A comparison study of superovulation strategies for C57BL/6J and B6D2F1 mice in CRISPR-Cas9 mediated genome editing
Xue Zhao A # , Johnny X. Huang A B # * , Hailong Zhang A , Xueyang Gong A , Jinhua Dong A , Hong-Lin Ren C and Zengshan Liu A C
A B # * , Hailong Zhang A , Xueyang Gong A , Jinhua Dong A , Hong-Lin Ren C and Zengshan Liu A C
A School of Life Science and Technology, Weifang Medical University, Weifang, Shandong 261053, China.
B Institute for Molecular Bioscience, The University of Queensland, Brisbane, Qld 4072, Australia.
C Key Laboratory of Zoonosis Research, Ministry of Education, Institute of Zoonosis, College of Veterinary Medicine, Jilin University, Xi An Da Lu 5333, Changchun 130062, China.
Handling Editor: Xiaolong Wang
Reproduction, Fertility and Development 33(14) 772-781 https://doi.org/10.1071/RD21199
Published online: 9 November 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Reproductive techniques such as superovulation and in vitro fertilisation (IVF) have been widely used in generating genetically modified animals. The current gold standard for superovulation in mice is using coherent treatments of equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG). An alternative method using inhibin antiserum (IAS) instead of eCG has been recently reported. Here, we evaluate different superovulation strategies in C57BL/6J and B6D2F1 mice. Firstly, we found that using 5-week-old C57BL/6J and 4-week-old B6D2F1 donors could achieve better superovulation outcomes. Then, we compared eCG–hCG, IAS–hCG and eCG–IAS–hCG with different dosages in both mouse strains. Significantly increased numbers of oocytes were obtained by using IAS–hCG and eCG–IAS–hCG methods. However, low fertilisation rates (36.3–38.8%) were observed when natural mating was applied. We then confirmed that IVF could dramatically ameliorate the fertilisation rates up to 89.1%. Finally, we performed CRISPR-Cas9 mediated genome editing targeting Scn11a and Kcnh1 loci, and successfully obtained mutant pups using eCG–hCG and IAS–hCG induced zygotes, which were fertilised by either natural mating or IVF. Our results showed that IAS is a promising superovulation reagent, and the efficiency of genome editing is unlikely to be affected by using IAS-induced zygotes.
Keywords: assisted reproductive technology, CRISPR, embryo manipulation, fertilisation, genome editing, inhibin, in vitro fertilisation, superovulation.
Introduction
Superovulation, in vitro fertilisation (IVF) and embryo transfer are critical steps for assisted reproductive technologies (ARTs). These techniques were established in the mid-20th century and were expended for experimental purposes such as the production of transgenic mice to reduce the number of animals used (Gates 1956; Whittingham 1968; Nagy 2003). Many efforts have been made to optimise these ARTs over the past decades, and a few standards have been established for these techniques. For instance, a superovulation regime including an injection of equine chorionic gonadotropin (eCG) for stimulating follicular development and a consecutive injection of human chorionic gonadotropin (hCG) for ovulating induction has been considered as the gold standard in mice and other small experimental animals (Davis and Rosenwaks 2001; Nagy 2003; Behringer et al. 2018). However, different gonadotropin dosages may be used in different mouse strains to achieve better superovulation outcomes (Luo et al. 2011; Wu et al. 2013). The response to eCG and hCG treatment also varies among inbred and hybrid mouse strains (Byers et al. 2006). In addition, some factors such as age, body weight, and hormone injection time may also affect the efficiency of superovulation.
Superovulation may jeopardise the development of oocytes, resulting in abnormal blastocyst formation and fetal development (Ertzeid and Storeng 2001; Van der Auwera and D’Hooghe 2001). Nevertheless, it has been widely used for generating genetically modified (GM) animals due to the little effect it has on fertilisation and early cleavage. In recent years, CRISPR-Cas9 mediated generation of GM mice has been widely reported (Shen et al. 2013; Wang et al. 2013; Yang et al. 2013; Sander and Joung 2014). The common procedure is to deliver CRISPR-Cas9 system and single-stranded oligodeoxynucleotide (ssODN) carrying objective arrangements into mouse zygotes using pronuclear microinjection. Together with more and more genome editing tools emerging, advanced superovulation technologies have also been developed. For example, inhibin antiserum (IAS) has been shown to induce more efficient superovulation in mice and other species (Kishi et al. 1996; Ishigame et al. 2005; Takeo and Nakagata 2015, 2016; Hasegawa et al. 2016; Wuri et al. 2020). The antiserum neutralises inhibin, which is a nonsteroidal hormone that acts on pituitary cells to suppress follicle-stimulating hormone (FSH) production, resulting in increased FSH level and follicular development. Furthermore, it has been reported that a combination of IAS and eCG may yield nearly 100 oocytes from a single female C57BL/6 mouse (Takeo and Nakagata 2016).
In this study, we investigated many factors that may affect superovulation in C57BL/6J and B6D2F1 (a hybrid strain yield from natural mating of C57BL/6 and DBA/2J mice) mice, including age, body weight, injection time, and the dosage of eCG treatment. We further investigated the performance of IAS in the two mouse strains. Then, superovulated oocytes fertilised either by natural mating or IVF were used for generating GM mice by CRISPR-Cas9 technology, targeting sodium voltage-gated channel alpha subunit 11 gene (Scn11a) and potassium voltage-gated channel subfamily H member 1 gene (Kcnh1). Scn11a gene encodes NaV1.9, which is a subtype of the voltage-gated sodium channels contributed to sensory neuron excitability and pain signalling (Dib-Hajj et al. 2015). Kcnh1 gene encodes the voltage-gated KV10.1 potassium channel, which is predominantly expressed in the central nervous system. Kcnh1 mutations are highly related to genetic diseases in human (Simons et al. 2015; Fukai et al. 2016). Our results showed that IAS–hCG and eCG–IAS–hCG methods significantly increased the efficiency of superovulation in both mouse strains compared to traditional eCG–hCG method, and IVF was more reliable in increasing the fertilisation rates than natural mating. Furthermore, CRISPR-Cas9-based GM production using superovulated zygotes was successful. C57BL/6J mice with mutant alleles were obtained from eCG–hCG and IAS–hCG induced oocytes, which were fertilised by natural mating or IVF, and the birth and mutation rates were not affected by different superovulation approaches.
