The tables have turned: taxonomy, systematics and biogeography of the Acropora hyacinthus (Scleractinia: Acroporidae) complex
Sage H. Rassmussen A * , Peter F. Cowman B C D , Andrew H. Baird C , Augustine J. Crosbie B C , Andrea M. Quattrini E , Victor Bonito F , Frederic Sinniger G , Saki Harii G , Patrick C. Cabaitan H , Nur Fadli I , Chun-Hong Tan J , Julia Yun-Hsuan Hung
A * , Peter F. Cowman B C D , Andrew H. Baird C , Augustine J. Crosbie B C , Andrea M. Quattrini E , Victor Bonito F , Frederic Sinniger G , Saki Harii G , Patrick C. Cabaitan H , Nur Fadli I , Chun-Hong Tan J , Julia Yun-Hsuan Hung  C , Teina Rongo K , Danwei Huang L , Tuikolongahau Halafihi M and Tom C. L. Bridge B C *
C , Teina Rongo K , Danwei Huang L , Tuikolongahau Halafihi M and Tom C. L. Bridge B C *
A
B
C
D
E
F
G
H
I
J
K
L
M
Handling Editor: Allen Collins
Abstract
Genomic data have revealed that traditional coral taxonomy based on skeletal morphology does not accurately reflect the true diversity of, or systematic relationships within, the order Scleractinia. Here, we apply an integrated taxonomic approach combining molecular analysis and morphological comparison of type material with specimens collected from across the Indo-Pacific to revise the taxonomy of a clade within the species-rich and ecologically dominant reef coral genus Acropora, which includes the species Acropora hyacinthus (Dana, 1846) and related species (termed the ‘hyacinthus species complex’). Using a collection of specimens comprising preserved tissues, field images and skeletal vouchers collected from 22 regions spanning the Indian and Pacific Oceans, we generated a phylogenomic reconstruction using targeted capture of ultraconserved elements (UCEs) and exons, combined with examination of morphological characters, to generate primary species hypotheses (PSHs) for the clade. We then tested PSHs by calling Single Nucleotide Polymorphism (SNPs) from the genomic dataset to provide additional lines of evidence to support the delineation of species within the clade and revise the taxonomy of the group. Our integrated approach recovered 16 lineages sufficiently delineated to be designated as distinct species. Based on comparison of our specimens to type material and geographical distributions, we remove nine species from synonymy: A. turbinata (Verrrill, 1864), A. surculosa (Dana, 1846), A. patella (Studer, 1878), A. flabelliformis (Milne-Edwards, 1860), A. conferta (Quelch, 1886), A pectinata (Brook, 1892), A. recumbens (Brook, 1892), A. sinensis (Brook, 1893) and A. bifurcata Nemenzo, 1971. We also describe five new species: A. harriottae sp. nov. from south-eastern Australia, A. tersa sp. nov. from eastern Australia and the Western Pacific, A. nyinggulu sp. nov. from the eastern Indian Ocean, Indo-Australian Archipelago and southern Japan, A. uogi sp. nov. from the western Pacific and A. kalindae sp. nov. from north-eastern Australia. Our data reveal that the species richness within this clade of Acropora is far greater than currently assumed due to both overlooked provincialism across the Indo-Pacific as well as lumping of distinct sympatric species based on superficial morphological similarity. Given the key ecological role tabular Acropora play on Indo-Pacific reefs our findings have significant implications for reef conservation and management, for example, A. harriottae sp. nov. is restricted to a small geographical region of south-eastern Australia and is therefore at comparatively high risk of extinction.
ZooBank: urn:lsid:zoobank.org:pub:6C42546C-9253-4639-9FF4-D8D80808D78C
Keywords: coral reef, Indo-Pacific, integrative taxonomy, phylogenomics, species delimitation, tabular growth form, target capture, taxonomic revision, ultraconserved elements.
Introduction
Species are the fundamental units of biological organisation, therefore the capacity to correctly identify species is critical for research, conservation and management of the natural world (Thomson et al. 2018). However, a significant portion of species on Earth are not yet formally described (Bickford et al. 2007; Mora et al. 2011; Appeltans et al. 2012). Furthermore, molecular phylogenomics is revealing that many ‘species’ delineated based on morphological characters are actually species complexes or even distantly related lineages that have evolved similar morphological characters independently (Fukami et al. 2004; Bickford et al. 2007; Jörger and Schrödl 2013). Consequently, many of the morphological characters traditionally used to delineate species and higher taxonomic groups (e.g. genera, families) are homoplasies, resulting in an inability to accurately identify independently evolving species based solely on morphological characters in many taxa (Appeltans et al. 2012; Adams et al. 2014).
In biodiversity hotspots such as coral reefs, as few as 9% of species have been described (Fisher et al. 2015). This is attributable to both a lack of taxonomic research in hyper-diverse invertebrate groups (Cardoso et al. 2011; Plaisance et al. 2011) and a high occurrence of putatively ‘cryptic’ species in marine ecosystems (Pante et al. 2015b; Pearman et al. 2016; Bongaerts et al. 2021). The inability to correctly identify taxa can have cascading effects through the science and management of reef organisms (Bortolus 2008). For example, incorrectly identifying several species as one can result in underestimates of diversity, overestimate of abundances and inaccurate conclusions regarding the conservation status, ecology and biology of reef organisms (Plaisance et al. 2011; Sheets et al. 2018; Gómez-Corrales and Prada 2020). Furthermore, potentially rare or endemic species will be overlooked (Bickford et al. 2007; Pante et al. 2015a; Cros et al. 2016; Sheets et al. 2018; Gómez-Corrales and Prada 2020), increasing the possibilities of threatened species going extinct without our knowledge (Pimm et al. 2014), and limiting our ability to assess the effects of biodiversity losses on the wider ecosystem (Hending 2024).
Reef-building corals of the genus Acropora Oken 1815 (Anthozoa: Hexacorallia: Scleractinia) are the most abundant and taxonomically diverse corals on most Indo-Pacific reefs (Wallace 1999; Veron et al. 2016). Species of Acropora exhibit a diverse range of morphologies that contribute to the structural complexity of coral reefs, which in turn enhances biodiversity (Hongo and Kayanne 2011; Graham and Nash 2013). Approximately 400 extant nominal species of Acropora have been described (Hoeksema and Cairns 2025), making this the most species-rich extant genus of reef corals. However, taxonomic revisions of the genus in the late 20th Century based solely on qualitative morphology (Veron and Wallace 1984; Wallace 1999) recognised only one-third or less of nominal species as valid: the most recent revision of the genus by Wallace (1999) recognised 109 valid species (excluding species of Isopora Studer, 1878, which was elevated from subgenus to genus rank by Wallace et al. 2007); Veron et al. (2016) recognised 163, and WoRMS currently lists 140 Acropora species as valid (Hoeksema and Cairns 2025). The high number of synonymies was based primarily on the poorly tested hypothesis that morphological variation within species was due to habitat-mediated plasticity rather than interspecific differences (Veron and Pichon 1976; Veron and Wallace 1984; Veron 1995; Wallace 1999). However, phylogenomic data are increasingly revealing that these taxonomic decisions based on a single line of evidence underestimate the true diversity of Acropora, and that many, if not most, synonymies are likely to be incorrect (Ramírez-Portilla et al. 2022; Bridge et al. 2024).
Acropora contains a greater variety of growth forms than any other coral genus (Wallace 1999). Species with a tabular or plating colony morphology are often abundant and ecologically significant components of shallow-water coral reefs across the Indo-Pacific (Hongo and Kayanne 2011; Nakabayashi et al. 2019; Ortiz et al. 2021). The fast growth rates of tabular Acropora species enable them to rapidly recover after disturbances, and with many reefs exposed to ever more frequent disturbances tabular Acropora are becoming increasingly dominant in many Indo-Pacific coral assemblages (Johns et al. 2014; Morais et al. 2024). Tabular Acropora also provide key ecosystem services including providing a canopy that acts as shelter for fish and other organisms at different lifecycle stages (Baird and Hughes 2000; Pratchett et al. 2008; Kerry and Bellwood 2015, 2016). Consequently, tabular Acropora are of increasing interest to reef managers (Ortiz et al. 2021) and are often the focus of reef restoration activities (Boström-Einarsson et al. 2020; Ortiz et al. 2021).
Of the 140 currently accepted Acropora species (Hoeksema and Cairns 2025), 20 have a predominantly tabular growth form. In the most common and widespread of these is A. hyacinthus (Dana, 1846), originally described from a single specimen collected in Fiji by James Dana on the United States Exploratory Expedition of 1838–1842. The species is currently considered to occur from the Pitcairn Island group in the central-south Pacific, across the Pacific Ocean as far north as Honshu in Japan and south to Lord Howe Island, and across the Indian Ocean as far north as the northern Red Sea and as far south as subtropical Western Australia and South Africa (Veron et al. 2016). However, molecular data suggest there are at least six distinct evolutionary lineages within specimens identified as A. hyacinthus in the central-western Pacific Ocean alone (Ladner and Palumbi 2012; Suzuki et al. 2016; Sheets et al. 2018; Nakabayashi et al. 2019). However, from a taxonomic perspective these lineages remain lumped together as ‘cryptic species’ within A. hyacinthus, and no effort has been made to look for morphological characters that could delineate these lineages or attempt to resolve their taxonomic identity by comparison to the type material of the numerous nominal species currently synonymised with A. hyacinthus.
The growing body of evidence indicating that the current taxonomy does not reflect the true species richness of tabular Acropora demonstrates the need for a formal taxonomic revision of the group is necessary. Although it is clear that the current concept of A. hyacinthus encompasses multiple evolutionary lineages, whether these lineages are indeed cryptic or if they have morphological differences that have been overlooked due to the assumption of extensive morphological plasticity remains unknown. Indeed, a recent taxonomic revision of the A. tenuis (Dana, 1846) complex, another putatively widespread Acropora species with extensive evidence of ‘cryptic’ diversity (Cooke et al. 2020; Rosser et al. 2020; Zayasu et al. 2021; Matias et al. 2023) revealed that the species comprised numerous distinct species each with a much smaller geographic range across the Indo-Pacific (Bridge et al. 2024).
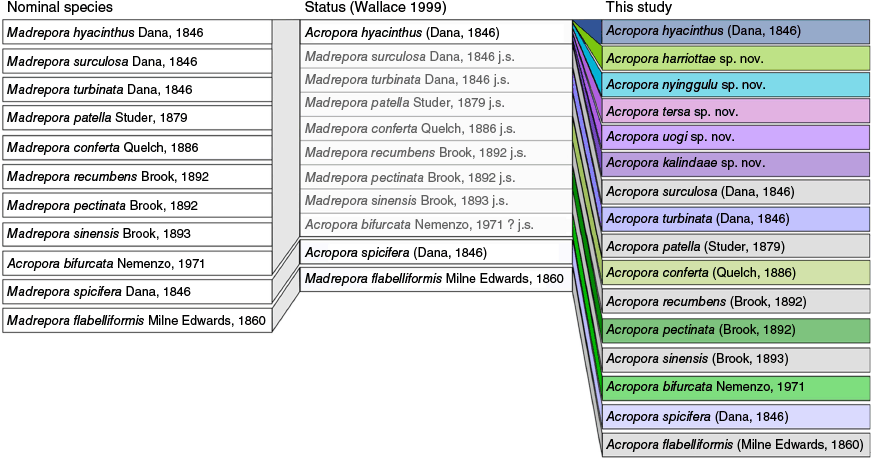
At least 38 nominal species of Acropora with a tabular growth form similar to that of A. hyacinthus have been described to date (Supplementary Table S1), mostly from the 19th Century (28 species) and all based solely on morphological features. Of these, 23 were synonymised in taxonomic revisions during the late 20th Century (Veron and Wallace 1984; Veron and Hodgson 1989; Wallace 1999, Supplementary Table S1). Owing to a lack of phylogenetically informative molecular markers in Acropora (see Cowman et al. 2020), these traditional taxonomic works still underpin most contemporary research on reef corals. Currently there are nine nominal species of Acropora that are considered subjective junior synonyms of A. hyacinthus (Fig. 1; Hoeksema and Cairns 2025).
Nomenclature history for tabular Acropora in the ‘hyacinthus complex’ and genetically related taxa as presented in the current study. First panel shows nominal species and authority. The middle panel shows each nominal species status according to the Wallace (1999) revision where many nominal taxa were synonymised with Acropora hyacinthus, with synonymised or unresolved taxa shaded in grey. The last panel shows the status of each nominal species resulting from this revision, including new species described. Colours in the final panel represent species investigated in the current study, and species in grey reflect species that were not sequenced in the current study, although taxonomic decisions were made because of this revision. Throughout, j.s. indicates species that are considered a junior synonym of A. hyacinthus, and question marks indicate that the species could not be adequately tested with the material available at the time.
Wallace (1999) also used morphological characters to examine the systematics and patterns of evolution of Acropora species through time based on transformation of skeletal characteristics and assigned most accepted species into ‘species groups’. These groups were originally based solely on morphological similarity (Wallace 1978; Veron and Wallace 1984) and were not intended to reflect evolutionary relationships. However, Wallace (1999) used a range of morphological characters to investigate phylogenetic relationships within Acropora and to develop hypotheses regarding the evolution of species and species groups based on transformation of skeletal characters. Most of the nominal species examined in the current paper, including A. hyacinthus, were included in the ‘hyacinthus group’, which also included A. microclados (Ehrenberg, 1834), A. cytherea (Dana, 1846), A. anthocercis (Brook, 1893), A. paniculata (Verrill, 1902), A. tanegashimensis Veron, 1990 and A. indonesia Wallace, 1997 by Wallace (1999). Few studies have since tested the validity of the evolutionary relationships inferred from the morphological analyses of Wallace (1999), and those that do indicate that at least some of these morphological groups, including the ‘hyacinthus group’, are not monophyletic (Cowman et al. 2020; Bridge et al. 2024).
Resolving the incongruence between morphological and molecular phylogenies and developing a robust, species-level taxonomy for Acropora has become time critical. Despite evidence that some tabular Acropora species are capable of rapid recovery relative to other coral taxa after disturbance (Morais et al. 2024), Acropora are generally considered highly susceptible to climate change, particularly the effects of mass bleaching events (Hughes et al. 2017, 2018). Throughout the Indo-Pacific, bleaching of any one single ‘species’ of tabulate Acropora from the 2016–17 events (e.g. A. hyacinthus; Hoogenboom et al. 2017; Hughes et al. 2017, 2018) was highly variable both within and between reefs. Although such patchiness might reflect spatial variability in environmental stress (Hoogenboom et al. 2017; Gardner et al. 2019), it is also highly possible that this conclusion is confounded by the poor capacity to resolve species within this tabulate complex (Gold and Palumbi 2018; Rose et al. 2021). Efforts to fast-track reef recovery through restoration currently focus on propagating ‘species’ of ‘A. hyacinthus’ (e.g. Morikawa and Palumbi 2019; Suggett et al. 2019), with the success dependent on confidently resolving how functional diversity is driven by species and within-species genotypic variation (Baums et al. 2019; Morikawa and Palumbi 2019). The ecological importance and increasing research interest in species within the ‘Acropora hyacinthus complex’ therefore provides further justification for a taxonomic revision of the group.
Here, we conduct a formal taxonomic revision of the A. hyacinthus complex, which occurs in Clade VI within the Acropora phylogeny sensu Cowman et al. (2020) using an integrated approach combining morphological and phylogenomic analysis. We collected 139 tabular Acropora specimens from across the Indo-Pacific and employed a targeted sequence approach to capture ~2500 UCE and exon loci (Cowman et al. 2020) to reconstruct a phylogeny for the group. We used the topology of the phylogeny to identify monophyletic lineages that could potentially represent distinct species, and then examined whether the morphological characters within and between each specimen supported the groupings indicated to identify primary species hypothesis (PSH). We then tested whether our PSHs were supported by additional lines of evidence by subjecting our PSHs to numerous species delimitation analyses based on Single Nucleotide Polymorphism (SNP) loci that were extracted from the target capture data, including discriminant analysis of principle components (DAPC), STRUCTURE, t-distributed stochastic neighbour embedding (t-SNE) and Bayes factor delimitation (BFD*) with SNAPP to provide the most robust possible conclusions regarding species delimitation. Finally, we applied names to the lineages identified by collating and examining the type material for all nominal Acropora species to assign nominal species names to resolved lineages where possible. Based on our analysis, we confirm the validity of 11 nominal species, 2 of which are currently accepted and 9 of which we remove from synonymy. We also describe five new species from the Indo-West Pacific.
Methods
Sampling
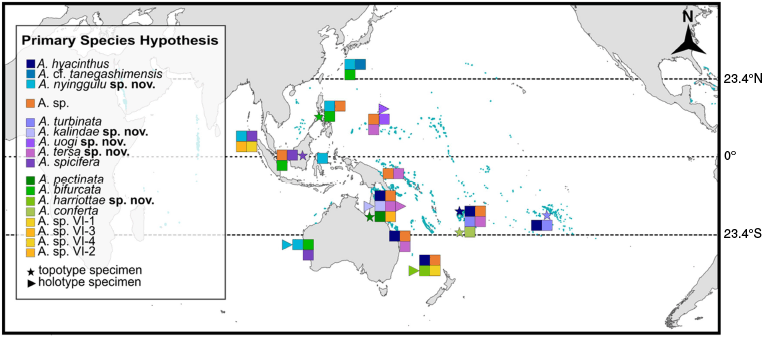
Sampling was conducted to obtain morphological and geographic representation for tabular Acropora with colonies (n = 139) sampled by SCUBA diving or snorkelling from 22 regions across the Indo-Pacific (Fig. 2; Supplementary Table S2). We defined regions as areas with arbitrary geographic boundaries containing coral reefs, of which one could reasonably make inferences regarding distribution of species. We sampled across a broad geographic range extending from the Society Islands, French Polynesia in the east to the Red Sea and western Indian Ocean in the west and aimed to collect the full range of morphological variability among tabular Acropora from each location to ensure sufficient genetic and morphological variation was captured for all known and unknown species to allow for a comprehensive taxonomic revision of this group. In the Indian Ocean, we conducted extensive sampling at Christmas Island and the Cocos (Keeling) Islands in the Australian Indian Ocean Territory, the Seychelles in the western Indian Ocean as well as the Red Sea, and smaller collections from other locations in the Western Indian Ocean; however, no specimens from the A. hyacinthus complex were recorded in these regions. Sampling efforts included searching for topotypes for all nominal species that shared morphological affinity with A. hyacinthus, in particular, the junior synonyms and other species listed as occurring in the hyacinthus species group of Wallace (1999). A topotype is defined as a specimen that closely resembles the type material and was collected as close as possible to the type locality. Character states were determined for each topotype, and these characters where then compared to the same character states for the name-bearing type for each nominal species (Supplementary Table S3 and ‘Acropora trait matrix’ section). We located topotypes for: A. bifurcata (Philippines), A. conferta (Fiji), A. hyacinthus (Fiji), A. pectinata (Great Barrier Reef), A. spicifera (Singapore) and A. turbinata (French Polynesia) (Fig. 2). Plates were prepared to illustrate the topotype with their respective type and to illustrate type material for all nominal species examined in this study (Supplementary Slides 1–32).
Map of geographic regions where samples were collected. Species listed are according to the Maximum Likelihood phylogeny Primary Species Hypothesis and coloured squares indicate species listed in the key. Stars indicate location of topotype material for nominal taxa, and triangles indicate collection location of holotype specimens for novel species described in the current study.
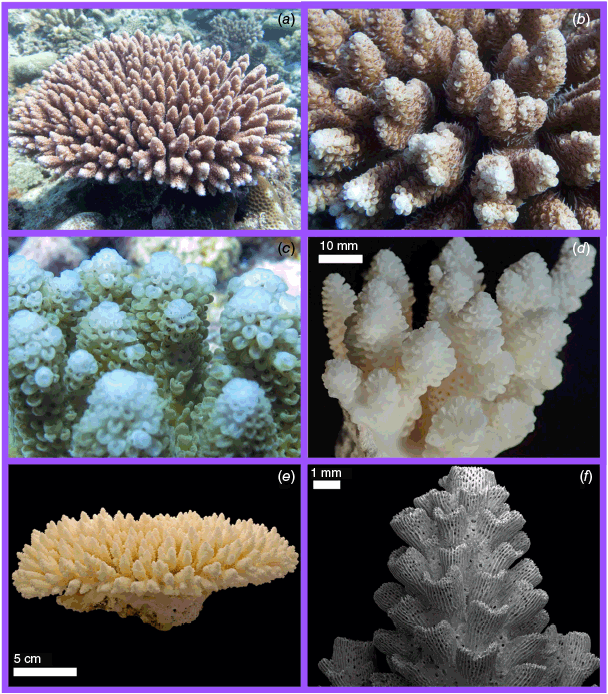
High-resolution photographs of each colony in situ, including both whole-colony and close-up images, were taken before a voucher specimen (~15–25-cm diameter) was collected using a hammer and chisel. From this fragment, a 1–2-cm subsample was preserved in 100% undenatured ethanol for molecular analysis. The remaining fragment was then bleached in sodium hypochlorite, rinsed in freshwater to remove tissue and then dried before imaging and morphological analysis.
DNA extractions and sequencing
To obtain a phylogeny in which we could characterise species-level relationships, we utilised target capture of UCE and exon regions of the genome (see Quattrini et al. 2018; Cowman et al. 2020). DNA was extracted from tissue samples according to a modified approach of the SDS-based method (Wilson et al. 2002) following Bridge et al. (2024). DNA quality was assessed using a Nanodrop spectrophotometer, with 260/280 ratios ranging from 1.8 to 2.1 and 260/230 ratios ranging from 1.4 to 3.2. A Qubit 2.0 fluorometer was also used to measure the DNA concentration of each sample. Samples that passed initial quality checks were sent to Arbor Biosciences (Ann Arbor, MI, USA) for further quality assessment, library preparation and sequencing following the methods outlined in Quattrini et al. (2018) and Bridge et al. (2024). A modified KAPA Hyper Prep Kit (Kapa Biosystems) protocol was used to process samples, outlined in Bridge et al. (2024). A hexacoral-specific bait set that was subset for 1132 UCE and 1365 exon loci in Scleractinia specifically [hexa-v2-scleractina] (Cowman et al. 2020), was used to target-enrich libraries in pools of either 8 or 12 samples, which were subsequently sequenced on a single lane of Illumina HiSeq. 3000 and generated 150-bp paired-end (PE) reads. As both UCE probes designed from genomic sources, and exons probes designed from transcriptomes provide similar phylogenetic resolution (Quattrini et al. 2018; Cowman et al. 2020) and many UCE loci have been shown to map to coding regions (Van Dam et al. 2021), we hereafter refer to the UCE and exon captures data exclusively as UCEs.
