Seed germination requirements of an Australian semi-arid floodplain Acacia species, Acacia stenophylla
William Higgisson A * , Breanna Reynolds A , Yasmin Cross A and Fiona Dyer A
A * , Breanna Reynolds A , Yasmin Cross A and Fiona Dyer A
A Centre for Applied Water Science, Institute for Applied Ecology, University of Canberra, University Drive, Bruce, Canberra, ACT 2617, Australia.
Marine and Freshwater Research 73(5) 615-623 https://doi.org/10.1071/MF21226
Submitted: 3 August 2021 Accepted: 8 January 2022 Published: 14 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Plants that occur on floodplains in dryland regions often use floods to disperse and germinate seeds, which establish during and following flooding events. Acacia stenophylla (river cooba) is a perennial tree, common in the riparian habitats of the Murray–Darling Basin, Australia. The aims of this study were to (1) determine the relationship between seed germination and seedling establishment of A. stenophylla and hydrological conditions, (2) determine the buoyancy of its seeds and, hence, ability to disperse by hydrochory and (3) provide recommendations on the hydrological requirements for A. stenophylla recruitment. Seedling recruitment data collected between 2014 and 2020 on the floodplain of the lower Lachlan River were compared with time since flooding. In a glasshouse experiment, seeds of A. stenophylla within their pods and with their pods removed were exposed to one of five experimental treatments (rainfall, soaked soil, and inundated for 20 and 40 days), over a period of 50 days. A. stenophylla germinated during and following flooding and following high rainfall. Seeds in pods floated for 8 days. A. stenophylla establishes during and following flooding and requires 1 month of flooding followed by flood recession to maximise seed germination. This research contributes to our broader understanding of the reproductive biology of one of the less studied Acacia species.
Keywords: Acacia, dispersal, flooding, floodplain, recruitment, seed germination, seedling establishment, seedpod.
Introduction
Floodplain vegetation that occurs in arid and semi-arid areas provides important biological diversity and ecosystem services in otherwise dry landscapes (Bennett et al. 2014). Floodplain vegetation provides essential refugia and pathways for invertebrates (Sheldon et al. 2002), migratory birds (Roshier et al. 2002), fish (Arthington and Balcombe 2011) and mammals (Hamilton et al. 2015). Floodplains in arid and semi-arid areas are a source of energy for long periods after floods recede (Baldwin et al. 2013). Changes in climate and the reallocation of water resources have contributed to these systems becoming drier and less resilient (Colloff and Baldwin 2010). It is, therefore, important to understand the hydrological requirements of the tree species that make up these ecosystems.
The flow regime of rivers is important in structuring and sustaining floodplain and riparian vegetation communities (Stromberg et al. 2007). In semi-arid and arid regions, precipitation is intermittent, evapotranspiration is high (Kalma and Franks 2003) and groundwater declines seasonally (Wittenberg 2003). Overbank flows are, therefore, particularly important in structuring and sustaining floodplain vegetation communities in such regions. Flood waters transport propagules, drown and scour plants, and alter resource availability (Bendix and Hupp 2000). Floodplain and riparian plants often require flooding for certain life-stages, including growth, flowering and germination (Lytle and Poff 2004). For example, Eucalyptus camaldulensis (river red gum) and Eucalyptus largiflorens (black box) require flooding to germinate (George 2004; Doody et al. 2014).
Across the semi-arid floodplain environments of the Murray–Darling Basin (MDB), five woody species dominate, namely, Eucalyptus camaldulensis, Eucalyptus largiflorens, Eucalyptus coolabah, Acacia stenophylla (river cooba) and Duma florulenta (tangled lignum), providing important structure and habitat. The distribution of these species often reflects the frequency of floods and droughts (Doody et al. 2014). In such systems where there are few woody species, changes to the inundation patterns have the potential to have profound effects on the structure of the riparian areas. In response, the provision of water to support vegetation communities is becoming increasingly common, but it is often limited by a lack of knowledge of the water requirements of key species. We generally have good understanding of the water requirements of E. camaldulensis, E. largiflorens (Doody and Overton 2009), Duma florulenta (Higgisson et al. 2018) and, to a lesser extent, E. coolabah (Good et al. 2014; Balcombe et al. 2021) from a range of field and experimental studies, but we have limited empirical evidence for A. stenophylla (Casanova 2015).
Acacia is predominantly a southern hemisphere genus containing in excess of 1350 species (Maslin et al. 2003), of which approximately two-thirds are found in Australia. Acacia is often associated with arid and semi-arid landscapes, but a notable few are strongly associated with riparian areas, including A. stenophylla (Cunningham et al. 1981). Acacias are known for the hard coating on their seeds, which requires softening or scarification before germination can occur, and studies have shown large differences in germination responses to different pre-treatments (Clemens et al. 1977; Brown et al. 2003; Walters et al. 2004; Kulkarni et al. 2007).
Acacia stenophylla (A.Cunn. ex Benth., Fabaceae) is a bushy tree that typically grows to between 4 and 12 m tall (Maslin and McDonald 2004), and sometimes up to 20 m (Hall 1972), and lives for more than 50 years (Thomson 1987). The species is commonly found in inland semi-arid and arid riverine habitats of eastern Australia along the river systems of the MDB (Australia’s Virtual Herbarium 2020). It occurs along the margins of floodplains, drainage lines and ephemeral wetlands (Cunningham et al. 1981), usually growing in heavy alkaline clays subject to occasional flooding (Maslin and McDonald 2004). Cunningham et al. (1981) described that seedlings of A. stenophylla emerge along flood lines, after flooding events, and suggested that germination is related to flooding. Although A. stenophylla has been observed to germinate between 10 and 28 days, under commercial propagation (with seed pod removed and seeds placed in near boiling water for 1 min; Maslin and McDonald 2004), the natural inundation conditions suitable for its germination are not known (see Roberts and Marston 2011).
