Intraspecific variation in diel patterns of rocky reef use suggests temporal partitioning in Port Jackson sharks
Nathan Charles Bass A E , Joanna Day A B , Tristan L. Guttridge C , Nathan A. Knott D and Culum Brown A
A E , Joanna Day A B , Tristan L. Guttridge C , Nathan A. Knott D and Culum Brown A
A Macquarie University, Department of Biological Sciences, North Ryde, NSW 2109, Australia.
B Taronga Institute of Science and Learning, Taronga Conservation Society Australia, Mosman, NSW 2088, Australia.
C Saving the Blue, Cooper City, FL 33328, USA.
D NSW Department of Primary Industries, Fisheries Research, 4 Woollamia Road, Huskisson, NSW 2540, Australia.
E Corresponding author. Email: nathancbass@mail.com
Marine and Freshwater Research - https://doi.org/10.1071/MF20204
Submitted: 29 June 2020 Accepted: 26 March 2021 Published online: 13 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Limited information exists about the temporal residency patterns of marine predators, especially at the individual level. Temporal partitioning of resources can reduce intra-specific competition, but this has seldom been examined in predators in marine ecosystems. Here, we used 8 years of acoustic telemetry data from 27 receivers deployed in a large coastal embayment to examine the temporal residency of 51 Port Jackson sharks (Heterodontus portusjacksoni), during their breeding season. We found that the residency lengths of male and female Port Jackson sharks on breeding reefs differed throughout the breeding season, with males showing longer residency at the start of the season and females showing longer residency at the end of the season. Port Jackson sharks also showed a 24-h or diel periodicity in their detection patterns. Although the majority of individuals were nocturnal, a small proportion of sharks was detected more frequently during the day, possibly to reduce competition for resources. Surprisingly, there was no difference in the sex ratio nor the size of diurnal and nocturnal individuals. This study provides long-term insight into the temporal residency patterns of mesopredatory sharks at a breeding site and, more broadly, our results highlight the importance of studying temporal variation at the individual level in movement ecology studies.
Keywords: elasmobranchs, marine, ecology.
Introduction
Examining the ways in which individuals use their environments on spatial and temporal scales is important to understanding the processes underpinning the development of different behavioural strategies (Chesson 2000). Spatial and temporal niche partitioning among ecologically similar species (e.g. Swanson et al. 2016) and between predator and prey (e.g. Wu et al. 2018) has been well studied. Additionally, variation in the space use of individuals within a species has been examined in a variety of taxa in the contexts of distribution (Zupcic-Moore et al. 2017), movement patterns (Lei and Booth 2017) and territoriality (de Souza et al. 2018). However, few studies have examined variation in the daily activity patterns of individuals within the same species (Kadri et al. 1997; Alanärä et al. 2001; Fingerle et al. 2016). This is important because time-sharing resources, such as resting locations or foraging grounds, can reduce intraspecific competition. Fingerle et al. (2016), for example, found that juvenile Arctic charr (Salvelinus alpines) distributed their activity over a greater portion of the 24-h cycle in response to higher conspecific density. Similarly, daily feeding patterns and foraging times of Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) vary among individuals of different sizes and dominance ranks respectively (Kadri et al. 1997; Alanärä et al. 2001). Together, these studies suggest that individuals may exhibit temporal niche partitioning in response to increased intraspecific competition, and highlight the importance of examining the behaviour of individuals on both spatial and temporal scales.
Understanding spatial and temporal variation in the behaviour of predators has important implications for the development of complex behavioural patterns, as well as the ecology and evolution of the populations, species and the communities in which they live (Austin et al. 2004). The majority of studies examining the movement ecology of predatory fishes, particularly elasmobranchs, focus on spatial components of movements in species occurring in tropical or subtropical areas (Chapman et al. 2015). For example, Munroe et al. (2016) studied the residency and movements of juvenile Australian blacktip sharks (Carcharhinus tilstoni) in northern Australia and found that individuals were highly variable in their space use throughout the year. Previous studies on the variation in movement behaviour of shark species over the course of the day have typically focused on the diel variation in activity levels and home-range size (Speed et al. 2010). Comparatively little is known about the way in which the timing and duration of residency events vary among marine predators.
Many coastal species of sharks appear to show periodicity in their presence on subtidal reefs within their home ranges, with some species showing 24-h periodicity relating to circadian rhythms (Papastamatiou et al. 2009; Speed et al. 2011; Barnett et al. 2012), whereas others show 8–12-h periodicity in detections relating to tidal movements (Papastamatiou et al. 2009; Field et al. 2011; Speed et al. 2011). In the case of blacktip reef sharks (Carcharhinus melanopterus, Papastamatiou et al. 2009) and grey reef sharks (Carcharhinus amblyrhynchos, Field et al. 2011), individuals showed both 24-h periodicity and 8–12-h periodicity. This is understandable, given the importance of tide and diel cycles in driving the behaviour of marine organisms (e.g. Collatos et al. 2020).
Studies on the relative abundances of individuals throughout the day have typically compared only the number of detections between day and night (Garla et al. 2006; Clarke et al. 2011) or have grouped all individuals together to look at relative hourly abundances of the species (Conrath and Musick 2010; Bessudo et al. 2011; Speed et al. 2011, 2016; Barnett et al. 2012), without considering intraspecific variation in residency behaviour. Carlson et al. (2008) and Heupel et al. (2006) both found that individuals demonstrated different patterns with respect to hourly detection patterns. Heupel et al. (2006) suggested that further research was needed into the behaviour patterns that led to the observed differences among individuals. Field et al. (2011) found that grey reef sharks exhibited two general patterns of reef attendance and suggested that these patterns may be reflective of individual foraging or behavioural strategies to reduce or avoid intraspecific competition. However, it is not clear how wide-spread temporal partitioning might be among apex and mesopredators in marine ecosystems.
Port Jackson sharks are a model species to examine the temporal partitioning in residency behaviour of a benthic shark species given their high levels of site fidelity (Bass et al. 2017), propensity to form breeding aggregations at known locations (Powter and Gladstone 2009; Bass et al. 2017) and their low stress response to capture, handling and tagging (Frick et al. 2009). The objective of this study was to use acoustic telemetry data to investigate the individual variation in patterns of temporal residency of 51 Port Jackson sharks in Jervis Bay over an 8-year period from 2012 to 2019. The high levels of site fidelity exhibited by Port Jackson sharks, combined with the large number of tagged individuals and multi-year nature of the study, allowed us to examine variation in the residency behaviour of individuals over multiple breeding seasons. Using this extensive dataset, we generated residency events, which are defined as the continuous use of a single receiver site, and calculated their lengths using the VTrack package (ver. 1.12, see https://cran.r-project.org/web/packages/VTrack/index.html; Campbell et al. 2012). It is worth noting that these sharks spend the majority of their time on a single breeding reef within the bay and each reef is covered by a single receiver. We then examined the influence of demographic variables, such as sex and length, on the length of residency events throughout the breeding season at breeding aggregation sites. Specifically, we hypothesised that males would exhibit longer residency events at the beginning of the season while they intercept receptive females as they arrive at the breeding ground. Conversely, we predicted that females would have the longest residency events at the end of the breeding season while they are laying eggs and males are departing for their southern migration. Second, we aimed to identify and describe cyclical patterns of detection at the sites at which individuals were most frequently detected within Jervis Bay. We anticipated that both diel and tidal cycles would influence reef attendance, but given the nocturnal nature of Port Jackson sharks (Kadar et al. 2019; Kelly et al. 2020), that diel rhythms would dominate. Finally, we expected that there would likely be multiple cyclical patterns of reef attendance exhibited by different individuals to help alleviate potential consequences of high conspecific densities and associated intraspecific competition.
