Suppression of Sertoli cell tumour development during the first wave of spermatogenesis in inhibin α-deficient mice
Jenna T. Haverfield A B , Peter G. Stanton A C , Kate L. Loveland A B C , Heba Zahid A D , Peter K. Nicholls A C , Justine S. Olcorn A C , Yogeshwar Makanji A , Catherine M. Itman E , Evan R. Simpson A C and Sarah J. Meachem A B FA Hudson Institute of Medical Research, 27–31 Wright Street, Clayton, Vic. 3168, Australia.
B Department of Anatomy and Developmental Biology, Monash University, Wellington Road and Blackburn Road, Clayton, Vic. 3800, Australia.
C Department of Biochemistry and Molecular Biology, Monash University, Wellington Road and Blackburn Road, Clayton, Vic. 3800, Australia.
D Faculty of Applied Medical Science, Taibah University, Al Madinah Al Monawarah, Universities Road, Medina, 30001, Saudi Arabia.
E Priority Research Centres for Reproductive Science and Chemical Biology, School of Environmental and Life Sciences, Faculty of Science and Information Technology, University of Newcastle, University Drive, Callaghan, NSW 2308, Australia.
F Corresponding author. Email: sarah.meachem@princehenrys.org
Reproduction, Fertility and Development 29(3) 609-620 https://doi.org/10.1071/RD15239
Abstract
A dynamic partnership between follicle-stimulating hormone (FSH) and activin is required for normal Sertoli cell development and fertility. Disruptions to this partnership trigger Sertoli cells to deviate from their normal developmental pathway, as observed in inhibin α-knockout (Inha-KO) mice, which feature Sertoli cell tumours in adulthood. Here, we identified the developmental windows by which adult Sertoli cell tumourigenesis is most FSH sensitive. FSH was suppressed for 7 days in Inha-KO mice and wild-type littermates during the 1st, 2nd or 4th week after birth and culled in the 5th week to assess the effect on adult Sertoli cell development. Tumour growth was profoundly reduced in adult Inha-KO mice in response to FSH suppression during Weeks 1 and 2, but not Week 4. Proliferative Sertoli cells were markedly reduced in adult Inha-KO mice following FSH suppression during Weeks 1, 2 or 4, resulting in levels similar to those in wild-type mice, with greatest effect observed at the 2 week time point. Apoptotic Sertoli cells increased in adult Inha-KO mice after FSH suppression during Week 4. In conclusion, acute FSH suppression during the 1st or 2nd week after birth in Inha-KO mice profoundly suppresses Sertoli cell tumour progression, probably by inhibiting proliferation in the adult, with early postnatal Sertoli cells being most sensitive to FSH action.
Additional keywords: FSH, cancer, testis.
Introduction
A dynamic partnership between follicle-stimulating hormone (FSH) and activin is fundamental to establish an appropriately sized, mature Sertoli : germ cell niche (Boitani et al. 1995; Meehan et al. 2000; Barakat et al. 2008). Disturbances to this partnership can trigger Sertoli cells to deviate from their normal developmental pathway and perturb normal testis growth in adulthood. One example is inhibin α-knockout (Inha-KO)-deficient mice, which develop focally invasive Sertoli cell tumours by 4 weeks of age (Matzuk et al. 1992). The stimulatory effect of pathological circulating activin levels on FSH secretion from the pituitary in this model leads to conditions under which a subset of proliferating Sertoli cells is prevented from differentiating in adulthood (Matzuk et al. 1992). Although unopposed activin action is thought to drive tumour formation in Inha-KO mice, it has been unequivocally shown that FSH augments tumour growth, because deficiency in both Inha and the FSH β-subunit (FSHβ) results in slower-growing and less-haemorrhagic tumours (Kumar et al. 1996, 1999). However, when and how FSH acts to stimulate Sertoli cell tumour growth in Inha-KO mice is not known.
During normal Sertoli cell development from fetal life until around puberty, FSH stimulates expansion of the Sertoli cell population in many species by promoting Sertoli cell proliferation (Orth 1984; Arslan et al. 1993; Schlatt et al. 1995; Singh and Handelsman 1996; Buzzard et al. 2002; Allan et al. 2004; Meachem et al. 2005a, 2005b) and preventing Sertoli cell apoptosis (Abel et al. 2000; Chausiaux et al. 2008). Sertoli cells are first responsive to FSH during late fetal development (Orth 1984, 1982) and changes in FSH levels during postnatal development have profound effects on Sertoli cell number in adulthood. For example, adult Sertoli cell numbers increase 130%–220% in rats treated with recombinant human FSH during postnatal life (Meachem et al. 1996) and are reduced 30%–40% in FSHβ-KO (Wreford et al. 2001) and FSH receptor (FSHR)-KO (O’Shaughnessy et al. 2012) mice. There are also age-dependent differences in Sertoli cell responsiveness to FSH (Boitani et al. 1995; Buzzard et al. 2003; Meachem et al. 2005a); Sertoli cell proliferation is most dependent on FSH after birth, with the greatest response at 9 days post partum (d.p.p.) in rats, with little to no response by 18 d.p.p., coincident with the timing of puberty.
Similarly, there is an important transition in activin responses during Sertoli cell development, which occurs after the 1st week post partum (Boitani et al. 1995; Buzzard et al. 2003). Initially, only SMAD3 is required to transduce the activin signal, which drives gene expression in proliferating Sertoli cells (Li et al. 2007b; Itman et al. 2009), by a mechanism yet to be established. Sertoli cells transition to a state in which activin signals are transduced by SMAD2 and SMAD3, and this drives expression of genes corresponding to their differentiated status (Itman et al. 2009).
For over a century it has been believed that adult Sertoli cell differentiation is fixed for life, a phenomenon called ‘terminal’ differentiation (Sertoli 1878), with a loss in proliferative ability considered a defining feature of the differentiated state (Sharpe et al. 2003). This largely stems from data in rats (Steinberger and Steinberger 1971; Orth 1982) and mice (Kluin et al. 1984; Vergouwen et al. 1991; Baker and O’Shaughnessy 2001), where Sertoli cells permanently cease proliferation between 15 and 21 d.p.p. (rats) or between 12 and 20 d.p.p. (mice). However, research efforts over the past decade, largely from the seasonal-breeding Djungarian hamster, have challenged this dogma by revealing that adult Sertoli cells are capable of re-entering the cell cycle and dedifferentiating (Meachem et al. 2005b; Tarulli et al. 2006, 2013; for a review, see Tarulli et al. 2012), a phenomenon regulated by FSH. Moreover, it was recently reported that adult Sertoli cell numbers increase between 30 and 70 d.p.p. in normal wild-type (WT) mice (Hazra et al. 2013). However, to date, Sertoli cells have not been observed to proliferate in vivo per se in the adult rodent, or primate testis, under normal conditions.
In the present study we determined how alterations in FSH and activin action during early postnatal and post-pubertal development affect the Sertoli cell population and tumour progression in the adult mouse. To do this, we took advantage of a model in which Sertoli cell proliferation is abnormally sustained in adulthood, namely the Inha-KO mouse. The aims of the present study were to: (1) investigate whether short-term FSH suppression can affect Sertoli tumour development; (2) identify the particular developmental windows most sensitive to perturbations in FSH action; (3) elucidate whether FSH-induced effects are a result of changes in proliferation and/or apoptosis; and (4) test the ability of adult Sertoli cells to proliferate in WT mice. To do this, FSH action was selectively suppressed by immunoneutralisation in Weeks 1, 2 or 4 after birth to reflect early postnatal, prepubertal and post-pubertal periods of development, respectively. The effects of early pre- and postnatal FSH suppression on adult Sertoli cell proliferation and apoptosis were assessed using flow cytometry, whereas Sertoli cell tumour growth was monitored via quantitative histological analysis.
Materials and methods
Mice
Male Inha-KO mice were maintained by heterozygote breeding on a C57Bl/6 background at the Monash University Central Animal Services (Melbourne, Vic., Australia) under a fixed 12-h light–dark cycle with free access to food and water. For timed matings, heterozygous males were caged with heterozygous females overnight, and the visualisation of a copulatory plug the next morning was recorded as 0.5 days postcoitum (d.p.c.). Natural birth occurred between 19 and 21 d.p.c., which was counted as Day 0 d.p.p. All animal experimentation was approved by the Monash Medical Centre Animal Ethics Committee and adhered to the National Health and Medical Research Council of Australia code of practice for the care and use of animals.
Acute suppression of FSH
To selectively suppress FSH action, Inha-KO mice and their WT littermates were administered daily subcutaneous injections of an ovine polyclonal antiserum raised against rat FSH (FSHAb; 3 mg kg−1; Meachem et al. 1998, 1999, 2005b) or a control sheep immunoglobulin (ConAb; 3 mg kg−1) for 7 days during 0–7 d.p.p. (Week 1), 7–14 d.p.p. (Week 2) or 21–28 d.p.p. (Week 4; n = 4–6 KO and n = 4–6 WT animals per group). The 3 mg kg−1 per day dose is 1.5-fold higher than that administered previously to adult rats, which achieved a level of serum FSH neutralisation >90% (reaching the assay detection limit; Meachem et al. 1998). The dose used in the present study was increased by 1.5-fold to account for elevated serum FSH levels in Inha-KO mice (Matzuk et al. 1992). All mice were killed by CO2 inhalation at 35 d.p.p. All endpoints were assessed at 35 d.p.p. for three reasons: (1) tumours are observed histologically from 4 weeks after birth (Matzuk et al. 1992); (2) at this age, one full spermatogenic wave has occurred; and (3) mice at this age are relatively healthy (they suffer from a cachexia-like wasting syndrome from 6–12 weeks of age; Matzuk et al. 1992).