Acacia stenophylla seeds develop in pods that are 10–20 cm long and 10 mm wide. These pods, when green, can be thick and hold the seeds vertically while hanging from the tree (Cunningham et al. 1981). As the seeds begin to ripen, the seed pods become a brown moniliform, which are highly constricted between each individual seed and break easily once mature (Ahmad et al. 2016). A. stenophylla is known to produce very large seed crops (Turnbull 1986); however, the seed pods are rarely indehiscent, making them some of the most difficult seeds from the Acacia genera to collect and grow (Maslin and McDonald 2004). The dispersal mechanism of the seeds is unknown, but given the distribution of the species along rivers, it is likely that water is involved and hydrochory may be important.
The aim of this study was to describe the hydrological conditions under which the riverine tree species A. stenophylla germinates and establishes. Data collected during a long-term monitoring project in the lower Lachlan River were used to investigate seedling abundance in relation to time since flooding. To further understand the response of A. stenophylla to hydrological conditions, a glasshouse experiment was undertaken to test the role of flood duration and soil moisture on seed germination and the ability of the seeds to float.
Materials and methods
Field-based study
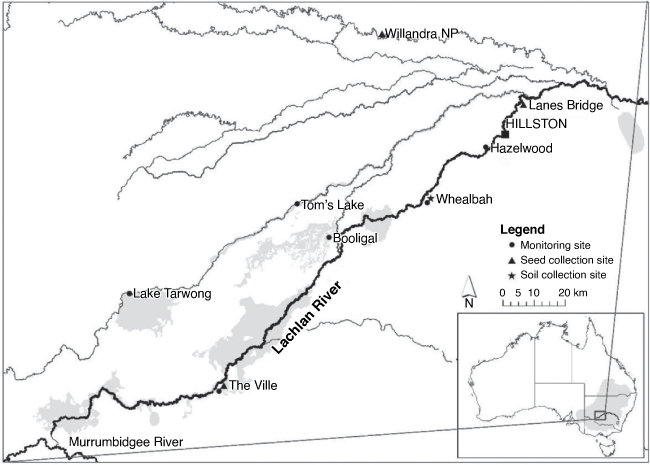
The present study used seedling recruitment data collected from sites in the lower Lachlan River System in austral spring and autumn each year between 2014 and 2020 as part of the Australian Commonwealth-funded Long-term Intervention Monitoring project (from 2014 to 2019) and Monitoring, Evaluation and Research Program (from 2019 to 2020; see Dyer et al. 2020). The monitoring sites consisted of two replicate 0.4-ha plots spaced ~100 m apart at each site. One site (Lake Tarwong) consisted of two pairs of replicate 0.4-ha plots because this site included two floodplain vegetation communities. Sites were distributed across wetlands and riparian zones along the lower Lachlan River, which have different watering probabilities (Dyer et al. 2020). Plants less than 20 cm tall at the time of survey were used in this study and are described here as seedlings. As part of this study, plots where seedlings were recorded at least once over the 12 trips (2014–2020) were used in the analysis, which included a total of 14 plots from 6 monitoring sites (Fig. 1).
Glasshouse experiment
The seed of A. stenophylla was collected from mature trees growing at three sites along the Lachlan River and Willandra Creek, New South Wales (Fig. 1). The seeds were stored in dry conditions from collection to the commencement of the experiment (~4 weeks). Soil for the experiment was collected in December 2016 from the floodplain of the lower Lachlan River near Whealbah Billabong, south of Hillston, where A. stenophylla is known to grow (Fig. 1). The soil was sterilised by pasteurisation at the Research School of Biology facility at the Australian National University in Canberra, by steaming it to a temperature of 64°C for 2 h.
An experiment was conducted in the University of Canberra glasshouse to investigate (a) the conditions required for germination of the seeds of A. stenophylla with and without pods and (b) the buoyancy of the seeds of A. stenophylla with and without pods. Prior to the commencement of the experiment, 750 seeds were manually removed from their seed pods. A further 750 seeds were retained within their pods. Each group of 750 were then counted into batches of 30 seeds. The average size of a single A. stenophylla seed pod was 21.5 mm and the average size of the seeds without pods were 7 mm long (from measuring 10 random seed pods and seeds).
Prior to the experiment, fifty 740-mL plastic containers, in total, were filled to a depth of 50 mm with sterilised soil. In total, 30 seeds within pods were placed on the soil in 25 containers (30 seeds in each) and 30 seeds without pods were placed on the soil in the other 25 containers. For the seeds with and without pods, the containers were then randomly allocated to one of five treatments (five containers per treatment – with and without pods). The treatments were as follows: (1) a (experimental) control treatment (Control) that had no water added throughout the experiment; (2) local rainfall conditions during January and February 2016 at Hillston (airport; Rainfall); (3) permanently soaked soil (Soaked); (4) inundation for 20 days, followed by recession of water (I20); and (5) inundation for 40 days, followed by recession of water (I40). These treatments replicated the local rainfall, soaked soil and flooded conditions that may be expected where A. stenophylla occurs. The control treatment was an experimental control and was not included in any of the analysis.
The experiment commenced on 8 January 2020 and was conducted over 50 days. A. stenophylla seeds typically mature in October–December (Marcar et al. 1995). The experiment was, therefore, run from January to February to coincide with the fall of the matured seeds. Throughout the experiment, the containers were randomly repositioned on the bench in the glasshouse each week. The rainfall treatment was watered using the same daily rainfall from the closest local weather station to the sites where the seeds were collected (Hillston) in 2016 (Bureau of Meteorology 2016) from January to February to replicate the seasonal rainfall. The rainfall regime in January and February 2016 was used because it was close to typical for the January to February time period; the mean long-term (120 years) and 2016 rainfall for January to February at Hillston were both ~58 mm (Bureau of Meteorology 2016). The daily rainfall throughout the experiment was 2.8 mm on Day 1, 1 mm on Day 9, 2 mm on Day 10, 1 mm on Day 17, 17.6 mm on Day 18, 12.4 mm on Day 22, 1.4 mm on Day 23, 20.1 mm on Day 26 and 0.2 mm on Day 28. The amount of water added to each container was calculated by multiplying the daily rainfall amount (mm) by the surface area of the container (cm2).
