Modulation of key sterol-related genes of Nicotiana benthamiana by phosphite treatment during infection with Phytophthora cinnamomi
Aayushree Kharel A , Mark Ziemann B , James Rookes
A , Mark Ziemann B , James Rookes  A and David Cahill
A and David Cahill  A *
A *
A
B
Abstract
Phytophthora cinnamomi is a globally destructive pathogen causing disease in over 5000 plant species. As sterol auxotrophs, Phytophthora species rely on host-derived phytosterols for reproduction, yet the effects of pathogen infection on plant sterol biosynthesis remains unclear. We utilised a soil-free plant growth system to analyze the impacts of P. cinnamomi on Nicotiana benthamiana roots, a new model for studying P. cinnamomi–plant root interactions. Our results show that P. cinnamomi successfully infected all ecotypes tested, but infection was inhibited by the systemic chemical, phosphite. While phosphite is traditionally associated with the activation of plant defence mechanisms, we show that phosphite also modulates plant immune receptors and phytosterol biosynthesis. qPCR analyses revealed a two-fold upregulation of the N. benthamiana elicitin receptor, Responsive to Elicitins (REL), and its co-receptor, suppressor of BIR1-1 (SOBIR) during P. cinnamomi infection when compared with infected, phosphite-treated plants. Furthermore, key genes related to plant sterol biosynthesis were upregulated in their expression during pathogen infection but were suppressed in phosphite-treated and infected plants. Notably, the cytochrome P450 family 710 (CYP710A) gene encoding a C22-sterol desaturase, involved in stigmasterol production, a phytosterol known to be linked to plant susceptibility to pathogens, was downregulated in phosphite-treated plants, independent of infection status. These findings reveal novel insights into the role of phosphite in modulating plant immune responses and sterol metabolism, with potential in managing diseases caused by P. cinnamomi.
Keywords: elicitin receptor, Nicotiana benthamiana, phosphite, Phytophthora cinnamomi, plant-pathogen interactions, soil-free plant growth system, sterol metabolism, sterol-related genes.
Introduction
Plant disease poses significant risks to global food security and environmental sustainability through loss of primary productivity and biodiversity. The oomycete genus Phytophthora comprises over 200 formally described species that threaten agriculture and natural ecosystems (Brasier et al. 2022). Among these, Phytophthora cinnamomi is a globally distributed soil-borne plant pathogen from Clade 7 (Burgess et al. 2017; Yang et al. 2017) that infects over 5000 plant species (Hardham and Blackman 2018) leading to die-back, root rot, plant death, and reductions in biodiversity. Also known as the ‘biological bulldozer’ (Kamoun et al. 2015), P. cinnamomi infects key crops such as Persea americana (avocado) (Engelbrecht and Van den Berg 2013), Castanea spp. (chestnut) (Fernandes et al. 2021) and Macadamia spp. (Akinsanmi et al. 2017). In Australia, P. cinnamomi is a severe threat to natural ecosystems (Cahill et al. 2008), putting more than 60 plant species at high risk of extinction (McDougall et al. 2024) and endangering numerous native flora and fauna (Dundas et al. 2016). Despite extensive research, its ability to infect and kill a wide range of plant species remains poorly understood. Investigating plant–pathogen interactions at the cellular and molecular levels has nevertheless provided valuable insights into the mechanisms underlying its pathogenicity.
During these interactions, a complex interplay of molecules from both the plant and the pathogen determines the outcome of the infection. Among these molecules, sterols have been recognised as key players in signal transduction, communication, and exchanges between partners, as reported by Wang et al. (2012) and Der et al. (2024). This is particularly critical in Phytophthora–plant interactions, where Phytophthora species, as sterol auxotrophs (Wang et al. 2021), depend on host-derived phytosterols for survival and proliferation. While Phytophthora infection has been shown to upregulate phytosterol synthesis (Shen et al. 2016; Evangelisti et al. 2017), advancing our understanding of how Phytophthora influences phytosterol biosynthesis and the regulatory mechanisms involved during P. cinnamomi infection remain poorly understood.
Current management strategies to control the spread of P. cinnamomi are limited (Shearer et al. 2007; Sena et al. 2018). The pathogen’s inability to synthesise sterols renders azole-based fungicides ineffective (Schuster et al. 2024), and its rapidly evolving genome complicates targeting its effectors (Shands et al. 2024). An alternative strategy could exploit Phytophthora’s sterol-auxotrophic nature. Our recent study of sterol sensing and recruitment genes in P. cinnamomi (Kharel et al. 2024a), revealed the central roles of sterol-sensing domain (SSD)-containing proteins (SCPs), and the β-cinnamomin elicitin, respectively. These new insights offer potential targets for novel control measures. In tandem, Huang and Joosten (2024) emphasise that understanding the plant receptors that interact with elicitins is equally promising for developing advanced disease resistance strategies and enhancing crop protection. For example, the identification of elicitin receptor genes, elicitin response (ELR), in wild potato (Solanum tuberosum) and their expression in cultivated varieties via transformation (Du et al. 2015) enabled recognition of elicitins from P. infestans that significantly enhanced disease resistance in otherwise susceptible crops. Similarly, the gene for elicitin receptor, responsive to elicitins (REL) was identified in Nicotiana benthamiana (Chen et al. 2023), where the recognition of elicitins by these receptor-like proteins (RLPs) led to pattern-triggered immunity (PTI) through mechanisms such as reactive oxygen species (ROS) bursts, calcium influx, and mitogen-activated protein kinases (MAPKs) activation (Chaparro-Garcia et al. 2011; Adachi et al. 2015). Thus, investigating the impact of P. cinnamomi on elicitin-associated plant receptors coupled with an analysis of sterol biosynthesis in the host plant will provide deeper insights into the role of phytosterols during plant–Phytophthora interactions.
Sterol biosynthesis in plants is a complex, multi-step process initiated by the mevalonate (MVA) pathway (Du et al. 2022), which produces critical isoprenoid intermediates. The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), a key rate-limiting enzyme in this pathway, is fundamental to sterol biosynthesis by providing necessary precursors (Li et al. 2014). Other major regulatory steps include catalysis by sterol methyltransferase (SMT), which converts cycloartenol to Δ5 C24-alkyl sterols such as campesterol and β-sitosterol. These sterols serve as essential precursors for downstream metabolic processes, including the synthesis of stigmasterol, a stress-related sterol (Valitova et al. 2024) that accumulates during pathogen attack. This conversion from β-sitosterol to stigmasterol is facilitated by C22-sterol desaturase, encoded by the cytochrome P450 family 710 gene (CYP710A) (Morikawa et al. 2006). To prevent sterol toxicity, excess sterols are converted into conjugated forms, including non-toxic sterol esters, by acyltransferases such as phospholipid acyltransferase (PSAT) (Ferrer et al. 2017). While the regulation of phytosterol synthesis is well understood, it can be influenced by chemical treatments, including azole (Benton and Cobb 1997) and morpholine (Rahier and Taton 1997) fungicides, which alter phytosterol levels. However, the potential impact of phosphite, a widely used control measure against disease caused by P. cinnamomi (Aberton et al. 1999; Gunning et al. 2013), on plant sterol biosynthesis remains to be explored.
Phosphite, a salt of phosphorous acid, is a systemically mobile chemical recognised as the most effective fungicide for managing Phytophthora diseases (Hardy et al. 2001; Mohammadi et al. 2021) and has emerged as a biostimulant that enhances plant performance under stress (Gómez-Merino and Trejo-Téllez 2015). Phosphite enhances resistance against P. cinnamomi by increasing defense-related metabolites in planta (Jackson et al. 2000; Gunning et al. 2013). Additionally, phosphite directly inhibits hyphal growth (King et al. 2010) and sporulation (Wilkinson et al. 2001), thereby slowing disease spread. Proteomic studies support the dual role of phosphite, as exemplified by Andronis et al. (2024), who describe its interference with P. cinnamomi metabolism while enhancing plant photosynthesis, carbon fixation, and lipid metabolism. Despite these insights, the full mode of action of phosphite remains elusive, particularly regarding its potential effects on sterol biosynthesis.
To investigate the influence of P. cinnamomi on phytosterol biosynthesis and the potential modifications introduced by phosphite, it is essential to use a model system that closely mimics natural infection conditions. Here, we use N. benthamiana as a model plant to study the interaction between the roots of a susceptible host and P. cinnamomi. While there remains ongoing debate about its ecological and taxonomic identity (Cauz-Santos et al. 2022; Ranawaka et al. 2023), as proposed by Bally et al. (2018), N. benthamiana remains a valuable model organism for plant-pathology research. In this paper, we refer to the different isolates/accessions of N. benthamiana as ecotypes to maintain consistency with previous studies and simplify the discussion by avoiding unresolved taxonomic debates.
Research using N. benthamiana as a host for P. cinnamomi has primarily focused on leaf assays (Belisle et al. 2019; He et al. 2024), even though P. cinnamomi is a root pathogen that infects through the root tip. Studies by Wang et al. (2019) and Gao et al. (2023) report significant differences in transcriptome and proteome profiles between leaves and roots under abiotic stress, underscoring the anatomical and functional differences that result in distinct pathogen behaviours and plant defence responses. Therefore, despite challenges like root inaccessibility and gene expression variability during sampling (Balmer and Mauch-Mani 2013; Fröschel 2021), root inoculation is essential for accurately studying pathogen dynamics of the root pathogen P. cinnamomi. As proposed by Allardyce et al. (2012), this approach better reflects natural conditions and emphasises the need for specialised growth systems tailored to root-pathogen studies.
In this study, N. benthamiana was utilised as a model host to investigate the impact of P. cinnamomi infection on plant sterol biosynthesis, and the influence of phosphite in this interaction. We established a soil-free growth system to accurately simulate root inoculations. Phosphite treatment of the inoculated plants significantly restricted pathogen spread, confining pathogen growth to the root cortex, while untreated but inoculated plants suffered extensive damage, and the pathogen extended into vascular tissue. Gene expression analysis indicated that infection with P. cinnamomi led to upregulation of the elicitin receptor REL and its co-receptor suppressor of BIR1-1 (SOBIR) genes, but phosphite treatment did not alter the expression of these genes. Additionally, we linked the influence of phosphite to sterol biosynthesis by analysing the expression profiles of key sterol-related genes. This approach allowed us to assess how phosphite treatment impacts the plant sterol synthesis pathway and how they are involved in the response to P. cinnamomi.
Materials and methods
Growth and maintenance of Phythophthora cinnamomi
We used a Phythophthora cinnamomi isolate (Du109, A2 mating type) from a culture collection held at Deakin University that was originally obtained from the Anglesea region, Victoria (isolated by Barry Schroeter, Deakin University). Isolates were grown and maintained on clarified V8 juice, cV8 agar [10% clarified Campbell’s V8 vegetable juice (Camden, NJ, USA), 0.1% CaCO3, 1.5% bacteriological agar] at 24°C in the dark, with subculturing performed every 5–6 days.
Comparison of Nicotiana benthamiana ecotypes in response to infection by P. cinnamomi using agar plug and zoospore inoculum techniques
To assess potential ecotypic differences in roots of Nicotiana benthamiana Domin root in response to P. cinnamomi infection, we initially used plugs of P. cinnamomi. This approach, similar to that described by Coelho et al. (2021), was selected for its efficiency and ease in isolating numerous plugs, enabling us to quickly evaluate the ecotypic responses. After identifying the ecotypic response, we transitioned to zoospore inoculation to better simulate the natural mode of infection and assess gene expression via qPCR in the presence or absence of phosphite.
Ecotypes of N. benthamiana
Seeds of N. benthamiana ecotypes Laboratory (LAB) and South Australia (SA) were purchased from Herbalistics Pty Ltd (Queensland, Australia). The LAB ecotype is the most widely used N. benthamiana line for research purposes globally. Seeds for north Western Australia (NWA), Western Australia (WA) and Northern Territory (NT) were the gift of Prof. Peter Waterhouse (Queensland University of Technology, Queensland, Australia). SA, NWA, WA, and NT are considered wild-type strains, collected from different geographic regions of Australia, as detailed in Bally et al. (2018).
