Synthesis of a Gal-β-(1→4)-Gal disaccharide as a ligand for the fimbrial adhesin UcaD
Eric D. Boittier A B , Norbert Wimmer A B , Alexandria K. Harris A B , Mark A. Schembri A B and Vito Ferro A B *
A B *
A School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Qld 4072, Australia.
B Australian Infectious Diseases Research Centre, The University of Queensland, Brisbane, Qld 4072, Australia.
Australian Journal of Chemistry 76(1) 30-36 https://doi.org/10.1071/CH22158
Submitted: 10 July 2022 Accepted: 25 August 2022 Published: 8 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The disaccharide Gal-β-(1→4)-Gal was recently identified as a ligand for the adhesin UcaD, a fimbrial protein used by Proteus mirabilis to adhere to exfoliated uroepithelial cells and colonise the urinary tract. To facilitate further studies, Gal-β-(1→4)-Gal was synthesised as the α-methyl glycoside via glycosylation of methyl 2,3,6-tri-O-benzoyl-α-d-galactopyranoside with 2,3,4,6-tetra-O-acetyl-d-galactopyranosyl trichloroacetimidate, followed by deprotection. The disaccharide was fully characterised by NMR spectroscopy. Earlier attempts to use a thiogalactoside as the glycosyl acceptor were hindered by intermolecular aglycone transfer side reactions.
Keywords: bacterial adhesins, disaccharide synthesis, galactopyranoside, Gal-β-(1→4)-Gal, glycosylation, intermolecular aglycone transfer, Proteus mirabilis, uropathogenic Escherichia coli.
Introduction
Urinary tract infections (UTIs) affect over 150 million people globally per year[1,2] and are a major precursor to sepsis, a disease with a mortality rate of ~25%.[1] Despite effective antibiotic treatment, ~30% of patients who contract an initial UTI suffer a recurrent infection.[2] The major cause of UTI is uropathogenic Escherichia coli (UPEC), but additional pathogens also frequently cause this disease, including other Gram-negatives such as Klebsiella pneumoniae, Enterobacter spp. and Proteus mirabilis.[3] These pathogens are all associated with increasing antibiotic resistance, necessitating the urgent development of new therapeutics to treat and prevent UTI.[3,4] One emerging treatment aims to prevent the colonisation of UPEC by interrupting pathways associated with bacterial adhesion to the cell surface of the host.[2,3,5–7] Bacteria target and adhere to the extracellular matrix of host cells through recognition of specific structures, often specific carbohydrates, on the cell surface.[6,7] This recognition is mediated through binding interactions between adhesins (proteins on the pili/fimbriae of the bacteria) and the host glycans.[6,7] We initiated investigation of the receptor specificity of P. mirabilis UCA fimbriae, which mediate adherence to human exfoliated uroepithelial cells and colonisation of the bladder and kidneys in experimental mice.[8,9] Our analyses employing carbohydrate microarray screening revealed that the disaccharide Gal-β-(1→4)-Gal binds to the P. mirabilis fimbrial tip adhesin UcaD (dissociation constant, KD 125.7 ± 7.4 nM).[10] Interestingly, Gal-β-(1→4)-Gal did not bind to the related UPEC Ucl fimbrial adhesin UclD.[2,10–13] The anomeric isomer Gal-α-(1→4)-Gal is known to bind to the UPEC fimbrial adhesin PapG, the receptor-binding component of P fimbriae that mediate colonisation of the upper urinary tract.[5] In order to confirm the binding results and to allow further study, we set out to synthesise milligram quantities of pure Gal-β-(1→4)-Gal disaccharide.
Results and discussion
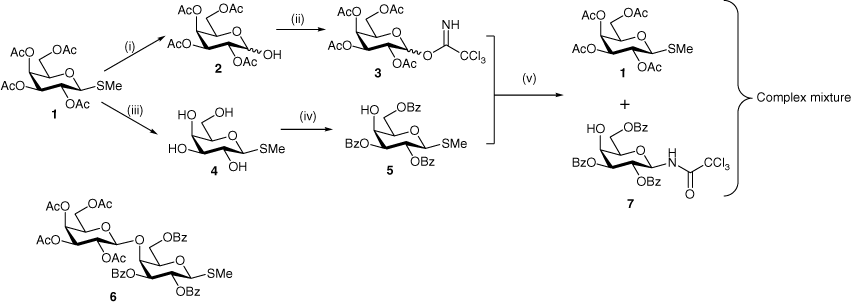
We envisaged that synthesis of the target disaccharide would be readily achievable via glycosylation of a suitably protected d-galactosyl acceptor with a free 4-OH group with any number of d-galactosyl donors bearing participating protecting groups. Given the availability of significant quantities of the protected thiogalactoside 1, a synthetic route was devised to prepare both a glycosyl donor 3 and a glycosyl acceptor 5 from this same starting material (Scheme 1). A productive glycosylation between 3 and 5 would provide disaccharide 6, which on deprotection would furnish the desired Gal-β-(1→4)-Gal disaccharide as the β-methyl thioglycoside. This route seemed attractive because the thioglycoside functionality could serve as a glycosyl donor for further transformations, if desired. For the glycosyl donor, the trichloroacetimidate was chosen, as this leaving group can be activated under mild Lewis acidic conditions that should not activate the thioglycoside moiety of the acceptor.
