From an irrigation system to an ecological asset: adding environmental flows establishes recovery of a threatened fish species
Ivor Stuart A B I , Clayton Sharpe C D E , Kathryn Stanislawski F , Anna Parker G and Martin Mallen-Cooper E HA Kingfisher Research, 177 Progress Road, Eltham, Vic. 3095, Australia.
B Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
C CPS Enviro, 972 Irymple Road, Irymple, Vic. 3498, Australia.
D New South Wales National Parks and Wildlife Service, PO Box 363, Buronga, NSW 2739, Australia.
E Institute for Land, Water and Society, Charles Sturt University, NSW 2640, Australia.
F Parks Victoria, PO Box 3100, Bendigo MC, Vic. 3554, Australia.
G North Central Catchment Management Authority, PO Box 18, Huntly, Vic. 3551, Australia.
H Fishway Consulting Services, 8 Tudor Place, Saint Ives, NSW 2075, Australia.
I Corresponding author. Email: ivor.stuart@delwp.vic.gov.au
Marine and Freshwater Research 70(9) 1295-1306 https://doi.org/10.1071/MF19197
Submitted: 28 May 2019 Accepted: 6 July 2019 Published: 26 July 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Worldwide, riverine fish are the target of environmental water because populations have declined in lotic river habitats following river regulation. Murray cod is an endangered Australian riverine fish with remaining populations associated with lotic river reaches with instream habitat, including some creeks operated as part of irrigation systems. Our objectives were to develop a life history model, apply the building block method of environmental flows to enhance the abundance of juvenile Murray cod and promote population recovery. From 2008 to 2018 we evaluated changes to Murray cod juvenile abundance before and after implementation of a perennial environmental flow regime that began in 2013. During the first year of the environmental flow, larvae were collected as evidence of spawning. Murray cod abundance can be enhanced with environmental flows that target: (1) an annual spring spawning or recruitment flow with no rapid water level drops; (2) maximising hydrodynamic complexity (i.e. flowing habitats that are longitudinally continuous and hydrodynamically complex); and (3) an annual base winter connection flow. Recognition that incorporating hydraulics (water level and velocity) at fine and coarse time scales, over spatial scales that reflect life histories, provides broader opportunities to expand the scope of environmental flows to help restore imperilled fish species in regulated ecosystems.
Additional keywords: Australia, building block method, Gunbower Creek, Murray cod, recruitment, water management.
Introduction
In many parts of the world, environmental flows (i.e. water quantity, timing and discharge) are being returned to rivers, wetlands and flood plains to restore the health of native fish, birds and trees (Acreman et al. 2014; Horne et al. 2017). Riverine fish are commonly the target of environmental flows because many populations have declined following river regulation (Koster et al. 2017; Belmar et al. 2018). Typically, environmental flows restore specific aspects of river hydrology, such as more natural flood frequency, discharge, magnitude (i.e. volume), duration, rate of change and timing, all components that can be targeted to benefit native fish (Poff and Zimmerman 2010; Arthington et al. 2018; Poff 2018).
Two additional components of environmental flows that are increasingly recognised as influential in restoring riverine fish communities are enhanced hydrodynamic complexity (Guse et al. 2015) and improved longitudinal and lateral connectivity (Perkin et al. 2015). Integrating these aspects into environmental flow objectives appears particularly effective in restoring long reaches of lotic (flowing water) habitats for riverine species in south-west North American streams (Dudley and Platania 2007; Wilde and Urbanczyk 2013), lowland rivers of Australia’s Murray–Darling Basin (MDB; Tonkin et al. 2019) and lowland rivers of Europe (Daufresne et al. 2015).
For planning and implementing environmental flows, there is a broad suite of approaches depending on the desired ecosystem–societal outcomes (Tharme 2003; Acreman and Dunbar 2004; King and Brown 2006). In Australia and South Africa, there has been emphasis on protecting entire aquatic habitats, often where the ecology of biota is poorly known, with holistic environmental flow methodologies (King et al. 2003; Arthington et al. 2006). The building block methodology (BBM) relies on expert consensus to build a flow regime based on clear ecological and social outcomes for each flow component (King and Louw 1998; King et al. 2000, 2003). By clearly identifying flow–ecology relationships, an environmental flow regime can be built from a starting point of zero and then hydrological elements added to restore specific ecosystem functions (Acreman et al. 2014; Moyle 2014).
Provision of environmental flows to maintain lotic refuges within irrigation systems, or within rivers and creeks used primarily as irrigation conduits, is an emerging theme for re-establishing fish assemblages in North America (Cowley et al. 2007; Carlson et al. 2019), France (Aspe et al. 2016) and Japan (Onikura et al. 2009). Within many natural rivers and other systems that are used as conduits for irrigation, a different approach to restoring an environmental flow regime is required because the starting flows have entirely consumptive objectives (Kingsford 2000). Two examples of these hydrological differences are during: (1) high-demand periods, when irrigation conduits are characterised by rapid increases and decreases in discharge that trigger abrupt short-term (e.g. hourly) fluctuations in water levels (Thoms and Cullen 1998); and (2) the ‘off-irrigation season’, when flows are discontinued and there can be channel dewatering (King and O’Connor 2007). Fish from low-gradient perennial rivers are not adapted to such rapid fluctuations or intermittency, and to mitigate these effects the missing flow components should be added to the consumptive flow regime (Cushman 1985).
At a fine temporal scale (e.g. hourly), rapid changes in flows downstream of dams and weirs can reduce the abundance and reproductive success of fish (Cushman 1985; Zimmerman et al. 2010). The challenge of planning and implementing subdaily environmental flows to maintain particular hydrological conditions is highlighted by fish that spawn in nests, including North American smallmouth bass Micropterus dolomieu, for which rapid water level fluctuations may cause the guarding male to abandon the eggs (Clark et al. 2008), and South American pirarcu Arapaima gigas, which require a specific set of hydrological conditions to access floodplain nesting habitats (Castello 2008). In Australia, Murray cod Maccullochella peelii also spawn in nests, which are cleaned hard surfaces such as logs or rocks (Balon 1975), and their distribution and abundance has declined following river regulation, combined with overfishing, habitat removal, abstraction of water, cold water release from impoundments and poor water quality (Rowland 2004; Koehn and Todd 2012; Baumgartner et al. 2014).
Where self-sustaining populations of Murray cod remain, certain elements of the flow regime seem especially influential, including: (1) no rapid major drops in water levels during the spring spawning season, which may cause reduced survival of eggs and larvae; and (2) perennial maintenance of lotic habitats (>0.25 m s–1 mean channel velocity), especially during winter, when flows in many irrigation systems typically cease, which may increase the exposure of age-0+ fish to higher rates of predation and reduced survival (Saddlier et al. 2008; Koehn and Nicol 2014). This ecological understanding has created an opportunity to recover Murray cod by implementing environmental flows to restore lost perennial lotic habitats. Demonstrating benefits within irrigation systems may also enable greater insight into whether the perceived conflicts (e.g. maintaining reliable irrigation delivery) or modifying an entirely consumptive flow regime to gain ecological outcomes can be resolved.
Our objectives were to develop a conceptual model of Murray cod life history and apply the BBM of environmental flows to enhance the survival of eggs, larvae and juvenile Murray cod, promoting population recovery. We hypothesised that the abundance of Murray cod would increase in years following environmental flows. The three building blocks of flow we used were: (1) irrigation water for consumptive users; (2) environmental flows to buffer rapid, short-term (hourly) drops in water height during intense consumptive irrigation demand periods, which also overlap the spring nesting and spawning season; and (3) permanent lotic habitats (>0.25 m s–1) to enhance overwintering survival of juvenile fish when irrigation flows historically entirely cease and the system dries into a series of disconnected pools. From 2008 to 2018, we evaluated changes to Murray cod abundance before and after implementation of a perennial environmental flow regime in a major irrigation system.
