Carbon sources supporting Australia’s most widely distributed freshwater fish, Nematalosa erebi (Günther) (Clupeidae: Dorosomatinae)
Bradley J. Pusey A B E , Timothy D. Jardine C , Leah S. Beesley A , Mark J. Kennard B , Tze Wai Ho D , Stuart E. Bunn B and Michael M. Douglas A
A B E , Timothy D. Jardine C , Leah S. Beesley A , Mark J. Kennard B , Tze Wai Ho D , Stuart E. Bunn B and Michael M. Douglas A
A National Environmental Science Program, The University of Western Australia, Stirling Highway, Crawley, WA 6009, Australia.
B Australian Rivers Institute, Griffith University, Kessels Road, Nathan, Qld 4111, Australia.
C School of Environment and Sustainability, Toxicology Centre, University of Saskatchewan, Preston Road, Saskatoon, SK, S7N5B3, Canada.
D Biological Sciences, The University of Western Australia, Stirling Highway, Crawley, WA 6009, Australia.
E Corresponding author. Email: bpusey@westnet.com.au
Marine and Freshwater Research 72(2) 288-298 https://doi.org/10.1071/MF20014
Submitted: 10 January 2020 Accepted: 3 May 2020 Published: 11 June 2020
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Both brown (detrital-based) and green (algal-based) food pathways support freshwater food webs, although the importance of either source may vary within species, regions and different phases of the flow regime. The bony bream (Nematalosa erebi Clupeidae: Dorosomatinae) is one of Australia’s most widely distributed freshwater fish species and is a key component of freshwater food webs, especially in northern Australia. We sought to better define the feeding habits of this species, previously classified as a detritivore, algivore or zooplanktivore (or combinations thereof), by undertaking meta-analyses of published accounts based on stomach content analysis and 13C and 15N stable isotope analysis. Stomach content analysis clearly indicated that detritus was the dominant food item, although benthic algae could be an important dietary component in some habitats (inland river flood plains) and during the wet season. Zooplankton were important for small fish (i.e. juveniles <100 mm in length). When data were pooled across a large number of locations, stable isotope analysis indicated that detritus derived from terrestrial vegetation was better aligned isotopically with values for both adult and juvenile bony bream, whereas algae were comparatively 13C enriched, indicating the latter source was not the dominant contributor to the biomass of this species. However, using site-specific data and a regression approach, a significant relationship was revealed between algal carbon and that of large fish, suggesting that carbon derived from benthic algae contributed ~20% of the carbon of adult bony bream. Zooplankton contributed a similar amount. Zooplankton provided the majority of carbon for small fish. We contend that detritus derived from terrestrial vegetation is the likely remaining carbon source for large bony bream, and this interpretation was supported by the outcomes of multiple regression analyses. Although previous studies of aquatic food webs in northern Australia have emphasised the importance of high-quality algal basal resources, this study indicates that terrestrial sources may be important for some species and demonstrates the need to better consider the circumstances that cause biota to switch between different food sources.
Additional keywords: algivory, aquatic food webs, detritivory, northern Australia, zooplanktivory.
Introduction
Most plant matter ends up as detritus and most community food webs contain both detrital and living primary producer energy channels (brown and green food chains respectively; Moore et al. 2004; Rooney et al. 2006). Early models of aquatic ecosystem function emphasised the importance of terrestrial or aquatic vascular plant material in supporting the biomass of aquatic consumers via a detrital breakdown pathway (Vannote et al. 1980; Junk et al. 1989). Qualification of this viewpoint has included the inclusion of microbiota as both conditioners of detritus that make nutrients and energy more available and as constituents, which are themselves consumed (e.g. France 2011). By contrast, while not discounting the importance of terrestrial inputs, Thorp and Delong (1994) emphasised the importance of algal production in supporting consumer biomass. The use of stable isotopes and fatty acid markers in food web studies has largely confirmed the importance of autochthonous algal production in aquatic food webs (Lewis et al. 2001; Bunn et al. 2003; Guo et al. 2016a, 2016b; Brett et al. 2017). Algal carbon is easier to digest and assimilate than that of vascular plant material (Brett et al. 2017). Moreover, algae contain higher quantities of polyunsaturated fatty acids (PUFA), which are essential for metazoan growth (Guo et al. 2016a).
Douglas et al. (2005) proposed that most biomass of tropical northern Australian rivers was ultimately derived from algal production. This hypothesis is largely supported by subsequent research, although other sources, such as terrestrially derived detritus, may also be important (Bunn et al. 2013; Pettit et al. 2017). Elsewhere, several experimental and field-based studies have revealed that some aquatic consumers are supported by carbon derived from detritus and attached microbes (e.g. McGoldrick et al. 2008; Reid et al. 2008; Brett et al. 2009; Solomon et al. 2011; Belicka et al. 2012). Further, fatty acid profiles of some primary consumer organisms indicate a detrital origin by microbial processors (Belicka et al. 2012), and some aquatic organisms may possess the capacity to convert some fatty acids into more physiologically active forms (Murray et al. 2014; Guo et al. 2016b). Brett et al. (2017) suggested that the extent to which terrestrial carbon supports upper trophic level production may depend on the simultaneous availability of essential biomolecules derived from algae and concluded that there is no doubt that terrestrially derived carbon is ingested and assimilated by herbivores, but that it is done so at much reduced efficiency. Clearly, an algal–detrital dichotomy oversimplifies the complex relationships present within aquatic food webs (Taylor and Batzer 2010; Jardine et al. 2015).
Detritivorous fishes are an important component of tropical aquatic food webs (Lowe-McConnell 1975; Goulding et al. 1988; Flecker 1996), transferring basal production to higher trophic levels and frequently forming the major prey of piscivorous fishes of socioeconomic importance (Winemiller 2004). Although detritivorous fishes are common in tropical regions globally, detritivory is less evident in temperate regions (Egan et al. 2018). Coates (1993) stated that truly detritivorous fishes are absent from freshwaters of the Australasian region, tropical or otherwise, but subsequent studies have revealed that detritus may comprise a significant fraction of the stomach contents of several benthic foraging species, particularly in tropical northern Australia (Pusey et al. 2000; Bishop et al. 2001; Kennard et al. 2001).
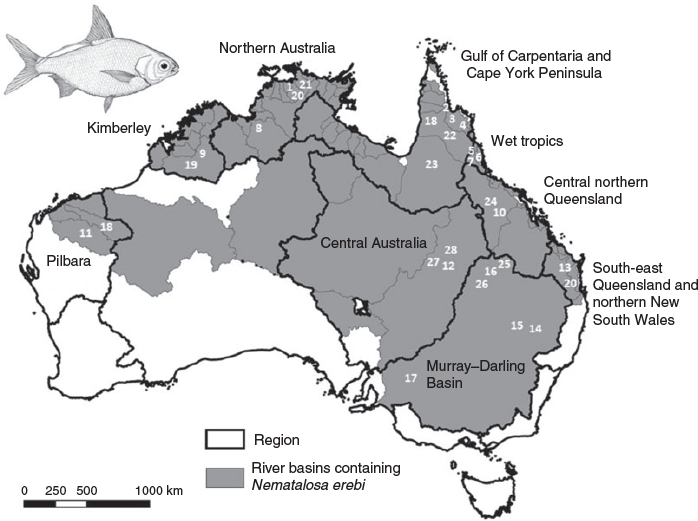
The bony bream (Nematalosa erebi (Günther)), a clupeid gizzard shad, is one of Australia’s most widely distributed freshwater fish species. It has been previously characterised as a detritivore, algivore or planktivore (or combinations thereof; for a review of its biology, see Pusey et al. 2004). This species is primarily tropical or subtropical, although its distribution extends as far south as 35°S in the Murray–Darling River system (Fig. 1). The species occurs in a great variety of perennial and intermittent aquatic habitat types and may achieve very high levels of abundance. N. erebi is itself consumed by many higher-order consumers, including piscivorous fishes, crocodiles and birds such as cormorants and pelicans; thus, it is an important component in the food webs of Australian rivers, particularly those of northern Australia.
|
In this study, we sought to better define the feeding habits and role of N. erebi in aquatic food webs by reference to published dietary information and more recent stable isotope analysis of food web structure. We specifically sought to determine whether N. erebi was reliant on allochthonous production (i.e. terrestrial vegetation, TVEG, via a detrital pathway) or autochthonous algal production. We hypothesised that algae were the main source of carbon for this species.
