Development of explicit criteria identifying potentially inappropriate polypharmacy in older adults in New Zealand primary care: a mixed-methods study
Lisheng Liu 1 2 , Jeff Harrison
1 2 , Jeff Harrison  1 *
1 *
1 School of Pharmacy, Faculty of Medical and Health Sciences, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.
2 Primary, Public and Community Health, MidCentral District, Te Whatu Ora, PO Box 2056, Palmerston North 4440, New Zealand.
Journal of Primary Health Care 15(1) 38-47 https://doi.org/10.1071/HC22135
Published: 24 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: The link between polypharmacy, risk of potentially inappropriate medication exposure, and avoidable medicines-related harm is well recognised. Not all polypharmacy is harmful, and contemporary multimodal approaches to managing long-term conditions are evidence-based and commonplace. What is needed is a focus on reducing inappropriate medication prescribing in polypharmacy.
Aim: This study aims to develop the New Zealand criteria, a set of New Zealand-specific potentially inappropriate medication indicators to correct for older adults with polypharmacy.
Methods: A mixed-methods approach was used. An expert panel group comprising four clinical pharmacists, two general practitioners, one geriatrician, and two nurse practitioners generated a collection of ideas via the nominal group technique, which combined with published criteria from literature, provided the list of potential criteria. These potential criteria were reviewed, validated, and ranked for importance via a two-round modified Delphi analysis with the same panel.
Results: The nominal group technique generated 35 indicators, of which 23 were rated as important. Fifty-nine of 91 indicators from literature were rated as relevant and important. This generated 82 indicators for the modified Delphi analysis, from which 61 achieved consensus. Overall, 21 unique criteria were judged ‘very important’, 31 were judged ‘important’, and nine were judged ‘somewhat important’. No indicators were judged ‘low importance’.
Discussion: The New Zealand criteria provides 61 medication indicators, which New Zealand experts recommend should prompt formal, documented review. The criteria can be used to systematically identify patients at the highest risk of avoidable medication-related harm for proactive review.
Keywords: aged, Delphi technique, geriatrics, inappropriate prescribing, pharmaceutical preparations, polypharmacy, potentially inappropriate medication list, surveys and questionnaires.
| WHAT GAP THIS FILLS |
| What is already known: Expert consensus-based explicit criteria have been developed internationally to identify potentially inappropriate medications that should be avoided or prescribed with caution for older adults due to their association with adverse outcomes. To date, no similar criteria have been published within the New Zealand healthcare setting. |
| What this study adds: The NZ criteria are explicit criteria for older adults with polypharmacy, which is tailored to New Zealand healthcare’s unique pharmacopoeia, clinical practice, and prescribing patterns. Some potentially inappropriate medications identified in the NZ criteria were less commonly identified in internationally developed criteria. |
Introduction
Polypharmacy is the concomitant prescribing of multiple medications for an individual.1
Polypharmacy often occurs in individuals with comorbidities; for example, for individuals with established hypertension or heart failure, combination treatment with multiple medications is often evidence-based and beneficial.2,3 Polypharmacy can also occur from potentially inappropriate prescribing; for example, prescribing medications not based on best practice evidence, or prescribing cascades, where one medication is prescribed to treat the adverse effect caused by another.1
Although it is acknowledged that polypharmacy is increasing in younger adults,4 polypharmacy in older adults is common, and represents a particular concern due to the higher likelihood of multiple co-morbidity1 accompanied by age-related reductions in physiological reserve and changes to physiological processes, which increase susceptibility to medicines-related harm.
The prevalence of polypharmacy is increasing as population demographics change with more older adults living with comorbidities.1 In the retrospective study by Nind et al. of New Zealand adults reported that in 2018, the percentage of adults prescribed five or more medications was 9.93%, whereas adults prescribed ≥10 medications was 1.92%. This represents a 4.10 and 7.11% increase respectively from 2014 rates.4 It has been projected that by 2048, approximately 26% of New Zealanders will be aged >65 years, compared to 16% in 2020.5
Polypharmacy has been associated with an increased risk of potentially inappropriate medication use. In the study by Price et al. of older adults, the number of medications taken was predictive of potentially inappropriate medication exposure (odds ratio 35.03; 95% confidence interval 34.37–35.71 for ≥10 medications vs zero to two medications).6
It should be highlighted that polypharmacy should not be perceived as invariably harmful or unsafe. In the retrospective study by Payne et al., in individuals diagnosed with six or more conditions, those taking four to six medications were no more likely to experience an unplanned hospital admission than those taking one to three medications (odds ratio 1.00; 95% confidence interval 0.88–1.14).7 The study highlighted the importance of a clinical review to assess polypharmacy within the context of each medication.