Background
Murray cod conceptual life history model
Endemic to Australia’s MDB, Murray cod is a nationally endangered fish, growing to 1.5 m long and 50 kg, with an estimated maximum life span of 48 years (Anderson et al. 1992). Populations tend to remain in riverine environments that preserve lotic conditions and high loadings of large woody debris (Koehn et al. 2009; Lyon et al. 2019). Movement occurs at a range of spatial scales, for example within river valleys (hundreds of kilometres) and within reaches (tens of kilometres), and some fish move long distances (e.g. more than 200 km) during major increases in flow and floods (Reynolds 1983; Jones and Stuart 2007; Koehn et al. 2009; Leigh and Zampatti 2013). In winter–spring, adult fish move to lotic spawning habitats, where these are available and accessible (Saddlier et al. 2008; Koehn et al. 2009).
Spawning occurs annually, mainly from mid-spring (October–November) and occasionally early summer (December–January), during a range of river flow conditions, and migration from home sites to lotic spawning sites is common (Saddlier et al. 2008; Koehn et al. 2009). From mid-spring (October–November), when water temperature exceeds 18°C, Murray cod spawn their eggs, in a confined group, usually on hard surfaces, such as clay ledges, undercut banks, rocks, hollow logs and in earthen hatchery ponds, confirming their spawning flexibility (Rowland 2004). The male fish guards the nest for up to 18 days and the nest is usually in the upper water column, potentially close to the surface (Cadwallader 1977; Rowland 1998).
After hatching and brood guarding, the larvae disperse from the nest and are regularly captured drifting downstream (Humphries 2005; Koehn and Harrington 2005; King et al. 2009); there is little information on settlement patterns, but juvenile fish often inhabit littoral or woody debris habitats where there can be rapid growth (Koehn and Harrington 2005, 2006; Tonkin et al. 2017). Early juvenile recruitment (i.e. survival to 1 year old) in the southern MDB has only been recorded in lotic habitats in river and creek channels and coincides with stable or rising flows in lotic habitats, including moderate and major rises contained within the channel, and overbank floods in the lower reaches of the Murray River (Ye and Zampatti 2007; King et al. 2009).
Impacts of river regulation
Mallen-Cooper and Zampatti (2018) reassessed the effects of flow regulation in the Murray River, Australia, and concluded that changes in hydraulics or hydrodynamics (changes in hydraulics over time and space) were a major factor in the decline of species like the Murray cod, whereas changes in hydrology (i.e. discharge) and flood plain inundation had been overemphasised. The major hydraulic impact identified was a change from lotic habitats in river channels to lentic weir pools, which was further confirmed by modelling (Bice et al. 2017). Full lotic conditions include creating ‘hydraulic complexity’, which is a flowing habitat that is longitudinally continuous and hydrodynamically complex (e.g. fast (>0.25 m s–1) and slow water with local eddies, vortices, gyres, turbulence and recirculation), with structural complexity (e.g. large woody debris).
Of the remaining self-sustaining populations of Murray cod in southern Australia, all are in lotic water habitats, including Mullaroo Creek, the Edward–Wakool System, mid- and upper Murray and Ovens rivers, Broken River, Yarra River, Chowilla anabranch and the lower Darling River, and this provides insights into the conditions that favour recruitment (Rowland 1998; Koehn and Harrington 2005; Saddlier et al. 2008; Lieschke et al. 2016; Tonkin et al. 2017). A key feature of these systems is that the hydraulics of the river channel are similar to natural, characterised by: (1) hydraulic diversity (fast- and slow-flowing water), which is largely permanent or with short periods of zero flow outside the spawning season; (2) a spring (October–December) hydrology that has no rapid or major declines in flows during the spawning season; (3) largely perennial base flows in winter; (4) abundant large woody habitat; (5) a natural temperature regime; and (6) good water quality with few anoxic black water events (Koehn 2005).
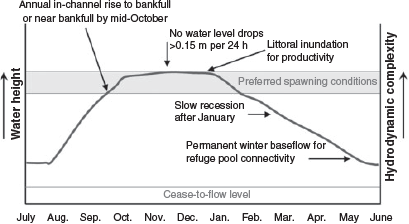
When flows are regulated for irrigation delivery they are contained wholly within the channel, but the key difference to natural flows is that there are often sudden changes in demand and use that cause widely fluctuating water levels within the channel (Mallen-Cooper and Zampatti 2018). These changes, especially sudden drops of sufficient magnitude (i.e. >0.5 m), could cause Murray cod to abandon nests in the upper half of the water column, where hollow logs and undercut banks are often located (Koehn et al. 2009). Where self-sustaining Murray cod populations remain, there are also largely perennial winter flows, reflecting a major difference to systems where flows are optimised for irrigation efficiency, which often have zero flow and few large persistent waterholes during non-irrigation periods, typically during winter, to minimise water losses. An effective way to address these changes is with an environmental flow regime that incorporates changes in hydraulics (water level and water velocity) that affect Murray cod life history. A conceptual model is shown in Fig. 1. In this context, hydrology can be viewed as a tool to achieve hydrodynamic objectives (Mallen-Cooper and Zampatti 2018).
|
Environmental flow
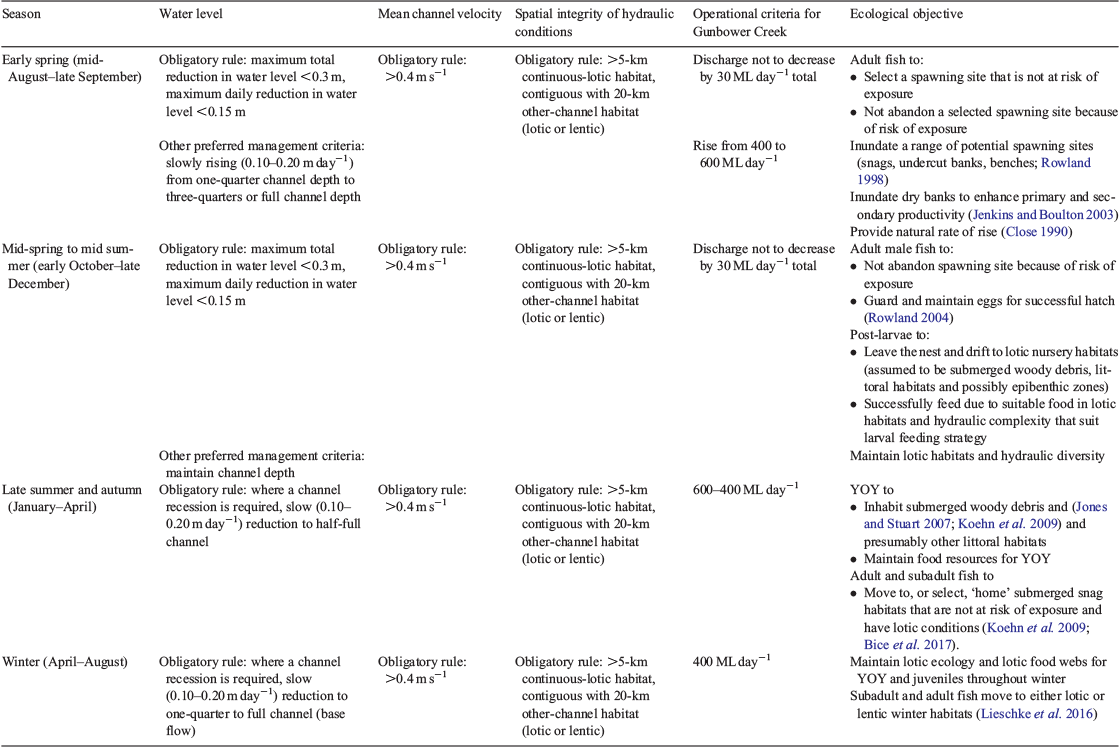
The conceptual environmental flow regime for Murray cod has three key components (Table 1; Fig. 1): (1) winter and early spring flows that are either high or rising, without sharp decreases in levels; (2) a spring and early summer long, steady peak without any sharp decreases in levels (i.e. <0.15 m variation per day, <0.3 m in total) to inundate benches and stimulate primary productivity processes, which enables Murray cod to nest and spawn, and for larvae to emerge, potentially drift downstream and feed in a productive littoral zone (Rowland 2004; Tonkin et al. 2017); and (3) a winter base flow to maintain lotic habitats to improve feeding conditions for early stage juvenile fish, and to avoid zero flows where fish are forced into remnant pools and potentially exposed to greater mortality. Gunbower Creek was operated for irrigation efficiency until 2012 and, from then on (2013–18), the environmental flow was applied each year to support the ecology of Murray cod spawning and juvenile survival.
