Fish biodiversity and inferred abundance in a highly valued coastal temperate environment: the inner Queen Charlotte Sound, New Zealand
Rodelyn Jaksons A B , Peter Bell C , Peter Jaksons A and Denham Cook C D *
C D *
A Sustainable Production Group, The New Zealand Institute for Plant and Food Research Limited, Canterbury Agriculture and Science Centre, 74 Gerald Street, Lincoln 7608, New Zealand.
B School of Mathematics and Statistics, University of Canterbury, Ilam, Christchurch 8041, New Zealand.
C Seafood Production Group, The New Zealand Institute for Plant and Food Research Limited, 293 Akersten Street, Port Nelson 7010, New Zealand.
D University of Waikato Coastal Marine Field Station, 58 Cross Road, Sulphur Point, Tauranga 3114, New Zealand.
Marine and Freshwater Research 73(7) 940-953 https://doi.org/10.1071/MF21247
Submitted: 27 August 2021 Accepted: 21 April 2022 Published: 26 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Context: The inner Queen Charlotte Sound–Tōtaranui is a focal and emblematic coastal area in New Zealand that is valued by diverse stakeholders. Fish diversity in the region is not well characterised.
Aims: This study sought to provide an inventory of local fish populations, determine the relative abundance of all species observed, and quantify fish biodiversity (including teleost, elasmobranch, syngnathid, chimaera, and cephalopod) in the region.
Methods: Baited remote underwater video, a spatially balanced acceptance sampling design, and Bayesian spatio-temporal analysis approaches using integrated nested Laplace approximation (INLA) were employed.
Key results: In total, 35 species were observed over 3 years. Average site-specific levels of species abundance were low (∼3) with only modest levels of biodiversity (Shannon–Wiener value = 0.65, Simpsons index = 0.51). On the basis of spatial residuals, greater species diversity was identified in western arms of the sound.
Conclusions: These findings provide a useful insight into the biodiversity of fish in the region, and baseline information on the relative abundance of a variety of fish species.
Implications: These findings characterise the contemporary status of fish populations in the inner Queen Charlotte Sound and present a useful framework for ongoing investigations of fish populations in this, and other, inshore marine environments.
Keywords: assemblage, BRUV, INLA, inshore, marine, modelling, spatial, survey.
Introduction
Coastal marine environments possess considerable societal and ecological value. They contribute to the livelihoods of the maritime and tourism sectors, energy production and mining industries, food production and capture industries, and provide significant cultural value and recreational opportunities (Costanza et al. 1997). This value is emphasised by the fact that urbanisation, economic development, and associated high population densities associate with coastal environments across the globe (Inman and Brush 1973). Because of this perceived value and utility, coastal habitats are generally recognised to be suffering a range of anthropogenic-derived environmental stressors such as climatic variations, nutrient stress, sedimentation, overfishing, habitat degradation and the spread of invasive species (Nixon 1995; Bax et al. 2003; Harley et al. 2006; Peterson and Lowe 2009).
Efforts to manage these marine environments seek to balance social, biological, and economic considerations to maximise the benefits from these valuable environments. Yet, efforts and resources are disproportionally directed at discrete areas generally deemed to possess significant biodiversity value, often at the expense of reduced coverage across habitats deemed less significant or valuable, yet potentially subject to the greatest levels of degradation (Roberts et al. 2003; Willis et al. 2003). Conversely, fisheries management is often focused on the analysis of data gathered for single-species stocks spread across large reporting areas (Mace et al. 2014). To address these challenges integrated monitoring and management strategies need to be developed that cover multiple species over a broad scale, and modelled or researched to infer or deliver finer-scale spatial resolution. To achieve this, the application of cost-effective tools that facilitate the rapid assessment of patterns of marine diversity and abundance are required. Once effective baselines have been established, ongoing research and increasingly rich datasets can then be incorporated or adapted to investigate broader ecosystem-level phenomena, i.e. infer and rank the relative threats of localised pressures (e.g. over fishing, sedimentation, and their cumulative impacts, Halpern et al. 2019; Li and Convertino 2021). These strategies can then be adapted to facilitate ongoing decision-making, track outcomes and adapt programs to optimise regional recovery.
Despite New Zealand’s brief history of settlement, coastal activity, and development, the same range of environmental stressors as experienced globally is also reported across its coastal marine environment (Thrush et al. 2004; Rodil et al. 2013). These stressors are anecdotally considered to have imposed abrupt change on various coastal marine environments and their fauna; however, a paucity of baseline information describing many regions and their faunal populations presents challenges to those wishing to quantitatively consider and evaluate the consequences of these supposed changes. One such example of an affected New Zealand marine environment is the Queen Charlotte Sound–Tōtaranui (Fahey and Coker 1992; Parnell et al. 2007; Urlich and Handley 2020; Watson et al. 2020).
The Queen Charlotte Sound comprises an extensive network of inlets and bays, and is located on the north-eastern margin of New Zealand’s South Island. The sheltered waterways, coastal forests, mountainous landscape, marine life (including marine mammals and birds), and the historic importance of the region, combine with a relatively small standing population (of ∼5000 people) to create a highly valued natural environment and destination. Economic activities associated with the region include ecotourism, notable recreational fishing effort, historical commercial fishing effort, fin fish aquaculture in the outer regions of the sounds, forestry, and maritime operations (port facilities, passenger ferries and cargo shipping). The Queen Charlotte Sound therefore represents an environment where multiple stakeholders derive economic and societal value from a blue economy. Yet, the activities of each of these stakeholders may potentially influence the natural diversity and abundance of fish because of the extractive pressures or environmental modifications (e.g. habitat degradation, water quality, anthropogenic noise) that associate with their activity. Despite the value of this region, research focus is limited, and few characterisations of the mobile species assemblages within this coastal environment have been undertaken or described in the scientific literature (with the exceptions of Cole et al. 2000; Davidson 2001; Francis et al. 2011; Beentjes and Carbines 2012; Handley 2016; Urlich and Handley 2020). This study therefore aimed to provide a necessary characterisation of fish biodiversity in the inner Queen Charlotte Sound.
