Control of African lovegrass by flupropanate in a flora conservation context
Carl R. Gosper A * , Julia Cullity B and Grazyna Paczkowska B
A * , Julia Cullity B and Grazyna Paczkowska B
A
B
Abstract
Managing widespread invasive plants to support biodiversity conservation is a significant challenge that requires weed control methods that have lesser impacts on co-occurring native species than ongoing weed invasion. African lovegrass (Eragrostis curvula) is a perennial grass invasive in many regions globally. There is a lack of effective control options, particularly in diverse native vegetation where application of broad-spectrum herbicide has risks of unacceptable off-target impacts.
We tested the effectiveness of flupropanate (sodium 2,2,3,3-tetra-fluoropropionate) in controlling African lovegrass in a conservation context in Mediterranean-climate south-western Australia, testing two application rates and measuring target and off-target impact.
Cover and condition (alive or dead) of plant species were measured in replicate plots in a ‘before-after-control-impact’ design. A small sample of endangered Grevillea curviloba individuals was deliberately treated with flupropanate.
Flupropanate significantly reduced African lovegrass cover, with greater reduction at the higher application rate. No significant off-target effects could be detected at a community or plant functional group level nor in the deliberate exposure of G. curviloba.
The results of this study indicate that flupropanate is effective in controlling African lovegrass in conservation settings in south-western Australia, including where G. curviloba is present. These findings contribute to the growing body of knowledge on the use of flupropanate for invasive grass management.
Possible off-target impacts on a single species in this study, and stronger evidence from other sources, suggest that robust testing of the susceptibility of conservation-listed flora to flupropanate should precede application in the habitat of these species.
Keywords: asset protection, Eragrostis curvula, invasive grass, off-target impact, selective herbicide, south-western Australia, threatened flora, weed management.
Introduction
Weed invasions have substantial impacts on biodiversity (Pyšek et al. 2012) and are regarded as a significant threat to many threatened Australian plants (Burgman et al. 2007). Effective weed control methods are thus needed to achieve flora conservation objectives. The application of herbicides is one method widely used to manage weed impacts in conservation contexts, and in some situations, programs involving herbicide applications have achieved impressive reductions in weed populations and have occasionally led to the restoration of native flora (Mason and French 2007; Gooden et al. 2009; Kaiser-Bunbury et al. 2015). However, significant adverse impacts of herbicides on off-target species have been recorded (Kaiser-Bunbury et al. 2015), including on threatened flora (Matarczyk et al. 2002). Furthermore, successful control of one weed species has often led to replacement by different weed species, an undesirable biodiversity conservation outcome (Mason and French 2007; Pearson et al. 2016) (noting that such outcomes may meet some objectives of species-led weed management; Timmins and Owen 2001). Objectives for asset- or site-led weed management for biodiversity conservation (Timmins and Owen 2001) include weed control methods that have lesser impacts on co-occurring native species than ongoing weed invasion and, over time, an improvement in metrics measuring native species occurrence in addition to metrics of decreased target weed abundance.
African lovegrass (Eragrostis curvula (Schrad.) Nees), a long-lived tussock grass that occurs naturally in southern Africa, has become a significant weed globally (Roberts et al. 2021) and is considered among the highest impact grass environmental weeds in Australia (Van Klinken and Friedel 2017). African lovegrass is competitively superior to native grasses under a range of environmental conditions and higher African lovegrass biomass is associated with lower richness of co-occurring species (Firn et al. 2010, 2018). African lovegrass has been introduced widely through seed contamination and deliberately as a pasture species and for soil stabilisation but has spread further to become a weed in both agricultural and environmental settings (Firn 2009; Van Klinken and Friedel 2017). As a perennial grass using the C4 photosynthetic pathway, African lovegrass grows actively through the warmer months of the year. African lovegrass has the capacity to resprout strongly from the base of tussocks following fire and other disturbances, and is spread primarily via dispersal of seed by machinery, wind and animals.
