Comparison of sperm motility subpopulation structure among wild anadromous and farmed male Atlantic salmon (Salmo salar) parr using a CASA system
Carina Caldeira A B E , Almudena García-Molina A , Anthony Valverde B C , Daznia Bompart A , Megan Hassane B , Patrick Martin D and Carles Soler A BA PROISER R+D, Av. Catedrático Agustín Escardino, 9, Building 3 (CUE), Floor 1, 46980 Paterna, Spain.
B University of Valencia, Faculty of Biological Sciences, Campus Burjassot, C/ Dr Moliner 50, 46100 Burjassot, Spain.
C Technological Institute of Costa Rica, San Carlos Campus, School of Agronomy, 223-21001 Alajuela, Costa Rica.
D Conservatoire National du Saumon Sauvage, Larma, 43 300 Chanteuges, France.
E Corresponding author. Email: carina.caldeira@proiser.com
Reproduction, Fertility and Development 30(6) 897-906 https://doi.org/10.1071/RD17466
Submitted: 31 October 2017 Accepted: 17 January 2018 Published: 13 April 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Atlantic salmon (Salmo salar) is an endangered freshwater species that needs help to recover its wild stocks. However, the priority in aquaculture is to obtain successful fertilisation and genetic variability to secure the revival of the species. The aims of the present work were to study sperm subpopulation structure and motility patterns in wild anadromous males and farmed male Atlantic salmon parr. Salmon sperm samples were collected from wild anadromous salmon (WS) and two generations of farmed parr males. Sperm samples were collected from sexually mature males and sperm motility was analysed at different times after activation (5 and 35 s). Differences among the three groups were analysed using statistical techniques based on Cluster analysis the Bayesian method. Atlantic salmon were found to have three sperm subpopulations, and the spermatozoa in ejaculates of mature farmed parr males had a higher velocity and larger size than those of WS males. This could be an adaptation to high sperm competition because salmonid species are naturally adapted to this process. Motility analysis enables us to identify sperm subpopulations, and it may be useful to correlate these sperm subpopulations with fertilisation ability to test whether faster-swimming spermatozoa have a higher probability of success.
Additional keywords: Bayesian method, CASA-Mot system.
Introduction
Atlantic salmon (Salmo salar) is a cultured fish species with a high economic value, which generates significant revenue from both wild catches and fish farming (Hindar et al. 2006). However, the total annual catch of wild Atlantic salmon in the North Atlantic has shown a marked decline during recent decades from approximately 12 000 t in 1973 down to 1200 t in 2016 (NASCO 2016). The commercial response to this problem is in the conservation, restoration, enhancement and rational management of wild salmon in the North Atlantic. By focusing on biology, the aims of scientific projects are to increase fish survival and improve their behavioural adaptation to natural conditions. In a conservation program, it is essential to secure the revival of the species by ensuring genetic variability (Rurangwa et al. 2004), using wild broodstock from the local habitat. The priority in reproduction for restocking is to ensure that all males contribute towards successful fertilisation. However, it is important to know the differences in sperm motility among males in order to maintain the genetic integrity of the broodstock used (McGinnity et al. 1997, 2003; Rurangwa et al. 2004). In addition, knowledge of sperm characteristics (velocity, optimal activation time and subpopulation structures) will help improve fertilisation procedures (Bobe and Labbé 2010; Fauvel et al. 2010).
In fish species with external fertilisation, the quest for reproductive success among competing males can lead to several adaptations, including behavioural, morphological and physiological, to enhance the competitiveness of their spermatozoa (Beatty et al. 1969; Parker 1970; Smith 1984; Järvi 1990; Lahnsteiner et al. 1993; Gage et al. 1995; De Gaudemar and Beall 1999). The physiological quality of spermatozoa differs between competing males because of differences in investment in gametes and sperm production (Ball and Parker 1996; Taborsky 1998; Vladić and Järvi 2001). Sperm quality is generally correlated with fertilising ability, which is often used as a determining factor in studies on sperm competition (Stoss 1983; Bencic et al. 1999; Levitan 2000). During spawning, the number of spermatozoa released and their swimming speed can affect the probability of fertilisation (Stoltz and Neff 2006; Taborsky 1998). Faster swimming cells may be able to reach the egg first, increasing the probability of successful fertilisation (Gage et al. 2004; Stoltz and Neff 2006). Atlantic salmon is an anadromous species that migrates up rivers from the sea in order to breed. In the case of this species, spawning males are characterised by intense sexual competition, which confers strong selective pressure on their reproductive physiology (Vladić and Järvi 2001). The wild salmon population can exhibit a wide natural variation in sperm traits leading to sperm competition (Gage et al. 1995; Gage et al. 2004). However, mature parr males also participate in the spawning, which means that they need to invest more in sperm production than anadromous males (Parker 1998).
Sperm quality can be assessed using simple methods, such as individual analysis of motility (Billard and Cosson 1992) and morphology (Holstein et al. 1988; Munkittrick et al. 1992), or sophisticated approaches involving molecular tools (Cabrita et al. 2014). Motility is the parameter that is most used to assess sperm quality because it directly reflects the fertilising ability of the spermatozoa. Initially, the percentage of motile spermatozoa and/or the duration of their motility were evaluated using subjective visual estimates. However, computer-aided sperm analysis (CASA-Mot) systems were developed to provide more reliable, repeatable and objective measurements of sperm movement (Rurangwa et al. 2004). Even though CASA technology was designed for mammalian species (Rurangwa et al. 2004), it is well adapted to fish spermatozoa (Gallego et al. 2013; Kime et al. 2001), which are characterised by a short period of vigorous motility after activation. In general, fish spermatozoa remain active for less than 2 min in most aquatic species and, in the case of salmonids specifically, the duration of sperm motility is around 20–30 s (Kime et al. 2001).
CASA-Mot systems provide a large amount of data based on the kinematic parameters of each spermatozoon. Applying subpopulation analysis to such data allows for the analysis of groups of spermatozoa with similar motility features and to estimate of sperm quality for each male (Gil Anaya et al. 2015; Soler et al. 2014). A subpopulation characterised by rapid linear movement has been proposed as an indicator of high-quality spermatozoa (Martínez-Pastor et al. 2005, 2011; Ferraz et al. 2014). Variations in subpopulation distributions have been reported for several species, including boar (Flores et al. 2009; Quintero-Moreno et al. 2004), bull (Muiño et al. 2009; Valverde et al. 2016), red deer (Martínez-Pastor et al. 2005), stallion (Quintero-Moreno et al. 2003), cat (Contri et al. 2012; Gutiérrez-Reinoso and García-Herreros 2016), dog (Núñez-Martínez et al. 2006), fowl (García-Herreros 2016), rooster (García-Herreros 2016), human (Santolaria et al. 2016; Vásquez et al. 2016; Yániz et al. 2016), gilthead seabream (Beirão et al. 2011) and steelhead (Kanuga et al. 2012). This statistical methodology has improved knowledge regarding sperm quality, although it is still used primarily as a research tool (Gil Anaya et al. 2015).
