Hidden reservoirs of hospital-associated infections
Claire GorrieMicrobiology Australia 38(3) 140-142 https://doi.org/10.1071/MA17051
Published: 17 August 2017
Klebsiella pneumoniae (Kp) is a Gram-negative bacterium that is ubiquitous in the environment and is of increasing concern in public health. Kp can be carried asymptomatically as a commensal organism and can cause opportunistic infections in susceptible individuals; this is further complicated by an increasing incidence of multi-drug-resistant (MDR) strains. Given Kp can be carried asymptomatically, and can cause infections, it is possible that asymptomatic carriage acts as a reservoir for infection. Our recent work in Melbourne confirms this is often true. Individuals who tested positive for carriage of Kp, on admission to ICU, were over five times more likely to develop an infection during their hospital stay, compared to non-carriers. Whole genome sequence analyses revealed extensive diversity amongst the Kp infection-causing strains. These results indicate the majority of opportunistic infections are caused by patients’ own microbiome strains that are already present on ICU admission. As such, screening of individuals on admission may enable clinicians to identify who is most at risk of developing infections during their hospital stay, and who is harboring drug-resistant strains that could transmit to others.
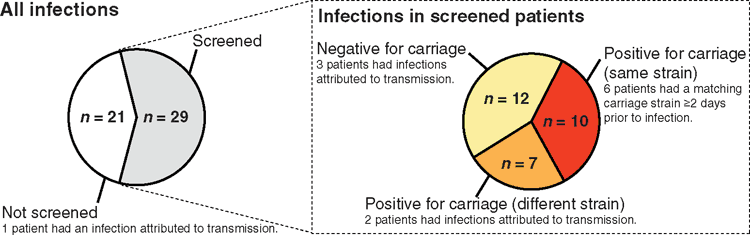
During a one-year cohort study conducted at the Alfred hospital intensive care unit (ICU) in Melbourne, 498 patients were screened for gut carriage of Kp shortly following admission and were monitored for Kp infection1. The frequency of gut carriage was 10% overall, but only 6% amongst those who had no recent contact with healthcare prior to being admitted to ICU. Fifty ICU patients (1.85% of admissions) had one or more infections attributed to Kp, and 29 of these individuals were also screened for carriage. Bacterial isolates underwent whole genome sequencing (WGS) and comparative analyses were conducted, using strict thresholds of genomic similarity to identify potential transmission and to explore whether carriage strains matched those causing infections. Ten patients (34% of infection patients who were screened) were carrying Kp strains that matched their infecting strain (<0.0005% DNA sequence divergence); in six of these cases, the infection occurred at least two days after carriage was detected. Additionally, six patients developed infections with strains that were near-identical to strains previously detected in other ICU patients, consistent with intra-hospital transmission (Figure 1).
Not all infections could be attributed to prior carriage or transmission, mainly because not all ICU patients were screened for carriage (screening required informed consent from the patient or a close relative, which was not always obtainable). So for many patients it is not possible to tell whether they were carrying their infecting strain on ICU admission. However, the WGS analysis showed that these individuals were typically infected with unique strains; not closely related to strains elsewhere in the hospital. Hence it is likely that these unexplained infections were caused by the patients’ own strains than new strains that they acquired in the hospital.
This study suggests that routine screening for Kp could identify patients at high risk of developing infections during their hospital stay. Some hospitals already have routine screening procedures of patients in place for carriage of various MDR bacteria, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, or carbapenem-resistant Enterobacteriaceae; some have reported a corresponding reduction in hospital-associated infections2–5. During the one-year period of this study, Kp infections were diagnosed by the clinical microbiology laboratory in 320 patients across Alfred Health (unpublished observations). Of these, 11% were caused by strains carrying extended spectrum beta-lactamase (EBSL) genes that confer resistance to third generation cephalosporins (unpublished observations), and a small number of these were additionally resistant to carbapenems (CP-R) (unpublished observations), which are typically the last line drug for treatment of Gram-negative bacterial pathogens6. Importantly, these genotypically ESBL and CP-R isolates displayed corresponding resistant phenotypes, when they underwent antimicrobial susceptibility profiling (unpublished observations). Whilst MDR infections are still in the minority, they are the most difficult to treat, having been estimated to cost billions of dollars in additional treatment costs and hospital stays, as well as thousands of deaths globally every year that would have been preventable had the infections not proved resistant to treatment7–9. Hence a screening strategy that looks for gut carriage of any MDR Gram-negative bacteria, which would detect Kp but also other common causes of hospital infections, could be of greatest clinical utility.
