Effect of a combined exercise and nutrition program on sarcopenia in older adults: a randomised controlled trial in primary care
Sunghwan Ji A B # , Ji Yeon Baek A # , Jin Go C , Chang Ki Lee D , Sang Soo Yu E , Hee-Won Jung A , Eunju Lee A * Il-Young Jang
A B # , Ji Yeon Baek A # , Jin Go C , Chang Ki Lee D , Sang Soo Yu E , Hee-Won Jung A , Eunju Lee A * Il-Young Jang  A *
A *
A
B
C
D
E
# These authors contributed equally to this paper
Abstract
Sarcopenia, marked by the progressive decline of skeletal muscle mass and strength, is a significant health issue in aging populations. Primary care-based interventions may offer effective management strategies, yet randomised controlled trials evaluating these interventions are limited.
We conducted an unblinded, parallel-group randomised controlled trial at a public health centre in Korea responsible for primary care, enrolling participants aged ≥65 years with sarcopenia. Participants were randomised to either an intervention group (receiving a 12-week program of group exercises and nutritional support) or a control group (receiving lifestyle management education). The primary outcome was the change in gait speed over the intervention period. Secondary outcomes included changes in physical performance, grip strength, muscle mass, quality of life and frailty index.
A total of 86 participants were enrolled and randomised (intervention group: n = 43; control group: n = 43). The intervention group demonstrated significant improvements in gait speed (0.14 m/s (95% CI: 0.10–0.18) vs −0.04 m/s (95% CI: −0.08–0.00), P < 0.001), Short Physical Performance Battery scores, grip strength, quality of life and frailty index compared with the control group.
The 12-week exercise and nutrition intervention yielded significant gains in physical performance, grip strength, quality of life and frailty reduction among community-dwelling older adults with sarcopenia. These findings support the value of community-based, multicomponent interventions for managing sarcopenia. The trial is registered at Clinical Research Information Service (http://cris.nih/go.kr, registration number: KCT0008952).
Keywords: exercise, frailty, geriatrics, nutrition, older adults, preventive medicine, primary health, sarcopenia.
Introduction
Sarcopenia, characterised by a progressive decline in skeletal muscle mass and strength, poses a significant health concern, particularly in aging populations (Cruz-Jentoft and Sayer 2019). This condition is associated with a variety of adverse outcomes, including increased risk of falls (Yeung et al. 2019), disabilities (Phillips et al. 2017), deteriorating quality of life (Tsekoura et al. 2017) and even mortality (Brown et al. 2016). To date, various interventions have been explored to combat sarcopenia, with exercise and nutritional interventions standing out as the most recommended strategies according to numerous guidelines (Dent et al. 2018; Chen et al. 2020; Lim et al. 2022). These approaches are supported by a body of research highlighting their effectiveness in populations with sarcopenia (Bernabei et al. 2022; Shen et al. 2023). Given that lifestyle modification is the most important intervention, primary care-based approaches are crucial.
Korea has recently become a super-aged society, with >20% of its population aged ≥65 years as of 2025. This demographic shift is occurring at an unprecedented speed, outpacing other aging countries, such as Japan, Italy and Germany (Ji et al. 2023). The country operates a universal healthcare system through its National Health Insurance, and public health centres function as primary care hubs, particularly in rural areas. In response to the growing concern over sarcopenia in Korea’s aging population, community-based public health centres have begun implementing programs aimed at addressing this issue. However, randomised controlled trials (RCTs) combining exercise and nutritional interventions specifically for sarcopenia are extremely limited in Korea. Existing Korean studies have tended to be disease-specific and delivered outside of the primary care, (Oh et al. 2020; Yun et al. 2021) or conducted using outdated diagnostic criteria that do not reflect current international consensus (Park et al. 2017). This highlights a critical gap in the evidence base for primary care-based intervention programs for sarcopenia, particularly in the context of Korea.
To bridge this knowledge gap, we conducted a RCT to examine whether a primary care-based 12-week interventional program, consisting of group exercise and nutritional support, could significantly improve gait speed, physical performance, grip strength and muscle mass compared with usual care, which involved a one-time lifestyle modification education session. In addition, we assessed its impact on activities of daily living, quality of life and the reduction of frailty burden, based on the understanding that sarcopenia is a systemic condition closely related to frailty (Cesari et al. 2014). This approach aligns with the World Health Organization’s Integrated Care for Older People framework, which emphasises community-level interventions to preserve functional capacity through key pillars, such as physical activity and nutritional care (WHO Guidelines Approved by the Guidelines Review Committee 2017).
Methods
Trial design and study populations
This trial was implemented at the PyeongChang Health Centre and County Hospital, a public primary care facility, and recruited participants from the Aging Study of Pyeongchang Rural Area (ASPRA), an ongoing prospective cohort of community-dwelling older adults (Baek et al. 2021a). In the Korean health system, public health centres are core primary care facilities, especially in rural areas. These centres serve as the primary access points for health care, offering preventive and chronic disease management services. All Korean citizens are eligible to receive care at these facilities, and adults aged ≥65 years are entitled to most services free of charge. This study leveraged this infrastructure by utilising public health centre personnel and resources – including a primary care physician, nurse, physiotherapist and nutritionist – to deliver the intervention. The program was developed in collaboration with the PyeongChang Health Centre and County Hospital and local community stakeholders.
