A phenotypic marker for quantifying heat stress impact during microsporogenesis in rice (Oryza sativa L.)
Krishna S. V. Jagadish A B E , Peter Craufurd B C , Wanju Shi A D and Rowena Oane AA International Rice Research Institute, DAPO Box 7777, Metro Manila, Philippines.
B Plant Environment Laboratory, University of Reading, Cutbush Lane, Shinfield, Reading, RG2 9AF, UK.
C International Crops Research Institute for the Semiarid Tropics, Patancheru, Andhra Pradesh 502324, India.
D College of Agronomy, Hunan Agricultural University, Changsha, Hunan 410128, China.
E Corresponding author. Email: k.jagadish@irri.org
Functional Plant Biology 41(1) 48-55 https://doi.org/10.1071/FP13086
Submitted: 6 April 2013 Accepted: 3 July 2013 Published: 30 July 2013
Abstract
Gametogenesis in rice (Oryza sativa L.), and particularly male gametogenesis, is a critical developmental stage affected by different abiotic stresses. Research on this stage is limited, as flowering stage has been the major focus for research to date. Our main objective was to identify a phenotypic marker for male gametogenesis and the duration of exposure needed to quantify the impact of heat stress at this stage. Spikelet size coinciding with microsporogenesis was identified using parafilm sectioning, and the panicle (spikelet) growth rate was established. The environmental stability of the marker was ascertained with different nitrogen (75 and 125 kg ha–1) and night temperature (22°C and 28°C) combinations under field conditions. A distance of –8 to –9 cm between the collar of the last fully opened leaf and the flag leaf collar, which was yet to emerge was identified as the environmentally stable phenotypic marker. Heat stress (38°C) imposed using the identified marker induced 8–63% spikelet sterility across seven genetically diverse rice genotypes. Identifying the right stage based on the marker information and imposing 6 consecutive days of heat stress ensures that >95% of the spikelets in a panicle are stressed spanning across the entire microsporogenesis stage.
Additional keywords: flag leaf, heat stress, microsporogenesis, rice, spikelet, tetrad formation.
Introduction
Rice is becoming increasingly exposed to adverse climatic conditions such as heat, cold, and water deficit stress, resulting in significant yield losses (Wassmann et al. 2009). Further, global climate models predict water deficit stress-affected rice area to double by 2100, and accompanied by a simultaneous increase in temperature of 2.0–4.5°C (IPCC 2007): the result will be serious damage to global rice production. The probability of these combined stresses damaging rice crops in the major rice-growing regions in Southern and South-east Asia has been recently mapped (Wassmann et al. 2009). The rice scientific community has intensified efforts to develop rice varieties capable of withstanding these adverse conditions (heat stress, Yoshida et al. 1981; Jagadish et al. 2010a, 2010b, 2011; drought stress, Bernier et al. 2007; Kumar et al. 2008; Venuprasad et al. 2008; cold stress, Andaya and Mackill 2003; Ji et al. 2011) to sustain rice production under future adverse climates. Rice is extremely sensitive to these stresses, in particular, during the reproductive – gametogenesis and flowering stages – and exposure can result in increased spikelet sterility, which in turn, reduces grain yield.
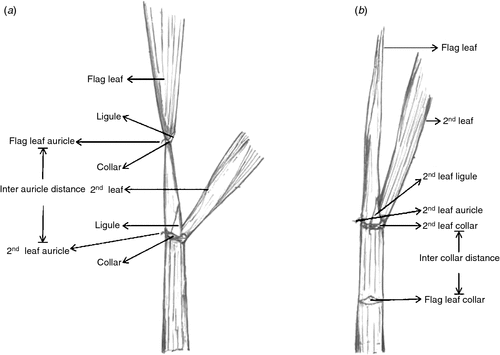
Because of the ease with which flowering can be studied, the majority of studies concentrating on different abiotic stresses have focussed on this stage. Microsporogenesis, the highly sensitive stage to heat stress (Matsui et al. 2000), has not been studied extensively due to the lack of a precise phenotypic marker. However, a marker based on inter auricle distance has been identified for cold stress phenotyping (Satake and Hayase 1970), and extended to quantify the impact of cold (Oliver et al. 2005, 2007) and drought stress (Ji et al. 2010; Liu and Bennett 2011) during young microspore and bi-nucleate pollen stages. The classic work by Satake and Hayase (1970) used different inter auricle distances and stress exposure duration to examine just two contrasting rice entries, indicating the challenge in identifying a robust marker that could be used across many genotypes. In more recent studies mentioned above, the inter auricle distance after the flag leaf emergence (Fig. 1a) has been employed, wherein a proportion of spikelets at the top of the panicle would have progressed beyond the target stage, diluting the estimation of panicle tolerance with stress escape. To overcome a similar phenomenon and to account for the asynchronous floral development in a rice panicle, marking protocol has been used to quantify heat stress impact during anthesis (Jagadish et al. 2008, 2010a). More importantly, cold (Thakur et al. 2010) and heat stress (Hedhly 2011) are known to delay and enhance most developmental stages, respectively, making it impractical to extend progress achieved with cold stress phenotyping directly to study heat stress. Hence, a phenotypic marker to quantify heat stress impact at microsporogenesis stage needs to be identified and extensively tested for extending its applicability across different rice genotypes and environmental conditions.