Explicit criteria have been effectively used as part of clinical medication reviews. Explicit criteria catalogue potentially inappropriate medications that should be avoided or prescribed cautiously in older adults, due to their association with increased adverse outcomes.8
The systematic review by Motter et al. identified 36 different explicit criteria developed internationally using literature review and expert consensus.9 Examples include the seminal Beers Criteria,10 the Screening Tool of Older Persons’ Prescriptions and Screening Tool to Alert doctors to Right Treatment criteria,11 and country-specific criteria, such as the French criteria.12 Each criterion varies broadly in the potentially inappropriate medications determined to be important. Several reasons could explain the variations between criteria. First, pharmacotherapy in older adults is complex, and there is often insufficient evidence to guide medicine therapy decisions. Second, differences between settings, medication formularies, and medication availability may result in differences between country-specific criteria.
To date, there have been no similar criteria published within the New Zealand healthcare setting. The Best Practice Advocacy Centre has advocated adopting the New Zealand Pill Pruner, or the internationally developed Beers Criteria.13 However, there are limitations to the utility of these criteria. The Pill Pruner is a condensed version of the Screening Tool of Older Persons’ Prescriptions and Screening Tool to Alert doctors to Right Treatment criteria. It has not been updated since 2009 or trialled in primary care.14 Meanwhile, international criteria are less generalisable to the New Zealand healthcare context. Additionally, some international criteria are derived outside of primary care so may not reflect prescribing for community-dwelling older adults. Furthermore, international criteria may not be practical to use due to their complexity, or only describe a sub-set of important potential risks.
To address the need for an effective approach towards identifying potentially inappropriate medication prescribing for older adults (aged ≥65 years) with polypharmacy, this study aims to develop the New Zealand (NZ) criteria. The NZ criteria are explicit criteria of potentially inappropriate medication indicators to correct for older adults with polypharmacy, which is tailored to New Zealand healthcare’s unique pharmacopoeia, clinical practice, and prescribing patterns.
Methods
To assemble the NZ criteria, a mixed-methods approach was selected. The nominal group technique (NGT), combined with published literature and modified Delphi analysis, was used to identify potentially inappropriate medications to correct for older adults with polypharmacy.
The study adhered to the Declaration of Helsinki principles and received approval from the Auckland Health Research Ethics Committee on 16 September 2021 (AH3396).
Nominal group technique
NGT is a structured method for idea generation using an expert panel group in a face-to-face environment.15 Thirteen clinicians across New Zealand were contacted by an email invitation letter to participate in the panel group. The identification of potential panel members was through purposive sampling, based on their prominent standing within the New Zealand healthcare system and their expertise in the pharmacotherapy of older adults. Panel members were required to be registered clinicians, currently involved in the clinical management of older adults, who had experience and knowledge in reducing inappropriate polypharmacy for older adults.
The panel group represented the opinions of geriatric medicine, pharmacy, nursing and general practice. Nine clinicians from primary and secondary healthcare settings, including four clinical pharmacists, two general practitioners, one geriatrician, and two nurse practitioners, consented to participate in the panel group.
The duration of the NGT meeting was one and a half hours. The author (LL) commenced and facilitated the NGT by describing the steps of the NGT, the panel group goals, contributions from each panel member, and how the results will be utilised.
Panel members were requested to submit potentially inappropriate medication indicators for older adults with polypharmacy that they thought were important to correct. Panel members were prompted to combine similar indicators, including double nominations and synonyms. If the panel group agreed an indicator was sufficiently dissimilar, it was included. The panel group then reviewed the collected indicators to discuss clarity of meaning. Subsequently, panel members were invited to write down and vote for the seven indicators from the collection they believed were the most important to correct. The votes were tallied by the author (LL) and one panel member to identify the indicators rated most important to correct by the panel group.
Modified Delphi analysis
Indicators collected from either the NGT or published literature were analysed using the modified Delphi technique by the panel group. Modified Delphi analysis is a method for using group consensus to collect information regarding a topic.16
Before commencing the modified Delphi analysis, the author (LL) examined the systematic review of explicit criteria by Motter et al. to identify potentially inappropriate medication indicators in literature to supplement the findings of the panel group.9 Overall, 907 medications and medication classes were identified from published criteria, with wide variability and limited overlap. Therefore, it was determined impractical to include all indicators from published criteria in the modified Delphi analysis.
