Outcomes of dermoscope-guided surgical procedures in primary care: case-control study
Antonio Chuh 1 2 6 , Vijay Zawar 3 , Gabriel Sciallis 4 , Regina Fölster-Holst 51 Department of Family Medicine and Primary Care, The University of Hong Kong and Queen Mary Hospital, Pokfulam, Hong Kong
2 Hong Kong Society of Primary Care Dermoscopy, Hong Kong
3 Department of Dermatology, Dr Vasantrao Pawar Medical College, Nashik, India
4 Department of Dermatology, Mayo Medical School, Minnesota, USA
5 Universitätsklinikum Schleswig-Holstein, Campus Kiel, Dermatologie, Venerologie und Allergologie, Germany
6 Corresponding author. Email: antonio.chuh@yahoo.com.hk
Journal of Primary Health Care 11(1) 54-63 https://doi.org/10.1071/HC18064
Published: 8 March 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: No research has been found regarding outcomes of dermoscope-guided surgical procedures in primary care.
AIM: To establish whether outcomes of dermoscope-guided procedures performed in primary care settings differ from outcomes for similar procedures, performed without the use of a dermoscope.
METHODS: A retrospective case-control study design was used. All records of dermoscope-guided procedures performed over a 6-month period were retrieved. For each study procedure, the record of the most recent control procedure without dermoscopy guidance performed on a sex-and-age matched patient was retrieved from before we began performing dermoscope-guided procedures. Primary outcomes were: local inflammation and infections within 2 weeks’ post procedure; relapse in 6 months; and obvious scars in 6 months. Pain affecting activities of daily living in the first week after the procedure was the secondary outcome.
RESULTS: Records of 39 dermoscope-guided procedures and 39 control procedures were retrieved. No significant difference in local inflammation and infections in 2 weeks was found; relapse in 6 months after the study procedures was significantly lower for dermoscope-guided than control procedures (risk ratio (RR): 0.22; 95% confidence interval (CI): 0.05–0.95), and there were fewer obvious scars for dermoscope-guided procedures than control procedures (RR: 0.52; 95% CI: 0.32–0.83), with the number of small lesions (<4 mm) leaving scars in study procedures particularly less than that for control procedures (RR: 0.30; 95% CI: 0.13–0.67). There was no difference in the secondary outcome of pain affecting activities of daily living in the first week following the procedure.
CONCLUSION: In primary care, dermoscope-guided procedures achieved better outcomes than similar procedures without dermoscope guidance. Performing dermoscope-guided procedures in primary care might lower medical costs.
KEYWORDS: Dermoscopy; general practice; laser procedures; primary health care; skin biopsy; skin microscopy
| WHAT GAP THIS FILLS |
| What is already known: Dermoscope-guided surgical procedures have been reported previously in specialist settings. The outcomes of these procedures performed in primary care settings have not been investigated. |
| What this study adds: Dermoscope guidance in 39 procedures achieved significantly less relapse and less obvious scars than 39 control procedures without dermoscope guidance performed on age-and-sex matched controls. |
Introduction
Dermoscopes are instruments used to examine the skin clearly by magnification and epiluminescence – ablating reflections from the skin surface to allow deeper structures to be visualised. They are widely used to diagnose skin cancers and other skin diseases. Dermoscope-guided surgical procedures are relatively new. We have previously reported dermoscope-guided (DG) excisional biopsy,1 DG punch biopsy2 and DG suturing.3 We aimed to find out if outcomes of DG procedures performed in primary care differ from outcomes for similar procedures, performed without the use of a dermoscope. We report here a case-control study comparing local inflammation, infections and pain after DG surgical procedures and after usual surgical procedures.
Methods
Setup of the procedures
The setting is a solo general practice where the general practitioner (GP) has a special interest in dermatology (AC). Patients can attend our care without referrals. The practice is affiliated with a university teaching hospital.
The room required for DG surgical procedures is the same as for minor operations. One properly trained clinician and one or two trained assistants is all that is required. They must be able to approach both sides of the patient lying on the couch. For practices with only one consultation room, DG surgical procedures can be performed if these requirements are met.
