The melatonin system is expressed in the ovine uterus: effect of the day of the oestrous cycle and undernutrition
C. Sosa A * , E. Laurenzana A , V. de Brun B , A. Meikle B and J. A. Abecia C
A * , E. Laurenzana A , V. de Brun B , A. Meikle B and J. A. Abecia C
A Departamento de Bioquímica y Biología Molecular y Celular, Universidad de Zaragoza, Facultad de Veterinaria, Zaragoza, Spain.
B Laboratorio de Endocrinología y Metabolismo Animal, Facultad de Veterinaria, UdelaR, Montevideo, Uruguay.
C Instituto de Investigación en Ciencias Ambientales (IUCA), Universidad de Zaragoza, Facultad de Veterinaria, Zaragoza, Spain.
Reproduction, Fertility and Development 35(11) 563-574 https://doi.org/10.1071/RD22194
Published online: 9 June 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Context: Melatonin influences female reproduction, but expression of the melatonin system has not been characterised in the ovine uterus.
Aims: We aimed to determine whether synthesising enzymes (arylalkylamine N-acetyltransferase (AANAT) and N-acetylserotonin-O-methyltransferase (ASMT)), melatonin receptors 1 and 2 (MT1 and MT2), and catabolising enzymes (myeloperoxidase (MPO) and indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and 2)), are expressed in the ovine uterus, and if they are influenced by the oestrous cycle (Experiment 1) or by undernutrition (Experiment 2).
Methods: In Experiment 1, gene and protein expression was determined in sheep endometrium samples collected on days 0 (oestrus), 5, 10 and 14 of the oestrous cycle. In Experiment 2, we studied uterine samples from ewes fed either 1.5 or 0.5 times their maintenance requirements.
Key results: We have demonstrated the expression of AANAT and ASMT in the endometrium of sheep. AANAT and ASMT transcripts, and AANAT protein were more elevated at day 10, then decreased to day 14. A similar pattern was observed for MT2, IDO1, and MPO mRNA, which suggests that the endometrial melatonin system might be influenced by ovarian steroid hormones. Undernutrition increased AANAT mRNA expression, but seemed to decrease its protein expression, and increased MT2 and IDO2 transcripts, whereas ASMT expression was unaffected.
Conclusions: The melatonin system is expressed in the ovine uterus and is affected by oestrous cycle and undernutrition.
Implications: The results help explain the adverse effects of undernutrition on reproduction in sheep, and the success of exogenous melatonin treatments in improving reproductive outcomes.
Keywords: AANAT, ASMT, IDO, melatonin, melatonin receptor, MPO, sheep, undernutrition, uterus.
Introduction
Melatonin (N-acetyl-5-methoxy tryptamine) is an essential hormone involved in numerous physiological processes, such as the regulation of circadian rhythms, seasonal reproduction in photoperiod-sensitive species, and free radicals scavenging and antioxidation (Reiter et al. 2014). In sheep, exogenous melatonin modulates the neuroendocrine system through the regulation of GnRH and LH secretion (Forcada et al. 2007), influences endometrial and luteal secretory function (Abecia et al. 2002, 2003), improves embryo viability in adverse nutritional conditions (Vázquez et al. 2010, 2013), and influences colostrum and milk composition (Abecia et al. 2020a, 2021), among others.
In addition to the photoperiod-regulated pineal synthesis of melatonin, differing amounts of locally produced melatonin occur in extra-pineal tissues including reproductive organs (Acuña-Castroviejo et al. 2014). The synthesis of melatonin from serotonin occurs in two enzymatic steps that are driven by the arylalkylamine N-acetyltransferase (AANAT) and the N-acetylserotonin-O-methyltransferase (ASMT) (Tan et al. 2015; Zhao et al. 2019). The expression of those enzymes in a tissue suggests a capacity for the synthesis of melatonin, which has been recently shown in the uterus of several animal species (mice, He et al. 2015; goats, Tao et al. 2018; gilts, Bae et al. 2020), but its expression in the ovine uterus has not been reported. Therefore, our first hypothesis was that the ovine uterus has the capacity to synthesise melatonin. On the other hand, melatonin is catabolised through non-, pseudo- and enzymatic processes that can also produce active metabolites. Myeloperoxidase (MPO) and indoleamine 2,3-dioxygenase isoforms 1 and 2 (IDO1 and 2) are the main enzymes involved in its metabolism (Tan et al. 2015).
Melatonin acts through endocrine, paracrine and autocrine pathways (Acuña-Castroviejo et al. 2014) and, although its free radical scavenging is receptor-independent, it exerts many of its biological actions through binding to specific receptors, mainly the G protein-coupled melatonin receptors subtypes 1 and 2 (MT1 and MT2, encoded by the MTNR1A and MTNR1B genes, respectively) (Dubocovich and Markowska 2005; Reiter et al. 2014). Several tissues in the female reproductive tract might be sensitive to melatonin. In sheep, melatonin receptors have been demonstrated in the ovary (Xiao et al. 2018, 2019) and the oviduct (Hu et al. 2020), but not in the uterus. Uterine physiology is under the endocrine control of the ovarian steroid hormones oestradiol (E2) and progesterone, whose fluctuating concentrations dictate the dynamics of oestrous cycles and modulate the reproductive functions. Cyclic variations in uterine gene expression of melatonin receptors have been reported in other species (Zhao et al. 2002). Thus, our second hypothesis was that uterine melatonin production and uterine sensitivity to melatonin changes during the oestrous cycle in sheep.
In addition, undernutrition can alter the uterine environment (Abecia et al. 2006; Meikle et al. 2018), and our previous research has shown that, in sheep, treatment with exogenous melatonin can reverse some of those adverse effects (Vázquez et al. 2013; Abecia et al. 2019). Melatonin implants improved embryo quality in anoestrus, particularly in undernourished ewes, which exhibited a significant increase in viability rate (Vázquez et al. 2010). Moreover, we reported changes in the endometrial sensitivity to steroids in undernourished ewes caused by exogenous melatonin (Vázquez et al. 2013). Thus, we additionally hypothesised that the melatonin system in sheep uterus is affected by the level of nutrition.
This study involved two experiments with the aims of identifying the genes involved in melatonin metabolism and function (i.e. melatonin synthesising and catabolising enzymes, and melatonin receptors) in the ovine uterus, and assessing the effects of the day of the oestrous cycle and undernutrition.
Material and methods
Animals
Two experiments that involved multiparous adult Rasa Aragonesa ewes were performed at the experimental farm of the University of Zaragoza (Zaragoza, Spain; 41°41′N, 0°52′W) and followed a protocol approved by the Ethics Committee of the University of Zaragoza, which met the requirements of the European Union for Scientific Procedure Establishments.
Twelve ewes that had a mean (±s.d.) body weight (BW) of 61.6 ± 1.8 kg and body condition (BC) of 2.9 ± 0.3 (0 = thin, 5 = obese; Russel et al. 1969) were synchronised in oestrus by intravaginal progestagen sponges (fluorogestone acetate 30 mg, Sincropart, CEVA Salud Animal, Barcelona, Spain) for 12 days. Oestrus day (day 0) was 2 days after pessary removal. On days 0, 5, 10 and 14 of the oestrous cycle, three ewes were subjected to general anesthesia induced by sodium thiopental (Tiobarbital, Braun Medical, Jaen, Spain) and sacrificed by a euthanasia agent (T-61, MSD, Madrid, Spain). The reproductive tract was placed on ice and samples were taken immediately from the middle third of the uterine horn, ipsilateral to the ovary bearing the most follicles or corpora lutea. One uterine section was fixed in 4% w/v paraformaldehyde in phosphate-buffered saline (PBS) and embedded in paraffin for immunostaining. In an adjacent sample, endometrium was dissected from myometrium, snap frozen in liquid N2, and stored at −80°C.
Eight ewes that had a mean BW of 63.1 ± 4.9 kg and BC of 3.1 ± 0.4 (Russel et al. 1969) were synchronised in oestrus as in Experiment 1. At the time of pessary insertion, ewes were allocated to one of two groups to be fed diets that provided either 1.5 (Control group; n = 4) or 0.5 (undernourished, Low group; n = 4) times the daily requirements for maintenance (Alderman and Cottrill 1993), and given ad libitum access to water. The daily diets consisted of either 0.55 kg or 0.1 kg of pellets (85% barley and 15% soybean) and either 0.8 kg or 0.5 kg of barley straw for the control and low nutrition groups, respectively. Those regimens were maintained until day 14 of the oestrous cycle, when ewes were euthanised for organ extraction. At the end of the experiment (day 14), the BW and BC of the control ewes did not differ significantly from the initial values; however, in the Low group, BW and BC decreased significantly (P < 0.05), from 62.0 ± 2.1 kg to 55.0 ± 2.3 kg, and from 3.0 ± 0.2 to 2.2 ± 0.2, respectively.