The containers in the soaked treatment were watered daily for 50 days to ensure that they remained waterlogged without being inundated above the surface of the soil. The inundated treatments (I20 and I40) were filled with water to ~50 mm above the soil surface. The water level in the inundated treatments was maintained for either 20 or 40 days depending on the treatment. On the 20th (I20) and 40th day (I40), the water was manually drawn down to the surface of the soil. No more water was added into the inundated treatments after the water was drawn down. The number of live germinants in each container were counted daily for a total of 50 days. To assess germination rates of the seed following the 50-day experiment, at the end of the 50-day period, all un-germinated seeds were soaked on soil for a further 20 days and any new germinants were then counted.
The buoyancy of A. stenophylla seeds was determined using the I20 and I40 treatments. All floating seeds were counted daily, with pod or without pod, and were recorded until all seeds were no longer floating. There were 10 replicates with pods and 10 replicates without pods for the first 20 days, at which stage all seeds had sunk.
The mean (±s.d.) minimum and maximum daily temperatures recorded in the glasshouse over the experiment were 18.8 ± 0.3 and 34.6 ± 0.7°C respectively, which are comparable to the long-term (1957–2020) average mean minimum and maximum temperatures recorded at Hillston (airport) during January and February (18.4 and 33.2°C respectively; Bureau of Meteorology 2016).
Data analysis
Field-based study
For each trip, time since last flood at a site was grouped into the following four flood classes: less than 6 months, 6–12 months, 1–2 years, and greater than 2 years since the last flood. Time since flooding data were estimated using field-based and satellite observations.
A linear mixed-effects model was used to model the abundance of A. stenophylla seedlings at a plot during each survey as a function of time since flooding, by using the R package lme4 (ver. 1.1-27.1, see https://github.com/lme4/lme4/; Bates 2005). The plots were considered the replicates that ranged from 10 to 24 per flood class. The flood class was considered the fixed effect and plot (ID) was considered the random effect to account for the non-independence of the data owing to the plots being repeatedly sampled. As the number of seedlings at a plot during a survey was not normally distributed and negatively skewed, the count data were represented in the model by using a Poisson distribution.
Glasshouse experiment
We tested the influence of the seed pod, the effect of the experimental treatment and the interaction between these two factors on the number of germinants by using a two-way ANOVA in the R package stats. The data were square-root transformed before analysis. We tested the effect of the seed pod and the treatment type, and the interaction between the seedpod and treatment type and report significance at P ≤ 0.05. A Tukey honest significance difference test was undertaken to determine where these significant differences occurred.
Results
Field-based study
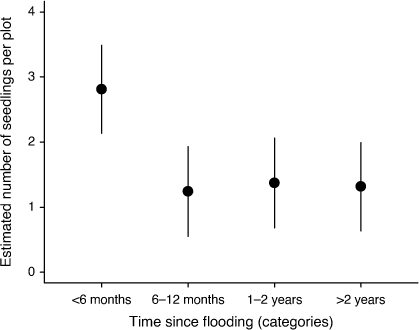
Plots flooded less than 6 months before surveys had a significantly greater number of A. stenophylla seedlings than did those in the other three flood classes, which had a greater time since flooding (as judged by the non-overlapping 95% confidence intervals, Fig. 2, and P < 0.001). This result suggests that the seeds of A. stenophylla germinate and the seedlings establish following flooding events. The number of seedlings in the flood classes 6–12 months, 1–2 years, and greater than 2 years were similar. Many of the seedlings in the flood class less than 6 months may have grown into the next growth size class (i.e. >20 cm) over the next 12 months or died.
|
Glasshouse experiment
Germination
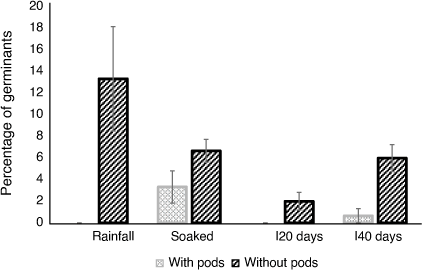
Seeds without pods germinated in all experimental treatments, with the greatest number being observed in the rainfall treatment. The seeds with pods germinated only in the soaked and I40 treatments and at much lower numbers (Fig. 3). There was a significant interaction effect of seed pod and treatment type (F(3, 32) = 3.72, P = 0.02). Seeds without pods had significantly greater numbers of germinants than did seeds with pods (F(2, 38) = 74.45, P < 0.001). The experimental treatment type also had a significant effect on the number of germinants (F(3, 35) = 4.93, P = 0.006). Treatment type Inundated 20 days had significantly fewer seeds germinate than did the Soaked treatment (P = 0.005) and the Rainfall treatment (P = 0.03).
|
Treatment type Rainfall with no pods had a significantly greater number of germinants than did Rainfall with pods (P < 0.001), Inundated 20 days with no pods (P = 0.005), Inundated 20 days with pods (P < 0.001), Inundated 40 days with pods (<0.001), and Soaked with pods (0.02).
Treatment type Soaked without pods had a significantly greater number of germinants than did Rainfall with pods (P = 0.002), Inundated 20 days with pods (P = 0.002) and Inundated 40 days without pods (P = 0.009). Treatment type Inundated 40 days without pods had a significantly greater number of germinants than did treatments Rainfall with pods (P = 0.003), Inundated 20 days with pods (P = 0.003) and Inundated 40 days with pods (P = 0.002).
