The Wonderful World of Poo: The Turdome and Beyond
Edouard C. Nice AA Department of Biochemistry and Molecular Biology, Monash University, Clayton, Vic. 3800, Australia. Email: ed.nice@monash.edu

Ed Nice is currently an Adjunct Professor at Monash University where he is Head of Clinical Biomarker Discovery and Validation (Department of Biochemistry and Molecular Biology) and a scientific advisor to the Monash Antibody Technologies Facility (MATF), for which he was director from 2009 to 2013. He also holds a Visiting Professorship at Sichuan University/West China Hospital and an adjunct position at Macquarie University, Sydney, Australia. His long-term research interests have been in protein and peptide micropurification, biomarker discovery and validation, SPR analysis, high throughput monoclonal antibody production and validation, and clinical biomarker assay development, with a strong translational focus on colorectal cancer, especially the development of faecal proteomics for colorectal cancer detection and surveillance. He is a founder member and past chair of the Australian Peptide Association, a founder member of the Protein and Peptide Society of Singapore and was awarded the 2012 Xiaoyu Hu Memorial Award by the Chinese Peptide Society in recognition of his significant achievements in peptide science. He has an active involvement in the Human Proteome Organization (HUPO), having been co-chair and treasurer of the successful HUPO 2010 meeting in Sydney, and is currently co-chair of the Pathology Pillar and the Human Cancer Proteome Project and leader of the HUPO ANZ Chromosome 7 initiative. |
Australian Journal of Chemistry 73(4) 257-263 https://doi.org/10.1071/CH19225
Submitted: 19 May 2019 Accepted: 1 August 2019 Published: 2 December 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Defecate: it is something we all do, it is something we joke about, yet for many in real life it is a subject that is taboo. However, it is now being realised that faeces are a veritable scientific goldmine, have many potential uses, and may even save your life! In this article I will review the history behind the use of faecal material and look at some of its emerging playing fields, in particular its role in medical diagnosis. I will discuss faecal proteomics and other omics technologies (Proteogenomics: The Omics Pipeline), including studies on the microbiome, in order to understand, diagnose, and treat gastrointestinal tract pathologies and other diseases, and show how these technologies will play a role in the move towards personalized medicine.
Introduction
Like it or not, poo is a fact of life: we all need to defecate. It has been calculated that annually 9 billion kilograms of poo are produced worldwide, with each of us producing on average six metric tons in our lifetime. Faeces contain 75 % water and 25 % solids, which comprise bacteria (25–54 %), protein and nitrogenous matter (2–25 %), carbohydrate and undigested plant matter (25 %), and lipid (2–15 %), opening the door for analysis of these specific components.
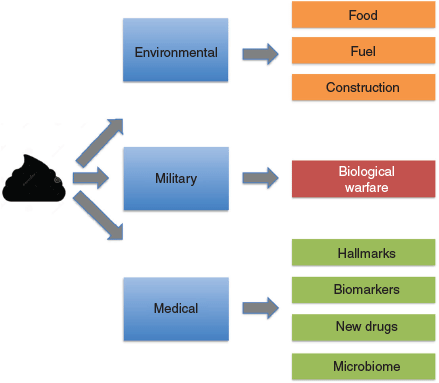
The complex coordination of multiple muscles and nerves controls when we defecate. Stool consistency depends on several factors including transit time, water adsorption, dietary factors (especially fibre and fluids), and gastrointestinal (GI) tract pathologies. Stool consistency can vary from hard lumps to a loose, watery stool (diarrhoea). However, for many poo is taboo. We tell jokes about it: a classic April Fool joke was a scientific paper on the optical isomer of TiHS, a brown amorphous substance, and the poo emoji ranks as one of the most frequently used. We quite happily change a baby’s nappy or pick up after our pooch but dislike actually looking at, or handling, our own poo. Yet poo has many uses (Fig. 1), and it is becoming obvious that poo is a veritable gold mine, opening the door to a range of interesting uses including medical diagnosis (Fig. 1).
|
While poo has been with us since time immemorial, it has a long (and sometimes chequered) history. Some of the earliest information on the GI tract and its contents has been revealed by palaeontology, especially from the study of coprolites (fossilised faeces). Coprolites were originally described by the Rev William Buckland in 1829. The name is derived from Greek: kopros meaning dung and lithos meaning stone. The oldest recorded samples (over 50000 years old) were discovered at El Salt in southern Spain. Using techniques including gas chromatography–mass spectrometry (GC-MS), they revealed, among other things, detailed dietary information including direct evidence of animal and plant intake by Neanderthals based on the identification of human faecal biomarkers in archaeological samples.[1] Meanwhile 14300 year old coprolites, discovered at Paisley Caves in Oregon, USA revealed interesting genetic and dietary information on the American Indians living there at that time [https://news.nationalgeographic.com/news/2014/06/140625-neanderthal-poop-diet-ancient-science-archaeology/]. MS studies revealed they were heavy meat eaters. Insights into the human microbiome, using pyrosequencing and bioinformatics, have also been obtained.[2] This study found that the ancient human microbiome more closely resembled that from current rural communities than those from cosmopolitan communities. Recently, using a complementary omics approach combined with microscopy, scientists analysed the stomach and small and large intestine content of the Iceman, a 5300-year-old European glacier mummy.[3] Again, detailed dietary information was obtained including the contents of his last meal.