Study area
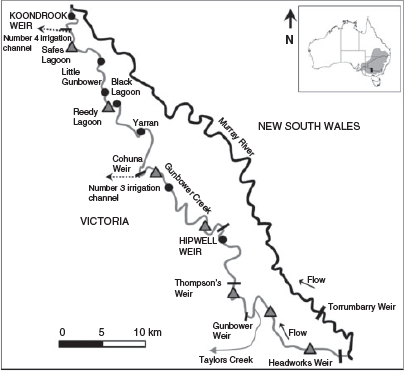
Gunbower Creek is a 126-km-long long anabranch of the mid-Murray River in north-central Victoria, Australia (–35.802080, 144.230813; Fig. 2). The creek was naturally a distributary channel with fringing wetlands, and during flow pulses and floods received water annually (Mallen-Cooper et al. 2014). Beginning in 1890, Gunbower Creek has been regulated and managed wholly for irrigation efficiency. The hydrology of Gunbower Creek was not considered for riverine fish, such as Murray cod, and these fish were affected by zero flows in winter, weir pools that create a lack of lotic reaches, much greater daily variation in water height than natural, with regular major reductions during the spring spawning season, reversed seasonality with high flows in summer and no end-of-system flow back to the Murray River.
|
Each year from 2001 to 2018, ~20 000 hatchery-bred Murray cod were stocked into Gunbower Creek, with fish being released along the entire length of the creek (G. Hodges, Victorian Fisheries, pers. comm.). Some years had higher stocking rates, with 120 000 fingerlings in 2012, 80 000 in 2014 and 100 000 in 2018.
Materials and methods
Hydrology
The daily discharge and river height data for Gunbower Creek were obtained from Goulburn–Murray Water (unpubl. data) and used to develop the environmental flow based on the conceptual model of Murray cod life history.
Larval sampling to confirm the occurrence and distribution of spawning
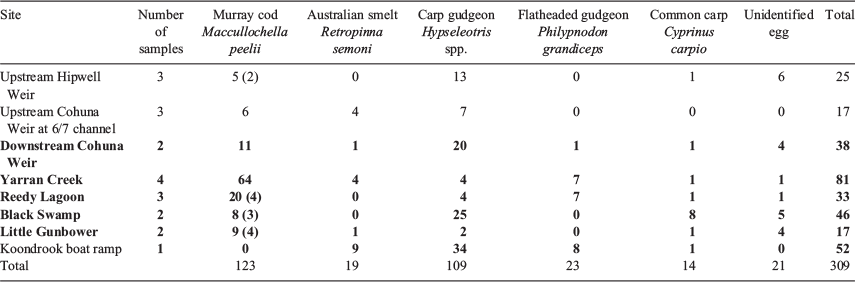
Sampling for Murray cod larvae was undertaken in Gunbower Creek during the breeding period in spring (October–December) 2013 to confirm the occurrence and spatial distribution of spawning for that year. Seven larval sampling sites were selected, with five sites from 5 km below Cohuna Weir to the downstream influence of Koondrook Weir pool (i.e. 23-km continuous lotic, 31-km continuous lentic) and two sites from slow-flowing weir pool environments from 20 km above Cohuna Weir (Fig. 2). Field sampling for fish larvae occurred on four occasions during the spring–summer Murray cod spawning season from 5 November to 10 December 2013; there were 16 sample nights with a maximum time between sampling events of 8 days.
At each site, three drift nets and four light traps were set immediately before dusk and retrieved the following morning (i.e. ~18 h soak time). The light traps, or modified quatrefoil traps, were constructed from clear Perspex, with a removable collecting dish and a 24-h yellow Cyalume light-stick (Cyalume Technologies Inc, West Springfield, MA, USA). Each light trap had a 3.0-mm mesh fitted across the opening to exclude small predatory fish and the light traps were set into littoral habitats (Vilizzi et al. 2008). The drift nets were 1.5 m long with an opening 0.5 m in diameter and the nets were constructed of 500-µm mesh that tapered into a removable reducing jar. Drift nets were suspended from snags to filter the top 0.5 m of the water column.
To provide an estimate of the volume of water filtered by each drift net, a measurement of water velocity was taken with a water velocity meter (FlowMate, Hong Kong SAR, PR China) in the mouth of each net upon deployment and retrieval. All larval samples were filtered through 500-µm mesh, preserved in >70% ethanol and returned to the laboratory for processing. All larval sampling in this study was carried out under permit number AEC13-27 from La Trobe University. Fish sampling was carrier out under permit number ACEC ENV/03/12/AEC from Griffith University.
Long-term sampling for Murray cod population structure
Boat electrofishing surveys were used to determine the relative abundance and size distribution of Murray cod 5 years before and 5 years after the environmental flow was implemented in 2013. Electrofishing was conducted at the same seven sites in Gunbower Creek in April–May (autumn) each year from 2008 to 2018, except in 2015. There were two sites downstream of Cohuna Weir and five upstream. None of the downstream sites was within 5 km of Cohuna Weir (which does not have a fishway) to avoid sampling any accumulations of fish. The 5.2-m electrofishing boats were equipped with 5.0-kVA electrofishing system (Model GPP 5.0 H/L; Smith–Root, Vancouver, WA, USA). The electrofisher was usually operated at 1000-V direct current (DC), 7.5 A pulsed at 120 Hz and 35% duty cycle. All boat electrofishing followed Sustainable Rivers Audit sampling protocols with 12 × 90-s ‘power-on’ replicates per site (Davies et al. 2010). Sampling was conducted during the day and all fish captured were weighed (g) and measured (total length, TL; mm) and then released.
Statistical analyses
Murray cod numbers through time
To determine any changes in Murray cod numbers over time, a mixed-model analysis was performed on catch per unit effort (CPUE) for two length categories, namely juveniles <400 mm TL and all Murray cod, from annual electrofishing among sites upstream and downstream of Cohuna Weir, and before (2008–13) and after (2013–18) implementation of the environmental flow. The juvenile size class was selected to ensure that the analyses focused on fish that were likely to be <3 years old and could thus be attributed to enhanced abundance in association with the environmental flow (Anderson et al. 1992).