RNA isolation and reverse transcription
A Quick-RNA Miniprep kit (Zymo Research, Orange, CA, USA) was used to extract total RNA. The concentration of RNA was measured based on its absorbance at 260 nm, purity was assessed based on the ratio of absorbance at 260/280 nm, and the integrity of the RNA was assessed by electrophoresis on a 1% agarose gel. All samples had A260/280 ratios between 1.8 and 2.1. A NZY M-MuLV First-Strand cDNA Synthesis Kit (NZYTech, Lisboa, Portugal) was used to reverse transcribe 1 μg total RNA to cDNA (one reaction per sample).
Quantitative real-time PCR (qPCR)
Quantitative real-time PCRs were performed using the NZYSpeedy qPCR Green master mix (NZYTech), with equimolar amounts of forward and reverse primers (400 nM, Table 1) and 2 μL cDNA. Samples were run in duplicate in two independent runs. Standard amplification conditions were 2 min at 95°C, and 40 cycles of 5 s at 95°C and 30 s at 60°C. At the end of each run, dissociation curves were analysed to confirm amplicon specificity and absence of contamination and or primer dimers. Ovis aries-specific intron-spanning primers were designed using the NCBI primer designing tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), and checked by BLAST analysis to verify gene specificity (Table 1). qPCR data was analysed by the 2−ΔΔCT method (Livak and Schmittgen 2001), where the changes in expression of the target genes relative to the housekeeping gene β-actin, were normalised with respect to a calibrator (pooled uterine cDNA from samples of each experiment).
| Gene | Accession number | Sense primer sequence | Antisense primer sequence | Amplicon length (bp) |
|---|---|---|---|---|
| AANATA | NM_001009461.1 | GCTGGTGCCCTTTTACCAGA | CAGTGCATCTCCGTGAAGGT | 90 |
| ASMT | NM_001306120.1 | GTGTCCCTGTACCCACGATG | AAGCTGATCCGCTCATCCTC | 104 |
| MT1 | NM_001009725.1 | GTCCGTTTTCAGCATCACGG | CAGGAACACGTAGCAGAGGG | 112 |
| MT2 | NM_001130938.1 | CCCAGAGGGGTTGTTTGTCT | TTCCCTGCGGAAGTTCTTGT | 100 |
| MPOB | XM_012185170.2 | CTCGGCGAACATTCCCTGTT | CGGTTGTGCTCCCTCACAAA | 99 |
| IDO1B | NM_001141953.1 | TATCTTCCAGCCTGCGCAAA | CAAGGGGCCTTTCCAAC | 116 |
| IDO2 | XM_012105992.3 | CGTCCTGTCCTCTGGGAATG | GCCGTGATGAGGTACTTGGT | 92 |
| β-actinA | NM_001009784 | CTCTTCCAGCCTTCCTTCCT | GGGCAGTGATCTCTTTCTGC | 178 |
AANAT, arylalkylamine N-acetyltransferase; ASMT, N-acetylserotonin-O-methyltransferase; MT1 and 2, melatonin receptors type 1 and 2; MPO, myeloperoxidase; IDO1 and 2, indoleamine 2,3-dioxygenase isoforms 1 and 2.
ASequences from Gonzalez-Arto et al. (2016).
BSequences from Martínez-Marcos et al. (2019).
Classic PCR
Classic PCR confirmed AANAT and ASMT mRNA in the ovine uterus. Reactions were carried out using NZYTaq II 2x Green Master Mix (NZYTech), forward and reverse primers at 250 nM, and 1.5 μL cDNA. Standard amplification conditions were 3 min at 95°C, followed by 35 cycles of 30 s at 94°C, 30 s at 58°C, and 15 s at 72°C, with a final extension of 5 min at 72°C. Primers were the same as those used for qPCR (Table 1). Amplified products were visualised by electrophoresis in 2% agarose gel.
Western blot
For protein extraction, samples of endometrium were mechanically disrupted in a radioimmunoprecipitation assay buffer (50 mM Tris pH 8, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, and protease inhibitor cocktail, Sigma-Aldrich, St. Louis, MO, USA). After a 2 h incubation at 4°C, samples were centrifuged and supernatants recovered and stored at −20°C. Total protein concentration was determined based on a BCA (bicinchoninic acid) Protein Assay kit (Thermo Fisher Scientific, MA, USA).
For immunoblotting, 50 μg of protein lysate were separated in a 12% SDS-PAGE gel and transferred onto PVDF membranes by a Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA). After blocking with 5% non-fat skim milk in 1% v/v Tween-20 PBS for 1 h, membranes were probed with polyclonal rabbit anti-AANAT (1:1000, Abcam, Cambridge, UK, Cat# ab3505) or anti-ASMT antibodies (1:200, Bioss Antibodies, Woburn, MA, USA, Cat# BS-6961R) overnight at 4°C. Following a 1 h incubation with a peroxidase-conjugated secondary goat anti-rabbit antibody (Santa Cruz Biotechnology, CA, USA), immunoblots were exposed to an enhanced chemiluminescence substrate (Clarity Western ECL substrate, Bio-Rad) and scanned in a VersaDoc imaging system (Bio-Rad). Each blot was performed twice. Densitometric quantification was performed by the Quantity One software (Bio-Rad).
Immunofluorescence
Tissue localisation of AANAT and ASMT was assessed by indirect immunofluorescence on paraffin 5-μm uterine transverse sections following the protocol of Zaqout et al. (2020), with slight modifications. Briefly, deparaffinised sections were exposed to a heat-mediated antigen retrieval with 0.01 M sodium citrate (pH 6.0), and blocked with 5% bovine serum albumin in 1X PBS/0.25% v/v Triton X-100 for 1 h at room temperature (RT). Rabbit anti-AANAT (1:100, Abcam, Cambridge, UK) or rabbit anti-ASMT (1:100, Bioss Antibodies, Woburn, MA, USA) were then applied to samples and incubated overnight in a humid chamber at 4°C. Negative controls were not incubated with primary antibodies. Sections were then rinsed with 1X PBS, incubated with Alexa Fluor 488 goat anti-rabbit IgG (1:400, Invitrogen, Carlsbad, CA, USA) for 1 h at RT, rinsed again, and treated with a solution containing 10 mM CuSO4 and 50 mM NH4Cl (pH 5) to reduce autofluorescence. Finally, the sections were washed with dd-H2O and mounted with ProLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen).
Statistical analysis
Normality was tested by Shapiro–Wilk tests. The differences between days of the oestrous cycle were assessed based on a one-way ANOVA and a Tukey’s multiple comparison test. Pairwise comparisons between nutritional treatments were assessed by a t-test. All statistical analyses were performed in GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). Results are presented as mean ± s.e.m. and were considered statistically different if P ≤ 0.05, and as tendency to differ if 0.05 < P ≤ 0.10.
