Cosmopolitan abyssal lineages? A systematic study of East Pacific deep-sea squat lobsters (Decapoda: Galatheoidea: Munidopsidae)
Paula C. Rodríguez-Flores A * , Charlotte A. Seid
A * , Charlotte A. Seid  B , Greg W. Rouse
B , Greg W. Rouse  B and Gonzalo Giribet
B and Gonzalo Giribet  A
A
A Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA.
B Scripps Institution of Oceanography, University of California San Diego, La Jolla, CA 92093, USA.
Invertebrate Systematics 37(1) 14-60 https://doi.org/10.1071/IS22030
Submitted: 1 July 2022 Accepted: 9 November 2022 Published: 11 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Munidopsid squat lobsters are among the most abundant decapods at abyssal depths and the most diverse squat lobster group in the East Pacific region. During recent cruises along the East Pacific, many deep-sea squat lobsters were collected. Among these, we described five new munidopsid species supported both by morphological characters and molecular phylogenetics: Munidopsis girguisi sp. nov., M. nautilus sp. nov., M. testuda sp. nov., M. cortesi sp. nov. and M. hendrickxi sp. nov. We also report new records of several Munidopsis species across the East Pacific that increase the species distribution ranges. Here, we reconstructed the phylogenetic relationships of the East Pacific species in relation to other Galatheoidea using one nuclear and two mitochondrial gene fragment(s); we also performed single locus species delimitation analyses to explore the species status of various East Pacific munidopsid taxa. The new taxa were photographed, illustrated and imaged with micro-computed tomography. The phylogenetic results show that: (1) Janetogalathea californiensis, previously included in the family Galatheidae, nests within Munidopsidae; (2) the phylogenetic position of Phylladiorhynchus and Coralliogalathea as belonging in Galatheidae is not supported; and (3) Munidopsis is paraphyletic, agreeing with recent systematic hypotheses. Short genetic distances and species delimitation analyses suggested that a clade mostly constituted by abyssal species might include fewer species than currently considered, as species show a wider geographic range than previously considered, conforming with traditional hypotheses of cosmopolitanisms in abyssal species.
ZooBank: urn:lsid:zoobank.org:pub:CED9EB18-7061-47A7-B2FF-7F1DAFCC7B12.
Keywords: crustaceans, long-distance dispersal, microCT, mitochondrial genes, morphology, new species, species delimitation, taxonomy.
Introduction
Squat lobsters in the family Munidopsidae are deep-sea dwellers, nearly absent from shallow waters, and represent the most abundant group of decapods at abyssal depths (Baba et al. 2008; Macpherson et al. 2010; Schnabel et al. 2011a). Munidopsids currently comprise nearly 300 species in 4 genera: Galacantha A. Milne-Edwards, 1880, Leiogalathea Baba, 1969, Munidopsis Whiteaves, 1874 and Shinkaia Baba & A.B. Williams, 1998 (Dong et al. 2021; Rodríguez-Flores et al. 2022a). Within this family, the global genus Munidopsis is the most speciose, with 270 species described to date. Additional species are added nearly every year (e.g. Rodríguez-Flores et al. 2018, 2022a; Dong et al. 2019, 2021; Marin 2020), suggesting that a great deal of diversity remains to be discovered. Munidopsids occur in deep-sea benthos across a latitudinal gradient in both hemispheres (Baba 2005) but few records are attributed to Antarctic waters (García Raso et al. 2008). Several species occur on seamounts, in cold-water coral reefs or as commensals of deep-sea echinoderms, whereas others are found in habitats supporting chemosynthesis such as hydrothermal vents, cold seeps or whale falls (Baba 2005; Macpherson 2007). Only Munidopsis serricornis (Lovén, 1852) inhabits open water shallower than 100 m, in Norway, and M. polymorpha Koelbel, 1892, occurs in shallow lava tubes in Lanzarote, Canary Islands, and these represent the species that occur at the shallowest depths in the family (Baba et al. 2008). Sampling these squat lobsters, as for other deep-sea fauna, is costly both in terms of time and funding. Moreover, several Munidopsis species are rare, known from only a few localities, few individuals or even only from the holotype (Rodríguez-Flores et al. 2022a). These factors have contributed to incomplete knowledge of species diversity and distribution patterns, making the assessment of intraspecific variation and consequent establishment of the basis for species delimitation within Munidopsis difficult.
Munidopsis species display high morphological disparity including several adaptations to life in the deep sea. The carapace is devoid of the typical striation of other galatheoidean squat lobsters, pigmentation is usually lacking and all species have reduced ocular orbits. Phylogenetic analyses have suggested that the genus Munidopsis is paraphyletic or polyphyletic (Ahyong et al. 2011; Rodríguez Flores 2021). Indeed, the sequential addition of taxa previously not included in the squat lobster tree of life has challenged the taxonomic status of several families, revealing that the current formal classification needs revision (Schnabel et al. 2011b; Rodríguez-Flores et al. 2022b). For example, members of Leiogalathea have an external habitus like that of the members of the squat lobster family Galatheidae, with well-developed eyes, a carapace dorsally covered by setose short striae and a triangular broad-on-base rostrum. However, previous studies (Ahyong et al. 2011; Schnabel et al. 2011b; Bracken-Grissom et al. 2013) placed Leiogalathea as the sister group to all other munidopsid taxa and this is currently classified within Munidopsidae. Given this fact, morphological convergence and homoplasy have contributed to a confusing taxonomic classification of squat lobsters, highlighting that systematics based solely on external characters of the carapace and rostrum should be cautiously considered
Studies on the biogeography of deep-sea squat lobsters have shown that the East Pacific diversity is apparently poor compared to that of the West Pacific basin (Macpherson et al. 2010; Schnabel et al. 2011a). Nevertheless, Munidopsidae remains the most diverse family of squat lobsters in this region, with more than 50 of the 73 known galatheoidean species from the East Pacific (Baba and Wicksten 2019). Other squat lobsters are poorly represented; for example Galatheidae includes only two species, Janetogalathea californiensis (Benedict, 1902), a common species occurring along the northern East Pacific, and Galathea paucilineata Benedict, 1902, only known from a damaged specimen collected in Galapagos. Two species of Phylladiorhynchus Baba, 1969 were recorded from Corral and Valparaiso in Chile but the systematic status in Galatheidae is dubious (Rodríguez-Flores et al. 2021, 2022b). Over 30 Munidopsis species from the East Pacific Munidopsidae represent East Pacific endemics, whereas the remaining species are found in the West and East Pacific, and few of these are distributed across the East Pacific and Atlantic (Jones and Macpherson 2007; Baba et al. 2008). Nine Munidopsidae species have been found in association with hydrothermal vents or cold seeps along the East Pacific including the endemic Munidopsis lentigo Williams & Van Dover, 1983, that is only known from the northern East Pacific Rise near Baja California. Several studies suggest that species with an abyssal distribution recorded from abyssal plains and seamounts have wider geographic ranges than species with bathyal distributions and those living in hydrothermal vents or cold seeps (Etter et al. 2005; McClain and Hardy 2010; Etter et al. 2011). Additionally, previous studies on squat lobsters have suggested rapid radiation or retardation in the molecular evolution of some deep-sea lineages (Machordom and Macpherson 2004; Cabezas et al. 2012; Rodríguez-Flores et al. 2020) coupled with a recent deep-sea colonisation. Therefore, poor genetic structure has been observed in bathyal and abyssal lineages of squat lobsters with cosmopolitan distributions whereas species from the continental shelf tend to have deeper genetic structure and display allopatry.
Thanks to an increased effort in deep-sea exploration during the past few decades (e.g. Hatch et al. 2020; Marlow et al. 2022; O’Hara et al. 2020; Salinas-de-León et al. 2020), the availability of deep-sea squat lobster material in museum collections has increased considerably. During the revision of squat lobster material recently sampled along the East Pacific, new taxa and records were identified. In an integrative taxonomic framework, we describe new deep-sea munidopsid squat lobsters from the East Pacific. We also reconstruct the phylogenetic relationships among East Pacific species based on two mitochondrial markers and one nuclear fragment (cytochrome c oxidase subunit I, 16S rRNA and 28S rRNA), aiming to explore the systematic position of the East Pacific munidopsids and Janetogalathea californiensis, currently in Galatheidae. Finally, we test species hypotheses within Munidopsidae based on morphology and single locus species delimitation methods to (1) test the taxonomic status of these species; (2) explore molecular and morphological diversity within the family; and (3) infer the effect of depth range in the geographic speciation pattern of this lineage.
Methods
Sampling
Museum specimens were collected during various oceanographic cruises. These included several expeditions by Charlotte A. Seid and Greg W. Rouse during the past decade that aim to explore and characterise East Pacific deep-sea (>200 m) biodiversity through the use of remotely operated vehicles (ROVs: Doc Ricketts, Hercules, SuBastian and Tiburon) and the human occupied vehicle (HOV) Alvin. During these expeditions, specimens were collected using suction samplers, hydraulic manipulators or other submersible-deployed devices. Specimens were maintained alive in chilled seawater, photographed using a handheld camera or photomicroscope, sampled for genetics into 95% ethanol for long-term storage at −20°C and vouchered for morphology (treated with 10% seawater formalin or 95% ethanol for at least 24 h and subsequently transferred to 50% ethanol for long-term archival).
Morphological examination
Specimens were examined using a Leica MZ 12.5 stereomicroscope. Drawings were made using a camera lucida and digitised with a Wacom Intuos Pro tablet. Terminology used for the species descriptions followed Baba et al. (2009, 2011). Size was indicated by postorbital carapace length. Rostrum length was taken from the base (frontal margin) to the tip of the rostrum; rostrum width was taken as the width of the base. Measurements of appendages were taken on dorsal (pereopod 1), lateral (antennule, pereopods 2–4) and ventral (antenna) midlines. Ranges of morphological and meristic variations were included in the description. Abbreviations used were as follows: Mxp, maxilliped; P1, pereopod; 1 (cheliped); P2–4, pereopods 2–4 (walking legs 1–3); M, male; F, female; ov., ovigerous. Type material of Munidopsis quadrata Faxon, 1893, M. carinipes Faxon, 1893, M. margarita Faxon, 1893 and M. townsendi Benedict, 1902 was examined and illustrated. All specimens were deposited in the Invertebrate Zoology collection at the Museum of Comparative Zoology (MCZ IZ), Cambridge, MA, USA; the Invertebrate Zoology collection at the California Academy of Sciences (CASIZ), San Francisco, CA, USA; the Benthic Invertebrate Collection at Scripps Institution of Oceanography (SIO-BIC), University of California San Diego, La Jolla, CA, USA; the Invertebrate Zoology collection at the Smithsonian National Museum of Natural History (USNM), Washington, DC, USA; the Museo de Zoología, Universidad de Costa Rica (MZUCR), San José, Costa Rica; and the Muséum national d’Histoire naturelle (MNHN), Paris, France.
Micro-CT imaging
Only new species with an intact external morphology were scanned, to avoid further damage to the types. We mounted specimens in plastic vials, without staining, dry and using polypropylene cotton. We used 15-mL Falcon tubes or 1.5-mL Eppendorf tubes, depending on the size of the specimens, sealed with Parafilm.
Micro-computed tomography (micro-CT) scans were conducted in a SkyScan 1273 scanner (Bruker MicroCT, Kontich, Belgium). The scanner was equipped with a Hamamatsu 130/300 tungsten X-ray source 40–130 kV and a 6-megapixel (3072 × 1944) flat-panel X-ray detector. Scanning parameters were as follows: source current, 100 µA; source voltage, 75 kV; exposure time, 1000 ms; frames averaged, 2–6; rotation step, 0.3; frames acquired over 180° = 960; no filter; no binning; activated flat field correction; and scanning time, 50–120 min. Computer images were reconstructed using the software NRecon (ver. 1.6.6.0, Bruker MicroCT, Kontich, Belgium). To enhance image contrast and compensate for ring and streak artefacts, the following reconstruction parameters were set: no smoothing; ring artefact correction, 5–11; and activated beam hardening correction. Three-dimensional-rendering was performed using CTvox software (ver. 2.2, Bruker).
DNA extraction, amplification and sequencing
Specimens used for DNA extraction were dissected under a stereomicroscope. Tissue subsampled from the 5th pereopod was digested overnight using the DNeasy Blood & Tissue kit (Qiagen) following the manufacturer’s protocol. Partial sequences of the mitochondrial gene cytochrome c oxidase subunit I (COI), 16S ribosomal RNA and nuclear 28S ribosomal RNA were amplified by polymerase chain reaction (PCR) using the following combination of primers: LCO 1490/HCO 2198 (Folmer et al. 1994) or alternatively a squat-lobster-specific primer 3′ from LCO 1490, tenuiCOIFwint/HCO 2198 (Rodríguez-Flores et al. 2019a); 16Sa/16Sbr (Palumbi 1991) or 16S1471/16S1472 (Crandall and Fitzpatrick 1996); and 28SBR/28Sphyf2 (Palero 2008; Rodríguez-Flores et al. 2022b). PuReTaq Ready-To-Go (RTG) PCR Beads (Cytiva) were employed for the DNA amplification, following these cycle conditions: 5 min of initial denaturation at 95°C followed by 35 cycles of 30 s of denaturation at 95°C, 45 s of annealing at 45–50°C, 1 min of extension at 72°C and a 10-min final extension at 72°C. Purification of the amplicons was carried out using ExoSAP-IT (Affymetrix). Sanger sequencing of both forward and reverse strands was performed by GeneWiz (Cambridge). Forward and reverse DNA sequences obtained for each specimen were checked and assembled using Sequencher (ver. 5.4, Gene Codes Corporation, Ann Arbor, MI, USA). The presence of pseudogenes was checked using either Sequencher or the web tool EXPASY (https://web.expasy.org/translate/) translating the nucleotide sequences to amino acids under the invertebrate mitochondrial genetic code. Ribosomal gene sequences were aligned with MAFFT (Katoh et al. 2002) using the iterative method L-INS, recommended for sequences with conserved domains flanked by long variable regions, such as in the dataset analysed here, especially for the 28S rRNA gene. A posteriori manual checking and correction of the alignments was carried out in AliView (ver. 1.26, see https://ormbunkar.se/aliview/; Larsson 2014). The alignment of the concatenated gene fragments was built in PAUP (ver. 4.0a, build 169, see https://paup.phylosolutions.com/; Swofford 2003). We also calculated the uncorrected genetic distance (p) between the new species and closest relatives using PAUP.
Phylogenetic analyses
Phylogenetic analyses were performed using two different data sets: (1) concatenated data of 152 taxa and (2) COI data alone that included a larger number of terminals available in public databases, with 212 sequences of 82 named species. We first retrieved sequences from previous publications from the NCBI database (Ahyong et al. 2011; Bracken-Grissom et al. 2013; Rodríguez-Flores et al. 2018, 2019a, 2022a, 2022b; Dong et al. 2019, 2021). In the first dataset (1), we included wide taxonomic sampling across Galatheoidea (Galatheidae, Munididae, Munidopsidae and Porcellanidae), providing a phylogenetic framework to test the systematic position of the East Pacific species. We rooted the tree with the Lithodidae Paralithodes camtschaticus (Tilesius, 1815) (see Supplementary Table S1 for details on the taxonomic sampling). For the second dataset (2) we selected Janetogalathea californiensis (Benedict, 1902) as the outgroup for Munidopsinae (sensu Ahyong et al. 2011). We ran MrBayes (ver. 3.2.1, see https://nbisweden.github.io/MrBayes/download.html; Huelsenbeck and Ronquist 2001) for the Bayesian inference (BI) analyses. We selected a partition scheme by gene and codon for COI in the concatenated dataset and by codon in the COI dataset, according to the results of Model Selection in W-IQ-TREE online (ver. 1, see http://iqtree.cibiv.univie.ac.at/; Trifinopoulos et al. 2016) based on a Bayesian information criterion (BIC). For the MrBayes analyses we set the parameters of the likelihood model as ‘mixed’ for the nucleotide substitution model and the rates as ‘invgamma’. Four Markov Chains Monte Carlo (MCMC) were run for 5 × 107 generations and sampling trees and parameters every 5000 generations for the estimation of the posterior probabilities. The initial 25% of the generations were discarded as burn-in. Bayesian analyses were run in the CIPRES portal (see http://www.phylo.org/; Miller et al. 2010). We also ran maximum likelihood analyses (ML) to compare both ML and BI results. The ML tree was inferred with W-IQ-TREE online (ver. 1; Trifinopoulos et al. 2016). Bootstrap support values were calculated with 1000 pseudoreplicates and the other parameters were set as default. Nodes were considered supported when bootstrap values (Bs) were higher than 70% and posterior probability (pP) higher than 0.95. Phylogenetic trees were plotted and edited in FigTree (ver. 1.4.4, see http://tree.bio.ed.ac.uk/software/figtree/). Posterior probabilities from the Bayesian Inference and bootstrap support from ML were included in the final tree.
Single locus species delimitation analyses
To test species hypotheses, we ran single locus species delimitation analyses using the COI dataset. The choice of this marker was based on utility in taxonomy and species delimitation in crustaceans, and broad data availability for further comparisons (i.e. Jones and Macpherson 2007). We employed several heuristic methods for these analyses: (1) distance-based methods: Assemble Species by Automatic Partitioning method (ASAP) (Puillandre et al. 2021) and Automatic Barcode Gap Discovery for primary species delimitation (ABGD); and (2) tree-based methods based on the Phylogenetic Species Concept: the Poisson Tree Process (PTP) (Zhang et al. 2013) and generalised mixed Yule coalescence (GMYC) (Fujisawa and Barraclough 2013). We compared the results of these methods with currently accepted species.
ASAP is an exploratory tool to identify the best partitions of species by implementation of a hierarchical clustering algorithm that is based on pairwise genetic distances. ASAP efficiently builds species partitions from single locus sequence alignments (Puillandre et al. 2021). We ran ASAP through the online server (see https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html). We selected simple distances (p-distances) as the substitution model to compute the distances. The probability under the group split was set to 0.01. The ABGD method delimits species in terms of the barcode gap between the intraspecific and interspecific genetic distances. This method detects the barcoding gap as the first significant gap beyond a given limit as proxy of species partition (Puillandre et al. 2012). ABGD was run online (see https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html). We set the parameter values as follows: Pmin= 0.001; Pmax= 0.1; steps = 10, Nb bins = 20. The parameter X (relative gap width) was initially set to 2.0 and subsequently modified according to the exploration analyses. The model of evolution was simple p-distance, based on a barcode gap of 2%.
The PTP model infers species boundaries by modelling speciation or branching events based on the number of substitutions in a given gene tree (Zhang et al. 2013). bPTP adds Bayesian support (BS) values to delimited species on the input tree file. As input, we used the unrooted ML tree obtained with IQTREE. We ran the analyses for 5 × 105 MCMC generations with a thinning set at 500, burn-in = 25% and removing outgroups to improve the delimitation results. The analyses were carried using the web tool platform bPTP server (see http://species.h-its.org/ptp/). We checked MCMC convergence after the run.
GMYC is a species delimitation method that uses a speciation and neutral coalescent model (Yule 1925; Hudson 1991). GMYC was run in R (ver. 4.1.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/) by using the ‘splits’ package (ver. 1.0-20, see http://r-forge.r-project.org/projects/splits). We obtained an ultrametric tree in BEAST (ver. 2.6, see https://www.beast2.org/; Bouckaert et al. 2019) for the GMYC analyses, using the package Beast Model test to select the substitution model fitting the data. We set a Relaxed log normal clock for the clock model since this allows rate heterogeneity among branches. As our purpose was only to obtain an ultrametric tree and not calibrate the molecular clock, we set the clock rate to 1.0. We set the tree prior to the Birth–Death model with a Birth rate distribution uniform (Lower = 0.0, Upper = 1000.0) and a Death rate distribution uniform (Lower = 0.0, Upper = 1.0.). Chain length for the MCMC run was 10 000 000 generations logging every 1000 generations. A maximum clade credibility tree was obtained, after discarding 25% of the trees, using TreeAnnotator (ver. 2.1.2, see https://www.beast2.org/treeannotator/; Drummond and Rambaut 2007). The bGMYC analysis was first run with the resulting maximum clade credibility (MCC) tree and subsequently with 100 randomly selected trees from the 7500 post-burn-in trees in the bGMYC package (ver. 1.0.2, see https://nreid.github.io/software/; Reid and Carstens 2012). We used the following uniform priors: Yule process rate change (0–5), coalescence process rate change (0–1.5), and threshold parameter or number of taxa (1–16). Then, a scale for these parameters (1, 1, 0.2) was fixed. The results were plotted to check for convergence.
Haplotype networks were built for selected clades including East Pacific samples in taxa with taxonomic uncertainty or potential species complexes. Haplotype networks were built with the R package pegas (ver. 1.1, see https://cran.r-project.org/package=pegas; Paradis 2010) using a parsimony network with the function haploNet. Maps for Munidopsis species were drawn in R by using the packages ggplot 2 (ver. 3.4.0, see https://ggplot2.tidyverse.org/; Kahle and Wickham 2013), rnaturalearthdata (ver. 0.1.0, see https://cran.r-project.org/package=rnaturalearthdata; South and South 2016) and gridExtra (ver. 2.3, B. Auguie, A. Antonov and M. B. Auguie, see https://cran.r-project.org/package=gridExtra).
Results
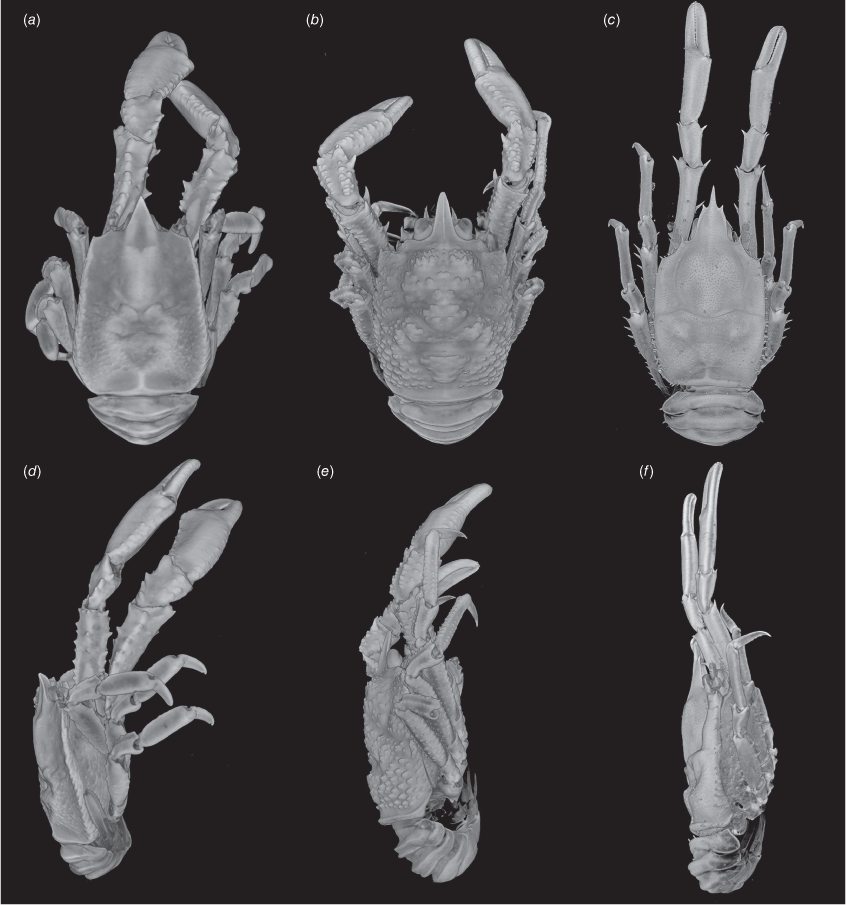
The overall results of our phylogenetic analyses and morphological examination converged on the presence of 5 new species in the eastern Pacific: Munidopsis girguisi sp. nov., Munidopsis nautilus sp. nov., Munidopsis testuda sp. nov., Munidopsis hendrickxi sp. nov. and Munidopsis cortesi sp. nov. Results of our analyses on morphological and molecular data suggest the need for establishing the following synonymies: Munidopsis alfredolaguardai Hendrickx & Ayón-Parente, 2013 and M. townsendi Benedict, 1902 are proposed as junior synonyms of Munidopsis aspera (Henderson, 1885); and Munidopsis asiatica Marin, 2020 is here proposed as a junior synonym of Munidopsis similis Smith, 1885. Janetogalathea californiensis is recovered as sister of Leiogalathea within the Munidopsidae clade, therefore this taxon should be reassigned from Galatheidae.
Phylogenetic results
According to model finder in IQ-TREE the best scheme and models obtained fitting our data were GTR+F+G4 for the first codon position, TN+F+I+G4 for the second and TIM3e+I+G4 for the third codon for COI, TVM+F+I+G4 for 16S rRNA and TVMe+I+G4 for 28S rRNA.
Results of ML and BI analyses yielded trees with congruent topologies. Munidopsidae was recovered as monophyletic with high support (Bs = 96% and pP = 1.00) (Fig. 1). Galatheidae was recovered as non-monophyletic since the genera Coralliogalathea Baba & Javed, 1974 and Phylladiorhynchus (both currently in Galatheidae) formed a clade unrelated to the remainder of the Galatheidae. This clade was sister group to Porcellanidae (although without support: Bs < 70% and pP < 0.95), both being sister group to a clade including Munididae + Galatheidae (excluding Coralliogalathea and Phylladiorhynchus) with relatively high support (Bs = 91% and pP = 0.96).
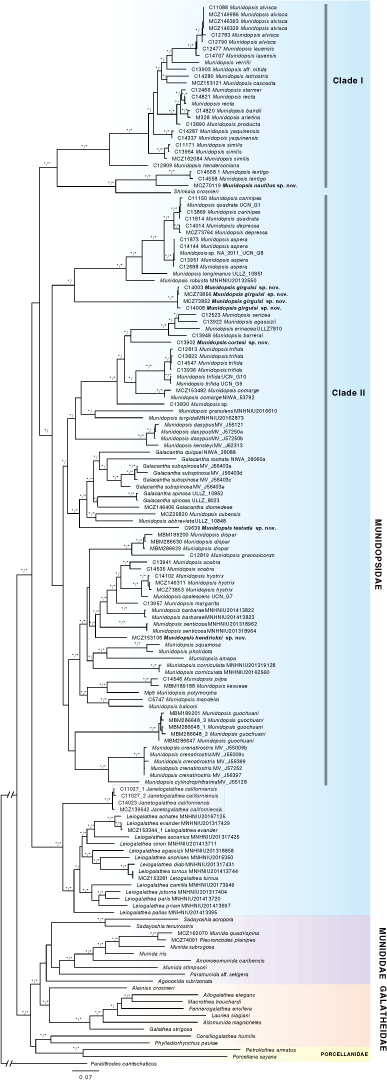
|
We did not obtain high support for the monophyletic clade including the family Munididae (Bs < 70% and pP < 0.95) although the clade with Munididae and most Galatheidae was well supported. The family Munidopsidae was recovered as sister group to the clade that includes the other galatheoids. This latter clade received a Bs = 71% and a pP = 1.00.