The relative absence of baseline biodiversity and species abundance information is commonly acknowledged for key New Zealand marine environments, which limits the ability of managers and observers to gauge any changes (i.e. improvements or declines) in the coastal environment (Jarvis and Young 2019). To address this issue, this study sought to employ a combination of baited remote underwater video (BRUV)-monitoring techniques, a spatially balanced acceptance sampling design, and Bayesian spatio-temporal analysis approaches using integrated nested Laplace approximation, or INLA, to develop a baseline dataset with the overarching intention of characterising the biodiversity in the region. Specific aims of the study included identifying the contemporary state of the region, including (1) an inventory of the species present in the region, (2) determinations of the relative abundance of each species, and (3) an assessment of the biodiversity and species richness throughout the inner sounds. Opportunistic assessments of highly valued ‘focal species’ were also undertaken following determinations of relative abundance. This combination of data was generated to establish quantitative estimates of fish abundance and diversity that address (in part) the absence of information describing the inshore fisheries of this nationally important coastal marine environment. Outcomes from this research are intended to support future monitoring efforts in the region and to identify a useful framework for BRUV-based surveys that are transferrable to other regions that also require baseline investigations.
Materials and methods
Study area and sampling design
The focal region of the study was the Queen Charlotte Sound–Tōtaranui, a drowned river valley comprising many rias that runs in a north–east to south–east direction before joining with the Cook Strait of New Zealand (centred on 41°13′45″S, 174°08′37″E, WGS84). Focusing on the innermost region of Queen Charlotte Sound across an area of ∼45 km2 (Fig. 1), topo-maps of the region were extracted from the NZ Coastlines topographic database (Topo 1:50 k) representing New Zealand’s boundary between land and sea (National Topographic Office 2017). Boundaries to the sampling region were defined by the mean high-water line of the inner sound to a line extending between Golden Point (41°13′44″S, 174°04′30″E, WGS84) and Motueka Bay (41°15′06″S, 174°04′33″E). Two sampling strata were generated from this region termed ‘shallow’ and ‘deep’, referring to nearshore locations of less than 25-m bathymetric depth, and locations greater than 25-m depth, throughout the sampling region. The maximum charted depth in the sampled area was 53 m. For detailed bio-physical descriptions of the Queen Charlotte Sound, please refer to Hadfield et al. (2014).
To select the sampling sites across all seasons and years, a spatial balanced probability sample was selected using balanced acceptance sampling (BAS; Robertson et al. 2013). The probabilities were set such that a similar number of sites would be selected in the ‘shallow’ and ‘deep’ strata. By using the BAS strategy, the 36 selected sampling sites were ensured to be spatially balanced, thus evenly distributed and avoiding clustering over space as well as over time.
Data collection
Sampling was performed seasonally over a continuous 3-year period between 2017 and 2020, starting on 1 September 2017 and ending on 28 May 2020. Each seasonal sample was performed as a single event over a 2-day period where observations from all 36 prescribed sampling sites were collected. Four BRUV rigs equipped with GoPro cameras (Hero3, 3+ Woodman Labs, California, USA) or GitUp cameras (Git1, GitUp Ltd, Shenzen, PR China) were deployed in sequence across the four closest waypoints. Following a minimum of 30-min soak time, each of the four BRUV rigs was retrieved and prepared for a subsequent deployment at the next set of neighbouring sampling points. Three, and on rare occasions up to four, sequential sets of deployment were performed during a sampling session. Sampling sessions were scheduled to occur within 5 h after sunrise, or 5 h prior to sunset on all occasions, so as to align with anecdotal daily behavioural rhythms (Helfman 1986).
The bait attached to the BRUV consisted of a pre-frozen standardised attractant placed in a burley canister, replaced immediately prior to each deployment. The bait comprised 300 g of minced pilchard (Sardinops sp.), minced greenshell mussel meat (Perna canaliculus), and tapwater (in a ratio 1:1:1 w/w), combined, and then ground using a 10-mm mincing pate. This mix of attractants was intended to represent the diversity of diet types (e.g. baitfish and shellfish) typical of the region and its fish populations. Logging temperature recorders (RBR SoloT, RBR Ltd, Canada) were attached to each rig to record bottom temperatures, and on select occasions, logging pressure sensors (RBRduetTD, RBR Ltd) were also deployed to corroborate bathymetric depth data. Immediately prior to deployment, sampling metadata were recorded (including start time, GPS waypoints, surface-water temperatures) and the BRUV lowered at the required GPS coordinates.
No ethical permits were required for this study, which complies with all relevant regulations.
Video analysis
Video footage from each site was analysed by a sole reviewer to eliminate bias among observers by using VLC media player (ver. 3.0, VideoLAN Org, Paris, France). The full length of video footage was reviewed, with data collection commencing as soon as video showed that the BRUV was stable on the benthos and ended when the rig was first disturbed during retrieval. Slight differences in observation time were then accounted for statistically (refer to the ‘Statistical analysis’ section). Video was reviewed at 1×, 1.5× or 2× magnification, depending on water clarity and fish densities. Repeated review of footage occurred when species proved difficult to identify or count.
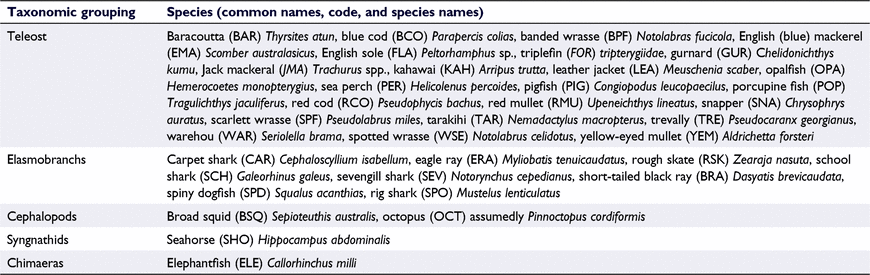
For each video recording and deployment, the time of first arrival (TTFA_SPC) for each species present in the recording was noted, along with a count of maximum number of fish occurring in any one video frame for each species (MaxN_SPC, where SPC represents the species code of the observed species; Table 1). When fish abundance was high (e.g. >100 individuals), it was often not possible to precisely determine MaxN. In these instances, MaxN was estimated to the nearest 10 value. Where a fish species could not be confidently identified, it was recorded alongside arrival time as unknown (UNK). For two highly valued species observed in this study, namely snapper (SNA) and blue cod (BCO), the combined residence time each species spent on screen (either individually, or as a group) was observed and recorded under the denotation Residence_SPC, although this metric was not investigated further. Variations associated with the BRUV deployment (i.e. rig landed in a vertical orientation) were noted. The substrate composition (i.e. rock, mud or silt) and structures (i.e. burrows or tracks) were recorded, along with counts of any identifiable benthic species (e.g. algae and invertebrates). The entirety of the footage between start and end times was examined.
|
Using the bait canister and the benthos as reference points, relative visibility was defined using a series of descriptive variables ranging from excellent to terrible (Excellent–Very Good; Good, OK or Poor; and Very Poor or Terrible). Visibility often varied during the footage because of variance in lighting, fish activity, or current; the change in relative classification was noted as this occurred. Sites where visibility was reduced to zero were recorded as NA.
