Review of options for creating and maintaining oxygen refuges for fish during destratification-driven hypoxia in rivers
Craig A. Boys A B G , Darren S. Baldwin C D , Iain Ellis
A B G , Darren S. Baldwin C D , Iain Ellis  E , Joe Pera
E , Joe Pera  F and Katherine Cheshire A
F and Katherine Cheshire A
A New South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Taylors Beach Road, Taylors Beach, NSW 2316, Australia.
B Institute for Land Water and Society, Charles Sturt University, Elizabeth Mitchell Drive, Thurgoona, NSW 2640, Australia.
C School of Environmental Science, Charles Sturt University, Elizabeth Mitchell Drive, Thurgoona, NSW 2640, Australia.
D Rivers and Wetlands, Lipsett Road, Thurgoona, NSW 2640, Australia.
E New South Wales Department of Primary Industries, 32 Enterprise Way, Buronga, NSW 2379, Australia.
F WaterNSW, 169 Macquarie Street, Parramatta, NSW 2150, Australia.
G Corresponding author. Email: craig.boys@dpi.nsw.gov.au
Marine and Freshwater Research 73(2) 200-210 https://doi.org/10.1071/MF20364
Submitted: 15 December 2020 Accepted: 1 June 2021 Published: 13 September 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Climate change, river regulation and water extraction create the conditions where destratification-driven hypoxia will become more common in rivers. Preventing this and the fish deaths that can result requires options that prevent stratification and create oxygen refuges for fish. Here we discuss aeration and mixing approaches that may help prevent fish deaths when flow-related measures are not available. The options were evaluated based on efficacy, environmental risk and readiness to be deployed cost-effectively. The options either promote mixing, aeration or both. Bubble diffusers and paddle wheels used commonly in aquaculture are unlikely to aerate already hypoxic pools. However, if deployed before stratification occurs, they may promote mixing and maintain aeration. In comparison, pumps with Venturi tubes or ultrafine oxygen bubble condensers both mix and aerate, making them suitable for use once hypoxic events are underway. Water jets are low cost and could be deployed quickly. Dosing reaches with calcium peroxide may be useful for emergency aeration, but requires further safety and efficacy testing. Flow management that maintains fish refuges and storage reserves during drought is the best way to guard against fish deaths, but if storage releases are not available, there are options for creating and maintaining oxygen refuges to minimise ecosystem damage.
Keywords: aeration, bubble diffusers, fish deaths, paddle wheels, stratification, ultrafine bubble condensers.
Introduction
Dissolved oxygen (DO) is one of the most important water quality parameters affecting the health of aquatic life (Wu 2002). Oxygen enters water by direct absorption from the atmosphere or by photosynthesis. Conversely, it is removed by respiration by organisms (including algae and bacteria) and the decomposition of organic matter. Fish absorb oxygen from the water into their bloodstream across their gills. Generally, a concentration of 5 mg L–1 DO is required for optimal health in high-density pond culture (Boyd and Hanson 2010). This translates to nature and, although some fish species can tolerate low DO better than others, and smaller fish typically tolerate it better than larger fish (McNeil and Closs 2007; Gilmore et al. 2018), most fish will become distressed once DO falls below 4 mg L–1 and will die at concentrations <2 mg L–1 (Gehrke 1988; Small et al. 2014). Even chronic and repeated exposure to non-lethal DO levels can make fish more susceptible to eventual disease and death (La and Cooke 2011). For example, daily reductions of DO to concentrations of 2–3 mg L–1 can inhibit many of the nitrifying bacteria that breakdown ammonia, which is toxic to fish (Sinha and Annachhatre 2007). Because DO levels can vary vertically and horizontally throughout a waterbody, fish can actively avoid habitats of low DO if suitable refuges of higher DO are available (Hasler et al. 2009).
Fluvial waterways can become hypoxic (low DO) or even anoxic (absence of DO) for a few reasons. One cause can be blackwater events that occur when organic matter is washed into floodplain rivers (Howitt et al. 2007). The source of carbon may be from a burnt catchment (Dahm et al. 2015) or accumulated plant material that is mobilised into rivers following an extended dry period (Hladyz et al. 2011). The high concentration of dissolved organic carbon (DOC) is a food source for bacteria, which can, in turn, fuel productivity at higher tropic levels (Mitrovic et al. 2014). However, particularly at higher temperatures, respiration of the DOC by bacteria can consume DO faster than it can be replenished, resulting in hypoxic blackwater (Kerr et al. 2013). Hypoxic blackwater was the cause of fish deaths in the Murray River (Australia) and its tributaries in 2010 and 2016, the former event lasting for close to 6 months and affecting 2000 km of river channel (Whitworth et al. 2012). Another factor that can lead to hypoxia in waterways involves algal blooms. During a large algal bloom, the DO concentration can change from supersaturation to hypoxic within a 24-h period as algae shift from photosynthesis during the day to respiration at night (see McDonnell and Kountz 1966).
In the summer of 2018–19, hypoxia was found to be the cause of successive large-scale fish deaths in the Lower Darling River (LDR) in the Murray–Darling Basin (MDB) Australia (New South Wales Department of Primary Industries 2019; Vertessy et al. 2019; Sheldon et al. 2021). However, in this instance the cause of the hypoxia was not related specifically to blackwater or algae. Instead, the fish deaths were triggered by destratification of the thermally stratified water column, which, in turn, mixed hypoxic (or anoxic) water in the deeper hypolimnion with surface water (Fig. 1).
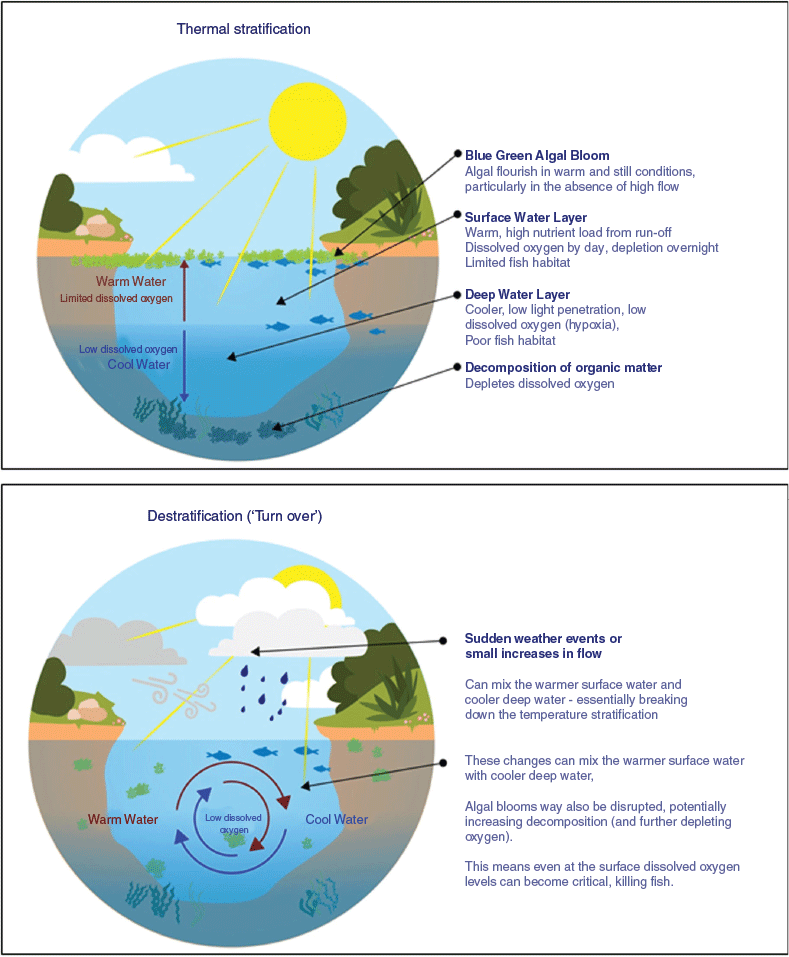
|
The proximate conditions leading to the event included extended drought and low tributary inflows, combined with river regulation and water extraction pressure, which culminated in there being little water left in the river channel and minimal reserves remaining in storage (Vertessy et al. 2019). During extended periods of zero flows and extreme summer day and night temperatures, the water column became thermally stratified (New South Wales Department of Primary Industries 2019). Although DO in the surface layer is replenished by atmospheric diffusion or photosynthetic organisms, in the stratified water column oxygenated surface water cannot mix with the colder, denser water trapped in the hypolimnion. Microbes in the sediments consume oxygen, making the hypolimnion hypoxic or even anoxic. Sediment oxygen demand can account for more than 50% of oxygen consumption in aquatic ecosystems and can be a major cause of DO deficit (Matlock et al. 2003; MacPherson et al. 2007). If the water column remains thermally stratified, fish can survive by inhabiting the oxygenated surface layer. However, if the thermocline breaks down (as it did on multiple occasions during the LDR events; Baldwin 2019; Sheldon et al. 2021), the hypoxic hypolimnion becomes mixed throughout the water column. The final DO of the water column following mixing is based on oxygen mass balance; that is, the relative difference in DO concentrations and volumes in the surface water compared with the hypolimnion. Because the thermocline in the Darling River is typically weak (Mitrovic et al. 2011), all that was required to cause destratification and hypoxia throughout the entire water column in the LDR in 2018–19 was a drop in surface water temperature following a rapid decrease in air temperature, with associated rainfall and wind (New South Wales Department of Primary Industries 2019; Sheldon et al. 2021). What resulted was the widespread and catastrophic death of millions of native fish, both large and small, predominately bony herring, Murray cod Maccullochella peelii, golden perch Macquaria ambigua and silver perch Bidyanus bidyanus (Sheldon et al. 2021).
