Children, snails and worms: the Brachylaima cribbi story
Andrew R ButcherSA Pathology
Microbiology and Infectious Diseases
SA, Australia
Email: andrew.butcher.cecc@gmail.com
Microbiology Australia 37(1) 30-33 https://doi.org/10.1071/MA16012
Published: 11 February 2016
Brachylaimids are parasitic trematode fluke worms that have a terrestrial life cycle involving land snails and slugs as the first and/or second intermediate hosts for the cercarial and metacercarial larval stages. A wide range of mammals, birds, reptiles and amphibians are the definitive hosts for the adult worm. Brachylaima spp. have been reported from most continents including Europe, Africa, Asia, North and South America and Australia. There are over 70 described species in the genus with seven species indigenous to Australia. Although Brachylaima spp. are a cosmopolitan terrestrial trematode they have not been recorded to infect humans other than the three Brachylaima cribbi infections reported in two children and an adult from South Australia.
The publication of a new species of brachylaimid, Brachylaima cribbi by Butcher and Grove in 2001 was the culmination of a 12-year scientific journey that would not have been possible without a broad scientific network of colleagues and some ‘scientific luck’, which enabled the establishment of a laboratory life cycle using parasite eggs recovered from the stool of an infected human1. My long-term association, as an active member of the Australian Society for Microbiology and the special interest groups played a significant role in having a network of colleagues to help find techniques and data to assist in the study of this parasite.
The publication of the first human Brachylaima sp. infections followed the detection of a fluke worm egg, in the stool of two South Australian children, who had never travelled overseas. The eggs were identified as belonging to a genus of trematode worm endemic in the local area2. After conversations with many local and interstate colleagues, a veterinary parasitology colleague Michael O’Callaghan remembered the post-doctoral work of Thomas Cribb in South Australia. He had studied and published work on a brachylaimid in South Australia that infected mice3,4. This provided the data on a local trematode that had an egg morphology matching the eggs detected in the children’s stools.