Within Munidopsidae, Munidopsis was recovered as polyphyletic, since Galacantha and Shinkaia were clustered within the genus. A clade including all the samples of Leiogalathea and Janetogalathea was recovered with maximal support. This clade was the sister group to the remaining Munidopsidae species and this grouping also received maximal support. The clade with the remaining Munidopsidae did not receive high support, and was split into what we have named Clade I and Clade II (see Fig. 1). Clade I, which received maximal support, included mostly bathyal and abyssal species (e.g. Munidopsis alvisca Williams, 1988; M. bairdii (Smith, 1884); M. recta Baba, 2005; M. verrilli Benedict, 1902; M. starmer Baba & de Saint Laurent, 1992; and M. producta Baba, 2005). Many internal relationships within Clade I were poorly supported. Clade I divided into two early subgroups, one composed of Munidopsis lentigo and Munidopsis nautilus sp. nov., and one with the remaining species. M. hendersoniana Faxon, 1893 was sister group to the remaining species within the latter clade.
Clade II included the remaining Munidopsidae species analysed (Fig. 1), most of which inhabit shelf and slope depths. Most of the deep relationships within this clade were poorly supported; however, some species-groups with congruent morphology were recognised with the molecular data, for example the aspera-group including species with elongated ocular peduncles [M. carinipes Faxon, 1893; M. quadrata Faxon, 1893; M. depressa Faxon, 1893; M. aspera (Henderson, 1885); M. longimanus (A. Milne Edwards, 1880); and M. robusta (A. Milne Edwards, 1880)]; the crenatirostris-group [M. crenatirostris Baba, 1988 + M. cylindrophthalma (Alcock, 1894)]; the genus Galacantha; the dasypus-group (M. dasypus Alcock, 1894 + M. kensleyi Ahyong & Poore, 2004); and the polymorpha-group (M. polymorpha + M. piipa Marin, 2020 + M. kexueae Dong, Gan & X Li, 2021).
For the most part, the new species described below were scattered across the phylogeny and not closely related. Munidopsis girguisi sp. nov. clustered as the sister taxon of the aspera-group but with low support (Bs = 88% and pP < 0.95). Munidopsis cortesi sp. nov. was included in the trifida-group constituted by species with a trifid rostrum: e.g. M. erinacea (A. Milne-Edwards, 1880); M. agassizii Faxon, 1893; M. barrerai Bahamonde, 1964; M. comarge Taylor, Ahyong & Andreakis, 2010; and M. trifida Henderson, 1885; however, the systematic position as sister group of the clade including M. sericea Faxon, 1893 + M. agassizii + M. erinacea was poorly supported (Bs = 82% and pP < 0.95). Munidopsis hendrickxi sp. nov. is related to the clade including M. scabra Faxon, 1893; M. hystrix; M. opalescens Benedict, 1902; M. barbarae (Boone, 1927); M. margarita; and M. senticosa Rodríguez-Flores, Macpherson & Machordom, 2018. Munidopsis testuda sp. nov. was sister group to a clade including M. abbreviata (A. Milne-Edwards, 1880) + M. cubensis Chace, 1942 plus all the Galacantha species. This grouping was highly supported (Bs = 98% and pP = 1).
Phylogenetic analyses of the COI fragment alone showed shallow divergences and wide distributional species ranges within the clade including bathyal and abyssal species (Clade I), contrasting with the deep divergences and allopatric distribution within the clade mostly containing species from the continental shelf and slope (Clade II) (Fig. 2). However, in a few cases Munidopsis species hypotheses were not supported by species delimitation analyses.
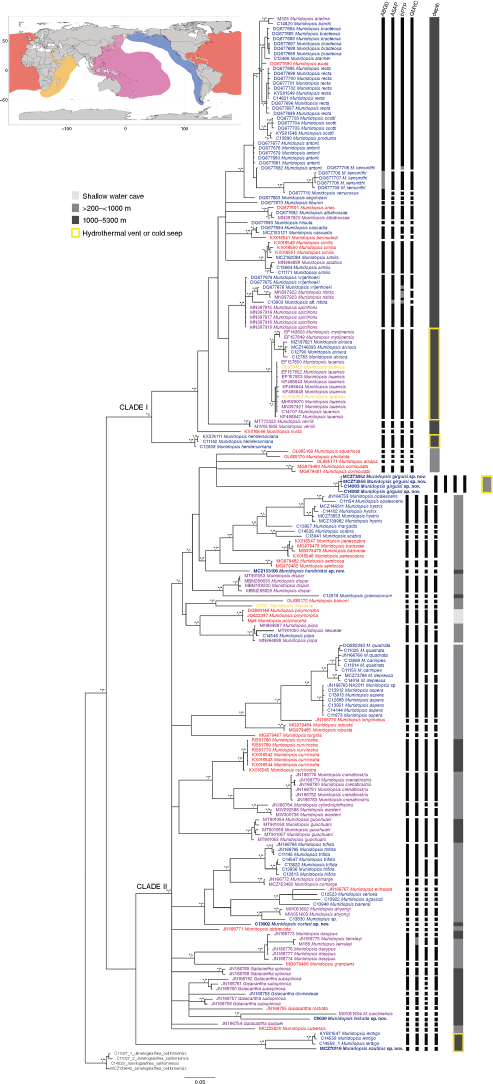
|
Species delimitation results
The different species delimitation analyses recovered largely congruent results but some methods tended to propose more species than others (see Fig. 2). ASAP recovered 10 species partition schemes, of which the best partition recovered 64 groups from the originally proposed 82 species including the outgroup. ABGD recovered 7 partitions when setting the parameter X = 1 after exploring the data and found the barcode gap width around this threshold. The initial species partition hypotheses found 57 groups (Partition with prior maximal distance P = 1.29 × 10−2; Barcode gap distance = 0.080; Distance Simple Dist MinSlope = 1.000000). The recursive analyses delimited up to 79 species, finding in all cases a barcode gap of ~0.08. bPTP results estimated a number of groups or species between 75 and 107 (mean 88) but only 79 of these presented support values higher than 50%. GMYC results were similar to the bPTP results, estimating 82 species with a confidence interval of 80–85 and a maximum likelihood of the GMYC model of 1438.268.
All these methods were congruent in suggesting that clade I (Fig. 2) comprised fewer species than currently accepted, i.e. all methods grouped multiple available names as single species. The clade including the mostly bathyal and abyssal species Munidopsis arietina Alcock & Anderson, 1894, M. bairdii, M. bracteosa Jones & Macpherson, 2007, M. recta, M. scotti Jones & Macpherson, 2007, M. antonii (Filhol, 1884) and M. exuta Macpherson & Segonzac, 2005 was resolved as a single species by all methods. The clade including M. vrijenhoeki Jones & Macpherson, 2007, M. nitida (A. Milne-Edwards, 1880) and M. spinifrons Dong, Xu, Li & Wang, 2019 was resolved as a single species by the distance-based methods but as four or five species by the tree-based methods. The hydrothermal vent species M. alvisca Williams, 1988 from the East Pacific, M. lauensis Baba & de Saint Laurent, 1992 and M. myojinensis Cubelio, Tsuchida, Hendrickx, Kado & Watanabe, 2007 from the West Pacific were considered a single species in all analyses and therefore this study supports the idea of a single species broadly distributed across hydrothermal vent sites across the Pacific and Indian Oceans; this is evidenced by the morphological conservatism between M. lauensis and M. alvisca that makes these species morphologically indistinguishable once multiple specimens per population are examined. An inability to distinguish species on a molecular basis occurs in M. similis and M. asiatica, two species that are extremely similar, and M. albatrossae Pequegnat & Pequegnat, 1973 and M. aries (A. Milne-Edwards, 1880), where the three specimens sampled from the Atlantic, East and West Pacific are not distinguished as different species. Additional species not supported by our species delimitation analyses include M. quadrata and M. carinipes (by all methods) and some methods also include M. depressa with these two species. Additionally, M. piipa and M. kexueae Dong, Gan & Li, 2021 are shown as a single species by some methods but not by GMYC, and M. kensleyi and M. dasypus are sometimes supported as one species and sometimes as two or three species. By contrast, M. scabra from California and Costa Rica, and Galacantha subspinosa Macpherson, 2007 (all sequences from GenBank) appear as multiple species.
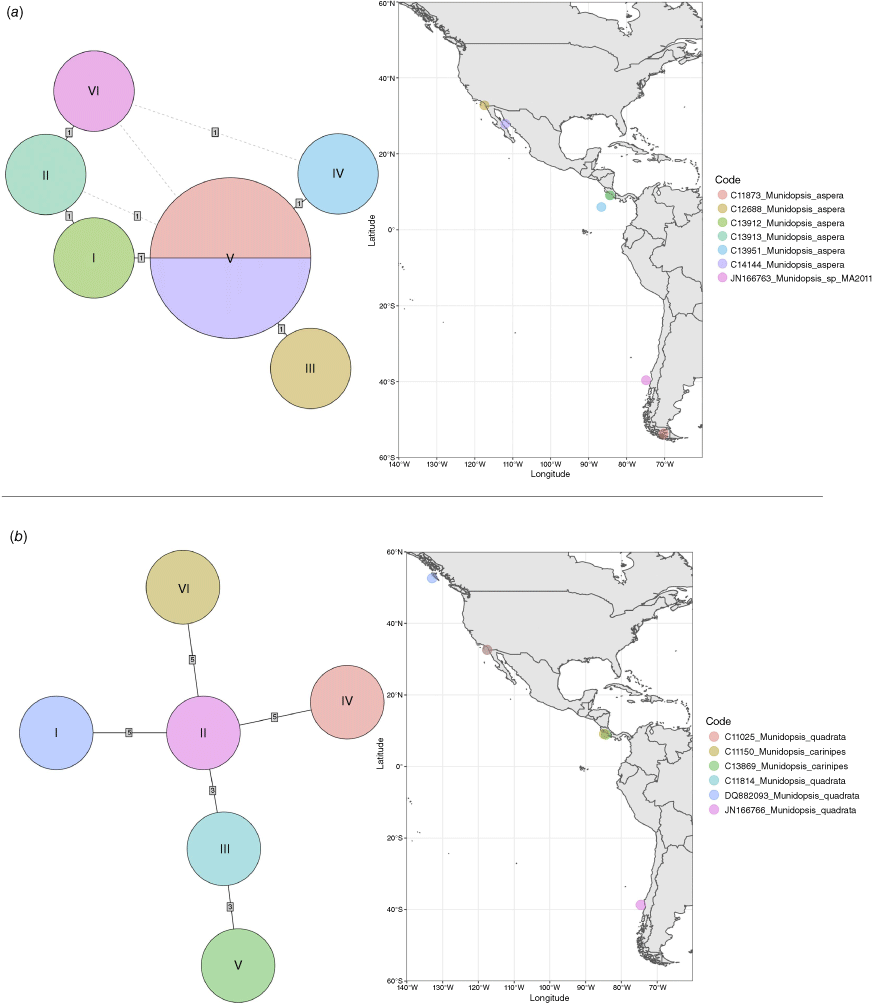
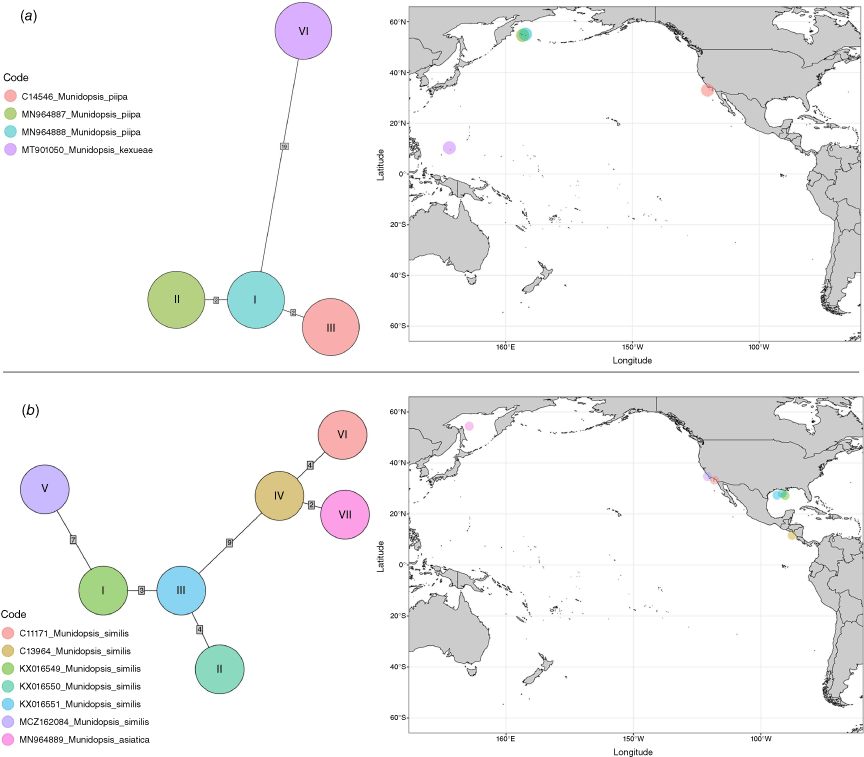
Haplotype networks
We reconstructed haplotype networks for the following selected clades: M. aspera; M. quadrata + carinipes; M. piipa + M. kexueae; and M. similis + M. asiatica (Fig. 3, 4). For Munidopsis aspera we included haplotypes from six individuals and a sequence from GenBank identified as Munidopsis sp. (from Chile) that nested within M. aspera. We found six haplotypes from these seven sequences, five of which were unique, and one of which was shared among individuals from Magellan Strait and the Gulf of California. These haplotypes were connected by a single mutational step. We found unique haplotypes for each of the six specimens analysed for M. quadrata and M. carinipes with a distance between haplotypes of three to five mutational steps. The haplotype network did not segregate the haplotypes among the two species sampled. For Munidopsis piipa from the Bering Sea and California, and M. kexueae from the West Pacific, haplotypes were also unique for each specimen and the haplotype network clearly separated the M. kexueae specimen from M. piipa, with 19 mutational steps between the two groups of haplotypes. This contrasted with some of the species delimitation analyses (Fig. 2) that found M. kexueae nested within M. piipa or assigned the four specimens as a single species. In the case of Munidopsis similis and M. asiatica, we found unique haplotypes for each specimen analysed, with a minimum distance between connected haplotypes of 2 and a maximum of 9 mutational steps. Interestingly, two of the most extreme haplotypes were from specimens from nearby localities in California. However, Munidopsis asiatica from the Sea of Okhotsk, NW Pacific, only differed from a specimen from the Pacific of Costa Rica by two mutational steps. Although all the samples from the Gulf of Mexico formed a group, one of the Pacific samples appeared closer to these than to the other Pacific samples.
|
|
Permits
Specimen collection and field operations in Costa Rica were performed under the following permits issued by CONAGEBIO (Comisión Nacional para la Gestión de la Biodiversidad), INCOPESCA (Instituto Costarricense de Pesca y Acuicultura) and SINAC (Sistema Nacional de Áreas de Conservación) under MINAE (Ministerio de Ambiente y Energía), Government of Costa Rica: INCOPESCA-CPI-003-12-2018, R-070-2018-OT-CONAGEBIO, SINAC-CUSBSE-PI-R-032-2018, SINAC-SE-CUS-PI-R-035-2017. In accordance with the Nagoya Protocol on Access and Benefit Sharing, DNA sequencing for this project was authorised by the Contract for the Grant of Prior Informed Consent between MINAE-SINAC-ACMC and Jorge Cortés Nuñez for the Basic Research Project: ‘FK190106 – Cuantificación de los vínculos biológicos, químicos y físicos entre las comunidades quimiosintéticas con el mar profundo circundante.’
Systematics
GALATHEOIDEA Samouelle, 1819
MUNIDOPSIDAE Ortmann, 1898
Genus Janetogalathea Baba & Wicksten, 1997
Janetogalathea californiensis (Benedict, 1902) family Munidopsidae stat. nov.
Material examined
Non-type specimens. USA: off San Diego, California, leg. Harim Cha, Kent Trego, R/V New Horizon student cruise; 28.x.2007, 32.67500°N, 117.36330°W, 175–300 m: 1 M 24.1 mm, 1 F 14.9 mm (SIO-BIC C11027). — USA: Crespi Knoll, off California, leg. Emily McLaughlin, Jessica Pruitt, R/V Falkor, ROV SuBastian dive S0169 Slurp 2, 13.x.2018, 33.09770°N, 117.86790°W, 484.2 m: 1 F 5.5 mm (SIO-BIC C14023). — USA: California, leg. E/V Nautilus, ROV Hercules dive H1534, Stn NA074-027-01, 14.vii.2018, 33.95930°N, 119.47540°W, 172.4 m: 1 F 9.5 mm (MCZ IZ-139642).
Diagnosis
Modified from Macpherson and Baba (2011). Carapace with transverse striae usually granulated with numerous short setae and scattered stiff long setae, laterally with 4–6 spines, cervical grooves distinct. Rostral spine flattish, subtriangular, lateral margin usually with 3–4 lateral spines, proximal small. With a pair of epigastric spines. Frontal margins slightly oblique. Orbit not distinctly excavated, without outer orbital angle. Anterolateral spine of carapace strong. Branchial margin with 4 spines. Abdominal somites 2–4 with 2 transverse ridges, unarmed. Telson incompletely subdivided. Sternite 3 narrow, expanded laterally, sternite 4 subtriangular. Eyes large, movable, cornea strongly dilated. Article 1 of antennule with 3 well-developed spines. Article 1 of antenna with distomesial spines short, fused with orbit. Short and thick flagellum on Mxp1. Mxp3 merus as long as ischium, subrhomboidal in lateral view. P1 slender, elongate. P2–4 long and slender; dactyli slender, curving, flexor margin with spine-like setae. Epipods absent from all pereopods.
|
Colouration
Carapace and abdomen reddish or light orange. P1–4 light orange.
Distribution
Baja California, Gulf of California, Mexico Channel Islands, California, USA. From 89- to 3993-m depth (see comments in Hendrickx 2021 and Hendrickx et al. 2011, regarding J. californiensis geographic distribution).
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Janetogalathea californiensis is now included in the family Munidopsidae due to evidence from molecular phylogenetics placing this as sister group of Leiogalathea, according to nuclear and mitochondrial data. We investigated the presence of the chief synapomorphy of Munidopsidae, the absence of a short or reduced flagellum on the exopod of the Mxp1. The Mxp 1 exopod presents a short, thick pseudoflagellum, as in Leiogalathea (Ahyong et al. 2010). However, the validity of this character as a synapomorphy for Munidopsidae is questionable. Both Leiogalathea and Janetogalahea also share the presence of a broad triangular or subtriangular rostrum, a well-developed cornea and a carapace with few to several transverse setose striae. Janetogalathea and Leiogalathea could arguably form a clade that could be assigned family rank, showing morphological convergence with squat lobsters in the family Galatheidae but this will be addressed in future studies.
The wide bathymetric distribution and dubious morphological identification of specimens from the Galapagos Islands (Arnés-Urgellés et al. 2020; Hendrickx 2021) suggest that Janetogalathea californiensis could constitute a species complex. A deeper study including genetic data across the species range should be conducted to test this hypothesis.
Genus Munidopsis Whiteaves, 1874
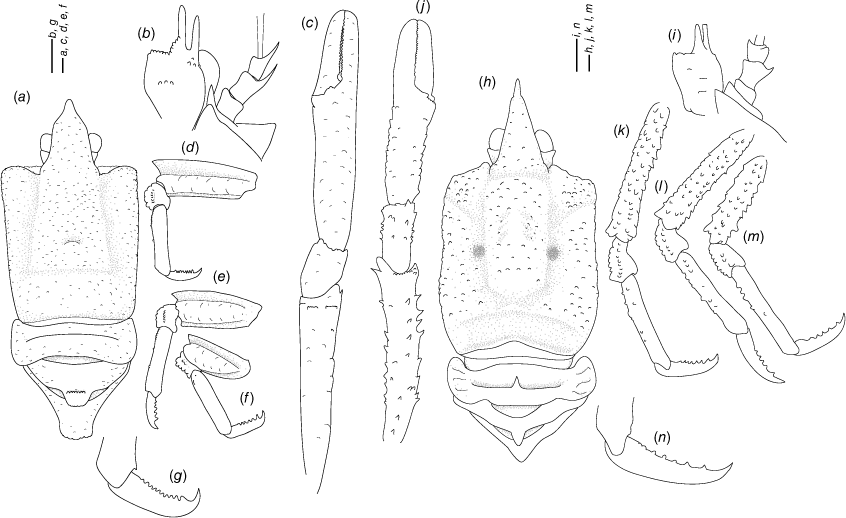
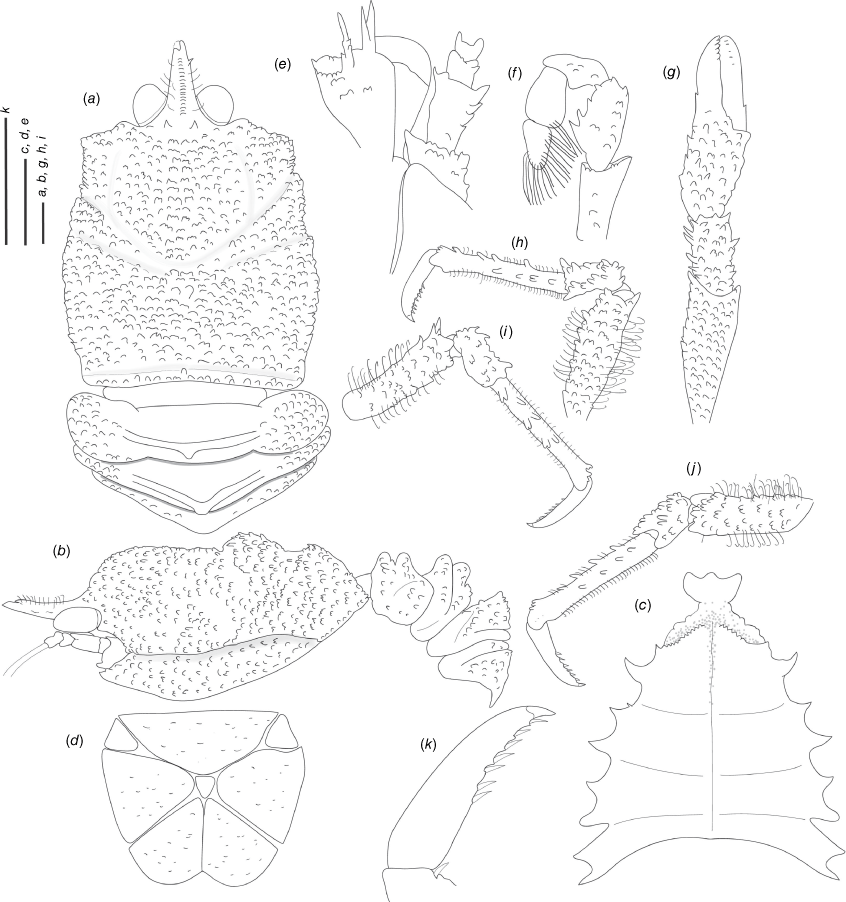
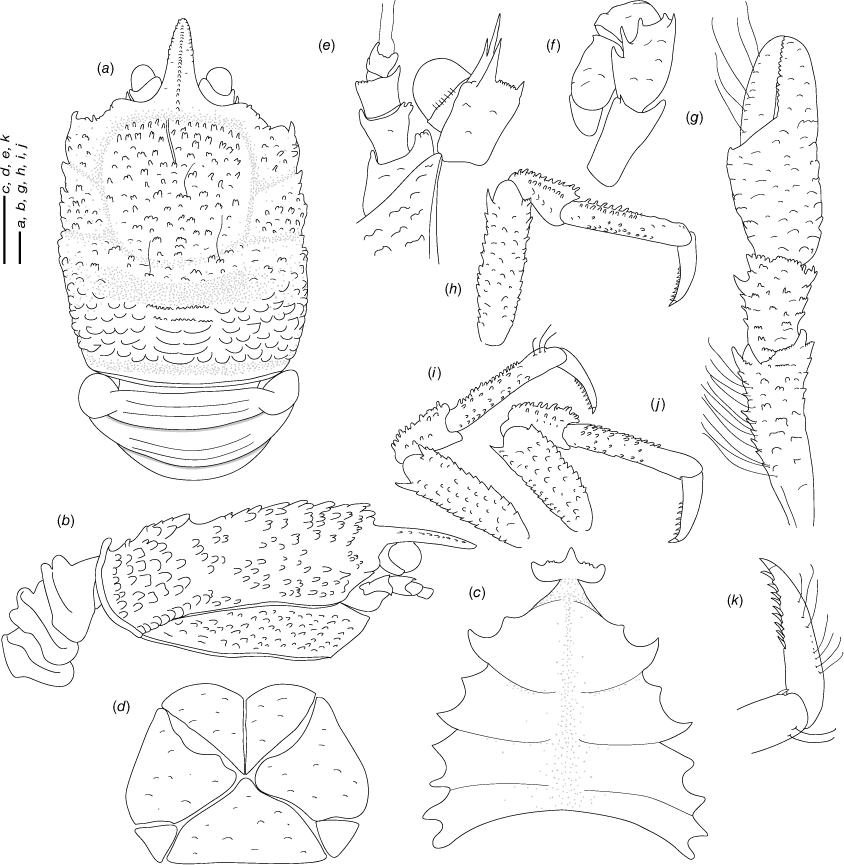
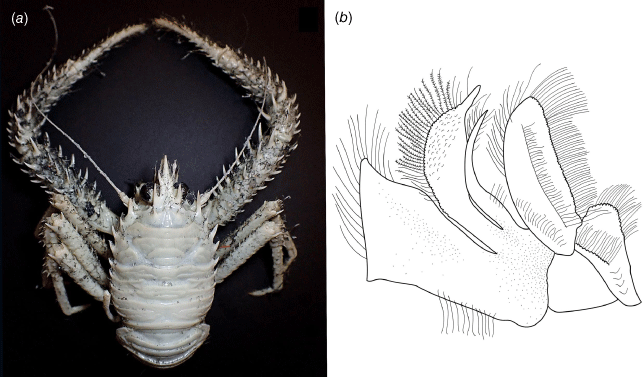
Munidopsis aspera (Henderson, 1885)
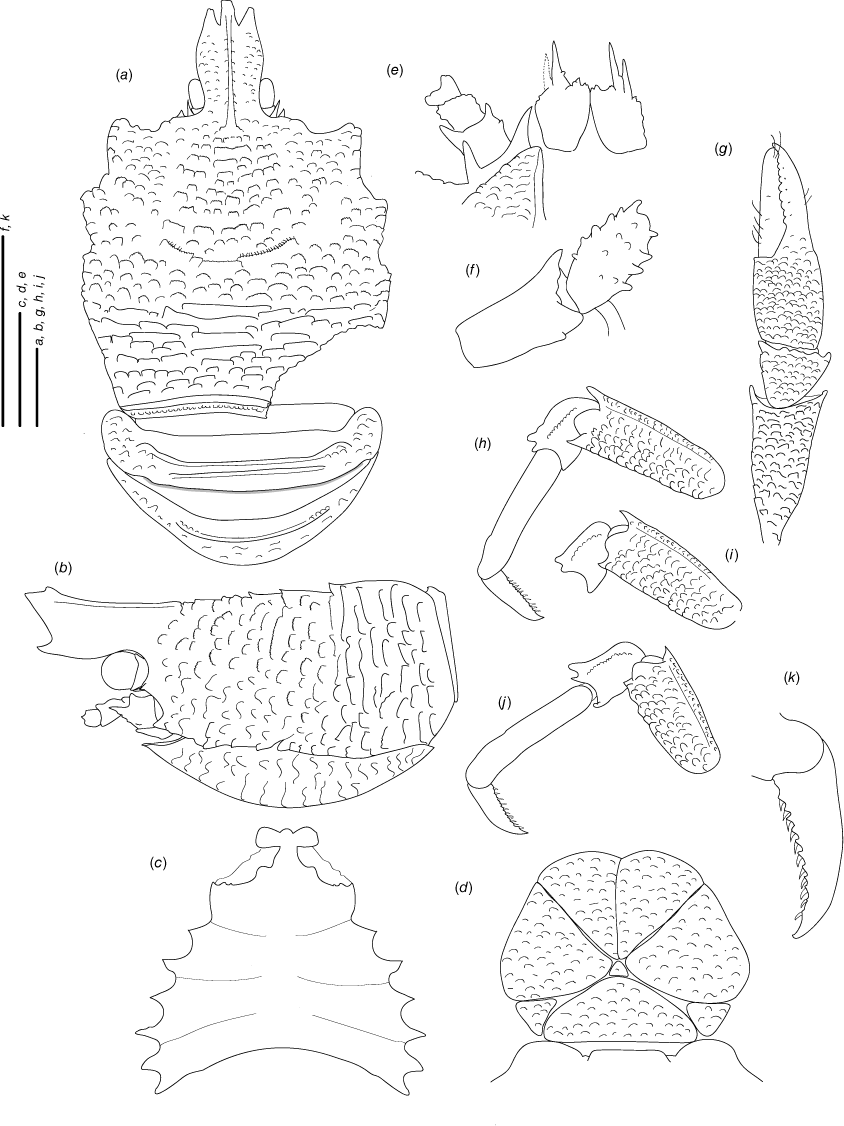
(Fig. 6a–j, 7a–e, Supplementary Fig. S1.)