Habitat characterisations
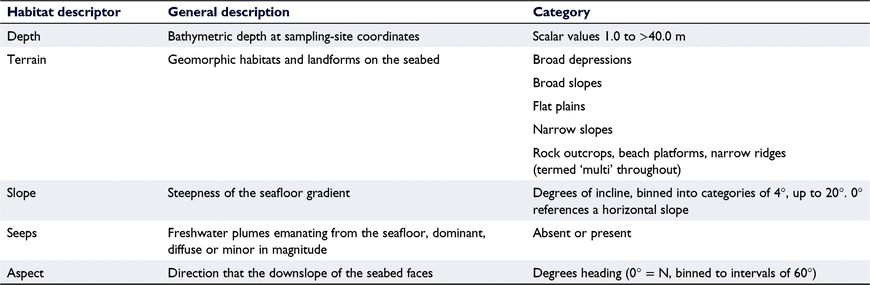
Habitat characteristics including terrain, slope, depth, aspect, and seeps were identified for each of the 36 sampling sites (Table 2). Identification of habitat characteristics was derived from seabed habitat maps provided by the Marlborough District Council, generated by Multibeam sonar (https://arcg.is/85bHT). Distance to shore was calculated as the minimum distance between the sampling point and the coastline. Habitat characteristics including depth, distance to shore, and terrain were retained for the primary analysis, with remaining characterisations excluded after exploratory analyses identified poor associations between these variables and outcomes.
|
Abundance and biodiversity metrics
The abundance index for each species was defined as the MaxN value (defined above). The total number of species observed in each analysed recording was then summed to define SpeciesCount. Derived biodiversity metrics including the Shannon–Wiener index (SW), and Simpson’s index (SI), were calculated according to the standard formulas (Magurran 2004). The SW indicates the diversity of the observed population. Low values of the SW index indicate low diversity or richness (randomness in species present), with progressively larger numbers indicating increased diversity. SI indicates the dominance, or evenness of any given species at the sampling site, where low values of SI indicate an assemblage of high diversity in species present, whereas high values of SI (e.g. 1) indicate low diversity (i.e. a mono-species assemblage).
Statistical analysis
Data produced from this study was analysed using Bayesian statistics by using R-INLA (ver. 21.6.11) in the R software package (ver. 4.0.5, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/; Rue et al. 2009). INLA was used for its fast computation of Bayesian models and the ability to fit the spatial Barrier model (Bakka et al. 2019). With all spatial data, Tobler’s first law of geography states that things in closer proximity are more alike than those that are further away. However, when it comes to fish abundance, then it is sensible to not only consider distance, but also barriers that would place objects further apart despite their close spatial proximity. Consider we have three sampling locations, where the first two locations are close to one another in distance but separated by a peninsula. Whereby a third location is further in distance from the first but does not have any physical barriers, then it is sensible to assume that first and third locations are more alike because there is no physical barrier that separates them. Therefore, the Barrier model that we applied differs from other spatial models because it considers connectivity of locations through a series of paths. The paths can then be altered so that paths between close points are cut-off if there is a physical barrier between them, such as in the aforementioned example. The Barrier model is particularly useful for coastal and convoluted waterways and has been used to analyse both biodiversity in coastal areas of Iceland and to identify vulnerable habitats of bottlenose dolphins in the Mediterranean (Jónsdóttir et al. 2019; Martínez-Minaya et al. 2019).
Three separate models were run to investigate species count, SI and SW, independently. To account for possible zero inflation (the large numbers of observations with no species detected), species count was assessed by each of the Poisson, negative binomial, Gaussian, and zero-inflated Poisson models. Model selection for species count was based on deviance information criterion (DIC) where lower values are preferred. Because the analyses showed that the Poisson likelihood had the best model fit, these results are discussed herein. The variables SW and SI were modelled using a Gaussian likelihood. The intensity parameter for SpeciesCount, and the mean parameter for SW index and SI were treated as a function of depth, temperature, and distance to shore. In addition, the models also consisted of random effects for sampling site, year, visibility, terrain and season. As logistical and technical challenges resulted in differences in the duration that each video recorded observations, an offset was included for the footage length for the species count variable, therefore turning the intensity parameter into rates per minute.
All of the models included spatially correlated errors and were assigned a penalised complexity Matérn prior, with the range parameter given P (range < 0.03) = 0.9, i.e. small to medium spatial variation is likely. The spatial variability or sill was given the prior P (spatial variability > 1) = 0.5. The linear fixed effects consisted of depth, temperature and distance to shore. For all response variables, the fixed effect and intercept priors were assigned a normal prior with a mean of zero and a precision of 0.001. The categorical variables were treated as random effects. The variables sampling site, year, visibility and terrain were treated as independent identically distributed normal random effects with means of zero and their corresponding precision parameters were given a gamma (0.01, 0.01) prior distribution. These predictors were treated as random effects because these variables had many factor levels. Although they are referred to as random effects, their effects can be considered as a shift in the intercept which allows one to see the change from the baseline. As the season variable was ordered and cyclical, they were given a Gaussian first-order random walk prior, which induced correlation or smoothed between nearby time points.
Results
Observation overview
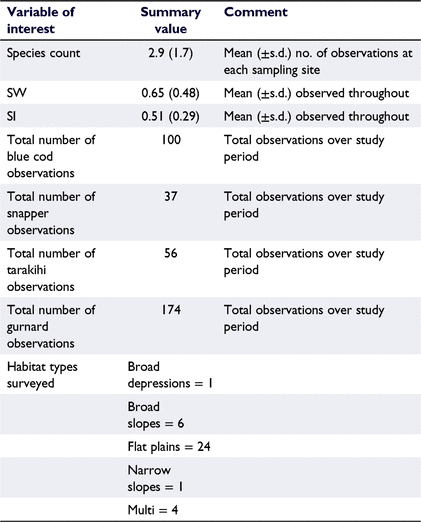
Throughout this study 35 different species of taxa were observed, primarily teleost fish followed by elasmobranch (sharks, skates and rays) and limited numbers of chimaeras, cephalopods, and sygnathids (seahorses and pipefish; Table 1), with a total of 5512 fish being observed across the 432 BRUV deployments (refer to Supplementary material). Among the teleost, spotted wrasse was the most numerically dominant species observed (MaxN = 8.45 ± 0.60, mean ± 95% CI, Table 3). Recreationally targeted and highly valued species including blue cod, snapper, tarakihi, and gurnard were observed infrequently, all with mean MaxN values of <0.5 across all observed sample sites. Spikey dogfish represented the most numerically dominant elasmobranch species (MaxN = 0.51 ± 0.11, mean ± 95% CI, Table 3). The mean duration lapsed before each species was observed in the footage (i.e. TTFA) varied, with species such as kahawai, blue cod and spotted wrasse being observed in <10 min from the beginning of the observation period, whereas other commonly observed teleost species including trevally, snapper and tarakihi weren’t typically observed until a mean duration of ∼20 min (Table 2). Commonly observed elasmobranchs (including rig, carpet shark, spiny dogfish, eagle ray and rough skate) were observed between 8 and 25 min, on average (refer to Supplementary material). Summary statistics of key raw variables of interest are presented below (Table 4), where after modelled outcomes are presented in the following sections.