|
Moreover, the major determinant of spikelet fertility with heat stress exposure in rice is known to be the male reproductive organ or pollen development and viability (Yoshida et al. 1981; Jagadish et al. 2010a). Our focus on microsporogenesis stage is based on the outcome of cross pollination experiment, i.e. heat stressed pistil pollinated with fresh pollen and vice versa wherein female reproductive organ did not reduce fertility even with 40°C exposure, whereas pollen exposed to 38°C led to a significant decline in spikelet fertility (Yoshida et al. 1981). Further, the majority of the physiological or molecular studies dealing with microsporogenesis have drawn conclusions based on a single genotype (Kerim et al. 2003; Hobo et al. 2008; Oliver et al. 2005, 2007; Endo et al. 2009). We hypothesise that spikelet size (length) coinciding with microsporogenesis varies with genotypes. Hence, it is essential to conduct systematic analysis of the microsporogenesis stage across a range of genotypes to identify the developmental stage just before microsporogenesis to impose precise heat stress phenotyping protocols, which is the main aim of the work presented here. The specific objectives of this work were to (i) identify a phenotypic marker for the entire microsporogenesis stage in rice and validate its effectiveness under heat stress exposure; (ii) estimate the rate of development of a panicle and the duration of exposure needed to quantify heat stress impact coinciding with microsporogenesis; and (iii) ascertain the stability of the identified marker under different environmental conditions.
Materials and methods
Crop husbandry
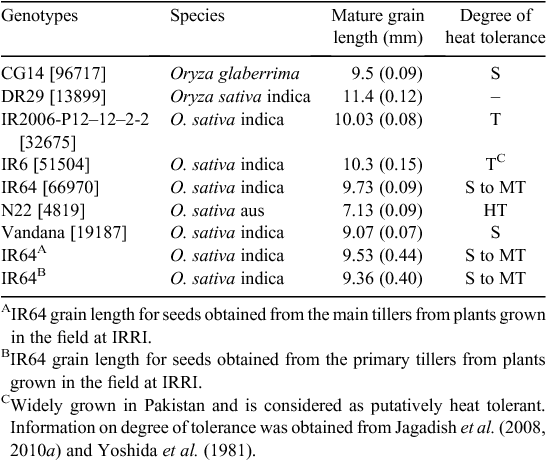
Rice (Oryza sativa L.) seeds of seven diverse genotypes (Table 1) were pre-treated at 50°C for 3 days to break dormancy. Seeds were direct sown in seeding trays and 14-day-old seedlings were transplanted into pots containing 6 kg of clay loam soil. Basal fertiliser (2.0 g (NH4)2SO4, 1.0 g KCL, and 1.0 g SSP) was added before transplanting and an additional 2.5 g of urea ((NH2)2CO) was added 30 days after transplanting. One plant per pot was grown under fully flooded conditions. Plants were maintained under controlled-greenhouse conditions with air temperature maintained at 29/21°C day/night (actual = 28.9°C (s.d. = ± 0.6)/22.2°C (s.d. ± 0.9)) and day/night RH at 75/80% (82% ( ± 10)/88% ( ± 4)) throughout the crop growth period. There were no major pest or disease problems except white flies (Bemissia spp.). Cypermethrin (Cymbush) at 0.42 g L–1 was sprayed at 15-day intervals, starting 30 days after transplanting, to manage whitefly infestation.
|
Stage and duration of stress exposure
To achieve the objective of identifying the ‘right stage’ and the ‘duration of stress exposure’ to quantify the impact of stress, information on (i) actual spikelet size (length) when tetrad and early microspore formation occurs (see ‘Sampling and sectioning’) and (ii) the time taken for >90% of the spikelets on a target panicle to complete microsporogenesis is essential (see ‘Spikelet growth’).