Material examined
Holotype (M. townsendi). ECUADOR: Galapagos Islands, Santa Cruz and San Cristobal Islands, leg. USFC Steamer Albatross, Stn 2818, field number USFC/A2818, 0.48330°S, 89.90830°W, 15.iv.1888, 717 m: ov. F 8.1 mm (USNM 26167).
Non-type specimens. Magellan Strait, A. Milne-Edwards det., Saint Laurent re-det.: 2 ov. F 10.2–10.9 mm (MNHN Ga301).
Magellan Strait, leg. Nerida Wilson, Greg Rouse, R/V Nathaniel Palmer, Stn SM1b-3, 10.iv.2013, 53.6022°S, 70.2456°W to 53.6025°S, 70.2322°W, 270–285 m: 1 M 13.4 mm, 1 ov. F 12.3 mm (SIO-BIC C11873).
ECUADOR: east of Galapagos Islands, leg. USFC Steamer Albatross, Stn 3402, 28.iii.1891, 0.95833°S, 89.05833°W, 421 fms (769.9 m): 1 M 6.5 mm, 3 ov. F 7.5–7.8 mm, 2 F 7.5–8.3 mm (MCZ IZ CRU-4557). — ECUADOR: east of San Salvador Island, Galapagos Islands, leg. USFC Steamer Albatross, Stn 3406, 3.iii.1891, 0.26666°S, 90.35833°W, 551 fms (1008 m): 1 M 4.8 mm, 1 F 5.1 mm (MCZ IZ CRU-4558).
COSTA RICA: The Thumb, leg. Greg Rouse, Allison Miller, R/V Falkor, ROV SuBastian dive S0217 B4-3, 10.i.2019, 9.04915°N, 84.39308°W, 1065 m; specimen not measured (SIO-BIC C13912), juvenile (SIO-BIC C13913). — COSTA RICA: Seamount 8, leg. Greg Rouse, Avery Hiley, R/V Falkor, ROV SuBastian dive S0226 Q7, 21.i.2019, 6.00765°N, 86.64906°W, 1310 m: 1 ov. F 11.2 mm (SIO-BIC C13951).
MEXICO: Guaymas Basin, Gulf of California, leg. Greg Rouse, Sigrid Katz, R/V Western Flyer, ROV Doc Ricketts dive D381, 11.iv.2012, 27.89591°N, 111.96700°W, 1064 m: 1 M 8.8 mm (SIO-BIC C14144).
USA: Pioneer Seamount, off San Francisco, California, leg. Craig McClain, David Clague, R/V Western Flyer, ROV Tiburon dive T1100, 18.vi.2006, 37.36141°N, 123.39300°W, 1229–1513 m: 1 M 6.75 mm (SIO-BIC C11953). — USA: off San Diego, California, leg. Greg Rouse and students, R/V Robert Gordon Sproul, otter trawl within oxygen minimum zone, 5.viii.2017, 32.7553°N, 117.4546°W, 570 m: 1 M 11.2 mm (SIO-BIC C12687), 1 ov. F 10.1 mm (SIO-BIC C12688), 1 M 11.2 mm (SIO-BIC C12524). — USA: off San Diego, California, leg. Greg Rouse, Avery Hiley and students, R/V Robert Gordon Sproul, SP1825, Stn 3 otter trawl, 2.ix.2018, 32.76463°N, 117.45417°W to 32.77180°N, 117.45325°W, 500 m: 1 ov. F 11.1 mm (SIO-BIC C13703). — USA: off San Diego, California, leg. Gabriella Berman, Sonja Huc, R/V Robert Gordon Sproul, SP2115 otter trawl, 7.viii.2021, 32.73467°N, 117.45100°W to 32.80017°N, 117.41567°W, 500 m: 1 ov. F 10 mm (SIO-BIC C14526).
Description
Carapace
Slightly longer than broad, widest at midlength; convex from side to side. Dorsal surface densely covered by acute denticles and denticulated tubercles, each denticle or tubercle with few short setae, hepatic and anterior branchial areas with denticles and some acute denticles. Regions well delineated by deep furrows, anterior and posterior cervical grooves distinct. Gastric region flattened. Posterior margin armed with tubercles, preceded by elevated ridge. Rostrum triangular to spiniform, setose, width 0.2–0.3× anterior width of carapace, directed slightly upwards, denticles and tubercles on margin and dorsal surface, 0.3× carapace length, 1.3–1.6× as long as broad. Frontal margin slightly concave behind ocular peduncle, blunt outer orbital angle above antennal peduncle, orbit delimited by several outer orbital denticles or tubercles. Lateral margins convex; anterolateral angle armed with acute tubercles; branchial margins, tuberculated, denticulated. Pterygostomian flap surface covered by denticles and granules, anterior margin blunt.
Sternum
Slightly longer than broad, maximum width at sternite 6. Sternite 3 broad, 2.2–2.6× wider than long, anterolaterally rounded, often serrated, anterior margin with median notch flanked by 2 lobes. Sternite 4 narrowly elongate anteriorly, anterior margin smooth; surface depressed in midline, smooth; greatest width 2.5–3.2× that of sternite 3, and 2.0–2.7× wider than long.
Abdomen
Tergites with tubercles and denticles in all surfaces, tergites 2–4 armed with a median broad spine covered by tubercles, tergites 2–3 with 1 elevated transverse ridge; tergites 4–6 lacking ridges; tergite 6 with weakly developed posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 9 plates; 1.4–1.5× as wide as long.
Eye
Eyestalk movable; peduncle elongated, densely covered by denticles and small tubercles, narrower than cornea length; cornea ovoid, length 0.7–0.9× that of peduncle; epistomial spine (below antennal spine, ventral to frontal margin) absent.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines, distolateral margin proximally armed with denticles; distomesial margin with denticles.
Antenna
Peduncle not exceeding eye, armed with denticles and granules; article 1 with distomesial spine and distolateral spine, distomesial spine reaching end of article 2. Article 2 unarmed or with minute distomesial and distolateral spine. Article 3 unarmed or with small lateral spine or with prominent distal denticles; article 4 unarmed.
Mxp3
Surface with granules and denticles. Ischium 1.3× longer than merus measured on extensor margin, distal margin serrated; flexor margin of merus with one prominent spine and several denticles, extensor margin with 3–4 spines including distal spine.
P1
Moderately slender, females 1.7–2.0, males 2.5–2.7× longer than carapace, cylindrical, with numerous tubercles and denticles. Merus 2.4–2.8× carpus length, with denticles and spines on all surfaces. Carpus 1.3–1.5× longer than broad, unarmed or with rows of spines on mesial, lateral margins and some distal stout spines. Palm with row of spines on all surfaces, moderately slender, 2.6× longer than carpus, 2.6–2.8× longer than broad. Fingers unarmed or armed with proximal small spines or denticles, 0.7–0.9× longer than palm, opposable margins nearly straight, not gaping, spoon-shaped; fixed finger without denticulate carina on distolateral margin.
P2–4
Moderately slender, coarsely tuberculated and denticulated in all surfaces, with fine distally curved setae on meri and carpi, cylindrical in cross section, slightly decreasing in size posteriorly. P2 merus moderately slender, 0.5–0.6× carapace length, nearly 4.0× longer than high and 1.5× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.9× length of P2 merus, P4 merus 0.9× length of P3 merus); extensor margin with granules and denticles along entire border, distal part cylindrical ending in 1–2 thick spines; flexor margin tuberculated; P2–4 carpi with 0–3 thick spines on extensor margin, acute tubercles on lateral sides; P2–4 propodi with acute tubercles on extensor margin and lateral sides, distal flattened, ending in 3 denticles, 5.2–6.0× as long as high, flattened in cross section, unarmed; dactyli short, 0.4–0.5× length of propodi; distal claw short, moderately curved; flexor margin distally strongly curved, unarmed.
Epipods
Absent from pereopods.
Eggs
Approximately 10–55 round eggs of 1 mm each.
Colouration
Body and pereopods whitish to light brownish, with light orange eyes.
Distribution
California, Gulf of California, to Strait of Magellan, from 166- to 1398-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis aspera was described from Port Churruca, Magellan strait, Chile (Henderson 1885), with additional records from Galapagos (Ecuador), Cocos Islands (Costa Rica) (Faxon 1895) and California (Schmitt 1921; Luke 1977). The species was poorly illustrated in the literature (Wicksten 2012) but considered widely distributed across the East Pacific (Baba et al. 2008). Munidopsis townsendi Benedict, 1902 was described from Galapagos and only the anterior part of the carapace and rostrum, and the left P1 were illustrated (Benedict 1902). Munidopsis alfredolaguardai was described from the Baja California Peninsula in the SW Gulf of California (Hendrickx and Ayón-Parente 2013) and later recorded from Chile (Guzman and Sellanes 2015). Munidopsis alfredolaguardai, M. aspera and M. townsendi are similar morphologically but undertaking a definitive comparison among species was difficult because of the absence of proper illustrations of M. aspera and M. townsendi. We analysed the morphology and genetics from material from the full range of M. aspera, including material from the type locality (Magellan Strait), Galapagos and Gulf of California, and the type of M. townsendi. We found morphological variation among specimens across the range of the species in carapace size, spinulation on cheliped palms, the number and size of the external denticles and tubercles on carapace, pereiopods, ocular peduncles and antennal articles. However, we did not elucidate genetic structure genetic differences across the distribution range for all the markers analysed (Fig. 1–3a), nor morphological differences between M. aspera and M. townsendi and an illustrated description of M. alfredolaguardai (Hendrickx and Ayón-Parente 2013; Guzman and Sellanes 2015). We therefore conclude that M. alfredolaguardai and M. townsendi are junior synonyms of M. aspera.
Munidopsis aspera belongs to a group of species sharing a frontal margin without delimited orbit, telson with 9–10 plates, eye movable, unarmed, with peduncle longer than cornea and epipods absent from all pereiopods. This group of species includes M. quadrata and M. carinipes from the Northern East Pacific, M. longimanus from the Gulf of Mexico and the Caribbean Sea, M. robusta from the Caribbean Sea, M. depressa from California, M. abdominalis (A. Milne Edwards, 1880) from the Caribbean Sea, M. alaminos Pequegnat & Pequegnat, 1970 from the Gulf of Mexico, M. brevimana (A. Milne Edwards, 1880) from the Caribbean Sea and M. riveroi Chace, 1939 from the Gulf of Mexico and the Caribbean Sea. Munidopsis aspera is easily distinguished from the remaining species of the group by having the flexor margin of the dactyli unarmed, and the carapace, pereopods, ocular peduncles and antennal articles densely covered by denticles and tubercles.
Munidopsis barrerai Bahamonde, 1964
Material examined
Non-type specimens. COSTA RICA: Las Gemelas Seamount, near Isla del Coco, leg. Greg Rouse, Avery Hiley, R/V Falkor, ROV SuBastian dive S0225 S7, 20.i.2019, 4.97945°N, 87.45870°W, 637 m: 1 F 14.5 mm (SIO-BIC C13948).
Genetic data
COI, 16S rRNA and 28S rRNA.
Distribution
Chile, Perú from 280- to 800-m depth and Costa Rica at 637-m depth.
Remarks
Munidopsis barrerai is newly recorded from Costa Rica. The general morphology of the specimen collected from Costa Rica agrees with the species described by Bahamonde (1964) and Guzman and Sellanes (2015). Unfortunately we did not have material from Chile for genetic comparisons between populations.
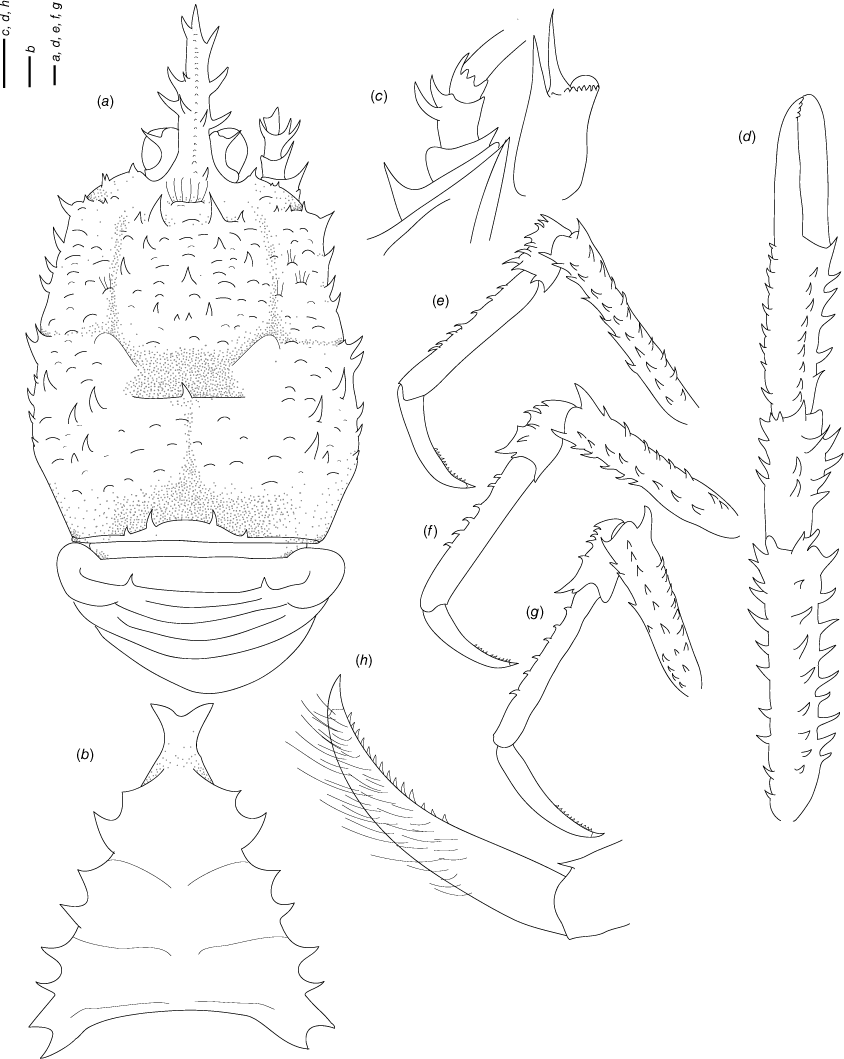
Munidopsis carinipes Faxon, 1893
(Fig. 8a–g, Supplementary Fig. S2.)
Material examined
Lectotype. PANAMA: Gulf of Panama, Azuero Peninsula, Morro de Puercos, leg. USFC Steamer Albatross, Stn 3353, 23.ii.1891, 7.10416°N, 80.56666°W, 695 fms (1271 m): 1 M 10.1 mm (MCZ IZ CRU-4561).
Paralectotype. Same collecting data as lectotype: 1 M 6.1 mm, 1 ov. F 8.2 mm (MCZ IZ-163058).
Non-type specimens. COSTA RICA: Jaco Scar, leg. Elena Perez, Jake Bailey, R/V Atlantis, HOV Alvin dive 4509, 3.iii.2009, 9.1172°N, 84.8425°W, 1459 m: 1 M 10.5 mm (SIO-BIC C11150). — COSTA RICA: Mound 11, leg. Victoria Orphan, Hang Yu, R/V Atlantis, HOV Alvin dive 4988, naturally occurring wood fall, 3.xi.2018, 8.92208°N, 84.30446°W, 1010 m: 1 M 9.9 mm (SIO-BIC C13869). — COSTA RICA: Mound 12, leg. Greg Rouse, Allison Miller, R/V Falkor, ROV SuBastian dive S0215 B3-7, 8.i.2019, 8.93313°N, 84.30750°W, 1011 m: 1 ov. F 10.0 mm (SIO-BIC C13916).
Diagnosis
Carapace dorsally smooth, porose, laterally unarmed, with deep dorsal furrows, cervical grooves indistinct. Rostrum broadly triangular, lateral margins subparallel, unarmed. Frontal margins slightly oblique, slightly concave behind ocular peduncle. Orbit not distinctly excavated, outer orbital angle blunt. Anterolateral angle unarmed. Branchial margin unarmed. Abdominal somites with transverse ridge, somites 3–5 armed with a broad spine. Telson divided into 9 plates. Sternite 3 moderately broad, anterolateral angles produced, sternite 4 subtriangular. Eyes unarmed, movable, epistomial spine absent. Article 1 of antennule with well-developed dorsolateral and distolateral spines. Article 1 of antenna with distomesial spine. Mxp3 merus subrhomboidal in lateral view. P1 slender, elongate, unarmed, fixed finger without denticulate carina on distolateral margin. P2–4 stout, unarmed; meri strongly carinated, with a ridge in extensor and flexor margin; dactyli moderately slender, curving, flexor margin with cuticular teeth along all margin decreasing proximally. Epipods absent from all pereopods.
Eggs
Approximately 20–35 eggs, 1 mm in diameter.
Colouration
Body and pereopods whitish, with light orange eyes.
Distribution
East Pacific, Panama and Costa Rica from 915- to 1459-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis carinipes belongs to a group of species sharing a frontal margin without a delimited orbit, telson with 9–10 plates, eye movable, unarmed, with peduncle longer than cornea, and epipods absent from all pereopods. The closest species is M. quadrata Faxon, 1893 from the Northeast Pacific. See the differences in the Remarks of that species.
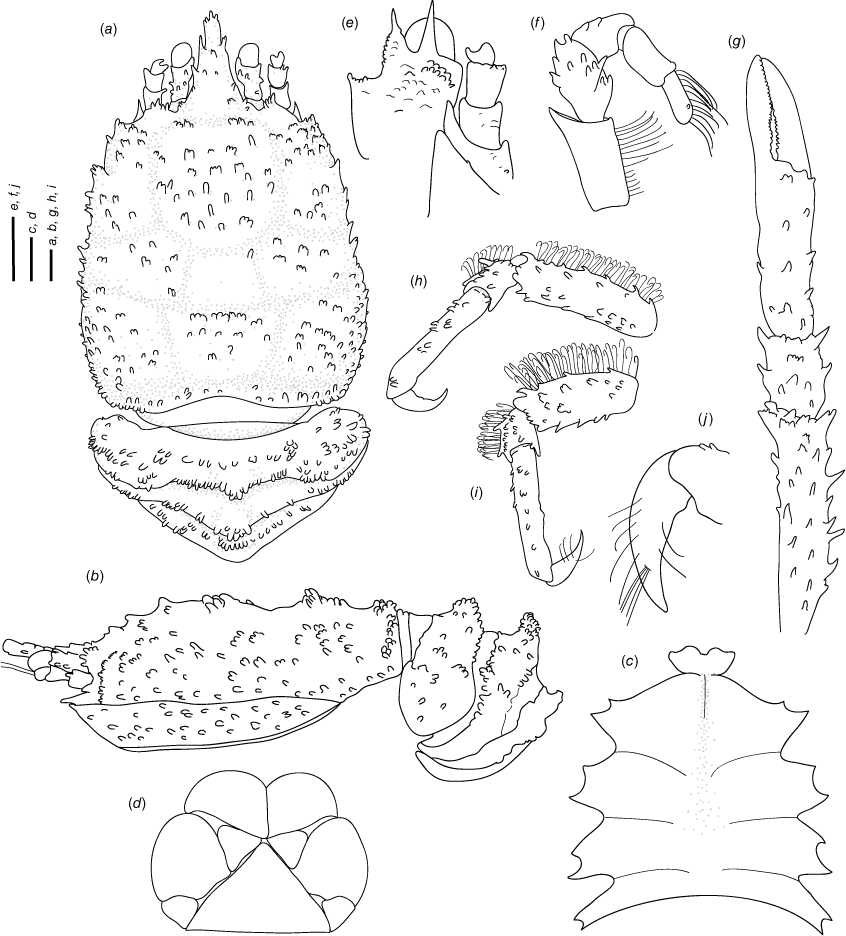
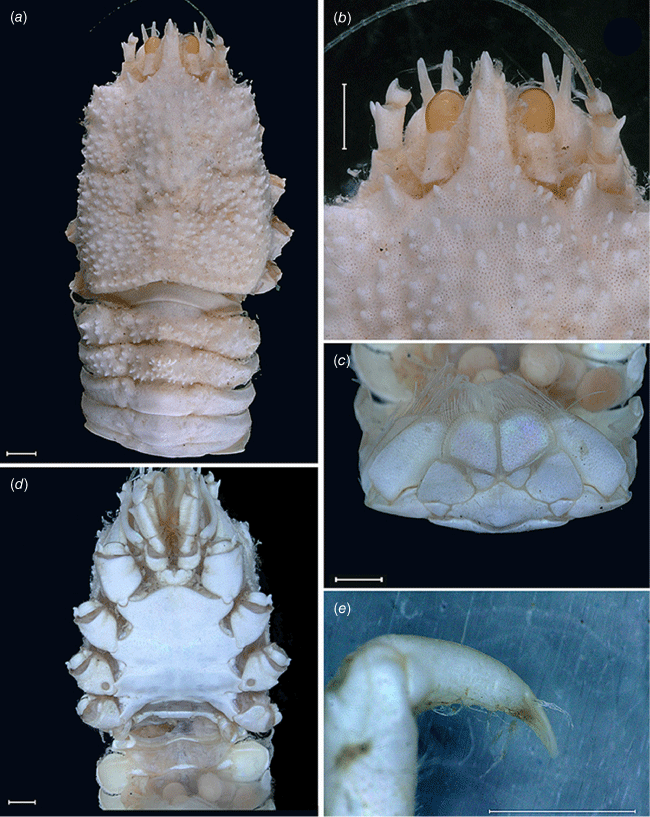
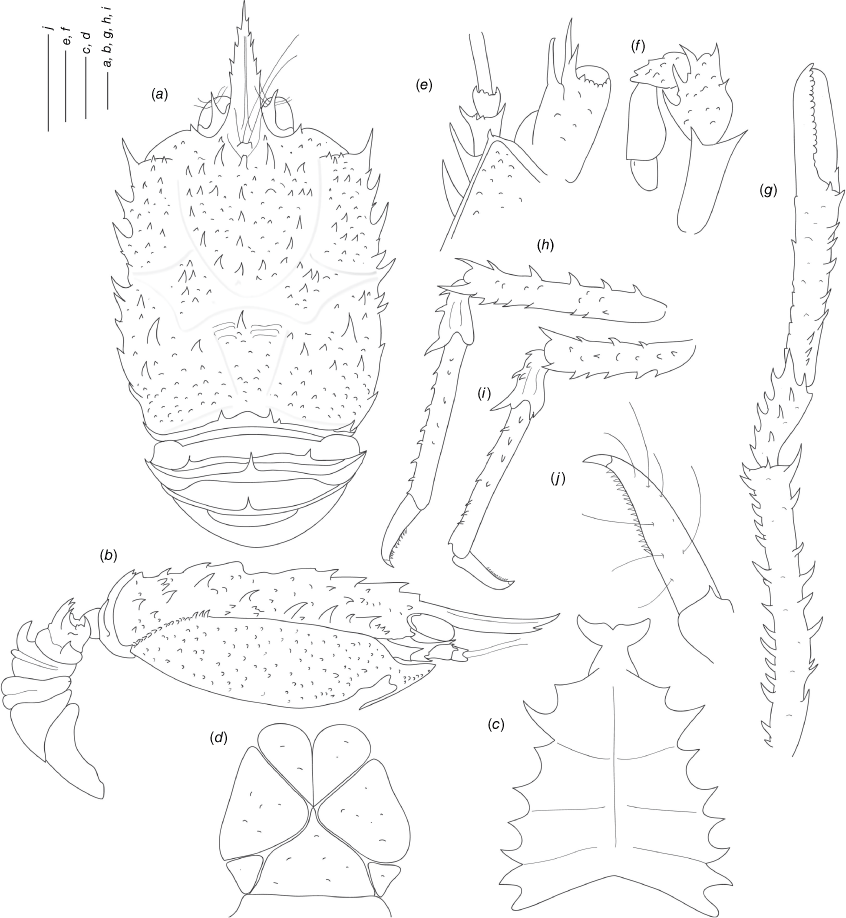
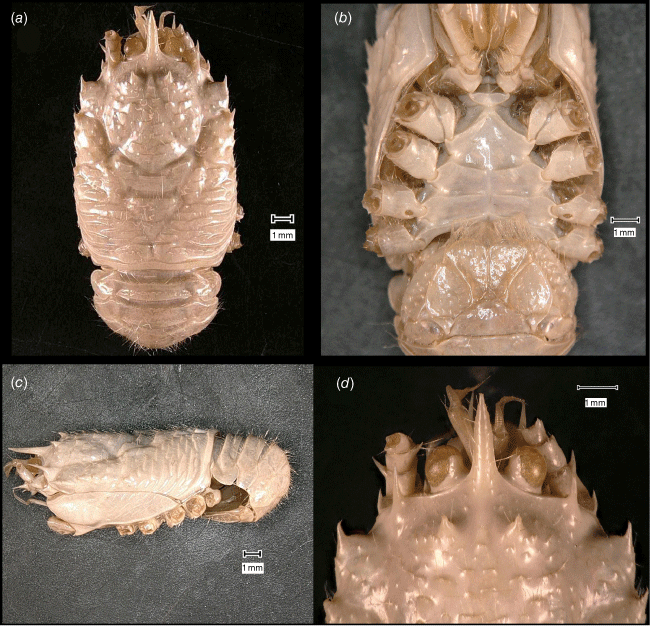
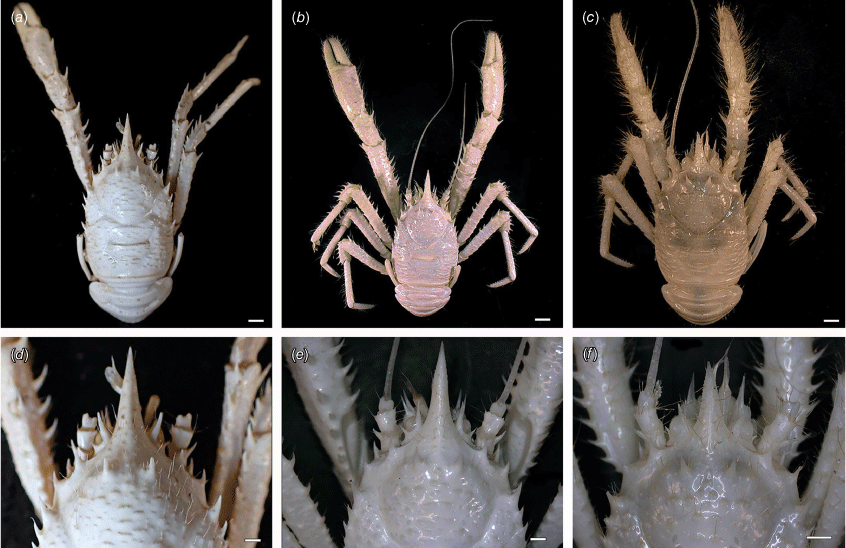
Munidopsis girguisi sp. nov.