Thiogalactoside 1 was thus converted into trichloroacetimidate 3[14] as a mixture of anomers (α/β = 2:1) in excellent yield following purification by flash chromatography. The thioglycoside was first hydrolysed with NBS in aqueous acetone and the resulting crude hemiacetal 2 was then treated with trichloracetonitrile and potassium carbonate to give 3. To prepare the glycosyl acceptor, thiogalactoside 1 was deacetylated under Zemplén conditions to give the tetrol 4[15,16] in essentially quantitative yield. Selective benzoylation of 4 with benzoyl chloride in pyridine at −78°C then gave the glycosyl acceptor 5[17] in a modest but acceptable 30% yield, similarly to analogous selective benzoylations of d-galactosides.[18–20] Also isolated were the tetrabenzoate[21,22] (21%) and mixed fractions containing the isomeric 3,4,6-tribenzoate (16%).
The glycosylation of acceptor 5 with donor 3 was next attempted at −78°C with BF3·Et2O as the promoter. The reaction was monitored by TLC and was stopped on disappearance of the starting materials. Following workup, attempts were made to isolate the major product(s) by flash chromatography. 1H NMR analysis indicated a complex mixture and the desired disaccharide 6 could not be detected. However, although no pure products could be isolated, mixed fractions of sufficient purity were obtained from which the products of intermolecular aglycone transfer,[23] 1 and 7, were identified by 1D and 2D NMR spectroscopy. The NMR signals for compound 1 matched those of the starting material used at the beginning of the synthesis, while compound 7 showed a diagnostic doublet for the trichloroacetamide proton at 7.76 ppm (J 9.0 Hz) with a COSY correlation to a doublet of doublets at 5.39 ppm (H-1, J1,29.3 Hz), the latter also showing an HSQC (heteronuclear single-quantum correlation) correlation to C1 at 80 ppm. Intermolecular aglycone transfers of this type are fairly common side reactions of glycosylations involving thioglycosides[24,25] and occur when there is a mismatch in reactivity between the glycosyl donor and acceptor. However, these mismatches are difficult to predict a priori.
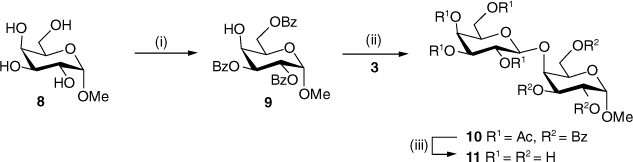
Intermolecular aglycone transfer side reactions can be minimised by changing the protecting groups[26] or the aglycone.[27] In this case, however, we decided to simply change the acceptor from a thiogalactoside (5) to an O-galactoside (9) in order to prepare the Gal-β-(1→4)-Gal disaccharide 11 as the target (Scheme 2). In the only previous chemical synthesis of 11, Cox et al.[28] obtained an inseparable mixture of 11 and the isomeric Gal-α-(1→4)-Gal-α-OMe (ratio ~7:6) in a combined yield of only 31% during the attempted synthesis of the latter via a Koenigs–Knorr glycosylation. Subsequently, Fujimoto et al.[29] used a transglycosylation reaction catalysed by β-galactanase (from Penicillium citrinium) but obtained 11 in only 18.8% yield along with the corresponding trisaccharide (3.5%). Millqvist-Fureby et al.[30] used crude glycosidase preparations from barley and snail to obtain 11 along with the β-(1→6) isomer but no yield was reported. Yamamoto et al.[31] used the β-1,3-galactosidase (from Bacillus circulans) but obtained 11 in only 0.23% yield. All the previous methods for the preparation of 11 are unsatisfactory and, apart from the 13C chemical shifts of the two anomeric carbons,[28] no characterisation data have been reported.
|
Methyl α-d-galactopyranoside 8 was thus selectively benzoylated in a similar fashion to tetrol 4 to give the tribenzoate 9[18,19] in good yield (51%) after purification by flash chromatography, along with the tetrabenzoate as the major by-product (29%). Glycosylation of acceptor 9 with trichloroacetimidate 3 proceeded smoothly at −20°C with TMSOTf as the promoter, to give the protected disaccharide 10[32,33] in 52% yield after purification by flash chromatography. Zemplén deacylation then furnished the target disaccharide 11 in 68% yield following recrystallisation from methanol, which was fully characterised by 1D and 2D NMR spectroscopy and high-resolution mass spectrometry (HRMS). The 13C NMR chemical shifts for the two anomeric carbons were in accordance with the literature.[28]
Conclusions
In conclusion, we synthesised a β-(1 → 4)-linked d-galabiose disaccharide as its α-methyl glycoside 11, fully characterised by NMR spectroscopy, for further testing as a ligand for the fimbrial adhesin UcaD. The synthesis was achieved by glycosylation of a benzoyl-protected methyl α-d-galactopyranoside with a d-galactopyranosyl trichloroacetimidate donor followed by deprotection, and represents a significant improvement in yield over past syntheses. Earlier attempts at disaccharide synthesis using a d-thiogalactoside acceptor with the same donor were thwarted by intermolecular aglycone transfer. Binding studies with UcaD and other fimbrial adhesins will be reported elsewhere in due course.
