Comprehensive evaluation of agronomic traits, physiological responses, and gene expression in chickpea cultivars under fungal stress
Samra Irum A B * , Muhammad Hayder Bin Khalid C D E * , Tanveer Hussain C E , Amjad Saeed C E , Imran Haider C E , Zaheer Ahmed F , Rashid Iqbal C E G , Noorah AlKubaisi H and Mohamed S. Elshikh H
C D E * , Tanveer Hussain C E , Amjad Saeed C E , Imran Haider C E , Zaheer Ahmed F , Rashid Iqbal C E G , Noorah AlKubaisi H and Mohamed S. Elshikh H
A
B
C
D
E
F
G
H
Abstract
Chickpea (Cicer arietinum L.), a widely grown legume with significant economic importance, serves as an important nutrient source for humans. However, its production is severely constrained by Fusarium wilt, caused by the pathogen Fusarium oxysporum. Due to the high pathogenic variability, effective control remains challenging, and the plant’s defense responses are not yet fully understood. In this study, we provide novel insights by identifying cultivar-specific responses and uncovering novel gene expression profiles associated with Fusarium resistance, which advance current understanding beyond previous studies. An integrative approach combining agronomic, physiological, and molecular analyses was used to evaluate chickpea cultivars under fungal stress. We assessed the disease severity index (DSI) to quantify infection levels and evaluated various morphological traits, including plant height, root length, number of pods per plant, days to maturity, 100-seed weight, and shoot biomass, to determine the physical impact of fungal stress. Antioxidant enzyme activities, including superoxide dismutase (SOD), peroxidase (POD), and polyphenol oxidase (PPO), were significantly elevated, reflecting an enhanced antioxidative response to mitigate reactive oxygen species generated during pathogen attack. Biochemical parameters such as malondialdehyde (MDA), protein, and chlorophyll content were also measured, with increased MDA levels indicating increased lipid peroxidation under stress. Additionally, strong positive correlations among SOD, POD, PPO, and MDA highlight a coordinated antioxidant response that helps minimize oxidative damage. Similarly, the protein and chlorophyll contents exhibited significant correlations with enzyme activities, suggesting their roles in enhancing stress resilience. Moreover, real-time quantitative PCR analysis revealed changes in gene expression related to defense pathways, with significant upregulation of WRKY55 and MADS-Box transcription factor 23-like genes under fungal stress. This molecular response aligns with the physiological data, depicting the role of both antioxidant enzymes and gene expression in chickpea’s defense mechanisms. This integrative analysis of agronomic traits, antioxidant responses, and gene expression under fungal stress conditions provides valuable insights for enhancing chickpea resilience against Fusarium wilt. Despite these findings, further research is needed to explore additional genetic factors contributing to resistance and to validate these biomarkers across diverse chickpea germplasms. Future studies should focus on applying these insights to breeding programs to develop Fusarium-resistant cultivars suitable for various agro-climatic conditions.
Keywords: antioxidants, crop protection, defense mechanism, disease, Fusarium oxysporum, gene expression, oxidative stress, physiology.
Introduction
Legumes are highly nutritious crops due to their high protein content, making them a key dietary crop all over the world. Chickpea (Cicer arietinum L.) is a diploid crop that undergoes self-pollination and has a genome size of 738 megabases, containing 28,269 genes (2n = 16) (Varshney et al. 2013; Agarwal et al. 2022). Chickpea accounts for 15% of the global legume yield and produces an annual output of 17.2 million metric tons. (Chandora et al. 2020). There are primarily two types of chickpeas: desi type (with small, dark seed colors) which makes up 85% of global chickpea production, and kabuli type, which is distinguished by its larger size and light-colored seeds, comprising 15% of global chickpea yield (Faridy et al. 2020). Chickpea possess a rich nutritional profile, with substantial protein (19.3%), carbohydrates (43.3%), and essential minerals such as iron and zinc. Additionally, they also contain vitamins A, B9, B2, and B6, along with dietary fiber, folate, beta-carotene, micronutrients, flavonols, proanthocyanidins, and health-promoting fatty acids (Sabbavarapu et al. 2013;Shan et al. 2025). These bioactive compounds exhibit anti-inflammatory and anti-diabetic properties, which are associated with improved health and disease prevention (Gupta et al. 2017; Faridy et al. 2020; Mahbub et al. 2021;Chen et al. 2025). Consequently, chickpea serve as an excellent dietary complement to cereal grains, particularly for individuals in developing countries (Sabbavarapu et al. 2013; Rachwa-Rosiak et al. 2015; Kumar et al. 2021). Pakistan is the third-largest producer of chickpea globally, following India and Turkey. It contributes 7% of global chickpea production (Thudi et al. 2016; Maurya and Kumar 2018). Moreover, chickpea cultivation in Pakistan covers almost 73% of the country’s overall pulse-producing area (Kinfe et al. 2015; Jan et al. 2020; Rafiq et al. 2020; Ullah et al. 2020). The chickpea crop adds 4.75% to the national economy, with an annual yield of 760,000 million tons (Chandio et al. 2016).
Biotic stresses are a significant factor in decreased chickpea productivity (Ankati et al. 2021). One of the major biotic stresses affecting chickpea during growth is wilt disease, caused by the pathogen Fusarium oxysporum f. sp. cicer. This stressor can lead to severe yield loss, crop failure, or a notable decrease in the quality of vulnerable cultivars, especially under favorable conditions. F. oxysporum can be transmitted through soil and seeds and may survive in the soil for up to 6 years, even in the absence of a susceptible host (Jendoubi et al. 2017). Fusarium wilt symptoms can be observed in various plant tissues, including leaves and roots. When infected by the fungal pathogen, plants become pale and lose their leaves. Moreover, the roots show a brown discoloration in internal tissues, leading to flagging and wilting, which result in severe yield losses (Nirmaladevi et al. 2016). Chickpea wilt can reduce yield by 10–50% annually (Caballo et al. 2019).
The utilization of resistant cultivars has been identified as the most pragmatic and implementable approach to managing Fusarium wilt. Developing and cultivating resistant varieties is considered one of the cost-effective strategies for disease control (Bharadwaj et al. 2022). Recent research has identified multiple Fusarium wilt resistance genes in chickpea (foc-1, foc-2, foc-3, foc-4, foc-5) that are mapped to linkage groups LG2 and LG5 in chickpea, providing key targets for breeding programs (Jendoubi et al. 2017). Marker-assisted selection (MAS) and genome-wide association studies (GWAS) have further accelerated the introgression of Fusarium wilt resistance genes into elite chickpea varieties, particularly through the use of wild Cicer species as genetic donors (Singh and Vyas 2021). Advanced breeding approaches, including single crosses between Desi and Kabuli types, have facilitated the transfer of resistance traits while maintaining agronomic performance (Jendoubi et al. 2017). The development of new varieties is of utmost importance due to the potential vulnerability of prevailing resistant varieties to novel physiological races of fungal pathogens (Yadav et al. 2023; Liu et al. 2025).
Despite these efforts, there is a lack of comprehensive studies integrating agronomic performance, physiological responses, and gene expression profiling under F. oxysporum stress across diverse chickpea genotypes. In the present study, we conducted a multi-dimensional evaluation of chickpea germplasm under F. oxysporum stress, combining agronomic traits evaluation, physiological stress markers, and cultivar-specific gene expression patterns. This integrative approach enables the identification of novel resistance-associated genes and physiological markers that can inform future breeding efforts. To our knowledge, this is the first report from Pakistan that provides such a holistic evaluation of F. oxysporum stress response in chickpea, offering new insights into cultivar-specific tolerance mechanisms and molecular targets for resistance breeding.
Materials and methods
Experimental design and plant material
A total of 15 chickpea genotypes, including Punjab-2008, Punjab-Noor 2009, Parbat, Dashat, CM-2008, Bittle-2016, Noor-2013, Wanhar 2000, CM-98, Bittle-98, and Paidar-91, were selected. The chickpea plants were grown under controlled glasshouse conditions with a photoperiod of 16 h of light (250 μmol m−2 s−1 light intensity) and 8 h of darkness. The temperature was maintained at 22–24°C during the day and 16–18°C at night, with relative humidity set at 60%. Soil composition consisted of a homogeneous mixture of sterilized sand and loamy soil in a 1:3 ratio, ensuring optimal aeration and nutrient availability. Glasshouse screening was performed at the vegetative and reproductive stages to ascertain the susceptibility and tolerance of chickpea lines to fungal pathogens and to comprehend the mechanism that underlies the fungal stress tolerance in chickpea. All treatments lasted for 30 days, with weekly monitoring of plant responses.
Fungal inoculum preparation
The F. oxysporum strain was cultured on potato dextrose agar (PDA, sigma) media at 28 ± 2°C for 3–5 days in a growth chamber with 70% humidity and a 12 h light/dark cycle. Spore formation was induced by subjecting the fungal culture to near-ultraviolet light at 25 ± 1°C. For inoculum preparation, 10 drops were added to 10 mL of sterile distilled water and thoroughly mixed. A small amount of the prepared mixture was then sprinkled on the fungal plate to gently scrape the fungal mycelium using a sterilized loop. The spore count was determined using a hemocytometer, and the spore suspension was adjusted to 5 × 106 spores mL−1 (Ankati et al. 2021).
Fungal stress on chickpea plants
The healthy chickpea seeds were surface sterilized and sown in 20 × 35 cm2 pots filled with a homogeneous mixture of sterilized sand and soil in a 1:3 ratio. The soil was autoclaved at 121°C for 30 min before use to prevent contamination. Pathogenicity testing was performed to confirm the virulence of the pathogen and its role in inducing vascular wilt disease symptoms. The root dip inoculation method was employed to inoculate 25-day-old seedlings, which were at the three-leaf stage (Nirmaladevi et al. 2016). Chickpea seedlings were carefully uprooted from the pots with minimal disturbance to the root structures. Subsequently, roots were rinsed with sterile water to eliminate any soil residues. The root part (1 cm) was trimmed and immersed in a freshly prepared F. oxysporum spore suspension for 30 min under gentle agitation. Following the treatment, the seedlings were transplanted into pots filled with a sand–soil mixture. Plants were irrigated with sterile distilled water and maintained under glasshouse conditions, with temperature settings of 22–24°C (day) and 16–18°C (night), relative humidity of 60%, and a 16-h light/8-h dark photoperiod. Symptoms of vascular wilt infection began to appear 15–20 days post-inoculation (dpi). To validate that vascular wilt was caused solely by the F. oxysporum spore suspension, a parallel control group of healthy seedlings was immersed in sterile distilled water and transplanted similarly. The experiment followed a randomized complete block design (RCBD), with three replications per genotype.
Disease severity index (DSI)
The disease severity was evaluated during both the vegetative and reproductive stages. For the reproduction stage, data on wilted plants were collected at physiological maturation (90–100 days after sowing). Resistance and susceptibility levels of each variety were determined using a standard rating scale ranging from 1 to 9 (Ali et al. 2012). The scale was based on the percentage of plants exhibiting symptoms such as necrosis, chlorosis, and wilt, with the following parameters: 1 = highly resistant (0–10 wilted plants), 3 = resistant (11–20% mortality), 5 = moderately resistant (21–30% mortality), 7 = susceptible (31–50% plant death), and 9 = highly susceptible (more than 50% death). The incidence of chickpea wilt was determined by expressing the number of infected plants as a relative percentage of the total plants assessed.
Evaluation of morphological traits
After fungal stress, the chickpea genotype was evaluated for morphological traits, including root length (RL), root biomass (RB), and shoot biomass (SB). At maturity, additional parameters such as plant height (PH), the number of primary (NPB) and secondary branches (NSB), number of pods per plant (NPP), days to maturity (DM), and seed weight (SW) were determined, and all the measurements were taken in replicates.
