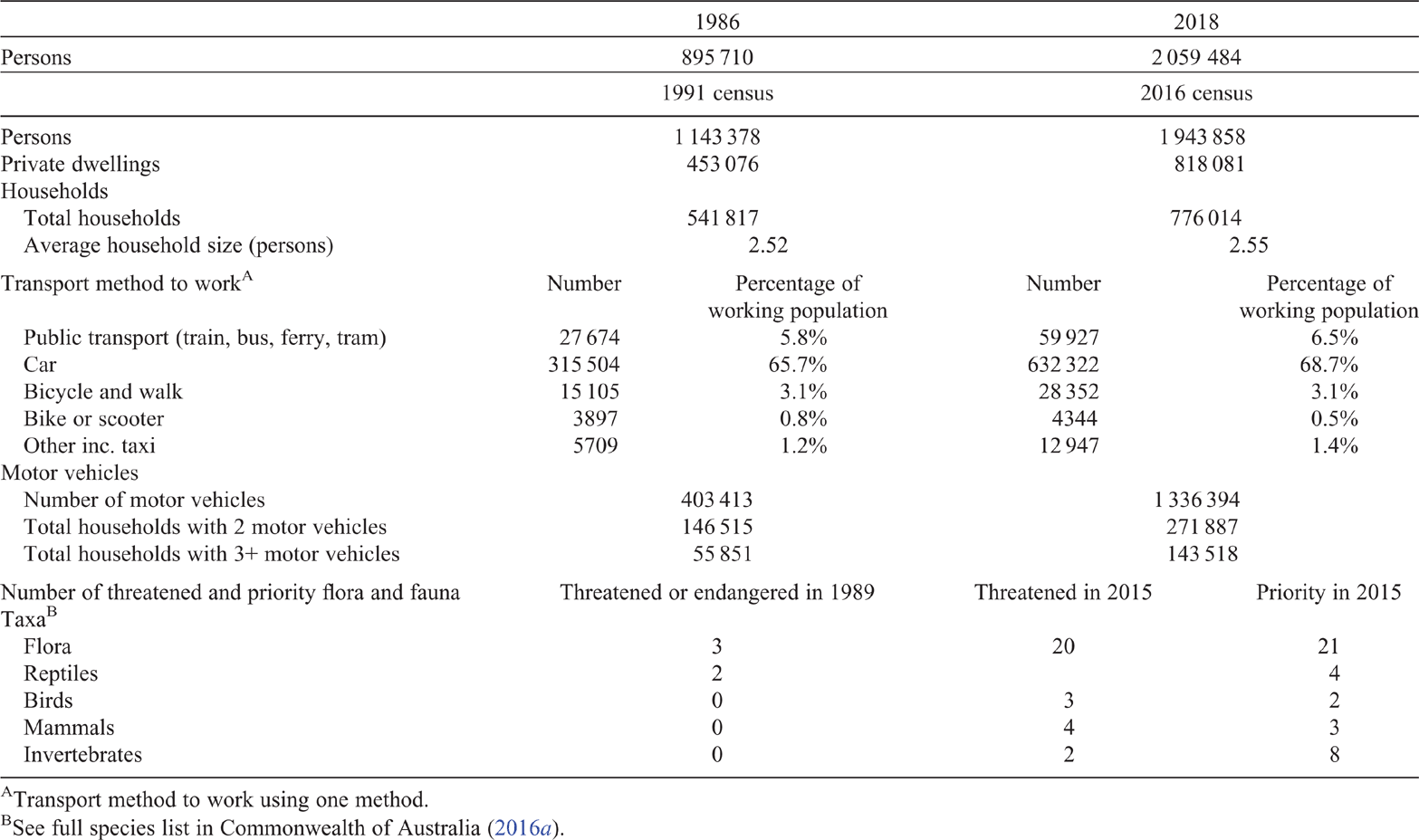
Perth is located on the Swan Coastal Plain (SCP) in South-western Australia (see Fig. 1), where the dominant vegetation community is Banksia woodlands (BWs). The South Western Australian Floristic Region (SWAFR) in which the SCP sits is one of only two recognised global biodiversity hotspots in Australia (Marchese 2015), and one of the world’s five Mediterranean Climate Ecosystems, which is characterised by cool, wet winters and hot, dry summers (Cowling et al. 1996). However, a shift in climatic conditions have been occurring regionally since the 1970s (Bates et al. 2008). Climate change is a major challenge to the conservation and restoration of the BWs ecosystem and the species it supports. Long-term declines in rainfall have occurred since the mid-1970s, with close to 14% less winter rainfall as of 2004 (Bates et al. 2008) (see Fig. 2), and average temperatures have risen by 0.15°C per decade over the same time period (Bates et al. 2008; CSIRO and BOM 2015). The warming and drying trends, and extreme events, throughout the SWAFR region, together with concurrent land transformation (e.g. land clearing, habitat fragmentation and increased urban infrastructure) (CSIRO and BOM 2015) are expected to drive declines in distribution and diversity of floral (Fitzpatrick et al. 2008; Yates et al. 2010) and faunal species (Ruthrof et al. 2018). Some authors highlight that without intervention, plant extinctions are predicted to exceed 2000 species in South-western Australia, making it one of the most vulnerable biodiversity hotspots globally (Malcolm et al. 2006). This risk of species loss increases the importance of the protection and conservation of intact, and restoration of degraded, BWs areas.
|
The most severe land transformations on the SCP include land clearing for agriculture, mining, silviculture, housing, and associated urban infrastructure. Mapping of remnant BWs has been conducted at varying spatial and taxonomic scales to help capture areas most affected and identify fine-scale variation in BWs species composition. By 2016, between 50 and 60% of the original extent of the woodlands had been cleared (Fig. 1a) (Commonwealth of Australia 2016a). Decline in BWs extent is most profound in the Perth metropolitan area with 72% estimated to have been cleared (Commonwealth of Australia 2016a). Current rates of clearing are estimated at 0.34% loss (by area) per year overall, but are much greater in the Perth metropolitan area, at ~1.2% annually (Commonwealth of Australia 2016a). In addition to clearing, much remaining BWs bushland in the metropolitan area is now highly fragmented (Fig. 1) and degraded, decreasing the connectivity between remnant patches and increasing the risk of invasion by introduced species. In 2016, BWs were listed as a Threatened Ecological Community (TEC) under Australia’s national environmental law, the Environmental Protection and Biodiversity Conservation Act 1999 (EPBC Act) given the overall decline in the system’s geographic extent, and its highly fragmented and degraded nature. These threats, and the persistent risks described in the listing advice make this a critical time to evaluate our understanding of the structure, composition and functioning of the BWs ecological community. Ecological communities comprise multiple ecological processes and species interactions; however, mechanisms of ecological functioning are rarely reported in detail within TEC listings (Saunders et al. 2020). Lack of information on ecological processes and species interactions can prevent effective conservation and recovery efforts for TECs (Saunders et al. 2020).
Currently, the primary purpose of clearing is to accommodate a rapidly expanding urban population. Thirty years ago, when Perth’s population was ~0.9 million people, BWs were considered common across the SCP (Hopper and Burbidge 1989). However, by 2018, the population had grown 130% (to >2 million people) (Table 1) and, for example, the clearing of 90 ha of ecologically significant remnant BWs bushland and adjacent wetland for transport networks was an issue that differentiated party political election campaigns at the state government level (Gaynor et al. 2018). The community response to this clearing of remnant bushland demonstrates the need for well-informed debate about the long-term protection, conservation and restoration of BWs within a growing urban environment. This paper summarises the scientific understanding of the structure and functioning of SCP BWs and the threats facing them and lists research priority actions (Fig. 3).
|
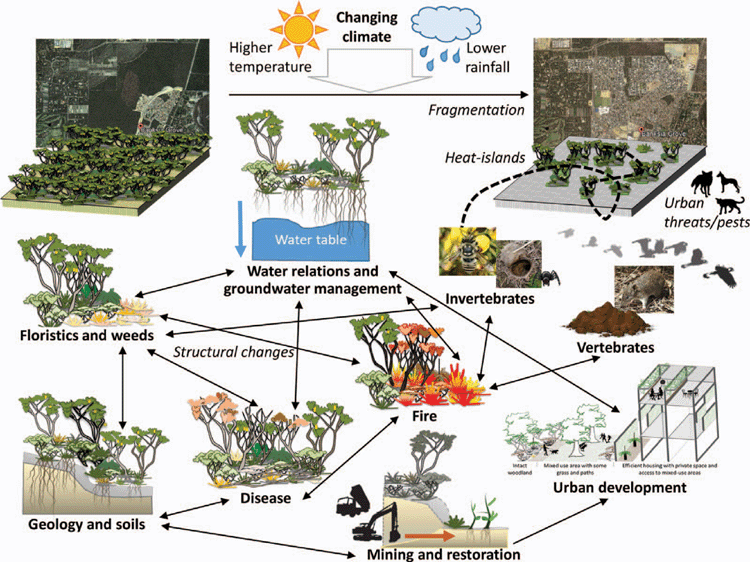
The last major review evaluating the state of BWs, their ecology, functioning and ecosystem management occurred in 1989 (Pate 1989), following a symposium held by the Royal Society of Western Australia. Extensive land transformation, including habitat fragmentation, plant pathogen spread, and invasive species proliferation have continued to cause substantial declines in regional biodiversity (Commonwealth of Australia 2016b). Given these threats to BWs and the species contained therein, we feel it is of critical importance to review advances in knowledge and science communication made since 1989. In this review, we have maintained a similar structure, covering the topics of (1) floristics and weeds, (2) geology and soils, (3) water relations and groundwater management, (4) invertebrates, (5) vertebrates, (6) disease, (7) fire, (8) mining and restoration, and 9) urban development (Fig. 3).
The 1989 review included two sections omitted from this review; the impact of horticulture on BWs and forestry on the SCP. Clearing for agriculture and horticulture has been noted as mainly a past threat (see ‘Approved Conservation Advice for the BWs’ in Commonwealth of Australia 2016a). With regard to forestry, we briefly explore the impacts of Pinus pinaster (maritime pine) plantations in sections dealing with water relations and groundwater management, mining and their association with fauna. We also present a case study of interactions between species and ecological processes, and conclude with future directions needed for SCP BWs research to inform practice and policy development and provide support for their protection, conservation and management.
Methods
This review commenced by inviting 33 BWs researchers from multiple universities and government sectors, to participate by providing expertise in one or more of the nine listed topics. Twenty-nine researchers responded and filled out an online Zoho form (see https://www.zoho.com/forms/, accessed 29 January 2021) to provide a snapshot of peer-reviewed scientific literature and documents published since 1989, which was used to guide the review paper structure. The form listed conservation and research questions posed in the 1989 Banksia Woodlands Symposium and participants were asked (1) whether these questions had been answered over the last 30 years through peer-reviewed publications; (2) where the gaps in knowledge remain and; (3) what are the future directions in research and conservation management for BWs (see ‘Banksia Woodland Review questions’ section of the Supplementary material for more details). The review was then developed through workshops, smaller writing groups (all 29 researchers were divided into groups based on expertise, and charged with drafting specific sections), internal review and follow-up meetings to identify the most pertinent information to BWs ecosystem research, practice and policy development.
Topics
Floristics and weeds
The SCP BWs ecological community is a low woodland dominated (or co-dominated) by Banksia (Proteaceae) trees, most commonly B. attenuata R.Br and B. menziesii R.Br., sometimes with scattered eucalypts and other tree species present within or above the Banksia canopy. The understorey is rich in plant species, including sclerophyllous shrubs, sedges, rushes and geophytes (for the full ecological community definition see Commonwealth of Australia 2016a). The TEC listing observes that ‘many understorey species are locally endemic and most do not occur across the full range of the ecological community’ and that surveys have recorded more than 600 native plant taxa (Commonwealth of Australia 2016a). Banksia woodlands are highly variable in composition across their range and, as such, the community provides habitat for many native flora, fungi and fauna, with remaining patches providing important wildlife corridors and refuges in a mostly fragmented landscape.
Dodd and Griffin (1989) provided general descriptions of BWs vegetation and recognised that floristic types, vegetation associations and their environmental variation had yet to be determined. Further, they recognised that rare and endemic flora of BWs had not been fully assessed, with only three declared rare flora species (all orchids) recognised at the time (Hopper and Burbidge 1989). The mapping of the BWs vegetation community is challenging because it is one of many ecosystems that co-occur in a matrix throughout the SCP making the extent and number of species contained within BWs difficult to quantify.
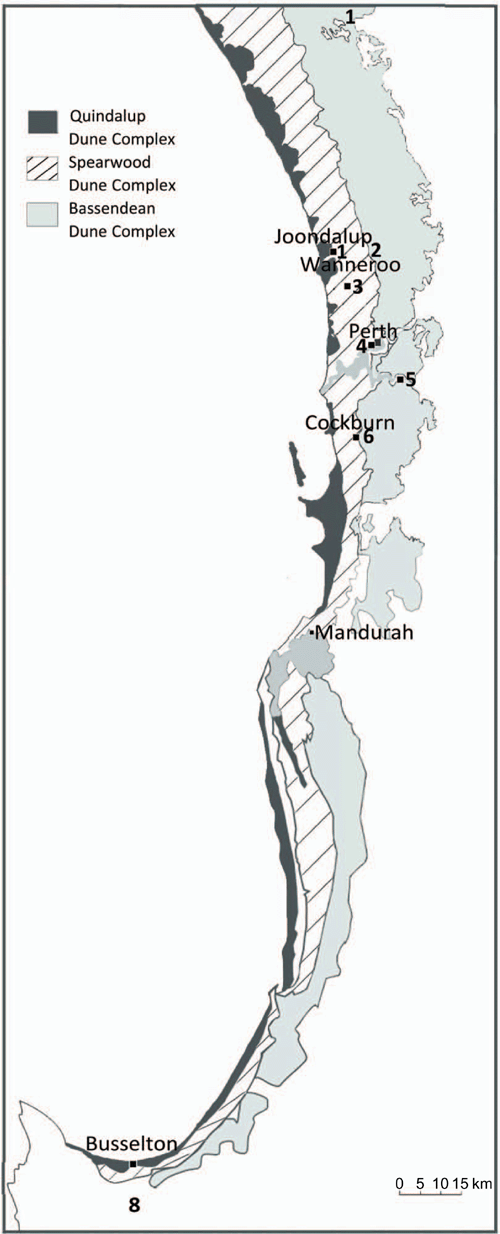
Not long after the 1989 BWs symposium, several floristic surveys were conducted that encompassed much of the SCP (Griffin and Keighery 1989; Gibson et al. 1994; Griffin 1994; Department of Environmental Protection 1996; Government of Western Australia 2000). Among these, Gibson et al. (1994) classified floristic data (species presence or absence) from five hundred and nine 100-m2 quadrats between Busselton and the Moore River (Fig. 1a) to derive 43 Floristic Community Types (FCTs), of which 10 FCTs were recognised as BWs types (Table 2). An extra 613 quadrats were added and analysed in 1996, which identified a further two BWs FCTs on the SCP (Department of Environmental Protection 1996; Government of Western Australia 2000); Table 2). Floristic surveys conducted between Moore River and Jurien Bay on the northern SCP identified an additional 10 BWs FCTs (Griffin and Keighery 1989; Griffin 1994), although these have not been integrated with the southern SCP classification. In all these surveys, floristic variation across quadrats was consistently correlated with soil characteristics, including soil moisture regimes, which are related to depth to groundwater (Zencich et al. 2002; Groom 2004), and soil type and age, which is linked to the degree of soil leaching and available nutrients (McArthur et al. 2004). In total, 20 BWs FCTs have been identified on the SCP and these form the basis of current conservation assessments, with 3 presently listed as TECs and 4 as Priority Ecological Communities at state level (Keighery and Keighery 2016). The Commonwealth listing assessed BWs of the SCP and adjoining areas in their entirety (Commonwealth of Australia 2016a). These adjoining areas include the Whicher Scarp (south of Busselton, Fig. 4), adjacent to the southern boundary of the SCP, which has seven recognised Banksia-dominated FCTs, three of which are listed as Priority Ecological Communities at the state level (Keighery et al. 2008; Commonwealth of Australia 2016a).

|
|
Although 80 reported threatened flora species occur on the SCP (see FloraBase of the Western Australian Herbarium at https://florabase.dpaw.wa.gov.au/, accessed 2 June 2020), it is not clear how many of these are restricted to or strongly favour BWs. Keighery and Keighery (2016) reported 20 ‘uncommon’ species that are completely, or mostly, confined to the BWs of the Perth area, including five threatened and seven priority flora species under state legislation. The Commonwealth of Australia (2016a) listed 20 threatened and 21 priority species ‘likely to occur’ in BWs of the SCP, although more research is needed to clarify the fidelity of these species to BWs (Table 1). Most (but not all) of the threatened species have approved conservation advice or recovery plans in place which includes assessment of their conservation status based on their known distribution, population sizes, habitat, reproductive ecology and threats. However, studies into the conservation biology of individual threatened species are limited, with rare orchids being the best studied group, especially in terms of reproductive ecology and population genetics (e.g. Hopper and Brown 2007; Swarts et al. 2009; Swarts et al. 2010; Menz et al. 2015). Other taxa have received less research attention (e.g. Stace 1995; Monks 1999; Close et al. 2006; Nield et al. 2009), with some threatened species, such as Tetraria australiensis C.B. Clarke (a perennial, tufted sedge) having no published biological information. Ramalho et al. (2014) observed that most native species (70%) in 30 BWs sites were only found in a small proportion of those sites, which is consistent with the high geographic species turnover known for the SWAFR (Hopper and Gioia 2004; Jones et al. 2016; Gibson et al. 2017; Tsakalos et al. 2018).
Keighery (1989) conducted the first and only comprehensive survey of introduced (non-native) plants in BWs, collating data on the taxonomy, life-form and origin of 120 naturalised plant species found across 100 sites. The invasive traits of those species (e.g. rate of spread, degree of impact, fecundity, response to disturbance), and appropriate management approaches, were still largely unknown then. In the 30 years since the compilation of an introduced plant list in BWs by Keighery (1989), an inventory of introduced species of the SCP is available through floristic databases (Keighery et al. 2012). Most of the floristic surveys conducted since 1989 have recorded introduced species presence, and so the list of BWs exotic flora is likely to be much larger now, but has yet to be collated. In terms of introduced species abundance, Ramalho et al. (2014) identified 11 species with ≥5% cover in at least one plot; all but two of these species were on the list of Keighery (1989).
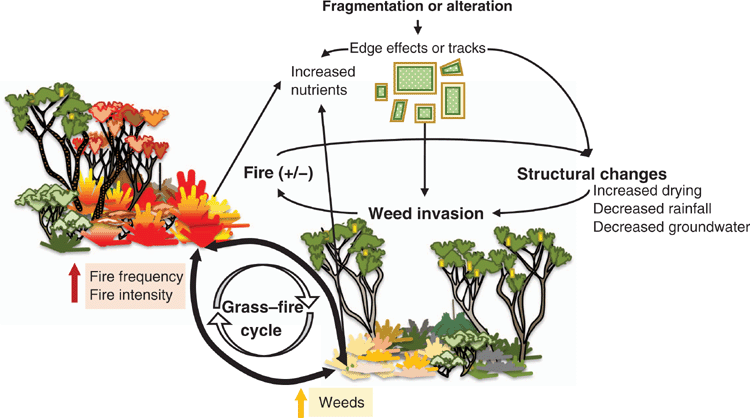
The key drivers of plant invasion in BWs have been partially investigated, although there is little published information about the invasive traits of most species. Ramalho et al. (2014) explored the effects of fragmentation, remnant age, herbivory, and proximity to the city centre on plant invasion. The study indicated that a decline in native herbaceous species abundance was associated with increased abundance of invasive introduced herbaceous species (Ramalho et al. 2014). Similarly, Fisher et al. (2006, 2009a, 2009b) investigated the effects of introduced species seed bank, fire, and soil nutrient status on plant invasion and found that invasive species alter the nutrient composition of soils and create conditions unconducive for native species. The role of fire in promoting invasion was also investigated by Milberg and Lamont (1995), Ruthrof et al. (2003), Ruthrof (2004) and Brown et al. (2016), among others. Ehrharta calycina Sm. (perennial veldt grass), an invasive perennial grass originating from southern Africa, is the most serious and hence best understood of the invasive plants in BWs (Brown and Brooks 2003). The ability of this species to produce abundant seed, maintain a large soil seed bank, and quickly regenerate following fire or other disturbances often within the first growing season contribute to its competitive advantage over many native understorey species, with frequent fire facilitating its progressive domination (Fisher et al. 2009a, 2009b). Additionally, nutrient levels are typically higher under and within E. calycina plants (compared with natives), suggesting differences in nutrient acquisition and recycling (Fisher et al. 2006). Although case studies of successful management strategies exist (Brown and Brooks 2003), comprehensive regional mapping of invasive plant species is lacking, and therefore large-scale conservation efforts are constrained.
The lack of a comprehensive map of FCTs and species distribution (both native and introduced) in BWs and a limited understanding the spatial patterns of β-diversity have constrained conservation planning efforts on the SCP. Such information is important for the assessment of individual FCTs and of TEC threats, particularly in the context of impact assessment studies, and is also crucial to help guide prioritisation for integration of new areas into the conservation reserve network. Conservation of BWs, with their high number of FCTs and predicted high β-diversity, requires a network of proximate conservation reserves throughout the SCP, while targeting specific FCTs in order to represent most vegetation complexes, and threatened flora (Ramalho et al. 2013). The current model prioritizes conservation at the city fringe and where urban expansion is unlikely. The Bush Forever Project (Government of Western Australia 2000) aimed to create such a network within the Perth metropolitan area. However, because of development pressures and high economic land values, most Bush Forever sites have not as yet been embedded in the conservation network (Dhakal 2014). Some of these lands have been partially cleared, and others remain formally unprotected. Also, offset policies allow the clearing of urban and peri-urban BWs in ‘exchange’ for land conservation in rural areas (because of differences in their availability for protection), although some consider that they are unlikely to conserve the same floristic assets that are cleared (Thorn et al. 2018).
