Individual variation influences avoidance behaviour of invasive common carp (Cyprinus carpio) and native buffalo (Ictiobus) to stroboscopic and acoustic deterrents
Paul A. Bzonek A B C , Jaewoo Kim B and Nicholas E. Mandrak A B
A B C , Jaewoo Kim B and Nicholas E. Mandrak A B
A Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks Street, Toronto, ON, M5S 3B2, Canada.
B Department of Biological Sciences, University of Toronto – Scarborough, 1265 Military Trail, Scarborough, ON, M1C 1A4, Canada.
C Corresponding author. Email: paul.bzonek@mail.utoronto.ca
Marine and Freshwater Research 72(11) 1682-1688 https://doi.org/10.1071/MF21051
Submitted: 12 February 2021 Accepted: 6 May 2021 Published: 9 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Uncontrolled biological invasions are reducing freshwater ecosystem diversity and resilience. Research is needed to evaluate whether non-structural deterrents are feasible within lock or canal environments. This study examined common carp (Cyprinus carpio) and buffalo hybrid (Ictiobus) movement patterns in response to acoustic and stroboscopic deterrents. Twelve strobe lights and one underwater speaker were deployed across the centre of a ship slip, with an environment analogous to a navigation canal. Common carp (n = 6) and Ictiobus (n = 4) were implanted with acoustic telemetry tags, and their behaviours were examined under control, stroboscopic, and acoustic stimuli for 60-min trial periods. Trials were run during the day and night for six straight days. Linear models determined that the stroboscopic and acoustic stimuli altered fish positioning within the ship slip, but produced weaker avoidance responses than those observed elsewhere. Weak responses were likely due to a strong preference for the open end of the ship slip and a lack of acoustic refuge during the acoustic treatment. Avoidance responses also differed widely among individuals of the same species, with fish expressing repeatability of avoidance radius size across trials. Ambient and stimulus sound-pressure levels should be carefully considered when deploying acoustic deterrents.
Keywords: non-physical barrier, invasive species, dispersal behaviour, inter-individual variation.
Introduction
Biological invasions are a driving force behind the loss of biodiversity in freshwater ecosystems (Ricciardi and MacIsaac 2011). These invasions are largely uncontrolled (Strayer 2010) and present a serious threat to ecosystem resilience (Downing et al. 2012). Because fish dispersal is largely confined to the waterways that they inhabit, deterrents placed along a dispersal route can halt or limit the range expansion of alien fishes (Noatch and Suski 2012). Halting range expansions before species have invaded a novel environment is a far more ecologically and economically effective strategy than managing an invasive species once it has become established in a new environment (Leung et al. 2002).
Acoustic, stroboscopic, or combined deterrent technologies have been tested in the field (Maiolie et al. 2001; Ruebush et al. 2012; Patrick et al. 2014), but studies have lacked the resolution to describe important components of deterrent avoidance behaviour such as avoidance radius, repeatability of individual responses, or habituation after repeated exposures (but see Dennis and Sorensen 2020). Here, we use acoustic telemetry to track deterrent responses within a realistic field environment.
Common carp and Ictiobus were used as study species. Common carp is a fast-growing, globally invasive species of significant management interest (Weber and Brown 2009). Management efforts often aim to exclude common carp from ecologically important marsh habitat (Caskenette et al. 2018). Bigmouth buffalo (Ictiobus cyprinellus) is a microphagous feeder native to the Laurentian Great Lakes (COSEWIC 2009). Within the Great Lakes basin, bigmouth buffalo hybridises with two other Ictiobus species, smallmouth and black buffaloes (Bart et al. 2010), making the three species difficult to distinguish; therefore, these fish are referred to as Ictiobus in this paper.
We made two predictions regarding how common carp and Ictiobus would respond to averse stroboscopic and acoustic stimuli. First, we predicted that each stimuli would produce an avoidance response, similar to the responses seen in laboratory settings (Murchy et al. 2016; Zielinski and Sorensen 2017). Second, we predicted that, on repeated exposures, individuals would exhibit evidence of habituation (Dennis and Sorensen 2020), thus reducing avoidance radius size.