The most common method for control of African lovegrass in conservation settings is application of the non-selective herbicide glyphosate, although mechanical removal can be effective for low-density infestations (Firn 2009; Blakely et al. 2022). Preventing spread is also an important African lovegrass management strategy (Firn 2009). African lovegrass control in conservation settings is challenging as there are risks of off-target impacts in the use of broad-spectrum herbicides. As an alternative to glyphosate, flupropanate (sodium 2,2,3,3-tetra-fluoropropionate; marketed under several product names) has been demonstrated to be effective for African lovegrass control in some eastern Australian agricultural and environmental situations (Campbell and Nicol 1998). While flupropanate may have potentially less uniform impact on co-occurring native vegetation than glyphosate (Campbell et al. 2002), off-target impacts on vegetation ranging from tree seedlings to desirable pasture species have been recorded (McLaren et al. 2008; Lusk et al. 2017; Blakely et al. 2022). Flupropanate has a low contact activity and is therefore mainly absorbed into the soil and taken up by plant roots after rain post-application. Flupropanate effectiveness is considered to be dependent on both temperature (acting faster with warmer conditions) and rainfall (requiring wet conditions for activation) (Lusk et al. 2017). Some residual soil effects are typical but may be short-lived (Bourdôt et al. 2017).
The aims of this study were to build on previous research by testing the efficacy of flupropanate to control African lovegrass in a different climatic and land use context, specifically to:
Determine if flupropanate remains effective in controlling African lovegrass when applied in a strongly Mediterranean climate, where herbicide effectiveness could plausibly be reduced by lower water availability over the warmer months of optimal African lovegrass growth.
Test any off-target effects on co-occurring native flora when flupropanate is applied to control African lovegrass in a conservation context.
Materials and methods
Study ecosystem and site
The Swan Coastal Plain, that forms part of the Southwest Australian Floristic Region biodiversity hotspot, supports an exceptional concentration of threatened flora, particularly on the relatively older (late Pliocene–middle Pleistocene) Bassendean sands and Guildford sediments (Gosper et al. 2021a, 2022) on the eastern side of the plain. Levels of weed invasion are relatively high on the comparatively fertile Guildford sediments (Gosper et al. 2021b), with African lovegrass particularly problematic on the heavier soils typical of this formation. In a weed prioritisation for the Department of Biodiversity, Conservation and Attractions’ (DBCA) Swan Region, African lovegrass was ranked with high ecological impact and rapid invasiveness (https://www.dbca.wa.gov.au/management/threat-management/weeds). African lovegrass invasion impacts a range of threatened flora on the Swan Coastal Plain, including Grevillea curviloba (Western Australian conservation status of endangered; Fig. 1), Darwinia foetida (endangered), Synaphea sp. Fairbridge Farm (D. Papenfus 696) (critically endangered), Synaphea sp. Pinjarra (R. Davis 6578) (critically endangered) and Synaphea sp. Pinjarra Plain (A. S. George 17182) (endangered), and threatened ecological communities such as Corymbia calophylla – Kingia australis woodlands on heavy soils (critically endangered) and Corymbia calophylla – Xanthorrhoea preissii woodlands and shrublands (endangered).
(a) African lovegrass (Eragrostis curvula) invasion impacting a population of the endangered Grevillea curviloba; (b) habitat of roadside G. curviloba plants that were regularly slashed and that were tested for tolerance to flupropanate. Photos: Carl Gosper, Julia Cullity.

An area near Bullsbrook, north-east of Perth in south-western Australia, was chosen for the study, having an extensive African lovegrass infestation adjoining and in similar habitat (soils, remnant vegetation) to key threatened flora in which African lovegrass control is required. The site, in a narrow (less than 100 m wide), linear rail easement vested in the Perth Transport Authority and the City of Swan, had been historically cleared of large trees but retained scattered mature native shrubs over a mixed ground layer of dense African lovegrass, invasive perennial veldt grass (Ehrharta calycina), invasive annual grasses, and native and invasive herbs, geophytes and graminoids.
Experimental treatments
An experiment was set up as a repeated measures ‘before-after-control-impact’ design. Three sets of replicate 10 × 10 m plots were established and sampled in 2019 prior to application of each of three treatments, with these treatments being a no herbicide control, and lower (1.5 mL L−1) and higher (3.0 mL L−1 – label rate) flupropanate application rates. Plot corners were marked with steel droppers and recorded with a Global Positioning System (GPS) device. Liquid herbicide (mixed with dye) was applied by a ground-based operator with backpack-based spray equipment as would be undertaken in a management scenario, by targeted spot spray to runoff of live African lovegrass plants. Due to high African lovegrass density, exposure of co-occurring native and other invasive flora to flupropanate was inevitable and because flupropanate uptake primarily occurs via the roots, even large shrubs and small trees may have been exposed. Treatments were applied in late spring (November) 2019, when African lovegrass was actively growing (Roberts et al. 2021), with follow-up spot spraying applied as required in November 2020.