Materials and methods
Animals
C57BL/6J and B6D2F1 mouse strains were purchased from Australian BioResources (ABR). All the mice were housed in a specific-pathogen-free (SPF) facility under a 12-h dark–light cycle (light from 07:00 to 19:00) at 22 ± 1°C with food and water. All animal procedures were conducted at the University of Queensland and were approved by the Animal Care Committee at the University of Queensland Animal Ethics Committee (approval no. IMB/009/17). The National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council 2011) has been followed in this study.
Superovulation
Superovulation reagents eCG (brand name: Folligon) and hCG (brand name: Chorulon) were purchased from Intervet Inc. IAS was purchased from the Center for Animal Resources and Development Institute, Japan. Stock solutions (1000 IU/mL) of eCG and hCG were prepared using 0.9% sodium chloride and were kept at −20°C. They were diluted to desired concentrations using the same solvent before each injection. IAS was stored at −20°C and was used directly without dilution. According to the provider, the IAS had a titre of 1:1 000 000, which is the final dilution of the IAS required to bind 50% 125I-labelled bovine inhibin (32 kDa, 5000 c.p.m.) at 4°C for 24 h incubation (Wang et al. 2001; Takeo and Nakagata 2015, 2016).
In general, superovulation was performed by the administration of different dosages of eCG, IAS or a mixture of eCG and IAS into immature female mice (3–6 weeks old) intraperitoneally. The mice were then administered 5 IU hCG at 47 h after the injection of eCG or/and IAS. For natural mating, injected female mice were mated 1:1 to males overnight. Male mice for breeding were maintained in individual cages in age from 3 to 7 months. In order to obviate any day-to-day variation, we only used proven stud males for breeding and kept the interval between each mating for a minimum of 7 days. The female mice were euthanised by cervical dislocation on the second morning at 09:00 after the administration of hCG. The oviducts were quickly collected and cumulus–oocyte complexes were isolated under the microscope. In the case of natural mating, the harvested oocytes were cultured overnight in EmbryoMax Advanced KSOM medium (Merck Millipore, MR-101-D) at 37°C under 5% CO2 overnight to the 2-cell stage and the fertilisation rate was calculated using the following equation:
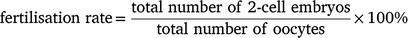
Age and weight analysis
Initial experiments involved determination of the age and weight range of female donors for C57BL/6J and B6D2F1 strains that would achieve the best superovulation results. Immature female donors at 3, 4, 5 and 6 weeks old were chosen for evaluation. In the first experiment, three female mice of each age group were injected with 5 IU eCG intraperitoneally, and then were administered 5 IU hCG 47 h later. Injected female mice were mated 1:1 to the proven stud males for natural mating overnight, and the oocytes were collected as described in the previous section. The same experiment was then repeated twice, with three and four donor mice of each age group used in the second and third experiments respectively. Eventually a total number of 10 donor mice from each age group were used. Further experiments were performed to test different dosages of eCG (1.25 IU, 2.5 IU, 5 IU, 10 IU and 20 IU) using 5-week-old C57BL/6J donors (n = 4 for each dosage) and 4-week-old B6D2F1 donors (n = 4 for each dosage) at the same time.
Hormone injection timing analysis
The injection interval of eCG and hCG was set at 47 h. Five-week-old C57BL/6J donors were randomly divided into three groups (n = 10 per group). The first group was administered eCG at 13:00 on the first day, followed by hCG injections at 12:00 on the third day. The second group was injected eCG at 15:00 on the first day, followed by hCG injections at 14:00 on the third day. The third group had eCG injections at 17:00 on the first day and hCG injections at 16:00 on the third day. After hCG injections, female mice were set to 1:1 mating with males overnight and all oocytes were harvested as described in the previous section on the fourth day at 9:00. Further experiments were carried out using 4-week-old B6D2F1 donors on the same days with the same experimental design.
Comparison of different superovulation strategies
Five superovulation strategies were tested, with the administration of different dosages of eCG, IAS or a mixture of eCG and IAS into 5-week-old C57BL/6J donors and 4-week-old B6D2F1 donors. The first strategy was the administration of 5 IU eCG into C57BL/6J (n = 10) and B6D2F1 (n = 10) donors. The second, third and fourth strategies were the administration of 50, 100 and 200 μL IAS into C57BL/6J (n = 5, 10 and 5) and B6D2F1 (n = 5, 5 and 5) donors. The fifth strategy was the administration of a mixture of 2.5 IU eCG and 100 μL IAS into C57BL/6J (n = 5) and B6D2F1 (n = 5) donors. All the female mice were then administered 5 IU hCG at 47 h after the injection of eCG or/and IAS. Injected female mice were mated 1:1 to males overnight and all oocytes were harvested as described in the previous section. The above experiment was performed at the same time.