Chlorophyll and protein content
To determine the chlorophyll content, fresh leaves (0.3 g) were homogenized in 80% acetone. The supernatant was subjected to centrifugation at 8000g for 10 min, and absorbance was recorded at 663 and 645 nm. Chlorophyll content, expressed as milligrams per gram (mg g−1) of leaf fresh weight, was calculated using the following formula:
where A represents the absorbance at a specific wavelength (nm), and LC refers to the light path length in the cell. For protein content estimation, chickpea leaves were homogenized in phosphate buffer (50 mM, pH 7.8) and centrifuged at 21,913g for 10 min. Bradford reagent and phosphate buffer were added to the supernatant, and the mixture was allowed to stand for 5 min at room temperature for color development. The absorbance was measured at 595 nm using a spectrophotometer.
Evaluation of antioxidant enzyme
To measure SOD activity, 0.2 g of plant tissue was homogenized in a medium containing polyvinylpyrrolidone (PVP, 1 mM) and Na2EDTA (0.1 mM) in phosphate buffer (pH 7.8). The homogenate was centrifuged at 4°C for 10 min at 21,913g. SOD activity was assayed using the enzyme extract (supernatant), and was determined by its capacity to inhibit the photochemical reduction of nitro blue tetrazolium (NBT). The absorbance was measured at 560 nm after 20 min of exposure to light. One unit of SOD is defined as the number of enzymes that reduced inhibition (50%) of NBT reduction (Labidi et al. 2021).
For POD activity, leaf tissues were crushed in potassium phosphate buffer (pH 7.0), EDTA (0.1 mM), and dithiothreitol (DTT, 1 mM). The homogenate was centrifuged for 20 min at 11,181g to collect enzyme extract (supernatant). The reaction solution included phosphate buffer (pH 7.0), guaiacol (20 mM), H2O2 (40 mM), and enzyme extract (0.1 mL). The absorbance was recorded at 470 nm every 20 s, and one unit of POD activity was observed as a change in optical density (OD) of 0.01 units per minute (Arbona et al. 2003).
To assess PPO activity, fresh leaf samples (0.5 g) were homogenized in phosphate buffer (5 mL, pH 6.8). The homogenate was centrifuged at 4°C for 20 min at 11,181g to collect enzyme extract (supernatant). The assay was performed by mixing enzyme extract (500 μL), with buffer (1500 μL) and pyragallol (50 mM). The reaction mixture was incubated for 5 min at 30°C and the reaction was terminated by adding sulfuric acid (5%), and absorbance was measured at 420 nm (Sabarre and Yagonia-Lobarbio 2021).
Malondialdehyde (MDA) content, as an indicator of lipid peroxidation, was determined using the thiobarbituric acid (TBA) method. Leaf samples were mixed with trichloroacetic acid (10%, TCA) having TBA (0.65%) and incubated at 95°C for 25 min. After cooling, the mixture was centrifuged at 4°C for 10 min at 11,181g. The absorbance of the supernatant was recorded at 450, 532, and 600 nm. The MDA concentration was calculated and expressed as micromoles per liter (μmol L−1 ) (Zhang et al. 2021).
Selection of tolerant and susceptible cultivar
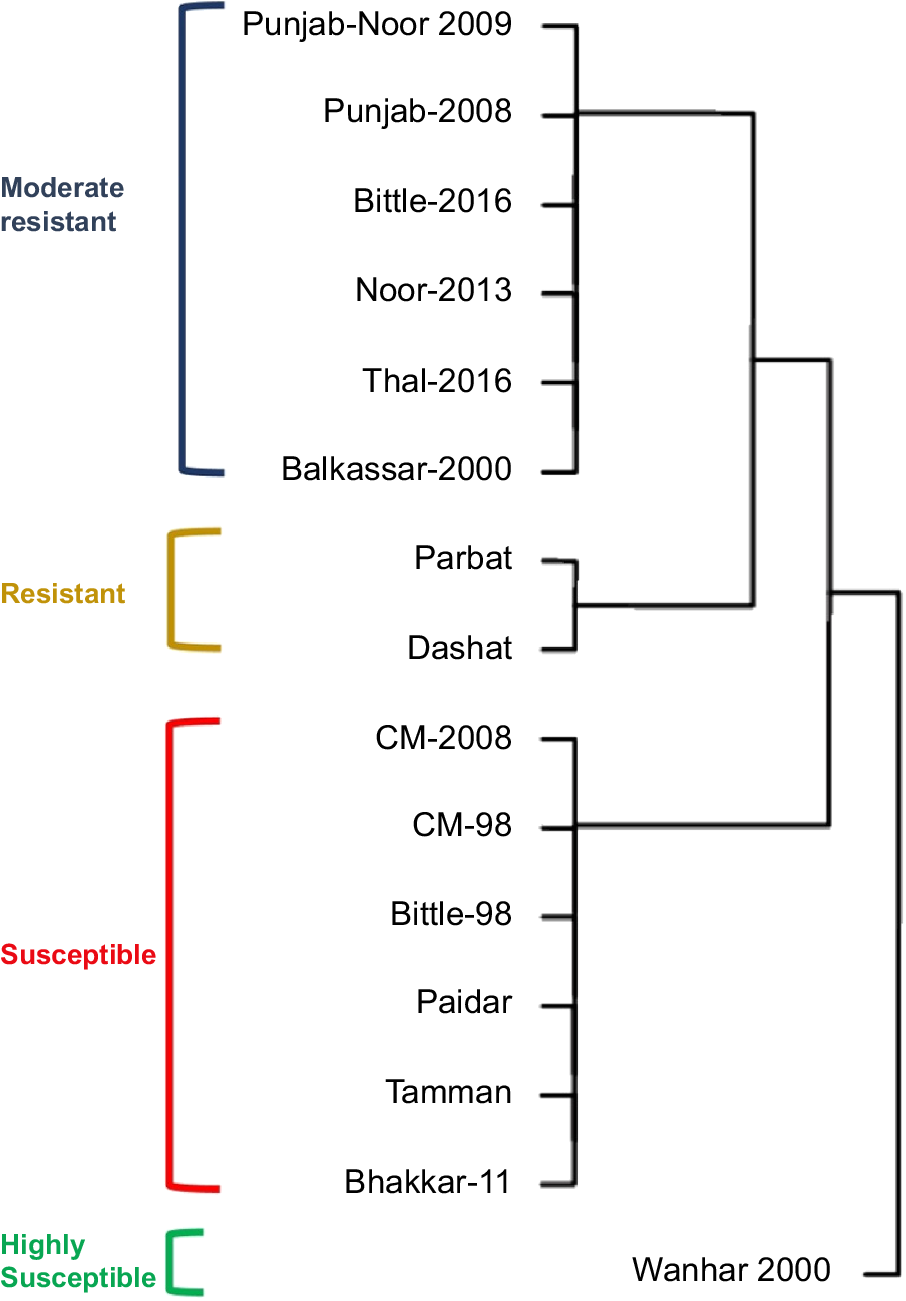
The efficacy of 15 genotypes of chickpea under fungal stress was evaluated based on morphological and physiological characteristics gleaned from glasshouse experiments at both the vegetative and pod-filling stages. The genotypes were characterized using Agglomerative Hierarchical Cluster (AHC) analysis in R studio. Dissimilarities among the genotypes were computed using Euclidean distances and Ward’s minimum variance method. This analysis facilitated the identification of tolerant and susceptible cultivars based on their cluster groupings.
Real-time-based expression analysis
Chickpea leaves were collected from disease-infected and control plants 2 weeks post-infection. For gene expression analysis, total RNA was extracted from lead samples using an Invitrogen Pure Link™ RNA kit, according to the manufacturer’s instructions. RNA integrity and concentration were assessed using a NanoDrop spectrophotometer and verified via agarose gel electrophoresis. First-strand cDNA was prepared using a Thermo-Scientifc ReverAid reverse transcriptase kit, with 1 μg of RNA as a template. For expression studies, primers targeting pathogenesis-related protein-5 (LOC101510320), WRKY55 (LOC101502928), MADS-box transcription factor 23-like (LOC101509359), sucrose transport protein SUC4 (LOC101496824) fungal genes were designed, and the internal reference gene Actin was used (Supplementary Table S1). Real-time PCR was performed on the Applied Biosystem StepOne Plus™ system with three technical replicates per sample and Actin as a reference gene. The real-time PCR reaction was carried out using maxima SYBR Green qPCR master mix (2X), forward and reverse primers (10 μM), and cDNA (1:100) from both infected and control samples. Reaction conditions included initial denaturation at 95°C for 5 min followed by 32 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and final extensions at 72°C for 5 min. Melt curve analysis was performed with the following steps: 95°C for 30 s, 58°C for 30 s, and a gradual increase to 95°C in continuous mode. The data were analyzed, where cycle threshold (Ct) values were recorded, normalized to a housekeeping gene, and processed to determine relative expression levels using the 2−ΔΔCt method (Irum et al. 2024).
Statistical analysis
All data were analyzed using R studio. Experiments were conducted in a completely randomized design (CRD) with three biological replicates per treatment. The results were expressed as mean ± standard error (s.e.), and statistical significance was determined at P < 0.05. For correlation analyses, Pearson’s correlation coefficients (r) were calculated to assess the relationships between morphological traits, antioxidant enzyme activities, and gene expression levels under control and stress conditions. Prior to conducting parametric tests, the assumptions of normality and homogeneity of variances were verified using the Shapiro–Wilk test, Levene’s test, and t-test, respectively. qPCR data were analyzed using the 2−ΔΔCt method, and gene expression differences were evaluated using Student’s t-test.
Results
Evaluation of DSI
The study evaluated the DSI of 15 chickpea genotypes at both the seedling and reproductive stages to categorize their resistance to wilt disease (Fig. 1). A t-test (P = 0.05) indicated significant variation in disease incidence among these chickpea lines, allowing them to be grouped as resistant, tolerant, or susceptible based on their disease responses at each stage. At the seedling stage, seven genotypes demonstrated a strong resistance to wilt, showing minimal disease incidence, and four genotypes exhibited a moderate response and were classified as tolerant. The remaining four genotypes were identified as susceptible, with noticeably higher disease incidence, averaging around 29%. The disease incidence within tolerant lines varied between 11% and 17%, suggesting a partial but stable response to the pathogen during early plant development. Overall, these findings indicate that 54% of tested genotypes exhibited resistance at the seedling stage, underscoring variability in wilt disease resilience among the lines tested. As the plants progressed to the reproductive stage, disease incidence increased in nearly all genotypes, showing a decline in average resistance levels to 32%. Despite this increase, seven genotypes maintained resistance, though with slightly higher incidence rates than observed at the seedling stage. Tolerant genotypes at this stage exhibited disease incidence between 11% and 20%, whereas four genotypes were fully susceptible, with an average disease incidence rising to approximately 57%. This trend of increased disease susceptibility at maturity, especially among lines that shifted from tolerant to susceptible, indicates a stage-dependent nature of wilt resistance and highlights the importance of screening at the reproductive phase to capture the full disease response profile. The resistant genotypes displayed slower disease incidence relative to susceptible lines, which exhibited rapid disease advancement. Some genotypes that appeared resistant or tolerant at the seedling stage displayed significantly higher susceptibility as they matured. For instance, several previously resistant genotypes showed incidence increases from 20% at the seedling stage to nearly 50% by the reproductive stage. This stage-dependent variation suggests that screening at the reproductive stage may offer a more accurate assessment of wilt resistance, capturing potential increases in disease incidence that occur closer to physiological maturity. The average disease incidence across all genotypes was 29% at the seedling stage, which increased to 57% at the reproductive stage. Tolerant genotypes consistently showed intermediate disease incidence values between 11% and 20% throughout both stages. In contrast, highly susceptible lines exhibited DSIs ranging from 4 to 5 at both vegetative and reproductive stages, underscoring the stark variation in disease responses. Although resistant lines retained some ability to limit disease progression, an overall increase in susceptibility with plant age suggests that physiological changes or environmental factors may influence disease vulnerability at later stages.