Sequence processing and alignments
Demultiplexed reads were processed according to the Phyluce pipeline (ver. 1.7.3, see https://github.com/faircloth-lab/phyluce; Faircloth 2016; see also https://phyluce.readthedocs.io/en/latest/tutorials/tutorial-1.html), following modifications outlined in Cowman et al. (2020) for trimming and assembling reads. Briefly, reads were cleaned using illumiprocessor (ver. 2.0.9, see https://github.com/faircloth-lab/illumiprocessor; Faircloth et al. 2012) for Trimmomatic (ver. 0.36, see http://www.usadellab.org/cms/index.php?page=trimmomatic; Bolger et al. 2014) and assembled with SPAdes (ver. 3.12, see https://github.com/ablab/spades; Bankevich et al. 2012). Assembled contig sequences were then matched to the hexacoral-v2-scleractina bait set at 70% minimum identity and 70% minimum coverage using ‘phyluce_assembly_match_contigs_to_probes’. Taxon specific loci were then extracted into FASTA files using ‘phyluce_assembly_get_match_counts’ and ‘phyluce_assembly_get_fastas_from_match_counts’. Loci were aligned with the standalone version of MAFFT (ver. 7.4.8, see https://mafft.cbrc.jp/alignment/software/; Katoh et al. 2002), and were both edge trimmed using ‘phyluce_align_get_trimmed_alignments_from_untrimmed’ and internally trimmed with ‘phyluce_align_get_gblocks_trimmed_alignments_from_untrimmed’ in Gblocks (ver. 0.91b, see http://phylogeny.lirmm.fr/phylo_cgi/one_task.cgi?task_type=gblocks; Castresana 2000). Both 50 and 75% matrices were generated for each alignment (edge and internally trimmed) using ‘phyluce_align_get_only_loci_with_min_taxa’.
Initially, samples from this study were processed alongside samples from Cowman et al. (2020) to obtain the phylogenetic position of tabulate Acropora – specifically the Acropora hyacinthus complex – within the six-clade structure previously outlined by Cowman et al. (2020). This allowed the use of five specimens of Acropora aff. downingi at the base of Clade VI as an appropriate outgroup for all our subsequent analyses that focused on the A. hyacinthus complex independently (Supplementary Table S2).
Phylogenomic reconstruction
The program IQ-TREE (ver. 2.1, see https://github.com/iqtree/iqtree2; Minh et al. 2020a) was used to perform a Maximum Likelihood (ML) analysis on each of the alignment matrices. A species tree was inferred with a partitioned analysis with ModelFinder (see http://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017) invoked to choose the best substitution model and partitioning scheme with the settings ‘-m TESTMERGE –merge-model GTR –merge-rate G –rcluster 10’. For each alignment we calculated ultrafast bootstrap (UFBoot) support approximation with 1000 replicates (Minh et al. 2013; Hoang et al. 2018), which provides a fast and effective measure of node support for large datasets. In general, UFBoot values >95% are considered strong clade support, whereas values between 90 and 95% are considered moderate support.
To assess topological concordance among gene trees and site patterns, we calculated gene concordance factors (gCF) and site concordance factors (sCF). Unlike parametric bootstrap measures, concordance factors are not measures of statistical support but can be viewed as descriptors of topological variation that relate to biological parameters (Lanfear and Hahn 2024). Gene concordance factor (gCF) is a measure of the percentage of gene trees that could have contained the branch of interest (known as decisive gene trees; see Minh et al. 2020a) that do contain that branch. It is calculated by comparing individual gene trees inferred from each UCE locus to the species tree inferred from the concatenated UCE alignment (Minh et al. 2020b). Site concordance factors (sCF) are based on quartet analysis. For each branch in the species tree, sCF measures the proportion of informative sites (those decisive under maximum parsimony) that support the corresponding unrooted quartet topology. Because there are three possible unrooted quartet topologies for any given set of four taxa, random chance alone would result in a sCF of ~33% (Minh et al. 2020b). As such, sCF values >34% sCF indicate some decisiveness for a branch arrangement, and higher values indicated a higher proportion of alignment sites that agree with that branch topology (Minh et al. 2020b). Unlike sCF, gCF values can range from 0 to 100%. A gene tree can disagree with the species tree if one or more of the subtending clades is not monophyletic, in which case gCF values can reach zero. This often occurs when gene trees are estimated from alignments with few informative sites, where there is insufficient phylogenetic signal to resolve relationships, or where topologies contain short branches that increase the likelihood of incomplete lineage sorting (Minh et al. 2020b). Concordance factors were calculated in IQ-TREE 2 following an online tutorial (see https://www.robertlanfear.com/blog/files/concordance_factors.html).
IQ-TREE 2 was then run on each locus to produce individual bootstrapped gene trees. The program newick_utils (ver. 1.6, see https://github.com/tjunier/newick_utils; Junier and Zdobnov 2010) was run on each treefile to collapse any branches with lower than 30% bootstrap support and TreeShrink (ver. 1.3.9, see https://github.com/uym2/TreeShrink; Mai and Mirarab 2018) was run to identify and remove samples reconstructed on long branches from associated alignments. The resulting ‘shrunk’ fasta alignments were then re-processed through IQ-TREE 2 and combined before processing through ASTRAL (ver. 5.7.1, see https://github.com/smirarab/ASTRAL; Zhang et al. 2018) to calculate local posterior probability (LPP) for the final species tree. The LPP is a probability measure that each branch is true based on the given set of gene trees.
SNP calling
Single nucleotide polymorphisms were extracted from the combined UCE datasets using a modified script from Erickson et al. (2021), which was adapted from previous taxonomic and population genetic studies (Zarza et al. 2016; Derkarabetian et al. 2019). Briefly, for each of the identified subclades the individual taxon with the highest number of recovered loci from ‘phyluce_assembly_get_match_counts’ was used as a reference for SNP calling within that clade. For each reference individual, a fasta of UCE contigs was created using ‘phyluce_assembly_get_match_counts’ and ‘phyluce_assembly_get_fastas_from_match_counts’. The reference fasta files were then indexed using BWA (ver. 0.7.17, see https://github.com/lh3/bwa; Li and Durbin 2009). BAM files were subsequently created by mapping individual reads to the reference individual using bwa-mem (ver. 0.7.17, see https://github.com/bwa-mem2/bwa-mem2; Li 2013). Reads were sorted with SAMtools (ver. 1.12, see https://github.com/samtools/samtools; Li et al. 2009), and duplicates removed using Picard (ver. 2.18.29, Broad Institute, see https://github.com/broadinstitute/picard). BAM files were then realigned with GATK (ver. 3.8, see https://gatk.broadinstitute.org/hc/en-us; McKenna et al. 2010) and filtered at >75% missing data using VCFtools (see https://github.com/vcftools/vcftools; Danecek et al. 2011). A STRUCTURE formatted file (.STR, see https://web.stanford.edu/group/pritchardlab/structure.html) was generated with the script ‘adegenet_from_vcf.py’ in seqcap_pop (see http://github.com/mgharvey/seqcap_pop; Harvey et al. 2016), selecting all SNPs for downstream analysis. Due to low capture of SNP genotypes, the STRUCTURE files were filtered using poppr (ver. 2.9.4, see https://cran.r-project.org/package=poppr; Kamvar et al. 2014) to remove loci with <80% complete genotypes and individual samples with >20% missing SNP data.
Species delimitation (STRUCTURE, DAPC, t-SNE, SNAPP)
All specimens were assigned to a Primary Species Hypothesis (PSH) (Puillandre et al. 2012), based on an initial species assignment according to the ML phylogeny (Supplementary Fig. S1) and an assessment of the original descriptions and type material for all the ~400 nominal species of Acropora. Following Cowman et al. (2020), a series of open nomenclature (ON) qualifiers were used to indicate the level of certainty in the given epithet. Topotypes were assigned to a nominal species with no qualifier. The qualifier cf. (conferret) was given to specimens that closely resemble the type but were not sampled from the type locality, suggesting that future work will likely confirm the identity of the species. The qualifier aff. (affinis) was given to specimens that had morphological affinities with the type of a nominal species, but future work will likely confirm is a different species. Additionally, lineages that were not similar to any of the type material were identified with the qualifier sp. followed by the Clade (I–IV) sensu Cowman et al. (2020) and either the voucher number if it was a single specimen (e.g. A. sp. VI-PN02) or a numerical identifier (e.g. A. sp. VI-1; Supplementary Fig. S1).
To identify optimal genetic clusters (K) within each subclade, the genetic clustering methods STRUCTURE and Discriminant Analysis of Principle Components (DAPC) were employed using the filtered SNP dataset. These analyses were conducted to assess contemporary population genetic structure and identify potentially independently evolving lineages within each subclade that could be traced back to known or unknown species (see Erickson et al. 2021; Ramírez-Portilla et al. 2022). STRUCTURE analysis was run on each subclade using StrAuto (ver. 1.0, see https://vc.popgen.org/software/strauto/; Chhatre and Emerson 2017) for 1 × 106 generations, 2.5 × 105 burn in and five replicates for each value K that has been shown to be ideal settings in similar datasets (Erickson et al. 2021), with the maximum K for each subclade chosen to be the number of PSH identified from the phylogenetic analysis plus one. Results were visualised using pophelper (ver. 1.0.10, see https://github.com/royfrancis/pophelper; Francis 2017) and optimal KSTRUCTURE determined based on Evanno calculations of ΔK and Mean L(K). DAPC analysis was performed in RStudio (ver. 2023.09.01, Posit Software, PBC, Boston, MA, USA, see https://posit.co/products/open-source/rstudio/) using the adegenet package (ver. 2.1.10, https://cran.r-project.org/package=adegenet; Jombart 2008) function ‘find.clusters’ initially run to determine the optimal KDAPC required to minimise the Bayesian Information Criterion (BIC) score.
To determine if clusters identified were truly indicative of species-level divergence, and not population-level structure we performed several clustering analysis methods on our data modified from Derkarabetian et al. (2019). Each analysis was performed on subclades separately for higher resolution on finer scale species structure. Firstly, we executed t-Distributed Stochastic Neighbour Embedding (t-SNE; van der Maaten and Hinton 2008), a nonlinear dimensionality reduction algorithm that clusters similar objects and repels dissimilar objects with high probability in a two- or three-dimensional space. Subsequently, clustering analysis was performed on the t-SNE output by running: (1) PAM clustering with the optimal Kgap determined by gap statistic calculated using factoextra (ver. 1.0.7, A. Kassambara and F. Mundt, see https://CRAN.R-project.org/package=factoextra); and (2) hierarchical clustering analysis (HCA) with the R package mclust (ver. 5.4.1, see https://CRAN.R-project.org/package=mclust; Scrucca et al. 2016), which determined optimal KHCA and clustered specimens.
Second, to identify the highest supported species delimitation model within each subclade we applied a Bayes Factor Delimitation with genomic data (BFD*; Leaché et al. 2014) approach using the program SNAPP (ver. 1.5.2, see https://www.beast2.org/snapp/; Bryant et al. 2012) through BEAST (ver. 2, see https://www.beast2.org/; Bouckaert et al. 2014). BFD* provides a statistically rigorous framework for species delimitation, allowing for the quantification of uncertainty and the assessment of the relative support for different species delimitation models (Leaché et al. 2014). For each subclade, we performed path sampling with 48 steps (MCMC = 100,000, burnin = 10,000) across multiple species hypothesis models based on ML phylogeny topologies, biogeography, and STRUCTURE results (Supplementary Table S4) following parameters in Quattrini et al. (2019) and Leaché et al. (2014). Models were ranked on their marginal likelihood (MLE) and Bayes Factors (BF) were calculated [2* model 1 MLE – model 2 MLE] comparing alternate species models with the STRUCTURE models, with a positive BF value indicating support of model 1 and vice-versa. Topologies for the highest supported species hypothesis models were visualised in DensiTree (ver. 2.2.7, see https://github.com/rbouckaert/DensiTree).
Species delineation
Here, we define species as separately evolving lineages, as outlined by De Queiroz (2007). Under this unified species concept, species are defined according to a combination of genetic, ecological, geographic and morphological characteristics that reflect their distinct evolutionary trajectories. In this study, we applied this criterion by defining species boundaries where multiple independent lines of evidence support a lineage as distinct. Specifically, a lineage was considered distinct if it was, at a minimum, supported by half of following lines of evidence:
internal node support (UFBoot) for a lineage in the 50% edge-trimmed ML phylogeny was >80,
sCF for a lineage in the 50% edge-trimmed ML phylogeny was >34,
internal node support (LPP) in the Astral phylogeny was >70,
a population showed little admixture in STRUCTURE, with more than 80% genetic ancestry assigned to a single cluster,
DAPC resolved a lineage as a distinct cluster (KDAPC),
t-SNE with PAM or HCA clustering resolved a lineage as a distinct cluster (K),
BFD* proposed a most likely population model (highest BF support) that supported a lineage as distinct,
morphological comparison of specimens to type material and original descriptions proved specimens to be morphologically unique, and
lineages are geographically separated, or sympatric and occurring in separate subclades as inferred by our phylogenies.
Results
UCE and exon capture
Through target capturing of UCEs, we enriched 144 individuals with a total of 2354 loci (2,474,864 bp). The average number of loci enriched per sample was 1153 ± 122 (range 746–1545, Supplementary Table S5). Alignments spanned 1233 loci across the 50% complete alignment matrix and the percentage of parsimony informative sites was 6.71%.
Maximum Likelihood phylogeny
Our phylogenetic reconstructions of the ‘hyacinthus complex’ places this morphological group in Clade VI (Fig. 3). By focusing on this morphological complex, we resolve concordant topologies across all reconstructions that support four subclades (designated as HA, HB, HC and HD; Fig. 3) with varying levels of support (UFBoot, gCF and sCF; LPP) across alignments (Supplementary Fig. S1). Only one subclade (HA) was not resolved among all reconstructions because it formed a paraphyletic group in the internally trimmed 75% complete matrix tree (Supplementary Fig. S1). One individual exhibited ‘rogue’ behaviour, switching clades in the edge trimmed 75% matrix phylogeny from HB to Clade HA (KM71, Supplementary Fig. S1). The inconsistencies within each clade across reconstructions likely stemmed from low loci resolution in the 75% complete matrices (413 loci) resulting in discordant species level topologies and low support for clade topologies in the 75% matrix phylogenies. Similarly, as our taxa were closely related, we found the edge trimmed alignments resolved higher support and concordance. Due to higher resolution (1173 loci), and strong node support the results below are shown according to the edge trimmed 50% complete matrix phylogenies.
ASTRAL ML phylogeny generated with edge trimmed 50% complete matrix. The bars on the right indicate the four clades (HA–HD) resolved throughout the phylogenetic reconstructions (Supplementary Fig. S1). Shaded nodes within each clade show the Primary Species Hypothesis resolved from this phylogeny. Inset tree to the left shows the six clade Acropora phylogeny resolved in Cowman et al. (2020) with the specimens in the current study resolved in Clade VI of this genus reconstruction. Node support depicts local posterior probability (LPP) with key in the bottom left corner indicating support levels. Branches terminated by a star indicate holotypes, whereas those terminated by a circle indicate topotypes.
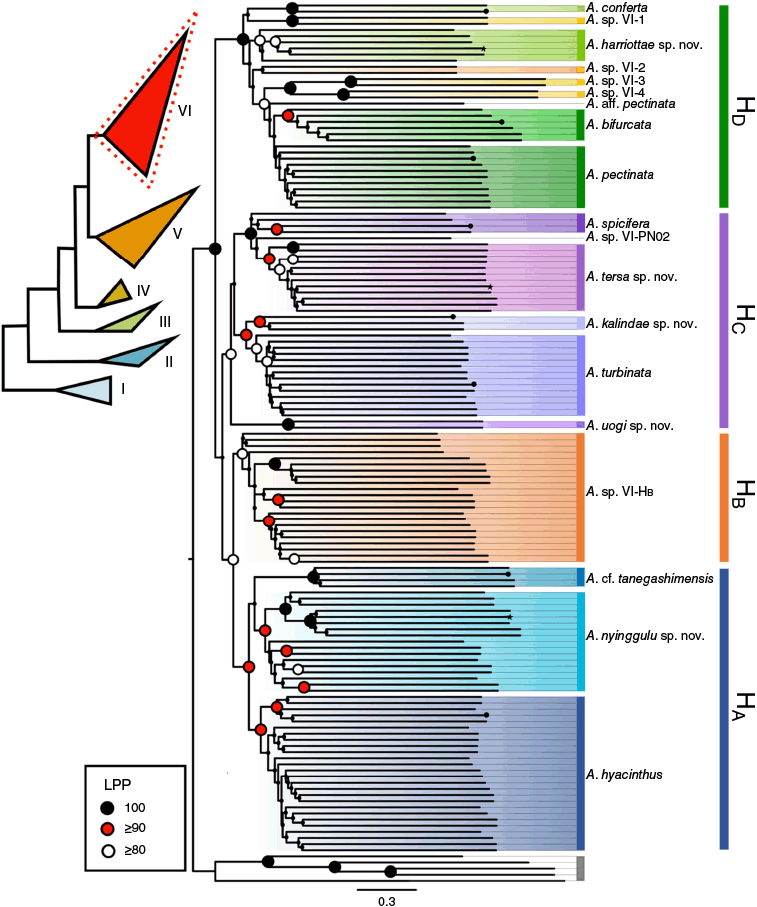
Across the ML and Astral phylogenetic reconstructions we recovered high node support for the four subclades (100% UFBoot; >37% sCF; >89% Local Posterior Probability). Across all reconstructions, gene concordance factors (gCF) were consistently low (<11%; Supplementary Fig. S1), which is not unusual because single loci and short branch lengths in UCE datasets can be uninformative (Minh et al. 2020a). In the ML phylogeny, high UFBoot support (100%) and sCF (≥37%) across subclades HA–HC recovered a total of nine lineages proposed for primary species hypothesis (Fig. 2). Subclade HD resolved a further eight lineages, although with varying levels of support (UFBoot 78–100%, sCF > 33, Fig. 3, Supplementary Fig. S1) with the A. pectinata lineage displaying discordant topologies and non-monophyly across phylogenies.
Out of the 17 lineages, 6 lineages contained a reference topotype specimen (Fig. 1, Table 1). Eleven lineages contained samples from a single biogeographic region and only one lineage (Clade HB) included samples found in greater than three biogeographic regions (Table 1, Supplementary Fig. S3). A lineage in Clade HC contained a single specimen (A. sp. VI-PN02, Fig. 3) that did not match any type material.
| Species | Status | Biogeography | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Central Pac. | Eastern Aus. | Western Pac. | Japan | Coral Triangle | Western Aus. | ||||
| HA | A. hyacinthus | n. | x | x | |||||
| A. nyinggulu | nov. | x | x | x | |||||
| A. cf. tanegashimensis | n. | x | |||||||
| HB | A. sp. VI-HB | unres. | x | x | x | x | |||
| HC | A. kalindae | nov. | x | ||||||
| A. turbinata | n. | x | |||||||
| A. uogi | nov. | x | |||||||
| A. spicifera | n. | x | x | ||||||
| A. tersa | nov. | x | x | x | |||||
| HD | A. pectinata | n. | x | ||||||
| A. bifurcata | n. | x | x | x | |||||
| A. sp. VI-4 | unres. | x | |||||||
| A. sp. VI-3 | unres. | x | |||||||
| A. sp. VI-2 | unres. | x | |||||||
| A. harriottae. | nov. | x | |||||||
| A. sp. VI-1 | unres. | x | |||||||
| A. conferta | n. | x | |||||||
Biogeographic distribution table of PSH based on clades resolved from ML and MSC phylogenetic reconstructions. Status of species is nominal (n.), novel species (nov.) and unresolved (unres.). Topotype designation for nominal species is indicated by x in bold and underlined (x).
Species delimitation (PSH assignments, STRUCTURE, DAPC, t-SNE, SNAPP)
After categorising SNP data to the recovered subclades (HA–HD) and filtering individuals with >20% missing data, two samples were removed (HC = 29-8257; HD = 19.GBR.112) and an average of 34 loci were removed per dataset. Additional filtering steps to detect and remove non-polymorphic loci resulted in a final species delimitation dataset for each subclade as follows; 47 samples with 1481 SNPs for HA, 20 samples with 713 SNPs for HB, 28 samples with 985 SNPs for HC and 28 samples with 843 SNPs for HD.
The genetic clustering resolved by STRUCTURE analysis supported an optimal ΔK = 2 clusters each in clade, HA, HB and HC. Within Clade HA, the optimal ΔK = 2 with A. hyacinthus and A. cf. tanegashimensis forming one cluster, whereas A. nyinggulu sp. nov. formed a second cluster (Fig. 4). Clade HB resolved ΔK = 2; however, all individuals had >50% ancestry to a single cluster (Supplementary Fig. S2). Clade HC individuals also resolved ΔK = 2, with clusters in congruence with the monophyletic clades in the ML phylogeny (Fig. 4), although interestingly A. turbinata and A. tersa sp. nov. each displayed >97% ancestry to distinct lineages showing high support for these two PSHs, whereas A. kalindae sp. nov., A. uogi sp. nov. and A. spicifera each displayed mixed ancestry among the two clusters. The STRUCTURE analysis for Clade HD supporting an optimal ΔK = 3 resolved three clear lineages with the first containing the A. sp. VI-3 and A. sp. VI-4 specimens, the second combining A. pectinata and A. bifurcata, and the third containing A. harriottae sp. nov., A conferta, A. sp. VI-1 and A. sp. VI-2 (Fig. 4). In all STRUCTURE analyses, a proportion of admixture was evident between PSHs. Interestingly, where we identified a second most probable KSTRUCTURE for subclades HA and HD – indicated where Evanno plots displayed a second ΔK peak or an alternate high Mean L(K) score – we found clustering in alignment with initially identified PSHs. For subclade HA at KSTRUCTURE = 5 we found A. cf. tanegashimensis to contain a portion (>25%) of unique genotypes, and within A. hyacinthus we found the population from the central Pacific to also contain >20% of a distinct genotype not found in significant proportions in the Great Barrier Reef (GBR) populations (HA K = 5, Fig. 4). For subclade HD at KSTRUCTURE = 5 we resolved clear clusters for A. harriottae and A. conferta that were not identified in the ΔK = 3 clusters (HD K = 5. Fig. 4).
Results from STRUCTURE analysis for subclades (a) HA (K = 2 and K = 5), (b), HC (K = 2), and (c) HD (K = 3 and K = 5). Bars are coloured according to majority ancestry and PSH have been grouped and labelled.