N. benthamiana seed pre-treatment and seedling growth
Seeds of N. benthamiana were surface sterilised following the method used for Arabidopsis thaliana L., as described by Islam et al. (2020).Seeds were placed in a microcentrifuge tube and treated with 50% v/v ethanol in water containing 5% hydrogen peroxide solution for 5 min followed by three rinses with sterile distilled water. Sterilised seeds were then transferred to a 9 cm sterile Petri plate lined with two layers of sterile, round filter paper (Advantec 90 mm), and the seeds were covered with an additional two layers of filter paper. To maintain moisture and facilitate seed imbibition, 10 mL of sterile water was added to each plate. The plates were then sealed with a parafilm and placed in an incubator (Thermoline Scientific, Australia) at 21°C under cool white fluorescent light (100 μmol photons m−2s−1) with a 16/8-h light/dark cycle for 48 h, with the cycle restarting every 24 h to maintain light/dark exposure.
Plants were initially grown in a sand-based tube system following the protocol outlined by Islam et al. (2020). Briefly, commercial propagation sand (Bunnings, Australia) was sieved using a 5 mm sieve to ensure uniform particle size. The sand was autoclaved for sterilisation and subsequently added to 5 mL plastic disposable pipette tips (T-5000-C, Axygen, Vic, Australia) until 2 mm from the top. To moisten the sand, 1 mL of tap water was added. A single, imbibed seed was then placed in each tube, at a depth of approximately 3 mm from the surface. An additional 400 μL of water was added to ensure the seeds were adequately covered by the sand. The tubes containing the seeds were arranged on a rack and placed inside a Saxon Mini Green House without a seed tray (Bunnings, Australia), which held 1 L of tap water supplemented with 1/3 strength of Total Horticultural Concentrate (THC) nutrient solutions A and B (THC, Excel Distributors, Reservoir, Australia). The nutrient solution was refreshed every 5 days. To maintain humidity, the lid and base of the greenhouse were sealed with tape. The entire setup was transferred to a plant growth chamber under previously described conditions.
After 2 weeks, once the seeds had germinated, 400 μL of liquid fertiliser (Yates Thrive, Australia) was added. Any seeds that had not germinated at this time were discarded. The greenhouse was resealed with tape and returned to the growth chamber for an additional 3 weeks before transfer to a soil-free plant growth system (SPS) (Gunning and Cahill 2009).
N. benthamiana transfer to a soil-free plant growth system
To transfer 5-week-old plants from sand tubes to the soil-free system without damaging the root system, individual plants were carefully removed from the growth tubes. To remove plants from sand, each tube was briefly submerged in distilled water to loosen the sand, followed by gentle tapping of the tube to dislodge the plants while maintaining root integrity. The roots were then immediately submerged in water within a beaker to remove any remaining sand particles before transferring them to the SPS.
The SPS setup was based on the method described by Gunning and Cahill (2009), with slight modifications. The base of each polycarbonate unit was filled with 800 mL water and 1/3 strength THC solutions A and B (THC, Excel Distributors, Reservoir, Australia). Chromatography paper 1803C (Filtech, Australia) was placed on the front and back panels of each tray and saturated with tap water using a pressurised spray bottle. Three cotton wool square pads (Swisspers, Australia), halved lengthwise, were positioned on top of the filter paper and saturated with water. A 5 cm wide strip of chromatography paper was placed over the cotton layer to prevent root penetration into the cotton. Plants removed from sand tubes were carefully positioned with their stem-root intersections resting on the cotton layer, ensuring that the aerial parts remained above the cotton while the roots were in contact with the chromatography paper. Each tray accommodated 8–10 plants, evenly spaced to avoid overcrowding.
Another layer of 5 cm wide chromatography paper, followed by a layer of cotton pad, both saturated with water, was placed over the stem–root intersection. The front and back of the tray were then clipped together using fold-back clips and placed within the polycarbonate unit. Since the plants were previously within a mini greenhouse chamber, to maintain similar conditions, each unit was covered with a thin, transparent plastic sheet (Bunnings, Australia). The units were placed in the incubator under the previously described growth conditions.
For anatomical observation, SPS trays were placed horizontally on a bench at room temperature for 2 days. Root health was recorded by noting the absence of root tip browning, overall root length, and branching patterns. Relative chlorophyll concentration, as an indicator of plant health, was measured using a chlorophyll meter (502 SPAD, Konica Minolta, Osaka, Japan), which assesses light transmittance through leaf tissue based on chlorophyll’s spectral absorbance. Chlorophyll concentration was measured in two leaves per plant for 20 plants per ecotype and analysed using a t-test.
Inoculation of N. benthamiana roots with agar plugs that contain P. cinnamomi
N. benthamiana plants were cultured in the SPS for 2 days, after which the polycarbonate tray containing the plants was placed horizontally on a bench at room temperature and opened. The tray was misted with water to keep the roots from drying out. A strip of parafilm was positioned below the zone of elongation of the main root of each plant. The zone of elongation was inoculated with a 5 mm diameter plug of P. cinnamomi, with the mycelial surface facing down, taken from a 5-day-old cV8 agar plate containing P. cinnamomi. The growth tray was securely closed, and the SPS unit was returned to the plant growth conditions.
Control roots of all ecotypes were inoculated with plugs taken from a sterile cV8 agar plate. After 24 h, parafilm and agar plugs from mock-inoculated, or P. cinnamomi-inoculated roots were carefully removed using forceps.
To validate successful infection with P. cinnamomi, infected roots of P. cinnamomi were subcultured onto Phytophthora-selective medium (PARPH) plates, and incubated at 24°C for 2 days (Allardyce et al. 2012). Plugs from the growing edge of the culture were then transferred to 10% cV8 agar plates and incubated for 5 days. Mycelia were scraped, collected from the agar plates, and frozen using liquid nitrogen. Genomic DNA of P. cinnamomi was extracted using the CTAB method described by Kharel et al. (2024b). The extracted DNA was then amplified by nested PCR, using CIN3A and CINITS4 as outer primers, and CIN3B and CIN2R as inner primers (Williams et al. 2009) (see Supplementary Table S1).
Parameters monitored for response of different ecotypes to P. cinnamomi infection
Four parameters were selected to assess plant health and to characterise the interaction of P. cinnamomi with root tissue: (1) root tissue fresh weight and dry weight; (2) root length; (3) visible lesion length; and (4) P. cinnamomi colonisation as observed through microscopy. These parameters were measured according to Allardyce et al. (2012), with slight modifications to suit a different host plant. A total of 10 control and 10 inoculated plants were used per ecotype at each time point, with each parameter independently tested in triplicate.
Individual plants were gently removed from the SPS units 7 days post inoculation (dpi), and the roots were harvested. The roots were then placed on a plastic weigh tray on a top-loading balance to determine their fresh weight. Following this, the roots were wrapped in aluminium foil and placed in an oven at 50°C. After 3 days, the dry roots were weighed again. Mean and standard deviation values were calculated and reported in mg.
The SPS units were positioned horizontally on the laboratory bench, and the front panel was carefully removed. The cotton pad from the top layer of each plant was then taken off, and images were captured using a digital camera. The length of the main root for both control and inoculated plants, and visible lesion length was measured on Days 1, 3, 5, and 7 post-inoculation using ImageJ software. After taking the images, the plants were returned to the growth chamber, ensuring no damage to the root system. Percentage of visible lesion length was calculated with Eqn (1):
To evaluate colonisation, entire roots were harvested on Days 1, 3, 5, and 7 post-inoculation. The roots were surface sterilised with 70% ethanol for 30 s and washed twice with sterile water. The roots were then sequentially placed onto PARPH plates, which were incubated at 24°C. The following day, the plates were examined under a light microscope to identify the root regions where the pathogen emerged, root images were captured and ImageJ software was used to measure this length. Percentage of root colonisation was calculated with Eqn (2):
Phosphite treatment study
This study focused on the LAB ecotype of N. benthamiana grown in a sand system. To examine how phosphite affects the interaction between N. benthamiana and P. cinnamomi, and its influence on plant sterol-related gene expression, we used four treatment groups: (1) surfactant only (mock-inoculated with water); (2) surfactant plus phosphite (mock-inoculated with water); (3) surfactant only (P. cinnamomi zoospore inoculated); and (4) surfactant plus phosphite (P. cinnamomi zoospore inoculated).
The protocol followed the method described by Gunning et al. (2013). For treatment groups (1) and (3), 0.1% surfactant (Wetter 1000, Apparent Ag, Australia) was foliar sprayed (Canyon Corp Pty Ltd, Australia), 48 h before inoculation. For treatment groups (2) and (4), we applied a foliar spray mixture of 0.1% surfactant and 6 g L−1 phosphite (Phosphite 600 systemic fungicide, Apparent Ag, Australia), 48 h prior to inoculation.
To prevent surfactant and/or phosphite runoff into the plant roots and its subsequent absorption by the sand, an absorbent paper barrier was used. Following treatment, the absorbent paper was removed, and the plants were left for 15 min before being returned to controlled growth conditions.
Infection of N. benthamiana roots with P. cinnamomi zoospores
Zoospores of P. cinnamomi were produced following the method described by Kharel et al. (2024b) and zoospore concentration was adjusted to 5 × 105 zoospores mL−1 with sterile water. Root inoculation of the plants followed the method described by Islam et al. (2020). Inoculation took place 48 h post adjuvant or phosphite application (Gunning et al. 2013). A 400 μL zoospore suspension was carefully pipetted against the side wall of the plant growth tube above the sand surface for plants of treatment groups (3) and (4). Inoculated plants (eight plants per replicate per treatment) were harvested immediately (0 h) and at 24 h and 48 h post inoculation (hpi). To harvest the plants, the same method described previously was used whereby the tubes were submerged in water and the plants were gently tapped out. For mock-inoculated plants of treatment groups (1) and (2), 400 μL of sterile water was added and plants were harvested similarly.
To confirm root inoculation, roots from eight plants per treatment were sampled at 24 hpi and placed on PARPH medium to confirm typical morphology of P. cinnamomi hyphae. Whole plant images were captured using a digital camera 7 days after inoculation.
RNA extraction and reverse transcription quantitative PCR (RT qPCR)
At 0, 24, and 48 hpi, roots from each treatment group were harvested, flash-frozen in liquid nitrogen, and stored at −80°C. The frozen root material was ground into a fine powder using a mortar and pestle, and total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, CA, USA) following the manufacturer’s protocol. The extracted RNA was qualitatively visualised by agarose gel electrophoresis and the total RNA was quantified (NanoDrop 1000 spectrophotometer, Thermo Fisher Scientific, United States), and the RNA purity was confirmed by determining the absorbance ratio of A260/280 and A260/230. cDNA was synthesised using QuantiTect Reverse Transcription Kit (Qiagen, Australia).
RT qPCR was performed to analyse the expression of genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), sterol methyltransferase 1a and 1b (SMT1a and SMT1b), cytochrome P450, family 710 (CYP710A), and phospholipid acyltransferase (PSAT). The responsive to elicitin (REL) gene was selected as an elicitin receptor, with SOBIR as its co-receptor, to evaluate their transcript levels. Transcript levels of the F-box and protein phosphatase 2A (PP2A) were used as reference genes due to their consistent expression across time points in transcriptome data.