Sample collection
Eighteen hours before mice were killed, they were given an intraperitoneal injection of 5-ethynyl-2′-deoxyuridine (EdU; Life Technologies, Carlsbad CA, USA) at a dose of 5 mg kg−1 in sterile saline. Following CO2 asphyxiation, mice were decapitated to collect trunk blood (~1 mL), from which serum was isolated (~0.1 mL) and stored at −80°C for hormone analysis. Both testes were excised. Approximately 50 mg of one testis was collected using a scalpel and snap frozen at −80°C for later quantitative polymerase chain reaction (qPCR) analysis, with the remainder of the testis placed into phenol red and fetal calf serum (FCS)-free Dulbecco’s modified Eagle’s medium (DMEM) for flow cytometry analysis. The second testis was fixed in Bouin’s solution for less than 5 h, weighed and processed in paraffin wax for quantitative histological tumour analysis. For all analyses, four to six testes per group were used.
Histological tumour analysis
The entire Bouin’s-fixed testis from each mouse (n = 4–6 mice per group) was serially sectioned at 5 μm (~600 total sections per testis) and adhered to Superfrost Plus slides (Thermo Scientific, Waltham, MA, USA). To obtain an unbiased representation of tumour growth, six sections per testis were systematically selected using a uniform random sampling approach (Meachem et al. 1997). Briefly, sections were selected from a random start, and every 100th section thereafter was taken for analysis until a total of six sections were collected. Sections were stained with haematoxylin and eosin and imaged at ×20 magnification on an Aperio Scan Scope XT (Vista, CA, USA). For each section, the area of focal tumour growth was measured as a percentage of total testis area using ImageJ software (http://rsbweb.nih.gov/ij/, accessed 1 February 2013). The percentage of total tumour area for each testis was calculated by averaging the percentage of tumour area from the six sections analysed per testis.
Flow cytometry
Freshly dissected testes were decapsulated and incubated in 3 mL FCS-free DMEM (Life Technologies) containing 0.5 mg mL−1 collagenase and 1 mg mL−1 deoxyribonuclease I (#C0130 and #DN25, respectively; Sigma-Aldrich, St Louis, MO, USA) for 30 min at 35°C on an orbital shaker at 130 r.p.m. To remove interstitial cells, dissociated tubules were resuspended in 3 mL DMEM with 1 mg mL−1 collagenase, 0.5 mg mL−1 deoxyribonuclease I and 1 mg mL−1 hyaluronidase type II (#H2126; Sigma-Aldrich) for 20 min at 35°C and 130 r.p.m. To produce a single cell suspension, tubules were resuspended in 1 mL TrypLE Express (Life Technologies) for 15 min at 35°C and 130 r.p.m. Phosphate-buffered saline (PBS) + 10% FCS was added to quench the TrypLE Express reaction and cells were then filtered through a 70-μm cell strainer and counted to collect 1 × 106 cells per testis for flow cytometry staining and analysis. Dissociated cells were resuspended in 2% paraformaldehyde fixative for 10 min, then washed in PBS–1% bovine serum albumin (BSA) and resuspended in 100 μL Click-iT saponin-based permeabilisation and wash reagent (Life Technologies) for 10 min all at room temperature. EdU incorporation to monitor proliferation was detected using the Click-iT EdU Alexa Fluor 647 Flow Cytometry Assay (Life Technologies). Before antibody staining, cells were blocked with 10% normal goat serum for 45 min on ice. Cells were then stained with mouse anti-β III tubulin antibody (TUJ1) (βIII-tubulin; MMS-435P; Covance, Princeton, NJ, USA; dilution 1 : 16 000) as a marker of Sertoli cells and rabbit anti-cleaved caspase 3 (#9664; Cell Signaling, Beverly, MA, USA; dilution 1 : 400) as a marker of apoptosis. Negative controls were performed by incubating cells in either mouse or rabbit IgG isotype control at a concentration equivalent to that of the primary antibody. Secondary antibodies (goat anti-mouse 488 or goat anti-rabbit 405; Life Technologies; 1 : 5000 dilution) were applied for 1 h on ice. All serum and antibodies were diluted in PBS–1% BSA. Cell populations (50 000–100 000) were analysed using a BD FACS CantoTM II Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with 405-, 488- and 635-nm excitation lasers. Data were analysed using FlowJo software (Tree Star, Ashland, OR, USA).
Immunofluorescent colocalisation
To monitor proliferation, KO tissues (n = 3 per group) treated with ConAb or FSHAb were examined with antibodies against proliferating cell nuclear antigen (PCNA; a marker of S phase proliferation; M087901-2; Dako, Glostrup, Denmark; 1 : 1000 dilution) and GATA binding protein 4 (GATA4) (a marker of somatic cells; 14-9980-82; eBioScience; 1 : 100 dilution) using a protocol modified from Haverfield et al. (2013). Briefly, sections were dewaxed in histolene (2 × 5 min) and 100% ethanol (2 × 5 min) before rehydration in a graded series of ethanol (90%, 70%, 50% and 30% ×5 min each) and finally in H2O (5 min). Antigen retrieval was performed by heating slides in 1 mM EDTA–NaOH, pH 8, in a 1000-W microwave at 100% power for 10 min, before cooling at room temperature for 1 h. To block non-specific staining, sections were treated with 10% normal goat serum (Chemicon, Temecula, CA, USA) diluted in neat CAS block (Invitrogen, Paisley, UK) for 20 min, before incubation with the primary antibody overnight at room temperature. Samples were incubated with the secondary antibody (goat anti-rabbit Alexa-488; Molecular Probes, Eugene, OR, USA; dilution 1 : 200) in the dark for 1 h at room temperature. Sections were mounted with FluorSave (Calbiochem, La Jolla, CA, USA) and immunofluorescence was visualised using a confocal microscope (Fluoview FV300; Olympus, Mt Waverley, Vic., Australia). Enumeration of the number of tubule cross-sections stained with GATA4 only or GATA4/PCNA double staining was performed using a systematic uniform random sampling method within an unbiased frame (Meachem et al. 1998).
Activin A and B serum concentrations
Activin A was measured using a specific ELISA (Oxford Bio-Innovations, Oxfordshire, UK; Knight et al. 1996), using a protocol modified from Okuma et al. (2006). Recombinant activin A standard and samples, diluted in 5% BSA–PBS (pH 7.4), were treated with sodium dodecyl sulfate (SDS; final concentration 3%) and boiled for 3 min. Once cooled, samples were treated with H2O2 (final concentration 2%) and incubated for 30 min at room temperature. Samples were added to duplicate wells in E4 antibody-coated plates and incubated for 1 h at room temperature. Plates were then probed with biotinylated-E4 antibody and incubated overnight at room temperature. After washing with PBS with 0.5% Tween (pH 7.4), a PolyS streptavidin–horseradish peroxidase (HRP; Sanquin, Amsterdam, The Netherlands) conjugate diluted in 1% casein solution (Sanquin) was added to the wells and incubated at room temperature for 1 h. After further washes with PBS with 0.5% Tween, HRP activity was detected with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Life Technologies) by measuring absorbance at 450 nm. Sample preparation was similar for activin B standard and sample, except that after boiling in SDS, 50 µL Triton assay diluent (25 mM Tris, 0.15M NaCl, 5% Triton-X100, 0.1% NaN3 and 10% BSA) was added to each tube. Samples were added to duplicate wells in 46A/F antibody-coated plates together with biotinylated-46A/F antibody and incubated for 1 h at room temperature. Detection following the addition of streptavidin-HRP and TMB was the same as for activin A (Ludlow et al. 2009). The sensitivity of the activin A and B activin ELISAs was 5 and 8.6 pg mL−1, respectively. Because of the small volume (~0.1 mL) of serum obtainable from mice, direct measurement of FSH concentrations was not possible in the present study, because at least 1 mL serum is required to remove the assay interfering bound and unbound fraction of the FSHAb from the serum by using HPLC (Meachem et al. 1998). Therefore, we chose to measure the relative mRNA levels of FSH-regulated genes as an indirect assessment of FSH levels.
RNA isolation and quantitative reverse transcription–polymerase chain reaction
The relative mRNA levels of four known FSH-regulated genes, namely Smad3, steroidogenic acute regulatory protein (Star), insulin-like growth factor-binding protein 3 (Igfbp3) and 17-beta-hydroxysteroid dehydrogenase 1 (Hsd17b1), were measured. These genes were selected because they feature the most significant change in expression when FSH is acutely suppressed in rats (Meachem et al. 2005a). Total RNA extraction from frozen mouse testes and reverse transcription was performed as described previously (McCabe et al. 2010). Smad3, Star, Igfbp3 and Hsd17b1 transcript levels were measured using SYBR green gene expression assays according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). Oligonucleotide primers were designed using Primer-BLAST (National Centre for Biotechnology Information, Bethesda, MD, USA) for accession numbers NM_016769.4 (Smad3; forward: AGTTGGACGAGCTGGAGAAG; reverse: GGCAGTAGATAACGTGAGGGA), NM_011485.4 (Star; forward: TTGGGCATACTCAACAACCA; reverse: TGATGACCGTGTCTTTTCCA), NM_008343.2 (Igfbp3; forward: GGAAACCCATAACCGAGTGAC; reverse: TCCCACAGTTCCCAAGTAGATC) and NM_010475.1 (Hsd17b1; forward: GTTATGAGCAAGCCCTGAGC; reverse: AAGCGGTTCGTGGAGAAGTA) obtained from Sigma Genosys (Castle Hill, NSW, Australia). All reactions were amplified using an Applied Biosystems 7900HT Fast-Start Real-time PCR system at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 60 s at 62°C. All samples (n = 5 animals per group) were assayed in triplicate and candidate gene expression was normalised against that of β-actin (NM_007393.3; oligonucleotide pairs; forward: AGGCTGTGCTGTCCCTGTAT; reverse: AAGGAAGGCTGGAAAAGAGC), which is unaffected by FSH perturbations (Haverfield et al. 2014).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0d (GraphPad Software, La Jolla, CA, USA). Data were analysed using one-way analysis of variance or, in the case of non-parametric data, a Kruskal–Wallis test for multiple comparisons between groups, followed by Tukey’s post hoc test. Statistical significance was accepted at P < 0.05. Data are expressed as the mean ± s.d.