Percent disease severity index (DSI) of chickpea genotypes against Fusarium oxysporum. The DSI was assessed at both the seedling and reproductive stages based on wilt symptoms (chlorosis, necrosis, and plant death). Genotypes (15) were categorized as resistant, moderately resistant, susceptible, or highly susceptible. Bars represent mean values (±s.e.) from three replicates.
Assessment of morphological traits of chickpea genotypes
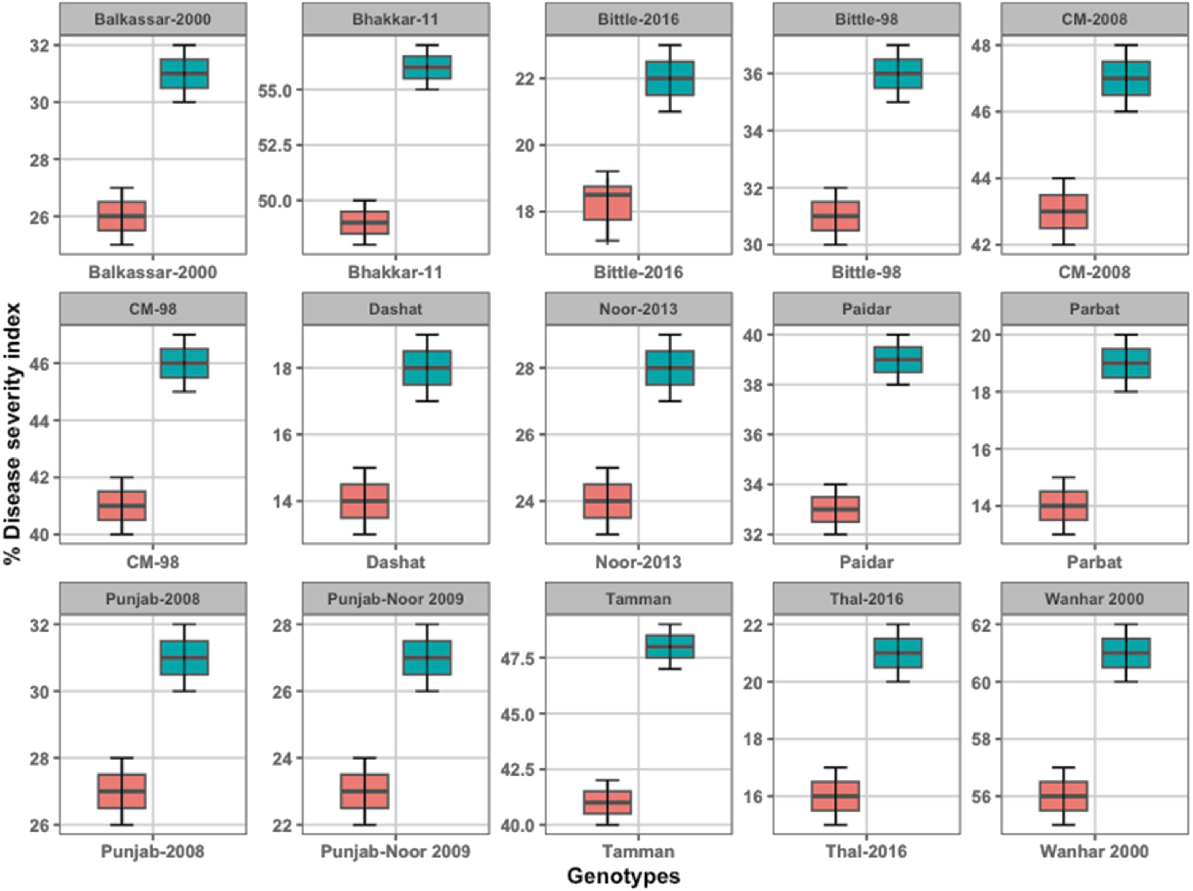
During the maturity stage, multiple agronomic traits were evaluated across different chickpea genotypes under both stress and control conditions. These parameters included plant height, number of primary and secondary branches, root length, number of pods per plant, days to maturity, shoot biomass, and 100-seed weight. Each of these traits provides insight into the plant’s growth, adaptability, and yield potential under varying environmental conditions. Plant height was recorded as the vertical distance from the soil surface to the apex of the plant. Both primary and secondary branch counts were documented as indicators of vegetative branching, and root length represented the extent of the root system’s growth. Under stress conditions, specifically, plant height and the number of primary and secondary branches were reduced, likely due to limited water availability affecting vegetative growth. For instance, Punjab-Noor 2009, a moderately resistant genotype, had a plant height of 45 cm and only 1.5 primary branches under stress, whereas under control conditions, the plant height reached 51 cm with 2.55 branches. This pattern was observed across most genotypes, demonstrating that stress negatively impacted vegetative traits. Root length was also influenced by stress, as plants adapted to seek deeper water reserves. Genotypes such as Punjab-2008 displayed a relatively stable root length across stress (5.5 cm) and control (6 cm) conditions, suggesting a level of fungal resilience in its root architecture. On the contrary, more susceptible genotypes, such as Wanhar 2000, exhibited a sharper decline in root length under stress, highlighting its vulnerability to disease. The number of pods per plant also declined under stress. For example, the resistant genotype Parbat had 48 pods under stress but increased to 55 under control. This decline in pod production under stress indicates the impact of diseases on reproductive performance and seed development. Additionally, days to maturity were generally shorter under stress conditions across genotypes, with plants maturing faster in an attempt to complete their life cycle before resources were depleted. For instance, Tamman matured 84 days under stress compared to 160 days under control, demonstrating an adaptive survival strategy under fungal conditions. Shoot biomass and 100-seed weight also showed significant reductions in stressed plants. Lower biomass under stress indicates reduced photosynthetic activity and reduced vegetative growth, as seen in Noor-2013, which had a biomass of 31 g under stress compared to 34 g under control. Similarly, 100-seed weight, an indicator of seed size and quality, was lower under stress conditions, reflecting reduced nutrient allocation to seeds. Bittle-2016’s seed weight decreased from 30 g under control to 18 g under stress, emphasizing disease impact on seed formation and filling. The interaction between genotype and stress was significant for most traits, indicating genetic variability in fungus responses. Resistant genotypes, such as Parbat and Dashat, maintained relatively stable performance across most traits under stress, whereas susceptible genotypes such as Wanhar 2000 and CM-98 exhibited pronounced declines (Fig. 2). These results highlight the importance of selecting disease-resistant genotypes for breeding programs aimed at enhancing chickpea resilience in stress environments. Overall, the study demonstrated that disease stress adversely affects morphological and yield-related traits in chickpeas, with genotypic differences playing a key role in disease adaptation.
Comparison of morphological traits (a–h) of chickpea genotypes under control and fungal stress conditions. Traits such as plant height, root length, shoot biomass, and pod number were measured at maturity. Bars represent mean values (±s.e.) from three replicates. Small circles with various colors depict the chickpea genotypes. In the violin plot, the large red circle indicates the mean, whereas the boxplot shows the median (center line), along with the minimum, maximum, and error bars.
Correlation among morphological traits under control and stress conditions
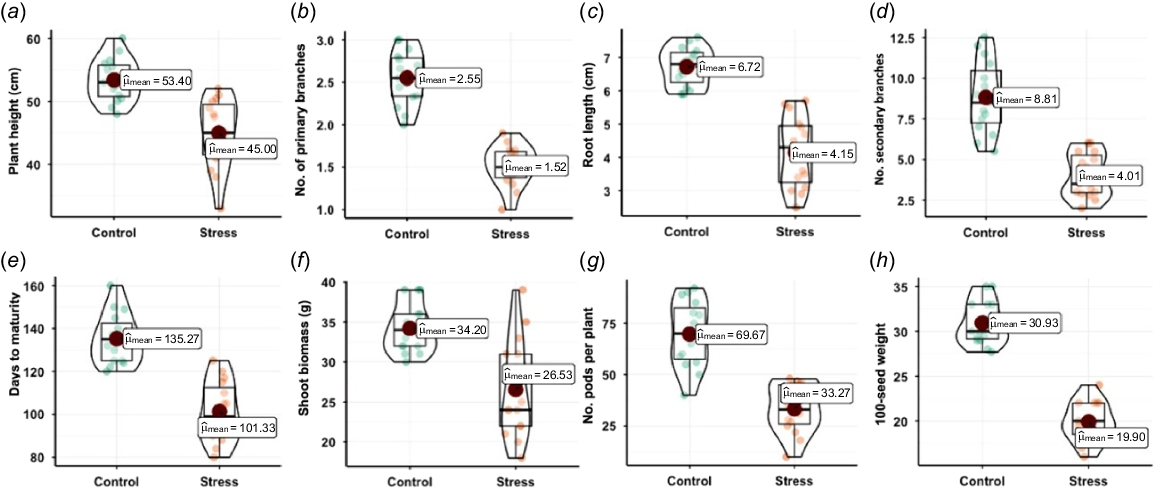
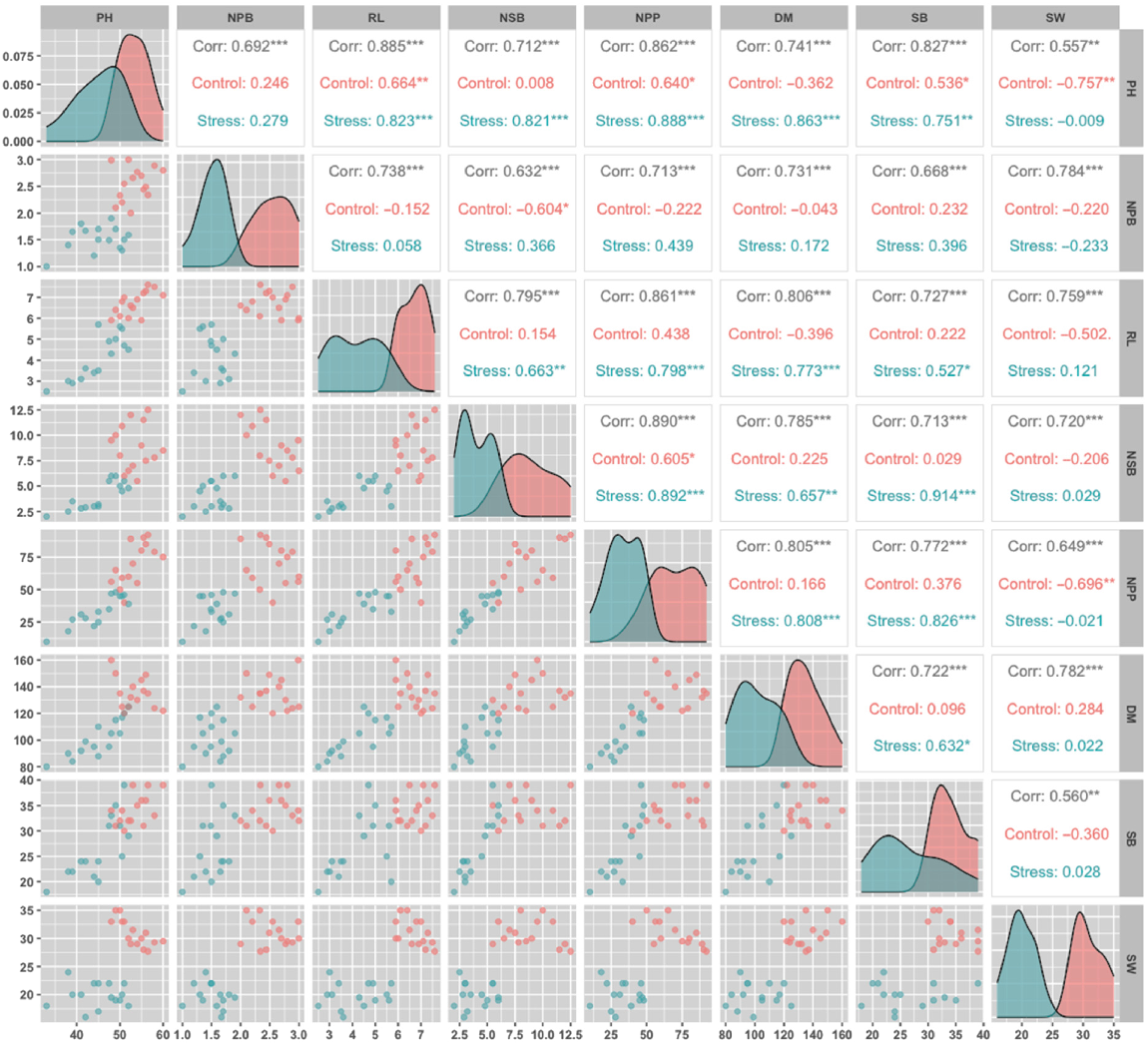
A Pearson correlation analysis was conducted to understand the relationship among several growth and yield parameters: plant height (PH), number of primary branches (NPB), root length (RL), number of secondary branches (NSB), number of pods per plant (NPP), days to maturity (DM), shoot biomass (SB), and 100-seed weight (SW). The results indicate significant correlations, with distinct trends under control and stress conditions. Under control conditions, RL exhibited moderate to high positive correlations with PH (r = 0.738, P < 0.001), indicating that taller plants tend to have longer roots. Similarly, NSB showed a strong correlation with NPP (r = 0.861, P < 0.001), suggesting that secondary branching contributes significantly to pod production. The relationship between DM and SB was also significant (r = 0.805), highlighting that prolonged maturity is associated with increased SB. Under stress conditions, however, these correlations shifted. RL showed a high positive correlation with SB (r = 0.798, P < 0.001), implying that RL is crucial for biomass accumulation under stress. Similarly, NSB’s correlation with SB remained high (r = 0.914, P < 0.001), reinforcing the importance of secondary branching in enhancing stress tolerance. Notably, SW exhibited a negative correlation with NPP (r = −0.696) under control conditions, but this relationship weakened under stress, suggesting a shift in resource allocation (Fig. 3). Overall, traits such as RL, NSB, and SB showed stronger correlations under stress conditions, making them valuable selection criteria for breeding programs focused on stress tolerance. The observed correlations highlight the importance of these traits in improving resilience and yield stability in challenging environments.