Materials and methods
Study sites
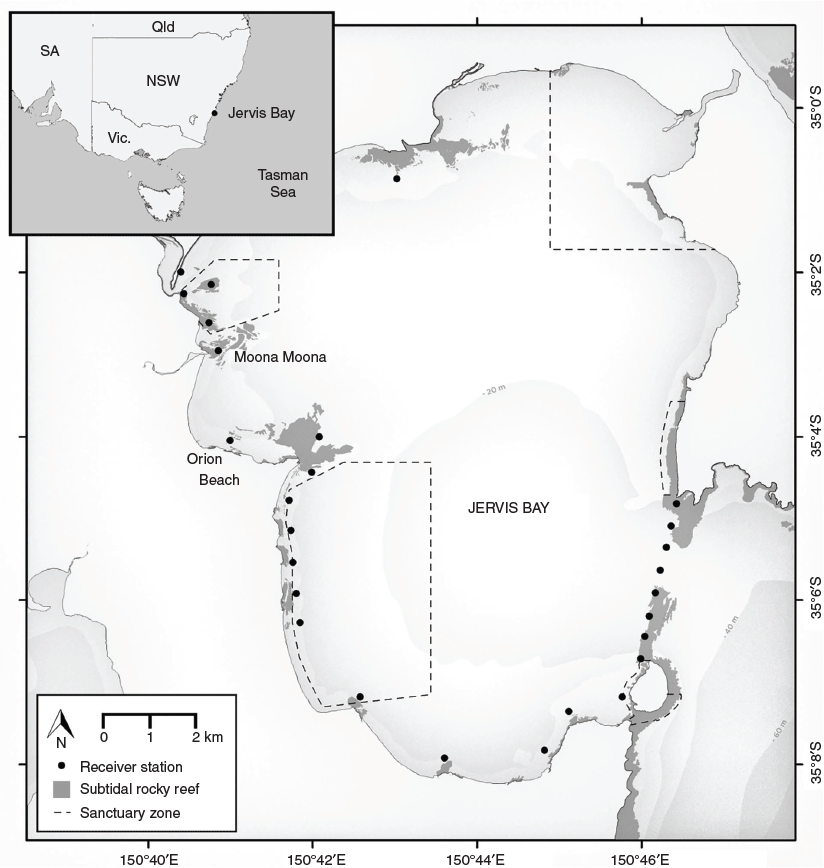
This study was conducted in Jervis Bay, which forms the central part of the Jervis Bay Marine Park, a multi-use marine park that allows recreational fishing and boating within its boundaries, on the southern coast of New South Wales, Australia. Jervis Bay covers 102 km2 and contains a wide variety of marine habitats, including shallow and deep rocky reefs, sandy benthos, seagrass communities and mangroves (Fig. 1). Previous studies have found that Jervis Bay supports large aggregations of adult Port Jackson sharks during their breeding season (Powter and Gladstone. 2009; Bass et al. 2017). Port Jackson sharks are long lived and return to the same breeding sites each year (Bass et al. 2017), with females exhibiting higher levels of reproductive philopatry than do males (Day et al. 2019). Within a given season, both sexes show unusually high fidelity to a single reef within the bay (Bass et al. 2017).
In this study, adult Port Jackson sharks were captured from two breeding aggregation sites within Jervis Bay, namely, Orion Beach (n = 32) and Moona Moona Creek (n = 19), between 2012 and 2014 and fitted with acoustic transmitters. An acoustic array was deployed by New South Wales Department of Primary Industries in 2011 to monitor the movements of acoustically tagged marine organisms within the Jervis Bay Marine Park. The array consists of 19 receivers on all of the rocky reefs within Jervis Bay and a further eight receivers across the mouth of Jervis Bay (Fig. 1). The detection ranges of the receivers were estimated to be 250 m (50% detection probability, Swadling et al. 2020) and were downloaded on an annual basis. Ferguson et al. (2013) found that V9 acoustic transmitters showed a 1.2–2.6% reduction in detection frequency at night at two subtidal rocky reefs in Jervis Bay, suggesting that variation in detection probabilities between day and night is unlikely to contribute significant variation to observed detection frequencies.
Tagging procedures
Individual sharks were captured by hand on snorkel and transported by kayak to a tub containing sea water on the shore (~100-m distance). The individuals were then measured, weighed, sexed and tagged with passive integrated transponder (FDXB transponders, Microchips Australia) tags for individual identification. Individuals were then sedated in a solution of tricaine methanesulfonate (MS-222; 150 mg mL–1) and a V16-x acoustic transmitter (Vemco, Halifax, NS, Canada; battery life 2805 days (7.7–10 years), delay 90–180 s) was implanted into their peritoneal cavity through a 2.5–4-cm incision. The incision was sutured using interrupted suture knots and super glue (Mulcahy 2003). The procedure took less than 10 min from sedation to release. Individuals typically recovered within 10 min and were released at their site of capture once they were able to swim under their own power. All capture and tagging procedures were conducted in accordance with an Animal Research Authority permit (2012/009) granted by the Macquarie University Animal Ethics Committee and two NSW fisheries permits (P08/0010-3.1 and P08/0010-4.2).
Statistical analysis
Detections from each receiver were downloaded by Fisheries NSW by using VUE software (ver. 2.6.2, Vemco, Halifax, NS, Canada) and then uploaded and stored on the Animal Tracking Facility database (part of the Australian Integrated Marine Observing System). Acoustic telemetry data were downloaded from the IMOS database to analyse the movements of Port Jackson sharks within Jervis Bay (IMOS Animal Tracking Database, see https://animaltracking.aodn.org.au, accessed 1 January 2020).A Data were filtered to remove single detections of a transmitter at a receiver within a 24-h period because these may represent tag collisions and not necessarily true detections of the individuals at a given site.
Data processing and analyses were performed using RStudio (ver. 1.0.136, R Foundation for Statistical Computing). Figures were generated using the packages ‘dplyr’ (ver. 1.0.5, H. Wickham, R. François, L. Henry, K. Müller and RStudio, see https://CRAN.R-project.org/package=dplyr) and ‘ggplot2’ (ver. 3.3.3, see https://cran.r-project.org/package=ggplot2 and https://ggplot2.tidyverse.org.; Wickham 2016). Data for total length were mean-centred for each sex independently to account for variation between the sizes of males and females and, thus, mean-centred body lengths ranged from 0 (the smallest individual) to 1 (the largest individual) for each sex.
Length of residency
The residency lengths of Port Jackson sharks were estimated using the event analyser function in the R package ‘VTrack’ (Campbell et al. 2012). A residency event began when a tagged individual was detected by the receiver two or more times and terminated when an individual was not detected within a pre-set timeout window or if the individual was detected at a different receiver. Thus, each residency event is likely to consist of periods of rest and movement within the range of the receiver. However, given the highly sedentary nature of Port Jackson sharks (McLaughlin 1969; Powter and Gladstone 2009), we expected that residency lengths will be a good proxy for resting duration. For this study, the timeout window for residency events was set to 15 min. This is considered ample time for a Port Jackson shark to swim out of the detection range of a receiver on the basis of previously reported swim speeds (Ryan et al. 2015). Further, the timeout window of 15 min should also be sufficient to account for variation in both the detection probabilities throughout the range of the receiver and the detection probabilities during the day and the night.