However, was this a true human infection that resulted in the establishment of mature gravid worms producing the eggs detected in their stool or was it a spurious infection? The answer to this question was resolved 18 months later when an elderly lady from the mid-north of South Australia presented with chronic diarrhoea. Microscopic examination of her stool detected Brachylaima eggs, which matched the morphology of the eggs detected in the two children. In addition, on the first day following treatment with praziquantel a gravid degenerate adult Brachylaima worm was recovered from her stool5. This provided the evidence that a Brachylaima sp. was infecting humans and completing its life cycle. However, due to the advanced state of decomposition of the worm there was insufficient morphological detail to establish the species identification.
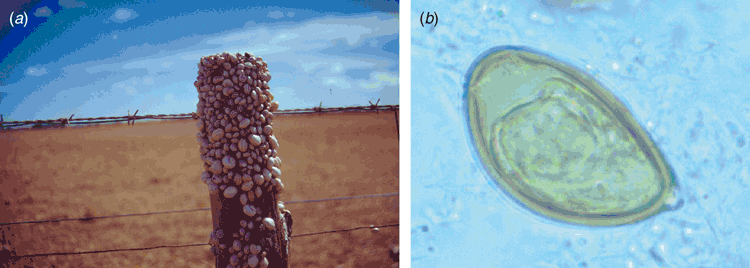
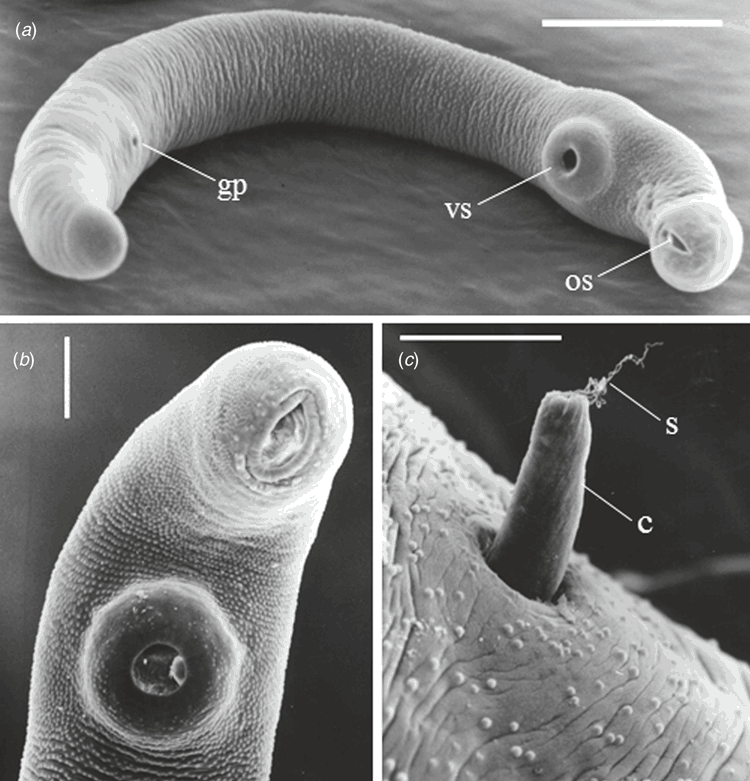
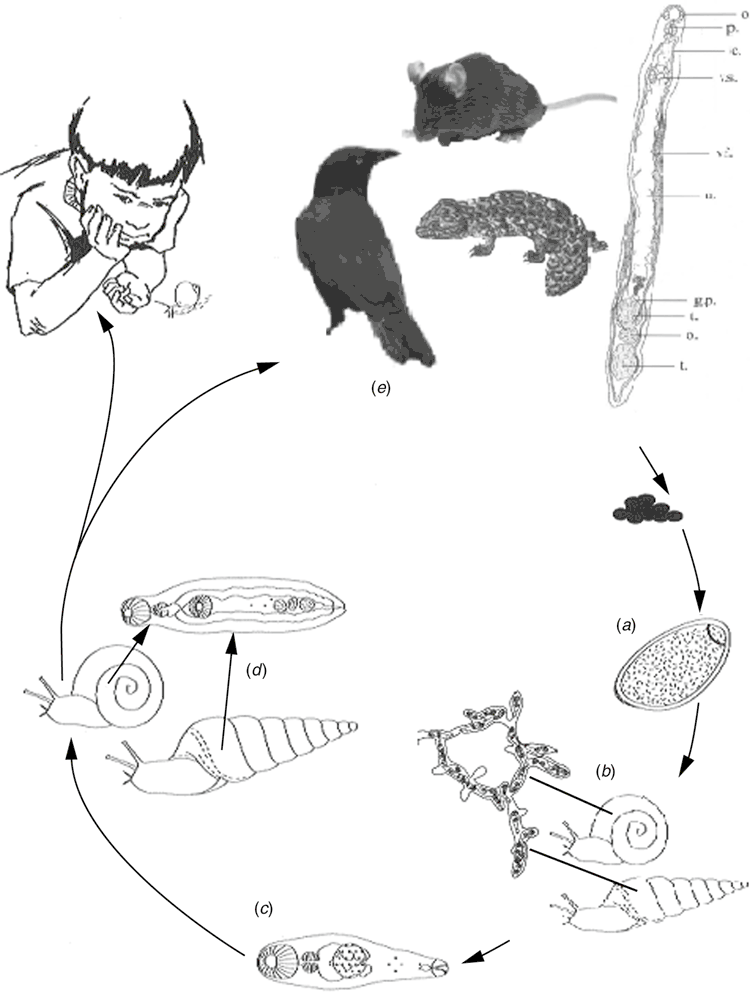
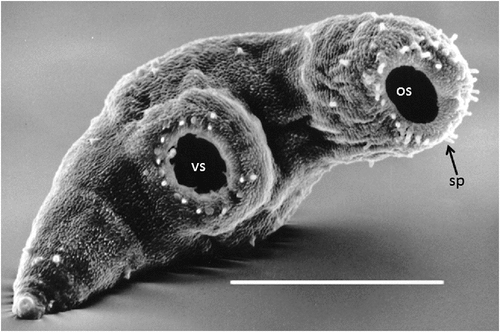
Previous to the finding of the third case described above I had commenced the cultivation of introduced European helicid and hygromiid land snails (Figure 1a) in homemade terrariums using techniques published for snail farming6. My office in the diagnostic Microbiology laboratory had now become part snail culture laboratory. These snails were used to study the larval stages in an attempt to understand how humans could have become infected. At the same time I had obtained a copy of an article by Mas-Coma and Montoliu detailing the life cycle of Brachylaima ruminae from the duodenum of a rodent from the Mediterranean island of Formentera (Spain)7. This article provided methods for the establishment of a laboratory life cycle. With these methods in hand and my snail cultures thriving I set myself the task to attempt to establish a laboratory life cycle using eggs recovered from the stool of the third patient. Faecal sediment of the patient’s stool containing washed concentrated eggs was smeared on wet filter paper and was fed to laboratory snails in Petri dishes overnight. Eight weeks later, when the snails were exposed to moisture in a Petri dish, cercariae by the thousands were observed emerging from the snails (Figure 2). Cercariae were collected in water and used to infect the common brown garden snails Cornu aspersum (Helix aspersa) via the respiratory orifice. After 8–10 weeks mature metacercariae were harvested from kidneys of the snails. Mice were inoculated orally with metacercariae. Seven weeks later eggs were detected in mouse faeces. Gravid adult Brachylaima worms were recovered from the small intestine for characterisation and morphological identification (Figure 3). Eggs from mouse faeces and dissected worms were used to continue the life cycle (Figure 4) in the laboratory to study the life cycle kinetics.
|