Results
Effects of FSH suppression on adult testis weights
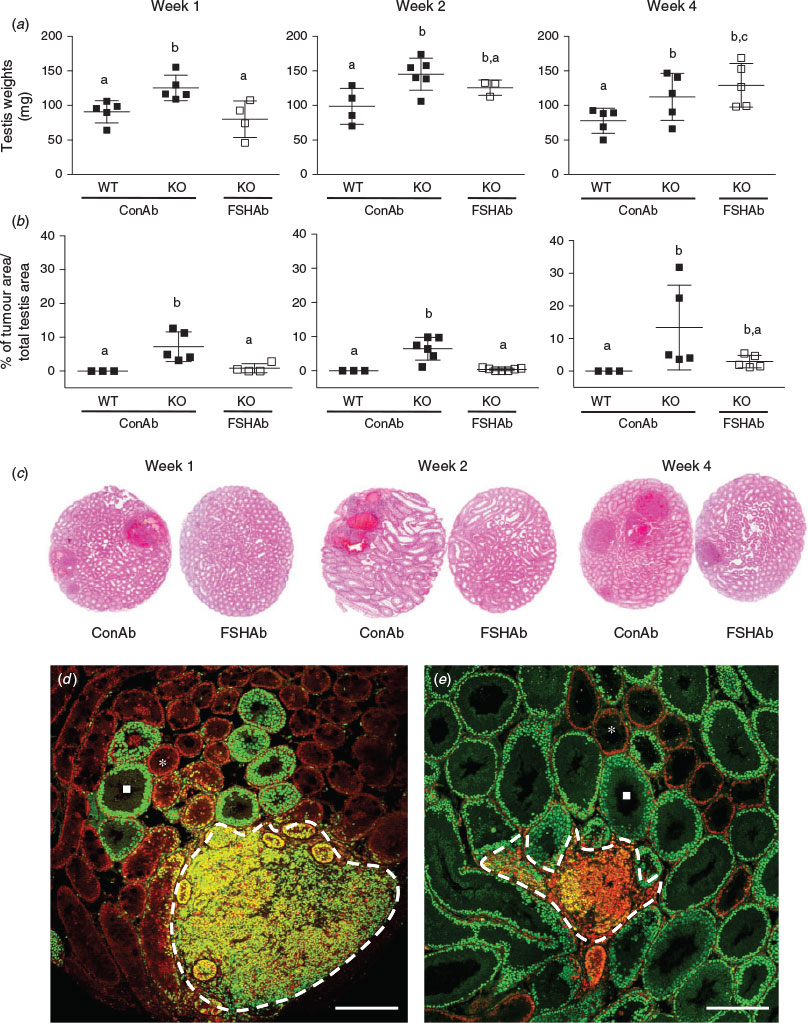
We first sought to examine the effects of FSH suppression during Weeks 1, 2 or 4 on adult testis weight. Inha-KO mice treated with FSHAb during Week 1 exhibited a 36% decrease (P = 0.02) in testis weight at 35 d.p.p. compared with age-matched Inha-KO mice treated with ConAb, with testis weight comparable to that of WT mice (P = 0.6; Fig. 1a). Inha-KO mice treated with FSHAb during Weeks 2 and 4 showed no significant change in testis weights at 35 d.p.p. compared with ConAb-treated Inha-KO mice (P = 0.2 and 0.4, respectively; Fig. 1a). Testis weights were increased 38%–49% in Inha-KO mice treated with ConAb during Weeks 1, 2 and 4 compared with age-matched WT mice receiving identical treatment with ConAb (P < 0.05; Fig. 1a).
Effects of FSH suppression on Sertoli cell tumour development
Next, we examined the effects of FSH suppression during Weeks 1, 2 or 4 on the extent of Sertoli cell tumour development in the adult testis. Inha-KO mice treated with FSHAb during Week 1 exhibited significantly reduced tumour areas at 35 d.p.p. compared with age-matched Inha-KO mice treated with ConAb (tumour area 0.8% vs 7.2%, respectively; P = 0.02; Fig. 1b). Similarly, Inha-KO mice treated with FSHAb during Week 2 exhibited a marked reduction in tumour area at 35 d.p.p. compared with Inha-KO mice treated with ConAb (0.4% vs 6.5%, respectively; P = 0.0005; Fig. 1b). However, Inha-KO mice treated with FSHAb during Week 4 did not exhibit reduced tumour area at 35 d.p.p. compared with Inha-KO mice treated with ConAb (P = 0.1), presumably because of the large standard deviation in tumour area in Inha-KO mice treated with ConAb (Fig. 1b). WT mice treated with ConAb during Weeks 1, 2 and 4 had no observable tumours at 35 d.p.p. (Fig. 1b). Representative images of tumour area at 35 d.p.p. in Inha-KO mice treated with either ConAb or FSHAb during Weeks 1, 2 or 4 are shown in Fig. 1c. Further qualitative immunofluorescent investigations support these data by showing a marked reduction in the area of colocalisation of PCNA, a marker of proliferative cells, and GATA4, a marker of Sertoli cells, at 35 d.p.p. in Inha-KO mice following FSHAb treatment (Fig. 1d; images representative of Week 2 treatment group). Interestingly, we also observed that the tumours in ConAb-treated Inha-KO mice were surrounded by abnormal Sertoli cell-only seminiferous tubules that lacked germ cells, as determined by lack of PCNA immunostaining (Fig. 1d). Subsequent quantification revealed a marked reduction in the percentage of abnormal Sertoli cell-only tubules in Inha-KO mice at 35 d.p.p. following treatment with FSHAb during Week 2 compared with age-matched Inha-KO mice treated with ConAb (7.5% vs 20.5%, respectively; Fig. 1d, e).
Effects of FSH suppression on Sertoli cell numbers
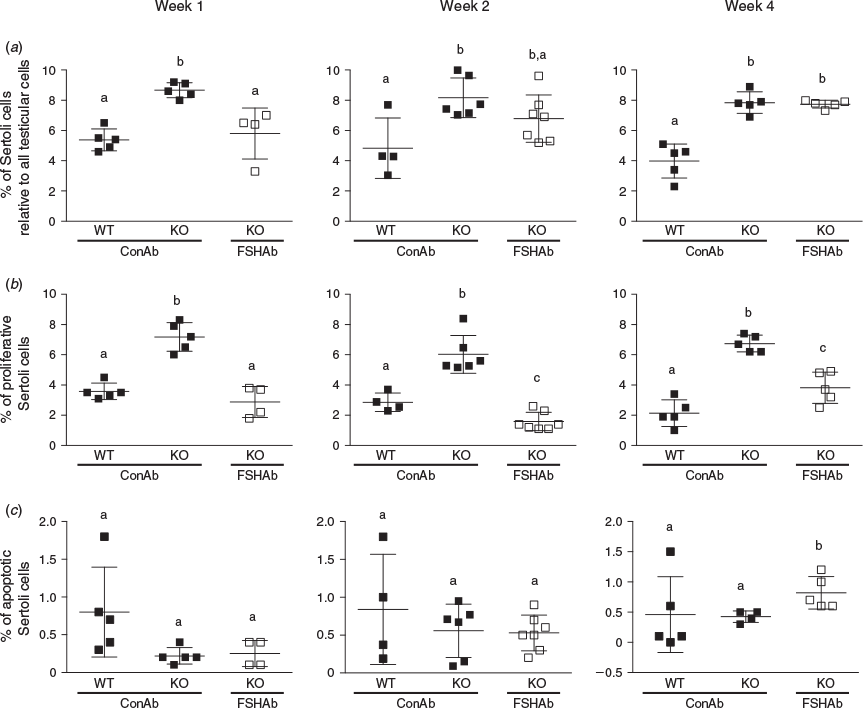
To test the effect of FSH suppression on Sertoli cell numbers, we used an established Sertoli cell-specific marker, namely βIII-tubulin. Inha-KO mice treated with FSHAb during Week 1 had fewer βIII-tubulin-positive cells (33% reduction; P = 0.008) at 35 d.p.p. compared with ConAb-treated Inha-KO mice (Fig. 2a), resulting in numbers analogous to those in WT mice (P = 0.6; Fig. 2a). There was no change in Sertoli cell numbers at 35 d.p.p. in Inha-KO mice treated with FSHAb during Weeks 2 and 4 (P = 0.1 and 0.8, respectively) compared with ConAb-treated Inha-KO mice (Fig. 2a). Sertoli cell numbers were increased (28%–32%) in Inha-KO mice treated with ConAb during Weeks 1, 2 and 4 compared with age-matched WT mice (P < 0.05; Fig. 2a).