In these analyses, years were nested within pre- and post-environmental flow, and sites were nested within upstream and downstream of Cohuna Weir locations. log(count + 1) data were used and all assumptions were verified using visual inspection of the residual plots. All analyses were performed using SAS/STAT (2013; SAS Institute, Cary, NC, USA).
Murray cod sizes
Total catch data were pooled from all sites above and below Cohuna Weir to demonstrate Murray cod length distributions in each section of the creek, and a two-tailed Kolmogorov–Smirnov goodness-of-fit test was performed to compare the two pooled distributions, with P < 0.05 considered significant (McKillup 2005).
Results
Hydrology
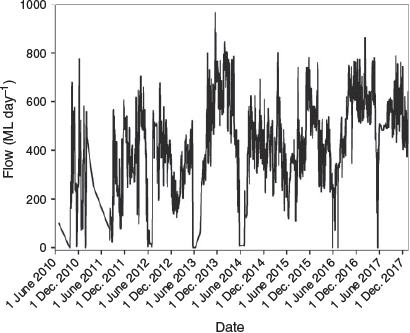
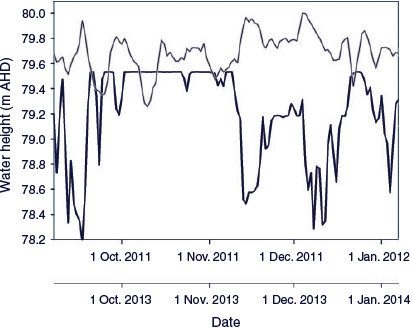
From 2002 to 2013, before implementation of winter base flows the maximum duration of zero flows below Cohuna Weir was 59 days in 2007, followed by 42 days in 2009. Since 2014, after implementing winter base flows as part of the environmental flow plan, the maximum duration of zero flows was 2 days (Fig. 3). The mean number of zero flow days before and after the winter base flows (from 2002 to 2017) was 15.5 and 1.5 days respectively. In 2011, between 1 September and 31 December before implementation of spring spawning flows, the mean daily drop in water height was 0.17 m and the maximum was 0.64 m (Fig. 4). From 2013 to 2018, following implementation of environmental flows, between 1 September and 31 December the mean drop in water height was 0.05 m and the maximum was 0.16 m. No water level data were available for 2012.
|
|
The environmental flow was implemented downstream of Cohuna Weir from 13 September to 30 November 2013. In 2013, the total volume of environmental flows embedded into the irrigation delivery schedule was 19 013 ML, which was targeted to mitigate short-term hourly or daily fluctuations in water levels during the spring breeding period and provided interannual connectivity of flows, including the winter period. The environmental flow was repeated in each year from 2014 to 2018.
Owing to the 3-day travel time of flows along Gunbower Creek, water managers had difficulty in achieving a relatively stable flow below Cohuna Weir and providing for irrigation orders. Hence, the variation in water level over the critical Murray cod spawning period was <0.5 m below Cohuna Weir and <0.1 m at Yarran Creek (~6 km below Cohuna Weir; Fig. 3); this was less than that for a similar period in 2011, when there was a 1–2-m variation per day in water level below Cohuna Weir.
Hydraulics
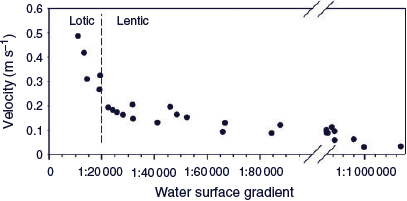
A HEC-RAS (US Army Corps of Engineers, Davis, CA, USA) computer model of the hydraulics of flows in Gunbower Creek was run using 53 cross-sections for the 126-km-long stream. Four flow scenarios were used that included inflows of 1000, 2000, 3000 and 3870 ML day–1. Typical irrigation flows were then included within the upper, middle and lower reaches of Gunbower Creek. Seven detailed cross-sections were included to estimate mean channel velocity, which was compared with water surface gradient. Lotic (flowing water) habitats were nominally defined as having mean water velocities >0.25 m s–1, noting that 0.3 m s–1 is likely required for full lotic habitats to develop (Mallen-Cooper and Zampatti 2018). A water surface gradient steeper than 1 : 20 000 was required to achieve lotic conditions (Fig. 5).
|
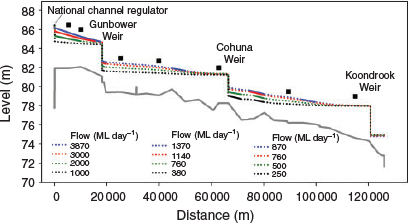
Water level surface profiles are shown in Fig. 6 for each of the modelled flows, with lotic and lentic habitats identified using data from Fig. 5. Three trends are apparent: (1) the upper reach, between Headworks and Gunbower weirs, has lotic habitats at high flows but none at low flow; (2) the middle reach, between Gunbower and Cohuna weirs, has little lotic habitat and almost none at low flows; and (3) the downstream reach, below Cohuna Weir, has lotic habitats at all flows, notably including very low flows of 250 ML day–1. The model excluded Thompson’s and Hipwell Road weirs in the middle reach between Cohuna and Gunbower weirs; these weirs would reduce lotic habitats further by providing several kilometres of additional backwater.
|
Murray cod larvae
Of the 5 species of fish larvae collected, Murray cod dominated, representing 40% of total catch, with 123 collected in November and December 2013 (Table 2). The majority of Murray cod larvae (89%) were collected from the light traps set in littoral habitats (e.g. partially inundated sedges, saplings, macrophytes and fallen branches) adjacent to the drift nets set in the central channel. The majority (68%) of Murray cod larvae were collected at two sites below Cohuna Weir: at Yarran Creek and at Reedy Lagoon. These sites had high-quality physical habitat (dense abundant large woody debris and littoral macrophytes) and fast lotic water (>0.3 m s–1). The size range of Murray cod larvae was 7–17 mm (mean (±s.d.) 11.4 ± 1.1 mm) and based on their developmental stage (Serafini and Humphries 2004), spawning was estimated to occur from 21 October until at least 27 November 2013. During this period, flows were 600–1000 ML day–1 (i.e. near bankfull) and water level varied by <0.5 m (Fig. 7).
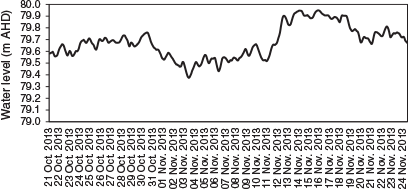
|
Juvenile and adult Murray cod
From annual boat electrofishing between 2008 and 2018, 215 Murray cod were collected, with a size and weight range of 70–1100 mm TL and 3.4–19 060 g. There were significant variations in the catch among the years within the pre- and post-environmental flow periods (F = 3.5, d.f. = 7, 32, P < 0.0001), but the post-environmental flow average of 4.3 Murray cod per site per year was significantly higher than 0.71 per site per year pre-environmental flow (F = 26.9, d.f. = 1, 32, P < 0.0001; Fig. 8).