Contemplation of human toilet habits over time also reveals interesting information. Of our current world population of around 7.6 billion, around 900 million (approx. 12 %) still openly defecate, although toilets have been in operation since at least early Egyptian times. Toilets have been found in Egyptian tombs and archaeological evidence suggests that the rich had toilets in their homes. King Tut is credited with inventing toilet paper to reduce the stench from the workers in the enclosed space of the pyramids. Networks of drainage systems and flushing toilets have been found in the Indus Valley (2600–1900 BC) and Minoan civilizations (2000 to 1600 BC). The Romans built sewers to collect both rainwater and sewage and even had a sewer goddess: Cloacina. Wealthy Romans had their own toilets but there were also communal public lavatories. Examples of this can be seen at archaeological sights such as Pompeii (visited by many delegates attending the 27th European Peptide Conference in Sorrento).
While an ancient latrine, dating back to the Western Han Dynasty (206 BC to 24 AD), was discovered in a tomb in Shangqiu county, Henan province, China in 2000, the invention of the flushing toilet as we know it (cited as one of the hundred ideas which changed the world)[4] is usually accredited to Sir John Harrington in 1596. The first patent was granted to watchmaker and inventor Alexander Cumming in 1775. Thus, contrary to common belief, Thomas Crapper was NOT the inventor of the flushing toilet, although he did make improvements, and his name will forever be associated with bowel movements. The development of the flushing toilet has continued through the centuries with many refinements. In the 1800s the Germans developed a model with a shelf (‘Flachspüler’, also known as ‘lay and display’). This was said to enable regular inspection of stools, offering defence against intestinal disease, water-borne parasites, or worm-riddled, undercooked bratwurst. Now we even have the high-tech SMART Toilet found in over 80 % of Japanese households that combine calming music, heated seats, fragrance control, and built-in bidets with a cleaning and drying function. It appears feasible that in the future such toilets will be capable of performing analytical tests such as the faecal occult bowel test (FOBT), which detects blood in the stool, in situ.[5] Toilets to cope with zero gravity during space missions (probably the world’s most expensive toilets with a price tag in the millions of dollars) are also being developed. World Toilet Day (19th November), a United Nations sanctioned day founded in 2001 by Jack Sim, a Singapore philanthropist, was established to help improve world sanitation.
So, how has faeces contributed/is contributing to society over the ages (Fig. 1)? It plays an extensive role in the food cycle, being used in many third world countries as a fertiliser which unfortunately also contributes to a prevalence of viruses such as Hepatitis B and C in such countries, as well as parasites. There are gourmet foods such as civet coffee (Kopi Luwak) made from beans harvested from civet faeces or Black Ivory Coffee, harvested from elephant dung (the world’s most expensive coffee). At the other end of the food chain, recent experiments have shown that it is possible to create food from faecal materials. Japanese scientist Mitsuyuki Ikeda has developed the ‘pooburger’ made from soya, steak sauce essence, and protein extracted from microbes from human faeces. A similar concept has also been used to make sausages and probiotics. The use of microbial fermentation is currently being considered for dealing with human excrement during space missions.
Another use of human faecal material is fuel. Faeces can be simply dried and used directly, or used to generate ‘biogas’. Biogas can be used for heating or to power generators and micro-turbines to produce electricity, or as vehicle fuel (Colorado uses human waste to power all its city vehicles). Faecal material can also be used for construction. Material has been extracted from faeces and used for the production of bricks.[6] It was proposed that this approach could significantly reduce the carbon footprint of this industry.
Over the decades poo has also played a role as a biological weapon [https://www.vice.com/en_au/article/vdx4ad/brown-death-a-history-of-poop-as-a-weapon-111]. The Scythians, a central Eurasian nomadic people (9th century BC to 4th century AD) used poisoned arrows dipped into a mixture of snake venom, human blood, and faeces. If wounded, the arrows could cause gangrene and tetanus. In the middle ages, faeces from bubonic plague victims were catapulted over castle walls in an attempt to cause infection (perhaps the first example of biological warfare). During the Vietnam War, the Viet Cong developed a weapon known as punji sticks, made by sharpening bamboo sticks, which were then dipped in human excrement and concealed in a pit camouflaged with foliage as a trap designed to cause infection if enemy soldiers fell on them.