Effects of FSH suppression on Sertoli cell proliferation
Suppression of FSH in Inha-KO mice during Weeks 1, 2 or 4 resulted in a significant reduction in the number of EdU-positive Sertoli cells at 35 d.p.p. compared with their ConAb-treated counterparts (P = 0.0003, 0.0001 and 0.0005, respectively), reaching levels comparable to those in ConAb-treated WT mice (P > 0.05; Fig. 2b). In testes from 35 d.p.p. Inha-KO mice, compared with ConAb treatment, the proportion of EdU-positive Sertoli cells was reduced from 7.2% to 2.9% following FSHAb treatment during Week 1, from 6.0% to 1.6% following FSHAb treatment during Week 2 and from 6.7% to 3.8% following FSHAb treatment during Week 4 (Fig. 2b). Fold-change calculations revealed that FSH suppression during Week 2 results in the greatest reduction (3.8-fold) in Sertoli cell proliferation at 35 d.p.p. across all injection periods (Fig. 2b). As expected, EdU-positive Sertoli cell numbers were significantly increased (50%–68%) in Inha-KO mice treated with ConAb during Weeks 1, 2 and 4 compared with ConAb-treated WT mice (Fig. 2b).
Proliferative ability of adult Sertoli cells in WT mice
It has long been considered that Sertoli cells in the adult testis are a strictly homogeneous ‘terminally’ differentiated population. In the present study, WT mice treated with ConAb during Weeks 1, 2 and 4 of postnatal life had 3.6%, 2.9% and 2.2% EdU-positive 35 d.p.p. Sertoli cells, respectively (Fig. 2b). These data demonstrate that at least a subset of Sertoli cells in the adult testis is in the cell cycle and exhibits proliferative activity.
Effects of FSH suppression on Sertoli cell apoptosis
Treatment of Inha-KO mice with FSHAb during Weeks 1 and 2 had no significant effect on the percentage of cleaved caspase 3-positive Sertoli cells at 35 d.p.p. (P = 0.8 and 0.9, respectively) compared with corresponding ConAb-treated Inha-KO mice (Fig. 2c). However, Inha-KO mice treated with FSHAb during Week 4 exhibited a 50% increase in cleaved caspase 3-positive Sertoli cells at 35 d.p.p. compared with ConAb treatment (P = 0.03; Fig. 2c). These findings show that FSH plays an important role in regulating Sertoli cell apoptosis during the 4th week of postnatal life in mice, but not at the earlier time points studied. No significant differences in the percentage of cleaved caspase 3-positive Sertoli cells were observed between WT and Inha-KO mice treated with ConAb during Weeks 1, 2 or 4 (P = 0.06, 0.4 and 0.9, respectively; Fig. 2c).
Effects of FSH suppression on activin A and activin B
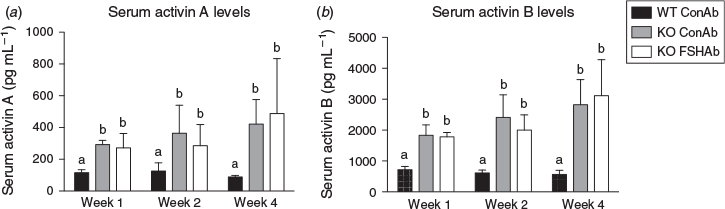
Levels of activins A and B at the 35 d.p.p. time point were significantly elevated in Inha-KO mice treated with ConAb (2.5- and 4.7-fold, respectively) compared with WT mice treated with ConAb across all treatment groups (Fig. 3). Inha-KO mice treated with FSHAb did not show differences in levels of activin A or activin B at 35 d.p.p. in any group; furthermore, levels did not differ from those in ConAb-treated KO mice (P = 0.8 and 0.7, respectively; Fig. 3).
Effects of FSH suppression on gene expression
Acute suppression of FSH in Inha-KO mice during Weeks 1, 2 or 4 had no effect on the relative mRNA levels of the known FSH-regulated genes Star, Hsd17b1, Igfbp3 and Smad3 in 35 d.p.p. testes compared with ConAb treatment (see Fig. S1, available as Supplementary Material to this paper).
Discussion
An important role for FSH in the growth of Sertoli cell tumours has been demonstrated in Inha-KO mice with combined deletion of FSHβ (Kumar et al. 1996, 1999). In the present study we expand on this finding by selectively suppressing FSH action during distinct windows throughout first wave of spermatogenesis in the Inha-KO mouse to identify when FSH has the greatest effect on tumour growth and the cellular kinetics underpinning how FSH augments tumour growth. The results show that FSH action during the 2nd week of postnatal life has the greatest effect on Sertoli cell tumour growth and acts by promoting Sertoli cell proliferation, not apoptosis. Overall, we have demonstrated that transient inhibition of FSH for only 7 days in early postnatal life profoundly suppresses tumourigenesis in Inha-KO mice, as shown by little to no observable tumour development, resembling that of the WT testis.
The present study shows that FSH drives Sertoli cell tumour progression in Inha-KO mice by regulating proliferation, at least within the first 2 weeks of life. These findings are consistent with the period in which FSH stimulates Sertoli cell proliferation in normal rodents during the first wave of spermatogenesis (Meachem et al. 2005a). However, the supraphysiological levels of FSH alone are unlikely to underpin the onset of tumourigenesis, because neonatal treatment of animals with exceedingly high levels of recombinant human FSH does not result in Sertoli cell tumours, but instead increases the adult Sertoli cell population (Meachem et al. 1996). Similarly, neoplastic changes have not been reported in studies treating gonadotrophin-deficient boys with recombinant human FSH (Raivio et al. 1997, 2007). Therefore, we speculate that tumourigenesis in this model is the result of the concerted actions of both FSH and activin, which is pathologically elevated in the Inha-KO mouse (Matzuk et al. 1992). This is supported by evidence showing activin can only stimulate Sertoli cell proliferation in conjunction with FSH in postnatal testis fragment cultures (Boitani et al. 1995). Moreover, increasing systemic activin A concentrations in adult mice using an adeno-associated virus system does not induce Sertoli cell proliferation in vivo (Nicholls et al. 2012). Furthermore, excess activin and FSH signalling synergistically activate the SMAD3 tumourigenic pathway in Sertoli cells (Li et al. 2007a, 2007b; Looyenga and Hammer 2007). Given that not all testicular cells become tumourigenic in the Inha-KO mouse, it is plausible that the exclusive expression of the FSH receptor (FSHR) on Sertoli cells (Griswold 1998) makes them uniquely vulnerable to aberrant activin signalling. Establishing the Sertoli cell FSHR expression profile throughout pre- and postnatal life in the Inha-KO mouse is an important next step towards pinpointing the specific temporal contribution of FSH to the pathogenesis of Sertoli cell tumours.
The present study also showed that FSH supports Sertoli cell survival in the Inha-KO mouse in the 4th week of life, but not within the first 2 weeks. Indeed, that FSH is not a survival factor for Sertoli cells in postnatal and prepubertal life has been demonstrated in rats (Meachem et al. 2005a) and mice (Mazeyrat et al. 2001); however, the present study is the first to document that FSH is an anti-apoptotic factor for postpubertal Sertoli cells. Although others have shown that Sertoli cell apoptosis results from gonadotrophin insufficiency in rats (Yagi et al. 2007) and mice (Chausiaux et al. 2008), these results are likely confounded by the effects of androgens, which are also anti-apoptotic in Sertoli cells in vitro (Tesarik et al. 1998). In addition to inducing apoptosis, FSH inhibition altered the proliferative status of postpubertal Sertoli cells. Together, these findings show that adult mouse Sertoli cells are not a stable population, and their cellular kinetics are adaptable to a changing endocrine environment. This differs from earlier work showing the absence of overt Sertoli cell division or degeneration in hormonally manipulated adult rats (Russell and Clermont 1977; Muffly et al. 1994; McLachlan et al. 1995), but supports a growing body of evidence in other species, such as hamsters (Meachem et al. 2005b; Tarulli et al. 2006) and humans (Tarulli et al. 2013), indicating that postpubertal Sertoli cells are modifiable in terms of their differentiation status. Moreover, this inherent plasticity of adult Sertoli cells has been shown in mice by their ability to dedifferentiate into granulosa-like cells in the absence of doublesex and mab-3 related transcription factor-1 (DMRT1) (Matson et al. 2011). The combined use of in vivo (immunohistochemistry) and ex vivo (flow cytometry) labelling of proliferating cells now affords a level of sensitivity that can detect previously unidentified proliferating mouse Sertoli cells. The potential for the adult Sertoli cell to be replenished opens up novel therapeutic implications to rescue a failing Sertoli cell population in syndromes of infertility, testicular insult or following oncological treatment.
The findings from the present study show that Sertoli cells exhibit a developmental switch in their responsiveness to FSH action in the Inha-KO mouse. This is consistent with the age-dependent responsiveness of Sertoli cells to the mitogenic actions of FSH (Boitani et al. 1995; Meehan et al. 2000; Buzzard et al. 2003; Meachem et al. 2005a) and activin (Itman et al. 2009) in the developing normal rodent testis. With regard to FSH, why Sertoli cells at certain ages are more sensitive to the actions of FSH than others is poorly understood. The number of FSHRs is unlikely to contribute to differences in FSH sensitivity because numbers per Sertoli cell are constant from postnatal Day 2 to 21, with the highest number of receptors measured in adulthood (Bortolussi et al. 1990). Furthermore, FSH binding affinity to its receptor does not change from Day 2 to −60 (Ketelslegers et al. 1978). Although a sharp rise in FSH levels occurs from Day 10 to 16 in mice, these levels persist into adulthood (Barakat et al. 2008). Therefore, FSH levels alone cannot account for the differences in proliferation and tumour growth across groups in the present study. We speculate that these differences are caused by changes in intricate interactions between FSH and growth factors and/or cytokines throughout development, via agonistic or antagonistic effects with FSH or its receptor. Data supporting this speculation are limited, but a range of factors both interact with FSH and regulate Sertoli cell proliferation, including insulin and insulin-like growth factor-1 (Kanematsu et al. 1991; Soldani et al. 1994; Pitetti et al. 2013), glial cell line-derived neurotrophic factor (Hu et al. 1999), interleukin-1 (Petersen et al. 2002), activin (Boitani et al. 1995; Buzzard et al. 2003; Nicholls et al. 2012) and inhibin (Robertson et al. 1985; Buzzard et al. 2004), which also antagonises activin action (Hedger and Winnall 2012).