Experimental
General methods
Melting points were determined on a DigiMelt MSRS apparatus. Optical rotations were determined on a JASCO P-2000 polarimeter at ambient temperature and are given in units of 10−1 degrees cm2 g−1. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 MHz spectrometer at 20°C. The residual solvent peaks (CDCl3: δH 7.26 and δC 77.16 ppm or CD3OD: δH 3.31 and δC 49.0 ppm) served as internal standards. Coupling constants in hertz (Hz) were measured from one-dimensional spectra. The analyses of 1H and 13C NMR spectra were assisted by gCOSY and HSQC experiments. Analytical low-resolution (LR)MS and HRMS were performed in positive or negative ion electrospray ionisation (ESI) mode on a Bruker HCT spectrometer and a Bruker micrOTOFQ spectrometer, respectively. All reagents and solvents were obtained from Merck, Australia, and were used without further purification, except EtOAc, n-hexane, MeOH and DCM, which were distilled prior to use. Reactions were monitored by analytical thin layer chromatography (TLC) on silica gel 60 F254 plates and visualised by charring with anisaldehyde/H2SO4 stain in ethanol. Flash chromatography was performed on silica gel under positive pressure with specified solvent systems.
2,3,4,6-Tetra-O-acetyl-d-galactopyranosyl trichloroacetimidate (3)
(a) Methyl 2,3,4,6-tetra-O-acetyl-1-thio-β-d-galactopyranoside 1 (1.20 g, 3.2 mmol) was dissolved in a mixture of acetone (30 mL) and water (2.5 mL) and was cooled to −20°C. A solution of N-bromosuccinimide (2.22 g, 12.5 mmol) in acetone (10 mL) was added dropwise and the mixture was stirred at −20°C for 25 min. Aqueous 20% NaS2O3/NaHCO3 (1:1, 50 mL) was added, and then the mixture was diluted with EtOAc and washed with water (3 × 50 mL). The combined aqueous phase was extracted with EtOAc (3 × 50 mL) and the combined organic phase was washed with brine (1 × 100 mL), dried (MgSO4), filtered and concentrated under vacuum to give the hemiacetal 2 as a clear syrup (1.00 g, 90%), used without further purification in the next step. The 1H NMR spectrum was in accordance with the literature.[14] (b) The crude hemiacetal (865 mg, 2.4 mmol) was dissolved in DCM (10 mL) and trichloroacetonitrile (0.7 mL, 7.2 mmol, 3 equiv.) was added at rt with stirring. After 15 min, the mixture was cooled to 0°C, potassium carbonate (1.46 g, 10.6 mmol) was added and the reaction was stirred overnight at rt. The mixture was concentrated under vacuum and the residue was purified by flash chromatography (6:1 toluene/EtOAc → 3:1 toluene/EtOAc → EtOAc) to give the trichloroacetimidate 3 as a colourless oil (1.13 g, 92%) as a mixture of anomers (α/β = 2:1). The 1H NMR spectrum was in accordance with the literature.[14] 1H NMR (500 MHz, CDCl3) α-anomer: δ 8.66 (s, 1H, NH), 6.60 (d, 1H, J1,2 3.5 Hz, H-1), 5.56 (dd, 1H, J3,4 3.2 Hz, J4,5 1.4 Hz, H-4), 5.48 (dd, J2,3 10.9 Hz, H-3), 5.37 (dd, H-2), 4.46–4.42 (m, 1H, H-5), 4.17 (dd, 1H, A part of ABX, J5,6a 6.6 Hz, J6a,6b 11.3 Hz, H-6a), 4.08 (dd, 1H, B part of ABX, J5,6b 6.7 Hz), 2.17, 2.03, 2.02, 2.01 (4s, 4 × 3H, 4 × Me); β-anomer: δ 8.71 (s, 1H, NH), 5.84 (d, 1H, J1,2 8.2 Hz, H-1), 5.49 (dd, 1H, J2,3 10.4 Hz, H-2), 5.46 (dd, J3,4 3.4 Hz, J4,5 1.3 Hz, H-4), 5.12 (dd, H-3), 4.22–4.09 (m, 3H, H-5, 6a, 6b), 2.18, 2.04, 2.02, 2.00 (4s, 4 × 3H, 4 × Me).
Methyl 1-thio-β-d-galactopyranoside (4)
Methyl 2,3,4,6-tetra-O-acetyl-1-thio-β-d-galactopyranoside 1 (1.11 g, 2.9 mmol) was dissolved in dry MeOH/THF (4:1, 50 mL). NaOMe in MeOH (0.7 M, 0.5 mL) was added dropwise, with stirring, at 0°C under a nitrogen atmosphere. The reaction was stirred at rt for 24 h and was then neutralised with Amberlite® IR120 (H+ form). The suspension was filtered and the resin was washed with MeOH. The filtrate and washings were evaporated under vacuum to give the tetrol 4 as a fine, white powder (0.61 g, 99%), mp 173–174°C (lit.[15] 174–175°C), [α]D +9.5 (c 0.9, H2O; lit.[15] +10.7, H2O). The 1H NMR spectrum was in accordance with the literature.[16] 1H NMR (500 MHz, CD3OD): δ 4.21 (d, 1H, J1,2 9.5 Hz, H-1), 3.89 (dd, 1H, J4,5 1.1 Hz, J3,4 3.3 Hz, H-4), 3.75 (dd, 1H, A part of ABX, J5,6a 6.8 Hz, J6a,6b 11.4 Hz, H-6a), 3.68 (dd, B part of ABX, 1H, J5,6b 5.2 Hz, H-6b), 3.58 (dd, 1H, J2,3 9.4 Hz, H-2), 3.53 (ddd, 1H, H-5), 3.47 (dd, 1H, H-3), 2.20 (s, 3H, SMe). 13C NMR (125 MHz, CD3OD): δ 87.9 (C-1), 80.7 (C-5), 76.2 (C-3), 70.8 (C-4), 70.6 (C-2), 62.7 (C-6), 12.0 (SMe). ESMS: m/z 233.0 [M + Na]+.