Previously published primers for HMGCR, designed by Atsumi et al. (2018), and for F-box and PP2A, designed by Liu et al. (2012), were utilised in this study. For the remaining targets, primers were designed using the PrimerBLAST tool, following the parameters described in Kharel et al. (2024a). Primers were designed to achieve a product amplification range of 70–200 bp, with a GC content ranging between 40 and 60%, and a length of 18–25 nucleotides. The primer melting temperatures were 60 ± 1°C. The specificity and efficiency of each primer pair were tested separately and the primer sequence is in Table S1. Due to the incomplete genome annotation of N. benthamiana, we retrieved sequences for individual genes for A. thaliana and used the Sol Genomics Network (https://solgenomics.net/) and the Nicotiana benthamiana Genome and Transcription Sequencing Consortium (https://sefapps02.qut.edu.au/) to find homologous genes in N. benthamiana. The A. thaliana sequence for AtCYP710A1 was used as a reference to identify Nb5.1tr6219645, which was determined to be a probable cytochrome P450 710A1. For sequences corresponding to AtCYP710A2, AtCYP710A3, and AtCYP710A4, Sol Genomics provided the same homologous sequence, Nb5.1tr6219645, in N. benthamiana. Similarly, the A. thaliana sequence for PSAT At1G04010 was used as a reference to identify Nb5.1tr6226238. Sequences for Nb5.1tr6219645 and Nb5.1tr6226238 were verified using BLAST analysis against the N. benthamiana genome. Additionally, PCR product size confirmation was performed to ensure amplification specificity. Sequences for Smt1a and Smt1b were obtained from Atsumi et al. (2018), while sequences for REL and SOBIR were sourced from Chen et al. (2023) and Huang et al. (2021), respectively.
Experimental design for gene expression analysis was carried out as described by Kharel et al. (2024a), in accordance with the minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines (Taylor et al. 2019).
Data analysis
Data were analysed using GraphPad Prism software (ver. 8.0.0). Statistical analyses were performed, and the significance of differences were determined using Tukey’s multiple comparison test.
Visualisation of pathogen colonisation through microscopy
Root tissue was examined on each side of the lesion front in infected tissue, and at the inoculation site in mock-inoculated roots. To visualise P. cinnamomi within infected tissues, whole roots were stained with 0.5% toluidine blue in citrate buffer solution (pH 4.0) for 10 min (Cahill et al. 1989), then washed and mounted in water. Toluidine blue was used due to its ability to differentiate plant and Phytophthora structures based on their chemical composition (Phillips et al. 1987). Slides were examined using a light microscope (Axioscope, Zeiss Pty Ltd, Göttingen, Germany), and images were captured using a digital camera attached to the microscope.
For the phosphite treatment study, root samples at 0, 24, and 48 hpi were collected and preserved in 10% neutral buffered formalin. The samples were then paraffin-embedded, and transverse sections of 10 μm thickness were prepared. The sections were stained with toluidine blue and visualised using a light microscope. Root processing was conducted by the Monash Histology Platform (Monash University, Victoria, Australia).
Results
Establishment of a soil-free plant growth system for investigating N. benthamiana root interactions with P. cinnamomi
Seeds of all ecotypes of N. benthamiana (LAB, SA, NT, NWA, and WA) were successfully grown in a sand-based system and maintained in SPS units (Fig. S1). Plants in the SPS developed roots that were white, clean, and lesion-free, exhibiting continuous growth throughout the experiment, as observed in the mock-inoculated roots (Fig. 1a). Microscopic examination of mock-inoculated roots showed uniformly blue-stained cells without dark-stained regions (figure not included).
Visualisation of the interaction of different ecotypes of the model host plant Nicotiana benthamiana grown in a soil-free plant growth system (SPS), with the root pathogen, Phythophthora cinnamomi. Since all ecotypes exhibited similar results, the images presented are of the LAB ecotype and are representative of three biological replicates. The primary root (Pr) of different N. benthamiana ecotypes was mock- or pathogen-inoculated with an agar plug placed at the elongation zone of the root, and images were captured 7 days post-inoculation (dpi). (a) Roots of mock-inoculated LAB ecotype, with the root tip (Rt) and lateral roots (Lr) labelled. (b) Visible lesion development (brown colouration) in a primary root of N. benthamiana following inoculation with P. cinnamomi. The region between the white arrows indicates the extent of the visible lesioned area. (c–f) Following inoculation, roots were harvested, stained with toluidine blue, and placed longitudinally on microscope slides for examination, with images taken from the elongation zone near the root tip. (c) At 1 dpi, a visible point of penetration (Pp) was observed along the plant cell wall (Cw) as P. cinnamomi hyphae (Hy) grew along the root cell surface. (d) At 3 dpi, both intracellular hyphae (In. Hy) within plant cells, and intercellular hyphae (It. Hy) between host cells were visible. (e) By 5 dpi, extensive spread of hyphae throughout the root was observed, with hyphal swelling (Hs) present. (f) At 7 dpi, chlamydospores (Ch) and hyphal swellings were present within the root cells (Scale = 10 μm).
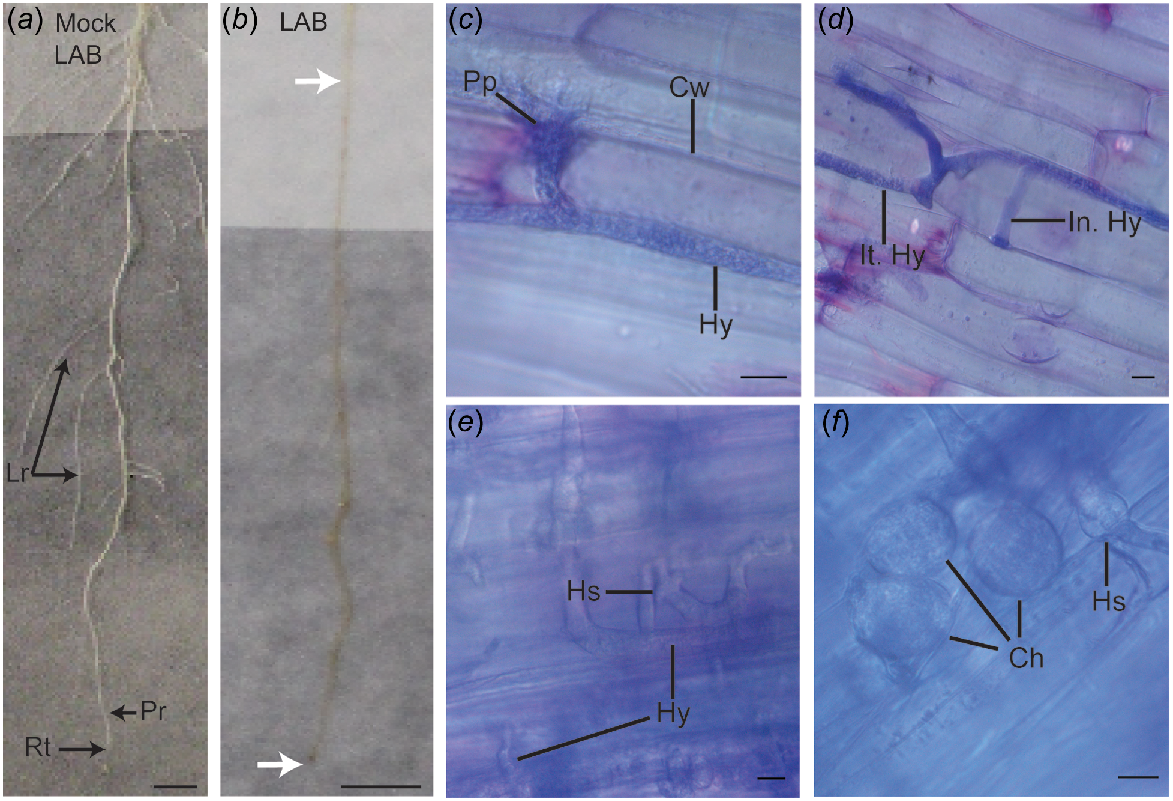
All pathogen-inoculated roots developed visible brown lesions at the inoculation site by 1 dpi. By 3 dpi, lesions extended beyond the initial inoculation site and spread to the lateral roots by 5 dpi, with progression monitored up to 7 dpi (Fig. 1b). Since all ecotypes exhibited similar results, the images presented are of the LAB ecotype and are representative of three biological replicates. The interaction between N. benthamiana and P. cinnamomi was examined microscopically using a light microscope. At 1 dpi, the pathogen had penetrated the root epidermal layer at the elongation zone (Fig. 1c), with the point of penetration staining darker, likely due to the accumulation of plant phenolics, similar to the observations made by Ruiz Gómez et al. (2015). Microscopic images revealed the typical coenocytic hyphae, characteristic of Phytophthora species. At 3 dpi, hyphae had penetrated into the root cortex, and intracellular hyphae were clearly visible (Fig. 1d). At 5 dpi, extensive branching and spread of the pathogen within the plant cells were observed, along with hyphal swellings (Fig. 1e). Plant cells also stained dark blue, indicating the accumulation of tannins and polyphenols (O’Brien et al. 1964). As shown in Fig. 1f, a cluster of chlamydospores with intact cytoplasmic content was visible in the root cortex at 7 dpi.
Following inoculation, the percentage of total root length that was visibly lesioned for different ecotypes was quantified (Fig. S2a). By Day 7, nearly 50% of the root length was lesioned in all ecotypes although, due to the fine structure of N. benthamiana roots, this percentage may be an underestimate of the total lesion length. To address this, observations were made of where P. cinnamomi emerged when roots were plated onto PARPH selective medium (Fig. S2b). This method revealed that pathogen colonisation exceeded visible lesion length. By 7 dpi, all ecotypes showed over 60% of root colonization by the pathogen.
At 7 dpi, fresh root (Fig. S2c) and dry root (Fig. S2d) biomass showed a significant reduction in inoculated plants compared to non-inoculated controls within each ecotype. Due to inherent growth differences among ecotypes, direct comparisons between the ecotypes were not made. Control plants consistently exhibited higher biomass than those that were inoculated. Infection of roots by P. cinnamomi halted further growth and significantly reduced biomass across all ecotypes.
To confirm that the necrotic lesions were caused by P. cinnamomi and to validate the results, infected roots were placed onto PARPH selective medium. The pathogen was successfully isolated and identified as P. cinnamomi using nested PCR (Fig. S3). P. cinnamomi was not isolated from the mock-inoculated plants.
Phosphite application enhanced resistance of N. benthamiana to P. cinnamomi
The LAB ecotype was used for the phosphite treatment experiments, with plants grown in a sand-based system and inoculated with a zoospore suspension of P. cinnamomi. Microscopic examination of N. benthamiana roots revealed successful colonisation of the host tissue and establishment of infection following inoculation with P. cinnamomi zoospores (Fig. S4).
