Dry storage of mammalian spermatozoa and cells: state-of-the-art and possible future directions
P. Loi A F , D. A. Anzalone A , L. Palazzese A , A. Dinnyés B C D , J. Saragusty A and M. Czernik A EA Laboratory of Embryology, Faculty of Veterinary Medicine, University of Teramo, Teramo, TE 64100, Italy.
B BioTalentum Ltd, Gödöllő, 2100 Gödöllő, Hungary.
C HCEMM-USZ, StemCell Research Group, University of Szeged, Szeged, Hungary.
D Sichuan University, College of Life Sciences, Chengdu, China.
E Institute of Genetics and Animal Biotechnology of the Polish Academy of Sciences, Jastrzebiec, Poland.
F Corresponding author. Email: ploi@unite.it
Reproduction, Fertility and Development 33(2) 82-90 https://doi.org/10.1071/RD20264
Published: 8 January 2021
Journal Compilation © IETS 2021 Open Access CC BY-NC-ND
Abstract
This review provides a snapshot of the current state-of-the-art of drying cells and spermatozoa. The major successes and pitfalls of the most relevant literature are described separately for spermatozoa and cells. Overall, the data published so far indicate that we are closer to success in spermatozoa, whereas the situation is far more complex with cells. Critical for success is the presence of xeroprotectants inside the spermatozoa and, even more so, inside cells to protect subcellular compartments, primarily DNA. We highlight workable strategies to endow gametes and cells with the right combination of xeroprotectants, mostly sugars, and late embryogenesis abundant (LEA) or similar ‘intrinsically disordered’ proteins to help them withstand reversible desiccation. We focus on the biological aspects of water stress, and in particular cellular and DNA damage, but also touch on other still unexplored issues, such as the choice of both dehydration and rehydration methods or approaches, because, in our view, they play a primary role in reducing desiccation damage. We conclude by highlighting the need to exhaustively explore desiccation strategies other than lyophilisation, such as air drying, spin drying or spray drying, ideally with new prototypes, other than the food and pharmaceutical drying strategies currently used, tailored for the unique needs of cells and spermatozoa.
Keywords: biobanks, cells, reversible desiccation, spermatozoa.
Introduction
Backing-up cell lines and germplasm in biobanks has always been a fundamental pillar of basic and applied research and clinical applications. Lately, though, there has been an exponential growth in the number of biobanks due to developments in medical research and an increased awareness of the loss of biodiversity across the globe. In medical research, applications associated with somatic stem cells, embryonic and induced pluripotent stem cells and embryonic and somatic stem cells, clustered under the broad heading of ‘cell and tissue regeneration therapies,’ are a global priority (Zhao and Ikeya 2018; Ortuño-Costela et al. 2019). Therefore, thousands of groups worldwide are deriving and storing countless numbers of multipotent cell lines for therapeutic purposes (Carpenter 2015). In addition to the standard use of multipotent cells for therapy, cell lines are becoming central in what has become known as ‘personalised medicine’. In essence, first cell lines and now organoids have been established, mostly from cancer patients, and used to assess the most effective anticancer therapies for a specific tumour epi- or genotype, thus refining therapies and making them more effective (Elbadawy et al. 2020). Furthermore, cell lines are unparalleled tools for in vitro toxicity testing. Finally, biobanks support human infertility treatments.
On the biodiversity front, not-for-profit organisations, and even constellations of individual research groups (including our own), are collecting and storing DNA, tissues, cells, seeds, gametes and embryos from organisms spanning the plant and animal kingdoms, primarily those threatened with extinction. The best known organisations are the Frozen Ark Consortium (https://www.frozenark.org, accessed 19 October 2020) and the Smithsonian Institute, but the reality is that this is a global multicentre effort to store DNA, cells and germplasm from threatened animals (Comizzoli 2015). Having biobanks scattered in different countries, with each maintaining the right to hold samples, as established in the Nagoya Protocol, is an obstacle to the exchange of genetic material between countries, thus reducing the global efforts against biodiversity loss (Comizzoli and Holt 2016).
This short overview clearly articulates the rationale behind the sharp increase in the number of biobanks worldwide. Cells, germplasm, tissues and DNA are usually stored frozen in liquid nitrogen (LN), the gold standard for long-term preservation. Indeed, LN storage is effective, but is associated with adverse side effects, particularly related to safety issues, and a large carbon footprint because of the production and transportation of LN, storage and high maintenance costs (Saragusty et al. 2020). Therefore, more economical alternative storage methods would considerably alleviate the costs and hazards associated with the creation and, in particular, the maintenance of biobanks, making them more affordable and accessible worldwide.
Life can be reversibly suspended in two ways: (1) by practically halting macromolecular interactions through a change the physical state of water (i.e. freezing or vitrification); or (2) by extracting the intracellular water (i.e. drying).
It may come as a surprise to many, but freeze drying was successfully attempted in the very first paper that demonstrated the survival of fowl spermatozoa cryopreserved with glycerol (Polge et al. 1949). In the final section of that article, the authors claimed that high proportions of water could be subtracted from fowl spermatozoa without affecting their morphological and functional properties. Fowl spermatozoa were frozen to –79°C, rewarmed to –25°C and then vacuum-dried to remove up to 90% of the total water (Polge et al. 1949). However, many crucial technical passages were omitted in the paper; moreover, the findings were further complicated by the presence of very high concentrations of glycerol (20–30%) in the drying medium, a substance that remains liquid and, more importantly, very toxic at room temperature.
Since then, ‘cryo-’ and ‘lyo-’ preservation strategies diverged, with the latter remaining on the back burner and the former becoming the most widely used and reliable preservation approach. Although cryopreservation protocols were continuously optimised and expanded from spermatozoa to many other types of cells, including, more recently, embryos and oocytes, a few scientists attempted to perfect the drying protocol (i.e. lyophilisation). Informed by studies on model membranes (Mouradian et al. 1985), lyophilisation experiments were performed on anucleate blood cells, erythrocytes and platelets (Wolkers et al. 2001; Arav and Natan 2012) with encouraging results. It is not known why anucleate rather than nucleated cells were selected. One reason may be the strong interest in dry blood for use in the battlefield, a primary interest in army research. Another possible reason is that scientists feared that the nucleus and DNA could not withstand the dehydration stress. It may also be that these cells best resembled the liposomes used to study the basic mechanisms of drying. Unfortunately, dried blood never made it into clinical trials.