A consensus was reached between the authors (JH and LL) that the Beers Criteria were rigorously developed using systematic literature review and evaluation of evidence through Delphi consensus. Additionally, the Beers Criteria are widely used in clinical practice and have been selected by researchers to develop other criteria.8 Consequently, indicators from the 2019 Beers Criteria were collected to supplement the findings of the NGT for the modified Delphi analysis.
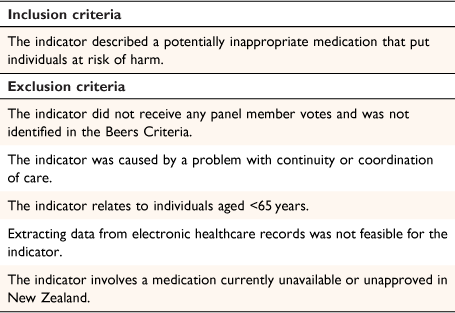
The gathered indicators were filtered according to the study’s inclusion and exclusion criteria (Table 1). The exclusion criteria removed indicators highly uncommon in New Zealand clinical practice in which the yield was likely too small to justify inclusion. Indicators caused by continuity or coordination of care complications were excluded because they reflect broader healthcare system failures rather than person-specific harm. Indicators that could not be extracted from electronic healthcare records were also excluded. Excluded indicators and the rationale for exclusion were presented to the panel group before commencing the modified Delphi analysis.
|
A two-round modified Delphi analysis was conducted with the panel members 2 weeks following the NGT. The authors determined an acceptable consensus level was an agreement between at least six out of nine panel members (≥66%).
The round one questionnaire was emailed to panel members and included indicators identified from the NGT and Beers Criteria. Panel members were requested to indicate on a four-point Likert scale (1 = ‘low importance’, 2 = ‘somewhat important’, 3 = ‘important’, 4 = ‘very important’) the importance of each indicator to correct for older adults with polypharmacy. Panel members were provided 2 weeks to complete and return the questionnaire.
The second-round questionnaire was emailed to panel members 2 weeks following the conclusion of the first round. The second round included the same indicators as the first round, but also analysed the panel group response from round one. The median Likert scale score and interquartile range allowed each panel member to reconsider their initial rating for indicators that had not yet reached group consensus based on the panel group response. Indicators that had reached group consensus in round one remained in the second-round questionnaire; however, they were flagged as having already reached group consensus and no longer voted on. Panel members were provided 2 weeks to complete round two.
All indicators that had reached group consensus in the two-round modified Delphi analysis had their mean Likert score and consensus percentage calculated to rank the indicators by their importance to causing harm.
Results
Nominal group technique
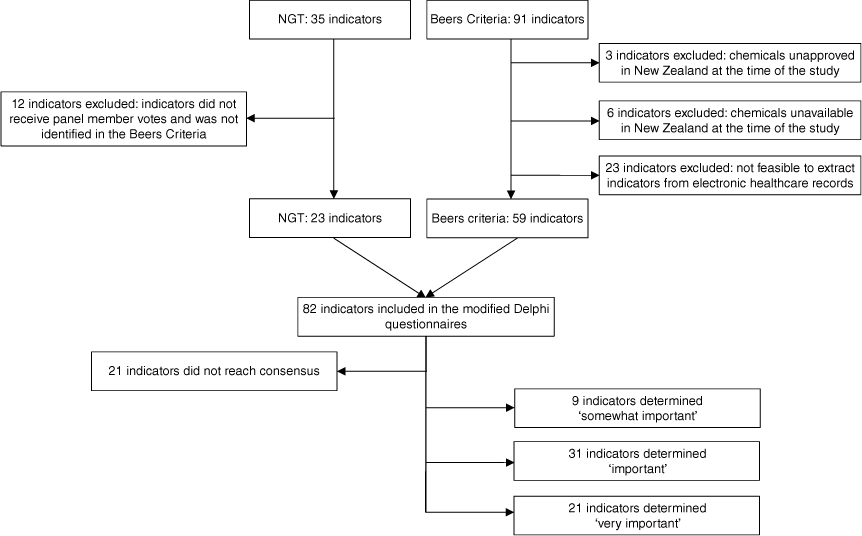
Thirty-five indicators were identified and voted on by the panel members in the NGT. From the 35 indicators, 23 indicators were included in the modified Delphi analysis based on the study’s inclusion and exclusion criteria (Fig. 1) (Supplementary Table S1). Twelve indicators identified in the NGT were excluded from the modified Delphi analysis because they did not receive any panel member votes (Supplementary Table S2).