A new concept of data analysis based on probabilistic modelling with Bayesian networks (BNs) has been proposed to improve the study of associations between variables (Thompson et al. 2004; Daly et al. 2011). A BN approach represents relationships of independence and dependence among variables and provides a probabilistic distribution between them (Yet et al. 2014). The networks established by BN methodology can be applied to almost any type of investigation and help researchers make assumptions with regard to the problem being studied (Jensen and Nielsen 2007).
Frequentist and Bayesian analyses are two different statistical approaches that can be used in biological studies. Frequentist methods consider a fixed parameter of interest and a random parameter of the sampled data. The results, expressed as P-values, provide uncertainty about the hypothetical collection of data, helping to reject the original parameter value. Thus, the inability to learn and adapt to new information is a weakness of the frequentist approach. The Bayesian method is completely different in that the data are fixed and the parameter is random or uncertain. In this case, the result identifies all possible parameter values and gives a probability plot for these values. Bayesian estimates are influenced by previously held beliefs and information resulting from a clinical test. The traditional frequentist and Bayesian approaches produce analogous results when there is no useful a priori information (Ashby and Smith 2000; Carlin and Louis 2000).
In the present study, fish sperm samples were collected to evaluate motility patterns and the sperm subpopulation structure in wild anadromous male fish and two generations of offspring of farmed mature parr males of Atlantic salmon, using two different statistical approaches. First, an analysis of subpopulations was conducted using motility variables to identify the different sperm subpopulations and clarify their physiology from each group of male fish. Subsequently, the BN method was used to investigate the relative importance of this statistical analysis when determining sperm quality based on motility.
Materials and methods
Broodstock conditions
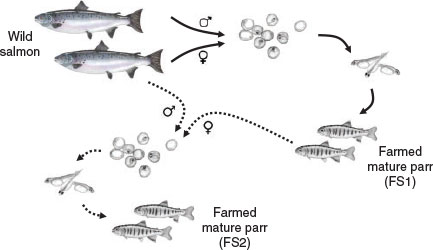
Wild and cultured male Atlantic salmon were sampled at the Conservatorie National du Saumon Sauvage (Chanteuges, France). The hatchery station is located in the Allier River, which is a tributary of the Loire River. Anadromous fish were captured in the wild and maintained in the hatchery. Wild salmon breeders (WS) were used to produce two different types of offspring (Fig. 1). A cross between wild male and female salmon produced one generation of farmed salmon (FS1), whereas a second generation of salmon offspring (FS2) was produced by crossing wild anadromous male (WS) and farmed salmon female fish (FS1). In the hatchery, photoperiod and temperature were adjusted to simulate the environmental conditions of the area.
|
Sperm collection
Sperm samples were collected from mature WS and farmed parr (FS1 and FS2) males during the reproductive season, from October to December. Six specimens from each type of mature male were selected at random (n = 18). Sexually mature males were anaesthetised (MS-222; 1 mL L−1) before sperm collection by abdominal massage. Care was taken to avoid contamination of the samples with faeces and urine by cleaning the genital area with fresh water and drying it thoroughly. Samples were stored at 4°C in plastic tubes until analysis.
Sperm motility evaluation
Sperm motility was assessed using a CASA-Mot system (Integrated Semen Analysis System (ISAS) v1; Proiser R&D) coupled with a phase contrast microscope (UOP; Proiser R&D) with a ×10 negative phase contrast objective. Video files were recorded and analysed at a rate of 200 frames s−1 (MQ003MG-CM; XIMEA) 5 and 35 s after activation. Sperm motility was activated by mixing a drop of ejaculate with 2 µL distilled water in a commercially produced chamber (Spermtrack; Proiser R&D; depth 10 µL). Each sample was analysed in triplicate. In the present study, particle area (µm2) and several motility parameters were considered, namely: total motility (MOT; %), defined as the percentage of motile cells within the sample; curvilinear velocity (VCL; µm s−1), defined as the velocity of a sperm head along the real curvilinear trajectory; straight line velocity (VSL; µm s−1), defined as the velocity of a sperm head along the straight line between the first and last detected position; angular path velocity (VAP; µm s−1), defined as the velocity of sperm head along a derived, smoothed trajectory path; straightness (STR; %), defined as the linearity of the angular path distance and calculated as VSL/VAP; and linearity (LIN; %), defined as the ratio of the straight line distance to the real path distance (VSL/VCL). Other parameters that were also determined using the ISAS v1 system included wobble (WOB; %), the amplitude of lateral head movement (ALH; µm) and beat cross frequency (BCF; Hz). Software settings were adjusted for salmon sperm analysis: 2–90 µm for the head area and 6 µm for connectivity.
Statistical analysis
The data obtained from the analysis of all kinematic parameters were first tested for normality and homoscedasticity using the Shapiro–Wilk and Kolmogorov–Smirnov tests respectively. Clustering procedures were performed to identify sperm subpopulations from the complete set of motility data (Vicente Fiel et al. 2013). The first step was to perform a principal component analysis (PCA). The number of principal components (PC) that should be used in the next step of the analysis was determined using the Kaiser criterion, namely selecting only those with an eigenvalue (variance extracted for that particular PC) >1. The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA. All sperm cells within each generation and time after activation were clustered using a non-hierarchical clustering procedure (k-means model and Euclidean distance). This analysis enabled the identification of sperm subpopulations and the detection of outliers.
The effects of clusters between treatments for measuring motility parameters were analysed using the Kruskal–Wallis test, followed by the Mann–Whitney paired non-parametric U-test when significant differences were found. The effects of generation, time after activation and individual male fish on the relative distribution frequency of sperm subpopulations was analysed using Chi-squared and Mantel-Haenszel Chi-squared tests. After characterising sperm subpopulations, one-way analysis of variance (ANOVA) was used to explore relationships between the proportions of each sperm subpopulation in the sample. Results are presented as median values with the interquartile range (IQR) or as the mean ± s.d. Statistical significance was set at P < 0.05 (two-sided). All data were analysed using InfoStat Software v.2017 (Nacional University of Córdoba, Argentina) for Windows, as described previously (Di Rienzo et al. 2017).
Differences in sperm motility indicators were estimated using a model that included the effects of generation and time after activation as permanent effects. All analyses were performed using Bayesian methodology. The posterior median of the difference between generations (D), the highest posterior density region at 95% (HPD95%) and the probability (P) of the difference being positive when D > 0 or negative when D < 0 were calculated. Bounded uniform priors were used for all effects. Residuals were normally distributed a priori with mean of 0 and a variance of σ2e. The priors for the variances were also bounded uniformly. Features of the marginal posterior distributions for all unknowns were estimated using Gibbs sampling. Convergence was tested using the Z criterion of Geweke (Sorensen and Gianola 2002) and Monte Carlo sampling errors were computed using the time series procedures described in Geyer (1992). The Rabbit program, developed by the Institute for Animal Science and Technology (Valencia, Spain), was used for all procedures.