A linear mixed model (LMM) with a Gaussian distribution was generated using the ‘lme4’ package (ver. 1.1-23, see https://cran.r-project.org/package=lme4; Bates et al. 2015) and was used to examine variation in the duration of residency events between the sex and size of individual sharks, as well as between months and years. An interaction term between month and sex was also included in candidate models to account for the seasonal variation between residency and movement of males and females (Bass et al. 2017). Residency lengths (min) were log-transformed to normalise the data and improve model fit. Candidate models were generated and their Akaike information criteria (AICc) were compared using the ‘MuMIn’ package (ver. 1.43.15, K. Bartoń, see https://cran.r-project.org/package=MuMIn). The model with the lowest AICc was used to examine the effects of mean-centred fork length, sex and month on residency length (sensu Burnham et al. 2011). Individual ID was set as a random factor to account for pseudoreplication of individuals in the analysis and year was set as a random factor to account for variation between the total number of tagged individuals detected over the course of the study. P-values for the general linear model were estimated using the ‘Anova’ function in the ‘car’ package (ver. 3.0-7, see https://cran.r-project.org/package=car and https://socialsciences.mcmaster.ca/jfox/Books/Companion/; Fox and Weisberg 2019).
Cyclical patterns of reef attendance
Lomb–Scargle periodograms were generated for each individual to identify cyclical patterns in reef attendance, by using the ‘lomb’ package (ver. 1.2, see https://cran.r-project.org/package=lomb; Ruf 1999). The Lomb–Scargle algorithm is suitable for incomplete and unequally spaced time series data and has been shown to have better periodicity detection frequency and accuracy in the presence of noise than do other methods (Ruf 1999). The majority of Port Jackson sharks are not present in Jervis Bay outside of the breeding season and there is variation in the arrival and departure dates among individuals and among years (Bass et al. 2017). Consequently, the detection data of individuals are unequally spaced and incomplete throughout the year (Fig. S1 of the Supplementary material). For the purpose of this analysis, individuals were restricted to their favoured site for each breeding season. The ‘favoured site’ for each individual was defined as the site at which they were most frequently detected throughout the breeding season (on average, more than 85% of detections; Table S1 of the Supplementary material). Detections of individuals at their favoured site were pooled into hourly bins for each individual and the Lomb–Scargle algorithm was sampled from periods of 2 to 100 h, with an oversampling factor of 5 and α of 0.01.
Hourly detection patterns
Rao’s test for homogeneity was used to determine whether there was significant variation in the hourly detection patterns among individuals. Rao’s spacing test was then used to determine whether detection data for each individual was uniformly distributed over a 24-h period to determine whether each individual was detected at a particular time of day. Rao’s spacing test and Rao’s test for homogeneity were performed using the ‘circular’ package (ver. 0.4-93, C. Agostinelli and U. Lund, see https://cran.r-project.org/package=circular). Given that there was significant variation between the hourly detection patterns of individuals and detections of most individuals were not uniformly distributed over the day, we used hierarchical clustering, based on a Euclidean distance matrix, to group individuals on the basis of their hourly detection patterns. K-means clustering, using the silhouette method, was used to determine the optimal number of clusters and assign individuals to those clusters. A heat map was then generated to visually compare the hourly detection patterns among the clusters. Logistic regression was used to determine whether mean-centred total length influenced the hourly detection cluster of individuals. A chi-square test of association was used to determine whether the proportion of males and females differed among the clusters.
Results
Residency lengths
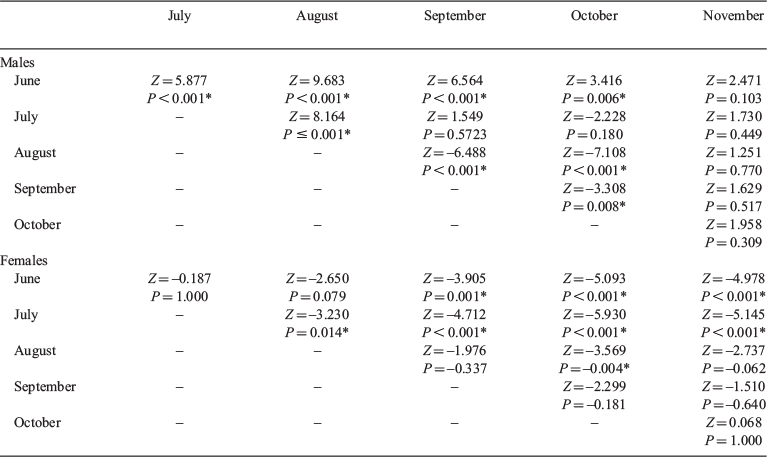
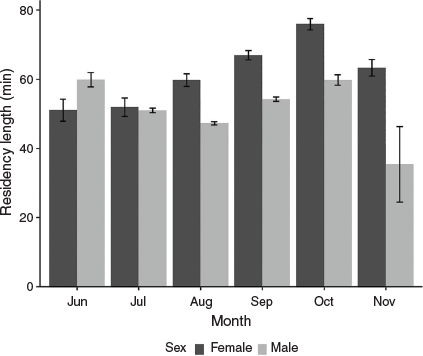
In total, 115 046 residency events were recorded from the 51 tagged individuals over the 8 breeding seasons. The maximum residency length of Port Jackson sharks at their favoured sites ranged from 254 to 3180 min (4.2–53.0 h) and the average residency length of Port Jackson sharks at their favoured sites was 55.2 ± 0.3 min (Table 1; median = 20.0 min). The best model explaining variation in residency length included sex, month and the interaction between these factors as fixed effects and individual ID and year as random effects (ΔAICc = 1.93, AICc weight = 0.724; Table 2). The GLMM that contained both individual ID and year as random effects explained significantly more variance than did the same model without the random effects (ΔAICc = 11 918.46, weight = 1.00). The random effects contributed to explain 11.8% of the variance within the model, whereas the fixed effects explained 1.3% of the variation. There was a significant interaction between sex and month (χ2 = 76.567, d.f. = 5, P < 0.001). Post hoc Tukey contrasts showed that female Port Jackson sharks exhibited significantly shorter residency lengths at the beginning of the breeding season (June, mean ± s.e. = 51.1 ± 3.18 min; July, 51.9 ± 2.69 min) than at end of the breeding season (September, mean ± s.e. = 67.0 ± 1.36 min; October, 76.0 ± 1.66 min; November, 63.3 ± 2.36 min; Fig. 2, Table 3). By contrast, male Port Jackson sharks showed the highest residency lengths in June (mean ± s.e. = 59.9 ± 2.07 min) and October (mean ± s.e. = 59.8 ± 1.52 min), with the lengths of residency periods being lower over the middle months of the breeding season (July, mean ± s.e. = 51.0 ± 0.686 min; August, 47.3 ± 0.516 min; September, 54.3 ± 0.652 min; Fig. 2, Table 3). The lengths of residency events of males in November were typically lower than in all of the other months (mean ± s.e. = 24.9 ± 2.98 min).