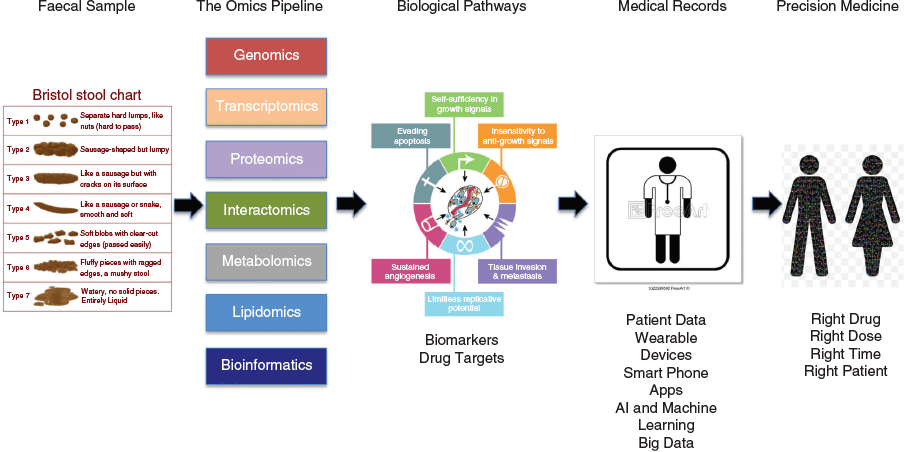
However, perhaps one of the most important uses of poo is in the medical field (Fig. 2). Doctors throughout the centuries have used faecal samples for investigation, or even as a therapeutic. The Egyptians used medicines based on dried infant faecal material, while the ancient Greeks included faeces as part of their medical examinations.[7] The Ebers Papyrus, a document from Ancient Egypt dating back to 1500 BC, contains more than 50 prescriptions for medicines in which faeces are the active ingredient. Faeces (Purisha), also formed part of the basic doctrine of Indian Ayurveda medicine while the fourth-century Chinese medical doctor Ge Hong documented faecal microbiota transplantation (FMT) for treating diarrhoea. FMT was subsequently described during the 16th century Ming dynasty by the Chinese physician Lee Shizhen who used fermented faeces and faecal extracts to treat abdominal pain and diarrhoea, and in the 17th century by the Italian anatomist and surgeon Fabricius ab Acquapendente for the treatment of GI tract pathologies in animals. It is said that stool inspection aided the diagnosis of porphyria (purple stools) responsible for the madness of the UK monarch, King George III. Burkitt (usually associated with Burkitt’s lymphoma) collected stool samples in Africa and India[8] to investigate the role of dietary fibre on stool composition and transit times, and its role in disease. Stool composition formed the basis for the Bristol Stool Chart (Fig. 2), developed in 1997 by a team at the British Royal Infirmary in Bristol, UK. This defines seven stool types based on shape, texture, and consistency that can be used to aid medical diagnosis. Interestingly it has recently been reported that there is an association between stool consistency based on the Bristol Stool Chart[9] and the number of bacterial species present, the ratio of two of the major phyla (Bacteroidetes and Firmicutes), the enterotypes (stratification of the human gut microbiota), and genus abundance.
|
It is now realised that faecal proteomics (affectionately called Turdomics in a presentation at the 7th Australian Peptide Conference/4th International Peptide Symposium held in Cairns in 2007), combined with other omics technologies (Proteogenomics, The Omics Pipeline (Fig. 2)) can play an important role in unravelling the complex biology behind GI tract pathologies, particularly an understanding of the hallmarks of cancer,[10] identifying potential biomarkers and biomarker panels, assisting in drug development, and interrogating the microbiome[11,12] (Fig. 2). These topics will be addressed in the remainder of this article.
Diseases of the GI Tract
The GI tract stretches from the mouth to the anus, via the oesophagus, stomach, small intestine (duodenum, jejunum, and ileum), large intestine (appendix, caecum, colon, and rectum), and anus. The salivary glands, liver, pancreas, and gallbladder feed into it. Unfortunately several major pathologies affect the GI tract. These include colourectal cancer (CRC), familial adenomatous polyposis (FAP), irritable bowel disease (IBD), coeliac disease (CD), and Clostridium difficile infection (CDI).
Globally, CRC is the third most common cancer accounting for around 10 % of all cases.[13] In Australia it is equally prevalent in men and women and affects ~58/100000 people. Tumours can be graded using staging systems such as the American Joint Committee on Cancer (AJCC), tumour/node/metastasis (TNM), or Dukes’ staging systems. For example, the AJCC system defines Stage I tumours as being localised within the bowel wall, stage II having invaded beyond the muscularis propria, stage III spreading to the lymph nodes, while in stage IV the tumour has metastasised to distant organs. If tumours can be detected at Stage 1, CRC is essentially curable by simple surgical resection. However, by the time the tumour has metastasised (~20 % of patients with CRC already have metastases at diagnosis) the 5-year survival rate is less than 10 %.[14] There is therefore a currently unmet clinical need for accurate and reliable biomarkers for early detection.
Tumours arise from a multistep carcinogenic process that begins with hyperproliferation of the normal epithelium leading to the development of adenomas (polyps), which in turn are believed to be precursors of carcinomas. These histological changes are associated with the accumulation of several genetic and epigenetic changes involving the activation of oncogenes and the inactivation of tumour suppressor genes including KRAS, APC, DCC, Smad4, and p53.[15] This process typically takes between 10 to 15 years, with transition from carcinoma to metastatic CRC taking only a further 2–3 years. There is, therefore, an excellent window of opportunity for early detection before the cancer has begun to spread.
FAP is an autosomal dominant inherited condition leading to the early onset of numerous adenomatous polyps, mainly in the epithelium of the large intestine.[16] These frequently progress to CRC by the age of 35–40, but only account for less than 1 % of all cases. However, about a third of all CRC cases observed before 35 years of age are hereditary.
IBD, which includes Crohn’s Disease and ulcerative colitus, affects around 400/100000 people in Australia. In ulcerative colitis, inflammation is usually confined to the inner lining of the gut, while in Crohn’s disease inflammation can spread through the whole wall.[17] These are often painful and debilitating diseases that can lead to life-threatening complications as well as an increased risk of colon cancer. Currently, between 40 and 60 % of patients do not benefit from the available treatments, indicating a considerable unmet need for new, more effective therapies and personalised treatment (precision medicine).
Coeliac disease is an autoimmune disorder, triggered by eating gluten, which causes inflammation and damage to the small intestine.[18] It affects over 1 % of the population in Australia. A strict, lifelong gluten free diet is currently the only recognised medical treatment for coeliac disease. Diagnosis of the presence of the disease and improved management (e.g. immunotherapy) are urgent clinical needs.