Results
General results
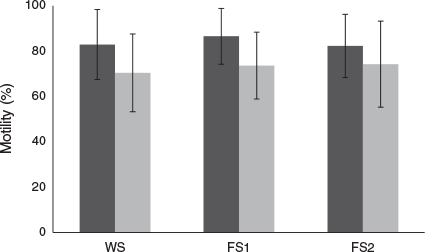
Sperm motility of WS and both FS1 and FS2 parr males was observed over time and the percentage of motile spermatozoa in samples from all fish evaluated was remarkably high (Fig. 2). The percentage of motile spermatozoa 5 s after activation was 82.9 ± 15.5%, 86.5 ± 12.3% and 82.3 ± 14.0% for WS and FS1 and FS2 parr males respectively. The percentage motile spermatozoa in samples 35 s after activation decreased to 70.4 ± 17.2%, 73.6 ± 14.7% and 74.2 ± 19.0% for WS and FS1 and FS2 parr males respectively. Farmed parr males showed lower variability in the percentage of motile cells 5 s after activation.
|
A preliminary analysis using Pearson’s correlation coefficient revealed that the nine CASA-Mot kinematic variables had negative correlations with time, whereas the provenance of the male fish had no substantial effect on these variables (data not shown). In addition, velocity (VCL, VSL and VAP), progressivity (LIN and STR), ALH and BCF showed the strongest correlations with time, with values for all parameters decreasing over time (P < 0.0001).
PCA and subpopulations
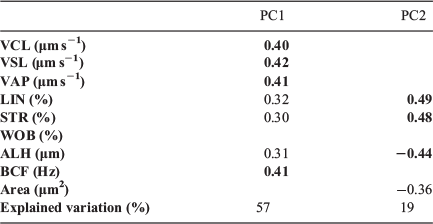
The PCA was performed to reduce the dimensionality of the present study, which included nine CASA-Mot kinematic variables (VCL, VSL, VAP, LIN, STR, WOB, ALH, BCF and area) for anadromous males and farmed parr males for each time after activation (5 and 35 s). Two PCs with a smaller number of variables were selected and explained 76% of the total variance of all data (Table 1). PC1, designated ‘velocity’, was positively related to the velocity parameters (VCL, VSL, VAP) and BCF, whereas PC2, designated ‘linearity’, was positively related to progressivity parameters (LIN, STR) and negatively related to ALH and area.
|
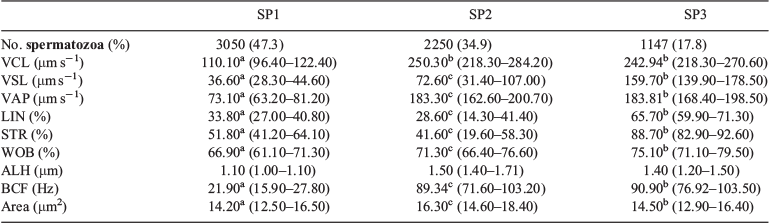
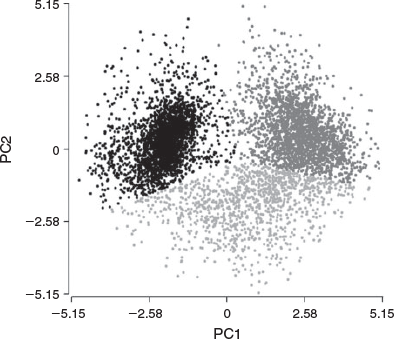
These two PCs were used to identify three well-defined subpopulations (SP1, SP2 and SP3; Fig. 3), characterised by median values of the nine CASA-Mot parameters (Table 2). SP1 included spermatozoa characterised by low speed (VCL, VSL and VAP) and low linear trajectories (LIN and STR). This subpopulation represented 47.3% of the total sample and was considered as a slow non-linear subpopulation. SP2 accounted for 34.9% of total motile cells and was characterised by high velocity but low linear trajectories; this group was designated as a fast and non-linear subpopulation. Spermatozoa in SP3 had both high velocity and linear trajectories, and this group was designated as a fast and linear subpopulation and accounted for 17.8% of all cells. Non-parametric comparisons showed the existence of statistically significant differences (P < 0.0001) between the three subpopulations for eight of the CASA-Mot variables. ALH was the only variable that did not differ significantly between SP1, SP2 and SP3.
|
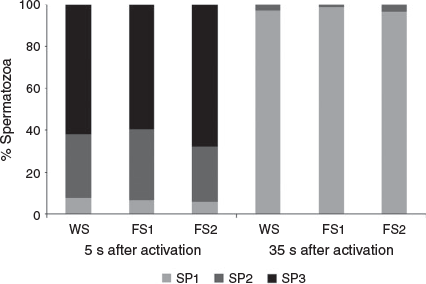
The percentage of spermatozoa in each subpopulation varied over time in the WS, FS1 and FS2 groups (Fig. 4). The proportional size of SP1 increased significantly 35 s after activation, whereas the opposite was observed for SP2 and SP3. At the beginning of the analysis, most of the spermatozoa from all analysed males were classified as either SP2 or SP3 (i.e. 30.4% and 61.8% respectively for WS males; 33.8% and 59.7% respectively for FS1 males; and 26.4% and 67.7% respectively for FS2 males). In the second period (35 s after activation), the proportion of these subpopulations declined substantially to values less than 4% and most spermatozoa were then classified as SP1 (i.e. 97.0%, 98.6% and 96.4% in the WS, FS1 and FS2 groups respectively). There were significant differences in the percentage of spermatozoa in each subpopulation between the two periods after activation in each of the WS, FS1 and FS2 groups (P < 0.0001).
|
Bayesian analysis
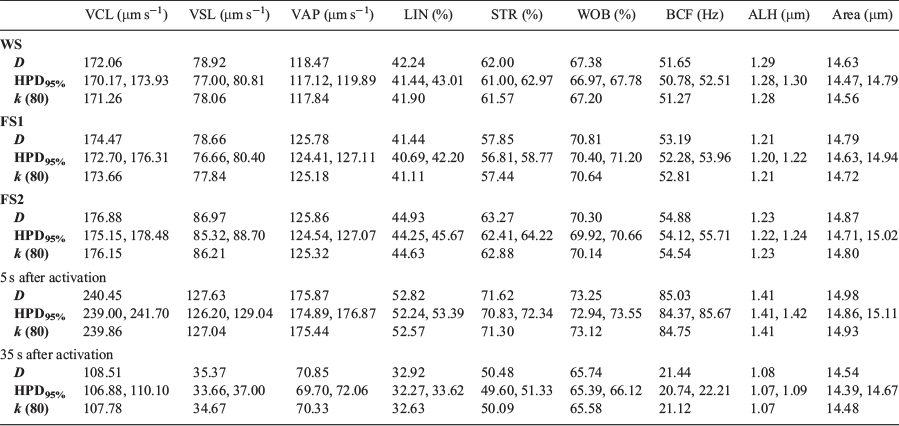
Probabilistic modelling with the Bayesian method describes each treatment separately and provides the probability of each possible combination within treatments (i.e. WS, FS1 and FS2; and 5 and 35 s) for the nine CASA-Mot descriptors. Descriptive analysis of all variables showed variations according to the provenance of the male fish and the time period studied (Table 3). WS males were characterised by smaller particle areas (median 14.6 µm), as well as the lowest velocity (172.1, 78.9 and 118.5 µm s−1 for VCL, VSL and VAP respectively) and linearity (41.9% for LIN). Farmed parr males showed higher sperm speeds, although spermatozoa from FS2 offspring were the fastest. In the FS2 group, the median values of the velocity variables VCL, VSL and VAP were 176.9, 87.0 and 125.9 µm s−1 respectively. In addition, the FS2 group had a high particle area (median 14.9 µm2). With regard to the time after activation (5 or 35 s), the velocity and progressivity parameters, as well as particle area declined over time. For example, 5 s after activation VCL and LIN were 240.5 µm s−1 and 52.8% respectively, declining to 108.5 µm s−1 and 32.9% respectively 35 s after activation. The area of motile cells also decreased from 15.0 µm2 at 5 s to 14.5 µm2 at 35 s.