Materials and methods
Sources of dietary information: stomach content analysis
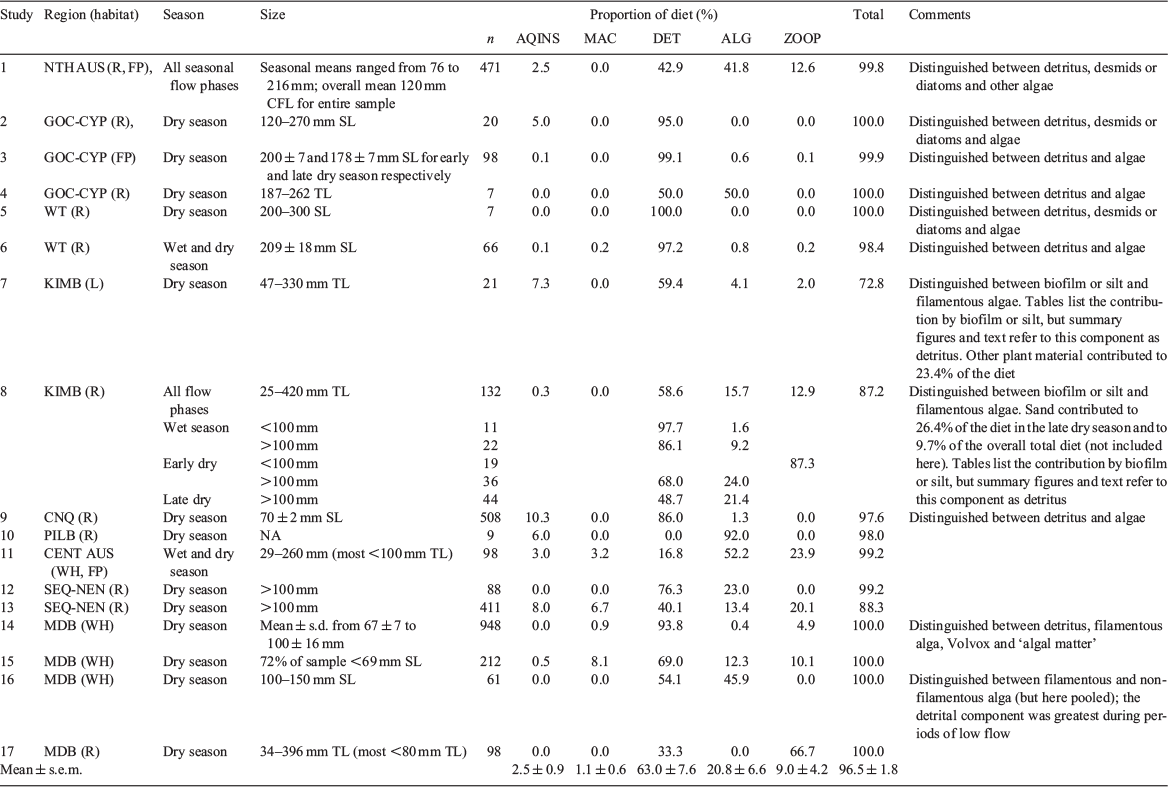
Dietary information for N. erebi was available from 17 individual studies, drawn from the published literature, 1 university thesis, 2 consultancy reports to state government and 1 unpublished dataset (mean ± s.e.m. sample size 191.5 ± 61.6; see Table 1; Fig. 1). The data used in this study were drawn from a larger dataset being used to examine the trophic ecology of Australian freshwater fishes in which diet was apportioned to proportional contributions within 15 categories (for details concerning diet summarisation and data treatment, see Kennard et al. 2001). Here we present information for only five categories, namely aquatic insects, aquatic macrophytes, detritus, algae (including filamentous algae, diatoms and biofilm) and zooplankton, because these collectively accounted for a mean ± s.e.m. 96.5 ± 1.8% of the diet across studies (Table 1). We also included some measure of the size of the fish examined in each study, either as the mean ± s.e.m. size or size range. The lower limit of the size range of individuals included was >100-mm standard length (SL) for eight studies (i.e. adults; cf. Table 1), whereas four studies were largely dominated by individuals <100 mm SL (i.e. juveniles; studies 9,14,15 and 17 in Table 1). These latter studies were undertaken within the Murray–Darling Basin, with the exception of one undertaken in the Burdekin River of Central North Queensland (Fig. 1). Most studies attempted to distinguish between detritus per se and microalgae mixed in with small detrital particles. Only 4 of the 17 studies reviewed (studies 1, 6, 8 and 11 in Table 1) included data from both wet season and dry season sampling periods. In the remaining 13 studies, dry season samples were collected as part of a one-off sampling event.
|
Sources of stable isotope information
Information was sourced on carbon and nitrogen stable isotope (SI) values of bony bream tissue (fin or muscle) and three potential food sources (benthic algae (primarily periphyton), TVEG and zooplankton) from 11 separate food web studies undertaken in northern, eastern and central Australia and the northern portion of the Murray–Darling Basin (Fig. 1) in which the authors have been individually or collectively involved and that included N. erebi (Beesley 2006; Blanchette et al. 2014; Bunn et al. 2003; Jardine et al. 2012a, 2012b, 2013, 2015, 2017; L. S. Beesley, B. J. Pusey, M. M. Douglas, C. A. Canham, C. S. Keogh, O. P. Pratt, M. J. Kennard, and S. A. Setterfield, unpubl. data; S. E. Bunn’s three unpublished data sets). These studies were intended to examine nutrient and energy transfer between a variety of basal sources, many organisms (including many species of fish) and trophic levels. Only three of these studies were undertaken in the dry season only (Blanchette et al. 2014; Jardine et al. 2012a; Bunn, unpubl. data). We excluded any data that did not allow us to distinguish between fish of different size classes (i.e. <100 and >100 mm SL). The manner in which samples were collected and analysed was largely consistent across studies (for detailed methods, see Jardine et al. 2012a) with the exception of particulate organic matter for which different particle sizes (i.e. coarse particulate organic matter, CPOM, and fine particulate organic matter, FPOM) were not consistently differentiated or collected. By contrast, all studies collected dead leaves of riparian trees (i.e. TVEG; primarily Melaleuca and Eucalyptus spp.), and these species contribute most allochthonous carbon inputs to freshwater systems in the study area. For those samples in which SI information was available for TVEG, CPOM and FPOM, δ13C values of CPOM and FPOM differed from TVEG by less than +1 and +2–3‰ respectively, and differences in δ15N were less than +1‰. These differences accord well with similar comparisons elsewhere (e.g. Finlay and Kendall 2007). In total, SI information was available for fish collected from 120 separate locations (i.e. sites). δ13C and δ15N values for putative source material for each site were estimates based on the mean of at least three samples. Similarly, information from at least 3, but often up to 20, individuals for each size class of N. erebi was used to estimate mean δ13C and δ15N values of fish at each site.