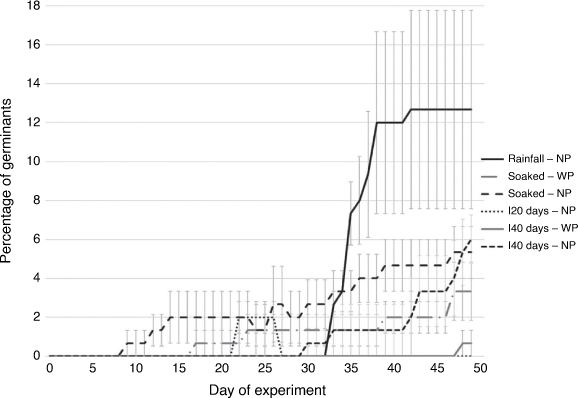
Seeds without pods in the rainfall treatment began germinating on Day 34 (Fig. 4). This was following the 3 days (Days 18, 22 and 26) with the greatest rainfall during the 50-day experiment and the seeds continued to germinate until Day 42. No further rainfall occurred after Day 28 in the rainfall treatments. The seeds in the Soaked treatment without pods began germination on Day 10 and continued to germinate up to Day 47. The seeds without pods in the Inundated 20 days treatment began germinating on Day 23 and germinated until Day 27. A few of the seeds without pods in the Inundated 40 days treatment began germinating on Day 31 while the seeds were still submerged. Seeds in the Inundated 40 days without pod treatment continued to germinate up until Day 50 (Fig. 4). In the treatments with pods, germination started on Day 18 in the Soaked treatment and continued to germinate until Day 47. In the Inundated 40 days treatment with pods, germination occurred on Day 49, 9 days after the water had receded.
|
|
Following the experiment, all seeds in each treatment were soaked for a further 20 days. The seeds with pods in the Rainfall and Inundated 20 days treatments and the seeds without pods in the Inundated 20 days and Inundated 40 days treatments had no further germinants after the 20-day soaking period. The seeds with pods in the Soaked and Inundated 40 days treatment had two and three further germinants respectively. The seeds without pods in the Rainfall and Soaked treatments had 12 seeds and 1 seed germinate respectively, following the 20-day soaking period.
Seed buoyancy
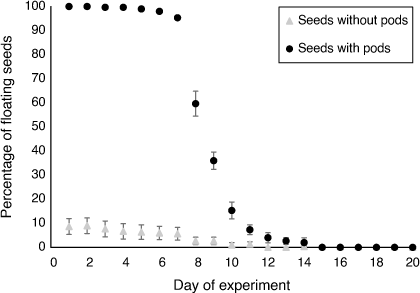
The majority (95%) of A. stenophylla seeds with pods floated for 7 days. By the 8th day of the experiment, the percentage of floating seeds dropped to 56%. The last day that a seed with pods was recorded floating was on Day 14 (Fig. 5). In contrast, the majority of the seeds without pods sunk on the first day of the experiment, with only 8.7% of the seeds still floating on Day 2. All the seeds without pods had sunk by Day 12; however, on Day 14, one seed rose and began floating again until Day 19 (Fig. 5).
Discussion
Seed germination after flood recession is a common trait of riparian vegetation (Catford and Jansson 2014). The results of our study showed that A. stenophylla also possesses this trait. The field-based study presented here demonstrated that A. stenophylla seedlings are more likely to occur directly after a flood event. The glasshouse study is consistent with this result, suggesting that seed germination in A. stenophylla coincides with flood recession and prolonged high soil moisture. This result is also congruent with a study by Maxwell et al. (2016), which found seedling growth in A. stenophylla to be greatest on soaked soil.
Germination rates of A. stenophylla seeds varied between the seeds with and without pods. The best conditions for maximum germination of A. stenophylla seeds with pods was on soaked soil. Germinants from seeds with pods were also recorded in the I40 treatments, although germination percentage was less than 1% (Fig. 3). This is comparable to the results of an unpublished pilot study reported in Roberts and Marston (2011), in which less than 10% of the seeds with pods germinated in moist, aerated soil conditions after 20 weeks. However, no seeds germinated in completely submerged or alternatively submerged and drained soil in the Roberts and Marston (2011) study. In the present study, germination of seeds with pods in the Soaked treatment started on Day 18 and continued until Day 50, whereas germination in the I40 with pods treatment commenced on Day 48 of the experiment, following flood recession. This suggests that the seeds within pods of A. stenophylla require moist conditions for at least 20 days to germinate. The seed pod may require soaking to break down and swell open, allowing water to enter around the seed. Bonney and Miles (1994) reported that the seeds of A. stenophylla are difficult to remove from their pods and seeds are usually removed manually or require soaking in water for several days.
Seeds without pods germinated in all treatments (except the control), demonstrating that seeds of A. stenophylla without pods can germinate on soaked soil, following large rainfall events, and during or following inundation. The Rainfall treatment had the greatest number of germinants for seeds without pods (13.3%). This indicates that once the pod has been removed or opened, A. stenophylla is able to germinate under local rainfall conditions.
The seeds in the Rainfall treatment were periodically subject to rainfall conditions, with the containers drying between rainfall events. Germination occurred on Day 34 in the Rainfall without pods treatment. This was following the three largest rainfall events of the experiment, which occurred on Days 18, 22 and 26. It is likely that these three rainfall events maintained high soil moisture for at least 16 days. The seed of most Acacia species has a thick impermeable seed-coat (Murray 1986) and soaking of seeds has previously shown to increase germination rates in other Acacia species (Milton and Hall 1981). Acacia nilotica seeds, for example, exhibit dormancy until they are immersed in water for a prolonged time (Warrag and Eltigani 2005).