Aggregation and validation of proposed criteria
The Beers Criteria identified another 91 indicators not identified in the NGT. From the 91 indicators, 59 indicators were included in the modified Delphi analysis, based on the study’s inclusion and exclusion criteria (Supplementary Table S1). Thirty-two indicators from the Beers Criteria were excluded from the modified Delphi analysis. Three indicators were excluded because the chemical described was unapproved by the New Zealand Medicines and Medical Devices Safety Authority at the time of the study.17 Six indicators were excluded because the chemical described was unavailable in New Zealand at the time of the study.17 Twenty-three indicators were excluded because extracting data from electronic healthcare records was not feasible (Supplementary Table S2).
Nine indicators from the NGT were duplicated in the Beers Criteria and were included in the modified Delphi analysis (Supplementary Table S1).
Modified Delphi analysis
Eighty-two indicators comprising 23 indicators from the NGT and 59 indicators from the Beers Criteria were included in the two-round modified Delphi analysis (Supplementary Table S1).
In round one, 23 indicators achieved the consensus threshold of ≥66%. Another 38 indicators achieved the consensus threshold in round two. After two rounds, 61 indicators achieved the consensus threshold, whereas 21 indicators did not achieve the consensus threshold (Supplementary Table S3).
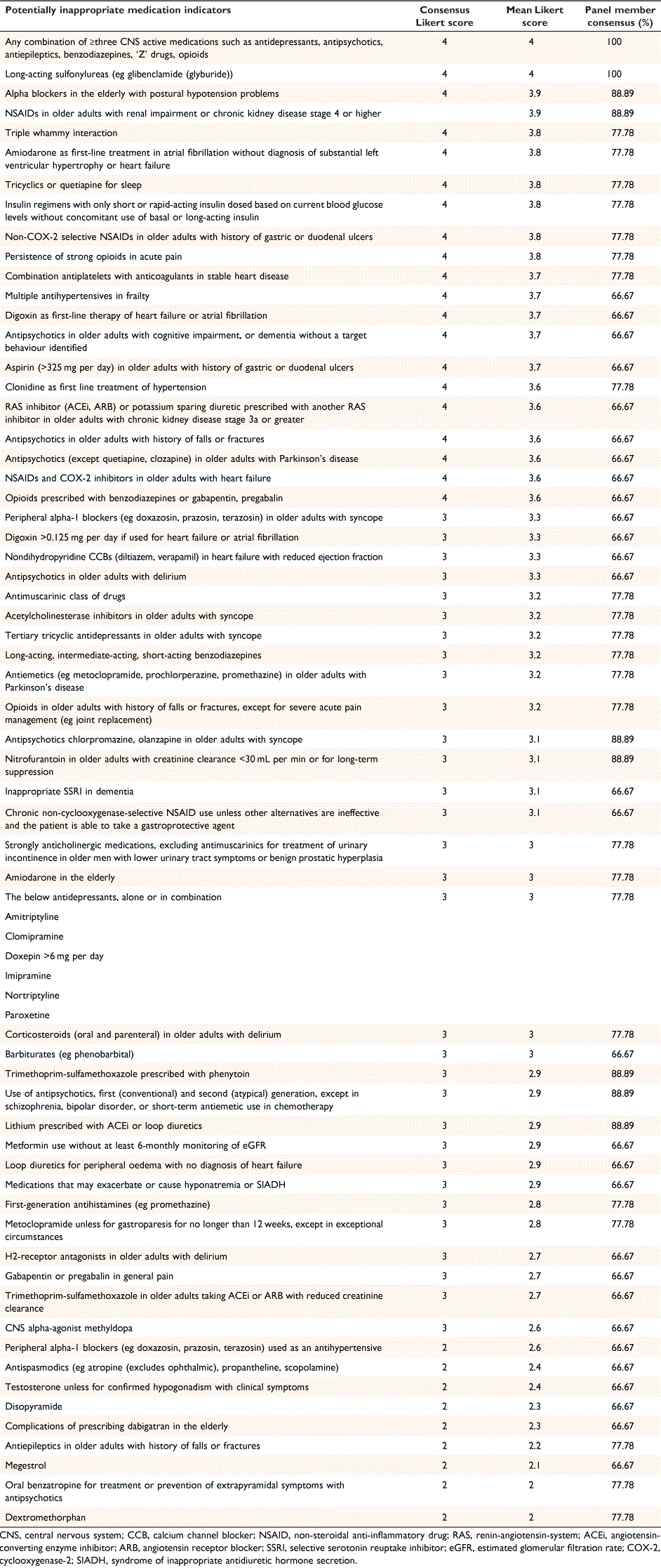
Twenty-one indicators attained consensus Likert score 4: ‘very important’, 31 indicators attained consensus Likert score 3: ‘important’, nine indicators attained consensus Likert score 2: ‘somewhat important’, and zero indicators attained consensus Likert score 1: ‘low importance’, regarding how important the indicator is to correct (Table 2).
|
Discussion
This study successfully developed the NZ criteria, a collection of 61 potentially inappropriate medication indicators to correct for older adults with polypharmacy, as identified by a panel of New Zealand expert clinicians.