A parallel-group, unblinded RCT with a 1:1 allocation was conducted, with participants recruited through the ASPRA cohort. Eligibility criteria for ASPRA included age ≥65 years, being registered in the National Healthcare Service, being ambulatory with or without assistance, living at home and being capable of providing informed consent independently or through proxies. Exclusions were made for individuals residing in nursing homes and hospitals, or receiving nursing home-level care at home. ASPRA participants underwent an annual Comprehensive Geriatric Assessment with sarcopenia assessment conducted by trained nurses at regional community health posts or PyeongChang Health Centre and County Hospital. The specific protocol is demonstrated in a previous paper (Baek et al. 2021a).
A total of 1955 older adults were screened in the ASPRA database, with 290 identified as having sarcopenia within 3 months. Exclusions were applied to individuals who declined participation; were unable to walk alone for >5 min without assistance; had significant hearing impairment, hindering group intervention participation; were hospitalised for ≥2 weeks in the past 3 months; were receiving treatment for uncontrolled congestive heart failure or metastatic cancer; or had an expected life expectancy of <1 year. Following informed consent, measurements were taken from 102 participants according to the study protocol (1st measurement), excluding those not meeting sarcopenia criteria at the initial measurement (n = 16). Eligible participants were randomly assigned to either the combined intervention group or the control group. The assignment flow chart is described in Fig. 1.
Study flowchart. ASPRA, Aging Study of Pyeongchang Rural Area; CHF, congestive heart failure; ITT, Intention-to-treat.
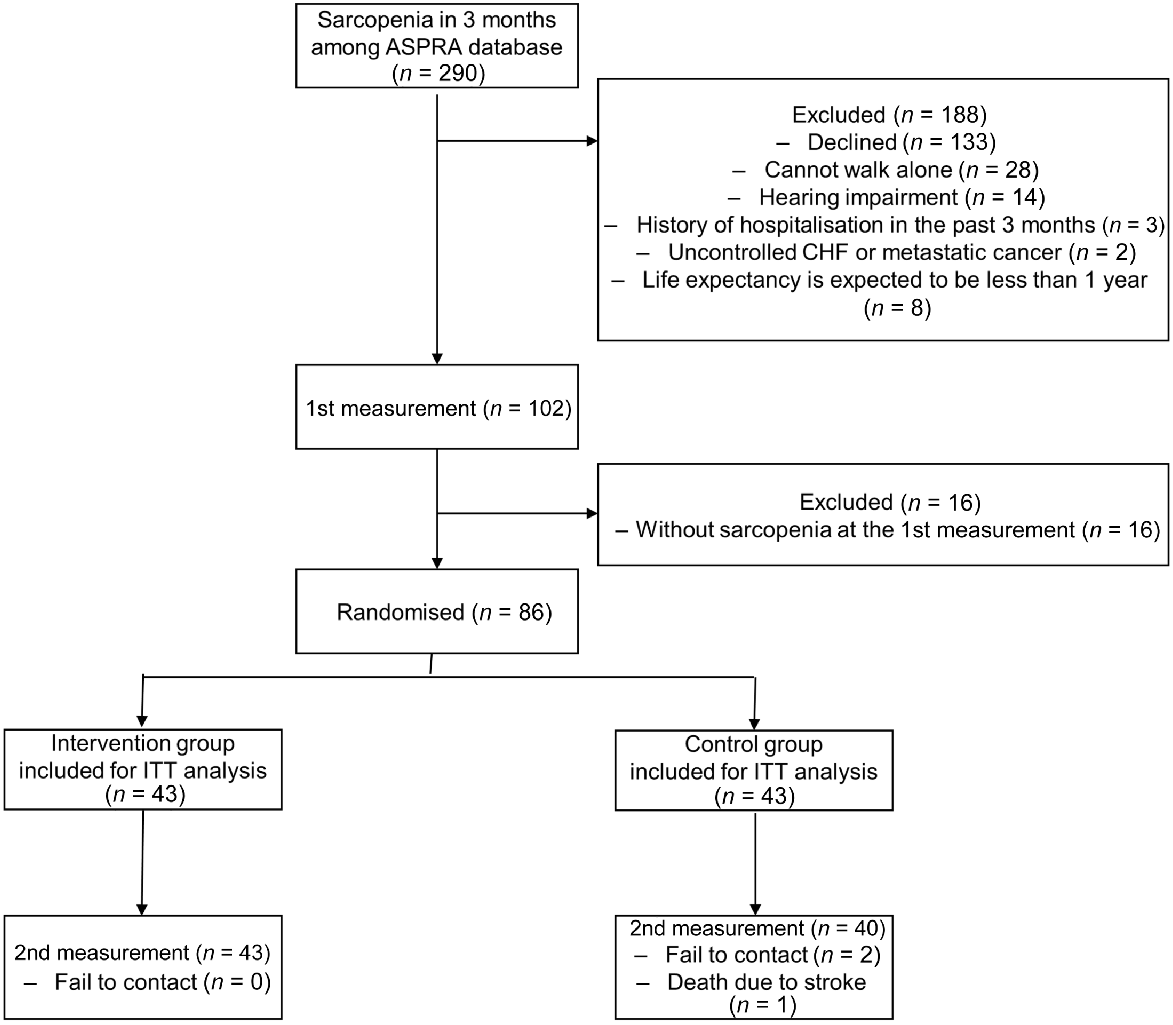
Informed consent was obtained from all individual participants included in the study. The trial protocol followed the Declaration of Helsinki, and received approval from the Asan Medical Centre Institutional Review Board (IRB number: 2023-1180). The trial is registered at Clinical Research Information Service (http://cris.nih/go.kr, registration number: KCT0008952).