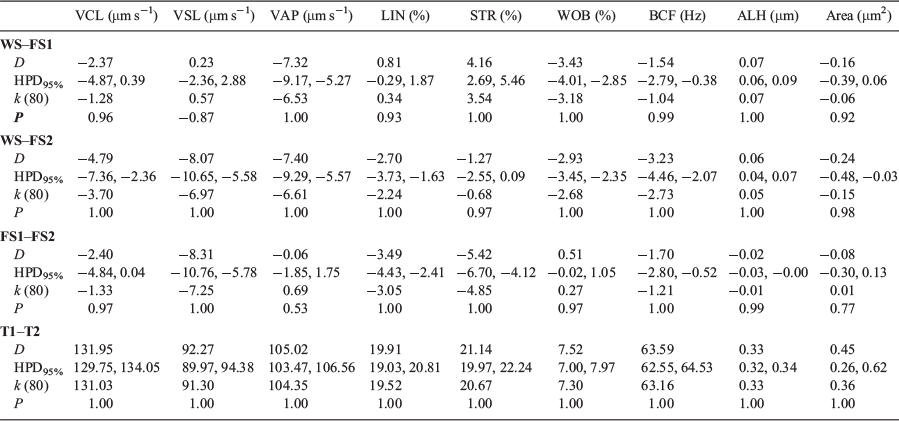
Investigating relationships between WS and FS1 and FS2 male revealed that the farmed fish had higher values for sperm velocity variables (VCL, VSL and VAP) compared with anadromous males (Table 4). Compared with WS males, VCL and VAP were higher in the FS1 males (median 2.4 µm s−1 and 7.3 µm s−1 respectively) and VCL, VSL and VAP were higher in FS2 males (median 4.8, 8.1 and 7.4 µm s−1 respectively). Among the farmed mature parr male offspring, those in the FS2 group had the highest VCL (>2.40 µm s−1) and VSL (>8.31 µm s−1). In contrast, linearity (LIN and STR) was lower in FS1 males (i.e. decreases of 0.8% for LIN and 4.2% for STR) but higher in FS2 males (i.e. an increase of 2.7% for LIN and 1.27% for STR) compared with WS males. The area was greater for both FS1 and FS2 males compared with WS males, but was highest for the FS2 group, being 0.24 µm2 greater than in WS males. Values for all nine CASA-Mot variables decreased over time. The velocity (VCL, VSL and VAP) and linearity (LIN and STR) parameters were higher 5 s after activation, as was sperm area, which was 0.45 µm2 higher for the same time period.
Discussion
Male Atlantic salmon are characterised by intense sexual competition, with a strong selective pressure on male reproductive features, which could lead to multiple paternity (Weir et al. 2010). It is known that parr males invest more in the physiological competitiveness of spermatozoa during spawning (Vladić and Järvi 2001; Gage et al. 2004). In a natural population, females may mate with many males, including small mature parr and large anadromous males (Weir et al. 2010). In the present study, analysis of the percentage of motile spermatozoa showed that small mature males, such as the farmed parr males, produced competitive spermatozoa with a similar proportion of motile cells. This may be explained by the greater concentrations of ATP in farmed parr male ejaculates (Vladić and Järvi 2001), indicating greater sperm vigour.
Sperm motility analysis using a CASA-Mot system provides information about a large number of kinematic variables for each individual spermatozoon. This objective analysis generates a great deal of data that should be examined statistically using techniques that allow for spermatozoa to be classified into subcategories with certain motility characteristics. The cluster approach conducted for several species confirms that ejaculate samples are heterogeneous in that they contain spermatozoa with different motility patterns (Abaigar et al. 1999; Quintero-Moreno et al. 2003, 2004; Chantler et al. 2004; Miró et al. 2005; Núñez-Martínez et al. 2006; Martínez-Pastor et al. 2008; Muiño et al. 2008; Beirão et al. 2009; Dorado et al. 2010, 2011; Contri et al. 2012; Kanuga et al. 2012). In the present study, we identified three different sperm subpopulations in samples from wild anadromous Atlantic salmon and two groups of farmed parr male offspring. However, the proportions of the different subpopulations varied with the time after activation. The distribution of motile spermatozoa in each subpopulation was used to evaluate differences between the provenance of the male and/or time. These results demonstrated that there was some variability within the groups of males, which could be reflect a connection between variability in sperm velocity and male genetic quality (McGinnity et al. 1997, 2003; Fleming et al. 2000; Fitzpatrick et al. 2007; Beirão et al. 2009, 2011; Kanuga et al. 2012). Fertilisation success is correlated with sperm velocity and progressivity (Gage et al. 2004; Casselman et al. 2006; Tuset et al. 2008; Kanuga et al. 2012). The presence of a fast and linear sperm subpopulation may be advantageous for fecundity and the selection of high-quality spermatozoa (Kime et al. 2001; Rurangwa et al. 2004; Martínez-Pastor et al. 2005, 2011; Beirão et al. 2009; Ferraz et al. 2014). High proportions of fast spermatozoa (SP2 and SP3 in the present study) at the beginning of activation (5 s after activation) may be considered biologically important because of the limited time spermatozoa have to locate and enter the egg’s micropyle (Kime et al. 2001; Rurangwa et al. 2004). In the case of salmonids, the eggs are large with a diameter of approximately 5 mm and, during the first 30 s, motile spermatozoa swim a distance of 3–4.9 mm around the egg (Perchec et al. 1993). However, fast spermatozoa with non-linear progressivity could play an important role in the successful fertilisation of salmonid eggs. Trout spermatozoa exhibit circular trajectories, which maximise the chances of sperm–egg contact during the short period of sperm motility (Cosson et al. 1989). In this regard, cluster analysis to identify sperm subpopulations based on velocity and progressivity could be proposed as an indicator of high-quality breeders.