Priority research
Despite the general understanding that BWs floristic variation is influenced by regional north–south (climate-related), east–west (nutrient availability-related), and local (topography-related) environmental gradients (Commonwealth of Australia 2016a), three major knowledge gaps are yet to be resolved:
-
The characterisation of BWs species turnover patterns and relationships with soil or landform.
-
Mapping BWs floristic communities as currently understood.
-
Consolidation of the huge amount of existing floristic data available across disparate sources (consultants, academics, government agencies and community groups) in a publicly accessible information system that would enable updates and improvement in classification and mapping over time, as well as identification of poorly known areas and communities.
Geology and soils
Semeniuk and Glassford (1989) identified two primary areas as critical to our understanding of the dune systems of the SCP: (1) a systematic description of the dunes (landform, stratigraphy, soils, interrelationships and age structure), and (2) how these systematic descriptions inform delineations of vegetation habitats. A few large-scale studies describing dune zonation based on mineralogical analysis and soil physical characteristics have been published in the last 30 years (Bastian 1996; Turner et al. 2018). Three main dune units are distinguished within the region based on parent sand deposits: the Quindalup (~5000 years old), Spearwood (20 000–80 000 years old) and Bassendean (<120 000 years old) Dune Systems (Fig. 4) (McArthur and Bettenay 1974; Turner et al. 2018). These three dune systems represent a natural chronosequence that formed as a result of receding ocean levels and subsequent dissolution of parent material (Bastian 1996). The SWAFR region is classified as an ‘old climatically buffered infertile landscape’ (Hopper et al. 2016), and soil nutrient content, specifically nitrogen (N) and phosphorus (P), and pH all decline from youngest to oldest dune (Turner et al. 2018).
A critical component of soil research that has emerged since 1989 is the understanding of preferential flow pathways and hydraulic attributes of surface soils (Salama et al. 2005), which affect both land and aquifer management. Land management practices are highly reliant on an understanding of surface soil properties such as hydrophobicity and seasonal changes in hydraulic conductivity (Rye and Smettem 2015, 2017, 2018). Similarly, surface soil hydraulic properties have direct impacts on aquifer response and water level patterns (Salama et al. 2005), which in turn, are likely to affect vegetation composition and distribution (see ‘Water relations and ground water management’).
Distinct ecohydrological habitats are known to be critical to many plant species (Canham et al. 2009) and vegetation composition and production is tied to soil properties that vary across the SCP (Turner et al. 2018). A deeper understanding of the complex relations between plant species distribution and soil physical and chemical properties are therefore vital to the conservation of many species in BWs. For example, many species are known to have landform-specific requirements for persistence, such as dune swale positions that have a shallow depth to the water table and dune top species that require better drained soils (Zencich et al. 2002; Canham et al. 2012 and see ‘Water relations and groundwater management’). Thus, the consideration and study of the distribution of thin subsurface clay bands and palaeosols that help increase water retention and nutrient availability on isolated small areas is required. The intensity of interactions between species and plant functional types within a specific landform may change due to altered soil physical and chemical characteristics, for example, some dune swales may have increased nutrient contents due to agricultural run-off that may affect the spatial structure and species composition.
Research on soil biology and plant root associations has greatly increased since 1989. Plant species native to BWs are known to have numerous root adaptations, growth forms and complex soil–root interactions related with low soil fertility (Dodd and Griffin 1989; Lamont 2003). Banksia spp. and most other Proteaceae spp. around the world are non-mycorrhizal but have proteoid (cluster) roots that are specialised to ‘mine’ particularly scarce elements like phosphorus and certain micronutrients (tested by Lambers et al. 2011 in BWs). Cluster roots also occur in some Fabaceae, and dauciform roots of Cyperaceae are similar in structure and function. Mycorrhizal associations between roots and fungal species are also very common in BWs as a means of acquiring soil nutrients, especially with increasing time since fire (Pate and Bell 1999). Mycorrhizal mediated strategies for nutrient acquisition likely play a critical role in plant–plant interactions, in particular competition for limited soil nutrients during periods of high surface soil moisture (Bell et al. 1995). Similarly, BWs plant species affect the activity and distribution of soil microbial communities (Marschner et al. 2005). Throughout BWs, ~20% of woody shrub species demonstrate strong arbuscular mycorrhizal associations, whereas ~13% of herbaceous species exhibit either AM or orchid-type mycorrhizal associations (Pate 1994). Similarly, many species within BWs are known to have ectomycorrhizal associations that may promote nutrient acquisition (Lambers et al. 2008). Mycorrhiza clearly play a critical role in species maintenance and reproduction, such as the critical link between orchid germination and mycorrhiza (Bonnardeaux et al. 2007). However, the distribution, diversity and abundance of soil mycorrhizal species throughout intact BWs is not well understood, nor are the spatio-temporal associations between various soil microbes and plant and animal species, climate and soil types.
Impacts of disturbance, such as mining and soil nutrient changes due to agriculture, introduced species and intensified land use, on microbial species diversity and persistence is poorly quantified. Birnbaum et al. (2017) found that soil freshly stripped from intact woodlands suffers large declines in soil microbial activity that may take several years to recover. Many species of mycorrhizal fungi and soil-dwelling biota likely exist in BWs, and their response to disturbances will likely vary. However, more research is needed to improve our understanding of the structure and functioning of soil microbial systems.
Priority research
Many advances in our understanding of BWs soils have been made, but critical gaps still exist including the following:
-
A large regional classification that delineates specific above and below-ground species composition as it relates to landform and soil physical and chemical properties is needed to determine whether certain soil characteristics are linked with composition and distribution of floral and faunal species.
-
Research on the small-scale patterns of soils within the chrono-sequence (redistribution of material in recent times, plant–microbe–soil feedbacks, impact of altered hydrological wetting cycles and fire regimes) is essential to understanding plant communities and their species distributions.
-
The study of soil microbial communities in greater depth by using new methodology (e.g. eDNA metabarcoding), to enumerate species composition and examine ecological functioning and responses or resilience to disturbance.
Water relations and groundwater management
The dunal systems that carry BWs have experienced a drying trend due to reduced precipitation (CSIRO and BOM 2015) and increased groundwater exploitation (Sommer and Froend 2011). Groundwater levels have dropped 1.8 m between 1998 and 2016 within the Perth metropolitan region (Department of Water and Environmental Regulation 2019), causing declines in groundwater dependent vegetation (Froend and Sommer 2010). Over the last 30 years, there has been improved understanding of the importance of groundwater, including responses of vegetation to reduced access, and management of groundwater resources to minimise impacts on vegetation (Barron et al. 2014; Sommer and Froend 2014; Rohde et al. 2017). Bounded by the Swan River (south), Moore River and Gingin Brook (north), Darling Scarp (east) and the south-west Australian coast (west) (Fig. 4), the Gnangara Groundwater Mound (GGM) is a large sand mound underlying seasonal and permanent wetlands, pine plantations and extensive areas of BWs that contains groundwater which supplies the city with fresh scheme water. (McFarlane et al. 2012). McFarlane et al. (2012) documented lessons learned in managing groundwater levels on the GGM, in response to declining rainfall in this region determined that a multi-agency approach to land and water planning is necessary as interactions between land use, land management, and water extraction became crucial to meet competing social, economic, and environmental values. The environmental impact of groundwater extraction has instigated several studies on water relations of BWs plants, particularly the tree species presumed vulnerable to altered groundwater levels. Drying of the vadose (unsaturated) zone due to reduced rainfall and increased temperature has received much less attention. Many BWs plant species, including Banksia spp. in higher landscape positions, do not rely on access to groundwater (Zencich et al. 2002), and the impact of drying soils might be crucial for their persistence.
Dodd and Griffin (1989) outlined the seasonal variation in transpiration of Banksia canopy species, which were positively correlated with increased evaporative demand (highest in summer). Since 1989, our understanding of understorey and overstorey species water utilisation and the interactions of species within the system has increased, and has progressed towards understanding differences between species in BWs and their vulnerability to reduced water availability. Published studies vary from physiological studies of single plant water use (Pate et al. 1998), to community responses to groundwater drawdown by monitoring of long-term sites (Froend and Sommer 2010). The most studied mass decline in BWs vegetation (thus far), as a consequence of groundwater drawdown, occurred during the summer of 1990–1991 (Groom et al. 2000a), which led to a procession of studies aimed at understanding the BWs vegetation dependency on groundwater, and response to changes in groundwater availability (Groom et al. 2000a, 2000b, 2001). Groom et al. (2000b) was one of the first published cases correlating mortality in BWs understorey species with depth to groundwater. Myrtaceous species found at sites of shallow depth to water table (<1 m) were observed to have the greatest reduction in population size as groundwater levels declined (Groom et al. 2000b). At two long-term monitoring sites, high rates of drawdown (50 cm year−1) resulting from groundwater abstraction and drought caused rapid changes in BWs floristic composition, reflected in a 33% dissimilarity to pre-abstraction floristics over 12 years (Froend and Sommer 2010). Slower water table drawdown (9 cm year−1) caused a more gradual change in floristic composition over 33 years (Froend and Sommer 2010). The predominant biophysical driver of shifts in the BWs floristics was depth to the water table, and these shifts were more often a result of reduction in the abundance of more susceptible species and increases in more drought-tolerant ones, rather than by replacement over the study period (Froend and Sommer 2010).
Several studies (Zencich et al. 2002; Veneklaas and Poot 2003; Groom 2004; Froend and Sommer 2010; Sommer and Froend 2014; Muler et al. 2018b) have highlighted species differences in water use patterns relating to rooting depth relative to groundwater depth. Understorey shrubs with relatively deep roots (e.g. Adenanthos cygnorum Diels, Eremaea pauciflora (Endl.) Druce and Stirlingia latifolia (R.Br.) Streud.) can be dependent on groundwater where accessible (Groom 2004). They are likely to be affected by declining groundwater in these areas, especially where drawdown is rapid. However, shrubs with shallow roots take up water from the unsaturated zone and therefore are sensitive to site water balance (mainly determined by rainfall and evaporation conditions rather than changes in groundwater). Muler et al. (2018a), identified five plant functional types based on differences in water relation traits between fifteen common, dominant BWs species on the GGM adapted to summer water deficit stress, highlighting that species without deep and extensive root systems that may or may not provide access to groundwater require high levels of drought tolerance. The five functional types identified were: species with leaf dehydration adaptations (group A), drought tolerator species (group B), species with intermediate values (group C), deep-rooted species without a water table (group D) and deep-rooted species with water table present (group E) (Muler et al. 2018a). However, variations in microclimatic conditions due to altering vegetation structure throughout BWs and interannual variation in climatic conditions may make the strength of traits used to define the five functional groups vary both spatially and temporally (Veneklaas and Poot 2003) as well as plant phenotypic plasticity to a changing climate (Muler et al. 2018a).
The importance of groundwater for deep-rooted overstorey Banksia spp. during dry summer months was first demonstrated by Pate et al. (1998) using stable isotopes. The seasonal importance of groundwater varies spatially with habitat type: Banksia trees use more groundwater in summer if it is within reach of their root system (Zencich et al. 2002). Veneklaas and Poot (2003) concluded that deep-rootedness allows dominant Banksia spp. to access soil moisture throughout the entire soil profile, in contrast with shrubs and herbs with shallow roots that experience greater drought stress during summer in BWs and only have access to surface soil moisture. Froend and Drake (2006) provided physiological confirmation that BWs tree species occurring in drier (higher) parts of the dunal landscape are less vulnerable to drought. Canham et al. (2009) expanded these insights reporting that in wetter parts of the landscape, there were no between-species differences in vulnerability to water stress (for the key canopy species: B. attenuata, B. menziesii, B. ilicifolia R.Br. and B. littoralis R.Br.). However, species with broad ecohydrological ranges (B. attenuata and B. menziesii) were more resistant to drought when growing at dune crest sites due to acclimations to periodic dry downs, than species at wetter sites with shallow groundwater, such as B. ilicifolia and B. littoralis, which are restricted to sites with a shallow water table.
Reduced soil water availability not only decreases transpiration rates, but also causes foliage temperatures to increase more than air temperatures. Bader et al. (2014) found that extreme spring temperatures preceded the mortality of B. menziesii, suggesting a temperature- or humidity-related threshold, and thus warmer and drier conditions associated with climate change in the future may result in increased plant mortality. High mortality during drought has also been reported in a seedling experiment of B. attenuata, B. menziesii and Eucalyptus todtiana F.Muell. over multiple drought events (Benigno et al. 2014) and in larger B. attenuata and B. menziesii trees following drought (Challis et al. 2016).
The importance of understanding ecohydrological processes that support vegetation and fauna habitat is critical in the face of a drying climate. Burgess et al. (2000) demonstrated hydraulic lift by B. prionotes roots in response to gradients in water potential from high to low water content across soil strata, which suggests that deep-rooting overstorey Banksia have the potential to create ecologically important hydrological conditions in shallow soil. Increasing surface soil moisture through hydraulic lift of groundwater by overstorey species may provide improved conditions for understorey species and facilitate their survival and growth. Evidence for the indirect use of redistributed groundwater by understorey plants exists in the context of ecological restoration, but the mechanisms behind these interactions remain unclear and are likely to be complex (Muler et al. 2018b). For example, evidence suggests phreatophytic Banksia spp. provide a benefit to seedlings until water becomes limited in late summer (Muler et al. 2018b).
Management interventions to prevent vegetation change or restore original floristics of BWs are considered extremely difficult after ecohydrological habitats have passed a critical threshold. Assessments of alternative vegetation states for BWs were identified over a multi-decadal drying of the Mediterranean-type BWs landscape (Sommer and Froend 2014). Plant community composition varied across a range of groundwater depths suggesting that as groundwater depth increases, the degree of habitat specificity decreases. Over a 35-year period, the composition and structure of vegetation was consistent in spite of progressive increases in depth to the water table. However, the narrow range of groundwater depths defining discrete states suggests small changes in water table depth may cause rapid shifts to alternative vegetation states, which will likely be exacerbated by a continued drying trend.
Understanding ecohydrological processes in water-limited BWs requires consideration of temporal and spatial vegetation patterns of water use. On the GGM, understorey evapotranspiration was considered a major component of the water budget of BWs and therefore an essential influence on recharge to the aquifer (Farrington et al. 1989). To increase aquifer recharge it was agreed in the early 2000s that pine plantations on the mound would be progressively removed (McFarlane et al. 2012). Farrington et al. (1989) suggested that a reduction in understorey cover, potentially through a controlled burning program, would keep evaporation rates of the ground flora at a low level, and possibly allow groundwater recharge rates to increase and raise water tables. However, the relationship between vegetation and evaporative loss are likely more complex. For example, a recent assessment of the spatial and temporal variability in evapotranspiration using remotely sensed data across northern BWs by Sommer et al. (2016) showed vegetation in shallow groundwater habitats had higher evapotranspiration rates during the growth season (spring and summer) than in deeper groundwater habitats, suggesting that the former was not physiologically constrained by water deficit. Inter-annual variability in actual evapotranspiration correlated with rainfall and, during low rainfall years, peaked one month earlier relative to higher rainfall years. Therefore, remote sensing can give an indication of where groundwater is supporting BWs and thus may be a valuable tool in identifying areas of high conservation value.
Priority research
Groundwater extraction and climate change will likely continue to exert significant pressure on water availability to BWs vegetation. Despite the surge in studies on BWs vegetation water relations and response to changes in water availability, more information is needed on:
-
Soil–plant–atmosphere interactions that would inform water availability for vegetation and groundwater management models.
-
Identification of management triggers to mitigate threshold-type responses in vegetation.
-
Resilience and restoration of ecohydrological habitats (e.g. increased understanding of plant–plant interactions through time).
-
Interactions between water deficit, fire, weeds, and other ecological pressures.
Invertebrates
Majer (1989) reviewed the available literature of invertebrate fauna of BWs and highlighted that there were few published studies. He suggested that there were differences in the invertebrate fauna of BWs compared to adjacent vegetation types and there were likely to be species endemic to BWs (Abbott 1982; Rossbach and Majer 1983; Majer 1989). Negative effects of fragmentation, land clearance and habitat modification on composition and persistence of BWs invertebrate communities were highlighted with urbanisation, weeds, and the frequency and season of fire identified as major threats (Majer 1989).
A census of the invertebrate fauna throughout BWs has not been conducted. Invertebrate studies since 1989 have largely been functionally not taxonomically focused. Examples of functional studies are the role of invertebrates as pollination vectors for specific plant species (Newman et al. 2013; Phillips et al. 2015), ants as indicators of disturbance (Burbidge et al. 1992), and changes in butterfly and moth assemblage as a result of fragmentation (e.g. Dover and Rowlingson 2005; Williams 2009; Williams 2011). Arachnids have been largely studied in BWs with regard to their interaction with fire (Mason et al. 2019) and weeds (Mason et al. 2016, 2018b).
The effects of habitat fragmentation and other disturbances on invertebrate communities are complex and likely to differ between invertebrate species and biogeographic associations (Harvey et al. 1997; Williams 2011; Newman et al. 2013; Phillips et al. 2015; Mason et al. 2016). Habitat fragmentation has been linked to declines in species diversity, with larger reserves containing a greater diversity and species richness of rare species of butterflies and day flying moths than smaller reserves (e.g. butterfly Ogyris idmo in Koondoola bushland; Williams 2009, 2011). In addition, edge effects associated with fragmentation are known to affect thynnid wasp persistence (genera Zaspilothynnus) (Newman et al. 2013) whereas predation of mygalomorph spiders (genera Aname and Teyl) is significantly influenced by the proportion of surrounding parkland (Mason et al. 2018a). Likewise, plant host species density affects butterfly species persistence within remnants (Williams 2011). A study of butterflies in BWs found 13 taxa now primarily use exotic plant species as hosts in and around urban fragments (e.g. butterflies Vanessa kershawi often breeds on widespread capeweed, Arctotheca calendula (L.) K.Lewin and Geitoneura minyas on Ehrharta spp.) (Williams 2009). Burbidge et al. (1992) found that ant species that preferred litter cover and were solitary foragers were indicative of larger, undisturbed BWs sites. Conversely, Dover and Rowlingson (2005) found that western jewel butterflies (Hypochrysops halyaetus) preferred degraded areas within high quality BWs due to foraging and roosting behaviours. The abundance of trapdoor spiders (Nemesiid spp.) in BWs was negatively correlated with the presence of invasive perennial veldt grass and rabbit disturbance (Mason et al. 2016). Each of these studies highlights how changes in these woodlands can influence invertebrate abundance and distribution.
Recent research by Phillips et al. (2015) has highlighted the significant impact that specialist invertebrate–plant relationships can have on plant species persistence in BWs. The small form of thynnine wasp (Macrothynnus insignis), has a specialised pollinator relationship with the once more common orchid, Caladenia huegelii Rchb.f. The decline of this thynnine wasp in fragmented BWs is contributing to the increasing rarity of the orchid, and the species is now facing an almost complete loss of pollination services (Phillips et al. 2015). Many of these relationships remain undocumented or studied.
Changes to disturbance regimes and quality of habitat also affect invertebrate populations. For example, mygalomorph spiders are less abundant in BWs after high intensity fires as compared to low intensity fires (Mason et al. 2018b). Most invertebrate taxa are approximately half as abundant in the first year after fire than in areas unburnt for 22 years (Bamford 1992). By contrast, ants are extremely abundant in the first few years after fire, with lower and slightly declining levels of abundance more than 4 years after fire (Bamford 1992).
Not discussed in 1989 was the presence and condition of subterranean fauna communities within underground aquifers under the SCP. Studies of stygofauna in Western Australia began in the early 1990s (e.g. Knott 1993), including surveys of the GGM (see ‘Water relations and groundwater management’) (Knott 1993). Nearly all stygofauna found in Western Australia are invertebrates, mostly crustaceans, though a few larger vertebrate species are also found (Humphreys 2006; Halse 2008). Underneath BWs, the GGM feeds the threatened ecological communities of stygofauna found within the Yanchep caves. These communities contain high levels of diversity, with each cave containing 30–40 species, compared to a global average of 6 species (Jasinska et al. 1996; English et al. 2003). At least 100 species of fauna are known from these six caves, including 6 Gondwanan relics (English et al. 2003). These communities are threatened by drought, climate change, increased groundwater use, and land clearing altering hydrology. Most species are unable to survive if their aquifer dries out, for example, when the stream in Gilgie Cave dried out in 1996 no fauna survived, and levels of recolonisation have been extremely low (English et al. 2003). Although our knowledge of the stygofauna communities found beneath BWs has grown over the last 30 years, we still lack a basic understanding of their biology, ecology, and management.
Over the last 30 years, we have increased our understanding of the functional role that invertebrates play in BWs. Despite this, there are still major gaps in our understanding of invertebrate biology, and their interactions with other species within BWs. Although some invertebrate taxa have been investigated in some detail (e.g. moths, thynnine wasps, trapdoor spiders), many taxa have been poorly studied. For many threatened and non-threatened invertebrates, their management requirements are largely unknown (Braby 2018). Knowledge of their identity, abundance, diversity, distribution, fidelity and reliance on BWs is essential to their conservation. Although this is not a problem unique to BWs, initial studies would suggest BWs are home to several endemic or short range endemic invertebrate species, some of which are likely to be threatened (Abbott 1982; Mason et al. 2018b). Similarly, the role of invasive invertebrates (e.g. Portuguese millipede (Ommatoiulus moreletii), European house borer (Hylotrupes bajulus) and European honeybee (Apis mellifera) and their impacts on both native plant and faunal species is largely understudied, though likely has consequences for ecosystem functioning.