Definition of sarcopenia
The definition of sarcopenia used in this study adheres to the Asian Working Group on Sarcopenia (Chen et al. 2020) and Korean Working Group on Sarcopenia (Baek et al. 2023) guidelines. It was defined as the presence of low muscle mass in conjunction with either low grip strength or slow gait speed. Muscle mass was evaluated using bioelectrical impedance analysis at frequencies of 5, 50 and 500 kHz. The appendicular muscle mass divided by height squared (ASM/h2) was calculated by summing the lean mass of the upper and lower extremities, and dividing it by the height squared. Low muscle mass was identified as ASM/h2 <7.0 kg/m2 in men and <5.7 kg/m2 in women. Grip strength was measured using a handgrip dynamometer (T.K.K 5401 Grip-D; Takei, Tokyo, Japan), with low grip strength defined as <28 kg in men and <18 kg in women. Gait speed assessment involved participants walking 7 m at their usual pace, with trained nurses measuring the transit time of 4 m, excluding the 1.5-m acceleration and deceleration intervals, calculated from the time taken to cover 4 m. Slow gait speed was defined as <1 m/s.
Interventions
Participants in the Intervention group engaged in a 12-week exercise and nutritional intervention program facilitated by the public health centre. The exercise regimen consisted of biweekly group sessions, 24 sessions over 12 weeks. Each 60-min session began and ended with a 5-min warm-up and cool-down period, respectively, emphasising muscle stretching. These sessions were facilitated by community trainers from the public health centre, trained in delivering exercise regimens tailored to older adults. The core session, lasting 50 min, comprised 30 min of resistance training and 20 min of aerobic exercise. Resistance training alternated between upper and lower body workouts, with trainers determining initial loads based on each participant’s one-repetition maximum strength. Aerobic exercises, performed on treadmills, targeted an intensity range of 50−70% of participants’ maximum oxygen uptake, with personalised adjustments made over time. Participants were provided with written exercise guidelines and encouraged to engage in 60 min of daily independent exercise. A detailed program description using the Consensus on Exercise Reporting Template guidelines is detailed in Supplementary material S1.
Regarding nutrition, participants received tailored education at the beginning of the intervention from public health centre nutritionists. Additionally, participants were supplied with a protein-rich nutritional powder (DanbaegChaeum™ by Dr. WELLNESS, Seoul, Korea) intended for twice-daily consumption between meals. Each serving, mixed with 140 mL of water, contained 25 g of content, providing 95 kcal, including 13 g of protein, 1.4 g of fat and 8 g of carbohydrates. Adherence rate was defined as the number of attended exercise sessions divided by the total number of scheduled sessions (24 per participant). Nutritional supplements were provided only during attended sessions, and only if the participant had consumed the previously distributed supplements. Therefore, this definition reflects adherence not only to the exercise component, but also to the nutritional supplementation component of the intervention.
In contrast, participants in the control group received standard exercise and nutritional education, which is the usual care provided by primary care-based public health centres. This consisted of written materials on exercise and diet, supplemented by a brief one-time consultation with a dietitian, but without any follow up, group exercise sessions or provision of nutritional supplements.
A nurse was responsible for conducting the Comprehensive Geriatric Assessment throughout the intervention, whereas the geriatrician oversaw the program’s design, monitoring and overall management, serving as the program lead.
Measurements
Participants were assessed both at the first and second measurements of the intervention by trained nurses who were blinded to the participants’ interventional statuses. These assessments covered demographic information (including age, gender, bodyweight, height, education level and eligibility for medical aid based on a monthly income under US$500), existing health conditions and lifestyle factors, such as exercise and diet. Data on 12 conditions diagnosed by doctors were collected: hypertension, diabetes, stroke, dyslipidaemia, thyroid disorders, coronary artery disease, asthma, allergies, arthritis, chronic kidney disease, liver disease and pulmonary tuberculosis. The frequency of taking prescribed medications was noted. The presence of depressive symptoms was evaluated using the Patient Health Questionnaire-9, with a score >21 indicating depression (Levis et al. 2019). Cognitive functions were assessed using the Mini-Cog test, where a score of ≤3 suggested cognitive impairment (Wright and Harrell 2022). Disability was identified by difficulties in performing at least one of seven activities of daily living (ADLs) and 10 instrumental ADLs (IADLs; Won et al. 2002). Gait speed was measured by a single trained nurse in accordance with international guidelines, which recommend a 4-m walking distance, a clear acceleration/deceleration zone and timing with a stopwatch (Cruz-Jentoft et al. 2019). The Short Physical Performance Battery, with scores ranging from 0 to 12 was utilised to measure physical function (Guralnik et al. 1994). The Clinical Frailty Scale was determined using its Korean version (Ko et al. 2021).