We generated histograms of the frequency distributions for δ13C and δ15N for the three food sources and both size classes of N. erebi across all sites to assess the extent of spatial variation in isotope values and the extent of overlap in isotope values for different potential food sources. Broad distributions (i.e. high variance) indicate high spatial variation. We also assessed whether δ13C or δ15N of individual source materials varied independently using Pearson’s correlation. Gradient-based approaches where isotope variation of producers and consumers is measured at multiple locations have proved useful for determining the importance of different food sources exhibiting large spatial variation in isotope values (Rasmussen 2010; Jardine et al. 2012a). This approach, in contrast to a mixing model approach, does not require potential sources to be distinct at all sites, does not require a priori knowledge of the extent of isotope trophic discrimination and does not require spatial variation in isotope values of different consumers to be independent (Moore and Semmens 2008; Rasmussen 2010). From a practical viewpoint, a gradient-based approach can maximise the number of locations used in analyses because it does not require all three potential sources of carbon to have been measured at every site.
Such an approach is well suited to the present case, where data were collected from multiple locations within many rivers. We plotted δ13C values of each size class of N. erebi against δ13C values of algae, TVEG and zooplankton. We used simple linear regression to assess the strength of the relationship between consumer (i.e. N. erebi) δ13C values and food source (i.e. algae, TVEG and zooplankton) δ13C values and report statistical significance at the α = 0.05 level. We estimated whether the slopes of the relationship between isotope values were significantly different from 1 or 0 (i.e. not within the 95% confidence limits of the estimated slope). A close dependency on one source or the other should see N. erebi muscle δ13C values aligned with spatial variation in δ13C for that potential source (i.e. values should fall along a line denoting a 1 : 1 relationship or slope = 1). Conversely, if no significant relationship (i.e. slope = 0) is detected between consumer and food sources isotope values, then it is assumed that source is unlikely to be important. Slopes significantly different from both 0 or 1 indicate a mixed feeding model (i.e. more than one source contributes to the carbon or nitrogen assimilated into body mass; Jardine et al. 2012a). An important assumption in this approach is that isotope ratios in the consumer organism are in equilibrium with the measured source materials (i.e. isotope values do not reflect feeding at some other time or location). To a large degree, this assumption underpins most food web studies undertaken in natural environments. We similarly examined and tested the relationship between δ15N values of potential food sources and those of N. erebi tissue. We also performed multiple regression analyses for each size class and both isotopes for those locations for which information concerning all three potential sources was available (δ13C: n = 29 and 35 for small and large fish respectively; δ15N: n = 28 and 29 for small and large fish respectively). Finally, we estimated δ15N trophic enrichment factors (i.e. Δδ15N = δ15Nconsumer – δ15Nsource) for each source and age class combination and, from these data, estimated overall trophic enrichment taking into account the estimated proportional contribution of each source derived from the slopes of the lines relating δ13C variation of source and consumers.
Where appropriate, data are given as the mean ± s.e.m.
Results
Stomach contents analysis
Across all studies, the mean contribution of detritus to the diet was 63 ± 8% and that of algae was 20.8 ± 9.0% (Table 1). Zooplankton contributed a further 9 ± 4%, whereas aquatic insect larvae and aquatic macrophytes formed only a minor fraction of the diet (2.5 and 1.1% respectively). Detritus was the dominant dietary component in most studies except two undertaken in arid zone rivers, where algae contributed 90% and 52% to the diet (Studies 10 and 11 respectively, Table 1) and another undertaken in the Gulf Cape York Peninsula region (Study 4, Table 1) in which detritus and algae were codominant. Consumption of zooplankton was greatest in arid zone or southern regions (i.e. the Murray–Darling Basin); however, high consumption of zooplankton was also recorded in northern regions (i.e. the Kimberley and north Australia). All studies in which zooplankton contributed more than 1% of the diet (seven studies) were either dominated by or included fish <100 mm SL. For example, zooplankton comprised 87.3% of the diet in a seasonal subsample comprised entirely of small fish within one study undertaken in the Kimberley region (Study 8, Table 1). Similarly, a high contribution of zooplankton was recorded in the most southern study available (in the Murray–Darling Basin; Study 17, Table 1) and in which the sample was dominated by small individuals. Aquatic insect larvae (chironomid larvae) comprised ~10% of the diet in another study (Study 9, Table 1) undertaken in a large shallow sand bed river. Individuals included in that study were also small (mean ± s.e.m. SL 70 ± 2 mm). Thus, consumption of zooplankton and, to a lesser extent, aquatic insect larvae was limited to individuals of small size. Consumption of detritus and algae was greatest in larger individuals (i.e. >100 mm SL).
Stable isotope analyses
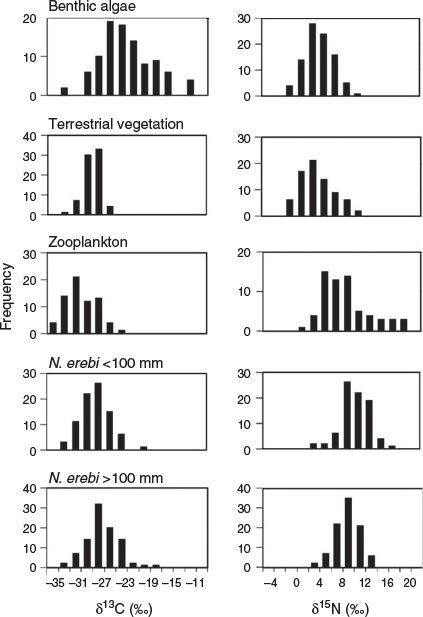
A wide range of δ13C values from –34.6 to –12.0‰ (mean –23.0 ± 0.5‰) was recorded for benthic algae (Fig. 2). TVEG was relatively depleted in 13C and varied little (mean δ13C –29.1 ± 0.2‰; range –33.3 to –26.5‰). Zooplankton δ13C values ranged from –38.2 to –23.5‰ and were typically highly depleted in 13C (mean δ13C –31.1 ± 0.4‰). Large N. erebi exhibited an intermediate range of δ13C values (ranging from –33.3 to –18.5‰; mean 13C –27.4 ± 0.3‰) and were more depleted in 13C compared with algae (as were small N. erebi: range –33.7 to –20.9‰; mean δ13C –28.4 ± 0.3‰). The δ13C values of algae, TVEG and zooplankton varied independently of one another (r < 0.20, P > 0.05 for all comparisons).
|
Algae and TVEG had similar mean values and variability in δ15N values (mean δ15N 4.5 ± 0.3 and 4.3 ± 0.2‰ respectively; Fig. 2). By contrast, zooplankton were comparatively enriched in 15N (mean δ15N 9.2 ± 0.5‰) and some samples were highly enriched (maximum 18.9‰). Large N. erebi were similarly enriched in 15N (mean δ15N 9.0 ± 0.2‰) and small N. erebi were slightly more enriched in 15N than larger fish and zooplankton (mean δ15N 10.8 ± 0.3‰). The δ15N values of algae, TVEG and zooplankton did not vary independently of one another (r = 0.60, 0.51 and 0.93 for algae and TVEG, algae and zooplankton, and TVEG and zooplankton respectively; P < 0.001 for all).
