Sourcing phages for compassionate use
Jessica C Sacher A , Jan Zheng A and Shawna McCallin B CA Phage Directory, 151 Ted Turner Drive NW, Atlanta, GA 30303, USA
B Unit of Regenerative Medicine, Department of Musculoskeletal Medicine, Service of Plastic, Reconstructive, and Hand Surgery, University Hospital of Lausanne (CHUV), Switzerland
C Tel: +417 9791 9543, Email: shawna.mccallin@gmail.com
Microbiology Australia 40(1) 24-27 https://doi.org/10.1071/MA19012
Published: 22 February 2019
Antibiotic resistance is a phenomenon that knows no geographical borders, so addressing this crisis is a worldwide public health priority. While total global resistance rates are difficult to estimate and vary between countries, an international report asserts that the development of new antibacterials is essential to ensuring the future ability to treat bacterial infections1. Bacteriophage (phage) therapy is a likely contributor to resolving potentially devastating effects of antibiotic resistance, yet no phage product currently holds a marketing authorisation that would permit their free use in clinical medicine outside of former countries of the Soviet Union, where phage therapy is a long-standing practice2,3. In the interim, the compassionate use of phage therapy (cPT) remains a possible treatment avenue for cases of antibiotic failure, and several competency centres, physicians, and researchers have achieved therapeutic benefits with this option. As antibiotic resistance continues to rise, there is much to be done in order to streamline cPT efforts, particularly in terms of phage sourcing, in order to reach more patients in an efficient, effective, and safe manner. This article highlights how cPT can be coordinated, and describes the experience of cPT in Australia.
Compassionate treatment denotes the use of unapproved medicines outside of clinical trials for the treatment of patients for whom approved therapeutic options have been exhausted unsuccessfully. The premise of compassionate use is stated in Article 37 of the ‘Helsinki Declaration of Ethic Principles for Medical Research Involving Human Subjects’, an international guideline for research on human subjects4. Both Articles 3 and 37 concern individuals at the end of their therapeutic options. Official oversight of compassionate use programs varies from country-to-country, but is handled by regulatory agencies, such as the FDA in the USA or the EMA in the European Union5–7. In Australia, access to unapproved therapeutic goods can be attained through a Special Access Scheme (SAS), which is divided into three categories (A: notification for the seriously ill; B: application; C: notification of use of specified products)8. The Second Australian report on Antimicrobial Use and Resistance in Human Health has identified 14 target pathogens for which resistance rates to certain antibiotics varied widely from <1 to 96% of isolates, indicating an existing need for alternative treatment options, such as cPT9.
The use of phage for compassionate treatment of antibiotic-resistant infections is increasing, with >20 published papers from experimental treatment centres, pilot studies, and case reports since 2000, reviewed in10–15. A diversity of infections have been treated by cPT, including endocarditis16, diabetic toe ulcers14,17, abdominal cysts18, prostatitis15,19,20, otitis15 and osteomyelitis12, encompassing both local and systemic routes of administration. One case report described the treatment of a refractory urinary tract infection caused by a multidrug-resistant strain of Pseudomonas aeruginosa in Australia21. Administration of a six-phage personalised cocktail directly into the bladder resulted in sterile urine cultures for this pathogen after only eight days (although antibiotics were administered six days after phage therapy started and may have contributed to the final effect). The case required the cooperation of international researchers and physicians from Australia, France, and Georgia to obtain therapeutic phages and to coordinate treatment. For this treatment to be possible, phages were prepared by the renowned Eliava Phage Therapy Center in Tbilisi and sent on-site. This is one of the few studies to date that documents multiple aspects of human treatment that are critical for understanding phage therapy, including viable phage and bacterial counts and phage sensitivity testing, and should serve as an example for future reporting criteria21,22. This case, like others, highlights the need for close collaboration between physicians and phage researchers, sometimes at an international level, in order to provide know-how and facilitate access to therapeutic phages for compassionate means until approved alternatives become available.
Finding phages for cPT
Access to phages with activity against the patient’s bacterial isolate is evidently essential for compassionate use, and while phages are ubiquitous in nature, environmental isolation requires starting the phage selection process from scratch and having sufficient infrastructure and resources. Given the high level of fundamental and translational research conducted on phages, many phages suitable for cPT already exist in both academic and state research institutions. The phage community is a highly cooperative and supportive research area; many of its members have voiced their willingness to share both their experience and their phages for cPT cases, and several have already done so. Another source of phages for compassionate use are small biotech companies that are in the process of developing clinical-grade phage products, such as Pherecydes Pharma in France, Adaptive Phage Therapeutics in the USA, and Ampliphi Biosciences, which is based both in the USA and Australia.