|
|
Cyclical patterns of residency
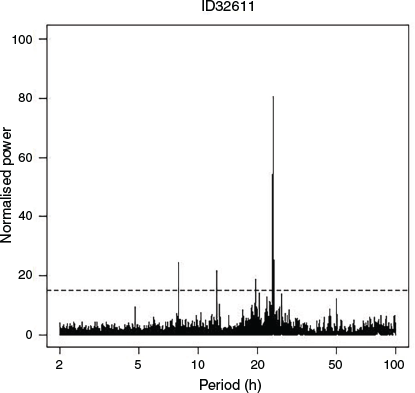
Adult Port Jackson sharks showed high levels of periodicity in their attendance patterns at their favoured sites (Table 1). The majority of individuals (n = 43) demonstrated significant periodicity at an interval of ~24 h (Table 1, e.g. Fig. 3). Several individuals also showed significant peaks ~8 h (n = 14) and 12 h (n = 12; Table 1). Collectively, these results suggest that the attendance patterns of Port Jackson sharks at breeding reefs generally follow a 24-h cyclical pattern, which is consistent with their responding to day–night light regimes with some minor variation, perhaps through interactions with tidal regimes.
|
Hourly detection patterns
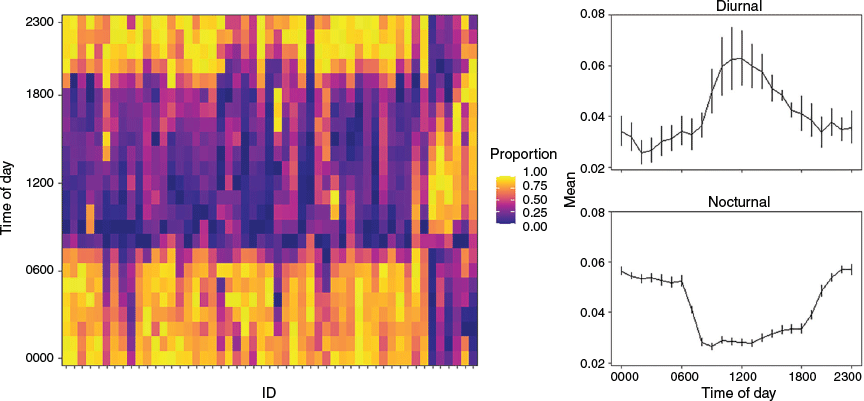
Rao’s tests for homogeneity found that there were significant differences among the circular distributions of the hourly detection patterns of individuals (test statistic = 7686.820, d.f. = 53, P < 0.001). The hourly detection frequencies for the majority of the tagged Port Jackson sharks (except ID32583) were non-homogeneous (Table 1). Hierarchical clustering identified two hourly detection patterns, namely, nocturnal (n = 40; Fig. 4) and diurnal (n = 11; Fig. 4). There was no significant difference between the mean centred length of individuals who were detected at night and those that were detected during the day (z = 0.06, P = 0.952). Additionally, the proportions of males and females in each cluster were not significantly different among clusters (χ2 = 0.975, d.f. = 1, P = 0.323).
Discussion
This study presents the first analysis of the temporal variation of residency in a benthic shark species. Throughout eight breeding seasons, we found that the lengths of residency events of males and females at their favoured sites varied throughout the breeding season. The majority of Port Jackson sharks demonstrated 24-h periodicity, suggesting a strong influence of diel period on their observed attendance patterns; however, some individuals exhibited periodicity of ~8–12 h, suggesting that tidal factors may influence the attendance patterns of some individuals on breeding reefs. Additionally, individuals fitted into two general hourly detection patterns at their favoured sites, namely, diurnal (n = 11) or nocturnal (n = 40). The constituents of the two groups were not influenced by sex or size. This is broadly consistent with the hypothesis that individuals may partition space use on a temporal scale to reduce intraspecific competition for resting sites on the breeding reefs. Our results highlighted the importance of studying temporal variation among conspecifics, so as to understand the ways in which individuals within a population can mediate intraspecific competition for resources, such as access to mates, which has implications for the study of movement ecology across a broad range of taxa. To our knowledge, this is the first such study in marine ecosystems.
Variation in the residency lengths of male and female Port Jackson sharks has important implications for the reproductive biology of Port Jackson sharks. This variation was primarily driven by the longer residency events of males at the beginning of the breeding season and the longer residency events of females thereafter, particularly at the end of the season. Previous studies examining the arrival and departure of male and female Port Jackson sharks found that male Port Jackson sharks arrive at and depart from breeding aggregation sites in Jervis Bay before females do (Bass et al. 2017; J. Pini-Fitzsimmons, S. Newson and C. Brown, unpubl. data). The longer residency events of male Port Jackson sharks early in the season may reflect the increased time spent waiting on reefs to intercept females as they arrive at the aggregation sites during this period. Alternatively, individuals may exhibit spatial or temporal variation in residency behaviour in response to intraspecific competition (Papastamatiou et al. 2018) and, thus, males may exhibit increased residency event lengths at the start of the breeding season to ensure that they do not miss mating opportunities. Similarly, we have observed that females tend to stay on the breeding reefs after males have left as they lay their eggs in rocky crevices. Females may exhibit longer residency events because the water warms up to maximise their fecundity and increase the rate of egg development, a behaviour observed in other elasmobranch species (Economakis and Lobel 1998; Wearmouth and Sims 2008). Further, with fewer remaining males, females are less likely to be sexually harassed later in the breeding season, which may also prolong female residency events on the subtidal rocky reefs. Sexual segregation on a spatial scale is a common behaviour observed across many species of elasmobranchs (Wearmouth and Sims 2010), including other horned sharks (Meese and Lowe 2020). It is thought to result from intraspecific competition, differences in pre- and post-copulatory reproductive strategies or differences in habitat or energetic requirements between the sexes (Sims 2003; Wearmouth and Sims 2008; Jacoby et al. 2010). However, much of the data on sexual segregation in sharks come from differences in sex ratios of fisheries capture data and do not allow for the testing of ecological hypothesis (Sims 2003). Here, we show that Port Jackson sharks exhibit sexual segregation on a temporal scale and that this variation may reflect contrasting reproductive strategies of male and female Port Jackson sharks.
Although the presence of temporal sexual segregation in Port Jackson sharks may alleviate the pressures of intraspecific competition for resources (e.g. access to mates, food, resting sites) between males and females, there is still increased competition among individuals owing to their high levels of residency at specific sites and the long residency events, as shown by this study. This potential for competition is further increased by the strong 24-h periodicity in attendance at their favoured reefs. To mediate this increased pressure for resting sites, food resources or access to mates, individuals within the population may exhibit temporal variation in their use of particular sites or resources (Kadri et al. 1997; Alanärä et al. 2001; Fingerle et al. 2016). Here, we found that Port Jackson sharks exhibited two different hourly detection patterns at their favoured sites throughout the breeding season. Although the majority of individuals were primarily detected on the breeding reefs during the night, a small proportion was primarily detected during the day. Given that the Port Jackson sharks are aggregating during their breeding season, individuals may compete for access to mates, access to limited refuging or high-quality microhabitats that improve the ability of females to avoid sexual harassment by males or to secure their eggs (e.g. caves). This potential competition for access to limited microhabitats may lead to temporal partitioning in residency behaviour. Given that research on the visual system of the Port Jackson shark found that they are typically well adapted for nocturnal vision (Peel et al. 2020), understanding the factors that influence the diel residency patterns of individuals is interesting. Understanding why some individuals are resident during the day or night is a clear priority for future research.