To monitor the response of N. benthamiana to P. cinnamomi infection, with or without phosphite treatment, both visual and microscopic observations were conducted. Control plants sprayed with surfactant-only and mock-inoculated with water displayed white, lesion-free roots and undamaged leaves (Fig. S5a), similar to those treated with a surfactant-phosphite mixture and mock-inoculated with water (Fig. S5b). In contrast, plants sprayed with surfactant-only and inoculated with P. cinnamomi zoospores developed necrotic root and stem lesions, along with wilted leaves (Fig. S5c). However, plants treated with a surfactant-phosphite mixture and inoculated with P. cinnamomi zoospores showed minimal lesion development, and their leaves remained healthy (Fig. S5d). Phosphite treatment significantly reduced lesion length and root colonisation (Fig. S5e, f), though P. cinnamomi was still isolated from all infected plants. Responses of N. benthamiana to P. cinnamomi zoospore inoculation, with or without foliar phosphite treatment, are presented in Table 1.
| Whole roots (at 7 days post inoculation, dpi) | Control A | Phosphite-treated A | P. cinnamomi infected A | Phosphite-treated and P. cinnamomi infected A | |
|---|---|---|---|---|---|
| Root condition | White and undamaged roots | White and undamaged roots | Extensive necrosis | Limited necrotic areas | |
| % of visible root lesion B | NA | NA | 43.3 ± 9.2 | 2.3 ± 0.1 | |
| % of root colonisation C | NA | NA | 65.2 ± 5.7 | 3.0 ± 1.2 | |
| Plant dry weight (mg) | 78.2 ± 10.6 | 79.3 ± 6.6 | 42.2 ± 5.1 | 74.0 ± 9.3 | |
| Symptoms on stems | Green and non-necrotic stem | Green and non-necrotic stem | Brown and necrotic lesions spread to the stem | Green and non-necrotic stem | |
| Chlorophyll content (SPAD units) | 30.5 ± 2.1 | 31.1 ± 4.2 | 20.8 ± 3.9 | 30.6 ± 5.8 | |
| Chlorosis and wilting A | No chlorosis and no wilting | No chlorosis and no wilting | Severe chlorosis and pronounced wilting | Minimal to no chlorosis, and no wilting | |
| Root thin sections (microscopic observations) | |||||
| 1 dpi | No pathogen | No pathogen | Hyphae in the epidermal layer | Hyphae not visible in the epidermal layer | |
| 5 dpi | No pathogen | No pathogen | Intra- and intercellular hyphae along the cortex | Hyphae in the epidermal layer | |
| 7 dpi | No pathogen | No pathogen | Chlamydospore present, hyphae in the endodermal layer and vascular tissue | No chlamydospores and hyphae restricted to cortex | |
| Intact cells and no lignification | Intact cells and no lignification | Cell integrity disrupted, lignification at the site of pathogen penetration | Intact cells and no lignification | ||
Thin transverse root sections were collected and visualised with toluidine blue stain under bright-field microscopy to monitor disease progression. As shown in Fig. 2a, e, control root sections treated with surfactant and mock-inoculated with water displayed intact cell walls and well-defined cellular structures, with no signs of damage. Similar cellular structures were observed in phosphite-treated plants (Fig. 2b, f). In P. cinnamomi infected roots, hyphae were observed along the epidermal layer at 1 dpi (Fig. 2c). By 5 dpi, the hyphae had advanced along the cortex and were visible in cells near the endodermis, bypassing the lignified cell walls (Fig. 2g). As the pathogen progressed towards the vascular tissue, formation of phenolic compounds was evident by the excessive blue staining within the cells. Intracellular and intercellular hyphae were present in the cortex and vascular tissues at 7 dpi (Fig. S6a, b), and chlamydospores were visible within the root cells (Fig. S6c), confirming the susceptibility of N. benthamiana to P. cinnamomi.
Visualisation of thin root sections of Nicotiana benthamiana stained with toluidine blue, showcasing the impact of foliar phosphite treatment on the altered invasion of Phytophthora cinnamomi upon zoospore inoculation. Plants were foliar sprayed with 0.1% surfactant 48 h prior to mock (control) or P. cinnamomi inoculation (P. cinn). For phosphite-treated plants, a mixture of phosphite and surfactant was applied as a foliar spray 48 h before mock (Phos) or P. cinnamomi zoospore inoculation (Phos + P. cinn). (a–d) Samples collected at 1 day post inoculation (dpi), and (e–h) at 5 dpi. (a) and (e) Control plants sprayed only with surfactant and mock-inoculated with water, showing clear distinction of plant cell types including epidermis (Ep), cortex (cr), endodermis (Ed), protoxylem (Px) and xylem (Xy). Few of the nuclei (Nu) visible in the plant cells within each section are labeled. (b) and (f) Plants foliar sprayed with phosphite and mock-inoculated displayed similar characteristics when compared to the control plants. (c) Roots infected with P. cinnamomi zoospores at 1 dpi showed darkly stained P. cinnamomi hyphae (Hy) along the epidermis. (g) Infected roots at 5 dpi with lignified cell walls (Lcw). P. cinnamomi hyphae had advanced to the cortex. (d) Plants foliar sprayed with phosphite and roots infected with P. cinnamomi showed no clear invasion of the pathogen at 1 dpi. (h) At 5 dpi, P. cinnamomi was localised along the middle lamella through cell walls in the cortex, near the epidermis (Scale = 10 μm).
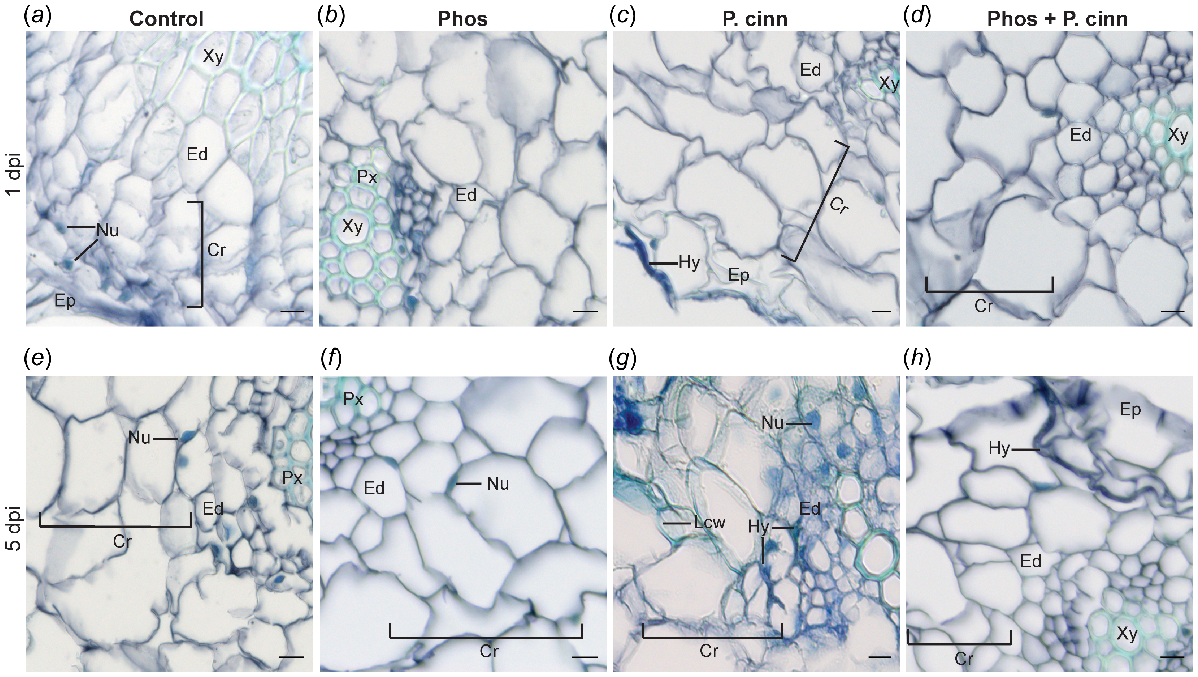
In contrast, in pathogen-inoculated and phosphite-sprayed N. benthamiana, the root sections did not contain visible hyphae at 1 dpi (Fig. 2d). While hyphae may have been present, they were not visible in the sections visualised under the conditions used. By 5 dpi, P. cinnamomi hyphae were present but restricted to the epidermal layer (Fig. 2h). The root cells remained intact and comparable to the control. Root sections visualised at 7 dpi showed the pathogen was confined to the cortex (image not shown) and there was no evidence of cell damage.
Impact of phosphite treatment and P. cinnamomi infection on elicitin receptor and sterol-related gene expression of N. benthamiana
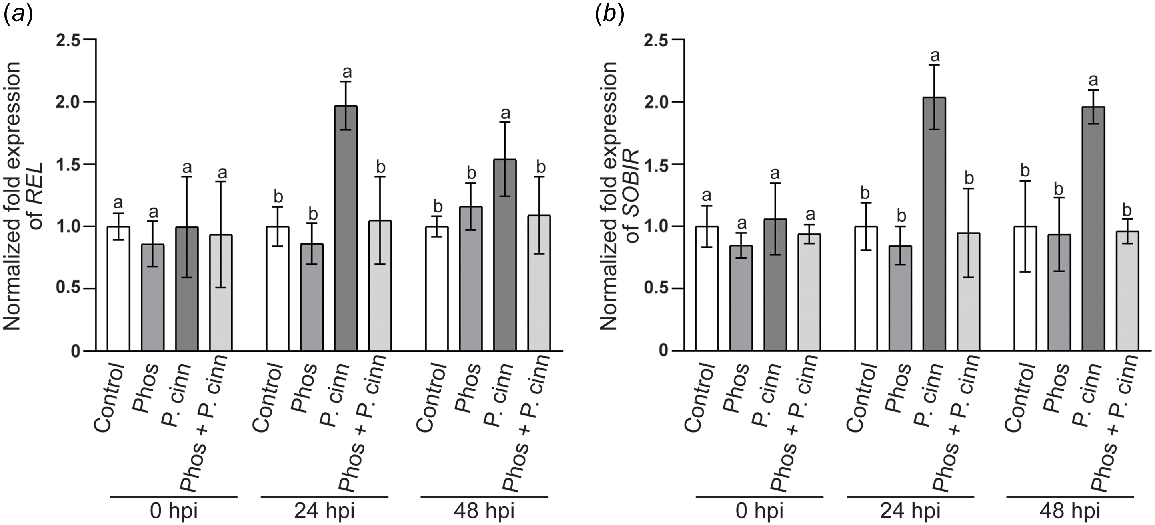
For RT qPCR analyses, samples were collected at 0, 24, and 48 hpi. Similar to the microscopy study, a comparison was made between P. cinnamomi inoculated and uninoculated plants, with or without phosphite. Transcript levels of two key modulators in the interaction with the apoplastic elicitins of P. cinnamoni were monitored: (1) the elicitin receptor, REL (Fig. 3a); and (2) its co-receptor, SOBIR (Fig. 3b). Transcript levels at 0 hpi did not differ significantly between treatments, confirming that the basal expression levels of these genes were comparable across samples. Additionally, phosphite application alone did not affect the expression of either gene at 24 or 48 hpi. In contrast, inoculation with P. cinnamomi resulted in a marked increase in the transcript levels of both genes, with a near two-fold increase observed at 24 hpi. At 48 hpi, transcript levels remained significantly higher than the control. However, in phosphite-treated N. benthamiana plants inoculated with P. cinnamomi, the transcript levels of REL and SOBIR remained comparable to control values at 24 hpi and 48 hpi.
Gene expression analysis of Nicotiana benthamiana elicitin receptor, (a) responsive to elicitin (REL) and its co-receptor, (b) SOBIR, at various time points following phosphite treatment, with or without Phythophthora cinnamomi infection. Plants were foliar sprayed with 0.1% surfactant 48 h prior to mock (control) or P. cinnamomi inoculation (P. cinn). For phosphite-treated plants, a phosphite-surfactant mixture was foliar applied 48 h prior to mock (Phos) or P. cinnamomi zoospore inoculation (Phos + P. cinn). Following inoculation, roots were collected at 0, 24 and 48 h post inoculation (hpi). Transcript abundance was standardised by comparison with two housekeeping genes and normalised to expression levels of surfactant sprayed plants (control). Values represent mean with standard deviation of three independent repeats, and different letters above the bar indicates significant differences (P < 0.05, one-way ANOVA, Tukey multiple comparison).
Additionally, the transcript levels of key phytosterol biosynthesis and homeostasis genes were monitored. HMGCR (Fig. 4a), Smt1a (Fig. 4b), Smt1b (Fig. 4c), and PSAT (Fig. 4e) showed no significant differences between control and phosphite-sprayed N. benthamiana in the absence of P. cinnamomi at all time points. Notably, the transcript levels of CYP710A (Fig. 4d) were slightly reduced compared to the control plants upon phosphite treatment.
Gene expression analysis of Nicotiana benthamiana key sterol-related genes, (a) 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (b) Sterol methyltransferase 1a (Smt1a) (c) Smt1b (d) Cytochrome P450, family 710 (CYP710A) (e) Phospholipid sterol acyltransferase (PSAT), at various time points following phosphite treatment, with or without Phytophthora cinnamomi infection. Plants were foliar sprayed with 0.1% surfactant 48 h prior to mock (control) or P. cinnamomi inoculation (P. cinn). For phosphite-treated plants, a phosphite-surfactant mixture was foliar applied 48 h prior to mock (Phos) or P. cinnamomi zoospore inoculation (Phos + P. cinn). Following inoculation, roots were collected at 0, 24, and 48 h post inoculation (hpi). Transcript abundance was standardised by comparison with two housekeeping genes and normalised to expression levels of surfactant sprayed plants (control). Values represent mean with standard deviation of three independent repeats, and different letters above the bar indicates significant differences (P < 0.05, one-way ANOVA, Tukey multiple comparison).