Outcomes
The primary outcome of the study was the change in gait speed over 12 weeks, which is a recommended endpoint in clinical trials (Reginster et al. 2021). Secondary outcomes included the changes in the Short Physical Performance Battery, grip strength, ASM/h2, Clinical Frailty Scale, ADL, IADL and 45-item frailty index from baseline to 12 weeks. The 45 items of the frailty index are listed in Supplementary Table S1.
Sample size calculation
The sample size calculation was based on the effect size (Cohen’s d) of 0.65 observed in gait speed before and after a 12-week intervention conducted in China (Zhu et al. 2019). Assuming an alpha error of 0.05 and a power of 80%, with an intergroup sample size ratio of 1:1, the power calculation was conducted for 39 participants in each group. Taking into account a dropout rate of 10% (adherence rate of 90%), the minimum required sample size was determined to be 86 participants (43 participants per group).
Randomisation and blinding
We used simple randomisation, stratified by age (≥75 years or <75 years) and gender, as both factors may interact with the intervention (Aragon et al. 2023; Noh and Park 2023; Sánchez et al. 2025), without the use of blocks. Participants were assigned randomly by a study coordinator who used a computer-generated list to allocate 43 individuals each to either the control or intervention groups. The details of these allocations were concealed within sequentially numbered, sealed envelopes. These envelopes were opened in order as new participants joined the study. Due to the nature of the intervention and its implementation in a public health context, it was not possible to blind the study. However, the trained nurses who conducted outcome assessments, as well as the statistician responsible for data analysis, were blinded to group allocation.
Statistical analyses
We compared baseline continuous and categorical variables between the two groups using t-tests and chi-squared tests, respectively. Differences in the mean changes for primary and secondary outcomes at 12 weeks from baseline were analysed using linear mixed models. In these models, time and group, along with their interactions, were considered fixed factors, whereas individual participants were treated as random factors. The analysis was performed using the intention-to-treat approach. Effect sizes were calculated using Cohen’s d. Additional exploratory subgroup analyses were conducted based on age (≥80 years or <80 years), gender, living situation (living alone vs not) and frailty index (≥0.2 vs <0.2). All statistical analyses were performed using R Software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria) and were two-tailed with a significance level set at P < 0.05.
This study was reported in accordance with the Consensus Reporting Items for Studies in Primary Care guidelines to enhance the transparency and contextual relevance of a primary care setting. The completed Consensus Reporting Items for Studies in Primary Care checklist is provided in the Table S2.
Results
Enrollment and baseline characteristics
In total, 86 individuals were eligible, enrolled and randomised into the intervention and control groups (43 individuals each; Fig. 1). Participants were recruited from September 2023 to November 2023. During the study, three participants from the control group were lost to follow up due to failure to make contact (n = 2) and death due to stroke (n = 1). In the intervention group, the mean adherence rates were 86.86%. No adverse events were reported throughout the duration of the study.
The baseline characteristics of the two groups were similar, and no variables were statistically different (Table 1). The mean age was 78.93 ± 5.78 years in the intervention group and 78.98 ± 6.89 years in the control group. Twenty-four (55.8%) individuals were women in both groups. The mean Short Physical Performance Battery score was 7.44 ± 2.05 and 7.42 ± 2.01 for the intervention and control groups, respectively. The mean frailty index was 0.27 ± 0.13 and 0.28 ± 0.12 for the intervention and control groups, respectively.
| Intervention (n = 43) | Control (n = 43) | P-value | ||
|---|---|---|---|---|
| Age (years) | 78.93 ± 5.78 | 78.98 ± 6.89 | 0.97 | |
| Female | 24 (55.8) | 24 (55.8) | 1.00 | |
| Body mass index (kg/m2) | 22.64 ± 2.23 | 23.21 ± 2.76 | 0.30 | |
| Skeletal muscle index (kg/m2) | 5.79 ± 0.77 | 5.63 ± 0.90 | 0.38 | |
| Gait speed (m/s) | 0.65 ± 0.23 | 0.65 ± 0.22 | 0.97 | |
| Short physical performance battery | 7.44 ± 2.05 | 7.42 ± 2.01 | 0.96 | |
| Grip strength (kg) | 21.19 ± 7.25 | 19.43 ± 8.03 | 0.29 | |
| Mini-Cog ≤ 3 | 29 (67.4) | 35 (81.4) | 0.22 | |
| No. of chronic conditions | 1.79 ± 1.12 | 1.60 ± 1.18 | 0.46 | |
| No. of medications | 3.44 ± 2.92 | 3.00 ± 1.96 | 0.41 | |
| Fall in the past year | 9 (20.9) | 18 (41.9) | 0.06 | |
| ADL impairment (%) | 17 (39.5) | 12 (27.9) | 0.36 | |
| IADL impairment (%) | 16 (37.2) | 23 (53.5) | 0.19 | |
| Clinical frailty scale | 3.65 ± 0.78 | 3.84 ± 0.90 | 0.30 | |
| Frailty index | 0.27 ± 0.13 | 0.28 ± 0.12 | 0.77 |
Data are represented as means (standard deviations) or numbers (%).