Sampling and sectioning
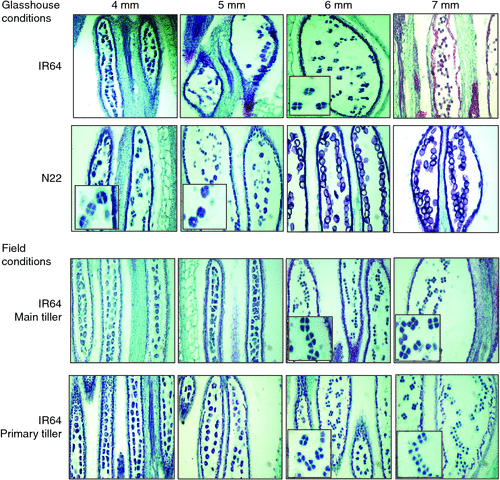
Plants were regularly monitored following panicle initiation (by dissecting) to collect spikelets with sizes varying from 3 to 7 mm for identifying the appropriate spikelet length coinciding with tetrad and early microspore formation. A total of 10–15 spikelets of size 3 to 7 mm were collected from the main and first primary tiller at random from four replicate plants. Detached panicles were placed in water-filled Petri-plates and spikelets were separated with the help of forceps and standard ruler and categorised according to their length and transferred immediately into FAA fixative (10% (v/v) formaldehyde, 50% (v/v) absolute ethanol, 5% (v/v) acetic acid) and dehydrated through a graded ethanol series and embedded using paraffin (Paraplast Plus; Sigma Chemical Co., St Louis, MO, USA). Serial sections of 10 μm thickness were obtained by a microtome (Leica RM2135, Singapore) and placed on Superforst Plus microscope slides (Fisher Scientific, Hampton, NH, USA) and incubated at 45°C for 48 h. Sections were dewaxed in xylene, rehydrated through a graded ethanol series, and stained with 2% safranin dissolved in 50% ethanol. This was followed by ethanol washing and subsequent staining of sections with 0.05% fast green in 80% acetone. The samples were then mounted and oven-dried at 65°C for 24 h. Sections were viewed under an Axioplan 2 microscope (Carl Zeiss, Oberkochen, Germany) and images taken using a DP70 camera attached to the microscope. A similar exercise was conducted using spikelets of mega-rice variety IR64 collected from both main and primary tillers separately from plants grown in the field to test the application of this marker under field conditions.
Spikelet (panicle) growth
To ascertain the duration of heat stress exposure needed to ensure that stress affects at least 90% of the spikelets in the panicle during their microsporogenesis stage, spikelet growth rate was used as an indirect measure of panicle growth rate (see Fig. S1a, available as Supplementary Material to this paper). Preliminary analyses were conducted and the length of all the spikelets on the panicle was measured at different times related to the distance (starting at −15 to +5 cm) between the collar (illustrated in Fig. 1b) of the fully opened leaf and the flag leaf collar (identified by gently running the thumb and forefinger along the main tiller), which was yet to emerge. Knowing the actual length of the spikelet coinciding with tetrad formation (through the sectioning exercise), and the length of all the spikelets across the whole panicle, we identified the approximate distance between the collar of the fully opened leaf and flag leaf when 5% of the spikelets (at the tip of the panicle) would have undergone tetrad formation and with nearly 90–95% yet to undergo these processes (Fig. S1b).
Tillers identified to be at an appropriate distance (see ‘Results’) were tagged and dissected and the size of each of the spikelets on the panicle was measured across all seven varieties. In total, eight tillers with approximately the same distance between the fully opened leaf and the yet-to-emerge flag leaf collar were tagged on the same day. Two tillers were dissected on four consecutive days, including the day when the tillers were tagged, and all spikelets on each of the panicles were measured to (i) estimate the rate of spikelet growth and (ii) check the duration needed for >90% of the spikelets on the panicles to have undergone microsporogenesis. This was used to determine the duration of stress exposure needed for standardising phenotyping protocols.