(Fig. 9a–k, 10a, b, g, 11a, d, Supplementary Fig. S3.)
Material examined
Holotype. USA: California, leg. E/V Nautilus, ROV Hercules dive H1455, Stn NA066-152, 8.viii.2015, 33.66222°N, 118.56974°W, 535 m: 1 M 18 mm (MCZ IZ-73856).
Paratypes. Same collecting data as holotype: 1 M 4 mm, 1 F 10 mm (MCZ IZ-163059). — USA: California, leg. E/V Nautilus, ROV Hercules dive H1455, Stn NA066-145, 8.viii.2015, 33.66009°N, 118.57134°W, 558 m: 1 F broken 7 mm (MCZ IZ-73852). — USA: Point Dume, off Malibu, California, leg. Charlotte Seid, Emily McLaughlin, R/V Falkor, ROV SuBastian dive S0163, Slurp 1, 8.x.2018, 33.94187°N, 118.84381°W, 719 m: 14 M 11.6–16.7 mm, 4 ov. F 12.3–15.6 mm, 3 F 9.9–11.2 mm (ethanol-treated specimens, SIO-BIC C14008), 4 M 16.6–20.0 mm 2 ov. F 12.3–13.4 mm (formalin-fixed specimens, SIO-BIC C14008).
Non-type specimens. USA: California, leg. E/V Nautilus, ROV Hercules dive H1455, Stn NA066-151, 8.viii.2015, 33.66017°N, 118.56964°W, 554.6 m: 1 M 3 mm, 1 F 4.7 mm (MCZ IZ-73844). — USA: California: seep, leg. E/V Nautilus, ROV Hercules dive H1455, Stn NA066-150, 8.viii.2015, 33.6604°N, 118.5695°W, 554 m: 1 M 4.5 mm, 1 F 2.0 mm (broken) (MCZ IZ-74029). — USA: Point Dume, off Malibu, California, leg. Charlotte Seid, Emily McLaughlin, R/V Falkor, ROV SuBastian dive S0164, Slurp 4, 9.x.2018, 33.94186°N, 118.843986°W, 723 m, 1 ov. F 12.3 mm (SIO-BIC C14010). — USA: Point Dume, off Malibu, California, leg. Charlotte Seid, Emily McLaughlin, R/V Falkor, ROV SuBastian dive S0164, Slurp 3, 9.x.2018, 33.94187°N, 118.84376°W, 723 m, 2 M 14.5–15.6 mm (formalin-fixed specimens, SIO-BIC C14012), 5 M 12.3–15.6 mm, 1 ov. F 13.4 mm, 1 F 12.3 mm (ethanol-treated specimens, SIO-BIC C14012). — USA: Redondo Knoll, off California, leg. Charlotte Seid, Emily McLaughlin, R/V Falkor, ROV SuBastian dive S0167, 11.x.2018, 33.68120°N, 118.57140°W to 33.6898°N, 118.57497°W), 498–578 m: 1 F 6.7 mm (SIO-BIC C14038). —USA: Santa Monica Mound and Fossil Hill, off California, leg. Greg Rouse, R/V Western Flyer, ROV Doc Ricketts dive D1250, 7.ii.2020, 33.83851°N, 118.69038°W to 33.84447°N, 118.68830W, 623–863 m, 1 M 16.7 mm (SIO-BIC C14442), 1 M 12.3 mm 3 ov. F 11.2–14.5 mm (SIO-BIC C14443). — USA: Santa Monica Mound, off California, leg. Greg Rouse, R/V Western Flyer, ROV Doc Ricketts dive D1252, 8.ii.2020, 33.79938°N, 118.64631°W to 33.79947°N, 118.64698°W, 789–807 m, 1 ov. F 10.0 mm (SIO-BIC C14445). — USA: Lasuen Knoll, off California, leg. Greg Rouse, Nicolas Mongiardino Koch, R/V Falkor, ROV SuBastian dive S0449, SCB-236, slurp 2, 2.viii.2021, 33.38816°N, 118.00555°W, 382 m, 1 M 13.4 mm (SIO-BIC C14553). — USA: Lasuen Knoll, off California, leg. Greg Rouse, Nicolas Mongiardino Koch, R/V Falkor, ROV SuBastian dive S0449, SCB-235, 2.viii.2021, 33.38818°N, 118.00556°W, 382 m, 2 F 9.9–8.8 mm (SIO-BIC C14554). — USA: Rosebud whalefall, off San Diego, California, leg. Greg Rouse, R/V Western Flyer, ROV Doc Ricketts dive D1253, 9.ii.2020, 32.77687°N, 117.48807°W, 845 m: 1 M 13.4 mm (SIO-BIC C14437). — USA: California, leg. Robert C. Vrijenhoek, R/V Western Flyer, ROV Doc Ricketts dive D476, 21.v.2013, 33.843400°N, 118.68900°W, 664 m, 6 M 5.4–10.8 mm, 3 ov. F 11.5–12.3 mm, 4 F 7.0–11.4 mm (USNM 1463927). — USA: California, leg. Robert C. Vrijenhoek, R/V Western Flyer, ROV Doc Ricketts dive D631, 24.vi.2014, 33.90430°N, 118.73400°W, 535 m, 2 M 3.1–4.0 m, 2 F 3.7–12.5 mm (USNM 1487201), 2 M 3.1–3.3 mm, 2 F 3.3–5.7 mm (USNM 1487195).
COSTA RICA: Jaco Summit, leg. Greg Rouse, Allison Miller, R/V Falkor, ROV SuBastian dive S0213, 6.i.2019, 9.17341°N, 84.80380°W, 730–820 m: 2 specimens not measured (tissue SIO-BIC C13897 ex MZUCR 3760-01).
Etymology
Named for Prof. Peter Girguis, Chief Scientist of the R/V Falkor ‘Backyard Deep’ cruise FK181005, during which most of the paratypes were collected. The type locality off Los Angeles matches Prof. Girguis’ ‘Angeleno’ origins. The presence of filamentous bacteria resonates with Prof. Girguis’ research in deep-sea microbiology, and the tripoint carapace marking resembles the ABISS autonomous lander deployed by the Girguis lab on this cruise. We honor Prof. Girguis’ enthusiasm for collecting these animals and his kind, inclusive leadership as Chief Scientist.
Diagnosis
Carapace quadrangular, dorsally smooth, with pair of epigastric scales, with dorsal deep furrows and rugae, cervical grooves indistinct. Rostrum broadly triangular, lateral margins convergent, unarmed. Frontal margins slightly oblique. Orbit not distinctly excavated, outer orbital angle with a minute spine. Anterolateral angle armed with a small spine. Branchial margin serrated, unarmed. Pterygostomian flap with rugae. Abdominal somites unarmed. Telson divided into 7–8 plates. Sternite 3 anterolaterally rounded, anterior margin with median notch flanked by 2 lobes, sternite 4 subtriangular. Eyes unarmed, movable, epistomial spine present. Article 1 of antennule with dorsolateral process mesially concave. Article 1 of antenna with well-developed distolateral spine. Mxp3 merus subrhomboidal in lateral view. P1 moderately stout, with some spines, fixed finger without denticulate carina on distolateral margin. P2–4 stout, unarmed; meri carinated, propodi paddle-shaped; dactyli stout, curving, flexor margin with cuticular teeth along all margin decreasing proximally. Epipods absent from all pereopods.
Description
Carapace
Quadrangular, slightly longer than broad, widest at posterior part; slightly convex from side to side. Dorsal surface smooth, with two epigastric produced scales, hepatic and anterior branchial areas with scarce rugae; posterior cardiac and intestinal region with few rugae. Regions well delineated by deep furrows, anterior and posterior cervical grooves indistinct. Gastric region slightly elevated. Posterior cardiac region constricted by lateral furrows, forming notches; posterior margin unarmed, preceded by deep transverse depression. Rostrum broadly triangular, dorsally concave, width 0.15–0.25× anterior width of carapace, horizontal, lateral margins coarsely serrated, ventrally convex and carinated, 0.2–0.3× carapace length, 1–1.5× as long as broad. Frontal margin serrated, oblique behind ocular peduncle, blunt outer orbital angle above antennal peduncle, outer orbital spine and process (antennal spine) minute. Lateral margins carinated, with numerous rugae, preceded by a depression, nearly straight proximally, oblique distally; anterolateral spine minute. Pterygostomian flap surface with large and short rugae, anteriorly acute.
Sternum
Slightly longer than broad, maximum width at sternite 6. Sternite 3 moderately broad, 1.8–2.5× wider than long, anterolaterally rounded, anterior margin with median notch flanked by 2 lobes. Sternite 4 narrowly elongate anteriorly; surface depressed in midline, smooth; greatest width 2–3× that of sternite 3 and 2.0× wider than long.
Abdomen
Unarmed; tergite 2 with 2 elevated transverse ridges, smooth; tergites 3–5 lacking posterior ridge; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 7–8 plates, 1.2× as wide as long.
Eye
Eyestalk movable, partially concealed by rostrum; peduncle smooth, longer than cornea length; cornea subglobular; lateral surface contiguous to epistomial spine, ventral to frontal margin.
Antennule
Article 1 of peduncle with dorsolateral process mesially concave, distally serrated; distomesial margin produced and squamate.
Antenna
Peduncle not exceeding eye; article 1 with strong distolateral spine, distomesial angle unarmed, not reaching end of article 2, partially concealed by pterygostomian flap. Article 2 with well-developed distomesial and distolateral spines. Article 3 longer than article 2, with well-developed distomesial and distolateral spines, often double distolateral or distomesial spines. Article 4 unarmed. Flagellum longer than carapace.
Mxp3
Surface smooth. Flexor margin of merus with 2 spines, proximal larger, small distal spine; extensor margin with 1 distal spine. Ischium slightly longer than merus measured on extensor margin, unarmed. Crista dentate, finely denticulate. Dactylus, propodus and carpus unarmed.
P1
Moderately stout, with some granules and scales, females 1.7–1.9, males 1.8–2.2× longer than carapace. Merus 1.9–2.0× carpus length, with some spines at all surfaces, mesial stronger, including a few distal stout spines, dorsal margin proximally carinated. Carpus 1.5–1.8× longer than broad, with few spines at all surfaces, distal spines absent. Palm stout, slightly longer than carpus, 1.5–1.7× longer than broad, with row of small spines on mesial and lateral margins. Fingers unarmed, 0.7–0.9× longer than palm, opposable margins nearly straight, slightly gaping, spooned; fixed finger without denticulate carina on distolateral margin.
P2–4
Stout, coarsely granulated, devoid of setae, slightly decreasing in size posteriorly. P2 merus stout, 0.3–0.5× carapace length, nearly 3.0× longer than high and 1.1–1.2× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.8 length of P2 merus, P4 merus 0.9 length of P3 merus); extensor margin of P2–4 meri carinated, with small spines along entire border, distal part flattish ending in thick spine; flexor margin granulate ending in a thick spine; carpi with one small spine on extensor margin, granulated carina along lateral side; P2–4 propodi paddle-shaped, 2.6–2.7× as long as high, flattened in cross-section, extensor margin granulated; dactyli 0.6–0.7× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 11–13 min dactylar teeth decreasing in size proximally, each with slender corneous spine, ultimate tooth closer to dactylar angle than to penultimate tooth.
Epipods
Absent from pereopods.
Eggs
Approximately 10–45 eggs, 0.6–1 mm in diameter.
Colouration
Carapace and pereopods pink or varying shades of orange; carapace with a white tripoint marking on many of the California specimens.
Distribution
California and Costa Rica from 381- to 845-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
This new species appears to be covered by filamentous bacteria on the carapace, abdomen and chelipeds when alive but these bacteria were missing after fixation. Other squat lobster species living in hydrothermal vents and cold seeps exhibit this kind of epibiotic bacteria (e.g. Goffredi et al. 2008). Munidopsis girguisi sp. nov. resembles Munidopsis denudata Macpherson, 2007 from the Solomon Islands and M. inermis Faxon, 1893 from Panama. However, the new species is easily distinguished from these species by the following characters:
The new species has deep furrows on the dorsal surface of the carapace and rugae on the pterygostomian flap, whereas these surfaces are smooth in M. denudata and in M. inermis.
The frontal margin of the carapace is unarmed in M. denudata and M. inermis whereas this is armed with a minute antennal spine in the new species.
The anterolateral angle is unarmed in M. denudata and M. inermis whereas this angle is armed with a small spine in the new species.
The new species has an acute triangular rostrum whereas M. denudata has a broadly triangular rostrum.
The flexor margin of the Mxp3 is armed with one strong proximal spine and one small distal spine in M. denudata whereas this margin is armed with two proximal spines in the new species.
P1–4 have spines in the new species, whereas these are unarmed in M. denudata and M. inermis.
The dactyli flexor margin is unamred in Munidopsis inermis whereas this margin is armed with minute spines in the new species.
Munidopsis granosicorium Williams & Baba, 1989
Material examined
Non-type specimens. COSTA RICA: Parrita Seep, leg. Lisa Levin, Kris Krasnosky, R/V Atlantis, HOV Alvin dive 4924, 7.vi.2017, 9.03168°N, 84.62100°W, 1402 m: 1 ov. F 6.1 mm (SIO-BIC C12819).
Description
Carapace
As long as broad, widest at midlength; heavily sculptured, ridged longitudinally; convex from side to side. Dorsal surface densely covered by denticles and tubercles, each denticle or tubercle with a few short setae, epigastric denticles strongly serrated, hepatic and anterior branchial areas with denticles and some acute granules. Regions well delineated by deep furrows including distinct anterior and posterior cervical grooves. Gastric region elevated, with a longitudinal ridge interrupted by cervical groove. Cardiac region elevated, including prominent longitudinal ridge. Intestinal region with longitudinal ridge, preceded by a depression. Posterior margin unarmed, preceded by transverse depression. Rostrum narrowly triangular, width 0.23× anterior width of carapace, directed slightly upwards, few spines on margin, dorsally carinate, 0.33× carapace length, 1.4× as long as broad. Basis of rostrum armed with pair of minute spines in line with base of eye, peri-epigastric. Frontal margin slightly concave behind ocular peduncle, blunt outer orbital angle above antennal peduncle, outer orbital spine and process (antennal spine) absent. Lateral margins nearly straight; anterolateral angle unarmed; branchial margins unarmed. Pterygostomian flap surface covered by denticles and granules, anterior margin blunt, unarmed.
Sternum
1.2× longer than broad, maximum width at sternite 7. Sternite 3 broad, 2× wider than long, anterolaterally rounded, anterior margin with median lobe flanked by 2 lobes. Sternite 4 narrowly elongated anteriorly, anterior margin serrated; surface depressed in midline, smooth; greatest width 3× that of sternite 3 and 2.0× wider than long.
Abdomen
Tergites with granules, tergites 2–4 armed with a median broad spine, tergites 2–3 with 2 elevated transverse ridges, lateral part of dorsal surfaces covered by granules and scales; tergites 4–6 lacking posterior ridge; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 8 plates; 1.2× as wide as long.
Eye
Eyestalk immovable; peduncle small, shorter than cornea length; cornea globular, prominent, exposed; epistomial spine absent.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines, dorsolateral double, distolateral proximally armed with denticles; distomesial margin granulate.
Antenna
Peduncle slightly exceeding eye, armed with denticles and granules; article 1 with distomesial spine, distolateral with granules and denticles. Article 2 with well-developed distomesial and distolateral spine, lateral margin with produced denticles. Article 3 with a small lateral spine, distolateral and distomesial angles unarmed. Article 4 unarmed.
Mxp3
Surface with granules and denticles. Ischium as long as merus measured on extensor margin, distal margin serrated; flexor margin of merus with 4 spines decreasing in size distally, distal margin armed with one spine; extensor margin with distal spine.
P1
Moderately slender, with numerous granules and denticles, each scale with few short setae, 1.6× longer than carapace. Merus 1.75× carpus length, with few distal stout spines. Carpus 1.8× longer than broad, with rows of spines on mesial, lateral margins, mesial stronger, distal spines stout. Palm with row of spines on all surfaces, slightly longer than carpus, 1.6× longer than broad. Fingers armed with a small spine on midlength margins, as long as palm, opposing margins nearly straight, not gaping, spooned; fixed finger without denticulate carina on distolateral margin.
P2–4
Moderately slender, coarsely tuberculated or denticulated on all surfaces, with fine distally curved setae in meri, short setae in propodi, cylindrical in cross-section, slightly decreasing in size posteriorly. P2 merus moderately slender, 0.5× carapace length, nearly 2.2× longer than high and 0.9× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.9× length of P2 merus, P4 merus 0.95× length of P3 merus); extensor margin with granules and denticles along entire border, distal part cylindrical ending in 1–2 thick spines; flexor margin granulated, ending in thick spine; P2–4 carpi with denticles and spines on extensor margin, acute tubercles on lateral sides; P2–4 propodi with acute tubercles on extensor margin and lateral sides, distal flattened, ending in 3 flattened denticles, 7.2–7.6× as long as high, trianguloid in cross-section, unarmed; dactyli 0.5–0.6× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 7–8 min teeth only at distal-half margin, ultimate tooth closer to dactylar angle than to penultimate tooth.
Epipods
Present on P1–3.
Eggs
2 eggs of 1.9–2 mm in diameter.
Colouration
Carapace and pereopods whitish, with brownish setae. Eyes and Mpx3 light orange. Eggs light orange.
Distribution
Vancouver Island, Washington at 2020-m depth. Newly registered from Costa Rica at 1402-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
The specimen described from Costa Rica fits well with the description of M. granosicorium from Vancouver Island (Williams and Baba 1989). However, there is some morphological variation in the relative width of the rostrum, with this being lender in the Vancouver specimen and broader in the Costa Rica specimen. There are also fewer but large eggs in the specimens from Costa Rica compared to those from Vancouver, suggesting lecithotrophic larval cycle that in turn implies poor long distance dispersal ability (Baco et al. 2016). Given the geographical distance between the two known populations, these likely correspond to different species but an analysis of more material would be necessary to confirm this. Munidopsis granosicorium belongs to the group of species with the dorsal surface of the carapace covered by tubercles, granules or denticles, epigastric processes, the lateral margins of the carapace unarmed, a triangular rostrum, the frontal margin concave behind the ocular peduncle, eyes with the ocular peduncle short and fixed, without an eye-spine and the telson composed of 7–8 plates. This species closely resembles M. follirostris Khodkina, 1973 from Chile, M. sonne Baba, 1995 from Fiji, M. tuberosa Osawa, Lin & Chan, 2008 from southwestern Taiwan and the South China Sea, and M. dispar from the Mariana Trench. However, M. granosicorium is easily distinguished from these species by the following characters:
M. granosicorium has the carapace heavily sculptured, with a longitudinal ridge on the gastric and cardiac regions, whereas the carapace lacks these longitudinal ridges in the other species.
M. granosicorium has the rostrum broadly triangular whereas M. follirostris, M. sonne and M. tuberosa have the rostrum constricted between the eyes.
The rostrum is dorsally carinated in M. granosicorium but the dorsal carina is absent in M. tuberosa.
Abdominal somites 2–5 are armed with a median broad spine in M. granosicorium whereas these spines are absent in M. dispar, M. sonne and M. tuberosa.
The lateral margin of the antennular peduncle is unarmed in M. granosicorium, whereas this is armed with small processes in M. tuberosa.
M. granosicorium, M. follirostris and M. sonne have epipods in P1–3 whereas M. dispar lacks epipods on pereopods.
The closest species to M. granosicorium sequenced in this study is M. dispar. The genetic divergence for M. dispar and M. granosicorium ranged from 14.7 to 16.5% for COI. Unfortunately, we do not have genetic data for M. follirostris, M. sonne or M. tuberosa.