ADL, activity of daily living; IADL, instrumental activity of daily living; NA, not applicable.
Outcomes
Regarding the primary outcome, the intervention group demonstrated a significant improvement in gait speed compared with the control group (mean change: 0.14 m/s (95% CI: 0.10–0.18) vs −0.04 m/s (95% CI: −0.08–0.00); P < 0.001). For secondary outcomes, the intervention group showed significant improvements in the Short Physical Performance Battery, grip strength, EQ-5D and frailty index, whereas the skeletal muscle index and Clinical Frailty Scale did not significantly improve compared with the control group. There was no difference between the two groups in the number of impaired ADL and IADL tasks. Detailed results of changes in each outcome by group are shown in Table 2.
| Mean | Change | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow up | Mean change (95% CI) | P-value of interaction | Effect size (within-group) | Effect size (between-group) | ||
| Primary outcome | |||||||
| Gait speed (m/s) | |||||||
| Intervention | 0.65 | 0.79 | 0.14 (0.10, 0.18) | <0.001 | 0.61 | 0.82 | |
| Control | 0.65 | 0.61 | −0.04 (−0.08, 0.00) | −0.21 | |||
| Secondary outcome | |||||||
| SPPB | |||||||
| Intervention | 7.44 | 8.65 | 1.21 (0.81, 1.61) | <0.001 | 0.60 | 0.74 | |
| Control | 7.42 | 7.09 | −0.33 (−0.75, 0.08) | −0.17 | |||
| Grip strength (kg) | |||||||
| Intervention | 21.2 | 24.2 | 3.02 (1.71, 4.34) | 0.005 | 0.41 | 0.35 | |
| Control | 19.4 | 19.7 | 0.29 (−1.07, 1.65) | 0.04 | |||
| SMI (kg/m2) | |||||||
| Intervention | 5.79 | 5.95 | 0.16 (0.05, 0.26) | 0.22 | 0.20 | 0.11 | |
| Control | 5.63 | 5.69 | 0.06 (−0.04, 0.17) | 0.07 | |||
| Clinical frailty scale | |||||||
| Intervention | 3.65 | 3.49 | −0.16 (−0.34, 0.02) | 0.24 | −0.22 | −0.17 | |
| Control | 3.84 | 3.83 | −0.01 (−0.20, 0.18) | −0.01 | |||
| No. of impaired ADL tasks (0–7) | |||||||
| Intervention | 0.67 | 0.47 | −0.21 (−0.61, 0.20) | 0.12 | −0.18 | −0.35 | |
| Control | 0.35 | 0.60 | 0.25 (−0.17, 0.66) | 0.33 | |||
| No. of impaired IADL tasks (0–10) | |||||||
| Intervention | 1.28 | 1.35 | 0.07 (−0.57, 0.70) | 0.68 | 0.03 | −0.06 | |
| Control | 1.70 | 1.96 | 0.26 (−0.39, 0.92) | 0.10 | |||
| EQ-5D | |||||||
| Intervention | 0.76 | 0.84 | 0.08 (0.05, 0.12) | <0.001 | 0.67 | 0.73 | |
| Control | 0.74 | 0.73 | −0.01 (−0.05, −0.03) | −0.06 | |||
| Frailty index | |||||||
| Intervention | 0.27 | 0.23 | −0.04 (−0.07, −0.01) | 0.003 | −0.32 | −0.44 | |
| Control | 0.28 | 0.30 | 0.02 (−0.01, 0.05) | 0.16 | |||
Bold: P-value <0.05 comparing follow up with baseline.
ADL, activity of daily living; IADL, instrumental activity of daily living; NA, not applicable; SMI, skeletal muscle index; SPPB, Short Physical Performance Battery.
We performed exploratory subgroup analyses based on several factors: age (≥80 years or <80 years), gender, frailty index (≥0.2 or <0.2) and living situation (living alone vs not). Although no significant interactions were observed, the data suggest that individuals with a frailty index of ≥0.2 showed a more pronounced response, as shown in Supplementary Fig. S1.
Discussion
In this study, we observed that a primary care-based 12-week exercise and nutritional program improved the primary outcome, gait speeds, in older adults with sarcopenia. Furthermore, secondary outcomes of the Short Physical Performance Battery, grip strength, EQ-5D and frailty index were improved significantly. The skeletal muscle index, Clinical Frailty Scale, and the number of impaired ADL and IADL tasks were not significantly different between the intervention and control groups.
