Small but mighty: microorganisms offer inspiration for mine remediation and waste stabilisation
Alan Levett A B , Emma J Gagen A and Gordon Southam AA School of Earth and Environmental Sciences, University of Queensland, Brisbane, Qld 4072, Australia
B Tel: +61 4 3419 8225, +61 7 3365 6899, Email: alan.levett@uqconnect.edu.au
Microbiology Australia 40(4) 190-194 https://doi.org/10.1071/MA19055
Published: 14 November 2019
Understanding the natural microbiological mechanisms that promote iron cycling in iron ore mine environments may provide novel tools for the remediation of the fragile, iron-rich duricrust ecosystems associated with these environments as well as provide a solution for the stabilisation of hillslopes and tailings (waste) dams. A diverse array of microfossils is frequently identified throughout metre-scale duricrusts (canga; >50 wt.% Fe) that cap iron ore deposits in Brazil, shedding light on the intimate role of microorganisms in the evolution of these crusts. Nanoscale secondary ion mass spectrometry revealed that carbon and nitrogen biosignatures are occasionally preserved, and typically associated with the cell envelope structures of microfossils. The microfossils are 1–5 μm in length, with filamentous and rod-shaped morphologies commonly preserved1,2. When examined using backscatter electron scanning electron microscopy, canga shows a complex microstructure from repeated dissolution and re-precipitation of iron oxide minerals. Geochronology3, geochemistry4 and microbiology5 provide insights into the past and present-day role of microorganisms in the evolution of canga. These dynamic biogeochemical processes in canga contribute to the continuous formation of new iron cements, preserving some of world’s longest-lived continuously exposed surfaces. Harnessing and accelerating the biogeochemical cycling of iron may contribute to the development of novel technologies for mine remediation and waste stabilisation.
To supply the world with building resources, mountains are literally moved. The iron ore mining provinces in Australia and Brazil are vast, with iron ore reserves in each country totalling 10s of billions of tonnes6. China’s financial development in the last decade has required an exponential production of iron ore: more than 880 and 440 million tonnes of iron ore are now extracted from Australian and Brazilian mines each year respectively6. The increased extraction of the iron ore creates serious environmental risks and mine remediation challenges.
The massive iron ore provinces in Australia and Brazil are primarily produced by extensive weathering of 2 billion-year-old banded iron formations7, leaching away the silica and enriching the relatively immobile iron. During weathering in these systems, a hard ferruginous duricrust, referred to as canga, forms at the surface. Canga caps the iron ore deposits3 and is extremely resistant to erosion, thereby protecting the iron ore below it from erosion during the relentless weathering over billions of years8. Cosmogenic isotope studies have revealed canga to be one of the longest-lived continuously exposed surfaces in the world9,10. The key to the longevity of canga is the redox cycling of iron. In canga, Fe(III) oxide minerals are continuously dissolved by the flora and microbiota associated with canga, driving iron into solution (Reaction 1). However, Fe2+(aq) is unstable; it oxidises and re-precipitates to form new iron-rich cements in the presence of oxygen (Reactions 2 and 3). Therefore, chemical weathering of canga is partly responsible for the generation of new iron oxide cements that contribute to the long-term stability of canga and its extreme resistance to erosion11. Canga continues to evolve today via the biogeochemical cycling of iron3.