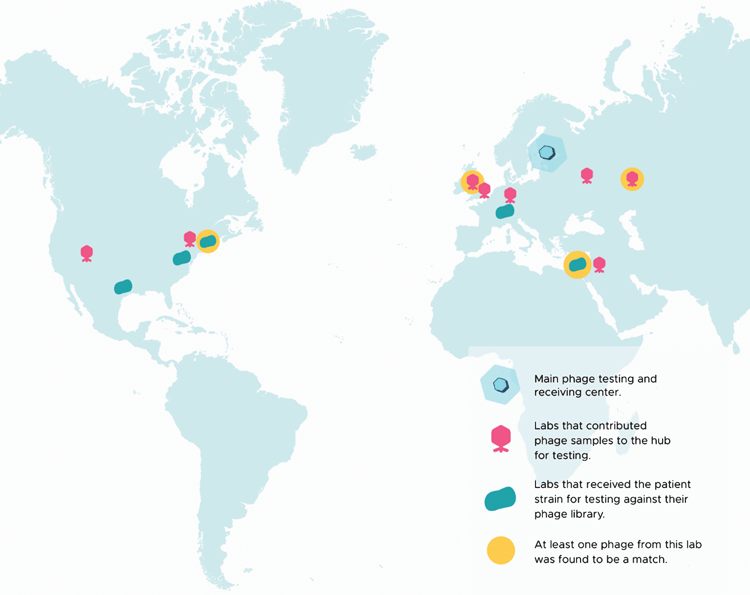
An initiative to organise sharing for cPT, called Phage Directory, was founded by two of the authors in 201723. This organisation was created in response to a need for Burkholderiacepacia phages for the authorised cPT treatment of a 25-year-old cystic fibrosis patient in Pittsburgh, PA (USA); the lack of readily-available phages delayed cPT and the patient passed away. Currently, the academic phage laboratories and phage banks registered on Phage Directory represent more than 20 different countries, including labs at Monash University and the University of Adelaide in Australia, and contribute phages that target >30 host genera. An example of how the Phage Directory network has facilitated phage sharing for cPT is presented here (Figure 1).
In late 2018, Phage Directory coordinated the sourcing of Klebsiella pneumoniae phages for a patient in Helsinki, Finland by sending an electronic alert to its network of registered labs and phage collections. This was in response to a request from a phage laboratory in Finland that was working with the patient’s medical team. Within one week, nine academic labs and one phage biotech had offered to test or share phages, and within three weeks, more than 175 Klebsiella phages had been tested (Figure 1). At least six of these phages were found to be active against the patient’s isolate in vitro. This process is ongoing; phages have not yet been administered to the patient, as appropriate preparations are currently being made. This example illustrates the willingness of the phage community to participate in such efforts and shows how phage sharing can be expedited through central coordination.
cPT in Australia
After the published case from 2011, no cPT uses were reported in Australia until 2017. Ampliphi Biosciences then announced the intravenous administration of their phage preparation against Staphylococcus aureus, AB-SA01, for an endocarditis infection under Category A of the SAS framework8,24. Since then, the company has established an expanded access agreement to compassionately use AB-SA01, and another product against P. aeruginosa, in collaboration with Western Sydney Local Health District and Westmead Institute for Medical Research25. They have now reported treating 13 patients suffering from serious S. aureus infections with an 83% success rate26. While these data have not yet been formally documented in peer-reviewed publications, they have been publicly presented both at scientific conferences and as press releases. The AB-SA01 product was previously tested in phase l clinical trials for topical administration and for chronic rhinosinusitis, and it is likely that the positive phase l data, along with positive results in cPT, will support phase ll trials that could lead to marketing authorisation for this product.