Case study – high-temperature stress
Seeds of all seven varieties were obtained from the International Rice Research Institute gene bank and plants were grown under greenhouse conditions (as detailed above) and moved into controlled-temperature chambers (Thermoline Inc., Sydney, NSW, Australia) for imposing heat stress coinciding with tetrad formation and the early microsporogenesis stage by employing the identified marker. A detailed description of the greenhouse and growth chamber conditions, including technical details, has been given elsewhere (Jagadish et al. 2010a, 2011). Briefly, the main tillers of the plants were tagged and regularly monitored for the marker distance. Once the distance between the collar of the fully opened leaf and the yet-to-emerge flag leaf collar was close to the target range, a set of four replicate pots of each of the seven varieties was moved into the chambers maintained at 39°C (actual = 38.94 ± 0.12) and RH at 75% (74.31 ± 5) from 0800 to 1430 hours for 4 consecutive days. Immediately following the completion of exposure, plants were moved back to the control conditions in the greenhouse and maintained till maturity. Similarly, another set of four replicate pots for each variety was left in the glasshouse for the entire period as true controls. Twenty days after flowering, the tagged main tillers from both the control and stressed plants were cut and percent spikelet fertility was estimated by carefully pressing the spikelets between the thumb and the forefinger (Prasad et al. 2006). The data was analysed using Genstat ver. 13 (VSN International, Rothamsted Experimental Station, Hemel Hempstead, UK).
Validating environmental stability of the marker
The environmental stability of the marker was validated using 16 temperature-controlled chambers under field conditions (each chamber measured 6 × 3 × 2 m in length, width, and height, respectively; see Fig. S2) set up to study the interaction between high night temperature stress and two different levels of nitrogen in cv. N22. There were two inlet and two outlet fans installed in the front frame and the back frame, respectively, to minimise the differences in RH and CO2 concentration within the chamber compared with the ambient by constant but mild air exchange. During daytime (0600–1800 hours), the chambers were open, exposing the plants to natural conditions. At night (1800–0600 hours), the chambers were closed manually and the air conditioners (CW-1805V, Matsushita Electric Philippines Corp., Taytay, Rizal, Philippines) were programmed to automatically impose control (22°C) and stress (28°C) treatments, following Shi et al. (2013). The temperature and RH were monitored every minute and averaged over 30 min (HOBO, Onset computer Corp., Bourne, MA, USA). Nearly 5 cm of standing water was maintained throughout the experiment to ensure a leak-proof covering of the tents for the whole night. Temperature treatments started from the panicle initiation stage ~31 days after transplanting and continued up to physiological maturity. Moderate (75 kg N ha–1) and high (125 kg N ha–1) nitrogen levels were applied and, as a result, each of the four combinations was replicated in four chambers each. Nitrogen in the form of urea was applied in four splits with total amount (40% as basal, 20% at mid-tillering, 30% at panicle initiation, and 10% at heading). For both the nitrogen levels, phosphorus (15 kg P ha–1), potassium (20 kg K ha–1), and zinc (2.5 kg Zn ha–1) were applied and incorporated in all plots 1 day before transplanting. Finally, 11–18 days after imposing the temperature treatments, 16–20 panicles per treatment were selected randomly based on the identified marker and dissected for measuring spikelet length following the protocol detailed above.
Results and discussion
By employing the inter auricle distance as a phenotypic marker and imposing 3–4 days of cold or drought stress (Oliver et al. 2005, 2007; Ji et al. 2010), young microspore and bi-nucleate pollen stage were targeted. However, tetrad formation and early microspore generation has also been identified to be most sensitive to cold stress (Nishiyama 1970, 1976). Further, targeting a precise stage during microsporogenesis in a panicle would realistically expose the more mature spikelet on the panicle (bi/tri nucleate stage or beyond) and younger spikelets (at tetrad formation stage or microspore mother cell stage) to stress. So a clear demarcation of the stress coinciding specifically with the target stage across the entire panicle is not possible. For example, stress imposed targeting tetrad stage will invariably expose other spikelets at young microspore or bi-nucleate stage to stress, depending on their position along the panicle. Hence, we targeted the entire microsporogenesis stage of a panicle using the inter collar distance to come up with a phenotypic marker for use across a wide range of genotypes and to extend its application in heat stress tolerance breeding programs.