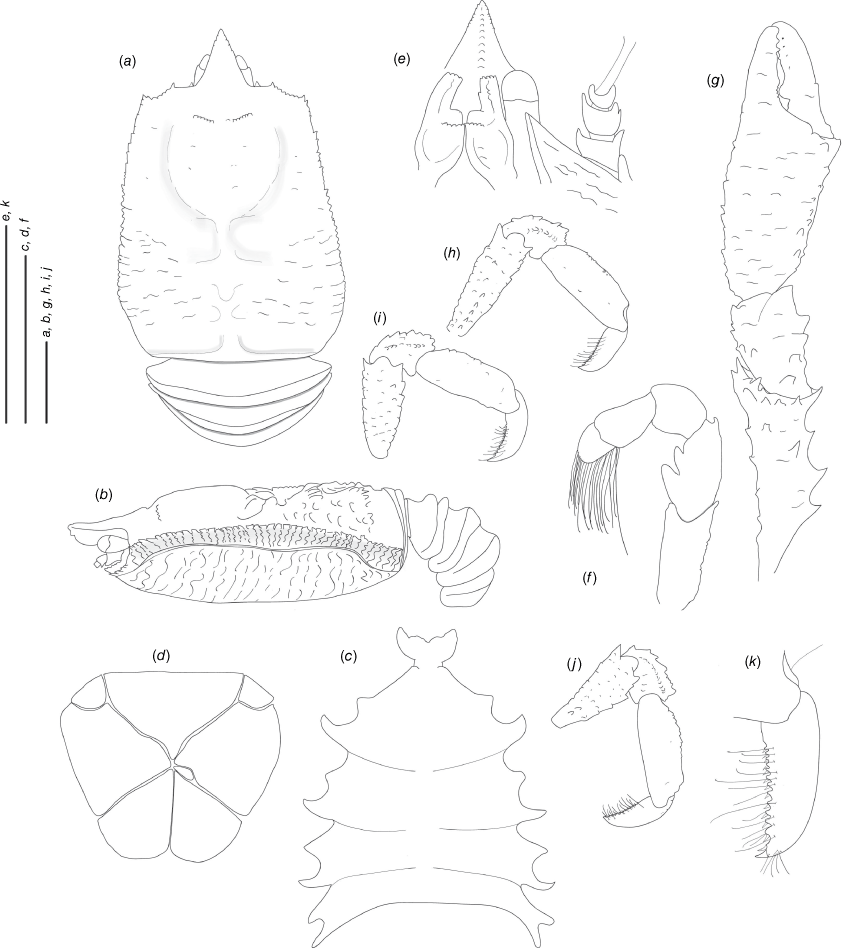
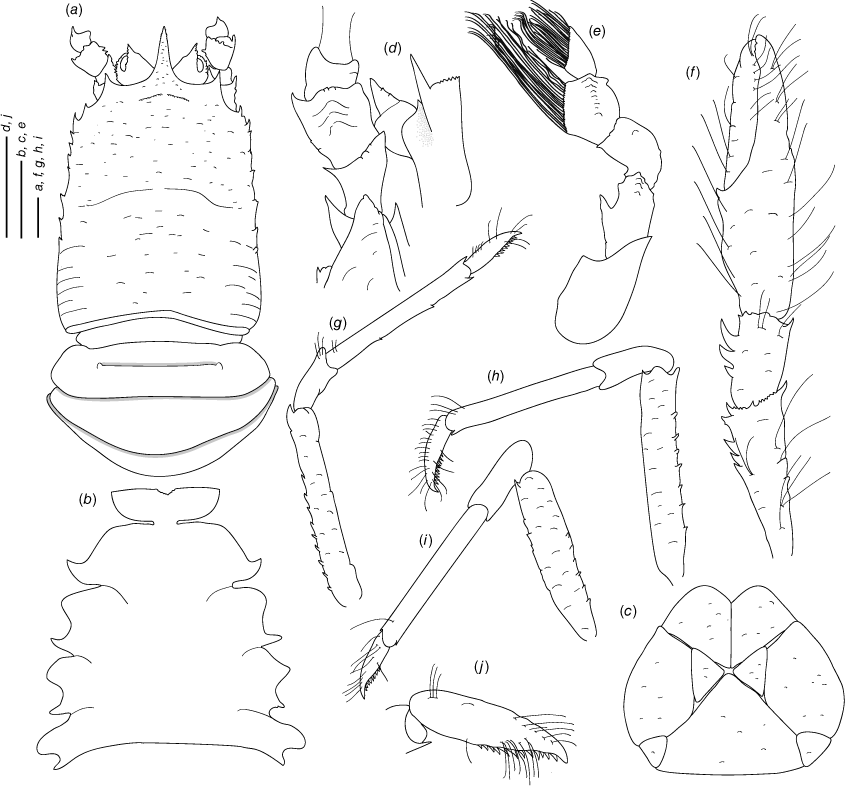
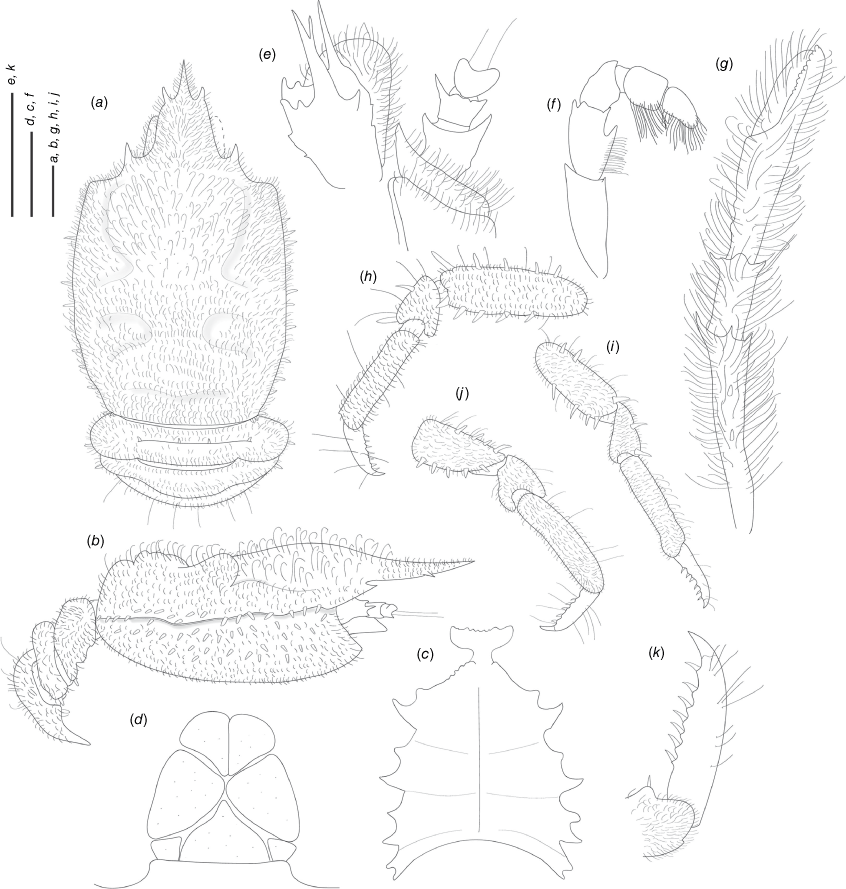
Munidopsis hendrickxi sp. nov.
(Fig. 10c, d, 11b, e, 13a–k.)
Material examined
Holotype. USA: California, E/V Nautilus, ROV Hercules dive H1795, Stn NA117-012-01-B-MCZ: 14.x.2019, 35.51857°N, 122.63877°W, 3188 m: 1 M 9.1 mm (MCZ IZ-153106).
Etymology
Named after Michel Hendrickx, crustacean researcher at the Universidad Autonoma of Mexico, in recognition of dedication to the study of squat lobsters and other crustaceans from the Americas.
Diagnosis
Carapace dorsally covered by denticulate tubercles and scales, with dorsal deep furrows and rugae, cervical grooves distinct. Rostrum narrowly triangular, lateral margins subparallel, unarmed. Frontal margins slightly concave. Orbit slightly excavated, outer orbital angle with a blunt lobe. Anterolateral angle armed with a spine. Branchial margin unarmed. Abdominal somites unarmed. Telson divided into 7 plates. Sternite 3 anterolaterally produced, anterior margin serrated with a median acute lobe, sternite 4 narrowly subtriangular. Eyes unarmed, immovable, epistomial spine present. Article 1 of antennule with well-developed dorsolateral and distolateral spines, distolateral double. Article 1 of antenna with distomesial blunt process, distolateral with denticules. Mxp3 merus subrhomboidal in lateral view. P1 moderately slender, with some spines, fixed finger without denticulate carina on distolateral margin. P2–4 moderately slender, unarmed; meri carinated, dactyli slender, curving, flexor margin with minute teeth along all margins decreasing proximally. Epipods present on P1–2.
Description
Carapace
Slightly longer than broad, widest at midlength; moderately convex from side to side. Dorsal surface covered by denticulate tubercles and scales, each tubercle and scale with a few short setae and some long, thick setae; hepatic and anterior branchial areas with scales and some acute tubercles; posterior cardiac and intestinal region covered by larger scales. Regions well delineated by furrows including distinct anterior and posterior cervical grooves. Gastric region slightly elevated. Posterior margin preceded by elevated ridge. Rostrum narrowly triangular, directed downwards slightly, distally covered with tubercles on lateral margins; dorsal surface longitudinally carinate, with granules along midline, 0.3× as long as remaining carapace length, 1.7× longer than broad, 1.4× as long as broad. Frontal margin slightly concave behind ocular peduncle, outer orbital angle produced into blunt lobe above antennal peduncle, outer orbital spine and process (antennal spine) absent. Lateral margins straight; anterolateral spine well developed; anterior branchial margin with 2–3 small spines. Pterygostomian flap surface covered by granules and scales, anterior margin blunt, unarmed.
Sternum
As long as broad, maximum width at sternites 6 and 7. Sternite 3 broad, 2.6× wider than long, anterolaterally produced, anterior margin serrated with a median acute lobe. Sternite 4 narrowly elongate anteriorly; surface depressed in midline, smooth; greatest width 2.5× that of sternite 3 and 1.6× times wider than long.
Abdomen
Unarmed; tergites 2–3 with 2 elevated transverse ridges, lateral parts of dorsal surfaces smooth; tergites 4–6 lacking posterior ridge; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 7 plates; 1.3× as wide as long.
Eye
Eyestalk immovable; peduncle short and fixed, shorter than cornea length, wider than cornea width; cornea globular; lateral surface contiguous to small epistomial spine, ventral to frontal margin.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines, distodorsal spine double; distomesial margin with spine distinct, slightly dentate.
Antenna
Peduncle exceeding eye; article 1 with distomesial blunt process, distolateral process with denticles. Article 2 with well-developed distolateral spine, distomesial surface with denticles. Article 3 with distomesial spine well-developed, distolateral angle with denticles. Article 4 unarmed. Flagellum longer than carapace.
Mxp3
Merus with 3 large spines along flexor margin, proximal larger; 2 small distal spines on extensor margin. Ischium as long as merus measured on extensor margin, with distal flexor and extensor spines. Crista dentata finely denticulate. Dactylus, propodus and carpus unarmed.
P1
Moderately slender, covered by denticles and scales, and fine, long setae, 1.9× longer than carapace. Merus 1.8× carpus length, with rows of spines on mesial, lateral margins and some distal stout spines. Carpus 1.5× longer than broad, with rows of spines on mesial, lateral margins and some distal stout spines. Palm unarmed, stout, slightly longer than carpus, 1.2× longer than broad. Fingers unarmed, 0.9× longer than palm, opposing margins straight, not gaping, spooned, fixed finger without denticulate carina on distolateral margin.
P2–4
Moderately slender, covered by denticles, devoid of setae, cylindrical in cross-section, slightly decreasing in size posteriorly. P2 merus moderately slender, 0.5× carapace length, nearly 3.5× longer than high and 1.2× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.9× length of P2 merus, P4 merus 0.8× length of P3 merus); extensor margin of P2–4 meri carinate, with denticles along entire border, distal part flattish ending in thick spine; flexor margin denticulate, distal spine strong; carpi with one thick distal spine on extensor margin, granulated carina along lateral side; P2–4 propodi 5.0–6.0 times as long as high, trianguloid in cross-section, armed with numerous denticles on flexor and extensor margins, and dorsal and ventral surface; dactyli 0.5–0.6× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 6–9 minute teeth only at distal-half margin, decreasing in size proximally, ultimate tooth at midlength between penultimate tooth and dactylar angle.
Epipods
Present on P1–2.
Colouration
Body light orange, whitish eyes.
Distribution
California, at ~3188-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis hendrickxi sp. nov. belongs to the group of species with the dorsal carapace surface covered in tubercles, granules or denticles, lateral margins of the carapace unarmed, rostrum triangular, frontal margin concave behind the ocular peduncle, eyes with the ocular peduncle short and fixed, lacking an eye-spine and the telson composed of 7–8 plates. The new species morphologically resembles Munidopsis tuberosa Osawa, Lin & Chan, 2008 from southwestern Taiwan and the South China Sea, and M. dispar from the Mariana Trench. However, the new species can be easily distinguished from the other by the following characters:
The new species has a small spine on the anterolateral margin of the carapace, whereas this margin is unarmed in the other species.
The new species has different tuberculation in the gastric area (denticles) from that in the cardiac area (long scales) whereas the carapace ornamentation is homogeneous in the other species.
The new species has epipods on P1–2, whereas epipods are absent from the pereopods in M. dispar and present on P1–3 in M. tuberosa.
The new species and M. dispar diverge on 11.6–12.8% for COI. Unfortunately, there are no molecular data available for M. tuberosa.
Munidopsis hystrix Faxon, 1893
Material examined
Lectotype. MEXICO: Nayarit, off Tres Marias Islands, leg. USFC Steamer Albatross, Stn 3425, 18.iv.1891, 21.31666°N, 106.40000°W, 680 fms (1244 m): 1 M 17.1 mm (MCZ IZ-CRU-4549).
Paralectotypes. Same collecting data as lectotype: 7 M 8.8–18.7 mm, 1 ov. F 16.4 mm, 2 F 8.8–12.2 mm (MCZ IZ-163060).
Non-type specimens. MEXICO: western slope of Concepcion Canyon, Guaymas Basin, leg. Greg Rouse, Sigrid Katz, R/V Western Flyer, ROV Doc Ricketts dive D383 A2, 12.iv.2012, 26.88295°N, 111.658622°W, 823 m: 1 M 8.9 mm (SIO-BIC C14357). — MEXICO: leg. E/V Nautilus, ROV Hercules dive H1665, Stn NA092-058, 13.xi.2017, 19.24652°N 110.87116°W, 819 m: 1 M 15.3 mm (MCZ IZ-146311).
USA: California, leg. E/V Nautilus, ROV Hercules dive H1455, Stn NA066-148, 8.viii.2015, 33.66012°N, 118.56919°W, 556 m: 1 F 8.5 mm (MCZ IZ-73853). — USA: California, leg. E/V Nautilus, ROV Hercules dive H1460, Stn NA067-011, 15.viii.2015, 33.45408°N, 118.67693°W, 702 m: 1 juvenile 2 mm (MCZ IZ-73638). — USA: California, leg. E/V Nautilus, ROV Hercules dive H1542, Stn NA075-002, 25.vii.2016, 33.74604°N, 118.61636°W, 795 m: damaged specimen (MCZ IZ-139982). — USA: Santa Monica Mound, off California, leg. Emily McLaughlin, Jessica Pruitt, R/V Falkor, ROV SuBastian dive S0172, Slurp 4, 15.x.2018, 33.83468°N, 118.67614°W, 780 m: 1 F 11.20 mm (SIO-BIC C14031). — USA: Emery Knoll, off California, leg. Emily McLaughlin, Jessica Pruitt, R/V Falkor, ROV SuBastian dive S0175, Slurp 4, 17.x.2018, 33.03611°N, 118.39808°W, 665 m: 1 F 3.5 mm (SIO-BIC C14037). — USA: off San Diego, California, leg. Greg Rouse and students, R/V Robert Gordon Sproul SP1913, otter trawl, 14.vii.2019, 32.8093°N, 117.4673°W to 32.8739°N, 117.5154°W, 700 m: 1 ov. F 19.9 mm (SIO-BIC C14102), 1 M 20 mm (SIO-BIC C14111). — USA: Forty Mile Bank, off San Diego, California, leg. Greg Rouse, Kaila Pearson, E/V Nautilus, ROV Hercules dive H1845, cruise NA124, 3.xi.2020, 32.60044°N, 118.02825°W to 32.59787°N, 118.01624°W, 1111–650 m: 1 F 11.1 mm (SIO-BIC C14470).
Diagnosis
Carapace spinose, dorsally covered by small spinules and setose scales, dorsal deep furrows, cervical grooves indistinct. Gastric region covered with spines, 1–2 pairs of well-developed epigastric spines, median cardiac spine well developed, preceded by a deep depression. Rostrum narrowly triangular, dorsally carinated, margin armed with 2–3 well-developed spines. Frontal margins concave. Orbit distinctly excavated, outer orbital angle delimited by antennal spine. Anterolateral angle armed with a strong spine. Anterior branchial margin armed with 3–4 well-developed spines; posterior branchial area dorsally armed with 3–4 pairs of well-developed spines, margin armed with small spines. Posterior carapace margin with 1–2 pairs of spines, preceded by a depression. Abdominal somites 2–4 with 2 transverse ridges, somite 2 armed with a pair of spines. Telson divided into 7 plates. Sternite 3 narrow, anterolateral angles produced, sternite 4 subtriangular. Eyes armed with dorsal spine, movable, epistomial spine absent. Article 1 of peduncle with well-developed dorsolateral and distolateral spines. Article 1 of antenna with distolateral spine. Mxp3 merus 0.5–0.6× longer than ischium, subrhomboidal in lateral view. P1 slender, elongate; meri and carpi with well-developed spines on all surfaces, fixed finger without denticulate carina on distolateral margin. P2–4 slender, spinose; dactyli slender, curving, flexor margin with teeth only at distal-half margin. Epipods absent from all pereopods.
Distribution
Galapagos Islands, Perú, Gulf of California, Baja California and California, from 436- to 1244-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis hystrix belongs to the group of species having a frontal margin with a delimited orbit, movable eye with small median spine projecting from upper surface, the abdomen armed with spines and the telson composed of 7–8 plates. This group of species includes M. hystrix, M. opalescens and M. margarita Faxon, 1893 from the East Pacific. These three species are morphologically and genetically similar. Differences relating to this complex are described in Remarks of M. margarita.
Munidopsis margarita Faxon, 1893
Material examined
Lectotype. ECUADOR: Galápagos, Off Galapagos Islands, leg. USFC Steamer Albatross, Stn 3404, 28.iii.1891, 1.05000°S, 89.46666°W, 385 fms (704 m): M 7.5 mm (MCZ IZ CRU-4551).
Paralectotype. Same collecting data as lectotype: 1 F 6.5 mm (MCZ IZ-163061).
Non-type material. COSTA RICA: Seamount 6, leg. Greg Rouse, Avery Hiley, R/V Falkor, ROV SuBastian dive S0227, B3-7, 22.i.2019, 7.67944°N, 85.91141°W, 578 m: 1 F broken 8.9 mm (SIO-BIC C13957).
Description
Carapace
Spinose, slightly broader than long, widest at midlength; moderately flattened from side to side. Dorsal surface densely covered in spines and a few scales, each scale with a few setae; pair of epigastric spines strong, median processes with long setae, hepatic and anterior branchial areas with spinules; posterior cardiac and intestinal region covered with spinules and scales. Regions well delineated by deep furrows including distinct anterior and posterior cervical grooves. Gastric region elevated. Posterior margin armed with 3 pairs of spines, 2 median pairs (median pair the strongest) and 1 lateral pair, close to the carapace margin, posterior margin preceded by a depression. Rostrum triangular, width 0.2× anterior width of carapace, directed slightly upwards, dorsally carinate, margin distally armed with 5 small spines; 0.4× carapace length, 1.8× as long as broad. Frontal concave behind ocular peduncle, blunt outer orbital angle above antennal peduncle, ending in a small outer orbital spine (antennal spine). Lateral margins straight; anterolateral spine strong; anterior branchial margin with 6 spines, each pair constituting a strong spine followed by a small spine; 5–6 branchial spines behind posterior branch of cervical groove. Pterygostomian flap surface covered in granules, anteriorly acute, armed with a strong spine.
Sternum
0.9× as long as broad, maximum width at sternite 7. Sternite 3 broad, 3.2× wider than long, anterolaterally rounded, anterior margin with median notch flanked by 2 lobes. Sternite 4 narrowly elongated anteriorly; surface depressed in midline, smooth; greatest width 2.3× that of sternite 3 and 1.7× wider than long.
Abdomen
Tergites 2–3 armed with posterolateral broad spines, tergite 2 with 3 spines and 2 elevated transverse ridges, tergite 3 armed with 1 median spine, tergites 3–6 lacking a posterior ridge; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 7 plates; 1.2× as wide as long.
Eye
Eyestalk movable; peduncle forming a median spine, projecting from upper surface and exceeding cornea, peduncle shorter than cornea length; cornea subglobular; epistomial spine absent.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines; distomesial margin slightly produced and granulated.
Antenna
Peduncle slightly exceeding eye. Article 1 with strong distolateral spine, not reaching end of article 2. Article 2 with well-developed distolateral spine, distomesial margin granulated. Article 3 with well-developed distomesial and distolateral spine. Article 4 unarmed.
Mxp3
Surface with granules. Ischium as long as merus measured on extensor margin; flexor margin of merus with 3 strong spines subequal in size; extensor margin with 2 small spines.
P1
Slender, spinose, with numerous spines. Merus 2.7× carpus length, with rows of strong spines at lateral and mesial margins. Carpus 0.9× longer than broad, with rows of strong spines on dorsal, mesial and lateral surfaces. Palm armed with row of strong spines on mesial margin, lateral margin irregular, with few spines, slender, 2.3× longer than carpus, 1.2× longer than broad. Movable finger armed with a basal spine, 0.9× longer than palm, opposable margins nearly straight, not gaping, spooned; fixed finger without denticulate carina on distolateral margin.
P2–4
Slender, spinose, cylindrical in cross-section. P2 merus slender, 0.6× carapace length, 4× longer than high and 1.1× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.9× length of P2 merus, P4 merus 0.8× length of P3 merus); extensor margin of P2 and 4 meri with strong spines on all surfaces, distal part flattish, terminating in a thick spine; flexor margin with spines; carpi with spines on extensor margin, distal spine strong, carina along lateral side; P2 and 4 propodi 5.1–5.2× as long as high, trianguloid in cross-section, armed with small spines on lateral and extensor surfaces; dactyli 0.5× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 11–14 teeth at midlength, ultimate tooth at midlength between penultimate tooth and dactylar angle.
Epipods
Absent from pereopods.
Colouration
Carapace and eyes deep orange, P1–4 colourless.
Distribution
Previously known from Galapagos at 704-m depth. Newly registered from Costa Rica, at 578-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis margarita belongs to the group of species having a frontal margin with a delimited orbit, movable eye with small median spine projecting from upper surface, the abdomen armed with spines and the telson composed of 7–8 plates. This group includes M. hystrix, M. opalescens and M. margarita, and these are morphologically and genetically close. These species can be distinguished based on the following morphological characters:
The spines on the carapace and rostrum margin are more prominent in M. opalescens and M. margarita than in M. hystrix.
The rostrum is spiniform in M. hystrix but triangular in M. opalescens and M. margarita.
Munidopsis margarita has abdominal tergites 2–3 armed with posterolateral spines, whereas these spines are absent in the other species.
Munidopsis margarita has abdominal tergite 2 armed with 3 or more spines whereas this tergite is armed with a single median spine in the other species.
Genetic divergences among these species ranged from 6.8 to 10% for COI.
Munidopsis nautilus sp. nov.
(Fig. 16a–j, Supplementary Fig. S4.)
Material examined
Holotype. ECUADOR: off Galapagos Islands, leg. E/V Nautilus, ROV Hercules, ROV Hercules dive H1433, Stn NA063-016, ROV Hercules dive H1433, 20.vi.2015, 0.73720°N, 85.89091°W, 2600 m: 1 ov. F 11.0 mm (MCZ IZ-70119).
Etymology
Named after the Exploration Vessel Nautilus from which the exploration of the Galapagos Rift region was conducted, yielding these specimens. The name is a substantive in apposition.
Diagnosis
Carapace dorsally smooth, pair of epigastric scales, cervical grooves weakly distinct. Rostrum narrowly triangular, lateral margins subparallel, unarmed. Frontal margins concave. Orbit excavated, outer orbital angle with a spine. Anterolateral angle armed with a small spine. Branchial margin armed with spines. Abdominal somites unarmed. Telson divided into 9 plates. Sternite 3 anterolaterally blunt, anterior margin nearly straight with a median notch, sternite 4 widely triangular. Eyes unarmed, immovable, epistomial spine present. Article 1 of antennule with well-developed dorsolateral and distolateral spines. Article 1 of antenna with distomesial and distolateral spines. Mxp3 merus rectangular in lateral view, with dorsal carina. P1 moderately slender, with some spines, ventral pad in palm, fixed finger without denticulate carina on distolateral margin. P2–4 slender, unarmed; meri and propodi cylindrical, dactyli stout, curving, flexor margin with teeth along all margins decreasing proximally. Epipods absent from all pereopods.
Description
Carapace
1.2× longer than broad, widest posteriorly; weakly convex from side to side. Dorsal surface smooth, covered in small scales, each with a few short setae and some thick long setae; pair of epigastric produced and elevated scales. Regions not delineated, anterior and posterior cervical grooves weakly distinct. Mid-dorsal ridge medially interrupted. Posterior margin unarmed, angular, emarginated medially. Rostrum narrowly triangular, dorsally flattened, slightly directed downwards, 0.2× as long as remaining carapace length, 1.7× longer than broad, lateral margins serrated anteriorly. Frontal margin concave behind ocular peduncle, outer orbital angle produced into a spine above antennal peduncle, outer orbital spine strong, much larger (twice as large) than anterolateral spine. Lateral margins straight; anterolateral spine small; branchial margins armed with spines. Pterygostomian flap surface irregular, anterior margin acute.
Sternum
Slightly longer than broad, maximum width at sternite 7. Sternite 3 broad, 3.2× wider than long, anterolateral angle blunt, anterior margin nearly straight with a median notch. Sternite 4 narrowly elongated anteriorly; surface flattened, smooth; greatest width 2.9× that of sternite 3 and 1.8× wider than long.
Abdomen
Unarmed; tergite 2 with anterior transverse ridge, lateral partsof dorsal surfaces smooth; tergites 3–6 lacking ridges; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 9 plates; 1.2× as wide as long.
Eye
Eye immovable; flattened peduncle recovering all cornea surfaces, laterally and ventrally spinose, projected on dorsal surface in an acute triangular medial spine, exceeding midlength of rostrum, with serrated lateral margins; epistomial spine absent.
Antennule
Article 1 of peduncle with well-developed dorsolateral and small distolateral spines, distodorsal spine lateral margin strongly concave; distomesial margin dentate.
Antenna
Peduncle clearly exceeding eye. Article 1 with distomesial and distolateral spines. Article 2 with well-developed distolateral and distomesial spines, distal surface with dorsal carina. Article 3 with distomesial angle unarmed, distolateral spine well-developed, distal surface with denticles, dorsal surface carinated. Article 4 unarmed. Flagellum thick, longer than carapace.
Mxp3
Ischium as long as merus measured on extensor margin, with distal flexor and extensor spines. Crista dentata finely denticulate. Merus with 2 spines along flexor margin, proximal strong, distal small; extensor margin unarmed, dorsal surface carinated. Dactylus and carpus unarmed; propodus dorsal surface armed with a longitudinal carina.
P1
Slender, with few scales and long setae, 2.2× longer than carapace. Merus 1.9× carpus length, with row of well-developed spines on mesial margin and some distal stout spines, extensor margin serrated with few spines including strong distal spine. Carpus 1.5× longer than broad, with row of well-developed spines on mesial margin and some distal stout spines. Palm armed with distal spine mesial margin, slightly longer than carpus, pad of setae on ventral surface, 1.7× longer than broad. Fingers unarmed, 1.2× longer than palm, opposing margins straight, not gaping, spooned, fixed finger without denticulate carina on distolateral margin. Cheliped pad oval on ventral side of fixed finger.
P2–4
Slender, covered in setose scales, cylindrical in cross-section, slightly decreasing in size posteriorly. P2 merus slender, 2.0× carapace length, 6.5× longer than high and 1.2× length of P2 propodus. P3 meri longer than P2 and P4 (P3 merus 1.2× length of P2 merus, P4 merus 0.8× length of P3 merus); extensor margin of P2–4 meri with spines along entire border, distal ending in thick spine in P2–3; flexor margin serrated, distal spine strong; carpi unarmed, lateral side smooth; P2–4 propodi 7.5–9.0× as long as high, cylindrical in cross-section, extensor margin unarmed, flexor margin armed with 0–3 spines, distal spine strong; dactyli highly setose, 0.38–0.4× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 11–12 teeth at distal half margin, ultimate tooth at midlength between penultimate tooth and dactylar angle.
Epipods
Absent from all pereopods.
Colouration
Unknown.
Distribution
Off Galapagos, at 2600-m depth.
Genetic data
COI and 28S rRNA.
Remarks
The new species is morphologically similar to M. lentigo from the East Pacific Rise (Williams and Van Dover 1983). We have found the following morphological differences between both species:
The new species presents produced epigastric scales whereas these epigastric scales are absent or weakly marked in M. lentigo.
The spines on the branchial margin of the carapace are smaller than the outer orbital spine in the new species than in M. lentigo.
The rostrum is narrowly triangular in the new species, whereas this is more strap-like in M. lentigo.