Demographic factors, such as size and sex, do not appear to influence the hourly detection patterns that individuals display. Research on temporal partitioning in fish has found that the residency patterns of brown trout are influenced by the social rank of individuals (Alanärä et al. 2001). Alanärä et al. (2001) found that dominant individuals preferentially fed during the night time, which maximised their foraging success while minimising predation risk, and subordinate individuals foraged during the day time. Given the large detection ranges of the acoustic receivers used in this study, it was impossible to determine the extent to which individual hourly detection patterns were influenced by the presence of conspecifics (Mourier et al. 2017). It would be valuable for future studies to quantify the behaviour, and particularly interactions, of individuals by using fine-scale acoustic receivers (Bass 2012; Mourier et al. 2017), Vemco positioning systems (VPS, Mourier et al. 2017) or novel proximity receiver technology (Guttridge et al. 2010), to determine whether individuals are competing for the same resources or whether they are simply utilising different parts of the reef. Further, future studies could utilise biologging tags incorporating tri-axial sensors to validate resting behaviour or further quantify the resting times of individuals throughout the breeding season (Kadar et al. 2019).
Port Jackson sharks have previously been shown to exhibit high levels of residency and site fidelity to specific sites (McLaughlin and O’Gower 1971; Powter and Gladstone 2009). Although the residency lengths found in the present study (mean maximum residency length = 15.4 h) were considerably lower than are previously reported resting durations based on visual observations (27 h, Powter and Gladstone 2009), they highlighted the propensity of Port Jackson sharks to exhibit long periods of resting behaviour (McLaughlin 1969). Although data on the mean duration of resting events of other elasmobranch species are rare, the maximum residency lengths of Port Jackson sharks in the present study are similar to those observed in other benthic species. For example, Huveneers et al. (2006) reported that banded wobbegongs (Orectolobus halei) were found in the same area for up to 1.8 days. Similarly, Castro (2000) observed that adult nurse sharks (Ginglymostoma cirratum) rest in the same locations for several days. These low levels of activity may reflect their low metabolic rate. Research on nurse sharks found that they exhibit low standard metabolic rates and spend the majority of their time resting to optimise energy efficiency (Whitney et al. 2016). Luongo and Lowe (2018) measured oxygen consumption in juvenile California horn sharks (Heterodontus francisci) and found that they exhibited standard metabolic rates similar to those of nurse sharks. Although juvenile Port Jackson sharks exhibited slightly higher oxygen consumption rates than did California horn sharks and nurse sharks, they still had relatively low standard metabolic rates compared with other shark species (Gervais 2019). Like nurse sharks, adult Port Jackson sharks may limit unnecessary energy usage to maximise available energy for reproduction.
Understanding the factors that influence temporal and spatial behaviour is key to predicting how populations and species may vary in their movement ecology. In elasmobranchs, individual variation in behaviour is typically studied in relation to habitat use (e.g. Clarke et al. 2011) or foraging behaviour (e.g. Matich and Heithaus 2015; Papastamatiou et al. 2018; Matich et al. 2019). Matich and Heithaus (2015) suggested that intraspecific variation in the habitat use of juvenile bull sharks could be caused by intrinsic factors such as habitat-use preferences, foraging strategies or even personalities. Although there has been limited research on personality in sharks (Jacoby et al. 2014; Byrnes and Brown 2016), variation in personality traits could drive variation in the residency, habitat use, movement patterns and social behaviour of individuals (Finger et al. 2016; Finger et al. 2018; Dhellemmes et al. 2020). In other taxa, personality traits, such as aggression and boldness, have been shown to drive intraspecific variation in the foraging behaviour, intraspecific niche variation, habitat preferences, home range utilisation and dispersal in individuals (Bergmüller and Taborsky 2010; Cote et al. 2010; Brown and Irving 2014; Spiegel et al. 2017). Additionally, social factors, such as dominance rank, also influence diel behavioural variation in other taxa (Fingerle et al. 2016). Linking personality traits, such as boldness and aggression, and social factors, such as dominance ranks and network position, to individual variation in the habitat use and movements of elasmobranchs is a priority for future research and is critical to better understanding the role that individual variation plays in the ecological role of populations and species.
Although our work focussed on Port Jackson sharks, which are of little conservation concern, they do play key roles in the ecosystems in which they live. Therefore, these findings have important implications for the communities in which Port Jackson sharks live because they tend to dominate these communities, particularly during the breeding season. Port Jackson sharks not only exhibit a ‘top down’ influence on the ecosystems as predators but may also exhibit an important ‘bottom up’ influence on the ecosystem, with their eggs providing a massive influx of nutrients into the recipient ecosystem. By developing a greater understanding of the temporal partitioning of males and females on a seasonal scale, we will be better able to predict the impact of their roles within the ecosystem. Moreover, sexual and intraspecific variation in movement behaviour has important ecological and evolutionary implications for the development of complex behavioural patterns (Austin et al. 2004). In this study, we have shown that individuals and sexes within a population may partition their use of particular locations within their home range on a daily and seasonal scale. Few studies examining movement and habitat use in sharks and rays have taken both temporal and spatial variability into account. Partitioning resources in this way may substantially enhance the carrying capacity of given habitats, which has implications for marine conservation and management more broadly. Although we detected two different clusters of habitat use over a 24-h period, we have little idea what the fitness implications might be for individuals displaying these traits. Moreover, the findings of this study may be applied to improving the conservation and management of similar species that exhibit predictable temporal patterns and site fidelity, because these are species that are often most vulnerable to overexploitation. Last, our results highlighted that studies grouping intraspecific variation in behaviour into a population mean essentially disregards the variation on which natural selection and evolutionary processes operate (Judson 1994; Zollner and Lima 1999; Austin et al. 2004).
Conclusions
In summary, we examined the residency behaviour of 51 acoustically tagged Port Jackson sharks over eight consecutive breeding seasons and found that individuals exhibited variation in both the timing and length of their residency events on subtidal rocky reefs. Whereas the majority of individuals exhibited nocturnal hourly detection patterns, a small proportion of individuals exhibited diurnal hourly detection patterns at subtidal rocky reefs within Jervis Bay. It is possible that this variation in hourly detection patterns reduces male–male competition for access to females and reduces competition for resting or oviposition sites for females. Second, we found that males typically exhibit longer residency events at the beginning of the breeding seasons, whereas females exhibit longer residency events for the latter part of the season, which is reasonably consistent with our current knowledge of their breeding behaviour. These results highlight the importance of considering the ways in which individuals within populations and sexes within species utilise the areas in which they live. Understanding the biological and social drivers of this intraspecific variation in temporal residency behaviour remains a priority for future research.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was funded by Sea World Research and Rescue Foundation Inc., the Australian Research Council (LP140100319), Taronga Conservation Society Australia and the Department of Biological Sciences, Macquarie University.
Acknowledgements
The authors thank Sue Newson from the Jervis Bay Marine Discovery and Research Centre and various volunteers for assistance with tagging in the field. The authors thank NSW DPI for access to data from the NSW DPI Jervis Bay Receiver Array.