CDI is a symptomatic infection caused by the spore-forming bacterium C. difficile present in faeces.[19] Symptoms include watery diarrhoea, puss in the stools, fever, loss of appetite, nausea, and abdominal pain, tenderness, or cramping caused by severe inflammation. Once hospitalised, the overall rate of mortality among CDI patients is around 8.5 %. FMT has been reported to be effective in treating CDI, with an incredible success rate of over 90 %.
Clearly there are many urgent requirements to help address these serious diseases. As will be discussed below, faecal proteomics offers several potential solutions.
Faecal Proteomics
Recent advances in MS now facilitate the targeted discovery and validation of potential GI tract biomarkers as well as revealing the complex biology around health and disease [https://medicalfuturist.com/the-complex-world-of-cancer-pathways]. These techniques can be used to mine deeply into the faecal proteome to reveal candidate proteins suitable for sensitive and specific quantitative multiplexed analyses, leading to the development of novel clinical assays.[11] These, in turn, will contribute to personalised/precision medicine where, rather than using the traditional ‘one size fits all’ approach in which diseases are treated based on an average drug response, treatments are based on a comprehensive understanding of an individual’s own biology allowing delivery of the right drug at the right dose to the right patient at the right time.[20]
The principal behind faecal proteomics is that as a stool passes down the GI tract it constantly samples the cellular environment it is exposed to, including epithelial tissues as well as tumours or other lesions and their attendant micro-environment. It will contain components (e.g. proteins, peptides, lipids, and carbohydrates) present due to leakage, exfoliation, and/or secretion. Importantly these components should be present at higher relative concentrations than in blood or urine due to the close proximity of sampling and lack of dilution.
Early studies used animal models to show proof of principal. Ang et al. used ApcMin/+ mice as a model system for human CRC.[21] These mice have a mutant tumour suppressor gene which encodes a nonsense mutation at codon 850 of Apc, resulting in truncation of the Apc protein. These mice are highly susceptible to spontaneous intestinal adenoma and polyp formation and, as in humans, older mice typically show visible signs of rectal bleeding.[22] Using orthogonal separation techniques (1D SDS PAGE, reverse phase (RP)-HPLC, size exclusion chromatography (SEC)) on stools recovered from young, aged, or control C57Bl6 mice followed by nano-RP-HPLC/electrospray ionisation (ESI)-IT/tandem mass spectrometry (MS/MS) (LCQ-Deca) 336 proteins were identified (115 proteins of murine origin, 201 from faecal bacteria, 18 associated with food intake, and 2 of apparent parasitic origin), of which a number were murine homologues of proteins that had been previously described as being associated with CRC (colourectal cancer associated proteins: CCAP). Comparison of these data with that of Oleksiewicz et al.[23] who used C57Bl6 mice followed by 2D-PAGE and MALDI-TOF MS, showed all 16 murine proteins identified in the Oleksiewicz study were present, except mouse tissue kallikrein.
Encouraged by these results, human faecal samples were next investigated. Using a hypothesis-driven approach, proteins previously implicated as CCAP identified from the literature were targeted by excision from 1D-SDS-PAGE based on their predicted molecular weight followed by directed identification and relative quantification using multiple reaction monitoring (MRM). Nineteen of these proteins were detected in the faeces from a patient with CRC. Comparison of these 19 CCAP between 5 CRC patients and 5 healthy volunteers revealed haemoglobin, myeloperoxidase, S100A9, filamin A, and l-plastin to only be present in the samples from the CRC patients.[24] Potential CCAP can also be identified by direct in silico studies and meta analysis (a recent PubMed search using the keywords Protein Biomarkers and Colourectal Cancer gave 18316 hits).
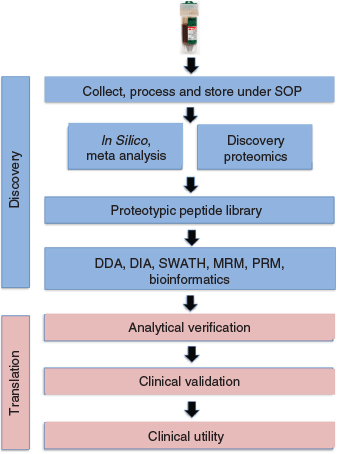
Next, a new paradigm for the discovery and validation of potential biomarkers for CRC was developed (Fig. 3). This pipeline can be readily modified for other GI tract pathologies simply by using the appropriate clinical samples. It is possible to investigate biomarkers or biomarker panels not only for early detection of disease, but also prognosis, monitoring of disease, or drug monitoring. The protocol contains both discovery and translational components (Fig. 3). Importantly, samples must be collected, processed, and stored under strict standard operating procedures (SOP) to prevent bias. While some esoteric methods have been used historically to recover uncontaminated stool samples (e.g. manual recovery using a gloved hand in a manner analogous to that used for the murine experiments (where the samples were recovered manually using forceps, as the animal, not surprisingly, usually defecates when picked up), or the Katmandu method (designed for field situations where a functional toilet is not available: A. Wilks, pers. comm.)), several commercial kits are now available to facilitate recovery (e.g. EasySampler® Stool Collection Kit, GP Medical Devices, Denmark). Attempts to ‘piggyback’ on residual samples collected for FOBT/FIT tests were unsuccessful due to the presence of substances such as chelators, and/or stabilisers (e.g. bovine serum albumin or rabbit immunoglobulin) which interfered with the MS analysis.