Priority research
Information on BWs invertebrates is severely deficient, research areas where more information is needed include:
-
Conducting a census of the BWs invertebrate fauna to gather basic information about their identity, abundance, distribution, fidelity, function and reliance on BWs.
-
Understanding of invertebrate biology and their interactions with other species in BWs.
-
Improving our understanding about how we can manage threatened and endangered invertebrates, including stygofauna communities found beneath BWs.
-
Improving our understanding of invasive invertebrates and their impact on native and endemic species as well as their impact on the BWs ecosystem.
Vertebrates
How and Dell (1989) highlighted that although BWs amphibian, reptile and bird communities had been relatively resistant to anthropogenic impacts, mammal communities had been severely affected, with many species locally extinct. They also noted negative effects of weed invasion on reptiles, without citation, and mentioned the lack of knowledge of BWs bat communities (How and Dell 1989).
Much research has been conducted on BWs vertebrate communities since 1989; however, many knowledge gaps remain. Taxonomic work has clarified the species status of some reptiles that occur primarily in BWs (Kay and Keogh 2012; Doughty and Oliver 2013). Knowledge of finer-scale distributions of mammals, birds, reptiles and frogs has increased, due to research conducted for the Gnangara Sustainability Strategy (Bamford and Huang 2009; Davis 2009a; Valentine et al. 2009) and in urban remnants within the Perth metropolitan area (How and Dell 1994, 2000; How 1998; Davis et al. 2013). This research confirmed the dire state of BWs mammal communities (Wilson et al. 2012). It also highlighted that reptile and frog communities, while more resilient to anthropogenic threats, are also susceptible to fragmentation, with remnant woodland size and species richness positively related in all groups except skinks and frogs (How and Dell 2000).
Frogs and reptiles
Frog populations have been found to be reasonably resilient to the impacts of fire, although the abundance of two species (western banjo frog, Limnodynastes dorsalis, and the turtle frog, Myobatrachus gouldii) was positively related to time since fire (Bamford 1992). A study of the responses of frogs to hydrological changes as a consequence of reduced rainfall, highlighted a range of responses from ‘unlikely to be affected’ (M. gouldi), to ‘likely to be severely affected’ (Glauert’s frog, Crinia glauerti, and Günther’s toadlet, Pseudophryne guentheri) (Bamford and Huang 2009). A study of genetic variation in the frog M. gouldi found low genetic divergence between populations (Vertucci et al. 2017). Similarly, weak population genetic structuring for the scincid lizard, Ctenotus fallens, suggests historically high levels of connectivity among recently fragmented urban populations (Krawiec et al. 2015). Our understanding of the distribution of BWs frogs is still limited, and deserves greater attention.
Fire is suggested to be the biggest threat to remnant BWs reptile communities, particularly those in small, unconnected fragments (How and Dell 2000). Although reptiles have a range of post-fire responses (Valentine et al. 2012; Davis and Doherty 2015), generally, the rarest species have been found only in long unburnt sites. This suggests that, although reptile communities typically recover rapidly (<3 years) post fire (How and Dell 2000; Davis and Doherty 2015), the retention of long unburnt habitat remains critical for reptile conservation in BWs (Wilson et al. 2014). There are four Australian squamates (lizards and snakes) found primarily in BWs (Ctenotus ora, Lerista lineata, Diplodactylus polyophthalmus and Neelaps calonotus) that were classified as threatened using the IUCN Red List during a recent assessment of all reptile species by Tingley et al. (2019). However, three of these species are currently listed as WA Priority 3 fauna (Ctenotus ora, Lerista lineata, and Neelaps calonotus). Range extensions have been reported for several species of reptiles recorded in BWs (Davis and Bamford 2005; Davis and Wilcox 2008; Thompson et al. 2008) and it is likely that further fauna surveys will document the extension of the distribution of other reptiles in the region. A review of the distribution and threats to L. lineata recommended an urgent re-evaluation of its conservation status, as its long-term survival is tenuous at best (Maryan et al. 2015). How and Dell (1989) also suggested the potential for weeds to be a threat to reptile communities, but no research has been conducted on this topic in BWs. A recent paper highlighted the threat posed to reptile populations by anticoagulant rodenticides (Lettoof et al. 2020).
Birds
Research on BWs bird communities has focused on responses to fragmentation and urbanisation, fire, and the plant pathogen Phytophthora cinnamomi, as well as specific studies on the endangered Carnaby’s cockatoo (Calyptorhynchus (Zanda) latirostris) and southern boobook (Ninox boobook). Studies on fragmentation and urbanisation found large variations in the impact on individual bird species over the past 60 years (Recher and Serventy 1991; Davis et al. 2013). All species that had declined or become locally extinct were dependent on bushland and unlikely to persist in small or isolated remnants (Davis et al. 2013), with ground foragers disproportionately represented, perhaps due to weed invasion (Recher and Serventy 1991). Nectarivorous bird pollinators can functionally connect some plant species in fragmented urban BWs, with evidence of widespread pollen dispersal between remnant and restored B. menziesii populations (Ritchie et al. 2019). Most bird species recovered reasonably rapidly post-fire, although one species (western thornbill, Acanthiza inornata) may have disappeared from Kings Park (one of the largest urban BWs reserves) following a fire (Recher 1997), suggesting that fire poses a threat to some bird communities in isolated remnants (Recher 1997; Davis 2009b). Conversely, in large intact expanses of BWs, only two species (splendid fairy-wren, Malurus splendens and yellow-rumped thornbill, Acanthiza chrysorrhoa) have been found to be affected by fire (Davis 2009a), indicating that fire effects on bird communities may be somewhat ephemeral in contiguous woodlands (Davis 2009b). The plant pathogen P. cinnamomi (see ‘Plant disease’ below) was found to indirectly affect BWs bird communities with three bird species less abundant and one species more abundant in diseased sites (Davis et al. 2014). In BWs north of Perth, 10 bird species dependent on wetland vegetation have been identified as likely to be affected by falling groundwater tables due to reduced rainfall and extraction (Davis 2009b). Several studies found that C. latirostris use of BWs was related to Banksia seed availability (Johnston et al. 2019), which peaked in relatively long unburnt sites (11–30 years post fire) where P. cinnamomi was absent (Davis et al. 2014; Valentine et al. 2014; Johnston et al. 2016). Pine plantations near Perth have provided an important food source for C. latirostris since the 1940s (Stock et al. 2013). Demographic models projected significant declines in the SCP C. latirostris populations proportionate to the area of BWs and pine plantations cleared in the future (Stock et al. 2013; Williams et al. 2016, 2017). Birds are also affected by the use of rodenticides on the SCP, with one study of dead N. boobook in urban BWs finding that most birds contained sub-lethal concentrations of anticoagulant rodenticide (Lohr 2018).
Mammals
Research on mammals has focused on fire effects, surveys and reviews on the past and current status of populations in BWs. These studies confirm significant losses of mammal species from BWs, with losses greatest in isolated remnants. Very few small to medium-sized ground dwelling mammals remain in remnants around Perth, and they are largely restricted to peri-urban areas (How and Dell 2000). However, the Quenda (Isoodon fusciventer) persists in wetland and riparian areas of some remnants within the Perth metropolitan area (Howard et al. 2014) and BWs reserves with wetlands north of Perth (Wilson et al. 2012).They have also been reintroduced into several BWs urban and peri-urban reserves within the last decade (e.g. Ryan et al. 2020). Ground-dwelling mammals, except for the honey possum (Tarsipes rostratus), were also found to be scarce in extensive woodlands north of Perth (Valentine et al. 2009; Wilson et al. 2012), with Boonanarring Reserve (Fig. 4) supporting most small ground-dwelling mammals (Moore et al. 2016). A study of the relationship between mammals and fire found that the introduced house mouse (Mus musculus), was more abundant in recently burnt woodlands, whereas T. rostratus were more frequently detected at sites 20–26 years post fire (Valentine et al. 2009). Species-specific studies have also examined the diets of the western brush wallaby (Notamacropus irma), and western grey kangaroo (Macropus fuliginosus), (Wann and Bell 1997), home ranges of N. irma (Povh et al. 2019) and ecology of the ash-grey mouse (Pseudomys albocinereus) (Smith et al. 2019). How and Dell (1989) noted a lack of information on bats and since this study, there has been very little research on bats in BWs. However, there has been a new record of the western false pipistrelle (Falsistrellus mackenziei) (Hosken and O’Shea 1994) and a radio-tracking study identified that long-eared bats (Nyctophilus major and N. geoffroyii) roost primarily in dead Banksia and Melaleuca preissiana Schauer trees (Hosken 1996).
The research conducted on BWs vertebrates since 1989 has aided conservation through identifying threatened species and threats that need to be ameliorated. Urban BWs remnants are unlikely to support populations of mammals and reptiles in the long term unless they are functionally connected. This indicates that the continuing clearance and fragmentation of BWs remains the primary threat to vertebrate populations and only large contiguous woodlands are likely to retain populations in the long term, even for wide-ranging species. Inappropriate fire regimes remain another threat to vertebrate populations in remnants and a significant threat in contiguous woodlands. In 2010, over 60% of remaining BWs in the Gnangara Groundwater System had been burnt <7 years previously, and only ~3% had not been burnt in >25 years (Wilson et al. 2014). Yet long unburnt patches support the most diverse vertebrate communities, and several species appear restricted to them (Wilson et al. 2014). Wilson et al. (2014) concluded that changes to current fire management practices may assist in maintaining populations of some vertebrate species.
Falling groundwater tables, through reduced rainfall, and extraction, also pose a threat to BWs vertebrates, with research already identifying those species most susceptible to this threat (Wilson et al. 2012). The dearth of small ground-dwelling mammals suggests predation is not the sole threat to these species, with fire, weeds and interactions among threats likely driving population declines. Research conducted since 1989 has identified much of what is required to conserve BWs vertebrates. However, ongoing investment in research is required to address increasing threats (e.g. urban sprawl and associated human impacts), in an integrated conservation approach.
Priority research
Research is needed in the following areas:
-
The interactions between multiple threats to BWs vertebrates, including land transformation and climate change, so synergistic effects can be better understood and improve our ability to conserve vertebrate populations.
-
Understanding traits of species persisting in BWs, particularly those persisting in remnants.
-
Identification of mechanisms underpinning species responses to fragmentation, and then the development of strategies to ameliorate fragmentation effects, including the permeability of the urban matrix to individual species.
-
Knowledge of extinction debt in BWs remnants and its relationships with remnant size, and it would be beneficial to revisit sites surveyed by How and Dell (2000).
-
An examination of the genetic structure of vertebrate populations, and how this relates to various threats in order to facilitate the conservation of populations, as well as the genetic variability they require to adapt to climate change.
-
Understanding human–wildlife interactions (including those related to reintroductions) and ways in which community support and engagement can be utilised to promote effective species conservation.
-
Investigations of how vertebrates in urban BWs remnants, especially feral cats, spread diseases.
Plant disease
With the existence of only 30 publications, plant diseases of BWs were described as a neglected area of research in 1989, and in need of a systematic survey (Shearer and Hill 1989). Considerable research has been undertaken on plant diseases in BWs over the last 30 years. Although other pathogens, such as Australian honey fungus (Armillaria luteobubalina), have been recorded in BWs of the Spearwood dune system (Shearer 1994), the most prolific and serious pathogen is Phytophthora cinnamomi. Many of the life history characteristics, survival mechanisms and spread mechanisms for this water mould have been described (Shearer and Dillon 1996a, 1996b). Other Phytophthora spp. (such as P. multivora) are now commonly detected within BWs, but comparatively less is known about their pathogenicity and influence on the plant community (Scott et al. 2009; Ireland 2011; Barber et al. 2013; Burgess et al. 2017, 2019). Phytophthora cinnamomi disease centres are more commonly found in deeper soils where they can alter the root system to provide refugia for persistence (Hill et al. 1994; Shearer et al. 2010). Colonies originate from fine roots and spread by water movement, erosion or anthropogenic or potential animal vector (native and non-native species) soil movement and root to root contact (Shearer 1990; Cahill et al. 2008). Phytophthora cinnamomi may persist indefinitely at infested sites through infected asymptomatic hosts plants, thick-walled oospores, chlamydospores and stromata (Crone et al. 2013; Jung et al. 2013). Soil abiotic factors affect the occurrence of P. cinnamomi; disease expression is rare on the calcareous, alkaline Quindalup and Spearwood Dune systems of the SCP (Shearer and Dillon 1996a) (Fig. 4). However, P. multivora and other species are detected frequently from these dune systems and are more tolerant of alkaline soils (Scott et al. 2009). Crushed limestone is used for pedestrian paths in BWs as it is assumed to reduce the spread of P. cinnamomi. The higher levels of phosphorus and calcium in the Quindalup Dunes are thought to promote host defence, although, this is understudied (Shearer and Crane 2014).
The effects of P. cinnamomi on above-ground biota are becoming increasingly defined (Shearer et al. 2004). Many common plant families in BWs are susceptible to P. cinnamomi, including Proteaceae, Fabaceae, Ericaceae, Xanthorrhoeaceae, and Zamiaceae, causing mortality by hydraulic failure, leading to changes in plant species abundance and community structure (Shearer and Dillon 1996a, 1996b; Kinloch and Wilson 2009). The change in plant community composition and structure, and potential localised loss of key species can have flow- on effects for fauna dependent on specific habitat and food sources (Davis et al. 2014; Johnston et al. 2016). Fire plays a major role in the ecological functioning of BWs (see ‘Fire’ below); however, very little is known about the response to, and potential impact of, fire on plant diseases (Moore et al. 2014). Moore et al. (2014) indicated that fire within P. cinnamomi-infested communities has the potential to increase the severity and the extent of disease in native ecosystems in the SWAFR. Predicted climate change in the SWAFR, with longer drier periods, will potentially increase fire frequency, which in turn could worsen plant deaths when conditions are warm and wet (Moore et al. 2014). Managers need to consider these interactions when developing management strategies.
Increased understanding of the biology and ecology of P. cinnamomi has enabled tailored management strategies. Phosphite can be applied as a foliar spray or injected into the plant stem (typically of trees) and is the most commonly used control method (Komorek et al. 1997). In addition, the application of calcium sulphate to soil has been shown to augment and prolong the effect of phosphite application (Stasikowski et al. 2014). Management of P. cinnamomi, not specific to BWs, by the Department of Biodiversity, Conservation and Attractions (DBCA) has occurred through aerially spraying of phosphite, for a rapid treatment of plant communities containing endangered plant species, and in areas where ruggedness of the terrain makes application by hand expensive (O’Gara et al. 2005). Some 400 ha of native woodland and shrubland in south-western Australia have been sprayed regularly at recommended rates of between 12 and 24 kg ha–1 (Hardy et al. 2001; Barrett and Rathbone 2018). Phosphite initiates an immune response in the plant and alleviates symptoms while decreasing the spread of the pathogen; however, P. cinnamomi is not eradicated (Shearer and Fairman 2007; Shearer and Crane 2014; Barrett and Rathbone 2018). The pathogen can, in fact, sporulate from lesions contained by the immune response initiated by phosphite (Wilkinson et al. 2001). Fungicide treatments are infrequently used in natural vegetation with mechanical and chemical (e.g. glyphosate) vegetation removal being an effective eradication and containment strategy (Hill 1995). Vegetation removal, disease suppression (i.e. metalaxyl) and fumigation (metham-sodium) have been combined to eradicate spot infestations; though no large-scale methods have been developed. The increased knowledge about how the pathogen proliferates and spreads has been helpful in implementing logistical control methods (Project Dieback 2019). Methods, such as hygiene washes and restricted movement of soils from infected areas (Bell 2002; Cahill et al. 2008), have been developed, yet their effectiveness is debatable as it is very hard to enforce these measures. Effort is required to ensure all land users and managers follow hygiene protocols to minimise spread of the pathogen and other Phytophthora spp. into non-infested areas. Despite concerns of potential negative long-term impacts of phosphite application on the abundance of Phytophthora sensitive Proteaceae species and species composition (Lambers et al. 2013), a recent long-term (7–16 years) study of Kwongan sites within the SWAFR by Barrett and Rathbone (2018) indicated there was no evidence of adverse effects on Phytophthora sensitive species or change in species assemblages.
Priority research
Key areas for investigation include the following:
-
Whether the effectiveness of eradication treatments in spot infestations of Phytophthora spp. will result in suppression or significant spread if not managed.
-
Response of P. cinnamomi, P. multivora and the two species together in their different life cycle stages to varying climatic conditions and disturbance types.
-
Response of flora, fauna, and fungi to the effects of P. cinnamomi under varying climatic conditions and disturbance types, such as drought plus heavy summer rainfall and varying fire frequency and intensities.
-
The biology, ecology and pathology of native and introduced species of Phytophthora present in BWs, their host ranges, ecological roles and their interactions with P. cinnamomi.
-
The response of P. cinnamomi-affected woodlands to restoration that aims to provide ecosystem services and functions similar to those before impact. Opportunities exist to select resistant or tolerant species through seed collection following impact and reintroduction by tube stock or broadcast seeding.
-
A better understanding of other plant diseases and disease-causing organisms present in BWs and how they may be affected by disturbances such as climate change and fire.
Fire
In the 30 years since Hopkins and Griffin (1989) summarised fire ecology and fire management in BWs, most of the issues they raised have only become more complex. These include: substantial expansion of the wildland–urban interface (Australian Bureau of Statistics 2019); weed–fire interactions (Fisher et al. 2009b); a drying and warming climate and the frequency of extreme events (Andrys et al. 2017). The broad categories of fire science knowledge gaps identified by Hopkins and Griffin (1989) also remain relevant today, including:
-
Fuel dynamics and wildfire risk in relation to fire history.
-
Tolerance of native and introduced species to varying fire frequency, intensity, and season.
-
How fragmentation and altered landscapes influence fire regimes and management strategies.
Fire management
Fire within BWs is a natural process with evidence of its occurrence dating back to 2.7 × 106–2.5 × 106 years BP (Hassell and Dodson 2002). Fossil charcoal evidence of aboriginal use of fire within BWs dates back at least 30 000 years BP (Hallam 2014). Fire occurrence in BWs remains driven by human activity: based on 12 years of Department of Fire and Emergency Services data (2004–2012; excluding 5.5% of fires occurring on public land), Plucinski (2014) attributed just over 1% of fires in the Perth metropolitan region to natural causes, (i.e. lightening or spontaneous combustion), 55% to arson, 30% to accidents (including 2.5% to escaped prescribed burns), and 14% to unknown causes. The majority of fires occurred October–April, fires were more likely on weekends than weekdays and more ignitions occurred under higher fire danger conditions. Although unplanned ignitions dominated the number of fires, the majority of area burned was from planned managed fires (Plucinski 2014).
DBCA’s risk-based framework elaborates bushfire management objectives across four Fire Management Areas (FMAs) and 13 broad fuel types (Howard et al. 2020). BWs are recognised as one of these fuel types, but, noting the absence of a specific fuel accumulation and fire behaviour model, the framework adapts the Dry Eucalypt Forest Fire Model (Cheney et al. 2012) for BWs. Public lands within each fuel type are classified into four FMAs ‘defined by the primary intent of fuel management, which is a function of potential fire behaviour and the type and distribution of assets characteristic of the area’ (Howard et al. 2020). Based on fuel accumulation and fire behaviour models, the framework identifies a threshold fire intensity that precludes effective suppression action under the 95th percentile fire danger conditions to derive management objectives within each FMA. These objectives are expressed as proportion of FMA area with treated fuels that will not support a head fire of intensity above that threshold. This results in objectives of 60% for the Settlement-Hazard Separation FMA (within 1 km of settlements), and 30% in the Landscape Risk Reduction FMA within 5 km of private property for BWs (Howard et al. 2020). Five-year-old fuel is indicated as a representative intensity threshold for open eucalypt forest, but Howard et al. (2020) do not provide a value for BW. If, for example, an indicative threshold fuel age of 8–10 years is used for BWs (following Burrows and McCaw 1990), objectives would correspond to 13–17- and 27–33-year burn rotations in BWs at 0–1 and 1–5 km from settlements or private property respectively.
Most DBCA prescribed burning in BWs occurs in spring, with smaller amounts in autumn, usually aiming for low intensity fire with minimal canopy scorch and some spatial patchiness (Densmore and Clingan 2019). The placement of burns in the landscape addresses strategies both targeting infrastructure, such as reducing intensity of fire close to assets (Florec et al. 2020; Howard et al. 2020), as well as breaking up landscape continuity by developing a matrix of mixed fuel ages thus creating options to suppress wildfires under elevated fire weather conditions (Conservation and Parks Commission 2018).
Fuel dynamics in relation to fire
Fuel loads and structure in BWs are known to be affected by fire regimes components, such as varying fire interval, this relationship was recognised in 1989 but with little evidence available (Hopkins and Griffin 1989). Subsequently, fuel characteristics and accumulation dynamics of BWs were described by Burrows and McCaw (1990) who concluded that BWs fuel accumulation stabilises at ~8–10 years post fire. This remains the only published study of BWs fuel dynamics. Fuel dynamics interactions are likely to be complex. For instance, Ryan et al. (2020) recently showed that reintroduced digging mammals could have a significant effect on small-scale fuel distributions. Bioturbation by mammals may have potential value as a complimentary tool for reducing litter fuel loads, and potentially, fire risk (Ryan et al. 2020).