Seed dormancy is commonly induced by the impermeability of the seed (Barton 1965). Unfavourable environmental conditions such as the lack of moisture availability, can lead to seeds delaying germination for preferential conditions (Sweedman and Merritt 2006). This is a common trait in many Australian plants, which inhibits germination until substantial rain has fallen (Murray 1986). The results of this study indicated that seed germination is delayed in A. stenophylla until large or successive rainfall events occur, or during and following a flood event, and suggest that A. stenophylla may have a persistent soil seedbank. Such persistent soil seedbanks appear to be an Acacia genus trait (Richardson and Kluge 2008; Gibson et al. 2011). Some seeds with and without pods resisted germination during the 50-day experimental period and germinated during the 20 days on soaked soil following the experiment. This suggests that seeds of A. stenophylla germinate with continued contact with moisture beyond the 50-day experimental period reported here.
Germination rates for A. stenophylla without pods were higher in the I40 treatment than in the I20 treatment, showing that extended flooding improves germination rates. Inundation has also been found to improve germination rates of other riverine Acacia species such as Acacia salicina and Acacia parvipinnula (Stone et al. 2020). Two germinants from seeds without pods were present while fully submerged in the water in the I40 treatment from Day 31, and the number of germinants increased following recession, demonstrating that A. stenophylla seeds are able to germinate under water to a depth of 50 mm and after flood events. This strategy ensures high and prolonged soil moisture conditions, increasing seedling survival and growth (Maxwell et al. 2016). A. stenophylla seedlings have been shown to have a significant tolerance to flooding and can survive up to 90 days under submergence (Maxwell et al. 2016). The present study demonstrated that this tolerance to flooding and anoxic soil conditions is also present during seed germination and seedling establishment.
The majority of A. stenophylla seeds with pods floated for 9 days, with some seeds floating for up to 14 days. Once seed pods begin absorbing water, they become saturated, causing a reduction in buoyancy, eventually causing them to sink (Nilsson et al. 2010). Most seeds without pods sank on the first day of the experiment, suggesting that once the seed pod has cracked open and the seed is released, the seed is unlikely to disperse long distances by hydrochory. The longer seeds float, the further the seed may disperse before germination (Boedeltje et al. 2004). Seeds without pods also germinated quicker and in higher numbers than do the seeds with pods. The results indicated that seed pods of A. stenophylla increase seed floating time and inhibit germination. A study by Capon and Brock (2006) showed that during major flood events, seeds from species of the Fabaceae family had a significant decline in the germination rate; however, after flood water had receded, the seed germination rates of these species returned to normal. The results from the present field study support this, with the number of seedlings being much greater at sites more recently flooded.
A study by Higgisson et al. (2020) observed high levels of gene flow among populations of A. stenophylla in the Lachlan River Catchment and concluded that hydrochory appears to be an important dispersal mechanism. The results of the present study demonstrated that seed pods of A. stenophylla can float for up to 14 days. In addition, an oily film was observed on the water surface in the inundated treatments for the seeds with pods, while seeds were floating. This oily film was persistent for most of the flooding duration period. Seeds of other species that are contained within a pod and known to use water as a dispersal mechanism, will often release oils from their pod (Howe and Smallwood 1982). These observations and results indicated that A. stenophylla pods disperse by nautochory, i.e. the dispersal by floating on the surface of water currents (Nilsson et al. 2010). This dispersal method is likely to connect habitat patches along rivers and on floodplains (Nilsson et al. 1991).
Parrots and galahs are reported to eat the seeds of A. stenophylla from the pod (Bonney and Miles 1994). Acacia seeds that have been ingested have been found to germinate faster than do undigested seeds (Milton and Hall 1981; Barnes 2001). However, the dispersal of A. stenophylla by birds is unlikely, because the seed is large (Doran and Turnbull 1997) and is not fleshy or arillate (Khan and Sahito 2017), which is a typical requirement for vertebrate dispersal (Fenner 2000).
Conclusions
Water resource development has altered the natural flow regime of most of the major rivers globally (Grill et al. 2019). This has changed the frequency and duration of flood events (Nilsson and Berggren 2000). Because rainfall is patchy in arid and semi-arid parts of Australia, floodwater may be the only water plants receive during droughts (Brock et al. 2006).
Our research has provided insight into the water requirements of a key life-history stage of an important floodplain species. In doing so, it contributes to our broader understanding of the reproductive biology of one of the less studied Acacia species. There are thought to be two main dispersal syndromes for Acacia seeds, birds and ants (O’Dowd and Gill 1986), with germination being typically fire-, heat- or disturbance-triggered (Gibson et al. 2011). However, these generalisations are based on a very limited proportion of the more than 1350 Acacia species for which there is understanding. The results of this study suggest that like many riverine and floodplain species (Capon 2007), A. stenophylla disperses during flooding events and seeds germinate and seedlings establish following flood events, suggesting soaking as the germination trigger for this Acacia species. Roberts and Marston (2011) reported that A. stenophylla requires a flood event for a duration of 3 months every 3 years for population maintenance. The results of the present study indicated that A. stenophylla requires a flood event for a duration of approximately 1 month to provide appropriate conditions for seed dispersal and germination.
Data availability
The data used as part of this study may be made available on reasonable request.
Conflicts of interest
Fiona Dyer is an Associate Editor of Marine and Freshwater Research but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This research was partially funded by the Commonwealth Environmental Water Office (CEWO).
Acknowledgements
The authors thank the NSW National Parks and Wildlife Service, Department of Planning, Industry and Environment, for granting permission to collect the seed and soil samples for the present study. The authors thank Tom Ross, Liam Allan, Jack and Chris Reynolds and Alica Tschierschke for their help at different stages of the project. The authors also thank the reviewers for providing thorough and constructive feedback during the review process.