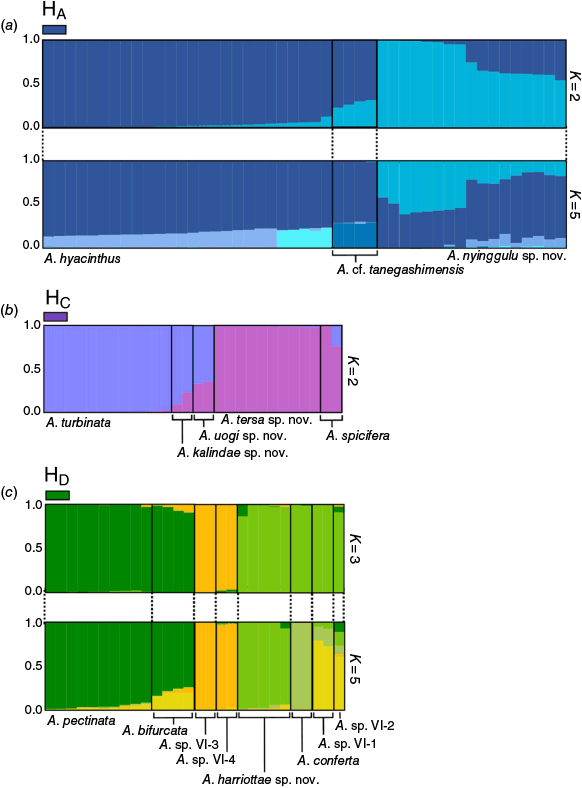
The DAPC analysis followed a similar pattern to that of STRUCTURE. Both clade HA and HC resolved the same clustering determined by ΔK in STRUCTURE (KDAPC = 2), wherease HB DAPC analysis proposed a most likely KDAPC = 1 cluster, in line with PSH assessment of a single species as discussed above. This single genetic cluster delineation was echoed by subsequent t-SNE analysis (Kgap and KHCA); however, we found discordance across the STRUCTURE, DAPC and t-SNE analyses with a lack of population structure preventing us from making further inferences on the boundaries of this hypothesised lineage. Also, the specimens within Clade HB display a range of morphologies, further blurring the lines. Considering this, the following results will focus on the remaining three subclades where species resolution was found. The DAPC analysis for HD proposed a most likely KDAPC = 1, combing all PSH into a single genetic cluster.
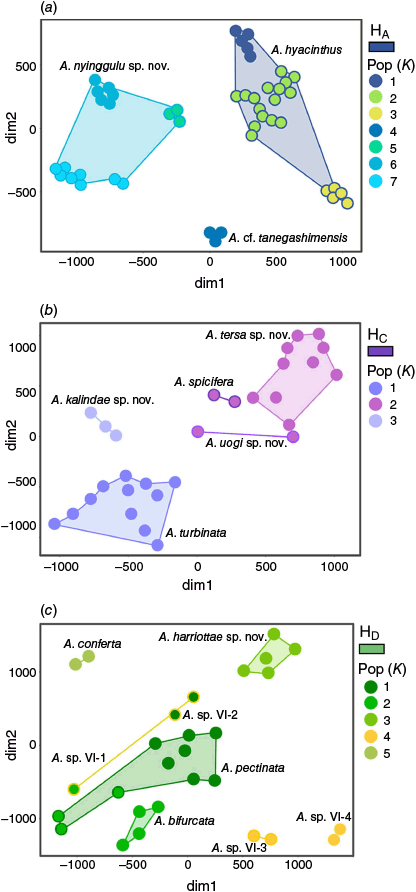
For the t-SNE analysis the general clustering of each subclade was concordant with initial PSH assignments and highlighted geographic patterns among the datasets (Fig. 5). For subclade HA, A. hyacinthus formed three populations in HCA, approximately represented sampling efforts from the central Pacific, southern GBR and northern GBR (Fig. 5). Both clustering methods successfully resolved A. cf. tanegashimensis as a single genetic entity, wherea A. nyinggulu was again split into biogeographic clusters with groups representing sampling in Western Australia, the Coral Triangle and Okinawa in Japan (Fig. 5). For subclade HC, HCA resolved A. kalindae and A. turbinata as distinct clusters, while combining the remaining PSH under one cluster (Fig. 5). This pattern was mirrored by PAM clustering, with the distinction of A kalindae and A. turbinata forming one single cluster and the single specimen for A. uogi switching groups. For HD, HCA designated five clusters successfully resolving A. conferta and A. harriottae and clustering A. sp. VI-3 and A. sp. VI-4 as one population, although with visual distinctions between these PSH clusters. The remaining PSH were all grouped into two populations with mixed alignments to the PSH designations (Fig. 5). These results were mirrored by PAM clustering.
Results from the t-SNE analysis showing clustering of specimens according to most likely population (K) determined by Hierarchical Clustering Analysis for clades HA (a), HC (b) and HD (c). Circles are coloured according to HCA populations, and outlines and convex hulls represent PSH as labelled.
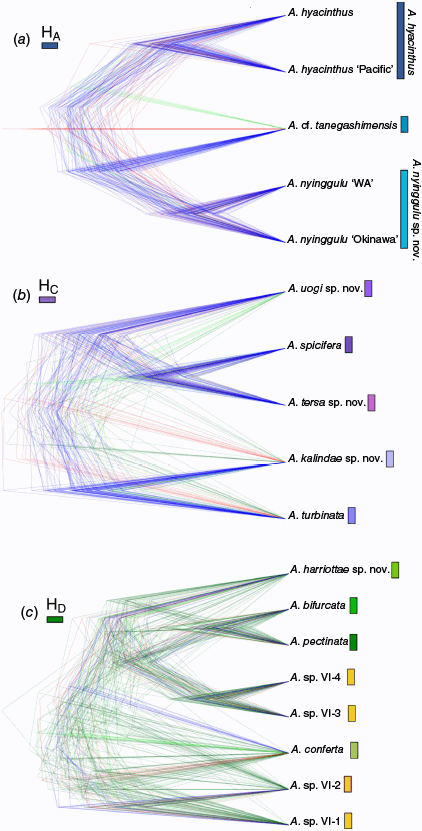
Within the BFD* analysis (HA, HC and HD), the population models with the highest Bayes Factor (BF) support were those that represented the most diverse species delimitation models, in congruence with PSH for HC and HD clades, although supporting a further split in PSH for Clade HA (Fig. 6). Support of a five species model in subclade HA (MLE = −28,797, BF = −1676) included splitting Acropora hyacinthus into separate central Pacific and Great Barrier Reef populations, reflecting the STRUCTURE (K = 5, Fig. 4a) results. Similarly, A. nyinggulu was split geographically, with a distinction between the Western Australian population and the Coral Triangle and Japan populations (A. nyinggulu ‘WA’ and A. nyinggulu ‘Okinawa’ respectively, Fig. 6). The highest supported model for both clades HC (MLE = −12,733, BF = −899) and HD (MLE = −13,625, BF = −1783) were congruent with PSH designated from phylogenomic data.
Results from Bayes Factor Delimitation with genomic data (BFD*). Topologies displayed represent the highest supported species model for subclades HA (a), HC (b) and HD (c). Branch colours represented the support of that topology with blue representing the most likely topology, red the second most likely and green the remaining topologies. Branch tips are labelled according to the most probable BFD* model, and coloured bars indicate PHS according to the ML phylogeny.
Species delineation
We defined species boundaries where multiple independent lines of evidence supported a lineage as evolutionarily distinct. Twelve out of the 17 lineages resolved were supported by five or more lines of evidence (Table 2) and thus considered distinct species. Four lineages were poorly supported outside of our phylogenies and BFD* analysis and therefore remain unresolved. One morphologically variable lineage (represented by all specimens in Clade HB) was only supported in the phylogenies, with further study required to resolve species boundaries.
| ML e50 | Astral | STR | DAPC | t-SNE | BFD* | Morphology | Geography | LOE number | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UFBoot | sCF | LPP | PAM | HCA | |||||||||
| HA | A. hyacinthus | – | Y | Y | Y | Y | *Y | *Y | *Y | Y | Y | 9 | |
| A. nyinggulu sp. nov. | – | Y | – | Y | Y | *Y | *Y | *Y | Y | – | 7 | ||
| A. cf. tanegashimensis | Y | Y | – | – | – | Y | Y | Y | Y | – | 6 | ||
| HB | A. sp. VI–HB | Y | Y | Y | – | – | – | – | – | – | – | 3 | |
| HC | A. kalindae sp. nov. | Y | Y | Y | – | – | – | Y | Y | Y | Y | 7 | |
| A. turbinata | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 | ||
| A. uogi sp. nov. | Y | – | Y | – | – | – | – | Y | Y | Y | 5 | ||
| A. spicifera | Y | – | Y | – | – | – | – | Y | Y | Y | 5 | ||
| A. tersa sp. nov. | Y | – | Y | Y | Y | Y | Y | Y | Y | Y | 9 | ||
| HD | A. harriottae sp. nov. | Y | Y | Y | Y | – | – | Y | Y | Y | – | 7 | |
| A. bifurcata | Y | Y | – | – | – | – | – | Y | Y | Y | 5 | ||
| A. pectinata | Y | – | – | Y | – | – | – | Y | Y | Y | 5 | ||
| A. sp. VI-3 | Y | Y | Y | – | – | – | – | Y | – | – | 4 | ||
| A. sp. VI-4 | Y | Y | Y | – | – | – | – | Y | – | – | 4 | ||
| A. sp. VI-1 | Y | Y | – | – | – | – | – | Y | – | – | 3 | ||
| A. sp. VI-2 | Y | Y | – | – | – | – | – | Y | – | – | 3 | ||
| A. conferta | Y | – | – | Y | – | – | Y | Y | Y | Y | 6 | ||
| Number of supported lineages | 15 | 12 | 10 | 7 | 4 | 5 | 8 | 16 | 12 | 9 | |||
Each lineage is listed next to the corresponding subclades (HA, HB, HC, HD). The analyses performed in this study are listed along the top rows. Any box containing a ‘Y’ indicates the results of this analysis supports the corresponding lineage as distinct. Species delimitations are made when five or more lines of evidence (LOE) support a lineage as distinct.
Taxonomic account
Here, we propose a revised taxonomy for the Acropora hyacinthus complex as outlined below. Of the 17 lineages recovered in the phylogeny, 6 represent nominal species (Fig. 7), only 2 of which were considered valid by the most recent revision of the genus (Wallace 1999): A. hyacinthus (Dana, 1846) and A. spicifera (Dana, 1846). (Supplementary Table S1). We therefore remove four species from synonymy with A. hyacinthus; A. turbinata (Verrill, 1864), A. pectinata (Brook, 1892), A. conferta (Quelch, 1886) and A. bifurcata Nemenzo, 1971. Acropora. bifurcata is currently listed as ‘accepted’ at WoRMS (Hoeksema and Cairns 2025), however, it was formally synonymised by Veron and Hodgson (1989) and considered a potential synonym of A. hyacinthus by Wallace (1999) but included in Veron (2000). Of the 11 other lineages, we describe five as new species (Acropora nyinggulu sp. nov., Acropora tersa sp. nov., Acropora uogi sp. nov., Acropora harriottae sp. nov. and Acropora kalindae sp. nov.) and six are unresolved (A. sp. VI-1, A. sp. VI-2, A. sp. VI-3, A sp. VI-4, A. sp. VI-HB and A. cf. tanegashimensis, Fig. 8). The specimens we identify as A. cf. tanegashimensis, from the Ryukyu Islands, show some morphological affinity to A. tanegashimensis Veron, 1990 from the subtropical Japanese island of Tanegashima; however, given the geographic distance (~500 km) and habitat differences (corals growing on rocky substrate at high-latitude v. a fringing reef on Sesoko Island, Okinawa) between the type locality and the collection location of our specimens, a topotype is required to confirm the identity of this species. Additionally, the removal of A. pectinata (Brook, 1892) from synonymy with A. hyacinthus highlighted the fact that A. pectinata Veron, 2000 is an invalid junior homonym; therefore, as per Article 60.3 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature 1999), we designate Acropora floresensis as a nomen novum (replacement name) for A. pectinata Veron, 2000 (see A. floresensis taxonomic account below). The lineage Acropora sp. VI-HB (Fig. 8), which encompasses all Clade HB, was poorly resolved across all analyses despite a large sample size (n = 22) and is therefore considered unresolved. It is possible that this subclade represents a single species with a large morphological variation and a broad geographic range; however, further work is required to resolve the taxonomy of this clade.
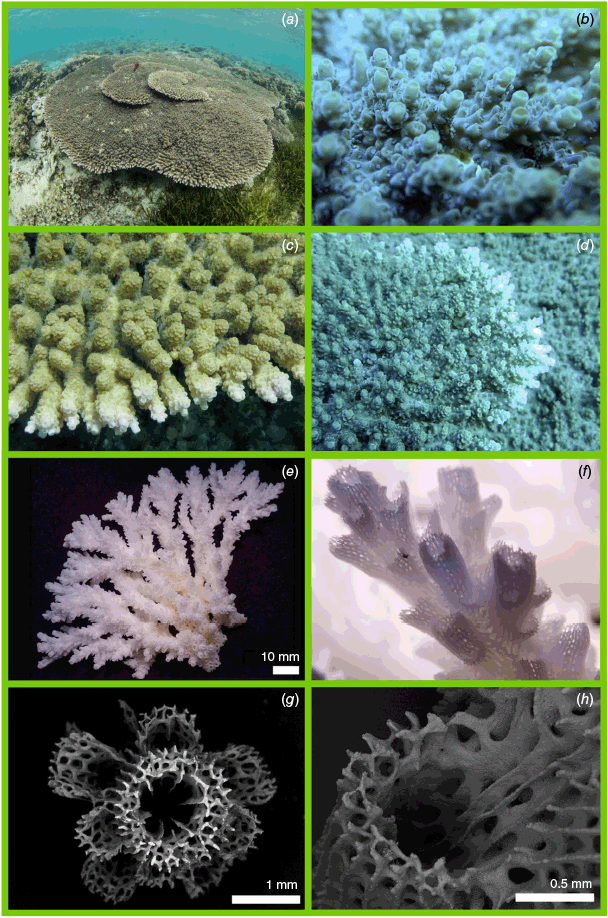
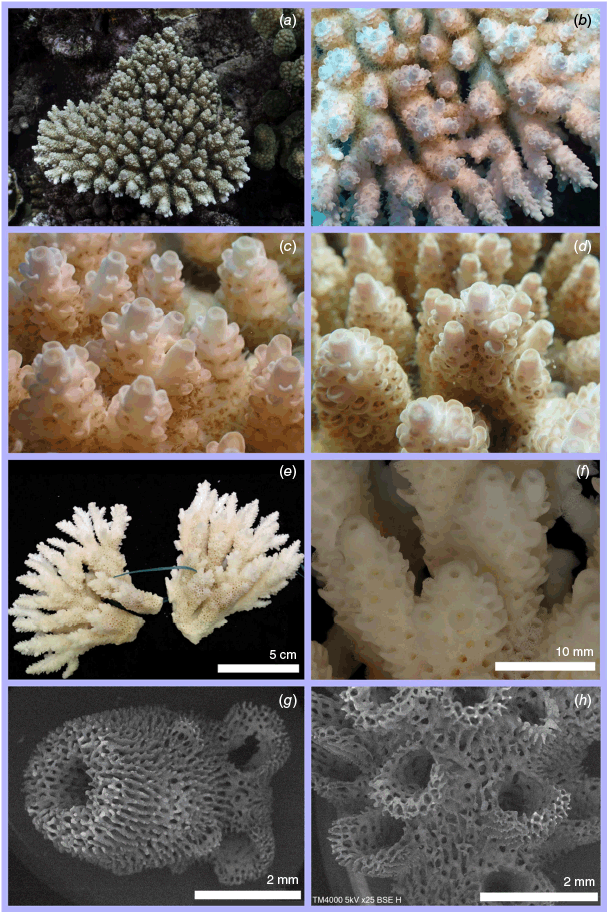
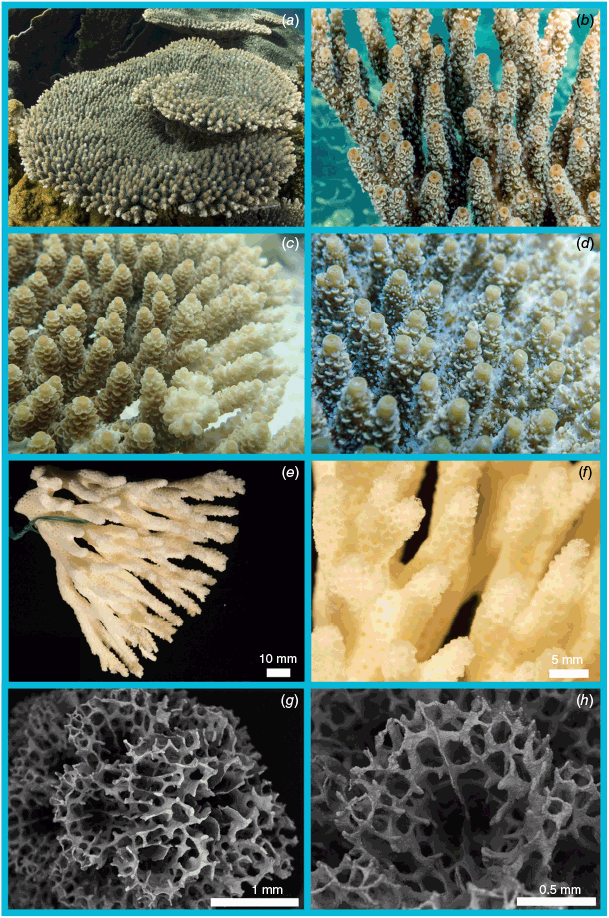
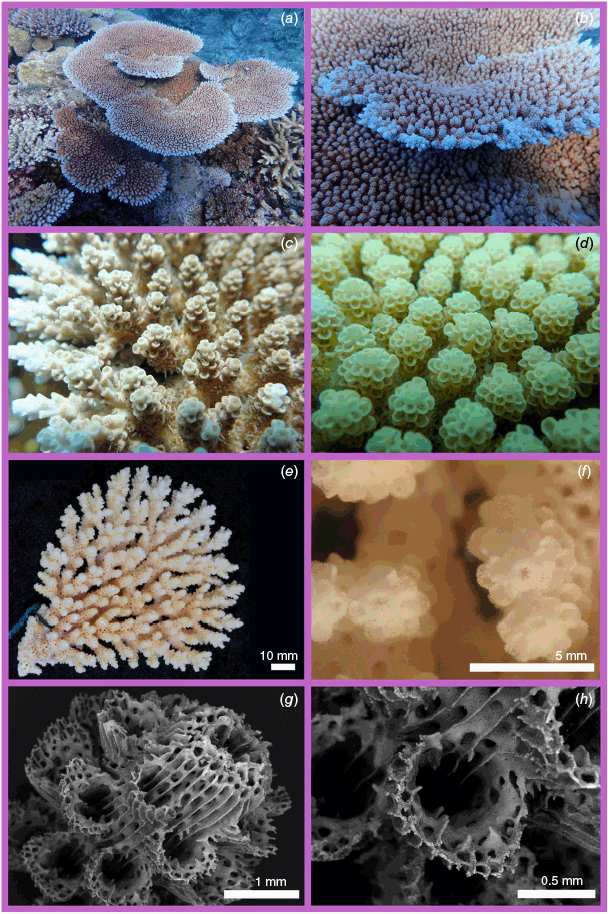
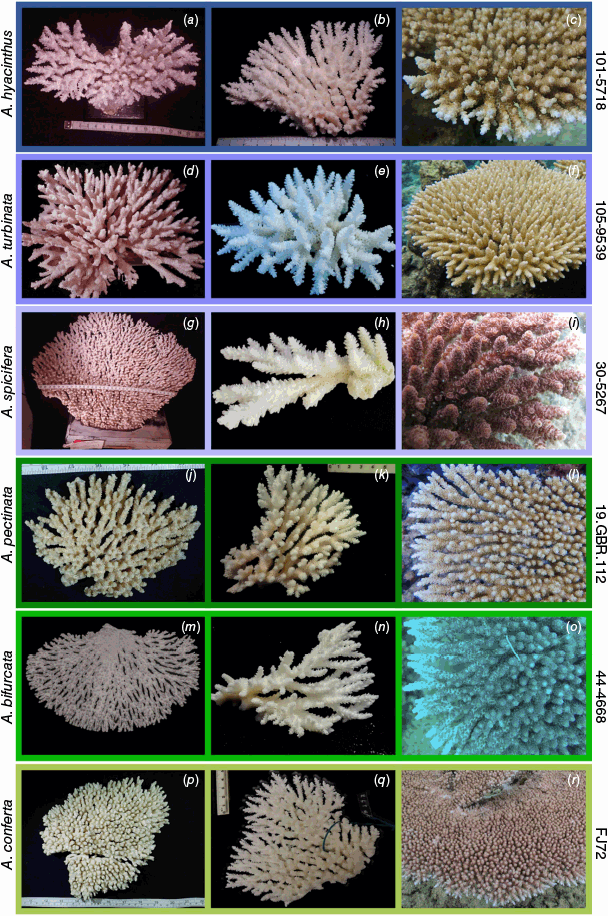
Nominal species of tabular Acropora resolved in the present study. (a) Acropora hyacinthus (Dana, 1846) holotype USNM 246, Fiji, (b, c) and topotype specimen 101-5718, Fiji; (d) Acropora turbinata (Verrill, 1864) holotype YPM: 2017, Tahiti, (e, f) and topotype specimen 105-9539, Society Islands, French Polynesia; (g) Acropora spicifera (Dana, 1846) lectotype USNM: 244, Singapore, (h, i) and topotype specimen 30-5267, Singapore Strait, Singapore; (j) Acropora pectinata (Brook, 1892) lectotype, NHM: 1892.6.8.154, Thursday Island, Torres Straits, (k, l) and topotype specimen 19.GBR.112, 12-040 Reef, Far-North Great Barrier Reef, Australia; (m) Acropora bifurcata Nemenzo, 1971 holotype MSI-UP: U.P.C.-1295, Mindoro, Philippines, (n, o) and topotype specimen 44-4668, Philippines; (p) type specimen for Acropora conferta (Quelch, 1886) holotype NHM: 1885.2.1.12, Fiji, (q, r) and topotype specimen FJ72, Fiji.