Correlation analysis of morphological traits of chickpea genotypes under fungal stress and control conditions. PH = plant height (cm), NPB = number of primary branches, RL = root length (cm), NSB = number of secondary branches, NPP = number of pods per plant, DM = days to maturity, SB = shoot biomass (g), and SW = 100-seed weight (g). *** highly significant (P < 0.001), **highly significant (P < 0.01), * significant (P < 0.05). Pearson correlation coefficients (R-values) illustrate relationships between plant height, branching, root traits, pods per plant, and biomass. Strong correlations (P < 0.05) indicate interdependence among traits under stress.
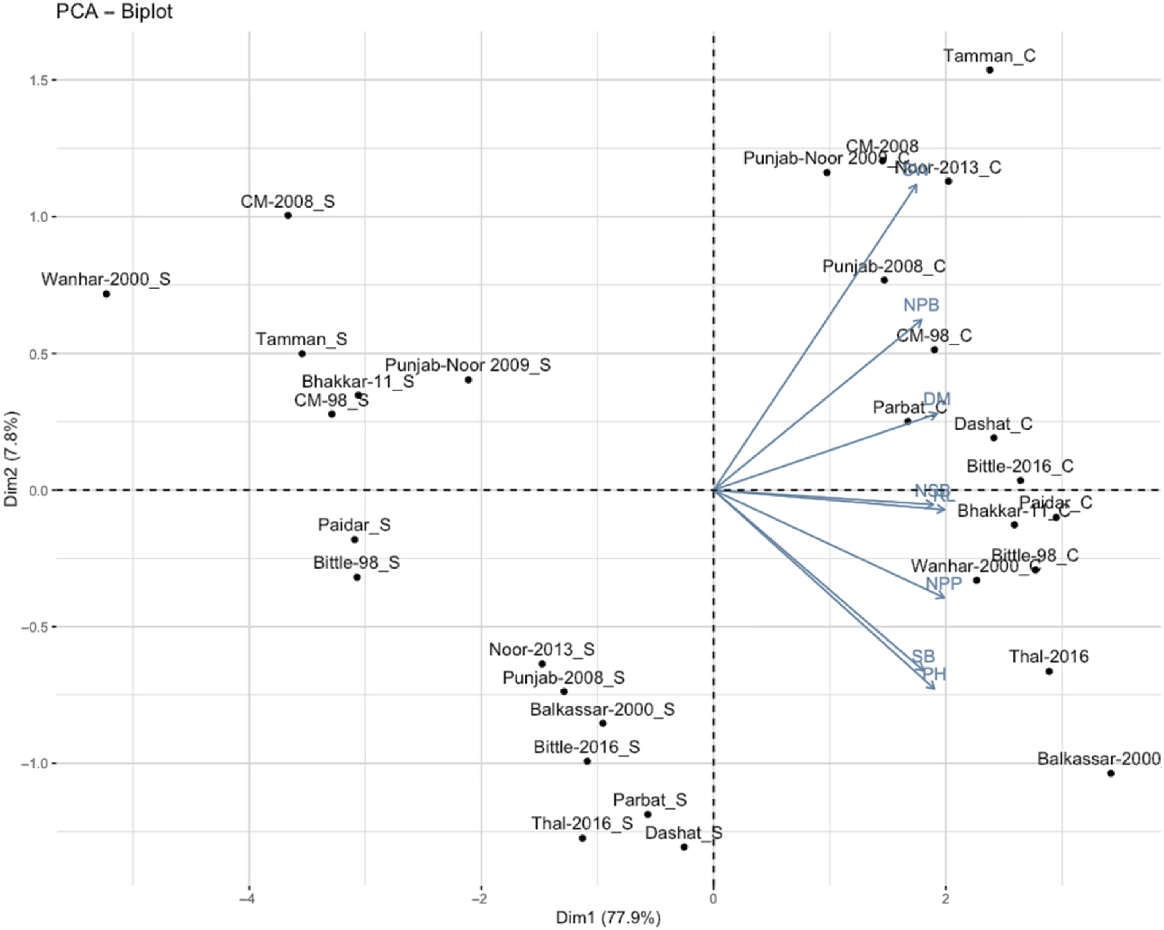
Principal component analysis (PCA) of morphological traits
PCA is a statistical method used to reduce dimensionality while retaining most of the variation present in the dataset. To evaluate the range of genotypes and the association between morphological traits, PCA based on the correlation matrix was used to study the variation pattern among the genotypes under stress conditions. In this analysis, the morphological traits included PH, NBP, RL, NSB, NPP, DM, SB, and SW. The PCA biplot in Fig. 4 illustrates the first two principal components, which collectively explain 90% of the total variation in the morphological traits. In this study, PC1 accounted for 87.60% of the variance, and PC2 explained 2.8%. PH, SB, and NPP displayed strong contributions to the first principal component (PC1), indicating their significant role in the differentiation of the genotypes. DM and RL were positively associated with each other and were primarily influenced by the second principal component (PC2). The genotypes show a clear grouping based on their trait values, with certain genotypes clustering closely together, indicating similar trait profiles, whereas others are dispersed, showing unique characteristics. For instance, ‘Tamman_C’ and ‘CM-2008_C’ were positively associated with traits like PH and NPB, whereas ‘Thal-2016_S’ and ‘Dashat_S’ displayed an inverse relationship, positioning them opposite on the biplot (Fig. 4).
PCA of morphological traits of chickpea genotype. Chickpea plants were evaluated under control (C) and fungal stress (S). PH = plant height, NPB = number of primary branches, RL = root length, NSB = number of secondary branches, NPP = number of pods per plant, DM = days to maturity, SB = shoot biomass, SW = 100-seed weight (g). PCA biplot shows the distribution of genotypes based on plant height, branching, root length, pods per plant, and maturity. PC1 and PC2 explain 90% of the total variance, highlighting key traits influencing genotype clustering.
Effect of fungal stress on chlorophyll, protein content, and antioxidant enzyme level
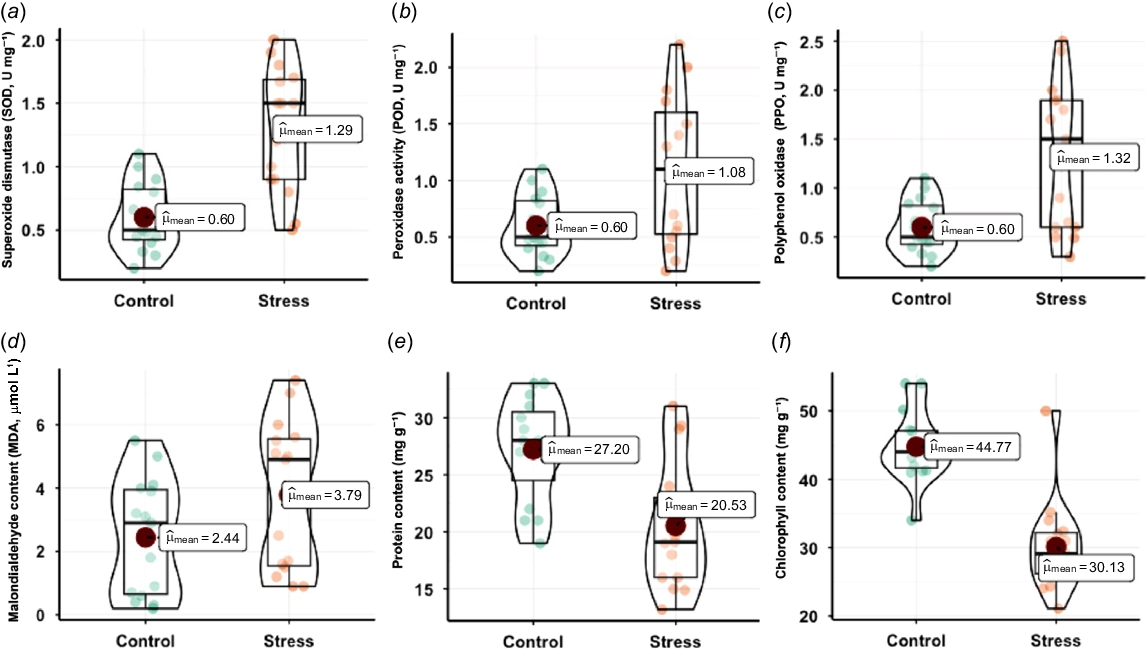
The statistical analysis of antioxidant enzyme activities and biochemical parameters under control and fungal stress conditions revealed significant shifts indicative of oxidative stress responses. Each parameter’s mean and distribution between conditions were evaluated, emphasizing the quantitative variation (Fig. 5). SOD activity showed a substantial increase under stress, with the mean rising from 0.60 in control to 1.29. POD activity also demonstrated a marked increase, with the mean shifting from 0.60 in control to 1.08 under disease conditions. The elevated POD levels under stress were statistically significant, reflecting the enzyme’s involvement in the stress response. PPO activity increased significantly, with a mean value rising from 0.60 in control to 1.32 under stress conditions. This statistically significant increase suggests the upregulation of PPO in response to oxidative stress. Each of these antioxidant enzymes (SOD, POD, and PPO) displayed statistically significant elevations in activity under fungal stress compared to control conditions, indicating a consistent enhancement of the antioxidant defense system. The mean protein content is 27.20 in control conditions. However, under stress conditions, the mean protein content decreases to 20.53, indicating a reduction in protein synthesis or stability in response to stress. The mean chlorophyll content under control conditions is 44.77. It reduces to 30.13 in disease conditions, suggesting that stress negatively impacts chlorophyll content, likely affecting photosynthetic efficiency.