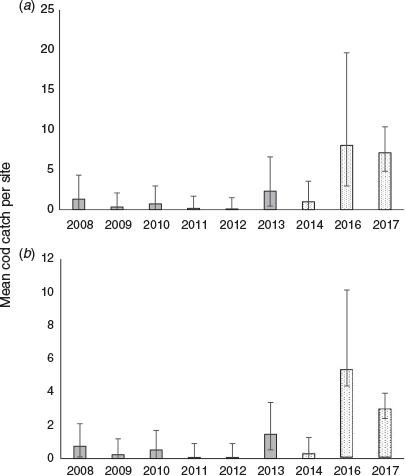
|
The electrofishing catch of juvenile (<400 mm TL) Murray cod differed between pre- and post-environmental flow depending on location (upstream or downstream of Cohuna Weir) within Gunbower Creek (location × period = 6.4, d.f. = 1, 32, P < 0.02). There were always more juvenile Murray cod below Cohuna Weir, even before implementation of the environmental flow, but after implementation of the environmental flow the magnitude of the difference was much larger (Fig. 9, 10). For example, there was an average of 4.9 juvenile Murray cod per site after implementation of the environmental flow (>2013), compared with 0.8 before implementation of the environmental flow. Above Cohuna Weir, there was an average of 0.71 juvenile cod per site after implementation of the environmental flow, compared with 0.15 per site before implementation.
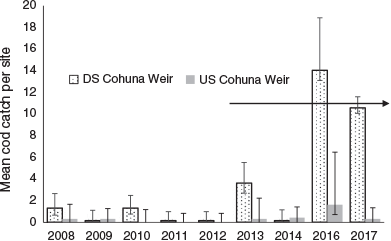
|
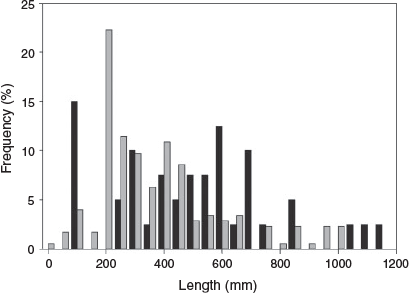
|
Discussion
In rivers where the flow regime is regulated for irrigation, there are often strict flow schedules determined by downstream users, and these do not generally consider environmental objectives (Carlson et al. 2019). The flow regime of Gunbower Creek reflects variable irrigation demand and typifies an altered hydrology more commonly observed below large dams and hydropower facilities, where short-term flow variability is magnified when there is high demand (Smokorowski et al. 2011).
Spatial context of hydrodynamics and habitat
In Gunbower Creek, the environmental flows were applied over a total spatial scale of 54 km. During spring there was 23 km of lotic water and 31 km of pool habitats, whereas during winter there was 7 km of lotic habitat and 47 km of pool habitat. For Murray cod, the minimum spatial scale that supports a population is not specifically known, which is essential information for managing freshwater fish at a scale that matches life history (Fausch et al. 2002). Within anabranch habitats, populations appear to be maintained with 11 km of lotic habitat and 30 km of pool habitat (Saddlier et al. 2008). Hence, we propose that the 23-km lotic and 31-km lentic habitats below Cohuna Weir may be an appropriate starting point for defining a minimum scale to enable spawning and recruitment for Murray cod. Larger scales may be required in rivers with higher discharge and velocities, where larval drift could be over a greater spatial scale, whereas the maintenance of metapopulations may also require consideration of large scales for population resilience.
The spatial scale for larval drift and survival is unknown, but in lotic rivers there can be a 5- to 7-day drifting phase, where larvae may travel tens of kilometres, and this is considered obligatory (Humphries and Lake 2000; Koehn and Harrington 2005, 2006). By contrast, in Gunbower Creek, relatively few larvae were caught in drift nets, with most appearing to actively disperse into shallow littoral habitats, where they were caught in light traps, immediately after leaving the nest. Hence, the spatial scale for restoring Murray cod may be more flexible than first thought and include the common short (i.e. ~30–70 km) interweir distances of the MDB where permanent lotic habitats can be restored.
Explanations for enhanced abundance
There are three explanations for the increased abundance of Murray cod juveniles: immigration, stocking and our hypothesis of natural recruitment. Immigration into the study area could occur from downstream, upstream or large overbank floods from the Murray River (Fig. 2). However, the most downstream weir (Koondrook) has no fishway and the upstream weir has undershot gates that can cause high mortality of larvae (Baumgartner et al. 2006), although juveniles and subadults could potentially pass these gates. The irrigation area is largely protected from flooding, although some floodwater can come through the Gunbower Forest from the Murray River into Gunbower Creek. Regardless, the most telling evidence of poor immigration is the scarcity, over many years, of Murray cod juveniles collected before the environmental flow; during this time, the population was represented mostly by a low abundance of large fish (>500 mm TL), indicative of consistently poor annual recruitment.
Between 20 000 and 120 000 hatchery-bred Murray cod fingerlings have been stocked into Gunbower Creek each year since 2001. However, relatively few juvenile fish (i.e. <400 mm TL) were detected in the 5 years of sampling before the environmental flows. This is consistent with several previous studies where stocking of Murray cod fingerlings into large and small rivers, some of which included over-winter flows, was of limited benefit; natural recruitment was still the main driver behind population recovery (Koehn and Todd 2012; Forbes et al. 2016; Thiem et al. 2017). The present study did not differentiate stocked and natural recruits, but recruitment of juveniles, in either case, was enhanced. Despite years of stocking, recruitment was very poor without an environmental flow regime.
The most likely explanation for increased Murray cod abundance is that the environmental flow regime improved survival of eggs, larvae or juveniles (stocked or natural). Egg survival was not measured among years, but our hypothesis is that this was enhanced by the reductions in water level fluctuations reducing nest abandonment by the male, which is particularly significant for nests in the upper water column. Stable or rising water levels in spring, with no sudden decreases, more closely resemble the conditions of rivers where Murray cod regularly recruit (Rowland 1998; Koehn and Harrington 2005; Saddlier et al. 2008; Lieschke et al. 2016; Tonkin et al. 2017). The nest-guarding American smallmouth bass M. dolomieu is another example of a fish for which the magnitude of water level fluctuations is critical to determining reproductive success (Clark et al. 2008).
Larval survival could have been enhanced by the hydrodynamic complexity and productivity of shallow littoral nursery habitats, which promoted feeding and survival without rapid water level fluctuations (Tonkin et al. 2017). Finally, survival of juveniles (stocked or natural) could have been promoted by the establishment of perennial lotic habitats, especially over winter. In Gunbower Creek, the flow regime that was managed wholly for irrigation historically included a rapid draw down each autumn, where the system contracted into disconnected pools for up to 3 months over winter. Murray cod do not naturally inhabit ephemeral or intermittent systems, so fish exposed to these conditions could have poor feeding success, be stressed and have reduced survival. The environmental flow regime, with perennial lotic habitats, more closely resembles the natural hydrological and hydraulic regime of Murray cod habitats (Close 1990; Mallen-Cooper and Zampatti 2018).
Further experimental work is required to explore the effects of each hydrological element: (1) spawning, egg and larval survival rates with spring environmental flows; (2) the value of winter lotic habitats for the survival of young stocked or naturally spawned fish; and (3) the underlying reasons for greater larval and stocked fish abundance (e.g. changes in primary productivity; Tonkin et al. 2017). Nevertheless, there is immediate scope for broader application of annual environmental flows to help recover Murray cod in other rivers where the flow regime is dominated by irrigation needs, such as in the Goulburn, Campaspe, Loddon, Murrumbidgee, Edward–Wakool, Gwydir and lower Darling systems.