|
The initial processing step in the faecal proteomics protocol (Fig. 3) used an orthogonal multidimensional fractionation process, using acidic (0.1 % triflouroacetic acid (TFA)) extracts, to mine as deeply as possible into the proteome. Quantitation can be achieved using either data dependent (DDA), targeted (e.g. MRM/selected reaction monitoring (SRM)/parallel reaction monitoring (PRM))[25] or data independent (DIA) analysis (e.g. SWATH[26]). Using bioinformatics, MS/MS data from these samples is then searched to identify proteotypic peptides capable of uniquely identifying a specific protein, and a library generated which can be used to identify potential candidates for MRM/SRM analysis. This will typically lead to several potential candidates. Potential biomarkers and biomarker panels can then be optimised and verified, and candidate panels selected, on a new sample set (analytical validation) before clinical validation on a larger, statistically relevant, cohort leading to an assay with clinical utility (the degree to which the use of a test leads to improved patient outcomes and cost-effective care) (Fig. 3). The majority of biomarker studies to date have been based on a triangular strategy in which the number of samples required increases as one moves from discovery to validation and clinical utility. However, a recent study[27] proposes a ‘rectangular’ plasma proteome profiling strategy, in which the proteome patterns of large cohorts are correlated with their phenotypes in health and disease. The development of SRM/MRM assays has been greatly facilitated by a study from The Institute of Systems Biology in Seattle, USA[28] who developed an SRM Atlas containing validated SRM data enabling quantification of 99.7 % of the 20277 annotated human proteins predicted from the human genome.
In an initial experiment designed to show proof of principal, a MRM assay for several non-redundant human proteins present in faeces was developed.[29] Multiplexed analysis, using multiple MS/MS transition ions, was performed on 73 proteins in a single assay (min 3 transitions each) using unfractionated faecal samples (using a modified extraction protocol including sonication and acetone precipitation, suspension in 8 M urea, reduction and alkylation, and solid phase extraction (SPE)) from eight CRC patients and seven normal volunteers. Twenty four proteins were identified which were consistently found in all samples, including serum albumin, which was significantly elevated in the CRC samples and showed potential as a biomarker. Of course, serum albumin would be masked in blood-based assays. Nine proteins were found only in CRC patients, indicating the potential of this approach for the analysis of specific CRC biomarkers. Absolute quantitation, using C-terminal isotopically labelled synthetic peptides (obtained from JPT Peptides, Germany, who now have a catalogue containing > 400 000 light and heavy validated proteotypic peptides covering essentially all proteins of the human body) for haemoglobin (the basis of the FOBT test) and carcinoembryonic antigen 5 (a low abundance CCAP) was also demonstrated. These studies[29] led to the identification of a 5 biomarker panel with better apparent selectivity and sensitivity than the current FOBT.
This approach was recently confirmed in a large case controlled study[30] in which 315 stool samples from one series of 12 patients with CRC and 10 people with no apparent symptoms of colorectal disease (control samples) and a second series of 81 patients with CRC, 40 with advanced adenomas, and 43 with non-advanced adenomas, as well as 129 controls, were analysed by MS/MS. Eight hundred and thirty four human proteins were identified in total, of which 29 were statistically significantly enriched in CRC versus control stool samples in both series. A third cohort of 72 FIT samples from 14 patients with CRC, 16 with advanced adenomas, and 18 with non-advanced adenomas, as well as 24 controls was used for antibody-based assays for 4 selected proteins identified from the MS/MS studies. The above studies, together with data generated from microbiome studies (see below[31]) indicate the number of human proteins in the faecal proteome to be around 600–800 at the current levels of instrument sensitivity. As would be expected, there was significant overlap between the proteins of human origin identified. Taken together, the above studies clearly show the potential of faecal proteomics for identifying potential biomarkers/biomarker panels for monitoring GI tract pathologies and developing the appropriate clinical assays. Such assays will clearly be used to support precision medicine initiatives.
Attempts to analyse the faecal peptidome appear to have been less successful. The presence of, for example, truncated forms of haemoglobin, argue that the corresponding peptide fragments should be present. However, inspection of a typical RP-HPLC profile of a 0.1 % CH3CN faecal extract (see fig. 2B in Ang and Nice[24]) showed a broad elevated baseline with none of the sharp, early eluting peaks that would have been anticipated for peptide components, with few MS identifications in the early eluting fractions.[29] Rather, TOF-MS indicated the presence of a major polymeric component in these fractions that may have been suppressing other signals.
The Faecal Microbiome
Faecal proteomics is rapidly emerging as an important platform for analysis of the microbiome.[31] While in our earlier studies the microbiota were considered to be a potential hurdle in the analysis of the faecal data,[32] it is now recognised that the microbiome contains important useful information in its own right on both health and disease, and may provide a means of studying host–microbe interactions. The microbiome, which is often called the ‘forgotten organ’, is essential for maintaining human health and physiology, but has now been associated with several diseases including cancer, IBD, coeliac disease, obesity, cardiac disease, psoriasis, asthma, autism,[33] CDI, and cystic fibrosis.[34]
While earlier studies used mainly genomic- or transcriptomic-based approaches, faecal proteomics is now filling a new niche.[35] The initial non-targeted, shotgun MS/MS-based metaproteomics study of the faecal microbiome used stool samples from two identical twins.[36] Several thousand proteins were identified, of which ~30 % were of human origin. In a recent study using a protocol combining an efficient integrated sample preparation/fractionation step (Simple and Integrated Spintip-based PROteomics Technology, SISPROT), high resolution MS, and bioinformatics, Zhang et al. performed the deepest metaproteomic analysis of the human gut microbiome to date.[31] Using stool samples (0.1 g stool material) from children with paediatric IBD, they identified an average of 20558 protein groups per sample. Their data revealed human and microbial changes that were not readily apparent using standard genomic approaches. Metaproteomic approaches have also been used to identify a stable and personalised functional microbiome,[37] while Li et al. used GC-MS, NMR spectroscopy, and liquid chromatography–mass spectrometry to determine the influence of microbial metabolites on liver metabolism using a mouse model.[38]
Something that would not have been readily predicted was that the first FDA approved clinical proteomics applications would be in the field of microbiology. The US FDA has now cleared two commercially available instruments for in vitro diagnostics, namely the Bruker Biotyper and the BioMérieux Vitek MS, both based on MALDI TOF MS. These instruments, which have now been placed in several thousands of laboratories worldwide, are highly efficient in terms of speed, cost, and efficiency, and are now replacing phenotypic microbial identification. Assays can be performed in minutes, with identification based on spectroscopic libraries. For example, Bruker developed an initial library around data from 26 laboratories of the International Mycobacterium Consortium, providing well characterised clinical strains from which reference entries were derived from more than 470 genera. This library is being continually expanded as new data becomes available. Alternatively, laboratories can derive their own customised libraries. Artificial intelligence (AI)/machine learning can assist in the analysis of the complex data generated.