There was renewed interest in the freeze drying of nucleated cells after lyophilised mouse spermatozoa produced viable pups following their injection into mature oocytes (Wakayama and Yanagimachi 1998). That was a radical turning point. Since then, these findings were reproduced in other species, confirming that dry spermatozoa can contribute to embryo development in vitro (Keskintepe et al. 2002; Kwon et al. 2004; Olaciregui et al. 2017; Palazzese et al. 2018; Anzalone et al. 2019) and, to a lesser extent and in a limited number of species, to offspring in the mouse (Wakayama and Yanagimachi 1998), rabbit (Liu et al. 2004), rat (Hirabayashi et al. 2005), horse (Choi et al. 2011) and hamster (Muneto and Horiuchi 2011). On the back of these encouraging results, scientists raised the stakes, demonstrating that even somatic cells kept dry at room temperature for 8 years were able to contribute to embryo development after nuclear transfer (Loi et al. 2008). Met initially with scepticism, the findings of that study were successfully reproduced in mice (Ono et al. 2008) and pigs (Das et al. 2010). Together, these achievements have increased the interest in the dry storage of cells and germplasm, but have also highlighted the need for a radical revision of the procedures thus far used to dry nucleated cells.
In this review we summarise the most relevant information available to date, and how to use this information for further progress, for spermatozoa and cells separately. We then touch on the methods available thus far for water subtraction and analyse whether there is room for improvement in our concluding remarks.
Spermatozoa: current situation
Spermatozoa are very special cells, with a lower water content than all other cells of the body and, more importantly, with a unique DNA packaging; no wonder they were the first cell type to be successfully frozen and the first to be dried. What can be drawn from the published data?
Dried spermatozoa lack viability owing to extensive membrane and structural damage (more or less, depending on the method used); however, as established by Wakayama and Yanagimachi (1998), cell viability is not related to nuclear viability. Spermatozoa are almost invariably dried through lyophilisation and maintain their capacity to contribute to embryo development and, to a lesser extent, to produce offspring. Here, mice remain unbeaten in terms of frequencies of blastocyst and term development, whereas offspring from dried spermatozoa are limited to only five species (see above). The limit of offspring from dried spermatozoa to five species only, in our view, is that the nuclear organisation in spermatozoa of different species makes them more or less vulnerable to the drying process and the culprit here cannot be anything else but DNA damage. A strict relationship exists between single- and, more so, double-stranded DNA damage and the chances of successfully contributing to development. In our study with freeze-dried epididymal ram spermatozoa, we found a cut-off of approximately 2% double-strand breaks, over which there was a total loss of fertility (Palazzese et al. 2018). Why there are so many differences in the susceptibility of spermatozoa from different species to withstand lyophilisation is unknown. However, some hints may come from recent data on the DNA organisation of spermatozoa, notably nucleosome retention.
In contrast to what was thought just a few years ago, an important proportion of the spermatozoa’s DNA skips the tight compaction induced by protamine (1 and 2) binding, retaining a nucleosomal organisation (Samans et al. 2014). The proportion of sperm DNA that retains a nucleosomal organisation varies widely across species, ranging from 1% to 15% (Samans et al. 2014). In mice, it accounts for only 1% (Erkek et al. 2013). Conversely, human and bovine spermatozoa have higher genome domains, in the range 10–15% (Samans et al. 2014), that maintain a nucleosomal organisation. It is highly probable that DNA in regions with nucleosomal organisation is more ‘open’ and therefore far more vulnerable to dehydration stress than its protaminised counterpart. This is confirmed by the extensive DNA damage and ensuing repairing activity in freeze-dried somatic cells (Iuso et al. 2013). The mouse haploid genome in spermatozoa is therefore more resistant than in other species investigated so far. This difference may explain why dried spermatozoa work so well in this species.
To summarise, if we can preserve DNA integrity in dried spermatozoa, it is very likely that they will support normal development following intracytoplasmic sperm injection (ICSI), but the data show that we are still far from attaining this goal.
Somatic cells: current situation
Compared with spermatozoa, very few data are available on the dry preservation of somatic cells. The first report of dried cells being successfully used for nuclear transfer (Loi et al. 2008) was followed by confirmatory papers (Ono et al. 2008; Das et al. 2010), and shortly after by a few reports claiming that dried cells, specifically cord blood stem cells, survived lyophilisation and were able to grow into colonies again (Natan et al. 2009; Buchanan et al. 2010). While waiting for these studies to be replicated, our experience with lyophilised cells is that viability is irreparably lost once rehydrated; however, as established earlier in spermatozoa (Wakayama and Yanagimachi 1998), the nuclear compartment remains functional following nuclear transfer into enucleated oocytes. This is indeed striking. No animal has been produced so far from embryos produced from dried somatic cells. However, their genome has been preserved through the establishment of stem cells from blastocysts developed after the nuclear transfer of lyophilised mouse cells (Ono et al. 2008). This is a surprise. If our previous reasoning that a nucleosome chromatin configuration is prohibitive for withstanding lyophilisation in spermatozoa, it is striking that somatic cells have a functional genome following rehydration given that all their genome is wrapped around nucleosomes. Clearly, the DNA damage in dry cells is massive. In our previous work, lyophilised sheep cells transplanted into enucleated oocytes were assessed for the incorporation of phosphorylated histone H2A histone family member X (γH2AX), a histone variant involved in the DNA repair machinery recruited to sites of double-strand DNA breaks (Podhorecka et al. 2010). The data showed a positivity for γH2AX throughout the nuclear compartment, indicating extensive DNA damage in dry somatic cell nuclei (Iuso et al. 2013). In that study we also investigated the DNA repair potential of oocytes, with surprising results. Positive expression of γH2AX was comparable in intensity even when four somatic nuclei (equivalent to eight spermatozoa) were injected into an oocyte (Iuso et al. 2013). Thus, oocytes have an incredible capacity to repair DNA in the sense that the DNA repairing capacity might fix the DNA damage caused in the cells by the drying stress. To summarise, dehydration stress is something eukaryotic cells are not equipped to cope with.
Anhydrobiotic organisms, like tardigrades and midges, among others, are able to withstand water deprivation thanks to the expression and intracellular accumulation of a plethora of compounds, ranging from sugars and osmolytes to a complex family of proteins (for review, see Loi et al. 2013; Kikuta et al. 2017; Sogame and Kikawada 2017; Miyata et al. 2019). Unfortunately, none of the genes encoding these compounds is present in the human genome; therefore, the only way to protect cells against dehydration is to provide them with suitable ‘xeroprotectants’, borrowed from anhydrobiotes using a ‘biomimicry’ approach. In support of this strategy, preliminary but encouraging results are being published on this topic, with fibroblasts surviving dehydration thanks to the heterologous expression of trehalose (Eroglu et al. 2000) or late embryogenesis abundant (LEA) proteins (Hand et al. 2011; Li et al. 2012; Czernik et al. 2020).
To freeze or not to freeze before water extraction?
As noted above, the default approach for water subtraction in spermatozoa, and even in the first reports on somatic cells, has been lyophilisation. Under vacuum, water needs to solidify through freezing so it can be removed by sublimation. However, spermatozoa are almost invariably frozen in LN at –196°C. Thus, it is not surprising that we all follow the methods described in the first report on freezing spermatozoa (Wakayama and Yanagimachi 1998). Deep freezing represents a major mechanical and osmotic stress for spermatozoa, especially because no cryoprotectants, such as glycerol or ethylene glycol, can be added before freezing. These cryoprotectants remain liquid, and toxic, at room temperature and are therefore unsuitable for use in dry storage. Consequently, scientists started investigating solutions to this problem using milder cold temperatures to solidify water prior to sublimation (Restrepo et al. 2019; Wakayama et al. 2019; Palazzese et al. 2020) with better results. After all, the aforementioned anhydrobiotes do not resort to freezing before dehydration; simply put, once a reduction in environmental water is sensed, they activate a timely, regulated gene expression leading to the accumulation of xeroprotectants in their cells.
