Detecting co-cultivation induced chemical diversity via 2D NMR fingerprints
Larissa Buedenbender A C D , Anthony R Carroll A E and D İpek Kurtböke BA Griffith School of Environment, Griffith University, Gold Coast Campus, Qld 4222, Australia
B Genecology Research Centre, School of Science and Engineering, University of the Sunshine Coast, Maroochydore, Qld 4558, Australia. Tel: +61 7 5430 2819, Email: ikurtbok@usc.edu.au
C Present address: GEOMAR Centre for Marine Biotechnology (GEOMAR-Biotech), Research Unit Marine Natural Products Chemistry, GEOMAR Helmholtz Centre for Ocean Research Kiel, 24106 Kiel, Germany
D Tel: +49 431 600 4424, Email: larissa.buedenbender@alumni.griffithuni.edu.au
E Tel: +61 7 5552 9187, Email: a.carroll@griffith.edu.au
Microbiology Australia 40(4) 186-189 https://doi.org/10.1071/MA19054
Published: 11 November 2019
Rediscovery of already known compounds is a major issue in microbial natural product drug discovery. In recent years, progress has been made in developing more efficient analytical approaches that quickly identify known compounds in a sample to minimise rediscovery. In parallel, whole genome sequencing of microorganisms has revealed their immense potential to produce secondary metabolites, yet the majority of biosynthetic genes remain silent under common laboratory culturing conditions. Therefore, increased research has focused on optimising culturing methods to activate the silent biosynthetic gene clusters. Co-cultivation of different microbial strains can activate biosynthetic gene clusters that remain silent under standard laboratory fermentations involving mono-cultures, hence, the technique has great potential for natural product drug discovery. However, innovative methods are still needed to evaluate the success of any co-cultured fermentation end-product. Here, the application of HSQC-TOCSY NMR spectra and subsequent PCoA to identify changes in the metabolite diversity induced through co-cultivation is described.
In nature, microorganisms occur in dynamic communities and thus co-cultivation allows for interspecific interactions that resemble natural microbial ecosystems more closely and trigger the production of cryptic metabolites1. Numerous examples of successful co-cultivation induced natural products, most often polyketides and non-ribosomal peptides, have been published and reviewed in the literature2–7. Such examples highlight the potential of co-culturing for natural product drug discovery. Chemometric-profiling approaches have been used to assess the induction of microbial metabolites through co-cultures5. Application of liquid chromatography coupled to UV or MS detectors is often used, but these techniques depend on the presence of chromophores or the ability of the natural products to ionise. NMR fingerprinting techniques have also been reported, by which the crude extract of mono- and co-cultures were fractionated and 1H NMR spectra of the pure cultures profiles were compared to those of the co-culture2. The strengths of NMR profiling are that it provides insight into structural components of the extract, it is highly reproducible and provides universal detection8. We recently reported a technique using 2D heteronuclear single quantum correlation - total correlation spectroscopy (HSQC-TOCSY) NMR profiles to prioritise microbial strains that have higher potential to produce drug-like natural products9. Hereby, the advantage of using 2D NMR profiles is that chemical resolution is improved by spreading the structural information over two-dimensions and consequently allows assessment of unfractionated crude extracts.
For co-cultivation, four strains all originating from different species, namely Streptomyces sp. (USC-16018), Micromonospora sp. (USC-16046), Nocardia sp. (USC-16096) and Staphylococcus aureus (ATCC 157293), were chosen in order to assess a broad diversity of microbial strains originating from different genera. Liquid fermentation was favoured over the solid agar cultures as mixed fermentations can more easily be upscaled, while on agar cultures the interactions between the two strains are generally highly localised and therefore compound isolation becomes more challenging as often low quantities of the compounds are produced5. Mono- and co-cultures were fermented in liquid ASW-A media10 and after two weeks of incubation, the microbial cells were separated from the media and extracted with methanol (MeOH). The liquid media phases were partitioned with ethyl acetate (EtOAc) to extract the metabolites that were diffused into the media. The crude extracts of the mono- and co-cultures were profiled using 2D HSQC-TOCSY NMR experiments. The advantage of this approach is that metabolite changes such as de novo production, and up- or down-regulation of metabolites in crude extracts can readily be detected in the spectra without fractionation of the crude extract. NMR further provides insights about the structural classes in the extract. This is particularly valuable for untargeted analysis, where a chemical profile is first established to assess chemical diversity within a sample and subsequent chemometric analysis can be used to identify uniqueness and similarities in the metabolome and reduce redundancy within the set of samples.
