Is preovulatory follicle selection influenced by the production of oocyte-secreted factors?
Nicholas J. Anderson A and Michael W. Pankhurst
A and Michael W. Pankhurst  A *
A *
A
Abstract
The mammalian ovary ovulates only a fraction of the oocytes it produces, as more than 99% are discarded during development. Females devote a large amount of energy to pregnancy, lactation and subsequent parental care, hence there is strong imperative to produce highly competitive offspring. It would be evolutionarily advantageous if the mammalian ovary had developed a method to detect which developing ovarian follicles contain good-quality oocytes, and preferentially select them for ovulation. No such mechanism has been clearly identified to date. Oocyte-secreted factors (OSFs) such as BMP15 and GDF9, represent one form of communication from oocyte to follicle somatic cells. Herein we discuss the hypothesis that OSFs can increase the growth rate of ovarian follicles, which provides the follicle with a greater ability to compete for follicle dominance and selection for ovulation. Some limited evidence suggests that oocytes with higher OSF secretion produce higher quality embryos but further investigation is needed to firmly link the two concepts of OSFs providing an indication of oocyte quality, and OSFs increasing the chances of follicle selection for ovulation.
Keywords: BMP15, bone morphogenetic protein 15, folliculogenesis, GDF9, granulosa cell, growth differentiation factor 9, oocyte-secreted factors, ovulation, preovulatory follicle.
The ovarian follicle stores the dormant oocyte, supports oocyte maturation, produces reproductive hormones and ovulates the mature oocyte. Follicles are formed in the fetal ovary when newly-formed oocytes become encapsulated by somatic pre-granulosa cells to form a quiescent primordial follicle. In mammals, the ovaries establish a finite number of primordial follicles just before or after birth and this supply is slowly depleted throughout the lifespan (Tingen et al. 2009). Continuous activation of this ‘ovarian reserve’ of primordial follicles generates a continuous ‘production-line’ of developing follicles for ovulation. The average human female will be born with ~400,000 primordial follicles but will only ovulate ~400 oocytes between menarche and menopause (Wallace and Kelsey 2010; Monniaux et al. 2014). Therefore, >99% of follicles will never complete maturation and ovulation and will instead undergo atresia (degeneration).
The storage of dormant oocytes in mammalian ovaries arises from our evolutionary history. The mitotic expansion of oogonia in embryonic ovaries occurs in all vertebrate species (Pepling et al. 1999; Matova and Cooley 2001; Grier et al. 2016). Some species have periodically evolved the ability to maintain oogonial stem cells in adulthood, as in some lizards and amphibians (Guraya 1989a; Ogielska et al. 2010), but this is rare. Birds and mammals have retained the classical vertebrate ovary developmental programme; oogonia proliferate in the fetal ovary then transition into a finite pool of terminally differentiated oocytes that are incapable of further proliferation (Tingen et al. 2009; Johnson 2014). In mammalian and avian evolution, there has been a trend towards lower ovulation rates and smaller number of high-investment offspring. Consequently, a greater proportion of oocytes are discarded via atresia during folliculogenesis (Guraya 1989b; Etches and Petitte 1990; Ogielska et al. 2010). With decreasing ovulation rates and therefore decreased reproductive opportunities, the consequences of ovulating poor-quality oocytes increases substantially. It would have been a great evolutionary advantage if the ovary developed the ability to identify, and preferentially support, the development of follicles with high-quality oocytes. However, clear evidence to support this hypothesis has remained elusive.