So, the million dollar question is, do we need to freeze our cells or gametes before water subtraction? At the moment, the answer is that we do not know the best strategy for the desiccation of spermatozoa and cells. A recent review from our group addressing drying reported that even though lyophilisation is the most used approach, probably as a default, there are at least 10 other methodologies that have been used to dry spermatozoa and cells (Saragusty and Loi 2019) (Table 1).

|
Of the alternative methodologies, spin drying (owing to the uniformity in moisture content in samples) and rapid drying are of particular interest (Chakraborty 2011). Spray drying is the method of choice for drying thermally sensitive material in the food processing and pharmaceutical industries (Poozesh and Bilgili 2019). Moreover, these methods offer the advantage of providing the material to be dried (in our case, spermatozoa or cells) an external, protective matrix (Arpagaus et al. 2018). However, the aim of this review is not to critically compare the different drying methods (exhaustive reviews are already available; Walters et al. 2014), but simply to stress that the ideal method for drying spermatozoa or cells has yet to be established.
Future directions: spermatozoa
Spermatozoa, with their nuclear packaging, offer unique structural advantages to withstand drying, yet DNA damage is a major limiting factor. It may seem surprising that only 2% of double-strand breaks are enough to arrest development completely (at least with ram epididymal spermatozoa) but 2% is not uninfluential, if, as it might be, that DNA damage occurs at nucleosome organised domains. In fact, there is accumulating evidence that a nucleosomal DNA organisation is retained in sperm nuclei, and that nucleosome-organised domains mark crucial genes that are transcribed early in development (Saitou and Kurimoto 2014). Therefore, it is plausible that these domains are more vulnerable to water stress, whereas the protaminised DNA should be more resistant. Of course, experimental data precisely mapping sites of DNA damage in dry spermatozoa would be needed to support our claims. The protection afforded by compounds like sugars, osmolytes and antioxidants added to the drying medium is only partial, because the compounds only cover the external side of the sperm membrane. Xeroprotectants (for an updated list of xeroprotectants and mechanisms of action, see Loi et al. 2013) need to act also from the inside, protecting all cellular compartments. This limit has been long acknowledged, since trehalose was used to protect platelets or red blood cells (Crowe and Crowe 2000). Consequently, scientists have tried various ways to upload trehalose into cells for many years. Several papers have been published with encouraging results, including increased permeability during the temperature-induced lipid phase transition (Satpathy et al. 2004), electroporation (Zhou et al. 2010), the use of haemolysin (Patrick et al. 2017) and the activation of pre-existing ion channels (Elliott et al. 2006).
In spermatozoa, although electroporation was used a long time ago (Gagné et al. 1995), it was only recently that the temperature-induced lipid phase transition was used to induce trehalose uptake by cells (Zhang et al. 2016; Oldenhof et al. 2017). Given the short time frame and the reduced molecular movements caused by the cold temperature, the amount of trehalose taken up by spermatozoa is limited. In one of the few studies in which this issue was addressed, with a trehalose concentration of 250 mM in the drying medium the intracellular concentration was found 10-fold lower at 23 mM (Oldenhof et al. 2017). These values are far from the physiological trehalose concentrations found in anhydrobiotes. The trehalose concentration accounts for 2.9% of the dry weight of tardigrades (Wełnicz et al. 2011), but is much higher in Artemia cysts or in the nematode Aphelenchus avenae, in which trehalose concentrations can reach 18% and 15% of dry weight respectively (Clegg 1965; Madin and Crowe 1975). Quantitative studies of anhydrobiotes are valuable because they can provide us with a good indication of the effective concentrations of xeroprotectants, and can guide us in our drying studies (Hengherr et al. 2008).
Given that the amount of trehalose loaded into spermatozoa by diffusion or phase transition is limited, it is worthy exploring alternative strategies. One promising strategy may be the use of trehalose analogues, in which the hydrophilic hydroxyl groups of trehalose are turned into esters (Bragg et al. 2017). Another promising permeable version of trehalose is trehalose hexaacetate, a molecule synthesised several years ago that is lipophilic and can readily cross the membrane (Abazari et al. 2015). Membrane-permeable trehalose would, in theory, allow better control of its uptake (and removal) by changing concentrations and, even more, by longer exposure to trehalose gradients. Surprisingly, a recent report claimed that trehalose lacks any DNA protection activity (Ito et al. 2019); given that this report comes from an authoritative group, alternative compounds with antioxidant activity, like rosmarinic acid or melatonin, should be explored, as suggested by Mercati et al. (2020). However, in our opinion, natural xeroprotectants found in tardigrades, midges or other anhydrobiotic organisms should guide our choices.
As for the use of LEA proteins for drying spermatozoa, there are no data available at the moment. However, because of their size and the absence of translation machinery, the use of LEA proteins for the dry preservation of these cells may be complicated.
Future directions: cells
In contrast with spermatozoa, somatic cells have a high water content, a complex subcellular organisation and a chromatin organisation that is highly prone to drying damage. The limited data published on drying cells, compared with spermatozoa, indirectly confirms the difficulties with drying these cells.
The beneficial effect of trehalose in the drying medium was clear since the first report of the lyophilisation of somatic cells, granulosa cells in this case (Loi et al. 2008), and reconfirmed recently by Zhang et al. (2017). But, as described for spermatozoa, cells need protection from the inside to withstand water stress. If it is true that cell complexity is an obstacle, we can exploit the translational machinery of cells to express xeroprotectants, a path that scientists have started to pursue. One of the best candidates for protecting cells against desiccation stress are LEA proteins (Marunde et al. 2013). LEA proteins were first discovered in cotton seeds approximately 40 years ago (Galau and Dure 1981) and later in seeds and vegetative tissues of several other plants (Shih et al. 2008). A relatively recent survey counted as many as 1710 LEA proteins (http://forge.info.univ-angers.fr/~gh/Leadb/index.php?action=0andmode=0, accessed 19 October 2020). LEA proteins are highly hydrophilic and acquire a random coil conformation in water but become structurally organised during desiccation, a property that has led to them being described as ‘intrinsically disordered’ proteins (McCubbin et al. 1985). The properties and mechanisms of action of LEA proteins have been described in several authoritative reviews (Furuki and Sakurai 2018; Janis et al. 2018).