Materials and methods
Study site
To determine how stroboscopic and acoustic stimuli influence common carp and Ictiobus movement, fishes were monitored via acoustic telemetry within a large outdoor mesocosm. The mesocosm was a ship slip modified to retain and observe target fishes. The mesocosm was immediately adjacent to the Burlington Bay Canal under the Burlington Skyway Bridge at the western end of Lake Ontario in Canada. It is enclosed with concrete and corrugated metal sheathing on three sides and open to Hamilton Harbour on the fourth side, with a double net installed to prevent fishes from escaping or debris from entering. Because of its construction alongside the Burlington Bay Canal, the mesocosm simulated the environmental conditions of shipping canals within the Laurentian Great Lakes basin. The mesocosm is 107.5 m long, 34.5 m wide and 8.0 m deep and the substrate was predominantly silt. The stroboscopic stimulus was produced with 12 underwater strobe lights (Seebrite LED, I.A.S. Ltd, Vancouver, BC, Canada) that were spread equidistant across the width of the mesocosm (Supplementary material Fig. S1 available at the journal’s website). The strobe lights were suspended at alternating depths of 3 and 6 m with aircraft cable. The acoustic stimulus was produced with a single speaker (LubellLL-1424HP, Lubell Laboratories, OH, USA) placed in the centre of the mesocosm and suspended at a depth of 4 m with aircraft cable.
Experimental design
Alternating stimulus and control trials were conducted over day- and night-time periods. Trials were 1 h long to minimise within-trial behavioural habituation. Stroboscopic trials were conducted 23–26 June 2015, and acoustic trials were conducted from 26–29 June 2015. Day trials were operated at 0900 hours, 1100 hours, 1300 hours and 1500 hours. Night trials were operated at 2100 hours, 2300 hours, 0100 hours and 0300 hours. Control trials were the 1 h periods between treatment trials.
Study animals
This research was conducted under GWACC Animal Use Protocol 1522. Common carp (n = 13) and Ictiobus (n = 13) were collected by boat electrofishing or seining in Hamilton Harbour and Jordan Harbour, Lake Ontario. Fishes were held at the Aquatic Life Research Facility (Burlington, ON, Canada) before release. For acoustic tagging, individuals were anaesthetised with a Portable Electrosedation System (PES, Smith-Root Inc., Vancouver, BC, Canada; Kim et al. 2017) and then weighed, measured and implanted with a 19 mm HTI™ acoustic tag (Model 900 LV, Hydroacoustic Technology Inc., Seattle, WA, USA) via surgical incision. After a monitored recovery, individuals were transferred to holding tanks for 24 h before they were transferred to the mesocosm. Once in the mesocosm, fishes were acclimated for 60 h before the first experimental trial.
Aversive stimuli
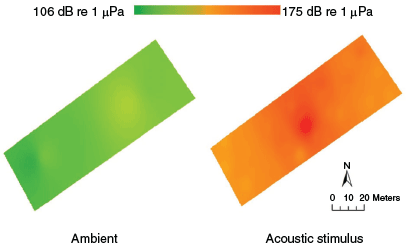
The stroboscopic stimuli were produced by 12 underwater strobe lights, which operated with a frequency of 1–20 Hz. The strobe light produced 51 µmol s–1 m2 of radiation between 400 and 700 nm at 1 m from the source. The acoustic stimulus was a 4-s loop combining a 200–1400-Hz sweep, a 200–1500-Hz band sweep, and a recording of a 50-hp outboard motor (Fig. S2) played with a sound pressure level of 175 dB re 1 µPa at 1 m the speaker (Fig. 1). This was the same stimulus as used in Bzonek et al. (2020). See methods in Supplementary material for details on acoustic profile measurements.
|
Telemetry tracking
To determine fish responses to the aversive stimuli, individuals were tracked with acoustic telemetry. In total, 12 hydrophones were deployed in an array such that tagged individuals could be triangulated between at least three hydrophones (Model 290 ATR, Hydroacoustic Technology Inc., Seattle, WA, USA) and two depth levels. This allowed for the accurate estimation of latitude and longitude for each tagged individual. Fish locations were estimated every 2.1–3.4 s. See methods in Supplementary material for details on position estimates and data filtration.