In the present study, a new statistical approach was used for the sperm motility analysis. The aim of the Bayesian analysis was to study the relationship between variables based on the probability of distribution. This distribution gives the probability that the variables are integrated into a range of specific values for that variable. However, it not only considers individual probabilities, but also probabilities for all variables jointly.
The results from the present study indicated that there is a relationship between the velocity and progressivity variables (VCL, VSL, VAP, LIN and STR) and particle area, the provenance of the male fish and time after activation. These results were consistent with the cluster analysis described above. An important advantage of the Bayesian analysis is the ability to make inferences. In the present study, wild anadromous males had small spermatozoa with low velocity and progressivity, whereas mature farmed parr males had larger spermatozoa with higher speeds. However, the FS2 group of offspring that came from a cross between wild males and farmed female FS1 fish had the fastest spermatozoa with higher progressivity. Genotype–environment interactions and growth conditions in the fish farm are important for sexual maturation in salmonids (Garant et al. 2003; Vladić et al. 2010). The combination of these factors determines the precocious maturation of Atlantic salmon parr males. For successful participation in spawning, precocious parr males invest more in their gonads with regard to sperm motility and quality (Vladić and Järvi 2001; Vladić et al. 2010).
As expected, sperm velocity declined to less than half over time, which is in accordance with the depletion of sperm ATP stores (Vladić and Järvi 2001). Despite spermatozoa remaining active 35 s after activation, motile cells did not show vigorous movement, thereby decreasing fertilising ability. A long duration of sperm motility could not be considered an advantage because, for salmonids, the eggs are fertilised within 30 s after activation (Ginsburg 1968; Kime et al. 2001). However, under natural conditions, the egg and micropyle of salmonids are surrounded by ovarian fluid and the pH of this fluid could enhance the swimming speed, trajectory and duration of sperm motility (Wojtczak et al. 2007). Therefore, the spermatozoa swimming behaviour is a determinant trait in Atlantic salmon reproduction.
Conclusions
In the present study, a Bayesian approach was used to identify the relationship between all sperm variables after identification of three sperm subpopulations based on motility parameters. These analyses confirmed some expectations. The provenance of male fish and time proved to be determinant variables in the characterisation of Atlantic salmon sperm physiology.
Different statistical methods for analysing sperm motility can be used to increase knowledge regarding fish sperm characteristics and sperm speed in a species, such as the structure of sperm subpopulations and sperm competition. However, it is important to obtain fertility data to understand the relationship between sperm velocity and sperm fertilisation ability. These approaches could help with sperm selection processes and fertility potential, and consequently improve artificial reproduction for fish species.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie project IMPRESS (GA No. 642893). AV is granted by the CONICIT and MICITT, Costa Rica.
References
Abaigar, T., Holt, W. V., Harrison, R. A. P., and del Barrio, G. (1999). Sperm subpopulations in boar (Sus scrofa) and gazelle (Gazella dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments. Biol. Reprod. 60, 32–41.| Sperm subpopulations in boar (Sus scrofa) and gazelle (Gazella dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhvFymtA%3D%3D&md5=4b952b68134a6a2b14a3285ba42c3b2fCAS |
Ashby, D., and Smith, A. F. M. (2000). Evidence-based medicine as Bayesian decision-making. Stat. Med. 19, 3291–3305.
| Evidence-based medicine as Bayesian decision-making.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3kt12rsQ%3D%3D&md5=717e080a73ec876fcda64cb12dbf2172CAS |
Ball, M. A., and Parker, G. A. (1996). Sperm competition games: external fertilization and ‘adaptive’ infertility. J. Theor. Biol. 180, 141–150.
| Sperm competition games: external fertilization and ‘adaptive’ infertility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283ps1eqsA%3D%3D&md5=91c76743190a20fa7c04ca1dd1b1c4afCAS |
Beatty, R. A., Bennett, G. H., Hall, J. G., Hancock, J. L., and Stewart, D. L. (1969). An experiment with heterospermic insemination in cattle. J. Reprod. Fertil. 19, 491–502.
| An experiment with heterospermic insemination in cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M3osFygsg%3D%3D&md5=5971342e2985d29b7677f850b619aeefCAS |
Beirão, J., Soares, F., Herráez, M. P., Dinis, M. T., and Cabrita, E. (2009). Sperm quality evaluation in Solea senegalensis during the reproductive season at cellular level. Theriogenology 72, 1251–1261.
| Sperm quality evaluation in Solea senegalensis during the reproductive season at cellular level.Crossref | GoogleScholarGoogle Scholar |
Beirão, J., Cabrita, E., Pérez-Cerezales, S., Martínez-Paramo, S., and Herráez, M. P. (2011). Effect of cryopreservation on fish sperm subpopulations. Cryobiology 62, 22–31.
| Effect of cryopreservation on fish sperm subpopulations.Crossref | GoogleScholarGoogle Scholar |
Bencic, D. C., Krisfalusi, J. G., Cloud, J. G., and Ingermann, R. L. (1999). ATP levels of Chinook salmon (Oncorhynchus tschawytscha) sperm following in vitro exposure to various oxygen tensions. Fish Physiol. Biochem. 20, 389–397.
| ATP levels of Chinook salmon (Oncorhynchus tschawytscha) sperm following in vitro exposure to various oxygen tensions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVerurk%3D&md5=d0b2829363214fcfeb7e51d35027cd67CAS |
Billard, R., and Cosson, M. P. (1992). Some problems related to the assessment of sperm motility in freshwater fish. J. Exp. Zool. 261, 122–131.
| Some problems related to the assessment of sperm motility in freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Bobe, J., and Labbé, C. (2010). Egg and sperm quality in fish. Gen. Comp. Endocrinol. 165, 535–548.
| Egg and sperm quality in fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktVentA%3D%3D&md5=a224f7af520f97b2a3ab3b9af43ce79bCAS |
Cabrita, E., Martínez-Páramo, S., Gavaia, P. J., Riesco, M. F., Valcarce, D. G., Sarasquete, C., Herráez, M. P., and Robles, V. (2014). Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 432, 389–401.
| Factors enhancing fish sperm quality and emerging tools for sperm analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXosFWiu78%3D&md5=df6ce8a3a1bec26a1e1e50e584b815a0CAS |
Carlin, B. P., and Louis, T. A. (2000). Empirical Bayes: past, present and future. J. Am. Stat. Assoc. 95, 1286–1289.
| Empirical Bayes: past, present and future.Crossref | GoogleScholarGoogle Scholar |
Casselman, S. J., Schulte-Hostedde, A., and Montgomerie, R. (2006). Sperm quality influences male fertilization success in walleye (Sander vitreus). Can. J. Fish. Aquat. Sci. 63, 2119–2125.
| Sperm quality influences male fertilization success in walleye (Sander vitreus).Crossref | GoogleScholarGoogle Scholar |
Chantler, E., Abraham-Peskir, J., and Roberts, C. (2004). Consistent presence of two normally distributed sperm subpopulations within normozoospermic human semen: a kinematic study. Int. J. Androl. 27, 350–359.