The impacts of longer intervals, weed contribution to fuels, spatial variation, and the contribution of climate and groundwater change on fuel dynamics remain unknown. Owing to the lack of development in understanding fuel and fire dynamics in BWs, many of the management and operational mechanisms currently used in BWs have been imported from other ecosystems (e.g. fire spread models developed in dry eucalyptus forests are used for BWs (Howard et al. 2020).
Tolerance of flora to varying fire regimes
Since 1989, several studies have investigated the tolerance of native BWs plant species to varying fire regimes. At least a quarter of native species are fire-sensitive, obligate seeders (Pate and Bell 1999; Mickle et al. 2010), with the slowest maturing species (from a sample of conservation priority taxa) taking between 4 and 7 years to reach maturity and 8 and 16 years to reach an age where a sufficient seed bank has accumulated for population persistence (Wilson et al. 2014), and therefore interpreted as vulnerable to shortened fire intervals. Season of fire effects have also been demonstrated, with fire in the wet winter–spring period shown to have negative effects on germination (Roche et al. 1998; Tangney et al. 2019), seedling recruitment (Hobbs and Atkins 1990; Roche et al. 1998), and post-fire flowering (Bowen and Pate 2004) for some species. Studies of population recovery following a high-intensity summer wildfire found that some resprouting plants are capable of regenerating within a few months of fire, before the wet winter period, and that smaller Banksia individuals are less tolerant of high-intensity fire than larger individuals (Bell et al. 1992; Miller et al. 2020). Densmore and Clingan (2019) found that higher intensity fire (wildfires) resulted in reduced Banksia tree survival and cone production relative to lower intensity fires (prescribed burns) within the same season. With the diversity of plant species and fire response types present in BWs (dominated by serotinous tree species like B. attenuata and B. menziesii and a wide range of resprouting and obligate seeding understorey species), and the effects of varying site, pre- and post-fire conditions, and fire event attributes, there remains much to be understood regarding the tolerance of native BWs species to varying fire regimes. Assessing demographic change in plant populations following fire is likely a particularly useful approach to this problem as it provides a mechanistic understanding of patterns of change (Miller et al. 2019; Tangney et al. 2020a).
Tolerance of fauna to varying fire regimes
Faunal research in relation to fire has focused on vertebrate food resources (especially for large parrots), structural habitat features, or indices of reptile and frog abundance (Bamford 1992; Valentine et al. 2012, 2014; Davis and Doherty 2015). Substantial gaps remain in understanding fire impacts, and how they vary by fire type (i.e. prescribed burn v. wildfire, varying season of fire and interval) for native fauna. For example, Valentine et al. (2014) investigated fruit production of the two most common Banksia tree species, a key food resource for endangered black cockatoos (primarily Carnaby’s cockatoo) across a time since fire chronosequence, finding that 20–35-year-old sites supported maximum fruit production. Densmore and Clingan (2019) showed that varying fire intensity also has significant consequences for the availability of woody fruits, which serve as cockatoo food resources, with severe wildfire depleting this resource substantially, whereas low severity prescribed burns have negligible impacts.
Effects of an altered and fragmented landscape
Several studies show that alteration and fragmentation of BWs can influence the fire regime through diverse pathways, including by altered herbivore density – total grazing pressure (Brown et al. 2016), weed invasion, and isolation of remnants (Ramalho et al. 2014). Ramalho et al. (2014) studied BWs remnant condition in 30 reserves in the Perth region, revealing interactions involving fire. They found that fire was most frequent in large remnants and in areas with greater human activity, and that native woody plant species richness was associated with higher fire frequency in these reserves (Ramalho et al. 2014).
Non-native species, especially grassy weeds, are known to increase with fire occurrence in BWs (Milberg and Lamont 1995; Ramalho et al. 2014; Brown et al. 2016). Ehrharta calycina (see ‘Floristics and weeds’) is recognised as a key contributor to this grass-fire cycle in BWs. Repeated prescribed burning in Kings Park (a large urban BWs reserve) during the mid-20th century was implicated in the shift from shrub-dominated to invasive-grass-dominated understorey vegetation by Crosti et al. (2007). Ehrharta calycina and other grassy weeds produce abundant seeds, germinate under a wide range of conditions, and, while many seeds may perish under wildfire temperatures, some are always sufficiently buried to survive (Smith et al. 1999; Fisher et al. 2009a). Prevalence of invasive plant species has been found to increase in long-isolated reserves regardless of fire history or greater rabbit abundance (Ramalho et al. 2014; Brown et al. 2016), specifically in areas that experience low soil heating during fire (Tangney et al. 2020b). Several case studies demonstrate the efficacy of weed management (i.e. herbicide application) immediately post-fire in constraining weeds and promoting native vegetation (Brown et al. 2016). Native herbivore interactions with fire, either by reducing fuel loads or reducing post-fire vegetation recovery, have also been identified (Brown et al. 2016; Ryan et al. 2020). Further work is required to understand the extent of these interactions.
Priority research
Many issues raised by Hopkins and Griffin (1989) regarding fire ecology and management remain relevant today. In particular, the development of a BWs specific fuel accumulation and fire behaviour model and an understanding of the effectiveness and impacts of prescribed burning on both wildfire risk and biodiversity values requires the following:
-
Fuel dynamics and variation according to fire history, spatial extent of BWs and changing climate conditions.
-
Explicit description of fire behaviour and a model suitable to predict fire behaviour in BWs.
-
The role of environmental variables (including varying weed cover, total grazing pressure, digging animals, climate, and soil types) on fire responses, fuel characteristics, fire behaviour, and wildfire risk.
-
Quantification of the grass–fire cycle, including its impacts on wildfire and native biodiversity, and documentation of effective management options that interrupt this cycle.
-
Integration of fire science, fine-scale meteorology and remote sensing, and human health impacts to reveal potential trade-offs in differing fire management strategies.
-
Detailed species traits and responses to varying fire regime elements, including:
-
– fire season, including effects of burning in various seasons,
-
– fire intensity,
-
– fire interval (or frequency), and
-
– fire patchiness under different conditions.
-
To support land managers in avoiding unwanted fire effects, research incorporating interactions between all of these elements, which is complex and largely unstudied, is required.
Mining and restoration
The focus of mining-related activities in Gozzard and Mouritz (1989) was on the method of extraction and quality of limestone and sand resources. The use of extracted resources at the time was largely for local housing and infrastructure development and exports of sand for glass production (Gozzard and Mouritz 1989). Following mining, pits were converted to landfill waste disposal and suburban development areas, with some rehabilitated to native vegetation. However, little attention in the review was given to post-mining restoration practices. Since 1989, there has been a significant increase in the mining of base raw materials (BRM) occurring in areas that support BWs (e.g. sand, limestone, clay, hard rock and gravel aggregates). Increases in BRM demand are mainly driven by Perth’s population increase with concomitant urban development. It is estimated that an average dwelling and supporting infrastructure in the Perth region requires ~663 tonnes (Mg) of BRM including 151 Mg of hard rock, 255 Mg of sand, 102 Mg of clay and 155 Mg of limestone (Western Australian Planning Commission 2012). Deposits of BRM were thought of as abundant in 1989 (Gozzard and Mouritz 1989). However, a recent analysis showed the occurrence of economically viable deposits is limited, increasingly constrained by environmental and land-use considerations (Western Australian Planning Commission 2012).
Since 1989, research on BWs restoration following mining has increased dramatically, resulting in the publication of a book devoted to BWs restoration (Stevens et al. 2016). The conservation of intact woodlands is critical to maintain ecosystem function and habitat, especially for rare and endangered flora and fauna. However, continuous degradation over the last 30 years has made restoration efforts more critical than ever.
Broadly speaking, the restoration requirements of individual mines depend upon the type of mining (e.g. hard rock pit voids v. sand strip mining), the nature of the surrounding environment (e.g. urban v. intact woodland), and the agreed-upon post-mining or next land-use (based on land-use hierarchies, and agreed-upon completion criteria). Restoration typically comprises designing and reconstructing appropriate landforms, spreading of topsoil, and additional seeding and planting where required. Large advances have been made in the efficacy of post-mining restoration efforts in the last 30 years, which has aided post-pine, post-agricultural and greenfield land restoration, though large gaps in knowledge still exist and efficiency of efforts requires improvement.
Soils
Post-mining environments can be challenging to restore because of extreme changes in abiotic and biotic conditions (Stevens et al. 2016). In the past two decades, advances in soil profile reconstruction and topsoil transfer practices have substantially improved the quality and quantity of plant species restored (Stevens et al. 2016; Waryszak et al. 2021). Mineral sand extraction is a common mining activity in BWs where 5–50-m dunes are mined to within approximately four meters of the water table (Rokich 2016). This extreme alteration in soil profile leads to multiple problems for restoration, the most critical of which is a hardening process of the subsurface soil layer that constricts plant root development (Rokich et al. 2000, 2001; Benigno et al. 2013). Soil reconstruction using various sand materials before redistributing topsoil, such as soil ripping (Rokich et al. 2001) and the application of organic subsurface mulches (Benigno et al. 2013) have been found to improve seedling survival.
Appropriate handling of topsoil is arguably the most important ingredient for the successful establishment of BWs because of many species having topsoil-stored seeds (Rokich et al. 2000; Fowler et al. 2015). Specifically, the depth at which topsoil is stripped and redistributed across post-mine sites and the timing of transfer have a large effect on both the richness and abundance of species that can establish from seed stored in the topsoil (Rokich et al. 2000). The quality of topsoil utilised is also critical to native seed persistence. A high occurrence of seed from invasive species in topsoils may lead to highly competitive environments that result in increased native seedling mortality (Fisher et al. 2009b).
Seeds and seed technology
Significant advances have been made in seed, collection, handling, storage, and usage techniques and technologies, spurred on by the demand for seeds for mine site restoration. Fundamental to the increased effectiveness of seed has been the ability to release seed dormancy and stimulate germination at much higher rates than previously possible. Early research into the seed germination ecology of BWs species provided insights into the effects of temperature and light on germination (Bell et al. 1993, 1995). In the 1990s investigations into the role of smoke in stimulating germination (Dixon et al. 1995; Roche et al. 1997) culminated in research establishing and identifying an active chemical contained within smoke responsible for germination stimulation, karrikinolide (Flematti et al. 2004). Karrikinolide was identified as a predominant chemical responsible for promoting germination in a wide range of native and introduced seeds (Flematti et al. 2004; Dixon et al. 2009) with other chemicals now identified for other specific families within BWs (e.g. organic compound cyanohydrins – Haemodoraceae) (Flematti et al. 2013).
Similar advancements have been achieved in understanding germination of seeds of BWs species that require specific treatments to break dormancy and promote germination (Bell et al. 1995; Roche et al. 1998; Bell 1999; Turner et al. 2006; Merritt et al. 2007). For example, dry heat is used to release dormancy in the iconic red and green kangaroo paw (Anigozanthos manglesii D.Don) (Tieu et al. 2001). Similarly, stratification (Turner et al. 2006), and wetting-drying cycles (Baker et al. 2005) have been found to improve seed germination rates of multiple species. Using this information, seed may be treated for dormancy and used in tandem with topsoil transfer efforts that occur at broad-acre scales (Stevens et al. 2016)
Challenges remain with regard to germinating seeds of some of the important understorey species in BWs because of dormancy-breaking requirements that are still unclear. Reliable methods of dormancy-breaking are yet to be established for species of Ericaceae (those that disperse seeds encased in woody, indehiscent fruits), Cyperaceae, Restionaceae, and Rutaceae (Merritt et al. 2007).
Restoration genetics
Prior to the 1989 review, genetic issues of relevance to BW restoration were largely unknown. An appreciation of these issues began with the development of restoration ecology as a strong scientific discipline from the early 1990s (Bradshaw 1993). This coincided with the development of genetic markers which increased the understanding of genetic variation and its spatial structuring among populations within species (Linhart and Grant 1996). Today, restoration genetics is a mature scientific discipline concerned with researching and understanding genetic issues potentially affecting the practise of ecological restoration (Williams et al. 2014; Mijangos et al. 2015). A key issue is seed sourcing, with implicit acknowledgment that natural selection is the primary selection pressure that leads to genetic differentiation, and assumptions that local seeds are best adapted to the local restoration site (Hufford and Mazer 2003). To investigate how local is local, genetic markers have been used since 2000 to generate genetically informed seed sourcing guidelines for many SCP species (Krauss et al. 2013; Krauss 2016). Recent investigations into adaptive genetic variation has facilitated the assessment of genetic resilience to environmental stressors. By detecting selection and adaptation-related candidate genes, the role of genetic diversity in buffering BWs species against the effects of climate change (He et al. 2016), and the environmental drivers in trait differentiation within and between species (He et al. 2019), may be revealed. Recent ecological genetic studies have also confirmed the return of pollinator services and reproductive functionality in post-mining restoration populations of Banksias (Ritchie and Krauss 2012; Frick et al. 2014), as well as the genetic integration and connectivity with nearby remnant populations (Ritchie et al. 2019).
Priority research
Research is required in the following areas:
-
Develop a complete understanding of what processes (physical, chemical and biological) are driving subsurface soil hardening in post-mining restoration sites.
-
Determine the conditions for the successful germination and establishment of the many species within BWs that are unknown.
-
Investigate and develop seed enhancement technologies to maximise seed use for restoration of BWs (e.g. Brown et al. 2019).
-
Refine best practise seed-sourcing guidelines using provenance trials to test if ‘local-is-best’, or whether there are benefits from implementing a climate-adjusted provenancing may prove more successful with expected climate change (Prober et al. 2015).
-
Investigate new opportunities that exist from the rapid development of high-throughput DNA sequencing technologies. These include rapid and comprehensive eDNA assessment of above- and below-ground biological communities (e.g. soil biota) pre-and post-restoration, as well as gene expression analysis for assessment of genetic resilience to environmental stressors of targeted plant species or communities.
-
Identify faunal species that passively recolonise restoration areas to identify species that will, and will not, benefit from restoration activities.
-
Develop techniques to move towards holistic restoration of BWs that specifically facilitates the recolonization of soil biota and fauna, particularly keystone and threatened species and those critical for ecosystem services (e.g. pollinators).
Urban development
In 1989, Poole (1989) correctly predicted that Perth would grow immensely and that regional planning strategies would be unsuccessful at containing urban sprawl (Table 1; Fig. 1). The Perth and Peel metropolitan regions currently extend over 130 km along the coast and may reach 170 km by 2050 (Weller 2009). Indeed, Perth has the lowest population density of any mainland capital city in Australia with 1000 people km–2 (by comparison Sydney has 1900, Melbourne 1500, and Adelaide 1300) (Hunn 2017). In 1989, Perth had a population of ~0.9 million people. The city was one of the fastest growing in Australia with an annual growth rate of 3% in the late 1980s that declined to 1.4% in the early 1990s (Australian Bureau of Statistics 1996). Projections at the time indicated that the city would reach 1.9 million people by 2021 (Poole 1989); however, Perth’s population attained over 2 million in 2018 (Australian Bureau of Statistics 2019).
Perth has had five main planning strategies since 1990, which have tried to address urban sprawl and the need for a coordinated regional open space system. In 1990, the Metroplan (Department of Planning and Development 1990), proposed a regional parks network for conservation and recreational use in the Perth metropolitan area (MacCallum and Hopkins 2011). The parks network was established in law and vested in the Conservation Commission of Western Australia in 1997 with an emphasis on the protection of the Swan River and Darling Scarp (Fig. 1b). Metroplan was the first Perth plan to specifically identify the importance of protecting urban green spaces, mainly in terms of the benefits to people nearby (Pauli and Boruff 2016). In 2000, the Bush Forever Project was endorsed for bushland protection and management on the coastal plain portion of the Perth metropolitan region (Davis and Harford-Mills 2016) (see the ‘Floristics and weeds’). Similarly, the Network City Plan (Western Australian Planning Commission 2004) anticipated significant population growth (MacCallum and Hopkins 2011), and again encouraged a more compact urban form. Importantly, it introduced an urban growth boundary and 60% infill target for new housing (Curtis 2006). Released in 2010, Directions 2031 (Western Australian Planning Commission 2010) followed the spirit of Network City Plan, although it reduced the infill target to 47% and expanded the planning area to include the Peel region.
Some large BWs areas have been secured within the Perth metropolitan area regional network established in 1997 (e.g. Jandakot, Beeliar, Canning River and Yellagonga Regional Parks, Fig. 4). Ramalho et al. (2013) identified that several Bush Forever sites remain outside the conservation estate and that, in order to further protect remaining BWs FCTs, especially those in the northern section of the metropolitan region, they should be placed in the conservation estate.
The form and distribution of public open space (POS) in urban developments also requires consideration (Bolleter 2017). One key rule currently determining the proportion of land released per development to be set aside for POS has been inherited from the Stephenson and Hepburn (1955) plan. This plan suggested a minimum area of developable land (10%) should be allocated to POS for recreational purposes, based on the number of people the POS was intended to serve. However, biodiversity conservation was not considered in the establishment of these development rules. At the same time, suburban areas are based on the ‘Liveable Neighbourhoods’ design code which calls for the creation of smaller, but more accessible parks (Western Australian Planning Commission 2007). The trade-off of park size against accessibility makes many of these parks too small to support self-sustaining BWs (Ramalho et al. 2014).
Finally, it is important to model the integration of conservation and reserve planning scenarios with alternative growth patterns to optimise protection of BWs. Urban development in Perth typically follows the dispersed sites (sprawl) and transportation-corridor (strip) models which according to Forman (2010), cause extensive degradation of natural areas. Some of strategies and scenarios to integrate conservation and planning could include securing land tenure for remaining BWs, directing development towards already cleared areas (introduced pine plantations, abandoned agricultural lands), and steering development towards higher density, more connected communities requiring less land and physical infrastructure. Bolleter (2016) suggested alternatives to the current infill approaches, such as transit-oriented development and small scale, ad hoc ‘background’ infill to deliver Perth’s infill development targets and increase suburban amenity. Background infill refers to small infill development projects that yield fewer than five group dwellings, and result from the subdivision of individual suburban lots (Department of Planning and Western Australian Planning Commission 2014). Alternatives to this infill include residential densification around redesigned, ecologically enhanced urban parks in inner and middle ring suburbs (Bolleter and Ramalho 2014).
Priority research
Research is required in the following areas:
-
Model the integration of conservation and reserve planning scenarios with alternative growth patterns to optimise protection of BWs. Analyse the growth patterns of the dominant urban development types outlined in Bolleter (2018) for use and application in Perth.
-
Research strategies and scenarios to include securing land tenure for remaining BWs, directing development towards already cleared areas.
-
The development of alternative strategies for achieving urban densification in Perth’s existing suburbs that are reconciled with suburban lifestyle aspirations of both existing and future residents.
-
Overcoming social and environmental barriers (and identifying enablers) for expansion of BWs flora into streetscapes and urban landscaping as a coordinated approach across the Perth metropolitan area.
Interactions
Ecological systems are complex, with many factors being influenced by changing climate and land management practices. Many interrelated processes and relationships exist among biotic and abiotic components of BWs occurring across a range of scales, from the small-scale (metre) interactions between plant species and invertebrates (discussed in Lamont 1989) to large scale (kilometre) processes, such as the breakdown in pollinator networks due to land fragmentation. A key example in BWs is the interaction between weeds, native species and fire. Land clearing can adversely affect native species diversity and promote weed invasion because of increasing areas of disturbance, fragmentation of habitat (edge effects, Fig. 5) and increased presence of weed species decreases the likelihood of persistence of many native species (Standish et al. 2012; Stanbury et al. 2018). The relationship between weed species and fire also provides a challenge to the maintenance of native species assemblages. Fire can have a negative or positive effect on weed invasion and establishment: some weeds species are supressed or killed by fire, while other weed species benefit from fire (see ‘Floristics and weeds’). Increased weed growth following fire can also increase the continuity and availability of fuels for future fires, driving a positive feedback loop of frequent high-intensity fires in a weed dominated state (grass-fire cycle Fig. 5, see ‘Fire’). Weeds on the SCP are also found to modify soil nutrients within BWs (see ‘Floristic and weeds’). BWs vegetation has adapted to the nutrient poor soils of the SCP, and Fisher et al. (2006) demonstrated how the introduced weed species, E. calycina and Pelargonium capitatum (L.) Aiton significantly increase soil nutrient content, which further decreases the competitive ability of native plants.
The positive feedback of fire and weed invasion is detrimental to native plant structure and faunal communities associated with the floristic community (see ‘Vertebrates’ and ‘Invertebrates’). Many BWs plant species are also sensitive to changes in climatic conditions, such as increased occurrence of heat wave events (Ruthrof et al. 2018) and severe droughts (Challis et al. 2016). The impacts of climate change other than increased fire frequency (e.g. heatwaves and drought) are yet unknown for invasive weed species in BWs. However, increased stress on native plant populations will likely provide greater opportunity for weed species invasion if they are tolerant to changes in climatic conditions. Subsequently, the replacement of BWs plants with weed species could lead to further faunal reductions, through loss of habitat (Davis et al. 2013, 2014; Mason et al. 2018b, 2019) and food resources (e.g. (Valentine et al. 2014; Mason et al. 2016).
Future directions
In addition to key priorities for research set throughout this paper (and in Table S1 in the Supplementary material), the increase of existing pressures and new challenges will drive the need for continued research on the ecological functioning and structure of BWs and their associated species into the future. Here we outline the anticipated future landscape and the necessary policy and practice to support research, conservation and management of BWs.