Australia is the second driest continent on Earth, and the rivers of the MDB are heavily regulated and water extraction can typically be in excess of 70% of surface run-off (Thoms and Sheldon 2000). In the summer of 2018–19, the country was in the grip of one of the worst droughts in recent history (Dey et al. 2019). The LDR was typical of many river systems in the northern MDB that had either dried up completely or become a series of shallow disconnected pools. The LDR was under a high algal alert and remaining refuge pools had started to become hypoxic overnight (in the absence of photosynthesis). The first fish deaths in the LDR occurred in December 2018 and, with a long, hot summer predicted with zero rainfall, river managers needed a way of reducing the risk of further fish deaths in the remaining refuge pools. Most town weir pools and upstream impoundments in the northern MDB were reduced to critical levels, with many towns having to truck in water for town supply. Therefore, delivering an emergency environmental flow to the LDR to dilute hypoxic conditions was not an option. Other options were needed that could create oxygen refuges for fish until summer passed and temperatures subsided in autumn. This was relatively uncharted territory for current fisheries and river managers in the MDB because, although they had recent experience in managing hypoxic events relating to blackwater (Kerr et al. 2013), there were no documented cases where destratification-driven hypoxia had been successfully managed.
Climate change, river regulation and high levels of water extraction will mean that many parts of the world, including south-eastern Australia, will experience far less rainfall, more extreme summer temperatures and extended periods of low and zero river flow (Hughes 2003; Dey et al. 2019). This increases the likelihood that destratification-driven hypoxic events, as seen in the LDR, will become more common. Managing these events will require river managers to be better prepared to prevent stratification establishing that can subsequently lead to destratification-driven hypoxia and have options available with which to reoxygenate already hypoxic reaches.
Here we discuss several chemical and mechanical options that could assist in the emergency management or the prevention of destratification-driven hypoxic fish deaths. The feasibility of the different options is discussed using the available literature from both nature and intensive aquaculture. Because the review was undertaken in response to the LDR fish deaths, in parts we make specific mention of the feasibility of options within that specific context. This provides illustrative examples, but the recommendations and information provided could be equally applied to other rivers in other parts of the world. It is hoped that through this discussion we can learn from recent events so that fisheries and river managers may respond quickly to future incidences where there is a high likelihood of destratification-driven hypoxic fish deaths. Where we have identified that uncertainty remains about the suitability of some options, the opportunity should be taken to address these knowledge gaps.
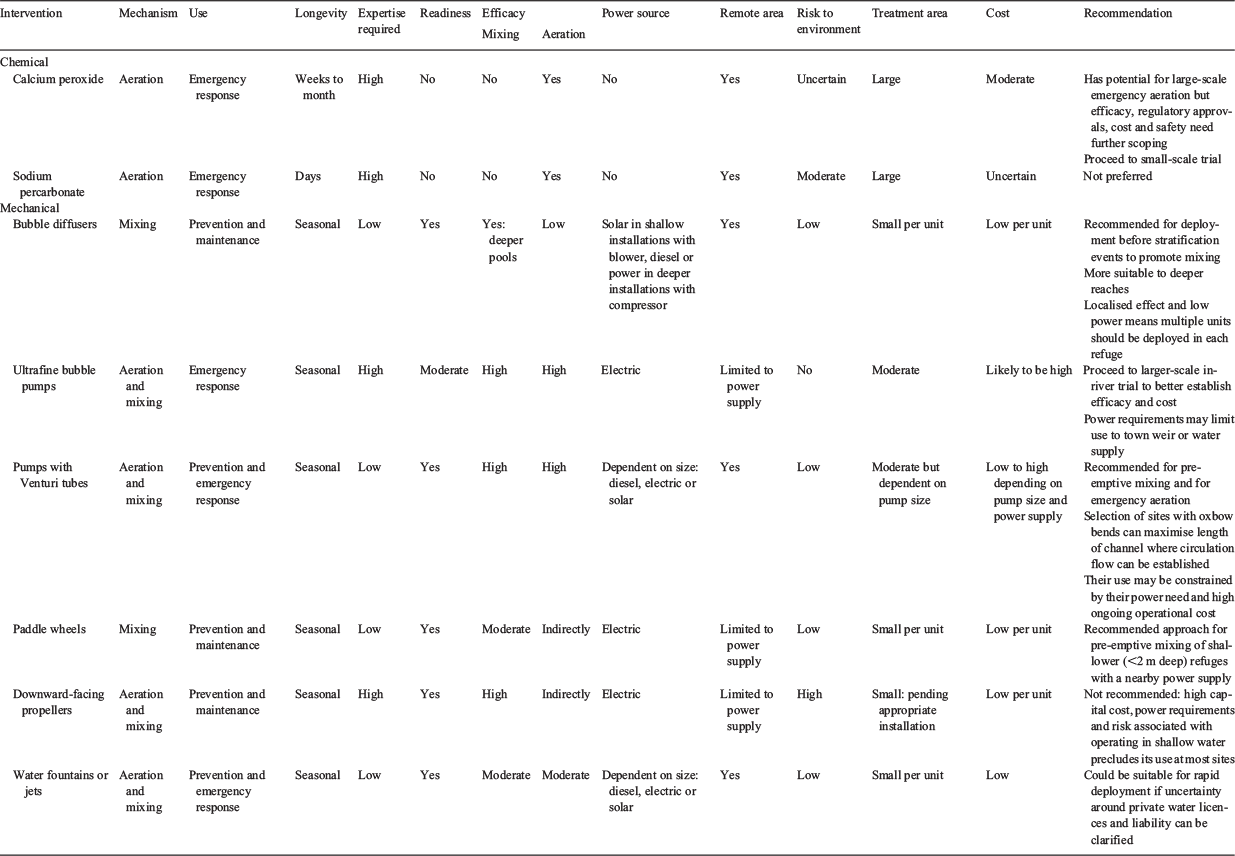
The options considered included two chemical treatments, namely calcium peroxide and sodium percarbonate, and various mechanical mixing and aeration techniques, such as bubble diffusers, ultrafine oxygen bubble, Venturi pumps, paddle wheels, downward-facing propellers and water fountains. Consideration was given to their readiness, efficacy, risk of doing more environmental harm, ability to be deployed in remote areas (with limited electrical power), robustness under extreme environmental conditions (heat, dust, vermin and vandalism), cost-effectiveness, whether technical expertise was required, the size of the area that could be treated and the length of time re-oxygenation would last.