| Consistent presence of two normally distributed sperm subpopulations within normozoospermic human semen: a kinematic study.Crossref | GoogleScholarGoogle Scholar |
Contri, A., Zambelli, D., Faustini, M., Cunto, M., Gloria, A., and Carluccio, A. (2012). Artificial neural networks for the definition of kinetic subpopulations in electroejaculated and epididymal spermatozoa in the domestic cat. Reproduction 144, 339–347.
| Artificial neural networks for the definition of kinetic subpopulations in electroejaculated and epididymal spermatozoa in the domestic cat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlKqu7jJ&md5=2840220ca9ae0fabdbdce0699bec52f5CAS |
Cosson, M. P., Billard, R., and Letellier, L. (1989). Rise of internal Ca2+. Cell Motil. Cytoskeleton 14, 424–434.
| Rise of internal Ca2+.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhvFWgug%3D%3D&md5=6bab1a6c0ba425a071404396d412db6fCAS |
Daly, R., Shen, Q., and Aitken, S. (2011). Learning Bayesian networks: approaches and issues. Knowl. Eng. Rev. 26, 99–157.
| Learning Bayesian networks: approaches and issues.Crossref | GoogleScholarGoogle Scholar |
De Gaudemar, B., and Beall, E. (1999). Reproductive behavioural sequences of single pairs of Atlantic salmon in an experimental stream. Anim. Behav. 57, 1207–1217.
| Reproductive behavioural sequences of single pairs of Atlantic salmon in an experimental stream.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2sbgs1Siuw%3D%3D&md5=a40d6f15e4d712a0397ead97bd570be3CAS |
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., and Robledo, C. W. (2017). InfoStat versión 2017. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available at http://www.infostat.com.ar
Dorado, J., Molina, I., Munoz-Serrano, A., and Hidalgo, M. (2010). Identification of sperm subpopulations with defined motility characteristics in ejaculates from Florida goats. Theriogenology 74, 795–804.
| Identification of sperm subpopulations with defined motility characteristics in ejaculates from Florida goats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjosVGjug%3D%3D&md5=848432ace5bfb74478988d5372307cf8CAS |
Dorado, J., Gálvez, M. J., Murabito, M. R., Muñoz-Serrano, A., and Hidalgo, M. (2011). Identification of sperm subpopulations in canine ejaculates: effects of cold storage and egg yolk concentration. Anim. Reprod. Sci. 127, 106–113.
| Identification of sperm subpopulations in canine ejaculates: effects of cold storage and egg yolk concentration.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MfjtVGlsA%3D%3D&md5=325d06515949b8a832244833b15b1039CAS |
Fauvel, C., Suquet, M., and Cosson, J. (2010). Evaluation of fish sperm quality. J. Appl. Ichthyol. 26, 636–643.
| Evaluation of fish sperm quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKrtL%2FE&md5=231dbac3bf8c5e70ed3eb1aab3215058CAS |
Ferraz, M. A., Morato, R., Yeste, M., Arcarons, N., Pena, A. I., Tamargo, C., Hidalgo, C. O., Muino, R., and Mogas, T. (2014). Evaluation of sperm subpopulation structure in relation to in vitro sperm–oocyte interaction of frozen–thawed semen from Holstein bulls. Theriogenology 81, 1067–1072.
| Evaluation of sperm subpopulation structure in relation to in vitro sperm–oocyte interaction of frozen–thawed semen from Holstein bulls.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cvptVCnsg%3D%3D&md5=7f99026e9538198c3e2cb48cc33ae66dCAS |
Fitzpatrick, J. L., Desjardins, J. K., Milligan, N., Montgomerie, R., and Balshine, S. (2007). Reproductive-tactic-specific variation in sperm swimming speeds in a shell-brooding cichlid. Biol. Reprod. 77, 280–284.
| Reproductive-tactic-specific variation in sperm swimming speeds in a shell-brooding cichlid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Onsb0%3D&md5=d56c7fa8fdeb011b96ab662164168c1eCAS |
Fleming, I. A., Hindar, K., Mjølnerød, I. B., Jonsson, B., Balstad, T., and Lamberg, A. (2000). Lifetime success and interactions of farm salmon invading a native population. Proc. Biol. Sci. 267, 1517–1523.
| Lifetime success and interactions of farm salmon invading a native population.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrgslCgsw%3D%3D&md5=2cfc6ae50bdc2c681548b5a95eaad212CAS |
Flores, E., Fernandez-Novell, J. M., Pena, A., and Rodríguez-Gil, J. E. (2009). The degree of resistance to freezing–thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa. Theriogenology 72, 784–797.
| The degree of resistance to freezing–thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKgsL3I&md5=496d422be59ef925c11455803d62482aCAS |
Gage, M. J. G., Stockley, P., and Parker, G. A. (1995). Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): theoretical and empirical investigations. Philos. Trans. R. Soc. Lond., B 350, 391–399.
| Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): theoretical and empirical investigations.Crossref | GoogleScholarGoogle Scholar |
Gage, M. J. G., Macfarlane, C. P., Yeates, S., Ward, R. G., Searle, J. B., and Parker, G. A. (2004). Spermatozoa traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47.
| 1:CAS:528:DC%2BD2cXjsFemsw%3D%3D&md5=db4e15d712c272563bb8884a3f568a55CAS |
Gallego, V., Carneiro, P. C. F., Mazzeo, I., Vílchez, M. C., Peñaranda, D. S., Soler, C., Pérez, L., and Asturiano, J. F. (2013). Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software. Theriogenology 79, 1034–1040.
| Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svjslSktQ%3D%3D&md5=ec9534b50819c2ff1ab02a6511b237efCAS |
Garant, D., Dodson, J. J., and Bernatchez, L. (2003). Differential reproductive success and heritability of alternative reproductive tactics in wild Atlantic salmon (Salmo salar L.). Evolution 57, 1133–1141.
García-Herreros, M. (2016). Sperm subpopulations in avian species: a comparative study between the rooster (Gallus domesticus) and Guinea fowl (Numida meleagris). Asian J. Androl. 18, 889–894.
Geyer, C. J. (1992). Practical Markov chain Monte Carlo (with discussion). Stat. Sci. 7, 473–483.
| Practical Markov chain Monte Carlo (with discussion).Crossref | GoogleScholarGoogle Scholar |
Gil Anaya, M. C. G., Calle, F., Pérez, C. J., Martın-Hidalgo, D., Fallola, C., Bragado, M. J., Garcıa-Marın, L. J., and Oropesa, A. L. (2015). A new Bayesian network-based approach to the analysis of sperm motility: application in the study of tench (Tinca tinca) semen. Andrology 3, 956–966.
| A new Bayesian network-based approach to the analysis of sperm motility: application in the study of tench (Tinca tinca) semen.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC28%2Fot1Okug%3D%3D&md5=aaba825b9dd60c95a1b0a21f1b92e2ddCAS |
Ginsburg, A. S. (1968). Fertilization in fishes and the problem of polyspermy (Ed. T. A. Detlaf.). (Nauka Press: Moscow.) [Translated from Russian by the Israell Program for Scientific Translations, Jerusalem 1972].