This study is significant, as it was based on a real-world, community health centre-based combined exercise and nutrition program, closely reflecting the ongoing intervention system in Pyeongchang County in Korea. Although exercise and nutrition are the most effective interventions for sarcopenia (Park and Roh 2023), and interventions in primary care are important (Won 2023), current sarcopenia interventions in primary care and community settings in Korea are lacking. This study could serve as a model to expand this community health centre-based intervention nationwide. Importantly, the observed improvement in gait speed (0.14 m/s) exceeded the threshold for clinically meaningful change in older adults, as defined by Perera et al. (Perera et al. 2006). Furthermore, our subgroup analysis, which shows comparable effects, even in frail participants (frailty index ≥0.2) or older individuals (age ≥80 years), can provide useful results for policymaking.
To the best of our knowledge, this study is the first study that shows the effect of public health exercise and nutritional intervention in community-dwelling Korean older adults. In Korea, which is rapidly approaching a super-aged society, the prevalence of sarcopenia among older adults has been reported to be as high as 46.8% (Baek et al. 2021b). Given these statistics, addressing sarcopenia is becoming increasingly important in Korean populations. Despite the increasing importance of sarcopenia in the Korean population, there has been a lack of RCTs evaluating the effects of exercise and nutrition on sarcopenia within this demographic. Our study is the first RCT in Korea regarding exercise and nutrition using the updated sarcopenia criteria based on the Asian Working Group on Sarcopenia 2019 and Korean Working Group on Sarcopenia guidelines. A comprehensive summary of studies on the effects of exercise on sarcopenia in the Korean population is provided in Table S2. Among the four studies, Oh et al. (2020) used the Asian Working Group on Sarcopenia 2014 criteria and demonstrated that an antigravity treadmill can be effective for patients with sarcopenia post-hip fractures. Yun et al. (2021) defined sarcopenia solely using muscle mass, with participants being patients in a nursing hospital with moderate Alzheimer’s disease, which is not generalisable to community dwellers. Park et al. (2017) and Seo et al. (2021) conducted RCTs in the community; however, Park et al. (2017) defined sarcopenia only using muscle mass and weight without considering physical performance or grip strength, whereas Seo et al. (2021) included only women.
Notably, our intervention led to improvements not only in sarcopenia-related parameters – such as gait speed, physical performance, grip strength and muscle mass – but also in the frailty index. Although the Clinical Frailty Scale did not show a significant change, this finding is consistent with previous reports, indicating that this judgement-based categorical tool has limited sensitivity to short-term interventions (Dent et al. 2019). In contrast, the observed reduction in the frailty index more sensitively reflects an overall decrease in frailty burden. This aligns with the understanding that sarcopenia and physical frailty are closely related, often overlapping conditions that share common risk factors, pathophysiology and intervention strategies (Cesari et al. 2014). Previous trials demonstrated similar outcomes, showing that multicomponent interventions combining exercise and nutrition can improve both sarcopenia and frailty in community-dwelling older adults (Chan et al. 2017; Ji et al. 2025). Clinical guidelines, including those from the International Conference on Frailty and Sarcopenia Research, highlight physical activity alongside adequate nutritional support as the cornerstone of frailty management (Dent et al. 2019), which mirrors evidence-based recommendations for sarcopenia. Thus, within primary care settings, a shared approach to both conditions is not only practical, but also supported by current clinical evidence. Our findings reinforce the idea that targeting sarcopenia through muscle-focused interventions can also reduce frailty burden, underscoring the value of integrated care strategies in aging populations.
This study had several limitations. First, as it was conducted in a single community in Korea, the generalisability of the findings should be approached with caution. Second, the study only compared results before and after the 12-week intervention without long-term follow up. Future studies should investigate the long-term effects and the sustained impact of the intervention. Third, despite the relatively high adherence rate among study participants, three participants from the control group were lost to follow up, requiring imputation of missing values. Finally, the relatively small sample size necessitates caution when interpreting the results of the subgroup analysis.
In conclusion, the primary care-based 12-week exercise and nutrition intervention significantly improved physical performance, grip strength and quality of life, and reduced the frailty burden among community-dwelling older adults with sarcopenia in Korea. This study offers valuable evidence supporting the effectiveness of community-based interventions for managing sarcopenia.
Declaration of funding
This research received support from multiple sources, including the Asan Institute for Life Science, Asan Medical Centre (grant number: 2023IF0005) and the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR20C0026). This research was also supported by a grant of the MD-Phd/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
References
Aragon AA, Tipton KD, Schoenfeld BJ (2023) Age-related muscle anabolic resistance: inevitable or preventable? Nutrition Reviews 81(4), 441-454.
| Crossref | Google Scholar |
Baek JY, Lee E, Oh G, Park YR, Lee H, Lim J, Park H, Park CM, Lee CK, Jung H-W, Jang I-Y, Kim DH (2021a) The aging study of pyeongchang rural area (ASPRA): findings and perspectives for human aging, frailty, and disability. Annals of Geriatric Medicine and Research 25(3), 160-169.
| Crossref | Google Scholar |
Baek JY, Lee E, Jung H-W, Jang I-Y (2021b) Geriatrics fact sheet in Korea 2021. Annals of Geriatric Medicine and Research 25(2), 65-71.