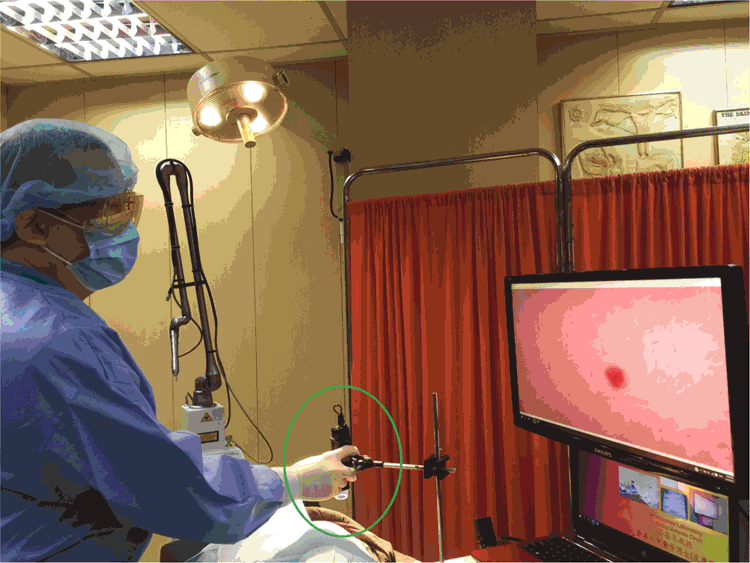
Hand-held dermoscopes are inadequate for DG surgical procedures. The middle and right models with cross-polarisation in Figure 1 are suitable. We secure the dermoscope by clamping it to a sturdy steel stand, so that the receiver is vertically above but not touching the surgical field (Fig. 2), so that the dermoscope does not get into the way of scalpels, laser tips and other surgical equipment. We then connect the dermoscope to a computer outputting signals to a monitor. The depth of the tissue inspected is adjusted by the extent of polarisation. The magnification is governed by the distance between the dermoscope receiver and the surgical field – longer distances result in lower magnifications.
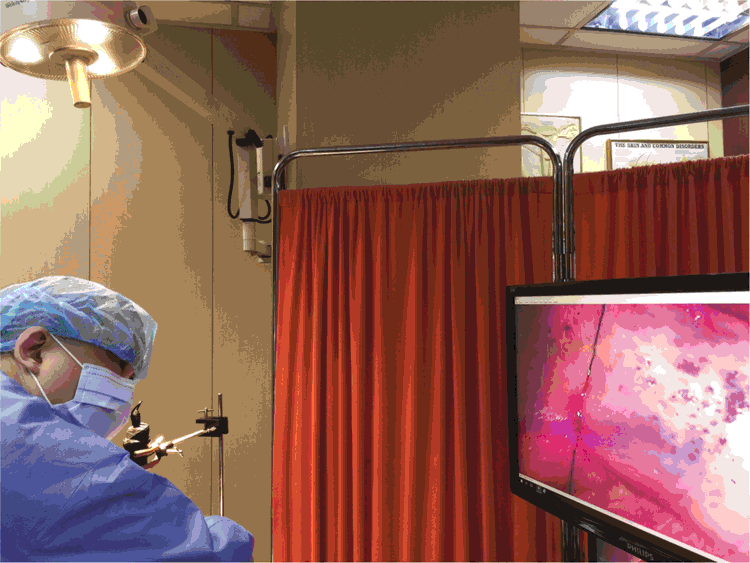
After administration of perilesional anaesthetic agent, we fix our eyes mainly on the monitor during the procedures. The heads of surgical instruments such as forceps can be visualised even after insertion into the surgical field, and can no longer be visualised by the naked eye. Suturing from inside the mucosal surfaces is also possible (Fig. 3). The risk of infection transmission should be considered. In this case study, we placed a microscope slide between the dermoscope and the skin in examinations.4,5 During dermoscope-guided surgical procedures (DGSP), the head of the dermoscope was not in contact with any tissue.
|
Case-control study
When we started performing DG surgical procedures, we had no academic intention. We published three case reports subsequently1–3 and then decided to scientifically investigate patient outcomes from DR surgical procedures. We searched our records system and retrieved all reports of DGSP performed over a 6-month period. For each procedure (one patient could have multiple procedures), we retrieved the record of the most recent same or similar procedure performed, without dermoscope guidance, on a sex-and-age (±5 years) matched control patient. Our interventions for the study and control procedures were with dermoscope guidance and without dermoscope guidance. We used retrospective data for both cases and controls.
Our primary outcomes were: (i) local inflammation and infection requiring treatment within 2 weeks after the procedures, (ii) incomplete removal of lesions or relapse of lesions within 6 months; and (iii) obvious scars (visible at a distance of 50 mm for perfect vision) within 6 months. Our secondary outcome was pain affecting activities of daily living in the first week following the procedure. We also asked the study participants whether they would opt for DG surgical procedures if indicated in the future.
We obtained informed written consent from all patients or parents or legal guardians for patients aged 18 years and younger to have the procedures done and to have their data included and analysed in this study.