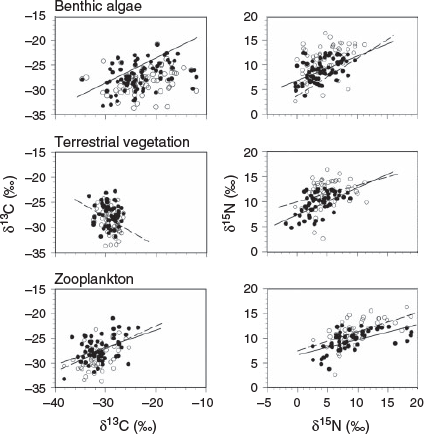
Fig. 3 plots isotope variation (δ13C and δ15N) in large and small N. erebi against variation in isotope values of putative dietary components across a large number of sites (for sample sizes and regression statistics, see Table 2). Variation in δ13C of large N. erebi was significantly positively related to variation in δ13C of both algae and zooplankton, and the slopes for these relationships (0.22 and 0.33 respectively) were both significantly different from 0 and 1, suggesting a mixed feeding strategy with algae and zooplankton together accounting for approximately one-half of assimilated carbon. No significant relationship between δ13C of large N. erebi and terrestrial carbon was detected. Variation in δ13C of small N. erebi was not significantly related to variation in benthic algal δ13C values, but was significantly positively correlated with zooplankton δ13C, with the slope of this relationship (0.52) being significantly different from 0 and 1 (Table 2), again suggesting a mixed feeding model with approximately one-half of assimilated carbon being derived from this source (Fig. 3; Table 2).
|

|
δ15N variation in both large and small N. erebi was significantly positively correlated with variation in all putative food sources (i.e. algae, TVEG and zooplankton), perhaps not surprisingly given variation within sources was also significantly positively correlated. However, δ15N variation in large N. erebi was most strongly correlated with variation in δ15N of TVEG (Table 2). Trophic enrichment (δ15Nconsumer – δ15Ndiet) in large N. erebi averaged 4.9 ± 0.3, 6.0 ± 0.3 and 0.5 ± 0.2‰ for algae, TVEG and zooplankton respectively and 6.2 ± 0.4, 6.7 ± 0.4 and 1.5 ± 0.4‰ respectively in small N. erebi. However, when scaled for the different proportional contributions by each putative food source, the estimated mean trophic enrichment for all sources was 4.1 ± 0.3‰ and 3.2 ± 0.4‰ for large and small N. erebi respectively. The results of multiple regression analyses based on data available from the reduced set of locations (Table 3) strongly supported the outcomes of simple linear regression analysis. That is, δ13C values of small fish (<100 mm SL) were most strongly related to those of zooplankton, whereas those of the larger size class were most strongly related to TVEG values and then benthic algae. The δ15N isotope information was less informative than that for δ13C (as was the case for simple linear regression) in that only zooplankton exhibited a significant relationship with δ15N values of small fish.
Discussion
Stomach content analysis from multiple studies indicates that N. erebi is zooplanktivorous as a juvenile before transitioning to a primarily detrital diet with increasing size. These ontogenetic changes in diet mirror similar changes observed in a closely related clupeid, the American gizzard shad Dorosoma cepedianum (Smoot and Findlay 2010). When based on all samples, stable isotope information also suggested that detritus derived from TVEG, and zooplankton, provided a large fraction of the assimilated carbon. On average, the δ13C values of both small and large N. erebi (–28.5 ± 0.3 and –27.4 ± 0.3‰ respectively) were very closely aligned to that of TVEG, depleted in 13C with respect to algal δ13C values (–23.0 ± 0.5‰) and enriched compared with zooplankton (–31.1 ± 0.4‰). Collectively, these data do not support a significant contribution by benthic algae to carbon assimilation in large N. erebi. However, regression analyses using site-specific data revealed a significant positive relationship between algal carbon and that of large fish, suggesting that benthic algae may also form an important carbon source for this size class of N. erebi.
Important methodological considerations for both stomach content and stable isotope analyses must be considered before accepting the generality of these findings. First, most studies, particularly those undertaken in northern Australia, examined diet during the dry season only, whereas the consumption of algae was greatest in those few studies undertaken over a long period and that included either wet seasons or periods immediately following a wet season. Thus, the contribution of algae to the diet of N. erebi could conceivably be higher than reported here. Second, trituration of ingested material within the muscular gizzard renders most material to a fine paste and it is highly likely that, despite the best intentions of researchers, algal material, other than filamentous algae, may not be readily identifiable (i.e. distinguished from detritus) and reliably quantified when examined macroscopically. Third, in aggregating stable isotope information across studies and locations, any spatial variation in the relationship between algal isotope values and those of the consumer is likely obscured. As a consequence, conclusions regarding the importance of detritus derived from TVEG and a minimal contribution by benthic algae warrant further scrutiny.
Indeed, isotope values for the putative food sources of algae and zooplankton varied greatly and the range of values overlapped substantially for all sources. Such large variation in δ13C is not unexpected given the range of different potential pathways by which carbon is available for uptake (i.e. atmospheric v. dissolved) and the wide array of factors that affect carbon fractionation in aquatic systems (Finlay 2004; Barnes et al. 2007). Similarly, baseline values of δ15N vary extensively in space due to variation in the taxonomic composition of producers, isotope distinction between various sources (i.e. N2, NO3, NH3) and variation in the efficiency with which they are used (Akiyama et al. 1997). However, it is notable that δ15N values of TVEG (riparian species primarily within Myrtaceae) and benthic algae in the present study were highly correlated despite their taxonomic distinctiveness. This finding suggests that both derived their nitrogen from the same source (i.e. that dissolved within the stream or groundwater).
Rather than being an impediment to interpreting relationships between sources and consumers, the presence of spatial variation in algal and zooplankton δ13C values helps identify the source of carbon sustaining N. erebi. Our data suggest a mixed feeding strategy in both large and small individuals. A significant relationship between δ13C values of algae and large fish with a slope (0.22) significantly different from both 0 and 1 indicates that algae contribute approximately one-quarter (range, as defined by confidence intervals, 10–35%) of the carbon assimilated by this size class. Carbon derived from benthic algal production is important in freshwater ecosystems globally (Roach 2013), including in Australia and especially in northern regions and arid zones (Bunn et al. 2003, 2006; Douglas et al. 2005; Leigh et al. 2010; Jardine et al. 2012a, 2012b). However the present study suggests that benthic algal carbon contributed little to the biomass of small N. erebi, which were, in contrast, more reliant on zooplankton carbon (52%; range 26–77%). Zooplankton also contributed substantially to the biomass of large N. erebi (33%; range 11–54%). Stomach content analysis for small N. erebi also identified zooplankton as an important dietary component. Medeiros and Arthington (2011) reported a significant correlation between spatial variation in δ13C values for N. erebi (and other fish species) and zooplankton that is consistent with the findings of stomach content analysis (Medeiros and Arthington 2008). Further, Jardine et al. (2015) found that zooplankton accounted for 50% of assimilated carbon in small (~1 g) N. erebi, declining to 25% in fish as large as 500 g. Phytoplankton are typically highly depleted in 13C (Vuorio et al. 2006) and are the most likely source of carbon for zooplankton in the present study. In contrast to benthic algal production, planktonic algal production likely contributes to carbon assimilation in small individuals by their consumption of zooplankton.