A major challenge not acknowledged in 1989 is climate change, affecting both the abiotic conditions and biotic responses in BWs. By 2030, rainfall is predicted to decline a further 6% over the south-west of Western Australia and the annual number of days above 35°C to increase from 28 (1971–2000) to 36 (Department of Water and Environmental Regulation 2019). The regional impacts of climate change may also be exacerbated by local scale changes, driven by land use change, such as the heat island effect that occurs due to conversion from vegetated ecosystems to hard surface developments (MacLachlan et al. 2017). New developments in the Perth region have lower percentage canopy cover of trees and higher percentage cover of hot reflective surfaces (Fig. 1, 6), which may adversely affect inhabitants (Duncan et al. 2019). When urban temperatures are high, inhabitants utilise air conditioners and refrigerators at higher capacity, increasing energy costs and exacerbating heat island effects.
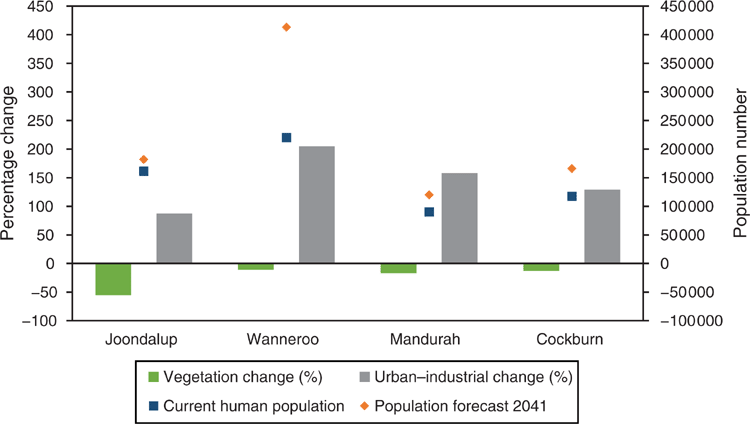
|
Higher density housing will be an essential component of future growth plans that account for both a growing population and conservation of BWs in Perth (Fig. 3). The incorporation of biophilic and environmental sensitive design in plans for urban development will likely contribute to reductions in heat island effects. Perth has a vision to be a leading waterwise city by 2030 and the current state government has outlined that 100% of government-led urban development in Perth and Peel will be waterwise by 2030, working with local governments to reduce irrigation rates and adopt water-sensitive urban designs to account for a growing population (Department of Water and Environmental Regulation 2019). Conserving and protecting BWs from conversion to open-use green spaces will help to reduce water usage and increase recharge.
Alterations in species composition (Scholze et al. 2006; Forbes et al. 2018) and extinctions (Yates et al. 2010) are likely to occur due to changing climate, which means BWs floristics and faunistics are likely to shift over time, which in turn would affect local FCT classification, regional FCT distributions, conservation assessments and protection (Boitani et al. 2015). These challenges are intensified by the pressure of a growing urban environment (Fig. 1, 6; Table 1). To prevent further biodiversity loss, we require knowledge of how, when and why these shifts occur within this ecological community (Saunders et al. 2020). Therefore, strengthening the current and future reserve systems to protect the full complement of BWs biodiversity and their key sustaining processes is critical. Conserving small patches and remnant vegetation that may be perceived as insignificant can provide a larger landscape matrix through which to optimise the retention of biodiversity and functionality of the ecological community (Wintle et al. 2019). Further, it is important that adequate resources are available for ongoing research and management of existing reserves in urban areas where they are particularly vulnerable to altered fire regimes, weed invasion, disease, incursion by feral predators and herbivores, and human disturbance.
Extensive restoration and rehabilitation will be essential in the coming decades to conserve BWs. In 1989, ‘restoration ecology’ was not yet a discipline (Young et al. 2005). Since 1990, considerable research in conservation has progressed hand-in-hand with a need to restore the ecological function of degraded BWs. Ongoing research and implementation of ecological restoration (Stevens et al. 2016) and reintroductions of fauna are also critical for returning areas of BWs following clearing and maintaining ecosystem processes in disturbed BWs (Ritchie et al. 2019; Ryan et al. 2020). Although much progress has been made in re-establishing BWs, technological advances in large-scale biodiverse restoration are vital to the development and achievement of successful ecological restoration in order to compensate for clearing.
Environmental offsets are a key policy instrument that has become prominent recently, and are particularly relevant for BWs because ongoing urban development has been a major cause of woodland loss on the SCP (Thorn et al. 2018). Offsets policy aims to assess development proposals in relation to their likely effect on biodiversity and the potential to avoid, mitigate or offset this loss. Offset schemes require developers to invest in other actions that compensate for the losses caused by the developments after successfully and comprehensively implementing the mitigation hierarchy (Maron et al. 2012). Offsets are supposed to be instituted only when all other options to avoid and reduce adverse environmental impacts have been exhausted. In Western Australia, 39% of offsets studied by May et al. (2017) were considered effective in delivering their planned outcome, with authors concluding that although there have been recent efforts to advance offset implementation and effectiveness, there is need for further improvement. The 2016 Federal Register listing of BWs (Commonwealth of Australia 2016a) and its identification as habitat for species listed as Threatened nationally such as Carnaby’s cockatoos, are important factors strengthening the need for protection of BWs.
The public now and into the future will play a large role in protecting and conserving BWs. The ecological community is of great significance to many locals, including the indigenous inhabitants of the SCP, the Noongar people, and their language groups. In 1989, Hopper and Burbidge (1989) concluded ‘…few would consider these communities (BWs) to be of serious conservation concern’. In 2017, Perth residents actively protested a development that would have cleared substantial BWs and wetlands, bringing about publicity of BWs, Beeliar wetlands and Aboriginal heritage sites and their protection in Perth (Gaynor et al. 2018). If we ‘care for Country’, and place significance on this unique ecological community, we are more likely to secure what is left for future generations. ‘Place’ narratives are a central part of Australian Indigenous culture and knowledge, and the significance of place is critical to Aboriginal people’s health and wellbeing (Kingsley et al. 2013).
Studies have shown that sights of nature, or proximity to nature and greenspace is important for health and mental wellbeing for all people (Zylstra et al. 2014; Wood et al. 2017). Thus, the need to demonstrate the nature of BWs and the value they add to society remains as critical now as it was in 1989. ‘Nature’ can be reframed, not as a problem or constraint to urban infrastructure, but as an asset that can be utilised to improve the urban context by incorporating the principles of biodiversity sensitive urban design. Human health within sustained urban growth is dependent upon a healthy natural environment, and carefully planned development, utilising sound scientific, social and traditional knowledge can incorporate increasing human populations while conserving the natural environment and minimising impacts on our threatened ecological communities.
Australian cities are known to support more nationally threatened animal and plant species than non-urban areas throughout Australia (Ives et al. 2016). However, our cities continue to expand, with state governments reporting that greenfield development rates account for ~20% of growth in Sydney, 30% in Melbourne and as much as 70% in Perth (Infrastructure Australia 2019; University of NSW City Futures Research Centre Astrolabe Group 2019). Banksia Woodlands provide vital habitat for numerous species of which over 20 are nationally Threatened (Commonwealth of Australia 2016b). Perth is predicted to grow to 3 million by 2050 and 6.6 million by 2061 (Australian Bureau of Statistics 2018), potentially meaning an increase of 1486 km2 of suburban area (Bolleter 2015), further increasing pressures on already threatened species.
The utilisation of scientific research will be critical for informing the environmental protection of BWs while balancing and managing the competing land uses on the SCP. The policy environment within which BWs conservation and management operates is complex, with relevant policies and regulations at local, regional, state and federal levels, covering woodlands on private and public lands. Policies relate to woodland protection, management, fire, weeds, and particular species conservation concern. Therefore, there is an ever-greater need for scientists to work as part of cross-disciplinary efforts and with diverse stakeholder groups to protect this ecosystem from further declines.
This review has brought together a diverse group (in terms of both age and gender) of individuals from multiple agencies and universities as the new generation of ecological researchers currently working in the conservation and management of these woodlands. The challenges faced and the knowledge necessary to overcome them requires a transdisciplinary approach and we recognise that in going forward a greater diversity of people and perspectives need to be included in the discussion and research of BWs, specifically greater communication among researchers, local, state and national government agencies, non-government organizations, community and Noongar people, and private businesses. Given the last 30 years of urban growth, BWs now require an urban ecology perspective, involving a greater variety of stakeholders from differing cultural, ethnic and professional backgrounds. Continued funding in research is necessary to better anticipate emerging processes threatening the environment and, through the integration of science into adaptive management programs, lessen their impact. Greater investment allocation to on-ground management agencies is required to effectively integrate scientific knowledge gained and implement management programs to restore, conserve and maintain remaining remnant BWs areas.
Conclusion
Following the federal 2016 TEC listing of BWs (Commonwealth of Australia 2016a), clear strategies need to be developed and applied in order to overthrow the predictions made in 1989 of ‘total destruction or near destruction plus degradation of the remnants’ (Burbidge 1989). We suggest the priority research areas that we have derived from the BWs studies reviewed here will be integrated into research aimed at sustainable nature conservation alongside sustainable population growth in one of Australia’s fastest growing cities. Gaining accurate data on an ongoing basis will provide the evidence required to better understand impacts, such as from climate change. Effective information transfer between research, management and policy development would facilitate effective conservation. This review brought together researchers from a range of institutions and scientific disciplines. However, to preserve BWs and the species this ecosystem supports, a greater diversity of professionals and stakeholders including community groups need to be engaged in discussions about appropriate ways forward for conservation, management and restoration efforts so that the growing city of Perth and BWs may coexist. Our hope is that this review serves as a foundation for future collaborative efforts between a greater diversity of research, management, community organisations and planning entities. Preservation of these unique ecosystems and their associated species is dependent on scientifically informed management in conjunction with novel approaches to accommodate a growing human population.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of Funding
This research did not receive any specific funding. A. L. Ritchie was supported by The Australian Research Council (LP170100075) in the preparation of this article.
Acknowledgements
The authors thank David Merritt and Richard Silberstein for their contributions to the manuscript. The authors thank the reviewers for their helpful and thorough critique of the manuscript. The authors acknowledge the Traditional Owners of country throughout Western Australia and their continuing connection to land, sea and community. We pay our respects to them, their culture and to their Elders past and present.
References
Abbott I (1982) The distribution of earthworms in the Perth metropolitan area. Records of the Western Australian Museum 10, 1–34.Andrys J, Kala J, Lyons TJ (2017) Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059). Climate Dynamics 48, 1723–1747.
| Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059).Crossref | GoogleScholarGoogle Scholar |
Australian Bureau of Statistics (1988) 2102.0 – Census of Population and Housing, 1986. ABS, Canberra, ACT, Australia.
Australian Bureau of Statistics (1993) 2101.0 – Census of Population and Housing, 1991. ABS, Canberra, ACT, Australia.
Australian Bureau of Statistics (1996) 4102.0 – Australian Social Trends, 1996. ABS, Canberra, ACT, Australia.
Australian Bureau of Statistics (2017) 1001.0 – Australian Bureau of Statistics -- Annual Report, 2016-17. ABS, Canberra, ACT, Australia.
Australian Bureau of Statistics (2018) Population Projections, Australia, 2012 to 2101. Vol. 2019. ABS, Canberra, ACT, Australia.
Australian Bureau of Statistics (2019) 3218.0 - Regional Population Growth, Australia, 2017–18. ABS, Canberra, ACT, Australia.
Bader MKF, Ehrenberger W, Bitter R, Stevens J, Miller BP, Chopard J, Rüger S, Hardy GESJ, Poot P, Dixon KW, Zimmermann U, Veneklaas EJ (2014) Spatio‐temporal water dynamics in mature Banksia menziesii trees during drought. Physiologia Plantarum 152, 301–315.
| Spatio‐temporal water dynamics in mature Banksia menziesii trees during drought.Crossref | GoogleScholarGoogle Scholar |
Baker K, Steadman K, Plummer J, Merritt D, Dixon K (2005) The changing window of conditions that promotes germination of two fire ephemerals, Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae). Annals of Botany 96, 1225–1236.
| The changing window of conditions that promotes germination of two fire ephemerals, Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae).Crossref | GoogleScholarGoogle Scholar | 16199485PubMed |
Bamford MJ (1992) The impact of fire and increasing time after fire upon Heleioporus eyrei, Limnodynastes dorsalis and Myobatrachus gouldii (Anura: Leptodactylidae) in Banksia woodland near perth, western Australia. Wildlife Research 19, 169–178.
| The impact of fire and increasing time after fire upon Heleioporus eyrei, Limnodynastes dorsalis and Myobatrachus gouldii (Anura: Leptodactylidae) in Banksia woodland near perth, western Australia.Crossref | GoogleScholarGoogle Scholar |
Bamford MJ, Huang N (2009) The occurrence and status of frogs in the Gnangara Sustainability Strategy study area. Report, Department of Environment and Conservation, Perth, WA, Australia.
Barber P, Paap T, Burgess T, Dunstan W, Hardy GSJ (2013) A diverse range of Phytophthora species are associated with dying urban trees. Urban Forestry & Urban Greening 12, 569–575.
| A diverse range of Phytophthora species are associated with dying urban trees.Crossref | GoogleScholarGoogle Scholar |
Barrett S, Rathbone D (2018) Long‐term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43, 360–374.
| Long‐term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi.Crossref | GoogleScholarGoogle Scholar |
Barron O, Froend RH, Hodgson G, Ali R, Dawes W, Davies P, McFarlane D (2014) Projected risks to groundwater-dependent terrestrial vegetation caused by changing climate and groundwater abstraction in the Central Perth Basin, Western Australia. Hydrological Processes 28, 5513–5529.
| Projected risks to groundwater-dependent terrestrial vegetation caused by changing climate and groundwater abstraction in the Central Perth Basin, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bastian L (1996) Residual soil mineralogy and dune subdivision, Swan Coastal Plain, Western Australia. Australian Journal of Earth Sciences 43, 31–44.
| Residual soil mineralogy and dune subdivision, Swan Coastal Plain, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bates BC, Hope P, Ryan B, Smith I, Charles S (2008) Key findings from the Indian Ocean Climate Initiative and their impact on policy development in Australia. Climatic Change 89, 339–354.
| Key findings from the Indian Ocean Climate Initiative and their impact on policy development in Australia.Crossref | GoogleScholarGoogle Scholar |
Bell DT (1999) The process of germination in Australian species. Australian Journal of Botany 47, 475–517.
| The process of germination in Australian species.Crossref | GoogleScholarGoogle Scholar |
Bell RW (2002) Restoration of degraded landscapes: principles and lessons from case studies with salt-affected land and mine revegetation. Chiang Mai University Journal of Natural Sciences 1, 1–21.
Bell DT, Loneragan WA, Ridley WJ, Dixon KW, Dixon IR (1992) Response of tree canopy species of Kings Park, Perth, Western Australia to the severe summer wildfire of January 1989. Journal of the Royal Society of Western Australia 75, 35–39.
Bell DT, Plummer JA, Taylor SK (1993) Seed germination ecology in southwestern Western Australia. Botanical Review 59, 24–73.
| Seed germination ecology in southwestern Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bell DT, Rokich DP, McChesney CJ, Plummer JA (1995) Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia. Journal of Vegetation Science 6, 797–806.
| Effects of temperature, light and gibberellic acid on the germination of seeds of 43 species native to Western Australia.Crossref | GoogleScholarGoogle Scholar |
Benigno SM, Dixon KW, Stevens JC (2013) Increasing soil water retention with native‐sourced mulch improves seedling establishment in postmine Mediterranean sandy soils. Restoration Ecology 21, 617–626.
| Increasing soil water retention with native‐sourced mulch improves seedling establishment in postmine Mediterranean sandy soils.Crossref | GoogleScholarGoogle Scholar |
Benigno SM, Dixon KW, Stevens JC (2014) Seedling mortality during biphasic drought in sandy Mediterranean soils. Functional Plant Biology 41, 1239–1248.
| Seedling mortality during biphasic drought in sandy Mediterranean soils.Crossref | GoogleScholarGoogle Scholar | 32481073PubMed |
Birnbaum C, Bradshaw LE, Ruthrof KX, Fontaine JB (2017) Topsoil stockpiling in restoration: Impact of storage time on plant growth and symbiotic soil biota. Ecological Restoration 35, 237–245.
| Topsoil stockpiling in restoration: Impact of storage time on plant growth and symbiotic soil biota.Crossref | GoogleScholarGoogle Scholar |
Boitani L, Mace GM, Rondinini C (2015) Challenging the scientific foundations for an IUCN Red List of Ecosystems. Conservation Letters 8, 125–131.
| Challenging the scientific foundations for an IUCN Red List of Ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bolleter J (2015) ‘Scavenging the Suburbs: Auditing Perth for 1 million infill dwellings.’ (UWA Publishing: Perth, WA, Australia)
Bolleter JA (2016) Background noise: a review of the effects of background infill on urban liveability in Perth. Australian Plants 53, 265–278.
| Background noise: a review of the effects of background infill on urban liveability in Perth.Crossref | GoogleScholarGoogle Scholar |
Bolleter J (2017) Fringe benefits? A review of outer suburban development on Perth’s fringes in relation to state government goals concerning the natural environment and efficient transport connectivity. Australian Plants 54, 93–114.
| Fringe benefits? A review of outer suburban development on Perth’s fringes in relation to state government goals concerning the natural environment and efficient transport connectivity.Crossref | GoogleScholarGoogle Scholar |
Bolleter JA (2018) The consequences of three urbanisation scenarios for northern Australia. Australian Plants 55, 103–125.
| The consequences of three urbanisation scenarios for northern Australia.Crossref | GoogleScholarGoogle Scholar |
Bolleter J, Ramalho C (2014) The potential of ecologically enhanced urban parks to encourage and catalyze densification in greyfield suburbs. Journal of Landscape Architecture 9, 54–65.
| The potential of ecologically enhanced urban parks to encourage and catalyze densification in greyfield suburbs.Crossref | GoogleScholarGoogle Scholar |
Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K (2007) Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111, 51–61.
| Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions.Crossref | GoogleScholarGoogle Scholar | 17289365PubMed |
Bowen BJ, Pate JS (2004) Effect of season of burn on shoot recovery and post-fire flowering performance in the resprouter Stirlingia latifolia R. Br. (Proteaceae). Austral Ecology 29, 145–155.
| Effect of season of burn on shoot recovery and post-fire flowering performance in the resprouter Stirlingia latifolia R. Br. (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Braby MF (2018) Threatened species conservation of invertebrates in Australia: an overview. Austral Entomology 57, 173–181.
| Threatened species conservation of invertebrates in Australia: an overview.Crossref | GoogleScholarGoogle Scholar |
Bradshaw A (1993) Restoration ecology as a science. Restoration Ecology 1, 71–73.
| Restoration ecology as a science.Crossref | GoogleScholarGoogle Scholar |
Brown K, Brooks K (2003) ‘Bushland Weeds: a Practical Guide to their Management.’ (Environmental Weeds Action Network Inc.: Perth, WA, Australia)
Brown K, Paczkowska G, Gibson N (2016) Mitigating impacts of weeds and kangaroo grazing following prescribed fire in a Banksia woodland. Ecological Management & Restoration 17, 133–139.
| Mitigating impacts of weeds and kangaroo grazing following prescribed fire in a Banksia woodland.Crossref | GoogleScholarGoogle Scholar |
Brown VS, Ritchie AL, Stevens JC, Harris RJ, Madsen MD, Erickson TE (2019) Protecting direct seeded grasses from herbicide application: can extruded pellet formulations be used in restoring natural plant communities? Restoration Ecology 27, 488–494.
| Protecting direct seeded grasses from herbicide application: can extruded pellet formulations be used in restoring natural plant communities?Crossref | GoogleScholarGoogle Scholar |
Burbidge A (1989) Banksia woodlands: Summary and conclusions. Journal of the Royal Society of Western Australia 71, 117–118.
Burbidge A, Leicester K, McDavitt S, Majer J (1992) Ants as indicators of disturbance at Yanchep national Park, Western Australia. Journal of the Royal Society of Western Australia 75, 89–95.
Burgess SS, Pate JS, Adams MA, Dawson TE (2000) Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Annals of Botany 85, 215–224.
| Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes.Crossref | GoogleScholarGoogle Scholar |
Burgess TI, White D, McDougall KM, Garnas J, Dunstan WA, Català S, Carnegie AJ, Worboys S, Cahill D, Vettraino A, Stukely MJC, Liew ECY, Paap T, Bose T, Migliorini D, Williams B, Brigg F, Crane C, Rudman T, Hardy GESJ (2017) Distribution and diversity of Phytophthora across Australia. Pacific Conservation Biology 23, 150–162.
| Distribution and diversity of Phytophthora across Australia.Crossref | GoogleScholarGoogle Scholar |
Burgess TI, McDougall KL, Scott PM, Hardy GES, Garnas J (2019) Predictors of Phytophthora diversity and community composition in natural areas across diverse Australian ecoregions. Ecography 42, 565–577.
| Predictors of Phytophthora diversity and community composition in natural areas across diverse Australian ecoregions.Crossref | GoogleScholarGoogle Scholar |
Burrows ND, McCaw WL (1990) Fuel characteristics and bushfire control in banksia low woodlands in Western Australia. Journal of Environmental Management 31, 229–236.
| Fuel characteristics and bushfire control in banksia low woodlands in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279–310.
| Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control.Crossref | GoogleScholarGoogle Scholar |
Canham CA, Froend RH, Stock WD (2009) Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia. Plant, Cell & Environment 32, 64–72.
| Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Canham CA, Froend RH, Stock WD, Davies M (2012) Dynamics of phreatophyte root growth relative to a seasonally fluctuating water table in a Mediterranean-type environment. Oecologia 170, 909–916.
| Dynamics of phreatophyte root growth relative to a seasonally fluctuating water table in a Mediterranean-type environment.Crossref | GoogleScholarGoogle Scholar | 22692384PubMed |
Challis A, Stevens J, Mcgrath G, Miller B (2016) Plant and environmental factors associated with drought-induced mortality in two facultative phreatophytic trees. Plant and Soil 404, 157–172.
| Plant and environmental factors associated with drought-induced mortality in two facultative phreatophytic trees.Crossref | GoogleScholarGoogle Scholar |
Cheney NP, Gould JS, McCaw WL, Anderson WR (2012) Predicting fire behaviour in dry eucalypt forest in southern Australia. Forest Ecology and Management 280, 120–131.
| Predicting fire behaviour in dry eucalypt forest in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Close DC, Messina G, Krauss SL, Rokich DP, Stritzke J, Dixon KW (2006) Conservation biology of the rare species Conospermum undulatum and Macarthuria keigheryi in an urban bushland remnant. Australian Journal of Botany 54, 583–593.
| Conservation biology of the rare species Conospermum undulatum and Macarthuria keigheryi in an urban bushland remnant.Crossref | GoogleScholarGoogle Scholar |
Commonwealth of Australia (2016a) Approved Conservation Advice (incorporating listing advice) for the Banksia Woodlands of the Swan Coastal Plain ecological community. Approved 26 August 2016; Listing effective from 16 September 2016. Report, Department of Environment and Energy, Canberra, ACT, Australia.