|
|
Species abundance
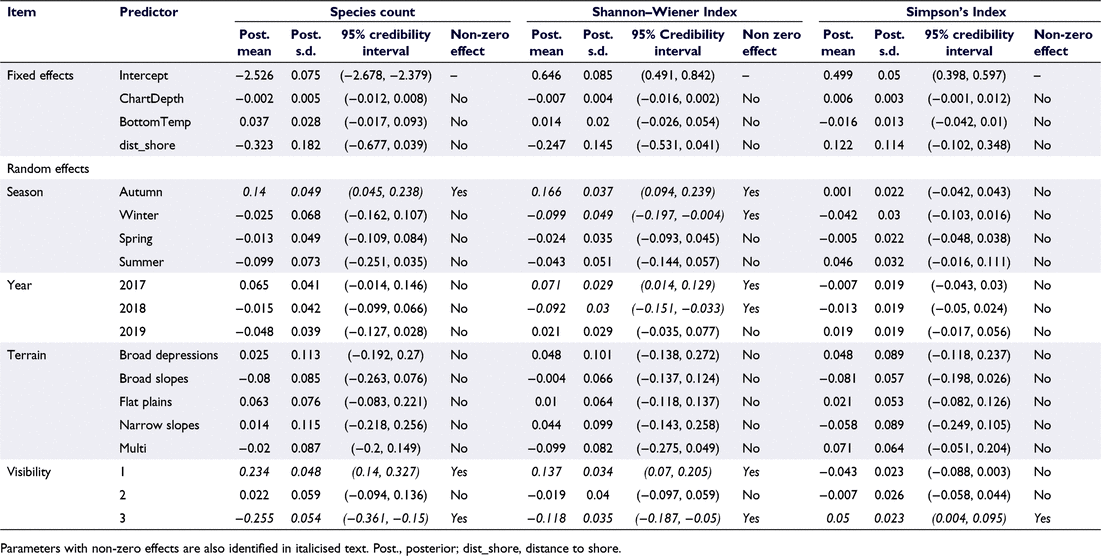
Modelled analysis of SpeciesCount identified that the credible intervals of the predictor’s distance to shore, depth, temperature, year and terrain included zero, suggesting no statistical support for an association of these predictors with species richness outcomes (Table 5). For the seasonal predictor, the 95% credible interval results suggest that austral autumn is positively associated with SpeciesCount (Table 5, Fig. 2a). For visibility, the 95% credible interval indicates a purely positive effect on SpeciesCount when visibility was good (visibility score = 1) and a negative association when visibility was bad (visibility score = 3; Table 5, Fig. 2b). The spatial interpolation of the model residuals does not indicate any areas of above-average SpeciesCount (Fig. 2c–e).
|
Fish biodiversity
Summary statistics describing the biodiversity of the study region were identified to be 0.65 and 0.51 for the SW and SI respectively (Table 4). Our model suggests that key variables including depth, bottom temperature and distance to shore were not associated with the SW biodiversity metric (Table 5). The effect for season shows that austral autumn has the highest positive effect on the SW index, with its 95% credible interval showing support for a purely positive effect (Fig. 3a). The effect for year shows that 2018 had, on average, a lower SW value (Fig. 3b). On average, the SW index was highest for visibility score one, and lower for visibility score three (Fig. 3c). Higher than expected SW values, identified by high residual exceedance probabilities, were detected in sampling sites located in the western arm of the Queen Charlotte Sound (Fig. 3d–f).
For SI, all of the credible intervals for predictors included zero; therefore, one could not rule out a zero or no effect. Furthermore, the model residuals do not indicate any areas of above-average SI (Fig. 4a–c).
Discussion
Fish biodiversity and abundance
The present investigation identified 35 different species of fish (including teleost fishes, cephalopods, and chondrichthyans) distributed through the inner-most coastal environment of the Queen Charlotte Sound. Each site sampled typically observed ∼12 or 13 individuals throughout the duration of recording, although, on occasions, observations of up to 100 individuals were registered (refer to Supplementary material). Typically, each observation identified between one and five different species, and the duration period employed (∼30 min) captured a broad variety of fish species.
Throughout the period of observation, the species counts observed in the inner Queen Charlotte Sound varied seasonally but not annually. This suggests that although the presence of various species fluctuates on a regular basis, the species assemblage present in inner Queen Charlotte Sound was stable across the surveyed period. When comparing abundance metrics with comparable coastal locations, measures of species count were lower than those observed during underwater visual census techniques (diver surveys) in both inshore and offshore rocky reef ecosystems from north-eastern regions of New Zealand, lower than species richness observations in temperate southern hemisphere sites (Tasmania, Australia) of comparable latitude, and lower than measures of fish abundance and species richness observed in more northern surveys of coastal fish abundance and diversity in Australia (Edgar and Barrett 1999; Holmes et al. 2013; Parsons et al. 2016). However, comparisons of high- and low-latitude locations (akin to the prior comparison) are fraught as one considers the well documented decrease in species richness that occurs with increasing latitude across a range of marine systems (McClatchie et al. 1997; Francis et al. 2011). It must also be noted that these survey metrics cited were also derived by underwater visual-census (diver-based) techniques and therefore the ability to perform direct comparisons are equivocal.
More poignant comparisons with estuarine and harbour systems throughout New Zealand are also lower than expected species counts and individuals in the current study. Species richness values of 11 have been uniformly reported in another study sampling the Queen Charlotte Sound (including geographically nearby sampling sites), yet the mean number of species observed during our observations was less than three (Francis et al. 2011). The lower values observed in our study may plausibly relate to two factors, including (1) the differences in sampling techniques, whereby Francis et al. (2011) used beach seining techniques and (2) the commonly accepted notion that estuarine ecosystems support high primary and secondary productivity, as well as diverse fish and invertebrate populations (the inner Queen Charlotte Sound and the sites sampled in this study did not have significant freshwater or estuarine characteristics; Beck et al. 2001). Despite the apparent and uniformly depressed determinations of species counts in this study, we stress the paucity of historical data describing species and abundance counts in the Queen Charlotte Sound region, which makes it challenging to determine whether these low values are typical of the region. This highlights that the development of baseline data is a critical step in being able to detect future changes, even if past changes cannot be clearly identified.