One medical area that has the potential to be supported by faecal proteomics/metaproteomics is FMT.[12] This could include assistance in diagnosis as well as in the understanding of disease mechanisms using suitable biomarkers, determination of the type of transplant material by analysing the structure, function, and composition of the microbiota in stool samples, help in the identification of optimal donors by comparative studies between recipients and donors, and monitoring the efficacy of the procedure. This is becoming increasingly important as several studies have suggested that the success of FMT is critically dependent on the microbial diversity and composition of the donor stool, and a super-donor phenomenon has been proposed.[39] Clearly a more personalised donor selection rather than a one size fits all approach is required. Importantly, changes in microbiota diversity, function, and structure before and after FMT could be monitored by proteomics coupled with genomic (e.g. DNA sequencing) and transcriptomic methods (proteogenomics) to evaluate therapeutic effect.[12]
Stool biobanks are already being developed to support FMT. Faecal proteomics will be useful in supporting these and maintaining quality control (similar biobanks, containing both normal and disease-associated faecal samples will also be required to support research studies).
The Future: Towards Precision/Personalised Medicine
Faecal proteomics clearly has a role to play in the detection and monitoring of GI tract diseases, and those that may be associated with dysregulation of the healthy microbiome. However, in many ways this technology platform is still only coming of age. Like most major recent scientific advances, faecal proteomics has been technology driven. This will continue to be the case, with development of more sensitive and rapid instrumentation (including multiplexed MS platforms) and the use of robotics for high throughput sample preparation. This will be mirrored by advances in bioinformatics and methods for the analysis of Big Data (e.g. improved AI/machine learning algorithms). More comprehensive libraries will be developed to support both SWATH and MALDI TOF MS analysis of microbiota. Faecal biobanks will become a valuable health resource, but will require the development and general acceptance of suitable SOPs under suitable regulatory control to ensure quality. While several commercial companies are already offering faecal assays based on existing clinical analysers (e.g. DiaSorin, an Italian multinational biotechnology company who have several faecal assays including Calprotectin, H. pyllori, C. difficile, Rotavirus) or MALDI TOF MS for microbial peptides, it is anticipated that commercially available clinical MS assays will begin to permeate the market. Interestingly, a recent study showed excellent correlation between clinical measurements using a DiaSorin assay and those measured by LC-MS/MS, with good agreement with quality assurance samples.[40]
Faecal proteomics is, and will continue to be, part of the pipeline for precision medicine (Fig. 2). It is currently part of a large Personalized Omics Profiling approach,[41] the hPOP study, initiated at Stanford and run under the auspices of the human proteome organisation (HUPO), where blood, urine, and faecal samples are collected at the annual HUPO meetings for comprehensive proteogenomic analysis. Likewise, faecal proteomics was part of the 100K Wellness Project, initiated at The Institute for Systems Biology, Seattle, where participants submitted blood, urine, saliva, and faecal samples every three months for metabolite, proteomic, and gut microbiome testing.[42] Two major hurdles will have to be overcome in the roll out of precision medicine. The first is the data privacy issue. Many people have concerns about Internet and Cloud security due of the sensitive nature of some of the data involved (e.g. patients medical records). In Australia, more than 2.5 million people (~10 % of the population), opted out of the recently introduced My Health Record system for such reasons. The second problem is that of compliance with faecal testing. A recent review has shown this is typically less than 45 % (in Australia it is around 32 % for men and 36 % for women).[43] Education is urgently required using effective advertising campaigns. In Queensland, Australia, a humorous approach has been adopted by Queensland Health , using an actor (Shane Jacobson) who starred in a hilarious comedy movie (Kenny: https://www.youtube.com/watch?v=ZHjc0cu9CgU) in which he played the part of a plumber at a portable toilet (portaloo) rental firm who knew all things lavatorial. He now advocates bowel screening for the over 50s in an advert with the punch line ‘Make your number 2 your number 1 priority with a bowel cancer screening kit’ (https://www.smh.com.au/national/queensland/jacobsons-bowel-cancer-ad-20160909-4kes9.html). How effective this approach will be remains to be seen, with some favouring a more shock approach as used on cigarette packaging in many countries or the successful, but somewhat controversial, ‘Grim Reaper’ adverts aired in Australia in the late 1980s to promote public awareness of the dangers of AIDs. Only by changing from a ‘poo is taboo’ to a ‘poo helps you’ mentality can we prevent or manage serious GI tract diseases like colorectal cancer.