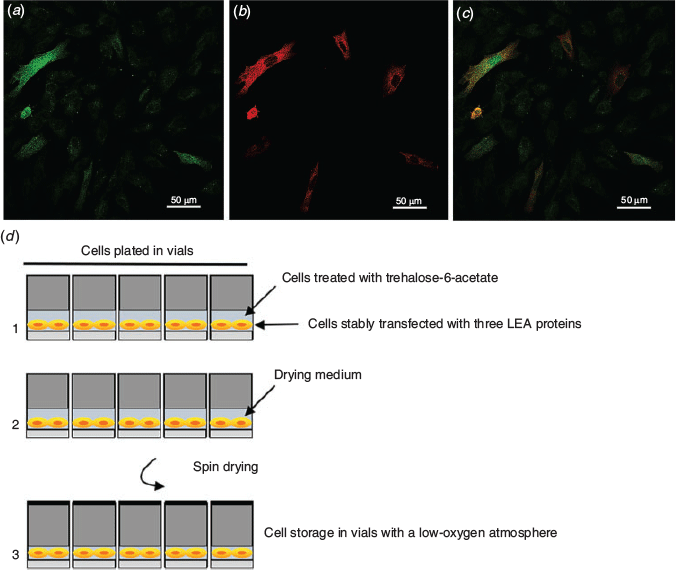
The landmark paper demonstrating the protective effects of LEA proteins in mammalian cells was published 8 years ago (Li et al. 2012). The results were convincing. It is a remarkable fact that LEA proteins are expressed and localise to specific organelle compartments, such as the mitochondria, and protect them against water deprivation. We have extended that original study. In addition to the LEA proteins AfrLEA3m (mitochondria) and AfrLEA2 (cytoplasm and nucleus) already assessed in somatic cells (Li et al. 2012), we expressed two additional LEA proteins in fibroblasts, namely pTag-RAB17-GFP-N, Zea mays dehydrin-1dhn, targeting the nucleus and cytoplasm, and pTag-WCOR410-RFP, Triticum aestivum cold acclimation protein WCOR410, that binds to membranes (Hand et al. 2011). Encouragingly, we had shown a robust protective synergy when all three LEA proteins were expressed in somatic cells before partial dehydration (AfrLEA3m, RAB17, WCOR410; Czernik et al. 2020). Although in that study the proportion of water subtraction was limited (23%) and the study duration was limited to 4 h, the control fibroblasts died but the LEA protein-expressing cells survived and recovered very quickly once cultured under normal conditions (Czernik et al. 2020). This is indeed a proof-of-principle that LEA proteins confer cells with a tolerance to water stress that typically kills them. Thus, heterologous expression of a cocktail of LEA proteins that would specifically protect the DNA, membranes and cell organelles appears to be the best course to follow for the induction of reversible drying in mammalian cells (Fig. 1). This path is further justified by the fact that trehalose seems to play a secondary role in desiccation tolerance in tardigrades, with many of them incapable of synthesising the sugar (Guidetti et al. 2011).
Key molecules responsible for desiccation tolerance in model anhydrobiotes, like midges and tardigrades, are being identified (Yamada et al. 2020). Most of the information collected from these models is enlightening. A new class of proteins, cytosolic abundant heat soluble (CAHS) and secreted abundant heat soluble (SAHS) proteins, collectively described as tardigrade-specific intrinsically disordered proteins (TDPs), has been thoroughly characterised recently in the tardigrade Hypsibius dujardini (Boothby et al. 2017). Thus, the number of candidate xeroprotectants to be expressed and tested for reversible drying in mammalian cells is expanding, and the new candidates promise to further the current state-of-the-art. Fig. 1 summarises a possible approach for drying fibroblasts expressing a cocktail of three LEA proteins and loaded with a permeable version of trehalose to improve the resistance to the desiccation stress before being subjected to spin drying.
Concluding remarks
This review addresses the induction of reversible drying in cells and male gametes, focusing on the biological side of the issue. There is another technological factor of utmost importance for success: the process for water subtraction. Lyophilisation has been the master strategy used so far, but some elements are suggesting that it may not be entirely appropriate. As discussed above, the safest approach to induce desiccation tolerance in cells and gametes is to use the ‘tricks’ used by anhydrobiotic organisms. It is worth noting that even though tardigrades survive happily in LN when in a dry state, freezing is not a prerequisite for drying, as in any other anhydrobiotic organisms. Therefore, it is mandatory that alternative solutions for water subtraction from cells and spermatozoa are explored. Ideally, the alternative solutions for water extraction should be compatible with cell viability but, understanding the difficulties that the challenge poses, we would be happy for now if DNA integrity was guaranteed at the very least.
In a previous article (Saragusty and Loi 2019), we described 10 different alternative drying methodologies to lyophilisation that have already been tested (Table 1). Some of the techniques are certainly promising, although the number of replicates in each is too low to enable definitive conclusions to be drawn. Of significance though are the daring experiments into air drying oocytes’ chromosomes, leading to the maintenance of meiotic competence (Graves-Herring et al. 2013).
If we look at cell drying, after the first pioneering report on lyophilisation, alternative methods were rapidly adopted, such as air drying, microwave drying or spin drying (Hand et al. 2011; Graves-Herring et al. 2013; Loi et al. 2013; Czernik et al. 2020). Of significance, is the recent report on microwave-assisted drying of ovarian tissue slices (Lee et al. 2019). Technologically, lyophilisation was, and still is, accomplished using equipment borrowed from the food processing industry. It is easy to see that the requirements for processing tiny cells or gametes, suspended in volumes in the order of hundreds of microlitres, differ markedly from those of the lyophilisation of large volumes of milk or coffee. It is our feeling that the current status of drying cells and gametes has to be seen as ground zero, and the best solution in our view would be to design reliable prototypes of drying (bench-top) devices with the specific needs of cells and gametes in mind. Finally, another technical step, so far relatively unexplored but, in our view, of major importance, is the rehydration phase (imbibition). So far, the subtracted volume of water has been added in a single step, probably causing osmotic and mechanical damage. Ongoing experiments in our laboratory clearly indicate that slow progressive rehydration may further improve outcomes.
Thus, in conclusion, although there are positive indications from the published literature on the drying of cells and gametes, we need to explore, in collaboration with engineers from the food and pharmaceutical industries, the most promising drying methods, such as air, spin or spray drying, ideally with dedicated prototype drying equipment fit to use with our samples (Fig. 2). These efforts, together with the use of the most effective xeroprotectants and desiccation media, will surely tell us whether it is realistic to store dry cells or germplasm, or whether we must dump the idea for good.

|
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors’ work reported herein received funding from the European Union (EU) Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement (No. 734434; DRYNET) and the Narodowe Centrum Nauki (NCN; Grant no. 2016/21/D/NZ3/02610) to M. Czernik, A. Dinnyés and P. Loi. P. Loi and M. Czernik received support from the project ‘DEMETRA (MIUR)’ Department of Excellence: 2018–2022. A. Dinnyés has received support from the EU Horizon 2020 Research and Innovation Program under Grant agreement no. 739593 and Chinese Hungarian Bilateral Project (2018-2.1.14-TÉT-CN-2018-00011, Chinese No. 8-4).