Spikelet length and microsporogenesis
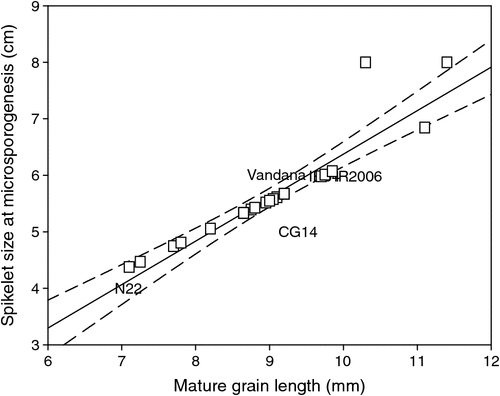
The set of seven diverse genotypes selected for this exercise had varying mature grain length ranging from 7.1 to 11.4 mm (Table 1). Spikelet length at microsporogenesis varied with genotype, and was strongly correlated with final mature grain length with four out of five tested entries within the 95% confidence interval (Fig. 2). N22 had the smallest mature grain length of 7.1 mm and also underwent microsporogenesis with the smallest spikelet length at 4 mm (Fig. 3). In the longer grain varieties, namely, IR2006-P12–12–2-2 and IR64 (9.7–10 mm), spikelet size of 6 mm coincided with tetrad formation and early microspore formation (Figs 3, S3). We were unable to identify the spikelet length coinciding with microsporogenesis among genotypes with mature grain length of >10 mm (DR29 and IR6) since tetrad and early microspore formation probably occurred with developing spikelet length beyond 7 mm, the size at which we had restricted our analyses (Fig. S3). Results from samples obtained from the field followed the glasshouse results with IR64 spikelets at size 6–7 mm coinciding with microsporogenesis (Fig. 3). This indicated the consistency and repeatability of results across controlled environments and field conditions, as well as across different tillers.
|
|
Mature grains of 15 popular rice varieties grown across Africa, South Asia, South-east Asia, and Latin America were obtained and mature grain length was used to predict possible spikelet length at microsporogenesis, with IR64 as the reference. We noted that almost all these entries except Sahel 329 were within the 10 mm range, similar to IR2006 and IR64, and the spikelet lengths coinciding with microsporogenesis were well extrapolated within the tested range, with a high correlation, including both the predicted and observed data (r = 0.81; Fig. 2). On the basis of these results and the predicted spikelet lengths, we conclude that the developing spikelet length coinciding with microsporogenesis stage varies with genotypes and has a close relationship with mature grain size. Hence, the marker can be employed to study responses to heat stress at the microsporogenesis stage across most of the widely grown rice varieties.
Rate of panicle development and validation of the marker
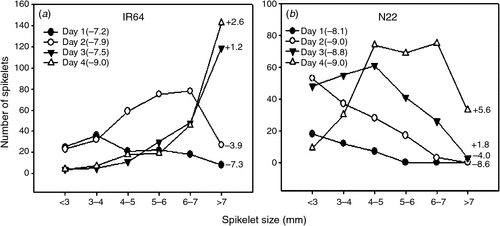
A highly dynamic growth rate of the panicles was observed, with an average increase of >2.5 cm day–1 across all seven genotypes studied (Figs 4, S4). The highest rate of panicle growth was seen with N22 having a 3.65 cm day–1 decrease in collar distance of the fully opened leaf and yet-to-emerge flag leaf (Fig. 4). A similar rapid increase in panicle size in N22 over 4 days is shown in Fig. S1a, b, with a close relationship between the panicle and collar growth rate and hence collar distance was used as a proxy for identifying the tillers at the right stage and determining panicle growth rate. Accordingly, the spikelet size increase followed the panicle growth pattern and, within 4 days of observation, >90% of the spikelets that were yet to undergo microsporogenesis passed through this stage (Figs 4, S4).
|
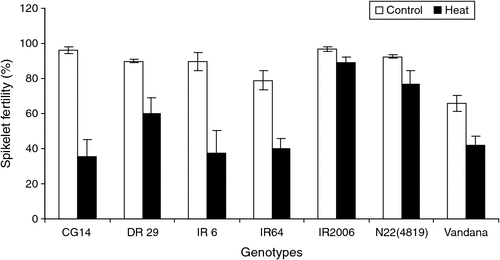
The marker (distance between the collar of the fully opened leaf with the yet-to-emerge flag leaf collar at −8 to −9 cm) can be more reliably used for stress phenotyping with entries having mature grain size of <10 mm or slightly longer, irrespective of the variation in spikelet size coinciding with microsporogenesis across genotypes. For longer grain sizes, we recommend preliminary analysis to confirm the effectiveness before using the marker. The phenotypic marker (−8 to −9 cm) was validated by exposing all seven genotypes to heat stress and a significant decrease in spikelet fertility was recorded across entries (P < 0.001), and temperature treatment (P < 0.001), with a significant interaction (P < 0.01). Induced percent spikelet sterility ranged from 63% (CG14) to as low as 8 and 16% in IR2006 and N22 respectively (Fig. 5). The sterility recorded was comparable with that noted by Yoshida et al. (1981) in DR 29 (33%) but other genotypes demonstrated a wide variation in response to stress, indicating an opportunity to exploit this variation in breeding varieties tolerant of heat stress at the microsporogenesis stage. Additionally, this case study provides evidence for the effectiveness in using the identified phenotypic marker for imposing abiotic stress at this complex sensitive stage in rice. N22, which is known to be highly heat tolerant (Prasad et al. 2006; Jagadish et al. 2008, 2010a), was on par with IR2006, indicating its tolerance during both critical developmental stages. Breeding programs using N22 as a donor for developing heat-tolerant rice varieties for the flowering stage could induce higher tolerance even for the microsporogenesis stage.