Results
AANAT and ASMT are expressed in the ovine uterus
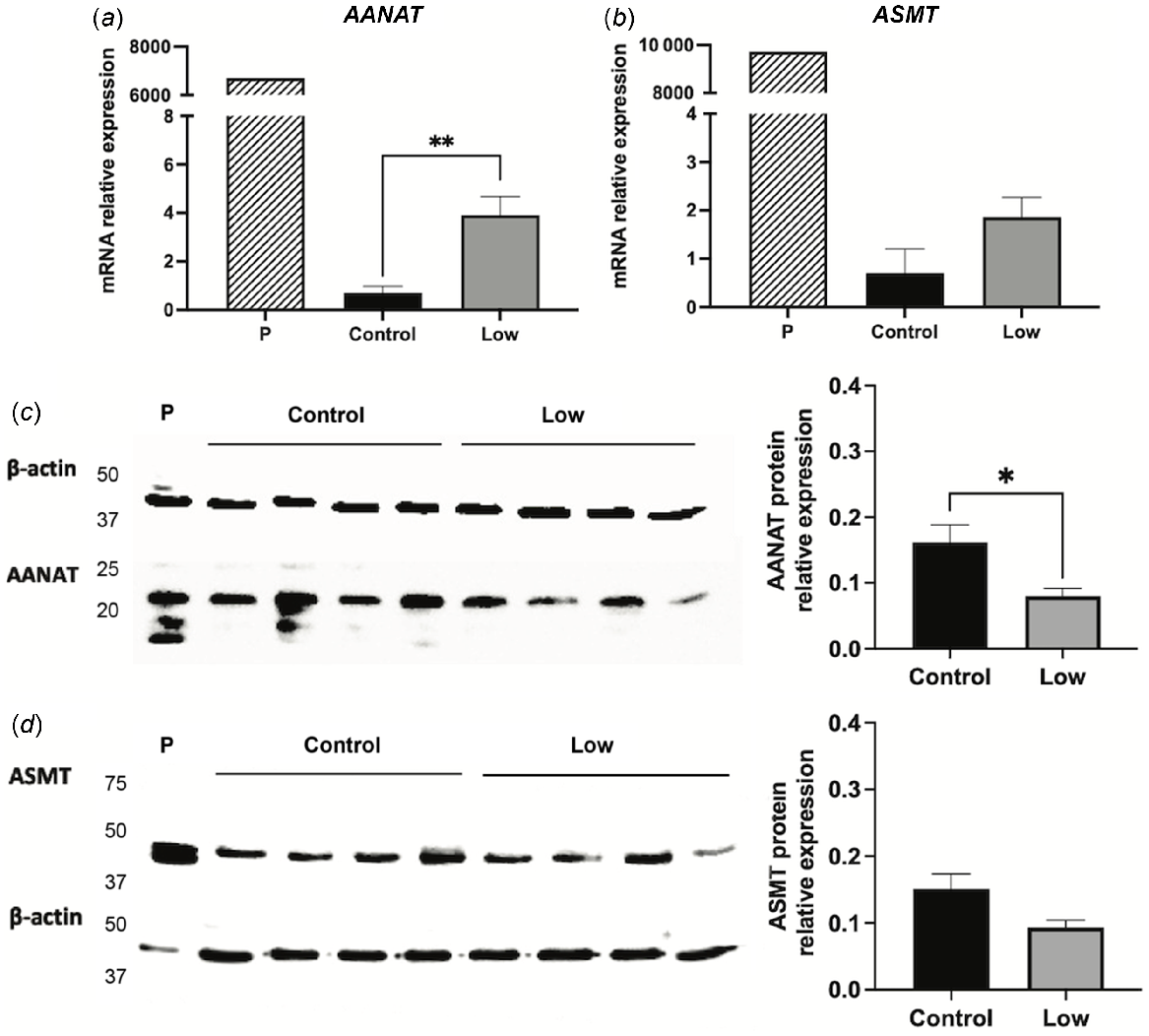
The expression of AANAT and ASMT mRNA in the sheep endometrium was demonstrated based on specific primers for quantitative (Figs 1a and 2a) and classic PCR (Figs 1b and 2b). Sheep pineal gland served as a positive control, and it had the highest expression levels of the two genes. In qPCR, the threshold cycle (Ct) values obtained for AANAT and HIOMT pineal expression were approximately 16, whereas in the endometrium they ranged between 28 and 32. The PCR products from the uterine samples were unambiguously at the expected size for each primer set and of the same size as that of pineal gland. Uterine protein expression for AANAT and ASMT was confirmed by Western blot and immunofluorescence after binding to specific antibodies. AANAT displayed a clear band of about 23 kDa (compatible with the expected size of the protein) in the pineal and uterine samples and, in most cases, a less intense band at about 18 kDa (Fig. 1c). ASMT produced a single band of about 45 kDa, compatible with the expected molecular weight of ASMT, and a less intense band in the pineal gland and in the uterus of remarkable expression (Fig. 2c). The expression of the two proteins occurred at all stages of the oestrous cycle (Experiment 1, Figs 1 and 2) and under nutritional restriction (Experiment 2).
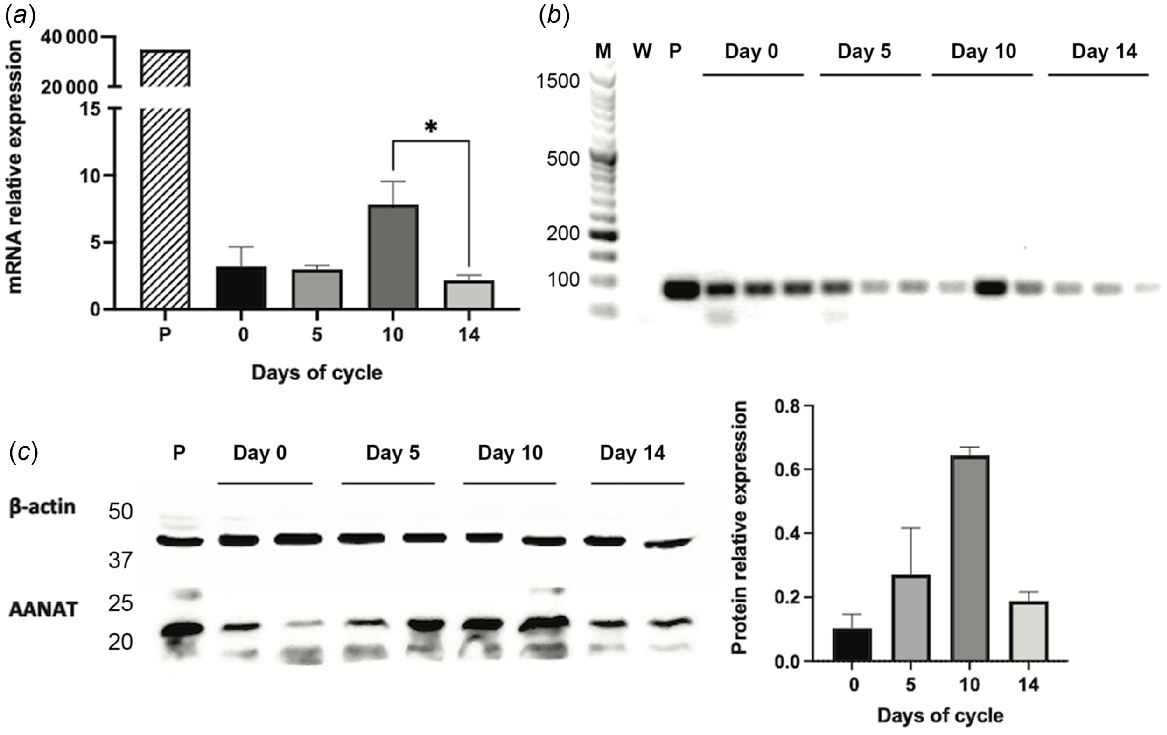
Arylalkylamine N-acetyltransferase (AANAT) expression in Rasa Aragonesa sheep endometrium on days 0 (oestrus), 5, 10 and 14 of the oestrous cycle. Expression in pineal gland (P) served as a positive control. (a) AANAT mRNA relative expression as determined by qPCR (n = 3); (b) mRNA expression by classic PCR (M = MW ladder; W = water); (c) Western blot and densitogram analysis of AANAT protein expression (n = 2). *P < 0.05.