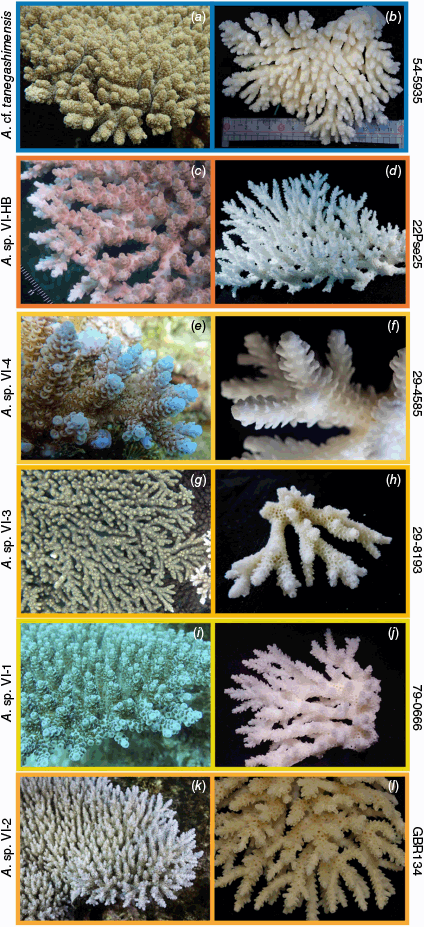
Unresolved lineages of tabular Acropora from the present study. (a, b) Acropora cf. tanegashimensis, 54-5935, Ryukyu Islands, Japan. (c, d) Acropora sp. VI-HB, 22Pse25, Orpheus Island, Great Barrier Reef, Australia. (e, f) Acropora sp. VI-4, 29-4585, Aceh, Indonesia. (g, h) Acropora sp. VI-3, 29-8193, Aceh, Indonesia. (i, j) Acropora sp. VI-1, 79-0666, Solitary Islands, NSW, Australia. (k, l) Acropora sp. VI-2, GBR134, Myrmidon Reef, GBR, Australia.
Our results show the taxonomic diversity of the A. hyacinthus complex is much higher than previously thought, and that the geographic ranges of most of the species in this complex are far smaller than currently assumed. By sampling over a broad area covering the Indian and Pacific Oceans we show that A. hyacinthus, first described by James Dwight Dana in 1846 from a specimen collected from Fiji, likely occurs only in the western and central South Pacific (eastern Australia, Fiji, Tonga and the Cook Islands). Although A. hyacinthus is currently thought to have a wide geographic range spanning most of the Indo-Pacific, our results show that specimens identified as A. hyacinthus from other regions (e.g. the Indian Ocean and Red Sea) are not A. hyacinthus. Secondly, we confirm the validity of four species previously synonymised with A. hyacinthus: A. bifurcata Nemenzo, 1971, A. conferta (Quelch, 1886), A. pectinata (Brook, 1892) and A. turbinata (Verrill, 1864). All four species are resolved as distinct molecular and morphological lineages, none of which are in the same subclade as A. hyacinthus (Fig. 3). Not only are these species distinct from A. hyacinthus, but A. hyacinthus sensu Veron and Wallace (1984) and Wallace (1999) is a polyphyletic species group. Although we aimed to sample topotypes for all nominal species currently synonymised with A. hyacinthus, we were unable to obtain topotypes of Madrepora patella Studer, 1878 from Bougainville, M. surculosa Dana, 1846 from Fiji and M. recumbens Brook, 1892 from the Great Barrier Reef. Nonetheless, examination of the type material for these species suggests they are distinct from the holotype of A. hyacinthus (Dana, 1846) (see A. hyacinthus taxonomic account below) and we therefore formally remove them from synonym with A. hyacinthus (Dana, 1846).
The fact that A. hyacinthus is restricted to the south-western Pacific also enables us to remove from synonymy a further two nominal species with type localities well outside its range: A. flabelliformis (Milne-Edwards, 1860) from the Indian Ocean and A. sinensis (Brook, 1893) from Taiwan. In addition to this geographical evidence, the type specimens of both A. flabelliformis and A. sinensis differ morphologically from that of A. hyacinthus (Dana, 1846), as outlined below.
Wallace (1999) included six species in the ‘hyacinthus’ morphological group in addition to A. hyacinthus: A. tanegashimensis Veron, 1990; A. anthocercis (Brook, 1893); A. cytherea (Dana, 1846), A. microclados (Ehrenberg, 1834); A. paniculata (Verrill, 1902) and A. indonesia Wallace, 1997. However, our re-examination of the type material shows that some of these species have been misinterpreted and that they are unlikely to be closely related to A. hyacinthus. Below, we discuss species included in the ‘hyacinthus group’ by Wallace (1999) to illustrate the characters that delineate them from the new species we describe.
The Taxonomic Account includes the following sections:
descriptions of new species;
nominal species sequenced in this study;
nominal species not sequenced in this study but subjected to nomenclatural acts; and
other nominal species relevant to, but not sampled, in this study e.g. comments on other nominal species included in the ‘hyacinthus group’ by Wallace (1999).
When referring to specimens examined, the institution abbreviations are: QMT, Queensland Museum Tropics, Townsville, Queensland, Australia; WAM, Western Australian Museum, Perth, Western Australia, Australia; AM, Australian Museum, Sydney, New South Wales, Australia; NHM, Natural History Museum, London, United Kingdom; USNM, United States’ National Museum of Natural History – Smithsonian Institution, Washington DC, United States of America; YPM, Peabody Museum of Natural History at Yale University, New Haven, Connecticut, United States of America; UoG, University of Guam, Guam, United States of America; MNHN, Muséum national d’Histoire naturelle, Paris, France; MSI-UP, Marine Science Institute, University of the Philippines, Diliman, Quezon City, Philippines; FMF-SKU, Faculty of Marine and Fisheries, Syiah Kuala University, Banda Aceh, Aceh, Indonesia; RUMF, Ryukyu University Museum (Fujukan), Nishihara, Japan.
Acropora harriottae Baird & Rassmussen, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:CE031C1B-2949-40A1-BCF4-E2217899A797
HOLOTYPE. QMT G85302; North Solitary Island, New South Wales, Australia, 8 m (−29.9274, 153.3893) Col. AHB (Fig. 9b, d–h). PARATYPES. QMT G85165 North Solitary Island, New South Wales, Australia, 8 m (−29.9294, 153.3904) Col. AHB; QMT G85216 Lord Howe Island, New South Wales, Australia, 1 m (−31.5264, 159.0507) Col. AHB (Fig. 9c)
QMT: G7359 Moreton Bay, Queensland, Australia; G24194, G79851, G79853 Lord Howe Island, New South Wales, Australia; G85202 North Solitary Island, New South Wales, Australia. G825048, G82513, G82530, G82543, G82549, G82589 Keppel Islands, Queensland, Australia. AM: G14839 Lord Howe Island, New South Wales, Australia. Southern Cross University: record 443; Peter Harrison private collection.
Part of colony, greatest length 11 cm, width 7 cm and height 3 cm. Branches: final branch length 5–12 mm; 3–5 mm in diameter; axial dominated; terete. Axial corallites: tubular, some with a slight taper; outer diameter 1.5–2.0 mm; inner diameter 0.8–1.0 mm; height 2.0–2.5 mm; 2 synapticular rings; porous; primary septa all present up to 1/4 R; secondary septa absent. Radial corallites: labellate with flaring lips; mixed sizes; mostly touching; 4–6 radials on the branch circumference; primary septa absent; secondary septa absent. Coenosteum: the same on and between radial corallites; costate; no spinules.
Colony morphology: a side-attached plate. Colour: grey, with a white growing margin.
G85165: the final branches are tapered; radial corallites are more regularly distributed than in the holotype, the colony colour is dark brown. G85216: colony is a centrally attached table, axial corallites no larger than 1.5 mm in height; colour is light brown with pinkish margin.
Subtidal, growing on rocks in the Solitary Island to 8-m depth, in the lagoon on Lord Howe Island, and on the fringing reefs of the Keppel Islands.
Specimens of this species occur in subclade HD within Clade VI of the Acropora phylogeny (sensu Cowman et al. 2020).
This species has previously been recorded as A. hyacinthus (Dana, 1846) in the Solitary Islands by Peter Harrison and Vicki Harriott (record 443; Harrison private collection, Southern Cross University) and on Lord Howe Island by Veron and Done (1979) (AM G14839). Throughout the range of A. harriottae from Lord Howe Island in the south to the Keppel Islands in the north it co-occurs with several other tabular species with labellate radial corallites including A. hyacinthus (Dana, 1846) and other currently unidentified tabulate Acropora species. More research is needed to determine how to distinguish these species in the field.
The molecular data confirm that this species is present on Lord Howe Island and in the Solitary Islands. In addition, specimens with morphological affinities to the holotype have been collected in Moreton Bay in subtropical south-east Australia, and the Keppel Islands in the southern Great Barrier Reef.
Acropora kalindae Crosbie, Baird, Bridge & Rassmussen sp. nov.
ZooBank: urn:lsid:zoobank.org:act:80907CEB-77D0-4BC1-BBD9-07FF8736F345
Acropora anthocercis Veron & Wallace, 1984 (non Brook, 1893)
HOLOTYPE. QMT: G78731 Queensland, Myrmidon Reef, 5 m (−18.255532, 147.383883). Col. TCLB. (Fig. 10a, e, f, g, h), PARATYPE. G81385 Queensland, Myrmidon Reef, 6 m (−18.258283, 147.400039). Col. AHB (Fig. 10b, c).
QMT: G28044, G28045, G28047, G28049, G28050, G28052, G28053, G28054, G28056, G28057, G28058, G28061, G28063, G28415, G28417, G28418, G28420, G28421, G28422, G28423, G28424, G28425, G28426, G28427, G28428, G29888, G29889 (Veron and Wallace, Fig. 775), G29890, G29891, G29892, G29893, G29894, G29895, G29897, G30843, G30845, G30848, G30849, G30851, G34866, G84966 Great Barrier Reef.
Part of colony, 2 fragments; 1: maximum diameter 10 cm, width 8 cm wide and height 5 cm. 2: maximum diameter 12 cm, width 9 cm and height 5 cm. Branches: primary branches horizontal; primary branch length indeterminant, primary branch diameter 10–15 mm; final branch length 10–30 mm; final branch diameter 8–15 mm; branch angle primary v. final 45–90°; final branches round, tapering and not fused; radial crowding intermediate; no naked branches; two or more incipient axials surround the axial corallite on central final branches. Axial corallites: conical, openings round; outer diameter 2.0–3.0 mm; inner diameter 0.8–1.0 mm; height 2.0–4.0 mm; 3 synapticular rings; septa in 2 cycles; primary septa 3/4 R; porous; 2 directives in some axials. Radial corallites: mixed shapes and sizes, on final branches primarily labellate with round openings, occasionally labellate with an extended outer wall or immersed, becoming appressed tubular to sub-immersed on primary branches; height 2.0–2.5 mm; outer diameter 1.5–2.0 mm; inner diameter 1.0–1.5 mm; angle to branch 30°–60°; >10 radials on the branch circumference; 2 septal cycles; primary septa to 1/4 R; 1–2 directive septa in most radials. Coenosteum: Same on and between radials, costate with simple spines.
Colony morphology: side attached plate; primary branches fused into solid plate towards colony centre. Colour: Colony pale pinkish-brown. Axial and radial corallites pale pink–cream with brown polyps. Polyps and long, semi-transparent directive tentacle extended during the day.
Colony morphology: side attached plate; primary branches fused solid in colony centre. Colour: Colony pale pinkish-brown. Axial and radial corallites pale pink–cream with brown polyps. Tentacles including a single long, semi-transparent directive extended during the day.
G81385: Final branches thinner (6–10 mm) and have fewer incipient axial corallites than holotype. Axial corallites are smaller, outer diameter 1.5–2.0 mm; inner diameter 0.8–1.0 mm; height 1.5–2.0 mm.
This species has previously been identified as A. anthocercis (Brook, 1893) on the GBR by Veron and Wallace (1984). However, examination of the lectotype (NHM 1892.6.8.235) from the Palm Islands and the descriptions of A. anthocercis (Brook, 1892 as A. coronata; Brook 1893) reveal that the species has numerous morphological differences to the specimens examined by Veron and Wallace (1984) and those in the present study. The lectotype of A. anthocercis (1892.6.8.235) is digitate with terete branches ~5 mm in diameter, whereas A. kalindae has tapering branches as per the description of A. anthocercis by Veron and Wallace (1984) and Wallace (1999). The axial corallites of A. anthocercis are tubular and were described by Brook (1893) as ‘2 or more frequently 3 mm diameter and about 4 mm exsert’; in contrast, Veron and Wallace (1984) describe the axial corallites of A. anthocercis as ‘characteristically large and protuberant, frequently up to 8 mm exsert. They taper from 3 to 5 mm thick at their base to 2–2.5 mm at their tips’ – a description consistent with A. kalindae but not with the lectotype of A. anthocercis. The radial corallites of the A. anthocercis lectotype are nariform, appressed tubular or tubular with rounded openings, whereas the radial corallites on the specimens figured by Veron and Wallace (1984) as A. anthocercis and our samples of A. kalindae are primarily labellate. Both A. anthocercis and A. kalindae often have multiple axial corallites on a single final branch, a potential explanation for why A. kalindae was identified as A. anthocercis by Veron and Wallace (1984). Interestingly, most specimens in the QMT collection come from a small region that includes Myrmidon Reef on the GBR shelf-edge north-east of Townsville (the type locality for A. kalindae) and the outer-shelf reefs of the far northern GBR between Princess Charlotte Bay and Torres Strait. Qualitative surveys by one of the authors (TB) in 2021 also indicated that A. kalindae was common in the far northern GBR.
Acropora nyinggulu Bridge & Rassmussen sp. nov.
ZooBank: urn:lsid:zoobank.org:act:2C85D17C-31AD-429F-AEBD-577ECE9673E4
Acropora spicifera Veron, 1986 (non Dana, 1846)
Acropora spicifera Veron & Marsh, 1988 (non Dana, 1846)
HOLOTYPE. WAM Z100478, 2 m (−23.142994, 113.749810) Col. TCLB (Fig. 11a, b, e, f). PARATYPE. WAM Z100479, 1 m (−23.15291/113.7614), Col. TCLB. Both specimens collected from Coral Bay, Nyinggulu (Ningaloo Reef), Western Australia, Australia.
QMT: G39762, G39764, G39770, G39771, G40470, G47008, G51538, G52447, G52449, G52450, G52452, G52454, G52456, G52457, G84313, G84317, G83595, G85312, G85314, G85313 Western Australia, Australia; G51269 Northern Territory, Australia; G46695, G46696 Bali, Indonesia; G48523 Alor Islands, Indonesia; G50170, G55429, INDO4256, INDO4278 Sulawesi; G36823, G47774 Akajima, Japan; G50065 Dongsha Atoll, South China Sea; MSI-UP collection: 45-3610, 45-3742 Bohol, Philippines; FMF-SKU: 29-8190, 29-4461 Aceh, Indonesia; RUMF: ZG-05459, ZG-05460, ZG-05461, Okinawa, Japan.
Colony fragment taken from the edge of the colony, diameter 10 × 10 cm, 3 cm in height. Branches: final branches 5–20 mm in length and 3–6 mm in diameter; axial dominated; predominantly terete; final branch density 1.5 cm–2. Axial corallites: tubular, terete; outer diameter 2.0–2.4 mm; inner diameter 0.7–0.9 mm; 2 synapticular rings; porous; primary septa all present up to 1/2 R; secondary septa sometimes present up to 1/4 R. Radial corallites: labellate, becoming immersed with increasing distance down axial corallite; mostly one size; mostly touching; 6–8 radials on the branch circumference; primary septa vary between corallites, up to 1/4 R in some but absent in others; secondary septa poorly developed or absent. Coenosteum; the same on and between radial corallites; costate; no spinules.
Colony morphology: tabular, with multiple tiers. Colour: dark green–brown with yellow axials; directive tentacles extended during the day.
WAM Z100479: Final branches shorter than in the holotype (5–10 mm in height), and lower walls of radial corallites are also shorter.
Subtidal: occurs in on reef crests, back-reef margins and fore-reef slopes to depths of at least 15 m. It is most common in shallow depths less than 6 m where it may be the dominant species.
A. nyinggulu sp. nov. occurs in subclade HA in Acropora Clade VI sensu Cowman et al. (2020). It is sister to A. cf. tanegashimensis, with both species forming a clade that is sister to A. hyacinthus.
Previously recorded on Western Australian reefs as A. spicifera (Dana, 1846), which co-occurs with A. nyinggulu in Western Australia but is much less common, initially by Veron (1986) and subsequently by other Western Australian reef scientists (e.g. Veron and Marsh 1988). The images of A. spicifera in Veron (1986) are A. nyinggulu and the author states that the species is not found on the east coast of Australia but extends eastwards to Fiji. This might be because Dana’s original description of A. spicifera includes syntypes collected from Singapore and Fiji, rather than specific records of the species from the South Pacific. Wallace (1999) discussed taxonomic uncertainties surrounding A. spicifera and concluded that Dana’s syntypes represented different species, designating USNM 244 from Singapore as the lectotype. Wallace (1999) identifies Dana’s paralectotype USNM 234 as A. millepora and discusses morphological similarities between A. spicifera and A. millepora. Both these species are morphologically distinct from A. nyinggulu, particularly since A. millepora is corymbose and has longer branches. Radial corallite morphology distinguishes A. nyinggulu from other table species, both in the field and in museum collections, as the radial corallites are more appressed, and the lower lip is less flaring than other tabular species, including A. spicifera (Supplementary Table S3). In some specimens the radial corallites are almost immersed. Our molecular analyses show that A. nyinggulu is distinct from specimens of A. spicifera collected from the east coast of Malaysia (145-0335) and Singapore (30-5267) (the type locality of A. spicifera), which are recovered in Clade HC, sister to A. tersa. Although the distribution of A. nyinggulu extends into south-east Asia, it has not been found in Singapore, providing further evidence that the species from Western Australia is not A. spicifera.
The specimens figured as A. spicifera by Wallace (1999) vary widely in gross morphology and likely include multiple species, although one of these specimens (G51538, from Western Australia) is likely A. nyinggulu. The images illustrating A. hyacinthus in Wallace (1999) also include a specimen of A. nyinggulu from Akajima Island, Japan (G47774), although as discussed below further work is required to determine whether the north-west Pacific specimens represent a distinct sister species. The account of A. spicifera on the Corals of the World website (Veron et al. 2016) includes images of A. nyinggulu from the Houtman-Abrolhos Islands, as well as images of A. spicifera from Brunei and another unknown species.
Acropora nyinggulu dominates intertidal reef flats and back-reef margins at Ningaloo Reef and the Houtman Abrolhos Islands, Western Australia, where it forms very large colonies >3 m in diameter and extensive monospecific stands as illustrated by Veron (1986). Examination of specimens in the QMT suggest A. nyinggulu is common on reefs elsewhere in Western Australia. Although it is most common in shallow depths, it can occur to depths of at least 15 m. At these depths, the species can develop an unusual morphology where branches become sparse and project upwards rather than horizontally – a morphology that is particularly common in the Houtman-Abrolhos Islands (Supplementary Fig. S3). In isolation, these colonies could appear to represent a different species; however, it is possible to observe the morphological transition from the unusual deep-water morphology to the standard tabular morphology at sites where the species is abundant throughout its depth range. These observations are confirmed by our molecular phylogeny, which includes a specimen with this morphology from the Houtman Abrolhos-Islands (WA31/G84313) within the A. nyinggulu clade. Although we have observed colonies with considerable variation in branch length, we have not observed this extreme morphology outside of the Houtman Abrolhos Islands.
Molecular species delimitation indicates some genetic structure between populations in Western Australia and those from Sulawesi (Indonesia), Luzon (Philippines) and the Ryukyu Islands (Japan), suggesting that specimens from the latter regions might represent a distinct sister species. However, neither molecular nor morphological analyses consistently delineated two distinct groups. Additional sampling is needed to resolve this issue, potentially in conjunction with additional molecular analyses such as haplowebs (e.g. Ramírez-Portilla et al. 2022).
Coastal western Australia, east to the Arafura Sea and north through the Indo-Australian Archipelago to Okinawa, Japan, and west to the Andaman Sea.
This species is named after the local Indigenous name – Nyinggulu (Ningaloo) – for the region where the holotype was collected. The Traditional Owners, the Baiyungu and Yinnigurrura Peoples, occupied the region for over 30,000 years and we name the species ‘nyinggulu’ because the species is particularly abundant in the region. We thank the Traditional Owners and the Nganhurra Thanardi Garrbu Aboriginal Corporation for allowing us to work on their Country and granting permission to use this name.
Acropora tersa Rassmussen, Bridge & Baird, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:EE1319B0-5118-4363-A459-92CDBAA0A681
HOLOTYPE. QMT G78594 Little Stevens Reef, Great Barrier Reef, Queensland, Australia, 5 m (−20.599890, 150.038144) Col. SHR (Fig. 12a, b, e–h). PARATYPES. QMT G85041, south-east Pelorus Island, Great Barrier Reef, Queensland, Australia, 4 m (−18.5614/146.5011) Col. AHB (Fig. 12c); QMT G83221, south-east Pelorus Island, Great Barrier Reef, Queensland, Australia, 2 m (−18.5614/146.5011) Col. AJC & TCLB.
QMT: G27616, G27619, G32749, G43502, G43520, G435325, G43530, G43548, G43550, G46057, G78575, G78580, G78586, G78596, G83219, G83220, G83222, G83769 Great Barrier Reef, Australia; G35634, G53594, G81222 Papua New Guinea; G61865, G77993, G77854 Palau; G78290, G27928 Fiji.
Fragment taken from the edge of the colony, 9 × 10 cm in diameter, 2 cm high. Branches: final branches 5–12 mm in length and 2–4 mm in diameter; axial dominated; terete; final branch density 2.5 cm–2. Axial corallites: tubular, terete; outer diameter 1.2–1.8 mm; inner diameter 0.6–0.8 mm; 2 synapticular rings; porous; primary septa all present up to 1/2 R; secondary septa sometimes present up to 1/4 R. Radial corallites: labellate with square lips; appressed towards the axial, inner diameter 0.4–0.8 mm, outer diameter 0.6–0.10 mm, often touching, forming a neat rosette around the axial; primary septa vary between corallites, up to 1/4 R in some corallites but absent in most; secondary septa absent. Coenosteum: costate on the axial and radials, becoming reticulate along the branches in between corallites, no spinules.
Colony morphology: tabular, composed of tiered plates with tightly reticulate basal branches. Colour: light pink with a white growing margin.
G85041: terminal branch density of 2 cm–2; darker pink in colour compared to the holotype; primary branches are almost entirely fused. G83221: terminal branch density of 3 cm–2; pinkish-brown in colour and a large flat plating colony compared to the tiered tables of the holotype.