When to mix and when to aerate?
First, before considering the available approaches, it is important to distinguish between the objective of mixing water layers to promote secondary aeration versus direct aeration. When flow in a river has ceased for an extended period, often the best approach is to ‘restart the flow’ by managed releases from upstream storages in order to promote thermal mixing. However, this may not always be possible. For example, in the example of the LDR fish deaths of 2018–19, there was insufficient water held in river storage to allow for this. If managed flow releases are not an option, the next best mitigating measure can often be pre-emptive in nature, involving mechanical mixing of water to avoid stratification in the first place. The mixing must be applied early and maintained throughout the summer to avoid restratification and the associated risk of hypoxia. Pre-emptive mixing can be targeted at refuges deemed valuable and at most risk of hypoxic fish deaths. Pre-emptive mixing will ensure that oxygen absorbed from the atmosphere is sufficiently dispersed throughout the water column. Once the river has already become thermally stratified and hypoxic in the hypolimnion, then rapid destratification can create a risk of ‘hypoxic turnover’, as occurred at the time of the LDR fish deaths (New South Wales Department of Primary Industries 2019). Therefore, care must be taken not to ‘turn over’ the entire refuge pool upon the initiation of mixing efforts. Fish are then better able to avoid the area that is being mixed until DO levels rise again. Once the water is destratified, manual mixing must be maintained continuously to ensure stratification does not reoccur.
In pools that have already become thermally stratified for extended periods, it may be more efficient and safer to use aeration devices to boost DO levels and create ‘pockets’ of suitable water where fish could congregate within the otherwise hypoxic pool. The effectiveness of aeration can be dependent on the time of day. During the day the surface water is likely to be supersaturated with DO by photosynthesising algae. Under this situation, surface aerators (such as paddle wheels) can actually reduce DO because there is a net loss of oxygen to the atmosphere (Moore and Whitis 1999). Instead, surface aeration can be more beneficial after sunset, once surface oxygen has been consumed by respiring algae, and continued until late morning.
With these considerations in mind, several different chemical and mechanical options for mixing and aerating were compared, with the main findings summarised in Table 1 and discussed below.
Chemical aeration options
Calcium peroxide
Calcium peroxide (CaO2) is a sparingly soluble calcium salt that breaks down slowly (potentially over months) to produce oxygen through the decomposition of peroxide (Hanh et al. 2005). It has been effectively used in aquaculture to maintain oxygen levels, mostly through offsetting sediment oxygen demand rather than directly adding oxygen to the water column. This occurs because it both dissolves and reacts slowly, therefore accumulating on the sediment surface rather than being dispersed through the water column (e.g. Hanh et al. 2005). It appears to be a promising option, although there is still uncertainty as to whether it would be effective across large river reaches (tens to hundreds of kilometres).
For the previous reasons, using calcium peroxide is likely to be low risk to the environment, although this may require further confirmation using small-scale application and evaluation before large-scale deployment. Calcium peroxide has been shown to inhibit algal growth either through direct peroxide toxicity (e.g. Bauzá et al. 2014) or indirectly through the coordination and precipitation of phosphorus by calcium (Cho and Lee 2002). If the calcium peroxide reacted in the surface water, in the presence of a strong algal blooms, it could cause significant algal die-off, which would subsequently decrease DO levels. However, because most of the calcium peroxide will accumulate at the sediment surface, and not in the photic zone where the algal bloom is concentrated, the risk of such an algal bloom die-off is considered relatively low.
When the use of calcium peroxide was considered as a response to the LDR fish deaths, it was seen as attractive from the perspective that it could deployed in remote areas relatively efficiently by air drop (although the logistics of this was not evaluated in any great detail). For example, if the 40-km affected reach of the LDR is used as an example, it would have required an estimated 1600 tonnes (Mg) to raise the DO concentration of the water by 4 mg L–1. That assumes that 22% by weight is converted to dioxygen (i.e. 100% efficiency) and 8000 ML of water was to be treated. Assuming the top 1 m of water has a DO concentration of 6 mg L–1 and the bottom 3 m is anoxic, DO in the water column would be expected to increase to 5.5 mg L–1. However, there is a chance that more calcium peroxide, and repeated dosing, may have been required if most of the calcium peroxide is preferentially oxidised by the sediment.
The use of chemicals in nature requires regulatory approval, which would take time to obtain and should therefore be investigated well ahead of when it may be needed. For this reason, calcium peroxide was not considered ready for use as an emergency response option during the LDR fish deaths.
Sodium percarbonate
Sodium percarbonate (2(Na2CO3)·3H2O2) is a water-soluble compound that rapidly breaks down (minutes to hours) to produce peroxide and carbonate or bicarbonate; the former then decomposes to form oxygen (e.g. Zhang et al. 2011). Typically found in commercial bleaches and used as a water sanitiser in aquaculture (e.g. Pedersen and Jokumsen 2017), the speed of its breakdown means that sodium percarbonate can quickly increase DO. However, this also means sodium percarbonate is likely to be less effective in the longer-term maintenance of DO. Like calcium peroxide, it could be deployed across large scales in remote areas efficiently (e.g. by air drop). Using the 40-km reach of the LDR affected by the fish deaths as an example, it would have required ~2250 Mg of sodium percarbonate to raise the DO concentration by 4 mg L–1. This assumes that 15% by weight is converted to dioxygen (100% efficiency), with the remaining assumptions as per the earlier calcium peroxide example. The requirement for frequent and repeated dosing would need to be considered in any evaluation of cost. As with calcium peroxide, the use of sodium percarbonate would require regulatory approval.
More concerning in relation to the use of sodium percarbonate is the potential environmental risk. Sodium percarbonate would likely increase the alkalinity of the water in reaches where pH levels can already be quite high due to algal blooms. In reaches where fish deaths have occurred, ammonium from decomposing fish can be readily converted to ammonia at higher pH. Ammonia is toxic to fish and may result in more fish deaths. Furthermore, the rapid production of peroxide in the surface layer will kill algae and has the potential to cause a rapid die-off of the algal bloom. Decomposition of the dead algae would further deplete DO. This combination of risks meant that sodium percarbonate was not considered a viable option for aeration during the LDR fish deaths.
Mechanical mixing and aeration
Bubble diffusers
A bubble diffuser is placed at the bottom of a waterbody, where it releases millimetre-sized bubbles that rise to the surface. In doing so, the diffusers draw deep water to the surface and, over time, this creates circular mixing, which prevents or breaks down thermal stratification and prevents hypoxic water accumulating in deeper layers. Bubble diffusers are sometimes used in large reservoirs (Mobley and Brock 1995; Cox et al. 1998; Sahoo and Luketina 2006) and intensive aquaculture (Colt et al. 2010). Although the bubble stream can enhance mixing, because the bubbles are relatively large and do not persist in the water column, they are not very efficient at releasing oxygen into the water (Navisa et al. 2014). Therefore, their benefit does not come from direct oxygenation from the bubbles per se, but rather the mixing of surface and bottom waters, which can have the secondary effect of improving oxygen levels.