Unfortunately, we only have one specimen of the new species, therefore we cannot discuss intraspecific variation. However, Munidopsis lentigo and the new species are highly divergent genetically, with a genetic distance between 12 and 13% for COI.
Munidopsis piipa Marin, 2020
Material examined
Non-type specimens. USA: California, Pioneer Seamount, 7.ii.1950, 37.35000°N, 123.41666°W, 1097–822.96 m: 1 F 20.6 mm (CAS-IZ 190354). — USA: California, Pioneer Seamount, 7.ii.1950, 37.349957°N, 123.430026°W, 805.–988 m: 1 M 10.5 mm (CAS-IZ 190356). — USA: Forty Mile Bank, off San Diego, California, leg. Greg Rouse and Nicolas Mongiardino Koch, R/V Falkor, ROV SuBastian dive S0446, SCB-172, 31.vii.2021, 32.6029°N, 118.02571°W, 1035 m: 1 F 13.4 mm (SIO-BIC C14546). — USA: California, leg. Robert C. Vrijenhoek, R/V Western Flyer, ROV Tiburon dive T665, 2.v.2004, 33.10070°N, 120.96000°W, 870 m, 1 specimen not sexed, not measured (USNM 1463979).
Distribution
Bering Sea (Piip submarine volcano) at 984-m depth, California, from 805- to 1097-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Newly recorded for the East Pacific. Munidopsis piipa belongs to a group of species with a rostral spine short and spiniform, frontal margin of the carapace straight and telson with 10–12 plates. This group includes Munidopsis ariadne Macpherson, 2011 from the eastern Mediterranean, Munidopsis goodridgii Alcock & Anderson, 1899, Munidopsis karukera Macpherson, Beuck & Freiwald, 2016 from the Caribbean Sea, Munidopsis maunga Schnabel & Bruce, 2006, Munidopsis milleri Henderson, 1885 from the Philippines, Munidopsis polymorpha from shallow caves in the Canary Islands, Munidopsis spinipes MacGilchrist, 1905 from the Bay of Bengal and Munidopsis kexueae Dong, Gan & Li, 2021 from seamounts near the Yap Trench, West Pacific.
Munidopsis piipa closely resembles M. kexueae both morphologically and genetically. The descriptions of these species almost overlapped during the time of revisions and publication, therefore the species were not compared before our work. Phylogenetic and some species delimitation analyses do not support these species hypotheses, considering M. piipa and M. kexueae as a single taxon. However, haplotype networks demonstrate large genetic distances between the two species, with up to 4% of divergence for COI or 19 mutational steps (Fig. 4). The percentage of divergence between these species is low compared to other squat lobsters in the family, (e.g. Rodríguez-Flores et al. 2019b, Leiogalathea); although some species of the abyssal clade have been delimited based on similar genetic distance values (Jones and Macpherson 2007; Dong et al. 2019). According to the original species descriptions (Marin 2020; Dong et al. 2021), spinulation on the carapace margins and relative width of the rostrum are different in both taxa. However, these morphological and genetic differences could be part of intraspecific variation and population genetic structure, as very few specimens have been analysed to date. More data, including the analyses of more specimens, would be desirable to solve this taxonomic problem.
Munidopsis quadrata Faxon, 1983
(Fig. 8j–n, Supplementary Fig. S2.)
Material examined
Lectotype. MEXICO: Nayarit, off Tres Marias Islands, leg. USFC Steamer Albatross, Stn 3425, 18.iv.1891, 21.316660°N, 106.40000°W, 680 fms (1244 m): 1 M 9.8 mm (MCZ IZ CRU-4560).
Paralectotype. MEXICO: Nayarit, off Tres Marias Islands, leg. USFC Steamer Albatross, Stn 3424, 18.iv.1891, 21.25000°N, 106.38333°W, 676 fms (1236 m): 1 ov. F 9.9 mm (MCZ IZ CRU-4559).
Non-type specimens. USA: San Diego Trough, off California, leg. Robert Hessler, Spencer Luke, R/V Melville, Stn SIO69-486, 13.xii.1969, 32.41670°N, 117.49670°W, 1250 m: 6 M 6.6–10.0 mm (SIO-BIC C638). — USA: San Diego Trough, off California, leg. Harim Cha, Kent Trego and students, R/V New Horizon, otter trawl, 27.x.2007, 32.60330°N, 117.54180°W, 1210 m: 1 ov. F 9.9 mm (SIO-BIC C11814), 1 ov. F 7.8 mm (SIO-BIC C11025).
Diagnosis
Carapace granulated, dorsally covered in small granules, laterally unarmed, with dorsal deep furrows, cervical grooves indistinct. Gastric and cardiac region with a longitudinal protuberance. Rostrum triangular, broad base and lateral margins convergent, serrated, directed upwards. Frontal margins slightly oblique, slightly concave behind ocular peduncle. Orbit not distinctly excavated, outer orbital angle blunt. Anterolateral angle unarmed or armed with a small spine. Branchial margin unarmed. Abdominal somites with transverse ridge, somites 2–5 armed with a spine. Telson divided into 9 plates. Sternite 3 moderately broad, anterolateral angles produced, sternite 4 subtriangular. Eyes unarmed, movable, epistomial spine absent. Article 1 of peduncle with well-developed dorsolateral and distolateral spines. Article 1 of antenna with distomesial spine. Mxp3 merus 0.5–0.6× longer than ischium, subrhomboidal in lateral view. P1 slender, elongate; meri and carpi with small spines on all surfaces, fixed finger without denticulate carina on distolateral margin. P2–4 slender, spinose; dactyli moderately slender, curving, flexor margin with dactylar teeth along all margins decreasing proximally. Epipods absent from all pereopods.
Eggs
Approximately 35–55 eggs of 1 mm.
Colouration
Unknown.
Distribution
Northeast Pacific, from Mexico to Canada.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis quadrata belongs to a group of species with a frontal margin without delimited orbit, telson with 9–10 plates, eye movable, unarmed, with peduncle larger than cornea. The most closely related species is M. carinipes from Panama and Costa Rica. Indeed, there is no support for considering these species as different taxa according to molecular data (Fig. 2, 3b). However, both taxa are morphologically highly divergent, and the distribution ranges do not overlap. Both species are easily distinguished by the following characters:
The anterolateral angle of the carapace is often armed with a small spine in M. quadrata whereas this angle is always unarmed in M. carinipes.
The dorsal surface of the carapace is porose and smooth in M. carinipes whereas this is densely covered in granules in M. quadrata.
Abdominal somite 2 is armed with a median broad spine in M. quadrata whereas this spine is absent in M. carinipes.
Abdominal somites 3–5 are armed with a median small spine in M. quadrata, whereas these spines are much broader in M. carinipes.
The rostral margins are subparallel in M. carinipes whereas these are convergent in M. quadrata.
The P1 meri and carpi are spinose in M. quadrata whereas these are unarmed in M. carinipes.
The P2–4 meri are dorsally and ventrally carinated and smooth in M. carinipes, whereas these are cylindrical in cross-section, spinose and not carinated in M. quadrata.
The walking legs P2–4 are more slender in M. quadrata (propodus 6–7× as long as high) than in M. carinipes (propodus 4–5× as long as high).
All these morphological characters are consistent for all specimens examined from Panama and Costa Rica (M. carinipes), and from Mexico and California (M. quadrata). Therefore we decided to maintain the species status of both taxa, despite the lack of genetic support. Explanatory hypotheses for the molecular and morphological incongruence in this case would be: (1) recent speciation including incomplete lineage sorting for the analysed markers, (2) hybridisation or introgression between both species, or (3) extreme phenotypic variability. The lack of morphotypes of M. carinipes in M. quadrata populations and vice versa led to the prevalence of hypotheses (1) and (2) over hypothesis (3). To test these hypotheses, we would need broader population sampling and nuclear markers or ultimately genomic data.
Munidopsis similis Smith, 1885
(Fig. 8e, f, 18a–f.)
Material examined
Holotype. USA: Nantucket Shoals, leg. United States Fish Commission, Albatross R/V, Stn 2192, 5.viii.1884, 39.77500°N, 70.24580°W, 1939 m, 1 ov. F 16.7 mm (USNM 8255).
Non-type specimens. USA: Gay Head–Bermuda transect, Atlantic Ocean, leg. R/V Chain 088, Howard L. Sanders, Stn 210A, 2.ii.1969, 39.716644°N, 70.766679°W, 2024 to 2064 m, 1 M 17.5 mm (MCZ IZ-81478). — USA: Hudson Canyon, leg. R/V Knorr 35, Stn 326, 18.xi.1973, 39.200000°N, 71.783333°W, 2288 to 2297 m: 3 M 12.3–22.1 mm, 1 ov. F 18.3 mm, 3 F 12.1–17.9 mm (MCZ IZ-127820). — USA: Hudson Canyon, Atlantic, leg. R/V Knorr 35, Stn 325, 18.xi.1973, 39.221611°N, 71.890043°W, 1919 to 1974 m: 1 M 14.5 mm (MCZ IZ-81527). — USA: rise off New England, Atlantic, leg. R/V Atlantis II 086, Stn 432, 41′ Gulf of Mexico shrimp trawl, 19.iii.1975, 39.52000°N, 70.33333°W, 2450 to 2350 m, 1 M 20.4 mm, 1 F 12.3 mm broken (MCZ IZ-39189). — USA: Atlantic Ocean, leg. R/V Chain 124, Stn 501, 4.vii.1975, 38.33666°N, 70.21833°W, 3287 to 3314 m: 1 M broken (MCZ IZ-81526).
USA: West Cortes Bank, California, leg. R/V Atlantis II, HOV Alvin dive 1508, cruise AT112, Leg 19, Stn MEA 85-5, 19.i.1985, 32.21823°N 119.25998°W, 1538–1678 m, 1 ov. F 12.1 mm (CAS-IZ 190355). — USA: San Diego Trough, off California, leg. Lisa Levin, Larry Lovell and students, R/V Robert Gordon Sproul, otter trawl, 2.x.2005, 32.60000°N, 117.50830°W, 1215 m: 1 M 11.2 mm (SIO-BIC C10923). — USA: San Diego Trough, off California, leg. Lisa Levin and students, R/V New Horizon, otter trawl, 31.x.2009, 32.59600°N, 117.48300°W, 1200 m: 1 not measured (SIO-BIC C11171). — USA: California, leg. E/V Nautilus, ROV Hercules dive H1829, Stn NA123-015-02-B-MCZ, 17.x.2020, 34.87917°N, 121.74157°W, 1295 m: 1 F 12.1 mm (MCZ IZ-162084).
COSTA RICA: Mound Jaguar, leg. Greg Rouse, Avery Hiley, R/V Falkor, ROV SuBastian dive S0230 S3, 25.i.2019, 9.65882°N, 85.88289°W, 1895 m: 1 M 5.8 mm (SIO-BIC C13964).
Diagnosis
Carapace with numerous short scales each with long, stiff setae, laterally with 3–4 spines, cervical grooves distinct. Rostral spine triangular, slender, often dorsally carinated, lateral margin often serrated. Pair of epigastric spines, often with additional small spines. Frontal margins slightly oblique. Orbit not distinctly excavated; outer orbital angle produced into a strong antennal spine. Anterolateral spine of carapace strong. Branchial margin with 3–4 spines. Abdominal somites with transverse ridge, unarmed. Telson divided into 10 plates. Sternite 3 moderately broad, anterolateral angles produced, sternite 4 subtriangular. Eyes fixed, armed with mesial and lateral spines, mesial larger, epistomial spine absent. Article 1 of peduncle with well developed subequal dorsolateral and distolateral spines. Article 1 of antenna with distomesial spines and distolateral spines. Mxp3 merus as long as ischium, subrhomboidal in lateral view. P1 slender, elongate, spinose, fixed finger with denticulate carina on distolateral margin. P2 not reaching end of P1. P2–4 long and slender; dactyli moderately slender, curving, flexor margin with teeth along entire margin decreasing proximally. Epipods absent from all pereopods.
Colouration
Carapace, abdomen, eyes and pereopods whitish.
Distribution
Northwest Atlantic Ocean, Bering Sea and Northeast Pacific, including Costa Rica, from 1390- to 3314-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis similis was described from southern New England and recorded from the Middle Atlantic Bight, West of Iceland and the Gulf of Mexico (Smith 1885; Hansen 1908; Wenner 1982; Coykendall et al. 2017). Marin (2020) described a new taxon, M. asiatica, from the Bering Sea, reported as morphologically and genetically very similar to M. similis (see Marin 2020). We discovered new records of M. similis for the Northeast Pacific (USA and Costa Rica). Our analyses demonstrate a lack of genetic structure for COI among the Atlantic and East Pacific populations of M. similis, including the Bering Sea population corresponding to M. asiatica, therefore there is no molecular evidence for considering these as two separate taxa (Fig. 2, 4b). We found morphological variation at the individual level among and within the Atlantic and East Pacific populations in the relative width of the rostrum in relation to length, the presence or absence of a dorsal carina and the presence or absence of small additional spines on the epigastric and hepatic dorsal carapace (see Supplementary Fig. S8). Variations within these characters were considered to be morphological differences between both species (Marin 2020). However, when several specimens of different populations are examined, this variation becomes intraspecific. Therefore, due to the lack of morphological and molecular support, we propose M. asiatica as a junior synonym of M. similis.
Munidopsis testuda sp. nov.
Material examined
Holotype. ECUADOR: Galapagos Archipelago, leg. R/V Thomas Washington, Stn PLUM 02 WT, Rock Dredge D-08, 23.i.1990, 1.11683°N, 88.26283°W, 1000–917 m: ov. F 15.1 mm, 2 eggs (SIO-BIC C9639).
Etymology
From the Latin testudo (meaning ‘turtle’), referring to Galapagos Islands but also the squamate carapace of the species, like a turtle shell.
Diagnosis
Carapace dorsally covered in granules and scales, wider scales at posterior, with dorsal deep furrows, cervical grooves distinct. Rostrum broad, dorsally elevated, spade-shaped, dorsally carinate, distally armed with 2 spines. Frontal margin nearly straight, spine below antennal angle. Orbit not excavated, outer orbital angle with a blunt lobe. Anterolateral angle armed with a spine. Branchial margin armed with a spine. Abdominal somites unarmed. Telson divided into 7 plates. Sternite 3 anterolaterally rounded, anterior margin with median lobe flanked by 2 lobes, sternite 4 narrowly subtriangular. Eyes unarmed, movable, epistomial spine present, cornea elongated. Article 1 of antennule with dorsolateral and distolateral spines, distolateral double. Article 1 of antenna with strong distomesial spine and distolateral spines. Mxp3 merus rectangular in lateral view. P1 stout, unarmed, fixed finger without denticulate carina on distolateral margin. P2–4 stout, unarmed; meri carinated, dactyli slender, curving, flexor margin with minute teeth along all margins decreasing proximally. Epipods present on P1–2.
Description
Carapace
Slightly broader than long, widest at midlength; moderately convex from side to side. Dorsal surface densely covered in granules and scales, each scale with a few short setae; hepatic and anterior branchial areas with scales and some acute granules; posterior cardiac and intestinal region covered in larger scales. Regions well delineated by deep furrows including distinct anterior and posterior cervical grooves. Gastric region slightly elevated. Posterior margin unarmed, preceded by elevated ridge. Rostrum broad, dorsally elevated, spade-shaped (lateral margins constricted between eyes), width 0.2× anterior width of carapace, directed slightly upwards, dorsally carinate, distally armed with 2 broad spines; 0.4× carapace length, 1.8× as long as broad. Frontal margin nearly straight behind ocular peduncle, blunt outer orbital angle above antennal peduncle, outer orbital spine and process (antennal spine) absent; spine below antennal angle, ventral to frontal margin, close to epistomial spine. Lateral margins straight; anterolateral spine broad; anterior branchial margin with one broad spine; one broad branchial spine behind posterior branch of cervical groove. Pterygostomian flap surface covered in granules and scales, anteriorly acute.
Sternum
0.9× as long as broad, maximum width at sternite 6. Sternite 3 broad, 3.2× wider than long, anterolaterally rounded, anterior margin with median lobe flanked by 2 lobes. Sternite 4 narrowly elongated anteriorly; surface depressed in midline, smooth; greatest width 2.3× that of sternite 3 and 1.7× wider than long.
Abdomen
Unarmed; tergite 2 with 2 elevated transverse ridges, lateral parts of dorsal surfaces covered in granules and scales; tergites 3–6 lacking a posterior ridge; tergite 6 with weakly produced posterolateral lobes and nearly transverse posteromedian margin. Telson composed of 8 plates; 1.5× as wide as long.
Eye
Eyestalk movable, partially concealed by rostrum; peduncle covered with a few granules, shorter than cornea length; cornea ovoid and elongated; lateral surface contiguous to epistomial spine, epistomial spine ventral to frontal margin.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines; distomesial margin slightly produced and granulated.
Antenna
Peduncle slightly exceeding eye. Article 1 with strong distomesial spine and distolateral spines, not reaching end of article 2. Article 2 with well developed distomesial and distolateral spines, distomesial margin granulated. Articles 3–4 unarmed.
Mxp3
Surface with granules. Ischium as long as merus measured on extensor margin; flexor margin of merus with 3 spines subequal in size and a smaller distal spine; extensor margin with 6 granules or blunt spines.
P1
Stout, with numerous minute granules and scales, each scale with few short setae, 1.3× longer than carapace. Merus 2.2× carpus length, with distal stout spines. Carpus 0.9× longer than broad, with some distal stout spines, a few acute granules along dorsal side. Palm unarmed, stout, slightly longer than carpus, 1.2× longer than broad. Fingers unarmed, 1.2× longer than palm, opposing margins nearly straight, not gaping, spooned; fixed finger without denticulate carina on distolateral margin.
P2–4
Stout, coarsely granulated, devoid of setae, cylindrical in cross-section, slightly decreasing in size posteriorly. P2 merus stout, 0.6× carapace length, 2.7× longer than high and 1.1× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.9× length of P2 merus, P4 merus 0.85× length of P3 merus); extensor margin of P2–4 meri carinate, with small granules along entire border, distal part flattish ending in thick spine; flexor margin granulated; carpi with one thick distal spine on extensor margin, granulated carina along lateral side; P2–4 propodi 5.1–5.2× as long as high, trianguloid in cross-section, unarmed; dactyli 0.5× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 11–12 dactylar teeth at entire margin, each with slender corneous spine, ultimate tooth closer to penultimate tooth than to dactylar angle.
Epipods
Present on P1–2.
Colouration
Unknown.
Distribution
Galapagos Islands, at 917–1000-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
The new species represents a highly divergent lineage that shares a trifid rostrum with the trifida-group (Fig. 1, 2): e.g. M. trifida from East Pacific, M. ahyongi Dong & Li, 2021 and M. comarge from the West Pacific. M. testuda sp. nov., however, does not have a close relationship or share a common ancestor with this group of species (Fig. 1, 2) and differs from this group in having epipods on P1–2 instead of having pereopods without epipods or only in P1, and a carapace that is as long as broad instead of broader than long. The new species is closely related to M. carolinensis Dong & Li, 2021 from the Caroline Ridge in the tropical West Pacific and M. expansa Benedict, 1902 from off Florida and the Caribbean Sea. However, the new species can be distinguished from the other species by the following morphological characters:
The rostrum is triangular in M. carolinensis and M. expansa whereas this is spade-shaped and constricted between the eyes in the new species.
The scales on the carapace and pereopods are smaller and less dense in M. carolinensis and M. expansa than in the new species.
The new species has an additional pair of spines apart from the epistomial spines, each lateral to the eyestalk and ventral to the frontal margin, whereas this pair of spines is absent in M. carolinensis and M. expansa.
The new species has epipods on pereopods 1–2 whereas M. carolinensis has pereopods without epipods.
Munidopsis testuda sp. nov. and M. carolinensis diverge on 18% for COI. Unfortunately, no molecular data are available for M. expansa.
Munidopsis cortesi sp. nov.
(Fig. 20g, h, 16a–j.)
Material examined
Holotype. COSTA RICA: Jaco Summit, leg. Greg Rouse, Allison Miller, R/V Falkor, ROV SuBastian dive S0213, Slurp 4, 6.i.2019, 9.17402°N, 84.79990°W, 742 m: ov. F 5.2 mm (tissue SIO-BIC C13902 ex MZUCR 3761-01).
Etymology
Named for Prof. Jorge Cortés Núñez, Universidad de Costa Rica, for his invaluable collaboration on deep-sea research and his efforts to create awareness of the deep regions of Costa Rica. We honour Prof. Cortés’ academic retirement after more than 40 years of research in marine biodiversity and ecology, and we celebrate his continued dedication to promoting ocean conservation.
Diagnosis
Carapace pubescent, dorsally smooth, with deep dorsal furrows, cervical grooves indistinct. Rostrum broadly triangular, trifid distally. Frontal margins concave. Orbit distinctly excavated, outer orbital angle with a strong spine. Anterolateral unarmed. Branchial unarmed. Abdominal somites 2–4 armed with posterolateral teeth, abdominal somite 3 armed with 4 teeth. Telson divided into 7 plates. Sternite 3 anterolaterally rounded, anterior margin with median serrated shallow notch flanked by 2 lobes, sternite 4 subtriangular. Eyes unarmed, movable, epistomial spine absent. Article 1 of antennule with dorsolateral and distolateral spines. Article 1 of antenna with well-developed distomesial spine. Mxp3 merus subrhomboidal in lateral view. P1 slender, pubescent, fixed finger without denticulate carina on distolateral margin. P2–4 stout, pubescent; meri armed with acute teeth on flexor and extensor margins, propodi flattish in cross-section; dactyli slender, curving, flexor margin with cuticular teeth along all margins decreasing proximally. Epipods absent from all pereopods.
Description
Carapace
1.2× longer than wide; moderately convex from side to side; hirsute, dorsally unarmed, densely covered in short, fine setae, long distally curved setae on gastric region. Regions well delineated by deep furrows, cervical grooves indistinct. Gastric region slightly elevated. Posterior cardiac region elevated and constricted by lateral furrows, forming notches; posterior margin posteriolaterally armed with small teeth, preceded by deep transverse depression. Rostrum broad, width 0.3× anterior width of carapace, 0.4× carapace length; trifid distally; apex directed slightly upwards; lateral proximal margin weakly oblique, 1.4× as long as broad. Frontal margin concave behind ocular peduncle, antennal (outer orbital) spine strong. Lateral margins unarmed; slightly convex; widest at midlength, anterolateral angle unarmed. Pterygostomian flap densely covered with acute teeth, viewed in dorsal view, anterior margin blunt, unarmed.
Sternum
1.2× longer than broad, maximum width at sternite 5. Sternite 3 broad, 2.9× wider than long, anterolaterally rounded, anterior margin with median serrated shallow notch flanked by 2 lobes. Sternite 4 narrowly elongated anteriorly; surface depressed in midline, smooth; greatest width 2.7× that of sternite 3 and 2.0× wider than long.
Abdomen
Tergites with short, fine, dense setae; tergites 2–4 armed with posterolateral teeth; tergite 3 with elevated ridge, armed with 4 teeth; tergites 3–6 without elevated ridges; tergite 6 with posterior margin not produced. Telson composed of 7 plates, as wide as long.
Eye
Ocular peduncle unarmed; surface densely setose, movable, partially concealed by rostrum. Cornea subglobular, slightly wider than peduncle.
Antennule
Article 1 of peduncle with subequal dorsolateral and distolateral spines, distolateral spine bifid; distomesial margin with 2 pairs of blunt processes, distal surface dentate; distolateral margin armed with one spine.
Antenna
Peduncle exceeding eye; Article 1 with distomesial spine not reaching end of article 2, distolateral angle rounded. Articles 2–3 with well-developed distolateral spine, distomesial spine smaller. Article 4 unarmed. Flagellum longer than carapace.
Mxp3
Merus with 2 large spines along flexor margin, proximal larger than distal; small distal spine on extensor margin. Ischium longer than wide, with distal flexor and extensor spines. Crista dentata finely denticulate. Dactylus, propodus and carpus unarmed.
P1
Slender, densely covered in short, fine setae and long setae, 1.4× longer than carapace. Merus 2.4× carpus length, with some stout distal spines. Carpus 2.4× longer than broad, with some stout distal spines. Palm unarmed, slender, slightly longer than carpus, 2.6× longer than broad. Fingers unarmed, 0.85× longer than palm, opposing margins nearly straight, not gaping, spooned, fixed finger without denticulate carina on distolateral margin.
P2–4
Stout, densely covered in short fine setae and some long distally curved setae, cylindrical in cross-section, slightly decreasing in size posteriorly. P2 merus stout, 0.55× carapace length, nearly 3.1× longer than high and 1.3× length of P2 propodus. P2–4 meri decreasing in length posteriorly (P3 merus 0.7× length of P2 merus, P4 merus 0.9× length of P3 merus); extensor margin of P2–4 meri with acute teeth along entire border, distal part trianguloid; flexor margin with acute teeth along entire border, distal strongest; carpi P2–3 with two thick teeth on extensor margin, distal strongest, flexor margin unarmed; P2–4 propodi 4.3–4.4× as long as high, flattish in cross-section, unarmed; dactyli 0.7–0.9× length of propodi; distal claw short, moderately curved; flexor margin distally curved, with 6–8 dactylar teeth decreasing in size proximally, each with slender corneous spine, ultimate tooth at midlength between penultimate tooth and dactylar angle.
Epipods
Absent from pereopods.
Colouration
Carapace, abdomen and pereopods orange and brownish. Ocular orbits orange.
Distribution
Costa Rica at 742-m depth.
Genetic data
COI, 16S rRNA and 28S rRNA.
Remarks
Munidopsis cortesi sp. nov. belongs to a group of species sharing a flattened, distally trifid rostrum and unarmed carapace surface, and lacking pereopodal epipods. This group includes M. acuminata Benedict, 1902 from off South Carolina, north-western Atlantic; M. alcocki Ahyong, 2014 from the Indian Ocean, M. aurantia Lin & Chan, 2011 from Taiwan, M. kareenae Ahyong, 2013 from New Zealand, M. modesta Benedict, 1902 from Galapagos Islands, M. nias Ahyong, 2014 from the eastern Indian Ocean, M. pubescens Macpherson, 2007 from Madagascar, M. serricornis from the North Atlantic Ocean, M. tuerkayi Macpherson, Beuck & Freiwald, 2016 from the Caribbean Sea and M. treis Ahyong & Poore, 2004 from the West Pacific Ocean.
This new species is very different and easily distinguished from the other species of the group by the following characters:
The new species has an unarmed anterolateral angle of the carapace and unarmed carapace margins whereas the other species have at least the anterolateral margin armed.