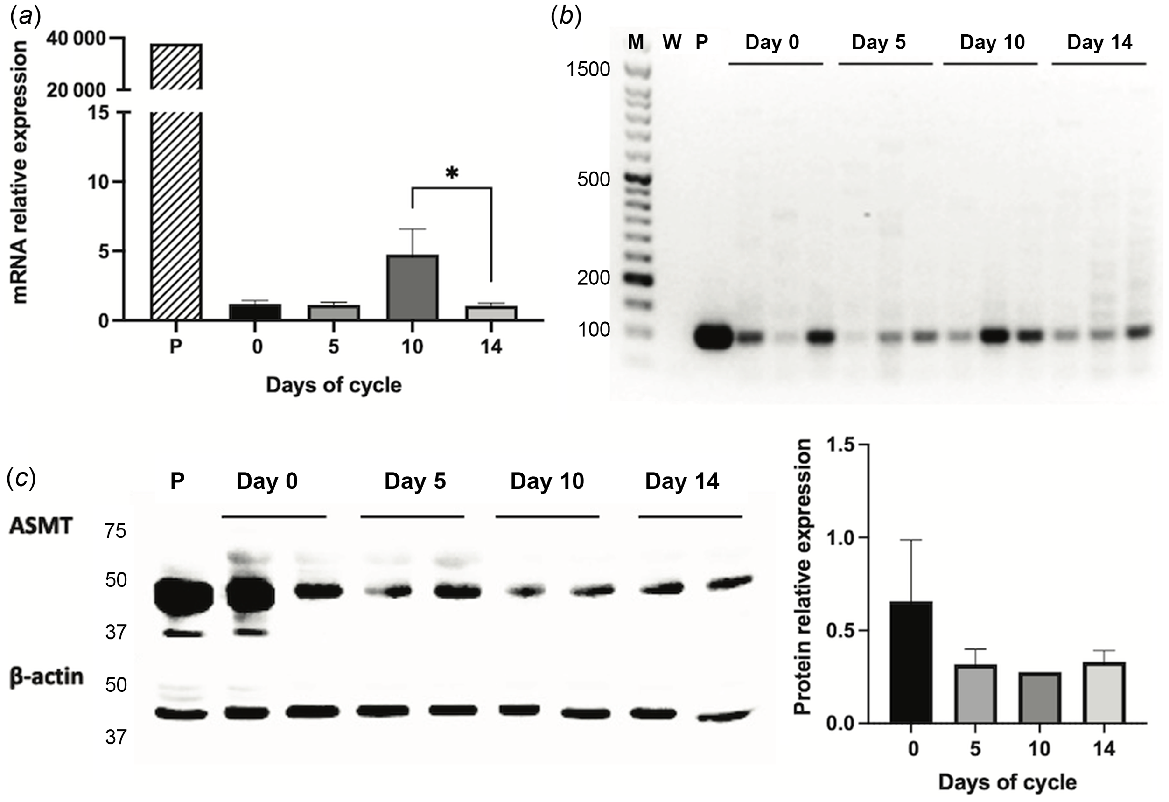
N-acetylserotonin-O-methyltransferase (ASMT) expression in Rasa Aragonesa sheep endometrium on days 0 (oestrus), 5, 10 and 14 of the oestrous cycle. Expression in pineal gland (P) served as a positive control. (a) ASMT mRNA relative expression as determined by qPCR (n = 3); (b) mRNA expression by classic PCR (M = MW ladder; W = water); (c) Western blot and densitogram analysis of ASMT protein expression (n = 2). *P < 0.05.
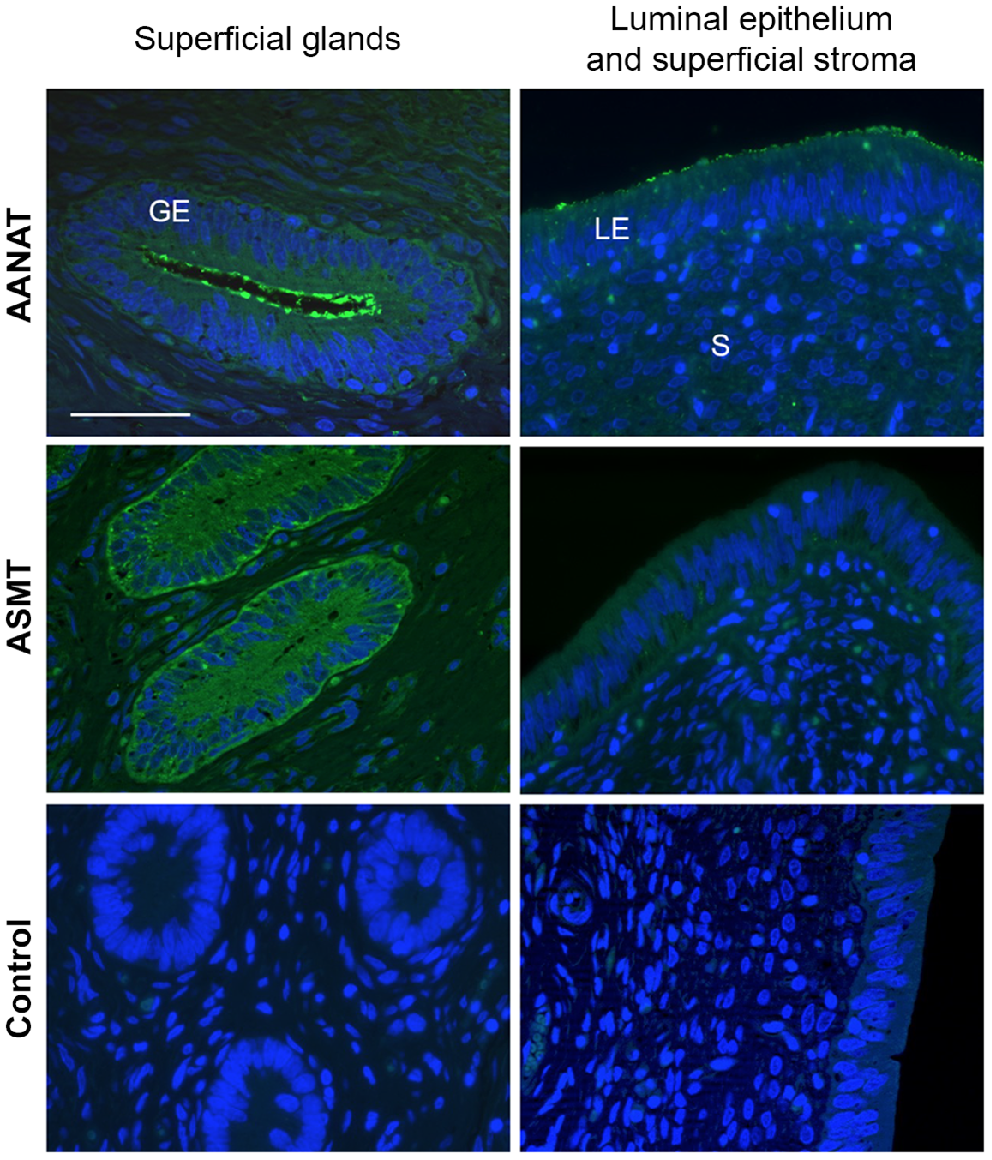
As revealed by immunofluorescence, both AANAT and ASMT were expressed cytoplasmically mainly in the luminal epithelium and stratum compactum (i.e. glandular epithelia and stroma next to the luminal epithelium, Fig. 3), but were almost absent in the glands and stroma near myometrium. We noted that AANAT was strongly detected in the apical membrane of epithelial cells, mostly in the superficial glands, and to a lesser extent, in the luminal epithelium. In contrast, ASMT was more evenly distributed in the epithelial cells.
Immunofluorescence staining of AANAT and ASMT proteins in the ovine endometrium. Representative microphotographs of day 10 of the oestrous cycle. AANAT and ASMT (green) were localised cytoplasmically mainly in the superficial glandular epithelium (GE), superficial stroma (S) and luminal epithelium (LE). Nuclei were counterstained with DAPI (blue). No specific staining (green colour) was observed in the negative control (no primary antibody). Scale bar = 50 μm for all microphotographs.
Effects of the oestrous cycle
Gene expressions of AANAT and ASMT were affected by the days of the oestrous cycle (P < 0.05), with higher concentrations at day 10 compared to day 14 (P < 0.05, Figs 1a and 2a). The protein expression of AANAT also seemed to be higher at day 10 than at day 14 (Fig. 1c), but the low sample number per day (n = 2) precluded reliable statistical analyses. In turn, ASMT protein level did not seem to vary (Fig. 2c).
For the melatonin receptors, the days of the oestrous cycle did not have a significant effect on the gene expression of MT1, but it affected the abundance of MT2 mRNA (P < 0.001), being higher at day 10 than at days 0, 5 and 14 (P < 0.01, Fig. 4).
Gene expression as assessed by qPCR of melatonin receptors and metabolising enzymes in the endometrium of Rasa Aragonesa sheep on days 0 (oestrus), 5, 10 and 14 of the oestrous cycle (n = 3). MT1 and 2, melatonin receptors 1 and 2; IDO1 and 2, indoleamine 2,3-dioxygenase isoforms 1 and 2; MPO, myeloperoxidase. *P < 0.05; **P < 0.01; ***P < 0.001.