References
Ahmad, R, Tehsin, Z, Malik, ST, Asad, SA, Shahzad, M, Bilal, M, Shah, MM, and Khan, SA (2016). Phytoremediation potential of hemp (Cannabis sativa L.): identification and characterization of heavy metals responsive genes. Clean 44, 195–201.| Phytoremediation potential of hemp (Cannabis sativa L.): identification and characterization of heavy metals responsive genes.Crossref | GoogleScholarGoogle Scholar |
Arthington, AH, and Balcombe, SR (2011). Extreme flow variability and the ‘boom and bust’ ecology of fish in arid‐zone floodplain rivers: a case history with implications for environmental flows, conservation and management. Ecohydrology 4, 708–720.
| Extreme flow variability and the ‘boom and bust’ ecology of fish in arid‐zone floodplain rivers: a case history with implications for environmental flows, conservation and management.Crossref | GoogleScholarGoogle Scholar |
Australia’s Virtual Herbarium (2020) Acacia stenophylla A.Cunn. ex Benth. In ‘New South Wales Flora Online’. (Council of Heads of Australasian Herbaria). Available at https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Acacia∼stenophylla [Verified 1 May 2020]
Balcombe, SR, Biggs, A, Prior, A, and Capon, SJ (2021). Seedling root growth experiments for two Australian dryland riparian eucalypts provide new insights for environmental watering. River Research and Applications 37, 1463–1470.
| Seedling root growth experiments for two Australian dryland riparian eucalypts provide new insights for environmental watering.Crossref | GoogleScholarGoogle Scholar |
Baldwin, DS, Rees, GN, Wilson, JS, Colloff, MJ, Whitworth, KL, Pitman, TL, and Wallace, TA (2013). Provisioning of bioavailable carbon between the wet and dry phases in a semi-arid floodplain. Oecologia 172, 539–550.
| Provisioning of bioavailable carbon between the wet and dry phases in a semi-arid floodplain.Crossref | GoogleScholarGoogle Scholar | 23124331PubMed |
Barnes, ME (2001). Seed predation, germination and seedling establishment of Acacia erioloba in northern Botswana. Journal of Arid Environments 49, 541–554.
| Seed predation, germination and seedling establishment of Acacia erioloba in northern Botswana.Crossref | GoogleScholarGoogle Scholar |
Barton, LV (1965). Dormancy in seeds imposed by the seed coat. Differenzierung und Entwicklung 15, 2374–2392.
| Dormancy in seeds imposed by the seed coat.Crossref | GoogleScholarGoogle Scholar |
Bates, D (2005). Fitting linear mixed models in R. R News 5, 27–30.
Bendix, J, and Hupp, CR (2000). Hydrological and geomorphological impacts on riparian plant communities. Hydrological Processes 14, 2977–2990.
| Hydrological and geomorphological impacts on riparian plant communities.Crossref | GoogleScholarGoogle Scholar |
Bennett, AF, Nimmo, DG, and Radford, JQ (2014). Riparian vegetation has disproportionate benefits for landscape‐scale conservation of woodland birds in highly modified environments. Journal of Applied Ecology 51, 514–523.
| Riparian vegetation has disproportionate benefits for landscape‐scale conservation of woodland birds in highly modified environments.Crossref | GoogleScholarGoogle Scholar |
Boedeltje, G, Bakker, JP, Ten Brinke, A, Van Groenendael, JM, and Soesbergen, M (2004). Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported. Journal of Ecology 92, 786–796.
| Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: the flood pulse concept supported.Crossref | GoogleScholarGoogle Scholar |
Bonney N, Miles A (1994) ‘What seed is that? A field guide to the identification, collection and germination of native seed in South Australia.’ (N. Bonney: Adelaide, SA, Australia)
Brock MA, Capon SJ, Porter JL (2006) Disturbance of plant communities dependent on desert rivers. In ‘Ecology of desert rivers’. (Ed. R Kingsford) pp. 100–132. (Cambridge University Press: New York, NY, USA)
Brown, J, Enright, N, and Miller, B (2003). Seed production and germination in two rare and three common co‐occurring Acacia species from south‐east Australia. Austral Ecology 28, 271–280.
| Seed production and germination in two rare and three common co‐occurring Acacia species from south‐east Australia.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2016) Daily rainfall (millimetres): HILLSTON AIRPORT. In ‘Climate Data Online’. Available at http://www.bom.gov.au/tmp/cdio/IDCJAC0009_075032_2016.pdf [Verified 7 February 2022]
Capon, SJ (2007). Effects of flooding on seedling emergence from the soil seed bank of a large desert floodplain. Wetlands 27, 904–914.
| Effects of flooding on seedling emergence from the soil seed bank of a large desert floodplain.Crossref | GoogleScholarGoogle Scholar |
Capon, SJ, and Brock, MA (2006). Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshwater Biology 51, 206–223.
| Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain.Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2015) Review of water requirements for key floodplain vegetation for the northern basin: literature review and expert knowledge assessment. Report to the Murray‒Darling Basin Authority, Charophyte Services, Lake Bolac, Vic., Australia.