IVF
Superovulation was performed using 5-week-old C57BL/6J donors and 4-week-old B6D2F1 donors. They were injected with 5 IU eCG (n = 10 for each strain) or 100 μL IAS (n = 5 for each strain), followed by 5 IU hCG after 47 h. Oocytes were then collected for IVF on the second day of hCG injections. Two male mice (12 weeks old) from each strain were euthanised by cervical dislocation. The cauda epididymides were collected and pre-incubated in a modified Krebs-Ringer Bicarbonate solution (Cat# 4002, Sigma–Aldrich) medium with 1.0 mg/mL polyvinyl alcohol (Cat# P8136, Sigma–Aldrich) and 0.75 mM methyl-β-cyclodextrin (Cat# C4555, Sigma–Aldrich) covered with light mineral oil (Cat# M8410, Sigma–Aldrich) at 37°C for 1 h to induce capacitation. Fertilisation was performed by adding 400–800 sperm/μL into EmbryoMax human tubal fluid (Cat# MR-070, Sigma–Aldrich) containing cumulus–oocyte complexes and cultured at 37°C, 5% CO2 for 3 h. The above experiment was performed at the same time. Fertilisation rate was calculated at 24 h after insemination using the following formula:
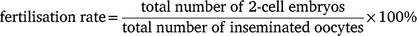
Production of genetically modified mice
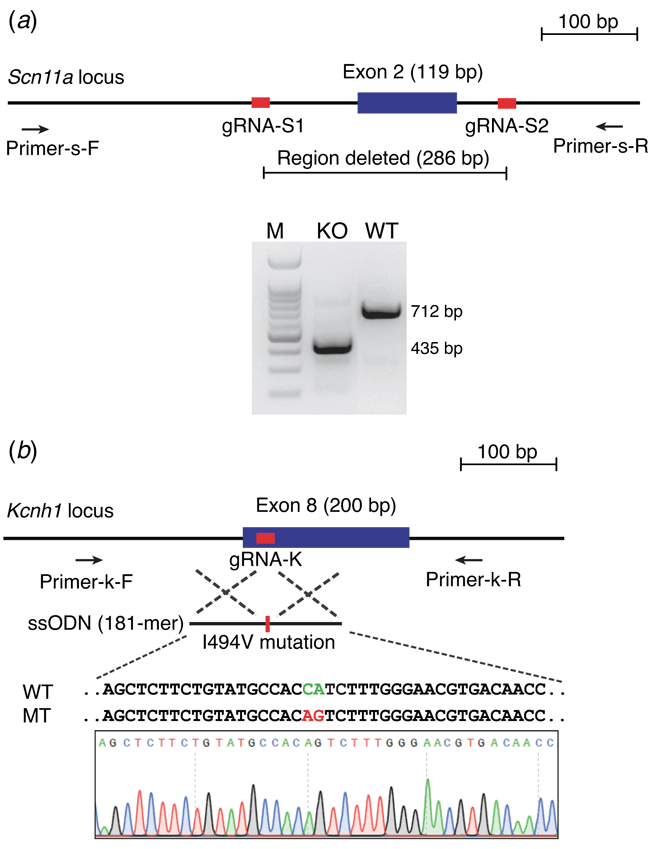
Guide RNAs (gRNAs) targeting Scn11a and Kcnh1 loci were selected using the online CRISPR design tool CRISPOR (Haeussler et al. 2016; http://crispor.tefor.net/). A pair of gRNAs was employed to delete exon 2 of Scn11a gene, while one gRNA and one 181-bp ssODN were used to target Kcnh1 gene and introduce c. 1480A > G (p.I494V) mutation. Polymerase chain reaction (PCR) primers and ssODN sequence used in this study are shown in Supplementary Table S1.
Genetically modified mice were produced using a previously described method (Yang et al. 2014). In brief, superovulation was performed as previously described using either eCG (5 IU) or IAS (100 μL) followed by 5 IU hCG injection. Cas9 protein, tracrRNA, crRNA and ssODN were purchased from Integrated DNA Technologies, Singapore. To prepare the Cas9 ribonucleoprotein (RNP), crRNA and tracrRNA were annealed to form an RNA duplex (gRNA), which was subsequently mixed with Cas9 protein, followed by incubation at 37°C for 15 min. For microinjection, the Cas9 RNP containing Cas9 protein (30 ng/μL), gRNA (10 ng/μL each) and ssODN (10 ng/μL) was injected into the pronuclei of zygotes. The injected zygotes were cultured overnight in EmbryoMax Advanced KSOM medium (Merck Millipore, MR-101-D) at 37°C under 5% CO2 to the 2-cell stage. Then, 25–31 2-cell-stage embryos were transferred to the oviducts of pseudopregnant CD1 females (n = 8) at 0.5 days post coitum. After natural delivery, pups with the desired mutant alleles were identified using PCR and Sanger sequencing (Sanger and Coulson 1975). The above experiments were repeated using the same design and a total number of 32 C57BL/6 donors and 16 CD1 foster mice were used. Birth and mutation rates were calculated using the following formulas:

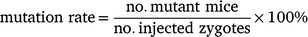
Statistics
Statistical analysis was performed using Prism 6.0 (GraphPad) software. Results are expressed as the mean or the mean ± standard deviation (s.d.). Data was analysed using two-way ANOVA test. P < 0.05 was considered statistically significant.
Results
Determination of age and weight ranges of female mice
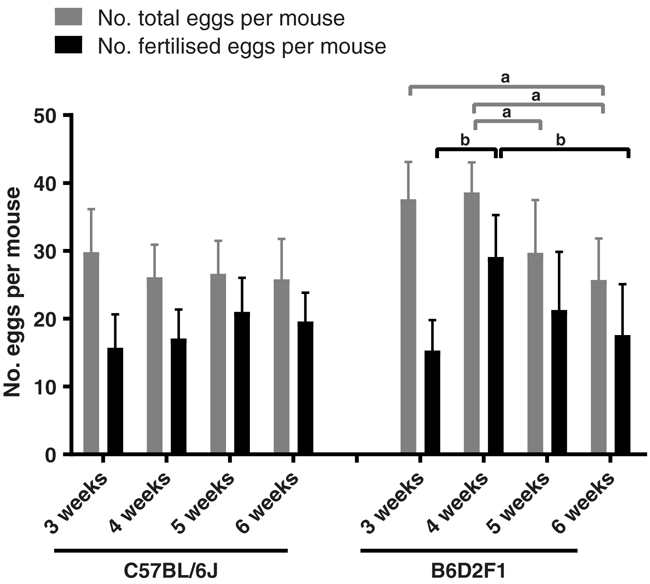
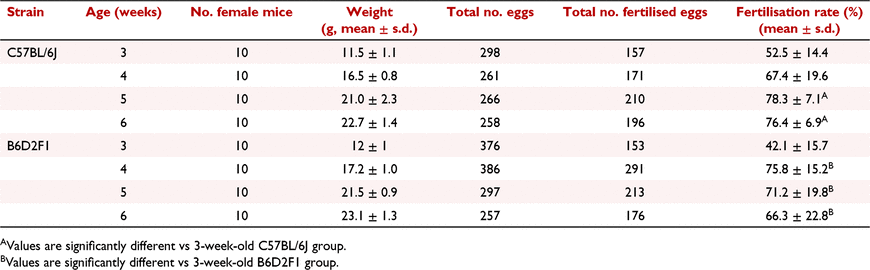
We first designed experiments to determine the age and weight ranges of female mice used for superovulation experiments. Many transgenic facilities use a specific age range (generally 3–6 weeks old) rather than the weight range when choosing the female donors. Thus, we set up our analysis using 3-, 4-, 5- and 6-week-old females of each strain and assessed the weight of each mouse at different ages (Table 1). The average weight of 3-week-old C57BL/6J mice was 11.5 g, and it rapidly increased to 21 g at week 5. The body weights of the 6-week-old mice were similar to those of the 5-week-old ones. In the case of the hybrid line B6D2F1, the body weights showed similar increasing pattern to the C57BL/6J mice, having the average weights of 12 g and 23.1 g at week 3 and week 6 respectively.