Potentially inappropriate medication indicators for older adults from internationally developed criteria align closely with the indicators identified as ‘very important’ or ‘important’ in the NZ criteria.9 Recurrently identified medication classes include benzodiazepines, non-steroidal anti-inflammatories, antipsychotics, first-generation antihistamines, and anticholinergics. The use of benzodiazepines is associated with cognitive impairment, delirium, and falls.18–20 Non-steroidal anti-inflammatories increase the risk of acute kidney injury, gastrointestinal bleeding, and coronary events.21–23 Antipsychotics increase the risk of sedation, orthostatic hypotension, falls, and fractures.24,25 In older adults with dementia, antipsychotics also increase the risk of ischaemic stroke.26 Lastly, anticholinergic medications, including first-generation antihistamines, are associated with cognitive impairment, urinary retention, and falls.27
Some indicators identified as ‘somewhat important’ in the NZ criteria were less frequently described in internationally developed criteria.9 Medication examples include dextromethorphan and megestrol. Dextromethorphan can be purchased over-the-counter in New Zealand pharmacies as a cough suppressant.17 Megestrol is indicated for the palliative treatment of endometrial and breast cancer, and cachexia in acquired immunodeficiency syndrome or advanced neoplastic disease.17 The finding suggests there may be a consensus between clinicians that although these indicators are potentially harmful, the risk may be less than other indicators or less commonly observed in their clinical practice.
Some indicators identified in internationally developed criteria were not selected as important by the panel group.9 Examples include short-acting nifedipine, indomethacin, pethidine, and oestrogen. Short-acting nifedipine and indomethacin were excluded because they were unapproved medications at the time of the research.17 Pethidine and oestrogen were excluded because they did not reach group consensus in the modified Delphi analysis. This result may highlight that among some clinicians, there is limited exposure to these medications and a need for further information to assess their safety.
This study adds to the existing literature by describing what is thought to be New Zealand’s first explicitly developed set of criteria of potentially inappropriate medications for older adults in primary care. The Health Quality and Safety Commission has recommended that New Zealand clinicians adopt internationally developed criteria such as the Beers Criteria, or the Australian prescribing indicators tool when prescribing for older adults.28 However, adapting internationally developed criteria for use in New Zealand clinical practice requires extensive modifications to exclude medications that are not available or not approved for government subsidy. For example, dronedarone and short-acting dipyridamole were identified in the Beers Criteria, but dronedarone is unavailable and short-acting dipyridamole remains a non-subsidised, unapproved medication in New Zealand.17 Lastly, international criteria may differ from national prescribing guidelines, as other country-specific criteria have noted.29 Therefore, internationally developed criteria cannot replace the need for a set of criteria developed using New Zealand expert consensus, such as in this study. The NZ criteria are up-to-date and suitable for use in New Zealand healthcare settings.
The explicit process to develop, validate, and rank the importance of the NZ criteria adopted a comprehensive triangulation method through the NGT, literature review, and modified Delphi analysis. The process to apply clinical expertise and Delphi analysis to reach group consensus was comparable to the development of other criteria.29–32 The approach is supported by the ‘wisdom of crowds’ notion that in the deficiency of robust randomised controlled trial evidence to guide prescribing decisions, the evidence generated from group consensus is preferred over individual opinions.33
The panel members brought both their clinical expertise and experience to bear in selecting potentially inappropriate medications and potentially inappropriate prescribing practice that is local, relevant, and important to contemporary New Zealand practice. Additionally, the panel members remained highly motivated throughout the study. There were no withdrawals, and both modified Delphi analysis rounds achieved a 100% response rate. The absence of attrition helped to achieve rigour in the Delphi analysis and reduced the likelihood of withdrawal bias.
Regarding limitations, there have been criticisms about the validity and reliability of information generated by the NGT and Delphi method.34 Concerns include limitations with method standardisation, difficulties classifying experts for panel group inclusion, and the imprecise concept of consensus.35,36 To minimise these limitations, the authors predefined the method before recruitment, including the selection of panel members, the consensus method, and the number of modified Delphi rounds.