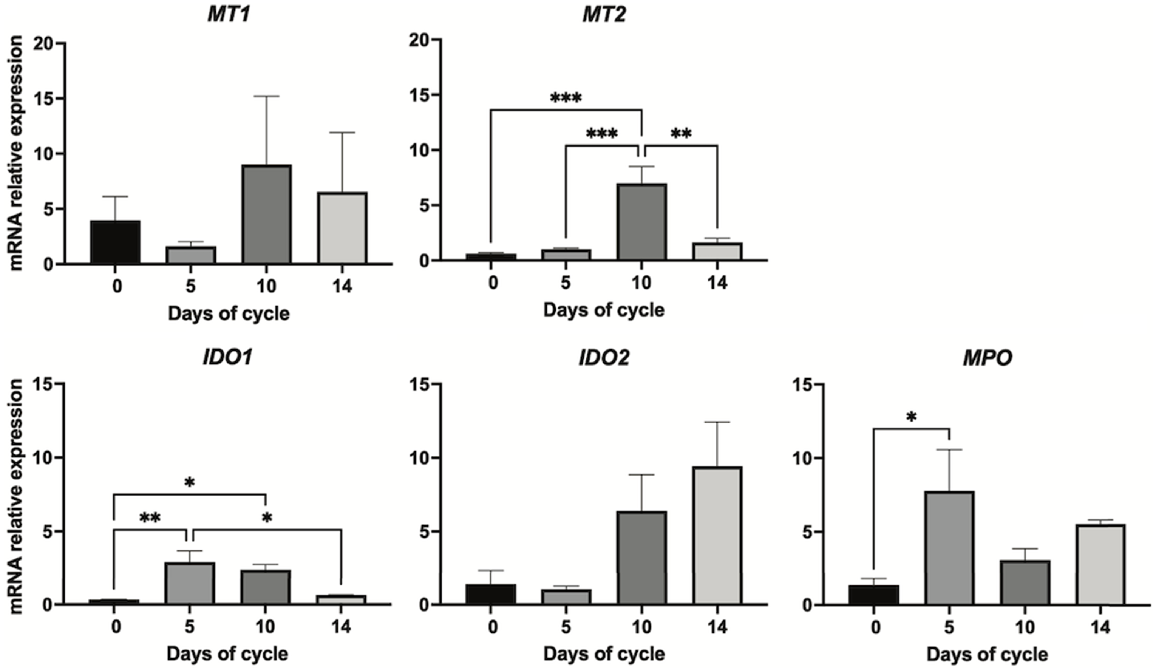
The oestrous cycle affected some of the melatonin-catabolising enzymes; specifically, IDO1 (P < 0.01) and MPO (P < 0.05, Fig. 4). Gene expression of IDO1 was upregulated from oestrus (day 0) to day 5 (P < 0.01) and to day 10 (P < 0.05), and decreased on day 14 (P < 0.05); however, the oestrous cycle did not regulate the isoform IDO2. For MPO, mRNA expression was lowest on day 0 and was significantly (P < 0.05) higher at day 5.
Effects of undernutrition
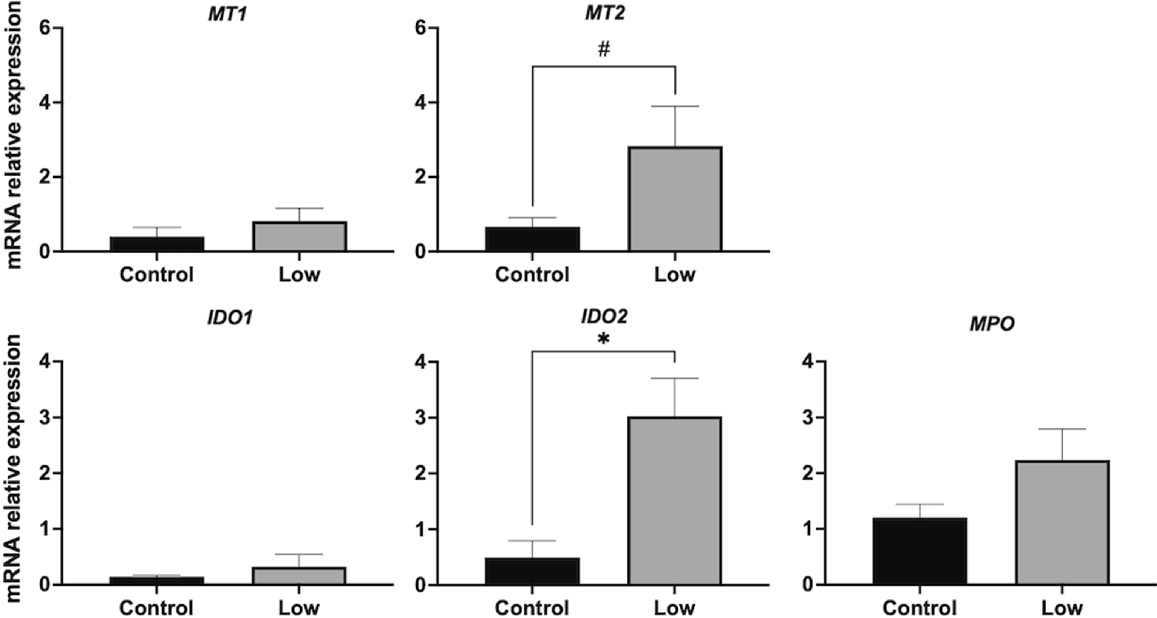
Undernutrition significantly (P < 0.01) upregulated AANAT transcript expression (Fig. 5a); however, protein expression of AANAT was significantly (P < 0.05) lower in undernourished ewes (Fig. 5c). ASMT was unaffected by the nutritional treatment at either the gene (Fig. 5b) or the protein level (Fig. 5d). Undernutrition did not influence the gene expression of MT1, but it tended (P = 0.06) to increase MT2 mRNA expression (Fig. 6). Similarly, undernutrition increased transcript expression of IDO2 (P < 0.05), but did not have a significant effect on IDO1 or MPO expression (Fig. 6).
Arylalkylamine N-acetyltransferase (AANAT) and N-acetylserotonin-O-methyltransferase (ASMT) uterine expression in Control (1.5 times the daily requirements for maintenance) and Low (undernourished, 0.5 times the daily requirements for maintenance) Rasa Aragonesa ewes on day 14 of the oestrous cycle. Expression in pineal gland (P) was used as a positive control. (a) AANAT and (b) ASMT mRNA expression as determined by qPCR (relative to β-actin) (n = 4). (c) AANAT and (d) ASMT Western blot and densitometry analysis of protein expression (n = 4). *P < 0.05; **P < 0.01.
Uterine mRNA expression of melatonin receptors and metabolising enzymes in Control (1.5 times the daily requirements for maintenance) and Low (undernourished, 0.5 times the daily requirements for maintenance) Rasa Aragonesa ewes on day 14 of the oestrous cycle (n = 4). MT1 and 2, melatonin receptors 1 and 2; IDO1 and 2, indoleamine 2,3-dioxygenase isoforms 1 and 2; MPO, myeloperoxidase. *P < 0.05; #P = 0.06.
Discussion
To our knowledge, this study has provided the first evidence of the expression of melatonin synthesising enzymes in the uterus of sheep. Gene expression was confirmed by quantitative and classic PCR, and protein expression was confirmed by Western blots using the pineal gland as a positive tissue, and by immunofluorescence. The activities of AANAT and ASMT are necessary in the pathway to convert tryptophan to melatonin, and its presence in the ovine uterus adds to the known non-pineal sources of melatonin. Although novel, the finding is not surprising because the two enzymes occur in female reproductive tissues including ovaries (rat and human, Itoh et al. 1997, 1999; sheep, Xiao et al. 2018, 2019), uterus and placenta (human, Iwasaki et al. 2005; mice, He et al. 2015; goats, Tao et al. 2018; gilts, Bae et al. 2020), and in the male reproductive tract (Tijmes et al. 1996; Gonzalez-Arto et al. 2016). Indeed, melatonin synthetic capacity might have been transferred to all eukaryotic cells from mitochondria that evolved from bacteria (Tan et al. 2013).
The presence of melatonin biosynthetic enzymes in the ovine uterus suggests the local production of melatonin. Unlike in pineal synthesis, melatonin produced in peripheral tissues is not released into circulation; rather, it acts locally in an autocrine and paracrine manner (Reiter et al. 2022). Uterine melatonin and its metabolites might protect embryos and the endometrium from oxidative stress by free radical scavenging. In addition to our study, melatonin receptors have been identified in the uterus (Schlabritz-Loutsevitch et al. 2003; Mosher et al. 2019; Bae et al. 2020) and in embryos (Sampaio et al. 2012; Casao et al. 2019), and they are targets of melatonin action. Melatonin plays important roles in uterine physiology; e.g. in decidualisation (Cui et al. 2021) and uterine contractility (Rahman et al. 2019), and in several processes from embryogenesis to birth (Carlomagno et al. 2018; Ivanov et al. 2021). Treatment with exogenous melatonin reduced the number of non-viable embryos recovered in sheep (Abecia et al. 2008) and, under adverse nutritional conditions, it improved embryo quality and modulated oviductal and uterine gene expression (Vázquez et al. 2013, 2019). We have localised AANAT and ASMT proteins mainly to the glandular and luminal epithelia, which is consistent with AANAT immunolocalisation in mice (He et al. 2015). This suggests that local synthesis of melatonin has a role in the uterine environment.