Commonwealth of Australia (2016b) Banksia Woodlands of the Swan Coastal Plain: a nationally protected ecological community. Report, Department of Environment and Energy, Canberra, ACT, Australia.
Conservation and Parks Commission (2018) Position Statement on prescribed burning on vested lands. Report, Conservation and Parks Commission, Perth, WA, Australia.
Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M (1996) Plant diversity in Mediterranean-climate regions. Trends in Ecology & Evolution 11, 362–366.
| Plant diversity in Mediterranean-climate regions.Crossref | GoogleScholarGoogle Scholar |
Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013) Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology 117, 112–123.
| Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species.Crossref | GoogleScholarGoogle Scholar | 23452949PubMed |
Crosti R, Dixon K, Ladd P, Yates C (2007) Changes in the structure and species dominance in vegetation over 60 years in an urban bushland remnant. Pacific Conservation Biology 13, 158–170.
| Changes in the structure and species dominance in vegetation over 60 years in an urban bushland remnant.Crossref | GoogleScholarGoogle Scholar |
CSIRO and BOM (2015) Climate change in Australia information for Australia’s natural resource management regions: Technical report, CSIRO and Bureau of Meteorology Australia, Canberra, ACT, Australia.
Curtis C (2006) Network city: retrofitting the Perth metropolitan region to facilitate sustainable travel. Urban Policy and Research 24, 159–180.
| Network city: retrofitting the Perth metropolitan region to facilitate sustainable travel.Crossref | GoogleScholarGoogle Scholar |
Davis RA (2009a) The impact of fire and Phytophthora dieback on birds in the Gnangara Sustainability Strategy. Report, Department of Environment and Conservation, Perth, WA, Australia.
Davis RA (2009b) Terrestrial avian diversity and threatening processes on the Gnangara groundwater mound, Western Australia. Report, Department of Environment and Conservation, Perth, WA, Australia.
Davis RA, Bamford MJ (2005) A range extension for Lerista lineopunctulata and a second record of Lerista lineata, near Yalgorup, Western Australia. Western Australian Naturalist 25, 59–60.
Davis RA, Doherty TS (2015) Rapid recovery of an urban remnant reptile community following summer wildfire. PLoS One 10, e0127925
| Rapid recovery of an urban remnant reptile community following summer wildfire.Crossref | GoogleScholarGoogle Scholar | 25992802PubMed |
Davis G, Harford-Mills G (2016) Examining 60 years of strategic planning in metropolitan Perth and Peel. Report, Committee for Perth, Perth, WA, Australia.
Davis RA, Wilcox J (2008) Range extension of the western heath dragon Rankinia adelaidensis and Gray’s legless lizard Delma grayii with notes on the distribution of southern Swan Coastal Plain reptiles. Western Australian Naturalist 26, 67–70.
Davis RA, Gole C, Roberts JD (2013) Impacts of urbanisation on the native avifauna of Perth, Western Australia. Urban Ecosystems 16, 427–452.
| Impacts of urbanisation on the native avifauna of Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Davis RA, Valentine LE, Craig MD, Wilson B, Bancroft WJ, Mallie M (2014) Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia. Biological Conservation 171, 136–144.
| Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Densmore VS, Clingan ES (2019) Prescribed burning in a Mediterranean-climate region mitigates the disturbance by bushfire to a critical food resource for an endangered bird, the Carnaby’s cockatoo. Fire Ecology 15, 36
| Prescribed burning in a Mediterranean-climate region mitigates the disturbance by bushfire to a critical food resource for an endangered bird, the Carnaby’s cockatoo.Crossref | GoogleScholarGoogle Scholar |
Department of Environmental Protection (1996) System 6 and part System 1 update program. Bushland plot and area records, EPA, Perth, WA, Australia.
Department of Planning and Development (1990) ‘Metroplan: A planning strategy for the Perth Metropolitan Region.’ (Art Gallery of Western Australia: Perth, WA, Australia)
Department of Planning, Western Australian Planning Commission (2014) Urban Growth Monitor: Perth Metropolitan, Peel and Greater Bunbury Regions. Report Western Australian Planning Commission.
Department of Water and Environmental Regulation (2019) Waterwise Perth. Report, Government of Western Australia, Perth, WA, Australia.
Dhakal SP (2014) Securing the future of urban environmental sustainability initiatives in Australia. Urban Policy and Research 32, 459–475.
| Securing the future of urban environmental sustainability initiatives in Australia.Crossref | GoogleScholarGoogle Scholar |
Dixon KW, Roche S, Pate JS (1995) The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101, 185–192.
| The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants.Crossref | GoogleScholarGoogle Scholar | 28306789PubMed |
Dixon K, Merritt D, Flematti G, Ghisalberti E (2009) Karrikinolide – a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Horticulturae 813, 155–170.
| Karrikinolide – a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture.Crossref | GoogleScholarGoogle Scholar |
Dodd J, Griffin E (1989) Floristics of the Banksia woodlands. Journal of the Royal Society of Western Australia 71, 89–90.
Doughty P, Oliver PM (2013) Systematics of Diplodactylus (Squamata: Diplodactylidae) from the south-western Australian biodiversity hotspot: redefinition of D. polyophthalmus and the description of two new species. Records of the Western Australian Museum 28, 44–65.
| Systematics of Diplodactylus (Squamata: Diplodactylidae) from the south-western Australian biodiversity hotspot: redefinition of D. polyophthalmus and the description of two new species.Crossref | GoogleScholarGoogle Scholar |
Dover JW, Rowlingson B (2005) The western jewel butterfly (Hypochrysops halyaetus): factors affecting adult butterfly distribution within native Banksia bushland in an urban setting. Biological Conservation 122, 599–609.
| The western jewel butterfly (Hypochrysops halyaetus): factors affecting adult butterfly distribution within native Banksia bushland in an urban setting.Crossref | GoogleScholarGoogle Scholar |
Duncan J, Boruff B, Saunders A, Sun Q, Hurley J, Amati M (2019) Turning down the heat: An enhanced understanding of the relationship between urban vegetation and surface temperature at the city scale. The Science of the Total Environment 656, 118–128.
| Turning down the heat: An enhanced understanding of the relationship between urban vegetation and surface temperature at the city scale.Crossref | GoogleScholarGoogle Scholar | 30504014PubMed |
English V, Jasinska E, Blyth J (2003) Aquatic root mat community of caves of the Swan Coastal Plain and the Crystal Cave crangonyctoid interim recovery plan 2003–2008. Report, Department of Conservation and Land Management, Western Australian Threatened Species and Communities Unit, Perth, WA, Australia.
Farrington P, Greenwood E, Bartle G, Beresford J, Watson G (1989) Evaporation from Banksia woodland on a groundwater mound. Journal of Hydrology 105, 173–186.
| Evaporation from Banksia woodland on a groundwater mound.Crossref | GoogleScholarGoogle Scholar |
Fisher JL, Veneklaas EJ, Lambers H, Loneragan WA (2006) Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum. Plant and Soil 284, 253–264.
| Enhanced soil and leaf nutrient status of a Western Australian Banksia woodland community invaded by Ehrharta calycina and Pelargonium capitatum.Crossref | GoogleScholarGoogle Scholar |
Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ (2009a) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biological Conservation 142, 2270–2281.
| Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland.Crossref | GoogleScholarGoogle Scholar |
Fisher JL, Loneragan WA, Dixon K, Veneklaas EJ (2009b) Soil seed bank compositional change constrains biodiversity in an invaded species-rich woodland. Biological Conservation 142, 256–269.
| Soil seed bank compositional change constrains biodiversity in an invaded species-rich woodland.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick MC, Gove AD, Sanders NJ, Dunn RR (2008) Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia. Global Change Biology 14, 1337–1352.
| Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD (2004) A compound from smoke that promotes seed germination. Science 305, 977
| A compound from smoke that promotes seed germination.Crossref | GoogleScholarGoogle Scholar | 15247439PubMed |
Flematti GR, Waters MT, Scaffidi A, Merritt DJ, Ghisalberti EL, Dixon KW, Smith SM (2013) Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Molecular Plant 6, 29–37.
| Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds.Crossref | GoogleScholarGoogle Scholar | 23180672PubMed |
Florec V, Burton M, Pannell D, Kelso J, Milne G (2020) Where to prescribe burn: the costs and benefits of prescribed burning close to houses. International Journal of Wildland Fire 29, 440–458.
| Where to prescribe burn: the costs and benefits of prescribed burning close to houses.Crossref | GoogleScholarGoogle Scholar |
Forbes CJ, Gillson L, Hoffman MT (2018) Shifting baselines in a changing world: Identifying management targets in endangered heathlands of the Cape Floristic Region, South Africa. Anthropocene 22, 81–93.
| Shifting baselines in a changing world: Identifying management targets in endangered heathlands of the Cape Floristic Region, South Africa.Crossref | GoogleScholarGoogle Scholar |
Forman R (2010) ‘Urban Regions: Ecology and Planning Beyond the City.’ (Cambridge University Press: Cambridge, MA, USA)
Fowler WM, Fontaine JB, Enright NJ, Veber WP (2015) Evaluating restoration potential of transferred topsoil. Applied Vegetation Science 18, 379–390.
| Evaluating restoration potential of transferred topsoil.Crossref | GoogleScholarGoogle Scholar |
Frick KM, Ritchie AL, Krauss SL (2014) Field of dreams: restitution of pollinator services in restored bird‐pollinated plant populations. Restoration Ecology 22, 832–840.
| Field of dreams: restitution of pollinator services in restored bird‐pollinated plant populations.Crossref | GoogleScholarGoogle Scholar |
Froend R, Drake P (2006) Defining phreatophyte response to reduced water availability: preliminary investigations on the use of xylem cavitation vulnerability in Banksia woodland species. Australian Journal of Botany 54, 173–179.
| Defining phreatophyte response to reduced water availability: preliminary investigations on the use of xylem cavitation vulnerability in Banksia woodland species.Crossref | GoogleScholarGoogle Scholar |
Froend R, Sommer B (2010) Phreatophytic vegetation response to climatic and abstraction-induced groundwater drawdown: examples of long-term spatial and temporal variability in community response. Ecological Engineering 36, 1191–1200.
| Phreatophytic vegetation response to climatic and abstraction-induced groundwater drawdown: examples of long-term spatial and temporal variability in community response.Crossref | GoogleScholarGoogle Scholar |
Gaynor A, Newman P, Jennings P (2018) ‘Never Again: Reflections on Environmental Responsibility after Roe 8.’ (UWA Publishing: Perth, WA, Australia)
Gibson N, Keighery BJ, Keighery GJ, Burbidge AH, Lyons MN (1994) A floristic survey of the Southern Swan Coastal Plain. Australian Heritage Commission. Report for the Australian Heritage Commission prepared by the Department of Conservation and Land Management and the Conservation Council of Western Australia, Inc.
Gibson N, Prober S, Meissner R, Van Leeuwen S (2017) Implications of high species turnover on the south-western Australian sandplains. PLoS One 12, e0172977
| Implications of high species turnover on the south-western Australian sandplains.Crossref | GoogleScholarGoogle Scholar | 28245232PubMed |
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sensing of Environment 202, 18–27.
| Google Earth Engine: Planetary-scale geospatial analysis for everyone.Crossref | GoogleScholarGoogle Scholar |
Government of Western Australia (2000) Bush Forever – keeping the bush in the city. Vol. 2: Directory of bush forever sites. Department of Environmental Protection, Report, Perth, WA, Australia.
Gozzard J, Mouritz M (1989) Mineral resources and mining of the Spearwood and Bassendean dune systems. Journal of the Royal Society of Western Australia 71, 109–110.
Griffin EA (1994) Floristic survey of northern sandplains between Perth and Geraldton. Technical report 144, Department of Agriculture and Food, Perth, WA, Australia.
Griffin EA, Keighery B (1989) ‘Moore River to Jurien Sandplain Survey.’ (Western Australian Wildflower Society: Perth, WA, Australia)
Groom PK (2004) Rooting depth and plant water relations explain species distribution patterns within a sandplain landscape. Functional Plant Biology 31, 423–428.
| Rooting depth and plant water relations explain species distribution patterns within a sandplain landscape.Crossref | GoogleScholarGoogle Scholar | 32688914PubMed |
Groom BPK, Froend RH, Mattiske EM (2000a) Impact of groundwater abstraction on a Banksia woodland, Swan Coastal Plain, Western Australia. Ecological Management & Restoration 1, 117–124.
| Impact of groundwater abstraction on a Banksia woodland, Swan Coastal Plain, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Groom P, Froend R, Mattiske E, Koch B (2000b) Myrtaceous shrub species respond to long-term decreasing groundwater levels on the Gnangara Groundwater Mound, northern Swan Coastal Plain. Journal of the Royal Society of Western Australia 83, 75–82.
Groom P, Froend R, Mattiske E, Gurner R (2001) Long-term changes in vigour and distribution of Banksia and Melaleuca overstorey species on the Swan Coastal Plain. Journal of the Royal Society of Western Australia 84, 63–70.
Hahs AK, McDonnell MJ, McCarthy MA, Vesk PA, Corlett RT, Norton BA, Clemants SE, Duncan RP, Thompson K, Schwartz MW, Williams NSG (2009) A global synthesis of plant extinction rates in urban areas. Ecology Letters 12, 1165–1173.
| A global synthesis of plant extinction rates in urban areas.Crossref | GoogleScholarGoogle Scholar | 19723284PubMed |
Hallam SJ (2014) ‘Fire and Hearth: a Study of Aboriginal Usage and European Usurpation in South-western Australia.’ (UWA Publishing: Perth, WA, Australia)
Halse S (2008) Literature review and monitoring program for stygofauna in the Gnangara groundwater system. Report, Department of Environment and Conservation, Perth, WA, Australia.
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133–139.
| The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems.Crossref | GoogleScholarGoogle Scholar |
Harvey M, Waldock J, How R, Dell J, Kostas E (1997) Biodiversity and biogeographic relationships of selected invertebrates from urban bushland remnants, Perth, Western Australia. Memoirs of the Museum of Victoria 56, 275–280.
| Biodiversity and biogeographic relationships of selected invertebrates from urban bushland remnants, Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hassell CW, Dodson JR (2002). Fire history of south-west Western Australia prior to European settlement in 1826–1829. In ‘Proceedings of the Fire in Ecosystems of South-West Western Australia: Impacts and Management’, 16–18 April 2002. Perth, WA, Australia’. (Eds I Abbott, N Burrows) Vol. 1, pp. 71–85. (Backhuys Publishers: Leiden, Netherlands)
He T, D’Agui H, Lim SL, Enright NJ, Luo Y (2016) Evolutionary potential and adaptation of Banksia attenuata (Proteaceae) to climate and fire regime in southwestern Australia, a global biodiversity hotspot. Scientific Reports 6, 26315
| Evolutionary potential and adaptation of Banksia attenuata (Proteaceae) to climate and fire regime in southwestern Australia, a global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar | 27210077PubMed |
He T, Lamont BB, Enright NJ, D’Agui HM, Stock W (2019) Environmental drivers and genomic architecture of trait differentiation in fire‐adapted Banksia attenuata ecotypes. Journal of Integrative Plant Biology 61, 417–432.
| Environmental drivers and genomic architecture of trait differentiation in fire‐adapted Banksia attenuata ecotypes.Crossref | GoogleScholarGoogle Scholar | 29993190PubMed |
Hill TCJ (1995) Evaluation of three treatments for eradication of Phytophthora cinnamomi from deep, leached sands in southwest Australia. Plant Disease 79, 122–127.
| Evaluation of three treatments for eradication of Phytophthora cinnamomi from deep, leached sands in southwest Australia.Crossref | GoogleScholarGoogle Scholar |
Hill T, Tippett J, Shearer B (1994) Invasion of Bassendean Dune Banksia woodland by Phytophthora cinnamomi. Australian Journal of Botany 42, 725–738.
| Invasion of Bassendean Dune Banksia woodland by Phytophthora cinnamomi.Crossref | GoogleScholarGoogle Scholar |
Hobbs RJ, Atkins L (1990) Fire-related dynamics of a Banksia woodland in south-western Western Australia. Australian Journal of Botany 38, 97–110.
| Fire-related dynamics of a Banksia woodland in south-western Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hopkins AJM, Griffin EA (1989) Fire in the Banksia woodlands of the Swan Coastal Plain. Journal of the Royal Society of Western Australia 71, 92–94.
Hopper SD, Brown AP (2007) A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators. Australian Systematic Botany 20, 252–285.
| A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators.Crossref | GoogleScholarGoogle Scholar |
Hopper S, Burbidge A (1989) Conservation status of Banksia woodlands on the Swan Coastal Plain. Journal of the Royal Society of Western Australia 71, 115–116.
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623–650.
| The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity.Crossref | GoogleScholarGoogle Scholar |
Hopper SD, Silveira FA, Fiedler PL (2016) Biodiversity hotspots and Ocbil theory. Plant and Soil 403, 167–216.
| Biodiversity hotspots and Ocbil theory.Crossref | GoogleScholarGoogle Scholar |
Hosken DJ (1996) Roost selection by the lesser long-eared bat, Nyctophilus geoffroyi, and the greater long-eared bat, N. major (Chiroptera: Vespertilionidae) in Banksia woodlands. Journal of the Royal Society of Western Australia 79, 211–216.
Hosken DJ, O’Shea JE (1994) Falsistrellus mackenziei at Jandakot. Western Australian Naturalist 19, 351
How RA (1998) Long-term sampling of a herpetofaunal assemblage on an isolated urban bushland remnant, Bold Park, Perth. Journal of the Royal Society of Western Australia 81, 143–148.
How RA, Dell J (1989) Vertebrate fauna of Banksia woodlands. Journal of the Royal Society of Western Australia 71, 97–98.
How RA, Dell J (1994) The zoogeographic significance of urban bushland remnants to reptiles in the Perth region, Western Australia. Pacific Conservation Biology 1, 132–140.
| The zoogeographic significance of urban bushland remnants to reptiles in the Perth region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
How RA, Dell J (2000) Ground vertebrate fauna of Perth’s vegetation remnants: Impact of 170 years of urbanization. Pacific Conservation Biology 6, 198–217.
| Ground vertebrate fauna of Perth’s vegetation remnants: Impact of 170 years of urbanization.Crossref | GoogleScholarGoogle Scholar |
Howard KH, Barrett G, Ramalho CE, Friend JA, Boyland RJI, Hudson J, Wilson B (2014) Community quenda survey 2012. Report, Worldwide Fund for Nature and Department of Parks and Wildlife, Perth, WA, Australia.
Howard T, Burrows N, Smith T, Daniel G, McCaw L (2020) A framework for prioritising prescribed burning on public land in Western Australia. International Journal of Wildland Fire 29, 314–325.
| A framework for prioritising prescribed burning on public land in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hufford KM, Mazer SJ (2003) Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology & Evolution 18, 147–155.
| Plant ecotypes: genetic differentiation in the age of ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Humphreys WF (2006) Aquifers: the ultimate groundwater-dependent ecosystems. Australian Journal of Botany 54, 115–132.
| Aquifers: the ultimate groundwater-dependent ecosystems.Crossref | GoogleScholarGoogle Scholar |
Hunn P (2017) Australian cities among the largest and least densely settled in the world. https://architectureau.com/articles/australian-cities-among-the-largest-and-least-densely-settled-in-the-world/ [Verified 21 February 2019]
Infrastructure Australia (2019) An Assessment of Australia's Future Infrastructure Needs: The Australian Infrastructure Audit 2019.Report, Australian Government, Canberra, ACT, Australia.
Ireland K (2011) Phytophthora ramorum: Susceptibility of Australian plants, potential geographic range and science into policy and management. PhD thesis, Murdoch University, Perth, WA, Australia.