|
Marker application
Our aim was to identify the right stage of the panicle so that 90% of the spikelets on the target panicle are exposed to stress at microsporogenesis and we successfully reached our target in CG14 and Vandana. However, a few later developing spikelets were still below the critical spikelet size (for example, in IR64 and N22) when the 4-day stress imposed was relieved (Fig S4). Hence, we recommend that the combination of identifying the marker at the right stage and imposing 6 days of heat stress will ensure that >90% of the spikelets are exposed to stress. Extended application of this marker to cold and particularly water stress could be slightly complex with the rate of growth reduction depending on the severity of cold stress or water limitation and hence the marker as well as the duration of exposure has to be applied with caution. Additionally, a high synchrony between male and female gametogenesis in rice is essential for normal gamete development, fertilisation, and finally seed-set (Zinn et al. 2010). Hence, we hypothesise that this phenotypic marker identified could be extended to study the impact of stress coinciding with either microsporogenesis (male gamete formation) or megasporogenesis (female gamete formation) or their combined effect on seed-set, through artificial cross-pollination studies as employed effectively in tomato (Peet et al. 1997).
Environmental stability of the marker
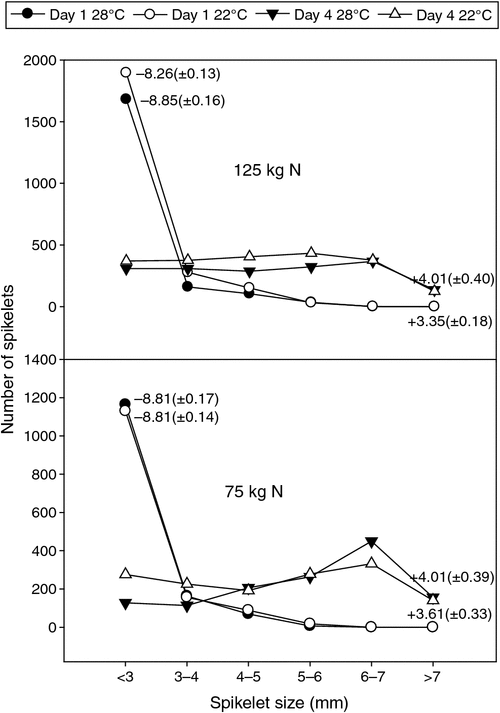
The proportion of N22 spikelets with ≤3 mm in length (with 4 mm coinciding with the tetrad formation) was ≥80%, coinciding with marker distance (–8 to –9 cm; Day 1), across all four combinations of nitrogen and night temperature treatments (Fig. 6). Across these treatments, only 5–8% of the spikelets were ≥4 mm on Day 1. Four days later, 80––90% of the spikelets were sized ≥4 mm. The per cent spikelets before undergoing tetrad formation and the per cent spikelets passing through the critical microsporogenesis stage in the subsequent 4 days under field conditions are highly comparable with the proportions obtained under controlled chamber conditions (Fig. 4b). Even though the total number of spikelets was significantly reduced with 75 kg N (mainly attributed to shortage of resources), the relative proportions of spikelet sizes on Day 1 (≤3 mm) and Day 4 (≥4 mm) did not vary significantly compared with 125 kg N. Moreover, 4 days after the right stage was identified, nearly 9–19% of the spikelets were ≤3 mm in length, a case which was also observed under controlled environments with N22. Hence, as recommended in the marker application section, stress exposure has to be extended to 6 days to ensure that >90% of the spikelets in the target panicle undergo microsporogenesis under stress conditions. In addition, the panicle length across the treatments did not change significantly (data not shown). The reliable performance of the identified marker under field conditions with different combinations of nitrogen and night temperatures (and light conditions – field vs chamber conditions) provides concrete support for its stability under different environmental conditions.