Results
We retrieved clinical records of 39 DG surgical procedures performed for 36 patients in our practice: 21 (58%) were male and 15 (42%) female. They were aged from 7 to 89 years (mean age 48.5 years, standard deviation 20.9 years). The procedures were 22 excisional biopsies (56% of all procedures), five (13%) suturing, five (13%) laser ablations, five (13%) cauteries and two (5%) punch biopsies. The procedure used was with dermoscope guidance.
We matched control procedures. Their intervention was without dermoscope guidance. For a punch biopsy on a patient with extra-mammary Paget’s disease, we paired a control procedure on a patient with squamous cell carcinoma in situ. For the excisional biopsy on scrotal tumoural calcinosis, we paired an excisional biopsy for a scrotal epidermoid cyst. For excisional biopsy of a juvenile xanthogranuloma, we paired a control of an excisional biopsy on a patient with neurofibromatosis type I to relieve compression of a neurofibroma on an adjacent nerve.
The age of patients on which control procedures were performed ranged from 11 to 87 years (mean age 49.4 years, standard deviation 23.2 years). There was no significant difference between patients in the intervention and control groups (z-score: –0.97; P = 0.33). There were 22 male patients in the control group (47%) and 24 females (53%).
Dermoscope-guided excisional biopsies
The commonest histopathological diagnoses were seborrhoeic keratosis (six lesions; 27% of all DG-excisional biopsies), benign melanocytic naevi (five lesions; 23%) and viral warts (three lesions; 14%).
One excisional biopsy for a 7-year-old boy revealed juvenile xanthogranuloma.1 When we first saw him he had a 1-year history of a solitary, firm and oval nodule on the anterior aspect of his right thigh, with enlargement during the previous 2 months. Dermoscopy with cross-polarisation revealed a well-structured mass with six to seven lobules (Fig. 3). Dermoscopic guidance achieved precise margins for complete excision and acceptable cosmetic outcome. Histopathology subsequently confirmed complete removal of the juvenile xanthogranuloma, with adequate margins. Immunophenotype of tumour cells was positive against cluster of differentiation 68 and negative against S100 proteins.
Dermoscope-guided suturing
Five (13% of all procedures) DG suturing procedures were performed, with four for accidental injuries with open wounds and one for a self-inflicted injury on the wrist. One suturing was for an 89-year-old female who had an accidental fall leading to open wounds on both sides of the nasal bridge, due to studs on her spectacles pressing into the sides of the nasal bridge.3 The left-sided wound was deep and wide, and was near the left lacrimal sac and nasolacrimal duct. Unguided or blind suturing might damage these tissues and cause permanent epiphora. During DG suturing, we could visualise the entire route travelled by our 19-gauge needle from entry to exit points. While our needle tip passed through mucosal surfaces, we re-focused and could see the mucosal surfaces on the monitor. We ultimately made two sutures. There was no post-operative epiphora and the final cosmetic outcome was satisfactory.
Dermoscope-guided laser ablation and electrocautery
The indications for these procedures were for viral warts (six), acrochordons (two), and molluscum contagiosum (one). These interventions are commonly performed and considered appropriate in our part of the world.6 We proceed only if the lesions cause symptoms affecting patients’ activities of daily living. We counsel all patients on the natural courses of these diseases, the risks of recurrence and the implications of the Köbner phenomenon, where applicable.
Dermoscope-guided punch biopsy
A 54-year-old female with pemphigus vulgaris that was suspected clinically and serologically had one of the two punch biopsies. Dermoscopy guided us to the most indurating point at the margin of an erosion. Histopathology and direct immunofluorescence studies confirmed the diagnosis. The other punch biopsy was for a male aged 60 years with an extensive indurating plaque extending from the left inguinal crease to the scrotal wall, root of penis and left thigh.2 We aimed to perform multiple biopsies, but financial constraints allowed only one biopsy, and a referral without a biopsy report to the public sector in our medical system would take many months, which would delay the management. After discussion with the patient, we decided to perform a DG punch biopsy on one site initially. During the procedure, we noted a site with reticular pattern and clumps of cherry-red dots, this was also the most severely indurated site. We performed a thick punch biopsy. Histopathology suggested extramammary Paget’s disease. Immunohistochemical staining revealed positivities against epithelial membrane antigen, cytokeratin 7 and cytokeratin 20, with negativities against S100 proteins, human melanoma black 45 and prostate-specific antigen. With these results, we promptly referred the patient for further investigations and management. Further investigation confirmed that our biopsy site was indeed the site with the highest yield of Paget cells and it had the most severe destruction of the architecture of the skin and underlying tissues. DG punch biopsy had elevated our precision as well as lowered the cost for the first procedure to define the diagnosis in the shortest possible time.