Methyl 1-thio-2,3,6-tri-O-benzoyl-β-d-galactopyranoside (5)
The tetrol 4 (0.96 g, 4.5 mmol) was dissolved in anhydrous pyridine (4 mL), cooled to −78°C and stirred for 30 min. A solution of benzoyl chloride (1.57 g, 1.30 mL, 11.2 mmol, 2.5 equiv.) in anhydrous pyridine (7 mL) was added dropwise with stirring over 1 h, under an atmosphere of nitrogen. After 3 h, the reaction was quenched with water (10 mL). The mixture was diluted with DCM (25 mL) and washed with saturated aqueous NaHCO3 (3 × 25 mL). The combined aqueous phase was re-extracted with DCM (3 × 25 mL). The combined organic phase was then washed with brine (50 mL), dried (MgSO4), filtered and concentrated under vaccuum. The residue was purified by flash chromatography (9:1 toluene/EtOAc → 5:1 toluene/EOAc → EtOAc) to give the tribenzoate 5 as a colourless solid (0.72 g, 30%); Rf = 0.43 (5:1 toluene/EtOAc); mp 136°C. [α]D +60 (c 0.6, CHCl3; lit.[17] +3.3). 1H NMR (500 MHz, CDCl3) δ 8.07–7.33 (m, 15H, Ph), 5.87 (dd, 1H, J1,2 J2,3 9.9 Hz, H-2), 5.42 (dd, 1H, J3,4 3.2 Hz, H-3), 4.71 (dd, 1H, A part of ABX, J5,6a 6.7 Hz, J6a,6b 11.5 Hz, H-6a), 4.65 (d, 1H, H-1), 4.47 (dd, 1H, B part of ABX, J5,6b 6.2 Hz, H-6b), 4.39–4.36 (m, 1H, H-4), 4.11 (bdd, 1H, H-5), 2.58 (bd, 1H, J 4.6 Hz, OH), 2.27 (s, 3H, Me). HRMS: m/z calcd for C28H26O8S [M + H]+: 523.14267; found: 523.14007. Also isolated was the tetrabenzoate by-product, methyl 1-thio-2,3,4,6-tetra-O-benzoyl-β-d-galactopyranoside[21,22] (0.60 g, 21%), Rf 0.6 (5:1 toluene/EtOAc); and mixed fractions containing methyl 1-thio-3,4,6-tri-O-benzoyl-β-d-galactopyranoside (0.38 g, 16%), Rf 0.3 (5:1 toluene/EtOAc).
Attempted glycosylation of 3 and 5
The tribenzoate 5 (272 mg, 0.52 mmol) was dissolved in dry DCM (2 mL) and was stirred under an atmosphere of nitrogen for 2 h in the presence of 4 Å molecular sieves. The mixture was cooled to −78°C and BF3·Et2O (14 μL, 0.12 mmol, 0.23 equiv.) was added. After 15 min, a solution of the trichloroacetimidate 3 (390 mg, 0.79 mmol, 1.5 equiv.) in dry DCM (1 mL) and added dropwise over 5 min. After 30 min, the reaction was neutralised with triethylamine to pH 7. The reaction was diluted with chloroform (5 mL) and filtered to remove the molecular sieves. These were washed with chloroform (4 × 20 mL). The combined organic phase was washed with sat. aqueous NaHCO3 (3 × 30 mL) and the combined aqueous phase was re-extracted with DCM (3 × 30 mL). The combined organic phase was washed with brine (50 mL), dried (MgSO4), filtered and concentrated under vaccuum. The residue was purified by flash chromatography (7:1 toluene/EtOAc → 3:2 toluene/EtOAc) to give a white foam (718 mg) containing a mixture of products. Further purification allowed the identification of the thioglycoside 1 and the trichloroacetamide 7. 1H NMR data for 2,2,2-trichloro-N-(2,3,6-tri-O-benzoyl-β-d-galactopyranosyl)acetamide (7): 1H NMR (500 MHz, CDCl3) δ 8.07–7.86, 7.61–7.32 (m, 15H, Ph), 7.76 (d, 1H, J1,NH 9.0 Hz, NH), 5.82 (dd, 1H, J1,2 9.3 Hz, J2,3 10.2 Hz, H-2), 5.60 (dd, 1H, J3,4 3.2 Hz, H-3), 5.39 (dd, 1H, H-1), 4.76 (dd, 1H, A part of ABX, J5,6a 7.1 Hz, J6a,6a 11.4 Hz, H-6a), 4.53 (dd, 1H, B part of ABX, J5,6b 5.9 Hz, H-6b), 4.41–4.37 (m, 1H, H-4), 4.24–4.20 (m, 1H, H-5), 2.94 (d, 1H, J4,OH 3.3 Hz, OH). HRMS: m/z calcd for C29H24Cl3NO9 [M + H]+: 636.05949; found: 636.05588. 1H NMR data for thioglycoside 1 was identical to an authentic sample: 1H NMR (500 MHz, CDCl3) δ 5.43 (dd, 1H, J3,4 3.4 Hz, J4,5 1.2 Hz, H-4), 5.26 (dd, 1H, J1,2 9.9 Hz, J2,3 10.1 Hz, H-2), 5.05 (dd, H-3), 4.39 (d, 1H, H-1), 4.16 (dd, 1H, A part of ABX, J5,6a 6.6 Hz, J6a,6b11.3 Hz, H-6a), 4.12 (dd, 1H, B part of ABX, J5,6b 6.6 Hz, H-6b), 3.96 (ddd, 1H, H-5), 2.19 (s, 3H, SMe), 2.15, 2.08, 2.05, 1.99 (4 × s, 4 × 3H, 4 × Me).