References
Alanärä, A., Burns, M. D., and Metcalfe, N. B. (2001). Intraspecific resource partitioning in brown trout: the temporal distribution of foraging is determined by social rank. Journal of Animal Ecology 70, 980–986.| Intraspecific resource partitioning in brown trout: the temporal distribution of foraging is determined by social rank.Crossref | GoogleScholarGoogle Scholar |
Austin, D., Bowen, W. D., and McMillan, J. I. (2004). Intraspecific variation in movement patterns: modeling individual behaviour in a large marine predator. Oikos 105, 15–30.
| Intraspecific variation in movement patterns: modeling individual behaviour in a large marine predator.Crossref | GoogleScholarGoogle Scholar |
Barnett, A., Abrantes, K. G., Seymour, J., and Fitzpatrick, R. (2012). Residency and spatial use by reef sharks of an isolated seamount and its implications for conservation. PLoS One 7, e36574.
| Residency and spatial use by reef sharks of an isolated seamount and its implications for conservation.Crossref | GoogleScholarGoogle Scholar | 23284819PubMed |
Bass, N. C. (2012). Social networking and site fidelity in Port Jackson sharks (Heterodontus portusjacksoni). B.Mar.Sc.(Hons) Thesis, Macquarie University, Sydney, NSW, Australia.
Bass, N. C., Mourier, J., Knott, N. A., Day, J., Guttridge, T., and Brown, C. (2017). Long-term migration patterns and bisexual philopatry in a benthic shark species. Marine and Freshwater Research 68, 1414–1421.
| Long-term migration patterns and bisexual philopatry in a benthic shark species.Crossref | GoogleScholarGoogle Scholar |
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bergmüller, R., and Taborsky, M. (2010). Animal personality due to social niche specialisation. Trends in Ecology & Evolution 25, 504–511.
| Animal personality due to social niche specialisation.Crossref | GoogleScholarGoogle Scholar |
Bessudo, S., Soler, G. A., Klimley, A. P., Ketchum, J. T., Hearn, A., and Arauz, R. (2011). Residency of the scalloped hammerhead shark (Sphyrna lewini) at Malpelo Island and evidence of migration to other islands in the Eastern Tropical Pacific. Environmental Biology of Fishes 91, 165–176.
| Residency of the scalloped hammerhead shark (Sphyrna lewini) at Malpelo Island and evidence of migration to other islands in the Eastern Tropical Pacific.Crossref | GoogleScholarGoogle Scholar |
Brown, C., and Irving, E. (2014). Individual personality traits influence group exploration in a feral guppy population. Behavioral Ecology 25, 95–101.
| Individual personality traits influence group exploration in a feral guppy population.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., Anderson, D. R., and Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65, 23–35.
| AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons.Crossref | GoogleScholarGoogle Scholar |
Byrnes, E. E., and Brown, C. (2016). Individual personality differences in Port Jackson sharks Heterodontus portusjacksoni. Journal of Fish Biology 89, 1142–1157.
| Individual personality differences in Port Jackson sharks Heterodontus portusjacksoni.Crossref | GoogleScholarGoogle Scholar | 27228221PubMed |
Campbell, H. A., Watts, M. E., Dwyer, R. G., and Franklin, C. E. (2012). V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Marine and Freshwater Research 63, 815–820.
| V-Track: software for analysing and visualising animal movement from acoustic telemetry detections.Crossref | GoogleScholarGoogle Scholar |
Carlson, J. K., Heupel, M. R., Bethea, D. M., and Hollensead, L. D. (2008). Coastal habitat use and residency of juvenile Atlantic sharpnose sharks (Rhizoprionodon terraenovae). Estuaries and Coasts 31, 931–940.
| Coastal habitat use and residency of juvenile Atlantic sharpnose sharks (Rhizoprionodon terraenovae).Crossref | GoogleScholarGoogle Scholar |
Castro, J. I. (2000). The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands. Environmental Biology of Fishes 58, 1–22.
| The biology of the nurse shark, Ginglymostoma cirratum, off the Florida east coast and the Bahama Islands.Crossref | GoogleScholarGoogle Scholar |
Chapman, D. D., Feldheim, K. A., Papastamatiou, Y. P., and Hueter, R. E. (2015). There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Annual Review of Marine Science 7, 547–570.
| There and back again: a review of residency and return migrations in sharks, with implications for population structure and management.Crossref | GoogleScholarGoogle Scholar | 25251267PubMed |
Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31, 343–366.
| Mechanisms of maintenance of species diversity.Crossref | GoogleScholarGoogle Scholar |
Clarke, C., Lea, J. S. E., and Ormond, R. F. G. (2011). Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea. Marine and Freshwater Research 62, 668–675.
| Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea.Crossref | GoogleScholarGoogle Scholar |
Collatos, C., Abel, D. C., and Martin, K. L. (2020). Seasonal occurrence, relative abundance, and migratory movements of juvenile sandbar sharks, Carcharhinus plumbeus, in Winyah Bay, South Carolina. Environmental Biology of Fishes 103, 859–873.
| Seasonal occurrence, relative abundance, and migratory movements of juvenile sandbar sharks, Carcharhinus plumbeus, in Winyah Bay, South Carolina.Crossref | GoogleScholarGoogle Scholar |
Conrath, C. L., and Musick, J. A. (2010). Residency, space use and movement patterns of juvenile sandbar sharks (Carcharhinus plumbeus) within a Virginia summer nursery area. Marine and Freshwater Research 61, 223–235.
| Residency, space use and movement patterns of juvenile sandbar sharks (Carcharhinus plumbeus) within a Virginia summer nursery area.Crossref | GoogleScholarGoogle Scholar |
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 365, 4065–4076.
| Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations.Crossref | GoogleScholarGoogle Scholar | 21078658PubMed |
Day, J., Clark, J. A., Williamson, J. E., Brown, C., and Gillings, M. (2019). Population genetic analyses reveal female reproductive philopatry in the oviparous Port Jackson shark. Marine and Freshwater Research 70, 986–994.
| Population genetic analyses reveal female reproductive philopatry in the oviparous Port Jackson shark.Crossref | GoogleScholarGoogle Scholar |
de Souza, D. C., Vieira, L. D., and da Silva Castro, A. L. (2018). Territoriality and home range of red legged seriema (Cariama cristata). Ornitologia Neotropical 29, 101–105.
Dhellemmes, F., Finger, J. S., Laskowski, K. L., Guttridge, T. L., and Krause, J. (2020). Comparing behavioural syndromes across time and ecological conditions in a free-ranging predator. Animal Behaviour 162, 23–33.
| Comparing behavioural syndromes across time and ecological conditions in a free-ranging predator.Crossref | GoogleScholarGoogle Scholar |
Economakis, A. E., and Lobel, P. S. (1998). Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean. Environmental Biology of Fishes 51, 129–139.
| Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, Central Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Ferguson, A. M., Harvey, E. S., Taylor, M. D., and Knott, N. A. (2013). A herbivore knows its patch: luderick, Girella tricuspidata, exhibit strong site fidelity on shallow subtidal reefs in a temperate marine park. PLoS One 8, e65838.
| A herbivore knows its patch: luderick, Girella tricuspidata, exhibit strong site fidelity on shallow subtidal reefs in a temperate marine park.Crossref | GoogleScholarGoogle Scholar | 23741515PubMed |
Field, I. C., Meekan, M. G., Speed, C. W., White, W., and Bradshaw, C. J. A. (2011). Quantifying movement patterns for shark conservation at remote coral atolls in the Indian Ocean. Coral Reefs 30, 61–71.
| Quantifying movement patterns for shark conservation at remote coral atolls in the Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Finger, J. S., Dhellemmes, F., Guttridge, T. L., Kurvers, R. H., Gruber, S. H., and Krause, J. (2016). Rate of movement of juvenile lemon sharks in a novel open field, are we measuring activity or reaction to novelty? Animal Behaviour 116, 75–82.