We also investigated the influence of P. cinnamomi infection on the transcript levels of these genes, in the absence of phosphite. Immediately after inoculation (at 0 hpi), transcript levels were comparable to the control, indicating similar baseline expression levels. At 24 hpi, the expression of HMGCR, Smt1a, CYP710A, and PSAT were upregulated in response to the infection, while Smt1b was downregulated. At 48 hpi, HMGCR expression peaked with a near 2-fold increase, and CYP710A expression increased by approximately 1.5-fold. In contrast, Smt1a expression decreased slightly compared to its value at 24 hpi, and PSAT returned to control levels by 48 hpi. Smt1b remained downregulated at 48 hpi.
In phosphite-treated plants inoculated with P. cinnamomi, HMGCR and Smt1a were significantly upregulated at 24 and 48 hpi, though to a lesser extent than in plants infected without phosphite. PSAT was transiently upregulated, and Smt1b was transiently downregulated at 24 hpi, with both returning to control levels by 48 hpi. CYP710A expression remained lower than the control but comparable to phosphite-only treated plants.
Discussion
This study establishes N. benthamiana as a useful model for examining the interactions of a higher plant species with P. cinnamomi. Our analysis of various N. benthamiana ecotypes consistently revealed susceptibility to P. cinnamomi, demonstrating the widespread vulnerability of this commonly distributed Australian plant species. Interestingly, the phylogenetic and population genetic analyses conducted by Chase et al. (2022) suggest that these ‘ecotypes’ or ‘accessions’ may in fact represent distinct species. If this reclassification is widely accepted, it could significantly increase the number of host species susceptible to P. cinnamomi, a concerning development given the pathogen’s destructive impact on global plant populations.
In our experiments, in the absence of phosphite treatment of host plants, P. cinnamomi rapidly invaded root cells, leading to necrotic lesion formation and extensive pathogen spread. Investigation into the sterol-related pathways of N. benthamiana following infection by P. cinnamomi revealed a significant upregulation of REL and SOBIR, genes linked to elicitin receptors. Additionally, key sterol biosynthesis genes, including CYP710A, which encodes for C22-sterol desaturase and is known to correlate with increased pathogen susceptibility (Valitova et al. 2024), were upregulated. However, phosphite treatment significantly reduced pathogen spread and disease progression. Despite P. cinnamomi infection, phosphite treatment of N. benthamiana hosts maintained relatively low levels of sterol-related gene expression. This stabilisation of gene expression and the consequent limitation in sterol availability may slow the pathogen spread (Kharel et al. 2024a) and provide the host additional time to activate defence mechanisms (Pérez-Zavala et al. 2024), potentially providing a strategic advantage in managing the infection.
With limited research on belowground plant–pathogen interactions (Rasmann and Agrawal 2008; De Coninck et al. 2015), this study lays an essential foundation for investigating root and soil-borne pathogens. While leaf inoculation studies with P. cinnamomi have yielded valuable insights (Rookes et al. 2008; Eshraghi et al. 2011), these models do not fully replicate natural root infections. Our system, building on previous advancements in soil-free growth for Tasmannia lanceolata (Sinhalagoda et al. 2023), Zea mays (Allardyce et al. 2012), and Lupinus angustifolius (Gunning and Cahill 2009) enables direct root inoculation, closely mimicking natural infection conditions. The presence of P. cinnamomi’s haustoria-like structures within N. benthamiana host cells indicated the biotrophic phase of the pathogen (Crone et al. 2013; Redondo et al. 2015), which transitioned to a necrotrophic phase marked by necrotic lesion formation and extensive host tissue damage due to saprophytic growth (Andronis et al. 2022). There have been few studies of ecotypic response of plant species to P. cinnamomi, and some have reported differences in response among populations (Robinson and Cahill 2003; Rodríguez-Romero et al. 2022). Notably, all N. benthamiana ecotypes tested, despite their morphological and physiological diversity (Bally et al. 2018), showed uniform susceptibility to P. cinnamomi. The microscopic observations between P. cinnamomi and the host plant are similar to those seen in other susceptible species, such as L. angustifolius (Islam et al. 2017) and Quercus ilex (Ruiz Gómez et al. 2015). The pathogen’s broad array of effectors, including elicitins, crinkling and necrosis (CRN) proteins, avirulence (Avr) proteins, and necrosis-inducing Phytophthora proteins (NPPs), likely contributes to its wide host range (Hardham and Blackman 2018; Evangelisti and Govers 2024).
Phosphite supplementation, known to enhance root growth and plant health (Mohammed et al. 2022), showed no phytotoxic effects on N. benthamiana plants at the concentration used. RT qPCR analysis revealed no significant differences in sterol-related genes between the control (surfactant only) and phosphite-treated plants, except for CYP710A, which was downregulated in phosphite-treated plants. These results align with previous studies by Machinandiarena et al. (2012) and Burra et al. (2014), which examined defense-related genes and found that transcript levels typically return to basal levels shortly after phosphite application, indicating that the treatment induces a rapid but transient effect on the plant transcriptome. Furthermore, Pérez-Zavala et al. (2024) reported that the effects of phosphite are tightly regulated, preventing unnecessary energy expenditure and growth penalties that could impact plant fitness. However, shifts in the plant’s metabolite profile after phosphite application, caused by those early transcriptional changes, as observed by Gunning et al. (2013), Burra et al. (2014) and Andronis et al. (2024), may play a critical role in priming the plant for resistance, enhancing its ability to suppress pathogen proliferation during subsequent infections.
Our study confirms the effectiveness of phosphite against the P. cinnamomi isolate used in this study. Phosphite application upregulates the expression levels of those genes that are involved in jasmonic acid and salicylic acid biosynthesis (Pérez-Zavala et al. 2024), thereby enhancing plant immunity (Liu et al. 2016) and reducing pathogen colonisation, as demonstrated in Ananus comosus (pineapple) (Anderson et al. 2012), Eucalyptus marginata, and Banksia grandis (Shearer and Fairman 2007). A recent proteomics study by Andronis et al. (2024) has shown that phosphite impacts pathways beyond defence, including photosynthesis, carbon fixation, and lipid metabolism, opening up avenues to explore how pathogen attack influences sterol biosynthesis and the role of phosphite in this process. The interactions of key sterol-related gene responses to P. cinnamomi infection in the roots of untreated and phosphite-treated N. benthamiana are shown schematically in Fig. 5. A significant upregulation of key sterol-related genes in response to P. cinnamomi infection was found in the host plant, but a distinct difference in response was observed in phosphite-treated plants.
Overview of gene expression changes in elicitin receptors and key sterol-related genes in Nicotiana benthamiana during interaction with P. cinnamomi, in the presence or absence of phosphite. Phythophthora cinnamomi cysts germinate on the root cell surface and penetrate the plant, secreting cell wall degrading enzymes (CWDEs) and apoplastic effectors, including cell wall-associated elicitins (Evangelisti and Govers 2024). Elicitins such as β-cinnamomin, containing microbe-associated molecular patterns (MAMPs), are secreted during early infection into the apoplast (Islam et al. 2019) and interact with the plant receptor-like protein (RLP), responsive to elicitins (REL), and its co-receptor, SOBIR, to initiate immune signalling (Derevnina et al. 2016). Elicitins also assist in sterol recruitment for the sterol-auxotrophic Phytophthora species, though their roles in sterol binding and receptor activation are independent (Dokládal et al. 2012). As the germ tube extends, cytoplasmic content flows into the growing hyphae, forming a plug to prevent backflow. Post-penetration, P. cinnamomi hyphae grow intra- or intercellularly, secreting effectors into the plant cytoplasm. The sterol biosynthesis pathway, from acetyl-CoA to phytosterols, which undergoes alterations during pathogen attack, is summarised above. The impact of phosphite treatment on this pathway in pathogen-inoculated plants is also shown. Multistep pathways are indicated with dotted black arrows while solid black arrows represent direct pathways. Gene expression changes relative to the control (surfactant only) are colour-coded: blue for below the normalised value of 1 and red for above. qPCR analysis was performed at 0, 24, and 48 h post-inoculation (hpi) with P. cinnamomi zoospores. (Not to scale).
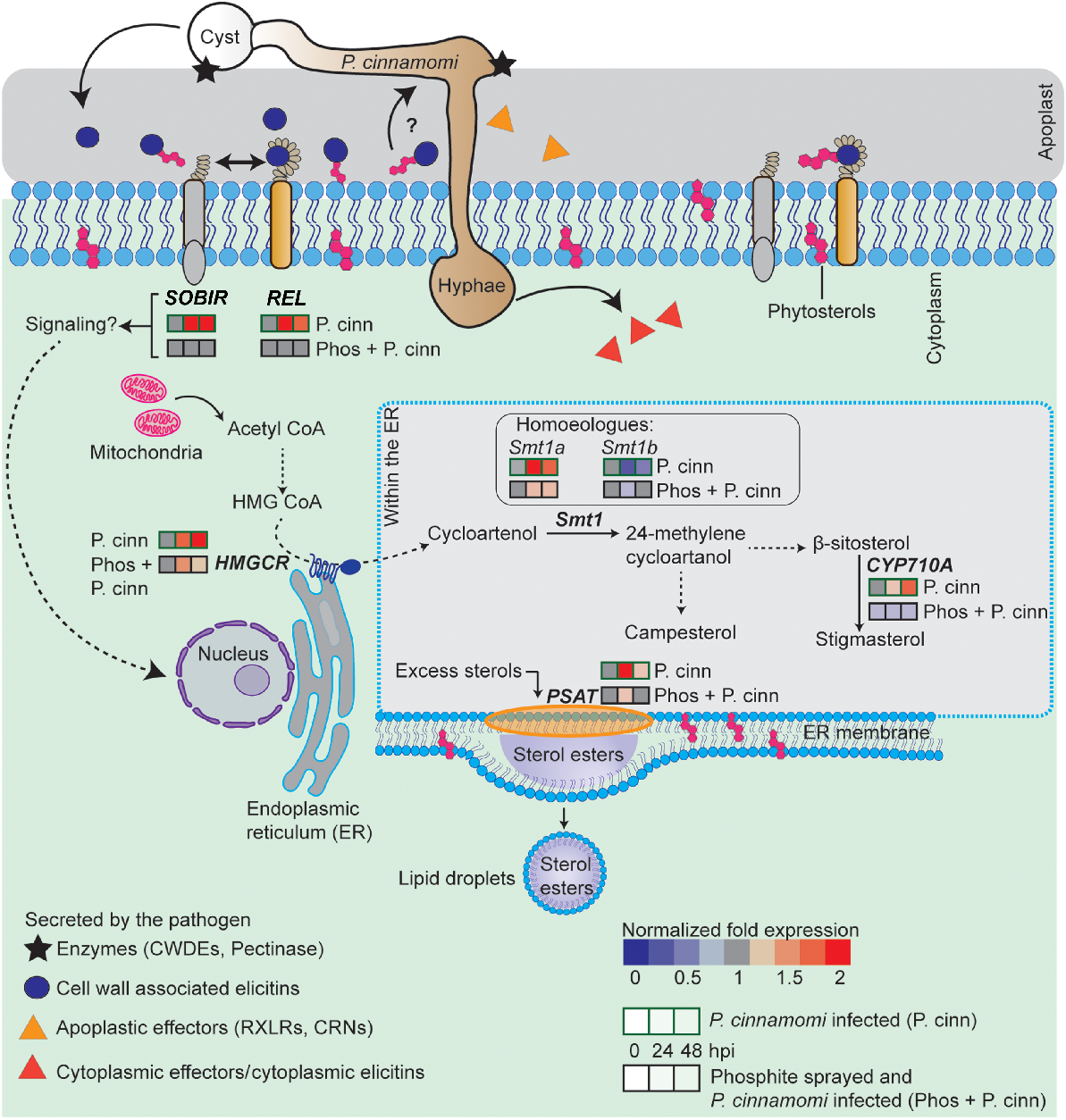
Our study provides new insights into the role of immune receptors ELR and SOBIR in response to P. cinnamomi infection. We observed constitutive expression of both receptors, which can be explained by the structural characteristics of RLPs, such as REL, that lack intracellular signalling domains but interact with the receptor-like kinase SOBIR (Domazakis et al. 2018). Both REL and SOBIR are key modulators of PTI in Phytophthora-mediated plant responses (Derevnina et al. 2016). The upregulation of these receptor genes at the time points observed is likely driven by elevated elicitin concentrations during early infection stages, as demonstrated by Horta et al. (2008) and Islam et al. (2019). Direct infiltration of elicitins into plant leaves has been shown to trigger protein-protein interactions between elicitins and these receptors, inducing a HR and localized cell death (Du et al. 2015; Chen et al. 2023). However, in our whole plant–pathogen interaction study, despite the upregulation of these receptors, the plants remained susceptible. This suggests the complexity of plant-pathogen interactions, involving multiple defence layers. Gaining a deeper understanding of these layers provides valuable insights that could contribute to developing more resilient plant defence strategies against Phytophthora infection.