The science and implementation of the environmental flow in Gunbower Creek was designed for a single species outcome, with each component of the flow regime sequentially added to support a specific function of Murray cod life history (King et al. 2000; Acreman et al. 2014). Although many authors have emphasised flows for holistic ecosystem recovery, we suggest that the basic components of the flow regime for Murray cod (perennial lotic habitats with more natural rates of rise and fall) reflect past natural conditions and can be viewed as a foundation hydrological and hydraulic regime from which other elements can be added to achieve multispecies outcomes. For example, in spring–summer, the addition of small water level variations may cue spawning of silver perch Bidyanus bidyanus (Tonkin et al. 2019) and a sharp flow rise can cause spawning of golden perch Macquaria ambigua (Sharpe and Stuart 2018).
Fishes that guard eggs, have adhesive eggs or require perennial lotic habitats encompass a large group of species in temperate (Teletchea et al. 2009) and tropical (Winemiller et al. 2008) rivers. These fish are at high risk from short-term fluctuations of water levels (causing nest abandonment or exposure of eggs) and loss of lotic habitats (Clark et al. 2008). Hydropower and irrigation have caused major changes to the hydrology and hydraulics of rivers globally, including short-term (hourly; Smokorowski et al. 2011) and long-term changes in discharge, water levels and hydrodynamic complexity (Nilsson et al. 2005; Schmutz and Moog 2018). Environmental flow is the most common tool for mitigating these impacts using hydrology (i.e. discharge) with daily, seasonal and yearly time steps (Baumgartner et al. 2014). The present study suggests that incorporating hydraulics (water level and velocity) at fine and coarse time scales, over spatial scales that reflect life histories, should be a transparent aim of managed flow regimes with environmental objectives.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this study was provided by North Central Catchment Management Authority and the Murray–Darling Basin Authority.
Acknowledgments
Sincere thanks to Khane Mason and Ross Stanton, Goulburn-Murray Water, for local support in implementing the environmental flows. The authors thank Steve Campbell-Brown for field assistance and Wayne Robinson, Charles Sturt University, for statistical analyses. John Koehn and Wayne Koster from the Arthur Rylah Institute and an anonymous referee provided valuable comments to improve the manuscript. Iain Ellis and the Murray Darling Freshwater Research Centre, Lower Basin Laboratory, of LaTrobe University, administered the collection and enumeration of fish samples.
References
Acreman, M. C., and Dunbar, M. J. (2004). Defining environmental river flow requirements? A review. Hydrology and Earth System Sciences Discussions 8, 861–876.| Defining environmental river flow requirements? A review.Crossref | GoogleScholarGoogle Scholar |
Acreman, M., Arthington, A. H., Colloff, M. J., Couch, C., Crossman, N. D., Dyer, F., Overton, I., Pollino, C. A., Stewardson, M. J., and Young, W. (2014). Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world. Frontiers in Ecology and the Environment 12, 466–473.
| Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world.Crossref | GoogleScholarGoogle Scholar |
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the lower Murray–Darling Basin, Australia, from thin-sectioned otoliths. Marine and Freshwater Research 43, 983–1013.
| Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the lower Murray–Darling Basin, Australia, from thin-sectioned otoliths.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H., Bunn, S. E., Poff, N. L., and Naiman, R. J. (2006). The challenge of providing environmental flow rules to sustain river ecosystems. Ecological Applications 16, 1311–1318.
| The challenge of providing environmental flow rules to sustain river ecosystems.Crossref | GoogleScholarGoogle Scholar | 16937799PubMed |
Arthington, A. H., Kennen, J. G., Stein, E. D., and Webb, J. A. (2018). Recent advances in environmental flows science and water management – innovation in the Anthropocene. Freshwater Biology 63, 1022–1034.
| Recent advances in environmental flows science and water management – innovation in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Aspe, C., Gilles, A., and Jacqué, M. (2016). Irrigation canals as tools for climate change adaptation and fish biodiversity management in Southern France. Regional Environmental Change 16, 1975–1984.
| Irrigation canals as tools for climate change adaptation and fish biodiversity management in Southern France.Crossref | GoogleScholarGoogle Scholar |
Balon, E. K. (1975). Reproductive guilds of fishes: a proposal and definition. Journal of the Fisheries Board of Canada 32, 821–864.
| Reproductive guilds of fishes: a proposal and definition.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Reynoldson, N., and Gilligan, D. M. (2006). Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Marine and Freshwater Research 57, 187–191.
| Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Conallin, J., Wooden, I., Campbell, B., Gee, R., Robinson, W. A., and Mallen-Cooper, M. (2014). Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems. Fish and Fisheries 15, 410–427.
| Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems.Crossref | GoogleScholarGoogle Scholar |
Belmar, O., Vila-Martínez, N., Ibáñez, C., and Caiola, N. (2018). Linking fish-based biological indicators with hydrological dynamics in a Mediterranean river: relevance for environmental flow regimes. Ecological Indicators 95, 492–501.
| Linking fish-based biological indicators with hydrological dynamics in a Mediterranean river: relevance for environmental flow regimes.Crossref | GoogleScholarGoogle Scholar |
Bice, C. M., Gibbs, M. S., Kilsby, N. N., Mallen-Cooper, M., and Zampatti, B. P. (2017). Putting the ‘river’ back into the lower River Murray: quantifying the hydraulic impact of river regulation to guide ecological restoration. Transactions of the Royal Society of South Australia 141, 108–131.
| Putting the ‘river’ back into the lower River Murray: quantifying the hydraulic impact of river regulation to guide ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, P. L. (1977). J. O. Langtry’s 1949–50 Murray River investigations. Fisheries and Wildlife Division, Melbourne, Vic., Australia.
Carlson, E. A., Cooper, D. J., Merritt, D. M., Kondratieff, B. C., and Waskom, R. M. (2019). Irrigation canals are newly created streams of semi-arid agricultural regions. The Science of the Total Environment 646, 770–781.
| Irrigation canals are newly created streams of semi-arid agricultural regions.Crossref | GoogleScholarGoogle Scholar | 30064103PubMed |
Castello, L. (2008). Nesting habitat of Arapaima gigas (Schinz) in Amazonian floodplains. Journal of Fish Biology 72, 1520–1528.
| Nesting habitat of Arapaima gigas (Schinz) in Amazonian floodplains.Crossref | GoogleScholarGoogle Scholar |
Clark, M. E., Rose, K. A., Chandler, J. A., Richter, T. J., Orth, D. J., and Van Winkle, W. (2008). Water-level fluctuation effects on centrarchid reproductive success in reservoirs: a modeling analysis. North American Journal of Fisheries Management 28, 1138–1156.
| Water-level fluctuation effects on centrarchid reproductive success in reservoirs: a modeling analysis.Crossref | GoogleScholarGoogle Scholar |
Close, A. (1990). The impact of man on the natural flow regime. In ‘The Murray’. (Eds N. Mackay and D. Eastburn.) pp. 61–76. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Cowley, D. E., Wissmar, R. C., and Sallenave, R. (2007). Fish assemblages and seasonal movements of fish in irrigation canals and river reaches of the middle Rio Grande, New Mexico (USA). Ecology Freshwater Fish 16, 548–558.
| Fish assemblages and seasonal movements of fish in irrigation canals and river reaches of the middle Rio Grande, New Mexico (USA).Crossref | GoogleScholarGoogle Scholar |
Cushman, R. M. (1985). Review of ecological effects of rapidly varying flows downstream from hydroelectric facilities. North American Journal of Fisheries Management 5, 330–339.
| Review of ecological effects of rapidly varying flows downstream from hydroelectric facilities.Crossref | GoogleScholarGoogle Scholar |
Daufresne, M., Veslot, J., Capra, H., Carrel, G., Poirel, A., Olivier, J. M., and Lamouroux, N. (2015). Fish community dynamics (1985–2010) in multiple reaches of a large river subjected to flow restoration and other environmental changes. Freshwater Biology 60, 1176–1191.