Statistical analysis
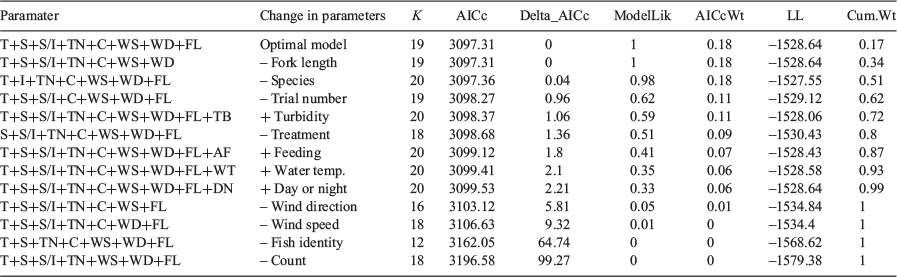
For all statistical analysis, fish positioning was summarised for each fish of each trial of each treatment. Analyses were performed in R version 4.0.2 (R Core Team 2013; Vienna, Austria). Model selection was used to identify the candidate models that best described the 5% avoidance radius size during trials. The 5% avoidance radius size was calculated by determining the radius around deterrents in which only the nearest 5% of detections are included for a given trial and a given fish. Such a metric was used because it captures the repulsive range of the deterrent, while still sensitive to infrequent deterrent passes. The sizes of the avoidance radii were then fit to candidate linear models that were compared with backwards selection using corrected Akaike’s information criterion (AICc). Fish identity was nested within species. All models were visually inspected for normality and homogeneity of variance through examination of residuals and quantile–quantile plot.
To determine the repeatability of fish avoidance, an intra-class correlation coefficient (ICC) was determined for the size of the avoidance radius. The optimal linear model was determined by AICc scores, where species and fish identity were fit as random effects. Adjusted R values were constructed with the ‘rptR’ function of the package rptR (Stoffel et al. 2017).
Results and discussion
Small sample sizes presented a major challenge for this study. All tags were tested and reported working before release, but many were never detected in the mesocosm, indicating a possible failure of the containment net and/or tag failure. Of the 13 tagged Ictiobus released into the mesocosm, 10 individuals were detected by the hydrophone array, and seven individuals continued to be detected throughout the duration of the study. Four individuals displayed regular movement indicative of a fish retaining its acoustic tag and were used in this study. Two of these four Ictiobus individuals stopped moving on the second last day of the experiment. Individuals that did not move for durations greater than 1 day were considered to have died or shed their tag. Every individual that stopped moving for the duration of a day remained stationary throughout the remainder of the experiment. Of the 13 tagged common carp individuals released into the mesocosm, six were detected by the hydrophone array. All six common carp individuals were used in the study, but two individuals were no longer detected by the hydrophone array after the first day of the experiment. Future studies should carefully evaluate the cost and effort of efficiently containing fish within their experimental arena, with the costs being associated with increased tagging sample size.