|
Conclusion
We identified a reliable phenotypic marker to identify the microsporogenesis stage in rice, based on final grain length. Results indicate a rapid increase in both spikelets and panicles during the microsporogenesis stage, and exposing the plants or identified tillers at the right stage for 6 days of stress would expose >90% of the spikelets to stress, thus, allowing precise estimation of stress impact on this complex developmental stage. Importantly, the identified marker was stable across different environmental conditions. This finding has to be further verified and extended to study multiple abiotic stress impacts on seed-set and to quantify other negative effects on megasporogenesis, male and female organ developmental asynchrony under heat stress, and more importantly, the contribution of the female reproductive organ to spikelet sterility under major abiotic stresses.
Acknowledgements
PB Malabanan and BA Enriquez are thanked for their technical assistance during the experiment. Bill Hardy from IRRI is thanked for editing the manuscript.
References
Andaya VC, Mackill DJ (2003) QTLs conferring cold tolerance at the booting stage of rice using recombinant inbred lines from a japonica × indica cross. Theoretical and Applied Genetics 106, 1084–1090.Bernier J, Kumar A, Ramaiah V, Spaner D, Atlin GN (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Science 47, 507–516.
| A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice.Crossref | GoogleScholarGoogle Scholar |
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant & Cell Physiology 50, 1911–1922.
| High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVajur7E&md5=e53c5089a89a6a798e4c81565ae7961aCAS |
Hedhly A (2011) Sensitivity of flowering plant gametophytes to temperature fluctuations. Environmental and Experimental Botany 74, 9–16.
| Sensitivity of flowering plant gametophytes to temperature fluctuations.Crossref | GoogleScholarGoogle Scholar |
Hobo T, Suwabe K, Aya K, Suzuki G, Yano K, Ishimizu T, Fujita M, Kikuchi S, Hamada K, Miyano M, Fujioka T, Kaneko F, Kazama T, Mizuta Y, Takahashi H, Shiono K, Nakazono M, Tsutsumi N, Nagamura Y, Kurata N, Watanabe M, Matsuoka M (2008) Various spatiotemporal expression profiles of anther-expressed genes in rice. Plant & Cell Physiology 49, 1417–1428.
| Various spatiotemporal expression profiles of anther-expressed genes in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVaiurrO&md5=9a7bb43057d79e9121d57873d2c6501dCAS |
IPCC (2007) Summary for policy makers. In ‘Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change’. (Eds SD Solomon, M Qin, Z Manning, M Chen, M Marquis, KB Avery, M Tignor, HL Miller) pp. 12–25. (Cambridge University Press: Cambridge)
Jagadish SVK, Craufurd PQ, Wheeler TR (2008) Phenotyping parents of mapping populations of rice (Oryza sativa L.) for heat tolerance during anthesis. Crop Science 48, 1140–1146.
| Phenotyping parents of mapping populations of rice (Oryza sativa L.) for heat tolerance during anthesis.Crossref | GoogleScholarGoogle Scholar |
Jagadish SVK, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ (2010a) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). Journal of Experimental Botany 61, 143–156.
| Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGgu7fJ&md5=824447bac181c9375fb1d2d3527a603aCAS |
Jagadish SVK, Cairns J, Lafitte R, Wheeler TR, Price AH, Craufurd PQ (2010b) Genetic analysis of heat tolerance at anthesis in rice. Crop Science 50, 1633–1641.
| Genetic analysis of heat tolerance at anthesis in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Omur7P&md5=adb9e9c55594b2836d6316860177fe83CAS |
Jagadish SVK, Muthurajan R, Rang ZW, Malo R, Heuer S, Bennett J, Craufurd PQ (2011) Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22). Rice 4, 1–11.
| Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22).Crossref | GoogleScholarGoogle Scholar |
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, Richards RA, Dolferus R (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell & Environment 33, 926–942.
| Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVagsb4%3D&md5=ab34326906c510141684c61d9ebd9f21CAS |
Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R (2011) Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiology 156, 647–662.
| Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvFWrsb0%3D&md5=e3716879b786ec48e4b494595ef41142CAS | 21502188PubMed |
Kerim T, Imin N, Weinman JJ, Rolfe BG (2003) Proteome analysis of male gametophyte development in rice anthers. Proteomics 3, 738–751.