Methyl 2,3,6-tri-O-benzoyl-α-d-galactopyranoside (9)
Methyl α-d-galactopyranoside 8 (940 mg, 4.84 mmol) was suspended in anhydrous pyridine (6.3 mL) at 10°C and benzoyl chloride (2.43 mL, 20.9 mmol, 4.3 equiv.) was dropwise added over 2 h. The temperature was raised to 20°C and stirring was continued for 3.5 h. The reaction was quenched by addition of water (7.5 mL) at 10°C over 10 min and left stirring overnight at rt. The suspension was diluted with toluene (40 mL) and the organic phase was washed with aqueous NaHCO3 solution (8%, w/v, 2 × 12 mL), aqueous HCl (1 M, 2 × 12 mL), water (5 × 30 mL) and brine (2 × 30 mL) and dried (MgSO4), and concentrated under vaccuum to give a colourless foam (2.33 g). Purification by flash chromatography (toluene/EtOAc 10:1→toluene/EtOAc 3:1) gave first the perbenzoylated by-product methyl 2,3,4,6-tetra-O-benzoyl-α-d-galactopyranoside as a white foam (850 mg, 29%; Rf 0.44, toluene/EtOAc, 10:1). Further elution then gave the tribenzoate 9 as white solid (1.26 g, 51%), Rf 0.18 (toluene/EtOAc, 3:1), mp 140–141°C (lit.[19] 139–140.5°C). [α]D +126 (c 0.5, CHCl3; lit.[18] +123). 1H NMR (500 MHz, CDCl3): δ 8.07–7.96, 7.60–7.34 (m, 15H, Ph), 5.76 (dd, 1H, J2,3 10.7 Hz, J3,4 3.1 Hz, H-3), 5.68 (dd, 1H, J1,2 3.6 Hz, H-2), 5.22 (d, 1H, H-1), 4.69 (dd, 1H, A part of ABX, J5,6a 5.9 Hz, J6a,6b 11.5, H-6a), 4.56 (dd, 1H, B part of ABX, J5,6b 6.8 Hz, H-6b), 4.42–4.39 (m, 1H, H-4), 4.36 (bdd, 1H, H5), 3.45 (s, 3H, Me), 2.48 (d, J 4.2 Hz, OH). HRMS: m/z calcd for C28H26O9 [M + H]+: 507.16551; found: 507.16252.
Methyl 2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-(1→4)-2,3,6-tri-O-benzoyl-α-d-galactopyranoside (10)
The alcohol 9 (103 mg, 203 μmol) was dissolved in dry DCM (1.5 mL) and stirred with freshly dried molecular sieves (4 Å) for 30 min, followed by dropwise addition of a stock solution of TMSOTf in dry DCM (36 μL/mL, 200 μL, 20 μmol, 0.2 equiv.) at rt. After 30 min, the mixture was cooled to −20°C and a stock solution of the trichloroacetimidate 3 in dry DCM (50 mg in 100 μL, 101 μmol) was added dropwise over 5 min. The mixture was then stirred at 0°C for 90 min. TLC indicated complete consumption of donor 3 and formation of product. To rearrange any possible orthoester, the suspension was again cooled to −20°C and more stock solution of TMSOTf in dry DCM (36 μL/mL, 400 μL, 40 μmol, 0.4 equiv. donor) was added dropwise over 5 min. The mixture was stirred at 0°C for 30 min, and then the reaction was neutralised by addition of Et3N (5 μL) at 0°C and stirring was continued at rt for 10 min. The mixture was diluted with CHCl3, centrifuged and the molecular sieves were washed with CHCl3 (10 × 10 mL). The combined supernatant was washed with water (2 × 20 mL), brine (30 mL), dried (MgSO4) and concentrated under vaccuum. The residue was purified by flash chromatography (hexane/EtOAc 2:1 → 3:2 → 1:1 → EtOAc, all containing 0.5% Et3N) to give disaccharide 10 as an off-white foam (44 mg, 52%), Rf 0.24 (hexane/EtOAc 2:1). [α]D +66 (c 0.4, CHCl3; lit.[32] +56.6; lit.[33] +73). 1H NMR (500 MHz, CDCl3): δ 7.94–8.10, 7.34–7.59 (m, 15H, Ph), 5.84 (dd, 1H, J2,3 10.7 Hz, J3,4 2.9 Hz, H-3), 5.55 (dd, 1H, J1,2 3.6 Hz, H-2), 5.35 (dd, 1H, J1′,2′ 7.9 Hz, J2′,3′ 10.5 Hz, H-2′), 5.29 (dd, 1H, J3′,4′ 3.4 Hz, J4′,5′ 1.0 Hz, H-4′), 5.09 (d, 1H, H-1), 4.91(dd, 1H, H-3′), 4.67 (d, 1H, H-1′), 4.67 (dd, 1H, A part of ABX, J5,6a 4.6 Hz, J6a,6b 11.8 Hz, H-6a), 4.50 (dd, 1H, B part of ABX, J5,6b 7.2 Hz, H-6b), 4.45 (dd, 1H, J4,5 1.0 Hz, H-4), 4.36 (ddd, 1H, H-5), 4.04 (dd, 1H, A part of ABX, J5′,6a′ 6.9 Hz, J6a′, 6b′ 11.4 Hz, H-6a′), 3.96 (dd, 1H, B part of ABX, J5′,6b′ 6.4 Hz, H-6b′), 3.70 (ddd, 1H, H-5′), 3.41 (s, 3H, OMe), 2.18, 2.16, 2.00, 1.95 (4 × s, 12H, 4 × OAc). HRMS: m/z calcd for C42H44O18 [M + Na]+: 859.24254; found: 859.23856.