References
Abazari, A., Meimetis, L. G., Budin, G., Bale, S. S., Weissleder, R., and Toner, M. (2015). Engineered trehalose permeable to mammalian cells. PLoS One 10, e0130323.| Engineered trehalose permeable to mammalian cells.Crossref | GoogleScholarGoogle Scholar | 26115179PubMed |
Anzalone, D. A., Palazzese, L., Iuso, D., Martino, G., and Loi, P. (2019). Corrigendum to ‘Freeze-dried spermatozoa: an alternative biobanking option for endangered species’ [Anim. Reprod. Sci. 190C (2018) 85–93]. Anim. Reprod. Sci. 202, 96.
| Corrigendum to ‘Freeze-dried spermatozoa: an alternative biobanking option for endangered species’ [Anim. Reprod. Sci. 190C (2018) 85–93].Crossref | GoogleScholarGoogle Scholar | 30683430PubMed |
Arav, A., and Natan, D. (2012). Freeze drying of red blood cells: the use of directional freezing and a new radio frequency lyophilization device. Biopreserv. Biobank. 10, 386–394.
| Freeze drying of red blood cells: the use of directional freezing and a new radio frequency lyophilization device.Crossref | GoogleScholarGoogle Scholar | 24849889PubMed |
Arpagaus, C., Collenberg, A., Rütti, D., Assadpour, E., and Jafari, S. M. (2018). Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 546, 194–214.
| Nano spray drying for encapsulation of pharmaceuticals.Crossref | GoogleScholarGoogle Scholar | 29778825PubMed |
Boothby, T. C., Tapia, H., Brozena, A. H., Piszkiewicz, S., Smith, A. E., Giovannini, I., Rebecchi, L., Pielak, G. J., Koshland, D., and Goldstein, B. (2017). Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 65, 975–984.e5.
| Tardigrades use intrinsically disordered proteins to survive desiccation.Crossref | GoogleScholarGoogle Scholar | 28306513PubMed |
Bragg, J. T., D’Ambrosio, H. K., Smith, T. J., Gorka, C. A., Khan, F. A., Rose, J. T., Rouff, A. J., Fu, T. S., Bisnett, B. J., Boyce, M., Khetan, S., and Paulick, M. G. (2017). Esterified trehalose analogues protect mammalian cells from heat shock. ChemBioChem 18, 1863–1870.
| Esterified trehalose analogues protect mammalian cells from heat shock.Crossref | GoogleScholarGoogle Scholar | 28722776PubMed |
Buchanan, S. S., Pyatt, D. W., and Carpenter, J. F. (2010). Preservation of differentiation and clonogenic potential of human hematopoietic stem and progenitor cells during lyophilization and ambient storage. PLoS One 5, e12518.
| Preservation of differentiation and clonogenic potential of human hematopoietic stem and progenitor cells during lyophilization and ambient storage.Crossref | GoogleScholarGoogle Scholar | 20824143PubMed |
Carpenter, L. (2015). Biobanks for induced pluripotent stem cells and reprogrammed tissues. In ‘Cord Blood Stem Cells and Regenerative Medicine’ (Eds C. Stavropoulus-Giokas, D. Charron, and C. Navarret.) pp. 179–194. (Academic Press: Oxford, UK.)
Chakraborty, N. (2011). Cryopreservation of spin-dried mammalian cells. PLoS One 6, e24916.
| Cryopreservation of spin-dried mammalian cells.Crossref | GoogleScholarGoogle Scholar | 21966385PubMed |
Choi, Y. H., Varner, D. D., Love, C. C., Hartman, D. L., and Hinrichs, K. (2011). Production of live foals via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse. Reproduction 142, 529–538.
| Production of live foals via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse.Crossref | GoogleScholarGoogle Scholar | 21846810PubMed |
Clegg, J. S. (1965). The origin of trehalose and its significance during emergence of encysted dormant embryos of Artemia salina. Comp. Biochem. Physiol. 14, 135–143.
| The origin of trehalose and its significance during emergence of encysted dormant embryos of Artemia salina.Crossref | GoogleScholarGoogle Scholar | 14288194PubMed |
Comizzoli, P. (2015). Biobanking efforts and new advances in male fertility preservation for rare and endangered species. Asian J. Androl. 17, 640–645.
| Biobanking efforts and new advances in male fertility preservation for rare and endangered species.Crossref | GoogleScholarGoogle Scholar | 25966625PubMed |
Comizzoli, P., and Holt, W. V. (2016). Implications of the Nagoya Protocol for genome resource banks composed of biomaterials from rare and endangered species. Reprod. Fertil. Dev. 28, 1145–1160.
| Implications of the Nagoya Protocol for genome resource banks composed of biomaterials from rare and endangered species.Crossref | GoogleScholarGoogle Scholar |
Crowe, J. H., and Crowe, L. M. (2000). Preservation of mammalian cells – learning nature’s tricks. Nat. Biotechnol. 18, 145–146.
| Preservation of mammalian cells – learning nature’s tricks.Crossref | GoogleScholarGoogle Scholar | 10657114PubMed |
Czernik, M., Fidanza, A., Luongo, F. P., Valbonetti, L., Scapolo, P. A., Patrizio, P., and Loi, P. (2020). Late embryogenesis abundant (LEA) proteins confer water stress tolerance to mammalian somatic cells. Cryobiology 92, 189–196.
| Late embryogenesis abundant (LEA) proteins confer water stress tolerance to mammalian somatic cells.Crossref | GoogleScholarGoogle Scholar | 31952948PubMed |
Das, Z. C., Gupta, M. K., Uhm, S. J., and Lee, H. T. (2010). Lyophilized somatic cells direct embryonic development after whole cell intracytoplasmic injection into pig oocytes. Cryobiology 61, 220–224.
| Lyophilized somatic cells direct embryonic development after whole cell intracytoplasmic injection into pig oocytes.Crossref | GoogleScholarGoogle Scholar | 20691649PubMed |
Elbadawy, M., Abugomaa, A., Yamawaki, H., Usui, T., and Sasaki, K. (2020). Development of prostate cancer organoid culture models in basic medicine and translational research. Cancers (Basel) 12, 777.
| Development of prostate cancer organoid culture models in basic medicine and translational research.Crossref | GoogleScholarGoogle Scholar |
Elliott, G. D., Liu, X.-H., Cusick, J. L., Menze, M., Vincent, J., Witt, T., Hand, S., and Toner, M. (2006). Trehalose uptake through P2X7 purinergic channels provides dehydration protection. Cryobiology 52, 114–127.
| Trehalose uptake through P2X7 purinergic channels provides dehydration protection.Crossref | GoogleScholarGoogle Scholar | 16338230PubMed |
Erkek, S., Hisano, M., Liang, C. Y., Gill, M., Murr, R., Dieker, J., Schübeler, D., van der Vlag, J., Stadler, M. B., and Peters, A. H. (2013). Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat. Struct. Mol. Biol. 20, 868–875.
| Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa.Crossref | GoogleScholarGoogle Scholar | 23770822PubMed |
Eroglu, A., Russo, M. J., Bieganski, R., Fowler, A., Cheley, S., Bayley, H., and Toner, M. (2000). Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 18, 163–167.
| Intracellular trehalose improves the survival of cryopreserved mammalian cells.Crossref | GoogleScholarGoogle Scholar | 10657121PubMed |
Fabre, A. L., Colotte, M., Luis, A., Tuffet, S., and Bonnet, J. (2014). An efficient method for long-term room temperature storage of RNA. Eur. J. Hum. Genet. 22, 379–385.
| An efficient method for long-term room temperature storage of RNA.Crossref | GoogleScholarGoogle Scholar | 23860045PubMed |
Furuki, T., and Sakurai, M. (2018). Physicochemical aspects of the biological functions of trehalose and group 3 LEA proteins as desiccation protectants. Adv. Exp. Med. Biol. 1081, 271–286.
| Physicochemical aspects of the biological functions of trehalose and group 3 LEA proteins as desiccation protectants.Crossref | GoogleScholarGoogle Scholar | 30288715PubMed |
Gagné, M., Pothier, F., and Sirard, M. A. (1995). The use of electroporated bovine spermatozoa to transfer foreign DNA into oocytes. Methods Mol. Biol. 48, 161–166.
| The use of electroporated bovine spermatozoa to transfer foreign DNA into oocytes.Crossref | GoogleScholarGoogle Scholar | 8528389PubMed |
Galau, G. A., and Dure, L. (1981). Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by reciprocal heterologous complementary deoxyribonucleic acid-messenger ribonucleic acid hybridization. Biochemistry 20, 4169–4178.
| Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by reciprocal heterologous complementary deoxyribonucleic acid-messenger ribonucleic acid hybridization.Crossref | GoogleScholarGoogle Scholar | 7284318PubMed |
Graves-Herring, J. E., Wildt, D. E., and Comizzoli, P. (2013). Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature. Biol. Reprod. 88, 139.
| Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature.Crossref | GoogleScholarGoogle Scholar | 23575153PubMed |
Guidetti, R., Altiero, T., and Rebecchi, L. (2011). On dormancy strategies in tardigrades. J. Insect Physiol. 57, 567–576.
| On dormancy strategies in tardigrades.Crossref | GoogleScholarGoogle Scholar | 21402076PubMed |
Hand, S. C., Menze, M. A., Toner, M., Boswell, L., and Moore, D. (2011). LEA proteins during water stress: not just for plants anymore. Annu. Rev. Physiol. 73, 115–134.
| LEA proteins during water stress: not just for plants anymore.Crossref | GoogleScholarGoogle Scholar | 21034219PubMed |
Hengherr, S., Heyer, A. G., Köhler, H. R., and Schill, R. O. (2008). Trehalose and anhydrobiosis in tardigrades – evidence for divergence in responses to dehydration. FEBS J. 275, 281–288.
| Trehalose and anhydrobiosis in tardigrades – evidence for divergence in responses to dehydration.Crossref | GoogleScholarGoogle Scholar | 18070104PubMed |
Hirabayashi, M., Kato, M., Ito, J., and Hochi, S. (2005). Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads. Zygote 13, 79–85.
| Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads.Crossref | GoogleScholarGoogle Scholar | 15984166PubMed |
Ito, D., Wakayama, S., Kamada, Y., Shibasaki, I., Kamimura, S., Ooga, M., and Wakayama, T. (2019). Effect of trehalose on the preservation of freeze-dried mice spermatozoa at room temperature. J. Reprod. Dev. 65, 353–359.
| Effect of trehalose on the preservation of freeze-dried mice spermatozoa at room temperature.Crossref | GoogleScholarGoogle Scholar | 31118350PubMed |
Iuso, D., Czernik, M., Di Egidio, F., Sampino, S., Zacchini, F., Bochenek, M., Smorag, Z., Modlinski, J. A., Ptak, G., and Loi, P. (2013). Genomic stability of lyophilized sheep somatic cells before and after nuclear transfer. PLoS One 8, e51317.
| Genomic stability of lyophilized sheep somatic cells before and after nuclear transfer.Crossref | GoogleScholarGoogle Scholar | 23308098PubMed |
Janis, B., Belott, C., and Menze, M. A. (2018). Role of intrinsic disorder in animal desiccation tolerance. Proteomics 18, 1800067.
| Role of intrinsic disorder in animal desiccation tolerance.Crossref | GoogleScholarGoogle Scholar | 30144288PubMed |
Kawase, Y., and Suzuki, H. (2011). A study on freeze-drying as a method of preserving mouse sperm. J. Reprod. Dev. 57, 176–182.
| A study on freeze-drying as a method of preserving mouse sperm.Crossref | GoogleScholarGoogle Scholar | 21551975PubMed |
Keskintepe, L., Pacholczyke, G., Machnicka, A., Norris, K., Curuk, M. A., Khan, I., and Brackett, B. G. (2002). Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol. Reprod. 67, 409–415.
| Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa.Crossref | GoogleScholarGoogle Scholar | 12135874PubMed |
Kikuta, S., Watanabe, S. J., Sato, R., Gusev, O., Nesmelov, A., Sogame, Y., Cornette, R., and Kikawada, T. (2017). Towards water-free biobanks: long-term dry-preservation at room temperature of desiccation-sensitive enzyme luciferase in air-dried insect cells. Sci. Rep. 7, 6540.
| Towards water-free biobanks: long-term dry-preservation at room temperature of desiccation-sensitive enzyme luciferase in air-dried insect cells.Crossref | GoogleScholarGoogle Scholar | 28747745PubMed |
Kwon, I. K., Park, K. E., and Niwa, K. (2004). Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa. Biol. Reprod. 71, 1430–1436.
| Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa.Crossref | GoogleScholarGoogle Scholar | 15215192PubMed |
Lee, P. C., Adams, D. M., Amelkina, O., White, K. K., Amoretti, L. A., Whitaker, M. G., and Comizzoli, P. (2019). Influence of microwave-assisted dehydration on morphological integrity and viability of cat ovarian tissues: first steps toward long-term preservation of complex biomaterials at supra-zero temperatures. PLoS One 14, e0225440.
| Influence of microwave-assisted dehydration on morphological integrity and viability of cat ovarian tissues: first steps toward long-term preservation of complex biomaterials at supra-zero temperatures.Crossref | GoogleScholarGoogle Scholar | 31800613PubMed |
Li, S., Chakraborty, N., Borcar, A., Menze, M. A., Toner, M., and Hand, S. C. (2012). Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc. Natl Acad. Sci. USA 109, 20859–20864.
| Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation.Crossref | GoogleScholarGoogle Scholar | 23185012PubMed |
Liu, J. L., Kusakabe, H., Chang, C. C., Suzuki, H., Schmidt, D. W., Julian, M., Pfeffer, R., Bormann, C. L., Tian, X. C., Yanagimachi, R., and Yang, X. (2004). Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol. Reprod. 70, 1776–1781.