The differential FSH responsiveness of Sertoli cells may also be due to effects further downstream. FSH acts via its G-protein-coupled receptor located exclusively on Sertoli cells to activate at least five signal transduction pathways (for a review, see Walker and Cheng 2005). FSH alone triggers both activation and inhibition of extracellular signal-regulated kinase (ERK) mitogen-activated protein kinases according to the age of Sertoli cells. For example, FSH stimulates cAMP-dependent ERK activation in 5 d.p.p. Sertoli cells, which supports a proliferative response by upregulating cyclin D1, but negatively regulates the ERK pathway in 19 d.p.p. Sertoli cells (Crépieux et al. 2001). FSH also targets the phosphatidylinositol 3-kinase–Akt–mammalian target of rapamycin complex 1 (mTORC1)-dependent pathway, which increases mRNA levels of the proto-oncogene c-Myc, thereby stimulating Sertoli cell cycle progression (Riera et al. 2012). c-Myc has been implicated in many other types of cancers (Lin et al. 2012) and, importantly, in a patient with a bilateral Sertoli cell tumour (Xiao et al. 2012). It is also possible other endocrine and paracrine factors alter FSH activity by targeting convergent signalling pathways with FSH. For example, FSH-stimulated proliferation is suppressed at puberty by activating AMP-activated protein kinase (Riera et al. 2012), an enzyme activated by retinoic acid and thyroid hormone, with levels of both of these increasing at puberty. FSH is also an important regulator of aromatase (Cyp19A1) transcription (McDonald et al. 2006; for a review, see Haverfield et al. 2011) and, indeed, disturbances to the levels of androgens and oestrogens have been implicated in the development of Sertoli cell tumours in Inha-KO mice (Shou et al. 1997; Burns et al. 2003). Thus, it is likely that an imbalance in activin, FSH, androgens and oestrogens, and the resulting changes in their independent or convergent downstream signalling pathways, may contribute to Sertoli cell tumourigenesis in our model.
To define the direct effects of FSH action on Sertoli cell tumourigenesis and cell population kinetics would provide valuable insight. This was beyond the scope of the present study because of fiscal constraints; however, we would predict the immediate affect of FSH inhibition on the Sertoli cell population (proliferation and survival) and tumour formation during the 1st, 2nd and 4th weeks of postnatal life would be even more profound than what is reported in the present study at Day 35.
An important finding from the present study was the demonstration that postpubertal Sertoli cells from WT mice are in the cell cycle. An average of 2.9% of adult Sertoli cells across all WT mice incorporated EdU, a thymidine analogue integrated during DNA synthesis. Although postpubertal mouse and rat adult Sertoli cells readily proliferate in culture (Ahmed et al. 2009; Chui et al. 2011; Nicholls et al. 2012), in vivo studies reported the absence of Sertoli cell proliferation in the normal adult rodent testis (Steinberger and Steinberger 1971; Russell and Clermont 1977; Orth 1984) which led to the classification of adult Sertoli cells as ‘terminally’ differentiated. Our finding challenges this long-standing classification and supports other data showing that adult Sertoli cells express a combination of genes and proteins that are not present in other ‘terminally’ differentiated cells. These include inhibitor of DNA binding proteins (Id proteins) (Sablitzky et al. 1998; Chaudhary et al. 2001, 2005), cyclin-dependent kinase inhibitor 1B (CDKN1B/p27kip1; Beumer et al. 1998; Ahmed et al. 2009) and several DNA repair proteins, including X-ray repair complementing defective repair in chinese hamster cells 5 (XRCC5) (KU86) and X-ray repair complementing defective repair in chinese hamster cells 6 (XRCC6) (KU70), poly (ADP-ribose) polymerase 1 (PARP1), tumor protein P53 binding protein 1 (TP53BP1) and phosphorylated X-ray repair cross-complementing protein 1 (XRCC1) and ATM serine/threonine kinase (ATM) ( Beumer et al. 1998; Choi et al. 2002; Anway et al. 2003; Hamer et al. 2003, 2004; Ahmed et al. 2009), although the percentage of adult Sertoli cells that express these markers has not been reported. Nonetheless, the ability of adult Sertoli cells to proliferate under endocrine stimulation (Tarulli et al. 2012) and their expression of factors associated with cell proliferation, differentiation and DNA repair suggest that differentiated adult Sertoli cells maintain the capacity to proliferate and regenerate. Future research efforts are needed to identify whether only a discrete population of Sertoli cells is capable of proliferating or whether all Sertoli cells can enter the cell cycle throughout spermatogenesis.
In conclusion, the data presented herein show that acute FSH suppression in the first 2 weeks of life in adult Inha-KO mice protects against the growth of Sertoli cell tumours. The cascade of events leading to the formation of Sertoli cell tumours in this model is not well understood; however, our results delineate a developmental-specific role for FSH in the pathogenesis of these tumours by regulating Sertoli cell kinetics. Our model has enabled us to identify how changes to the testicular endocrine environment during development contribute to Sertoli cell proliferation and tumourigenesis in adult life. Future studies aimed at targeting components involved in the FSH and activin partnership may elucidate mechanisms for alternative therapeutic interventions for Sertoli cell tumours in boys and men.
Acknowledgements
This work was supported by an Australian Postgraduate Award and Prince Henry’s Institute Graduate Excellence Award (to JTH), a National Health and Medical Research Council (Australia) Program Grant No. 494802 (to SJM, ERS and PGS), a C. J. Martin Fellowship to YM (No. 1016460), Senior Research Fellowships to KLL (No. 545916) and ERS (No. 550900) and by the Victorian Government’s Operational Infrastructure Support Program.
References
Abel, M. H., Wootton, A. N., Wilkins, V., Huhtaniemi, I., Knight, P. G., and Charlton, H. M. (2000). The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141, 1795–1803.| 1:CAS:528:DC%2BD3cXlt1WnsL0%3D&md5=1a1bbbdf6c073e4704e60b8a0c8f4994CAS | 10803590PubMed |
Ahmed, E. A., Barten-van Rijbroek, A. D., Kal, H. B., Sadri-Ardekani, H., Mizrak, S. C., van Pelt, A. M. M., and de Rooij, D. G. (2009). Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol. Reprod. 80, 1084–1091.
| Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlGmsrY%3D&md5=c12906eb5a5839b51e16e35ef8852474CAS | 19164176PubMed |
Allan, C. M., Garcia, A., Spaliviero, J., Zhang, F.-P., Jimenez, M., Huhtaniemi, I., and Handelsman, D. J. (2004). Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology 145, 1587–1593.
| Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1SjurY%3D&md5=47c5e13f4e8a5edf48a89c3f1ef62ceaCAS | 14726449PubMed |
Anway, M. D., Folmer, J., Wright, W. W., and Zirkin, B. R. (2003). Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol. Reprod. 68, 996–1002.
| Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFGks7k%3D&md5=6b7c6ca6d0f20c6cab73554ea444d7d5CAS | 12604653PubMed |
Arslan, M., Weinbauer, G. F., Schlatt, S., Shahab, M., and Nieschlag, E. (1993). FSH and testosterone, alone or in combination, initiate testicular growth and increase the number of spermatogonia and Sertoli cells in a juvenile non-human primate (Macaca mulatta). J. Endocrinol. 136, 235–243.
| FSH and testosterone, alone or in combination, initiate testicular growth and increase the number of spermatogonia and Sertoli cells in a juvenile non-human primate (Macaca mulatta).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXntlyqsg%3D%3D&md5=761e7b19b60043ee4fa064f8000f85bfCAS | 8459189PubMed |
Baker, P. J., and O’Shaughnessy, P. J. (2001). Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122, 227–234.
| Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFWjtLo%3D&md5=c687b718ebd32e58d65dd1d61c33e1f2CAS | 11467973PubMed |
Barakat, B., O’Connor, A. E., Gold, E., de Kretser, D. M., and Loveland, K. L. (2008). Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction 136, 345–359.
| Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1GmtL3O&md5=2a73564ba735d65bba236d11a7453de1CAS | 18515316PubMed |
Beumer, T. L., Roepers-Gajadien, H. L., Gademan, I. S., van Buul, P. P., Gil-Gomez, G., Rutgers, D. H., and de Rooij, D. G. (1998). The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 5, 669–677.
| The role of the tumor suppressor p53 in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlslWgtrg%3D&md5=59c78214e3fde230c77e5f2a4f348e8fCAS | 10200522PubMed |
Boitani, C., Stefanini, M., Fragale, A., and Morena, A. R. (1995). Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology 136, 5438–5444.
| 1:CAS:528:DyaK2MXps1Kisr8%3D&md5=3ff64665b9a1d0b60c61465ff679dc7fCAS | 7588293PubMed |
Bortolussi, M., Zanchetta, R., Belvedere, P., and Colombo, L. (1990). Sertoli and Leydig cell numbers and gonadotropin receptors in rat testis from birth to puberty. Cell Tissue Res. 260, 185–191.