Conflicts of Interest
ECN is a co-chair of the HUPO Human Pathology Pillar and the HUPO Cancer Proteome Project, and PI of the HUPO Australia/New Zealand Chr 7 C-HPP.
Acknowledgements
It is an honour to have been invited to contribute a manuscript to this Special Issue of the Australian Journal of Chemistry to honour the retirement of my long-time friend and colleague Prof Paul Alewood. We have spent many hours together over the years, often over a glass or two of (prophylactic) red wine, contemplating the usefulness of the Bristol Stool Chart. This research did not receive any specific funding.
References
[1] C. Sistiaga, C. Mallol, B. Galván, R. E. Summons, PLoS One 2014, 9, e101045.| Crossref | GoogleScholarGoogle Scholar |
[2] R. Y. Tito, D. Knights, J. Metcalf, A. J. Obregon-Tito, L. Cleeland, F. Najar, B. Roe, K. Reinhard, K. Sobolik, S. Belknap, M. Foster, P. Spicer, R. Knight, C. M. Lewis, PLoS One 2012, 7, e51146.
| Crossref | GoogleScholarGoogle Scholar | 23251439PubMed |
[3] F. Maixner, D. Turaev, A. Cazenave-Gassiot, M. Janko, B. Krause-Kyora, et al. Curr. Biol. 2018, 28, 2348.
| Crossref | GoogleScholarGoogle Scholar | 30017480PubMed |
[4] J. Osman, 100 Ideas that Changed the World 2011 (BBC Digital: London).
[5] P. Ranjitkar, Clin. Chem. 2018, 64, 1128.
| Crossref | GoogleScholarGoogle Scholar |
[6] A. Mohajerani, A. Ukwatta, T. Jeffrey-Bailey, M. Swaney, M. Ahmed, G. Rodwell, S. Bartolo, N. Eshtiaghi, S. Setunge, Buildings 2019, 9, 14.
| Crossref | GoogleScholarGoogle Scholar |
[7] C. F. Kleisiaris, C. Sfakianakis, J. Papathanasiou, J. Med. Ethics Hist. Med. 2014, 15, 6.
[8] D. P. Burkitt, A. R. P. Walker, N. S. Painter, Lancet 1972, 300, 1408.
| Crossref | GoogleScholarGoogle Scholar |
[9] D. Vandeputte, G. Falony, S. Vieira-Silva, R. Y. Tito, M. Joossens, J. Raes, Gut 2016, 65, 57.
| Crossref | GoogleScholarGoogle Scholar | 26069274PubMed |
[10] D. Hanahan, R. A. Weinberg, Cell 2011, 144, 646.
| Crossref | GoogleScholarGoogle Scholar | 21376230PubMed |
[11] C. S. Ang, M. S. Baker, E. C. Nice, Methods Enzymol. 2017, 586, 247.
| Crossref | GoogleScholarGoogle Scholar | 28137566PubMed |
[12] P. Jin, K. Wang, C. Huang, E. C. Nice, Expert Rev. Proteomics 2017, 14, 445.
| Crossref | GoogleScholarGoogle Scholar | 28361558PubMed |
[13] F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, A. Jemal, CA Cancer J. Clin. 2018, 68, 394.
| Crossref | GoogleScholarGoogle Scholar | 30207593PubMed |
[14] R. Etzioni, N. Urban, S. Ramsey, M. McIntosh, S. Schwartz, B. Reid, J. Radich, G. Anderson, L. Hartwell, Nat. Rev. Cancer 2003, 3, 243.
| Crossref | GoogleScholarGoogle Scholar | 12671663PubMed |
[15] E. R. Fearon, B. A. Vogelstein, Cell 1990, 61, 759.
| Crossref | GoogleScholarGoogle Scholar | 2188735PubMed |
[16] J. Groden, A. Thliveris, W. Samowitz, M. Carlson, L. Gelbert, et al. Cell 1991, 66, 589.
| Crossref | GoogleScholarGoogle Scholar | 1651174PubMed |
[17] B. A. Hendrickson, R. Gokhale, J. H. Cho, Clin. Microbiol. Rev. 2002, 15, 79.
| Crossref | GoogleScholarGoogle Scholar | 11781268PubMed |
[18] P. H. Green, B. Lebwohl, R. Greywoode, J. Allergy Clin. Immunol. 2015, 135, 1099.
| Crossref | GoogleScholarGoogle Scholar | 25956012PubMed |
[19] M. Rupnik, M. H. Wilcox, D. N. Gerding, Nat. Rev. Microbiol. 2009, 7, 526.
| Crossref | GoogleScholarGoogle Scholar | 19528959PubMed |
[20] P. Jin, J. Lan, K. Wang, M. S. Baker, C. Huang, E. C. Nice, Expert Rev. Proteomics 2018, 15, 231.
| Crossref | GoogleScholarGoogle Scholar | 29310484PubMed |
[21] C. S. Ang, J. Rothacker, H. Patsiouras, A. W. Burgess, E. C. Nice, J. Chromatogr. A 2010, 1217, 3330.
| Crossref | GoogleScholarGoogle Scholar | 19875126PubMed |
[22] A. R. Moser, H. C. Pitot, W. F. Dove, Science 1990, 247, 322.
| Crossref | GoogleScholarGoogle Scholar | 2296722PubMed |
[23] M. B. Oleksiewicz, H. O. Kjeldal, T. G. Klenø, Biomarkers 2005, 10, 29.