Comparison of the outcomes
For the primary outcomes (Table 1), we found no significant difference between study groups for localised inflammation or infection that required treatment, other acute complications within 2 weeks and subacute and chronic complications other than scarring within 6 months.
|
Incomplete removal of lesions or relapse within 6 months of the procedures were noted in two (6%) study procedures and nine (28%) control procedures (Risk Ratio (RR): 0.22, 95% confidence interval (CI): 0.05–0.95; P < 0.05). Obvious scars at 6 months were seen in 14 (36%) study and 27 (69%) control procedures (RR: 0.52, 95% CI: 0.32–0.83; P < 0.05).
For the 32 operations aiming to remove entire lesions (excisional biopsy, laser ablation and electrocautery), 20 lesions were <4 mm at the largest diameters. Out of these, five (25%) left obvious scars 6 months after surgery, significantly less than 10 (83%) lesions with scars out of 12 small lesions for the controls (RR: 0.30, 95% CI: 0.13–0.67; P < 0.05). For large lesions (≥4 mm), no significant difference was found (RR: 0.77, 95% CI: 0.40–1.47; P = 0.47).
For the secondary outcome, pain affecting activities of daily living in the first week was reported for six (17%) intervention and five (14%) control procedures. The difference was not significant (RR: 1.20, 95% CI: 0.40–3.58; P = 0.74). Most patients receiving DG procedures (32 (89%)) would choose DG procures again for treating similar diseases or injuries (Table 2).
Discussion
This research shows the usefulness of DG operations conducted in primary care. In the 6-month period of this study, we conducted 39 DG procedures. The findings in two of our primary outcomes – relapse in 6 months and scarring in 6 months – demonstrated the superiority of dermoscope guidance over usual care.
DG histologic sectioning, ex vivo, has been found to enhance yields of histopathological investigations.7,8 DG surgical procedures, in vivo, have been reported for incisional biopsies,9,10 excisional biopsy,11 nail ablation12 and in Mohs surgeries.13–16 However, these studies were conducted in specialist settings and might not be applicable to primary care.
The retrospective nature of our study is an advantage. If we performed a prospective study, the performance of the GP could be affected by awareness of participating in research. Being retrospective, our study achieved masking (blinding before assessments) as well as allocation concealment (blinding during assessments).
This study also had a low likelihood of selection bias. The study time period of 6 months was predetermined, and all DG procedures performed within this period were studied. The control procedures without dermoscope guidance were the most recent procedures performed for the same or most similar indications in age-and-sex pair-matched patients. The investigators had no freedom to choose the controls, leading to virtually no selection bias.
A potential limitation could be the confounding variable of the experience and skills of the GP. The last control procedure was performed before the first study procedure. As the GP has performed more operations, he gained more proficiency than when he started. However, the GP has been performing similar surgical procedures in the same setting for more than 20 years. His technique should have plateaued by the time he performed the control and study procedures.
The most important limitation of the study is the low numbers of intervention and control procedures performed by one GP only, although this did eliminate the issue of inter-clinician performance and variation across clinical settings. However, this limits the generalisability of the results to other clinical settings and to other parts of the world.
Although we tried to match each study procedure with a control procedure with similar diagnosis and disease severity, there were heterogeneities we were unable to eliminate completely. Another limitation is that owing to the retrospective nature of the methodology, we have limited the patient-assessed outcomes to pain in the first week only, and treated this as a secondary outcome only. Patient-assessed outcomes are at least as important as clinician-rated outcomes.
DG surgery is relatively novel and is virtually unreported in primary care settings apart from our previous publications. We continued to perform DG surgical procedures as this study was being undertaken. We naturally focus on advantages of these procedures and may not therefore detect its adverse aspects. As responsible clinicians, we wished to study our work and we believe that the case-control approach to studying the outcomes of DG surgical procedures was the most feasible and robust approach to take.
Further studies might recruit more patients and involve more GPs. This would check the validity of our findings and enable assessment of inter-clinician variability. The training of GPs and assistants, quality of the equipment and cost-effectiveness should also be explored.
Dermoscopes are within reach for many GPs. Our experience is that proficiency in DG surgical procedures is relatively easy to attain if a GP is competent in dermoscopy. Performing these procedures in primary care settings might also lower medical costs in the community.
Funding
This research did not receive any specific funding.
COMPETING INTERESTS
None.