| Crossref | Google Scholar |
Baek JY, Jung H-W, Kim KM, Kim M, Park CY, Lee K-P, Lee SY, Jang I-Y, Jeon OH, Lim J-Y (2023) Korean working group on sarcopenia guideline: expert consensus on sarcopenia screening and diagnosis by the Korean society of sarcopenia, the Korean society for bone and mineral research, and the Korean geriatrics society. Annals of Geriatric Medicine and Research 27(1), 9-21.
| Crossref | Google Scholar | PubMed |
Bernabei R, Landi F, Calvani R, Cesari M, Signore SD, Anker SD, Bejuit R, Bordes P, Cherubini A, Cruz-Jentoft AJ, Bari MD, Friede T, Ayestarán CG, Goyeau H, Jónsson PV, Kashiwa M, Lattanzio F, Maggio M, Mariotti L, Miller RR, Rodriguez-Mañas L, Roller-Wirnsberger R, Rýznarová I, Scholpp J, Schols AMWJ, Sieber CC, Sinclair AJ, Skalska A, Strandberg T, Tchalla A, Topinková E, Tosato M, Vellas B, Von Haehling S, Pahor M, Roubenoff R, Marzetti E (2022) Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ 377, e068788.
| Crossref | Google Scholar | PubMed |
Brown JC, Harhay MO, Harhay MN (2016) Sarcopenia and mortality among a population-based sample of community-dwelling older adults. Journal of Cachexia, Sarcopenia and Muscle 7(3), 290-298.
| Crossref | Google Scholar | PubMed |
Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E (2014) Sarcopenia and physical frailty: two sides of the same coin. Frontiers in Aging Neuroscience 6, 192.
| Crossref | Google Scholar | PubMed |
Chan D-D, Tsou H-H, Chang C-B, Yang R-S, Tsauo J-Y, Chen C-Y, Hsiao C-F, Hsu Y-T, Chen C-H, Chang S-F, Hsiung CA, Kuo KN (2017) Integrated care for geriatric frailty and sarcopenia: a randomized control trial. Journal of Cachexia, Sarcopenia and Muscle 8(1), 78-88.
| Crossref | Google Scholar | PubMed |
Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee W-J, Lee Y, Liang C-K, Lim J-Y, Lim WS, Peng L-N, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H (2020) Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. Journal of the American Medical Directors Association 21(3), 300-307.e302.
| Crossref | Google Scholar |
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. The Lancet 393(10191), 2636-2646.
| Crossref | Google Scholar |
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 48(1), 16-31.
| Crossref | Google Scholar | PubMed |
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, Bauer JM, Pahor M, Clark BC, Cesari M, Ruiz J, Sieber CC, Aubertin-Leheudre M, Waters DL, Visvanathan R, Landi F, Villareal DT, Fielding R, Won CW, Theou O, Martin FC, Dong B, Woo J, Flicker L, Ferrucci L, Merchant RA, Cao L, Cederholm T, Ribeiro SML, Rodríguez-Mañas L, Anker SD, Lundy J, Gutiérrez Robledo LM, Bautmans I, Aprahamian I, Schols JMGA, Izquierdo M, Vellas B (2018) International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. The Journal of nutrition, health and aging 22(10), 1148-1161.
| Crossref | Google Scholar | PubMed |
Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, Woo J, Aprahamian I, Sanford A, Lundy J, Landi F, Beilby J, Martin FC, Bauer JM, Ferrucci L, Merchant RA, Dong B, Arai H, Hoogendijk EO, Won CW, Abbatecola A, Cederholm T, Strandberg T, Gutiérrez Robledo LM, Flicker L, Bhasin S, Aubertin-Leheudre M, Bischoff-Ferrari HA, Guralnik JM, Muscedere J, Pahor M, Ruiz J, Negm AM, Reginster JY, Waters DL, Vellas B (2019) Physical frailty: ICFSR international clinical practice guidelines for identification and management. The Journal of nutrition, health and aging 23(9), 771-787.
| Crossref | Google Scholar | PubMed |
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology 49(2), M85-M94.
| Crossref | Google Scholar | PubMed |
Ji S, Jung H-W, Baek JY, Jang I-Y, Lee E (2023) Geriatric medicine in South Korea: a stagnant reality amidst an aging population. Annals of Geriatric Medicine and Research 27(4), 280-285.
| Crossref | Google Scholar | PubMed |
Ji S, Baek JY, Go J, Lee CK, Yu SS, Lee E, Jung H-W, Jang I-Y (2025) Effect of exercise and nutrition intervention for older adults with impaired physical function with preserved muscle mass (functional sarcopenia): a randomized controlled trial. Clinical Interventions in Aging 20, 161-170.
| Crossref | Google Scholar |
Ko R-E, Moon SM, Kang D, Cho J, Chung CR, Lee Y, Hong YS, Lee SH, Lee JH, Suh GY (2021) Translation and validation of the Korean version of the clinical frailty scale in older patients. BMC Geriatrics 21(1), 47.
| Crossref | Google Scholar | PubMed |
Levis B, Benedetti A, Thombs BD (2019) Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. Bmj 365, l1476.