Vegetation measurement
At each of the nine plots, species presence and condition (whether the whole plant was alive or dead) were recorded at 200-point intercepts with a 12 mm diameter pole distributed 0.25 m apart in a grid pattern along five equally spaced transects across the plot. This technique provided an objective measure of species abundance/cover as the number of intercepts with the species present, either alive and/or dead, out of 200. Any additional species present in the plot but not intercepted were also recorded and allocated a nominal cover value of 0.5 intercepts out of 200. Vegetation measurements were completed in 2019 (pre-treatment), 2020 (~1 year after the first treatment in 2019) and 2021 (~1 year after second treatment in 2020) immediately prior to herbicide application in that year. Consideration was given to normal modes of seasonal growth in vegetation measurements, therefore annual species or annually active species such as geophytes, were considered ‘alive’ regardless of whether senescence had begun, due to senescence being the expected condition in late spring. Annual or annually active species in the post-treatment samples would have germinated or sprouted after the previous herbicide treatment, therefore detection indicates that these were able to grow after habitat treatment, with the effect of treatments being assessed through changes in the number of intercepts. In contrast, any susceptibility of aboveground perennial species can be assessed through changes in the quantity of both live and dead intercepts.
Statistical analysis
Cover of plant species was aggregated into functional groups on the basis of growth form (annual, perennial groundcover (grass, herb and graminoid) and woody shrub) and status as native or invasive, using the information from the Western Australian Herbarium (1998–2024) (Supplementary Material S1). Functional groupings did not include cover of African lovegrass.
Summed plot live vegetation intercepts (cover) for African lovegrass, invasive annuals, invasive perennial groundcovers, native perennial groundcovers and native woody shrubs were analysed using the repeated measures ANOVA module in Statistica 7.1 (https://docs.tibco.com/products/spotfire-statistica), with a fixed factor of treatment and the fixed repeated measure of sample year (2019, 2020, 2021). Cover of African lovegrass was log10 transformed.
Ordination was used to explore changes in species composition over time and with treatments. The cover data were filtered to live touches only, singletons were removed, and separate data files prepared including and excluding cover of African lovegrass. Data were square-root transformed and non-metric multidimensional scaling applied using the Bray–Curtis dissimilarity metric in PRIMER analysis software (ver. 6.1.11, https://www.primer-e.com/software). PERMANOVA, using a design with a fixed factor of treatment, a random factor of site nested in treatment and the fixed repeated measure of sample year (Anderson et al. 2008), was used to test for differences among herbicide treatments and time.
Targeted exposure of Grevillea curviloba
Grevillea curviloba is one of the threatened species for which an effective non-broad-spectrum herbicide for African lovegrass control would be highly valuable. To test the effect of exposure to flupropanate of G. curviloba, three potentially ‘sacrificial’ plants were sprayed with the high dose 3.0 mL L−1 liquid flupropanate treatment, with three unsprayed controls. The ‘sacrificial’ plants were growing along a road verge and are periodically slashed (Fig. 1), and therefore artificially maintained in a prostrate growth form. Sample plants were marked by a metal tag attached to a peg inserted into the ground near the base of the plant, and canopy dimensions (north-south and east-west) and condition were assessed prior to spraying (November 2019) and at 1, 6, 12 and 24 months afterwards.
Results
Application of flupropanate resulted in a significant reduction in African lovegrass cover (Fig. 2), demonstrated by a significant year × treatment interaction. The higher application rate resulted in a greater decline in African lovegrass cover in the first year post-treatment and although a second application further reduced African lovegrass cover at both herbicide rates, the difference between application rates was maintained.
Effects of treatment with flupropanate (control – untreated; low rate – 1.5 mL L−1; high rate – 3.0 mL L−1) on cover of live African lovegrass (Eragrostis curvula). ANOVA results (in text box) are based on log10 transformed cover values (the number of point intercepts out of 200 with the species per plot) that were converted to proportional cover for graphical presentation. ***P < 0.001, **P < 0.01, *P < 0.05.