Overall biodiversity scores determined throughout the duration of sampling were quantified with SW scores of 0.646 and a SI of 0.499, although locations in the western reaches of the sound had higher than expected levels of diversity (SW scores). This indicates that sites in the Queen Charlotte Sound generally had low levels of species richness and moderate levels of species evenness, with a ∼50% probability that two species drawn at random would be the same. Comparisons, again to the north-eastern North Island, identified that these more northern and warmer temperate locations had SW indexes greater than 1 in various nearshore habitats (e.g. shallow and deep reef systems, kelp, turf and sponge habitats); however, it must be noted that much lower SW values (∼0.1) were observed in sand habitats. Species evenness was comparable, with the SI ranging between ∼0.5 and 0.6, but again with lower values (∼0.1) on sand habitats (Williams et al. 2008). Owing to differences in sampling design, these differences do not afford a direct comparison; however, they do identify that microscale habitat structure can have a measurable effect on diversity metrics.
On all three metrics of abundance and diversity, the relative visibility scores recorded during video analysis had a negative association with the statistical outcomes of this study (e.g. poor visibility associated with below-average outcomes). This represents an obvious limitation of any visual survey method and is commonly associated with BRUV systems (Mallet and Pelletier 2014; Bicknell et al. 2016). Whether this outcome (1) adversely influenced the ability of the analyser to interpret the video (i.e. a systematic sampling error), (2) influenced fish behaviour and their responses to the sampling rig, or (3) led to a more general displacement of fish from the general survey area, cannot readily be discerned. With respect to the last of these alternatives, the behavioural responses of fish to regions of poor water quality, or how the prevailing environmental conditions affect fish responses to (expectedly novel) BRUV systems, present an interesting line of future research that may present additional factors that need to be considered and accounted for during biodiversity surveys of fishes.
Relative abundance of valued species
Throughout this study, many species of high recreational and or commercial value were observed, albeit at low levels of relative abundance. Blue cod is one of these species, and was observed at a range of life stages (results not presented). A notable reduction of blue cod length has been associated with the Queen Charlotte Sound region over the past ∼100 years, and localised depletion of blue cod is commonly associated with the wider Queen Charlotte Sound region (Rapson 1956; Beentjes et al. 2018). Results from this study indicated that localised depletion is equally common in the innermost region of the Queen Charlotte Sound. Similarly, the relative abundance of snapper in the region was among the lowest of all species observed in the region. Although a commercial fishery once operated in the region, the occurrence of the species in the Queen Charlotte Sound is no longer considered a common feature (Davey et al. 2008; Handley 2016). Because the Queen Charlotte Sound arguably represents a habitat on the southern (cooler) edge of the natural distribution of snapper, its abundance is likely to be highly influenced by environmental and extractive pressures (Parsons et al. 2014). The relative abundances of other commonly valued species including tarakihi, gurnard, trevally and kahawai were also observed and defined in the current study. With limited knowledge of the abundance of each of these species in the region, it is hoped that this baseline data enable future comparisons.
In addition to our observations of species contemporarily valued in the region, it is worth noting that over the 3 years of sampling, there were no observations of large (>2 m length) predatory species such as sharks (excepting one large seven gilled shark), hāpuku (Polyprion oxygeneios), nor any rock lobster (Jasus edwardsii). Although observations of oceanic predators such as shark are rare within the innermost sounds, anecdotal descriptions identify that species such as groper and lobster were once common even in the inner Queen Charlotte Sound (Handley 2016).
The value of the spatial sampling technique and analytical models adopted
The selection of BAS sampling techniques provided a notable benefit to this investigation, providing the ability to investigate over a broad yet spatially balanced scale efficiently. The use of INLA approach also provided significant benefits to the investigation, which could highlight areas of unexpected increased abundance while simultaneously accounting for landscape barriers, valuable in the convoluted coastline of the Queen Charlotte Sound. The suite of descriptive variables collected and used to generate the model also proved to be appropriate predictors of species abundance and biodiversity (primarily the SW index), because most spatial analyses showed locations with high exceedance probabilities. The results of this study have provided a useful description of broad-scale fish abundance and biodiversity. Future studies could expand on this information by conducting further sampling in areas of close proximity to the original sampling sites to allow better estimation of small-scale regional variability, which in turn would provide additional information on habitat characteristics or similar phenomena associated with individual species. The use of the BAS-generated sampling points herein as a ‘master sample’ that can be subsampled in future, or on which with other spatial sampling designs and legacy observations can be incorporated, presents as a suitable option for future research (van Dam-Bates et al. 2018).
In this work, the primary focus was on assessing biodiversity in the inner Queen Charlotte Sound and an extended multivariate analysis of highly valued species was considered out of scope. Therefore, it is plausible that microscale habitat and environmental associations may be detectable for different species combinations using INLA or other statistical techniques, for which the data included as supplementary materials may be complimentary to other, more targeted, studies.
Future directions
In addition to the options for future research and study designs described in the above section, there is opportunity to interrogate the ecological drivers of fish presence in the Queen Charlotte Sound. Ongoing investigations of the interactions between fish presence with coastal processes (including sedimentation, habitat destruction and restoration activities) and climate level variation in the studied environment present obvious directions, upon which fish abundance and biodiversity may be able to be baselined on the present results. Notable predictions of comparably higher species diversity in western reaches of the Sound warrant further consideration and investigation of the habitat-associated factors that drive this observed phenomenon. Moreover, greater consideration of whether the low levels of biodiversity in the studied region associate with the greater isolation associated with the complex network of inlets and bays (often encountered in drowned river valleys) when compared with more exposed coastal locations, is warranted.
A key aim of this study was to provide a useful framework for future biodiversity surveys in the Queen Charlotte Sound and other coastal marine environments. One limitation of the current study was the resource-intensive and time-consuming requirement to review large volumes of video footage to enable data collection and analysis. The incorporation of automated or artificial intelligence-based computer vision-detection methodologies for the review of video footage presents as an opportunity to reduce this time and the associated cost and embed more common and frequent surveys across small- and large-scale coastal marine environments (e.g. Rauf et al. 2019; Ditria et al. 2020; Knausgård et al. 2020; Sheehan et al. 2020; Connolly et al. 2021; Lopez-Marcano et al. 2021). Further research should also seek to disentangle the effect of water visibility on the observability of fish in this, and likely other, camera-based surveys. Identifying whether the association between visibility and biodiversity metrics is a systematic sampling error, or biological reality, could be investigated using sonar-based monitoring systems that can identify fish even in areas of poor visibility. Expanded investigations incorporating drop cameras, capture surveys, underwater visual census, eco-physiological sampling, and environmental DNA (eDNA) could readily be incorporated to provide for ongoing patterns of abundance, diversity, movements, environmental response, and site fidelity of key target species in the coverage area, including cryptic species missed by BRUV systems.