References
[1] Chuh A, Klapper W, Zawar V, Fölster-Holst R. Dermoscope-guided excisional biopsy in a child with CD68+ and S100- juvenile xanthogranuloma. Eur J Pediat Dermatol. 2017; 27 134–7.[2] Chuh A, Zawar V, Fölster-Holst R. Dermoscope-guided lesional biopsy to diagnose EMA+ CK7+ CK20+ extramammary Paget’s disease with an extensive lesion. J Eur Acad Dermatol Venereol. 2018; 32 e92–4.
| Dermoscope-guided lesional biopsy to diagnose EMA+ CK7+ CK20+ extramammary Paget’s disease with an extensive lesion.Crossref | GoogleScholarGoogle Scholar | 28846155PubMed |
[3] Chuh A. Dermatoscope-guided suturing for an open wound adjacent to the lacrimal sac and the nasolacrimal duct. Australas J Dermatol. 2018; 59 153–4.
| Dermatoscope-guided suturing for an open wound adjacent to the lacrimal sac and the nasolacrimal duct.Crossref | GoogleScholarGoogle Scholar | 28891080PubMed |
[4] Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013; 3 33–4.
| Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion.Crossref | GoogleScholarGoogle Scholar | 24282661PubMed |
[5] Chuh A. Roles of epiluminescence dermoscopy beyond the diagnoses of cutaneous malignancies and other skin diseases. Int J Trop Dis Health. 2017; 24 1–10.
| Roles of epiluminescence dermoscopy beyond the diagnoses of cutaneous malignancies and other skin diseases.Crossref | GoogleScholarGoogle Scholar |
[6] Chuh AA, Wong WC, Wong SY, Lee A. Procedures in primary care dermatology. Aust Fam Physician. 2005; 34 347–51.
| 15887937PubMed |
[7] Malvehy J, Aguilera P, Carrera C, et al. Ex vivo dermoscopy for biobank-oriented sampling of melanoma. JAMA Dermatol. 2013; 149 1060–7.
| 23863988PubMed |
[8] Merkel EA, Amin SM, Lee CY, et al. The utility of dermoscopy-guided histologic sectioning for the diagnosis of melanocytic lesions: a case-control study. J Am Acad Dermatol. 2016; 74 1107–13.
| The utility of dermoscopy-guided histologic sectioning for the diagnosis of melanocytic lesions: a case-control study.Crossref | GoogleScholarGoogle Scholar | 26826889PubMed |
[9] Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013; 27 1299–303.
| 22449222PubMed |
[10] Bomm L, Benez MD, Maceira JM, et al. Biopsy guided by dermoscopy in cutaneous pigmented lesion – case report. An Bras Dermatol. 2013; 88 125–7.
| Biopsy guided by dermoscopy in cutaneous pigmented lesion – case report.Crossref | GoogleScholarGoogle Scholar | 23539018PubMed |
[11] Carducci M, Bozzetti M, de Marco G, et al. Preoperative margin detection by digital dermoscopy in the traditional surgical excision of cutaneous squamous cell carcinomas. J Dermatolog Treat. 2013; 24 221–6.
| Preoperative margin detection by digital dermoscopy in the traditional surgical excision of cutaneous squamous cell carcinomas.Crossref | GoogleScholarGoogle Scholar | 22390630PubMed |
[12] Bet DL, Reis AL, Di Chiacchio N, Belda W. Dermoscopy and onychomycosis: guided nail abrasion for mycological samples. An Bras Dermatol. 2015; 90 904–6.
| Dermoscopy and onychomycosis: guided nail abrasion for mycological samples.Crossref | GoogleScholarGoogle Scholar | 26734877PubMed |
[13] Gurgen J, Gatti M. Epiluminescence microscopy (dermoscopy) versus visual inspection during Mohs microscopic surgery of infiltrative basal cell carcinoma. Dermatol Surg. 2012; 38 1066–9.
| Epiluminescence microscopy (dermoscopy) versus visual inspection during Mohs microscopic surgery of infiltrative basal cell carcinoma.Crossref | GoogleScholarGoogle Scholar | 22676346PubMed |
[14] Marchetti MA, Marghoob AA. Dermoscopy. CMAJ. 2014; 186 1167
| Dermoscopy.Crossref | GoogleScholarGoogle Scholar | 24934889PubMed |
[15] Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014; 40 334–7.
| Dermoscopy to identify biopsy sites before Mohs surgery.Crossref | GoogleScholarGoogle Scholar | 24447179PubMed |
[16] Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014; 89 38–43.
| Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery.Crossref | GoogleScholarGoogle Scholar | 24626646PubMed |