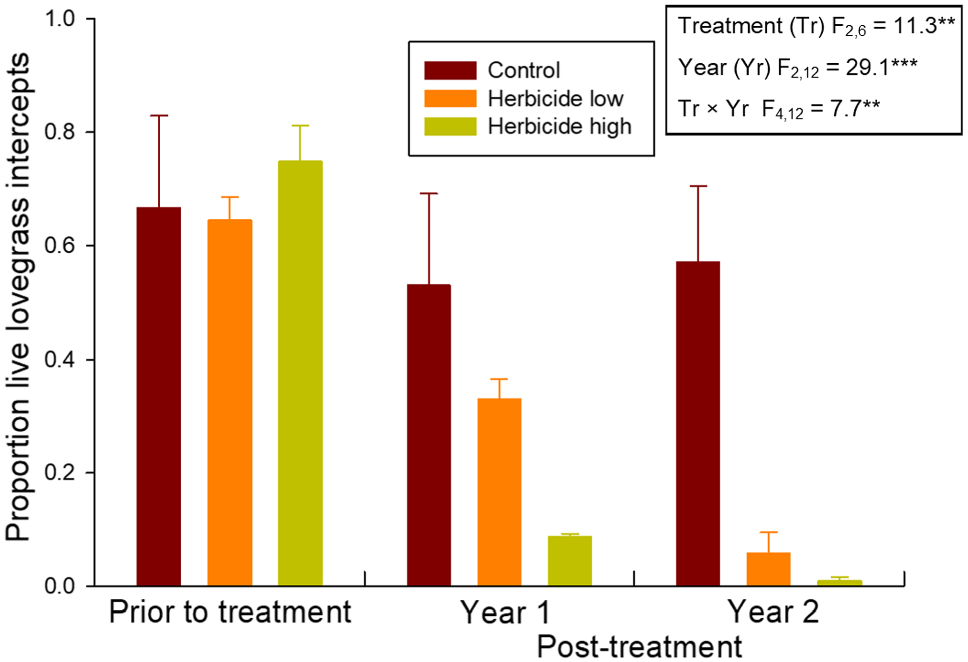
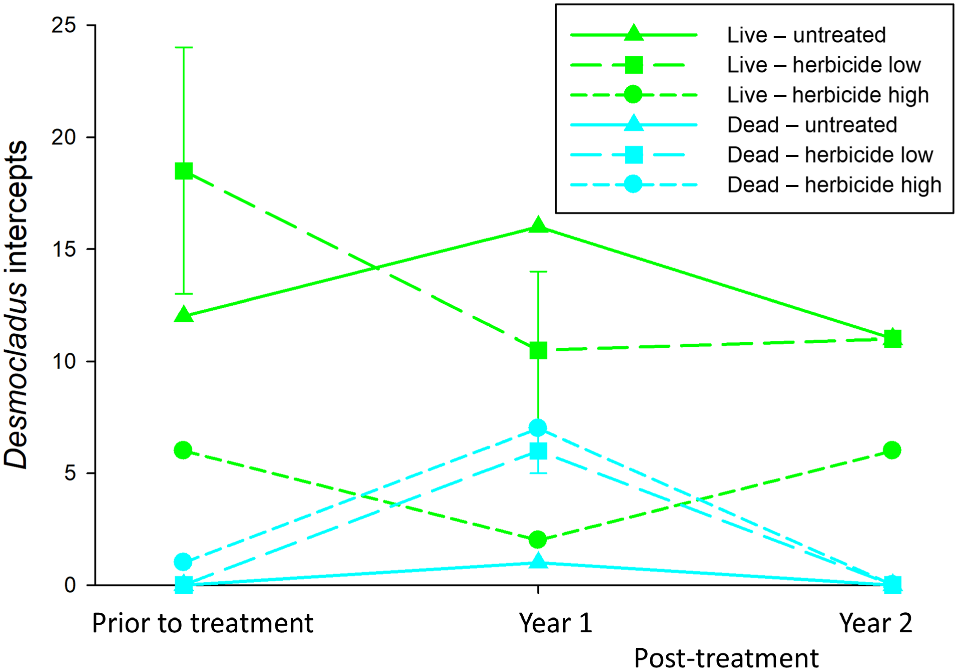
No effects of flupropanate treatment were detected for any of the invasive annual, invasive perennial groundcover, native perennial groundcover or native shrub functional groups (Table 1). For invasive annuals, there was a significant effect of Year, with greater cover in the two post-treatment years. While there were insufficient intercept data and a lack of replication across the sample plots for robust statistical analysis at the species level for native flora, there was a suggestion of an adverse impact to flupropanate in Desmocladus virgatus (Fig. 3). There was both an increase in dead intercepts and a decline in live intercepts in the first year post-treatment in all flupropanate-treated plots where this species was recorded, but this pattern of change in cover was not apparent in the control plot with D. virgatus. Live cover of D. virgatus recovered somewhat following the second year of treatment.