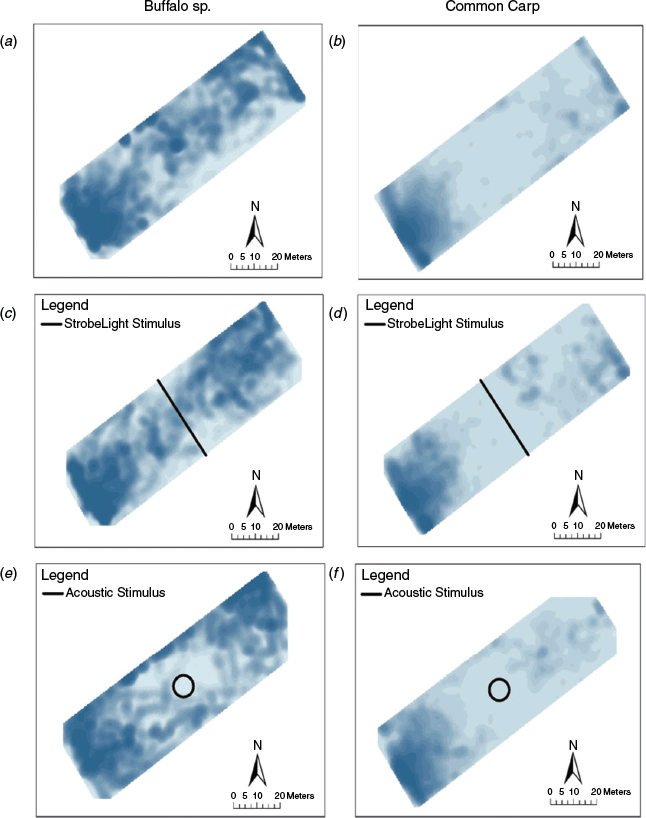
Across all treatments, common carp and Ictiobus displayed a strong preference for the open, western end of the mesocosm (Fig. 2). This may be due to the presence of plant growth on the containment nets, increased water flow through wave action at the open end of the mesocosm, consistent attempts to disperse through the block nets, or a combination of all of these reasons.
Fish avoidance exhibited a weak relationship with treatment type. Of the 13 models that described the 5% avoidance radius, seven were supported (delta AICc of <2; Table 1). The optimal model explained 36% of the variation (adj R2 = 0.36) and described the 5% avoidance radius with treatment, species, fish identity, number of detections, trial number, wind speed, wind direction and fork length (Supplementary material Table S1). There were similar levels of support (delta AICc <2) for models that did or did not include treatment. Thus, the stroboscopic and acoustic stimuli deployed in our study were not an effective deterrent for common carp.
Physical, low-head barriers have been used as a tool to manage sea lamprey migrations throughout the Laurentian Great Lakes, where they have been found to be ~94% effective at halting upstream migrations (Lavis et al. 2003). Non-structural deterrents may still be useful even if less effective than physical barriers because of their increased flexibility and range of applications (Noatch and Suski 2012). However, the weak avoidance responses found in this study would not be useful for management efforts. Caskenette et al. (2018) modelled that the partial exclusion of common carp in marshes can actually increase population growth rate because of improved habitat. Thus, an ineffective non-structural deterrent may be counter-productive to management goals.
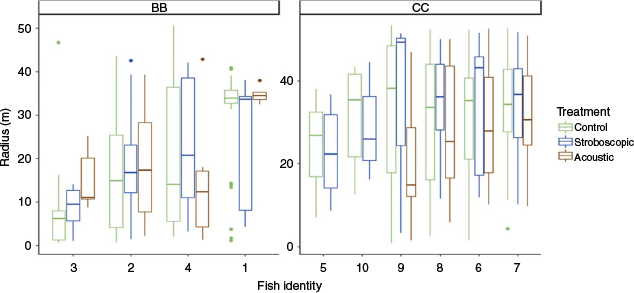
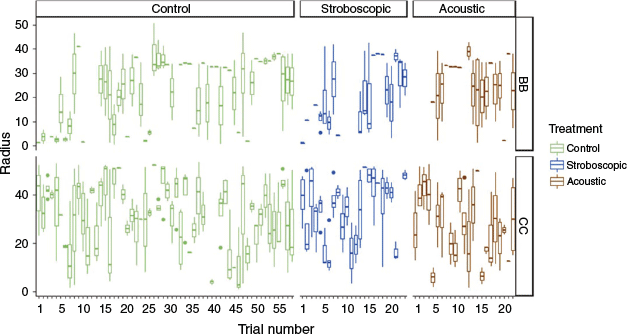
Individuals expressed repeatable deterrent avoidance behaviour (ICC = 0.50, CI = [0.323, 0.752]), with large differences among individuals of the same species (Fig. 3). Intraspecific variation in deterrent avoidance responses is rarely documented in the field but has important implications. Observing this trend in the field indicates that individual variation remains an important factor for deterrent effectiveness despite the added complexity of environmental variation and spatial heterogeneity. In this study, the deterrents consistently produced a weak response in some individuals, which made the deterrent ineffective.