| Proteome analysis of male gametophyte development in rice anthers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjvFGisbw%3D&md5=ac1eaa7536f1650e4f917f474777ad3cCAS | 12748952PubMed |
Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN (2008) Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Research 107, 221–231.
| Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations.Crossref | GoogleScholarGoogle Scholar |
Liu JX, Bennett J (2011) Reversible and irreversible drought-induced changes in the anther proteome of rice (Oryza sativa L.) genotypes IR64 and Moroberekan. Molecular Plant 4, 59–69.
| Reversible and irreversible drought-induced changes in the anther proteome of rice (Oryza sativa L.) genotypes IR64 and Moroberekan.Crossref | GoogleScholarGoogle Scholar | 20643753PubMed |
Matsui T, Omasa K, Horie T (2000) High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Production Science 3, 430–434.
| High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.).Crossref | GoogleScholarGoogle Scholar |
Nishiyama I (1970) Male sterility caused by cooling treatment at the meiotic stage in rice plants. IV: Respiratory activity of anthers following cooling treatment at the meiotic stage. Proceedings of the Crop Science Society of Japan 39, 65–70.
| Male sterility caused by cooling treatment at the meiotic stage in rice plants. IV: Respiratory activity of anthers following cooling treatment at the meiotic stage.Crossref | GoogleScholarGoogle Scholar |
Nishiyama I (1976) Male sterility caused by cooling treatment at the young microspore stage in rice plants. Proceedings of the Crop Science Society of Japan 45, 254–262.
| Male sterility caused by cooling treatment at the young microspore stage in rice plants.Crossref | GoogleScholarGoogle Scholar |
Oliver SN, Dongen JTV, Alfred SC, Mamun EA, Zhao X, Saini HS, Fernandes SF, Blanchard CL, Sutton BG, Geigenberger P, Dennis ES, Dolferus R (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell & Environment 28, 1534–1551.
| Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVynuw%3D%3D&md5=61187d8cd2d8b60481e114a0ae1ed2a6CAS |
Oliver SN, Dennis ES, Dolferus R (2007) ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant & Cell Physiology 48, 1319–1330.
| ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFyksrfO&md5=e0e918be0287c30e2cef7a2da64512b3CAS |
Peet MM, Willits DH, Gardner R (1997) Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress. Journal of Experimental Botany 48, 101–111.
| Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtlensrY%3D&md5=ee068b7a0146569fc840927767d00771CAS |
Prasad PVV, Boote KJ, Allen LH, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research 95, 398–411.
| Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Crossref | GoogleScholarGoogle Scholar |
Satake T, Hayase H (1970) Male sterility caused by cooling treatment at the young microspore stage in rice plants. Proceedings of the Crop Science Society of Japan 39, 468–473.
| Male sterility caused by cooling treatment at the young microspore stage in rice plants.Crossref | GoogleScholarGoogle Scholar |
Shi W, Muthurajan R, Rahman H, Selvam J, Peng S, Zou Y, Jagadish KSV (2013) Source–sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytologist 197, 825–837.
| Source–sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmslKlsA%3D%3D&md5=f17c73b41494cf5774ef1577adfdf5c3CAS | 23252708PubMed |
Thakur P, Kumar S, Malik JA, Berger JD, Nayyar H (2010) Cold stress effects on reproductive development in grain crops: an overview. Environmental and Experimental Botany 67, 429–443.
| Cold stress effects on reproductive development in grain crops: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVGmur3L&md5=3f83e933c5994a5205437fb9cca9053cCAS |
Venuprasad R, Sta Cruz MT, Amante M, Magbanua R, Kumar A, Atlin GN (2008) Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Research 107, 232–244.
| Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations.Crossref | GoogleScholarGoogle Scholar |
Wassmann R, Jagadish SVK, Sumfleth K, Pathak H, Howell G, Ismail A, Serraj R, Redoña E, Singh RK, Heuer S (2009) Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Advances in Agronomy 102, 91–133.
| Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation.Crossref | GoogleScholarGoogle Scholar |
Yoshida S, Satake T, Mackill DS (1981) High temperature stress in rice. IRRI Research Paper Series 67.
Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany 61, 1959–1968.
| Temperature stress and plant sexual reproduction: uncovering the weakest links.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsFGjsb8%3D&md5=ce84baf2fe4fd96620635590a97ad30cCAS | 20351019PubMed |