| Tr | Yr | Tr × Yr | ||
|---|---|---|---|---|
| d.f. | 2,6 | 2,12 | 4,12 | |
| Functional group | ||||
| Invasive annuals | 0.07 | 5.35* | 1.45 | |
| Invasive perennial groundcovers | 0.26 | 0.48 | 1.21 | |
| Native perennial groundcovers | 0.07 | 0.21 | 0.70 | |
| Native woody shrubs | 0.40 | 1.39 | 0.39 | |
Treatment (Tr) is the effect of levels of flupropanate, Year (Yr) is the effect of sample year (pre-treatment, year 1 and 2 post-treatment) and Tr × Yr is the interaction between these effects.
***P < 0.001, **P < 0.01, *P < 0.05.
Effects of treatment with flupropanate (control – untreated; low rate – 1.5 mL L−1; high rate – 3.0 mL L−1) on cover of Desmocladus virgatus, showing intercepts with live D. virgatus plants (green lines and symbols) and dead D. virgatus plants (cyan lines and symbols). D. virgatus was present in 4 of the 9 plots (two in the herbicide low treatment, with these data showing means ± s.e.) and occurred so infrequently that robust statistical analysis was not possible.
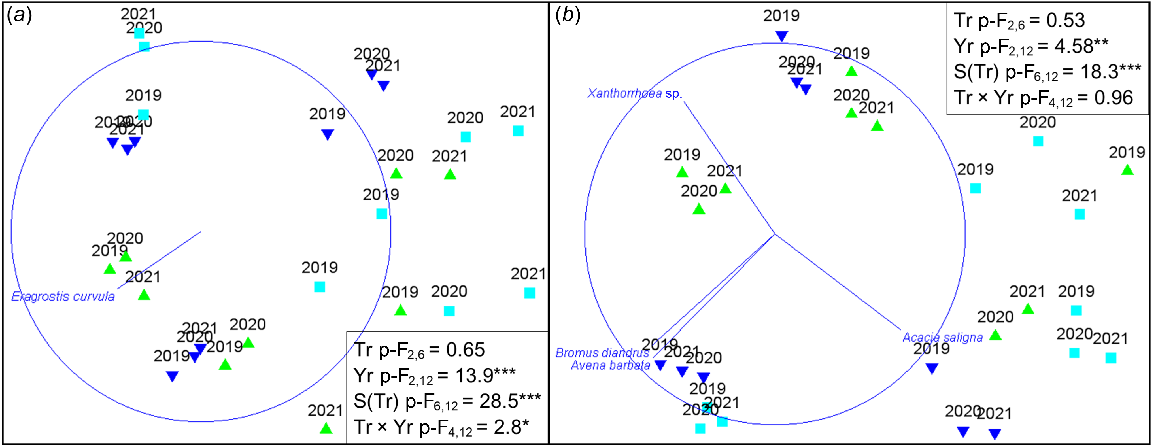
Flupropanate application had a significant effect on plant community composition when all species were included, as evidenced by a significant treatment × year interaction in PERMANOVA (Fig. 4a). In ordination, two of the three control plots remained highly similar in composition across all years, while all flupropanate-treated plots showed a consistent directional shift in location in multivariate space over time associated with lower African lovegrass cover. In contrast, using the same data except for excluding cover of the target weed African lovegrass, there were no significant effects of flupropanate application on community composition (Fig. 4b). Plots did vary in composition (significant site nested in treatment effect) and over time, and these were reflected in the associations of the perennial native shrubs Acacia saligna and Xanthorrhoea sp., and invasive annuals Bromus diandrus and Avena barbata with the location of sites in multivariate space respectively.