Conclusions
The ultimate future of phage therapy awaits the completion of randomised, controlled clinical trials in order to determine efficacy and attain marketing approvals. However, as there is no definitive date for when this may be accomplished, compassionate treatment options are an impactful way to address the clinical needs of patients suffering from intractable antibiotic-resistant infections today. In addition to the instances in Australia, cPT is also occurring around the world at phage therapy competency centres such as the Phage Therapy Unit at the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy in Poland27–30, the Eliava Phage Therapy Center in Tbilisi, Georgia, and the Center for Innovative Phage Applications and Therapeutics (IPATH) at the University of California San Diego School of Medicine10,18,31. cPT is also being done by independent medical teams through different access schemes in the USA, France, and Belgium32, and academic phage labs and biotech companies around the world are providing phages on behalf of patients. Collectively, this indicates an international inclination to support cPT. It is the hope of the authors that by streamlining the process of accessing and sharing therapeutic phages, cPT will be available to more patients in need.
Conflicts of interest
Two of the authors, Jessica C Sacher and Jan Zheng, are co-founders of Phage Directory.
Acknowledgements
This research did not receive any specific funding.
References
[1] O’Neill J. (2016) Tackling drug-resistant infections globally: final report and recommendations. Review on antimicrobial resistance. London, UK.[2] Kutateladze, M. (2015) Experience of the Eliava Institute in bacteriophage therapy. Virol. Sin. 30, 80–81.
| Experience of the Eliava Institute in bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 25662889PubMed |
[3] Sulakvelidze, A. et al. (2001) Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659.
| Bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 11181338PubMed |
[4] World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194.
| World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.Crossref | GoogleScholarGoogle Scholar | 24141714PubMed |
[5] Balasubramanian, G. et al. (2016) An overview of compassionate use programs in the European Union member states. Intractable Rare Dis. Res. 5, 244–254.
| An overview of compassionate use programs in the European Union member states.Crossref | GoogleScholarGoogle Scholar | 27904819PubMed |
[6] Bunnik, E.M. et al. (2017) The changing landscape of expanded access to investigational drugs for patients with unmet medical needs: ethical implications. J. Pharm. Policy Pract. 10, 10.
| The changing landscape of expanded access to investigational drugs for patients with unmet medical needs: ethical implications.Crossref | GoogleScholarGoogle Scholar | 28239479PubMed |
[7] Jarow, J.P. et al. (2017) Overview of FDA’s expanded access program for investigational drugs. Ther. Innov. Regul. Sci. 51, 177–179.
| Overview of FDA’s expanded access program for investigational drugs.Crossref | GoogleScholarGoogle Scholar | 28553565PubMed |
[8] Donovan, P. (2017) Access to unregistered drugs in Australia. Aust. Prescr. 40, 194–196.
| Access to unregistered drugs in Australia.Crossref | GoogleScholarGoogle Scholar | 29109604PubMed |
[9] (ACSQHC) ACoSaQiHC (2017) Aura 2017: second Australian report on antimicrobial use and resistance in human health. Sydney: ACSQHC.
[10] Aslam, S. et al. (2018) Bacteriophage treatment in a lung transplant recipient. J. Heart Lung Transplant. 37, S155–S156.
| Bacteriophage treatment in a lung transplant recipient.Crossref | GoogleScholarGoogle Scholar |
[11] Sybesma, W. et al. (2018) Silk route to the acceptance and re-implementation of bacteriophage therapy–part II. Antibiotics 7, 35.
| Silk route to the acceptance and re-implementation of bacteriophage therapy–part II.Crossref | GoogleScholarGoogle Scholar |
[12] Ferry, T. et al. (2018) Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J. Antimicrob. Chemother. 73, 2901–2903.
| Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages.Crossref | GoogleScholarGoogle Scholar | 30060002PubMed |
[13] Ferry, T. et al. (2018) Salvage debridement, antibiotics and implant retention (‘DAIR’) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect. Dis. 5, ofy269.
| 30474047PubMed |
[14] Fish, R. et al. (2018) Resolving digital staphylococcal osteomyelitis using bacteriophage–a case report. Antibiotics 7, 87.
| Resolving digital staphylococcal osteomyelitis using bacteriophage–a case report.Crossref | GoogleScholarGoogle Scholar |
[15] Patey, O. et al. (2018) Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11, 18.
| Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections.Crossref | GoogleScholarGoogle Scholar |
[16] Chan, B.K. et al. (2018) Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 60–66.
| Phage treatment of an aortic graft infected with Pseudomonas aeruginosa.Crossref | GoogleScholarGoogle Scholar | 29588855PubMed |
[17] Fish, R. et al. (2016) Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J. Wound Care 25, S27–S33.