Gutiérrez-Reinoso, M. A., and García-Herreros, M. (2016). Normozoospermic versus teratozoospermic domestic cats: differential testicular volume, sperm morphometry, and subpopulation structure during epididymal maturation. Asian J. Androl. 18, 871–878.
Hindar, K., Fleming, I. A., McGinnity, P., and Diserud, O. (2006). Genetic and ecological effects of salmon farming on wild salmon: modelling from experimental results. ICES J. Mar. Sci. 63, 1234–1247.
| Genetic and ecological effects of salmon farming on wild salmon: modelling from experimental results.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvVSlu7s%3D&md5=5cbe737ead25448492bf8428a9a9d488CAS |
Holstein, A. F., Roosen-Rumge, E. C., and Schirren, C. (1988). ‘Illustrated Pathology of Human Spermatogenesis.’ (Groose: Berlin.)
Järvi, T. (1990). The effects of male dominance, secondary sexual characteristics and female mate choice on the mating success of male Atlantic salmon, Salmo salar. Ethology 84, 123–132.
| The effects of male dominance, secondary sexual characteristics and female mate choice on the mating success of male Atlantic salmon, Salmo salar.Crossref | GoogleScholarGoogle Scholar |
Jensen, F., and Nielsen, T. (2007). ‘Bayesian Networks and Decision Graphs.’ 2nd edn. (Springer-Verlag: New York.)
Kanuga, M. K., Drew, R. E., Wilson-Leedy, J. G., and Ingermann, R. L. (2012). Subpopulation distribution of motile sperm relative to activation medium in steelhead (Oncorhynchus mykiss). Theriogenology 77, 916–925.
| Subpopulation distribution of motile sperm relative to activation medium in steelhead (Oncorhynchus mykiss).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383mt1CntA%3D%3D&md5=100c293f261c719e91d2ff8e193d9eb1CAS |
Kime, D. E., Van Look, K. J. W., McAllister, B. G., Huyskens, G., Rurangwa, E., and Ollevier, F. (2001). Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 130, 425–433.
| Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnptFCisQ%3D%3D&md5=040aa04b2aae5a6f6797ce47ed5ac8feCAS |
Lahnsteiner, F., Patzner, R. A., and Weismann, T. (1993). The spermatic ducts of salmonid fishes (Salmonidae, Teleostei). Morphology, histochemistry and composition of the secretion. J. Fish Biol. 42, 79–93.
| The spermatic ducts of salmonid fishes (Salmonidae, Teleostei). Morphology, histochemistry and composition of the secretion.Crossref | GoogleScholarGoogle Scholar |
Levitan, D. R. (2000). Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. Biol. Sci. 267, 531–534.
| Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3ks1ygtg%3D%3D&md5=487d5b6427cc9d8a580148d95428a702CAS |
Martínez-Pastor, F., Garcia-Macias, V., Alvarez, M., Herráez, P., Anel, L., and de Paz, P. (2005). Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process. Biol. Reprod. 72, 316–327.
| Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process.Crossref | GoogleScholarGoogle Scholar |
Martínez-Pastor, F., Cabrita, E., Soares, F., Anel, L., and Dinis, M. T. (2008). Multivariate cluster analysis to study motility activation of Solea senegalensis spermatozoa: a model for marine teleosts. Reproduction 135, 449–459.
| Multivariate cluster analysis to study motility activation of Solea senegalensis spermatozoa: a model for marine teleosts.Crossref | GoogleScholarGoogle Scholar |
Martínez-Pastor, F., Tizado, E. J., Garde, J. J., Anel, L., and de Paz, P. (2011). Statistical series: opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 75, 783–795.
| Statistical series: opportunities and challenges of sperm motility subpopulation analysis.Crossref | GoogleScholarGoogle Scholar |
McGinnity, P., Stone, C., Taggart, J. B., Cooke, D., Cotter, D., Hynes, R., McCamley, C., Cross, T., and Ferguson, A. (1997). Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES J. Mar. Sci. 54, 998–1008.
| Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment.Crossref | GoogleScholarGoogle Scholar |
McGinnity, P., Prodöhl, P., Ferguson, A., Hynes, R., Maoiléidigh, N. O., Baker, N., Cotter, D., O’Hea, B., Cooke, D., Rogan, G., Taggart, J., and Cross, T. (2003). Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. Biol. Sci. 270, 2443–2450.
| Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon.Crossref | GoogleScholarGoogle Scholar |
Miró, J., Lobo, V., Quintero-Moreno, A., Medrano, A., Pena, A., and Rigau, T. (2005). Sperm motility patterns and metabolism in Catalonian donkey sémen. Theriogenology 63, 1706–1716.
| Sperm motility patterns and metabolism in Catalonian donkey sémen.Crossref | GoogleScholarGoogle Scholar |
Muiño, R., Tamargo, C., Hidalgo, C. O., and Pena, A. I. (2008). Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein bulls: effects of cryopreservation and between-bull variation. Anim. Reprod. Sci. 109, 27–39.
| Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein bulls: effects of cryopreservation and between-bull variation.Crossref | GoogleScholarGoogle Scholar |
Muiño, R., Pena, A. I., Rodríguez, A., Tamargo, C., and Hidalgo, C. O. (2009). Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls. Theriogenology 72, 860–868.
| Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls.Crossref | GoogleScholarGoogle Scholar |
Munkittrick, T. W., Nebel, R. L., and Saacke, R. G. (1992). Effect of microencapsulation on accessory sperm in the zona pellucida. J. Dairy Sci. 75, 725–731.
| Effect of microencapsulation on accessory sperm in the zona pellucida.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK383jsFeksw%3D%3D&md5=cf20a236970f4b5ee77b5acd10470123CAS |
North Atlantic Salmon Conservation Organization (NASCO) (2016). Report of the twenty-second annual meeting of the Council of the North Atlantic Salmon Conservation Organization. Document no. CNL(05)50, NASCO, Edinburgh.
Núñez-Martínez, I., Moran, J. M., and Peña, F. J. (2006). A three-step statistical procedure to identify sperm kinematic subpopulations in canine ejaculates: changes after cryopreservation. Reprod. Domest. Anim. 41, 408–415.
| A three-step statistical procedure to identify sperm kinematic subpopulations in canine ejaculates: changes after cryopreservation.Crossref | GoogleScholarGoogle Scholar |
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in the insects. Biol. Rev. Camb. Philos. Soc. 45, 525–567.
| Sperm competition and its evolutionary consequences in the insects.Crossref | GoogleScholarGoogle Scholar |
Parker, G. A. (1998). Sperm competition and the evolution of ejaculates: towards a theory base. In ‘Sperm Competition and Sexual Selection’. (Eds T. R. Birkhead and A. P. Möller.) pp. 3–54. (Academic Press: London.)