The new species have acute teeth on the pterygostomian flap surface whereas these teeth are absent in the other species.
The new species have acute teeth on the flexor and extensor margins of the meri of P2–4, whereas these teeth are absent in the other species, and in all of these, the flexor margins of the meri are unarmed.
Discussion
Phylogenetic relationships of squat lobsters
Our phylogenetic results reiterate that the phylogenetic relationships of squat lobsters are poorly known at many levels. Although not part of this study, there is lack of consensus in the literature on the phylogenetic position of Galatheoidea within Anomura (Ahyong et al. 2009; Schnabel et al. 2011b; Bracken-Grissom et al. 2013; Tan et al. 2018). Also, conflicting results on the relationships of the families within Galatheoidea were recovered depending on the set of data used and the taxon sampling employed in analyses (Schnabel et al. 2011b; Bracken-Grissom et al. 2013; Roterman et al. 2018; Palero et al. 2019). Moreover, the validity of the current classification at the family level is brought into question, including the polyphyly of Galatheidae and the conflicting placement of some galatheid genera (Ahyong et al. 2011; Schnabel et al. 2011b; Bracken-Grissom et al. 2013; Rodríguez-Flores et al. 2022b). Our phylogenetic analyses agree with those obtained by Rodríguez-Flores et al. (2022b), whose results did not support the monophyly of Galatheidae. The systematic placement of Janetogalathea californiensis obtained in this work supports the hypothesis of the existence of an overall high-level of convergence and homoplasy within the group (Machordom and Macpherson 2004; Cabezas et al. 2012). The genus was initially assigned to Galatheidae, on the bases of the striation of the carapace and the presence of a triangular rostrum that is broad at the base and has 3–4 marginal spines (Ahyong et al. 2010; Baba et al. 2011). However, the conflicting position of Janetogalathea and other genera (Alainius Baba, 1991, Leiogalathea) lacking a trispinous or trilobate rostrum indicates that these characters are not true synapomorphies of Galatheidae and could therefore be the result of evolutionary convergence or another type of homoplasy. The inclusion of Leiogalathea in Munidopsidae was supported by the synapormophy of Munidopsidae having a reduced flagellum in the first maxilliped in Leiogalathea or the flagellum being absent in the remaining species of the family (Ahyong et al. 2010; Baba et al. 2011). The flagellum is also reduced in Janetogalathea as expected given the phylogenetic position as sister group to Leiogalathea.
By contrast, the more diverse genera within Galatheoidea (Munida Leach, 1820, Munidopsis and Galathea Fabricius, 1793) are non-monophyletic. Several authors have proposed that Munida is a composite taxon (Machordom and Macpherson 2004; Rodríguez-Flores et al. 2019a; Miranda et al. 2020) that includes more than 15 putative genera (Machordom pers. comm.). This is also the case for Munidopsis; indeed, a phylogeny using two mitochondrial genes and over 30 munidopsid taxa proposed the paraphyletic status (Ahyong et al. 2011). Our results fully agree with those of Ahyong et al. (2011) in recovering Munidopsis as non-monophyletic (Fig. 1) but neither Ahyong et al. (2011) nor our study recovered highly supported deep relationships, probably due to the low number of characters employed in the analyses and saturation of the mitochondrial genes (e.g. Blouin et al. 1998) or the existence of an explosive radiation that is reflected in short internodes in a phylogeny (Whitfield and Lockhart 2007). In this sense, including more molecular and morphological characters, and a more thorough taxonomic sampling in the phylogenetic reconstructions, may confirm how many independent lineages can be delimited at genus level (Rodríguez-Flores et al. 2021). What is evident is the presence of unique combinations of morphological characters in the different clades that point towards the existence of different genus-level lineages within Munidopsis. For example, the clade including only bathyal or abyssal species (Clade I, Fig. 1, 2) includes species lacking external pigmentation and ocular spines (e.g. M. aries, M. bairdii, M. alvisca); the trifida-clade that includes Munidopsis cortesi sp. nov. consists of species that present a trispinous rostrum; and the clade including M. lentigo contains species with unique carapace morphology and a pad of setae on the ventral surface of pereopod 1. All these clades are highly divergent, suggesting ancient splitting during the evolutionary history of munidopsids. Therefore this evidence, together with the lack of monophyly makes considering Munidopsis as a complex including several genus-level lineages reasonable. In conclusion, a great deal of work is needed to refine the squat lobster tree of life, ideally including more data and more complete taxon sampling to obtain a broader picture of the evolutionary history.
Species delimitation and speciation patterns
As a result of integrating molecular and morphological data for the East Pacific taxa, we have delimited five new species and propose three new junior synonyms.
Most species delimitation analyses have estimated a smaller number of species for the clade including only abyssal species within Munidopsis (Fig. 2) despite some of these being morphologically distinct. A total of 28 currently recognised species were analysed for this clade (Clade I), whereas some of these analyses only identified 12 species. Within clade I, a group of more than 10 species with very shallow divergences (M. arietina, M. antonii, M. bracteosa, M. bairdi, M recta, M. exuta, M. scotti, M. producta and M. starmer) (Fig. 2) is considered by some of the methods to be a single species. In clade I, there is also conflict in the morphological delimitation in 3 subclades that include pairs of allopatric species with very shallow divergences: (a) M. aries from the Atlantic + M. albatrossae from the Pacific, (b) M. bairdii from the Atlantic + M. arietina from the Pacific and (c) M. alvisca from the East Pacific + M. myojinensis + M. lauensis from the West Pacific (Macpherson and Segonzac 2005; Cubelio et al. 2007; Dong et al. 2019). Non-exclusive explanations for what may occur in this clade include the following: (1) original species descriptions are usually based on the morphological examination of only a few specimens due to the poor representation in collections (e.g. Jones and Macpherson 2007; Macpherson et al. 2016; Dong and Li 2021; Rodríguez-Flores et al. 2022a) that might have been a source of confusion regarding intraspecific variability and morphological differences among species. This general explanation could be applied to many other cases within Munidopsis apart from this clade; (2) large geographical distances between morphologically similar species that might lead to consideration of these as different taxa prior to the discovery of additional records that may suggest cosmopolitanism of a single morphotype; this could be the case for the pair of allopatric species M. aries vs M. albatrossae that are unclearly distinguished based on morphology but differ in geographical ranges (García Raso et al. 2008); (3) morphological disparity is high across Munidopsis (Rodríguez-Flores et al. 2022a) and also correlated with high intraspecific variation (see M. antonii in Baba 2005), and this high morphological disparity coupled with shallow divergences could be an indicator of a recent radiation of this clade; and (4) there might be a reduction in the rate of molecular evolution of the clade that results in shallow divergences when compared to other Munidopsis lineages, perhaps in relation to the adaptation to abyssal depths. In this sense, Rodríguez-Flores et al. (2022b) suggested that increased depth could reduce the rate of substitution in related squat lobsters. In this sense, there is evidence suggesting differences in functional loci in deep-sea fishes living at different depths (Gaither et al. 2018). Therefore, the effect of depth on the rate of molecular evolution of squat lobsters should be tested using thorough sampling, genomic data or a proxy of molecular evolution (e.g. CG content or transversion v. transition).
In any case some of these species likely constitute a single taxon with geographically structured genetic and morphological diversity. This may be the case for the clades mentioned earlier, and others, for example the species-group including M. nitida, M. vrijenhoeki and M. spinifrons from the Indian and Pacific oceans; and the species-group including M. bermudezi Chace, 1939 from the Atlantic and M. cascadia Ambler, 1980 from the East Pacific. We prefer not to make any taxonomic decisions in relation to any of these species, as a larger dataset including populations across all oceans, the study of intraspecific morphological variation and testing gene flow with nuclear markers should be considered to properly address these species hypotheses.
Limitations and caveats for these single-locus delimitation methods are broadly acknowledged (e.g. Sukumaran and Knowles 2017; Dellicour and Flot 2018). Population structure could be confounded with species divergences, especially for recent species. Introgression and other population processes such as incomplete lineage sorting could make species delimitation using a single mitochondrial locus difficult, in which case rapidly evolving nuclear genes can be much more informative. However, for deeply divergent species, single locus species delimitation seems to have relatively more power than for recently diverged ones (Rannala and Yang 2020). Another caveat of applying these methods to explore the species of Munidopsidae is that our data set contains many species, some of which are highly divergent with ancient speciation events and others of much more recent origin, and this prevents delimitation of all of these (Puillandre et al. 2021). Some of the methods might not be appropriate to analyse this set of data, for example there are many singletons that could not be suitable for these analyses. The nature of our data (many shallow and highly divergent species) resulted in a barcode gap that is barely discernible across the genetic distance data for ABGD; and other methods like bPTP or GMYC are probably over-splitting the real number of species (Dellicour and Flot 2018). Nonetheless, there is consensus in that we should consider different sources of data for species delimitation (e.g. Puillandre et al. 2012). Moreover, taking into account the agreement of several species delimitation methods (Dellicour and Flot 2018), the taxonomic status of the species included in this clade should be revised, including multiple sources of evidence and more robust data. However, some of the species considered in this clade might be a single species attaining an abyssal cosmopolitan distribution. This conclusion seems to be congruent with the general idea of cosmopolitanism in the abyssal depth (McClain and Hardy 2010). This hypothesis should be tested further.
Biogeographical considerations
Interestingly, after removing Janetogalathea from Galatheidae, the family Galatheidae would be represented in the East Pacific only by Galathea paucilineata (Benedict, 1902) from Galapagos (if considering Phylladiorhynchus as non-Galatheidae following Rodríguez-Flores et al. 2022b; Fig. 1). According to the original description (Benedict 1902), this species would fit well within the genus Leiogalathea. However, the rostrum and some legs are unknown and until more material of this taxon can be examined, we cannot disprove that G. paucilineata might be a Galathea species, a member of Leiogalathea or even a species of Janetogalathea (Arnés-Urgellés et al. 2020). Irrespectively, the family Galatheidae is poorly represented in the Americas in comparison with the East Atlantic and the West Pacific (Schnabel et al. 2011a). Also, Munididae, despite being the most diverse family of squat lobsters (Baba et al. 2008), is much less speciose in the East Pacific than Munidopsidae in the EP with only 21 species described or reported to date.
Munidopsidae, with more representatives in abyssal depths than Munididae – that has a peak of diversity in the continental shelf – seems to be more prone to cosmopolitanism and have wider geographical ranges than the other families. For example, the recent finding of M. lauensis in the Indian Ocean suggests long distance dispersal among hydrothermal vents between the Indian and Pacific oceans (Borda et al. 2013; Hwang et al. 2022). This fact together with the shallow divergence between this species and M. alvisca could indicate the existence of a single, widely distributed taxon in which hydrothermal vents could facilitate a type of stepping-stone dispersal. Cosmopolitanism is known for many deep-sea invertebrate species, for example nematodes at both genus- and species-level (Miljutin et al. 2010; Zeppilli et al. 2011), echinoderms (Thomas et al. 2020) and molluscs (Oliver 2015), among others. The abyss has often been perceived as a homogenous habitat lacking horizontal barriers to dispersal (e.g. Etter et al. 2005, 2011) but the existence of species complexes in abyssal depths has challenged the traditional view of general cosmopolitanisms for abyssal taxa (Schornikov 2005; Raupach et al. 2007; McClain and Hardy 2010; Brandão and Yasuhara 2013). In cases of taxa with pan-abyssal distributions, horizontal (geographic) barriers to gene flow seem to be less determinant than vertical (bathymetric) barriers (Etter et al. 2005; Breusing et al. 2020; McCowin et al. 2020; Prada and Hellberg 2021).
In the case of Munidopsis, some species are apparently distributed across small areas, whereas others have a much wider spatial distribution. These restricted distributions are more common in species from bathyal depths than for abyssal species, as is the case for species in Clade II, e.g. M. hystrix, M. scabra and M. depressa (Fig. 2), all endemic to the northern East Pacific. These contrasting distribution patterns could be related to the biological cycles and dispersal strategies of each species, although this could also be related to the rarity of some species (e.g. M. granosicorium is only known for the holotype and from the specimen described here from Costa Rica). The entire larval cycle of Munidopsis is only known for a few species, mainly from the abyssal clade, e.g. M. bermudezi, M. bairdii, M. curvirostra Whiteaves, 1874 and M. similis (Wenner 1982), all with planktotrophic larvae (Baba et al. 2011) and therefore able to disperse over long distances (Baco et al. 2016). However, hydrothermal vent species such as M. lentigo and M. subsquamosa Henderson, 1885 have been suggested to possibly have a lecithotrophic larva (Van Dover et al. 1985). The larval cycle for most species is unknown (Baba et al. 2011), however, and the only biological data available are body size and the relation to the size and number of eggs (Van Dover et al. 1985). If size and number of eggs are indicative of the type of life cycle, some munidopsids with a low number of large eggs (for example M. granosicorium, here analysed) might likely also have a lecithotrophic larval cycle with limited long-distance dispersal (Baco et al. 2016). Several studies at the population level (Etter et al. 2005; Zardus et al. 2006) have shown that bathyal species exhibit greater population structure than abyssal species, usually characterised by low genetic divergences among populations. This phenomenon may be influenced by the dispersal abilities of the species but also because of constraints for abyssal species in terms of vertical barriers in the deep sea.
Deep-sea squat lobsters from the East Pacific will continue to surprise us in many ways, for example with new taxa such as the new species described here, that represent ancient and highly divergent lineages. Also, new records demonstrate that deep species of Munidopsis could have wider ranges than previously considered, some perhaps being cosmopolitan and some of which have been able to cross the eastern Pacific Barrier (Lessios and Robertson 2006). Taxonomic knowledge is fundamental for investigating evolutionary and biogeographical questions regarding deep-sea taxa, in an era where the deep sea has become a target of economic interests as a source of multiple natural resources. Now more than ever there is a need to explore and study deep-sea species diversity and to understand these unique ecosystems before human activity has an irreversible impact.
Supplementary material
Supplementary material is available online.
Data availability
All genetic data are publicly available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Collecting information, images and life habitus pictures are available through the Harvard Museum of Comparative Zoology’s MCZbase (https://mczbase.mcz.harvard.edu/) and the Scripps Institution of Oceanography Benthic Invertebrate Collection database (https://sioapps.ucsd.edu/collections/bi/).
Conflicts of interest
G. Giribet is the Editor-in-Chief of Invertebrate Systematics and G. W. Rouse is Associate Editor of Invertebrate Systematics. Despite this relationship, these editors did not at any stage have Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Invertebrate Systematics encourages editors to publish in the journal and editors are kept totally separate from the decision-making process for their own manuscripts. The authors declare that they have no further conflicts of interest.
Declaration of funding
P. C. Rodríguez-Flores is funded by an E. O. Wilson Biodiversity Postdoctoral Fellowship at the Museum of Comparative Zoology, Harvard University. This project was partially funded by a LinnéSys Award (Systematic Association and Linnean Society). Shipping time, specimen collection or preliminary analyses were funded by the National Science Foundation (NSF) Office of Polar Programs Award 1043749 (N. Wilson, G. Rouse and R. Burton); NSF Division of Ocean Sciences Awards OCE-0826254 and OCE-1634172 (G. Rouse, L. Levin); the Monterey Bay Aquarium Research Institute; the Schmidt Ocean Institute; the Ocean Exploration Trust and National Oceanic and Atmospheric Administration Ocean Exploration and Research; and Scripps Institution of Oceanography.
Acknowledgements
We thank the staff of the Department of Invertebrate Zoology in the MCZ, especially J. Trimble and A. Baldinger, for support provided during the revision of the MCZ collection. We also thank C. Piotrowski from the California Academy of Sciences, San Francisco, for facilitating loans of specimens for this study, and K. Reed and M. Nizinski from the USNM for facilitating loans and hosting the corresponding author at the museum support centre of the Smithsonian Institution. We thank the captains, crew, submersible pilots, technicians and scientific participants on all research cruises who contributed to this study. We are grateful to E. Recuero, S. Derkarabetian, E. Macpherson, the Associate Editor and one reviewer for helpful comments on the manuscript draft.
References
Ahyong ST, Schnabel KE, Maas EW (2009) Anomuran phylogeny: new insights from molecular data. In ‘Decapod Crustacean Phylogenetics’. (Eds JW Martin, DL Felder, KA Crandall) Crustacean Issues, vol. 18, pp. 399–341. (CRC Press: Boca Raton, FL, USA)Ahyong, ST, Baba, K, Macpherson, E, and Poore, GCB (2010). A new classification of the Galatheoidea (Crustacea: Decapoda: Anomura). Zootaxa 2676, 57–68.
| A new classification of the Galatheoidea (Crustacea: Decapoda: Anomura).Crossref | GoogleScholarGoogle Scholar |
Ahyong, ST, Andreakis, N, and Taylor, J (2011). Mitochondrial phylogeny of the deep-sea squat lobsters, Munidopsidae (Galatheoidea). Zoologischer Anzeiger – a Journal of Comparative Zoology 250, 367–377.
| Mitochondrial phylogeny of the deep-sea squat lobsters, Munidopsidae (Galatheoidea).Crossref | GoogleScholarGoogle Scholar |
Arnés-Urgellés, C, Buglass, S, Ahyong, ST, Salinas-de-León, P, Wicksten, MK, and Marsh, L (2020). Arthropoda; crustacea; decapoda of deep-sea volcanic habitats of the galapagos marine reserve, tropical eastern pacific. Biodiversity Data Journal 8, e54482.
| Arthropoda; crustacea; decapoda of deep-sea volcanic habitats of the galapagos marine reserve, tropical eastern pacific.Crossref | GoogleScholarGoogle Scholar |
Baba, K (2005). Deep-sea chirostylid and galatheid crustaceans (Decapoda: Anomura) from the Indo-Pacific, with a list of species. Galathea Report 20, 1–317.
Baba , K, and Wicksten , MK (2019). Chirostyloidean squat lobsters (Crustacea: Decapoda: Anomura) from the Galapagos Islands. Zootaxa 4564, 391–421.
| Chirostyloidean squat lobsters (Crustacea: Decapoda: Anomura) from the Galapagos Islands.Crossref | GoogleScholarGoogle Scholar |
Baba, K, Macpherson, E, Poore, GCB, Ahyong, ST, Bermudez, A, Cabezas, P, Lin, C-W, Nizinski, M, Rodrigues, C, and Schnabel, KE (2008). Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomura – families Chirostylidae, Galatheidae and Kiwaidae). Zootaxa 1905, 1–220.
| Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomura – families Chirostylidae, Galatheidae and Kiwaidae).Crossref | GoogleScholarGoogle Scholar |
Baba K, Macpherson E, Lin CW, Chan TY (2009) ‘Crustacean Fauna of Taiwan. Squat lobsters (Chirostylidae and Galatheidae).’ (National Taiwan Ocean University: Keelung, Taiwan)
Baba K, Ahyong ST, Macpherson E (2011) Morphology of marine squat lobsters. In ‘The Biology of Squat Lobsters’. (Eds GCB Poore, ST Ahyong, J Taylor) pp. 1–38. (CSIRO Publishing: Melbourne, Vic., Australia)
Baco, AR, Etter, RJ, Ribeiro, PA, von der Heyden, S, Beerli, P, and Kinlan, BP (2016). A synthesis of genetic connectivity in deep‐sea fauna and implications for marine reserve design. Molecular Ecology 25, 3276–3298.
| A synthesis of genetic connectivity in deep‐sea fauna and implications for marine reserve design.Crossref | GoogleScholarGoogle Scholar |
Bahamonde, N (1964). Dos nuevos Munidopsis en aguas Chilenas Boletín del Museo Nacional de Historia Natural 28, 157–170.
Benedict, JE (1902). Descriptions of a new genus and forty-six new species of crustaceans of the family Galatheidae, with a list of the known marine species. Proceedings of the United States National Museum 26, 243–334.
| Descriptions of a new genus and forty-six new species of crustaceans of the family Galatheidae, with a list of the known marine species.Crossref | GoogleScholarGoogle Scholar |
Blouin, MS, Yowell, CA, Courtney, CH, and Dame, JB (1998). Substitution bias, rapid saturation, and the use of mtDNA for nematode systematics. Molecular Biology and Evolution 15, 1719–1727.
| Substitution bias, rapid saturation, and the use of mtDNA for nematode systematics.Crossref | GoogleScholarGoogle Scholar |
Borda, E, Kudenov, JD, Chevaldonné, P, Desbruyères, D, Blake, JA, Fabri, M-C, Hourdez, S, Shank, TM, Wilson, NG, Pleijel, F, Schulze, A, and Rouse, GW (2013). Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps. Proceedings of the Royal Society of London Series B: Biological Sciences 280, 20131876.
| Cryptic species of Archinome (Annelida: Amphinomida) from vents and seeps.Crossref | GoogleScholarGoogle Scholar |
Bouckaert, R, Vaughan, TG, Barido-Sottani, J, Duchêne, S, Fourment, M, Gavryushkina, A, Heled, J, Jones, G, Kühnert, D, De Maio, N, Matschiner, M, Mendes, FK, Müller, NF, Ogilvie, HA, du Plessis, L, Popinga, A, Rambaut, A, Rasmussen, D, Siveroni, I, Suchard, MA, Wu, CH, Xie, D, Zhang, C, Stadler, T, and Drummond, AJ (2019). BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15, e1006650.
| BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis.Crossref | GoogleScholarGoogle Scholar |
Bracken-Grissom, HD, Cannon, ME, Cabezas, P, Feldmann, RM, Schweitzer, CE, Ahyong, ST, Felder, DL, Lemaitre, R, and Crandall, KA (2013). A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda). BMC Evolutionary Biology 13, 128.
| A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda).Crossref | GoogleScholarGoogle Scholar |
Brandão, SN, and Yasuhara, M (2013). Challenging deep-sea cosmopolitanism: taxonomic re-evaluation and biogeography of ‘Cythere dasyderma Brady, 1880’ (Ostracoda). Journal of Micropalaeontology 32, 109–122.
| Challenging deep-sea cosmopolitanism: taxonomic re-evaluation and biogeography of ‘Cythere dasyderma Brady, 1880’ (Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Breusing, C, Johnson, SB, Tunnicliffe, V, Clague, DA, Vrijenhoek, RC, and Beinart, RA (2020). Allopatric and sympatric drivers of speciation in Alviniconcha hydrothermal vent snails. Molecular Biology and Evolution 37, 3469–3484.
| Allopatric and sympatric drivers of speciation in Alviniconcha hydrothermal vent snails.Crossref | GoogleScholarGoogle Scholar |
Cabezas, P, Sanmartín, I, Paulay, G, Macpherson, E, and Machordom, A (2012). Deep under the sea: unraveling the evolutionary history of the deep-sea squat lobster Paramunida (Decapoda, Munididae). Evolution 66, 1878–1896.
| Deep under the sea: unraveling the evolutionary history of the deep-sea squat lobster Paramunida (Decapoda, Munididae).Crossref | GoogleScholarGoogle Scholar |
Coykendall, DK, Nizinski, MS, and Morrison, CL (2017). A phylogenetic perspective on diversity of Galatheoidea (Munida, Munidopsis) from cold-water coral and cold seep communities in the western North Atlantic Ocean. Deep-Sea Research – II. Topical Studies in Oceanography 137, 258–272.
| A phylogenetic perspective on diversity of Galatheoidea (Munida, Munidopsis) from cold-water coral and cold seep communities in the western North Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Crandall, KA, and Fitzpatrick, JF (1996). Crayfish molecular systematics: using a combination of procedures to estimate phylogeny. Systematic Biology 45, 1–26.
| Crayfish molecular systematics: using a combination of procedures to estimate phylogeny.Crossref | GoogleScholarGoogle Scholar |
Cubelio, SS, Tsuchida, S, Hendrickx, ME, Kado, R, and Watanabe, S (2007). A new species of vent associated Munidopsis (Crustacea: Decapoda: Anomura: Galatheidae) from the Western Pacific, with notes on its genetic identification. Zootaxa 1435, 25–36.
| A new species of vent associated Munidopsis (Crustacea: Decapoda: Anomura: Galatheidae) from the Western Pacific, with notes on its genetic identification.Crossref | GoogleScholarGoogle Scholar |
Dellicour, S, and Flot, JF (2018). The hitchhiker’s guide to single‐locus species delimitation. Molecular Ecology Resources 18, 1234–1246.
| The hitchhiker’s guide to single‐locus species delimitation.Crossref | GoogleScholarGoogle Scholar |
Dong, D, and Li, X (2021). Two new species of the genus Munidopsis Whiteaves, 1874 (Crustacea: Anomura: Munidopsidae) from the Caroline Ridge, South of the Mariana Trench. Journal of Oceanology and Limnology 39, 1841–1853.
| Two new species of the genus Munidopsis Whiteaves, 1874 (Crustacea: Anomura: Munidopsidae) from the Caroline Ridge, South of the Mariana Trench.Crossref | GoogleScholarGoogle Scholar |
Dong, D, Xu, P, Li, XZ, and Wang, C (2019). Munidopsis species (Crustacea: Decapoda: Munidopsidae) from carcass falls in Weijia Guyot, West Pacific, with recognition of a new species based on integrative taxonomy. PeerJ 7, e8089.
| Munidopsis species (Crustacea: Decapoda: Munidopsidae) from carcass falls in Weijia Guyot, West Pacific, with recognition of a new species based on integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Dong, D, Gan, Z, and Li, X (2021). Descriptions of eleven new species of squat lobsters (Crustacea: Anomura) from seamounts around the Yap and Mariana Trenches with notes on DNA barcodes and phylogeny. Zoological Journal of the Linnean Society 192, 306–355.
| Descriptions of eleven new species of squat lobsters (Crustacea: Anomura) from seamounts around the Yap and Mariana Trenches with notes on DNA barcodes and phylogeny.Crossref | GoogleScholarGoogle Scholar |
Drummond, AJ, and Rambaut, A (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| BEAST: Bayesian evolutionary analysis by sampling trees.Crossref | GoogleScholarGoogle Scholar |
Etter, RJ, Rex, MA, Chase, MR, and Quattro, JM (2005). Population differentiation decreases with depth in deep‐sea bivalves. Evolution 59, 1479–1491.
| Population differentiation decreases with depth in deep‐sea bivalves.Crossref | GoogleScholarGoogle Scholar |
Etter, RJ, Boyle, EE, Glazier, A, Jennings, RM, Dutra, E, and Chase, MR (2011). Phylogeography of a pan‐Atlantic abyssal protobranch bivalve: implications for evolution in the deep Atlantic. Molecular Ecology 20, 829–843.
| Phylogeography of a pan‐Atlantic abyssal protobranch bivalve: implications for evolution in the deep Atlantic.Crossref | GoogleScholarGoogle Scholar |
Faxon, W (1895). XV. The stalk-eyed Crustacea. In ‘Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer “Albatross” during 1891, Lieut.-Commander Z.L. Tanner, U.S.N., commanding’. Memoirs of the Museum of Comparative Zoology at Harvard College 18, 1–230.