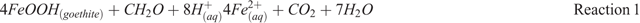


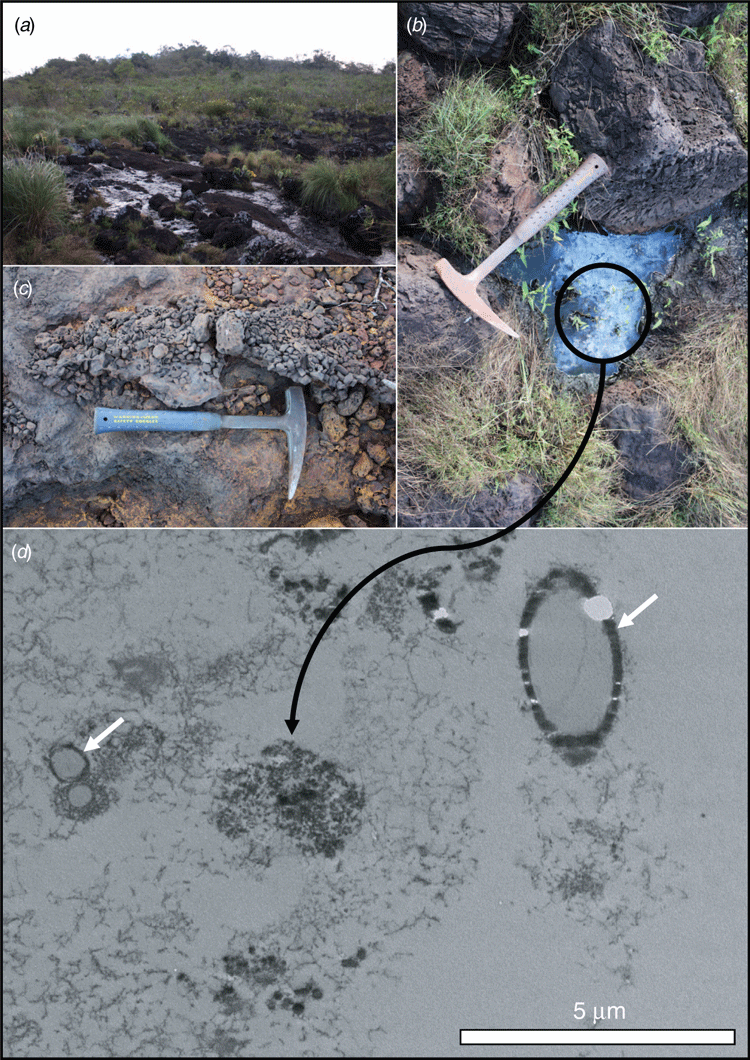
Our research aims to understand the role microorganisms play in the redox-based cycling of iron oxide minerals (Reactions 1–3) within iron-rich duricrusts. Understanding the biological processes that contribute to redox-based iron cycling within these duricrusts may provide new technologies to enhance the reformation of iron-rich duricrusts after the completion of iron ore mining. The accelerated re-cementation of iron-rich duricrusts will aim to restore functional hydrogeology12, provide a substrate for revegetation using native plants13,14 and assist in the development of new biotechnologies that aim to stabilise degraded slopes. Biological hotspots, including well developed biofilms in streams (Figure 1a), ephemeral pools (Figure 1b) and rhizospheric biomes, provide ideal sites to understand the modern-day biogeochemical cycling of iron. In these regions, microbially driven iron cycling is accelerated and new cements in the duricrust are formed at a relatively rapid rate. For example, organic acids exuded by plants and microbes may chelate iron, making it more readily available for iron-reduction. These environments also contain higher concentrations of carbon sources for direct iron reduction (Reaction 1). Microorganisms also play an integral role in cement formation. Their cell envelope structures act as active sites for mineral nucleation (Reaction 3)15. The question then becomes how can microbes growing at the micrometre scale be used to help to recreate these kilometre scale horizons?
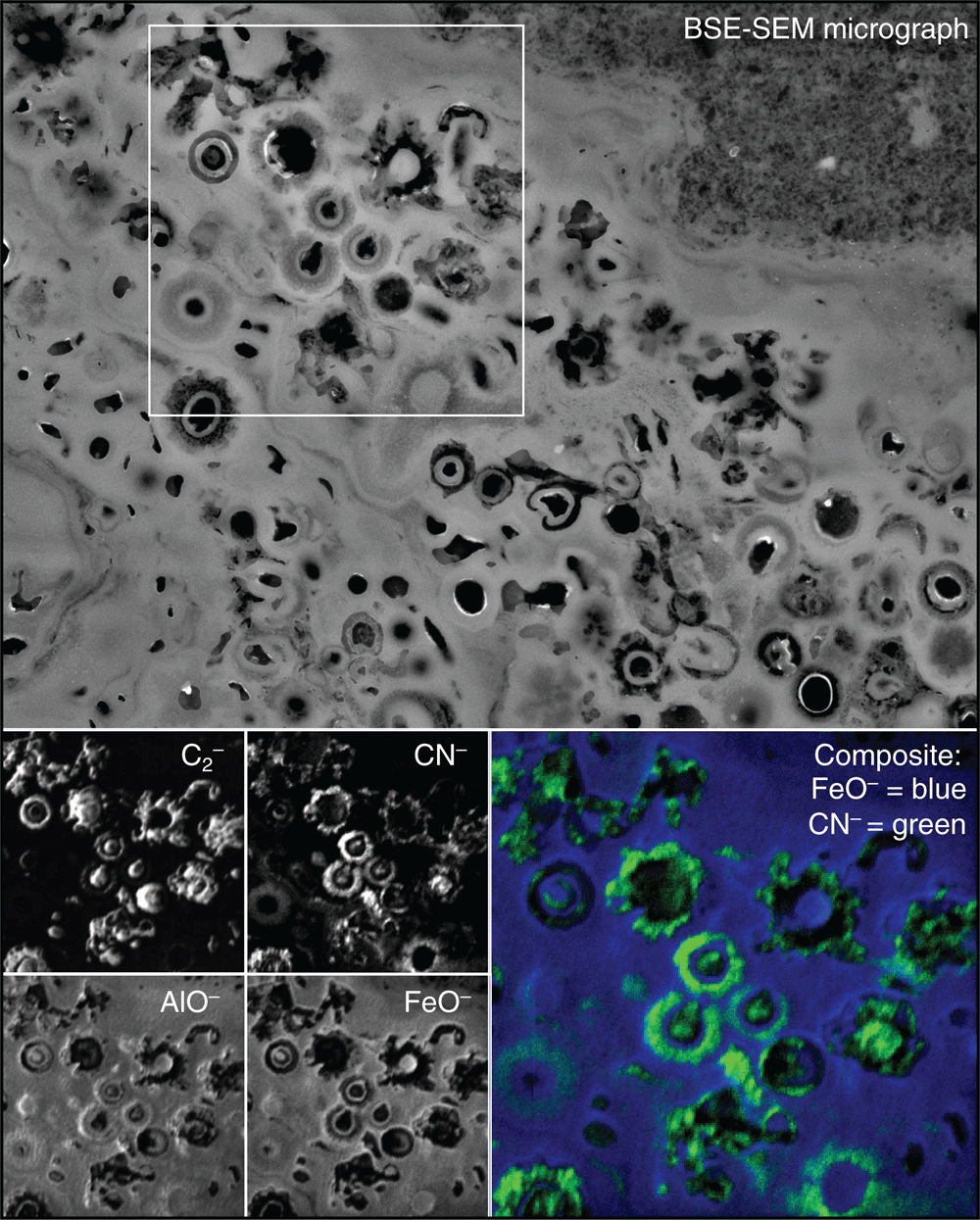
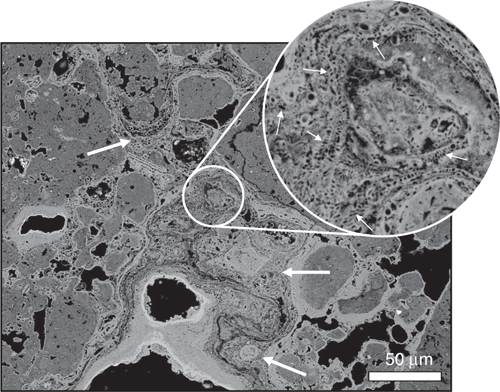