|
The superovulation was performed using a single dose of 5 IU eCG, followed by an injection of 5 IU hCG at 47 h. After the hCG injection, the females were mated 1:1 to fertility-proved male mice immediately. We then analysed the superovulation results of each age group (Table 1 and Fig. 1). The total numbers of oocytes harvested from superovulation were similar, with no significant difference among four age groups of C57BL/6J mice (Fig. 1, n = 10 per group). However, we observed that the mean fertilisation rates of 5–6-week-old mice were significantly higher than those of 3- and 4-week-old donors (P < 0.01, Table 1). In addition, 5-week-old C57BL/6J female mice could achieve slightly higher numbers of oocytes and better fertilisation rates than 6-week-old donors. Thus, we decided to use 5-week-old C57BL/6J donors for the subsequent experiments. In the case of B6D2F1 strain, more oocytes were obtained using the same superovulation method than the C57BL/6J strain. Total numbers of 376 and 386 oocytes were harvested from 3- and 4-week-old B6D2F1 donors (n = 10 per group) respectively, whereas the numbers decreased dramatically in the 5- and 6-week-old groups (Table 1). In addition, given the highest average fertilisation rate of 75.8%, 4-week-old B6D2F1 donors showed the best performance in superovulation compared to other groups (Table 1 and Fig. 1). Based on the above results, we used 4-week-old B6D2F1 donors in the experiments performed subsequently. Furthermore, we performed the experiments by using a dosage range of eCG for comparison. Superovulation was induced by injecting different dosages of eCG (1.25 IU, 2.5 IU, 5 IU, 10 IU and 20 IU) into the C57BL/6J and B6D2F1 mice. The results showed that both strains appeared to have better response to 5 IU eCG administration (Supplementary Table S2).
Hormone injection timing
Although the interval between eCG and hCG injections is commonly set at 47–49 h, the exact timing for injections varies from different facilities. To evaluate the effect of injection time point on superovulation, we set the injection interval at 47 h and tested three time points for eCG (13:00, 15:00 and 17:00) and hCG (12:00, 14:00 and 16:00) injections in C57BL/6J and B6D2F1 strains (Table 2). After hCG injections, female mice were set to 1:1 mating with males overnight and all oocytes were harvested on day 4 at 09:00. Our results showed that neither total oocyte numbers nor total fertilised egg numbers were affected by different injection time points.
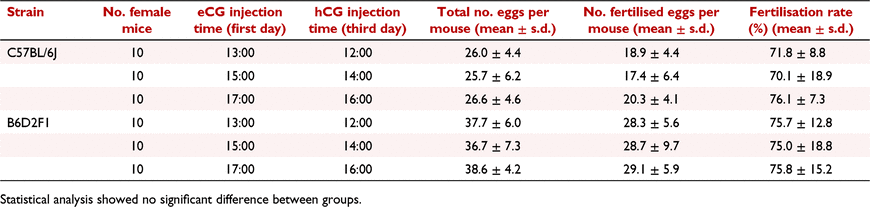
|
The comparison of eCG and IAS
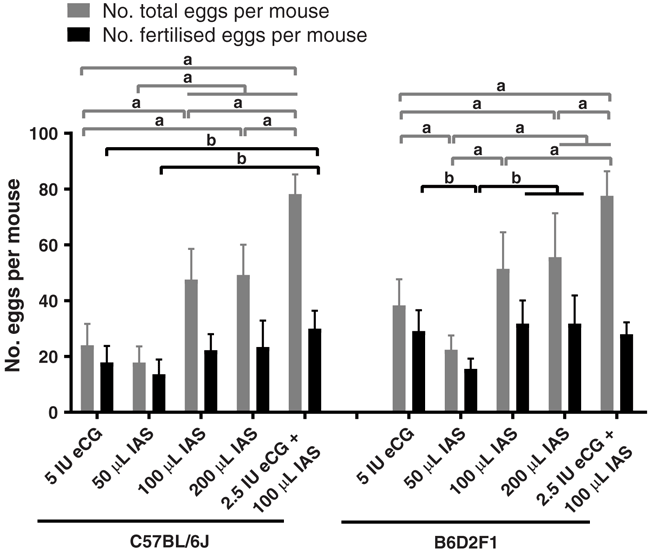
In order to achieve more zygotes using less numbers of animal donors, we evaluated the effect of different doses of eCG and IAS. We compared five types of administration, including 5 IU eCG, 50 μL IAS, 100 μL IAS, 200 μL IAS and a mixture of 2.5 IU eCG and 100 μL IAS in both C57BL/6J and B6D2F1 strains (Table 3). 50 μL IAS treatment gave similar superovulation results to 5 IU eCG in C57BL/6J female mice. However, the numbers of oocytes induced by 100 μL IAS were approximately two-fold higher than that induced by 5 IU eCG, and they were further increased by the combined injection of 2.5 IU eCG and 100 μL IAS (Table 3 and Fig. 2). In the case of the B6D2F1 strain, the superovulation results were slightly different to the C57BL/6J strain (Fig. 2). B6D2F1 females showed less sensitivity to IAS administration alone compared to C57BL/6J mice. Low amount IAS injections (50 μL) in B6D2F1 female mice showed less efficiency than 5 IU eCG administration (P < 0.05). The 100 μL IAS injection increased the number of oocytes, but it was not statistically significant. Although the 200 μL IAS injection gave greater numbers of oocytes compared to 5 IU eCG treatment (P < 0.05), it did not double the numbers (Fig. 2). Nevertheless, the combined injection of 2.5 IU eCG and 100 μL IAS achieved the highest numbers of oocytes in B6D2F1 mice, indicating the synergy effect of eCG and IAS. Intriguingly, we observed that in both C57BL/6J and B6D2F1 mice, the number of fertilised eggs obtained after natural mating did not correlate with the increasing number of oocytes, resulting in significantly low fertilisation rates in IAS (100 μL and 200 μL) and eCG–IAS combination treated groups (Fig. 2 and Table 3).