| Sertoli and Leydig cell numbers and gonadotropin receptors in rat testis from birth to puberty.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXit12qur0%3D&md5=faf9d48b52d2e8907f4ad726107106a1CAS | 2111226PubMed |
Burns, K. H., Agno, J. E., Chen, L., Haupt, B., Ogbonna, S. C., Korach, K. S., and Matzuk, M. M. (2003). Sexually dimorphic roles of steroid hormone receptor signaling in gonadal tumorigenesis. Mol. Endocrinol. 17, 2039–2052.
| Sexually dimorphic roles of steroid hormone receptor signaling in gonadal tumorigenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotFSrsb8%3D&md5=c94ba2b0dbe20624387bd3a5aba8079fCAS | 12855748PubMed |
Buzzard, J. J., Wreford, N. G., and Morrison, J. R. (2002). Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction 124, 633–641.
| Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1KltQ%3D%3D&md5=16fd649311baa272f0264fb05a55904eCAS | 12417001PubMed |
Buzzard, J. J., Farnworth, P. G., De Kretser, D. M., O’Connor, A. E., Wreford, N. G., and Morrison, J. R. (2003). Proliferative phase Sertoli cells display a developmentally regulated response to activin in vitro. Endocrinology 144, 474–483.
| Proliferative phase Sertoli cells display a developmentally regulated response to activin in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVWltro%3D&md5=16bee472c48677e2118528f5c05fb341CAS | 12538607PubMed |
Buzzard, J. J., Loveland, K. L., O’Bryan, M. K., O’Connor, A. E., Bakker, M., Hayashi, T., Wreford, N. G., Morrison, J. R., and de Kretser, D. M. (2004). Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology 145, 3532–3541.
| Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFykt7s%3D&md5=63bd04334e9c1122ee25938b0ab4f29dCAS | 15070852PubMed |
Chaudhary, J., Johnson, J., Kim, G., and Skinner, M. K. (2001). Hormonal regulation and differential actions of the helix-loop-helix transcriptional inhibitors of differentiation (Id1, Id2, Id3, and Id4) in Sertoli cells. Endocrinology 142, 1727–1736.
| 1:CAS:528:DC%2BD3MXjt1akt74%3D&md5=630ae1af0b07e123af965a415b05a022CAS | 11316735PubMed |
Chaudhary, J., Sadler-Riggleman, I., Ague, J. M., and Skinner, M. K. (2005). The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol. Reprod. 72, 1205–1217.
| The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslSntrY%3D&md5=bdd4874ae5a4f6d6d55bb0c6f7ea549aCAS | 15647457PubMed |
Chausiaux, O. E., Abel, M. H., Baxter, F. O., Khaled, W. T., Ellis, P. J. I., Charlton, H. M., and Affara, N. A. (2008). Hypogonadal mouse, a model to study the effects of the endogenous lack of gonadotropins on apoptosis. Biol. Reprod. 78, 77–90.
| Hypogonadal mouse, a model to study the effects of the endogenous lack of gonadotropins on apoptosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslE%3D&md5=e59e06be7e102d92ad12fa05ceb218deCAS | 17671269PubMed |
Choi, E. K., Lee, Y. H., Choi, Y. S., Kwon, H. M., Choi, M. S., Ro, J. Y., Park, S.-K., and Yu, E. (2002). Heterogeneous expression of Ku70 in human tissues is associated with morphological and functional alterations of the nucleus. J. Pathol. 198, 121–130.
| Heterogeneous expression of Ku70 in human tissues is associated with morphological and functional alterations of the nucleus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xns1Wiu7w%3D&md5=1dc2480bb4c8008413f3e88a06dc8b80CAS | 12210072PubMed |
Chui, K., Trivedi, A., Cheng, C. Y., Cherbavaz, D. B., Dazin, P. F., Huynh, A. L. T., Mitchell, J. B., Rabinovich, G. A., Noble-Haeusslein, L. J., and John, C. M. (2011). Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 20, 619–635.
| Characterization and functionality of proliferative human Sertoli cells.Crossref | GoogleScholarGoogle Scholar | 21054948PubMed |
Crépieux, P., Marion, S., Martinat, N., Fafeur, V., Vern, Y. L., Kerboeuf, D., Guillou, F., and Reiter, E. (2001). The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 20, 4696–4709.
| The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation.Crossref | GoogleScholarGoogle Scholar | 11498792PubMed |
Griswold, M. D. (1998). The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 9, 411–416.
| The central role of Sertoli cells in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvFyl&md5=171e22e4b3599291d36c604081bff1e6CAS | 9813187PubMed |
Hamer, G., Roepers-Gajadien, H. L., van Duyn-Goedhart, A., Gademan, I. S., Kal, H. B., van Buul, P. P. W., Ashley, T., and de Rooij, D. G. (2003). Function of DNA–protein kinase catalytic subunit during the early meiotic prophase without Ku70 and Ku86. Biol. Reprod. 68, 717–721.
| Function of DNA–protein kinase catalytic subunit during the early meiotic prophase without Ku70 and Ku86.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFGlurw%3D&md5=c980e6e2e99a7395c2a3c69e043c6ea9CAS | 12604618PubMed |
Hamer, G., Kal, H. B., Westphal, C. H., Ashley, T., and de Rooij, D. G. (2004). Ataxia telangiectasia mutated expression and activation in the testis. Biol. Reprod. 70, 1206–1212.
| Ataxia telangiectasia mutated expression and activation in the testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1Shsrc%3D&md5=2755419458318b8b554d6f4ee5e3c06aCAS | 14681204PubMed |
Haverfield, J. T., Ham, S., Brown, K. A., Simpson, E. R., and Meachem, S. J. (2011). Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis 1, 240–249.
| Teasing out the role of aromatase in the healthy and diseased testis.Crossref | GoogleScholarGoogle Scholar | 22319672PubMed |
Haverfield, J. T., Meachem, S. J., O’Bryan, M. K., McLachlan, R. I., and Stanton, P. G. (2013). Claudin-11 and connexin-43 display altered spatial patterns of organization in men with primary seminiferous tubule failure compared with controls. Fertil. Steril. 100, 658–666.e3.
| Claudin-11 and connexin-43 display altered spatial patterns of organization in men with primary seminiferous tubule failure compared with controls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovVCgsL8%3D&md5=001e903386ac52fc072c9660ab64d18aCAS | 23706332PubMed |
Haverfield, J. T., Meachem, S. J., Nicholls, P. K., Rainczuk, K. E., Simpson, E. R., and Stanton, P. G. (2014). Differential permeability of the blood–testis barrier during re-initiation of spermatogenesis in adult male rats. Endocrinology 155, 1131–1144.
| Differential permeability of the blood–testis barrier during re-initiation of spermatogenesis in adult male rats.Crossref | GoogleScholarGoogle Scholar | 24424039PubMed |
Hazra, R., Corcoran, L., Robson, M., McTavish, K. J., Upton, D., Handelsman, D. J., and Allan, C. M. (2013). Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol. Endocrinol. 27, 12–24.
| Temporal role of Sertoli cell androgen receptor expression in spermatogenic development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVymsg%3D%3D&md5=a4882ccea6c82475b571ebfefaa36117CAS | 23160479PubMed |
Hedger, M. P., and Winnall, W. R. (2012). Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol. Cell. Endocrinol. 359, 30–42.
| Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnslOjs7k%3D&md5=a9c34715a2c307e2db63d9aa5b2bb53aCAS | 21964464PubMed |
Hu, J., Shima, H., and Nakagawa, H. (1999). Glial cell line-derived neurotropic factor stimulates sertoli cell proliferation in the early postnatal period of rat testis development. Endocrinology 140, 3416–3421.
| Glial cell line-derived neurotropic factor stimulates sertoli cell proliferation in the early postnatal period of rat testis development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkslKrtb8%3D&md5=8dead86420877588c77639fbe278fac1CAS | 10433195PubMed |
Itman, C., Small, C., Griswold, M., Nagaraja, A. K., Matzuk, M. M., Brown, C. W., Jans, D. A., and Loveland, K. L. (2009). Developmentally regulated SMAD2 and SMAD3 utilization directs activin signaling outcomes. Dev. Dyn. 238, 1688–1700.
| Developmentally regulated SMAD2 and SMAD3 utilization directs activin signaling outcomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovV2nu78%3D&md5=9a066baa422087d533521c9460bfcce1CAS | 19517569PubMed |
Kanematsu, T., Irahara, M., Miyake, T., Shitsukawa, K., and Aono, T. (1991). Effect of insulin-like growth factor I on gonadotropin release from the hypothalamus–pituitary axis in vitro. Acta Endocrinol. (Copenh.) 125, 227–233.
| 1:CAS:528:DyaK3MXlvF2ltr8%3D&md5=efb13bbcc2a1a862b0039fd8662cab04CAS | 1910244PubMed |
Ketelslegers, J.-M., Hetzel, W. D., Sherins, R. J., and Catt, K. J. (1978). Developmental changes in testicular gonadotropin receptors: plasma gonadotropins and plasma testosterone in the rat. Endocrinology 103, 212–222.
| Developmental changes in testicular gonadotropin receptors: plasma gonadotropins and plasma testosterone in the rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXlt1KktbY%3D&md5=e437f2ebd97b55aa9fab7eb8dd46ea15CAS | 217637PubMed |
Kluin, P. M., Kramer, M. F., and Rooij, D. G. (1984). Proliferation of spermatogonia and Sertoli cells in maturing mice. Anat. Embryol. (Berl.) 169, 73–78.