|
Common carp or Ictiobus did not display evidence of habituation. Over the 6 days and nights of experimentation, the stimulus avoidance radius of any treatment did not significantly change in size (Fig. 4). A concern for non-structural deterrents is that deterrent effectiveness may decrease as fishes continue to interact with the deterrents over time. Mixed evidence for habituation to acoustic stimuli has been found. Silver carp expressed habituation or fatigue to complex acoustic tones after ~12 avoidance responses (Vetter et al. 2015), whereas bighead carp responded consistently to complex sound across repeated exposures (Vetter et al. 2017). When fish were placed in a dark environment, silver, bighead and common carps did habituate to sound (Zielinski and Sorensen 2017); however, when sound was coupled with an air curtain in a lit environment, bighead and common carps did not habituate to the stimuli (Dennis et al. 2019). When common carp individuals were exposed to sound trials within a lock, they habituated after the first exposure (Dennis and Sorensen 2020). Fish may not have expressed habituation in our study because of their already muted avoidance response. Alternately, fish may have reduced habituation by spending much of their time near the open end of the mesocosm, or they may not be susceptible to deterrent habitation within a large-scale environment. Future deterrent studies should continue to investigate the potential for stimulus habituation, and deterrent deployment strategies should consider designs that minimise the potential for habituation. For example, non-structural deterrents could be deployed in navigation locks to be activated only when the lock doors are open and upstream dispersal is possible.
|
Why are responses muted?
Common carp and Ictiobus expressed an avoidance response to the stroboscopic and acoustic stimuli, but at lower magnitudes than the responses reported elsewhere. The weaker stroboscopic responses may be due to differences in study species (Kim et al. 2019), strobe-light design (Sullivan et al. 2016) or study environment (Flammang et al. 2014).
The weak acoustic response may be due to the extreme sound-pressure levels throughout the mesocosm and a lack of acoustic refuge. Other studies that found successful acoustic-stimulus avoidance (Vetter et al. 2015; Murchy et al. 2016; Zielinski and Sorensen 2017) used much lower stimulus intensities. The acoustic stimulus in this study had a sound pressure of 175 dB re 1 µPa, which was 57 dB above the ambient conditions, whereas Vetter et al. (2015) and Zielinski and Sorensen (2017) had stimulus pressures of 150 dB re 1 µPa, respectively 30 dB and 70 dB above the ambient conditions. The quietest region of the mesocosm, the open western end, was 153 dB re 1 µPa during the acoustic treatment and was still of considerable biological magnitude, being louder than the loudest regions of the two cited examples, and near the stimulus sound pressure of other acoustic deterrents (Murchy et al. 2016, 2017). Goldfish, Carassius auratus, exposed to similar sound pressures (170 dB re 1μPa), and sound pressures as low as 130 dB re 1μPa, for 24 h expressed a temporary threshold-shift in hearing ability (Smith et al. 2004). Common carp has been found to avoid quieter acoustic gradients of 130–140 dB re 1 μPa in the laboratory (Zielinski and Sorensen 2017), but did not consistently avoid an acoustic stimulus of 145 dB re 1 μPa in the field (Dennis and Sorensen 2020). If fishes did not deem the quietest regions of the mesocosm to be a suitable acoustic refuge, they may have continued to search for an escape or further refuge (Bzonek et al. 2020), resulting in no preference for the quieter regions of the mesocosm. Romine et al. (2015) highlighted a similar concern after monitoring the responses of bigheaded carps to water-gun operations in an enclosed pond.