Methyl β-d-galactopyranosyl-(1→4)-α-d-galactopyranoside (11)
A solution of NaOMe in MeOH (0.7 M, 100 μL, 70 μmol) was added dropwise to a solution of disaccharide 10 (40 mg, 47 μmol) in dry MeOH (2.5 mL) at 0°C. The mixture was stirred at rt for 4.5 h under sonication and then was neutralised with Amberlite IR 120 (H+) to pH 7. The resin was removed by filtration and was washed under sonication with 50% aqueous MeOH (5 × 2 mL). The combined filtrate and washings were co-evaporated under vaccuum with 50% aqueous MeOH (3 × 5 mL). The residue was crystallised from MeOH to give disaccharide 11 as a solid (11.6 mg, 68%), Rf 0.18 (MeCN/H2O, 85:15). [α]D +118 (c 0.6, H2O). 1H NMR (500 MHz, D2O): δ 4.85 (bs, 1H, H-1), 4.58 (d, 1H, J1,2 7.8 Hz, H-1′), 4.22 (bs, 1H, H-4), 3.95 (bt, 1H, H-5), 3.89–3.92 (m, H-3, H-2, H-4′), 3.84 (dd, 1H, H-6′a or H-6a), 3.72–3.80 (m, 3H, 3 × H-6), 3.67 (ddd, J4′,5′ 0.8 Hz, H-5′), 3.66 (dd, J3,4 3.4 Hz, H-3′), 3.58 (dd, 1H, J2,3 9.9 Hz H-2′), 3.41 (s, 3H, Me). 13C NMR (125 MHz, D2O): δ 105.3 (C-1′), 100.4 (C-1), 79.2 (C-4), 76.1 (C-5′), 73.8 (C-3′), 72.4 (C-2′), 71.0, 70.9, 69.62, 69.58 (C-2, C-3, C-5, C-4′, assignments may be reversed), 61.9 (C-6 or C-6′), 61.8 (C-6 or C-6′), 56.1 (Me). The 13C NMR chemical shifts for C-1 and C-1′ matched those in the literature.[28] HRMS: m/z calcd for C13H24O11 [M + Na]+: 379.12163; found: 379.11989.
Supplementary material
Supplementary data: copies of NMR spectra for compounds 1, 3–5, 7, 9–11. Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
We thank the University of Queensland for financial support.
Acknowledgements
We thank Xinying Jia and Mehdi Mobli from the Centre for Advanced Imaging at The University of Queensland for helpful discussions.
References
[1] M Totsika, DG Moriel, A Idris, BA Rogers, DJ Wurpel, M-D Phan, DL Paterson, MA Schembri, Uropathogenic Escherichia coli mediated urinary tract infection. Curr Drug Targets 2012, 13, 1386.| Uropathogenic Escherichia coli mediated urinary tract infection.Crossref | GoogleScholarGoogle Scholar |
[2] CN Spaulding, RD Klein, S Ruer, AL Kau, HL Schreiber, ZT Cusumano, KW Dodson, JS Pinkner, DH Fremont, JW Janetka, H Remaut, JI Gordon, SJ Hultgren, Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528.
| Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist.Crossref | GoogleScholarGoogle Scholar |
[3] HM Zowawi, PNA Harris, MJ Roberts, PA Tambyah, MA Schembri, MD Pezzani, DA Williamson, DL Paterson, The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 2015, 12, 570.
| The emerging threat of multidrug-resistant Gram-negative bacteria in urology.Crossref | GoogleScholarGoogle Scholar |
[4] SJ Hancock, M-D Phan, Z Luo, AW Lo, KM Peters, NTK Nhu, BM Forde, J Whitfield, J Yang, RA Strugnell, DL Paterson, TR Walsh, B Kobe, SA Beatson, MA Schembri, Comprehensive analysis of IncC plasmid conjugation identifies a crucial role for the transcriptional regulator AcaB. Nat Microbiol 2020, 5, 1340.
| Comprehensive analysis of IncC plasmid conjugation identifies a crucial role for the transcriptional regulator AcaB.Crossref | GoogleScholarGoogle Scholar |
[5] JA Roberts, BI Marklund, D Ilver, D Haslam, MB Kaack, G Baskin, M Louis, R Möllby, J Winberg, S Normark, The Gal(α1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci U S A 1994, 91, 11889.
| The Gal(α1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract.Crossref | GoogleScholarGoogle Scholar |
[6] Linke D, Goldman A. Advances in Experimental Medicine and Biology. Dordrecht, Netherlands: Springer; 2011.