A big advantage of bubble diffusers is that they are relatively easy to obtain, are low-tech, low-cost and relatively easy to manufacture. They essentially consist of a blower (for shallow installations <2 m) or compressor (for deeper installations), air line and diffuser. Diffusers can be as simple in construction as tubing or a plate with holes drilled in it, or fine pore materials such as ceramic, carbon, porous plastic or a perforated membrane. These fine-pore diffusers produce fine bubbles with a diameter of ~2–5 mm (US Environmental Protection Agency 1989). Placing diffusers in the deepest part of the channel can facilitate greatest mixing because the bubbles entrain water over a greater vertical distance, although increasing depth also increases power consumption because greater air pressures are required (Boyd and Moore 1993). Depending on power requirements, the power supply can be from a main electrical supply or solar for more remote installations. When smaller, less-powerful, solar-powered blowers are used, more units need to be deployed in order to achieve the same result as fewer more-powerful units. These units should also run constantly to break down stratification and maintain mixing (Baldwin et al. 2021).
A disadvantage of bubble diffusers is that biofouling of the diffuser or the scaling of salts and minerals can significantly diminish their performance over time, particularly if the device is being used intermittently (Colt et al. 2010). Bubble diffusers can also pose some environmental risk that needs to be managed. Mixing water that has been thermally stratified for long periods can cause anoxic turnover, as anoxic water and sediments are entrained from the hypolimnion (Jones and Stokes 2004). To avoid this, it may be best to deploy these earlier in summer, before strong stratification has occurred and before all the oxygen has been consumed in the hypolimnion. To further manage the risk of fish deaths from anoxic turnover, small-scale turnover within larger refuges can be targeted. In these instances, the anoxic conditions would be transient and localised, and there would still be a nearby oxygen refuge for fish to escape. Finally, to further minimise the risk of transporting anoxic sediments, the diffusers should be placed off the riverbed.
In the case of the LDR fish deaths, a decision was made to deploy several solar-powered bubble diffusers where the fish deaths occurred to quickly provide some relief to stressed fish. Unfortunately, because many of these pools had already become anoxic, the timing of these deployments was unlikely to be as effective as it would have been if they had been deployed earlier. However, managers were faced with very little other option at such short notice. The deployment and performance of these bubble diffusers, as well as those deployed in other river systems of the MDB, are discussed in this issue of the Journal by Baldwin et al. (2021).
Ultrafine oxygen bubble pump
The diffusers just described release 2- to 5-mm sized bubbles, but technologies are available that can release much smaller bubbles, making them more effective at aerating water (Navisa et al. 2014). There is an industry standard (ISO 20480-1, see https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en) used to describe the size of bubbles, and they can range in decreasing size from millimetre to sub-millimetre (<1 mm) to microbubbles (<100 μm) to ultrafine bubbles (<1 μm). The advantage of ultrafine over millimetre-sized bubbles is that they have a much larger surface area to volume ratio, more than 95% of the of gas molecules in the suspension can exist in the dissolved phase (Kim et al. 2020) and they are more stable, remaining in the water column for longer (Thomas et al. 2021). They are therefore more efficient at increasing the DO concentration of hypoxic water and do so in a more energy-efficient way (Ovezea 2009). This means ultrafine bubble injection is becoming a more frequently used approach for aerating water in the aquaculture and waste water treatment industries (Duchène et al. 2001; Cheng et al. 2019). Ultrafine bubble pumps often use pure oxygen, either from compressed liquid gas or generated by a condenser, improving the efficiency with which oxygen is added to the water (Ovezea 2009).
Examples of their use in nature is rarer, but an ultrafine oxygen bubble pump has been used with some success to treat a hypoxic reach of the Swan River, Western Australia (Larsen et al. 2019). In this instance, up to 20 km of a tidally influenced river reach (<3 m deep) had its DO concentration enhanced using a 120-L s–1 centrifugal pump fitted with a proprietary (BOC Ltd) diffuser that injected vaporised liquid oxygen gas into the discharged water. Although this installation is a permanent one, the proprietors of that technology report that smaller, more mobile systems are also available (J. Pera, WaterNSW, pers. comm.). The biggest uncertainty that needs to be resolved is whether this technology can be deployed at much larger scales (many tens of kilometres) in a cost-effective manner. The cost of these systems has not been evaluated here, but is likely to be substantially higher than other lower-tech approaches such as Venturi pumps.
When ultrafine bubble pumps were considered for use during the LDR fish deaths, the decision was taken not to deploy them without first evaluating their environmental risk. It was thought there may be a risk that the highly efficient entrainment of significant levels of gas in the water column could lead to supersaturation of total dissolved gas. When total dissolved gas pressures exceed 110%, fish restricted to shallow water can develop gas bubble trauma (GBT), where emboli form in organs and gills (Pleizier et al. 2020). GBT can disrupt oxygen absorption and eventually kill fish. Fish deaths from GBT are frequently reported in nature as a result of supersaturation resulting from dam spill (Lutz 1995; Backman and Evans 2002) and algal blooms (Rensel and Whyte 2003), or in aquaculture when pumps entrain air (Boyd et al. 1994). GBT is exacerbated in warmer and shallower water (which is to be expected in drought refuges) because warm water can hold more dissolved gas and fish cannot dive deeper to greater pressures in order to reduce the release of gas from internal body fluids or organs. In the case of the LDR fish deaths, due to the perceived risk of GBT, the decision was made to first trial an ultrafine bubble pump in a pond at a state government hatchery. The result of that trial was promising, with a significant increase in DO concentration being achieved without an increase in total dissolved gas pressure to levels that would cause GBT (Baldwin et al. 2021).
Pumps with Venturi tubes
By establishing water currents in non-flowing waterbodies, water pumps promote mixing, destratification and the exchange of oxygen throughout the water column (Boyd 1998). Pump effectiveness will depend on the size and depth of the pool they are deployed in and the amount of water they exchange (Boyd 1998). Currents can be encouraged by drawing and releasing water at different depths or across a significant longitudinal distance. Because of this, their potential to displace water and encourage currents is far greater than with other mixing technologies, such as water wheels or bubble diffusers.
Aeration can be further improved with the addition of a Venturi tube to the pump (Baylar and Ozkan 2006). As water flows through a Venturi tube, it passes a constriction that accelerates the flow and creates a differential drop in pressure that sucks air through a hole. This entrains air into the water stream, effectively increasing its DO concentration. Venturi tubes are highly efficient, requiring less than 20% differential pressure to create this suction (Baylar and Ozkan 2006).
An advantage of adding a Venturi tube to a standard water pump is the potential to convert hypoxic water drawn from the hypolimnion layer into oxygenated water, therefore mixing and aerating at same time. By contrast, traditional water pumps that draw in hypoxic water would disperse this hypoxic water through the water column. Following the 2018–19 fish deaths in the LDR, Venturi water pumps were deployed at three sites with reasonable success in 2019 and 2020 (Baldwin et al. 2021). From this experience, it was found that the main limitation of using water pumps with Venturi tubes was that the pump itself can be expensive to operate and may not be as suitable for remote locations. The large Venturi pump used during the LDR fish deaths consisted of a 10- to 15-ML day–1 diesel pump (Baldwin et al. 2021). The pump required daily refuelling at a considerable cost. This cost and ongoing maintenance requirement could restrict its broadscale deployment. Smaller diesel electric and electric multiport Venturi systems have also been evaluated (Baldwin et al. 2021) in the LDR in 2019 and 2020. Although far less powerful (circulating 2–8 ML day–1) than the large diesel pump tested, they did show localised improvements in DO, with their performance exceeding what was recorded in nearby solar-powered bubble diffusers. As a mixing and aerating technology, we recommend the use of pumps with Venturi tubes as an emergency measure at high-value and high-risk refuge pools.