After primordial follicle activation, follicles develop through the primary, secondary/preantral and early antral stages of folliculogenesis without the need for endocrine support. Late in folliculogenesis, the granulosa cells in large antral follicles become dependent on follicle-stimulating hormone (FSH) stimulation for survival and the initiation of the final, rapid growth phase (Dierich et al. 1998). In this context, granulosa cells act as ‘gatekeepers’ of follicle survival. When the follicle is approaching its maximal size, the granulosa cells begin to express luteinising-hormone (LH) receptors (Xu et al. 1995). Thereafter, the follicle is referred to as ‘LH-dependent’ as it can now survive without FSH, if it receives LH-stimulation instead. Follicles are usually very large by the time they reach the LH-dependant stage and they have a high capacity for hormone production. In humans, one LH-dependent follicle can produce enough inhibin to suppress FSH secretion and thus initiate atresia in any other competing follicles in the cohort (Schipper et al. 1998; Mihm and Austin 2002). The fully-mature follicles are also the only follicles capable of producing enough oestrogen to stimulate the hypothalamus to initiate the preovulatory LH-surge (Karsch et al. 1973; Maeda et al. 2010). In mono-ovulatory species, the first follicle to achieve this overarching endocrine control of the hypothalamic-pituitary-gonadal axis in the follicular phase of the ovarian cycle is often referred to as the ‘dominant’ follicle.
In order for a follicle to emerge as the dominant follicle, it needs to have reached a certain size by the beginning of the follicular phase (2–6 mm diameter in humans) (Gougeon 1996). In humans, the dominant follicle (10–12 mm) will often emerge as the sole-remaining growing follicle by the seventh or eighth day of the follicular phase (Baerwald et al. 2003). In mono-ovulatory species, the number of these small antral follicles available in the early follicular phase is often ≥10 but only two to three follicles will be large enough to compete for follicle dominance by the mid-follicular phase (Baerwald et al. 2003; Haadsma et al. 2007; Adams et al. 2008). These larger follicles then compete to become the first to reach the LH-dependent stage and also need to be large enough to produce sufficient inhibin to cause FSH-suppression (Fauser and van Heusden 1997; Scaramuzzi et al. 2011; Baerwald et al. 2012). There is evidence from ultrasound studies that the dominant follicle was not always the largest antral follicle at the start of the follicular phase but became the largest follicle due to its rapid growth rate (Bodensteiner et al. 1996; Gastal et al. 1997; Jaiswal et al. 2004; Bashir et al. 2023). This also appears to occur in the poly-ovulatory mouse ovary, where multiple follicles are selected for ovulation, but seemingly only from the fastest-growing large antral follicles (Richard et al. 2024). Collectively, this suggests that there is variability in the ability of each follicle to compete for dominance.
Not all oocytes are generated equal. In older individuals, miscarriage rates and time-to-pregnancy both increase (Gnoth et al. 2003). Declining oocyte quality is a significant factor as IVF success rates increase when donor oocytes from young individuals are used (Stolwijk et al. 1997; Cimadomo et al. 2018). Even in young individuals, IVF is associated with delayed time to pregnancy and proportionately low numbers of live births (Malizia et al. 2009; Tarin et al. 2014), suggesting that when additional follicles are rescued from atresia and allowed to mature, many will be incapable of producing viable pregnancies. Chromosomal segregation failures (aneuploidy) and gamete DNA damage are both known oocyte defects that affect embryo survival (Munné et al. 2004; Adriaens et al. 2009; Titus et al. 2013) and investigation of additional oocyte defects continues. Therefore, it would clearly be an advantage if a follicle could detect when it contained a poor-quality oocyte, and if so, favour a pathway towards atresia rather than ovulation.
Oocytes communicate to somatic cells through oocyte-secreted factors (OSFs) (reviewed by Gilchrist et al. (2008)). The best characterised OSFs are the transforming growth factor β (TGFβ) superfamily members, bone morphogenetic protein (BMP15) and growth differentiation factor 9 (GDF9), which both appear to have oocyte-specific expression. Subtle differences exist between species but GDF9 tends to be expressed slightly earlier in folliculogenesis than BMP15, with little or no expression in primordial follicles, increasing expression at the primary or secondary stage and peak expression of both OSFs by the late preantral and early antral stages (Dube et al. 1998; Laitinen et al. 1998; Aaltonen et al. 1999; Elvin et al. 1999; Hayashi et al. 1999; Jaatinen et al. 1999; Galloway et al. 2000; Juengel et al. 2002).