Perchec, G., Cosson, J., André, F., and Billard, R. (1993). Spermatozoa motility of trout (Oncorhynchus mykiss) and carp (Cyprinus carpio). J. Appl. Ichthyol. 9, 129–149.
| Spermatozoa motility of trout (Oncorhynchus mykiss) and carp (Cyprinus carpio).Crossref | GoogleScholarGoogle Scholar |
Quintero-Moreno, A., Miro, J., Rigau, A. T., and Rodriguez-Gil, J. E. (2003). Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 59, 1973–1990.
| Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s7mslWquw%3D%3D&md5=55ce383b46b78ba3b26938b3b33d9130CAS |
Quintero-Moreno, A., Rigau, T., and Rodríguez-Gil, J. E. (2004). Regression analyses and motile sperm subpopulation structure study as improving tools in boar semen quality analysis. Theriogenology 61, 673–690.
| Regression analyses and motile sperm subpopulation structure study as improving tools in boar semen quality analysis.Crossref | GoogleScholarGoogle Scholar |
Rurangwa, E., Kime, D. E., Ollevier, F., and Nash, J. P. (2004). The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture 234, 1–28.
| The measurement of sperm motility and factors affecting sperm quality in cultured fish.Crossref | GoogleScholarGoogle Scholar |
Santolaria, P., Soler, C., Recreo, P., Carretero, R., Bono, A., Berné, J. M., and Yániz, J. L. (2016). Morphometric and kinematic sperm subpopulations in split ejaculates of normozoospermic men. Asian J. Androl. 18, 831–834.
Smith, R. L. (1984). ‘Sperm Competition and the Evolution of Animal Mating Systems.’ (Academic Press: London.)
Soler, C., García-Molina, A., Contell, J., Segarvall, J., and Sancho, M. (2014). Kinematics and subpopulations structure definition of blue fox (Alopex lagopus) sperm motility using the ISAS®v1 CASA system. Reprod. Domest. Anim. 49, 560–567.
| Kinematics and subpopulations structure definition of blue fox (Alopex lagopus) sperm motility using the ISAS®v1 CASA system.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cjosFSltA%3D%3D&md5=b010021ffe63f8d2c5b85ae4b5a131a1CAS |
Sorensen, D., and Gianola, D. (2002). Likelihood, Bayesian, and MCMC Methods in Quantitative Genetics. First Edition. (Springer-Verlag: New York.)
Stoltz, J. A., and Neff, B. D. (2006). Sperm competition in a fish with external fertilization: the contribution of sperm number, speed and length. J. Evol. Biol. 19, 1873–1881.
| Sperm competition in a fish with external fertilization: the contribution of sperm number, speed and length.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28ngvVaqug%3D%3D&md5=b810a52f70854a0735cde3b5bcfbdf59CAS |
Stoss, J. (1983). Fish gamete preservation and spermatozoan physiology. In ‘Fish Physiology. Vol. IX. Reproduction Part B. Behaviour and Fertility Control’. (Eds W. S. Hoar, D. J. Randall, and E. M. Donaldson.) pp. 305–350 (Academic Press: New York.)
Taborsky, M. (1998). Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol. Evol. 13, 222–227.
| Sperm competition in fish: ‘bourgeois’ males and parasitic spawning.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFyquw%3D%3D&md5=e641e0b80b8fac2a69499caa94ede256CAS |
Thompson, J. A., Love, C. C., Stich, K. L., Brinsko, S. P., Blanchard, T. L., and Varner, D. D. (2004). A Bayesian approach to prediction of stallion daily sperm output. Theriogenology 62, 1607–1617.
| A Bayesian approach to prediction of stallion daily sperm output.Crossref | GoogleScholarGoogle Scholar |
Tuset, V. M., Detrich, G. J., Wojtczak, M., Slowinska, M., De Monserrat, J., and Ciereszko, A. (2008). Relationships between morphology, motility and fertilization capacity in rainbow trout (Oncorhynchus mykiss) spermatozoa. J. Appl. Ichthyol. 24, 393–397.
| Relationships between morphology, motility and fertilization capacity in rainbow trout (Oncorhynchus mykiss) spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Valverde, A., Arenán, H., Sancho, M., Contell, J., Yániz, J., Fernández, A., and Soler, C. (2016). Morphometry and subpopulation structure of Holstein bull spermatozoa: variations in ejaculates and cryopreservation straws. Asian J. Androl. 18, 851–857.
| 1:CAS:528:DC%2BC1cXhtlWksLY%3D&md5=4fcaa3a8d99c41cd4484dca5b8717b74CAS |
Vásquez, F., Soler, C., Camps, P., Valverde, A., and García-Molina, A. (2016). Spermiogram and sperm head morphometry assessed by multivariate cluster analysis results during adolescence (12–18 years) and the effect of varicocele. Asian J. Androl. 18, 824–830.
Vicente-Fiel, S., Palacin, I., Santolaria, P., and Yaniz, J. L. (2013). A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer‐assisted fluorescence method (CASMA-F). Anim. Reprod. Sci. 139, 182–189.
| A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer‐assisted fluorescence method (CASMA-F).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sngs1Ogtg%3D%3D&md5=78cce6f6b62c0f921829357a3baa9328CAS |
Vladić, T. V., and Järvi, T. (2001). Sperm quality in alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle. Proc. Biol. Sci. 268, 2375–2381.
| Sperm quality in alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle.Crossref | GoogleScholarGoogle Scholar |
Vladić, T., Forsberg, L. A., and Järvi, T. (2010). Sperm competition between alternative reproductive tactics of the Atlantic salmon in vitro. Aquaculture 302, 265–269.
| Sperm competition between alternative reproductive tactics of the Atlantic salmon in vitro.Crossref | GoogleScholarGoogle Scholar |
Weir, L. K., Breau, C., Hutchings, J. A., and Cunjak, R. A. (2010). Multiple paternity and variance in male fertilization success within Atlantic salmon Salmo salar redds in a naturally spawning population. J. Fish Biol. 77, 479–493.
| 1:STN:280:DC%2BC3cjktFOgsQ%3D%3D&md5=77b4c36764b7f01eaa1db39cff44ba80CAS |
Wojtczak, M., Dietrich, G. J., Słowińska, M., Dobosz, S., Kuźmiński, M., and Ciereszko, A. (2007). Ovarian fluid pH enhances motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa. Aquaculture 270, 259–264.
| Ovarian fluid pH enhances motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXovFaqtbo%3D&md5=ed494a52f62d936968db81b84f33abccCAS |
Yániz, J. L., Vicente-Fiel, S., Soler, C., Recreo, P., Carretero, T., Bono, A., Berné, J. M., and Santolaria, P. (2016). Comparison of different statistical approaches to evaluate morphometric sperm subpopulations in men. Asian J. Androl. 18, 819–823.
Yet, B., Perkins, Z. B., Rasmussen, T. E., Tai, N. R., and Marsh, D. W. (2014). Combining data and meta-analysis to build Bayesian networks for clinical decision support. J. Biomed. Inform. 52, 373–385.
| Combining data and meta-analysis to build Bayesian networks for clinical decision support.Crossref | GoogleScholarGoogle Scholar |