Catford, JA, and Jansson, R (2014). Drowned, buried and carried away: effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytologist 204, 19–36.
| Drowned, buried and carried away: effects of plant traits on the distribution of native and alien species in riparian ecosystems.Crossref | GoogleScholarGoogle Scholar |
Clemens, J, Jones, P, and Gilbert, N (1977). Effect of seed treatments on germination in Acacia. Australian Journal of Botany 25, 269–276.
| Effect of seed treatments on germination in Acacia.Crossref | GoogleScholarGoogle Scholar |
Colloff, MJ, and Baldwin, DS (2010). Resilience of floodplain ecosystems in a semi-arid environment. The Rangeland Journal 32, 305–314.
| Resilience of floodplain ecosystems in a semi-arid environment.Crossref | GoogleScholarGoogle Scholar |
Cunningham G, Mulham W, Milthorpe P, Leigh J (1981) ‘Plants of western New South Wales.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Doody T, Overton I (2009) Environmental management of riparian tree health in the Murray–Darling Basin, Australia. In ‘River Basin Management, Vol. 124’. (Ed. CA Brebbia) p. 197. (WIT Press: Southampton, UK)
Doody, TM, Benger, SN, Pritchard, JL, and Overton, IC (2014). Ecological response of Eucalyptus camaldulensis (river red gum) to extended drought and flooding along the River Murray, South Australia (1997–2011) and implications for environmental flow management. Marine and Freshwater Research 65, 1082–1093.
| Ecological response of Eucalyptus camaldulensis (river red gum) to extended drought and flooding along the River Murray, South Australia (1997–2011) and implications for environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Doran J, Turnbull J (1997 ) ‘Australian trees and shrubs: species for land rehabilitation and farm planting.’ (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
Dyer F, Broadhurst B, Tschierschke A, Higgisson W, Allen H, Thompson R, Driver P, Bowen S, Lenehan J, Thiem J, Wright D (2020) Commonwealth environmental water office long term intervention monitoring project: Lower Lachlan River system 2018–19 technical report: final. Centre for Applied Water Science, Canberra, ACT, Australia.
Fenner M (2000) ‘Seeds the ecology of regeneration in plant communities’, 2nd edn. (CABI Publishing: Wallingford, UK)
George AK (2004) Eucalypt regeneration on the Lower Murray floodplain, South Australia. PhD thesis, School of Earth and Environmental Sciences, University of Adelaide, Adelaide, SA, Australia.
Gibson, MR, Richardson, DM, Marchante, E, Marchante, H, Rodger, JG, Stone, GN, Byrne, M, Fuentes‐Ramírez, A, George, N, and Harris, C (2011). Reproductive biology of Australian acacias: important mediator of invasiveness? Diversity & Distributions 17, 911–933.
| Reproductive biology of Australian acacias: important mediator of invasiveness?Crossref | GoogleScholarGoogle Scholar |
Good, MK, Clarke, PJ, Price, JN, and Reid, N (2014). Seasonality and facilitation drive tree establishment in a semi-arid floodplain savanna. Oecologia 175, 261–271.
| Seasonality and facilitation drive tree establishment in a semi-arid floodplain savanna.Crossref | GoogleScholarGoogle Scholar | 24469341PubMed |
Grill, G, Lehner, B, Thieme, M, Geenen, B, Tickner, D, Antonelli, F, Babu, S, Borrelli, P, Cheng, L, and Crochetiere, H (2019). Mapping the world’s free-flowing rivers. Nature 569, 215–221.
| Mapping the world’s free-flowing rivers.Crossref | GoogleScholarGoogle Scholar | 31068722PubMed |
Hall N (1972) ‘The use of trees and shrubs in the dry country of Australia.’ (Australian Government Publishing Service: Canberra, ACT, Australia)
Hamilton, BT, Roeder, BL, Hatch, KA, Eggett, DL, and Tingey, D (2015). Why is small mammal diversity higher in riparian areas than in uplands? Journal of Arid Environments 119, 41–50.
| Why is small mammal diversity higher in riparian areas than in uplands?Crossref | GoogleScholarGoogle Scholar |
Higgisson, W, Briggs, S, and Dyer, F (2018). Seed germination of tangled lignum (Duma florulenta) and nitre goosefoot (Chenopodium nitrariaceum) under experimental hydrological regimes. Marine and Freshwater Research 69, 1268–1278.
| Seed germination of tangled lignum (Duma florulenta) and nitre goosefoot (Chenopodium nitrariaceum) under experimental hydrological regimes.Crossref | GoogleScholarGoogle Scholar |
Higgisson, W, Gleeson, D, Broadhurst, L, and Dyer, F (2020). Genetic diversity and gene flow patterns in two riverine plant species with contrasting life-history traits and distributions across a large inland floodplain. Australian Journal of Botany 68, 384–401.
| Genetic diversity and gene flow patterns in two riverine plant species with contrasting life-history traits and distributions across a large inland floodplain.Crossref | GoogleScholarGoogle Scholar |
Howe, HF, and Smallwood, J (1982). Ecology of seed dispersal. Annual Review of Ecology and Systematics 13, 201–228.
| Ecology of seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Kalma JD, Franks SW (2003) Rainfall in arid and semi-arid regions. In ‘Understanding water in a dry environment’. (Ed. I Simmers) pp. 31–80. (CRC Press)
Khan, D, and Sahito, ZA (2017). Some observations on the phyllodes of the shoe string acacia (Acacia stenopylla A. Cunn. Ex. Benth.) growing in Karachi, Pakistan. FUUAST Journal of Biology 7, 131–137.
Kulkarni, M, Sparg, S, and Van Staden, J (2007). Germination and post-germination response of Acacia seeds to smoke-water and butenolide, a smoke-derived compound. Journal of Arid Environments 69, 177–187.
| Germination and post-germination response of Acacia seeds to smoke-water and butenolide, a smoke-derived compound.Crossref | GoogleScholarGoogle Scholar |
Lytle, DA, and Poff, NL (2004). Adaptation to natural flow regimes. Trends in Ecology & Evolution 19, 94–100.
| Adaptation to natural flow regimes.Crossref | GoogleScholarGoogle Scholar |
Marcar N, Crawford D, Leppert P, Jovanovic T, Floyd R, Farrow R (1995) ‘Trees for saltland: a guide to selecting native species for Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Maslin R, McDonald MW (2004) ‘Acaciasearch: Evaluation of acacia as a woody crop option for Southern Australia.’ (Rural Industries Research and Development Corporation: Canberra, ACT, Australia)
Maslin, B, Miller, J, and Seigler, D (2003). Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Australian Systematic Botany 16, 1–18.