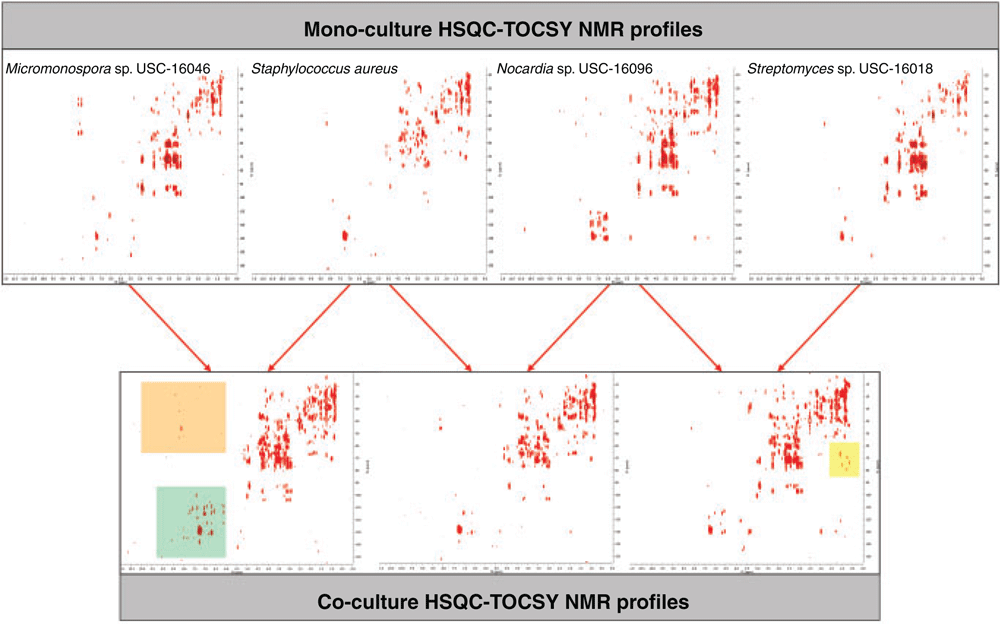
Visual inspection of the mono-culture profiles of the MeOH cell extracts readily depicted differences in microbial metabolomes at the genus level. Micromonospora sp. (USC-16046) displayed resonances of high intensity associated with peptides that were not detected in the other three mono-cultures. Resonances associated with polyketide fragments were observed in the Nocardia sp. (USC-16096) mono-culture as well as numerous peptide-associated NMR signals and diverse aromatic resonances. The Staphylococcus species (ATCC 157293) profile appeared chemically less diverse but indicated the presence of unique polyketide signals and Streptomyces sp. (USC-16018) had the least diverse mycelia extract (Figure 1). Comparison of the HSQC-TOCSY spectra of the MeOH cellular mono- and co-culture extracts showed clearly that the co-culture between Micromonospora sp. (USC-16046) and S. aureus (ATCC 157293) had triggered de novo secondary metabolite production. Numerous aromatic resonances not observed in the mono-cultures of the two strains as well as several peptide-associated peaks could be identified in the HSQC-TOCSY NMR profile (Figure 1). Additional polyketide fragment resonances were observed in the co-culture of Streptomyces sp. (USC-16018) and Nocardia sp. (USC-16096) (Figure 1). The EtOAc extracts showed several resonances of compounds that had diffused into the culture media (NMR spectra not depicted).