Paddle wheels
Paddle wheels are one of the most commonly used aeration and destratification devices in intensive aquaculture (Tanveer et al. 2018). These systems consist of a series of powered rotating paddles mounted on a floating pontoon. Aeration occurs through the constant splashing of the top 20 cm of water, a process that can entrain oxygen. This can create localised pockets of (partially) oxygenated water, but if a circulation flow can be generated the aerated plume can be dispersed. Paddle wheels can also stop the formation and even break down thermoclines in shallow (<2 m) waterbodies (Kerr et al. 2013). They have the advantage of being cost-effective, low maintenance and readily available through commercial suppliers.
Paddle wheels require a source of power, and this needs to be considered when deploying them in remote areas. More powerful diesel-operated units have been reported to be more effective than electric motor units at aerating water, but they are also more costly the run (Taparhudee et al. 2007). Solar-powered paddle wheels would be advantageous in remote settings, but due to the power requirements of paddle wheels, solar-powered systems do not appear to have proceeded past the conceptual design stage (Tanveer and Mayilsamy 2016).
The performance of paddle wheels has been extensively evaluated in the aquaculture context (for a review, see Tanveer et al. 2018). They have been shown to reduce the occurrence of hypoxia in ponds compared with ponds that are not aerated (Romaire and Merry 2007). The volume and depth of water to be aerated must be taken into account when designing an appropriate paddle wheel system (Omofunmi et al. 2016). Bigger-diameter paddles, certain blade configurations and faster speeds can increase the amount of oxygen transferred into the waterbody, but will require more power to operate (Ahmad and Boyd 1988; Moulick et al. 2005; Roy et al. 2015). Because of the trade-off between standard oxygen transfer rate and power consumption, the size and number of paddle wheels used should be optimised based on the size of the refuge pool requiring aeration and mixing.
In aquaculture, paddle wheels are typically used in ponds <2 m deep, raising questions over their ability to aerate deeper pools. However, there is some evidence of them effectively oxygenating pools that are 3.6 m deep with only a shallow (10-cm) paddle immersion (Moore and Whitis 1999). The vast amount of literature on their use is limited to intensive aquaculture ponds, and we only found one reference of the use of paddle wheels to mitigate hypoxia in river systems (Whitworth et al. 2013). During a 2010 blackwater event on the Murray River (Australia), a paddleboat was used as an aerator. Localised improvements in DO were reported and those authors observed that Murray cod ceased surface respiration when the paddle wheel was operated, with no fish deaths observed in the otherwise hypoxic reach. Although Whitworth et al. (2013) also mention the use of smaller paddle wheels (as used in aquaculture ponds), they do not report on their performance.
Downward-facing propellers
Downward-facing propellers work by drawing oxygenated surface water and pushing it deep to disrupt the thermocline and eventually oxygenate deeper waters. The technology was designed for reservoirs, where it has been shown to be effective (e.g. Morillo et al. 2009). The feasibility of using downward-facing propellers in shallow refuges was considered as part of the response to the LDR fish deaths, but was disregarded because it would have disturbed sediments in pools shallower than 6–7 m (J. Pera, pers. comm.). The propeller units themselves are not easily deployed like water paddles or airlift bubble diffusers and would cost significantly more to obtain and maintain. They would require constant power, which may limit sites where they would be effective, and there would ongoing operational costs. Because downward-facing propellers create down currents, there is a risk that bottom sediments would be disturbed, placing additional pressure on oxygen levels in the river. However, this would be dependent on how powerful the units are. Until further evaluation can prove otherwise, other more practical and cost-effective solutions outlined in this paper are preferable.
Water fountains or jets
In this approach, water is pumped from the river and sprayed through the air, to land back onto the surface of the water. Water jet aeration is an effective way of aeration, although the area treated is small, localised to the direct area of the jet–pool interface (Biń 1993). As the water jet passes through the air and plunges into a pool, it entrains air and creates a localised area of elevated DO concentration. The plunging water has benefits over other aeration systems in that it does not require an air compressor, it is simple to construct and operate and it does not have maintenance issues such as clogging diffusers. The performance of water jets can be further improved with the use of commercially available Venturi nozzles (Baylar and Ozkan 2006).
Some benefits of using water fountains or jets is that they are practical, relatively low cost compared with the other options described previously and could be deployed rapidly if existing irrigation, stock and domestic pumps are used. Their use also requires little to no prior training, and landholders, graziers and irrigators could all participate. As an emergency measure for treating hypoxic rivers, water jets were used during a hypoxic blackwater event in the Edwards River in 2000 (Baldwin et al. 2001). In this instance, local decapod crustacean (Cherax destructor) farmers used their fire-fighting pumps and hoses to create improvised water curtains to re-aerate their ponds. However, we could find no evidence outlining their effectiveness during this event. During the LDR fish death events, the use of water jets by private pump owners was hampered by uncertainty around the implications on water licences and liability should pump damage occur. Further consideration should be given to both these things so that policies can be put in place before future events.
There is a risk that water jets can lower DO in the water column if the water column is already heavily stratified. When the hypolimnion is hypoxic, care must be taken to elevate the intake (preferably into the top 1–2 m of water) to minimise the risk of entraining low-DO water or nutrient-rich sediments that could further deplete surface oxygen levels. In some instances, hypoxic water or nutrient-rich sediments may be evident as discoloured or stagnant-smelling water. However, in many instances it may not be possible to determine the oxygen levels of the water without appropriate monitoring equipment. Therefore, it is best to be conservative and avoid deeper water. As fish will likely accumulate around the water jet (C. Boys, pers. obs.), the jet must be a sufficient distance away from the pump intake to reduce the likelihood that fish may be sucked into the pump. Alternatively, a screen placed on the pump inlet can minimise the risk of fish entrainment.
Conclusion and recommendations
The catastrophic fish deaths that occurred in the LDR in the austral summer of 2018–19 highlighted the precarious state of the health of the MDB and its native fish populations. Climate change predictions of less rainfall and more extreme summer temperatures, combined with river regulation and the ever increasing demand for water resources, will mean that the management of rivers to prevent hypoxic fish death events is going to be an ongoing challenge (Hughes 2003; Dey et al. 2019). This challenge is not unique to Australia, with many other river systems throughout the world experiencing the same problem (Rabalais et al. 2001; Wang et al. 2012; Zhou et al. 2015). Preferably, catchment and water management policies should be in place to prevent river systems reaching a state where emergency interventions are required (Vertessy et al. 2019). In the case of the LDR fish deaths, once the deaths had started to occur, managers were left with little water and little time to respond to the unfolding emergency. To avoid this same scenario in the future, whether in the LDR or any river system, time must be taken to adequately prepare for future events in a changing climate. Thankfully, the LDR fish deaths prompted a detailed consideration of non-flow-dependent options for mitigating destratification-driven hypoxia and preventing fish deaths. By documenting these considerations in this paper, we hope that fisheries and other river managers, both in Australia and elsewhere, can be better prepared to respond to similar events during future droughts.