Thus, algae and zooplankton potentially contribute approximately one-half of the carbon assimilated by large N. erebi, whereas zooplankton contribute approximately one-half of the carbon assimilated by small N. erebi. What then accounts for the remaining fractions? Whereas spatial variation and correlation between source and consumer isotope values proved useful here for quantifying the contribution of algae and zooplankton, the minimal spatial variation in δ13C values of TVEG provided little scope for doing so. Nonetheless, stomach content analysis clearly indicates that detritus is the dominant food item, and the near absence of potential food items other than zooplankton or algae in stomach contents strongly suggests that we have not failed to consider or assess other potential sources. Moreover, the multiple regression analysis strongly supported a significant contribution by TVEG to N. erebi biomass. Thus, it seems most parsimonious to suggest that terrestrial detritus is, indeed, the missing source, despite the failure to detect a correlation between detrital δ13C values and those of fish, and the apparent poor nutritional quality of this food source (Brett et al. 2017). In addition, dead phytoplankton that have entered the detrital pool may have also contributed to the carbon assimilated by N. erebi.
It is rare for detritus not to have attached or embedded bacteria and fungi (Bowen 1987; Findlay et al. 2002). Detrital δ13C values do not change greatly with conditioning, and thus the isotope value of detritus, and of the microbial community living upon it, reflects its source origin (Finlay and Kendall 2007). As a result, δ13C values alone are unlikely to differentiate between carbon derived from detritus and that derived from microorganisms feeding upon that detritus. Given the refractory nature of vascular plant detritus, its nutritive value may be derived mostly from these attached organisms (France 2011) despite their low biomass relative to their substrate (Bowen 1987). Smoot and Findlay (2010) showed that the ingesta of the closely related facultative detritivore D. cepedianum contained eightfold more low-density material and was nutritionally enriched than the detrital or sediment material upon which it foraged. Moreover, the microbial biomass in ingesta was sevenfold greater than sediment. Smoot and Findlay (2010) suggested this living component of detritus was used as a food source by D. cepedianum. A similar comparison has not been performed for N. erebi. If, however, N. erebi possesses the same capacity to winnow detrital particles of differing quality, then it is possible that assimilation of carbon and nitrogen derived from microbiota feeding upon detritus is substantial. There is scant information on 15N fractionation by microorganisms, making any interpretation of enrichment patterns in consumers of this form of prey difficult (Vanderklift and Ponsard 2003); however, the high availability of microbial biomass within the detrital pool can exert a disproportionate effect on enrichment dynamics on higher-order consumers that feed from both brown and green food chains (Steffan et al. 2017). We estimated a trophic enrichment of 4.1 and 3.2‰ for large and small N. erebi respectively; these values are not dissimilar to the ~3‰ per trophic level increase reported by Vander Zanden and Rasmussen (2001) and Post (2002). Bunn et al. (2013) reported a trophic enrichment of 3.9 ± 1.4‰ for a range of Australasian herbivorous fishes.
Although the quality of the fine detrital fraction may not be as high as that of algae, and certainly not that of zooplankton, it is nonetheless an abundant food source. Moreover, if the higher-value microbial fraction can be separated from lesser-quality larger fractions, then its value is increased further. Fish faced with a diet of low or reduced quality, particularly of protein, can compensate by increasing consumption rates to meet both energy and essential nutrient demands provided the food source is not limiting, which is not usually the case for detritus. Notwithstanding the constraint imposed by the absence of intestinal structures enabling the processing of algae or detritus (e.g. the muscular gizzard is largely absent in fish <60 mm in length), switching between algal, detrital and zooplankton sources to achieve a blended diet across green and brown food chains may enable juvenile N. erebi >60 mm in length to achieve and maintain high growth and the intake of essential nutrients such as limiting amino acids and PUFAs.
This study has shown that detritus (with or without associated microbiota), algae and zooplankton are all important sources of carbon and nutrients for N. erebi. This species is almost ubiquitous across northern Australia and may dominate fish biomass (Pusey et al. 2017). It is itself consumed by many higher-order predators, some of which can move great distances, even across catchment boundaries in the case of water birds (Kingsford et al. 2010). Thus, the contribution of terrestrially derived carbon to N. erebi biomass, albeit occurring with low efficiency, may be translated up into higher trophic levels of aquatic food webs of northern Australia. Furthermore, the liberation of nutrients due to mass mortality of N. erebi in dry season waterholes of arid zone rivers contributes greatly to the production dynamics of dry season waters (Burford et al. 2008). N. erebi is clearly an important component of riverine food webs. Although not entirely dependent on detritus as a food source, detritus is an important component of the diet of N. erebi, and may thus contribute more to tropical and subtropical Australian aquatic food webs than previously considered. Our knowledge of the biology of N. erebi is scant, particularly in regard to the relationships among hydrological variation, reproduction and movement. Changes in flow regimes and connectivity between parts of the riverine landscape arising from the expansion of water resource use in northern Australia (Douglas et al. 2011; Pettit et al. 2017) and that affect the production dynamics of N. erebi have the potential to disrupt riverine food webs (Turschwell et al. 2019). The present study has shown that both detritus and algae are important sources of energy and nutrients for this common species, and hence for food web structure in general. Moreover, the findings support the assertion by Jardine et al. (2015) that a focus on an algal–detrital dichotomy is unhelpful and that a greater focus on the circumstances in which species switch between different food sources would provide a better appreciation of the way in which food webs are structured and how they may change in response to changes in hydrology. Furthermore, a greater focus on the carbon sources supporting zooplankton production is warranted because zooplankton are key to early life history development of N. erebi, and probably to that of most other freshwater fish species of the region.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Much of the research included here was supported by the Tropical Rivers and Coastal Knowledge (TRaCK) Programme, the Northern Environmental research Programme and the National Environmental Science Programme funded through the Australian Government’s Commonwealth Environment Research Facilities initiative, the Australian Government’s Raising National Water Standards Program, Land and Water Australia, the Fisheries Research and Development Corporation and the Queensland Government’s Smart State Innovation Fund.
Acknowledgements
The authors thank the two anonymous reviewers for their constructive and valuable contributions. We also thank the numerous people who have provided assistance in the field and the laboratory.
References
Akiyama, T., Oohara, I., and Yamamoto, T. (1997). Comparison of essential amino acid requirements with A/E ratio among fish species. Fisheries Science 63, 963–970.| Comparison of essential amino acid requirements with A/E ratio among fish species.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H., Bunn, S. E., and Gray, M. (1992). Tully–Millstream hydroelectric scheme, final report on additional limnological studies. Griffith University. Brisbane, Qld, Australia.
Atkins, B. (1984). Feeding ecology of Nematalosa erebi in the lower River Murray. B.Sc.(Hons) Thesis, University of Adelaide, Adelaide, SA, Australia.
Balcombe, S. R., Bunn, S. E., McKenzie‐Smith, F. J., and Davies, P. M. (2005). Variability of fish diets between dry and flood periods in an arid zone floodplain river. Journal of Fish Biology 67, 1552–1567.
| Variability of fish diets between dry and flood periods in an arid zone floodplain river.Crossref | GoogleScholarGoogle Scholar |
Barnes, C., Sweeting, C. J., Jennings, S., Barry, J. T., and Polunin, N. V. (2007). Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Functional Ecology 21, 356–362.
| Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation.Crossref | GoogleScholarGoogle Scholar |
Beesley, L. (2006). Environmental stability: its role in structuring fish communities and life history strategies in the Fortescue River, Western Australia. Ph.D. Thesis, The University of Western Australia, Perth, WA, Australia.