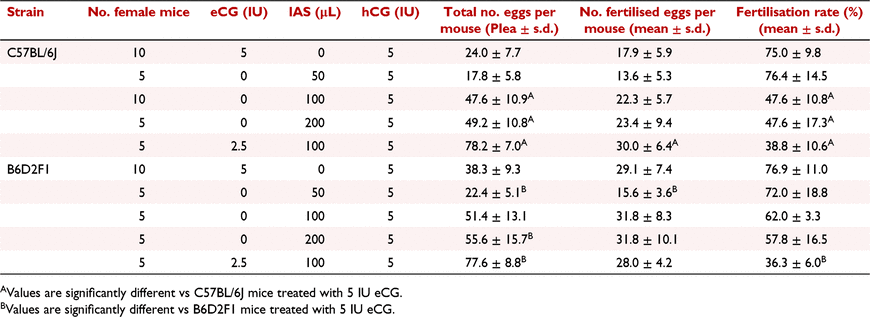
|
IVF vs natural mating
We noticed that the fertilisation rates were significantly reduced when natural mating was applied in both mouse strains. This could be due to the physical limitation of sperm activity in an in vivo environment. Thus, we performed IVF to fertilise eCG and IAS induced oocytes. The fertilisation rates of IVF were approximately 84.5–89.1%, regardless of mouse strains and the types of superovulation hormone used (Table 4), indicating better efficiency of IVF compared to natural mating.
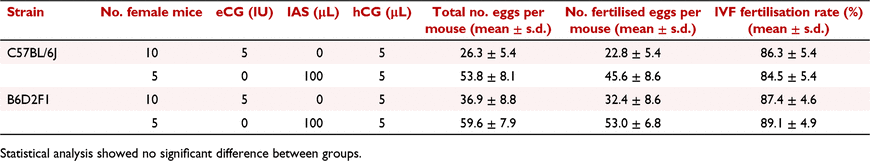
|
CRISPR-Cas9 genome editing using superovulated zygotes
Next, we evaluated superovulated C57BL/6J zygotes obtained from 5 IU eCG–5 IU hCG and 100 μL IAS–5 IU hCG strategies and fertilised by either IVF or natural mating for CRISPR-Cas9 genome editing, targeting and Scn11a and Kcnh1 loci. Targeting strategies and results were shown in Fig. 3 and Table 5. A total number of 577 zygotes obtained from different approaches were injected, and 453 2-cell embryos were transferred to 16 CD1 foster mothers. Thirteen mutant pups were obtained from the oocytes generated by eCG–hCG treatment, including six Scn11a knockout mice and seven Kcnh1 mutant mice, among which four mice were carrying correct Kcnh1 I494V mutant alleles. Eleven mutant pups (five Scn11a knockouts and six Kcnh1 mutants) were obtained from the oocytes created by IAS–hCG method, including two mice carrying precise Kcnh1 I494V mutation (Table 5).
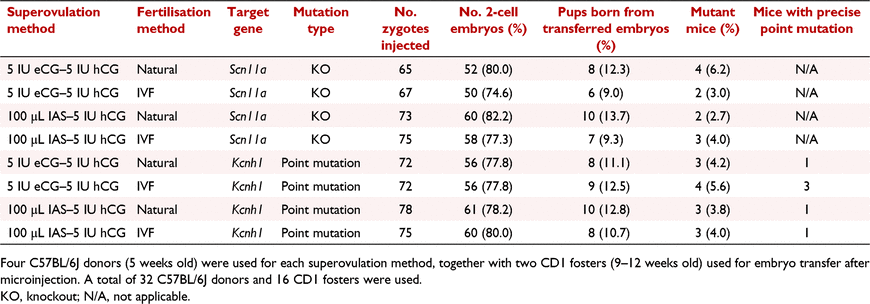
|
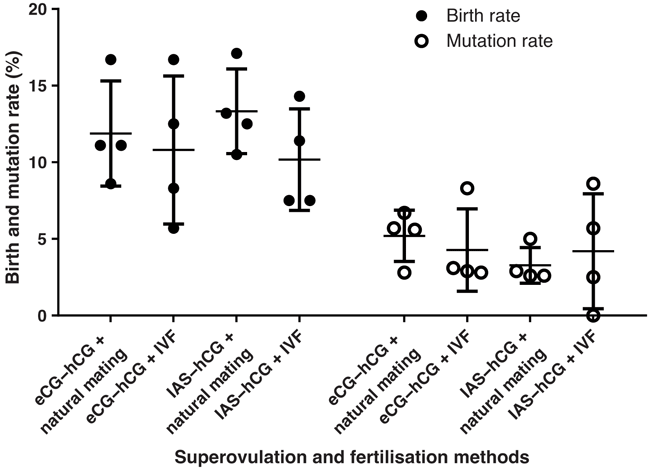
The average birth rate of oocytes obtained from eCG–hCG and natural mating was slightly higher than those from eCG–hCG with IVF method; however, they were not statistically significant (Fig. 4). The same trend was observed in the case of using IAS–hCG as the superovulation strategy (Fig. 4). In addition, although the average mutation rate of CRISPR-Cas9 mediated genome editing using zygotes generated by IVF was slightly lower than those obtained from natural mating while using eCG–hCG for superovulation, it was not statistically significant (Fig. 4). In the case of IAS–hCG induced groups, the average mutation rate of genome editing using IVF fertilised zygotes was slightly higher than those obtained from natural mating; however, it was not significant either (Fig. 4).