This study acknowledges that there is no ideal standard for selecting panel members. Although the authors predefined the selection criteria of panel members, differences between panel members, including knowledge and scope of practice, can influence the outcomes of the consensus approach, potentially limiting the reproducibility of the results. The panel size was also modest. Although a larger panel size could have provided more representation, the panel group would be more challenging to lead based on the study’s design and the resources available. Lastly, it is recognised that the consensus level for the modified Delphi analysis was set at 66%, which is below the level set in other comparable studies; however, the decision was pragmatic and made to ensure panel group consensus.
The NZ criteria were specifically developed to identify potentially inappropriate medications; however, clinicians should also consider potential prescribing omissions of clinically indicated medications in their practice. Potential prescribing omissions are prevalent in older adults with polypharmacy. They are associated with clinician reluctance to prescribe more medications due to concerns about increased medication interactions, adverse medication reactions, and reduced medication adherence.37,38
It should be noted that the NZ criteria are not designed to be applied punitively or substitute clinicians’ professional judgement. Instead, clinicians should adopt shared decision-making for each patient, weighing the unique risks and benefits of prescribing in conjunction with individual treatment goals, functional level, treatment response, values and preferences.39 Additionally, clinicians should exercise judgement to ensure patients do not receive a more harmful medication choice when avoiding medications from the criteria.
The NZ criteria are not intended to be a comprehensive record of all potentially inappropriate medications for older adults with polypharmacy. Instead, the criteria are intended to be a practical set of indicators where prescribing should be treated with caution or warrant greater scrutiny by clinicians.
The NZ criteria are designed for older adults with polypharmacy; however, research suggests that older adults without polypharmacy are also prescribed medications from the criteria. For example, data from 2019 indicated antipsychotic dispensing for older New Zealand adults had increased by 9% compared to 2018, with the highest rates (8%) belonging to adults aged ≥85 years.40 The implication is that some criteria can be useful for reducing potentially inappropriate prescribing for all older adults.
The NZ criteria can be helpful as an audit tool for comparing prescribing quality between clinics or measuring changes in prescribing quality within a clinic. Clinicians can also use the criteria as a resource to aid prescribing for older adults and improve the quality of medication reviews. Lastly, the criteria can be used as an educational and professional development tool for clinicians.
Further research could tailor the NZ criteria for use in specific settings, such as hospitals or aged care facilities. By way of example, given the high rates of antimicrobial use in adult hospital inpatients, anti-infective medication indicators may have more value in a hospital setting.41 Criteria tailored specifically for other settings have incorporated new indicators into existing criteria.32 The NZ criteria could also include new indicators for evaluation and external validation in specific healthcare settings.
The NZ criteria could support further updates. Internationally adopted criteria such as the Beers Criteria are updated on a 3-yearly cycle to remain current with evidence.10 Regular updates to the NZ criteria can ensure the criteria remains current with New Zealand’s prescribing trends, pharmacopoeia, and best practice evidence. Updates could also include the addition of potential prescribing omission indicators or offering pharmacological and non-pharmacological alternatives to potentially inappropriate medications.
Additional research may include a pharmacoepidemiological study to measure the correlation between potentially inappropriate medication use from the NZ criteria and harm in older adults. In comparable epidemiological studies, the use of potentially inappropriate medications from the Beers Criteria has been associated with an increased risk of hospitalisation.42
In the next steps of our research programme, the NZ criteria will be used to develop an information technology tool. The tool would identify older adults with polypharmacy prescribed potentially inappropriate medication indicators for priority intervention. The tool would be combined with educational outreach and medication review to develop a novel pharmacist-led intervention for primary care, which aims to optimise medication use and reduce potentially inappropriate prescribing for older adults with polypharmacy. A study to evaluate the feasibility of the intervention in primary care would be conducted before commencing a definitive randomised controlled trial evaluation.
Conclusion
The NZ criteria is a resource to reduce potentially inappropriate medication prescribing for older adults with polypharmacy. The criteria are New Zealand-specific, based on contemporary practice, as well as being consistent with international best-of-class measures, and reflect items of importance to clinicians. The criteria can be used to support targeted intervention where the risk of adverse medication events is believed to be highest.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this research will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest for this research.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors would like to thank the clinicians who participated in the panel group for their time and contribution.