Effects of treatment with flupropanate (  control – untreated;
control – untreated;  low rate – 1.5 mL L−1;
low rate – 1.5 mL L−1;  high rate – 3.0 mL L−1) on community composition based on plant cover (a) including all species; and (b) excluding cover of African lovegrass (Eragrostis curvula). Ordinations are non-metric multidimensional scaling with (a) stress 0.15, vector showing African lovegrass; (b) stress 0.13, vectors showing species with a Pearson’s correlation >0.8. PERMANOVA results (in text boxes) give pseudo-F values for effects of Treatment (Tr), sample Year (Yr), Site nested in Treatment (S(Tr)) and the interactions of Tr × Yr. ***P < 0.001, **P < 0.01, *P < 0.05.
high rate – 3.0 mL L−1) on community composition based on plant cover (a) including all species; and (b) excluding cover of African lovegrass (Eragrostis curvula). Ordinations are non-metric multidimensional scaling with (a) stress 0.15, vector showing African lovegrass; (b) stress 0.13, vectors showing species with a Pearson’s correlation >0.8. PERMANOVA results (in text boxes) give pseudo-F values for effects of Treatment (Tr), sample Year (Yr), Site nested in Treatment (S(Tr)) and the interactions of Tr × Yr. ***P < 0.001, **P < 0.01, *P < 0.05.
Intentional exposure of three G. curviloba individuals to flupropanate did not result in any mortality over the period of sampling nor any obvious change in plant condition. Similarly, the three control (no herbicide) individuals also survived.
Discussion
African lovegrass was effectively controlled by flupropanate, as has been found previously (Campbell and Nicol 1998), indicating that the strongly Mediterranean climate of the study area did not affect the herbicide’s effectiveness. Similarly to Bourdôt et al. (2017) with Nassella trichotoma, higher levels of weed control were achieved at the higher (3.0 mL L−1) flupropanate application rate. Our results indicate that flupropanate is likely to be effective in controlling African lovegrass in conservation contexts in south-western Australia, specifically where infestations co-occur with the endangered G. curviloba. Deliberate exposure of G. curviloba to flupropanate did not suggest off-target impacts, noting that the sample size of individuals tested was limited.
Flupropanate resistance in African lovegrass has been recorded in NSW (Powells 2022), emphasising the value of an integrated African lovegrass control program, as neither treatment rate in this study resulted in complete local removal. In situations where biodiversity assets are threatened by African lovegrass, we recommend rotation of herbicides of different mechanisms of action and consideration of physical removal treatments to reduce the potential for development of flupropanate herbicide resistance (Norsworthy et al. 2012). The off-target impacts of broad-spectrum herbicides are likely to be less significant, and physical removal more feasible and with less soil disturbance, after an initial flupropanate treatment to dramatically reduce the cover of African lovegrass (Fig. 2). As with all weed management programs, further considerations in managing African lovegrass with flupropanate include the potential for African lovegrass recolonisation following treatment in the context of the existing seed bank, propagule pressure and dispersal pathways, or replacement with other weeds.
As co-occurring flora species were in general either too infrequently encountered or did not occur in most plots, robust statistical analysis of the effects of flupropanate at the species level was not possible, therefore species were aggregated into functional groups and considered at the community level. There were no significant effects of flupropanate application on non-target groups or at the community level (with African lovegrass excluded), noting these findings refer to the effects of incidental exposure of non-target species to herbicide rather than targeted exposure. The greater cover of invasive annuals in post-treatment years is potentially explained by annual weeds increasing in abundance following African lovegrass control, as has been found elsewhere following invasive perennial grass control with flupropanate (Lusk et al. 2017). However, the increased invasive annual cover in post-treatment years was also found in control plots (no year × treatment interaction), suggesting that variable seasonal conditions may be a more parsimonious explanation.
There was, however, an indication of increased mortality in D. virgatus with flupropanate application, although more robust testing is required for confirmation. D. virgatus (Restionaceae) is a rhizomatous, tufted perennial herb and as a low-growing plant would readily have been exposed to herbicide applied to co-occurring African lovegrass. As some off-target effects of flupropanate were likely in this study and have been shown elsewhere (McLaren et al. 2008; Lusk et al. 2017), robust testing of conservation-listed flora’s susceptibility to the herbicide should precede any application in the habitat of these species.