Subtidal: occurs on reef crests, back reef margins and upper reef slopes to depths of ~8–10 m. On the Great Barrier Reef, it co-occurs with both A. hyacinthus and A. pectinata.
Occurs in subclade HC within Clade VI sensu Cowman et al. (2020), sister to A. spicifera, distinct from A. hyacinthus in subclade HA.
Often mistaken for A. hyacinthus on the GBR. Indeed, numerous specimens of A. tersa were identified as A. hyacinthus in the QMT collections, possibly because A. tersa has radial corallites arranged in neat rosettes, a feature noted by Veron and Wallace (1984) and Wallace (1999) as characteristic of A. hyacinthus. However, both the rosette-like arrangement and length of radial corallites are more uniform in A. tersa than in A. hyacinthus. The neat rosettes led to this species being referred to as ‘neat hyacinthus’ prior to its formal description here (e.g. Naugle et al. 2024).
In the field A. tersa can be identified by the compact and orderly arrangement of the radial corallites and final branches that end in one plane on the top of colonies (Fig. 12b). In addition, A. tersa colonies are generally pastel-like shades of pink, purple, blue and green. Colony colour is particularly useful for distinguishing A. tersa from A. pectinata where these two species co-occur, because A. pectinata has a pale coenosteum with contrasting dark brown polyps. Although A. pectinata has terminal branches of an even height, they are wider and less densely distributed across the upper surface of the colony than in A. tersa (Supplementary Table S3). Final branch density varies among habitats and generally decreases in lower-energy environments, but the final branches of A. tersa are consistently more densely distributed than those of A. pectinata when the two species co-occur. Nonetheless, the intraspecific variability in this character among habitats can cause difficulties for identification of specimens in collections if the location (e.g. depth or wave exposure) and the collection location of the specimen is unknown. The final branches also extend very close to or sometimes around the colony margin in A. tersa, whereas the colony margin in A. pectinata is pectinate. These characters are illustrated in Supplementary Fig. S4, which shows two specimens (A. pectinata (G84995) and A. tersa paratype (G85041)) co-occurring in the Palm Islands, Great Barrier Reef. The basal branches of both species also become more fused in higher-energy habitats, but the skeleton of A. tersa is consistently denser than A. pectinata for any given habitat where the species co-occur. Although A. hyacinthus can also have similar pink colony colour to A. tersa, where the two species co-occur they can be distinguished by numerous features: the axial corallite of A. tersa is smaller with an outer diameter range of 1.2–1.5 mm and is uniform in colour, whereas the outer diameter of the axial corallites of A. hyacinthus are larger ranging from 1.2 to 1.8 mm, have thicker walls, and are diagnostically white with a bright orange ring on top of the axial (see A. hyacinthus below). In addition, both the final branches and radial corallites of A. hyacinthus are more variable in shape and size – a character clearly visible in the holotype as well as the specimens examined below – giving the species an irregular appearance when compared to the ‘neat’ final branches and radial corallites of A. tersa.
In shallow and exposed reefs this species can form fused encrusting plates. In deeper areas of the reef the primary branches are more widely spaced than in colonies in shallow water.
Central-western Pacific Ocean, including Fiji, eastern Australia, the Bismarck Sea (Papua New Guinea) and Palau. On the Great Barrier Reef it is rare in the southernmost reefs (e.g. Capricorn-Bunker group) but becomes common in the central and northern regions.
Acropora uogi Randall, Burdick & Bonito, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:2AE8A941-09F0-4C8D-A005-5CB17AF44499
Acropora surculosa Randall & Myers, 1983, Randall, 2003 (non Dana)
HOLOTYPE. USNM DB-GU-0431 Pago Bay, Guam, Micronesia, 4 m (13.426056, 144.799240) Col. DB (Fig. 13a, b, e, f). PARATYPES. QMT G84925 Pohnpei, Micronesia, 2 m (6.823981, 157.918173) Col. AHB (Fig. 13c, d); G79945 Pohnpei, Micronesia, 6 m (6.823981, 157.918173) Col. TCLB.
UoG: UGI-716, UGI-3474, UGI-3475, Pago Bay, Guam, Micronesia; UGI-1137, Ipan, Guam, Micronesia.
Whole colony, corymbose, diameter 25 × 18 cm, 6 cm in height with final branches that form a flat, slightly convex upper surface. Branches: final branch length 12–25 mm; 5–12 mm in diameter; axial dominated; tapering. Axial corallites: tubular, some with a slight taper; outer diameter 1.7–2.2 mm; inner diameter 0.8–1.5 mm; height 0.8–1.2 mm; 2 synapticular rings; porous; primary septa present up to 1/4 R; secondary septa sometimes present up to 1/4 R. Radial corallites: labellate with round openings; 1.1–1.7 mm in diameter; touching; primary septa sometimes present up to 1/4 R; secondary septa absent. Coenosteum: costate with no spinules on the radials; reticulate with simple spinules between radials
G84925 and G79945: both paratypes share similar features; radial corallites labellate with flaring lips, radial corallites at almost 90° angle to the final branch; colonies are encrusting; cream in colour with white final branch tips that is also apparent on the edge of the holotype (DB-GU-0431); a single directive tentacle is extended during the day and is prominent in the field images.
Forms sturdy colonies on the upper seaward reef slope and reef margin habitats, and on reef flat platforms where there is good water circulation. Generally, a lavender or cream colour, commonly with a single directive tentacle extended during the day giving colonies a hairy appearance. The directive tentacle is conspicuously longer than the other tentacles. When the polyps are retracted the tentacular ring is darkly pigmented.
Occurs in subclade HC within Clade VI of the Acropora phylogeny (sensu Cowman et al. 2020), and in most phylogenetic reconstructions as sister to A. turbinata and A. kalinade.
Previously identified by Randall and Myers (1983) and Randall (2003) as A. surculosa on Guam and throughout Micronesia. However, comparison of specimens from the current study to the lectotype of A. surculosa (USNM 248) reveals that A. uogi has thicker and longer final branches than A. surculosa. In addition, the radial corallites of A. uogi are larger and labellate with a round opening, whereas those of A. surculosa are labellate with flaring lips (Supplementary Table S3).
Nominal species sequenced in this study
Acropora hyacinthus (Dana, 1846)
Madrepora hyacinthus Dana, 1846, p. 444, pl. 32, fig. 2.
Acropora hyacinthus (Dana) – Verrill (1902), p. 216.
Madrepora surculosa Dana, 1846. Here removed from synonymy with Acropora hyacinthus (Dana) contra Veron and Wallace (1984), p. 310.
Madrepora turbinata Verrill, 1864. Here removed from synonymy with Acropora hyacinthus (Dana) contra Wallace (1978, 1999), p. 256.
Madrepora patella Studer, 1878. Here removed from synonymy with Acropora hyacinthus (Dana) contra Veron and Wallace (1984), p. 310.
Madrepora conferta Quelch, 1886. Here removed from synonymy with Acropora hyacinthus (Dana) contra Veron and Wallace (1984), p. 310.
Madrepora pectinata Brook, 1892. Here removed from synonymy with Acropora hyacinthus (Dana) contra Wallace 1978 and Veron and Wallace (1984), p. 310.
Madrepora recumbens Brook, 1892. Here removed from synonymy with Acropora hyacinthus (Dana) contra Wallace (1978), p. 288.
Madrepora sinensis Brook, 1893. Here removed from synonymy with Acropora hyacinthus (Dana) contra Veron and Wallace (1984), p. 310.
Acropora bifurcata Nemenzo, 1971. Here removed from synonymy with Acropora hyacinthus (Dana) contra Veron and Hodgson (1989), p. 247.
USNM: 246 Madrepora hyacinthus holotype, Fiji; QMT: G85198, G85160 Solitary Islands, NSW, Australia; G85198, G85097, G85074 Lord Howe Island, Australia; G28686, G32756, G43499, G43503, G43506, G43509, G54325, G54355, G78572, G78581, G85074, G27595, G83226, G78563, G78585, G78561, G83771, G83772, G83779, G83776, G83773, G83774, G83770, G83778, G83783 Great Barrier Reef, Australia; G85097 Coral Sea, Australia; G34973, G58738, G61032 New Caledonia; G37560 Tuvalu; G27932, G28108, G31128 Fiji; G84272, G84274, G84279 Tonga; G78415, G78436, G48458 Cook Islands.
Veron and Wallace (1984) and Wallace (1999) describe A. hyacinthus as tabulate with short final branches with evenly sized labellate radials with a square or rounded lips arranged in a neat rosette around the axial corallite (Wallace 1999). Many species delineated here share these characters, explaining the rampant synonymy of tabular Acropora species by Wallace (1978, 1999) and Veron and Wallace (1984), and the concept of this species presented in Veron (2000) and Veron et al. (2016). For example, the field images for A. hyacinthus in Veron et al. (2016) include several tabular species, including A. hyacinthus but also A. nyinggulu from Western Australia, A. spicifera from Brunei and A. bifurcata from Indonesia, demonstrating that the characters used to define A. hyacinthus in these monographs are not taxonomically informative for delineating tabular Acropora species. Although specimens in this study display some variation in gross morphology, there are diagnostic characters visible in the field and in skeletons in Museum collections that can reliably delineate A. hyacinthus from related tabular species (Supplementary Table S3).
Previous molecular studies suggest that A. hyacinthus is a ‘species complex’ comprising at least six genetically distinct clades within the Indo-Pacific (Ladner and Palumbi 2012; Suzuki et al. 2016). However, there has been no attempt to resolve the taxonomy of these lineages. Here, we show that A. hyacinthus (Dana, 1846) is likely restricted to the south and south-western Pacific. Consequently, based on geographic range and morphological comparison of type material, we formally remove A. sinensis (Brook, 1893) stat. rev. from Taiwan and A. flabelliformis (Milne-Edwards, 1860) from the Indian Ocean from synonymy (discussed below) with A. hyacinthus (Dana, 1846), although topotypes are required to test the validity of A. sinensis and A. flabelliformis. Based on a comparison of type material and specimens sequenced in our phylogeny, we also remove A. turbinata (Verrill, 1864) stat. rev. from Tahiti, A. conferta (Quelch, 1886) stat. rev. from Fiji, A. pectinata (Brook, 1892) stat. rev. from the Torres Strait, A. bifurcata Nemenzo, from the Philippines 1971 stat. rev. from synonymy with A. hyacinthus. We also reinstate three other subjective junior synonyms of A. hyacinthus included in Wallace (1999): A. patella, A. surculosa and A. recumbens based on examination of the type material, which shows numerous differences from A. hyacinthus that we outline below.
Our data indicate that A. hyacinthus does not occur in the Indian Ocean or the Red Sea. Veron (2002) suggests that records of A. hyacinthus from the Red Sea are likely to be A. lamarcki Veron, 2000, A. parapharaonis Veron, 2000 or A. cytherea (Dana, 1846), yet Veron et al. (2016) lists A. hyacinthus as occurring in the Red Sea. Further research is required to resolve the taxonomy of tabular Acropora from these regions.
Although the holotype of A. hyacinthus is a small specimen, it nonetheless includes significant diagnostic characters of the species. Importantly, Veron and Wallace (1984) and Wallace (1999) suggest that A. hyacinthus is characterised by labellate radial corallites arranged in neat rosettes but that is neither consistent with the original description of Dana (1846) nor the holotype USNM 246, which is a vasiform plate with labellate square lipped radial corallites of uneven length, and proliferous final branches (Fig. 7a). Veron and Wallace (1984), the primary source of synonymies for A. hyacinthus, note that the holotype is a small colony yet to develop mature morphological characters; however, it still possesses numerous morphological features that can be identified in larger colonies collected in this study and in museum specimens. This species can be distinguished by the seemingly multidirectional growth and uneven length of the terminal branches, giving A. hyacinthus a ‘messy’ appearance that is apparent in the type (USNM 246) and in the specimens examined here. In some specimens, final branches fan out towards the edge of the colony (19.GBR.70 (G78585), 19.GBR.81 (G78561)).
Colonies of this species can be cream, brown or shades of pink, with the dominant colour varying among locations: In subtropical eastern Australia colonies are often uniformly cream, in the South Pacific (Tonga and Rarotonga, Cook Islands) colonies are predominantly light brown, whereas on the central Great Barrier Reef colonies are predominantly pink. An important field character of A. hyacinthus in both the South Pacific and the GBR is that the colour of the top of the axial corallite is darker (usually orange or pink) than the wall of the axial corallite, which is white (Fig. 7c). This feature is less apparent in specimens from the southern GBR and the Tasman Sea, where whole colonies are cream or brown in colour.
Acropora bifurcata Nemenzo, 1971 status revised
Acropora bifurcata Nemenzo, 1971, p. 147, pl. 2, fig. 1, 2.
Acropora cf. bifurcata – Ramirez-Portilla et al. (2022), pp. 462–465, 468, 470–471, fig. 1–4, table 1.
MSI-UP: U.P.C.-1295, Acropora bifurcata holotype, Puerto Galera, Mindoro, Philippines; QMT: G38015 Akajima, Japan; G41545, G47300, G48512, G50038 G50045, G50050, G53822, Indonesia; G84305 Western Australia; RUMF: ZG-05465, ZG-05466, ZG-05467, Okinawa, Japan; addition specimens collected in the field not yet incorporated into a Museum collection are discussed below.
A. bifurcata was listed as a nominal species of Acropora by Veron and Wallace (1984) (Table 2, p. 141) but was not mentioned in their taxonomic account of A. hyacinthus or any other species. The species was considered a junior synonym of A. hyacinthus by Veron and Hodgson (1989). Wallace (1999) considered the species a potential junior synonym of A. hyacinthus (Wallace 1999, p. 14 ‘?j.s. A. hyacinthus’) but concluded there was insufficient available information to resolve the status of the species. Veron (2000) includes a description of A. bifurcata; however, it was not included in Wallace et al. (2012). Our phylogeny demonstrates that A. bifurcata (HD) occurs in a separate clade to A. hyacinthus (HA), and morphological analyses provide additional evidence that these two species are distinct. Acropora hyacinthus has much longer final branches (up to 25 mm) and labellate radials with flaring lips, in comparison to the shorter final branches of A. bifurcata (up to 10 mm), which has labellate radial corallites with rounded lips (Supplementary Table S3). Here, contra Veron and Hodgson (1989, p. 247) we remove A. bifurcata from synonymy with A. hyacinthus (Dana 1846) and outline multiple lines of evidence to support this decision.
The specimens in the current study from Bohol, Philippines (44-4668) and Okinawa, Japan (ZG-05465, ZG-05466, ZG-05467) closely resemble the holotype in key morphological characters including terete final branches with blunt axial tip, bifurcated lips on the radial corallites and the tight reticulate mesh of the primary branches that fan out horizontally. Specimens from Western Australia, WA18 (G84305) and Malaysia (145-0344) differ primarily by their more open reticulate branching than the former specimens and the holotype described by Nemenzo (1971).
Ryukyu Islands, Philippines, Indonesia, Malaysia and Western Australia. Type locality: Puerto Galera, Mindoro, Philippines (13.513265, 120.955476).
The original description by Nemenzo (1971) describes the resemblance to A. pectinata (Brook, 1892), which is recovered as a close relative of this species in our molecular phylogeny. Although neither of these nominal taxa are A. hyacinthus, further investigations into potential boundaries between A. pectinata and A. bifurcata are warranted. Additionally, specimens RUMF-ZG-05465, RUMF-ZG-05466 and RUMF-ZG-05467 were initially included, as 18Oki32-34, in Ramírez-Portilla et al. (2022) and identified as A. cf. bifurcata based on morphological similarity to the type of A. bifurcata. Here, we confirming the identity of these specimens as A. bifurcata (Nemenzo 1971).
Acropora conferta (Quelch, 1886) status revised
Madrepora conferta Quelch, 1886, p. 164, pl. X, fig. 3c.
Acropora conferta (Dana) – Verrill (1902), p. 213.
NHM: 1885.2.1.12 Madrepora conferta holotype, Fiji; QMT: G78294, G78297 Fiji. G41296 America Samoa.
Brook (1893, p. 109) questioned the validity of Acropora conferta (Quelch, 1886), noting that the only character separating this species from A. hyacinthus was the shape of the colony. Wells (1954) considered A. conferta valid and extended its range to include much of the Pacific Ocean and possibly the Indian Ocean. Veron and Wallace (1984) synonymised A. conferta with A. hyacinthus because they argued that the morphology of the holotype of A. conferta (NHM 1885.2.1.12) was within the range of variation of A. hyacinthus. Although our specimens of A. conferta are similar to some specimens of A. hyacinthus, the molecular analysis of topotypes from Fiji indicates that the two species are distinct: A. conferta is in Clade HD, whereas A. hyacinthus is in Clade HA of the molecular phylogeny. Although similar in morphology, the species can be distinguished by the final branch lengths that reach just 10 mm in A. conferta, and up to 25 mm in A. hyacinthus. Consequently, we remove A. conferta from synonymy with A. hyacinthus.
Specimen G78297/FJ72 from Fiji matches Quelch’s holotype for A. conferta (NHM 1885.2.1.12). As per the original description, G78297 has coalescent primary branches, with some sections fusing together completely. Final branches are short and grow at right angles to primary branches, becoming longer and thinner at the edge of the colony. Axial corallites are no more than 2 mm wide and have six distinct primary septa. Radial corallites are small and labellate. Quelch (1886) remarked on the less distinct rosette formation of the corallites towards the colony edge, which is apparent in both G78297 and G78294, also from Fiji, which has irregular size and shape of corallites towards the colony edge. Both colonies collected in this study were rose-pink in colour with a distinct dark ring on top of the axial corallite similar to A. hyacinthus (Fig. 7c).
Acropora pectinata (Brook, 1892) status revised
Madrepora pectinata Brook, 1892, p. 460 – Brook (1893), p. 95, pl. XXVII, fig. D, E.
NHM: 1892.6.8.154 Madrepora pectinata lectotype and 1892.6.8.155 Madrepora pectinata syntype, Thursday Island, Torres Straits; 1892.6.8.156 Madrepora pectinata syntype, Capricorn Islands Great Barrier Reef, Australia; QMT: G28691, G37400, G46428, G54342, G54348, G54359, G59024, G78578, G78588, G78593, G78597, G83775, G83777, G83780, G83781, G83782, G83784, G83786, G84995, Great Barrier Reef, Australia.
Veron and Wallace (1984) state that A. pectinata (Brook, 1892) is a ‘very clear’ junior synonym of A. hyacinthus (Dana), with Wallace (1999) and Wallace et al. (2012) retaining this synonymy. In contrast, Wells (1954) (p. 420) suggested that A. pectinata (Brook, 1892) was a junior synonym of A. corymbosa (Lamarck, 1816), although the lectotype (NHM 1892.6.8.154) differs from the probable type of A. corymbosa (MNHN 0303-0-1), which has final branches that are longer, thinner and tapered compared to the shorter, terete and rounded final branches of A. pectinata. In addition, the probable type of A. corymbosa is from Mauritius, and is therefore unlikely to be a senior synonym for A. pectinata, with a type location of Thursday Island, Torres Strait. In our molecular phylogeny, A. pectinata occurs in Clade HD, whereas A. hyacinthus occurs in Clade HA. Therefore, we remove A. pectinata from synonymy with A. hyacinthus (Dana 1846) contra Veron and Wallace (1984, p. 310).
All specimens examined closely resemble the lectotype and original description of Brook (1892), forming open branching networks where individual branches are distinct. This characteristic anastomosing branching becomes more prominent (i.e. branches are more widely spaced) in specimens from lower-energy habitats such as protected bays and deeper water. Primary branches are between 5 and 12 mm in diameter, and final branches are <14 mm long, often clustered in groups of 2–5, and are no more than 6 mm apart along the branch. Axial corallites are tubular, terete and <2 mm in diameter. Radial corallites are labellate with a rounded or square lips and form a neat rosette similar to A. tersa. At the edge of the colony, branches are pectinate, and final branches are absent. Where this species co-occurs with A. hyacinthus and A. tersa on the GBR, it can be distinguished in situ by the colour of the colony: A. pectinata has pale cream coenosteum and dark brown polyps, compared with the often brightly pinks, blue and green of A. hyacinthus and A. tersa, and, in particular, by the contrast between the very light coloured coenosteum and dark coloured polyps. Colonies also have an irregular boundary, whereas A. hyacinthus often forms tiered plates.
Currently known only from the Great Barrier Reef and Torres Strait. Although the species is common in the central and northern GBR, there is one possible representative of A. pectinata (G78578) from the Pompey and Swains region in the southern GBR. However, further sampling and molecular analysis will be required to confirm its presence in this region. Type locality: Thursday Island, Torres Strait, Queensland, Australia.
Acropora spicifera (Dana, 1846)
Madrepora spicifera Dana, 1846, p. 442, pl 33, fig. 4a, b, 5 & pl. 31, fig. 6a–c.
Acropora spicifera (Dana) – Verrill, 1902, p. 218.
USNM: 244 Madrepora spicifera lectotype, Singapore; QMT: G84311 Western Australia, Australia; G50033 Bali, Indonesia; G50049, G50055, G50179 East Kalimantan, Indonesia; G53742 Halmahera, Indonesia; G71688 Raja Ampat, Indonesia; G49836 Riau, Indonesia; G50062 Seribu Islands, Indonesia; G50053, G50086, G50176 Sulawesi, Indonesia; G59144 Malaysia; G41022 Singapore; G50063 Pratas, South China Sea.
Acropora spicifera has a complicated taxonomic history, discussed briefly by Wallace (1999), primarily due to the likelihood that Dana’s type series, which includes specimens from Fiji and Singapore, consists of multiple species. Veron and Wallace (1984, p. 310) listed A. spicifera as a potential synonym of A. hyacinthus (Dana), although it was considered valid by subsequent authors (Veron 1986, 2000; Veron and Hodgson 1989; Wallace 1999; Wallace et al. 2012; Veron et al. 2016). In addition to issues with the identities of the syntype series, Dana also describes two variants (var. abbreviata from Singapore, and var. eucladia from an unknown locality). Dana’s syntype specimen from Singapore, USNM 244, was designated as the lectotype for A. spicifera by Wallace (1999), who considered another of the syntype series (USNM 234) to be A. millepora (Ehrenberg, 1834). Wallace (1999) suggests that the morphologies of A. hyacinthus and A. spicifera are sufficiently similar that they might be synonymous, but regarded them as distinct on the basis that the two species can be delineated on reefs in Western Australia. Although the specimens sequenced in our study suggest that A. spicifera does occur in Western Australia, it is less common than A. nyinggulu, described above, and most of the specimens identified as A. spicifera in the QMT collections are A. nyinggulu. Veron (1986, 2000), Wallace (1999) and Veron et al. (2016) all include images of A. nyinggulu as well as other species under their accounts of A. spicifera, highlighting the confusion regarding the taxonomic identity of this species.