| Proliferation of spermatogonia and Sertoli cells in maturing mice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c3gtFSmsA%3D%3D&md5=c10080b84bd857a2df4b808a85ad6d6eCAS | 6721222PubMed |
Knight, P. G., Muttukrishna, S., and Groome, N. P. (1996). Development and application of a two-site enzyme immunoassay for the determination of ‘total’ activin-A concentrations in serum and follicular fluid. J. Endocrinol. 148, 267–279.
| Development and application of a two-site enzyme immunoassay for the determination of ‘total’ activin-A concentrations in serum and follicular fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtVCrsrg%3D&md5=8e305a3b3948aa42df8d0df36ea37771CAS | 8699141PubMed |
Kumar, T. R., Wang, Y., and Matzuk, M. M. (1996). Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology 137, 4210–4216.
| 1:CAS:528:DyaK28XlvVSntbs%3D&md5=65364a976de3eea27d75c4d44044e342CAS | 8828479PubMed |
Kumar, T. R., Palapattu, G., Wang, P., Woodruff, T. K., Boime, I., Byrne, M. C., and Matzuk, M. M. (1999). Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol. Endocrinol. 13, 851–865.
| Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1ynsro%3D&md5=6c690655fcba74d60142ac1fa07586e5CAS | 10379885PubMed |
Li, Q., Graff, J. M., O’Connor, A. E., Loveland, K. L., and Matzuk, M. M. (2007a). SMAD3 regulates gonadal tumorigenesis. Mol. Endocrinol. 21, 2472–2486.
| SMAD3 regulates gonadal tumorigenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFGhu7bE&md5=5fc981135f358b9c8d0d150d8772cb33CAS | 17595316PubMed |
Li, Q., Kumar, R., Underwood, K., O’Connor, A. E., Loveland, K. L., Seehra, J. S., and Matzuk, M. M. (2007b). Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein. Mol. Hum. Reprod. 13, 675–683.
| Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVSgtL7M&md5=6932d6f72ac42f03a967d04c7e6ec50bCAS | 17704537PubMed |
Lin, C. Y., Lovén, J., Rahl, P. B., Paranal, R. M., Burge, C. B., Bradner, J. E., Lee, T. I., and Young, R. A. (2012). Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67.
| Transcriptional amplification in tumor cells with elevated c-Myc.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsValsLjO&md5=582f1e077c407e410a8ceed686960504CAS | 23021215PubMed |
Looyenga, B. D., and Hammer, G. D. (2007). Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol. Endocrinol. 21, 2440–2457.
| Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFGhu7bK&md5=45c743b360a1a7d35673aebbb6d73555CAS | 17652186PubMed |
Ludlow, H., Phillips, D. J., Myers, M., McLachlan, R. I., de Kretser, D. M., Allan, C. A., Anderson, R. A., Groome, N. P., Hyvönen, M., Duncan, W. C., and Muttukrishna, S. (2009). A new ‘total’ activin B enzyme-linked immunosorbent assay (ELISA): development and validation for human samples. Clin. Endocrinol. (Oxf.) 71, 867–873.
| A new ‘total’ activin B enzyme-linked immunosorbent assay (ELISA): development and validation for human samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1Sjtb3I&md5=a77907390babbef99062c28bd6c4b6eaCAS | 19486020PubMed |
Matson, C. K., Murphy, M. W., Sarver, A. L., Griswold, M. D., Bardwell, V. J., and Zarkower, D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104.
| DMRT1 prevents female reprogramming in the postnatal mammalian testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptV2itrY%3D&md5=278357003c9ced92403f6a0b229d55f0CAS | 21775990PubMed |
Matzuk, M. M., Finegold, M. J., Su, J. G., Hsueh, A. J., and Bradley, A. (1992). Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360, 313–319.
| Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhs1Gitw%3D%3D&md5=8e9c9a2d58decd9820b8d19edef4daa0CAS | 1448148PubMed |
Mazeyrat, S., Saut, N., Grigoriev, V., Mahadevaiah, S. K., Ojarikre, O. A., Rattigan, Á., Bishop, C., Eicher, E. M., Mitchell, M. J., and Burgoyne, P. S. (2001). A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis. Nat. Genet. 29, 49–53.
| A Y-encoded subunit of the translation initiation factor Eif2 is essential for mouse spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvFGmsbY%3D&md5=7f88a02c884227b4f56fc10ed7601592CAS | 11528390PubMed |
McCabe, M. J., Tarulli, G. A., Meachem, S. J., Robertson, D. M., Smooker, P. M., and Stanton, P. G. (2010). Gonadotropins regulate rat testicular tight junctions in vivo. Endocrinology 151, 2911–2922.
| Gonadotropins regulate rat testicular tight junctions in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsVyqtbc%3D&md5=064c517ce7a52ce3d8f7f57f229415cbCAS | 20357222PubMed |
McDonald, C. A., Millena, A. C., Reddy, S., Finlay, S., Vizcarra, J., Khan, S. A., and Davis, J. S. (2006). Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol. Endocrinol. 20, 608–618.
| Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol 3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xit1SnsLY%3D&md5=ee6d8fda450e60d693418e6c8e0c7ba8CAS | 16269516PubMed |
McLachlan, R. I., Wreford, N. G., de Kretser, D. M., and Robertson, D. M. (1995). The effects of recombinant follicle-stimulating hormone on the restoration of spermatogenesis in the gonadotropin-releasing hormone-immunized adult rat. Endocrinology 136, 4035–4043.
| 1:CAS:528:DyaK2MXns1WgtLc%3D&md5=4bdb8f7c59127aeab615ace722e7bdf8CAS | 7649112PubMed |
Meachem, S. J., McLachlan, R. I., de Kretser, D. M., Robertson, D. M., and Wreford, N. G. (1996). Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol. Reprod. 54, 36–44.
| Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXhtVSisLrP&md5=40a99bf4e350756154d9564da6901ef6CAS | 8837998PubMed |
Meachem, S. J., Wreford, N. G., Robertson, D. M., and McLachlan, R. I. (1997). Androgen action on the restoration of spermatogenesis in adult rats: effects of human chorionic gonadotrophin, testosterone and flutamide administration on germ cell number. Int. J. Androl. 20, 70–79.
| Androgen action on the restoration of spermatogenesis in adult rats: effects of human chorionic gonadotrophin, testosterone and flutamide administration on germ cell number.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvFaqtLo%3D&md5=8166c4d31eef4064931c83a1bbd09a6cCAS | 9292316PubMed |
Meachem, S. J., Wreford, N. G., Stanton, P. G., Robertson, D. M., and McLachlan, R. I. (1998). Follicle-stimulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J. Androl. 19, 725–735.
| 1:CAS:528:DyaK1MXktlKrsg%3D%3D&md5=bdf4d67e8532af3dfc8155ab3ecf5505CAS | 9876024PubMed |
Meachem, S. J., Mclachlan, R. I., Stanton, P. G., Robertson, D. M., and Wreford, N. G. (1999). FSH immunoneutralization acutely impairs spermatogonial development in normal adult rats. J. Androl. 20, 756–762.
| 1:CAS:528:DyaK1MXotVOrtLY%3D&md5=84744c8cf39357b8f6bf21d8722a2d9aCAS | 10591615PubMed |
Meachem, S. J., Ruwanpura, S. M., Ziolkowski, J., Ague, J. M., Skinner, M. K., and Loveland, K. L. (2005a). Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J. Endocrinol. 186, 429–446.
| Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVygt7%2FE&md5=6389c9acfe21d913bdd00e50568210b8CAS | 16135663PubMed |
Meachem, S. J., Stanton, P. G., and Schlatt, S. (2005b). Follicle-stimulating hormone regulates both sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biol. Reprod. 72, 1187–1193.
| Follicle-stimulating hormone regulates both sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslSntrg%3D&md5=e749a0eb5f24f7fdd2772bbc416b0587CAS | 15659702PubMed |
Meehan, T., Schlatt, S., O’Bryan, M. K., de Kretser, D. M., and Loveland, K. L. (2000). Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev. Biol. 220, 225–237.
| Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXit1ClsL4%3D&md5=61549610f1c222acbc66e016ba30c189CAS | 10753512PubMed |
Muffly, K. E., Nazian, S. J., and Cameron, D. F. (1994). Effects of follicle-stimulating hormone on the junction-related Sertoli cell cytoskeleton and daily sperm production in testosterone-treated hypophysectomized rats. Biol. Reprod. 51, 158–166.
| Effects of follicle-stimulating hormone on the junction-related Sertoli cell cytoskeleton and daily sperm production in testosterone-treated hypophysectomized rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXkt1OgtLs%3D&md5=5e4b1f99964c3b63f61802351f027814CAS | 7918871PubMed |
Nicholls, P. K., Stanton, P. G., Chen, J. L., Olcorn, J. S., Haverfield, J. T., Qian, H., Walton, K. L., Gregorevic, P., and Harrison, C. A. (2012). Activin signaling regulates Sertoli cell differentiation and function. Endocrinology 153, 6065–6077.
| Activin signaling regulates Sertoli cell differentiation and function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVahtbbM&md5=f6445740356872316889bd2e7879c771CAS | 23117933PubMed |
O’Shaughnessy, P. J., Monteiro, A., and Abel, M. (2012). Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS One 7, e35136.