| Freeze-dried sperm fertilization leads to full-term development in rabbits.Crossref | GoogleScholarGoogle Scholar | 14960482PubMed |
Loi, P., Matsukawa, K., Ptak, G., Clinton, M., Fulka, J., Nathan, Y., and Arav, A. (2008). Freeze-dried somatic cells direct embryonic development after nuclear transfer. PLoS One 3, e2978.
| Freeze-dried somatic cells direct embryonic development after nuclear transfer.Crossref | GoogleScholarGoogle Scholar | 18714340PubMed |
Loi, P., Iuso, D., Czernik, M., Zacchini, F., and Ptak, G. (2013). Towards storage of cells and gametes in dry form. Trends Biotechnol. 31, 688–695.
| Towards storage of cells and gametes in dry form.Crossref | GoogleScholarGoogle Scholar | 24169600PubMed |
Madin, K. A. C., and Crowe, J. H. (1975). Anhydrobiosis in nematodes: carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 193, 335–342.
| Anhydrobiosis in nematodes: carbohydrate and lipid metabolism during dehydration.Crossref | GoogleScholarGoogle Scholar |
Marunde, M. R., Samarajeewa, D. A., Anderson, J., Li, S., Hand, S. C., and Menze, M. A. (2013). Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J. Insect Physiol. 59, 377–386.
| Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein.Crossref | GoogleScholarGoogle Scholar | 23376561PubMed |
McCubbin, W. D., Kay, C. M., and Lane, B. (1985). Hydrodynamic and optical properties of the wheat germ Em protein. Can. J. Biochem. Cell Biol. 63, 803–811.
| Hydrodynamic and optical properties of the wheat germ Em protein.Crossref | GoogleScholarGoogle Scholar |
Mercati, F., Domingo, P., Pasquariello, R., Dall’Aglio, C., Di Michele, A., Forti, K., Cocci, P., Boiti, C., Gil, L., Zerani, M., and Maranesi, M. (2020). Effect of chelating and antioxidant agents on morphology and DNA methylation in freeze-drying in rabbit (Oryctolagus cuniculus) spermatozoa. Reprod. Domest. Anim. 55, 29–37.
| Effect of chelating and antioxidant agents on morphology and DNA methylation in freeze-drying in rabbit (Oryctolagus cuniculus) spermatozoa.Crossref | GoogleScholarGoogle Scholar | 31626708PubMed |
Miyata, Y., Tokumoto, S., Sogame, Y., Deviatiiarov, R., Okada, J., Cornette, R., Gusev, O., Shagimardanova, E., Sakurai, M., and Kikawada, T. (2019). Identification of a novel strong promoter from the anhydrobiotic midge, Polypedilum vanderplanki, with conserved function in various insect cell lines. Sci. Rep. 9, 7004.
| Identification of a novel strong promoter from the anhydrobiotic midge, Polypedilum vanderplanki, with conserved function in various insect cell lines.Crossref | GoogleScholarGoogle Scholar | 31065019PubMed |
Mouradian, R., Womersley, C., Crowe, L. M., and Crowe, J. H. (1985). Degradation of functional integrity during long-term storage of a freeze-dried biological membrane. Cryobiology 22, 119–127.
| Degradation of functional integrity during long-term storage of a freeze-dried biological membrane.Crossref | GoogleScholarGoogle Scholar | 3979081PubMed |
Muneto, N., and Horiuchi, T. (2011). Full-term development of hamster embryos produced by injecting freeze-dried spermatozoa into oocytes. J. Mamm. Ova Res. 28, 32–39.
| Full-term development of hamster embryos produced by injecting freeze-dried spermatozoa into oocytes.Crossref | GoogleScholarGoogle Scholar |
Natan, D., Nagler, A., and Arav, A. (2009). Freeze-drying of mononuclear cells derived from umbilical cord blood followed by colony formation. PLoS One 4, e5240.
| Freeze-drying of mononuclear cells derived from umbilical cord blood followed by colony formation.Crossref | GoogleScholarGoogle Scholar | 19381290PubMed |
Olaciregui, M., Luño, V., Domingo, P., González, N., and Gil, L. (2017). In vitro developmental ability of ovine oocytes following intracytoplasmic injection with freeze-dried spermatozoa. Sci. Rep. 7, 1096.
| In vitro developmental ability of ovine oocytes following intracytoplasmic injection with freeze-dried spermatozoa.Crossref | GoogleScholarGoogle Scholar | 28439073PubMed |
Oldenhof, H., Zhang, M., Narten, K., Bigalk, J., Sydykov, B., Wolkers, W. F., and Sieme, H. (2017). Freezing-induced uptake of disaccharides for preservation of chromatin in freeze-dried stallion sperm during accelerated aging. Biol. Reprod. 97, 892–901.
| Freezing-induced uptake of disaccharides for preservation of chromatin in freeze-dried stallion sperm during accelerated aging.Crossref | GoogleScholarGoogle Scholar | 29121172PubMed |
Ono, T., Mizutani, E., Li, C., and Wakayama, T. (2008). Nuclear transfer preserves the nuclear genome of freeze-dried mouse cells. J. Reprod. Dev. 54, 486–491.
| Nuclear transfer preserves the nuclear genome of freeze-dried mouse cells.Crossref | GoogleScholarGoogle Scholar | 18854641PubMed |
Ortuño-Costela, M. D. C., Cerrada, V., García-López, M., and Gallardo, M. E. (2019). The challenge of bringing iPSCs to the patient. Int. J. Mol. Sci. 20, 6305.
| The challenge of bringing iPSCs to the patient.Crossref | GoogleScholarGoogle Scholar |
Palazzese, L., Gosálvez, J., Anzalone, D. A., Loi, P., and Saragusty, J. (2018). DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development. J. Reprod. Dev. 64, 393–400.
| DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development.Crossref | GoogleScholarGoogle Scholar | 29973438PubMed |
Palazzese, L., Anzalone, D. A., Turri, F., Faieta, M., Donnadio, A., Pizzi, F., Matsukawa, K., and Loi, P. (2020). Whole genome integrity and enhanced developmental potential in ram freeze-dried spermatozoa at mild sub-zero temperature. Sci. Rep. 10, 18873.
| Whole genome integrity and enhanced developmental potential in ram freeze-dried spermatozoa at mild sub-zero temperature.Crossref | GoogleScholarGoogle Scholar | 33139842PubMed |
Patrick, J. L., Elliott, G. D., and Comizzoli, P. (2017). Structural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model. Theriogenology 103, 36–43.
| Structural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model.Crossref | GoogleScholarGoogle Scholar | 28772113PubMed |
Podhorecka, M., Skladanowski, A., and Bozko, P. (2010). H2AX phosphorylation: its role in DNA damage response and cancer therapy. J. Nucleic Acids 2010, 920161.