| Fish community dynamics (1985–2010) in multiple reaches of a large river subjected to flow restoration and other environmental changes.Crossref | GoogleScholarGoogle Scholar |
Davies, P. E., Harris, J. H., Hillman, T. J., and Walker, K. F. (2010). The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia. Marine and Freshwater Research 61, 764–777.
| The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Dudley, R. K., and Platania, S. P. (2007). Flow regulation and fragmentation imperil pelagic-spawning riverine fishes. Ecological Applications 17, 2074–2086.
| Flow regulation and fragmentation imperil pelagic-spawning riverine fishes.Crossref | GoogleScholarGoogle Scholar | 17974342PubMed |
Fausch, K. D., Torgersen, C. E., Baxter, C. V., and Li, H. W. (2002). Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat. Bioscience 52, 483–498.
| Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat.Crossref | GoogleScholarGoogle Scholar |
Forbes, J., Watts, R. J., Robinson, W. A., Baumgartner, L. J., McGuffie, P., Cameron, L. M., and Crook, D. A. (2016). Assessment of stocking effectiveness for Murray cod (Maccullochella peelii) and golden perch (Macquaria ambigua) in rivers and impoundments of south-eastern Australia. Marine and Freshwater Research 67, 1410–1419.
| Assessment of stocking effectiveness for Murray cod (Maccullochella peelii) and golden perch (Macquaria ambigua) in rivers and impoundments of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Guse, B., Kail, J., Radinger, J., Schröder, M., Kiesel, J., Hering, D., Wolter, C., and Fohrer, N. (2015). Eco-hydrologic model cascades: simulating land use and climate change impacts on hydrology, hydraulics and habitats for fish and macroinvertebrates. The Science of the Total Environment 533, 542–556.
| Eco-hydrologic model cascades: simulating land use and climate change impacts on hydrology, hydraulics and habitats for fish and macroinvertebrates.Crossref | GoogleScholarGoogle Scholar | 26188405PubMed |
Horne, A., Kaur, S., Szemis, J., Costa, A., Webb, J. A., Nathan, R., Stewardson, M., Lowe, L., and Boland, N. (2017). Using optimization to develop a ‘designer’ environmental flow regime. Environmental Modelling & Software 88, 188–199.
| Using optimization to develop a ‘designer’ environmental flow regime.Crossref | GoogleScholarGoogle Scholar |
Humphries, P. (2005). Spawning time and early life history of Murray cod, Maccullochella peelii peelii (Mitchell) in an Australian river. Environmental Biology of Fishes 72, 393–407.
| Spawning time and early life history of Murray cod, Maccullochella peelii peelii (Mitchell) in an Australian river.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., and Lake, P. S. (2000). Fish larvae and the management of regulated rivers. Regulated Rivers: Research and Management 16, 421–432.
| Fish larvae and the management of regulated rivers.Crossref | GoogleScholarGoogle Scholar |
Jenkins, K. M., and Boulton, A. J. (2003). Connectivity in a dryland river: short-term aquatic microinvertebrate recruitment following floodplain inundation. Ecology 84, 2708–2723.
| Connectivity in a dryland river: short-term aquatic microinvertebrate recruitment following floodplain inundation.Crossref | GoogleScholarGoogle Scholar |
Jones, M. J., and Stuart, I. G. (2007). Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river. Ecology Freshwater Fish 16, 210–220.
| Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river.Crossref | GoogleScholarGoogle Scholar |
King, A. J., and O’Connor, J. P. (2007). Native fish entrapment in irrigation systems: a step towards understanding the significance of the problem. Ecological Management & Restoration 8, 32–37.
| Native fish entrapment in irrigation systems: a step towards understanding the significance of the problem.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Mahoney, J. (2009). Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia. River Research and Applications 25, 1205–1218.
| Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
King, J., and Brown, C. (2006). Environmental flows: striking the balance between development and resource protection. Ecology and Society 11, art26.
| Environmental flows: striking the balance between development and resource protection.Crossref | GoogleScholarGoogle Scholar |
King, J., and Louw, D. (1998). Instream flow assessments for regulated rivers in South Africa using the building block methodology. Aquatic Ecosystem Health & Management 1, 109–124.
| Instream flow assessments for regulated rivers in South Africa using the building block methodology.Crossref | GoogleScholarGoogle Scholar |
King, J., Brown, C., and Sabet, H. (2003). A scenario-based holistic approach to environmental flow assessments for rivers. River Research and Applications 19, 619–639.
| A scenario-based holistic approach to environmental flow assessments for rivers.Crossref | GoogleScholarGoogle Scholar |
King, J. M., Tharme, R. E., and De Villiers, M. S. (2000). ‘Environmental Flow Assessments for Rivers: Manual for the Building Block Methodology.’ (Water Research Commission: Pretoria, South Africa.)
Kingsford, R. T. (2000). Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology 25, 109–127.
| Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2005). The loss of valuable Murray cod in fish kills: a science and management perspective. In ‘Management of Murray Cod in the Murray–Darling Basin. Statement, Recommendations and Supporting Papers’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) MDBC publication number 22/05, pp. 73–82. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Koehn, J. D., and Harrington, D. J. (2005). Collection and distribution of the early life stages of the Murray cod (Maccullochella peelii peelii) in a regulated river. Australian Journal of Zoology 53, 137–144.
| Collection and distribution of the early life stages of the Murray cod (Maccullochella peelii peelii) in a regulated river.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Harrington, D. J. (2006). Conditions and timing of the spawning of Murray cod (Maccullochella peelii peellii) and the endangered trout cod (M. macquariensis) in regulated and unregulated rivers. River Research and Applications 22, 327–342.
| Conditions and timing of the spawning of Murray cod (Maccullochella peelii peellii) and the endangered trout cod (M. macquariensis) in regulated and unregulated rivers.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Nicol, S. J. (2014). Comparative habitat use by large riverine fishes. Marine and Freshwater Research 65, 164–174.
| Comparative habitat use by large riverine fishes.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Todd, C. R. (2012). Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii. Fisheries Management and Ecology 19, 410–425.
| Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., McKenzie, J. A., O’Mahony, D. J., Nicol, S. J., O’Connor, J. P., and O’Connor, W. G. (2009). Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river. Ecology Freshwater Fish 18, 594–602.
| Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Amtstaetter, F., Dawson, D. R., Reich, P., and Morrongiello, J. R. (2017). Provision of environmental flows promotes spawning of a nationally threatened diadromous fish. Marine and Freshwater Research 68, 159–166.
| Provision of environmental flows promotes spawning of a nationally threatened diadromous fish.Crossref | GoogleScholarGoogle Scholar |
Leigh, S. J., and Zampatti, B. P. (2013). Movement and mortality of Murray cod (Maccullochella peelii) during overbank flows in the lower River Murray, Australia. Australian Journal of Zoology 61, 160–169.
| Movement and mortality of Murray cod (Maccullochella peelii) during overbank flows in the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Lieschke, J. A., Lyon, J. P., Moloney, P. D., and Nicol, S. J. (2016). Spatial partitioning in the use of structural woody habitat supports the cohabitation of two cod species in a large lowland river. Marine and Freshwater Research 67, 1835–1843.
| Spatial partitioning in the use of structural woody habitat supports the cohabitation of two cod species in a large lowland river.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., Bird, T. J., Kearns, J., Nicol, S., Tonkin, Z., Todd, C. R., O’Mahony, J., Hackett, G., Raymond, S., Lieschke, J., and Kitchingman, A. (2019). Increased population size of fish in a lowland river following restoration of structural habitat. Ecological Applications 29, e01882.
| Increased population size of fish in a lowland river following restoration of structural habitat.Crossref | GoogleScholarGoogle Scholar | 30946514PubMed |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., Stuart, I. G., and Sharpe, C. (2014). The Native Fish Recovery Plan – Gunbower and Lower Loddon. Report prepared for the North Central Catchment Management Authority. (Fishway Consulting Services: Sydney, NSW, Australia.) Available at http://www.nccma.vic.gov.au/sites/default/files/publications/native_fish_recovery_plan_gunbower_and_lower_loddon_2014.pdf [Verified 12 July 2019].