Important questions remain about how such information should be used. From an infection control standpoint, additional isolation measures could be taken to prevent transmission of MDR strains to other patients or healthcare workers. From a patient management standpoint, the drug susceptibility profile of known carriage strains could be used to guide the choice of antimicrobials used for therapy or prophylaxis2,10. First, this would help to avoid drugs that could select for overgrowth of resistant strains in the gut that could subsequently cause difficult-to-treat infections. Second, should an infection arise, foreknowledge of the likely strains and antimicrobial susceptibility profiles could be used to supplement empirical treatment protocols while awaiting laboratory confirmation of the susceptibility profile of the infecting organism.
The study demonstrates the value of WGS for fine-scale investigation of carriage-infection relationships, by clearly elucidating relationships between strains that cannot be distinguished by traditional laboratory typing techniques, e.g. multi-locus sequence typing or PFGE. As such, it is recommended that WGS approaches be implemented when investigating potential sources of infection, including for routine infection control as well as further research studies. Coupled with routine screening for carriage of MDR organisms, WGS could enable not just the identification of closely related strains but could also reveal the presence of mobile genes associated with antimicrobial resistance. Such genes can be readily transferred between species, hence knowledge of their presence could inform empirical treatment choices for Gram-negative infections generally and not just the isolated MDR organism. The WGS approach, with its continually developing sequencing and analyses platforms, is rapidly becoming more affordable and increasingly easy implement. Use of this technology in conjunction with, or in place of, traditional techniques could lead to much needed decreases in the numbers of hospital infections, particularly for difficult-to-treat MDR infections, leading to long term benefits both reducing the strain on the public health system and budget, but also for bettering patient outcomes.
Acknowledgements
Thank you to the team at Alfred Health who helped with various stages of this research; to the NHMRC for the funding grant that supported this work; to the team at the Wellcome Trust Sanger Institute for sequencing the genomes; and to my supervisors – Richard Strugnell, Adam Jenney, and Kathryn Holt.
References
[1] Gorrie, C.L. et al. (2017) Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. , .| Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients.Crossref | GoogleScholarGoogle Scholar |
[2] Ho, C. et al. (2013) Screening, isolation, and decolonization strategies for vancomycin-resistant Enterococci or extended spectrum beta-lactamase-producing organisms: a systematic review of the clinical evidence and health services impact. CADTH Technol. Overv. 3, e3202.
| 1:STN:280:DC%2BC3svjs1Gjtg%3D%3D&md5=97e7c6567b835aa1e2c32c6e6e81e743CAS |
[3] Glick, S.B. et al. (2014) Screening for methicillin-resistant Staphylococcus aureus: a comparative effectiveness review. Am. J. Infect. Control 42, 148–155.
| Screening for methicillin-resistant Staphylococcus aureus: a comparative effectiveness review.Crossref | GoogleScholarGoogle Scholar |
[4] Faron, M.L. et al. (2016) Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J. Clin. Microbiol. 54, 2436–2447.
| Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting.Crossref | GoogleScholarGoogle Scholar |
[5] Reyes, K. et al. (2016) Vancomycin-resistant Enterococci. Infect. Dis. Clin. North Am. 30, 953–965.
| Vancomycin-resistant Enterococci.Crossref | GoogleScholarGoogle Scholar |
[6] Nordmann, P. et al. (2011) Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17, 1791–1798.
| Global spread of carbapenemase-producing Enterobacteriaceae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlOmsr3O&md5=cf4b8ba60a08a67f48a05dd5b39d870aCAS |
[7] Centres for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States, 2013, Centres for Disease Control and Prevention, US Department of Health and Human Services.
[8] Gandra, S. et al. (2014) Economic burden of antibiotic resistance: how much do we really know? Clin. Microbiol. Infect. 20, 973–980.
| Economic burden of antibiotic resistance: how much do we really know?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2M7mtlOltQ%3D%3D&md5=f8eabcfe6c0fb2eaca170781f31f966fCAS |
[9] Boucher, H.W. et al. (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12.
| Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America.Crossref | GoogleScholarGoogle Scholar |
[10] Hussein, K. et al. (2013) Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J. Hosp. Infect. 83, 307–313.
| Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3s3ovFWisA%3D%3D&md5=4cb39476b7c4c63b1a3743b54c5313c1CAS |
Biography
Claire Gorrie is a PhD student at The University of Melbourne. Her research combines whole genome sequencing, bacterial genomics, and epidemiology to investigate carriage-infection dynamics of Klebsiella pneumoniae.