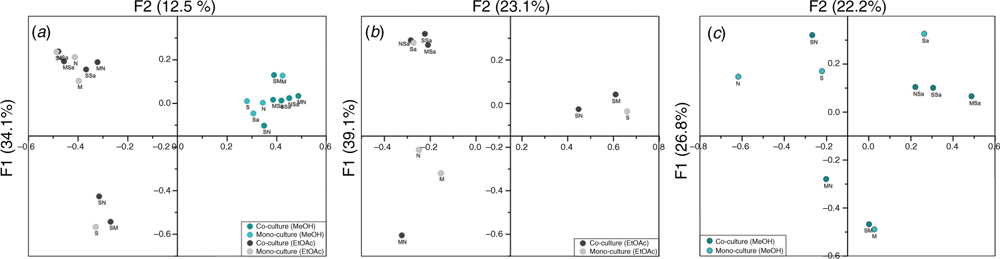
A chemometric metabolomics approach using multivariate analysis was implemented in order to dereplicate the dataset and identify significant correlations and differences in the secondary metabolomes. Peaks were picked from the HSQC-TOCSY NMR spectra regions of interest that were associated with polyketide, peptide, double bond, aromatic and heteroaromatic structure fragments as previously described9. After removal of resonances found in the media control, peaks between the samples were aligned using an Excel macro. Dice similarities were calculated and the generated correlation matrix was used for Principle Coordinate Analysis (PCoA; Figure 2). The first two principal coordinates (F1/F2) were able to explain more than 45% of the variation within the dataset.
Based on the position of the mono-cultures compared to the co-culture it could be determined which of the two strains was more dominant in the culture (Figure 2). The co-culture of Micromonospora sp. (USC-16046) and S. aureus (ATCC 157293) was distinct to both mono-cultures reiterating the uniqueness of this co-culture previously observed in the HSQC-TOCSY profile.
The HSQC-TOCSY NMR profiling approach proved to be very useful for the assessment of co-cultures. Not only did the profiles provide insight about de novo induction of secondary metabolites but in some cases also their suppression. The Micromonospora sp. (USC-16046) peptide resonances, which were dominant in all other MeOH co-cultures with the species, were highly suppressed in the co-culture with S. aureus (ATCC 157293), while aromatic resonances were significantly enhanced. Furthermore, it was clearly evident that the microorganisms diffuse secondary metabolites into the media that significantly differ from the metabolites found in their mycelia. Therefore, it is valuable to consider a range of extraction methods and solvents to obtain a complete spectrum of secondary metabolites in the culture.
Observations about the success and what type of structural fragments were found in the extracts could be made with minimal sample preparation in very little time as these 2D NMR spectra took only half an hour to be acquired. The inclusion of chemometric analysis can be a useful guide to detect unique samples, especially in larger datasets where sometimes similarities or differences in the NMR spectra cannot be identified solely by visual inspection. Only a few examples have been reported that utilise NMR profiles for the assessment of co-cultures2,11–13. Wu and co-workers implemented a 1H-NMR-based metabolomics approach to study metabolomic changes in a Streptomyces-fungus co-culture and found significant differences between mono- and co-culture extracts, but only in combination with UHPLC-TOF-MS they were able to identify the compound class12. The here presented HSQC-TOCSY profiling approach is to our knowledge the first account where 2D NMR profiling was used on co-cultivations. Due to the increased chemical shift resolution through spreading of the detected signals across two planes in 2D NMR spectra, assessment of unfractionated crude extracts was permitted and immediately revealed the structure type of the induced secondary metabolites. In the future, it will be interesting to pursue large-scale fermentation of the co-culture between S. aureus (ATCC-157293) and either Micromonospora sp. (USC-16046) and or Streptomyces sp. (USC-16018) in order to identify the co-culture induced de novo produced secondary metabolites.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was funded through an Australian Postgraduate Award provided by the Australian Government and a Griffith University International Postgraduate Research Scholarship awarded to Larissa Buedenbender.