Our results suggest that, within bounded environments, such as a canal, extreme sound intensities do not appear to act as an effective acoustic deterrent. Instead, acoustic deterrents should be deployed such that target regions provide access to acoustic refuge, with stimulus intensity optimised to avoid excessive sound-pressure levels. The use of multiple speakers, each operating at a moderate sound-pressure level, would allow for finer control in manipulating the acoustic environment. This study had small sample sizes and should be considered a pilot investigation. Although telemetry studies with small sample sizes have successfully described fish behaviour in the past (Laffargue et al. 2006), the findings presented here require additional empirical support to form more definitive conclusions. Future field studies should rigorously map sound-pressure levels before fish release to ensure sufficient acoustic refuge. Finally, ambient sound conditions should be considered when choosing deterrent stimuli. Noisy environments, such as those near dams, highways, construction, or loud industrial activities, may not be optimal for acoustic deterrent deployment.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This project was funded by the Fisheries and Oceans Canada Asian Carp Program.
Acknowledgements
We thank members of the Fisheries and Oceans Canada Asian Carp Program who aided in the maintenance and daily operation of the mesocosm: Cailtyn Bondy, Catherine Chandler, Bradley Doyle, and Kyle Crans. We also acknowledge all those that aided in the capture of wild fishes, and their maintenance at the Aquatic Life Research Facility: Alicia Mehlenbacher, Dave Marson, D’Arcy Campbell, Alex Price, and Asian Carp Program crew members. Tej Heer, Adrienne McLean, Fielding Montgomery, and members of the Mandrak lab provided helpful discussion of this work. This study was conducted according to the Animal Use Protocol approved by the Animal Care Committee at Canada Centre for Inland Waters.
References
Bart, H. L., Clements, M. D., Blanton, R. E., Piller, K. R., and Hurley, D. L. (2010). Discordant molecular and morphological evolution in buffalofishes (Actinopterygii: Catostomidae). Molecular Phylogenetics and Evolution 56, 808–820.| Discordant molecular and morphological evolution in buffalofishes (Actinopterygii: Catostomidae).Crossref | GoogleScholarGoogle Scholar | 20433933PubMed |
Bzonek, P. A., Kim, J., and Mandrak, N. E. (2020). Short-term behavioural response of common carp, Cyprinus carpio, to acoustic and stroboscopic stimuli. Management of Biological Invasions 11, 279–292.
| Short-term behavioural response of common carp, Cyprinus carpio, to acoustic and stroboscopic stimuli.Crossref | GoogleScholarGoogle Scholar |
Caskenette, A., Enders, E. C., Watkinson, D., and Wrubleski, D. (2018). Partial exclusion of spawning Cyprinus carpio to improve coastal marsh habitat may come at the cost of increased carp population growth. Ecological Modelling 385, 58–64.
| Partial exclusion of spawning Cyprinus carpio to improve coastal marsh habitat may come at the cost of increased carp population growth.Crossref | GoogleScholarGoogle Scholar |
COSEWIC (2009). COSEWIC Assessment and Status Report on the Bigmouth Buffalo Ictiobus cyprinellus, Great Lakes – Upper St Lawrence populations and Saskatchewan – Nelson River populations, in Canada. Ottawa, ON, Canada.
Dennis, C. E., and Sorensen, P. W. (2020). Common carp are initially repelled by a broadband outboard motor sound in a lock chamber but habituate rapidly. North American Journal of Fisheries Management 40, 1499–1509.
| Common carp are initially repelled by a broadband outboard motor sound in a lock chamber but habituate rapidly.Crossref | GoogleScholarGoogle Scholar |
Dennis, C. E., Zielinski, D., and Sorensen, P. W. (2019). A complex sound coupled with an air curtain blocks invasive carp passage without habituation in a laboratory flume. Biological Invasions 21, 2837–2855.
| A complex sound coupled with an air curtain blocks invasive carp passage without habituation in a laboratory flume.Crossref | GoogleScholarGoogle Scholar |
Downing, A. S., van Nes, E. H., Mooij, W. M., and Scheffer, M. (2012). The resilience and resistance of an ecosystem to a collapse of diversity. PLoS One 7, e46135.