| Crossref | GoogleScholarGoogle Scholar | 16097391PubMed |
[24] C. S. Ang, E. C. Nice, J. Proteome Res. 2010, 9, 4346.
| Crossref | GoogleScholarGoogle Scholar | 20684568PubMed |
[25] V. Vidova, Z. Spacil, Anal. Chim. Acta 2017, 964, 7.
| Crossref | GoogleScholarGoogle Scholar | 28351641PubMed |
[26] L. C. Gillet, P. Navarro, S. Tate, H. Röst, N. Selevsek, L. Reiter, R. Bonner, R. Aebersold, Mol. Cell Proteomics 2012, 11, O111.016717.
| Crossref | GoogleScholarGoogle Scholar | 22261725PubMed |
[27] P. E. Geyer, L. M. Holdt, D. Teupser, M. Mann, Mol. Syst. Biol. 2017, 13, 942.
| Crossref | GoogleScholarGoogle Scholar | 28951502PubMed |
[28] U. Kusebauch, D. S. Campbell, E. W. Deutsch, C. S. Chu, D. A. Spicer, et al. Cell 2016, 166, 766.
| Crossref | GoogleScholarGoogle Scholar | 27453469PubMed |
[29] C. S. Ang, J. Rothacker, H. Patsiouras, P. Gibbs, A. W. Burgess, E. C. Nice, Electrophoresis 2011, 32, 1926.
| Crossref | GoogleScholarGoogle Scholar | 21538981PubMed |
[30] L. J. W. Bosch, M. de Wit, T. V. Pham, V. M. H. Coupé, A. C. Hiemstra, et al. Ann. Intern. Med. 2017, 167, 855.
| Crossref | GoogleScholarGoogle Scholar |
[31] X. Zhang, W. Chen, Z. Ning, J. Mayne, D. Mack, A. Stintzi, R. Tian, D. Figeys, Anal. Chem. 2017, 89, 9407.
| Crossref | GoogleScholarGoogle Scholar | 28749657PubMed |
[32] H. Lin, Q. Y. He, L. Shi, M. Sleeman, M. S. Baker, E. C. Nice, Expert Rev Proteomics 2018, 16, 501.
| 30223687PubMed |
[33] Z. Y. Kho, S. K. Lal, Front. Microbiol. 2018, 9, 1835.
| Crossref | GoogleScholarGoogle Scholar | 30154767PubMed |
[34] G. Debyser, B. Mesuere, L. Clement, J. Van de Weygaert, P. Van Hecke, G. Duytschaever, M. Aerts, P. Dawyndt, K. De Boeck, P. Vandamme, B. Devreese, J. Cyst. Fibros. 2016, 15, 242.
| Crossref | GoogleScholarGoogle Scholar | 26330184PubMed |
[35] J. S. Lichtman, J. L. Sonnenburg, J. E. Elias, ISME J. 2015, 9, 1908.
| Crossref | GoogleScholarGoogle Scholar | 26057846PubMed |
[36] N. C. Verberkmoes, A. L. Russell, M. Shah, A. Godzik, M. Rosenquist, J. Halfvarson, M. G. Lefsrud, J. Apajalahti, C. Tysk, R. L. Hettich, J. K. Jansson, ISME J. 2009, 3, 179.
| Crossref | GoogleScholarGoogle Scholar | 18971961PubMed |
[37] C. A. Kolmeder, J. Salojärvi, J. Ritari, M. de Been, J. Raes, G. Falony, S. Vieira-Silva, R. A. Kekkonen, G. L. Corthals, A. Palva, A. Salonen, W. M. de Vos, PLoS One 2016, 11, e0153294.
| Crossref | GoogleScholarGoogle Scholar | 27070903PubMed |
[38] B. Li, K. Guo, L. Zeng, B. Zeng, R. Huo, Y. Luo, H. Wang, M. Dong, P. Zheng, C. Zhou, J. Chen, Y. Liu, Z. Liu, L. Fang, H. Wei, P. Xie, Transl. Psychiatry 2018, 8, 34.
| Crossref | GoogleScholarGoogle Scholar | 29382834PubMed |
[39] B. C. Wilson, T. Vatanen, W. S. Cu, J. M. O’Sullivan, Front. Cell. Infect. Microbiol. 2019, 9, 2.
| Crossref | GoogleScholarGoogle Scholar | 30719428PubMed |
[40] K. Spanaus, A. von Eckardstein, Clin. Chem. Lab. Med. 2017, 55, 1305.
| Crossref | GoogleScholarGoogle Scholar | 28245186PubMed |
[41] R. Chen, G. I. Mias, J. Li-Pook-Than, L. Jiang, H. Y. Lam, Cell 2012, 148, 1293.
| Crossref | GoogleScholarGoogle Scholar | 22424236PubMed |
[42] N. D. Price, A. T. Magis, J. C. Earls, G. Glusman, R. Levy, C. Lausted, D. T. McDonald, U. Kusebauch, C. L. Moss, Y. Zhou, S. Qin, R. L. Moritz, K. Brogaard, G. S. Omenn, J. C. Lovejoy, L. Hood, Nat. Biotechnol. 2017, 35, 747.
| Crossref | GoogleScholarGoogle Scholar | 28714965PubMed |
[43] C. Klabunde, J. Blom, J. L. Bulliard, M. Garcia, L. Hagoel, V. Mai, J. Patnick, H. Rozjabek, C. Senore, S. Törnberg, J. Med. Screen. 2015, 22, 119.
| Crossref | GoogleScholarGoogle Scholar | 25967088PubMed |