The phylogenetic placement of A. nyinggulu and A. spicifera in different subclades in our molecular phylogeny (HA and HC respectively) clearly demonstrates that these species are distinct, particularly since they co-occur in Western Australia and potentially elsewhere. However, there is some overlap in morphology that can lead to difficulties distinguish these species in the field. A feature that distinguishes A. spicifera from A. nyinggulu, especially in Western Australia, is the morphology of the radial corallites, which are wider with shorter walls in A. nyinggulu compared to A. spicifera. This feature is especially useful in identifying A. nyinggulu in museum collections, where it has often identified as A. spicifera. The axial corallites in A. nyinggulu are also wider (maximum 2.4 mm) than those of A. spicifera (maximum 1.5 mm) (Supplementary Table S3). In the field, A. spicifera often has a pinkish tinge, particularly on the radial corallite lip – a character it shares with other species in Clade HC. By contrast, A. nyinggulu is generally green or brown.
The lectotype of A. spicifera (USNM 244) from Singapore is a large flat plate with anastomosing primary branches and final branches with labellate radial corallites that are mostly touching, matching the description of Dana (1846). Although the specimens in the current study vary in morphology, our topotype from Singapore (30-5267) closely resembles the lectotype, with labellate radial corallites that are mostly touching (Supplementary Slides 1–32). A specimen in our study from the Houtman-Abrolhos Islands, Western Australia, G84311, has widely anastomosing branches and corallites that are widely spaced and with shorter corallite walls than the lectotype. This specimen is from 16 m deep at a high-latitude site (28.9°S), and therefore these morphological differences are not surprising. Furthermore, our field observations in numerous locations in the western Coral Triangle region where A. spicifera occurs (Singapore, Malaysia and Indonesia) suggest that the species is commonly more open-branching than the lectotype; however, the radial corallites of this species are distinctive regardless of the extent of plate fusion. Another specimen in this clade (29-8257), from Aceh, Indonesia, has primary branches that are almost completely fused, resembling Dana’s syntype M. spicifera var. abbreviata (USNM 235, 245).
Acropora turbinata (Verrill, 1864) status revised
Madrepora surculosa var. turbinata Dana, 1846, p. 445, pl. 32, fig. 5.
Madrepora turbinata (Dana): Verrill, 1864, p. 42.
Acropora turbinata (Dana): Verrill, 1902, pp. 219, 242.
Acropora HyaE sensu Ladner & Palumbi, 2012.
Madrepora turbinata Verrill, 1864. Here removed from synonymy with Acropora hyacinthus (Dana) contra Wallace (1999, p. 256). See also Bosserelle et al. (2014).
YPM: 2017, Acropora turbinata (Dana) Verrill, 1902, holotype, Tahiti; QMT: G33080, G53584, G54696 Tahiti; G44044 Mo’orea; G30102, G36017 Austral Islands; G36021, G36023, G36025 Niue; G63113 America Samoa; G78395, G79958, G79965, G79973 Tonga; G40713, G78422, G78428, G78430, G78449 Cook Islands; G28113, G28895 Fiji; G85303, G85270, G85181, G85182 Society Islands, French Polynesia; G57878 GBR, Australia.
Dana (1846) described this species as a form of Madrepora surculosa (Dana, 1846). It was raised to species rank by Verrill (1864); however, Dana (1846) is listed as the authority at WoRMS (Hoeksema and Cairns 2025). According to the ICZN code Article 10.2, the valid authority would be Verrill (1864) who first established this name as a valid species. Like M. surculosa, the holotype (YPM 2017) of M. turbinata is corymbose (Supplementary Table S3). Veron and Wallace (1984) included A. turbinata in their list of nominal Acropora, but omitted it from their taxonomic account. Wallace (1999, p. 256) included A. turbinata as a junior synonyms of A. hyacinthus (Dana) without further explanation.
This species is more corymbose than tabular, with final branches of the type (YPM 2017) reaching up to 45 mm long and 5 mm wide. Incipient axials are often present, although the extent of incipient axials is quite variable between colonies. Radial corallites are labellate with elongate lips, occasionally tubular-dimidiate. In the field the species is generally pale pink (referred to as ‘delicate rose-coloured“ by Verrill 1864) with dark brown polyps, similar to other specimens in Clade HC. A single directive tentacle is usually extended during the day. This species is abundant in many locations in the South Pacific, including Tonga, Rarotonga (Cook Islands) and Mo’orea (Society Islands, French Polynesia), and field images indicate it occurs as far east as the Gambier Archipelago. Many records of A. hyacinthus from French Polynesia are likely A. turbinata, as we have not found A. hyacinthus in the region. The species illustrated as A. hyacinthus in the field guide to the corals of Moorea by Bosserelle et al. (2014) is A. turbinata.
Nominal species not sequenced in this study but subjected to nomenclatural acts
Acropora flabelliformis (Milne-Edwards, 1860) status revised
Madrepora flabelliformis Milne-Edwards, 1860, p. 156.
Acropora flabelliformis (Milne-Edwards) Sheppard, 1987, p. 20.
The status of A. flabelliformis is unclear from recent revisions: Brook (1893) considered A. flabelliformis a junior synonym of A. microclados. However, the holotype differs significantly from Brook’s lectotype of A. microclados in the shape of the radials, which are labellate with rounded or flared openings, or labellate with an extended lip at right angles to the branch v. labellate with very short walls at an oblique angle to the branch in Brook’s lectotype A. microclados. Based on the gross morphology and shape of the radials, A. flabelliformis is likely to be in Clade VI sensu Cowman et al. 2020 but more closely related to A. cytherea-like species than the A. hyacinthus-like species. Veron and Wallace (1984) list A. flabelliformis in their nomenclature (p. 136), but not as a junior synonym of A. microclados despite their stated approach of maintaining previous synonymies (Veron and Pichon 1976). According to the World List of Scleractinia (Hoeksema and Cairns 2025), A. flabelliformis is a junior synonym of A. hyacinthus, following Sheppard (1987). Wallace (1999) suggested that A. flabelliformis was likely to be a junior synonym of A. hyacinthus but did not specifically list it as a junior synonym. Given that (1) the holotype is distinct to any of the species in the current study, and (2) the type locality of A. flabelliformis in the Indian Ocean is outside of the range of A. hyacinthus, we formally remove A. flabelliformis from synonymy with A. hyacinthus. Additional research is required to confirm the identity of this species.
Bruggemann (1879) and Sheppard (1987) suggest this species occurs in the Mascarene Islands in the Western Indian Ocean. Type location: Indian Ocean.
Acropora patella (Studer, 1879) status revised
Madrepora patella (Studer, 1879) p. 526, taf. 1 a–c.
Acropora patella (Studer) Veron & Wallace, 1984, p. 310.
Species removed from synonymy with A. hyacinthus contra Veron and Wallace (1984). Examination of the type material and a translation of Studer’s original description suggest that A. patella is distinct from A. hyacinthus (Supplementary Table S3). Distinguishing features of A. patella are the thick final branches up to 10 mm wide with crowded radial corallites that reach up to 5 mm in height (Supplementary Slides 1–32, Supplementary Table S3). Studer suggested that A. patella was similar to A. cytherea, although with crowded final branches. Although more research is required to confirm the identity of this species, examination of the type material and the original description both indicate that the species is not a junior synonym of A. hyacinthus.
Acropora recumbens (Brook, 1892) status revised
Madrepora recumbens Brook, 1892, p. 461, pl. XXVII, fig. F.
Acropora recumbens (Brook) Wallace, 1978, p. 288.
NHM: 1892.6.8.269. Madrepora recumbens lectotype, Rocky Island, Great Barrier Reef, Australia; 1892.6.8.270 syntype, Rocky Island, Great Barrier Reef, Australia.
Species removed from synonym with A. hyacinthus contra Wallace (1978). The lectotype of A. recumbens has primary branches up to 13 mm in diameter, tapering final branches and tubular radial corallites with oblique openings, which differs to A. hyacinthus that has branches <5 mm in diameter and labellate radial corallites with rounded lips (Supplementary Table S3). Indeed, none of the specimens sequenced in the current study have tubular radial corallites, a feature that indicates A. recumbens is likely a distant relative to A. hyacinthus.
Acropora sinensis (Brook, 1893) status revised
Madrepora sinensis Brook, 1893, p. 114, pl. XXXIII, fig. C.
NHM: 1870.5.9.11. Madrepora sinensis lectotype, Taiwan; 1870.5.9.12. Madrepora sinensis syntype, Taiwan.
Species removed from synonymy with A. hyacinthus contra Veron and Wallace (1984). The type locality of A. sinensis (Taiwan and China) is outside of the geographic range of A. hyacinthus, which occurs in the south-west Pacific. Furthermore, examination of the type material shows these species to be distinct morphologically (Supplementary Table S3): the lectotype of M. sinensis is caespitose v. the tabular–vasiform lectotype of A. hyacinthus, the axial corallite of M. sinensis can reach up to 3 mm diameter v. the 2 mm maximum of A. hyacinthus, and the final branches are 5–6 mm in diameter in M. sinensis v. 3–5 mm in A. hyacinthus (Supplementary Table S3).
Brook’s type series suggest this species occurs in Taiwan and southern China. Type location: Taiwan.
Brook suggested M. sinensis was similar to M. spicifera, whereas Veron and Wallace (1984) synonymised it citing ‘clear resemblance’ to A. hyacinthus. A comparison of the type material suggests all three species are all distinct (Supplementary Table S3).
Acropora surculosa (Dana, 1846) status revised
Madrepora surculosa Dana, 1846, p. 445, pl. 32, fig. 4a, 5.
Acropora surculosa (Dana) Crossland, 1952, p. 214.
USNM: 248, Acropora surculosa (Dana, 1846), lectotype, Fiji; 251, Acropora surculosa (Dana, 1846), syntype, Tahiti.
Species removed from synonymy with A. hyacinthus contra Veron and Wallace (1984). Examination of the type material and original description show A. surculosa and A. hyacinthus differ in numerous morphological characters (Supplementary Table S3). The lectotype of A. surculosa (248) is corymbose with curved final branches reaching up to 35 mm in length (Supplementary Table S3) v. A. hyacinthus that is tabular with final branches ranging from 8 to 25 mm in length. Veron and Wallace (1984) suggest that M. surculosa is possibly A. hyacinthus but with a colony morphology that was outside the range of the specimens they examined from the Great Barrier Reef, clearly indicating that they also observed differences in morphology between A. hyacinthus and M. surculosa. Comparison of the type material also show the similarities between A. surculosa and A. turbinata (Supplementary Table S3), the latter of which was originally described as a form of A. surculosa by Dana (1846). Neither of these nominal species are A. hyacinthus; however, the collection and sequencing of a topotype for A. surculosa is required to resolve the status of A. turbinata and A. surculosa.
Acropora floresensis Rassmussen, nom. nov.
ZooBank: urn:lsid:zoobank.org:act:32B97A21-7E57-4EBD-89A4-2B13C79CC15D
Acropora pectinatus Veron, 2000, p. 264 (incorrect spelling).
Acropora pectinatus Veron, 2002, pp. 44–46 (incorrect spelling).
Acropora pectinata Veron, 2000. – International Commission on Zoological Nomenclature (2011), p. 163.
QMT: HOLOTYPE G55801 Acropora pectinatus Flores, Indonesia. G49306 A. acuminata, East Kalimantan, Indonesia; G53696 A. acuminata, Togian Islands, Indonesia; G53757 A. acuminata, N. Sulawesi, Indonesia.
Described by Veron (2000) as Acropora pectinatus, although due to incorrect spelling of the specific epithet it was renamed Acropora pectinata Veron, 2000 following a ruling by the International Commission of Zoological Nomenclature (2011). This action by the commission did not, however, consider the availability of the names and explicitly stated that it ‘remains for subsequent workers to confirm availability of each name’. In bringing A. pectinata (Brook, 1892) out of synonymy, it became apparent that A. pectinata Veron, 2000 is an invalid junior homonym according to the Principle of Priority (Article 23 of the ICZN Code; International Commission on Zoological Nomenclature 1999). This also renders A. pectinatus Veron, 2000 a secondary homonym under Article 57.3 of the ICZN Code. In addition, according to Article 23.9 of the ICZN Code (International Commission on Zoological Nomenclature 1999), reversal of precedence – which would validate Veron’s junior homonym as the senior name – would only apply if the senior homonym had not been used as a valid name after 1899, and the junior homonym had been used as a valid name in a minimum of 25 works published by 10 authors in the preceding 50 years. Neither of these two criteria are met: at least 15 publications throughout the 20th Century referenced A. pectinata (Brook, 1892), whereas only two cite A. pectinatus Veron, 2000 prior to 2011, and another two works that referenced A pectinata Veron, 2000 after 2011. Consequently, we provide the nomen novum of A. floresensis in place of A. pectinata Veron, 2000 as per Article 60.3 of the ICZN Code.
Acropora pectinata (Veron, 2000) was placed in synonymy with A. acuminata (Verrill, 1864) by Wallace et al. (2012) following a comparison of the holotype of A. pectinata (G55801) with three specimens in the QMT collection identified as A. acuminata: G49306 from East Kalimantan, Indonesia, G53696 from the Togian Islands, Indonesia, and G53757 from N. Sulawesi, Indonesia, but surprisingly not with the holotype of A. acuminata (YPM 1807). The type locality of A. acuminata is the Kingsmill (Gilbert) Islands in the central Pacific, ~6000 km from type locality of A. floresensis or the specimens listed by Wallace et al. (2012). Moreover, examination of the holotype of A. acuminata suggest that the corallum was likely arborescent, whereas Wallace (1999) and Wallace et al. (2012) suggest the species is an arborescent table. Although the three specimens listed by Wallace et al. (2012) might be A. floresensis, we do not consider the species a junior synonym of A. acuminata because A. pectinata should never have been synonymised with A. acuminata without examination of specimens from the type localities of both species.
Other nominal species not sequenced but relevant to this study
Acropora anthocercis (Brook, 1893)
Madrepora anthocercis Brook, 1893, p. 106, pl. XIII, fig. C.
Madrepora coronata Brook, 1892, p. 456 (non Rehberg).
NHM 1892.6.8.235 Madrepora anthocercis lectotype, Palm Islands, Great Barrier Reef, Australia; 1892.6.8.236 Madrepora anthocercis paralectotype, Rocky Islet, Great Barrier Reef, Australia; 1892.6.8.237 Madrepora anthocercis paralectotype, Rocky Islet, Great Barrier Reef, Australia.
This species received little attention for almost 100 years after the original description of Brook, which was based on three specimens collected by W. Saville-Kent from the Great Barrier Reef. The species was not included in an account of GBR Acropora by Wallace (1978), and the only other record prior to Veron and Wallace (1984) is a single specimen from Palawan recorded by Nemenzo (1967). Veron and Wallace (1984) identify 63 specimens in their collection as A. anthocercis, with a distribution ranging from Torres Strait in the north, to Llewellyn Reef in the Capricorn-Bunker group in the south, as far east as Flinders Reef in the Coral Sea and Elizabeth Reef in the Tasman Sea. We have examined this collection and regard these 63 specimens as belonging to multiple species, which includes A. kalindae described above. Importantly, none of the specimens in the collection match the morphology of the lectotype (NHM 1892.6.8.235). In addition, none of the specimens examined by Veron and Wallace (1984) were collected from the type locality of the Palm Islands despite the extensive collections made from that location; likewise, extensive collecting throughout the Palm Islands by the authors have not located a single specimen resembling Brook’s lectotype. The lectotype of A. anthocercis is digitate and the branches have a reverse taper and are up to 12 mm in diameter, as opposed to the tapering branches described by Veron and Wallace (1984) and Wallace (1999). The axial corallites of the A. anthocercis lectotype are tubular and terete described by Brook (1893) as ‘2 or more frequently 3 mm diameter and about 4 mm exsert’; in contrast, Veron and Wallace (1984) describe the axial corallites of A. anthocercis as ‘characteristically large and protuberant, frequently up to 8 mm exsert. They taper from 3 to 5 mm thick at their base to 2–2.5 mm at their tips’. The radial corallites of the A. anthocercis lectotype are primarily nariform, appressed tubular or tubular with rounded openings rather than labellate. The clear morphological differences between Brook’s lectotype and any of the specimens in the Worldwide Acropora Collection at the QMT suggest that none of these specimens are A. anthocercis. Consequently, the only known specimen of this species is the lectotype and it has not been sampled since the 1890s.
Acropora microclados (Ehrenberg, 1834) nom. dub.
According to Brook (1893), who visited the Berlin Natural History Museum (ZMB) in the 1890s, Ehrenberg’s type series consisted of an unspecified number of specimens of two or three species, including Madrepora spicifera Dana, 1846 and M. flabelliformis Milne-Edwards, 1860. Brook suggested that the specimen he identified as M. flabelliformis is the only specimen in the type series that matches Ehrenberg’s description of A. microclados and designated this as the ‘type’. Brook described the specimen but gave no registration numbers for this or any other specimens in the type series. However, Brook’s description of the specimen indicates he was referring to ZMB-Cni-0845: ‘Corallum flabellate or shallow vasiform; the part preserved consists of a frond 26 cm high and 24 cm wide above, but tapering almost to a point at the base’.
In their treatment of A. microclados (Ehrenberg), Veron and Wallace (1984) state that there are ‘two type specimens in the ZMB collection (numbers 845 and 1054), number 845 from an unknown locality is in close agreement with coralla of the present series [i.e. A. microclados sensu Veron & Wallace, 1984 from the GBR] from shallow reef fronts’. However, ZMB-Cni-845, from the Gerresheim collection and therefore possibly from the ‘East Indies’ or the Red Sea, is closer to A. spicifera (Dana 1846) as suggested by Brook than A. microclados sensu Veron & Wallace (1984). In the catalogue of the Berlin Museum ZMB-Cni-1054 is listed as ‘cf microclados’, which suggests it was not part of Ehrenberg’s type series. Indeed, ZMB-Cni-1054 is almost certainly the Caribbean species A. palmata. Veron and Wallace (1984) do not appear to have read Brook’s account of the species. Wallace (1999) claims to have examined ZMB-Cni-1054 and designated this specimen as the lectotype (Table 1, p. 8). However, as discussed above ZMB-Cni-1054 is a flattened frond ~100 × 40 mm in diameter; it is almost certainly A. palmata and bears little resemblance to A. microclados sensu Veron and Wallace (1984) from the GBR or Wallace (1999), which is described as corymbose. Indeed, none of the specimens illustrated in Veron and Wallace (1984) or Wallace (1999) bear any similarity to ZMB-Cni-1054. Furthermore, Wallace suggests that A. microclados can be confused with Acropora nasuta or A. cerealis, which typically form corymbose colonies with nariform radial corallites. In other words, Wallace’s interpretation is very different from that of Brook and does not match the lectotype.
One of the authors (AHB) examined the collection at ZMB in Berlin in 2018 and imaged four specimens in the putative type series of A. microclados ZMB-Cni-845 (Brook’s ‘type’ for A. microclados); ZMB-Cni-847 (A. microclados sensu Veron & Wallace (1984)); ZMB-Cni-1054 (A. cf. palmata); and ZMB-Cni-1995 (A. cf. spicifera).
The tabular morphology and labellate radial corallites suggest that Brook’s type of A. microclados (ZMB-Cni-845) is likely to be in Clade VI. However, the corallites have very short lower wall, essentially hugging the branch, as opposed to the walls being at right angles to the branch in A. hyacinthus-like species. Based on the shape of the radials, A. microclados sensu Brook is potentially more closely related to A. pharaonis (Milne-Edwards & Haime, 1860) than to A. hyacinthus. However, although the discussion above highlights the conflicting opinions regarding the morphology of A. microclados, they are essentially irrelevant. Referring to the original description, Ehrenberg (1834) cites Ellis’ table 57 (see Supplementary Fig. S5) as potentially the only source of a specimen on which he based his description. Table 57 is an illustration of a specimen that is clearly two distinct species patched together and none of the specimens in the putative type series resemble either of these species. It seems Brook was incorrect to assume that the specimen now numbered 845 was the specimen on which Ehrenberg based his description. In particular, Ehrenberg describes the specimens as ‘creeping’, which matches the image in Ellis but not any of the other specimens in the putative type series.
Given this confusion in the literature, the lack of a definitive type and the inadequacy of the original description to allow for the designation of a neotype we consider Heteropora microclados Ehrenberg, 1834 nomen dubium.
Acropora tanegashimensis Veron, 1990
Acropora tanegashimensis Veron, 1990, p. 109, fig. 13, 14 & 73.
QMT: G32477 Acropora tanegashimensis holotype, Sumiyoshi, Tanegashima, Japan; Japan: G62308, G62322 Tanegashima; G62321, G62322 Shikoku; G62347, G62349 Wakayama.
Described from the high-latitude (30°N) island of Tanegashima in Japan, which harbours coral communities growing on rocky foreshores but does not support carbonate reef development. Veron (1990) notes that the species is similar to A. hyacinthus but is distinguished by having more crowded radial corallites, indistinct axial corallites and a different colour. Examination of the holotype of A. tanegashimensis G32477 reveals differences in the length of the radial corallites, which are shorter (1.5–1.7 mm in height) than in A. hyacinthus (1–3 mm in height), and the distribution of A. tanegashimensis in the subtropical north-west Pacific does not overlap with A. hyacinthus. Veron (1990) recorded the species only from Tanegashima; however, additional specimens in the QMT collections from Nishidomari, Shikoku (32.77°N, 132.73°E) and Kushimoto, Wakayama (33.48°N, 135.74°E) indicate this species occurs in other locations in subtropical Japan. Sugihara et al. (2015) lists A. tanegashimensis as occurring from Kushimoto, Wakayama to Tanegashima, noting on the rarity of this species in Tanegashima. We identify our specimens from Sesoko Island (26.64°N, 127.86°E) as A. cf. tanegashimensis because they are similar to A. tanegashimensis in that they tabular with labellate radial corallites with round lips (Supplementary Table S3); however, they differ from the holotype in characters in that the branches of our specimens are thinner, the axial corallites are taller and distinct from the radials, and the radials are less crowded (Supplementary Slides 1–32). We hypothesis that the specimens in our study are a tropical morph of A. tanegashimensis. Alternatively they might represent an undescribed species. Collection and sequencing of topotype of is A. tanegashimensis required to test these alternative hypotheses and confirm the distribution of A. tanegashimensis.