| The resilience and resistance of an ecosystem to a collapse of diversity.Crossref | GoogleScholarGoogle Scholar | 23029410PubMed |
Flammang, M. K., Weber, M. J., and Thul, M. D. (2014). Laboratory evaluation of a bioacoustic bubble strobe light barrier for reducing Walleye escapement. North American Journal of Fisheries Management 34, 1047–1054.
| Laboratory evaluation of a bioacoustic bubble strobe light barrier for reducing Walleye escapement.Crossref | GoogleScholarGoogle Scholar |
Kim, J., Doyle, B., and Mandrak, N. E. (2017). Electrosedation of freshwater fishes for the surgical implantation of transmitters. Canadian Journal of Zoology 95, 575–580.
| Electrosedation of freshwater fishes for the surgical implantation of transmitters.Crossref | GoogleScholarGoogle Scholar |
Kim, J., Bondy, C., Chandler, C. M., and Mandrak, N. E. (2019). Behavioural response of juvenile common carp (Cyprinus carpio) and juvenile channel catfish (Ictalurus punctatus) to strobe light. Fishes 4, 29.
| Behavioural response of juvenile common carp (Cyprinus carpio) and juvenile channel catfish (Ictalurus punctatus) to strobe light.Crossref | GoogleScholarGoogle Scholar |
Laffargue, P., Bégout, M. L., and Lagardère, F. (2006). Testing the potential effects of shellfish farming on swimming activity and spatial distribution of sole (Solea solea) in a mesocosm. ICES Journal of Marine Science 63, 1014–1028.
| Testing the potential effects of shellfish farming on swimming activity and spatial distribution of sole (Solea solea) in a mesocosm.Crossref | GoogleScholarGoogle Scholar |
Lavis, D. S., Hallett, A., Koon, E. M., and McAuley, T. C. (2003). History of and advances in barriers as an alternative method to suppress sea lampreys in the great lakes. Journal of Great Lakes Research 29, 362–372.
| History of and advances in barriers as an alternative method to suppress sea lampreys in the great lakes.Crossref | GoogleScholarGoogle Scholar |
Leung, B., Lodge, D. M., Finnoff, D., Shogren, J. F., Lewis, M. A., and Lamberti, G. (2002). An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings of the Royal Society B. Biological Sciences 269, 2407–2413.
| An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species.Crossref | GoogleScholarGoogle Scholar |
Maiolie, M. A., Harryman, B., and Ament, B. (2001). Response of free-ranging kokanee to strobe lights. In ‘Behavioral Technologies for Fish Guidance. American Fisheries Society Symposium’. (Ed. C. Coutant.) pp. 27–35. (American Fisheries Society: Bethesda, MD, USA.)
Murchy, K., Vetter, B., Brey, M., Amberg, J., Gaikowski, M., and Mensinger, A. (2016). Not all carp are created equal: impacts of broadband sound on common carp swimming behavior. Proceedings of Meetings on Acoustics Acoustical Society of America 27, 010032.
| Not all carp are created equal: impacts of broadband sound on common carp swimming behavior.Crossref | GoogleScholarGoogle Scholar |
Murchy, K. A., Cupp, A. R., Amberg, J. J., Vetter, B. J., Fredricks, K. T., Gaikowski, M. P., and Mensinger, A. F. (2017). Potential implications of acoustic stimuli as a non-physical barrier to silver carp and bighead carp. Fisheries Management and Ecology 24, 208–216.
| Potential implications of acoustic stimuli as a non-physical barrier to silver carp and bighead carp.Crossref | GoogleScholarGoogle Scholar |
Noatch, M. R., and Suski, C. D. (2012). Non-physical barriers to deter fish movements. Environmental Review 20, 71–82.
| Non-physical barriers to deter fish movements.Crossref | GoogleScholarGoogle Scholar |
Patrick, P. H., Mason, E., Powell, J., Milne, S., and Poulton, J. S. (2014). Evaluating the effectiveness of the Pickering Nuclear Generating Station fish diversion system barrier net. North American Journal of Fisheries Management 34, 287–300.
| Evaluating the effectiveness of the Pickering Nuclear Generating Station fish diversion system barrier net.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org/.