References
[1] De Roy, K. et al. (2014) Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ. Microbiol. 16, 1472–1481.| Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities.Crossref | GoogleScholarGoogle Scholar | 24274586PubMed |
[2] Dashti, Y. et al. (2014) Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 12, 3046–3059.
| Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163.Crossref | GoogleScholarGoogle Scholar | 24857962PubMed |
[3] Scherlach, K. and Hertweck, C. (2009) Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 7, 1753–1760.
| Triggering cryptic natural product biosynthesis in microorganisms.Crossref | GoogleScholarGoogle Scholar | 19590766PubMed |
[4] Cueto, M. et al. (2001) Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 64, 1444–1446.
| Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge.Crossref | GoogleScholarGoogle Scholar | 11720529PubMed |
[5] Bertrand, S. et al. (2014) Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 32, 1180–1204.
| Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery.Crossref | GoogleScholarGoogle Scholar | 24651031PubMed |
[6] Tashiro, Y. et al. (2013) Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 28, 13–24.
| Interspecies interaction between Pseudomonas aeruginosa and other microorganisms.Crossref | GoogleScholarGoogle Scholar | 23363620PubMed |
[7] Gonzalez, D.J. et al. (2011) Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry. Microbiology 157, 2485–2492.
| Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 21719540PubMed |
[8] Kurita, K.L. and Linington, R.G. (2015) Connecting phenotype and chemotype: high-content discovery strategies for natural products research. J. Nat. Prod. 78, 587–596.
| Connecting phenotype and chemotype: high-content discovery strategies for natural products research.Crossref | GoogleScholarGoogle Scholar | 25728167PubMed |
[9] Buedenbender, L. et al. (2018) HSQC–TOCSY fingerprinting for prioritization of polyketide- and peptide-producing microbial isolates. J. Nat. Prod. 81, 957–965.
| HSQC–TOCSY fingerprinting for prioritization of polyketide- and peptide-producing microbial isolates.Crossref | GoogleScholarGoogle Scholar | 29498849PubMed |
[10] Hou, Y. et al. (2012) Microbial strain prioritization using metabolomics tools for the discovery of natural products. Anal. Chem. 84, 4277–4283.
| Microbial strain prioritization using metabolomics tools for the discovery of natural products.Crossref | GoogleScholarGoogle Scholar | 22519562PubMed |
[11] Cheng, Y.F. et al. (2013) Production of citrate by anaerobic fungi in the presence of co-culture methanogens as revealed by 1H NMR spectrometry. Asian-Australas. J. Anim. Sci. 26, 1416–1423.
| Production of citrate by anaerobic fungi in the presence of co-culture methanogens as revealed by 1H NMR spectrometry.Crossref | GoogleScholarGoogle Scholar | 25049725PubMed |
[12] Wu, C. et al. (2015) Expanding the chemical space for natural products by Aspergillus-Streptomyces co-cultivation and biotransformation. Sci. Rep. 5, 10868.
| Expanding the chemical space for natural products by Aspergillus-Streptomyces co-cultivation and biotransformation.Crossref | GoogleScholarGoogle Scholar | 26040782PubMed |
[13] Bertrand, S. et al. (2014) Multi-well fungal co-culture for de novo metabolite-induction in time-series studies based on untargeted metabolomics. Mol. Biosyst. 10, 2289–2298.
| Multi-well fungal co-culture for de novo metabolite-induction in time-series studies based on untargeted metabolomics.Crossref | GoogleScholarGoogle Scholar | 24948000PubMed |
Biographies
Larissa Buedenbender completed her doctoral studies in marine actinomycete biodiscovery at Griffith University, Australia, under the supervision of Anthony R. Carroll and D. İpek Kurtböke in 2018. Following her PhD, she has secured a post-doctoral position at the GEOMAR Helmholtz Centre for Ocean Research Kiel, Marine Natural Products Chemistry Research Unit in Kiel, Germany, where she is currently conducting marine natural products research.
Anthony R Carroll received his PhD from Sydney University and has been conducting research in marine natural products chemistry since his postdoctoral fellowships at the University of Hawaii and at James Cook University, Australia. Fifteen years as head of natural products chemistry for the AstraZeneca/Griffith University drug discovery project expanded his interests to include high throughput purification and structure determination techniques and cheminformatics. Since 2008 he has held a faculty position at Griffith University, Gold Coast where he is currently a Professor.
D İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology and is senior lecturer at the University of the Sunshine Coast (USC), Queensland. She has also been an active member of the World Federation for Culture Collections (WFCC) including serving as the Vice-President of the Federation (2010–2013) and currently is the President of the Federation (2017–2020).