Expression of ELR and SOBIR peaked at 24 hpi but declined by 48 hpi, indicating pathogen-induced changes in receptor interaction. In contrast, phosphite treatment did not affect REL and SOBIR expression, indicating that phosphite may not directly impact these receptors. An in vitro study on Phytophthora conducted by Perez et al. (1995) reported increased elicitin production with phosphite, but there are limited follow-up studies to support this. Overall, our study highlights the importance of exploring plant immune receptors to enhance our understanding of pathogenesis. Recent advancements in the field by Witek et al. (2016) and Lin et al. (2022) further showcases the potential of using specific immune receptors that function as resistance (R) genes to enhance crop plant resistance against a range of Phytophthora diseases.
Shifting focus from receptor dynamics, our study also examined the impact of P. cinnamomi infection and phosphite treatment on sterol biosynthesis gene expression in plants. Our observation of HMGCR upregulation, the key gene of the MVA pathway, in N. benthamiana is consistent with findings from tobacco cell suspensions treated with Phytophthora elicitors (Chappell et al. 1991), suggesting that biotic stress may enhance HMGCR activity. The MVA pathway leads to the formation of squalene (Busquets et al. 2008), which is cyclized into cycloartenol, the first cyclic sterol precursor, and further converted into various phytosterols (Nes 2011). In addition to HMGCR, we observed the upregulation of Smt1a following infection with P. cinnamomi, which catalyses the conversion of cycloartenol to Δ5 C24-alkyl sterols.
The coordination between elevated HMGCR and Smt1a expression suggests a tightly regulated sterol biosynthesis pathway (Kim et al. 2014; Liao et al. 2020). Although we did not measure sterol content directly, these gene expression changes align with reported increases in sterol levels following similar HMGCR and Smt1a activity (Harker et al. 2003; Enfissi et al. 2005; Atsumi et al. 2018). Our study also reports that when there is an upregulation of Smt1a, the Smt1b is downregulated, supporting the study by Atsumi et al. (2018) who reported antagonistic regulation of these homoeologous genes. Excess sterols can disrupt cellular functions by inducing stress-related gene expression (Burciaga-Monge et al. 2022), necessitating tight regulation of sterol levels to prevent adverse effects on cell viability and plant performance. PSAT is involved in the formation of sterol esters, which accumulate as lipid droplets (Ferrer et al. 2017), and serve as a non-toxic storage form of sterols. Thus, a rise in free sterols possibly triggers allosteric regulation of PSAT by sterol end products (Kopischke et al. 2013), allowing PSAT to act as a sensor for free sterol levels in the membrane (Bouvier-Naveݩ et al. 2010), thereby playing a vital role in maintaining free sterol homeostasis.
Our study found that P. cinnamomi infection increased CYP710A expression, peaking at 48 hpi. This observation is consistent with studies by Fabro et al. (2008) and Griebel and Zeier (2010), which found elevated CYP710A expression in Arabidopsis infected by Pseudomonas syringae and Golovinomyces cichoracearum. Increased stigmasterol accumulation was associated with a higher stigmasterol:β-sitosterol ratio (Griebel and Zeier 2010), which enhanced pathogen multiplication and plant susceptibility, possibly due to alteration of the plant membrane sterol composition (Valitova et al. 2024). While it is unclear whether the rise in CYP710A expression results from an upregulation of sterol biosynthesis genes or the pathogen’s strategy to weaken plant defence, our findings confirm that P. cinnamomi infection upregulates CYP710A expression. In contrast, phosphite-treated plants showed downregulation of CYP710A expression, suggesting reduced enzyme activity. This downregulation indicates that phosphite treatment mitigates stigmasterol overaccumulation, which may contribute to reduced pathogen susceptibility of N. benthamiana in our case, however, the mechanism by which phosphite achieves this effect remains unknown.
In phosphite-treated plants inoculated with P. cinnamomi, upregulation of HMGCR, Smt1a and PSAT was observed, but the values remained largely below those observed in infected but untreated plants. There are two possible explanations for these observations. The first explanation is that phosphite application may act independently of the sterol pathway, preventing drastic fluctuations, keeping the plant in a primed state by enhancing the expression of defense-related genes (Machinandiarena et al. 2012). Such responses might include increased production of hydrogen peroxide, enhanced callose deposition, and the accumulation of salicylic acid and pathogenesis-related transcripts (Eshraghi et al. 2011; Massoud et al. 2012). This steady-state maintenance of the sterol biosynthesis pathway could be critical for disease resistance, enabling the plant to quickly activate defence mechanisms without being overwhelmed by pathogen-induced stress (Pérez-Zavala et al. 2024). The second explanation is that the reduced pathogen spread may be due to phosphite-induced defence responses, or the direct effects of phosphite on the pathogen, such as hyphal lysis (King et al. 2010), might account for the lack of significant changes in sterol-related pathways.
Thus, our study provides new insights into the impact of P. cinnamomi infection on sterol biosynthesis genes in N. benthamiana and has examined how phosphite modulates these responses. Our findings demonstrate that sterol biosynthesis, a crucial and ubiquitous process integral to plant immune responses, is significantly influenced by both infection and phosphite treatment. This research opens new avenues for exploring the role of sterols in pathogen interactions and stress responses. Additionally, the observation that phosphite reduced pathogen spread and prevented significant changes in sterol-related genes in planta underscores its potential as a strategic tool to enhance plant resilience, immunity, and growth, thereby enriching our understanding of phosphite’s effects on plant physiology.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
We thank Deakin University for providing a postgraduate research scholarship to AK. The work was supported by funds obtained from 2024 LES Blue Sky Fund, Deakin University and from the Centre for Sustainable Bioproducts, Deakin University.
Author contributions
All authors contributed to the conceptualisation and design of the study. AK conducted the experiments, data collection and performed the analyses. The initial draft of the manuscript was prepared by AK and extensively reviewed by DC, JR and MZ. All authors have read, provided valuable comments, and approved the manuscript.
Acknowledgements
We thank Professor Peter Waterhouse of Queensland University of Technology, Australia, for providing the different ecotypes of Nicotiana benthamiana under the material transfer agreement. We are grateful to Tejaswini Patlolla for assistance in the growth of N. benthamiana.
References
Aberton MJ, Wilson BA, Cahill DM (1999) The use of potassium phosphonate to control Phytophthora cinnamomi in native vegetation at Anglesea, Victoria. Australasian Plant Pathology 28, 225-234.
| Crossref | Google Scholar |
Adachi H, Nakano T, Miyagawa N, Ishihama N, Yoshioka M, Katou Y, Yaeno T, Shirasu K, Yoshioka H (2015) WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. The Plant Cell 27(9), 2645-2663.
| Crossref | Google Scholar | PubMed |
Akinsanmi O, Neal J, Drenth A, Topp B (2017) Characterization of accessions and species of Macadamia to stem infection by Phytophthora cinnamomi. Plant Pathology 66(2), 186-193.
| Crossref | Google Scholar |
Allardyce JA, Rookes JE, Cahill DM (2012) Defining plant resistance to Phytophthora cinnamomi: a standardized approach to assessment. Journal of Phytopathology 160(6), 269-276.
| Crossref | Google Scholar |
Anderson JM, Pegg KG, Scott C, Drenth A (2012) Phosphonate applied as a pre-plant dip controls Phytophthora cinnamomi root and heart rot in susceptible pineapple hybrids. Australasian Plant Pathology 41, 59-68.
| Crossref | Google Scholar |
Andronis CE, Jacques S, Lipscombe R, Tan K-C (2022) Comparative sub-cellular proteome analyses reveals metabolic differentiation and production of effector-like molecules in the dieback phytopathogen Phytophthora cinnamomi. Journal of Proteomics 269, 104725.
| Crossref | Google Scholar | PubMed |
Andronis CE, Jacques S, Lopez-Ruiz FJ, Lipscombe R, Tan K-C (2024) Proteomic analysis revealed that the oomyceticide phosphite exhibits multi-modal action in an oomycete pathosystem. Journal of Proteomics 301, 105181.
| Crossref | Google Scholar | PubMed |
Atsumi G, Kagaya U, Tabayashi N, Matsumura T (2018) Analysis of the mechanisms regulating the expression of isoprenoid biosynthesis genes in hydroponically-grown Nicotiana benthamiana plants using virus-induced gene silencing. Scientific Reports 8(1), 14804.
| Crossref | Google Scholar | PubMed |
Bally J, Jung H, Mortimer C, Naim F, Philips JG, Hellens R, Bombarely A, Goodin MM, Waterhouse PM (2018) The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annual Review of Phytopathology 56(1), 405-426.
| Crossref | Google Scholar |
Balmer D, Mauch-Mani B (2013) More beneath the surface? Root versus shoot antifungal plant defenses. Frontiers in Plant Science 4, 256.
| Crossref | Google Scholar |
Belisle RJ, McKee B, Hao W, Crowley M, Arpaia ML, Miles TD, Adaskaveg JE, Manosalva P (2019) Phenotypic characterization of genetically distinct Phytophthora cinnamomi isolates from avocado. Phytopathology 109(3), 384-394.
| Crossref | Google Scholar | PubMed |
Benton JM, Cobb AH (1997) The modification of phytosterol profiles and in vitro photosynthetic electron transport of Galium aparine L. (cleavers) treated with the fungicide, epoxiconazole. Plant Growth Regulation 22, 93-100.
| Crossref | Google Scholar |
Bouvier-Naveݩ P, Berna A, Noiriel A, Compagnon V, Carlsson AS, Banas A, Stymne S, Schaller H (2010) Involvement of the phospholipid sterol acyltransferase1 in plant sterol homeostasis and leaf senescence. Plant Physiology 152(1), 107-119.
| Crossref | Google Scholar |
Brasier C, Scanu B, Cooke D, Jung T (2022) Phytophthora: an ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 13(1), 12.
| Crossref | Google Scholar | PubMed |
Burciaga-Monge A, López-Tubau JM, Laibach N, Deng C, Ferrer A, Altabella T (2022) Effects of impaired steryl ester biosynthesis on tomato growth and developmental processes. Frontiers in Plant Science 13, 984100.
| Crossref | Google Scholar | PubMed |
Burgess TI, Scott JK, Mcdougall KL, Stukely MJC, Crane C, Dunstan WA, Brigg F, Andjic V, White D, Rudman T, Arentz F, Ota N, Hardy GESJ (2017) Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Global Change Biology 23(4), 1661-1674.
| Crossref | Google Scholar | PubMed |
Burra DD, Berkowitz O, Hedley PE, Morris J, Resjö S, Levander F, Liljeroth E, Andreasson E, Alexandersson E (2014) Phosphite-induced changes of the transcriptome and secretome in Solanum tuberosum leading to resistance against Phytophthora infestans. BMC Plant Biology 14, 254.