Discussion
Superovulation and IVF are important techniques for manipulating laboratory animals, especially in generating GM mouse strains. In the present study, we performed a comprehensive study investigating different superovulation methods and the factors that may affect the efficiency. We further compared the results of using zygotes obtained from different methods in generating GM mice by CRISPR-Cas9 strategy.
Previous studies have showed that factors such as mouse strain, age, body weight, hormone dosage and injection time may affect the results of superovulation (Davis and Rosenwaks 2001; Byers et al. 2006; Luo et al. 2011). We systematically investigated those factors using two commonly used strains in our laboratory, C57BL/6J and B6D2F1. We only used immature female mice (3–6 weeks old) for superovulation because adult females are less or nonresponsive to hormone treatment (Gates 1956; Luo et al. 2011). Our results showed that B6D2F1 mice are more responsive to eCG treatment than C57BL/6J mice, generating an average of 38.6 eggs per mouse in 4-week-old donors (Table 1). We also noticed that age and body weight are crucial to obtain better ovulation results in B6D2F1 mice, and most importantly, they may affect the fertilisation rates when using natural mating as the approach (Fig. 1). In B6D2F1 mice, the number of eCG–hCG superovulated oocytes decreased with the age and body weight, indicating the follicles become increasingly nonresponsive to hormone stimulation. The highest fertilisation rates were observed at 5 weeks in C57BL/6 mice and 4 weeks in B6D2F1 mice. Because the male mice used in the study were optimised for performance to exclude their influence on the number of fertilised oocytes, we considered the superovulation is female receptivity dependent and decided to use females at those ages with similar body weights for the experiments. Furthermore, we found that the injection time of hormones is unlikely to affect the superovulation results, provided the injection interval between eCG and hCG was kept at 47 h.
In recently years, many reports have shown that a newly developed superovulation reagent, IAS, can significantly increase the efficiency of superovulation in mice and other mammals (Kishi et al. 1996; Ishigame et al. 2005; Takeo and Nakagata 2015; Hasegawa et al. 2016; Mochida 2020). The method has also been used in generating various GM mouse models using CRISPR-Cas9 strategy (Nakagawa et al. 2016). In the present study, we evaluated the performance of IAS in the two mouse strains. We found IAS can significantly increase the maturation of follicle in both strains, especially in C57BL/6 mice by doubling the numbers of oocytes compared to eCG treatment. We also noticed that 100 μL of IAS was sufficient to achieve the peak of follicle maturation. Increasing the dose of IAS to 200 μL did not seem to further increase superovulation efficiency. However, the combined injection of 2.5 IU eCG and 100 IAS μL followed by hCG could further stimulate follicle maturation, giving a superior superovulation result of approximately 80 oocytes per mouse in both strains. Nevertheless, we noticed that the number of fertilised eggs was unlikely to be significantly affected by any superovulation methods while using natural mating for fertilisation (Table 3), resulting in very low fertilisation rates in IAS involved strategies. This may be due to the limitation of male reproductive ability. We found that IVF could achieve more fertilised oocytes with an average fertilisation rate of 84.5–89.1%, regardless of the mouse strain and the type of hormone used (Table 4). In addition, we noticed that the activity of IAS could be significantly reduced by a few freeze–thaw cycles (data not shown), which are not recommended while using IAS.
Some facilities may prefer natural mating in generating fertilised oocytes, while IVF could be an alternative option for some other facilities. There are pros and cons for each method. Natural mating does not need to sacrifice male mice, but IVF seems to be more stable in producing sufficient numbers of zygotes for microinjection. Previous studies have investigated the quality of IAS induced oocytes, which showed similar characterisation compared to normal oocytes (Wuri et al. 2020). We compared the outcomes of using superovulated zygotes either from natural mating or IVF for producing GM mice by CRISPR-Cas9 technology. C57BL/6J mice were administrated with eCG–hCG or IAS–hCG and the fertilised oocytes were generated by either mating or IVF. The two injection experiments we performed successfully produced Scn11a knockout and Kcnh1 I494V mice using both approaches. However, in the first injection experiment we were unable to obtain the desired GM mouse from IAS–hCG induced oocytes that were fertilised by IVF (Fig. 4). This could be a common occurrence as the surrogate mice may affect the birth rate. We repeated the injection experiment and successfully generated Kcnh1 I494V mice using IAS–hCG induced and IVF fertilised zygotes. Nevertheless, it is still uncertain whether he impact of an ultra-superovulation using IAS could influence early embryo development in mice. Recent studies have shown that superovulation could alter global DNA methylation in early mouse embryo development, which may result in the introduction of DNA methylation errors (Marshall and Rivera 2018; Tang et al. 2019; Yu et al. 2019). It is also reported that higher genome and Grb10 (an imprinted gene) methylation, and variable mRNA expression were observed during superovulation, together with reduced blastocyst cell numbers (Chen et al. 2018).
In summary, we have investigated a set of important factors that may affect superovulation in C57BL/6J and B6D2F1 mice. Novel superovulation methods such as IAS–hCG and eCG–IAS–hCG have been evaluated and GM mice were successfully generated using zygotes obtained from natural mating and IVF. Our results showed that IAS could be used as a reliable reagent for superovulation when generating GM mice using CRISPR-Cas9 technology. The data presented in the study could be a valuable guide for researchers who are interested in establishing GM mice producing facilities.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
JXH is supported by Weifang Medical University initial core funding (021862). ZL is supported by Shandong Provincial Natural Science Foundation (ZR2019MH103).