| Bacteriophage treatment of intransigent diabetic toe ulcers: a case series.Crossref | GoogleScholarGoogle Scholar |
[18] Schooley, R.T. et al. (2017) Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61, e00954-17.
| Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection.Crossref | GoogleScholarGoogle Scholar | 28807909PubMed |
[19] Letkiewicz, S. et al. (2009) Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis — case report. Folia Microbiol. (Praha) 54, 457–461.
| Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis — case report.Crossref | GoogleScholarGoogle Scholar | 19937220PubMed |
[20] Ujmajuridze, A. et al. (2018) Adapted bacteriophages for treating urinary tract infections. Front. Microbiol. 9, 1832.
| Adapted bacteriophages for treating urinary tract infections.Crossref | GoogleScholarGoogle Scholar | 30131795PubMed |
[21] Khawaldeh, A. et al. (2011) Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 60, 1697–1700.
| Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection.Crossref | GoogleScholarGoogle Scholar | 21737541PubMed |
[22] Abedon, S.T. (2017) Information phage therapy research should report. Pharmaceuticals (Basel) 10, 43.
| Information phage therapy research should report.Crossref | GoogleScholarGoogle Scholar |
[23] Phage Directory Atlanta Georgia 2017 https://phage.directory
[24] AmpliPhi Biosciences Corporation (2017) AmpliPhi Biosciences announces first intravenous treatment of a patient with AB-SA01 targeting Staphylococcus aureus [press release]. San Diego, CA, 11 September 2017.
[25] (2018) AmpliPhi to collaborate with Western Sydney Local Health District and Westmead Institute for Medical Research on expanded access for investigational bacteriophage therapeutics AB-SA01 and AB-PA01 [press release]. San Diego, CA, 19 March 2018.
[26] (2018) AmpliPhi Biosciences Announces presentation of positive clinical data from its expanded access program for serious S. aureus infections at IDWeek 2018 conference [press release]. San Diego, CA, 8 October 2018.
[27] Weber-Dąbrowska, B. et al. (2016) Bacteriophage procurement for therapeutic purposes. Front. Microbiol. 7, 1177.
| 27570518PubMed |
[28] Weber-Dabrowska, B. et al. (2000) Bacteriophage therapy of bacterial infections: an update of our institute’s experience. Arch. Immunol. Ther. Exp. (Warsz.) 48, 547–551.
| 11197610PubMed |
[29] Weber-Dabrowska, B. et al. (2001) Bacteriophage therapy for infections in cancer patients. Clin. Appl. Immunol. Rev. 1, 131–134.
| Bacteriophage therapy for infections in cancer patients.Crossref | GoogleScholarGoogle Scholar |
[30] Weber-Dabrowska, B. et al. (2003) Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man. Transplant. Proc. 35, 1385–1386.
| Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man.Crossref | GoogleScholarGoogle Scholar | 12826166PubMed |
[31] LaVergne, S. et al. (2018) Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 5, ofy064.
| Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection.Crossref | GoogleScholarGoogle Scholar | 29687015PubMed |
[32] Pirnay, J.P. et al. (2018) The magistral phage. Viruses 10, 64.
| The magistral phage.Crossref | GoogleScholarGoogle Scholar |
Biographies
Jessica Sacher completed her PhD at the University of Alberta, where she studied the interactions between the gut pathogen Campylobacter jejuni and its phages. In 2017, she co-founded Phage Directory as a way to help foster collaboration between medical and industry professionals, researchers, and regulators to accelerate phage research and its translational applications.
Jan Zheng is a product designer with a background in computer science, psychology, with a Master’s degree in Human-Computer Interaction from Carnegie Mellon University. In the past, he has worked with companies like Coca-Cola, Microsoft, and L’Oreal, to design and build better digital products and user experiences. He co-founded Phage Directory to apply his background in UX design and web engineering to make a difference in the areas of health and phage technology.
Shawna McCallin is a postdoctoral researcher at University Hospital of Lausanne (CHUV), Switzerland who has previously been involved in two phage clinical trials for E. coli diarrhea and S. aureus carriage. She has extensively studied commercial phage preparations from Russia and Georgia, with a focus on S. aureus phage diversity. Recently, she is developing a phage sensitivity assay for on-site testing and expanding her phage research to the microbiome.