At the same time as I was using my spare time to tinker with this life cycle experiment the former Institute of Medical and Veterinary Science (IMVS) was undergoing amalgamation with the Queen Elizabeth Hospital (TQEH) Laboratories. Professor David Grove was the Clinical Microbiologist Head of TQEH Microbiology Department and a world recognised expert clinical parasitologist. At that time Professor Grove offered me the opportunity to join the TQEH Microbiology team under the amalgamation program to continue the Brachylaima study and commence a PhD under his supervision. That is scientific luck, right place at the right time and the rest is now history, with the following years being the most simulating and productive science of my career.
Human cases
Since the publication of the three human cases to my knowledge there have been a further 12 laboratory confirmed human infections (A.R. Butcher and D.I. Grove, unpublished observations). Part of my PhD studies was the follow-up of all human infections with a standard questionnaire to obtain clinical history. The main presenting symptoms were mucoid, watery diarrhoea, abdominal pain, anorexia and weight loss or poor weight gain in children. All identified cases were in patients who lived in rural or semi-rural districts where helicid and hygromiid land snails infected with B. cribbi metacercariae were abundant. The likely infection source was the ingestion of raw snails. The majority of infections (80%) were in children under the age of two years. At this age the mouthing of objects is a common behavioural characteristic with parents of the infected children detailing their child’s consumption of snails. For the three adults infected they had all eaten home-grown garden vegetables contaminated with snails. The duration of symptoms ranged from 1 month to 2 years with the majority of infections diagnosed within 1–2 months of the first signs of symptoms. All patients were treated successfully with praziquantel with no adverse side-effects. The most commonly used dose in both adults and children was 20 mg/kg once per day for 3 days.
Laboratory diagnosis
Diagnosis of the infection relies on the detection of typical eggs in the faeces which are asymmetrical, being ovoid, with one side slightly flattened. They have a smooth shell, inconspicuous operculum, an abopercular knob or thickening and measure 26–32 μm by 16–17.5 μm. The eggs generally contain a well-developed miracidium (Fig. 1b). However, in chronic infections many of the eggs may be infertile, being of similar morphology but smaller size and lacking a developed miracidium. Infected land snails have been reported from coastal and inland Western Australia, South Australia and western Victoria8. Therefore, the potential exists for further human infections, especially in children living in these regional districts.