Several key recommendations can be made and Table 1 should serve as an important decision support tool moving forward. First, it is important to be clear on what the different technologies can achieve, because this will dictate when and where each should be used. Technologies may primarily mix (with secondary aeration), directly aerate or both. Options like bubble diffusers and paddle wheels are more suitable for mixing than for aerating. It is best to deploy them before stratification has become established and maintain their operation throughout the summer. They will then achieve aeration indirectly through the promotion of mixing. By contrast, pumps with Venturi tubes or ultrafine bubble oxygen injection are more efficient at aerating while they mix. This makes them more suitable for emergency deployment in pools where hypoxia has already become established.
Second, relying on what may initially appear to be the ‘cheapest’ option may not always result in an overall cost saving. Aeration and mixing efficiency tend to be inversely related to power consumption, and therefore cost. Smaller solutions, such as solar-powered bubble diffusers, may appear low cost per unit, but multiple units are needed in each pool in order to make a difference, and these may need to run for extended periods. Inversely, pumps that require diesel to operate may be capable of greater water, and therefore oxygen, exchange, but this comes at a greater ongoing operational cost. Detailed cost–benefit analysis of each option is required, considering initial capital outlay, staffing and operating costs per kilometre of river protected.
Third, it is important to avoid options that may cause more environmental risk. For example, downward-facing propellers, although effective mixers, are unlikely to be suitable in shallow river conditions due to the disturbance of anoxic sediments. A chemical like sodium percarbonate may crash algal blooms and raise pH, only exacerbating the impact of hypoxia in an already stressed ecosystem.
Finally, it is important to use the time between periods of high hypoxia risk to prepare. This may involve looking into the regulatory approval requirements needed to use broadscale chemical dosing. It may involve establishing policy guidelines that would enable private water users to use their existing pumping infrastructure to create water jets and localised refuges. Even identifying those priority reaches, and landholders within each reach that have the capacity to contribute to such action, may be of great benefit. It may involve undertaking further research and development into those more experimental approaches that show promise, such as calcium peroxide or ultrafine bubble pumps, including efficacy, safety and detailed cost–benefit analyses.
Conflicts of interest
Craig Boys is a guest editor of this special issue in Marine and Freshwater Research. Despite this relationship, he did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this Journal. Marine and Freshwater Research encourages its editors to publish in the Journal and they are kept totally separate from the decision-making process for their manuscripts. The authors declare that they have no further conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The manuscript was substantially improved after review by Natalie Moltschaniwskyj, Cameron Westaway, the Office of the NSW Chief Scientist and two anonymous reviewers.
References
Ahmad, T., and Boyd, C. E. (1988). Design and performance of paddle wheel aerators. Aquacultural Engineering 7, 39–62.| Design and performance of paddle wheel aerators.Crossref | GoogleScholarGoogle Scholar |
Backman, T. W., and Evans, A. F. (2002). Gas bubble trauma incidence in adult salmonids in the Columbia River Basin. North American Journal of Fisheries Management 22, 579–584.
| Gas bubble trauma incidence in adult salmonids in the Columbia River Basin.Crossref | GoogleScholarGoogle Scholar |
Baldwin, D. S. (2019). Stratification, mixing and fish deaths in the lower Darling River. Rivers and Wetlands, Albury, NSW, Australia.
Baldwin, D., Howitt, J., and Edwards, M. (2001). Blackwater event. Austasia Aquaculture 15, 21.
Baldwin, D. S., Boys, C. A., Rohls, A.-M., Ellis, I., and Pera, J. (2021). Field trials to determine the efficacy of aerators to mitigate hypoxia in inland waterways. Marine and Freshwater Research , .
| Field trials to determine the efficacy of aerators to mitigate hypoxia in inland waterways.Crossref | GoogleScholarGoogle Scholar |
Bauzá, L., Aguilera, A., Echenique, R., Andrinolo, D., and Giannuzzi, L. (2014). Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays. Toxins 6, 2657–2675.
| Application of hydrogen peroxide to the control of eutrophic lake systems in laboratory assays.Crossref | GoogleScholarGoogle Scholar | 25208009PubMed |
Baylar, A., and Ozkan, F. (2006). Applications of venturi principle to water aeration systems. Environmental Fluid Mechanics 6, 341–357.
| Applications of venturi principle to water aeration systems.Crossref | GoogleScholarGoogle Scholar |
Biń, A. K. (1993). Gas entrainment by plunging liquid jets. Chemical Engineering Science 48, 3585–3630.
| Gas entrainment by plunging liquid jets.Crossref | GoogleScholarGoogle Scholar |
Boyd, C. E. (1998). Pond water aeration systems. Aquacultural Engineering 18, 9–40.
| Pond water aeration systems.Crossref | GoogleScholarGoogle Scholar |
Boyd, C. E., and Hanson, T. (2010). Dissolved-oxygen concentrations in pond aquaculture. Global Aquaculture Advocate 2, 40–41.
Boyd, C. E., and Moore, J. M. (1993). Factors affecting the performance of diffused-air aeration systems for aquaculture. Journal of Applied Aquaculture 2, 1–12.
| Factors affecting the performance of diffused-air aeration systems for aquaculture.Crossref | GoogleScholarGoogle Scholar |
Boyd, C. E., Watten, B. J., Goubier, V., and Wu, R. (1994). Gas supersaturation in surface waters of aquaculture ponds. Aquacultural Engineering 13, 31–39.
| Gas supersaturation in surface waters of aquaculture ponds.Crossref | GoogleScholarGoogle Scholar |
Cheng, X., Xie, Y., Zhu, D., and Xie, J. (2019). Modeling re-oxygenation performance of fine-bubble–diffusing aeration system in aquaculture ponds. Aquaculture International 27, 1353–1368.
| Modeling re-oxygenation performance of fine-bubble–diffusing aeration system in aquaculture ponds.Crossref | GoogleScholarGoogle Scholar |
Cho, I., and Lee, K. (2002). Effect of calcium peroxide on the growth and proliferation ofMicrocystis aerusinosa, a water-blooming cyanobacterium. Biotechnology and Bioprocess Engineering; BBE 7, 231–233.
| Effect of calcium peroxide on the growth and proliferation ofMicrocystis aerusinosa, a water-blooming cyanobacterium.Crossref | GoogleScholarGoogle Scholar |
Colt, J., Kroeger, E., and Rust, M. (2010). Characteristics of oxygen flow through fine bubble diffusers used in the aquaculture hauling applications. Aquacultural Engineering 43, 62–70.
| Characteristics of oxygen flow through fine bubble diffusers used in the aquaculture hauling applications.Crossref | GoogleScholarGoogle Scholar |
Cox, J. D., Padley, M. B., and Hannon, J. (1998). Use of computational fluid dynamics to model reservoir mixing and destratification. Water Science and Technology 37, 227–234.
| Use of computational fluid dynamics to model reservoir mixing and destratification.Crossref | GoogleScholarGoogle Scholar |
Dahm, C. N., Candelaria‐Ley, R. I., Reale, C. S., Reale, J. K., and Van Horn, D. J. (2015). Extreme water quality degradation following a catastrophic forest fire. Freshwater Biology 60, 2584–2599.
| Extreme water quality degradation following a catastrophic forest fire.Crossref | GoogleScholarGoogle Scholar |
Dey, R., Lewis, S. C., Arblaster, J. M., and Abram, N. J. (2019). A review of past and projected changes in Australia’s rainfall. Wiley Interdisciplinary Reviews: Climate Change 10, e577.
| A review of past and projected changes in Australia’s rainfall.Crossref | GoogleScholarGoogle Scholar |
Duchène, P., Cotteux, E., and Capela, S. (2001). Applying fine bubble aeration to small aeration tanks. Water Science and Technology 44, 203–210.