Discussion
Our integrated approach demonstrates that the taxonomic diversity of the tabular Acropora is considerably higher than that proposed in the most recent taxonomic revisions of the group (Veron and Wallace 1984; Wallace 1999). Importantly, in contrast to Wallace (1999), Veron (2000), Wallace et al. (2012) and Veron et al. (2016), none of the species in the hyacinthus-complex has a geographic range spanning the Western Indian Ocean to the Central Pacific. Further investigations and focused sampling are required to determine if any additional species within this clade occur in under-sampled locations such as the western Indian Ocean and the northern Pacific. We also found numerous examples of distinct, sympatric species that were incorrectly synonymised. For example, A. hyacinthus sensu Veron & Wallace (1984) in eastern Australia is at least four distinct species; A. hyacinthus, A. pectinata, A. harriottae sp. nov. and A. tersa sp. nov. (Supplementary Fig. S6). Given the ecological dominance of tabular Acropora across the Indo-Pacific and their widespread use in experimental research and reef restoration, our results have implications for a range of basic and applied research questions.
Different species delimitation analyses were not always congruent in the number of species indicated. These inconsistencies were largely driven by STRUCTURE analysis, which had 10 fewer clusters than our initial PSH assignment, and 9 fewer clusters than the best BFD model. This may have been a result of two factors known to influence STRUCTURE analysis: (i) uneven sample size across populations (Gilbert 2016), and (ii) analysing closely related populations. Firstly, we found that PSH with a greater sample size (n > 5) were more likely to resolve a distinct population than PSH with less representation in some situation but not others. For example, A. turbinata (n = 12) and A. tersa (n = 11) in subclade HC both formed distinct genetic clusters, where A. kalinda (n = 3), A. uogi (n = 2) and A. spicifera (n = 1) did not. Similarly, within subclade HD, A. pectinata (n = 10) and A. harriottae (n = 5) both formed distinct genetic clusters compared to PSH with <5 samples, apart from A. conferta (n = 2), which formed a distinct lineage. Similarly, in subclade HA the larger sample sizes for A. hyacinthus (n = 26) and A. nyinggulu sp. nov. (n = 17) formed distinct genetic clusters, but the small A. cf. tanegashimensis (n = 4) population did not. Furthermore, the Evanno method of calculating ΔK has been shown to favour K = 2 in closely related populations (Evanno et al. 2005), which is consistent with our results for clades HA, HB and HC that all resolved K = 2 as the most likely number of clusters according to the peak ΔK score. Finally, as the minimum number of clusters (KSTRUCTURE) possible in STRUCTURE analysis is two it is likely that Clade HB is a single independently evolving lineage, consistent with our PSH.
Support for the PSHs among the other SNP-based automated species delimitation methods varied depending on the subclade. For subclade HA, all other methods supported a higher number of species than PSHs, generally splitting A. hyacinthus into distinct GBR and central Pacific populations. Similarly, A. nyinggulu was consistently split into three populations representing a Western Australian lineage, the Indo-Australian Archipelago (IAA) lineage and Ryukyu Islands lineage (Fig. 4, 5 and 6). However, the specimens in the current study and those examined from the Worldwide Acropora Collection (Wallace et al. 2012) from across the range sampled here (Western Australia, the IAA and Japan – see taxonomic account) do not exhibit clear morphological differences. Consequently, based on inconclusive evidence from molecular analyses and lack of prominent morphological distinctions, we consider A. nyinggulu to be a single species across its range. Further sampling of specimens from the Coral Triangle region is required to determine whether the Japanese and Western Australian species represent reciprocally monophyletic species or a single species with geographically structured populations.
Numerous studies using molecular data indicate that the taxonomy of Veron and Wallace (1984), Wallace (1999) and Veron (2000) do not accurately reflect genetic diversity within tabular Acropora (e.g. Ladner and Palumbi 2012; Suzuki et al. 2016; Sheets et al. 2018; Nakabayashi et al. 2019). However, these studies have generally focussed on population genetics rather than taxonomy and typically refer to the lineages identified as ‘cryptic species’ without conducting a taxonomic examination of whether these lineages show morphological differences. In contrast, Ramírez-Portilla et al. (2022) examined three co-occurring tabular Acropora in the Ryukyu Islands, Japan (referred to as A. cf. bifurcata, A. aff. cytherea and A. aff. hyacinthus) and found that the three species could be delineated based on molecular, morphological and biological evidence (i.e. the species did not hybridise – see also Furukawa et al. 2024). Indeed, because we included samples in the current study from Ramírez-Portilla et al. (2022; Supplementary Table S2) we can confirm that two of the species were A. nyinggulu (=A. aff. hyacinthus) and A. bifurcata (=A. cf. bifurcata).
Based on the information provided here, we inferred the likely species identifications for several of the putatively cryptic lineages (HyaB – E) identified by Ladner and Palumbi (2012) and Suzuki et al. (2016) by comparison of in text figures and geographic location with the species resolved herein. HyaB, figured in Suzuki et al. (2016, fig. 2). has a range spanning the north, south and west Pacific Ocean closely resembles specimens in lineages A. sp. VI-HB in the current study and could provide further evidence of the validity of this as a single species. HyaC, figured in both Ladner and Palumbi (2012; Supplementary Fig. S2) and Suzuki et al. (2016, fig. 2a–b) is a morphological match for A. tersa and the presence of this species within the Kuroshio Triangle could indicate a range expansion of this species slightly north of Palau in the western Pacific. HyaD, figured in Suzuki et al. (2016, fig. 2) is a morphological match for A. bifurcata in the current study. Interestingly, Ladner and Palumbi (2012) found this lineage in the Pacific spanning Australia, Samoa and Palmyra, which is outside of the range we present for A. bifurcata (Supplementary Fig. S6). As no images were provided for this lineage in Ladner and Palumbi (2012), we are unable to verify this identification. Finally, HyaE which was only found in Samoa by Ladner and Palumbi (2012; Supplementary Fig. S2) is A. turbinata. The colony figured matches the morphology of this species and the remarks in the text indicate that this lineage is easily identifiable with ‘thicker, longer and more distinct branches’, which is also reflected by the type material and the key to the types we present (Supplementary Table S3). Our re-evaluation of species identifications based on our revised taxonomic framework demonstrates that at least some putatively ‘cryptic’ species can be distinguished based on morphology and other lines of evidence. However, it also demonstrates the importance of providing clear figures, descriptions and ideally voucher specimens for all species or lineages when presenting data on putatively cryptic or taxonomically unresolved groups.
We found that the Acropora species identified here have much smaller geographic ranges than currently assumed. In contrast to the geographic ranges proposed by Wallace (1999), Veron (2000) and Veron et al. (2016), none of the 12 species examined here are geographically widespread across the Indo-Pacific. No specimens from this group were collected from further west than the eastern Indian Ocean (Western Australia and the Andaman Sea) despite extensive collections at locations including the Australian Indian Ocean Territory (Christmas and Cocos (Keeling) Islands) and the Red Sea. However, additional sampling in other regions in the Western Indian Ocean may reveal additional lineages belonging to this group. Examination of specimens in the Worldwide Acropora Collection at the Queensland Museum Tropics also revealed no specimens that matched the morphology of species identified here in the Western Indian Ocean, although one species with morphological affinities to A. tersa from the Maldives is figured in Wallace et al. (2012) as A. hyacinthus.
Introgression and hybridisation are proposed mechanisms to explain the presence of cryptic species and species richness of reef corals (Richards and Hobbs 2015; Mao et al. 2018), particularly in A. hyacinthus, which has been described as a ‘pseudocryptic complex’ that represents a global syngameon (Ladner and Palumbi 2012; Suzuki et al. 2016). However, recent evidence using more advanced molecular methods and a taxonomic approach (Ramírez-Portilla et al. 2022; Furukawa et al. 2024) suggests that this may be a genetic artefact, and that hybridisation in tabular Acropora (and other corals e.g. Bongaerts et al. 2021) is far less common than previously assumed. In this study, we found some evidence for hybridisation between species in the STRUCTURE analysis (Fig. 4) across all clades, with interspecific admixture present among multiple lineages. However, no individuals showing intraspecific admixture were found and thus we cannot disregard that this admixture may have been caused by (i) uneven sampling across populations (Gilbert 2016) or (ii) introgression caused by ‘secondary genomic admixture’ from a recent hybridising ancestor (Mao et al. 2018). Further phylogenomic analysis specifically investigating hybridisation, combined with other evidence such as breeding trials, are required to better understand the prevalence and also pre- and post-zygotic barriers to hybridisation in synchronous broadcast-spawning Acropora. Even if hybridisation is detected, it is now clear that species can still be delineated in lineages where ancestral or modern gene flow has occurred (Jackson et al. 2017).
Our integrated approach, combining type material and descriptions with topotype specimens, reveals that the taxonomic revisions of the late 20th Century (Veron and Wallace 1984; Wallace 1999) substantially underestimated the diversity of tabular Acropora. For example, at least four valid species (A. conferta, A. pectinata, A. bifurcata and A. turbinata) were synonymised with A. hyacinthus, with differences in morphology between the type material attributed to environmental or geographic variation within widespread species. Our results show that none of the synonymised species are sister to A. hyacinthus or fall within the same subclade in the molecular phylogeny. This result highlights the necessity of taxonomic revisions to not only highlight inconsistencies between molecular and morphological based taxonomy, but to also verify past decisions and develop a robust taxonomy for future coral biodiversity research.
Our findings have clear implications for understanding of extinction risk and informing conservation of Acropora (Bridge et al. 2020; Edgar 2025). For example, A. hyacinthus is currently listed as Endangered by International Union for Conservation of Nature (IUCN) Red List (Nuñez Lendo 2024) because it is highly susceptible to bleaching and disease and is predicted to decline in abundance by 62% by 2050. Although its population trend is listed as decreasing, the species is also considered to occur on most reefs across the Indian and Pacific Oceans (Supplementary Fig. S7). However, our results show A. hyacinthus is restricted to the south-west Pacific, and specimens previously considered A. hyacinthus are distinct species; consequently, its population size is likely to be far smaller than previous estimates. Similarly, A. spicifera, which is listed as Endangered (Nuñez Lendo 2024), is restricted to the western IAA and eastern Indian Ocean, a much smaller geographic range that than reported on the IUCN Red List (Supplementary Fig. S7). The increased species richness, including a number of potentially unnamed new species, and smaller geographic ranges of tabular Acropora species illustrated here suggests a high risk of ‘silent extinction’ (Howard and Bickford 2014) for these unnamed species and clearly illuminate the issues with conducting risk assessments without adequate data (Gutiérrez and Helgen 2013; Edgar 2025). Therefore, our results support previous studies (e.g. Bridge et al. 2020; Raja et al. 2021) that find that the IUCN Red List does not accurately reflect species extinction risk in corals. Consequently, the Red List provides little value for conservation prioritisation until focused taxonomic research can resolve uncertainties and inconsistencies in coral species classifications, particularly within species or species complexes with putatively large geographic distributions.
Data availability
Photographs of all specimens included in this study are available on the Queensland Museum website (see https://researchassets.qm.qld.gov.au/fotoweb/archives/5071-CoralBank-Research/). Molecular data are available at: BioProject: PRJNA1266366, BioSample, SAMN48781414-1546. Molecular data for specimens previously published by Cowman et al. (2020) are available at BioProject PRJNA601826, SRA GenBank SUB6852542, BioSample number SAMN13871686-1781; and specimens previously published by Ramírez-Portilla et al. (2022), BioProject:PRJNA665126 are available from the Dryad Digital Repository (Ramírez-Portilla et al. 2021).
Declaration of funding
This research was supported by an Australian Government Research Training Program (RTP) Fee Offset Scholarship to Sage H. Rassmussen, by the Australian Research Council (ARC) Centre of Excellence Programme (CE140100020) and the Japan Society for the Promotion of Science (JSPS) Invitational Fellowships for Research in Japan (L22550) to Andrew H. Baird, ARC DECRA Fellowships to Peter F. Cowman (DE170100516) and Tom C. L. Bridge (DE180100746), and the Queensland Museum’s Project DIG.
Acknowledgements
Thank you to all who assisted in field and lab work, and in providing and photographing specimens including Hanaka Mera, Jeremy Horowitz, Francesca Benzoni, Stefano Borghi, David Burdick and the captain and crew of the MV Kalinda. Thank you also to Lauriane Baraf for your help with SRA submission. We also acknowledge that the material from the Kamaran Islands, Yemen, was collected and provided by Francesca Benzoni. The authors are grateful also to E. Dutrieux (CREOCEAN), C. H. Chaineau (Total SA), R. Hirst and M. AbdulAziz (YLNG) and the permission of the Ministry of Oil and Minerals, Ministry of Transports (Maritime Affairs Authority), Ministry of Fish Wealth, and Ministry of Water and Environment (Environmental Protection Authority) for allowing and supporting research in Yemen. We thank S. Basheen (Professional Divers Yemen) and A. Caragnano for their help in logistics and field work respectively. We extend our thanks to the Nganhurra Thanardi Garrbu Aboriginal Corporation RNTBC for their consultation in the naming of a new species from Western Australia. We also extend our thanks to all national agencies and personnel that provided permission for conducting of this research. Finally, thank you to all the museum curators and collection managers that provided type material for examination.
Author contributions
The study was designed by Sage H. Rassmussen, Peter F. Cowman, Andrew H. Baird and Tom C. L. Bridge. Sample collection was collectively made possible with contributions by Sage H. Rassmussen, Andrew H. Baird, Tom C. L. Bridge, Augustine J. Crosbie, Victor Bonito, Frederic Sinniger, Saki Harii, TN, Patrick C. Cabaitan, Nur Fadli, Chun-Hong Tan, Teina Rongo, Danwei Huang and Tuikolongahau Halafihi. DNA extraction and preparation was performed by Julia Yun-Hsuan Hung, and sequencing made possible through Peter F. Cowman, Andrew H. Baird, Andrea M. Quattrini and Tom C. L. Bridge. Molecular data analysis was performed by Sage H. Rassmussen with assistance from Peter F. Cowman. Morphological data collection and analysis was performed by Sage H. Rassmussen. Taxonomic investigations were performed by Sage H. Rassmussen, with the assistance of Tom C. L. Bridge and Andrew H. Baird. All figures were created by Sage H. Rassmussen. Field and lab images were contributed by Sage H. Rassmussen, Andrew H. Baird, Augustine J. Crosbie and Tom C. L. Bridge. The manuscript was written by Sage H. Rassmussen, with significant edits and contributions made by Peter F. Cowman, Andrew H. Baird and Tom C. L. Bridge. All authors contributed to the final manuscript with relevant expertise.
References
Adams M, Raadik TA, Burridge CP, Georges A (2014) Global biodiversity assessment and hyper-cryptic species complexes: more than one species of elephant in the room? Systematic Biology 63(4), 518-533.
| Crossref | Google Scholar | PubMed |
Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko CB, Brandão SN, Bray RA, Bruce NL, Cairns SD, Chan TY, et al. (2012) The magnitude of global marine species diversity. Current Biology 22(23), 2189-2202.
| Crossref | Google Scholar | PubMed |
Baird AH, Hughes TP (2000) Competitive dominance by tabular corals: an experimental analysis of recruitment and survival of understorey assemblages. Journal of Experimental Marine Biology and Ecology 251(1), 117-132.
| Google Scholar |
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19(5), 455-477.
| Crossref | Google Scholar | PubMed |
Baums IB, Baker AC, Davies SW, Grottoli AG, Kenkel CD, Kitchen SA, Kuffner IB, LaJeunesse TC, Matz MV, Miller MW, Parkinson JE, Shantz AA (2019) Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications 29(8), e01978.
| Crossref | Google Scholar | PubMed |
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22(3), 148-155.
| Crossref | Google Scholar | PubMed |
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15), 2114-2120.
| Crossref | Google Scholar | PubMed |
Bongaerts P, Cooke IR, Ying H, Wels D, den Haan S, Hernandez-Agreda A, Brunner CA, Dove S, Englebert N, Eyal G, Forêt S, Grinblat M, Hay KB, Harii S, Hayward DC, Lin Y, Mihaljević M, Moya A, Muir P, Sinniger F, et al. (2021) Morphological stasis masks ecologically divergent coral species on tropical reefs. Current Biology 31(11), 2286-2298.e2288.
| Crossref | Google Scholar | PubMed |
Bortolus A (2008) Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. Ambio 37(2), 114-118.
| Crossref | Google Scholar | PubMed |
Boström-Einarsson L, Babcock RC, Bayraktarov E, Ceccarelli D, Cook N, Ferse SCA, Hancock B, Harrison P, Hein M, Shaver E, Smith A, Suggett D, Stewart-Sinclair PJ, Vardi T, McLeod IM (2020) Coral restoration – A systematic review of current methods, successes, failures and future directions. PLoS ONE 15(1), e0226631.
| Crossref | Google Scholar | PubMed |
Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10(4), e1003537.
| Crossref | Google Scholar | PubMed |
Bridge TCL, Luiz OJ, Kuo C-Y, Precoda K, Madin EMP, Madin JS, Baird AH (2020) Incongruence between life-history traits and conservation status in reef corals. Coral Reefs 39(2), 271-279.
| Crossref | Google Scholar |
Bridge TCL, Cowman PF, Quattrini AM, Bonito VE, Sinniger F, Harii S, et al. (2024) A tenuis relationship: traditional taxonomy obscures systematics and biogeography of the ‘Acropora tenuis’ (Scleractinia: Acroporidae) species complex. Zoological Journal of the Linnean Society 202(1), zlad062.
| Crossref | Google Scholar |
Brook G (1892) Preliminary descriptions of new species of Madrepora in the collection of the British Museum. Part II. Annals and Magazine of Natural History 10, 451-465.
| Crossref | Google Scholar |
Brook G (1893) ‘Catalogue of the madreporarian corals in the British Museum (Natural History). Vol. 1. The genus Madrepora.’ (Trustees of the British Museum: London, UK) Available at https://www.biodiversitylibrary.org/item/44856
Bruggemann F (1879) Corals: an account of the petrological, botanical, and zoological collections made in Kerguelen’s Land and Rodriguez during the Transit of Venus Expeditions, carried out by order of Her Majesty’s Government in the years 1874–75. Philosophical Transactions of the Royal Society of London 168, 569-579.
| Crossref | Google Scholar |
Bryant D, Bouckaert R, Felsenstein J, Rosenberg NA, RoyChoudhury A (2012) Inferring species trees directly from biallelic genetic markers: bypassing gene trees in a full coalescent analysis. Molecular Biology and Evolution 29(8), 1917-1932.
| Crossref | Google Scholar | PubMed |
Cardoso P, Borges PA, Triantis KA, Ferrández MA, Martín JL (2011) Adapting the IUCN Red List criteria for invertebrates. Biological Conservation 144(10), 2432-2440.
| Crossref | Google Scholar |
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17(4), 540-552.
| Crossref | Google Scholar | PubMed |
Chhatre VE, Emerson KJ (2017) StrAuto: automation and parallelization of STRUCTURE analysis. BMC Bioinformatics 18(1), 192.
| Crossref | Google Scholar | PubMed |
Cooke I, Ying H, Forêt S, Bongaerts P, Strugnell JM, Simakov O, Zhang J, Field MA, Rodriguez-Lanetty M, Bell SC, Bourne DG, van Oppen MJ, Ragan MA, Miller DJ (2020) Genomic signatures in the coral holobiont reveal host adaptations driven by Holocene climate change and reef specific symbionts. Science Advances 6(48), eabc6318.
| Crossref | Google Scholar | PubMed |
Cowman PF, Quattrini AM, Bridge TTCL, Watkins-Colwell GJ, Fadli N, Grinblat M, Roberts TE, McFadden CS, Miller DJ, Baird AH (2020) An enhanced target-enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Molecular Phylogenetics and Evolution 153, 106944.
| Crossref | Google Scholar | PubMed |
Cros A, Toonen RJ, Davies SW, Karl SA (2016) Population genetic structure between Yap and Palau for the coral Acropora hyacinthus. PeerJ 4, e2330.
| Crossref | Google Scholar | PubMed |
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, 1000 Genomes Project Analysis Group (2011) The variant call format and VCFtools. Bioinformatics 27(15), 2156-2158.
| Crossref | Google Scholar | PubMed |
De Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56(6), 879-886.
| Crossref | Google Scholar | PubMed |
Derkarabetian S, Benavides LR, Giribet G (2019) Sequence capture phylogenomics of historical ethanol-preserved museum specimens: unlocking the rest of the vault. Molecular Ecology Resources 19(6), 1531-1544.
| Crossref | Google Scholar | PubMed |
Edgar GJ (2025) IUCN Red List criteria fail to recognise most threatened and extinct species. Biological Conservation 301, 110880.
| Crossref | Google Scholar |
Ehrenberg CG (1834) Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen, und besonders des rothen Meeres, nebst einem Versuche zur physiologischen Systematik derselben. Abhandlungen der Königlichen Akademie der Wissenschaften, Berlin 1, 225-380 [In German].
| Google Scholar |
Erickson KL, Pentico A, Quattrini AM, McFadden CS (2021) New approaches to species delimitation and population structure of anthozoans: two case studies of octocorals using ultraconserved elements and exons. Molecular Ecology Resources 21(1), 78-92.
| Crossref | Google Scholar | PubMed |
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Molecular Ecology 14(8), 2611-2620.
| Crossref | Google Scholar | PubMed |
Faircloth BC (2016) PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32(5), 786-788.
| Crossref | Google Scholar | PubMed |
Faircloth BC, McCormack JE, Crawford NG, Harvey MG, Brumfield RT, Glenn TC (2012) Ultraconserved elements anchor thousands of genetic markers spanning multiple evolutionary timescales. Systematic Biology 61(5), 717-726.
| Crossref | Google Scholar | PubMed |
Fisher R, O’Leary RA, Low-Choy S, Mengersen K, Knowlton N, Brainard RE, Caley MJ (2015) Species richness on coral reefs and the pursuit of convergent global estimates. Current Biology 25(4), 500-505.
| Crossref | Google Scholar | PubMed |
Francis RM (2017) pophelper: an R package and web app to analyse and visualize population structure. Molecular Ecology Resources 17(1), 27-32.
| Crossref | Google Scholar | PubMed |
Fukami H, Budd AF, Paulay G, Solé-Cava A, Allen Chen C, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427(6977), 832-835.
| Crossref | Google Scholar | PubMed |
Furukawa M, Kitanobo S, Ohki S, Teramoto MM, Hanahara N, Morita M (2024) Integrative taxonomic analyses reveal that rapid genetic divergence drives Acropora speciation. Molecular Phylogenetics and Evolution 195, 108063.