Effect of Fusarium stress on antioxidant enzyme activity. (a) Levels of superoxide dismutase (SOD, U mg−1 protein), (b) peroxidase (POD, U mg−1 protein), (c) polyphenol oxidase (PPO, U mg−1 protein), (d) malondialdehyde (MDA, μmol L1 fresh weight), (e) total protein (mg g−1 fresh weight), and (f) chlorophyll content (mg g−1 fresh weight) were compared under control and stress conditions. Enzyme activities are expressed as specific activities per mg of total protein. Within the violin plot, the large red circle represents the mean. The boxplot displays the median (center line), along with the minimum, maximum, and error bars whereas small circles indicate the distribution of genotypes.
Association among antioxidant enzymes under fungal stress and control conditions
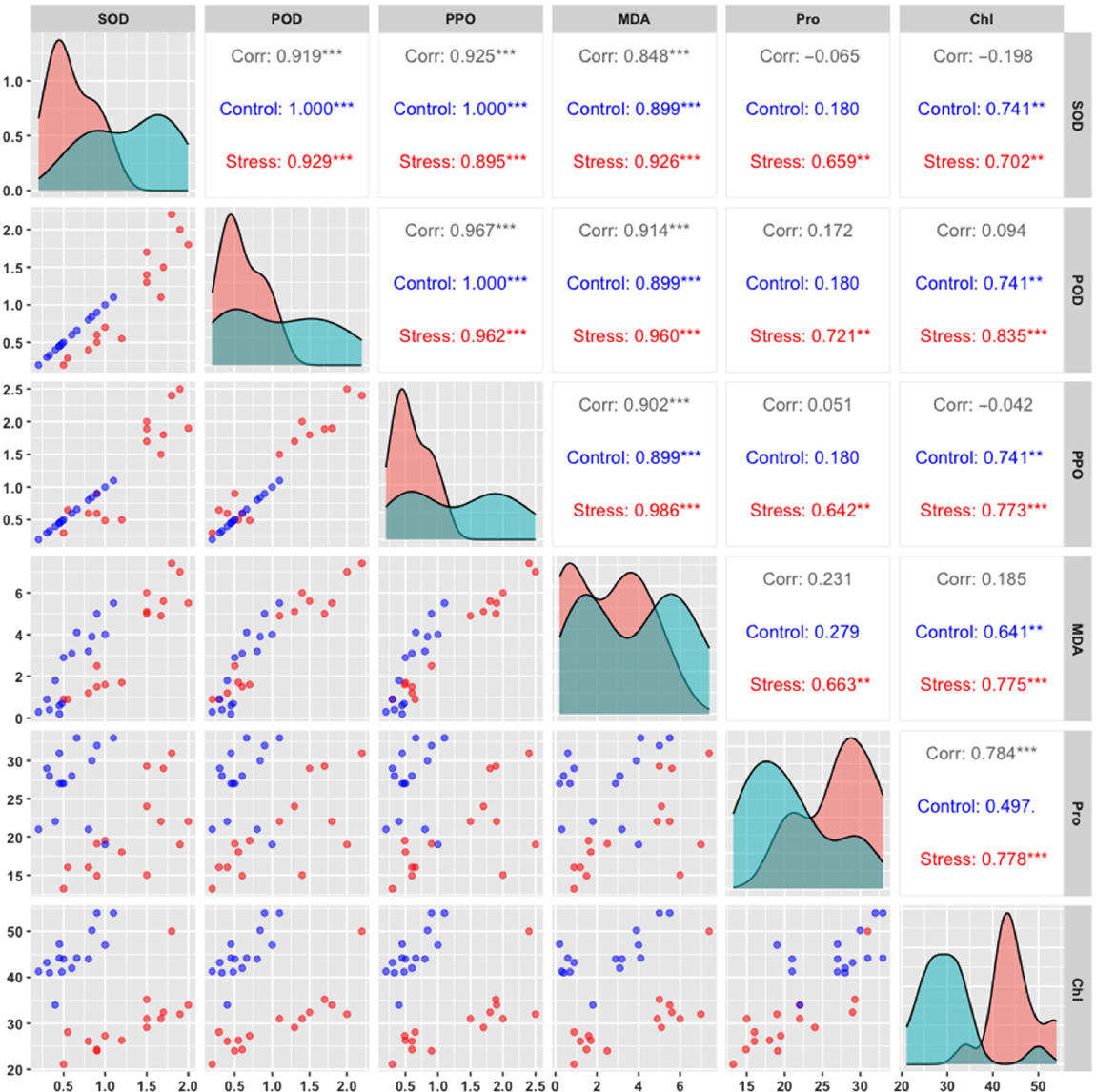
Based on the correlation analysis among antioxidant enzymes and biochemical parameters under control and fungal stress conditions in chickpea genotypes (Fig. 6), SOD activity showed a strong positive correlation with POD in both controls (r = 0.967***) and stress conditions (r = 0.962***), indicating that these enzymes might function synergistically in response to fungal stress. SOD also demonstrated a significant positive correlation with PPO under stress (r = 0.926***) and control (r = 0.899***) conditions, suggesting a coordinated antioxidant response involving both SOD and PPO across conditions. POD was strongly correlated with PPO in both conditions (control: r = 0.914***, stress: r = 0.960***), which further emphasizes the close association between these enzymes in managing oxidative stress. MDA, a marker for oxidative damage, displayed significant correlations with SOD (control: r = 0.899***, stress: r = 0.986***), indicating that increased oxidative damage is associated with upregulated SOD activity under stress. Protein content showed moderate positive correlations with SOD (r = 0.659**) and POD (r = 0.642**) under stress, suggesting that higher protein content may support antioxidant enzyme activity during stress. Chlorophyll content correlated positively with SOD (control: r = 0.741***, stress: r = 0.702***) and MDA (control: r = 0.641**, stress: r = 0.775***), highlighting the impact of oxidative stress on chlorophyll stability. These correlations reveal significant associations among antioxidant enzymes and biochemical parameters, emphasizing the coordinated antioxidative and protective responses in chickpea genotypes under fungal stress conditions.
Correlation between antioxidant enzymes, protein content, and chlorophyll level of chickpea genotypes under fungal stress and control conditions. Pearson’s correlation analysis shows relationships among SOD, POD, PPO, MDA, protein, and chlorophyll, highlighting coordinated stress responses in chickpea genotypes (P < 0.05). Superoxide dismutase (SOD), peroxidase activity (POD), polyphenol oxidase (PPO), malondialdehyde content (MDA), protein content (Pro), chlorophyll content (Chl). Asterisk *** denotes highly significant (P < 0.001), ** denotes highly significant (P < 0.01), * denotes significant (P < 0.05).
PCA assessment of antioxidant enzyme and chickpea genotypes
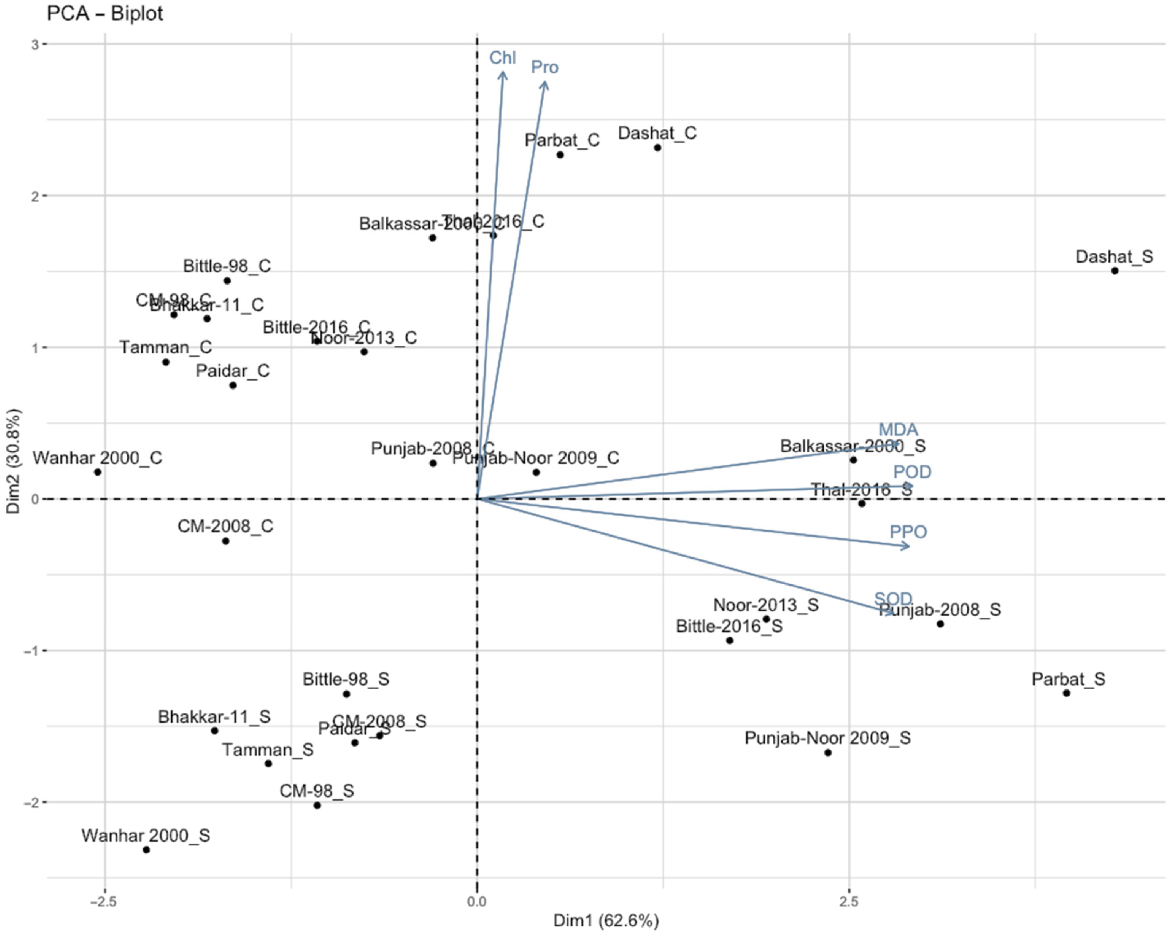
PCA was performed to assess the variation in antioxidant enzyme activities among different chickpea genotypes. The PCA biplot (Fig. 7) displays the relationships between genotypes and enzyme activities under fungal conditions. The first principal component (PC1) accounted for 62.6% of the total variance, indicating that this component captures the primary variability in antioxidant enzyme response across genotypes. Parameters such as MDA, POD, and PPO were positively loaded on PC1, showing a strong association among these antioxidant enzymes under stress. The second principal component (PC2) contributed 30.8% of the total variance, with SOD loading positively on this axis. Together, PC1 and PC2 explain 93.4% of the variation in the data, enabling a clear distinction among genotypes based on their antioxidant enzyme activity profiles. Genotypes clustered on the right side of the biplot exhibit high activities of POD and PPO enzymes, indicating a potential tolerance to fungal stress conditions, whereas those on the left, which are less associated with these parameters, may show lower tolerance. The distribution of genotypes on the biplot reveals diversity in antioxidant enzyme responses to fungal stress, suggesting variability in their ability to withstand disease conditions. This information could be valuable in identifying disease-tolerant chickpea genotypes for breeding programs aimed at improving crop resilience.
