Unprecedented toxic algal blooms impact on Tasmanian seafood industry
Gustaaf Hallegraeff A and Christopher Bolch BA Institute for Marine and Antarctic Studies
University of Tasmania
Private Bag 129
Hobart, Tas. 7001, Australia
Tel: +61 3 6226 2623
Email: Hallegraeff@utas.edu.au
B Institute for Marine and Antarctic Studies
University of Tasmania
Locked Bag 1370
Launceston, Tas. 7250, Australia
Tel: +61 3 6324 3815
Email: chris.bolch@utas.edu.au
Microbiology Australia 37(3) 143-144 https://doi.org/10.1071/MA16049
Published: 10 August 2016
While most microscopic algae provide food for filter-feeding shellfish and larvae of crustaceans and finfish, other so-called Harmful Algal Blooms (HABs) can have negative effects, causing severe economic losses to aquaculture, fisheries and tourism. Of greatest concern to human society are blooms of toxic HAB species that cause illness and death of fish, seabirds and mammals via toxins transferred through the food web. Unprecedented Alexandrium (Dinophyceae) blooms along the East Coast of Tasmania in 2012 and 2015, a previously low biotoxin risk area, led to major impacts on the local oyster, mussel, scallop and rock lobster industries. Four human hospitalisations also occurred from eating wild shellfish.
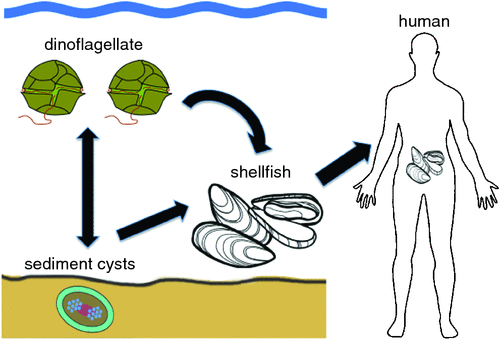
One of the first recorded fatal cases of human Paralytic Shellfish Poisoning (PSP) after eating shellfish contaminated with dinoflagellate toxins occurred in 1793, when Captain George Vancouver and his crew landed in British Columbia in an area now known as Poison Cove. He noted that it was taboo for local Indian tribes to eat shellfish when the sea became bioluminescent due to plankton blooms1. The causative organism, the dinoflagellate Alexandrium was formally described in 1936 and the neurotoxin it produces (saxitoxin, STX) chemically characterised in 19752,3 (Figure 1). Doses of 1 mg cause moderate symptoms in humans (tingling sensations around finger tips and lips) but doses of 10 mg can be lethal, resulting in death from respiratory paralysis. To prevent human poisonings, the United States Food and Drug Administration (US FDA) introduced a compulsory monitoring program in 1937 whereby seafood products are regularly tested in accredited laboratories using mouse bioassays4. Shellfish containing more than 0.8 mg PST/kg are deemed unsuitable for human consumption and prohibited from sale. Due to increasing concern over animal ethics, many algal toxins are now analysed using sophisticated Ultra Performance Liquid Chromatography (UPLC) and Liquid Chromatography Mass Spectrometry (LCMS) methods. These analyses are expensive ($500–$800/test) and when compounded by sample transport problems, result in frustrating delays for fishermen and regulators. In Tasmania, seafood biotoxin testing is conducted through the Tasmanian Shellfish Quality Assurance Program, using a specialist analytical laboratory in Sydney for routine testing – at an annual cost of up to $450 000.
|
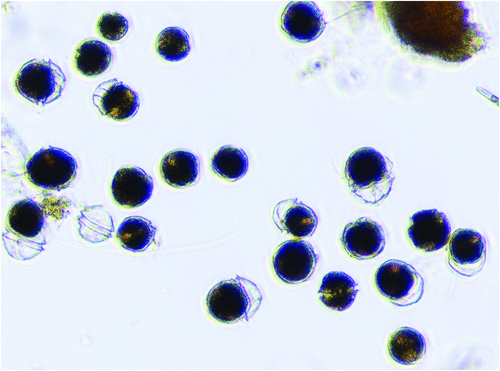
In October 2012 a shipment of blue mussels (Mytilus galloprovincialis) from the East Coast of Tasmania, Australia, was tested by Japanese import authorities and found to be contaminated with unacceptably high level of Paralytic Shellfish Toxins (10 mg PST/kg)5. Subsequent testing showed that oysters, scallops, clams, and abalone and rock lobster viscera were also contaminated along the entire Tasmanian East Coast. This led to precautionary fishery closures also for the high-value Southern Rock Lobster and abalone industries and an international shellfish product recall with losses of more than $23 million to the local economy. Following low toxicity events and no lengthy farm closures in 2013 and 2014, a more severe bloom event recurred during June–Oct 2015 (up to 300 000 dinoflagellate cells/L) resulting in >15 mg/kg PST in mussels, >6 mg/kg PST in oysters, and four human hospitalisations after consumption of wild shellfish by recreational collectors unaware of public health warnings. The causative dinoflagellate Alexandrium tamarense (Figure 2) had been previously detected in the area in very low cell concentrations over the past 10–15 years, but cultured strains had been mostly non-toxic or weakly toxic6. Accordingly, the area had been assigned a low biotoxin risk and monitored at low frequency, particularly in winter and autumn. Unexpectedly, all outbreaks since 2012 have been dominated by a highly toxic A. tamarense genotype never previously seen in bloom proportions in Australia. While we considered the possibility of ballast water introduction or climate-driven range extension7, preliminary molecular evidence suggests the causative dinoflagellate is a previously cryptic (rare) genotype in the area, now favoured by increased water column stratification associated with southward extension of the East Australian Current. Increased seafood and plankton monitoring now include Alexandrium quantitative molecular detection (qPCR) that offers more sensitive early detection of the causative dinoflagellate8 and routine immunoassay screening for toxins, in parallel with routine UPLC toxin testing9. The rapid immunoassay PSP test kits10 (Figure 3) provide an on-site or on-board qualitative yes/no result within 20 minutes, allowing producers to make on-farm harvest decisions prior to product processing and transport, thus reducing their business risk. Once validated at a national and international level, these tests can also be used by regulators as a pre-screening test to reduce the cost of testing negative samples, and improve public health outcomes.