| Rate of movement of juvenile lemon sharks in a novel open field, are we measuring activity or reaction to novelty?Crossref | GoogleScholarGoogle Scholar |
Finger, J. S., Guttridge, T. L., Wilson, A. D. M., Gruber, S. H., and Krause, J. (2018). Are some sharks more social than others? Short-and long-term consistencies in the social behavior of juvenile lemon sharks. Behavioral Ecology and Sociobiology 72, 17.
| Are some sharks more social than others? Short-and long-term consistencies in the social behavior of juvenile lemon sharks.Crossref | GoogleScholarGoogle Scholar |
Fingerle, A., Larranaga, N., and Steingrímsson, S. Ó. (2016). Density-dependent diel activity in stream‐dwelling Arctic charr Salvelinus alpinus. Ecology and Evolution 6, 3965–3976.
| Density-dependent diel activity in stream‐dwelling Arctic charr Salvelinus alpinus.Crossref | GoogleScholarGoogle Scholar | 27247761PubMed |
Fox, J., and Weisberg, S. (2019). ‘An R Companion to Applied Regression’, 3rd edn. (Sage: Thousand Oaks, CA, USA.)
Frick, L. H., Reina, R. D., and Walker, T. I. (2009). The physiological response of Port Jackson sharks and Australian swellsharks to sedation, gill-net capture, and repeated sampling in captivity. North American Journal of Fisheries Management 29, 127–139.
| The physiological response of Port Jackson sharks and Australian swellsharks to sedation, gill-net capture, and repeated sampling in captivity.Crossref | GoogleScholarGoogle Scholar |
Garla, R. C., Chapman, D. D., Shivji, M. S., Wetherbee, B. M., and Amorim, A. F. (2006). Habitat of juvenile Caribbean reef sharks, Carcharhinus perezi, at two oceanic insular marine protected areas in the southwestern Atlantic Ocean: Fernando de Noronha Archipelago and Atol das Rocas, Brazil. Fisheries Research 81, 236–241.
| Habitat of juvenile Caribbean reef sharks, Carcharhinus perezi, at two oceanic insular marine protected areas in the southwestern Atlantic Ocean: Fernando de Noronha Archipelago and Atol das Rocas, Brazil.Crossref | GoogleScholarGoogle Scholar |
Gervais, C. (2019). Physiological responses of developing Port Jackson sharks to predation and elevated temperatures. Ph.D. Thesis, Macquarie University, Sydney, NSW, Australia.
Guttridge, T. L., Gruber, S. H., Krause, J., and Sims, D. W. (2010). Novel acoustic technology for studying free-ranging shark social behaviour by recording individuals’ interactions. PLoS One 5, e9324.
| Novel acoustic technology for studying free-ranging shark social behaviour by recording individuals’ interactions.Crossref | GoogleScholarGoogle Scholar | 20174465PubMed |
Heupel, M. R., Semmens, J. M., and Hobday, A. J. (2006). Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Marine and Freshwater Research 57, 1–13.
| Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays.Crossref | GoogleScholarGoogle Scholar |
Huveneers, C., Harcourt, R. G., and Otway, N. M. (2006). Observation of localised movements and residence times of the wobbegong shark Orectolobus halei at Fish Rock, NSW, Australia. Cybium 30, 103–111.
Jacoby, D. M., Busawon, D. S., and Sims, D. W. (2010). Sex and social networking: the influence of male presence on social structure of female shark groups. Behavioral Ecology 21, 808–818.
| Sex and social networking: the influence of male presence on social structure of female shark groups.Crossref | GoogleScholarGoogle Scholar |
Jacoby, D. M., Fear, L. N., Sims, D. W., and Croft, D. P. (2014). Shark personalities? Repeatability of social network traits in a widely distributed predatory fish. Behavioral Ecology and Sociobiology 68, 1995–2003.
| Shark personalities? Repeatability of social network traits in a widely distributed predatory fish.Crossref | GoogleScholarGoogle Scholar |
Judson, O. P. (1994). The rise of the individual-based model in ecology. Trends in Ecology & Evolution 9, 9–14.
| The rise of the individual-based model in ecology.Crossref | GoogleScholarGoogle Scholar |
Kadar, J., Ladds, M., Mourier, J., Day, J., and Brown, C. (2019). Acoustic accelerometry reveals diel activity patterns in premigratory Port Jackson sharks. Ecology and Evolution 9, 8933–8944.
| Acoustic accelerometry reveals diel activity patterns in premigratory Port Jackson sharks.Crossref | GoogleScholarGoogle Scholar | 31462992PubMed |
Kadri, S., Metcalfe, N. B., Huntingford, F. A., and Thorpe, J. E. (1997). Daily feeding rhythms in Atlantic salmon II: size-related variation in feeding patterns of post-smolts under constant environmental conditions. Journal of Fish Biology 50, 273–279.
| Daily feeding rhythms in Atlantic salmon II: size-related variation in feeding patterns of post-smolts under constant environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Kelly, M. L., Murray, E. R., Kerr, C. C., Radford, C. A., Collin, S. P., Lesku, J. A., and Hemmi, J. M. (2020). Diverse activity rhythms in sharks (Elasmobranchii). Journal of Biological Rhythms 35, 476–488.
| Diverse activity rhythms in sharks (Elasmobranchii).Crossref | GoogleScholarGoogle Scholar | 32525441PubMed |
Lei, J., and Booth, D. T. (2017). Intraspecific variation in space use of a coastal population of lace monitors (Varanus varius). Australian Journal of Zoology 65, 398–407.
| Intraspecific variation in space use of a coastal population of lace monitors (Varanus varius).Crossref | GoogleScholarGoogle Scholar |
Luongo, S. M., and Lowe, C. G. (2018). Seasonally acclimated metabolic Q 10 of the California horn shark, Heterodontus francisci. Journal of Experimental Marine Biology and Ecology 503, 129–135.
| Seasonally acclimated metabolic Q 10 of the California horn shark, Heterodontus francisci.Crossref | GoogleScholarGoogle Scholar |
Matich, P., and Heithaus, M. R. (2015). Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas). Oecologia 178, 347–359.
| Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas).Crossref | GoogleScholarGoogle Scholar | 25669454PubMed |
Matich, P., Kiszka, J. J., Heithaus, M. R., Le Bourg, B., and Mourier, J. (2019). Inter-individual differences in ontogenetic trophic shifts among three marine predators. Oecologia 189, 621–636.
| Inter-individual differences in ontogenetic trophic shifts among three marine predators.Crossref | GoogleScholarGoogle Scholar | 30796523PubMed |
McLaughlin, R. H. (1969). The ecology of heterodont sharks. Ph.D. Thesis, University of New South Wales, Sydney, NSW, Australia.