McKillup, S. (2005). ‘Statistics Explained. An Introductory Guide for Life Scientists.’ (Cambridge University Press: Cambridge, UK.)
Moyle, P. B. (2014). Novel aquatic ecosystems: the new reality for streams in California and other Mediterranean climate regions. River Research and Applications 30, 1335–1344.
| Novel aquatic ecosystems: the new reality for streams in California and other Mediterranean climate regions.Crossref | GoogleScholarGoogle Scholar |
Nilsson, C., Reidy, C. A., Dynesius, M., and Revenga, C. (2005). Fragmentation and flow regulation of the world’s large river systems. Science 308, 405–408.
| Fragmentation and flow regulation of the world’s large river systems.Crossref | GoogleScholarGoogle Scholar | 15831757PubMed |
Onikura, N., Nakajima, J., Kouno, H., Sugimoto, Y., and Kaneto, J. (2009). Habitat use in irrigation channels by the golden venus chub (Hemigrammocypris rasborella) at different growth stages. Zoological Science 26, 375–381.
| Habitat use in irrigation channels by the golden venus chub (Hemigrammocypris rasborella) at different growth stages.Crossref | GoogleScholarGoogle Scholar | 19583495PubMed |
Perkin, J. S., Gido, K. B., Costigan, K. H., Daniels, M. D., and Johnson, E. R. (2015). Fragmentation and drying ratchet down Great Plains stream fish diversity. Aquatic Conservation 25, 639–655.
| Fragmentation and drying ratchet down Great Plains stream fish diversity.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L. (2018). Beyond the natural flow regime? Broadening the hydro-ecological foundation to meet environmental flows challenges in a non-stationary world. Freshwater Biology 63, 1011–1021.
| Beyond the natural flow regime? Broadening the hydro-ecological foundation to meet environmental flows challenges in a non-stationary world.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., and Zimmerman, J. K. (2010). Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55, 194–205.
| Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Reynolds, L. F. (1983). Migration patterns of five fish species in the Murray–Darling River system. Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. (1998). Aspects of the reproductive biology of Murray cod, Maccullochella peelii peelii. Proceedings of the Linnean Society of New South Wales 120, 147–162.
Rowland, S. (2004). Overview of the history, fishery, biology and aquaculture of Murray cod (Maccullochella peelii peelii). In ‘Management of Murray Cod in the Murray–Darling Basin. Statement, Recommendations and Supporting Papers’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) MDBC publication number 22/05, pp. 38–61. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Saddlier, S., O’Mahony, J., and Ramsey, D. (2008). Protection and enhancement of Murray cod populations. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Schmutz, S., and Moog, O. (2018). Dams: ecological impacts and management. In ‘Riverine Ecosystem Management’. (Eds S. Schmutz and J. Sendzimir.) pp. 111–127. (Springer: Cham, Switzerland.)
Serafini, L., and Humphries, P. (2004). Preliminary guide to the identification of larvae of fish, with a bibliography of their studies, from the Murray–Darling Basin. Identification and ecology guide number 48, Cooperative Research Centre for Freshwater Ecology, Albury, NSW, Australia.
Sharpe, C., and Stuart, I. (2018). Environmental flows in the Darling River to support native fish populations. CPS Enviro report to The Commonwealth Environmental Water Office. CPS Enviro, Irymple, Vic., Australia.
Smokorowski, K. E., Metcalfe, R. A., Finucan, S. D., Jones, N., Marty, J., Power, M., Pyrce, R. S., and Steele, R. (2011). Ecosystem level assessment of environmentally based flow restrictions for maintaining ecosystem integrity: a comparison of a modified peaking versus unaltered river. Ecohydrology 4, 791–806.
| Ecosystem level assessment of environmentally based flow restrictions for maintaining ecosystem integrity: a comparison of a modified peaking versus unaltered river.Crossref | GoogleScholarGoogle Scholar |
Teletchea, F., Fostier, A., Kamler, E., Gardeur, J.-N., Le Bail, P.-Y., Jalabert, B., and Fontaine, P. (2009). Comparative analysis of reproductive traits in 65 freshwater fish species: application to the domestication of new fish species. Reviews in Fish Biology and Fisheries 19, 403–430.
| Comparative analysis of reproductive traits in 65 freshwater fish species: application to the domestication of new fish species.Crossref | GoogleScholarGoogle Scholar |
Tharme, R. E. (2003). A global perspective on environmental flow assessment: emerging trends in the development and application of environmental flow methodologies for rivers. River Research and Applications 19, 397–441.
| A global perspective on environmental flow assessment: emerging trends in the development and application of environmental flow methodologies for rivers.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
Thoms, M., and Cullen, P. (1998). The impact of irrigation withdrawals on inland river systems. The Rangeland Journal 20, 226–236.
| The impact of irrigation withdrawals on inland river systems.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kitchingman, A., Lyon, J., Kearns, J., Hackett, G., O’Mahony, J., and Bird, T. (2017). Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river. Hydrobiologia 797, 289–301.
| Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Stuart, I., Kitchingman, A., Thiem, J. D., Zampatti, B., Hackett, G., Koster, W., Koehn, J., Morrongiello, J., Mallen-Cooper, M., and Lyon, J. (2019). Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river. Marine and Freshwater Research 70, 1333–1344.
| Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river.Crossref | GoogleScholarGoogle Scholar |
Vilizzi, L., Meredith, S. N., Sharpe, C. P., and Rehwinkel, R. (2008). Evaluating light trap efficiency by application of mesh to prevent inter- and intra-specific in situ predation on fish larvae and juveniles. Fisheries Research 93, 146–153.
| Evaluating light trap efficiency by application of mesh to prevent inter- and intra-specific in situ predation on fish larvae and juveniles.Crossref | GoogleScholarGoogle Scholar |
Wilde, G. R., and Urbanczyk, A. C. (2013). Relationship between river fragment length and persistence of two imperiled Great Plains cyprinids. Journal of Freshwater Ecology 28, 445–451.
| Relationship between river fragment length and persistence of two imperiled Great Plains cyprinids.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O., Agostinho, A. A., and Caramaschi, É. P. (2008). Fish ecology in tropical streams. In ‘Tropical Stream Ecology’. (Ed. D. Dudgeon.) pp. 107–146. (Elsevier: London, UK.)
Ye, Q., and Zampatti, B. (2007). Murray cod stock status – the Lower River Murray, South Australia. Stock status report to PIRSA Fisheries. SARDI Publication Number F2007-000211-1, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zimmerman, J. K., Letcher, B. H., Nislow, K. H., Lutz, K. A., and Magilligan, F. J. (2010). Determining the effects of dams on subdaily variation in river flows at a whole-basin scale. River Research and Applications 26, 1246–1260.
| Determining the effects of dams on subdaily variation in river flows at a whole-basin scale.Crossref | GoogleScholarGoogle Scholar |