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R, Valentine LE, Kendal D (2016) Cities are hotspots for threatened species. Global Ecology and Biogeography 25, 117–126.
| Cities are hotspots for threatened species.Crossref | GoogleScholarGoogle Scholar |
Jasinska EJ, Knott B, McComb AJ (1996) Root mats in ground water: a fauna-rich cave habitat. Journal of the North American Benthological Society 15, 508–519.
| Root mats in ground water: a fauna-rich cave habitat.Crossref | GoogleScholarGoogle Scholar |
Johnston TR, Stock WD, Mawson PR (2016) Foraging by Carnaby’s Black-Cockatoo in Banksia woodland on the Swan Coastal Plain, Western Australia. Emu – Austral Ornithology 116, 284–293.
| Foraging by Carnaby’s Black-Cockatoo in Banksia woodland on the Swan Coastal Plain, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Johnston TR, Stock WD, Mawson PR (2019) Implications of Banksia seed reward for conservation and management of Carnaby’s cockatoo on the Swan coastal plain, Western Australia. Australian Journal of Zoology 67, 12–18.
| Implications of Banksia seed reward for conservation and management of Carnaby’s cockatoo on the Swan coastal plain, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Jones MM, Gibson N, Yates C, Ferrier S, Mokany K, Williams KJ, Manion G, Svenning JC (2016) Underestimated effects of climate on plant species turnover in the Southwest Australian Floristic Region. Journal of Biogeography 43, 289–300.
| Underestimated effects of climate on plant species turnover in the Southwest Australian Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Jung T, Colquhoun IJ, Hardy GESJ, Woodward S (2013) New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomii different natural ecosystems in Western Australia. Forest Pathology 43, 266–288.
| New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomii different natural ecosystems in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Kay GM, Keogh JS (2012) Molecular phylogeny and morphological revision of the Ctenotus labillardieri (Reptilia: Squamata: Scincidae) species group and a new species of immediate conservation concern in the southwestern Australian biodiversity hotspot. Zootaxa 3390, 1–18.
| Molecular phylogeny and morphological revision of the Ctenotus labillardieri (Reptilia: Squamata: Scincidae) species group and a new species of immediate conservation concern in the southwestern Australian biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Keighery G (1989) Banksia woodland weeds. Journal of the Royal Society of Western Australia 71, 111–112.
Keighery GJ, Keighery BJ (2016) Floristics of the Banksia woodlands of the Swan Coastal Plain. In ‘Banksia Woodlands: a Restoration Guide for the Swan Coastal Plain’. (Eds JC Stevens, DP Rokich, VJ Newton, RL Barrett and KW Dixon) pp. 7–30. (UWA Publishing: Perth, WA, Australia)
Keighery B, Keighery G, Webb A, Longman V, Griffin E (2008) A floristic survey of the Whicher Scarp. Department of Environment Conservation, Perth, WA, Australia.
Keighery B, Keighery G, Longman V, Clarke K (2012) Native and weed flora of the Southern Swan Coastal Plain: 2005 Dataset. (Department of Biodiversity, Conservation and Attractions: Perth, WA, Australia) Available at: https://naturemap.dpaw.wa.gov.au/ [Verified 29 January 2021]
Kingsley J, Townsend M, Henderson-Wilson C, Bolam B (2013) Developing an exploratory framework linking Australian Aboriginal peoples’ connection to country and concepts of wellbeing. International Journal of Environmental Research and Public Health 10, 678–698.
| Developing an exploratory framework linking Australian Aboriginal peoples’ connection to country and concepts of wellbeing.Crossref | GoogleScholarGoogle Scholar | 23435590PubMed |
Kinloch J, Wilson B (2009) Phytophthora dieback risk assessment of Gnangara mound biodiversity. Report prepared for the Department of Environment and Conservation and the Gnangara Sustainability Strategy, Perth, WA, Australia.
Knott B (1993) Stygofauna from Cape Range peninsula, Western Australia: Tethyan relicts. Records of the Western Australian Museum 45, 109–127.
Komorek B, Shearer B, Smith B, Fairman R (1997) The control of Phytophthora in native plant communities. In ‘Control of Phytophthora and Diplodina canker in Western Australia. Final Report to the Threatened Species and Communities, Biodiversity Group, Environment Australia’. pp. 1–59. (Department of Conservation and Land Management: Perth, WA, Australia)
Krauss SL (2016) Seed sourcing. In ‘Banksia Woodlands: a Restoration Guide for the Swan Coastal Plain’. (Eds JC Stevens, DP Rokich, VJ Newton, RL Barrett, KW Dixon) pp. 117–145. (UWA Publishing: Perth, WA, Australia)
Krauss SL, Sinclair EA, Bussell JD, Hobbs RJ (2013) An ecological genetic delineation of local seed‐source provenance for ecological restoration. Ecology and Evolution 3, 2138–2149.
| An ecological genetic delineation of local seed‐source provenance for ecological restoration.Crossref | GoogleScholarGoogle Scholar | 23919158PubMed |
Krawiec J, Krauss SL, Davis RA, Spencer PB (2015) Weak genetic structuring suggests historically high genetic connectivity among recently fragmented urban populations of the scincid lizard, Ctenotus fallens. Australian Journal of Zoology 63, 279–286.
| Weak genetic structuring suggests historically high genetic connectivity among recently fragmented urban populations of the scincid lizard, Ctenotus fallens.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends in Ecology & Evolution 23, 95–103.
| Plant nutrient-acquisition strategies change with soil age.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Brundrett MC, Raven JA, Hopper SD (2011) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 348, 7–27.
| Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GESJ, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conservation Physiology 1, cot010
| Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar | 27293594PubMed |
Lamont B (1989) Biotic and abiotic interactions in Banksia woodland. Journal of the Royal Society of Western Australia 71, 99–100.
Lamont BB (2003) Structure, ecology and physiology of root clusters–a review. Plant and Soil 248, 1–19.
| Structure, ecology and physiology of root clusters–a review.Crossref | GoogleScholarGoogle Scholar |
Lawson DM, Lamar CK, Schwartz MW (2008) Quantifying plant population persistence in human‐dominated landscapes. Conservation Biology 22, 922–928.
| Quantifying plant population persistence in human‐dominated landscapes.Crossref | GoogleScholarGoogle Scholar | 18544094PubMed |
Lettoof D, Lohr M, Busetti F, Bateman P, Davis R (2020) Toxic time bombs: Frequent detection of anticoagulant rodenticides in urban reptiles at multiple trophic levels. The Science of the Total Environment 724, 138218
| Toxic time bombs: Frequent detection of anticoagulant rodenticides in urban reptiles at multiple trophic levels.Crossref | GoogleScholarGoogle Scholar | 32247128PubMed |
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics 27, 237–277.
| Evolutionary significance of local genetic differentiation in plants.Crossref | GoogleScholarGoogle Scholar |
Lohr MT (2018) Anticoagulant rodenticide exposure in an Australian predatory bird increases with proximity to developed habitat. The Science of the Total Environment 643, 134–144.
| Anticoagulant rodenticide exposure in an Australian predatory bird increases with proximity to developed habitat.Crossref | GoogleScholarGoogle Scholar | 29936157PubMed |
MacCallum D, Hopkins D (2011) The changing discourse of city plans: rationalities of planning in Perth, 1955–2010. Planning Theory & Practice 12, 485–510.
| The changing discourse of city plans: rationalities of planning in Perth, 1955–2010.Crossref | GoogleScholarGoogle Scholar |
MacLachlan A, Biggs E, Roberts G, Boruff B (2017) Urbanisation-induced land cover temperature dynamics for sustainable future urban heat island mitigation. Urban Science 1, 38
| Urbanisation-induced land cover temperature dynamics for sustainable future urban heat island mitigation.Crossref | GoogleScholarGoogle Scholar |
Majer J (1989) Terrestrial invertebrate fauna. Journal of the Royal Society of Western Australia 71, 95–96.
Malcolm JR, Liu C, Neilson RP, Hansen L, Hannah L (2006) Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology 20, 538–548.
| Global warming and extinctions of endemic species from biodiversity hotspots.Crossref | GoogleScholarGoogle Scholar | 16903114PubMed |
Marchese C (2015) Biodiversity hotspots: a shortcut for a more complicated concept. Global Ecology Conservation Biology 3, 297–309.
| Biodiversity hotspots: a shortcut for a more complicated concept.Crossref | GoogleScholarGoogle Scholar |
Maron M, Hobbs RJ, Moilanen A, Matthews JW, Christie K, Gardner TA, Keith DA, Lindenmayer DB, McAlpine CA (2012) Faustian bargains? Restoration realities in the context of biodiversity offset policies. Biological Conservation 155, 141–148.
| Faustian bargains? Restoration realities in the context of biodiversity offset policies.Crossref | GoogleScholarGoogle Scholar |
Marschner P, Grierson PF, Rengel Z (2005) Microbial community composition and functioning in the rhizosphere of three Banksia species in native woodland in Western Australia. Applied Soil Ecology 28, 191–201.
| Microbial community composition and functioning in the rhizosphere of three Banksia species in native woodland in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Maryan B, Gaikhorst G, O’Connell M, Callan S (2015) Notes on the distribution and conservation status of the Perth Lined Skink, Lerista lineata: a small lizard in a big city. Western Australian Naturalist 30, 12–29.
Mason LD, Wardell-Johnson G, Main BY (2016) Quality not quantity: conserving species of low mobility and dispersal capacity in south-western Australian urban remnants. Pacific Conservation Biology 22, 37–47.
| Quality not quantity: conserving species of low mobility and dispersal capacity in south-western Australian urban remnants.Crossref | GoogleScholarGoogle Scholar |
Mason L, Wardell-Johnson G, Luxton S, Bateman P (2018a) Predators show seasonal predilections for model clay spiders in an urban environment. Scientific Reports 8, 12444
| Predators show seasonal predilections for model clay spiders in an urban environment.Crossref | GoogleScholarGoogle Scholar | 30127351PubMed |
Mason LD, Bateman PW, Wardell-Johnson GW (2018b) The pitfalls of short-range endemism: high vulnerability to ecological and landscape traps. PeerJ 6, e4715
| The pitfalls of short-range endemism: high vulnerability to ecological and landscape traps.Crossref | GoogleScholarGoogle Scholar | 29740516PubMed |
Mason L, Bateman PW, Miller BP, Wardell‐Johnson GW (2019) Ashes to ashes: Intense fires extinguish populations of urban short‐range endemics. Austral Ecology 44, 514–522.
| Ashes to ashes: Intense fires extinguish populations of urban short‐range endemics.Crossref | GoogleScholarGoogle Scholar |
May J, Hobbs RJ, Valentine LE (2017) Are offsets effective? An evaluation of recent environemntak offsets in Western Australia. Biological Conservation 206, 249–257.
| Are offsets effective? An evaluation of recent environemntak offsets in Western Australia.Crossref | GoogleScholarGoogle Scholar |
McArthur WM, Bettenay E (1974) Development and distribution of soils of the Swan Coastal Plain, Western Australia. Report, CSIRO, Australia.
McArthur WM, Johnston DA, Snell LW (2004) ‘Reference Soils of South-western Australia.’ (Department of Agriculture, Western Australia on behalf of the Australian Society of Soil Science Inc., Perth: Perth, WA, Australia)
McCarthy MA, Thompson CJ, Williams NS (2006) Logic for designing nature reserves for multiple species. American Naturalist 167, 717–727.
| Logic for designing nature reserves for multiple species.Crossref | GoogleScholarGoogle Scholar |
McFarlane D, Strawbridge M, Stone R, Paton A (2012) Managing groundwater levels in the face of uncertainty and change: a case study from Gnangara. Water Science and Technology: Water Supply 12, 321–328.
| Managing groundwater levels in the face of uncertainty and change: a case study from Gnangara.Crossref | GoogleScholarGoogle Scholar |
Menz MH, Phillips RD, Anthony JM, Bohman B, Dixon KW, Peakall R (2015) Ecological and genetic evidence for cryptic ecotypes in a rare sexually deceptive orchid, Drakaea elastica. Botanical Journal of the Linnean Society 177, 124–140.
| Ecological and genetic evidence for cryptic ecotypes in a rare sexually deceptive orchid, Drakaea elastica.Crossref | GoogleScholarGoogle Scholar |
Merritt D, Turner S, Clarke S, Dixon K (2007) Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55, 336–344.
| Seed dormancy and germination stimulation syndromes for Australian temperate species.Crossref | GoogleScholarGoogle Scholar |
Mickle D, Valentine LE, Kuehs J, Swinburn M (2010) Post-fire juvenile period of plants in Banksia woodland on the northern Swan Coastal Plain. Report, Perth, WA, Australia.
Mijangos JL, Pacioni C, Spencer PB, Craig MD (2015) Contribution of genetics to ecological restoration. Molecular Ecology 24, 22–37.
| Contribution of genetics to ecological restoration.Crossref | GoogleScholarGoogle Scholar | 25377524PubMed |
Milberg P, Lamont BB (1995) Fire enhances weed invasion of roadside vegetation in southwestern Australia. Biological Conservation 73, 45–49.
| Fire enhances weed invasion of roadside vegetation in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Miller RG, Tangney R, Enright NJ, Fontaine JB, Merritt DJ, Ooi MK, Ruthrof KX, Miller BP (2019) Mechanisms of fire seasonality effects on plant populations. Trends in Ecology & Evolution 34, 1104–1117.
| Mechanisms of fire seasonality effects on plant populations.Crossref | GoogleScholarGoogle Scholar |
Miller RG, Tangney R, Enright NJ, Fontaine JB, Merritt DJ, Ooi MK, Ruthrof KX, Miller BP (2020) Fire seasonality mechanisms are fundamental for understanding broader fire regime effects. Trends in Ecology & Evolution 35, 869–871.
| Fire seasonality mechanisms are fundamental for understanding broader fire regime effects.Crossref | GoogleScholarGoogle Scholar |
Monks LT (1999) Conservation biology of the rare and threatened Dryandra ionthocarpa, D. mimica and D. serra. PhD thesis, Curtin University, Perth, WA, Australia.
Moore N, Barrett S, Howard K, Craig MD, Bowen B, Shearer B, Hardy G (2014) Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia. Australian Journal of Botany 62, 587–593.
| Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Moore TL, Burbidge AH, Sonneman T, Wilson BA (2016) Mammal assemblages in Boonanarring Nature Reserve, Dandaragan Plateau, Western Australia from 1986 and 2012. Journal of the Royal Society of Western Australia 99, 1–8.
Muler AL, Canham CA, van Etten EJ, Stock WD, Froend RH (2018a) Using a functional ecology approach to assist plant selection for restoration of Mediterranean woodlands. Forest Ecology and Management 424, 1–10.
| Using a functional ecology approach to assist plant selection for restoration of Mediterranean woodlands.Crossref | GoogleScholarGoogle Scholar |
Muler AL, van Etten E, Stock WD, Howard K, Froend R (2018b) Can hydraulically redistributed water assist surrounding seedlings during summer drought? Oecologia 187, 625–641.
| Can hydraulically redistributed water assist surrounding seedlings during summer drought?Crossref | GoogleScholarGoogle Scholar | 29752527PubMed |
Newman BJ, Ladd P, Brundrett M, Dixon KW (2013) Effects of habitat fragmentation on plant reproductive success and population viability at the landscape and habitat scale. Biological Conservation 159, 16–23.
| Effects of habitat fragmentation on plant reproductive success and population viability at the landscape and habitat scale.Crossref | GoogleScholarGoogle Scholar |
Nield AP, Ladd PG, Yates CJ (2009) Reproductive biology, post-fire succession dynamics and population viability analysis of the critically endangered Western Australian shrub Calytrix breviseta subsp. breviseta (Myrtaceae). Australian Journal of Botany 57, 451–464.
| Reproductive biology, post-fire succession dynamics and population viability analysis of the critically endangered Western Australian shrub Calytrix breviseta subsp. breviseta (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
O’Gara E, Howard K, Wilson B, Hardy GSJ (2005) Management of Phytophthora cinnamomi for biodiversity conservation in Australia: Part 2. National best practice guidelines. Report, Department of the Environment and Heritage, Canberra, ACT, Australia.
Pate JS (1989) Preface. Journal of the Royal Society of Western Australia 71, 83–84.
Pate JS (1994) The mycorrhizal association: just one of many nutrient acquiring specializations in natural ecosystems. Plant and Soil 159, 1–10.
| The mycorrhizal association: just one of many nutrient acquiring specializations in natural ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pate JS, Bell TL (1999) Application of the ecosystem mimic concept to the species-rich Banksia woodlands of Western Australia. Agroforestry Systems 45, 303-341
| Application of the ecosystem mimic concept to the species-rich Banksia woodlands of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pate JS, Jeschke D, Dawson TE, Raphael C, Hartung W, Bowen BJ (1998) Growth and seasonal utilisation of water and nutrients by Banksia prionotes. Australian Journal of Botany 46, 511–532.
| Growth and seasonal utilisation of water and nutrients by Banksia prionotes.Crossref | GoogleScholarGoogle Scholar |
Pauli N, Boruff B (2016) Natural environments, ecosystem services and green infrastructure: planning for Perth’s ‘Green’ Matrix. In ‘Planning Boomtown and Beyond’. pp. 238–276. (UWA Publishing: Perth, WA, Australia)
Phillips RD, Peakall R, Retter BA, Montgomery K, Menz MHM, Davis BJ, Hayes C, Brown GR, Swarts ND, Dixon KW (2015) Pollinator rarity as a threat to a plant with a specialized pollination system. Botanical Journal of the Linnean Society 179, 511–525.
| Pollinator rarity as a threat to a plant with a specialized pollination system.Crossref | GoogleScholarGoogle Scholar |
Plucinski MP (2014) The timing of vegetation fire occurrence in a human landscape. Fire Safety Journal 67, 42–52.
| The timing of vegetation fire occurrence in a human landscape.Crossref | GoogleScholarGoogle Scholar |
Poole MD (1989) Urban development. Journal of the Royal Society of Western Australia 71, 103–104.
Povh LF, Bencini R, Chambers BK, Kreplins TL, Willers N, Adams PJ, Wann J, Kobryn HT, Fleming PA (2019) Shedding light on a cryptic macropodid: Home ranges and habitat preferences of translocated western brush wallabies (Notamacropus irma). Australian Mammalogy 41, 82–91.
| Shedding light on a cryptic macropodid: Home ranges and habitat preferences of translocated western brush wallabies (Notamacropus irma).Crossref | GoogleScholarGoogle Scholar |
Prober SM, Byrne M, McLean EH, Steane DA, Potts BM, Vaillancourt RE, Stock WD (2015) Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Frontiers in Ecology and Evolution 3, 65
| Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Project Dieback (2019) ‘Dieback Public Map. Project Dieback. Dieback Information Delivery and Management System.’ (Gaia Resources Pty in association with South Coast NRM)
Ramalho CE, Barrett G, Glossop B, Mitchell D, Wilson B, Clarke K (2013) Spatial conservation prioritization in the Swan Region-a pilot study using Marxan. Report, Western Australian Department of Parks and Wildlife, Swan Region, Perth, WA, Australia.
Ramalho CE, Laliberté E, Poot P, Hobbs RJ (2014) Complex effects of fragmentation on remnant woodland plant communities of a rapidly urbanizing biodiversity hotspot. Ecology 95, 2466–2478.
| Complex effects of fragmentation on remnant woodland plant communities of a rapidly urbanizing biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Recher HF (1997) Impact of wildfire on the avifauna of Kings Park, Perth, Western Australia. Wildlife Research 24, 745–761.
| Impact of wildfire on the avifauna of Kings Park, Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Recher HF, Serventy DL (1991) Long term changes in the relative abundances of birds in Kings Park, Perth, Western Australia. Conservation Biology 5, 90–102.
| Long term changes in the relative abundances of birds in Kings Park, Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Ritchie AL, Krauss SL (2012) A genetic assessment of ecological restoration success in Banksia attenuata. Restoration Ecology 20, 441–449.
| A genetic assessment of ecological restoration success in Banksia attenuata.Crossref | GoogleScholarGoogle Scholar |
Ritchie AL, Dyer RJ, Nevill PG, Sinclair EA, Krauss SL (2019) Wide outcrossing provides functional connectivity for new and old Banksia populations within a fragmented landscape. Oecologia 190, 255–268.
| Wide outcrossing provides functional connectivity for new and old Banksia populations within a fragmented landscape.Crossref | GoogleScholarGoogle Scholar | 30919107PubMed |
Roche S, Koch JM, Dixon KW (1997) Smoke enhanced seed germination for mine rehabilitation in the southwest of Western Australia. Restoration Ecology 5, 191–203.
| Smoke enhanced seed germination for mine rehabilitation in the southwest of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Roche S, Dixon KW, Pate JS (1998) For everything a season: Smoke-induced seed germination and seedling recruitment in a Western Australian Banksia woodland. Austral Ecology 23, 111–120.
| For everything a season: Smoke-induced seed germination and seedling recruitment in a Western Australian Banksia woodland.Crossref | GoogleScholarGoogle Scholar |
Rohde MM, Froend R, Howard J (2017) A global synthesis of managing groundwater dependent ecosystems under sustainable groundwater policy. Ground Water 55, 293–301.
| A global synthesis of managing groundwater dependent ecosystems under sustainable groundwater policy.Crossref | GoogleScholarGoogle Scholar | 28419432PubMed |
Rokich DP (2016) Soil profile reconstruction. In ‘Banksia Woodlands: a Restoration Guide for the Swan Coastal Plain’. (Eds JC Stevens, DP Rokich, VJ Newton, RL Barrett, KW Dixon) pp. 83–90. (UWA Publishing, Perth, Western Australia: Perth, Australia)
Rokich DP, Dixon KW, Sivasithamparam K, Meney KA (2000) Topsoil handling and storage effects on woodland restoration in Western Australia. Restoration Ecology 8, 196–208.
| Topsoil handling and storage effects on woodland restoration in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rokich DP, Meney KA, Dixon KW, Sivasithamparam K (2001) The impact of soil disturbance on root development in woodland communities in Western Australia. Australian Journal of Botany 49, 169–183.
| The impact of soil disturbance on root development in woodland communities in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rossbach M, Majer J (1983) A preliminary survey of the ant fauna of the Darling Plateau and Swan Coastal Plain near Perth, Western Australia. Journal of the Royal Society of Western Australia 66, 85–90.