Surface sheens that occur on many of the pools and seeps in canga environments provide an insight into the contemporary biogeochemical cycling of iron (Figure 1b). Within these surface sheens, microorganisms and extracellular polymeric substances induce the precipitation of poorly crystalline iron oxide minerals at the nanoscale (Figure 1d). With high surface to volume ratios, the cell envelopes of microorganisms provide a surfeit of reactive sites for mineral nucleation (Figure 1d). Scaling-up nanoscale chemical processes for mine remediation remains a global challenge. However, the size and abundance of microorganisms as well as their ability to form biofilms16 represent an important aspect of bioremediation efforts. Critically for canga, as these biofilms are continuously exposed to iron solutions they become fossilised (Figure 2). The poorly crystalline iron oxides that nucleated on the cells’ surfaces transform to more stable phases (Reaction 4), creating biocements (Figure 2). In addition, microbial biofilms act as an organic scaffold that gives the iron-rich cements a ‘direction’ for growth, rather than simply coating and enlarging existing grains (Figure 2). Carbon and nitrogen biosignatures, revealed by nanoscale secondary ion mass spectrometry (nanoSIMS), may be preserved with mineral encrusted cell envelopes (Figure 3), likely as a result of the effectively irreversible complexation of aluminium with the cell envelope2. Combining these concepts, microbial growth on crushed rock surfaces could be enhanced to form natural biofilm-rock aggregates. Simultaneously, microbially-promoted iron reduction would be optimised, forming iron-rich solutions within anaerobic bioreactors that would be distributed throughout the mine remediation site. Finally, the biofilm-rock aggregates would be exposed to the iron-rich solutions, where the soluble iron would become immobilised as iron oxide precipitates on the microbial cells’ surfaces, mineralising the biofilms and forming new biocements. These processes could be repeated until the surface substrates were sufficiently stable to begin revegetation programmes.