| Testicular development in mice lacking receptors for follicle stimulating hormone and androgen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmt1ehtr4%3D&md5=d41094071ddb6af547d663797382e781CAS | 22514715PubMed |
Okuma, Y., O’Connor, A. E., Hayashi, T., Loveland, K. L., de Kretser, D. M., and Hedger, M. P. (2006). Regulated production of activin A and inhibin B throughout the cycle of the seminiferous epithelium in the rat. J. Endocrinol. 190, 331–340.
| Regulated production of activin A and inhibin B throughout the cycle of the seminiferous epithelium in the rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1WjtLw%3D&md5=6db37bd505a5ad46269bcde3697cb449CAS | 16899566PubMed |
Orth, J. M. (1982). Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat. Rec. 203, 485–492.
| Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s%2Fks1eqsg%3D%3D&md5=abded53ce2e8ce4cbf8f87761923db1dCAS | 7137603PubMed |
Orth, J. M. (1984). The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology 115, 1248–1255.
| The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlvVCntL8%3D&md5=9acf895b2e5103caa8887a93e67dfaf0CAS | 6090096PubMed |
Petersen, C., Boitani, C., Fröysa, B., and Söder, O. (2002). Interleukin-1 is a potent growth factor for immature rat sertoli cells. Mol. Cell. Endocrinol. 186, 37–47.
| Interleukin-1 is a potent growth factor for immature rat sertoli cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1ait7g%3D&md5=8c4beb9813a09c206701aefd058c11f0CAS | 11850120PubMed |
Pitetti, J.-L., Calvel, P., Zimmermann, C., Conne, B., Papaioannou, M. D., Aubry, F., Cederroth, C., Urner, F., Fumel, B., Crausaz, M., Docquier, M., Herrera, P. L., Pralong, F., Germond, M., Guillou, F., Jégou, B., and Nef, S. (2013). An essential role for insulin and IGF1 receptors in regulating Sertoli cells proliferation, testis size and FSH action in mice. Mol. Endocrinol. 27, 814–827.
| An essential role for insulin and IGF1 receptors in regulating Sertoli cells proliferation, testis size and FSH action in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsFSisL4%3D&md5=203d8b6898dcab53894d5c5acbcf71e1CAS | 23518924PubMed |
Raivio, T., Toppari, J., Perheentupa, A., McNeilly, A. S., and Dunkel, L. (1997). Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet 350, 263–264.
| Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlsVGhsLs%3D&md5=67a3a40d84057e75426ccd3109a3be50CAS | 9242808PubMed |
Raivio, T., Wikström, A. M., and Dunkel, L. (2007). Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur. J. Endocrinol. 156, 105–111.
| Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlyqur8%3D&md5=0495c98ab40b259d0110208849f4e85bCAS | 17218732PubMed |
Riera, M. F., Regueira, M., Galardo, M. N., Pellizzari, E. H., Meroni, S. B., and Cigorraga, S. B. (2012). Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am. J. Physiol. Endocrinol. Metab. 302, E914–E923.
| Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmsl2gtL4%3D&md5=9b1eebd22b4d6ac6c27b89bf88a9d71eCAS | 22275758PubMed |
Robertson, D. M., Foulds, L. M., Leversha, L., Morgan, F. J., Hearn, M. T., Burger, H. G., Wettenhall, R. E., and de Kretser, D. M. (1985). Isolation of inhibin from bovine follicular fluid. Biochem. Biophys. Res. Commun. 126, 220–226.
| Isolation of inhibin from bovine follicular fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtVKmtbs%3D&md5=5409aed7cfedc43dafdd692cb3b9ff89CAS | 3918529PubMed |
Russell, L. D., and Clermont, Y. (1977). Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat. Rec. 187, 347–365.
| Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXktVSmsrg%3D&md5=be018db72d97c7ee37b1ba0673d290d2CAS | 851237PubMed |
Sablitzky, F., Moore, A., Bromley, M., Deed, R., Newton, J., and Norton, J. (1998). Stage- and subcellular-specific expression of Id proteins in male germ and Sertoli cells implicates distinctive regulatory roles for Id proteins during meiosis, spermatogenesis, and Sertoli cell function. Cell Growth Differ. 9, 1015–1024.
| 1:CAS:528:DyaK1MXitlOk&md5=77632d140937de6f4d4ae9498f63a236CAS | 9869302PubMed |
Schlatt, S., Arslan, M., Weinbauer, G. F., Behre, H. M., and Nieschlag, E. (1995). Endocrine control of testicular somatic and premeiotic germ cell development in the immature testis of the primate Macaca mulatta. Eur. J. Endocrinol. 133, 235–247.
| Endocrine control of testicular somatic and premeiotic germ cell development in the immature testis of the primate Macaca mulatta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnsFGjtrs%3D&md5=473a553fb26e5d949e701ed49bce40e6CAS | 7655650PubMed |
Sertoli, E. (1878). Sulla sturttura dei canalicoli seminiferi dei testicolo. Arch. Sci. Med. (Torino) 2, 107–146, 267–295.
Sharpe, R. M., McKinnell, C., Kivlin, C., and Fisher, J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784.
| Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltFelt7c%3D&md5=37a446babf7b1da6681cb5d807983e1bCAS | 12773099PubMed |
Shou, W., Woodruff, T. K., and Matzuk, M. M. (1997). Role of androgens in testicular tumor development in inhibin-deficient mice. Endocrinology 138, 5000–5005.
| 1:CAS:528:DyaK2sXmslGrtrg%3D&md5=4a02d4f870bc5fa229408b6903369e5dCAS | 9348231PubMed |
Singh, J., and Handelsman, D. J. (1996). Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice. J. Endocrinol. 151, 37–48.
| Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmvVentL4%3D&md5=0cc424ca5dd31ab84941cb32350c68d4CAS | 8943767PubMed |
Soldani, R., Cagnacci, A., and Yen, S. S. (1994). Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur. J. Endocrinol. 131, 641–645.
| Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXivVGgs7o%3D&md5=dc289f734e932dd0b2a00ce1e82d8d1eCAS | 7804448PubMed |
Steinberger, A., and Steinberger, E. (1971). Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol. Reprod. 4, 84–87.
| 1:STN:280:DyaE38%2FitVWksg%3D%3D&md5=80a8430ce136fa87dbea8c0e95184744CAS | 5110903PubMed |
Tarulli, G. A., Stanton, P. G., Lerchl, A., and Meachem, S. J. (2006). Adult sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization. Biol. Reprod. 74, 798–806.
| Adult sertoli cells are not terminally differentiated in the Djungarian hamster: effect of FSH on proliferation and junction protein organization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjsl2jtb8%3D&md5=e5803141a05d50f19627205d68e9faecCAS | 16407497PubMed |
Tarulli, G. A., Stanton, P. G., and Meachem, S. J. (2012). Is the adult Sertoli cell terminally differentiated? Biol. Reprod. 87, 13.
| Is the adult Sertoli cell terminally differentiated?Crossref | GoogleScholarGoogle Scholar | 22492971PubMed |
Tarulli, G. A., Stanton, P. G., Loveland, K. L., Meyts, E. R.-D., McLachlan, R. I., and Meachem, S. J. (2013). A survey of Sertoli cell differentiation in men after gonadotropin suppression and in testicular cancer. Spermatogenesis 3, e24014.
| A survey of Sertoli cell differentiation in men after gonadotropin suppression and in testicular cancer.Crossref | GoogleScholarGoogle Scholar | 23687617PubMed |
Tesarik, J., Guido, M., Mendoza, C., and Greco, E. (1998). Human spermatogenesis in vitro: respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis. J. Clin. Endocrinol. Metab. 83, 4467–4473.
| Human spermatogenesis in vitro: respective effects of follicle-stimulating hormone and testosterone on meiosis, spermiogenesis, and Sertoli cell apoptosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnvFOrurk%3D&md5=f68f3bb71db06b4b063f97575d0d0c27CAS | 9851795PubMed |
Vergouwen, R. P. F. A., Jacobs, S. G. P. M., Huiskamp, R., Davids, J. G., and de Rooij, D. G. (1991). Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J. Reprod. Fertil. 93, 233–243.
| Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FhsFSmsg%3D%3D&md5=647c2c38f256affaec56ec3f6f1e25d7CAS |
Walker, W. H., and Cheng, J. (2005). FSH and testosterone signaling in Sertoli cells. Reproduction 130, 15–28.
| FSH and testosterone signaling in Sertoli cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXnt1ynsrk%3D&md5=b96ae9bcc93148e3e116eab854836717CAS | 15985628PubMed |
Wreford, N. G., Kumar, T. R., Matzuk, M. M., and de Kretser, D. M. (2001). Analysis of the testicular phenotype of the follicle-stimulating hormone β-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology 142, 2916–2920.
| 1:CAS:528:DC%2BD3MXkslelsb4%3D&md5=551efe809e5f4c5f3485bdd230f82b98CAS | 11416011PubMed |
Xiao, G.-Q., Granato, R. C., and Unger, P. D. (2012). Bilateral Sertoli cell tumors of the testis: a likely new extracolonic manifestation of familial adenomatous polyposis. Virchows Arch. 461, 713–715.
| 1:CAS:528:DC%2BC38XhslKgtrnN&md5=6ea5c631b8510bac428536fce06afd35CAS | 23090627PubMed |
Yagi, M., Takenaka, M., Suzuki, K., and Suzuki, H. (2007). Reduced mitotic activity and increased apoptosis of fetal Sertoli Cells in rat hypogonadic (hgn/hgn) testes. J. Reprod. Dev. 53, 581–589.
| Reduced mitotic activity and increased apoptosis of fetal Sertoli Cells in rat hypogonadic (hgn/hgn) testes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXosVylsLo%3D&md5=4fde10af9c2269383419ced70c7e6b64CAS | 17310077PubMed |