DBCA is further testing the effectiveness of flupropanate for control of other weed species of the Swan Coastal Plain and monitoring for off-target impacts on native flora in intact occurrences of two critically endangered threatened ecological communities, Banksia attenuata and/or Eucalyptus marginata woodlands on the eastern side of the Swan Coastal Plain and Corymbia calophylla – Kingia australis woodlands on heavy soils. The investigation of the trajectory of vegetation composition after weed control by flupropanate in native vegetation in good or better condition will be valuable, as in pasture settings replacement of the target weed by other weeds and bare ground has been the (undesirable) outcome (Lusk et al. 2017). In this study, insufficient time had elapsed after treatment to discern any patterns of vegetation change, with the dead thatch of African lovegrass remaining the dominant cover on treated plots. Additional research to improve the management of African lovegrass using flupropanate could include testing different integrated management approaches (e.g. altering the order and frequency of rotations of different herbicides and physical removal) and, as flupropanate enters the soil, the effects on soil biota (Brace et al. 2025).
Permits
The work was conducted under Section 40 Authorisation TFL 106-1920. Flupropanate application rates used in this study were within the upper and lower rates of an off-label permit for the control of tussock grasses (PER9792).
Data availability
Raw data are publicly available through the Department of Biodiversity, Conservation and Attractions’ Data Catalogue (https://data.bio.wa.gov.au/). https://doi.org/10.71726/hlmdqi80.
Author contributions
All authors conceptualised and designed the study, undertook data collection and revised the manuscript. CRG led the data analysis and writing.
Acknowledgements
We thank Anne Harris and David Mitchell for assistance with conceptualisation and fieldwork, and Sandra Williamson, Ebony Skey, Megan Young, John Dagnall, Amy Gaunt, Simon Caunter and Alan Jenkins for assistance with fieldwork. John Morrell from Arc Infrastructure and Brad Thompson from the City of Swan facilitated access to the study sites. We thank the two anonymous reviewers for constructive comments.
References
Blakely S, Vitelli M, Tully M, Johnson A-M, Colley J (2022) The advancing front of invasive lovegrasses across Australia’s rangelands. In ‘Proceedings of the 22nd Australasian Weeds Conference Adelaide September 2022,’ 25–29 September 2022, Adelaide Oval, Adelaide, South Australia. (Eds R Melland, C Brodie, J Emms, L Feuerherdt, S Ivory, S Potter) pp. 150–153. (Weed Management Society of South Australia)
Bourdôt GW, Jackman S, Saville DJ (2017) Plant mortality and seedling recruitment responses to flupropanate in grassland populations of Nassella trichotoma. New Zealand Plant Protection 70, 160-164.
| Crossref | Google Scholar |
Brace AJ, Ruthrof KX, Fontaine JB, Miller BP, Hopkins AJM (2025) How soil fungal communities respond to invasive plant species treatments in soil from Banksia woodland, south-western Australia. Australian Journal of Botany 73, BT24083.
| Crossref | Google Scholar |
Burgman MA, Keith D, Hopper SD, Widyatmoko D, Drill C (2007) Threat syndromes and conservation of the Australian flora. Biological Conservation 134(1), 73-82.
| Crossref | Google Scholar |
Campbell MH, Nicol HI (1998) Effects of wiping herbicides on serrated tussock (Nassella trichotoma (Nees) Arech.) and African lovegrass (Eragrostis curvula (Shrad.) Nees). Plant Protection Quarterly 13(1), 36-38.
| Google Scholar |
Campbell MH, Vere DT, Nicol HI (2002) Long-term control of serrated tussock (Nassella trichotoma (Nees) Arech.) by applying flupropanate at three-year or 10-year intervals. Plant Protection Quarterly 17(2), 58-63.
| Google Scholar |
Firn J (2009) African lovegrass in Australia: a valuable pasture species or embarrassing invader? Tropical Grasslands 43, 86-97.
| Google Scholar |
Firn J, MacDougall AS, Schmidt S, Buckley YM (2010) Early emergence and resource availability can competitively favour natives over a functionally similar invader. Oecologia 163, 775-784.
| Crossref | Google Scholar | PubMed |
Firn J, Ladouceur E, Dorrough J (2018) Integrating local knowledge and research to refine the management of an invasive non-native grass in critically endangered grassy woodlands. Journal of Applied Ecology 55(1), 321-330.