|

In tropical regions that experience monsoonal rainfall, timing of cement formation is also a logistical constraint. In the field, cements can form on relatively steep slopes (Figure 1c), indicating they can consolidate loose material relatively quickly. These cements may initially form on the scale of decades and continue to be reinforced over the ensuing centuries by the continued precipitation iron oxides. Therefore, understanding and accelerating the natural processes that have previously formed these concretions will provide tools for relatively rapid, long-term landform stabilisation.
One of the driving forces to develop novel strategies for iron ore mine remediation are the unique florae associated with ferruginous duricrusts in Australia and Brazil. More than 900 plant species have been identified on ironstone outcrops in south-western Australia, with 44 species considered ironstone specialists14. Similarly, the florae associated with iron ore provinces in Brazil are diverse and naturally rare, with several endemic plant species growing on canga substrates. Canga capping iron ore deposits in the Carajás Mineral Province, Pará, in northern Brazil, supports 856 plant species, while 946 plant species have been collectively identified on canga in the Quadrilátero Ferrífero (QF), Minas Gerais, in south-eastern Brazil13. Extraordinarily, only 96 species are common to both the Carajás and the QF13, highlighting the species richness of canga-associated florae. Accelerating the regeneration of canga-like substrates is essential for the restoration of these naturally rare ecosystems.
Brazil has suffered from two large-scale iron ore tailings dam collapses in recent years17,18, with disastrous environmental impacts and deaths occurring at each site. Promoting the natural biogeochemical cycling of iron oxide minerals within canga that has preserved iron ore mines in Australia and Brazil may serve as an effective strategy to remediate mine sites in the future. Increasingly, we are looking to our smallest companions, the mighty ‘microorganism’, to help solve our greatest environmental challenges.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge support from the Vale S.A.-UQ Geomicrobiology initiative and the Australian Research Council Linkage Program (LP140100805) to G. Southam. The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre of Microscopy and Microanalysis, at the University of Queensland. Alan Levett acknowledges the support from the Australian Government Research Training Program. We thank Linda Blackall and an anonymous reviewer for valuable suggestions that improved the manuscript.
References
[1] Levett, A. et al. (2016) Evidence of biogeochemical processes in iron duricrust formation. J. S. Am. Earth Sci. 71, 131–142.| Evidence of biogeochemical processes in iron duricrust formation.Crossref | GoogleScholarGoogle Scholar |
[2] Levett, A. et al. (2019) The role of aluminium in the preservation of microbial biosignatures. Geoscience Frontiers 10, 1125–1138.
| The role of aluminium in the preservation of microbial biosignatures.Crossref | GoogleScholarGoogle Scholar |
[3] Monteiro, H.S. et al. (2014) (U–Th)/He geochronology of goethite and the origin and evolution of cangas. Geochim. Cosmochim. Acta 131, 267–289.
| (U–Th)/He geochronology of goethite and the origin and evolution of cangas.Crossref | GoogleScholarGoogle Scholar |
[4] Spier, C.A. et al. (2019) Geochemistry of canga (ferricrete) and evolution of the weathering profile developed on itabirite and iron ore in the Quadrilátero Ferrífero, Minas Gerais, Brazil. Miner. Depos. 54, 983–1010.
| Geochemistry of canga (ferricrete) and evolution of the weathering profile developed on itabirite and iron ore in the Quadrilátero Ferrífero, Minas Gerais, Brazil.Crossref | GoogleScholarGoogle Scholar |
[5] Gagen, E.J. et al. (2018) Microbial diversity in actively forming iron oxides from weathered banded iron formation systems. Microbes Environ. 33, 385–393.
| Microbial diversity in actively forming iron oxides from weathered banded iron formation systems.Crossref | GoogleScholarGoogle Scholar | 30449766PubMed |
[6] United States Geological Survey (2018) Iron Ore Statistics and Information. https://minerals.usgs.gov/minerals/pubs/commodity/iron_ore/mcs-2018-feore.pdf (accessed 7 February 2018).