Conclusions
This study has generated a baseline dataset describing the presence, species richness, biodiversity scores, and relative abundance data of key fish species in the highly valued inner Queen Charlotte Sound region of New Zealand. Results have provided some indication that species richness and biodiversity scores (SW) are lower than should be predicted when compared with other temperate coastal marine ecosystems. These findings present an opportunity for managers and researchers to further investigate changes in fish populations within the region, explore ecological mitigation and remediation techniques, and support future decision making and management plan formulation.
Although there is considerable value and need for long-term observation, across broad, intermediate and narrow spatial scales, there is still much to be learned about the drivers of both spatial, seasonal and annual variation in marine environments. It is hoped that future studies employ long-term observational datasets (such as this) as the basis for exploration into the biological mechanisms associated with small- and large-scale biological phenomena in the context of the Australasian region and its associated fish biota.
Supplementary material
Supplementary material is available online.
Data availability
Summary data that support this study are available in the article and accompanying online Supplementary material. Rcode is available on request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was supported by a Strategic Science Investment Fund for Crown Research Institutes (Programme 1948) provided by the New Zealand Ministry of Business Innovation and Employment.
Author contributions
Conceptualisation was performed by D. Cook; data curation was performed by P. Bell, D. Cook, R. Jaksons; formal analysis was performed by P. Jaksons, R. Jaksons; investigation was performed by SF, P. Bell, D. Cook; methodology was performed by D. Cook, P. Jaksons; statistical methodology and analysis was performed by P. Jaksons, R. Jaksons; data visualisation was performed by P. Jaksons, R. Jaksons; writing of the draft was performed by D. Cook, P. Bell, R. Jaksons, P. Jaksons; review and editing of the text was performed by D. Cook, P. Bell, R. Jaksons, P. Jaksons.
Acknowledgements
We thank many staff from The New Zealand Institute for Plant and Food Research Limited, including Greg Knox, Sharon Ford, Gerard Janssen, Jason Hamill, and Alistair Jerrett, for their support in field activities and research program development. Notable thanks go to Shayne Olsen, Lochmara Lodge, and the Marlborough Sounds Wildlife Recovery Centre for their continued support and for accommodating the researchers during field activities. Additional thanks go to Oliver Wade (Marlborough District Council) and Eric Jorgenson (Marlborough Marine Futures) for their ongoing discussions and support for the research undertaken. This paper benefited from the input of three anonymous reviewers, who are thanked for their constructive considerations.
References
Bakka, H, Vanhatalo, J, Illian, JB, Simpson, D, and Rue, H (2019). Non-stationary Gaussian models with physical barriers. Spatial Statistics 29, 268–288.| Non-stationary Gaussian models with physical barriers.Crossref | GoogleScholarGoogle Scholar |
Bax, N, Williamson, A, Aguero, M, Gonzalez, E, and Geeves, W (2003). Marine invasive alien species: a threat to global biodiversity. Marine Policy 27, 313–323.
| Marine invasive alien species: a threat to global biodiversity.Crossref | GoogleScholarGoogle Scholar |
Beck, MW, Heck, KL, Able, KW, Childers, DL, Eggleston, DB, Gillanders, BM, Halpern, B, Hays, CG, Hoshino, K, Minello, TJ, Orth, RJ, Sheridan, PF, and Weinstein, MP (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633–641.
| The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates.Crossref | GoogleScholarGoogle Scholar |
Beentjes MP, Carbines GD (2012) Relative abundance, size and age structure, and stock status of blue cod from the 2010 survey in Marlborough Sounds, and review of historical surveys. New Zealand Fisheries Assessment Report 2012/43, Ministry for Primary Industries.
Beentjes MP, Page M, Sutton C, Olsen L (2018) Blue cod relative abundance, size and age structure, and stock status of blue cod from the 2017 survey in the Marlborough Sounds, and review of historical surveys. New Zealand Fisheries Assessment Report 2018/33, Ministry for Primary Industries.
Bicknell, AWJ, Godley, BJ, Sheehan, EV, Votier, SC, and Witt, MJ (2016). Camera technology for monitoring marine biodiversity and human impact. Frontiers in Ecology and the Environment 14, 424–432.
| Camera technology for monitoring marine biodiversity and human impact.Crossref | GoogleScholarGoogle Scholar |
Cole, RG, Villouta, E, and Davidson, RJ (2000). Direct evidence of limited dispersal of the reef fish Parapercis colias (Pinguipedidae) within a marine reserve and adjacent fished areas. Aquatic Conservation: Marine and Freshwater Ecosystems 10, 421–436.
| Direct evidence of limited dispersal of the reef fish Parapercis colias (Pinguipedidae) within a marine reserve and adjacent fished areas.Crossref | GoogleScholarGoogle Scholar |
Connolly, RM, Fairclough, DV, Jinks, EL, Ditria, EM, Jackson, G, Lopez-Marcano, S, Olds, AD, and Jinks, KI (2021). Improved accuracy for automated counting of a fish in baited underwater videos for stock assessment. Frontiers in Marine Science 8, 658135.
| Improved accuracy for automated counting of a fish in baited underwater videos for stock assessment.Crossref | GoogleScholarGoogle Scholar |
Costanza, R, d’Arge, R, de Groot, R, Farber, S, Grasso, M, Hannon, B, Limburg, K, Naeem, S, O’Neill, RV, Paruelo, J, Raskin, RG, Sutton, P, and van den Belt, M (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260.
| The value of the world’s ecosystem services and natural capital.Crossref | GoogleScholarGoogle Scholar |
Davey NK, Hartill B, Cairney DG, Cole RG (2008) Characterisation of the Marlborough Sounds recreational fishery and associated blue cod and snapper harvest estimates. New Zealand Fisheries Assessment Report 2008/31, Ministry for Primary Industries.