| Crossref | Google Scholar |
Busquets A, Keim V, Closa M, Del Arco A, Boronat A, Arró M, Ferrer A (2008) Arabidopsis thaliana contains a single gene encoding squalene synthase. Plant Molecular Biology 67, 25-36.
| Crossref | Google Scholar | PubMed |
Cahill D, Legge N, Grant B, Weste G (1989) Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 79(4), 417-424.
| Crossref | Google Scholar |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56(4), 279-310.
| Crossref | Google Scholar |
Cauz-Santos LA, Dodsworth S, Samuel R, Christenhusz MJM, Patel D, Shittu T, Jakob A, Paun O, Chase MW (2022) Genomic insights into recent species divergence in Nicotiana benthamiana and natural variation in Rdr1 gene controlling viral susceptibility. The Plant Journal 111(1), 7-18.
| Crossref | Google Scholar | PubMed |
Chaparro-Garcia A, Wilkinson RC, Gimenez-Ibanez S, Findlay K, Coffey MD, Zipfel C, Rathjen JP, Kamoun S, Schornack S (2011) The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana. PLoS ONE 6(1), e16608.
| Crossref | Google Scholar | PubMed |
Chappell J, VonLanken C, Vögeli U (1991) Elicitor-inducible 3-hydroxy-3-methylglutaryl coenzyme a reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures. Plant Physiology 97(2), 693-698.
| Crossref | Google Scholar | PubMed |
Chase MW, Cauz-Santos LA, Dodsworth S, Christenhusz MJM (2022) Taxonomy of the Australian Nicotiana benthamiana complex (Nicotiana section Suaveolentes; Solanaceae): five species, four newly described, with distinct ranges and morphologies. Australian Systematic Botany 35(5), 345-363.
| Crossref | Google Scholar |
Chen Z, Liu F, Zeng M, Wang L, Liu H, Sun Y, Wang L, Zhang Z, Chen Z, Xu Y, Zhang M, Xia Y, Ye W, Dong S, Govers F, Wang Y, Wang Y (2023) Convergent evolution of immune receptors underpins distinct elicitin recognition in closely related Solanaceous plants. The Plant Cell 35(4), 1186-1201.
| Crossref | Google Scholar | PubMed |
Coelho AC, Pires R, Schütz G, Santa C, Manadas B, Pinto P (2021) Disclosing proteins in the leaves of cork oak plants associated with the immune response to Phytophthora cinnamomi inoculation in the roots: a long-term proteomics approach. PLoS ONE 16(1), e0245148.
| Crossref | Google Scholar | PubMed |
Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013) Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology 117(2), 112-123.
| Crossref | Google Scholar | PubMed |
De Coninck B, Timmermans P, Vos C, Cammue BPA, Kazan K (2015) What lies beneath: belowground defense strategies in plants. Trends in Plant Science 20(2), P91-101.
| Crossref | Google Scholar |
Der C, Courty P-E, Recorbet G, Wipf D, Simon-Plas F, Gerbeau-Pissot P (2024) Sterols, pleiotropic players in plant–microbe interactions. Trends in Plant Science 29(5), 524-534.
| Crossref | Google Scholar | PubMed |
Derevnina L, Dagdas YF, De la Concepcion JC, Bialas A, Kellner R, Petre B, Domazakis E, Du J, Wu C-H, Lin X, Aguilera-Galvez C, Cruz-Mireles N, Vleeshouwers VGAA, Kamoun S (2016) Nine things to know about elicitins. New Phytologist 212(4), 888-895.
| Crossref | Google Scholar | PubMed |
Dokládal L, Obořil M, Stejskal K, Zdráhal Z, Ptáčková N, Chaloupkova R, Damborský J, Kašparovský T, Jeandroz S, Žd’árská M, Lochman J (2012) Physiological and proteomic approaches to evaluate the role of sterol binding in elicitin-induced resistance. Journal of Experimental Botany 63(5), 2203-2215.
| Crossref | Google Scholar | PubMed |
Domazakis E, Wouters D, Visser RGF, Kamoun S, Joosten MHAJ, Vleeshouwers VGAA (2018) The ELR-SOBIR1 complex functions as a two-component receptor-like kinase to mount defense against Phytophthora infestans. Molecular Plant-Microbe Interactions 31(8), 795-802.
| Crossref | Google Scholar | PubMed |
Du J, Verzaux E, Chaparro-Garcia A, Bijsterbosch G, Keizer LCP, Zhou J, Liebrand TWH, Xie C, Govers F, Robatzek S, van der Vossen EAG, Jacobsen E, Visser RGF, Kamoun S, Vleeshouwers , VGAA (2015) Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nature Plants 1(4), 15034.
| Crossref | Google Scholar |
Du Y, Fu X, Chu Y, Wu P, Liu Y, Ma L, Tian H, Zhu B (2022) Biosynthesis and the roles of plant sterols in development and stress responses. International Journal of Molecular Sciences 23(4), 2332.
| Crossref | Google Scholar | PubMed |
Dundas SJ, Hardy GESJ, Fleming PA (2016) The plant pathogen Phytophthora cinnamomi influences habitat use by the obligate nectarivore honey possum (Tarsipes rostratus). Australian Journal of Zoology 64(2), 122-131.
| Crossref | Google Scholar |
Enfissi EMA, Fraser PD, Lois L-M, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnology Journal 3(1), 17-27.
| Crossref | Google Scholar | PubMed |
Engelbrecht J, Van den Berg N (2013) Expression of defence-related genes against Phytophthora cinnamomi in five avocado rootstocks. South African Journal of Science 109(11/12), 8.
| Crossref | Google Scholar |
Eshraghi L, Anderson J, Aryamanesh N, Shearer B, McComb J, Hardy GESJ, O’Brien PA (2011) Phosphite primed defence responses and enhanced expression of defence genes in Arabidopsis thaliana infected with Phytophthora cinnamomi. Plant Pathology 60(6), 1086-1095.
| Crossref | Google Scholar |
Evangelisti E, Govers F (2024) Roadmap to success: how oomycete plant pathogens invade tissues and deliver effectors. Annual Review of Microbiology 78, 493-512.
| Crossref | Google Scholar |
Evangelisti E, Gogleva A, Hainaux T, Doumane M, Tulin F, Quan C, Yunusov T, Floch K, Schornack S (2017) Time-resolved dual transcriptomics reveal early induced Nicotiana benthamiana root genes and conserved infection-promoting Phytophthora palmivora effectors. BMC Biology 15, 39.
| Crossref | Google Scholar |
Fabro G, Di Rienzo JA, Voigt CA, Savchenko T, Dehesh K, Somerville S, Alvarez ME (2008) Genome-wide expression profiling Arabidopsis at the stage of Golovinomyces cichoracearum haustorium formation. Plant Physiology 146(3), 1421-1439.
| Crossref | Google Scholar | PubMed |
Fernandes P, Machado H, Silva MdC, Costa RL (2021) A histopathological study reveals new insights into responses of chestnut (Castanea spp.) to root infection by Phytophthora cinnamomi. Phytopathology 111(2), 345-355.
| Crossref | Google Scholar | PubMed |
Ferrer A, Altabella T, Arró M, Boronat A (2017) Emerging roles for conjugated sterols in plants. Progress in Lipid Research 67, 27-37.
| Crossref | Google Scholar | PubMed |
Fröschel C (2021) In-depth evaluation of root infection systems using the vascular fungus Verticillium longisporum as soil-borne model pathogen. Plant Methods 17(1), 57.
| Crossref | Google Scholar | PubMed |
Gao H, Ge W, Bai L, Zhang T, Zhao L, Li J, Shen J, Xu N, Zhang H, Wang G, Lin X (2023) Proteomic analysis of leaves and roots during drought stress and recovery in Setaria italica L. Frontiers in Plant Science 14, 1240164.
| Crossref | Google Scholar | PubMed |
Gómez-Merino FC, Trejo-Téllez LI (2015) Biostimulant activity of phosphite in horticulture. Scientia Horticulturae 196, 82-90.
| Crossref | Google Scholar |
Griebel T, Zeier J (2010) A role for β-sitosterol to stigmasterol conversion in plant–pathogen interactions. The Plant Journal 63(2), 254-268.
| Crossref | Google Scholar | PubMed |
Gunning T, Cahill DM (2009) A soil-free plant growth system to facilitate analysis of plant pathogen interactions in roots. Journal of Phytopathology 157(7–8), 497-501.
| Crossref | Google Scholar |
Gunning TK, Conlan XA, Parker RM, Dyson GA, Adams MJ, Barnett NW, Cahill DM (2013) Profiling of secondary metabolites in blue lupin inoculated with Phytophthora cinnamomi following phosphite treatment. Functional Plant Biology 40(11), 1089-1097.
| Crossref | Google Scholar | PubMed |
Hardham AR, Blackman LM (2018) Phytophthora cinnamomi. Molecular Plant Pathology 19(2), 260-285.
| Crossref | Google Scholar |
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133-139.
| Crossref | Google Scholar |
Harker M, Holmberg N, Clayton JC, Gibbard CL, Wallace AD, Rawlins S, Hellyer SA, Lanot A, Safford R (2003) Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy-3-methylglutaryl-CoA reductase. Plant Biotechnology Journal 1(2), 113-121.
| Crossref | Google Scholar | PubMed |
He H, Xu T, Cao F, Xu Y, Dai T, Liu T (2024) PcAvh87, a virulence essential RxLR effector of Phytophthora cinnamomi suppresses host defense and induces cell death in plant nucleus. Microbiological Research 286, 127789.
| Crossref | Google Scholar | PubMed |
Horta M, Sousa N, Coelho AC, Neves D, Cravador A (2008) In vitro and in vivo quantification of elicitin expression in Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 73(1–3), 48-57.
| Crossref | Google Scholar |
Huang WR, Joosten MHAJ (2024) Immune signaling: receptor-like proteins make the difference. Trends in Plant Science 30(1), 54-68.
| Crossref | Google Scholar | PubMed |
Huang WRH, Schol C, Villanueva SL, Heidstra R, Joosten MHAJ (2021) Knocking out SOBIR1 in Nicotiana benthamiana abolishes functionality of transgenic receptor-like protein Cf-4. Plant Physiology 185(2), 290-294.
| Crossref | Google Scholar | PubMed |
Islam MT, Rookes JE, Cahill DM (2017) Active defence by an Australian native host, Lomandra longifolia, provides resistance against Phytophthora cinnamomi. Functional Plant Biology 44, 386-399.
| Crossref | Google Scholar | PubMed |
Islam MT, Hussain HI, Russo R, Chambery A, Amoresano A, Schallmey A, Oßwald W, Nadiminti PP, Cahill DM (2019) Functional analysis of elicitins and identification of cell wall proteins in Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 107, 21-32.
| Crossref | Google Scholar |
Islam MT, Gan HM, Ziemann M, Hussain HI, Arioli T, Cahill D (2020) Phaeophyceaean (brown algal) extracts activate plant defense systems in Arabidopsis thaliana challenged with Phytophthora cinnamomi. Frontiers in Plant Science 11, 852.
| Crossref | Google Scholar | PubMed |
Jackson TJ, Burgess TI, Colquhoun I, Hardy GESJ (2000) Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathology 49(1), 147-154.
| Crossref | Google Scholar |
Kamoun S, Furzer O, Jones JD, Judelson HS, Ali GS, Dalio RJD, Roy SG, Schena L, Zambounis A, Panabières F, Cahill D, Ruocco M, Figueiredo A, Chen X-R, Hylvey J, Stam R, Lamour K, Gijzen M, Tyler BM, Grünwald NJ, Mukhtar MS, Tomé DF, Tör M, Van Den Ackerveken G, McDowell J, Daayf F, Fry WE, Lindqvist-Kreuze H, Meijer HJG, Petre B, Ristaino J, Yoshida K, Birch PRJ, Govers F (2015) The top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology 16(4), 413-434.