| Crossref | Google Scholar |
Gooden B, French K, Turner PJ (2009) Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia. Forest Ecology and Management 257, 960-967.
| Crossref | Google Scholar |
Gosper CR, Coates DJ, Hopper SD, Byrne M, Yates CJ (2021a) The role of landscape history in the distribution and conservation of threatened flora in the Southwest Australian Floristic Region. Biological Journal of the Linnean Society 133(2), 394-410.
| Crossref | Google Scholar |
Gosper CR, Kinloch J, Coates DJ, Byrne M, Pitt G, Yates CJ (2021b) Differential exposure and susceptibility to threats based on evolutionary history: how OCBIL theory informs flora conservation. Biological Journal of the Linnean Society 133(2), 373-393.
| Crossref | Google Scholar |
Gosper CR, Percy-Bower JM, Byrne M, Llorens TM, Yates CJ (2022) Distribution, biogeography and characteristics of the threatened and data-deficient flora in the Southwest Australian Floristic Region. Diversity 14(6), 493.
| Crossref | Google Scholar |
Kaiser-Bunbury CN, Mougal J, Valentin T, Gabriel R, Blüthgen N (2015) Herbicide application as a habitat restoration tool: impact on native island plant communities. Applied Vegetation Science 18(4), 650-660.
| Crossref | Google Scholar |
Lusk CS, Hurrell GA, Saville DJ, Bourdôt GW (2017) Changes in plant species composition after flupropanate application for nassella tussock control, in Canterbury hill-country pastures. New Zealand Journal of Agricultural Research 60(3), 263-276.
| Crossref | Google Scholar |
Mason TJ, French K (2007) Management regimes for a plant invader differentially impact resident communities. Biological Conservation 136(2), 246-259.
| Crossref | Google Scholar |
Matarczyk JA, Willis AJ, Vranjic JA, Ash JE (2002) Herbicides, weeds and endangered species: management of bitou bush (Chrysanthemoides monilifera ssp. rotundata) with glyphosate and impacts on the endangered shrub, Pimelea spicata. Biological Conservation 108(2), 133-141.
| Crossref | Google Scholar |
McLaren DA, Snell K, Butler K (2008) An assessment of native tree susceptibility to the simulated aerial application of the herbicide flupropanate, for management of exotic unpalatable grasses. In ‘Proceedings of the 16th Australian Weeds Conference,’ 18–22 May 2008, Cairns. (Eds RD van Klinken, VA Osten, FD Panetta, JC Scanlan) pp. 323–325. (Queensland Weeds Society)
Norsworthy JK, Ward SM, Shaw DR, Llewellyn RS, Nichols RL, Webster TM, Bradley KW, Frisvold G, Powles SB, Burgos NR, Witt WW, Barrett M (2012) Reducing the risks of herbicide resistance: best management practices and recommendations. Weed Science 60(SP1), 31-62.
| Crossref | Google Scholar |
Pearson DE, Ortega YK, Runyon JB, Butler JL (2016) Secondary invasion: the bane of weed management. Biological Conservation 197, 8-17.
| Crossref | Google Scholar |
Powells J (2022) Herbicide resistance in perennial pasture systems – the horse has bolted. In ‘Proceedings of the 22nd Australasian Weeds Conference Adelaide September 2022,’ 25–29 September 2022, Adelaide. (Eds R Melland, C Brodie, J Emms, L Feuerherdt, S Ivory, S Potter) pp. 17–20. (Weed Management Society of South Australia)
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Global Change Biology 18(5), 1725-1737.
| Crossref | Google Scholar |
Roberts J, Florentine S, van Etten E, Turville C (2021) Germination biology, distribution and control of the invasive species Eragrostis curvula [Schard. Nees] (African Lovegrass): a global synthesis of current and future management challenges. Weed Research 61(3), 154-163.
| Crossref | Google Scholar |
Van Klinken RD, Friedel HH (2017) Unassisted invasions: understanding and responding to Australia’s high-impact environmental grass weeds. Australian Journal of Botany 65(8), 678-690.
| Crossref | Google Scholar |
Western Australian Herbarium (1998–2024) Florabase – the Western Australian flora. Department of Biodiversity, Conservation and Attractions, Kensington. Available at https://florabase.dbca.wa.gov.au/ [verified 12 November 2024]