Effects of the oestrous cycle
Uterine physiology is under the cyclic regulation of ovary steroid hormones; therefore, we investigated whether the melatonin system was regulated as well. Gene expression of AANAT varied throughout the oestrous cycle, and seemed to be paralleled by the protein expression detected by Western blots. Although ASMT gene expression also changed during the oestrous cycle, protein expression did not seem to vary, which is consistent with a rather constitutive expression of this enzyme that occurs in the pineal gland in which ASMT exhibits little change in expression in response to dark and light (Ganguly et al. 2002). Expression of the AANAT and ASMT genes, and presumably the AANAT protein, was greatest in the late luteal phase (day 10), and lowest at oestrous and at the initiation of luteolysis (day 14), which suggests that oestrogen and or progesterone regulate these proteins. In the uterus of gilts, AANAT mRNA was upregulated at the end of the luteal phase (Bae et al. 2020). An inhibitory effect of oestrogens on melatonin synthesis has been demonstrated in rat pineal gland (Cardinali et al. 1974; Hernández-Díaz et al. 2001). Although, to our knowledge, that has not been demonstrated in extrapineal tissues, the results of our study indicated the lowest melatonin synthesis around oestrous, when oestrogen levels are highest. On the other hand, the upregulation of melatonin synthetic enzymes in the progesterone prevailing phase of the oestrous cycle is consistent with the roles of melatonin in early embryo development and implantation, when progesterone levels remain high in order to sustain pregnancy. Indeed, Bae et al. (2020) observed an increase in the gene expression of AANAT and ASMT in early pregnancy in the endometrium of gilts. Furthermore, crosstalk between melatonin and steroid hormones might occur (Cipolla-Neto et al. 2022), although its importance in the uterus has not been investigated thoroughly. In the ovine endometrium, for instance, exogenous melatonin seems to regulate progesterone receptor expression (Vázquez et al. 2013), although the mechanisms underlying this effect remain to be assessed.
In our study, the uterine expression of MT2 mRNA was influenced by the day of the oestrous cycle since there was a pronounced increase on day 10. The variation in melatonin receptor expression during the oestrous cycle was specific, as no differences were observed in MT1. In general, the results were similar to those reported in the uterus of gilts (Bae et al. 2020) because expression of MT1 remained constant, while expression of MT2 was highest in the late luteal phase. Oestradiol reduces the expression of the two melatonin receptors in the sheep oviduct (Hu et al. 2020). In line with our results, treatment with progesterone increased melatonin uterine binding in ovariectomised rats (Zhao et al. 2002). However, oestradiol showed a similar effect, which is difficult to reconcile with the low expression of MT2 mRNA at oestrus in the sheep uterus, although differences in species and experimental protocols probably account for the discrepancies. Indeed, to our knowledge, this is the first study to investigate MTs expression in the ovine uterus throughout the oestrous cycle.
Whether the strength of gene expression reflects protein abundance at the receptors is unknown but, if these findings corroborate at the protein level, the results of our study suggest an increase in uterine synthesis and sensitivity to melatonin in the luteal phase. In mice, melatonin acting via MT2 regulates the endometrial structure and glandular density, which improves uterus receptivity and suggests that melatonin is involved in the endometrial preparation for early gestation (He et al. 2015), and similar phenomena might occur in the ovine uterus. Nevertheless, interpretation of the results of our study is not straightforward because the binding of melatonin to each receptor subtype might activate different signalling pathways, and melatonin receptors can act individually or by forming homo- or heterodimers, and several events can regulate signal transduction (Dubocovich et al. 2010). Furthermore, another factor is the genetic variation of the melatonin receptors (Jockers et al. 2016). For instance, the polymorphisms of the MTNR1A gene sequence can influence reproductive performance in ewes and rams (Carcangiu et al. 2009; Abecia et al. 2020b; Starič et al. 2020).
Unlike melatonin synthesis, melatonin catabolism is less well understood and much more complex because it includes enzymatic, pseudoenzymatic, and direct transformations that occur after interactions with oxygen- and nitrogen-reactive species (Tan et al. 2015). Primarily, two isoforms of the indoleamine 2,3-dioxygenase (IDO1 and IDO2) and myeloperoxidase (MPO) are involved in the enzymatic cleavage of melatonin (Tan et al. 2007; Fatokun et al. 2013); however, neither IDO or MPO exhibit specificity for melatonin and have high affinity for a wide range of substrates including tryptophan (Ferry et al. 2005). In our study, the day of the oestrous cycle affected the gene expression of IDO1 and MPO in the sheep endometrium, with upregulation from oestrus to day 5, which was maintained up to day 10 in the case of IDO1. These results suggest a high rate of removal of melatonin from the cells, but also an increase in the bioactive melatonin metabolites. Enzymatic melatonin catabolism produces the metabolite N1-acetyl-N2-formyl-5-methoxykynuramine and its derivatives, which act as antioxidants and might mediate and amplify some of the functions of melatonin (Tan et al. 2007). In mammals, IDO1 acts as an immunomodulatory molecule that defends against infections and tumour invasion (Fatokun et al. 2013). In the uterus, that role might be important in the preparation of the endometrium for the arrival of an embryo. Indeed, IDO1 might contribute to the establishment of immune tolerance in early pregnancy so that the embryo is not rejected (Mellor et al. 2002; Ott 2019). The role of those enzymes in uterine physiology has not been identified and, probably, several individual and combined actions occur.
Effects of undernutrition
Undernutrition can impair reproduction in sheep (Abecia et al. 2006; Meikle et al. 2018) by delaying embryo development, increasing embryo mortality, and altering uterine gene expression in the oestrous cycle and early pregnancy (Abecia et al. 2006; Sosa et al. 2009; de Brun et al. 2021). Exogenous melatonin improves reproductive outcomes (Abecia et al. 2008, 2019), and modulates oviductal gene expression and uterine sensitivity to progesterone (Vázquez et al. 2013, 2019). Therefore, we sought to determine whether the uterine melatonin system is involved in these effects. Undernutrition to half maintenance energy requirements affected the expression of AANAT on day 14 of the oestrous cycle, but gene and protein expressions were uncoupled. Undernutrition increased AANAT gene expression, but the protein expression was reduced. Others have reported that changes in AANAT mRNA had no effect on AANAT protein expression or activity in cells from bovine pineal gland (Schomerus et al. 2000). Rather, AANAT activity seems to be influenced by changes in protein expression and other activating mechanisms such as cyclic AMP (Ganguly et al. 2002). The reduced AANAT protein expression in undernourished ewes in the late luteal phase might reduce endometrial melatonin production. Given the important roles of melatonin in uterine physiology, in the establishment of a receptive endometrium and or in embryo development, implantation, and survival (Carlomagno et al. 2018; Olcese 2020), this finding might contribute to the detrimental effects of undernutrition on reproduction in ewes. In our study, undernutrition did not have a similar effect on ASMT expression at the gene or at the protein level. Similarly, ASMT mRNA was higher at day 10 of the oestrous cycle, but its protein expression remained unchanged, showing stable integral expression.
In our study, uterine expression of MT2 mRNA was higher in undernourished ewes than it was in well-fed ewes, but MT1 gene expression did not differ significantly which suggests, perhaps, increased sensitivity to melatonin in the uterus of undernourished ewes by day 14 of the oestrous cycle. Possibly, this is a compensatory mechanism in response to reduced uterine melatonin (as reflected in the reduced expression of AANAT in these sheep), given melatonin’s roles in uterine remodellation, which is vital at the time of maternal recognition of pregnancy (i.e. day 14).