| Applying fine bubble aeration to small aeration tanks.Crossref | GoogleScholarGoogle Scholar | 11547985PubMed |
Gehrke, P. C. (1988). Acute cardio-respiratory responses of spangled perch, Leiopotherapon unicolor (Gunther 1859), to sublethal concentrations of zinc, temephos and 2,4-D. Australian Journal of Marine and Freshwater Research 39, 767–774.
| Acute cardio-respiratory responses of spangled perch, Leiopotherapon unicolor (Gunther 1859), to sublethal concentrations of zinc, temephos and 2,4-D.Crossref | GoogleScholarGoogle Scholar |
Gilmore, K. L., Doubleday, Z. A., and Gillanders, B. M. (2018). Testing hypoxia: physiological effects of long-term exposure in two freshwater fishes. Oecologia 186, 37–47.
| Testing hypoxia: physiological effects of long-term exposure in two freshwater fishes.Crossref | GoogleScholarGoogle Scholar | 29110076PubMed |
Hanh, D., Rajbhandari, B., and Annachhatre, A. (2005). Bioremediation of sediments from intensive aquaculture shrimp farms by using calcium peroxide as slow oxygen release agent. Environmental Technology 26, 581–590.
| Bioremediation of sediments from intensive aquaculture shrimp farms by using calcium peroxide as slow oxygen release agent.Crossref | GoogleScholarGoogle Scholar | 15974276PubMed |
Hasler, C. T., Suski, C. D., Hanson, K. C., Cooke, S. J., and Tufts, B. L. (2009). The influence of dissolved oxygen on winter habitat selection by largemouth bass: An integration of field biotelemetry studies and laboratory experiments. Physiological and Biochemical Zoology 82, 143–152.
| The influence of dissolved oxygen on winter habitat selection by largemouth bass: An integration of field biotelemetry studies and laboratory experiments.Crossref | GoogleScholarGoogle Scholar | 19199559PubMed |
Hladyz, S., Watkins, S. C., Whitworth, K. L., and Baldwin, D. S. (2011). Flows and hypoxic blackwater events in managed ephemeral river channels. Journal of Hydrology 401, 117–125.
| Flows and hypoxic blackwater events in managed ephemeral river channels.Crossref | GoogleScholarGoogle Scholar |
Howitt, J. A., Baldwin, D. S., Rees, G. N., and Williams, J. L. (2007). Modelling blackwater: predicting water quality during flooding of lowland river forests. Ecological Modelling 203, 229–242.
| Modelling blackwater: predicting water quality during flooding of lowland river forests.Crossref | GoogleScholarGoogle Scholar |
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Jones & Stokes (2004). Aeration technology feasibility report for the San Joaquin River deep water ship channel. Number J&S 03–405, Jones & Stokes, Sacramento, CA, USA.
Kerr, J. L., Baldwin, D. S., and Whitworth, K. L. (2013). Options for managing hypoxic blackwater events in river systems: a review. Journal of Environmental Management 114, 139–147.
| Options for managing hypoxic blackwater events in river systems: a review.Crossref | GoogleScholarGoogle Scholar | 23137913PubMed |
Kim, E., Choe, J. K., Kim, B. H., Kim, J., Park, J., and Choi, Y. (2020). Unraveling the mystery of ultrafine bubbles: Establishment of thermodynamic equilibrium for sub-micron bubbles and its implications. Journal of Colloid and Interface Science 570, 173–181.
| Unraveling the mystery of ultrafine bubbles: Establishment of thermodynamic equilibrium for sub-micron bubbles and its implications.Crossref | GoogleScholarGoogle Scholar | 32146244PubMed |
La, V. T., and Cooke, S. J. (2011). Advancing the science and practice of fish kill investigations. Reviews in Fisheries Science 19, 21–33.
| Advancing the science and practice of fish kill investigations.Crossref | GoogleScholarGoogle Scholar |
Larsen, S. J., Kilminster, K. L., Mantovanelli, A., Goss, Z. J., Evans, G. C., Bryant, L. D., and McGinnis, D. F. (2019). Artificially oxygenating the Swan River estuary increases dissolved oxygen concentrations in the water and at the sediment interface. Ecological Engineering 128, 112–121.
| Artificially oxygenating the Swan River estuary increases dissolved oxygen concentrations in the water and at the sediment interface.Crossref | GoogleScholarGoogle Scholar |
Lutz, D. S. (1995). Gas supersaturation and gas bubble trauma in fish downstream from a midwestern reservoir. Transactions of the American Fisheries Society 124, 423–436.
| Gas supersaturation and gas bubble trauma in fish downstream from a midwestern reservoir.Crossref | GoogleScholarGoogle Scholar |
MacPherson, T. A., Cahoon, L. B., and Mallin, M. A. (2007). Water column oxygen demand and sediment oxygen flux: patterns of oxygen depletion in tidal creeks. Hydrobiologia 586, 235–248.
| Water column oxygen demand and sediment oxygen flux: patterns of oxygen depletion in tidal creeks.Crossref | GoogleScholarGoogle Scholar |
Matlock, M. D., Kasprzak, K. R., and Osborn, G. S. (2003). Sediment oxygen demand in the Arroyo Colorado River. Journal of the American Water Resources Association 39, 267–275.
| Sediment oxygen demand in the Arroyo Colorado River.Crossref | GoogleScholarGoogle Scholar |
McDonnell, A. J., and Kountz, R. R. (1966). Algal respiration in a eutrophic environment. Journal – Water Pollution Control Federation 38, 841–847.
McNeil, D. G., and Closs, G. P. (2007). Behavioural responses of a south‐east Australian floodplain fish community to gradual hypoxia. Freshwater Biology 52, 412–420.
| Behavioural responses of a south‐east Australian floodplain fish community to gradual hypoxia.Crossref | GoogleScholarGoogle Scholar |
Mitrovic, S. M., Hardwick, L., and Dorani, F. (2011). Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. Journal of Plankton Research 33, 229–241.
| Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Mitrovic, S. M., Westhorpe, D. P., Kobayashi, T., Baldwin, D. S., Ryan, D., and Hitchcock, J. N. (2014). Short-term changes in zooplankton density and community structure in response to different sources of dissolved organic carbon in an unconstrained lowland river: evidence for food web support. Journal of Plankton Research 36, 1488–1500.
| Short-term changes in zooplankton density and community structure in response to different sources of dissolved organic carbon in an unconstrained lowland river: evidence for food web support.Crossref | GoogleScholarGoogle Scholar |
Mobley, M. H., and Brock, W. G. (1995). Widespread oxygen bubbles to improve reservoir releases. Lake and Reservoir Management 11, 231–234.
| Widespread oxygen bubbles to improve reservoir releases.Crossref | GoogleScholarGoogle Scholar |
Moore, J. M., and Whitis, G. N. (1999). Vertical water circulation capabilities of an electric paddle wheel aerator and dissolved oxygen loss due to daytime aeration. Journal of Applied Aquaculture 9, 25–36.
| Vertical water circulation capabilities of an electric paddle wheel aerator and dissolved oxygen loss due to daytime aeration.Crossref | GoogleScholarGoogle Scholar |
Morillo, S., Imberger, J., Antenucci, J. P., and Copetti, D. (2009). Using impellers to distribute local nutrient loadings in a stratified lake: Lake Como, Italy. Journal of Hydraulic Engineering 135, 564–574.
| Using impellers to distribute local nutrient loadings in a stratified lake: Lake Como, Italy.Crossref | GoogleScholarGoogle Scholar |
Moulick, S., Bandyopadhyay, S., and Mal, B. (2005). Design characteristics of single hub paddle wheel aerator. Journal of Environmental Engineering 131, 1147–1154.
| Design characteristics of single hub paddle wheel aerator.Crossref | GoogleScholarGoogle Scholar |
Navisa, J., Sravya, T., Swetha, M., and Venkatesan, M. (2014). Effect of bubble size on aeration process. Asian Journal of Scientific Research 7, 482–487.
| Effect of bubble size on aeration process.Crossref | GoogleScholarGoogle Scholar |
New South Wales Department of Primary Industries (2019). Lower Darling River Fish Death Event, Menindee 2018/19. NSW DPI, Port Stephens Fisheries Institute.