Davidson, RJ (2001). Changes in population parameters and behaviour of blue cod (Parapercis colias; Pinguipedidae) in Long Island–Kokomohua Marine Reserve, Marlborough Sounds, New Zealand. Aquatic Conservation: Marine and Freshwater Ecosystems 11, 417–435.
| Changes in population parameters and behaviour of blue cod (Parapercis colias; Pinguipedidae) in Long Island–Kokomohua Marine Reserve, Marlborough Sounds, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Ditria, EM, Lopez-Marcano, S, Sievers, M, Jinks, EL, Brown, CJ, and Connolly, RM (2020). Automating the analysis of fish abundance using object detection: optimizing animal ecology with deep learning. Frontiers in Marine Science 7, 429.
| Automating the analysis of fish abundance using object detection: optimizing animal ecology with deep learning.Crossref | GoogleScholarGoogle Scholar |
Edgar, GJ, and Barrett, NS (1999). Effects of the declaration of marine reserves on Tasmanian reef fishes, invertebrates and plants. Journal of Experimental Marine Biology and Ecology 242, 107–144.
| Effects of the declaration of marine reserves on Tasmanian reef fishes, invertebrates and plants.Crossref | GoogleScholarGoogle Scholar |
Fahey, BD, and Coker, RJ (1992). Sediment production from forest roads in Queen Charlotte Forest and potential impact on marine water quality, Marlborough Sounds, New Zealand. New Zealand Journal of Marine and Freshwater Research 26, 187–195.
| Sediment production from forest roads in Queen Charlotte Forest and potential impact on marine water quality, Marlborough Sounds, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Francis, MP, Morrison, MA, Leathwick, J, and Walsh, C (2011). Predicting patterns of richness, occurrence and abundance of small fish in New Zealand estuaries. Marine and Freshwater Research 62, 1327–1341.
| Predicting patterns of richness, occurrence and abundance of small fish in New Zealand estuaries.Crossref | GoogleScholarGoogle Scholar |
Hadfield M, Broekhuizen N, Plew D (2014) ‘A biophysical model for the Marlborough Sounds: Queen Charlotte Sound and Tory Channel.’ (NIWA: Christchurch, New Zealand)
Halpern, BS, Frazier, M, Afflerbach, J, Lowndes, JS, Micheli, F, O’Hara, C, Scarborough, C, and Selkoe, KA (2019). Recent pace of change in human impact on the world’s ocean. Scientific Reports 9, 11609.
| Recent pace of change in human impact on the world’s ocean.Crossref | GoogleScholarGoogle Scholar | 31406130PubMed |
Handley S (2016) ‘History of benthic change in Queen Charlotte Sound/Totaranui, Marlborough.’ (NIWA: Nelson, New Zealand)
Harley, CDG, Randall Hughes, A, Hultgren, KM, Miner, BG, Sorte, CJB, Thornber, CS, Rodriguez, LF, Tomanek, L, and Williams, SL (2006). The impacts of climate change in coastal marine systems. Ecology Letters 9, 228–241.
| The impacts of climate change in coastal marine systems.Crossref | GoogleScholarGoogle Scholar |
Helfman GS (1986) Fish behaviour by day, night and twilight. In ‘The behaviour of teleost fishes’. (Ed. TJ Pitcher) pp. 366–387. (Springer US: Boston, MA, USA)
| Crossref |
Holmes, TH, Wilson, SK, Travers, MJ, Langlois, TJ, Evans, RD, Moore, GI, Douglas, RA, Shedrawi, G, Harvey, ES, and Hickey, K (2013). A comparison of visual- and stereo-video based fish community assessment methods in tropical and temperate marine waters of Western Australia. Limnology and Oceanography: Methods 11, 337–350.
| A comparison of visual- and stereo-video based fish community assessment methods in tropical and temperate marine waters of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Inman, DL, and Brush, BM (1973). The coastal challenge. Science 181, 20–32.
| The coastal challenge.Crossref | GoogleScholarGoogle Scholar | 17769816PubMed |
Jarvis, RM, and Young, T (2019). Key research priorities for the future of marine science in New Zealand. Marine Policy 106, 103539.
| Key research priorities for the future of marine science in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Jónsdóttir, IG, Bakka, H, and Elvarsson, BT (2019). Groundfish and invertebrate community shift in coastal areas off Iceland. Estuarine, Coastal and Shelf Science 219, 45–55.
| Groundfish and invertebrate community shift in coastal areas off Iceland.Crossref | GoogleScholarGoogle Scholar |
Knausgård KM, Wiklund A, Sørdalen TK, Halvorsen K, Kleiven AR, Jiao L, Goodwin M (2020) Temperate fish detection and classification: a deep learning based approach. arXiv:2005.07518 [cs, eess]. Available at http://arxiv.org/abs/2005.07518. [Accessed 23 February 2021]
Li, J, and Convertino, M (2021). Temperature increase drives critical slowing down of fish ecosystems. PLoS ONE 16, e0246222.
| Temperature increase drives critical slowing down of fish ecosystems.Crossref | GoogleScholarGoogle Scholar | 34669703PubMed |
Lopez-Marcano, S, Jinks, E, Buelow, CA, Brown, CJ, Wang, D, Kusy, B, Ditria, E, and Connolly, RM (2021). Automatic detection of fish and tracking of movement for ecology. Ecology and Evolution 11, 8254–8263.
| Automatic detection of fish and tracking of movement for ecology.Crossref | GoogleScholarGoogle Scholar | 34188884PubMed |
Mace, PM, Sullivan, KJ, and Cryer, M (2014). The evolution of New Zealand’s fisheries science and management systems under ITQs. ICES Journal of Marine Science 71, 204–215.
| The evolution of New Zealand’s fisheries science and management systems under ITQs.Crossref | GoogleScholarGoogle Scholar |
Magurran AE (2004) ‘Measuring biological diversity.’ (Blackwells: Oxford, UK)
Mallet, D, and Pelletier, D (2014). Underwater video techniques for observing coastal marine biodiversity: a review of sixty years of publications (1952–2012). Fisheries Research 154, 44–62.
| Underwater video techniques for observing coastal marine biodiversity: a review of sixty years of publications (1952–2012).Crossref | GoogleScholarGoogle Scholar |
Martínez-Minaya, J, Conesa, D, Bakka, H, and Pennino, MG (2019). Dealing with physical barriers in bottlenose dolphin (Tursiops truncatus) distribution. Ecological Modelling 406, 44–49.
| Dealing with physical barriers in bottlenose dolphin (Tursiops truncatus) distribution.Crossref | GoogleScholarGoogle Scholar |
McClatchie, S, Millar, RB, Webster, F, Lester, PJ, Hurst, R, and Bagley, N (1997). Demersal fish community diversity off New Zealand: is it related to depth, latitude and regional surface phytoplankton? Deep Sea Research Part I: Oceanographic Research Papers 44, 647–667.
| Demersal fish community diversity off New Zealand: is it related to depth, latitude and regional surface phytoplankton?Crossref | GoogleScholarGoogle Scholar |
National Topographic Office (2017) NZ Coastlines topographic database. Available at https://data.linz.govt.nz/layer/51153-nz-coastlines-and-islands-polygons-topo-150k/ [Verified 7 March 2017]
Nixon, SW (1995). Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41, 199–219.
| Coastal marine eutrophication: a definition, social causes, and future concerns.Crossref | GoogleScholarGoogle Scholar |
Parnell, KE, McDonald, SC, and Burke, AE (2007). Shoreline effects of vessel wakes, Marlborough Sounds, New Zealand. Journal of Coastal Research 50, 502–506.