Belicka, L. L., Sokol, E. R., Hoch, J. M., Jaffé, R., and Trexler, J. C. (2012). A molecular and stable isotopic approach to investigate algal and detrital energy pathways in a freshwater marsh. Wetlands 32, 531–542.
| A molecular and stable isotopic approach to investigate algal and detrital energy pathways in a freshwater marsh.Crossref | GoogleScholarGoogle Scholar |
Bishop, K. A., Allen, S. A., Pollard, D. A., and Cook, M. G. (2001). Ecological studies on the freshwater fishes of the alligator rivers region, Northern Territory: autecology. Office of the Supervising Scientist Report 145, Supervising Scientist, Darwin, NT, Australia.
Blanchette, M. L., Davis, A. M., Jardine, T. D., and Pearson, R. G. (2014). Omnivory and opportunism characterize food webs in a large dry-tropics river system. Freshwater Science 33, 142–158.
| Omnivory and opportunism characterize food webs in a large dry-tropics river system.Crossref | GoogleScholarGoogle Scholar |
Bluhdorn, D. R., and Arthington, A. H. (1994). ‘The Effects of Flow Regulation in the Barker–Barambah Catchment. Volume 2: Biotic Studies and Synthesis.’ (Griffith University: Brisbane, Qld, Australia.)
Bowen, S. H. (1987). Composition and nutritional value of detritus. In ‘Detritus and Microbial Ecology in Aquaculture: Proceedings of the Conference on Detrital Systems for Aquaculture’, 26–31 August 1985, Como, Italy. (Eds D. J. W. Moriarty and R. S. V. Pullin.) pp. 192–216. (ICLARM: Manilla, Philippines.)
Brett, M. T., Kainz, M. J., Taipale, S. J., and Seshan, H. (2009). Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences of the United States of America 106, 21197–21201.
| Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production.Crossref | GoogleScholarGoogle Scholar | 19934044PubMed |
Brett, M. T., Bunn, S. E., Chandra, S., Galloway, A. W., Guo, F., Kainz, M. J., Kankaala, P., Lau, D. C., Moulton, T. P., Power, M. E., and Rasmussen, J. B. (2017). How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshwater Biology 62, 833–853.
| How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems?Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., Davies, P. M., and Winning, M. (2003). Sources of organic carbon supporting the food web of an arid zone floodplain river. Freshwater Biology 48, 619–635.
| Sources of organic carbon supporting the food web of an arid zone floodplain river.Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., Balcombe, S. R., Davies, P. M., Fellows, C. S., and McKenzie-Smith, F. J. (2006). Aquatic productivity and food webs of desert river ecosystems. In ‘Ecology of Desert Rivers’. (Ed. R. Kingsford.) pp. 76–99. (Cambridge University Press: Cambridge, UK.)
Bunn, S. E., Leigh, C., and Jardine, T. D. (2013). Diet–tissue fractionation of δ15N by consumers from streams and rivers. Limnology and Oceanography 58, 765–773.
| Diet–tissue fractionation of δ15N by consumers from streams and rivers.Crossref | GoogleScholarGoogle Scholar |
Burford, M. A., Cook, A. J., Fellows, C. S., Balcombe, S. R., and Bunn, S. E. (2008). Sources of carbon fuelling production in an arid floodplain river. Marine and Freshwater Research 59, 224–234.
| Sources of carbon fuelling production in an arid floodplain river.Crossref | GoogleScholarGoogle Scholar |
Coates, D. (1993). Fish ecology and management of the Sepik-Ramu, New Guinea, a large contemporary tropical river basin. Environmental Biology of Fishes 38, 345–368.
| Fish ecology and management of the Sepik-Ramu, New Guinea, a large contemporary tropical river basin.Crossref | GoogleScholarGoogle Scholar |
Douglas, M. M., Bunn, S. E., and Davies, P. M. (2005). River and wetland food webs in Australia’s wet–dry tropics: general principles and implications for management. Marine and Freshwater Research 56, 329–342.
| River and wetland food webs in Australia’s wet–dry tropics: general principles and implications for management.Crossref | GoogleScholarGoogle Scholar |
Douglas, M., Jackson, S., Pusey, B., Kennard, M., and Burrows, D. (2011). Northern futures: threats and opportunities for freshwater ecosystems. In ‘Aquatic Biodiversity of the Wet–Dry Topics of Northern Australia: Patterns, Threats and Future’. (Ed. B. J. Pusey.) pp 203–220. (Charles Darwin University Press: Darwin, NT, Australia.)
Egan, J. P., Bloom, D. D., Kuo, C. H., Hammer, M. P., Tongnunui, P., Iglésias, S. P., Sheaves, M., Grudpan, C., and Simons, A. M. (2018). Phylogenetic analysis of trophic niche evolution reveals a latitudinal herbivory gradient in Clupeoidei (herrings, anchovies, and allies). Molecular Phylogenetics and Evolution 124, 151–161.
| Phylogenetic analysis of trophic niche evolution reveals a latitudinal herbivory gradient in Clupeoidei (herrings, anchovies, and allies).Crossref | GoogleScholarGoogle Scholar | 29551522PubMed |
Findlay, S., Tank, J., Dye, S., Valett, H. M., Mulholland, P. J., McDowell, W. H., Johnson, S. L., Hamilton, S. K., Edmonds, J., Dodds, W. K., and Bowden, W. B. (2002). A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams. Microbial Ecology 43, 55–66.
| A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams.Crossref | GoogleScholarGoogle Scholar | 11984629PubMed |
Finlay, J. C. (2004). Patterns and controls of lotic algal stable carbon isotope ratios. Limnology and Oceanography 49, 850–861.
| Patterns and controls of lotic algal stable carbon isotope ratios.Crossref | GoogleScholarGoogle Scholar |
Finlay, J. C., and Kendall, C. (2007). Stable isotope tracing of temporal and spatial variability in organic matter sources to freshwater ecosystems. Stable Isotopes in Ecology and Environmental Science 2, 283–333.
| Stable isotope tracing of temporal and spatial variability in organic matter sources to freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Flecker, A. S. (1996). Ecosystem engineering by a dominant detritivore in a diverse tropical stream. Ecology 77, 1845–1854.
| Ecosystem engineering by a dominant detritivore in a diverse tropical stream.Crossref | GoogleScholarGoogle Scholar |
France, R. (2011). Leaves as ‘crackers’, biofilm as ‘peanut butter’: exploratory use of stable isotopes as evidence for microbial pathways in detrital food webs. Oceanological and Hydrobiological Studies 40, 110–115.
| Leaves as ‘crackers’, biofilm as ‘peanut butter’: exploratory use of stable isotopes as evidence for microbial pathways in detrital food webs.Crossref | GoogleScholarGoogle Scholar |
Goulding, M., Carvalho, M. L., and Ferreira, E. G. (1988). ‘Rio Negro, Rich Life in Poor Water. Amazonian Diversity and Foodchain Ecology as Seen Through Fish Communities.’ (SPB Academic Publishing: Amsterdam, Netherlands.)