In our study, undernutrition increased the expression of IDO2 mRNA in the uterus of sheep by day 14 of the oestrous cycle, which suggests an increase in melatonin catabolism that in addition to the impairment of melatonin synthesis (i.e. reduced AANAT expression), might result in a reduction in the melatonin available in the uterus of undernourished sheep. Furthermore, these results might explain why the treatment of undernourished ewes with exogenous melatonin in early pregnancy improves embryo survival (Vázquez et al. 2013; Abecia et al. 2019). Of the two indoleamine 2,3-dioxygenase isoforms, IDO2 was discovered most recently and less is known about its regulation and functions. IDO2 is thought to have less enzymatic activity than IDO1, and it might have non-enzymatic functions (Fatokun et al. 2013; Merlo, Mandik-Nayak 2016). Both isoforms serve immunomodulatory functions but, unlike IDO1, IDO2 mediates inflammatory responses (Merlo et al. 2022). In mice uterine stromal cells, IDO2 suppresses proliferation and promotes apoptosis (Li et al. 2015). Possibly, an increase in IDO2 in the uterus of undernourished sheep contributes to an unsuitable environment for embryo implantation, although the underlying mechanisms are unknown.
In conclusion, in this study, we have provided evidence of the expression of the melatonin system (i.e. receptors and synthetic and catabolic enzymes) in the endometrium of sheep throughout the oestrous cycle. The phase of the oestrous cycle affected the expression of most of the molecules studied, which suggests that they are under the influence of sexual steroid hormones, and links the melatonin system to the uterine physiology. Undernutrition altered the normal uterine pattern of expression of genes involved in sensitivity to melatonin and in the melatonin synthetic and catabolic pathways, which might help to explain the adverse effects of undernutrition on reproduction in sheep and the success of exogenous melatonin treatments for improving reproductive outcomes.
Acknowledgements
The authors thank Dr. Adriana Casao for providing some of the laboratory reagents used in this study, and Bruce MacWhirter for reviewing the English version of the manuscript. The authors acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza.
References
Abecia JA, Forcada F, Zúñiga O (2002) The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro. Veterinary Research Communications 26, 151-158.
| Crossref | Google Scholar |
Abecia JA, Forcada F, Valares JA, Zúñiga O, Kindahl H (2003) Effect of exogenous melatonin on in vivo and in vitro prostaglandin secretion in Rasa Aragonesa ewes. Theriogenology 60, 1345-1355.
| Crossref | Google Scholar |
Abecia JA, Sosa C, Forcada F, Meikle A (2006) The effect of undernutrition on the establishment of pregnancy in the ewe. Reproduction Nutrition Development 46, 367-378.
| Crossref | Google Scholar |
Abecia JA, Forcada F, Casao A, Palacín I (2008) Effect of exogenous melatonin on the ovary, the embryo and the establishment of pregnancy in sheep. Animal 2, 399-404.
| Crossref | Google Scholar |
Abecia JA, Forcada F, Vázquez M-I, Muiño-Blanco T, Cebrián-Pérez JA, Pérez-Pe R, Casao A (2019) Role of melatonin on embryo viability in sheep. Reproduction, Fertility and Development 31, 82-92.
| Crossref | Google Scholar |
Abecia JA, Garrido C, Gave M, García A-I, López D, Luis S, Valares JA, Mata L (2020a) Exogenous melatonin and male foetuses improve the quality of sheep colostrum. Journal of Animal Physiology and Animal Nutrition 104, 1305-1309.
| Crossref | Google Scholar |
Abecia JA, Mura MC, Carvajal-Serna M, Pulinas L, Macías A, Casao A, Pérez-Pe R, Carcangiu V (2020b) Polymorphisms of the melatonin receptor 1A (MTNR1A) gene influence the age at first mating in autumn-born ram-lambs and sexual activity of adult rams in spring. Theriogenology 157, 42-47.
| Crossref | Google Scholar |
Abecia JA, Luis S, Canto F (2021) Implanting melatonin at lambing enhances lamb growth and maintains high fat content in milk. Veterinary Research Communications 45, 181-188.
| Crossref | Google Scholar |
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cellular and Molecular Life Sciences 71, 2997-3025.
| Crossref | Google Scholar |
Bae H, Yang C, Lee JY, Park S, Bazer FW, Song G, Lim W (2020) Melatonin improves uterine-conceptus interaction via regulation of SIRT1 during early pregnancy. Journal of Pineal Research 69, e12670.
| Crossref | Google Scholar |
Carcangiu V, Mura MC, Vacca GM, Pazzola M, Dettori ML, Luridiana S, Bini PP (2009) Polymorphism of the melatonin receptor MT1 gene and its relationship with seasonal reproductive activity in the Sarda sheep breed. Animal Reproduction Science 116, 65-72.
| Crossref | Google Scholar |
Cardinali DP, Nagle CA, Rosner JM (1974) Effects of estradiol on melatonin and protein synthesis in the rat pineal organ. Hormone Research 5, 304-310.
| Crossref | Google Scholar |
Carlomagno G, Minini M, Tilotta M, Unfer V (2018) From implantation to birth: Insight into molecular melatonin functions. International Journal of Molecular Sciences 19, 2802.
| Crossref | Google Scholar |
Casao A, Pérez-Pé R, Cebrián-Pérez JA, Muiño-Blanco T, Forcada F, Abecia JA (2019) 125 Presence of melatonin receptors in ovine blastocysts. Reproduction, Fertility and Development 31, 188.
| Crossref | Google Scholar |
Cipolla-Neto J, Amaral FG, Soares JM, Jr, Congentino Gallo C, Furtado A, Cavaco JE, Gonçalves I, Santos CRA, Quintela T (2022) The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology 112, 115-129.
| Crossref | Google Scholar |
Cui L, Xu F, Wang S, Jiang Z, Liu L, Ding Y, Sun X, Du M (2021) Melatonin-MT1 signal is essential for endometrial decidualization. Reproduction 162, 161-170.
| Crossref | Google Scholar |
de Brun V, Loor JJ, Naya H, Graña-Baumgartner A, Vailati-Riboni M, Bulgari O, Shahzad K, Abecia JA, Sosa C, Meikle A (2021) The presence of an embryo affects day 14 uterine transcriptome depending on the nutritional status in sheep. b. Immune system and uterine remodeling. Theriogenology 161, 210-218.
| Crossref | Google Scholar |
Dubocovich ML, Markowska M (2005) Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27, 101-110.
| Crossref | Google Scholar |
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacological Reviews 62, 343-380.
| Crossref | Google Scholar |
Fatokun AA, Hunt NH, Ball HJ (2013) Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids 45, 1319-1329.
| Crossref | Google Scholar |
Ferry G, Ubeaud C, Lambert P-H, Bertin S, Cogé F, Chomarat P, Delagrange P, Serkiz B, Bouchet J-P, Truscott RJW, Boutin JA (2005) Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochemical Journal 388, 205-215.
| Crossref | Google Scholar |
Forcada F, Abecia JA, Casao A, Cebrián-Pérez JA, Muiño-Blanco T, Palacín I (2007) Effects of ageing and exogenous melatonin on pituitary responsiveness to GnRH in ewes during anestrus and the reproductive season. Theriogenology 67, 855-862.
| Crossref | Google Scholar |
Ganguly S, Coon SL, Klein DC (2002) Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell and Tissue Research 309, 127-137.
| Crossref | Google Scholar |
Gonzalez-Arto M, Hamilton TRdS, Gallego M, Gaspar-Torrubia E, Aguilar D, Serrano-Blesa E, Abecia JA, Pérez-Pé R, Muiño-Blanco T, Cebrián-Pérez JA, Casao A (2016) Evidence of melatonin synthesis in the ram reproductive tract. Andrology 4, 163-171.
| Crossref | Google Scholar |
He C, Wang J, Li Y, Zhu K, Xu Z, Song Y, Song Y, Liu G (2015) Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. Journal of Pineal Research 58, 300-309.
| Crossref | Google Scholar |
Hernández-Díaz FJ, Sánchez JJ, Abreu P, López-Coviella I, Tabares L, Prieto L, Alonso R (2001) Estrogen modulates α1/β-adrenoceptor-induced signaling and melatonin production in female rat pinealocytes. Neuroendocrinology 73, 111-122.
| Crossref | Google Scholar |
Hu J-J, Xiao L-F, Song L-L, Ge W-B, Duan H-W, Jiang Y (2020) The expression of melatonin receptors MT1 and MT2 is regulated by E2 in sheep oviduct. General and Comparative Endocrinology 286, 113135.
| Crossref | Google Scholar |
Itoh MT, Ishizuka B, Kudo Y, Fusama S, Amemiya A, Sumi Y (1997) Detection of melatonin and serotonin N-acetyltransferase and hydroxyindole-O-methyltransferase activities in rat ovary. Molecular and Cellular Endocrinology 136, 7-13.
| Crossref | Google Scholar |
Itoh MT, Ishizuka B, Kuribayashi Y, Amemiya A, Sumi Y (1999) Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Molecular Human Reproduction 5, 402-408.