Author contributions
JXH conceived and designed the study. XZ and JXH performed the experiments. JXH, XZ, HZ and XG analysed the data. JXH, JD, HLR and ZL prepared the manuscript.
Acknowledgements
The authors thank Professor Peter Koopman and Dr. Liang Zhao (The University of Queensland) for discussions and advice. They also thank the Queensland Facility for Advanced Genome Editing (QFAGE) for technical support.
References
Behringer, R, Gertsenstein, M, Nagy, KV, and Nagy, A (2018). Administration of gonadotropins for superovulation in mice. Cold Spring Harbor Protocols 2018, 24–27.| Administration of gonadotropins for superovulation in mice.Crossref | GoogleScholarGoogle Scholar |
Byers, SL, Payson, SJ, and Taft, RA (2006). Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 65, 1716–1726.
| Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs).Crossref | GoogleScholarGoogle Scholar | 16271754PubMed |
Chen, X, Huang, Y, Huang, H, Guan, Y, Li, M, Jiang, X, Yu, M, and Yang, X (2018). Effects of superovulation, in vitro fertilization, and oocyte in vitro maturation on imprinted gene Grb10 in mouse blastocysts. Archives of Gynecology and Obstetrics 298, 1219–1227.
| Effects of superovulation, in vitro fertilization, and oocyte in vitro maturation on imprinted gene Grb10 in mouse blastocysts.Crossref | GoogleScholarGoogle Scholar | 30251157PubMed |
Davis, OK, and Rosenwaks, Z (2001). Superovulation strategies for assisted reproductive technologies. Seminars in Reproductive Medicine 19, 207–212.
| Superovulation strategies for assisted reproductive technologies.Crossref | GoogleScholarGoogle Scholar | 11679901PubMed |
Dib-Hajj, SD, Black, JA, and Waxman, SG (2015). NaV1.9: a sodium channel linked to human pain. Nature Reviews Neuroscience 16, 511–519.
| NaV1.9: a sodium channel linked to human pain.Crossref | GoogleScholarGoogle Scholar | 26243570PubMed |
Ertzeid, G, and Storeng, R (2001). The impact of ovarian stimulation on implantation and fetal development in mice. Human Reproduction 16, 221–225.
| The impact of ovarian stimulation on implantation and fetal development in mice.Crossref | GoogleScholarGoogle Scholar | 11157810PubMed |
Fukai, R, Saitsu, H, Tsurusaki, Y, Sakai, Y, Haginoya, K, Takahashi, K, Hubshman, MW, Okamoto, N, Nakashima, M, Tanaka, F, Miyake, N, and Matsumoto, N (2016). De novoKCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures. Journal of Human Genetics 61, 381–387.
| De novoKCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures.Crossref | GoogleScholarGoogle Scholar | 26818738PubMed |
Gates, AH (1956). Viability and developmental capacity of eggs from immature mice treated with gonadotrophins. Nature 177, 754–755.
| Viability and developmental capacity of eggs from immature mice treated with gonadotrophins.Crossref | GoogleScholarGoogle Scholar | 13321955PubMed |
Haeussler, M, Schönig, K, Eckert, H, Eschstruth, A, Mianne, J, Renaud, JB, Schneider-Maunoury, S, Shkumatava, A, Teboul, L, Kent, J, Joly, JS, and Concordet, JP (2016). Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biology 17, 148.
| Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR.Crossref | GoogleScholarGoogle Scholar | 27380939PubMed |
Hasegawa, A, Mochida, K, Inoue, H, Noda, Y, Endo, T, Watanabe, G, and Ogura, A (2016). High-yield superovulation in adult mice by anti-inhibin serum treatment combined with estrous cycle synchronization. Biology of Reproduction 94, 21.
| High-yield superovulation in adult mice by anti-inhibin serum treatment combined with estrous cycle synchronization.Crossref | GoogleScholarGoogle Scholar | 26632610PubMed |
Ishigame, H, Medan, MS, Kawaguchi, M, Fukuda, A, Watanabe, G, Arai, KY, and Taya, K (2005). Induction of superovulation by immunoneutralization of endogenous inhibin in immature rats. Journal of Reproduction and Development 51, 559–566.
| Induction of superovulation by immunoneutralization of endogenous inhibin in immature rats.Crossref | GoogleScholarGoogle Scholar |
Kishi, H, Okada, T, Otsuka, M, Watanabe, G, Taya, K, and Sasamoto, S (1996). Induction of superovulation by immunoneutralization of endogenous inhibin through the increase in the secretion of follicle-stimulating hormone in the cyclic golden hamster. Journal of Endocrinology 151, 65–75.
| Induction of superovulation by immunoneutralization of endogenous inhibin through the increase in the secretion of follicle-stimulating hormone in the cyclic golden hamster.Crossref | GoogleScholarGoogle Scholar |
Luo, C, Zuñiga, J, Edison, E, Palla, S, Dong, W, and Parker-Thornburg, J (2011). Superovulation strategies for 6 commonly used mouse strains. Journal of the American Association for Laboratory Animal Science 50, 471–478.
| 21838974PubMed |
Marshall, KL, and Rivera, RM (2018). The effects of superovulation and reproductive aging on the epigenome of the oocyte and embryo. Molecular Reproduction and Development 85, 90–105.
| The effects of superovulation and reproductive aging on the epigenome of the oocyte and embryo.Crossref | GoogleScholarGoogle Scholar | 29280527PubMed |
Mochida, K (2020). Development of assisted reproductive technologies in small animal species for their efficient preservation and production. Journal of Reproduction and Development 66, 299–306.