PCA analysis of chickpea genotypes under control (C) and fungal disease (S) conditions. PC1 and PC2 explain 93.4% of the total variance, distinguishing stress-tolerant from susceptible genotypes. SOD = superoxide dismutase, POD = peroxidase activity, PPO = polyphenol oxidase, MDA = malondialdehyde content, Pro = protein content, Chl = chlorophyll content.
Cluster analysis of chickpea genotypes
Hierarchical clustering under disease conditions was performed and based on the chickpea performance. The 15 genotypes were divided into four groups: resistant, moderately resistant, susceptible, and highly susceptible (Fig. 8). Out of the 15 genotypes only two were categorized into the first group, six in the second group (moderately resistant), six genotypes in the third group (susceptible), and the fourth group contained one chickpea genotype.
Expression analysis of fungal susceptible and resistant genotypes
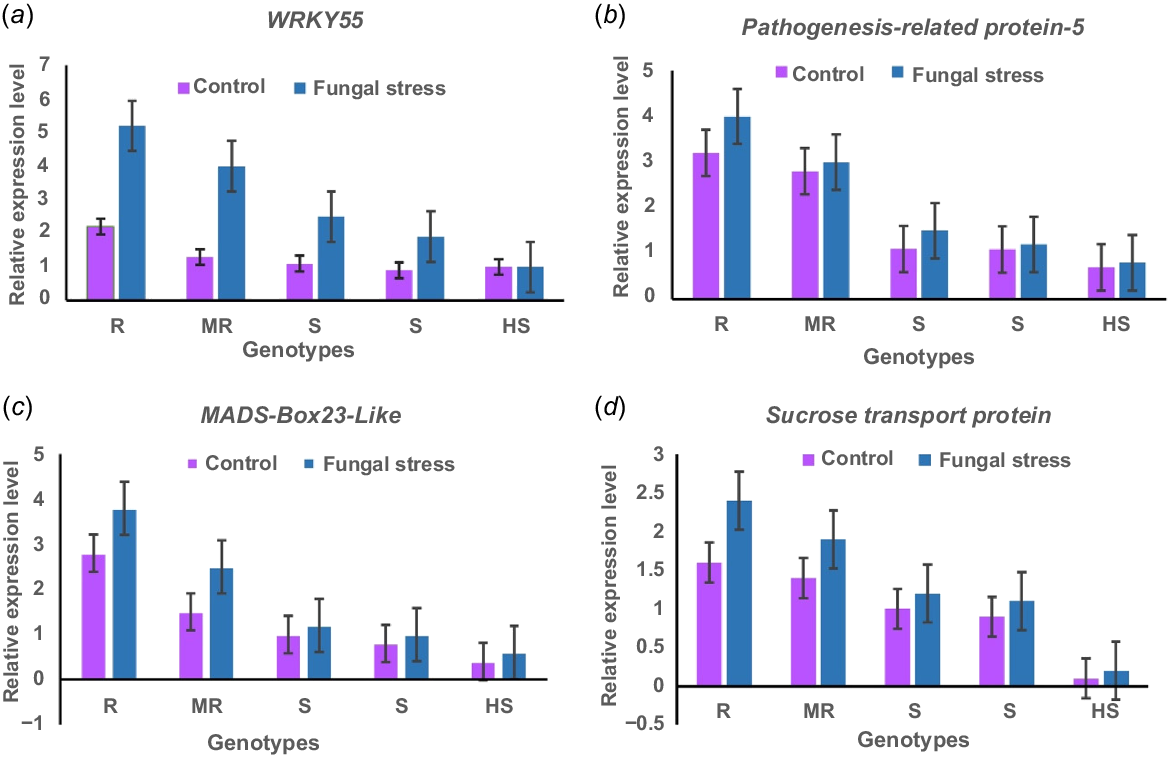
Real-time PCR-based expression revealed differential gene expression patterns across the five chickpea varieties, Parbat (R), Noor2013 (MR), Bittle 98 and Bhakkar-11 (both S), and Wanhar 2000 (HS), in response to fungal stress (Fig. 9). These variations in gene expression provide insights into the molecular mechanisms underlying resistance and susceptibility to fungal pathogens. The resistant genotype Parbat (R) showed an upregulation of WRKY55, pathogenesis-related protein-5, MADS-box transcription factor 23-like, and sucrose transport protein SUC4 target genes. Notably, WRKY55 showed a higher level of upregulation, emphasizing its potential role in providing resistance against fungal pathogens. Both MADS-box transcription factor 23-like and pathogenesis-related proteins exhibited significantly elevated expression levels, indicating a robust defense mechanism. Additionally, a moderate upregulation of sucrose transport protein SUC4 was also observed, suggesting its involvement in the plant’s adaptive response to fungal stress. In contrast, Noor2013 exhibited a more variable gene expression response under fungal stress. WRKY55 and pathogenesis-related protein showed moderate upregulation, though to a lesser degree than in the resistant variety, reflecting an intermediate defense capability. In contrast, sucrose transport protein SUC4 displayed minimal expression changes, suggesting a less critical role in the Noor-2013 defense mechanism compared to Parbat. In the susceptible varieties, Bittle 98 and Bhakkar-11, WRKY55 and pathogenesis-related protein showed minimal upregulation in response to fungal infection, indicating a weak defense response. These results imply that reduced upregulation of key genes may contribute to their vulnerability to fungal infection. Overall, the findings suggest that higher expression levels of WRKY55 and MADS-box transcription factor 23-like are associated with enhanced resistance in chickpea varieties, whereas lack of upregulation in susceptible varieties may contribute to increased vulnerability to fungal pathogens.
Real-time PCR-based expression analysis of stress-responsive genes in resistant, moderately resistant, susceptible, and highly susceptible chickpea genotypes. qPCR analysis of (a) WRKY55, (b) Pathogenesis-related protein-5, (c) MADS-Box-transcription factor 23-like, and (d) Sucrose transport protein SUC4 under control and stress conditions. Where, R = Parbat, MR = Noor2013, S = bittle 98 and Bhakkar-11, HS = Wanhar 2000. CaActin was used for the normalization of data. Bars represent mean fold changes (±s.e.).
Discussion
Plant diseases pose a significant challenge to global chickpea production by disrupting vital physiological processes, such as photosynthesis, cell division, water transport, and overall growth. Among phytopathogens, fungal infections are particularly damaging, accounting for up to 65% of the estimated 12.5% global crop losses due to biotic stress (Li et al. 2022). In recent years, biotic factors have increasingly contributed to yield stagnation or decline in chickpea (Caballo et al. 2019). Fusarium wilt, caused by Fusarium oxysporum f. sp. ciceris, remains a major constraint in chickpea production. This pathogen is transmitted through both seeds and soil, displaying two pathotypes and eight pathogenic races (Sabbavarapu et al. 2013; Pandey et al. 2016). The development of resistant chickpea lines remains the most effective strategy for managing Fusarium wilt. Breeding programs have successfully produced resistant lines and identified DNA markers associated with resistance to Fusarium wilt (Jendoubi et al. 2017). However, resistance is not always consistent across different environments or over time, underscoring the need for ongoing research. This study advances current understanding by integrating biochemical, physiological, and molecular responses, but also identifying WRKY55, MADS-box transcription factor 23-like, PR-5, and SUC4 as novel molecular markers linked to Fusarium resistance in chickpea.
The DSI demonstrated substantial variation in infection levels across chickpea cultivars that highlighted genotypic differences in susceptibility (Aslam et al. 2021). Increased DSI correlated with reductions in morphological traits, including plant height and root length, demonstrating the pathogen’s detrimental effect on growth and biomass production. Fusarium wilt restricts nutrient and water transport, leading to stunted growth, compromised root development, and reduced plant biomass, which affects plant health and productivity (Yadav et al. 2023). In resistant genotypes, the pathogen is managed more efficiently through antioxidant responses, whereas in susceptible genotypes, these mechanisms are either delayed or insufficient to combat the infection, leading to pronounced disease symptoms.
Antioxidant enzymes play a critical role in mitigating oxidative stress induced by fungal pathogens. We observed significant upregulation of SOD, POD, and PPO activities in resistance cultivars, indicating an upregulated antioxidative defense mechanism (Mahbub et al. 2021). The increased SOD activity is a direct response to the enhanced production of reactive oxygen species (ROS) triggered by fungal infection, converting superoxide radicals (O2−) into hydrogen peroxide (H2O2). POD and PPO function synergistically to detoxify H₂O₂, reinforce cell walls through lignification, and restrict pathogen invasion (Mahbub et al. 2021). WRKY55 regulate these enzyme activities by modulating oxidative stress responses, particularly through ROS-scavenging pathways. The positive correlations between these enzymes suggest a coordinated response that effectively mitigates oxidative stress, preventing further cellular damage and maintaining membrane integrity, restricting pathogen invasion (Kinfe et al. 2015). Beyond their individual enzymatic actions, these antioxidant systems are intricately connected with ROS-mediated signaling pathways (Huang et al. 2025; Sun et al. 2025), where transient ROS bursts can serve as secondary messengers to activate defense gene expression. Hormonal networks, particularly salicylic acid and jasmonic acid, likely modulate the antioxidant response, with salicylic acid generally promoting ROS accumulation for hypersensitive response activation and jasmonic acid orchestrating downstream stress tolerance responses (Zeyad et al. 2022). Thus, the interplay between ROS-scavenging enzymes and phytohormonal signaling cascades adds an additional regulatory dimension to the observed antioxidative patterns. Notably, chickpea exhibits a distinct defense strategy by showing particularly high PPO induction under Fusarium wilt, suggesting its significant role in long-term defense against the pathogen (Jameel et al. 2021). These findings are consistent with studies in other legumes, such as soybeans and lentil, where enhanced antioxidant activities were observed in disease resistant varieties (Matemu et al. 2021; Hernández-Ruiz et al. 2025). However, cultivar-specific analysis highlights that resistant genotypes exhibit a unique antioxidant enzyme expression pattern, further emphasizing genotypic variation in defense strategies.
Lipid peroxidation, as indicated by MDA content, increased under Fusarium wilt, showing oxidative damage to the cellular membrane (Farhana et al. 2022). The increased MDA levels indicate that Fusarium-induced ROS accumulation disrupts membrane lipid stability, triggering lipid peroxidation and initiating programmed cell death pathways in susceptible cultivars. (Thakur et al. 2023). The protein content displayed varied responses across genotypes, suggesting differential protein turnover as a part of the stress response (Wallace et al. 2016). The decreased protein content in some genotypes could be attributed to increased proteolytic activity under stress conditions, whereas other genotypes might retain or even increase protein levels as a protective mechanism. Chlorophyll content was significantly decreased in stressed chickpea plants, consistent with the photoinhibition typically observed under biotic stress (Alsamman et al. 2024). The decreased chlorophyll levels indicate impaired photosynthetic efficiency, which could further exacerbate stress-induced growth reduction (Li et al. 2025).
The correlation analysis of antioxidant enzymes and biochemical traits revealed key insights into the stress response. Positive correlations between SOD, POD, PPO, and MDA reinforce the notion that antioxidant enzymes work synergistically to counteract ROS damage. Additionally, significant associations between chlorophyll content and antioxidant enzyme activities suggest a linkage between oxidative stress response and photosynthetic efficiency (Costantini et al. 2021). These results imply that efficient regulation of the oxidative burst not only mitigates immediate cellular damage but may also preserve photosynthetic competence and growth potential during pathogenic stress. The combined action of these enzymes likely acts as a stabilizing force for cellular structures, ensuring functional continuity under stress conditions (Perez-Perez et al. 2021). Such correlation patterns not only highlight the interconnected nature of these physiological responses but also underscore the potential of these enzymes as biomarkers for Fusarium wilt tolerance. Unlike previous reports, this study integrates both biochemical and molecular data to propose a combined marker set for more accurate screening of resistant chickpea cultivars.