|

|
Acknowledgements
This work was funded by the Australian Government through Fishing Industry Research and Development Grant 2014/032.
References
[1] Vancouver, G. (1798). Voyage of Discovery to the North Pacific Ocean, and Round the world in the years 1791–95. London.[2] Sommer, H. et al. (1937) Relation of paralytic shellfish poison to certain plankton organisms of the genus Gonyaulax. Arch. Pathol. (Chic) 24, 537–559.
| 1:CAS:528:DyaA1cXhvVGjsw%3D%3D&md5=95c62aab7fa3d3ef38e70c562da35fbdCAS |
[3] Schantz, E.J. et al. (1975) The structure of saxitoxin. J. Am. Chem. Soc. 97, 1238–1239.
| The structure of saxitoxin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXht1Gms74%3D&md5=7bffd9e52a14f083713c2905c62c85b1CAS | 1133383PubMed |
[4] Sommer, H. and Meyer, K.F. (1937) Paralytic shellfish poisoning. Arch. Pathol. (Chic) 24, 560–598.
| 1:CAS:528:DyaA1cXhvVGjsg%3D%3D&md5=cc726706a98bc51ae06978f297cd1812CAS |
[5] Campbell, A., et al. (2013) Review of the 2012 paralytic shellfish toxin event in Tasmania associated with the dinoflagellate alga, Alexandrium tamarense. A SafeFish Review. FRDC Project 2012/060, Adelaide.
[6] Bolch, C.J.S. and de Salas, M. (2007) A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium tamarensis complex’ to Australasia. Harmful Algae 6, 465–485.
| A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium tamarensis complex’ to Australasia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsVGmtLg%3D&md5=0f3045278c65561ac546eaf13890411aCAS |
[7] Hallegraeff, G.M. (2010) Ocean climate change, phytoplankton community responses and harmful algal blooms: a formidable predictive challenge. J. Phycol. 46, 220–235.
| Ocean climate change, phytoplankton community responses and harmful algal blooms: a formidable predictive challenge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvF2rur0%3D&md5=e6b5dc980c1a9b078144d706f879f6e5CAS |
[8] Hosoi-Tanabe, S. and Sako, Y. (2005) Species-specific detection and quantification of toxic marine dinoflagellates Alexandrium tamarense and A. catenella by real-time PCR assay. Mar. Biotechnol. (NY) 7, 506–514.
| Species-specific detection and quantification of toxic marine dinoflagellates Alexandrium tamarense and A. catenella by real-time PCR assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGhtLjP&md5=0ac8dd54dd68939c634f3f2f71206cb6CAS | 16007374PubMed |
[9] Lawrence, J.F. et al. (2005) Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: collaborative study. J AOAC Int. 88, 1714–1732.
| 1:CAS:528:DC%2BD2MXhtlWqsLjM&md5=65a17c74d9b8b9855fc1e4347dcce460CAS | 16526455PubMed |
[10] Jawaid, W. et al. (2015) Development and validation of a novel lateral flow immunoassay (LFIA) for the rapid screening of paralytic shellfish toxins (PSTs) from shellfish extracts. Anal. Chem. 87, 5324–5332.
| Development and validation of a novel lateral flow immunoassay (LFIA) for the rapid screening of paralytic shellfish toxins (PSTs) from shellfish extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmvVygtbo%3D&md5=41e1883087467e705934d0a058e537c7CAS | 25893460PubMed |
Biographies
Gustaaf Hallegraeff is a Professor at the Institute of Marine and Antarctic Studies at the University of Tasmania. His research focuses on harmful algal blooms impacting on human health, the fish farm and shellfish industries, their stimulation by coastal eutrophication, climate change and global spreading via ship’s ballast water.
Christopher Bolch is a Senior Lecturer at the Launceston laboratories of the Institute of Marine and Antarctic Studies at the University of Tasmania. His research focuses on molecular biology, detection, diversity and biogeography of toxic and harmful algae, and their impact on marine and freshwater industries.