| Crossref | Google Scholar | PubMed |
Kharel A, Adcock J, Ziemann M, Rookes J, Cahill D (2024a) Sterol complex visualisation in Phytophthora cinnamomi and expression analysis of genes involved in sterol sensing, recruitment and conversion. Physiological and Molecular Plant Pathology 133, 102371.
| Crossref | Google Scholar |
Kharel A, Rookes J, Ziemann M, Cahill D (2024b) Viable protoplast isolation, organelle visualization and transformation of the globally distributed plant pathogen Phytophthora cinnamomi. Protoplasma 261, 1073-1092.
| Crossref | Google Scholar |
Kim Y-J, Lee OR, Oh JY, Jang M-G, Yang D-C (2014) Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiology 165(1), 373-387.
| Crossref | Google Scholar | PubMed |
King M, Reeve W, Van der Hoek MB, Williams N, McComb J, O’Brien PA, Hardy GESJ (2010) Defining the phosphite-regulated transcriptome of the plant pathogen Phytophthora cinnamomi. Molecular Genetics and Genomics 284, 425-435.
| Crossref | Google Scholar | PubMed |
Kopischke M, Westphal L, Schneeberger K, Clark R, Ossowski S, Wewer V, Fuchs R, Landtag J, Hause G, Dörmann P, Lipka V, Weigel D, Schulze-Lefert P, Scheel D, Rosahl S (2013) Impaired sterol ester synthesis alters the response of Arabidopsis thaliana to Phytophthora infestans. The Plant Journal 73(3), 456-468.
| Crossref | Google Scholar | PubMed |
Li W, Liu W, Wei H, He Q, Chen J, Zhang B, Zhu S (2014) Species-specific expansion and molecular evolution of the 3-hydroxy-3-methylglutaryl coenzyme a reductase (HMGR) gene family in plants. PLoS ONE 9(4), e94172.
| Crossref | Google Scholar | PubMed |
Liao P, Lung S-C, Chan WL, Bach TJ, Lo C, Chye M-L (2020) Overexpression of HMG-CoA synthase promotes Arabidopsis root growth and adversely affects glucosinolate biosynthesis. Journal of Experimental Botany 71(1), 272-289.
| Crossref | Google Scholar | PubMed |
Lin X, Olave-Achury A, Heal R, Pais M, Witek K, Ahn H-K, Zhao H, Bhanvadia S, Karki HS, Song T, Wu C-H, Adachi H, Kamoun S, Vleeshouwers VGAA, Jones JDG (2022) A potato late blight resistance gene protects against multiple Phytophthora species by recognizing a broadly conserved RXLR-WY effector. Molecular Plant 15(9), 1457-1469.
| Crossref | Google Scholar | PubMed |
Liu D, Shi L, Han C, Yu J, Li D, Zhang Y (2012) Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 7(9), e46451.
| Crossref | Google Scholar | PubMed |
Liu L, Sonbol F-M, Huot B, Gu Y, Withers J, Mwimba M, Yao J, He SY, Dong X (2016) Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nature Communications 7(1), 13099.
| Crossref | Google Scholar |
Machinandiarena MF, Lobato MC, Feldman ML, Daleo GR, Andreu AB (2012) Potassium phosphite primes defense responses in potato against Phytophthora infestans. Journal of Plant Physiology 169(14), 1417-1424.
| Crossref | Google Scholar | PubMed |
Massoud K, Barchietto T, Le Rudulier T, Pallandre L, Didierlaurent L, Garmier M, Ambard-Bretteville F, Seng J-M, Saindrenan P (2012) Dissecting phosphite-induced priming in Arabidopsis infected with Hyaloperonospora arabidopsidis. Plant Physiology 159(1), 286-298.
| Crossref | Google Scholar | PubMed |
McDougall KL, Barrett S, Velzeboer R, Cahill DM, Rudman T (2024) Evaluating the risk to Australia’s flora from Phytophthora cinnamomi. Australian Journal of Botany 72, BT23086.
| Crossref | Google Scholar |
Mohammadi MA, Cheng Y, Aslam M, Jakada BH, Wai MH, Ye K, He X, Luo T, Ye L, Dong C, Hu B, Priyadarshani SVGN, Wang-Pruski G, Qin Y (2021) ROS and oxidative response systems in plants under biotic and abiotic stresses: revisiting the crucial role of phosphite triggered plants defense response. Frontiers in Microbiology 12, 631318.
| Crossref | Google Scholar | PubMed |
Mohammed U, Davis J, Rossall S, Swarup K, Czyzewicz N, Bhosale R, Foulkes J, Murchie EH, Swarup R (2022) Phosphite treatment can improve root biomass and nutrition use efficiency in wheat. Frontiers in Plant Science 13, 1017048.
| Crossref | Google Scholar | PubMed |
Morikawa T, Mizutani M, Aoki N, Watanabe B, Saga H, Saito S, Oikawa A, Suzuki H, Sakurai N, Shibata D, Wadano A, Sakata K, Ohta D (2006) Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. The Plant Cell 18(4), 1008-1022.
| Crossref | Google Scholar | PubMed |
Nes WD (2011) Biosynthesis of cholesterol and other sterols. Chemical Reviews 111(10), 6423-6451.
| Crossref | Google Scholar | PubMed |
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59(2), 368-373.
| Crossref | Google Scholar |
Pérez-Zavala FG, Ojeda-Rivera JO, Herrera-Estrella L, López-Arredondo D (2024) Beneficial effects of phosphite in Arabidopsis thaliana mediated by activation of ABA, SA, and JA biosynthesis and signaling pathways. Plants 13(13), 1873.
| Crossref | Google Scholar | PubMed |
Perez V, Mamdouh AM, Huet J-C, Pernollet J-C, Bompeix G (1995) Enhanced secretion of elicitins by Phytophthora fungi exposed to phosphonate. Cryptogamie. Mycologie 16, 191-194.
| Crossref | Google Scholar |
Phillips D, Grant B, Weste G (1987) Histological changes in the roots of an avocado cultivar, Duke 7, infected with Phytophthora cinnamomi. Cytology and Histology 77(5), 691-698.
| Google Scholar |
Rahier A, Taton M (1997) Fungicides as tools in studying postsqualene sterol synthesis in plants. Pesticide Biochemistry and Physiology 57(1), 1-27.
| Crossref | Google Scholar |
Ranawaka B, An J, Lorenc MT, Jung H, Sulli M, Aprea G, Roden S, Llaca V, Hayashi S, Asadyar L, LeBlanc Z, Ahmed Z, Naim F, de Campos SB, Cooper T, de Felippes FF, Dong P, Zhong S, Garcia-Carpintero V, Orzaez D, Dudley KJ, Bombarely A, Bally J, Winefield C, Waterhouse PM (2023) A multi-omic Nicotiana benthamiana resource for fundamental research and biotechnology. Nature Plants 9, 1558-1571.
| Crossref | Google Scholar | PubMed |
Rasmann S, Agrawal AA (2008) In defense of roots: a research agenda for studying plant resistance to belowground herbivory. Plant Physiology 146(3), 875-880.
| Crossref | Google Scholar | PubMed |
Redondo MÁ, Pérez-Sierra A, Abad-Campos P, Torres L, Solla A, Reig-Armiñana J, García-Breijo F (2015) Histology of Quercus ilex roots during infection by Phytophthora cinnamomi. Trees 29, 1943-1957.
| Crossref | Google Scholar |
Robinson LH, Cahill DM (2003) Ecotypic variation in the response of Arabidopsis thaliana to Phytophthora cinnamomi. Australasian Plant Pathology 32, 53-64.
| Crossref | Google Scholar |
Rodríguez-Romero M, Cardillo E, Santiago R, Pulido F (2022) Susceptibility to Phytophthora cinnamomi of six holm oak (Quercus ilex) provenances: are results under controlled vs. natural conditions consistent? Forest Systems 31(2), e011.
| Crossref | Google Scholar |
Rookes JE, Wright ML, Cahill DM (2008) Elucidation of defence responses and signalling pathways induced in Arabidopsis thaliana following challenge with Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 72(4–6), 151-161.
| Crossref | Google Scholar |
Ruiz Gómez FJ, Navarro-Cerrillo RM, Sánchez-Cuesta R, Pérez-de-Luque A (2015) Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathology 64(3), 605-616.
| Crossref | Google Scholar |
Schuster M, Kilaru S, Steinberg G (2024) Azoles activate type I and type II programmed cell death pathways in crop pathogenic fungi. Nature Communications 15(1), 4357.
| Crossref | Google Scholar | PubMed |
Sena K, Crocker E, Vincelli P, Barton C (2018) Phytophthora cinnamomi as a driver of forest change: implications for conservation and management. Forest Ecology and Management 409, 799-807.
| Crossref | Google Scholar |
Shands AC, Xu G, Belisle RJ, Seifbarghi S, Jackson N, Bombarely A, Cano LM, Manosalva PM (2024) Genomic and transcriptomic analyses of Phytophthora cinnamomi reveal complex genome architecture, expansion of pathogenicity factors, and host-dependent gene expression profiles. Frontiers in Microbiology 15, 1341803.
| Crossref | Google Scholar | PubMed |
Shearer BL, Fairman RG (2007) A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australasian Plant Pathology 36(1), 78-86.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Australian Journal of Botany 55(3), 225-238.
| Crossref | Google Scholar |
Shen D, Chai C, Ma L, Zhang M, Dou D (2016) Comparative RNA-seq analysis of Nicotiana benthamiana in response to Phytophthora parasitica infection. Plant Growth Regulation 80, 59-67.
| Crossref | Google Scholar |
Sinhalagoda C, Wilson MD, Tran SN, Cahill D, Barry KM (2023) Screening native pepper, Tasmannia lanceolata (Poir.) A.C. Smith, for resistance against Phytophthora cinnamomi dieback. Australasian Plant Pathology 52(5), 427-437.
| Crossref | Google Scholar |
Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J (2019) The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends in Biotechnology 37(7), 761-774.
| Crossref | Google Scholar | PubMed |
Valitova J, Renkova A, Beckett R, Minibayeva F (2024) Stigmasterol: an enigmatic plant stress sterol with versatile functions. International Journal of Molecular Sciences 25(15), 8122.
| Crossref | Google Scholar | PubMed |
Wang K, Senthil-Kumar M, Ryu C-M, Kang L, Mysore KS (2012) Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant physiology 158(4), 1789-1802.
| Crossref | Google Scholar | PubMed |
Wang J, Qin Q, Pan J, Sun L, Sun Y, Xue Y, Song K (2019) Transcriptome analysis in roots and leaves of wheat seedlings in response to low-phosphorus stress. Scientific Reports 9(1), 19802.
| Crossref | Google Scholar | PubMed |
Wang W, Liu X, Govers F (2021) The mysterious route of sterols in oomycetes. PLoS Pathogens 17(6), e1009591.
| Crossref | Google Scholar | PubMed |
Wilkinson CJ, Holmes JM, Dell B, Tynan KM, McComb JA, Shearer BL, Colquhoun IJ, Hardy GESJ (2001) Effect of phosphite on in planta zoospore production of Phytophthora cinnamomi. Plant Pathology 50(5), 587-593.
| Crossref | Google Scholar |
Williams N, Hardy GESJ, O’Brien PA (2009) Analysis of the distribution of Phytophthora cinnamomi in soil at a disease site in Western Australia using nested PCR. Forest Pathology 39(2), 95-109.
| Crossref | Google Scholar |
Witek K, Jupe F, Witek AI, Baker D, Clark MD, Jones JD (2016) Accelerated cloning of a potato late blight–resistance gene using RenSeq and SMRT sequencing. Nature Biotechnology 34(6), 656-660.
| Crossref | Google Scholar | PubMed |
Yang X, Tyler BM, Hong C (2017) An expanded phylogeny for the genus Phytophthora. IMA Fungus 8, 355-384.
| Crossref | Google Scholar | PubMed |