McLaughlin, R. H., and O’Gower, A. K. (1971). Life history and underwater studies of a heterodont shark. Ecological Monographs 41, 271–289.
| Life history and underwater studies of a heterodont shark.Crossref | GoogleScholarGoogle Scholar |
Meese, E. N., and Lowe, C. G. (2020). Active acoustic telemetry tracking and tri-axial accelerometers reveal fine-scale movement strategies of a non-obligate ram ventilator. Movement Ecology 8, 8.
| Active acoustic telemetry tracking and tri-axial accelerometers reveal fine-scale movement strategies of a non-obligate ram ventilator.Crossref | GoogleScholarGoogle Scholar | 32071719PubMed |
Mourier, J., Bass, N. C., Guttridge, T. L., Day, J., and Brown, C. (2017). Does detection range matter for inferring social networks in a benthic shark using acoustic telemetry? Royal Society Open Science 4, 170485.
| Does detection range matter for inferring social networks in a benthic shark using acoustic telemetry?Crossref | GoogleScholarGoogle Scholar | 28989756PubMed |
Mulcahy, D. M. (2003). Surgical implantation of transmitters into fish. ILAR Journal 44, 295–306.
| Surgical implantation of transmitters into fish.Crossref | GoogleScholarGoogle Scholar | 13130160PubMed |
Munroe, S. E. M., Simpfendorfer, C. A., and Heupel, M. R. (2016). Variation in blacktip shark movement patterns in a tropical coastal bay. Environmental Biology of Fishes 99, 377–389.
| Variation in blacktip shark movement patterns in a tropical coastal bay.Crossref | GoogleScholarGoogle Scholar |
Papastamatiou, Y. P., Lowe, C. G., Caselle, J. E., and Friedlander, A. M. (2009). Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 90, 996–1008.
| Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll.Crossref | GoogleScholarGoogle Scholar | 19449694PubMed |
Papastamatiou, Y. P., Bodey, T. W., Friedlander, A. M., Lowe, C. G., Bradley, D., Weng, K., Priestley, V., and Caselle, J. E. (2018). Spatial separation without territoriality in shark communities. Oikos 127, 767–779.
| Spatial separation without territoriality in shark communities.Crossref | GoogleScholarGoogle Scholar |
Peel, L. R., Collin, S. P., and Hart, N. S. (2020). Retinal topography and spectral sensitivity of the Port Jackson shark (Heterodontus portusjacksoni). The Journal of Comparative Neurology 528, 2831–2847.
| Retinal topography and spectral sensitivity of the Port Jackson shark (Heterodontus portusjacksoni).Crossref | GoogleScholarGoogle Scholar | 32227480PubMed |
Powter, D. M., and Gladstone, W. (2009). Habitat-mediated use of space by juvenile and mating adult Port Jackson sharks, Heterodontus portusjacksoni, in Eastern Australia. Pacific Science 63, 1–14.
| Habitat-mediated use of space by juvenile and mating adult Port Jackson sharks, Heterodontus portusjacksoni, in Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Ruf, T. (1999). The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biological Rhythm Research 30, 178–201.
| The Lomb-Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series.Crossref | GoogleScholarGoogle Scholar |
Ryan, L. A., Meeuwig, J. J., Hemmi, J. M., Collin, S. P., and Hart, N. S. (2015). It is not just size that matters: shark cruising speeds are species-specific. Marine Biology 162, 1307–1318.
| It is not just size that matters: shark cruising speeds are species-specific.Crossref | GoogleScholarGoogle Scholar |
Sims, D. W. (2003). Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free‐ranging sharks. Journal of Fish Biology 63, 53–73.
| Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free‐ranging sharks.Crossref | GoogleScholarGoogle Scholar |
Speed, C. W., Field, I. C., Meekan, M. G., and Bradshaw, C. J. (2010). Complexities of coastal shark movements and their implications for management. Marine Ecology Progress Series 408, 275–293.
| Complexities of coastal shark movements and their implications for management.Crossref | GoogleScholarGoogle Scholar |
Speed, C. W., Meekan, M. G., Field, I. C., McMahon, C. R., Stevens, J. D., McGregor, F., Huveneers, C., Berger, Y., and Bradshaw, C. J. (2011). Spatial and temporal movement patterns of a multi-species coastal reef shark aggregation. Marine Ecology Progress Series 429, 261–275.
| Spatial and temporal movement patterns of a multi-species coastal reef shark aggregation.Crossref | GoogleScholarGoogle Scholar |
Speed, C. W., Meekan, M. G., Field, I. C., McMahon, C. R., Harcourt, R. G., Stevens, J. D., Babcock, R. C., Pillans, R. D., and Bradshaw, C. J. A. (2016). Reef shark movements relative to a coastal marine protected area. Regional Studies in Marine Science 3, 58–66.
| Reef shark movements relative to a coastal marine protected area.Crossref | GoogleScholarGoogle Scholar |
Spiegel, O., Leu, S. T., Bull, C. M., and Sih, A. (2017). What’s your move? Movement as a link between personality and spatial dynamics in animal populations. Ecology Letters 20, 3–18.
| What’s your move? Movement as a link between personality and spatial dynamics in animal populations.Crossref | GoogleScholarGoogle Scholar | 28000433PubMed |
Swadling, D. S., Knott, N. A., Rees, M. J., Pederson, H., Adams, K. R., Taylor, M. D., and Davis, A. R. (2020). Seagrass canopies and the performance of acoustic telemetry: implications for the interpretation of fish movements. Animal Biotelemetry 8, 8.
| Seagrass canopies and the performance of acoustic telemetry: implications for the interpretation of fish movements.Crossref | GoogleScholarGoogle Scholar |
Swanson, A., Arnold, T., Kosmala, M., Forester, J., and Packer, C. (2016). In the absence of a “landscape of fear”: How lions, hyenas, and cheetahs coexist. Ecology and Evolution 6, 8534–8545.
| In the absence of a “landscape of fear”: How lions, hyenas, and cheetahs coexist.Crossref | GoogleScholarGoogle Scholar | 28031805PubMed |
Wearmouth, V. J., and Sims, D. W. (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Advances in Marine Biology 54, 107–170.
| Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications.Crossref | GoogleScholarGoogle Scholar | 18929064PubMed |
Wearmouth, V. J., and Sims, D. W. (2010). Sexual segregation in elasmobranchs. Biologia Marina Mediterranea 17, 236–239.
Whitney, N. M., Lear, K. O., Gaskins, L. C., and Gleiss, A. C. (2016). The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre). Journal of Experimental Marine Biology and Ecology 477, 40–46.
| The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre).Crossref | GoogleScholarGoogle Scholar |
Wickham, H. (2016). ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag: New York, NY, USA.)
Wu, Y., Wang, H., Wang, H., and Feng, J. (2018). Arms race of temporal partitioning between carnivorous and herbivorous mammals. Scientific Reports 8, 1713.
| Arms race of temporal partitioning between carnivorous and herbivorous mammals.Crossref | GoogleScholarGoogle Scholar | 29379083PubMed |
Zollner, P. A., and Lima, S. L. (1999). Search strategies for landscape-level interpatch movements. Ecology 80, 1019–1030.
| Search strategies for landscape-level interpatch movements.Crossref | GoogleScholarGoogle Scholar |
Zupcic-Moore, J. R., Ruiz-Cooley, R. I., Paliza, O., Koch, P. L., and McCarthy, M. D. (2017). Using stable isotopes to investigate foraging variation and habitat use of sperm whales from northern Peru. Marine Ecology Progress Series 579, 201–212.
| Using stable isotopes to investigate foraging variation and habitat use of sperm whales from northern Peru.Crossref | GoogleScholarGoogle Scholar |
A Data were sourced from Australia’s Integrated Marine Observing System (IMOS) Animal Tracking Database (https://animaltracking.aodn.org.au). IMOS is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). It is operated by a consortium of institutions as an unincorporated joint venture, with the University of Tasmania as Lead Agent.