Ricciardi, A., and MacIsaac, H. J. (2011). ‘Fifty years of invasion ecology: The legacy of Charles Elton: Impacts of biological invasions on freshwater ecosystems.’ (Ed. D. M. Richardson.) (Blackwell Publishing: West Sussex, UK.)
Romine, J. G., Jensen, N. R., Parsley, M. J., Gaugush, R. F., Severson, T. J., Hatton, T. W., Adams, R. F., and Gaikowski, M. P. (2015). Response of Bighead Carp and Silver Carp to repeated water gun operation in an enclosed shallow pond. North American Journal of Fisheries Management 35, 440–453.
| Response of Bighead Carp and Silver Carp to repeated water gun operation in an enclosed shallow pond.Crossref | GoogleScholarGoogle Scholar |
Ruebush, B. C., Sass, G. G., Chick, J. H., and Stafford, J. D. (2012). In-situ tests of sound-bubble-strobe light barrier technologies to prevent range expansions of Asian carp. Aquatic Invasions 7, 37–48.
| In-situ tests of sound-bubble-strobe light barrier technologies to prevent range expansions of Asian carp.Crossref | GoogleScholarGoogle Scholar |
Smith, M. E., Kane, A. S., and Popper, A. N. (2004). Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water? The Journal of Experimental Biology 207, 3591–3602.
| Acoustical stress and hearing sensitivity in fishes: does the linear threshold shift hypothesis hold water?Crossref | GoogleScholarGoogle Scholar | 15339955PubMed |
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods in Ecology and Evolution 8, 1639–1644.
| rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models.Crossref | GoogleScholarGoogle Scholar |
Strayer, D. L. (2010). Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55, 152–174.
| Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future.Crossref | GoogleScholarGoogle Scholar |
Sullivan, B. G., Wilson, A. D. M., Gutowsky, L. F. G., Patrick, P. H., Sills, M., and Cooke, S. J. (2016). The behavioral responses of a warmwater teleost to different spectra of light-emitting diodes. North American Journal of Fisheries Management 36, 1000–1005.
| The behavioral responses of a warmwater teleost to different spectra of light-emitting diodes.Crossref | GoogleScholarGoogle Scholar |
Vetter, B. J., Cupp, A. R., Fredricks, K. T., Gaikowski, M. P., and Mensinger, A. F. (2015). Acoustical deterrence of silver carp (Hypophthalmichthys molitrix). Biological Invasions 17, 3383–3392.
| Acoustical deterrence of silver carp (Hypophthalmichthys molitrix).Crossref | GoogleScholarGoogle Scholar |
Vetter, B. J., Murchy, K. A., Cupp, A. R., Amberg, J. J., Gaikowski, M. P., and Mensinger, A. F. (2017). Acoustic deterrence of bighead carp (Hypophthalmichthys nobilis) to a broadband sound stimulus. Journal of Great Lakes Research 43, 163–171.
| Acoustic deterrence of bighead carp (Hypophthalmichthys nobilis) to a broadband sound stimulus.Crossref | GoogleScholarGoogle Scholar |
Weber, M. J., and Brown, M. L. (2009). Effects of common carp on aquatic ecosystems 80 years after ‘Carp as a Dominant’: ecological insights for fisheries management. Reviews in Fisheries Science 17, 524–537.
| Effects of common carp on aquatic ecosystems 80 years after ‘Carp as a Dominant’: ecological insights for fisheries management.Crossref | GoogleScholarGoogle Scholar |
Zielinski, D. P., and Sorensen, P. W. (2017). Silver, bighead, and common carp orient to acoustic particle motion when avoiding a complex sound. PLoS One 12, e0180110.
| Silver, bighead, and common carp orient to acoustic particle motion when avoiding a complex sound.Crossref | GoogleScholarGoogle Scholar | 28654676PubMed |