| Development of assisted reproductive technologies in small animal species for their efficient preservation and production.Crossref | GoogleScholarGoogle Scholar |
Nagy A (2003) ‘Manipulating the mouse embryo: a laboratory manual’. 3rd edn. (Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA)
Nakagawa, Y, Sakuma, T, Nishimichi, N, Yokosaki, Y, Yanaka, N, Takeo, T, Nakagata, N, and Yamamoto, T (2016). Ultra-superovulation for the CRISPR-Cas9-mediated production of gene-knockout, single-amino-acid-substituted, and floxed mice. Biology Open 5, 1142–1148.
| Ultra-superovulation for the CRISPR-Cas9-mediated production of gene-knockout, single-amino-acid-substituted, and floxed mice.Crossref | GoogleScholarGoogle Scholar | 27387532PubMed |
National Research Council 2011) ‘Guide for the care and use of laboratory animals’. 8th edn. (National Academies Press: Washington, DC) Available at
| Crossref |
Sander, JD, and Joung, JK (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology 32, 347–355.
| CRISPR-Cas systems for editing, regulating and targeting genomes.Crossref | GoogleScholarGoogle Scholar | 24584096PubMed |
Sanger, F, and Coulson, AR (1975). A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. Journal of Molecular Biology 94, 441–446.
| A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase.Crossref | GoogleScholarGoogle Scholar | 1100841PubMed |
Shen, B, Zhang, J, Wu, H, Wang, J, Ma, K, Li, Z, Zhang, X, Zhang, P, and Huang, X (2013). Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Research 23, 720–723.
| Generation of gene-modified mice via Cas9/RNA-mediated gene targeting.Crossref | GoogleScholarGoogle Scholar | 23545779PubMed |
Simons, C, Rash, LD, Crawford, J, Ma, L, Cristofori-Armstrong, B, Miller, D, Ru, K, Baillie, GJ, Alanay, Y, Jacquinet, A, Debray, F-G, Verloes, A, Shen, J, Yesil, G, Guler, S, Yuksel, A, Cleary, JG, Grimmond, SM, McGaughran, J, King, GF, Gabbett, MT, and Taft, RJ (2015). Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nature Genetics 47, 73–77.
| Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy.Crossref | GoogleScholarGoogle Scholar | 25420144PubMed |
Takeo, T, and Nakagata, N (2015). Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 10, e0128330.
| Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice.Crossref | GoogleScholarGoogle Scholar | 26024317PubMed |
Takeo, T, and Nakagata, N (2016). Immunotherapy using inhibin antiserum enhanced the efficacy of equine chorionic gonadotropin on superovulation in major inbred and outbred mice strains. Theriogenology 86, 1341–1346.
| Immunotherapy using inhibin antiserum enhanced the efficacy of equine chorionic gonadotropin on superovulation in major inbred and outbred mice strains.Crossref | GoogleScholarGoogle Scholar | 27242176PubMed |
Tang, SB, Yang, LL, Zhang, TT, Wang, Q, Yin, S, Luo, SM, Shen, W, Ge, ZJ, and Sun, QY (2019). Multiple superovulations alter histone modifications in mouse early embryos. Reproduction 157, 511–523.
| Multiple superovulations alter histone modifications in mouse early embryos.Crossref | GoogleScholarGoogle Scholar | 30884466PubMed |
Van der Auwera, I, and D’Hooghe, T (2001). Superovulation of female mice delays embryonic and fetal development. Human Reproduction 16, 1237–1243.
| Superovulation of female mice delays embryonic and fetal development.Crossref | GoogleScholarGoogle Scholar | 11387298PubMed |
Wang, H, Herath, CB, Xia, G, Watanabe, G, and Taya, K (2001). Superovulation, fertilization and in vitro embryo development in mice after administration of an inhibin-neutralizing antiserum. Reproduction 122, 809–816.
| Superovulation, fertilization and in vitro embryo development in mice after administration of an inhibin-neutralizing antiserum.Crossref | GoogleScholarGoogle Scholar | 11690542PubMed |
Wang, H, Yang, H, Shivalila, CS, Dawlaty, MM, Cheng, AW, Zhang, F, and Jaenisch, R (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918.
| One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering.Crossref | GoogleScholarGoogle Scholar | 23643243PubMed |
Whittingham, DG (1968). Fertilization of mouse eggs in vitro. Nature 220, 592–593.
| Fertilization of mouse eggs in vitro.Crossref | GoogleScholarGoogle Scholar | 5686738PubMed |
Wu, BJ, Xue, HY, Chen, LP, Dai, YF, Guo, JT, and Li, XH (2013). Effect of PMSG/hCG superovulation on mouse embryonic development. Journal of Integrative Agriculture 12, 1066–1072.
| Effect of PMSG/hCG superovulation on mouse embryonic development.Crossref | GoogleScholarGoogle Scholar |
Wuri, L, Agca, C, and Agca, Y (2020). Morphometric, subcellular, in vitro fertilisation and embryonic developmental assessment of mouse oocytes produced by anti-inhibin serum or pregnant mare serum gonadotrophin superovulation. Reproduction, Fertility and Development 32, 474–483.
| Morphometric, subcellular, in vitro fertilisation and embryonic developmental assessment of mouse oocytes produced by anti-inhibin serum or pregnant mare serum gonadotrophin superovulation.Crossref | GoogleScholarGoogle Scholar |
Yang, H, Wang, H, and Jaenisch, R (2014). Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nature Protocols 9, 1956–1968.
| Generating genetically modified mice using CRISPR/Cas-mediated genome engineering.Crossref | GoogleScholarGoogle Scholar | 25058643PubMed |
Yang, H, Wang, H, Shivalila, CS, Cheng, AW, Shi, L, and Jaenisch, R (2013). One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379.
| One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering.Crossref | GoogleScholarGoogle Scholar | 23992847PubMed |
Yu, B, Smith, TH, Battle, SL, Ferrell, S, and Hawkins, RD (2019). Superovulation alters global DNA methylation in early mouse embryo development. Epigenetics 14, 780–790.
| Superovulation alters global DNA methylation in early mouse embryo development.Crossref | GoogleScholarGoogle Scholar | 31060426PubMed |