| Crossref | Google Scholar | PubMed |
Gardner SG, Camp EF, Smith DJ, Kahlke T, Osman EO, Gendron G, Hume BCC, Pogoreutz C, Voolstra CR, Suggett DJ (2019) Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecology and Evolution 9(3), 938-956.
| Crossref | Google Scholar | PubMed |
Gilbert KJ (2016) Identifying the number of population clusters with structure: problems and solutions. Molecular Ecology Resources 16(3), 601-603.
| Crossref | Google Scholar | PubMed |
Gold Z, Palumbi SR (2018) Long-term growth rates and effects of bleaching in Acropora hyacinthus. Coral Reefs 37(1), 267-277.
| Crossref | Google Scholar |
Gómez-Corrales M, Prada C (2020) Cryptic lineages respond differently to coral bleaching. Molecular Ecology 29(22), 4265-4273.
| Crossref | Google Scholar | PubMed |
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32(2), 315-326.
| Crossref | Google Scholar |
Gutiérrez EE, Helgen KM (2013) Mammalogy: outdated taxonomy blocks conservation. Nature 495(7441), 314.
| Crossref | Google Scholar | PubMed |
Harvey MG, Smith BT, Glenn TC, Faircloth BC, Brumfield RT (2016) Sequence capture versus restriction site associated DNA sequencing for shallow systematics. Systematic Biology 65(5), 910-924.
| Crossref | Google Scholar |
Hending D (2024) Cryptic species conservation: a review. Biological Reviews 100, 258-274.
| Crossref | Google Scholar | PubMed |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35(2), 518-522.
| Crossref | Google Scholar | PubMed |
Hoeksema BW, Cairns S (2025) World List of Scleractinia. In ‘World Register of Marine Species (WoRMS)’. (Flanders Marine Institute) Available at https://www.marinespecies.org/scleractinia [Verified 14 July 2025]
Hongo C, Kayanne H (2011) Key species of hermatypic coral for reef formation in the northwest Pacific during Holocene sea-level change. Marine Geology 279(1), 162-177.
| Crossref | Google Scholar |
Hoogenboom MO, Frank GE, Chase TJ, Jurriaans S, Álvarez-Noriega M, Peterson K, Critchell K, Berry KLE, Nicolet KJ, Ramsby B, Paley AS (2017) Environmental drivers of variation in bleaching severity of Acropora species during an extreme thermal anomaly. Frontiers in Marine Science 4, 376.
| Crossref | Google Scholar |
Howard SD, Bickford DP (2014) Amphibians over the edge: silent extinction risk of data deficient species. Diversity and Distributions 20(7), 837-846.
| Crossref | Google Scholar |
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543(7645), 373-377.
| Crossref | Google Scholar | PubMed |
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, et al. (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359(6371), 80-83.
| Crossref | Google Scholar | PubMed |
International Commission on Zoological Nomenclature (2011) Coral taxon names published in ‘Corals of the world’ by J.E.N. Veron (2000): potential availability confirmed under Art. 86.1.2. Bulletin of Zoological Nomenclature 68(3), 162-166.
| Google Scholar |
Jackson ND, Carstens BC, Morales AE, O’Meara BC (2017) Species delimitation with gene flow. Systematic Biology 66(5), 799-812.
| Crossref | Google Scholar | PubMed |
Johns KA, Osborne KO, Logan M (2014) Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Coral Reefs 33(3), 553-563.
| Crossref | Google Scholar |
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24(11), 1403-1405.
| Crossref | Google Scholar | PubMed |
Jörger KM, Schrödl M (2013) How to describe a cryptic species? Practical challenges of molecular taxonomy. Frontiers in Zoology 10(1), 59.
| Crossref | Google Scholar | PubMed |
Junier T, Zdobnov EM (2010) The Newick utilities: high-throughput phylogenetic tree processing in the Unix shell. Bioinformatics 26(13), 1669-1670.
| Crossref | Google Scholar | PubMed |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14(6), 587-589.
| Crossref | Google Scholar | PubMed |
Kamvar ZN, Tabima JF, Grünwald NJ (2014) Poppr: an R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2, e281.
| Crossref | Google Scholar | PubMed |
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30(14), 3059-3066.
| Crossref | Google Scholar | PubMed |
Kerry JT, Bellwood DR (2015) The functional role of tabular structures for large reef fishes: avoiding predators or solar irradiance? Coral Reefs 34, 693-702.
| Crossref | Google Scholar |
Kerry JT, Bellwood DR (2016) Competition for shelter in a high-diversity system: structure use by large reef fishes. Coral Reefs 35, 245-252.
| Crossref | Google Scholar |
Ladner JT, Palumbi SR (2012) Extensive sympatry, cryptic diversity and introgression throughout the geographic distribution of two coral species complexes. Molecular Ecology 21(9), 2224-2238.
| Crossref | Google Scholar | PubMed |
Lanfear R, Hahn MW (2024) The meaning and measure of concordance factors in phylogenomics. Molecular Biology and Evolution 41, msae214.
| Crossref | Google Scholar | PubMed |
Leaché AD, Fujita MK, Minin VN, Bouckaert RR (2014) Species delimitation using genome-wide SNP data. Systematic Biology 63(4), 534-542.
| Crossref | Google Scholar | PubMed |
Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, 1303.3997 [Preprint, published 26 May 2013].
| Crossref | Google Scholar |
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25(14), 1754-1760.
| Crossref | Google Scholar | PubMed |
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16), 2078-2079.
| Crossref | Google Scholar | PubMed |
Mai U, Mirarab S (2018) TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19(5), 272.
| Crossref | Google Scholar | PubMed |
Mao Y, Economo EP, Satoh N (2018) The roles of introgression and climate change in the rise to dominance of Acropora corals. Current Biology 28(21), 3373-3382.e3375.
| Crossref | Google Scholar | PubMed |
Matias A, Popovic I, Thia JA, Cooke IR, Torda G, Lukoschek V (2023) Cryptic diversity and spatial genetic variation in the coral Acropora tenuis and its endosymbionts across the Great Barrier Reef. Evolutionary Applications 16(2), 293-310.
| Crossref | Google Scholar | PubMed |
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20(9), 1297-1303.
| Crossref | Google Scholar | PubMed |
Minh BQ, Nguyen MAT, von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30(5), 1188-1195.
| Crossref | Google Scholar | PubMed |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R (2020a) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37(5), 1530-1534.
| Crossref | Google Scholar |
Minh BQ, Hahn MW, Lanfear R (2020b) New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37(9), 2727-2733.
| Crossref | Google Scholar |
Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B (2011) How many species are there on earth and in the ocean? PLoS Biology 9(8), e1001127.
| Crossref | Google Scholar | PubMed |
Morais J, Tebbett SB, Morais RA, Bellwood DR (2024) Natural recovery of corals after severe disturbance. Ecology Letters 27(1), e14332.
| Crossref | Google Scholar | PubMed |
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proceedings of the National Academy of Sciences 116(21), 10586-10591.
| Crossref | Google Scholar | PubMed |
Nakabayashi A, Yamakita T, Nakamura T, Aizawa H, Kitano YF, Iguchi A, Yamano H, Nagai S, Agostini S, Teshima KM, Yasuda N (2019) The potential role of temperate Japanese regions as refugia for the coral Acropora hyacinthus in the face of climate change. Scientific Reports 9(1), 1892.
| Crossref | Google Scholar | PubMed |
Naugle MS, Denis H, Mocellin VJ, Laffy PW, Popovic I, Bay LK, Howells EJ (2024) Heat tolerance varies considerably within a reef-building coral species on the Great Barrier Reef. Communications Earth & Environment 5(1), 525.
| Crossref | Google Scholar |
Nemenzo F (1967) Systematic studies on Philippine shallow-water scleractinians: VI. Suborder Astrocoeniida. Natural and Applied Science Bulletin 20(1), 1-141.
| Google Scholar |
Nemenzo F (1971) Systematic studies on Philippine shallow-water scleractinians: VII. Additional forms. Natural and Applied Science Bulletin, University of the Philippines 23, 141-209.
| Google Scholar |
Nuñez Lendo CI (2024) Brush coral Acropora hyacinthus. In ‘The IUCN Red List of Threatened Species 2024’. e.T218764304A165910938. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/218764304/165910938 [Verified 14 July 2025]
Ortiz JC, Pears RJ, Beeden R, Dryden J, Wolff NH, Gomez Cabrera MDC, Mumby PJ (2021) Important ecosystem function, low redundancy and high vulnerability: the trifecta argument for protecting the Great Barrier Reef’s tabular Acropora. Conservation Letters 14(5), e12817.
| Crossref | Google Scholar |
Pante E, Puillandre N, Viricel A, Arnaud-Haond S, Aurelle D, Castelin M, Chenuil A, Destombe C, Forcioli D, Valero M, Viard F, Samadi S (2015a) Species are hypotheses: avoid connectivity assessments based on pillars of sand. Molecular Ecology 24(3), 525-544.
| Crossref | Google Scholar |
Pante E, Schoelinck C, Puillandre N (2015b) From integrative taxonomy to species description: one step beyond. Systematic Biology 64(1), 152-160.
| Crossref | Google Scholar |
Pearman JK, Anlauf H, Irigoien X, Carvalho S (2016) Please mind the gap – visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Marine Environmental Research 118, 20-30.
| Crossref | Google Scholar | PubMed |
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344(6187), 1246752.
| Crossref | Google Scholar | PubMed |
Plaisance L, Caley MJ, Brainard RE, Knowlton N (2011) The diversity of coral reefs: what are we missing? PLoS ONE 6(10), e25026.
| Crossref | Google Scholar | PubMed |
Pratchett MS, Berumen ML, Marnane MJ, Eagle JV, Pratchett DJ (2008) Habitat associations of juvenile versus adult butterflyfishes. Coral Reefs 27(3), 541-551.
| Crossref | Google Scholar |
Puillandre N, Modica MV, Zhang Y, Sirovich L, Boisselier M-C, Cruaud C, Holford M, Samadi S (2012) Large-scale species delimitation method for hyperdiverse groups. Molecular Ecology 21(11), 2671-2691.
| Crossref | Google Scholar | PubMed |
Quattrini AM, Faircloth BC, Dueñas LF, Bridge TCL, Brugler MR, Calixto-Botía IF, DeLeo DM, Forêt S, Herrera S, Lee SMY, Miller DJ, Prada C, Rádis-Baptista G, Ramírez-Portilla C, Sánchez JA, Rodríguez E, McFadden CS (2018) Universal target-enrichment baits for anthozoan (Cnidaria) phylogenomics: new approaches to long-standing problems. Molecular Ecology Resources 18(2), 281-295.
| Crossref | Google Scholar | PubMed |
Quattrini AM, Wu T, Soong K, Jeng M-S, Benayahu Y, McFadden CS (2019) A next generation approach to species delimitation reveals the role of hybridization in a cryptic species complex of corals. BMC Evolutionary Biology 19(1), 116.
| Crossref | Google Scholar | PubMed |
Quelch JJ (1886) Report on the reef-corals collected by H.M.S. ‘Challenger’ during the years 1873–76. Report on the scientific results of the Voyage of H.M.S. Challenger during the years 1873–1876. Zooology 16(46), 1-203.
| Google Scholar |
Raja NB, Lauchstedt A, Pandolfi JM, Kim SW, Budd AF, Kiessling W (2021) Morphological traits of reef corals predict extinction risk but not conservation status. Global Ecology and Biogeography 30(8), 1597-1608.
| Crossref | Google Scholar |
Ramírez-Portilla C, Baird A, Cowman P, Quattrini A, Harii S, Sinniger F, Flot J-F (2021) Solving the coral species delimitation conundrum. In Dryad, 8 October 2021. [Datasets] doi:10.5061/dryad.k98sf7m5x
Ramírez-Portilla C, Baird AH, Cowman PF, Quattrini AM, Harii S, Sinniger F, Flot J-F (2022) Solving the coral species delimitation conundrum. Systematic Biology 71(2), 461-475.
| Crossref | Google Scholar | PubMed |
Randall RH (2003) An annotated checklist of hydrozoan and scleractinian corals collected from Guam and other Mariana Islands. Micronesica 35(36), 121-137.
| Google Scholar |
Richards ZT, Hobbs J-PA (2015) Hybridisation on coral reefs and the conservation of evolutionary novelty. Current Zoology 61(1), 132-145.
| Crossref | Google Scholar |
Rose NH, Bay RA, Morikawa MK, Thomas L, Sheets EA, Palumbi SR (2021) Genomic analysis of distinct bleaching tolerances among cryptic coral species. Proceedings of the Royal Society of London – B. Biological Sciences 288(1960), 20210678.
| Crossref | Google Scholar | PubMed |
Rosser NL, Edyvane K, Malina AC, Underwood JN, Johnson MS (2020) Geography and spawning season drive genetic divergence among populations of the hard coral Acropora tenuis from Indonesia and Western Australia. Coral Reefs 39(4), 989-999.
| Crossref | Google Scholar |
Scrucca L, Fop M, Murphy TB, Raftery AE (2016) mclust 5: clustering, classification and density estimation using gaussian finite mixture models. The R Journal 8(1), 289-317.
| Crossref | Google Scholar | PubMed |
Sheets EA, Warner PA, Palumbi SR (2018) Accurate population genetic measurements require cryptic species identification in corals. Coral Reefs 37(2), 549-563.
| Crossref | Google Scholar |
Sheppard CR (1987) Coral species of the Indian Ocean and adjacent seas: a synonymized compilation and some regional distributional patterns. Atoll Research Bulletin 307, 1-32.
| Crossref | Google Scholar |
Studer T (1879) Zweite Abtheilung der Anthozoa Polyactinia, welche während der Reise A. M. S. Corvette Gazelle um die Erde gesammelt wurden. Monatsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin 1878, 525-550 [In German].
| Google Scholar |
Suggett DJ, Camp EF, Edmondson J, Boström-Einarsson L, Ramler V, Lohr K, Patterson JT (2019) Optimizing return-on-effort for coral nursery and outplanting practices to aid restoration of the Great Barrier Reef. Restoration Ecology 27(3), 683-693.
| Crossref | Google Scholar |
Sugihara K, Nomura K, Yokochi H, Shimoike K, Kajiwara K, Suzuki G, Kimura T (2015) 日本の有藻性イシサンゴ類~種子島編~ [‘Zooxanthellate scleractinian corals of Tanegashima Island, Japan’]. (国立環境研究所 生物·生態系環境研究センター [National Institute for Environmental Studies, Centre for Environmental Biology and Ecosystem Studies]: Tsukuba, Japan) Available at https://www.nies.go.jp/biology/data/coral.html [In Japanese]
Suzuki G, Keshavmurthy S, Hayashibara T, Wallace CC, Shirayama Y, Chen CA, Fukami H (2016) Genetic evidence of peripheral isolation and low diversity in marginal populations of the Acropora hyacinthus complex. Coral Reefs 35(4), 1419-1432.
| Crossref | Google Scholar |
Thomson SA, Pyle RL, Ahyong ST, Alonso-Zarazaga M, Ammirati J, Araya JF, Ascher JS, Audisio TL, Azevedo-Santos VM, Bailly N, Baker WJ, Balke M, Barclay M, Barrett RL, Benine RC, Bickerstaff J, Bouchard P, Bour R, Bourgoin T, Boyko CB, Breure A, Brothers DJ, Byng JW, Campbell D, Ceríaco L, Cernák I, Cerretti P, Chang CH, Cho S, Copus JM, Costello MJ, Cseh A, Csuzdi C, Culham A, D’Elía G, d’Udekem d’Acoz C, Daneliya ME, Dekker R, Dickinson EC, Dickinson TA, van Dijk PP, Dijkstra KB, Dima B, Dmitriev DA, Duistermaat L, Dumbacher JP, Eiserhardt WL, Ekrem T, Evenhuis NL, Faille A, Fernández-Triana JL, Fiesler E, Fishbein M, Fordham BG, Freitas A, Friol NR, Fritz U, Frøslev T, Funk VA, Gaimari SD, Garbino G, Garraffoni A, Geml J, Gill AC, Gray A, Grazziotin FG, Greenslade P, Gutiérrez EE, Harvey MS, Hazevoet CJ, He K, He X, Helfer S, Helgen KM, van Heteren AH, Hita Garcia F, Holstein N, Horváth MK, Hovenkamp PH, Hwang WS, Hyvönen J, Islam MB, Iverson JB, Ivie MA, Jaafar Z, Jackson MD, Jayat JP, Johnson NF, Kaiser H, Klitgård BB, Knapp DG, Kojima JI, Kõljalg U, Kontschán J, Krell FT, Krisai-Greilhuber I, Kullander S, Latella L, Lattke JE, Lencioni V, Lewis GP, Lhano MG, Lujan NK, Luksenburg JA, Mariaux J, Marinho-Filho J, Marshall CJ, Mate JF, McDonough MM, Michel E, Miranda V, Mitroiu MD, Molinari J, Monks S, Moore AJ, Moratelli R, Murányi D, Nakano T, Nikolaeva S, Noyes J, Ohl M, Oleas NH, Orrell T, Páll-Gergely B, Pape T, Papp V, Parenti LR, Patterson D, Pavlinov IY, Pine RH, Poczai P, Prado J, Prathapan D, Rabeler RK, Randall JE, Rheindt FE, Rhodin A, Rodríguez SM, Rogers DC, Roque FO, Rowe KC, Ruedas LA, Salazar-Bravo J, Salvador RB, Sangster G, Sarmiento CE, Schigel DS, Schmidt S, Schueler FW, Segers H, Snow N, Souza-Dias PGB, Stals R, Stenroos S, Douglas Stone R, Sturm CF, Štys P, Teta P, Thomas DC, Timm RM, Tindall BJ, Todd JA, Triebel D, Valdecasas AG, Vizzini A, Vorontsova MS, de Vos JM, Wagner P, Watling L, Weakley A, Welter-Schultes F, Whitmore D, Wilding N, Will K, Williams J, Wilson K, Winston JE, Wüster W, Yanega D, Yeates DK, Zaher H, Zhang G, Zhang Z-Q, Zhou H-Z (2018) Taxonomy based on science is necessary for global conservation. PLoS Biology 16(3), e2005075.
| Crossref | Google Scholar | PubMed |
Van Dam MH, Henderson JB, Esposito L, Trautwein M (2021) Genomic characterization and curation of UCEs improves species tree reconstruction. Systematic Biology 70(2), 307-321.
| Crossref | Google Scholar | PubMed |
van der Maaten L, Hinton G (2008) Visualizing data using t-SNE. Journal of Machine Learning Research 9(11), 2579-2605.
| Google Scholar |
Verrill AE (1864) List of the polyps and corals sent by the Museum of Comparative Zoology to other institutions in exchange, with annotations. Bulletin of the Museum of Comparative Zoology 1, 29-60.
| Google Scholar |
Verrill AE (1902) Notes on corals of the genus Acropora (Madrepora Lam.) with new descriptions and figures of types, and of several new species. Transactions of the Connecticut Academy of Arts and Sciences 11, 207-266.
| Crossref | Google Scholar |
Veron JEN (1990) New Scleractinia from Japan and other Indo-West Pacific countries. Galaxea 9, 95-173.
| Google Scholar |
Veron JEN (2002) New species described in Corals of the World. Australian Institute of Marine Science Monograph Series 11, 1-206.
| Google Scholar |
Veron JEN, Done TJ (1979) Corals and coral communities of Lord Howe Island. Marine and Freshwater Research 30(2), 203-236.
| Crossref | Google Scholar |
Veron JEN, Hodgson G (1989) Annotated checklist of the hermatypic corals of the Philippines. Pacific Science 43, 234-287.
| Google Scholar |
Veron JEN, Marsh LM (1988) Hermatypic corals of Western Australia: Records and annotated species list. Records of the Western Australian Museum 29(Supp.), 1-136.
| Google Scholar |
Veron JEN, Pichon M (1976) ‘Scleractinia of Eastern Australia. Part I. Families Thamnasteriidae, Astrocoeniidae, Pocilloporidae. Australian Institute of Marine Science Monograph Series Volume 1.’ (Australian Government Publishing Service: Canberra, ACT, Australia) 10.5962/bhl.title.60617
Veron JEN, Wallace CC (1984) Scleractinia of eastern Australia. Part V. Family Acroporidae. Australian Institute of Marine Science Monograph Series 6, 1-485.
| Google Scholar |
Veron JEN, Stafford-Smith MG, Turak E, DeVantier LM (2016) Acropora spicifera (Dana, 1846). In ‘Corals of the World’, version 0.01. Available at http://www.coralsoftheworld.org/species_factsheets/species_factsheet_summary/acropora-spicifera/?version=0.01 [Verified 14 July 2025]
Wallace CC (1978) The coral genus Acropora (Scleractinia: Astrocoeniina: Acroporidae) in the central and southern Great Barrier Reef province. Memoirs of the Queensland Museum 18, 273-319.
| Google Scholar |
Wallace CC, Chen CA, Fukami H, Muir PR (2007) Recognition of separate genera within Acropora based on new morphological, reproductive and genetic evidence from Acropora togianensis, and elevation of the subgenus Isopora Studer, 1878 to genus (Scleractinia: Astrocoeniidae; Acroporidae). Coral Reefs 26(2), 231-239.
| Crossref | Google Scholar |
Wallace CC, Done BJ, Muir PR (2012) Revision and catalogue of worldwide Staghorn corals ‘Acropora’ and ‘Isopora’ (Scleractinia: Acroporidae) in the Museum of Tropical Queensland. Nature 57(1), 1-257.
| Google Scholar |
Wilson K, Li Y, Whan V, Lehnert S, Byrne K, Moore S, Pongsomboon S, Tassanakajon A, Rosenberg G, Ballment E, Fayazi Z, Swan J, Kenway M, Benzie J (2002) Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture 204(3), 297-309.
| Crossref | Google Scholar |
Zarza E, Faircloth BC, Tsai WLE, Bryson RW, Jr, Klicka J, McCormack JE (2016) Hidden histories of gene flow in highland birds revealed with genomic markers. Molecular Ecology 25(20), 5144-5157.
| Crossref | Google Scholar | PubMed |
Zayasu Y, Takeuchi T, Nagata T, Kanai M, Fujie M, Kawamitsu M, Chinen W, Shinzato C, Satoh N (2021) Genome-wide SNP genotyping reveals hidden population structure of an acroporid species at a subtropical coral island: Implications for coral restoration. Aquatic Conservation: Marine and Freshwater Ecosystems 31(9), 2429-2439.
| Crossref | Google Scholar |
Zhang C, Rabiee M, Sayyari E, Mirarab S (2018) ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19(6), 153.
| Crossref | Google Scholar | PubMed |