Parsons, DM, Sim-Smith, CJ, Cryer, M, Francis, MP, Hartill, B, Jones, EG, Le Port, A, Lowe, M, McKenzie, J, Morrison, M, Paul, LJ, Radford, C, Ross, PM, Spong, KT, Trnski, T, Usmar, N, Walsh, C, and Zeldis, J (2014). Snapper (Chrysophrys auratus): a review of life history and key vulnerabilities in New Zealand. New Zealand Journal of Marine and Freshwater Research 48, 256–283.
| Snapper (Chrysophrys auratus): a review of life history and key vulnerabilities in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Parsons, DF, Suthers, IM, Cruz, DO, and Smith, JA (2016). Effects of habitat on fish abundance and species composition on temperate rocky reefs. Marine Ecology Progress Series 561, 155–171.
| Effects of habitat on fish abundance and species composition on temperate rocky reefs.Crossref | GoogleScholarGoogle Scholar |
Peterson, MS, and Lowe, MR (2009). Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources. Reviews in Fisheries Science 17, 505–523.
| Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources.Crossref | GoogleScholarGoogle Scholar |
Rapson AM (1956) Biology of the blue cod (Parapercis colias Forster) of New Zealand. PhD Thesis, Victoria University, Wellington, New Zealand.
Rauf, HT, Lali, MIU, Zahoor, S, Shah, SZH, Rehman, AU, and Bukhari, SAC (2019). Visual features based automated identification of fish species using deep convolutional neural networks. Computers and Electronics in Agriculture 167, 105075.
| Visual features based automated identification of fish species using deep convolutional neural networks.Crossref | GoogleScholarGoogle Scholar |
Roberts, CM, Branch, G, Bustamante, RH, Castilla, JC, Dugan, J, Halpern, BS, Lafferty, KD, Leslie, H, Lubchenco, J, McArdle, D, Ruckelshaus, M, and Warner, RR (2003). Application of ecological criteria in selecting marine reserves and developing reserve networks. Ecological Applications 13, 215–228.
| Application of ecological criteria in selecting marine reserves and developing reserve networks.Crossref | GoogleScholarGoogle Scholar |
Robertson, BL, Brown, JA, McDonald, T, and Jaksons, P (2013). BAS: balanced acceptance sampling of natural resources. Biometrics 69, 776–784.
| BAS: balanced acceptance sampling of natural resources.Crossref | GoogleScholarGoogle Scholar | 23844595PubMed |
Rodil, IF, Lohrer, AM, Hewitt, JE, Townsend, M, Thrush, SF, and Carbines, M (2013). Tracking environmental stress gradients using three biotic integrity indices: advantages of a locally developed traits-based approach. Ecological Indicators 34, 560–570.
| Tracking environmental stress gradients using three biotic integrity indices: advantages of a locally developed traits-based approach.Crossref | GoogleScholarGoogle Scholar |
Rue, H, Martino, S, and Chopin, N (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the Royal Statistical Society – B. Statistical Methodology 71, 319–392.
| Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations.Crossref | GoogleScholarGoogle Scholar |
Sheehan, EV, Bridger, D, Nancollas, SJ, and Pittman, SJ (2020). PelagiCam: a novel underwater imaging system with computer vision for semi-automated monitoring of mobile marine fauna at offshore structures. Environmental Monitoring and Assessment 192, 11.
| PelagiCam: a novel underwater imaging system with computer vision for semi-automated monitoring of mobile marine fauna at offshore structures.Crossref | GoogleScholarGoogle Scholar |
Thrush, SF, Hewitt, JE, Cummings, VJ, Ellis, JI, Hatton, C, Lohrer, A, and Norkko, A (2004). Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment 2, 299–306.
| Muddy waters: elevating sediment input to coastal and estuarine habitats.Crossref | GoogleScholarGoogle Scholar |
Urlich, SC, and Handley, SJ (2020). From ‘clean and green’ to ‘brown and down’: a synthesis of historical changes to biodiversity and marine ecosystems in the Marlborough Sounds, New Zealand. Ocean & Coastal Management 198, 105349.
| From ‘clean and green’ to ‘brown and down’: a synthesis of historical changes to biodiversity and marine ecosystems in the Marlborough Sounds, New Zealand.Crossref | GoogleScholarGoogle Scholar |
van Dam-Bates, P, Gansell, O, and Robertson, B (2018). Using balanced acceptance sampling as a master sample for environmental surveys. Methods in Ecology and Evolution 9, 1718–1726.
| Using balanced acceptance sampling as a master sample for environmental surveys.Crossref | GoogleScholarGoogle Scholar |
Watson, SJ, Neil, H, Ribó, M, Lamarche, G, Strachan, LJ, MacKay, K, Wilcox, S, Kane, T, Orpin, A, Nodder, S, Pallentin, A, and Steinmetz, T (2020). What we do in the shallows: Natural and anthropogenic seafloor geomorphologies in a drowned river valley, New Zealand. Frontiers in Marine Science 7, 579626.
| What we do in the shallows: Natural and anthropogenic seafloor geomorphologies in a drowned river valley, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Williams, GJ, Cameron, MJ, Turner, JR, and Ford, RB (2008). Quantitative characterisation of reef fish diversity among nearshore habitats in a northeastern New Zealand marine reserve. New Zealand Journal of Marine and Freshwater Research 42, 33–46.
| Quantitative characterisation of reef fish diversity among nearshore habitats in a northeastern New Zealand marine reserve.Crossref | GoogleScholarGoogle Scholar |
Willis, TJ, Millar, RB, Babcock, RC, and Tolimieri, N (2003). Burdens of evidence and the benefits of marine reserves: putting Descartes before des horse? Environmental Conservation 30, 97–103.
| Burdens of evidence and the benefits of marine reserves: putting Descartes before des horse?Crossref | GoogleScholarGoogle Scholar |