Ruthrof KX (2004) Invasion by Eucalyptus megacornuta of an urban bushland in southwestern Australia. Weed Technology 18, 1376–1380.
| Invasion by Eucalyptus megacornuta of an urban bushland in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Ruthrof KX, Loneragan WA, Yates CJ (2003) Comparative population dynamics of Eucalyptus cladocalyx in its native habitat and as an invasive species in an urban bushland in south-western Australia. Diversity & Distributions 9, 469–483.
| Comparative population dynamics of Eucalyptus cladocalyx in its native habitat and as an invasive species in an urban bushland in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Ruthrof KX, Breshears DD, Fontaine JB, Froend RH, Matusick G, Kala J, Miller BP, Mitchell PJ, Wilson SK, van Keulen M, Enright NJ, Law DJ, Wernberg T, Hardy GESJ (2018) Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Scientific Reports 8, 13094
| Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses.Crossref | GoogleScholarGoogle Scholar | 30166559PubMed |
Ryan C, Hobbs R, Valentine L (2020) Bioturbation by a reintroduced digging mammal reduces fuel loads in an urban reserve. Ecological Applications 30, e02018
| Bioturbation by a reintroduced digging mammal reduces fuel loads in an urban reserve.Crossref | GoogleScholarGoogle Scholar | 31596973PubMed |
Rye C, Smettem K (2015) Seasonal and interannual variability of the effective flow cross-sectional area in a water-repellent soil. Vadose Zone Journal 14, vzj2014.10.0141
| Seasonal and interannual variability of the effective flow cross-sectional area in a water-repellent soil.Crossref | GoogleScholarGoogle Scholar |
Rye C, Smettem K (2017) The effect of water repellent soil surface layers on preferential flow and bare soil evaporation. Geoderma 289, 142–149.
| The effect of water repellent soil surface layers on preferential flow and bare soil evaporation.Crossref | GoogleScholarGoogle Scholar |
Rye C, Smettem K (2018) Seasonal variation of subsurface flow pathway spread under a water repellent surface layer. Geoderma 327, 1–12.
| Seasonal variation of subsurface flow pathway spread under a water repellent surface layer.Crossref | GoogleScholarGoogle Scholar |
Salama RB, Silberstein R, Pollock D (2005) Soils characteristics of the Bassendean and Spearwood Sands of the Gnangara Mound (Western Australia) and their controls on recharge, water level patterns and solutes of the superficial aquifer. Water Air and Soil Pollution Focus 5, 3–26.
| Soils characteristics of the Bassendean and Spearwood Sands of the Gnangara Mound (Western Australia) and their controls on recharge, water level patterns and solutes of the superficial aquifer.Crossref | GoogleScholarGoogle Scholar |
Saunders ME, Bower DS, Mika S, Hunter JT (2020) Condition thresholds in Australia’s threatened ecological community listings hinder conservation of dynamic ecosystems. Pacific Conservation Biology
| Condition thresholds in Australia’s threatened ecological community listings hinder conservation of dynamic ecosystems.Crossref | GoogleScholarGoogle Scholar | [Published online early 13 November 2020]
Scholze M, Knorr W, Arnell NW, Prentice IC (2006) A climate-change risk analysis for world ecosystems. Proceedings of the National Academy of Sciences of the United States of America 103, 13116–13120.
| A climate-change risk analysis for world ecosystems.Crossref | GoogleScholarGoogle Scholar | 16924112PubMed |
Scott PM, Burgess T, Barber P, Shearer B, Stukely M, Hardy GSJ, Jung T (2009) Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia. Persoonia 22, 1
| Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia.Crossref | GoogleScholarGoogle Scholar | 20198133PubMed |
Semeniuk V, Glassford DK (1989) Bassendean and Spearwood dunes: their geomorphology, stratigraphy and soils as a basis for habitats of Banksia woodlands. Journal of the Royal Society of Western Australia 71, 87–88.
Seto KC, Fragkias M, Güneralp B, Reilly MK (2011) A meta-analysis of global urban land expansion. PLoS One 6, e23777
| A meta-analysis of global urban land expansion.Crossref | GoogleScholarGoogle Scholar | 21876770PubMed |
Shearer B (1990) Dieback of native plant communities caused by Phytophthora species-a major factor affecting land use in South-Western Australia.Land and Water Research News1990 5 1526
Shearer B (1994) The major plant pathogens occurring in native ecosystems of south-western Australia. Journal of the Royal Society of Western Australia 77, 113–122.
Shearer BL, Crane CE (2014) Phytophthora cinnamomi disease expression and habitat suitability of soils on a topographic gradient across a coastal plain from dunes to forested peneplain. Australasian Plant Pathology 43, 131–142.
| Phytophthora cinnamomi disease expression and habitat suitability of soils on a topographic gradient across a coastal plain from dunes to forested peneplain.Crossref | GoogleScholarGoogle Scholar |
Shearer B, Dillon M (1996a) Impact and disease centre characteristics of Phytophthora cinnamomi Infestations of Banksia woodlands on the Swan Coastal Plain, Western Australia. Australian Journal of Botany 44, 79–90.
| Impact and disease centre characteristics of Phytophthora cinnamomi Infestations of Banksia woodlands on the Swan Coastal Plain, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Shearer B, Dillon M (1996b) Susceptibility of plant species in Banksia woodlands on the Swan Coastal Plain, Western Australia, to infection by Phytophthora cinnamomi. Australian Journal of Botany 44, 433–445.
Shearer B, Fairman R (2007) A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australasian Plant Pathology 36, 78–86.
| A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years.Crossref | GoogleScholarGoogle Scholar |
Shearer B, Hill T (1989) Diseases of Banksia woodlands on the Bassendean and Spearwood dune systems. Journal of the Royal Society of Western Australia 71, 113–114.
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435–443.
| Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi.Crossref | GoogleScholarGoogle Scholar |
Shearer B, Dillon M, Kinal J, Buehrig R (2010) Temporal and spatial soil inoculum dynamics following Phytophthora cinnamomi invasion of Banksia woodland and Eucalyptus marginata forest biomes of south-western Australia. Australasian Plant Pathology 39, 293–311.
| Temporal and spatial soil inoculum dynamics following Phytophthora cinnamomi invasion of Banksia woodland and Eucalyptus marginata forest biomes of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Smith MA, Bell DT, Loneragan WA (1999) Comparative seed germination ecology of Austrostipa compressa and Ehrharta calycina (Poaceae) in a Western Australian Banksia woodland. Austral Ecology 24, 35–42.
| Comparative seed germination ecology of Austrostipa compressa and Ehrharta calycina (Poaceae) in a Western Australian Banksia woodland.Crossref | GoogleScholarGoogle Scholar |
Smith K, Fleming P, Kreplins T, Wilson B (2019) Population monitoring and habitat utilisation of the ash-grey mouse (Pseudomys albocinereus) in Western Australia. Australian Mammalogy 41, 170–178.
| Population monitoring and habitat utilisation of the ash-grey mouse (Pseudomys albocinereus) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Sommer B, Froend R (2011) Resilience of phreatophytic vegetation to groundwater drawdown: is recovery possible under a drying climate? Ecohydrology 4, 67–82.
| Resilience of phreatophytic vegetation to groundwater drawdown: is recovery possible under a drying climate?Crossref | GoogleScholarGoogle Scholar |
Sommer B, Froend R (2014) Phreatophytic vegetation responses to groundwater depth in a drying mediterranean‐type landscape. Journal of Vegetation Science 25, 1045–1055.
| Phreatophytic vegetation responses to groundwater depth in a drying mediterranean‐type landscape.Crossref | GoogleScholarGoogle Scholar |
Sommer B, Boggs DA, Boggs GS, van Dijk A, Froend R (2016) Spatio‐temporal patterns of evapotranspiration from groundwater‐dependent vegetation. Ecohydrology 9, 1620–1629.
| Spatio‐temporal patterns of evapotranspiration from groundwater‐dependent vegetation.Crossref | GoogleScholarGoogle Scholar |
Stace HM (1995) Protogyny, self-incompatibility and pollination in Anthocercis gracilis (Solanaceae). Australian Journal of Botany 43, 451–459.
| Protogyny, self-incompatibility and pollination in Anthocercis gracilis (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Stanbury KE, Stevens JC, Ritchie AL (2018) Legacy issues in post‐pine (Pinus pinaster) restoration environments: Weeds compromise seedling growth and function more than edaphic factors. Land Degradation & Development 29, 1694–1704.
| Legacy issues in post‐pine (Pinus pinaster) restoration environments: Weeds compromise seedling growth and function more than edaphic factors.Crossref | GoogleScholarGoogle Scholar |
Standish R, Fontaine JB, Harris R, Stock W, Hobbs R (2012) Interactive effects of altered rainfall and simulated nitrogen deposition on seedling establishment in a global biodiversity hotspot. Oikos 121, 2014–2025.
| Interactive effects of altered rainfall and simulated nitrogen deposition on seedling establishment in a global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Stasikowski P, McComb J, Scott P, Paap T, O’Brien P, Hardy GSJ (2014) Calcium sulphate soil treatments augment the survival of phosphite-sprayed Banksia leptophylla infected with Phytophthora cinnamomi. Australasian Plant Pathology 43, 369–379.
| Calcium sulphate soil treatments augment the survival of phosphite-sprayed Banksia leptophylla infected with Phytophthora cinnamomi.Crossref | GoogleScholarGoogle Scholar |
Stephenson G, Hepburn JA (1955) Plan for the metropolitan region of Perth and Fremantle, Western Australia: a report prepared for the Government of Western Australia. Government Printing Office, Perth, WA, Australia.
Stevens J, Rokich D, Newton V, Barrett R, Dixon K (2016) ‘Banksia Woodlands: a Restoration Guide for the Swan Coastal Plain. Floristics of the Banksia Woodlands of the Swan Coastal Plain.’ (UWA Publishing: Perth, WA, Australia)
Stock WD, Finn H, Parker J, Dods K (2013) Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape. PLoS One 8, e61145
| Pine as fast food: foraging ecology of an endangered cockatoo in a forestry landscape.Crossref | GoogleScholarGoogle Scholar | 23593413PubMed |
Swarts ND, Sinclair EA, Krauss SL, Dixon KW (2009) Genetic diversity in fragmented populations of the critically endangered spider orchid Caladenia huegelii: implications for conservation. Conservation Genetics 10, 1199–1208.
| Genetic diversity in fragmented populations of the critically endangered spider orchid Caladenia huegelii: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Swarts ND, Sinclair EA, Francis A, Dixon KW (2010) Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Molecular Ecology 19, 3226–3242.
| Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid.Crossref | GoogleScholarGoogle Scholar | 20618899PubMed |
Tangney R, Merritt DJ, Fontaine JB, Miller BP (2019) Seed moisture content as a primary trait regulating the lethal temperature thresholds of seeds. Journal of Ecology 107, 1093–1105.
| Seed moisture content as a primary trait regulating the lethal temperature thresholds of seeds.Crossref | GoogleScholarGoogle Scholar |
Tangney R, Merritt DJ, Callow JN, Fontaine JB, Miller BP (2020a) Seed dormancy interacts with fire seasonality mechanisms. Trends in Ecology and Evolution 35, 1057–1059.
| Seed dormancy interacts with fire seasonality mechanisms.Crossref | GoogleScholarGoogle Scholar | 33121751PubMed |
Tangney R, Merritt DJ, Callow JN, Fontaine JB, Miller BP (2020b) Seed traits determine species responses to fire under varying soil heating scenarios. Functional Ecology 34, 1967–1978.
| Seed traits determine species responses to fire under varying soil heating scenarios.Crossref | GoogleScholarGoogle Scholar |
Thompson SA, Thompson GG, Oates JE (2008) Range extension of the Western Heath Dragon, Rankinia adelaidensis adelaidensis (Squamata: Agamidae). Journal of the Royal Society of Western Australia 91, 207–208.
Thorn S, Hobbs RJ, Valentine LE (2018) Effectiveness of biodiversity offsets: An assessment of a controversial offset in Perth, Western Australia. Biological Conservation 228, 291–300.
| Effectiveness of biodiversity offsets: An assessment of a controversial offset in Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Tieu A, Dixon K, Meney K, Sivasithamparam K, Barrett R (2001) Spatial and developmental variation in seed dormancy characteristics in the fire-responsive species Anigozanthos manglesii (Haemodoraceae) from Western Australia. Annals of Botany 88, 19–26.
| Spatial and developmental variation in seed dormancy characteristics in the fire-responsive species Anigozanthos manglesii (Haemodoraceae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Tingley R, Macdonald SL, Mitchell NJ, Woinarski JCZ, Meiri S, Bowles P, Cox NA, Shea GM, Böhm M, Chanson J, Tognelli MF, Harris J, Walke C, Harrison N, Victor S, Woods C, Amey AP, Bamford M, Catt G, Clemann N, Couper PJ, Cogger H, Cowan M, Craig MD, Dickman CR, Doughty P, Ellis R, Fenner A, Ford S, Gaikhorst G, Gillespie GR, Greenlees MJ, Hobson R, Hoskin CJ, How R, Hutchinson MN, Lloyd R, McDonald P, Melville J, Michael DR, Moritz C, Oliver PM, Peterson G, Robertson P, Sanderson C, Somaweera R, Teale R, Valentine L, Vanderduys E, Venz M, Wapstra E, Wilson S, Chapple DG (2019) Geographic and taxonomic patterns of extinction risk in Australian squamates. Biological Conservation 238, 108203
| Geographic and taxonomic patterns of extinction risk in Australian squamates.Crossref | GoogleScholarGoogle Scholar |
Tsakalos JL, Renton M, Dobrowolski MP, Feoli E, Macintyre PD, Veneklaas EJ, Mucina L (2018) Community patterns and environmental drivers in hyper‐diverse kwongan scrub vegetation of Western Australia. Applied Vegetation Science 21, 694–722.
| Community patterns and environmental drivers in hyper‐diverse kwongan scrub vegetation of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Turner S, Merritt D, Ridley E, Commander L, Baskin J, Baskin C, Dixon K (2006) Ecophysiology of seed dormancy in the Australian endemic species Acanthocarpus preissii (Dasypogonaceae). Annals of Botany 98, 1137–1144.
| Ecophysiology of seed dormancy in the Australian endemic species Acanthocarpus preissii (Dasypogonaceae).Crossref | GoogleScholarGoogle Scholar | 17008351PubMed |
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. European Journal of Soil Science 69, 69–85.
| A climosequence of chronosequences in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
University of NSW City Futures Research Centre Astrolabe Group (2019) Australia’s Household Infrastructure Bill: Analysis Report, Sydney, NSW, Australia.
Valentine LE, Wilson BA, Reaveley A, Huang N, Johnson B, Brown P (2009) Patterns of ground-dwelling vertebrate biodiversity in the Gnangara Sustainability Strategy study area. Report, Department of Environment and Conservation, Perth, WA, Australia.
Valentine LE, Reaveley A, Johnson B, Fisher R, Wilson BA (2012) Burning in Banksia woodlands: How does the fire-free period influence reptile communities? PLoS One 7, e34448
| Burning in Banksia woodlands: How does the fire-free period influence reptile communities?Crossref | GoogleScholarGoogle Scholar | 22496806PubMed |
Valentine LE, Fisher R, Wilson BA, Sonneman T, Stock WD, Fleming PA, Hobbs RJ (2014) Time since fire influences food resources for an endangered species, Carnaby’s cockatoo, in a fire-prone landscape. Biological Conservation 175, 1–9.
| Time since fire influences food resources for an endangered species, Carnaby’s cockatoo, in a fire-prone landscape.Crossref | GoogleScholarGoogle Scholar |
Veneklaas EJ, Poot P (2003) Seasonal patterns in water use and leaf turnover of different plant functional types in a species-rich woodland, south-western Australia. Plant and Soil 257, 295–304.
| Seasonal patterns in water use and leaf turnover of different plant functional types in a species-rich woodland, south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Vertucci S, Pepper M, Edwards DL, Roberts JD, Mitchell N, Keogh JS (2017) Evolutionary and natural history of the turtle frog, Myobatrachus gouldii, a bizarre myobatrachid frog in the southwestern Australian biodiversity hotspot. PLoS One 12, e0173348
| Evolutionary and natural history of the turtle frog, Myobatrachus gouldii, a bizarre myobatrachid frog in the southwestern Australian biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar | 28296914PubMed |
Wann JM, Bell DT (1997) Dietary preferences of the black-gloved wallaby (Macropus irma) and the western grey kangaroo (M. fuliginosus) in Whiteman Park, Perth, Western Australia. Journal of the Royal Society of Western Australia 80, 55–62.
Waryszak P, Standish RJ, Ladd PG, Enright NJ, Brundrett M, Fontaine JB (2021) Best served deep: The seedbank from salvaged topsoil underscores the role of the dispersal filter in restoration practice. Applied Vegetation Science 24, e12539
| Best served deep: The seedbank from salvaged topsoil underscores the role of the dispersal filter in restoration practice.Crossref | GoogleScholarGoogle Scholar |
Weller R (2009) ‘Boomtown 2050: Scenarios for a Rapidly Growing City.’ (UWA Publishing: Perth, WA, Australia)
Western Australian Planning Commission (2004) ‘Network City: Community Planning Strategy for Perth and Peel.’ (Western Australian Planning Commission: Perth, WA, Australia)
Western Australian Planning Commission (2007) ‘Liveable Neighbourhoods: a Western Australian Government Sustainable Cities Initiative.’ (Western Australian Planning Commission: Perth, WA, Australia)
Western Australian Planning Commission (2010) Directions 2031 and beyond: metropolitan planning beyond the horizon. Report, Western Australian Planning Commission, Perth, WA, Australia.
Western Australian Planning Commission (2012) Basic raw materials: demand and supply study for the Bunbury–Busselton Region. Report, Western Australian Planning Commission, Perth, WA, Australia.
Wilkinson CJ, Holmes JM, Dell B, Tynan KM, McComb JA, Shearer BL, Coloquhoun IJ, Hardy GESJ (2001) Effect of phosphite on in planta zoospore production of Phytophthora cinnamomi. Plant Pathology 50, 587–593.
| Effect of phosphite on in planta zoospore production of Phytophthora cinnamomi.Crossref | GoogleScholarGoogle Scholar |
Williams MR (2009) Butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia: patterns of species richness. Pacific Conservation Biology 15, 32–46.
| Butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia: patterns of species richness.Crossref | GoogleScholarGoogle Scholar |
Williams MR (2011) Habitat resources, remnant vegetation condition and area determine distribution patterns and abundance of butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia. Journal of Insect Conservation 15, 37–54.
| Habitat resources, remnant vegetation condition and area determine distribution patterns and abundance of butterflies and day-flying moths in a fragmented urban landscape, south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Williams AV, Nevill PG, Krauss SL (2014) Next generation restoration genetics: applications and opportunities. Trends in Plant Science 19, 529–537.
| Next generation restoration genetics: applications and opportunities.Crossref | GoogleScholarGoogle Scholar | 24767982PubMed |
Williams MR, Yates CJ, Stock WD, Barrett GW, Finn HC (2016) Citizen science monitoring reveals a significant, ongoing decline of the Endangered Carnaby’s black-cockatoo Calyptorhynchus latirostris. Oryx 50, 626–635.
| Citizen science monitoring reveals a significant, ongoing decline of the Endangered Carnaby’s black-cockatoo Calyptorhynchus latirostris.Crossref | GoogleScholarGoogle Scholar |
Williams MR, Yates CJ, Saunders DA, Dawson R, Barrett GW (2017) Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris). Biological Conservation 210, 8–15.
| Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris).Crossref | GoogleScholarGoogle Scholar |
Wilson BA, Valentine LE, Reaveley A, Isaac J, Wolfe KM (2012) Terrestrial mammals of the Gnangara Groundwater System, Western Australia: History, status, and the possible impacts of a drying climate. Australian Mammalogy 34, 202–216.
| Terrestrial mammals of the Gnangara Groundwater System, Western Australia: History, status, and the possible impacts of a drying climate.Crossref | GoogleScholarGoogle Scholar |
Wilson BA, Kuehs J, Valentine LE, Sonneman T, Wolfe KM (2014) Guidelines for ecological burning regimes in Mediterranean ecosystems: A case study in Banksia woodlands in Western Australia. Pacific Conservation Biology 20, 57–74.
| Guidelines for ecological burning regimes in Mediterranean ecosystems: A case study in Banksia woodlands in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wintle BA, Kujala H, Whitehead A, Cameron A, Veloz S, Kukkala A, Moilanen A, Gordon A, Lentini PE, Cadenhead NCR, Bekessy SA (2019) Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proceedings of the National Academy of Sciences of the United States of America 116, 909–914.
| Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity.Crossref | GoogleScholarGoogle Scholar | 30530660PubMed |
Wood L, Hooper P, Foster S, Bull F (2017) Public green spaces and positive mental health–investigating the relationship between access, quantity and types of parks and mental wellbeing. Health & Place 48, 63–71.
| Public green spaces and positive mental health–investigating the relationship between access, quantity and types of parks and mental wellbeing.Crossref | GoogleScholarGoogle Scholar |
Yates CJ, McNeill A, Elith J, Midgley GF (2010) Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region. Diversity & Distributions 16, 187–201.
| Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Young TP, Petersen D, Clary J (2005) The ecology of restoration: historical links, emerging issues and unexplored realms. Ecology Letters 8, 662–673.
| The ecology of restoration: historical links, emerging issues and unexplored realms.Crossref | GoogleScholarGoogle Scholar |
Zencich SJ, Froend RH, Turner JV, Gailitis V (2002) Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer. Oecologia 131, 8–19.
| Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer.Crossref | GoogleScholarGoogle Scholar | 28547514PubMed |
Zylstra MJ, Knight AT, Esler KJ, Le Grange LL (2014) Connectedness as a core conservation concern: An interdisciplinary review of theory and a call for practice. Springer Science Reviews 2, 119–143.
| Connectedness as a core conservation concern: An interdisciplinary review of theory and a call for practice.Crossref | GoogleScholarGoogle Scholar |