[7] J Poole, CJ Day, M von Itzstein, JC Paton, MP Jennings, Glycointeractions in bacterial pathogenesis. Nat Rev Microbiol 2018, 16, 440.
| Glycointeractions in bacterial pathogenesis.Crossref | GoogleScholarGoogle Scholar |
[8] SK Wray, SI Hull, RG Cook, J Barrish, RA Hull, Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun 1986, 54, 43.
| Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis.Crossref | GoogleScholarGoogle Scholar |
[9] R Pellegrino, P Scavone, A Umpiérrez, DJ Maskell, P Zunino, Proteus mirabilis uroepithelial cell adhesin (UCA) fimbria plays a role in the colonization of the urinary tract. Pathog Dis 2013, 67, 104.
| Proteus mirabilis uroepithelial cell adhesin (UCA) fimbria plays a role in the colonization of the urinary tract.Crossref | GoogleScholarGoogle Scholar |
[10] SJ Hancock, AW Lo, T Ve, CJ Day, L Tan, AA Mendez, M-D Phan, NTK Nhu, KM Peters, AC Richards, BA Fleming, C Chang, DHY Ngu, BM Forde, T Haselhorst, KGK Goh, SA Beatson, MP Jennings, MA Mulvey, B Kobe, MA Schembri, Ucl fimbriae regulation and glycan receptor specificity contribute to gut colonisation by extra-intestinal pathogenic Escherichia coli. PLOS Pathog 2022, 18, e1010582.
| Ucl fimbriae regulation and glycan receptor specificity contribute to gut colonisation by extra-intestinal pathogenic Escherichia coli.Crossref | GoogleScholarGoogle Scholar |
[11] W Jiang, W Ubhayasekera, MM Pearson, SD Knight, Structures of two fimbrial adhesins, AtfE and UcaD, from the uropathogen Proteus mirabilis. Acta Crystallogr D Struct Biol 2018, 74, 1053.
| Structures of two fimbrial adhesins, AtfE and UcaD, from the uropathogen Proteus mirabilis.Crossref | GoogleScholarGoogle Scholar |
[12] DJ Wurpel, M Totsika, LP Allsopp, RI Webb, DG Moriel, MA Schembri, Comparative proteomics of uropathogenic Escherichia coli during growth in human urine identify UCA-like (UCL) fimbriae as an adherence factor involved in biofilm formation and binding to uroepithelial cells. J Proteomics 2016, 131, 177.
| Comparative proteomics of uropathogenic Escherichia coli during growth in human urine identify UCA-like (UCL) fimbriae as an adherence factor involved in biofilm formation and binding to uroepithelial cells.Crossref | GoogleScholarGoogle Scholar |
[13] DJ Wurpel, DG Moriel, M Totsika, DM Easton, MA Schembri, Comparative analysis of the uropathogenic Escherichia coli surface proteome by tandem mass-spectrometry of artificially induced outer membrane vesicles. J Proteomics 2015, 115, 93.
| Comparative analysis of the uropathogenic Escherichia coli surface proteome by tandem mass-spectrometry of artificially induced outer membrane vesicles.Crossref | GoogleScholarGoogle Scholar |
[14] DD Manning, CR Bertozzi, NL Pohl, SD Rosen, LL Kiessling, Selectin–saccharide interactions: revealing structure–function relationships with chemical synthesis. J Org Chem 1995, 60, 6254.
| Selectin–saccharide interactions: revealing structure–function relationships with chemical synthesis.Crossref | GoogleScholarGoogle Scholar |
[15] B Helferich, H Grünewald, F Langenhoff, Notiz über die Darstellung von Methyl-α-l-thio-arabinosid und von Methyl-β-d-thio-galaktosid. Chem Ber 1953, 86, 873.
| Notiz über die Darstellung von Methyl-α-l-thio-arabinosid und von Methyl-β-d-thio-galaktosid.Crossref | GoogleScholarGoogle Scholar |
[16] K Koike, M Sugimoto, S Sato, Y Ito, Y Nakahara, T Ogawa, Total synthesis of globotriaosyl-E and Z-ceramides and isoglobotriaosyl-E-ceramide. Carbohydr Res 1987, 163, 189.
| Total synthesis of globotriaosyl-E and Z-ceramides and isoglobotriaosyl-E-ceramide.Crossref | GoogleScholarGoogle Scholar |
[17] T Ogawa, M Matsui, Regioselective stannylation: Acylation of carbohydrates: coordination control. Tetrahedron 1981, 37, 2363.
| Regioselective stannylation: Acylation of carbohydrates: coordination control.Crossref | GoogleScholarGoogle Scholar |
[18] EJ Reist, RR Spencer, DF Calkins, BR Baker, L Goodman, Derivatives of 4-Amino-4-deoxy-d-glucose. J Org Chem 1965, 30, 2312.