Omofunmi, O. E., Adewumi, J. K., Adisa, A. F., and Alegbeleye, S. O. (2016). Development of a paddle wheel aerator for small and medium fish farmers in Nigeria. IOSR Journal of Mechanical and Civil Engineering 13, 50–56.
Ovezea, A. (2009). Saving energy: Using fine bubble diffusers. Filtration & Separation 46, 24–27.
| Saving energy: Using fine bubble diffusers.Crossref | GoogleScholarGoogle Scholar |
Pedersen, L.-F., and Jokumsen, A. (2017). The pros and cons of sodium percarbonate. Global Aquaculture Advocate 1, 1–4.
Pleizier, N. K., Algera, D., Cooke, S. J., and Brauner, C. J. (2020). A meta‐analysis of gas bubble trauma in fish. Fish and Fisheries 21, 1175–1194.
| A meta‐analysis of gas bubble trauma in fish.Crossref | GoogleScholarGoogle Scholar |
Rabalais, N. N., Turner, R. E., and Wiseman, W. J. (2001). Hypoxia in the Gulf of Mexico. Journal of Environmental Quality 30, 320–329.
| Hypoxia in the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar | 11285891PubMed |
Rensel, J., and Whyte, J. (2003). Finfish mariculture and harmful algal blooms. Manual on harmful marine microalgae. Monographs on Oceanographic Methodology 11, 693–722.
Romaire, R. P., and Merry, G. E. (2007). Effects of paddlewheel aeration on water quality in crawfish ponds. Journal of Applied Aquaculture 19, 61–75.
| Effects of paddlewheel aeration on water quality in crawfish ponds.Crossref | GoogleScholarGoogle Scholar |
Roy, S. M., Moulick, S., Mukherjee, C. K., and Mal, B. C. (2015). Effect of rotational speeds of paddle wheel aerator on aeration cost. American Research Thoughts 2, 3069–3087.
Sahoo, G. B., and Luketina, D. (2006). Response of a tropical reservoir to bubbler destratification. Journal of Environmental Engineering 132, 736–746.
| Response of a tropical reservoir to bubbler destratification.Crossref | GoogleScholarGoogle Scholar |
Sheldon, F., Barma, D., Baumgartner, L., Bond, N., Mitrovic, S., and Vertessy, R. (2021). Assessment of the causes and solutions to the significant 2018–2019 fish deaths in the Lower Darling River, New South Wales, Australia. Marine and Freshwater Research , .
| Assessment of the causes and solutions to the significant 2018–2019 fish deaths in the Lower Darling River, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Sinha, B., and Annachhatre, A. P. (2007). Partial nitrification – operational parameters and microorganisms involved. Reviews in Environmental Science and Bio/Technology 6, 285–313.
| Partial nitrification – operational parameters and microorganisms involved.Crossref | GoogleScholarGoogle Scholar |
Small, K., Kopf, R. K., Watts, R. J., and Howitt, J. (2014). Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes. PLoS One 9, e94524.
| Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes.Crossref | GoogleScholarGoogle Scholar | 24728094PubMed |
Tanveer, M., and Mayilsamy, S. (2016). A conceptual approach for development of solar powered aeration system in aquaculture farms. International Journal of Science, Environment and Technology 5, 2921–2925.
Tanveer, M., Roy, S. M., Vikneswaran, M., Renganathan, P., and Balasubramanian, S. (2018). Surface aeration systems for application in aquaculture: a review. International Journal of Fisheries and Aquatic Studies 6, 342–347.
Taparhudee, W., Benjaprasertsri, M., and Sattiti, B. (2007). Comparative study on paddle-wheel aerators using electric motors and diesel engines in Pacific white shrimp (Litopenaeus vannamei) culture ponds. Agriculture and Natural Resources 41, 522–530.
Thomas, B., Ohde, D., Matthes, S., Engelmann, C., Bubenheim, P., Terasaka, K., Schlüter, M., and Liese, A. (2021). Comparative investigation of fine bubble and macrobubble aeration on gas utility and biotransformation productivity. Biotechnology and Bioengineering 118, 130–141.
| Comparative investigation of fine bubble and macrobubble aeration on gas utility and biotransformation productivity.Crossref | GoogleScholarGoogle Scholar | 32886350PubMed |
Thoms, M. C., and Sheldon, F. (2000). Lowland Rivers: an Australian introduction. Regulated Rivers 16, 375–383.
| Lowland Rivers: an Australian introduction.Crossref | GoogleScholarGoogle Scholar |
US Environmental Protection Agency (1989). Design Manual: Fine Pore Aeration Systems. Number EPA/625/1–89/023, US EPA, Office of Research and Development Risk Reduction Engineering Laboratory, Cincinnati, OH, USA.
Vertessy, R., Barma, D., Baumgartner, L., Bond, N., Mitrovic, S., and Sheldon, F. (2019). Independent Assessment of the 2018/19 fish deaths in the lower Darling. Murray–Darling Basin Authority and Australian Government, Australia.
Wang, B., Wei, Q., Chen, J., and Xie, L. (2012). Annual cycle of hypoxia off the Changjiang (Yangtze River) Estuary. Marine Environmental Research 77, 1–5.
| Annual cycle of hypoxia off the Changjiang (Yangtze River) Estuary.Crossref | GoogleScholarGoogle Scholar | 22240466PubMed |
Whitworth, K. L., Baldwin, D. S., and Kerr, J. L. (2012). Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia). Journal of Hydrology 450–451, 190–198.
| Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia).Crossref | GoogleScholarGoogle Scholar |
Whitworth, K. L., Kerr, J. L., Mosley, L. M., Conallin, J., Hardwick, L., and Baldwin, D. S. (2013). Options for managing hypoxic blackwater in river systems: case studies and framework. Environmental Management 52, 837–850.
| Options for managing hypoxic blackwater in river systems: case studies and framework.Crossref | GoogleScholarGoogle Scholar | 23912322PubMed |
Wu, R. S. (2002). Hypoxia: from molecular responses to ecosystem responses. Marine Pollution Bulletin 45, 35–45.
| Hypoxia: from molecular responses to ecosystem responses.Crossref | GoogleScholarGoogle Scholar | 12398365PubMed |
Zhang, Y.-H., Xue, C.-M., and Guo, C.-H. (2011). Application sodium percarbonate to oxidative degradation trichloroethylene contamination in groundwater. Procedia Environmental Sciences 10, 1668–1673.
| Application sodium percarbonate to oxidative degradation trichloroethylene contamination in groundwater.Crossref | GoogleScholarGoogle Scholar |
Zhou, Y., Michalak, A. M., Beletsky, D., Rao, Y. R., and Richards, R. P. (2015). Record-breaking Lake Erie hypoxia during 2012 drought. Environmental Science & Technology 49, 800–807.
| Record-breaking Lake Erie hypoxia during 2012 drought.Crossref | GoogleScholarGoogle Scholar |