| Crossref | Google Scholar | PubMed |
Lim WS, Cheong CY, Lim JP, Tan MMY, Chia JQ, Malik NA, Tay L (2022) Singapore clinical practice guidelines for sarcopenia: screening, diagnosis, management and prevention. The Journal of Frailty & Aging 11(4), 348-369.
| Crossref | Google Scholar | PubMed |
Noh K-W, Park S (2023) Effects of resistance exercise on older individuals with sarcopenia: sex differences in humans. Exercise Science 32(3), 255-265.
| Crossref | Google Scholar |
Oh M-K, Yoo J-I, Byun H, Chun S-W, Lim S-K, Jang YJ, Lee CH (2020) Efficacy of combined antigravity treadmill and conventional rehabilitation after hip fracture in patients with sarcopenia. The Journals of Gerontology: Series A 75(10), e173-e181.
| Crossref | Google Scholar |
Park S-H, Roh Y (2023) Which intervention is more effective in improving sarcopenia in older adults? A systematic review with meta-analysis of randomized controlled trials. Mechanisms of Ageing and Development 210, 111773.
| Crossref | Google Scholar | PubMed |
Park J, Kwon Y, Park H (2017) Effects of 24-week aerobic and resistance training on carotid artery intima-media thickness and flow velocity in elderly women with sarcopenic obesity. Journal of Atherosclerosis and Thrombosis 24(11), 1117-1124.
| Crossref | Google Scholar | PubMed |
Perera S, Mody SH, Woodman RC, Studenski SA (2006) Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society 54(5), 743-749.
| Crossref | Google Scholar | PubMed |
Phillips A, Strobl R, Vogt S, Ladwig K-H, Thorand B, Grill E (2017) Sarcopenia is associated with disability status – results from the KORA-Age study. Osteoporosis International 28(7), 2069-2079.
| Crossref | Google Scholar | PubMed |
Reginster J-Y, Beaudart C, Al-Daghri N, Avouac B, Bauer J, Bere N, Bruyère O, Cerreta F, Cesari M, Rosa MM, Cooper C, Cruz Jentoft AJ, Dennison E, Geerinck A, Gielen E, Landi F, Laslop A, Maggi S, Prieto Yerro MC, Rizzoli R, Sundseth H, Sieber C, Trombetti A, Vellas B, Veronese N, Visser M, Vlaskovska M, Fielding RA (2021) Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clinical and Experimental Research 33(1), 3-17.
| Crossref | Google Scholar | PubMed |
Sánchez JLC, Gallardo-Gómez D, Alfonso-Rosa RM, Cruz BdP, Ramos-Munell J, del Pozo-Cruz J (2025) Effectiveness of different types of exercise based-interventions in sarcopenia: a systematic review and meta-analysis. Geriatric Nursing 63, 635-642.
| Crossref | Google Scholar |
Seo M-W, Jung S-W, Kim S-W, Lee J-M, Jung H-C, Song J-K (2021) Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: a randomized controlled trial. International Journal of Environmental Research and Public Health 18(13), 6762.
| Crossref | Google Scholar |
Shen Y, Shi Q, Nong K, Li S, Yue J, Huang J, Dong B, Beauchamp M, Hao Q (2023) Exercise for sarcopenia in older people: a systematic review and network meta-analysis. Journal of Cachexia, Sarcopenia and Muscle 14(3), 1199-1211.
| Crossref | Google Scholar | PubMed |
Tsekoura M, Kastrinis A, Katsoulaki M, Billis E, Gliatis J (2017) Sarcopenia and its impact on quality of life. In ‘GeNeDis 2016. Advances in experimental medicine and biology, Vol. 987’. (Ed. P Vlamos) pp. 213–218. (Springer) 10.1007/978-3-319-57379-3_19
Won CW (2023) Management of sarcopenia in primary care settings. Korean Journal of Family Medicine 44(2), 71-75.
| Crossref | Google Scholar | PubMed |
Won CW, Yang KY, Rho YG, Kim SY, Lee EJ, Yoon JL, Cho KH, Shin HC, Cho BR, Oh JR, Yoon DK, Lee HS, Lee YS (2002) The development of Korean activities of daily living(K-ADL) and Korean instrumental activities of daily living (K-IADL) scale. Journal of the Korean Geriatrics Society 6(2), 107-120.
| Google Scholar |
Wright AEH, Harrell HE (2022) Physical examination in the evaluation of dementia. Medical Clinics of North America 106(3), 471-482.
| Crossref | Google Scholar | PubMed |
Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB (2019) Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. Journal of Cachexia, Sarcopenia and Muscle 10(3), 485-500.
| Crossref | Google Scholar | PubMed |
Yun JH, Kim DH, Chang MC (2021) A simple bedside exercise method to enhance lower limb muscle strength in moderate alzheimer’s disease patients with sarcopenia. Healthcare 9(6), 680.
| Crossref | Google Scholar |
Zhu L-Y, Chan R, Kwok T, Cheng KC-C, Ha A, Woo J (2019) Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: a randomized controlled trial. Age and Ageing 48(2), 220-228.
| Crossref | Google Scholar | PubMed |