Real-time quantitative PCR analysis of defense-related genes confirmed chickpea response to fungal stress. The upregulation of genes involved in ROS scavenging, cell wall fortification, and pathogen defense correlated with the observed physiological and biochemical data (Shan et al. 2025). Notably, antioxidant enzyme-related genes showed increased expression levels, indicating a transcriptional response tailored to ROS detoxification (Maury et al. 2020). In the present study, WRKY55, MADS-box TF23-like, SUC4, and PR5 genes were selected based on their previously established roles in mediating defense responses against Fusarium oxysporum in chickpea (Caballo et al. 2019). These genes are associated with transcriptional regulation, sugar transport, and pathogenesis-related defense activation. Real-time PCR-based expression analysis was conducted across five chickpea varieties representing resistant to highly susceptible, enabling a comprehensive evaluation of genotype-specific defense mechanisms. This study establishes WRKY55 as a key regulator of Fusarium wilt resistance in chickpea, linking its expression to enhanced antioxidant enzyme activity. WRKY55 influences ROS homeostasis by activating key oxidative stress-related enzymes, leading to increased enzymatic defense responses. WRKY55 has been extensively studied in legumes, such as Medicago and soybean, where it plays a pivotal role in salicylic acid-mediated signaling (Mao et al. 2020; Zhao et al. 2024). Our findings suggest that WRKY55 not only regulates antioxidant defenses but also participates in pathogen recognition, potentially influencing broader defense signaling networks. Additionally, the significant upregulation of MADS-box 23-like in resistant genotypes suggests its involvement in defense activation (Castelán-Muñoz et al. 2019;Xin et al. 2025). MADS-box 23-like transcription factor has been identified as a novel component of the Fusarium defense response in chickpea. Although MADS-box genes have been linked to stress adaptation in Phyllostachys edulis (Zhang et al. 2018) and Arabidopsis thaliana (Castañón-Suárez et al. 2024), their specific role in Fusarium resistance in chickpea has remained largely unexplored. Results suggest that MADS-box genes may interact with WRKY55 to modulate stress responses, possibly through a hormone-dependent signaling network involving salicylic acid and jasmonic acid (Sun et al. 2024). The alignment of transcriptional and enzymatic responses further validates the functional significance of these genes in chickpea’s defense mechanism (Mahbub et al. 2021). The identification of WRKY55 and MADS-box transcription factors as key players in Fusarium wilt resistance has significant implications for chickpea breeding programs. These molecular markers can be integrated into marker-assisted selection (MAS) strategies to accelerate the development of stress-resilient chickpea varieties. Moreover, the enhanced activities of antioxidant enzymes (SOD, POD, PPO) and the associated gene expression patterns provide valuable phenotypic and molecular criteria for screening resistant genotypes.
This study provides an integrative analysis that links agronomic, physiological, and molecular findings in understanding Fusarium wilt resistance. By examining defense mechanisms differentially activated in resistant and susceptible genotypes, we present a cohesive framework for interpreting chickpea’s response to Fusarium infection. The integrative approach used in this study provides a comprehensive understanding of the multifaceted defense mechanisms in chickpea against Fusarium wilt (Kukreja et al. 2018). Given the genetic variability among chickpea cultivars, breeding efforts should focus on incorporating these resilience traits across diverse agro-climatic zones to ensure durable resistance (Foresto et al. 2023). The screening of chickpea varieties, leading to the identification of resistant varieties, is crucial for future plant disease management. The identified traits, such as enhanced antioxidant enzyme activity (SOD, POD, PPO) and upregulated WRKY55 expression, can serve as biomarkers for selecting Fusarium wilt-resistant chickpea genotypes in breeding programs. Integrating these molecular and physiological markers into MAS strategies can accelerate the development of resistant cultivars, improving chickpea yield stability under disease stress. Additionally, these findings can aid in genome editing approaches to enhance resistance in susceptible varieties. Thus, this study not only confirms previous findings but also introduces novel insights by providing cultivar-specific defense profiles and highlighting new candidate genes for resistance breeding. Given chickpea’s high protein content and significant role in global nutrition, this study’s findings offer valuable insights for breeders aiming to develop effective resistance breeding programs. Identifying fungal tolerance and understanding the genetic basis for resistance will be crucial in enhancing chickpea’s resilience to Fusarium wilt and other biotic stresses, thereby ensuring sustainable production and food security. Future research should further investigate the molecular pathways activated during fungal stress, with an emphasis on regulatory networks controlling antioxidant and defense responses. Expanding genomic and transcriptomic analyses across different chickpea genotypes will facilitate a deeper understanding of resistance mechanisms, ultimately contributing to sustainable agricultural practices and improved crop resilience against pathogenic threats. Our study provides new insights into the defense mechanisms activated by Fusarium wilt in chickpea, offering a more cohesive and integrative understanding of how resistant and susceptible genotypes differ in their response to the pathogen. By linking agronomic traits, physiological responses, and molecular markers, we have identified key factors that contribute to disease resistance in chickpea.
Declaration of funding
The authors acknowledge and appreciate the ongoing research funding program (ORF-2025-1091), King Saud Uni- versity, Riyadh, Saudi Arabia.
Author contributions
All authors contributed to conceptualization, literature review, resources, data curation, writing, original draft preparation, review and editing, and visualization. SI: supervision, project administration, and original draft writing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors acknowledge the research facilities provided by the International Islamic University Islamabad, Pakistan.
References
Agarwal C, Chen W, Varshney RK, Vandemark G (2022) Linkage QTL mapping and genome-wide association study on resistance in chickpea to Pythium ultimum. Frontiers in Genetics 13, 945787.
| Crossref | Google Scholar | PubMed |
Ali H, ul Haq MA, Shah TM, ur Rahman M, Chen W (2012) Validation of molecular markers for resistance among Pakistani chickpea germplasm to races of Fusarium oxysporum f. sp. ciceris. European Journal of Plant Pathology 132(2), 237-244.
| Crossref | Google Scholar |
Alsamman AM, Mousa KH, Istanbuli T, Abd El-Maksoud MM, Tawkaz S, Hamwieh A (2024) Unveiling the genetic basis of Fusarium wilt resistance in chickpea using GWAS analysis and characterization of candidate genes. Frontiers in Genetics 14, 1292009.
| Crossref | Google Scholar |
Ankati S, Srinivas V, Pratyusha S, Gopalakrishnan S (2021) Streptomyces consortia-mediated plant defense against Fusarium wilt and plant growth-promotion in chickpea. Microbial Pathogenesis 157, 104961.
| Crossref | Google Scholar | PubMed |
Arbona V, Flors V, Jacas J, García-Agustín P, Gómez-Cadenas A (2003) Enzymatic and Non-enzymatic antioxidant responses of carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant and Cell Physiology 44(4), 388-394.
| Crossref | Google Scholar | PubMed |
Aslam M, Shah JA, Hussain N, Ghaffar A, Abbas M, Hussan MF, Khan AA, Nadeem M, Irshad M (2021) Chickpea advanced lines screening for sources of resistance against two major diseases of chickpea “wilt and blight.”. Pakistan Journal of Phytopathology 33(2), 369-382.
| Crossref | Google Scholar |
Bharadwaj C, Jorben J, Rao A, Roorkiwal M, Patil BS, Jayalakshmi , Ahammed SK, Saxena DR, Yasin M, Jahagirdar JE, Sontakke PL, Pithia MS, Chudasama MK, Swarup I, Singh RK, Nitesh SD, Chitikineni A, Singh S, Singh I, Pratap A, Dixit GP, Srivastava AK, Varshney RK (2022) Development of high yielding Fusarium wilt resistant cultivar by pyramiding of “genes” through marker-assisted backcrossing in chickpea (Cicer arietinum L.). Frontiers in Genetics 13, 924287.
| Crossref | Google Scholar | PubMed |
Caballo C, Castro P, Gil J, Millan T, Rubio J, Die JV (2019) Candidate genes expression profiling during wilting in chickpea caused by Fusarium oxysporum f. sp. Ciceris race 5. PLoS ONE 14(10), e0224212.
| Crossref | Google Scholar | PubMed |
Castañón-Suárez CA, Arrizubieta M, Castelán-Muñoz N, Sánchez-Rodríguez DB, Caballero-Cordero C, Zluhan-Martínez E, Patiño-Olvera SC, Arciniega-González JA, García-Ponce B, Sánchez MdlP, Álvarez-Buylla ER, Garay-Arroyo A (2024) The MADS-box genes SOC1 and AGL24 antagonize XAL2 functions in Arabidopsis thaliana root development. Frontiers in Plant Science 15, 1331269.
| Crossref | Google Scholar |
Castelán-Muñoz N, Herrera J, Cajero-Sánchez W, Arrizubieta M, Trejo C, García-Ponce B, Sánchez MdlP, Álvarez-Buylla ER, Garay-Arroyo A (2019) MADS-box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Frontiers in Plant Science 10, 853.
| Crossref | Google Scholar |
Chandio AA, Yuansheng J, Magsi H (2016) Agricultural sub-sectors performance: an analysis of sector-wise share in agriculture GDP of Pakistan. International Journal of Economics and Finance 8(2), 156.
| Crossref | Google Scholar |
Chandora R, Gayacharan, Shekhawat N, Malhotra N (2020) Chapter 3 - Chickpea genetic resources: collection, conservation, characterization, and maintenance. In ‘Chickpea: crop wild relatives for enhancing genetic gains’. (Ed. M Singh) pp. 37–61. (Academic Press) doi:10.1016/B978-0-12-818299-4.00003-8
Chen Z, Song Y, Yan Y, Chen W, Ren T, Ma A, Jia Y (2025) Characterization of an epilactose-producing cellobiose 2-epimerase from Clostridium sp. TW13 and reutilization of waste milk. Food Chemistry 480, 143948.
| Crossref | Google Scholar |
Costantini M, Summo C, Centrone M, Rybicka I, D’Agostino M, Annicchiarico P, Caponio F, Pavan S, Tamma G, Pasqualone A (2021) Macro- and micro-nutrient composition and antioxidant activity of chickpea and pea accessions. Polish Journal of Food and Nutrition Sciences 71(2), 177-185.
| Crossref | Google Scholar |
Farhana , Munis MFH, Alamer KH, Althobaiti AT, Kamal A, Liaquat F, Haroon U, Ahmed J, Chaudhary HJ, Attia H (2022) ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium wilt. Journal of Fungi 8(7), 753.
| Crossref | Google Scholar | PubMed |
Faridy J-CM, Stephanie C-GM, Gabriela M-MO, Cristian J-M (2020) Biological activities of chickpea in human health (Cicer arietinum L.). A review. Plant Foods for Human Nutrition 75(2), 142-153.
| Crossref | Google Scholar | PubMed |
Foresto E, Carezzano ME, Giordano W, Bogino P (2023) Ascochyta blight in chickpea: an update. Journal of Fungi 9(2), 203.
| Crossref | Google Scholar | PubMed |
Gupta RK, Gupta K, Sharma A, Das M, Ansari IA, Dwivedi PD (2017) Health risks and benefits of chickpea (Cicer arietinum) consumption. Journal of Agricultural and Food Chemistry 65(1), 6-22.
| Crossref | Google Scholar | PubMed |
Hernández-Ruiz RG, Olivares-Ochoa XC, Salinas-Varela Y, Guajardo-Espinoza D, Roldán-Flores LG, Rivera-Leon EA, López-Quintero A (2025) Phenolic compounds and anthocyanins in legumes and their impact on inflammation, oxidative stress, and metabolism: comprehensive review. Molecules 30(1), 174.
| Crossref | Google Scholar |
Huang X, Tang X, Liao A, Sun W, Lei L, Wu J (2025) Application of cyclopropane with triangular stable structure in pesticides. Journal of Molecular Structure 1326, 141171.