[7] Goldich, S.S. (1973) Ages of Precambrian banded iron-formations. Econ. Geol. 68, 1126–1134.
| Ages of Precambrian banded iron-formations.Crossref | GoogleScholarGoogle Scholar |
[8] Gagen, E.J. et al. (2019) Biogeochemical processes in canga ecosystems: armoring of iron ore against erosion and importance in iron duricrust restoration in Brazil. Ore Geol. Rev. 107, 573–586.
| Biogeochemical processes in canga ecosystems: armoring of iron ore against erosion and importance in iron duricrust restoration in Brazil.Crossref | GoogleScholarGoogle Scholar |
[9] Monteiro, H.S. et al. (2018) Age and evolution of diachronous erosion surfaces in the Amazon: combining (U-Th)/He and cosmogenic 3He records. Geochim. Cosmochim. Acta 229, 162–183.
| Age and evolution of diachronous erosion surfaces in the Amazon: combining (U-Th)/He and cosmogenic 3He records.Crossref | GoogleScholarGoogle Scholar |
[10] Shuster, D.L. et al. (2012) Cosmogenic 3He in hematite and goethite from Brazilian “canga” duricrust demonstrates the extreme stability of these surfaces. Earth Planet. Sci. Lett. 329–330, 41–50.
| Cosmogenic 3He in hematite and goethite from Brazilian “canga” duricrust demonstrates the extreme stability of these surfaces.Crossref | GoogleScholarGoogle Scholar |
[11] Monteiro, H. et al. (2018) A combined (U‐Th)/He and cosmogenic 3He record of landscape armoring by biogeochemical iron cycling. J. Geophys. Res. Earth Surf. 123, 298–323.
| A combined (U‐Th)/He and cosmogenic 3He record of landscape armoring by biogeochemical iron cycling.Crossref | GoogleScholarGoogle Scholar |
[12] Toy, T.J. and Griffith, J.J. (2001) Changing surface-mine reclamation practices in Minas Gerais, Brazil. Int. J Surface Min. Reclamat. Environ. 15, 33–51.
[13] Mota, N.F.O. et al. (2018) Amazon canga: the unique vegetation of Carajás revealed by the list of seed plants. Rodriguésia 69, 1435–1488.
| Amazon canga: the unique vegetation of Carajás revealed by the list of seed plants.Crossref | GoogleScholarGoogle Scholar |
[14] Gibson, N. et al. (2012) Patterns of plant diversity in ironstone ranges in arid south western Australia. J. Arid Environ. 77, 25–31.
| Patterns of plant diversity in ironstone ranges in arid south western Australia.Crossref | GoogleScholarGoogle Scholar |
[15] Ferris, F.G. et al. (1988) Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms. Geology 16, 149–152.
| Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms.Crossref | GoogleScholarGoogle Scholar |
[16] Davey, M.E. and O’toole, G.A. (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867.
| Microbial biofilms: from ecology to molecular genetics.Crossref | GoogleScholarGoogle Scholar | 11104821PubMed |
[17] Segura, F.R. et al. (2016) Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ. Pollut. 218, 813–825.
| Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil).Crossref | GoogleScholarGoogle Scholar | 27524249PubMed |
[18] Hinman, P. (2019) Brazil: new vale mine disaster is one more corporate failure. Green Left Weekly (1207), 15.
Biographies
Alan Levett is a final year PhD candidate in the field of geomicrobiology at the University of Queensland. His research focuses on the biogeochemical cycling of iron within tropical iron ore systems. Alan’s research combines high-resolution microscopy, mass spectrometry (NanoSIMS) and synchrotron-based microspectroscopy techniques with ecogenomics to analyse past and present-day interactions of microorganisms with metals and minerals.
Dr Emma Gagen is a Research Fellow at the University of Queensland. Her research focuses on harnessing microbial processes for accelerated mine site rehabilitation (iron ore mines in Brazil, coal mines in central Queensland). Other projects she contributes to relate to microbial colonisation of meteorites, bacterial degradation of anhydrite, microbiology of sulphur rich environments and formation of seafloor iron-manganese crusts. Emma’s research interests extend to all areas of environmental microbiology and she is fascinated by the role microorganisms play in geochemical processes.
Professor Gordon Southam is an interdisciplinary researcher who has crossed traditional boundaries between biological and geological sciences. His research, using state-of-the-art microanalyses techniques (e.g. HR-TEM, FIB-SEM and synchrotron methods), focuses on the vital roles that bacteria play in mineral weathering, e.g. acid mine drainage systems, in the formation of mineral signatures in soils and sediments, and the genesis of authigenic secondary minerals, e.g. carbonates, iron oxides, metal sulphides and placer gold.