References
[1] Duerden M, Avery T, Payne R. Polypharmacy and medicines optimization. United Kingdom: The King’s Fund; 2013.[2] Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122 290–300.
| Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials.Crossref | GoogleScholarGoogle Scholar |
[3] Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol 2022; 79 e263–e421.
| 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines.Crossref | GoogleScholarGoogle Scholar |
[4] Nind J, Smith A, Devananda M, et al. A whole of population retrospective observational study on the rates of polypharmacy in New Zealand 2014 to 2018. Polypharmacy in New Zealand: what is the current status? Health Sci Rep 2021; 4 e263
| A whole of population retrospective observational study on the rates of polypharmacy in New Zealand 2014 to 2018. Polypharmacy in New Zealand: what is the current status?Crossref | GoogleScholarGoogle Scholar |
[5] StatsNZ. National population projections: 2020(base)–2073. New Zealand: New Zealand Government; 2020. Available at https://www.stats.govt.nz/information-releases/national-population-projections-2020base2073#:~:text=By%202030%2C%20it%20is%20expected,year%20to%20June%202073%20year [Accessed 4 November 2022].
[6] Price SD, Holman CDJ, Sanfilippo FM, et al. Are older Western Australians exposed to potentially inappropriate medications according to the Beers Criteria? A 13-year prevalence study. Australas J Ageing 2014; 33 E39–E48.
| Are older Western Australians exposed to potentially inappropriate medications according to the Beers Criteria? A 13-year prevalence study.Crossref | GoogleScholarGoogle Scholar |
[7] Payne RA, Abel GA, Avery AJ, et al. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol 2014; 77 1073–1082.
| Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care.Crossref | GoogleScholarGoogle Scholar |
[8] Bala SS, Chen TF, Nishtala PS. Reducing potentially inappropriate medications in older adults: a way forward. Can J Aging 2019; 38 419–433.
| Reducing potentially inappropriate medications in older adults: a way forward.Crossref | GoogleScholarGoogle Scholar |
[9] Motter FR, Fritzen JS, Hilmer SN, et al. Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria. Eur J Clin Pharmacol 2018; 74 679–700.
| Potentially inappropriate medication in the elderly: a systematic review of validated explicit criteria.Crossref | GoogleScholarGoogle Scholar |
[10] American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019; 67 674–694.
| American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults.Crossref | GoogleScholarGoogle Scholar |
[11] O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015Mar; 44 213–218.
| STOPP/START criteria for potentially inappropriate prescribing in older people: version 2.Crossref | GoogleScholarGoogle Scholar |
[12] Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 2007; 63 725–731.
| Potentially inappropriate medications in the elderly: a French consensus panel list.Crossref | GoogleScholarGoogle Scholar |
[13] Best Practice Advocacy Centre New Zealand. Managing medicines in older people. New Zealand: BPAC; 2012. Available at https://bpac.org.nz/bpj/2012/october/elderlyMedicines.aspx [Accessed 16 May 2021].
[14] Chieng JHC, Hughes L, Stewart A, et al. Introduction of the Pill Pruner to acute medical care: a simple medication guide to control polypharmacy. Australas J Ageing 2015; 34 58–61.
| Introduction of the Pill Pruner to acute medical care: a simple medication guide to control polypharmacy.Crossref | GoogleScholarGoogle Scholar |
[15] Gallagher M, Hares T, Spencer J, et al. The Nominal Group Technique: a research tool for general practice? Fam Pract 1993; 10 76–81.
| The Nominal Group Technique: a research tool for general practice?Crossref | GoogleScholarGoogle Scholar |
[16] Keeney S, Hasson F, McKenna H. The Delphi technique in nursing and health research. United Kingdom: Wiley-Blackwell; 2011.
[17] New Zealand Formulary. NZF. New Zealand: New Zealand Formulary; 2021. Available at www.nzf.org.nz [Accessed 1 December 2021].
[18] Zhong GC, Wang Y, Zhang Y, et al. Association between benzodiazepine use and dementia: a meta-analysis. PLoS One 2015; 10 e0127836
| Association between benzodiazepine use and dementia: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[19] Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011; 40 23–29.
| Which medications to avoid in people at risk of delirium: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[20] Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc 1999; 47 40–50.
| Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs.Crossref | GoogleScholarGoogle Scholar |
[21] Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ 2013; 346 e8525
| Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study.Crossref | GoogleScholarGoogle Scholar |
[22] Massó González EL, Patrignani P, Tacconelli S, et al. Variability among nonsteroidal anti-inflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum 2010; 62 1592–1601.
| Variability among nonsteroidal anti-inflammatory drugs in risk of upper gastrointestinal bleeding.Crossref | GoogleScholarGoogle Scholar |
[23] Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382 769–779.
| Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials.Crossref | GoogleScholarGoogle Scholar |
[24] Mackin P, Thomas SHL. Atypical antipsychotic drugs. BMJ 2011; 342 d1126
| Atypical antipsychotic drugs.Crossref | GoogleScholarGoogle Scholar |
[25] Lee SH, Hsu WT, Lai CC, et al. Use of antipsychotics increases the risk of fracture: a systematic review and meta-analysis. Osteoporos Int 2017; 28 1167–1178.
| Use of antipsychotics increases the risk of fracture: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[26] Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005; 330 445
| Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[27] Weiss BD, Lee JK. Aging: is your patient taking too many pills? J Fam Pract 2012; 61 652–661.
[28] Health Quality & Safety Commission New Zealand. Tools to guide which medicines should be considered for deprescribing. New Zealand: Health Quality & Safety Commission New Zealand; 2022. Available at https://www.hqsc.govt.nz/our-work/system-safety/reducing-harm/medicines/projects/appropriate-prescribing-toolkit/tools-to-guide-which-medicines-should-be-considered-for-deprescribing/ [updated 5 April 2022; Accessed 5 November 2022].
[29] Basger BJ, Chen TF, Moles RJ. Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method. BMJ Open 2012; 2 e001431
| Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method.Crossref | GoogleScholarGoogle Scholar |
[30] Winit-Watjana W, Sakulrat P, Kespichayawattana J. Criteria for high-risk medication use in Thai older patients. Arch Gerontol Geriatr 2008; 47 35–51.
| Criteria for high-risk medication use in Thai older patients.Crossref | GoogleScholarGoogle Scholar |
[31] Kim DS, Heo SI, Lee SH. Development of a list of potentially inappropriate drugs for the Korean elderly using the Delphi method. Healthc Inform Res 2010; 16 231–252.
| Development of a list of potentially inappropriate drugs for the Korean elderly using the Delphi method.Crossref | GoogleScholarGoogle Scholar |
[32] Nyborg G, Straand J, Klovning A, et al. The Norwegian General Practice – Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study. Scand J Prim Health Care 2015; 33 134–141.
| The Norwegian General Practice – Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study.Crossref | GoogleScholarGoogle Scholar |
[33] Campbell SM, Braspenning J, Hutchinson A, et al. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care 2002; 11 358–364.
| Research methods used in developing and applying quality indicators in primary care.Crossref | GoogleScholarGoogle Scholar |
[34] Cantrill JA, Sibbald B, Buetow S. The Delphi and nominal group techniques in health services research. Int J Pharm Pract 1996; 4 67–74.
| The Delphi and nominal group techniques in health services research.Crossref | GoogleScholarGoogle Scholar |
[35] Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015; 71 861–875.
| The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries.Crossref | GoogleScholarGoogle Scholar |
[36] Pagliari C, Grimshaw J, Eccles M. The potential influence of small group processes on guideline development. J Eval Clin Pract 2001; 7 165–173.
| The potential influence of small group processes on guideline development.Crossref | GoogleScholarGoogle Scholar |
[37] Galvin R, Moriarty F, Cousins G, et al. Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA). Eur J Clin Pharmacol 2014; 70 599–606.
| Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA).Crossref | GoogleScholarGoogle Scholar |
[38] Kuijpers MAJ, Van Marum RJ, Egberts ACG, et al. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol 2008; 65 130–133.
| Relationship between polypharmacy and underprescribing.Crossref | GoogleScholarGoogle Scholar |
[39] Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “there’s got to be a happy medium”. JAMA 2010; 304 1592–1601.
| Managing medications in clinically complex elders: “there’s got to be a happy medium”.Crossref | GoogleScholarGoogle Scholar |
[40] Best Practice Advocacy Centre New Zealand. National report: the use of antipsychotic medicines in older people. New Zealand: BPAC; 2020. Available at https://bpac.org.nz/report/2020/antipsychotic-medicines.aspx [Accessed 2 October 2021].
[41] Gardiner SJ, Basevi AB, Hamilton NL, et al. Point prevalence surveys of antimicrobial use in adult inpatients at Canterbury District Health Board hospitals. N Z Med J 2020; 133 18–33.
[42] Jano E, Aparasu RR. Healthcare outcomes associated with Beers’ Criteria: a systematic review. Ann Pharmacother 2007; 41 438–448.
| Healthcare outcomes associated with Beers’ Criteria: a systematic review.Crossref | GoogleScholarGoogle Scholar |