Guo, F., Kainz, M. J., Sheldon, F., and Bunn, S. E. (2016a). The importance of high-quality algal food sources in stream food webs – current status and future perspectives. Freshwater Biology 61, 815–831.
| The importance of high-quality algal food sources in stream food webs – current status and future perspectives.Crossref | GoogleScholarGoogle Scholar |
Guo, F., Kainz, M. J., Sheldon, F., and Bunn, S. E. (2016b). Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181, 449–462.
| Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams.Crossref | GoogleScholarGoogle Scholar | 26883960PubMed |
Hortle, K. G., and Person, R. G. (1990). Fauna of the Annan River system, far north Queensland, with reference to the impact of tin mining. I. Fishes. Marine and Freshwater Research 41, 677–694.
| Fauna of the Annan River system, far north Queensland, with reference to the impact of tin mining. I. Fishes.Crossref | GoogleScholarGoogle Scholar |
Jardine, T. D., Pettit, N. E., Warfe, D. M., Pusey, B. J., Ward, D. P., Douglas, M. M., Davies, P. M., and Bunn, S. E. (2012a). Consumer–resource coupling in wet–dry tropical rivers. Journal of Animal Ecology 81, 310–322.
| Consumer–resource coupling in wet–dry tropical rivers.Crossref | GoogleScholarGoogle Scholar | 22103689PubMed |
Jardine, T. D., Pusey, B. J., Hamilton, S. K., Pettit, N. E., Davies, P. M., Douglas, M. M., Sinnamon, V., Halliday, I. A., and Bunn, S. E. (2012b). Fish mediate high food web connectivity in the lower reaches of a tropical floodplain river. Oecologia 168, 829–838.
| Fish mediate high food web connectivity in the lower reaches of a tropical floodplain river.Crossref | GoogleScholarGoogle Scholar | 21983712PubMed |
Jardine, T. D., Hunt, R. J., Faggotter, S. J., Valdez, D., Burford, M. A., and Bunn, S. E. (2013). Carbon from periphyton supports fish biomass in waterholes of a wet–dry tropical river. River Research and Applications 29, 560–573.
| Carbon from periphyton supports fish biomass in waterholes of a wet–dry tropical river.Crossref | GoogleScholarGoogle Scholar |
Jardine, T. D., Woods, R., Marshall, J., Fawcett, J., Lobegeiger, J., Valdez, D., and Kainz, M. J. (2015). Reconciling the role of organic matter pathways in aquatic food webs by measuring multiple tracers in individuals. Ecology 96, 3257–3269.
| Reconciling the role of organic matter pathways in aquatic food webs by measuring multiple tracers in individuals.Crossref | GoogleScholarGoogle Scholar | 26909431PubMed |
Jardine, T. D., Rayner, T. S., Pettit, N. E., Valdez, D., Ward, D. P., Lindner, G., Douglas, M. M., and Bunn, S. E. (2017). Body size drives allochthony in food webs of tropical rivers. Oecologia 183, 505–517.
| Body size drives allochthony in food webs of tropical rivers.Crossref | GoogleScholarGoogle Scholar | 27896479PubMed |
Junk, W. J., Bayley, P. B., and Sparks, R. E. (1989). The flood pulse concept in river–floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106, 110–127.
Kennard, M. J. (1995). Factors influencing freshwater fish assemblages in floodplain lagoons of the Normanby River, Cape York Peninsula: a large tropical Australian river. Ph.D. Thesis, Griffith University, Brisbane, Qld, Australia.
Kennard, M. J., Pusey, B. J., and Arthington, A. H. (2001). Trophic Ecology of freshwater fishes in Australia. CRC Freshwater Ecology Scoping Study SCD6, CRC for Freshwater Ecology, Brisbane, Qld, Australia.
Kingsford, R. T., Roshier, D. A., and Porter, J. L. (2010). Australian waterbirds – time and space travellers in dynamic desert landscapes. Marine and Freshwater Research 61, 875–884.
| Australian waterbirds – time and space travellers in dynamic desert landscapes.Crossref | GoogleScholarGoogle Scholar |
Leigh, C., Burford, M. A., Sheldon, F., and Bunn, S. E. (2010). Dynamic stability in dry season food webs within tropical floodplain rivers. Marine and Freshwater Research 61, 357–368.
| Dynamic stability in dry season food webs within tropical floodplain rivers.Crossref | GoogleScholarGoogle Scholar |
Lewis, W. M., Hamilton, S. K., Rodríguez, M. A., Saunders, J. F., and Lasi, M. A. (2001). Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data. Journal of the North American Benthological Society 20, 241–254.
| Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data.Crossref | GoogleScholarGoogle Scholar |
Lowe-McConnell, R. H. (1975). ‘Fish Communities in Tropical Freshwaters: Their Distribution, Ecology, and Evolution.’ (Longman: London, UK.)
McGoldrick, D. J., Barton, D. R., Power, M., Scott, R. W., and Butler, B. J. (2008). Dynamics of bacteria–substrate stable isotope separation: dependence on substrate availability and implications for aquatic food web studies. Canadian Journal of Fisheries and Aquatic Sciences 65, 1983–1990.
| Dynamics of bacteria–substrate stable isotope separation: dependence on substrate availability and implications for aquatic food web studies.Crossref | GoogleScholarGoogle Scholar |
Medeiros, E. S., and Arthington, A. H. (2008). The importance of zooplankton in the diets of three native fish species in floodplain waterholes of a dryland river, the Macintyre River, Australia. Hydrobiologia 614, 19–31.
| The importance of zooplankton in the diets of three native fish species in floodplain waterholes of a dryland river, the Macintyre River, Australia.Crossref | GoogleScholarGoogle Scholar |
Medeiros, E. S., and Arthington, A. H. (2011). Allochthonous and autochthonous carbon sources for fish in floodplain lagoons of an Australian dryland river. Environmental Biology of Fishes 90, 1–17.
| Allochthonous and autochthonous carbon sources for fish in floodplain lagoons of an Australian dryland river.Crossref | GoogleScholarGoogle Scholar |
Medeiros, E. S., and Arthington, A. H. (2014). Fish diet composition in floodplain lagoons of an Australian dryland river in relation to an extended dry period following flooding. Environmental Biology of Fishes 97, 797–812.
| Fish diet composition in floodplain lagoons of an Australian dryland river in relation to an extended dry period following flooding.Crossref | GoogleScholarGoogle Scholar |
Moore, J. W., and Semmens, B. X. (2008). Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11, 470–480.
| Incorporating uncertainty and prior information into stable isotope mixing models.Crossref | GoogleScholarGoogle Scholar | 18294213PubMed |
Moore, J. C., Berlow, E. L., Coleman, D. C., de Ruiter, P. C., Dong, Q., Hastings, A., Johnson, N. C., McCann, K. S., Melville, K., Morin, P. J., and Nadelhoffer, K. (2004). Detritus, trophic dynamics and biodiversity. Ecology Letters 7, 584–600.
| Detritus, trophic dynamics and biodiversity.Crossref | GoogleScholarGoogle Scholar |
Morgan, D. L., Rowland, A. J., Gill, H. S., and Doupé, R. G. (2004). The implications of introducing a large piscivore (Lates calcarifer) into a regulated northern Australian river (Lake Kununurra, Western Australia). Lakes and Reservoirs: Research and Management 9, 181–193.
| The implications of introducing a large piscivore (Lates calcarifer) into a regulated northern Australian river (Lake Kununurra, Western Australia).Crossref | GoogleScholarGoogle Scholar |
Murray, D. S., Hager, H., Tocher, D. R., and Kainz, M. J. (2014). Effect of partial replacement of dietary fish meal and oil by pumpkin kernel cake and rapeseed oil on fatty acid composition and metabolism in Arctic charr (Salvelinus alpinus). Aquaculture 431, 85–91.
| Effect of partial replacement of dietary fish meal and oil by pumpkin kernel cake and rapeseed oil on fatty acid composition and metabolism in Arctic charr (Salvelinus alpinus).Crossref | GoogleScholarGoogle Scholar |
Pettit, N. E., Naiman, R. J., Warfe, D. M., Jardine, T. D., Douglas, M. M., Bunn, S. E., and Davies, P. M. (2017). Productivity and connectivity in tropical riverscapes of northern Australia: ecological insights for management. Ecosystems 20, 492–514.