References
[1] Butcher, A.R. and Grove, D.I. (2001) Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst. Parasitol. 49, 211–221.| Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvhslChtQ%3D%3D&md5=2c74f0e907711767bdd5222eae4228c2CAS | 11466482PubMed |
[2] Butcher, A.R. et al. (1996) Locally acquired Brachylaima sp. (Digenea: Brachylaimidae) intestinal fluke infection in two South Australian infants. Med. J. Aust. 164, 475–478.
| 1:STN:280:DyaK287pvFyntA%3D%3D&md5=22273d61cccd493b73815e8e70794965CAS | 8614338PubMed |
[3] Cribb, T.H. (1990) Introduction of a Brachylaima species (Digenea: Brachylaimidae) to Australia. Int. J. Parasitol. 20, 789–796.
| Introduction of a Brachylaima species (Digenea: Brachylaimidae) to Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M%2FlsFyrtg%3D%3D&md5=317e176ba9989dd9d7b56f9e10b5d312CAS | 2242962PubMed |
[4] Cribb, T.H. and O’Callaghan, M. (1992) An unusual trematode infecting domestic chickens. Aust. Vet. J. 69, 69–70.
| An unusual trematode infecting domestic chickens.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK383msV2msA%3D%3D&md5=3e3fadd5f47cdb40a63f813706a58f8eCAS | 1586322PubMed |
[5] Butcher, A.R. et al. (1998) First report of the isolation of an adult worm of the genus Brachylaima (Digenea: Brachylaimidae), from the gastrointestinal tract of a human. Int. J. Parasitol. 28, 607–610.
| First report of the isolation of an adult worm of the genus Brachylaima (Digenea: Brachylaimidae), from the gastrointestinal tract of a human.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3msFSqsQ%3D%3D&md5=972beaef489a5cd80ac56d9f22532b99CAS | 9602383PubMed |
[6] Baker, G.H. (1991) Production of eggs and young snails by adult Theba pisana (Müller) and Cernuella virgata (da Costa) (Mollusca: Helicidae) in laboratory culture and field populations. Aust. J. Zool. 39, 673–679.
| Production of eggs and young snails by adult Theba pisana (Müller) and Cernuella virgata (da Costa) (Mollusca: Helicidae) in laboratory culture and field populations.Crossref | GoogleScholarGoogle Scholar |
[7] Mas-Coma, S. and Montoliu, I. (1986) The life cycle of Brachylaima ruminae n. sp. (Trematoda: Brachylaimidae), a parasite of rodents. Z. Parasitenkd. 72, 739–753.
| The life cycle of Brachylaima ruminae n. sp. (Trematoda: Brachylaimidae), a parasite of rodents.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2Fps1Sjtg%3D%3D&md5=498ff02871dc0825ee7adbc013bf7382CAS | 3799006PubMed |
[8] Butcher, A.R. and Grove, D.I. (2003) Field prevalence and laboratory susceptibility of southern Australian land snails to Brachylaima cribbi sporocyst infection. Parasite 10, 119–125.
| Field prevalence and laboratory susceptibility of southern Australian land snails to Brachylaima cribbi sporocyst infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3szhvFWhsg%3D%3D&md5=6ffc695361abdccb8ec660ad8214c45dCAS | 12847918PubMed |
Biography
Dr Andrew Butcher is a retired medical scientist with 38 years experience as a diagnostic medical microbiologist with a special interest in parasitology. He has been an active member of the ASM Parasitology and Tropical Medicine Special Interest Group for many years being national convenor for 7 years. He has been involved in teaching diagnostic medical parasitology and one of the founding committee members of the ASM Parasitology Master Class.