| Overview of the generic status of Acacia (Leguminosae: Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Maxwell, A, Capon, SJ, and James, CS (2016). Effects of flooding on seedling establishment in two Australian riparian trees with contrasting distributions; Acacia stenophylla A. Cunn. ex Benth. and Casuarina cunninghamiana Miq. Ecohydrology 9, 942–949.
| Effects of flooding on seedling establishment in two Australian riparian trees with contrasting distributions; Acacia stenophylla A. Cunn. ex Benth. and Casuarina cunninghamiana Miq.Crossref | GoogleScholarGoogle Scholar |
Milton, S, and Hall, A (1981). Reproductive biology of Australian Acacias in the south-western Cape Province, South Africa. Transactions of the Royal Society of South Africa 44, 465–487.
| Reproductive biology of Australian Acacias in the south-western Cape Province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Murray DR (1986) Seed dispersal. In ‘Seed dispersal’. (Ed. DR Murray) pp. 49–85. (Academic Press)
Nilsson, C, and Berggren, K (2000). Alterations of riparian ecosystems caused by river regulation: dam operations have caused global-scale ecological changes in riparian ecosystems. How to protect river environments and human needs of rivers remains one of the most important questions of our time. Bioscience 50, 783–792.
| Alterations of riparian ecosystems caused by river regulation: dam operations have caused global-scale ecological changes in riparian ecosystems. How to protect river environments and human needs of rivers remains one of the most important questions of our time.Crossref | GoogleScholarGoogle Scholar |
Nilsson, C, Gardfjell, M, and Grelsson, G (1991). Importance of hydrochory in structuring plant communities along rivers. Canadian Journal of Botany 69, 2631–2633.
| Importance of hydrochory in structuring plant communities along rivers.Crossref | GoogleScholarGoogle Scholar |
Nilsson, C, Brown, RL, Jansson, R, and Merritt, DM (2010). The role of hydrochory in structuring riparian and wetland vegetation. Biological Reviews of the Cambridge Philosophical Society 85, 837–858.
| The role of hydrochory in structuring riparian and wetland vegetation.Crossref | GoogleScholarGoogle Scholar | 20233190PubMed |
O’Dowd DJ, Gill AM (1986) Seed dispersal syndromes in Australian Acacia. In ‘Seed dispersal’. (Ed. D Murray) pp. 87–121. (Academic Press: Sydney, NSW, Australia)
Richardson, DM, and Kluge, RL (2008). Seed banks of invasive Australian Acacia species in South Africa: role in invasiveness and options for management. Perspectives in Plant Ecology, Evolution and Systematics 10, 161–177.
| Seed banks of invasive Australian Acacia species in South Africa: role in invasiveness and options for management.Crossref | GoogleScholarGoogle Scholar |
Roberts J, Marston F (2011) ‘Water regime for wetland and floodplain plants: a source book for the Murray–Darling Basin.’ (National Water Commission: Canberra, ACT, Australia)
Roshier, D, Robertson, A, and Kingsford, R (2002). Responses of waterbirds to flooding in an arid region of Australia and implications for conservation. Biological Conservation 106, 399–411.
| Responses of waterbirds to flooding in an arid region of Australia and implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Sheldon, F, Boulton, AJ, and Puckridge, JT (2002). Conservation value of variable connectivity: aquatic invertebrate assemblages of channel and floodplain habitats of a central Australian arid-zone river, Cooper Creek. Biological Conservation 103, 13–31.
| Conservation value of variable connectivity: aquatic invertebrate assemblages of channel and floodplain habitats of a central Australian arid-zone river, Cooper Creek.Crossref | GoogleScholarGoogle Scholar |
Stone, L, Fryirs, K, and Leishman, M (2020). Simulating the effect of environmental flow duration on seedling emergence from riparian seed banks of the upper Hunter River, New South Wales. River Research and Applications 36, 607–619.
| Simulating the effect of environmental flow duration on seedling emergence from riparian seed banks of the upper Hunter River, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Stromberg, JC, Beauchamp, V, Dixon, M, Lite, S, and Paradzick, C (2007). Importance of low‐flow and high‐flow characteristics to restoration of riparian vegetation along rivers in arid south‐western united states. Freshwater Biology 52, 651–679.
| Importance of low‐flow and high‐flow characteristics to restoration of riparian vegetation along rivers in arid south‐western united states.Crossref | GoogleScholarGoogle Scholar |
Sweedman L, Merritt D (2006) ‘Australian seeds: a guide to their collection, identification and biology.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Thomson L (1987) Australian acacias for saline, alkaline soils in the hot, dry subtropics and tropics. In ‘Australian Acacias in Developing Countries’. pp. 66–69. (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
Turnbull JW (1986) ‘Multipurpose Australian trees and shrubs. Lesser-known species for fuelwood and agroforestry.’ (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
Walters, M, Midgley, JJ, and Somers, MJ (2004). Effects of fire and fire intensity on the germination and establishment of Acacia karroo, Acacia nilotica, Acacia luederitzii and Dichrostachys cinerea in the field. BMC Ecology 4, 3.
| Effects of fire and fire intensity on the germination and establishment of Acacia karroo, Acacia nilotica, Acacia luederitzii and Dichrostachys cinerea in the field.Crossref | GoogleScholarGoogle Scholar | 15068486PubMed |
Warrag, EI, and Eltigani, MA (2005). Breaking seed coat dormancy of Acacia nilotica seeds under simulated natural habitat conditions in Sudan. Tropical Ecology 46, 127–132.
Wittenberg, H (2003). Effects of season and man‐made changes on baseflow and flow recession: case studies. Hydrological Processes 17, 2113–2123.
| Effects of season and man‐made changes on baseflow and flow recession: case studies.Crossref | GoogleScholarGoogle Scholar |