| Crossref | Google Scholar |
Irum S, Rehman N, Inam S, Khan MZF, Khan MR (2024) Genome-wide identification and expression profiling of Pseudo-Response Regulator (PRR) gene family in tomato. Environmental and Experimental Botany 220, 105683.
| Crossref | Google Scholar |
Jameel S, Hameed A, Shah TM (2021) Biochemical profiling for antioxidant and therapeutic potential of Pakistani chickpea (Cicer arietinum L.) genetic resource. Frontiers in Plant Science 12, 663623.
| Crossref | Google Scholar |
Jan M, Haq TU, Sattar H, Butt M, Khaliq A, Arif M, Rauf A (2020) Evaluation and screening of promising drought tolerant chickpea (Cicer arietinum L.) genotypes based on physiological and biochemical attributes under drought conditions. Pakistan Journal of Agricultural Research 33(2), 662-672.
| Crossref | Google Scholar |
Jendoubi W, Bouhadida M, Boukteb A, Béji M, Kharrat M (2017) Fusarium wilt affecting chickpea crop. Agriculture 7(3), 23.
| Crossref | Google Scholar |
Kinfe E, Singh P, Fekadu T (2015) Physicochemical and functional characteristics of desi and kabuli chickpea (Cicer arietinum L.) cultivars grown in Bodity, Ethiopia and sensory evaluation of boiled and roasted products prepared using chickpea varieties. International Journal of Current Research in Biosciences and Plant Biology 2(4), 21-29 Available at https://idl-bnc-idrc.dspacedirect.org/handle/10625/54415.
| Google Scholar |
Kukreja S, Salaria N, Thakur K, Goutam U (2018) Fungal disease management in chickpea: current status and future prospects. In ‘Fungi and their role in sustainable development: current perspectives’. (Eds P Gehlot, J Singh) pp. 293–309. (Springer: Singapore) doi:10.1007/978-981-13-0393-7_17
Kumar R, Singh RK, Misra JP, Yadav A, Kumar A, Yadav R, Hegde VS, Kumar S, Yadav N (2021) Dissecting proteomic estimates for enhanced bioavailable nutrition during varied stages of germination and identification of potential genotypes in chickpea. Legume Research - An International Journal 45(9), 1082-1087.
| Crossref | Google Scholar |
Labidi O, Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM, Sleimi N (2021) Assessing of growth, antioxidant enzymes, and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Science & Nutrition 9(4), 2021-2031.
| Crossref | Google Scholar | PubMed |
Li Z, Liu X, Zhang M, Xing F (2022) Plant diversity and fungal richness regulate the changes in soil multifunctionality in a semi-arid grassland. Biology 11(6), 870.
| Crossref | Google Scholar | PubMed |
Li H, Tang Y, Meng F, Zhou W, Liang W, Yang J, Wang H, Guo J, Yang Q, Shao R (2025) Transcriptome and metabolite reveal the inhibition induced by combined heat and drought stress on the viability of silk and pollen in summer maize. Industrial Crops and Products 226, 120720.
| Crossref | Google Scholar |
Liu Y, Jia B, Ren Y, Xun W, Stefanic P, Yang T, Miao Y, Zhang N, Yao Y, Zhang R, Xu Z, Shen Q, Mandic-Mulec I (2025) Bacterial social interactions in synthetic Bacillus consortia enhance plant growth. iMeta 4(4), e70053.
| Crossref | Google Scholar |
Mahbub R, Francis N, Blanchard CL, Santhakumar AB (2021) The anti-inflammatory and antioxidant properties of chickpea hull phenolic extracts. Food Bioscience 40, 100850.
| Crossref | Google Scholar |
Mao P, Jin X, Bao Q, Mei C, Zhou Q, Min X, Liu Z (2020) WRKY transcription factors in Medicago sativa L.: genome-wide identification and expression analysis under abiotic stress. DNA and Cell Biology 39(12), 2212-2225.
| Crossref | Google Scholar |
Matemu A, Nakamura S, Katayama S (2021) Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 10(2), 316.
| Crossref | Google Scholar | PubMed |
Maury GL, Rodríguez DM, Hendrix S, Arranz JCE, Boix YF, Pacheco AO, Díaz JG, Morris-Quevedo HJ, Dubois AF, Aleman EI, Beenaerts N, Méndez-Santos IE, Ratón TO, Cos P, Cuypers A (2020) Antioxidants in plants: a valorization potential emphasizing the need for the conservation of plant biodiversity in cuba. Antioxidants 9(11), 1048.
| Crossref | Google Scholar | PubMed |
Maurya O, Kumar H (2018) Growth of chickpea production in India. Journal of Pharmacognosy and Phytochemistry 7(5), 1175-1177.
| Google Scholar |
Nirmaladevi D, Venkataramana M, Srivastava RK, Uppalapati SR, Gupta VK, Yli-Mattila T, Clement Tsui KM, Srinivas C, Niranjana SR, Chandra NS (2016) Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f. sp. lycopersici. Scientific Reports 6, 21367.
| Crossref | Google Scholar | PubMed |
Pandey MK, Roorkiwal M, Singh VK, Ramalingam A, Kudapa H, Thudi M, Chitikineni A, Rathore A, Varshney RK (2016) Emerging genomic tools for legume breeding: current status and future prospects. Frontiers in Plant Science 7, 455.
| Crossref | Google Scholar |
Perez-Perez LM, Huerta-Ocampo JÁ, Ruiz-Cruz S, Cinco-Moroyoqui FJ, Wong-Corral FJ, Rascón-Valenzuela LA, Robles-García MA, González-Vega RI, Rosas-Burgos EC, Corella-Madueño MAG, Del-Toro-Sánchez CL (2021) Evaluation of quality, antioxidant capacity, and digestibility of chickpea (Cicer arietinum L. cv Blanoro) stored under N2 and CO2 atmospheres. Molecules 26(9), 2773.
| Crossref | Google Scholar | PubMed |
Rachwa-Rosiak D, Nebesny E, Budryn G (2015) Chickpeas – composition, nutritional value, health benefits, application to bread and snacks: a review. Critical Reviews in Food Science and Nutrition 55(8), 1137-1145.
| Crossref | Google Scholar | PubMed |
Rafiq C, Mahmood M, Ahmad M, Ali I, Saleem M, Rasool I, Ali Z (2020) Differential response of elite chickpea genotypes under moisture stress conditions. Pakistan Journal of Agricultural Research 33(3), 422-428.
| Crossref | Google Scholar |
Sabarre DC, Jr, Yagonia-Lobarbio CF (2021) Extraction and characterization of polyphenol oxidase from plant materials: a review. Journal of Applied Biotechnology Reports 8(2), 83-95.
| Crossref | Google Scholar |
Sabbavarapu MM, Sharma M, Chamarthi SK, Swapna N, Rathore A, Thudi M, Gaur PM, Pande S, Singh S, Kaur L, Varshney RK (2013) Molecular mapping of QTLs for resistance to Fusarium wilt (race 1) and Ascochyta blight in chickpea (Cicer arietinum L.). Euphytica 193(1), 121-133.
| Crossref | Google Scholar |
Shan C, Dong K, Wen D, Cui Z, Cao J (2025) A review of m6A modification in plant development and potential quality improvement. International Journal of Biological Macromolecules 308, 142597.
| Crossref | Google Scholar | PubMed |
Singh C, Vyas D (2021) The trends in the evaluation of Fusarium wilt of chickpea. In ‘Chapter 6: Diagnostics of plant diseases’. (Ed. D Kurouski) pp. 1–15. (IntechOpen) doi:10.5772/intechopen.95612
Sun Y, Xie X, Jiang C-J (2024) Antioxidant agriculture for stress-resilient crop production: field practice. Antioxidants 13(2), 164.
| Crossref | Google Scholar |
Sun S, Xie W, Wang G, Zhang W, Hu Z, Sun X, DeLuca TH (2025) Evidence for phosphorus cycling parity in nodulating and non-nodulating N2-fixing pioneer plant species in glacial primary succession. Functional Ecology 39(4), 985-1000.
| Crossref | Google Scholar |
Thakur R, Sharma S, Devi R, Sirari A, Tiwari RK, Lal MK, Kumar R (2023) Exploring the molecular basis of resistance to Botrytis cinerea in chickpea genotypes through biochemical and morphological markers. PeerJ 11, e15560.
| Crossref | Google Scholar | PubMed |
Thudi M, Chitikineni A, Liu X, He W, Roorkiwal M, Yang W, Jian J, Doddamani D, Gaur PM, Rathore A, Samineni S, Saxena RK, Xu D, Singh NP, Chaturvedi SK, Zhang G, Wang J, Datta SK, Xu X, Varshney RK (2016) Recent breeding programs enhanced genetic diversity in both desi and kabuli varieties of chickpea (Cicer arietinum L.). Scientific Reports 6(1), 38636.
| Crossref | Google Scholar |
Ullah A, Shah TM, Farooq M (2020) Pulses production in Pakistan: status, constraints and opportunities. International Journal of Plant Production 14(4), 549-569.
| Crossref | Google Scholar |
Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar’an B, Millan T, Zhang X, Ramsay LD, Iwata A, Wang Y, Nelson W, Farmer AD, Gaur PM, Soderlund C, Penmetsa RV, Xu C, Bharti AK, He W, Winter P, Zhao S, Hane JK, Carrasquilla-Garcia N, Condie JA, Upadhyaya HD, Luo M-C, Thudi M, Gowda CLL, Singh NP, Lichtenzveig J, Gali KK, Rubio J, Nadarajan N, Dolezel J, Bansal KC, Xu X, Edwards D, Zhang G, Kahl G, Gil J, Singh KB, Datta SK, Jackson SA, Wang J, Cook DR (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nature Biotechnology 31(3), 240-246.
| Crossref | Google Scholar |
Wallace TC, Murray R, Zelman KM (2016) The nutritional value and health benefits of chickpeas and hummus. Nutrients 8(12), 766.
| Crossref | Google Scholar | PubMed |
Xin W, Zheng H, Yang L, Xie S, Xia S, Wang J, Wang M, Liu Y, Zou D, Liu H, Wang J (2025) Genome-wide association studies identify OsNLP6 as a key regulator of nitrogen use efficiency in rice. Plant Biotechnology Journal
| Crossref | Google Scholar |
Yadav RK, Tripathi MK, Tiwari S, Tripathi N, Asati R, Patel V, Sikarwar RS, Payasi DK (2023) Breeding and genomic approaches towards development of fusarium wilt resistance in chickpea. Life 13(4), 988.
| Crossref | Google Scholar |
Zeyad MT, Tiwari P, Ansari WA, Kumar SC, Kumar M, Chakdar H, Srivastava AK, Singh UB, Saxena AK (2022) Bio-priming with a consortium of Streptomyces araujoniae strains modulates defense response in chickpea against Fusarium wilt. Frontiers in Microbiology 13, 998546.
| Crossref | Google Scholar | PubMed |
Zhang Y, Tang D, Lin X, Ding M, Tong Z (2018) Genome-wide identification of MADS-box family genes in moso bamboo (Phyllostachys edulis) and a functional analysis of PeMADS5 in flowering. BMC Plant Biology 18(1), 176.
| Crossref | Google Scholar | PubMed |
Zhang Y, Luan Q, Jiang J, Li Y (2021) Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy. Frontiers in Plant Science 12, 735275.
| Crossref | Google Scholar |
Zhao Z, Wang R, Su W, Sun T, Qi M, Zhang X, Wei F, Yu Z, Xiao F, Yan L, Yang C, Zhang J, Wang D (2024) A comprehensive analysis of the WRKY family in soybean and functional analysis of GmWRKY164-GmGSL7c in resistance to soybean mosaic virus. BMC Genomics 25, 620.
| Crossref | Google Scholar | PubMed |