| Derivatives of 4-Amino-4-deoxy-d-glucose.Crossref | GoogleScholarGoogle Scholar |
[19] AC Richardson, JM Williams, Selective O-acylation of pyranosides. Chem Commun 1965, 1965, 104.
| Selective O-acylation of pyranosides.Crossref | GoogleScholarGoogle Scholar |
[20] CS Rye, SG Withers, Elucidation of the mechanism of polysaccharide cleavage by chondroitin AC lyase from Flavobacterium heparinum. J Am Chem Soc 2002, 124, 9756.
| Elucidation of the mechanism of polysaccharide cleavage by chondroitin AC lyase from Flavobacterium heparinum.Crossref | GoogleScholarGoogle Scholar |
[21] Y Ito, S Nunomura, S Shibayama, T Ogawa, Studies directed toward the synthesis of polysialogangliosides: the regio- and stereocontrolled synthesis of rationally designed fragments of the tetrasialoganglioside GQ1b+. J Org Chem 1992, 57, 1821.
| Studies directed toward the synthesis of polysialogangliosides: the regio- and stereocontrolled synthesis of rationally designed fragments of the tetrasialoganglioside GQ1b+.Crossref | GoogleScholarGoogle Scholar |
[22] Z Pakulski, D Pierożyński, A Zamojski, Reaction of sugar thiocyanates with Grignard reagents. New synthesis of thioglycosides. Tetrahedron 1994, 50, 2975.
| Reaction of sugar thiocyanates with Grignard reagents. New synthesis of thioglycosides.Crossref | GoogleScholarGoogle Scholar |
[23] F Belot, J-C Jacquinet, Intermolecular aglycon transfer of a phenyl 1-thiogalactosaminide derivative under trichloroacetimidate glycosylation conditions. Carbohydr Res 1996, 290, 79.
| Intermolecular aglycon transfer of a phenyl 1-thiogalactosaminide derivative under trichloroacetimidate glycosylation conditions.Crossref | GoogleScholarGoogle Scholar |
[24] HM Christensen, S Oscarson, HH Jensen, Common side reactions of the glycosyl donor in chemical glycosylation. Carbohydr Res 2015, 408, 51.
| Common side reactions of the glycosyl donor in chemical glycosylation.Crossref | GoogleScholarGoogle Scholar |
[25] M Govindarajan, Protecting group migrations in carbohydrate chemistry. Carbohydr Res 2020, 497, 108151.
| Protecting group migrations in carbohydrate chemistry.Crossref | GoogleScholarGoogle Scholar |
[26] T Zhu, G-J Boons, Intermolecular aglycon transfer of ethyl thioglycosides can be prevented by judicious choice of protecting groups. Carbohydr Res 2000, 329, 709.
| Intermolecular aglycon transfer of ethyl thioglycosides can be prevented by judicious choice of protecting groups.Crossref | GoogleScholarGoogle Scholar |
[27] Z Li, JC Gildersleeve, Mechanistic studies and methods to prevent aglycon transfer of thioglycosides. J Am Chem Soc 2006, 128, 11612.
| Mechanistic studies and methods to prevent aglycon transfer of thioglycosides.Crossref | GoogleScholarGoogle Scholar |
[28] DD Cox, EK Metzner, EJ Reist, A new synthesis of 4-O-α-d-galactopyranosyl-d-galactopyranose. Carbohydr Res 1978, 62, 245.
| A new synthesis of 4-O-α-d-galactopyranosyl-d-galactopyranose.Crossref | GoogleScholarGoogle Scholar |
[29] H Fujimoto, H Nakano, M Isomura, S Kitahata, K Ajisaka, Enzymatic synthesis of oligosaccharides containing Galβ→4Gal disaccharide at the non-reducing end using β-galactanase from Penicillium citrinum. Biosci Biotechnol Biochem 1997, 61, 1258.
| Enzymatic synthesis of oligosaccharides containing Galβ→4Gal disaccharide at the non-reducing end using β-galactanase from Penicillium citrinum.Crossref | GoogleScholarGoogle Scholar |
[30] A Millqvist-Fureby, DA MacManus, S Davies, EN Vulfson, Enzymatic transformations in supersaturated substrate solutions: II. Synthesis of disaccharides via transglycosylation. Biotechnol Bioeng 1998, 60, 197.
| Enzymatic transformations in supersaturated substrate solutions: II. Synthesis of disaccharides via transglycosylation.Crossref | GoogleScholarGoogle Scholar |
[31] Y Yamamoto, T Saito, K Ajisaka, Study of the regioselectivity in the transglycosylation to d-galactose derivatives using β-galactosidases of various origins. J Appl Glycosci 2004, 51, 335.
| Study of the regioselectivity in the transglycosylation to d-galactose derivatives using β-galactosidases of various origins.Crossref | GoogleScholarGoogle Scholar |
[32] S Hanessian, J Banoub, Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-di-saccharides. Carbohydr Res 1977, 53, C13.
| Chemistry of the glycosidic linkage. An efficient synthesis of 1,2-trans-di-saccharides.Crossref | GoogleScholarGoogle Scholar |
[33] P Fügedi, PJ Garegg, S Oscarson, G Rosén, BA Silwanis, Glycosyl 1-piperidinecarbodithioates in the synthesis of glycosides. Carbohydr Res 1991, 211, 157.
| Glycosyl 1-piperidinecarbodithioates in the synthesis of glycosides.Crossref | GoogleScholarGoogle Scholar |