Folmer, O, Black, M, Hoeh, W, Lutz, R, and Vrijenhoek, R (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
Fujisawa, T, and Barraclough, TG (2013). Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Systematic Biology 62, 707–724.
| Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets.Crossref | GoogleScholarGoogle Scholar |
Gaither, MR, Gkafas, GA, de Jong, M, Sarigol, F, Neat, F, Regnier, T, Moore, D, Grӧcke, DR, Hall, N, Liu, X, Kenny, J, Lucaci, A, Hughes, M, Haldenby, S, and Hoelzel, AR (2018). Genomics of habitat choice and adaptive evolution in a deep-sea fish. Nature Ecology & Evolution 2, 680–687.
| Genomics of habitat choice and adaptive evolution in a deep-sea fish.Crossref | GoogleScholarGoogle Scholar |
García Raso, JE, García Muñoz, JE, and Manjón-Cabeza, ME (2008). First record of Munidopsis albatrossae (Crustacea: Decapoda: Galatheidae) from Antarctic waters. Polar Biology 31, 1281–1285.
| First record of Munidopsis albatrossae (Crustacea: Decapoda: Galatheidae) from Antarctic waters.Crossref | GoogleScholarGoogle Scholar |
Goffredi, SK, Jones, WJ, Erhlich, H, Springer, A, and Vrijenhoek, RC (2008). Epibiotic bacteria associated with the recently discovered Yeti crab, Kiwa hirsuta. Environmental Microbiology 10, 2623–2634.
| Epibiotic bacteria associated with the recently discovered Yeti crab, Kiwa hirsuta.Crossref | GoogleScholarGoogle Scholar |
Guzman, GL, and Sellanes, J (2015). A review of the Munidopsidae Ortmann, 1898 (Decapoda, Galatheoidea) in Chilean waters, including new records for the Southeastern Pacific. Zootaxa 4021, 282–306.
| A review of the Munidopsidae Ortmann, 1898 (Decapoda, Galatheoidea) in Chilean waters, including new records for the Southeastern Pacific.Crossref | GoogleScholarGoogle Scholar |
Hansen HJ (1908) Crustacea Malacostraca. I. In ‘The Danish-Ingolf Expedition. Volume III’. (H. Hagerup: Copenhagen, Denmark)
Hatch, AS, Liew, H, Hourdez, S, and Rouse, GW (2020). Hungry scale worms: phylogenetics of Peinaleopolynoe (Polynoidae, Annelida), with four new species. ZooKeys 932, 27–74.
| Hungry scale worms: phylogenetics of Peinaleopolynoe (Polynoidae, Annelida), with four new species.Crossref | GoogleScholarGoogle Scholar |
Henderson, JR (1885). XXXIX. – Diagnoses of the new species of Galatheidea collected during the ‘Challenger’ expedition. Annals and Magazine of Natural History 16, 407–421.
| XXXIX. – Diagnoses of the new species of Galatheidea collected during the ‘Challenger’ expedition.Crossref | GoogleScholarGoogle Scholar |
Hendrickx, ME (2021). Squat lobsters (Crustacea: Decapoda: Anomura: Galatheoidea) from off the northwestern coast of the Baja California Peninsula, Mexico. Zootaxa 4965, 375–384.
| Squat lobsters (Crustacea: Decapoda: Anomura: Galatheoidea) from off the northwestern coast of the Baja California Peninsula, Mexico.Crossref | GoogleScholarGoogle Scholar |
Hendrickx, ME, and Ayón-Parente, M (2013). A new species of Munidopsis (Anomura, Galatheoidea, Munidopsidae) from the Gulf of California, Western Mexico. Crustaceana 86, 1304–1315.
| A new species of Munidopsis (Anomura, Galatheoidea, Munidopsidae) from the Gulf of California, Western Mexico.Crossref | GoogleScholarGoogle Scholar |
Hendrickx, ME, Ayón-Parente, M, and Serrano, D (2011). Registro adicional de Janetogalathea californiensis (Anomura: Galatheidae) del Golfo de California central, México, con notas de su distribución. Hidrobiológica 21, 89–94.
Hudson, RR (1991). Gene genealogies and the coalescent process. Oxford Surveys in Evolutionary Biology 7, 1–44.
Huelsenbeck, JP, and Ronquist, F (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755.
| MRBAYES: Bayesian inference of phylogenetic trees.Crossref | GoogleScholarGoogle Scholar |
Hwang, H, Cho, B, Cho, J, Park, B, and Kim, T (2022). New record of hydrothermal vent squat lobster (Munidopsis lauensis) provides evidence of a dispersal corridor between the Pacific and Indian oceans. Journal of Marine Science and Engineering 10, 400.
| New record of hydrothermal vent squat lobster (Munidopsis lauensis) provides evidence of a dispersal corridor between the Pacific and Indian oceans.Crossref | GoogleScholarGoogle Scholar |
Jones, WJ, and Macpherson, E (2007). Molecular phylogeny of the East Pacific squat lobsters of the genus Munidopsis (Decapoda: Galatheidae) with the descriptions of seven new species. Journal of Crustacean Biology 27, 477–501.
| Molecular phylogeny of the East Pacific squat lobsters of the genus Munidopsis (Decapoda: Galatheidae) with the descriptions of seven new species.Crossref | GoogleScholarGoogle Scholar |
Kahle, D, and Wickham, H (2013). ggmap: spatial visualization with ggplot2. The R Journal 5, 144–161.
| ggmap: spatial visualization with ggplot2.Crossref | GoogleScholarGoogle Scholar |
Katoh, K, Misawa, K, Kuma, K-i, and Miyata, T (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066.
| MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Crossref | GoogleScholarGoogle Scholar |
Larsson, A (2014). AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30, 3276–3278.
| AliView: a fast and lightweight alignment viewer and editor for large datasets.Crossref | GoogleScholarGoogle Scholar |
Lessios, HA, and Robertson, DR (2006). Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proceedings of the Royal Society of London – B. Biological Sciences 273, 2201–2208.
| Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier.Crossref | GoogleScholarGoogle Scholar |
Luke, SR (1977). Catalog of the benthic invertebrate collections of the Scripps Institution of Oceanography. I – Decapod Crustacea and Stomatopoda. Scripps Institution of Oceanography Reference Series 77-9, 1–72.
McClain, CR, and Hardy, SM (2010). The dynamics of biogeographic ranges in the deep sea. Proceedings of the Royal Society of London – B. Biological Sciences 277, 3533–3546.
| The dynamics of biogeographic ranges in the deep sea.Crossref | GoogleScholarGoogle Scholar |
McCowin, MF, Feehery, C, and Rouse, GW (2020). Spanning the depths or depth-restricted: three new species of Bathymodiolus (Bivalvia, Mytilidae) and a new record for the hydrothermal vent Bathymodiolus thermophilus at methane seeps along the Costa Rica margin. Deep-Sea Research – I. Oceanographic Research Papers 164, 103322.
| Spanning the depths or depth-restricted: three new species of Bathymodiolus (Bivalvia, Mytilidae) and a new record for the hydrothermal vent Bathymodiolus thermophilus at methane seeps along the Costa Rica margin.Crossref | GoogleScholarGoogle Scholar |
Machordom, A, and Macpherson, E (2004). Rapid radiation and cryptic speciation in squat lobsters of the genus Munida (Crustacea, Decapoda) and related genera in the South West Pacific: molecular and morphological evidence Molecular Phylogenetics and Evolution 33, 259–279.
| Rapid radiation and cryptic speciation in squat lobsters of the genus Munida (Crustacea, Decapoda) and related genera in the South West Pacific: molecular and morphological evidenceCrossref | GoogleScholarGoogle Scholar |
Macpherson, E (2007). Species of the genus Munidopsis Whiteaves, 1784 from the Indian and Pacific Oceans and reestablishment of the genus Galacantha A. Milne-Edwards, 1880 (Crustacea, Decapoda, Galatheidae). Zootaxa 1417, 1–135.
| Species of the genus Munidopsis Whiteaves, 1784 from the Indian and Pacific Oceans and reestablishment of the genus Galacantha A. Milne-Edwards, 1880 (Crustacea, Decapoda, Galatheidae).Crossref | GoogleScholarGoogle Scholar |
Macpherson E, Baba K (2011) Chapter 2. Taxonomy of squat lobsters. In ‘The Biology of Squat Lobsters’. (Eds GCB Poore, ST Ahyong, J Taylor) pp. 39–71. (CSIRO Publishing: Melbourne, Vic., Australia)
Macpherson, E, and Segonzac, M (2005). Species of the genus Munidopsis (Crustacea, Decapoda, Galatheidae) from the deep Atlantic Ocean, including cold-seep and hydrothermal vent areas. Zootaxa. 1095, 1–60.
| Species of the genus Munidopsis (Crustacea, Decapoda, Galatheidae) from the deep Atlantic Ocean, including cold-seep and hydrothermal vent areas.Crossref | GoogleScholarGoogle Scholar |
Macpherson, E, Richer de Forges, B, Schnabel, K, Samadi, S, Boisselier, MC, and Garcia-Rubies, A (2010). Biogeography of the deep-sea galatheid squat lobsters of the Pacific Ocean. Deep-Sea Research – I. Oceanographic Research Papers 57, 228–238.
| Biogeography of the deep-sea galatheid squat lobsters of the Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Macpherson, E, Beuck, L, and Freiwald, A (2016). Some species of Munidopsis from the Gulf of Mexico, Florida Straits and Caribbean Sea (Decapoda: Munidopsidae), with the description of two new species. Zootaxa 4137, 405–416.
| Some species of Munidopsis from the Gulf of Mexico, Florida Straits and Caribbean Sea (Decapoda: Munidopsidae), with the description of two new species.Crossref | GoogleScholarGoogle Scholar |
Marin, I (2020). Northern unicorns of the depths: diversity of the genus Munidopsis Whiteaves, 1874 (Decapoda: Anomura: Munidopsidae) in the northwestern Pacific Ocean, with descriptions of three new species along the Russian coast. Progress in Oceanography 183, 102263.
| Northern unicorns of the depths: diversity of the genus Munidopsis Whiteaves, 1874 (Decapoda: Anomura: Munidopsidae) in the northwestern Pacific Ocean, with descriptions of three new species along the Russian coast.Crossref | GoogleScholarGoogle Scholar |
Marlow, JJ, Anderson, RE, Reysenbach, AL, Seewald, JS, Shank, TM, Teske, AP, Wanless, VD, and Soule, SA (2022). New opportunities and untapped scientific potential in the abyssal ocean. Frontiers in Marine Science 8, 798943.
| New opportunities and untapped scientific potential in the abyssal ocean.Crossref | GoogleScholarGoogle Scholar |
Miljutin, DM, Gad, G, Miljutina, MM, Mokievsky, VO, Fonseca-Genevois, V, and Esteves, AM (2010). The state of knowledge on deep-sea nematode taxonomy: how many valid species are known down there? Marine Biodiversity 40, 143–159.
| The state of knowledge on deep-sea nematode taxonomy: how many valid species are known down there?Crossref | GoogleScholarGoogle Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA, USA. INSPEC Accession Number 11705685. (IEEE)
| Crossref |
Miranda I, Peres PA, Tavares MDS, Mantelatto FL (2020) New molecular data on squat lobster from the coast of São Paulo State (Brazil) (Anomura: Munida and Agononida) and insights on the systematics of the family Munididae. In ‘Deep-Sea Pycnogonids and Crustaceans of the Americas’. (Ed. ME Hendrickx) pp. 343–356. (Springer: Cham, Switzerland)
O’Hara, TD, Williams, A, Ahyong, ST, Alderslade, P, Alvestad, T, Bray, D, Burghardt, I, Budaeva, N, Criscione, F, Crowther, AL, Ekins, M, Eléaume, M, Farrelly, CA, Finn, JK, Georgieva, MN, Graham, A, Gomon, M, Gowlett-Holmes, K, Gunton, LM, Hallan, A, Hosie, AM, Hutchings, P, Kise, H, Köhler, F, Konsgrud, JA, Kupriyanova, E, Lu, CC, Mackenzie, M, Mah, C, MacIntosh, H, Merrin, KL, Miskelly, A, Mitchell, ML, Moore, K, Murray, A, O’Loughlin, PM, Paxton, H, Pogonoski, JJ, Staples, D, Watson, JE, Wilson, RS, Zhang, J, and Bax, NJ (2020). The lower bathyal and abyssal seafloor fauna of eastern Australia. Marine Biodiversity Records 13, 11.
| The lower bathyal and abyssal seafloor fauna of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Oliver, PG (2015). Deep-water Thyasiridae (Mollusca: Bivalvia) from the Oman Margin, Arabian Sea, new species and examples of endemism and cosmopolitanism. Zootaxa 3995, 252–263.
| Deep-water Thyasiridae (Mollusca: Bivalvia) from the Oman Margin, Arabian Sea, new species and examples of endemism and cosmopolitanism.Crossref | GoogleScholarGoogle Scholar |
Palero F (2008) Genètica evolutiva en llagostes de l’infraordre Achelata. PhD thesis, Universitat de Barcelona, Barcelona, Spain.
Palero, F, Rodríguez-Flores, PC, Cabezas, P, Machordom, A, Macpherson, E, and Corbari, L (2019). Evolution of squat lobsters (Crustacea, Galatheoidea): mitogenomic data suggest an early divergent Porcellanidae. Hydrobiologia 833, 173–184.
| Evolution of squat lobsters (Crustacea, Galatheoidea): mitogenomic data suggest an early divergent Porcellanidae.Crossref | GoogleScholarGoogle Scholar |
Palumbi S (1991) ‘Simple fool’s guide to PCR.’ (Department of Zoology and Kewalo Marine Laboratory, University of Hawaii: Hawaii, HI, USA)
Paradis, E (2010). pegas: an R package for population genetics with an integrated–modular approach. Bioinformatics 26, 419–420.
| pegas: an R package for population genetics with an integrated–modular approach.Crossref | GoogleScholarGoogle Scholar |
Prada, C, and Hellberg, ME (2021). Speciation‐by‐depth on coral reefs: Sympatric divergence with gene flow or cryptic transient isolation? Journal of Evolutionary Biology 34, 128–137.
| Speciation‐by‐depth on coral reefs: Sympatric divergence with gene flow or cryptic transient isolation?Crossref | GoogleScholarGoogle Scholar |
Puillandre, N, Lambert, A, Brouillet, S, and Achaz, G (2012). ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21, 1864–1877.
| ABGD, Automatic Barcode Gap Discovery for primary species delimitation.Crossref | GoogleScholarGoogle Scholar |
Puillandre, N, Brouillet, S, and Achaz, G (2021). ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21, 609–620.
| ASAP: assemble species by automatic partitioning.Crossref | GoogleScholarGoogle Scholar |
Rannala B, Yang Z (2020) Species delimitation. In ‘Phylogenetics in the Genomic Era’. (Eds C Scornavacca, F Delsuc, N Galtier) pp. 5.5:1–5.5:18. (Celine Scornavacca, Frédéric Delsuc and Nicolas Galtier) Available at https://hal.inria.fr/PGE/
Raupach, MJ, Malyutina, M, Brandt, A, and Wägele, JW (2007). Molecular data reveal a highly diverse species flock within the munnopsoid deep-sea isopod Betamorpha fusiformis (Barnard, 1920) (Crustacea: Isopoda: Asellota) in the Southern Ocean. Deep-Sea Research – II. Topical Studies in Oceanography 54, 1820–1830.
| Molecular data reveal a highly diverse species flock within the munnopsoid deep-sea isopod Betamorpha fusiformis (Barnard, 1920) (Crustacea: Isopoda: Asellota) in the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Reid, NM, and Carstens, BC (2012). Phylogenetic estimation error can decrease the accuracy of species delimitation: a Bayesian implementation of the general mixed Yule-coalescent model BMC Evolutionary Biology 12, 196.
| Phylogenetic estimation error can decrease the accuracy of species delimitation: a Bayesian implementation of the general mixed Yule-coalescent modelCrossref | GoogleScholarGoogle Scholar |
Rodríguez Flores PC (2021) Biodiversidad, biogeografía y patrones evolutivos en crustáceos (Anomura, Galatheoidea) de zonas tropicales y templadas. PhD thesis, Universitat de Barcelona, Barcelona, Spain.
Rodríguez-Flores, PC, Macpherson, E, and Machordom, A (2018). Three new species of squat lobsters of the genus Munidopsis Whiteaves, 1874, from Guadeloupe Island, Caribbean Sea (Crustacea, Decapoda, Munidopsidae). Zootaxa 4422, 569–580.
| Three new species of squat lobsters of the genus Munidopsis Whiteaves, 1874, from Guadeloupe Island, Caribbean Sea (Crustacea, Decapoda, Munidopsidae).Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Flores, PC, Machordom, A, Abelló, P, Cuesta, JA, and Macpherson, E (2019a). Species delimitation and multi-locus species tree solve an old taxonomic problem for European squat lobsters of the genus Munida Leach, 1820. Marine Biodiversity 49, 1751–1773.
| Species delimitation and multi-locus species tree solve an old taxonomic problem for European squat lobsters of the genus Munida Leach, 1820.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Flores, PC, Macpherson, E, and Machordom, A (2019b). Revision of the squat lobsters of the genus Leiogalathea Baba, 1969 (Crustacea, Decapoda, Munidopsidae) with the description of 15 new species. Zootaxa 4560, 201–256.
| Revision of the squat lobsters of the genus Leiogalathea Baba, 1969 (Crustacea, Decapoda, Munidopsidae) with the description of 15 new species.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Flores, PC, Macpherson, E, and Machordom, A (2021). Revision of the squat lobsters of the genus Phylladiorhynchus Baba, 1969 (Crustacea, Decapoda, Galatheidae) with the description of 41 new species. Zootaxa 5008, 1–159.
| Revision of the squat lobsters of the genus Phylladiorhynchus Baba, 1969 (Crustacea, Decapoda, Galatheidae) with the description of 41 new species.Crossref | GoogleScholarGoogle Scholar |
Rodríguez‐Flores, PC, Buckley, D, Macpherson, E, Corbari, L, and Machordom, A (2020). Deep‐sea squat lobster biogeography (Munidopsidae: Leiogalathea ) unveils Tethyan vicariance and evolutionary patterns shared by shallow‐water relatives Zoologica Scripta 49, 340–356.
| Deep‐sea squat lobster biogeography (Munidopsidae: Leiogalathea ) unveils Tethyan vicariance and evolutionary patterns shared by shallow‐water relativesCrossref | GoogleScholarGoogle Scholar |
Rodríguez-Flores, PC, Macpherson, E, and Machordom, A (2022a). New species of deep-sea squat lobsters (Decapoda: Anomura: Galatheoidea) from Guadeloupe, French West Indies, unveiled through integrative taxonomy. Journal of Crustacean Biology 42, ruab070.
| New species of deep-sea squat lobsters (Decapoda: Anomura: Galatheoidea) from Guadeloupe, French West Indies, unveiled through integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Flores, PC, Macpherson, E, Schnabel, K, Ahyong, ST, Corbari, L, and Machordom, A (2022b). Depth as a driver of evolution and diversification of ancient squat lobsters (Decapoda, Galatheoidea, Phylladiorhynchus). Molecular Phylogenetics and Evolution 171, 107467.
| Depth as a driver of evolution and diversification of ancient squat lobsters (Decapoda, Galatheoidea, Phylladiorhynchus).Crossref | GoogleScholarGoogle Scholar |
Roterman, CN, Lee, WK, Liu, X, Lin, R, Li, X, and Won, YJ (2018). A new yeti crab phylogeny: vent origins with indications of regional extinction in the East Pacific. PloS ONE 13, e0194696.
| A new yeti crab phylogeny: vent origins with indications of regional extinction in the East Pacific.Crossref | GoogleScholarGoogle Scholar |
Salinas-de-León, P, Martí-Puig, P, Buglass, S, Arnés-Urgellés, C, Rastoin-Laplane, E, Creemers, M, Cairns, S, Fisher, C, O’Hara, T, Ott, B, Raineault, NA, Reiswig, H, Rouse, G, Rowley, S, Shank, TM, Suarez, J, Watling, L, Wicksten, MK, and Marsh, L (2020). Characterization of deep-sea benthic invertebrate megafauna of the Galapagos Islands. Scientific Reports 10, 13894.
| Characterization of deep-sea benthic invertebrate megafauna of the Galapagos Islands.Crossref | GoogleScholarGoogle Scholar |
Schmitt, WL (1921). The marine decapod Crustacea of California with special reference to the decapod Crustacea collected by the United States Bureau of Fisheries Steamer Albatross in connection with the biological survey of San Francisco Bay during the years 1912–1913. University of California Publications in Zoology 23, 1–470.
Schnabel KE, Cabezas P, McCallum A, Macpherson E, Ahyong ST, Baba K, Poore GCB (2011a) Worldwide distribution patterns of squat lobsters. In ‘The Biology of Squat Lobsters’. (Eds GCB Poore, ST Ahyong, J Taylor) pp. 149–182. (CSIRO Publishing: Melbourne, Vic., Australia)
Schnabel, KE, Ahyong, ST, and Maas, EW (2011b). Galatheoidea are not monophyletic – molecular and morphological phylogeny of the squat lobsters (Decapoda: Anomura) with recognition of a new superfamily. Molecular Phylogenetics and Evolution 58, 157–168.
| Galatheoidea are not monophyletic – molecular and morphological phylogeny of the squat lobsters (Decapoda: Anomura) with recognition of a new superfamily.Crossref | GoogleScholarGoogle Scholar |
Schornikov, EI (2005). The question of cosmopolitanism in the deep-sea ostracod fauna: the example of the genus Pedicythere. Hydrobiologia 538, 193–215.
| The question of cosmopolitanism in the deep-sea ostracod fauna: the example of the genus Pedicythere.Crossref | GoogleScholarGoogle Scholar |
Smith, SI (1885). On some new or little known decapod Crustacea, from recent Fish Commission dredgings off the east coast of the United States. Proceedings of the United States National Museum 7, 493–511.
| On some new or little known decapod Crustacea, from recent Fish Commission dredgings off the east coast of the United States.Crossref | GoogleScholarGoogle Scholar |
South, A, and South, MA (2016). Package ‘rworldmap’. Mapping global data. The R Journal 3, 35–43.
Sukumaran, J, and Knowles, LL (2017). Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences 114, 1607–1612.
| Multispecies coalescent delimits structure, not species.Crossref | GoogleScholarGoogle Scholar |
Swofford DL (2003) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. (Sinauer Associates: Sunderland, MA, USA) Available at https://paup.phylosolutions.com/
Tan, MH, Gan, HM, Lee, YP, Linton, S, Grandjean, F, Bartholomei-Santos, ML, Miller, AD, and Austin, CM (2018). ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Molecular Phylogenetics and Evolution 127, 320–331.
| ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements.Crossref | GoogleScholarGoogle Scholar |
Thomas, EA, Liu, R, Amon, D, Copley, JT, Glover, AG, Helyar, SJ, Olu, K, Wiklund, H, Zhang, H, and Sigwart, JD (2020). Chiridota heheva – the cosmopolitan holothurian. Marine Biodiversity 50, 110.
| Chiridota heheva – the cosmopolitan holothurian.Crossref | GoogleScholarGoogle Scholar |
Tilesius, G (1815). De cancris Camtschaticis, oniscis, entomostracis et cancellis marinis microscopicis noctilucentibus, Cum tabulis IV. Aeneis et appendice adnexo de acaris et ricinis Camtschaticis. Mémoires de l’Académie Impériale des Sciences de St Pétersbourg 5, 331–405.
Trifinopoulos, J, Nguyen, LT, von Haeseler, A, and Minh, BQ (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44, W232–W235.
| W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis.Crossref | GoogleScholarGoogle Scholar |
Van Dover, CL, Factor, JR, Williams, AB, and Berg, CJ (1985). Reproductive patterns of decapod crustaceans from hydrothermal vents. Bulletin of the Biological Society of Washington 6, 223–227.
Wenner, EL (1982). Notes on the distribution and biology of Galatheidae and Chirostylidae (Decapoda: Anomura) from the Middle Atlantic Bight. Journal of Crustacean Biology 2, 360–377.
| Notes on the distribution and biology of Galatheidae and Chirostylidae (Decapoda: Anomura) from the Middle Atlantic Bight.Crossref | GoogleScholarGoogle Scholar |
Whitfield, JB, and Lockhart, PJ (2007). Deciphering ancient rapid radiations. Trends in Ecology & Evolution 22, 258–265.
| Deciphering ancient rapid radiations.Crossref | GoogleScholarGoogle Scholar |
Wicksten, MK (2012). Decapod Crustacea of the Californian and Oregonian zoogeographic provinces. Zootaxa 3371, 1–307.
Williams, AB, and Baba, K (1989). New squat lobsters (Galatheidae) from the Pacific Ocean: Mariana Back Arc Basin, East Pacific Rise, and Cascadia Basin. Fishery Bulletin 87, 899–910.
Williams, AB, and Van Dover, CL (1983). A new species of Munidopsis from submarine thermal vents of the east Pacific Rise at 21°N (Anomura: Galatheidae). Proceedings of the Biological Society of Washington 96, 481–488.
Yule, GU (1925). II. – A mathematical theory of evolution, based on the conclusions of Dr J. C. Willis, F. R. S. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 213, 21–87.
| II. – A mathematical theory of evolution, based on the conclusions of Dr J. C. Willis, F. R. S.Crossref | GoogleScholarGoogle Scholar |
Zardus, JD, Etter, RJ, Chase, MR, Rex, MA, and Boyle, EE (2006). Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939). Molecular Ecology 15, 639–651.
| Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939).Crossref | GoogleScholarGoogle Scholar |
Zeppilli, D, Vanreusel, A, and Danovaro, R (2011). Cosmopolitanism and biogeography of the genus Manganonema (Nematoda: Monhysterida) in the deep sea. Animals 1, 291–305.
| Cosmopolitanism and biogeography of the genus Manganonema (Nematoda: Monhysterida) in the deep sea.Crossref | GoogleScholarGoogle Scholar |
Zhang, J, Kapli, P, Pavlidis, P, and Stamatakis, A (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876.
| A general species delimitation method with applications to phylogenetic placements.Crossref | GoogleScholarGoogle Scholar |