| H2AX phosphorylation: its role in DNA damage response and cancer therapy.Crossref | GoogleScholarGoogle Scholar | 20811597PubMed |
Polge, C., Smith, A., and Parkes, A. (1949). Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 164, 666.
| Revival of spermatozoa after vitrification and dehydration at low temperatures.Crossref | GoogleScholarGoogle Scholar | 18143360PubMed |
Poozesh, S., and Bilgili, E. (2019). Scale-up of pharmaceutical spray drying using scale-up rules: a review. Int. J. Pharm. 562, 271–292.
| Scale-up of pharmaceutical spray drying using scale-up rules: a review.Crossref | GoogleScholarGoogle Scholar | 30910632PubMed |
Restrepo, G., Varela, E., Duque, J. E., Gómez, J. E., and Rojas, M. (2019). Freezing, vitrification, and freeze-drying of equine spermatozoa: impact on mitochondrial membrane potential, lipid peroxidation, and DNA integrity. J. Equine Vet. Sci. 72, 8–15.
| Freezing, vitrification, and freeze-drying of equine spermatozoa: impact on mitochondrial membrane potential, lipid peroxidation, and DNA integrity.Crossref | GoogleScholarGoogle Scholar | 30929788PubMed |
Saitou, M., and Kurimoto, K. (2014). Paternal nucleosomes: are they retained in developmental promoters or gene deserts? Dev. Cell 30, 6–8.
| Paternal nucleosomes: are they retained in developmental promoters or gene deserts?Crossref | GoogleScholarGoogle Scholar | 25026032PubMed |
Samans, B., Yang, Y., Krebs, S., Sarode, G. V., Blum, H., Reichenbach, M., Wolf, E., Steger, K., Dansranjavin, T., and Schagdarsurengin, U. (2014). Uniformity of nucleosome preservation pattern in mammalian sperm and its connection to repetitive DNA elements. Dev. Cell 30, 23–35.
| Uniformity of nucleosome preservation pattern in mammalian sperm and its connection to repetitive DNA elements.Crossref | GoogleScholarGoogle Scholar | 24998597PubMed |
Saragusty, J., and Loi, P. (2019). Exploring dry storage as an alternative biobanking strategy inspired by nature. Theriogenology 126, 17–27.
| Exploring dry storage as an alternative biobanking strategy inspired by nature.Crossref | GoogleScholarGoogle Scholar | 30508788PubMed |
Saragusty, J., Anzalone, D. A., Palazzese, L., Arav, A., Patrizio, P., Gosálvez, J., and Loi, P. (2020). Dry biobanking as a conservation tool in the Anthropocene. Theriogenology 150, 130–138.
| Dry biobanking as a conservation tool in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 31980207PubMed |
Satpathy, G. R., Török, Z., Bali, R., Dwyre, D. M., Little, E., Walker, N. J., Tablin, F., Crowe, J. H., and Tsvetkova, N. M. (2004). Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology 49, 123–136.
| Loading red blood cells with trehalose: a step towards biostabilization.Crossref | GoogleScholarGoogle Scholar | 15351684PubMed |
Shih, M. D., Hoekstra, F. A., and Hsing, Y. I. C. (2008). Late embryogenesis abundant proteins. In ‘Advances in Botanical Research’, Vol. 48. (Eds K. Jean-Claude and D. Michel.) pp. 212–255. (Elsevier: Amsterdam.)
Sogame, Y., and Kikawada, T. (2017). Current findings on the molecular mechanisms underlying anhydrobiosis in Polypedilum vanderplanki. Curr. Opin. Insect Sci. 19, 16–21.
| Current findings on the molecular mechanisms underlying anhydrobiosis in Polypedilum vanderplanki.Crossref | GoogleScholarGoogle Scholar | 28521938PubMed |
Wakayama, T., and Yanagimachi, R. (1998). Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 16, 639–641.
| Development of normal mice from oocytes injected with freeze-dried spermatozoa.Crossref | GoogleScholarGoogle Scholar | 9661196PubMed |
Wakayama, S., Ito, D., Kamada, Y., Yonemura, S., Ooga, M., Kishigami, S., and Wakayama, T. (2019). Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from –196°C to 150°C. Sci. Rep. 9, 5719.
| Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from –196°C to 150°C.Crossref | GoogleScholarGoogle Scholar | 30952922PubMed |
Walters, R. H., Bhatnagar, B., Tchessalov, S., Izutsu, K. I., Tsumoto, K., and Ohtake, S. (2014). Next generation drying technologies for pharmaceutical applications. J. Pharm. Sci. 103, 2673–2695.
| Next generation drying technologies for pharmaceutical applications.Crossref | GoogleScholarGoogle Scholar | 24916125PubMed |
Wełnicz, W., Grohme, M. A., Kaczmarek, Ł., Schill, R. O., and Frohme, M. (2011). Anhydrobiosis in tardigrades – the last decade. J. Insect Physiol. 57, 577–583.
| Anhydrobiosis in tardigrades – the last decade.Crossref | GoogleScholarGoogle Scholar | 21440551PubMed |
Wolkers, W. F., Walker, N. J., Tablin, F., and Crowe, J. H. (2001). Human platelets loaded with trehalose survive freeze-drying. Cryobiology 42, 79–87.
| Human platelets loaded with trehalose survive freeze-drying.Crossref | GoogleScholarGoogle Scholar | 11448110PubMed |
Yamada, T. G., Hiki, Y., Hiroi, N. F., Shagimardanova, E., Gusev, O., Cornette, R., Kikawada, T., and Funahashi, A. (2020). Identification of a master transcription factor and a regulatory mechanism for desiccation tolerance in the anhydrobiotic cell line Pv11. PLoS One 15, e0230218.
| Identification of a master transcription factor and a regulatory mechanism for desiccation tolerance in the anhydrobiotic cell line Pv11.Crossref | GoogleScholarGoogle Scholar | 32191739PubMed |
Zhang, M., Oldenhof, H., Sieme, H., and Wolkers, W. F. (2016). Freezing-induced uptake of trehalose into mammalian cells facilitates cryopreservation. Biochim. Biophys. Acta 1858, 1400–1409.
| Freezing-induced uptake of trehalose into mammalian cells facilitates cryopreservation.Crossref | GoogleScholarGoogle Scholar | 27003129PubMed |
Zhang, M., Oldenhof, H., Sydykov, B., Bigalk, J., Sieme, H., and Wolkers, W. F. (2017). Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity. Sci. Rep. 7, 6198.
| Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity.Crossref | GoogleScholarGoogle Scholar | 28740099PubMed |
Zhao, C., and Ikeya, M. (2018). Generation and applications of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Int. 2018, 9601623.
| Generation and applications of induced pluripotent stem cell-derived mesenchymal stem cells.Crossref | GoogleScholarGoogle Scholar | 30405724PubMed |
Zhou, X., Yuan, J., Liu, J., and Liu, B. (2010). Loading trehalose into red blood cells by electroporation and its application in freeze-drying. Cryo Letters 31, 147–156.
| 20687457PubMed |