| Productivity and connectivity in tropical riverscapes of northern Australia: ecological insights for management.Crossref | GoogleScholarGoogle Scholar |
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718.
| Using stable isotopes to estimate trophic position: models, methods, and assumptions.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Read, M. G., and Arthington, A. H. (1995). The feeding ecology of freshwater fishes in two rivers of the Australian Wet Tropics. Environmental Biology of Fishes 43, 85–103.
| The feeding ecology of freshwater fishes in two rivers of the Australian Wet Tropics.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Arthington, A. H., and Read, M. G. (2000). The dry season diet of freshwater fishes in monsoonal tropical rivers of Cape York Peninsula, Australia. Ecology Freshwater Fish 9, 177–190.
| The dry season diet of freshwater fishes in monsoonal tropical rivers of Cape York Peninsula, Australia.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Kennard, M. J., and Arthington, A. H. (2004). ‘Freshwater Fishes of North-eastern Australia’. (CSIRO Publishing: Melbourne, Vic., Australia.)
Pusey, B. J., Arthington, A. H., Stewart-Koster, B., Kennard, M. J., and Read, M. G. (2010). Widespread omnivory in freshwater fish assemblages of a hydrologically variable northern Australian river. Journal of Fish Biology 77, 731–753.
| 20701651PubMed |
Pusey, B. J., Burrows, D. W., Kennard, M. J., Perna, C. N., Unmack, P. J., Allsop, Q., and Hammer, M. P. (2017). Freshwater fishes of northern Australia. Zootaxa 4253, 1–104.
| Freshwater fishes of northern Australia.Crossref | GoogleScholarGoogle Scholar | 28609989PubMed |
Rasmussen, J. B. (2010). Estimating terrestrial contribution to stream invertebrates and periphyton using a gradient-based mixing model for δ13C. Journal of Animal Ecology 79, 393–402.
| Estimating terrestrial contribution to stream invertebrates and periphyton using a gradient-based mixing model for δ13C.Crossref | GoogleScholarGoogle Scholar | 20039981PubMed |
Rayner, T. S., Pusey, B. J., and Pearson, R. G. (2009). Spatio-temporal dynamics of fish feeding in the lower Mulgrave River, north-eastern Queensland: the influence of seasonal flooding, instream productivity and invertebrate abundance. Marine and Freshwater Research 60, 97–111.
| Spatio-temporal dynamics of fish feeding in the lower Mulgrave River, north-eastern Queensland: the influence of seasonal flooding, instream productivity and invertebrate abundance.Crossref | GoogleScholarGoogle Scholar |
Reid, D. J., Quinn, G. P., Lake, P. S., and Reich, P. (2008). Terrestrial detritus supports the food webs in lowland intermittent streams of south-eastern Australia: a stable isotope study. Freshwater Biology 53, 2036–2050.
| Terrestrial detritus supports the food webs in lowland intermittent streams of south-eastern Australia: a stable isotope study.Crossref | GoogleScholarGoogle Scholar |
Roach, K. A. (2013). Environmental factors affecting incorporation of terrestrial material into large river food webs. Freshwater Science 32, 283–298.
| Environmental factors affecting incorporation of terrestrial material into large river food webs.Crossref | GoogleScholarGoogle Scholar |
Rooney, N., McCann, K., Gellner, G., and Moore, J. C. (2006). Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269.
| Structural asymmetry and the stability of diverse food webs.Crossref | GoogleScholarGoogle Scholar | 16855582PubMed |
Smoot, J. C., and Findlay, R. H. (2010). Microbes as food for sediment-ingesting detritivores: low-density particles confer a nutritional advantage. Aquatic Microbial Ecology 59, 103–109.
| Microbes as food for sediment-ingesting detritivores: low-density particles confer a nutritional advantage.Crossref | GoogleScholarGoogle Scholar |
Solomon, C. T., Carpenter, S. R., Clayton, M. K., Cole, J. J., Coloso, J. J., Pace, M. L., Vander Zanden, M. J., and Weidel, B. C. (2011). Terrestrial, benthic, and pelagic resource use in lakes: results from a three-isotope Bayesian mixing model. Ecology 92, 1115–1125.
| Terrestrial, benthic, and pelagic resource use in lakes: results from a three-isotope Bayesian mixing model.Crossref | GoogleScholarGoogle Scholar | 21661572PubMed |
Steffan, S. A., Chikaraishi, Y., Dharampal, P. S., Pauli, J. N., Guédot, C., and Ohkouchi, N. (2017). Unpacking brown food-webs: animal trophic identity reflects rampant microbivory. Ecology and Evolution 7, 3532–3541.
| Unpacking brown food-webs: animal trophic identity reflects rampant microbivory.Crossref | GoogleScholarGoogle Scholar | 28515888PubMed |
Sternberg, D., Balcombe, S., Marshall, J., and Lobegeiger, J. (2008). Food resource variability in an Australian dryland river: evidence from the diet of two generalist native fish species. Marine and Freshwater Research 59, 137–144.
| Food resource variability in an Australian dryland river: evidence from the diet of two generalist native fish species.Crossref | GoogleScholarGoogle Scholar |
Taylor, A. N., and Batzer, D. P. (2010). Spatial and temporal variation in invertebrate consumer diets in forested and herbaceous wetlands. Hydrobiologia 651, 145–159.
| Spatial and temporal variation in invertebrate consumer diets in forested and herbaceous wetlands.Crossref | GoogleScholarGoogle Scholar |
Thorburn, D. C., Gill, H., and Morgan, D. L. (2014). Predator and prey interactions of fishes of a tropical Western Australia river revealed by dietary and stable isotope analyses. Journal of the Royal Society of Western Australia 97, 363–387.
Thorp, J. H., and Delong, M. D. (1994). The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems. Oikos 70, 305–308.
| The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Turschwell, M. P., Stewart-Koster, B., Pusey, B. J., Douglas, M., King, A., Crook, D., Boone, E., Allsop, Q., and Kennard, M. J. (2019). Flow-mediated predator-prey dynamics influences fish populations in a tropical river. Freshwater Biology 64, 1453–1466.
| Flow-mediated predator-prey dynamics influences fish populations in a tropical river.Crossref | GoogleScholarGoogle Scholar |
Unmack, P. (2013). Biogeography. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 25–48. (CSIRO Publishing: Melbourne, Vic., Australia.)
Vander Zanden, M. J., and Rasmussen, J. B. (2001). Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46, 2061–2066.
| Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies.Crossref | GoogleScholarGoogle Scholar |
Vanderklift, M. A., and Ponsard, S. (2003). Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136, 169–182.
| Sources of variation in consumer-diet δ15N enrichment: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 12802678PubMed |
Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R., and Cushing, C. E. (1980). The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37, 130–137.
| The river continuum concept.Crossref | GoogleScholarGoogle Scholar |
Vuorio, K., Meili, M., and Sarvala, J. (2006). Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshwater Biology 51, 807–822.
| Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (2004). Floodplain river food webs: generalizations and implications for fisheries management. In ‘Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries’, 11–14 February 2003, Phnom Penh, Cambodia, (Eds R. L. Welcomme and T. Petr.) Vol. 2, pp. 285–309. (Food and Agriculture Organization of the United Nations and Mekong River Commission, FAO Regional Office for Asia and the Pacific: Rome, Italy.)