| Crossref | Google Scholar |
Ivanov D, Mazzoccoli G, Anderson G, Linkova N, Dyatlova A, Mironova E, Polyakova V, Kvetnoy I, Evsyukova I, Carbone A, Nasyrov R (2021) Melatonin, its beneficial effects on embryogenesis from mitigating oxidative stress to regulating gene expression. International Journal of Molecular Sciences 22, 5885.
| Crossref | Google Scholar |
Iwasaki S, Nakazawa K, Sakai J, Kometani K, Iwashita M, Yoshimura Y, Maruyama T (2005) Melatonin as a local regulator of human placental function. Journal of Pineal Research 39, 261-265.
| Crossref | Google Scholar |
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, Cecon E, Zlotos DP (2016) Update on melatonin receptors: IUPHAR Review 20. British Journal of Pharmacology 173, 2702-2725.
| Crossref | Google Scholar |
Li D-D, Liu X-Y, Guo C-H, Yue L, Yang Z-Q, Cao H, Guo B, Yue Z-P (2015) Differential expression and regulation of Ido2 in the mouse uterus during peri-implantation period. In Vitro Cellular & Developmental Biology – Animal 51, 264-272.
| Crossref | Google Scholar |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408.
| Crossref | Google Scholar |
Martínez-Marcos P, Carvajal-Serna M, Lázaro-Gaspar S, Pérez-Pé R, Muiño-Blanco T, Cebrián-Pérez JA, Casao A (2019) Presence of melatonin-catabolizing non-specific enzymes myeloperoxidase and indoleamine 2,3-dioxygenase in the ram reproductive tract. Reproduction in Domestic Animals 54, 1643-1650.
| Crossref | Google Scholar |
Meikle A, de Brun V, Carriquiry M, Soca P, Sosa C, Adrien ML, Chilibroste P, Abecia JA (2018) Influences of nutrition and metabolism on reproduction of the female ruminant. Animal Reproduction 15(Suppl.1), 899-911.
| Crossref | Google Scholar |
Mellor AL, Chandler P, Lee GK, Johnson T, Keskin DB, Lee J, Munn DH (2002) Indoleamine 2,3-dioxygenase, immunosuppression and pregnancy. Journal of Reproductive Immunology 57, 143-150.
| Crossref | Google Scholar |
Merlo LMF, Mandik-Nayak L (2016) IDO2: a pathogenic mediator of inflammatory autoimmunity. Clinical Medicine Insights: Pathology 9s1, CPath.S39930.
| Crossref | Google Scholar |
Merlo LMF, Peng W, Mandik-Nayak L (2022) Impact of IDO1 and IDO2 on the B cell immune response. Frontiers in Immunology 13, 886225.
| Crossref | Google Scholar |
Mosher AA, Tsoulis MW, Lim J, Tan C, Agarwal SK, Leyland NA, Foster WG (2019) Melatonin activity and receptor expression in endometrial tissue and endometriosis. Human Reproduction 34, 1215-1224.
| Crossref | Google Scholar |
Olcese JM (2020) Melatonin and female reproduction: an expanding universe. Frontiers in Endocrinology 11, 85.
| Crossref | Google Scholar |
Ott TL (2019) Symposium review: immunological detection of the bovine conceptus during early pregnancy. Journal of Dairy Science 102, 3766-3777.
| Crossref | Google Scholar |
Rahman SA, Bibbo C, Olcese J, Czeisler CA, Robinson JN, Klerman EB (2019) Relationship between endogenous melatonin concentrations and uterine contractions in late third trimester of human pregnancy. Journal of Pineal Research 66, e12566.
| Crossref | Google Scholar |
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology 29, 325-333.
| Crossref | Google Scholar |
Reiter RJ, Sharma R, Rosales-Corral S, de Campos Zuccari DAP, de Almeida Chuffa LG (2022) Melatonin: a mitochondrial resident with a diverse skill set. Life Sciences 301, 120612.
| Crossref | Google Scholar |
Russel AJF, Doney JM, Gunn RG (1969) Subjective assessment of body fat in live sheep. The Journal of Agricultural Science 72, 451-454.
| Crossref | Google Scholar |
Sampaio RV, Conceição DSB, Miranda MS, Sampaio LdFS, Ohashi OM (2012) MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reproductive Biology and Endocrinology 10, 103.
| Crossref | Google Scholar |
Schlabritz-Loutsevitch N, Hellner N, Middendorf R, Müller D, Olcese J (2003) The human myometrium as a target for melatonin. The Journal of Clinical Endocrinology & Metabolism 88, 908-913.
| Crossref | Google Scholar |
Schomerus C, Korf H-W, Laedtke E, Weller JL, Klein DC (2000) Selective adrenergic/cyclic AMP-dependent switch-off of proteasomal proteolysis alone switches on neural signal transduction: an example from the pineal gland. Journal of Neurochemistry 75, 2123-2132.
| Crossref | Google Scholar |
Sosa C, Abecia JA, Carriquiry M, Vázquez MI, Fernández-Foren A, Talmon M, Forcada F, Meikle A (2009) Effect of undernutrition on the uterine environment during maternal recognition of pregnancy in sheep. Reproduction, Fertility and Development 21, 869-881.
| Crossref | Google Scholar |
Starič J, Farci F, Luridiana S, Mura MC, Pulinas L, Cosso G, Carcangiu V (2020) Reproductive performance in three Slovenian sheep breeds with different alleles for the MTNR1A gene. Animal Reproduction Science 216, 106352.
| Crossref | Google Scholar |
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of Pineal Research 42, 28-42.
| Crossref | Google Scholar |
Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary functionand evolution in eukaryotes. Journal of Pineal Research 54, 127-138.
| Crossref | Google Scholar |
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20, 18886-18906.
| Crossref | Google Scholar |
Tao J, Yang M, Wu H, Ma T, He C, Chai M, Zhang X, Zhang J, Ding F, Wang S, Deng S, Zhu K, Song Y, Ji P, Liu H, Lian Z, Liu G (2018) Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy 14, 1850-1869.
| Crossref | Google Scholar |
Tijmes M, Pedraza R, Valladares L (1996) Melatonin in the rat testis: evidence for local synthesis. Steroids 61, 65-68.
| Crossref | Google Scholar |
Vázquez MI, Forcada F, Casao A, Abecia JA, Sosa C, Palacín I (2010) Undernutrition and exogenous melatonin can affect the in vitro developmental competence of ovine oocytes on a seasonal basis. Reproduction in Domestic Animals 45, 677-684.
| Crossref | Google Scholar |
Vázquez MI, Forcada F, Sosa C, Casao A, Sartore I, Fernández-Foren A, Meikle A, Abecia JA (2013) Effect of exogenous melatonin on embryo viability and uterine environment in undernourished ewes. Animal Reproduction Science 141, 52-61.
| Crossref | Google Scholar |
Vázquez I, Sosa C, Carriquiry M, Forcada F, Meikle A, Abecia J (2019) Exogenous melatonin changes oviductal gene expression in pregnant undernourished ewes during the anestrous season. In ‘Animal reproduction – proceedings of the VII International Symposium on Animal Biology of Reproduction’. p. 148. (Brazilian College of Animal Reproduction)
Xiao L, Hu J, Zhao X, Song L, Zhang Y, Dong W, Zhang Q, Ma Y, Li F (2018) Expression of melatonin and its related synthase and membrane receptors in the oestrous corpus luteum and corpus luteum verum of sheep. Reproduction in Domestic Animals 53, 1142-1148.
| Crossref | Google Scholar |
Xiao L, Hu J, Song L, Zhang Y, Dong W, Jiang Y, Zhang Q, Yuan L, Zhao X (2019) Profile of melatonin and its receptors and synthesizing enzymes in cumulus-oocyte complexes of the developing sheep antral follicle – a potential estradiol-mediated mechanism. Reproductive Biology and Endocrinology 17, 1.
| Crossref | Google Scholar |
Zaqout S, Becker LL, Kaindl AM (2020) Immunofluorescence staining of paraffin sections step by step. Frontiers in Neuroanatomy 14, 582218.
| Crossref | Google Scholar |
Zhao H, Pang S, Poon A (2002) Variations of mt1 melatonin receptor density in the rat uterus during decidualization, the estrous cycle and in response to exogenous steroid treatment. Journal of Pineal Research 33, 140-145.
| Crossref | Google Scholar |
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Frontiers in Endocrinology 10, 249.
| Crossref | Google Scholar |