Folliculogenesis is abnormal in Gdf9−/− mice, because oocyte growth approaches full-size but the granulosa never exceeds one layer of cells, and theca cells are not recruited into the follicle (Dong et al. 1996). Bmp15−/− mice are fertile and their follicles develop to full size, but granulosa cell function and oocyte fertilisation rates are both attenuated (Yan et al. 2001). In sheep, the loss of either BMP15 or GDF9 signalling prevents follicles from developing beyond the primary/secondary stage, (Braw-Tal et al. 1993; Smith et al. 1997; Galloway et al. 2000; McNatty et al. 2007), similar to the effect seen in Gdf9−/− mice. In vitro experiments have shown that BMP15 and GDF9 act as potent granulosa cell mitogens (Hayashi et al. 1999; Gilchrist et al. 2004; McNatty et al. 2005; McIntosh et al. 2008; Fenwick et al. 2013), modulators of gonadotropin sensitivity (Otsuka et al. 2001; Orisaka et al. 2006) and stimulators of aromatase expression (Hobeika et al. 2019). Therefore, there appears to be an absolute requirement for an oocyte to produce at least one of these two OSFs (depending on species) to stimulate follicle growth beyond the preantral stage.
Gene dosage appears to be crucial for the function of BMP15, as heterozygote sheep with loss-of-function mutations in the Bmp15 gene have increased ovulation rates (Galloway et al. 2000). Similar effects are seen in sheep with homozygous loss of function mutations in Bmpr1a (Wilson et al. 2001), one of two possible type 1 receptors for BMP15, with the other being Bmpr1b (Chang et al. 2013). Initially, it was not clear how completely abolishing BMP15 signalling can lead to anovulation, while reducing gene-dose by half can lead to increased ovulation. It has now been determined that reduced BMP15 signalling slows granulosa cell proliferation, while oocyte growth continues at a normal rate (Wilson et al. 2001). By the antral follicle stage, growth is stunted, and follicles only reach about half the normal diameter by the time the granulosa cells attain LH receptors (McNatty et al. 2009; McNatty et al. 2017). In the animals with reduced BMP15 signalling, it required four to six of the stunted follicles to produce an equivalent quantity of oestrogen and inhibin to a single normal dominant follicle. Therefore, the reduced BMP15 does not generate healthier follicles but instead, a larger number of stunted follicles must cooperatively produce enough oestrogen to ovulate (McNatty et al. 2017). This demonstrates that when oocytes have reduced ability to produce OSFs, their follicles lose the ability to compete for dominance over the hypothalamic-pituitary-gonadal axis.
In the ovaries of wild-type animals, all oocytes have the capacity to produce BMP15 and GDF9, but levels can vary substantially from follicle to follicle (de Resende et al. 2012; Kristensen et al. 2022). In our own studies of wild-type mouse ovaries, we observed a relatively small proportion of oocytes with substantially elevated Bmp15 and Gdf9 mRNA (Fig. 1a). Differing levels of OSF production in different follicles would be expected to lead to variability in granulosa proliferation and therefore, variable growth rate. High levels of OSFs may give follicles a growth-advantage in the race to become the first follicle to attain LH-dependency. The follicle that ovulates each cycle is far from average, hence study designs to explore this phenomenon further should focus on the follicles growing at the upper end of the distribution curve, rather than those close to the median or mean.
Relative expression of Gdf9 and Bmp15 mRNA in individual mouse oocytes. (a) Distribution of Gdf9 and Bmp15 mRNA expression values for individual oocytes. (b) Scatter plot of Bmp15 and Gdf9 mRNA relative expression in each oocyte. Oocytes were dissected from 150 to 200 μm diameter follicles from three mouse ovaries (N = 72 oocytes) and were homogenised with RNA Gem extraction kit (MicroGem) and RT-qPCR was performed with SensiFAST SYBR No-ROX one-step kit (Bioline). Primers: Gdf9 (NM_008110.2) Fwd 5′ GATGTGACCTCCCTCCTTCAG 3′, Rev 5′ GCTAAACACTCCGTCCTCTGG 3′; Bmp15 (NM_009757.5) Fwd 5′ GAGCGAAAATGGTGAGGCTG 3′, Rev 5′ AGTTTCCACATGGCAGGAGAG 3′. Data obtained from Richard (2022). Bars represent median and interquartile range.
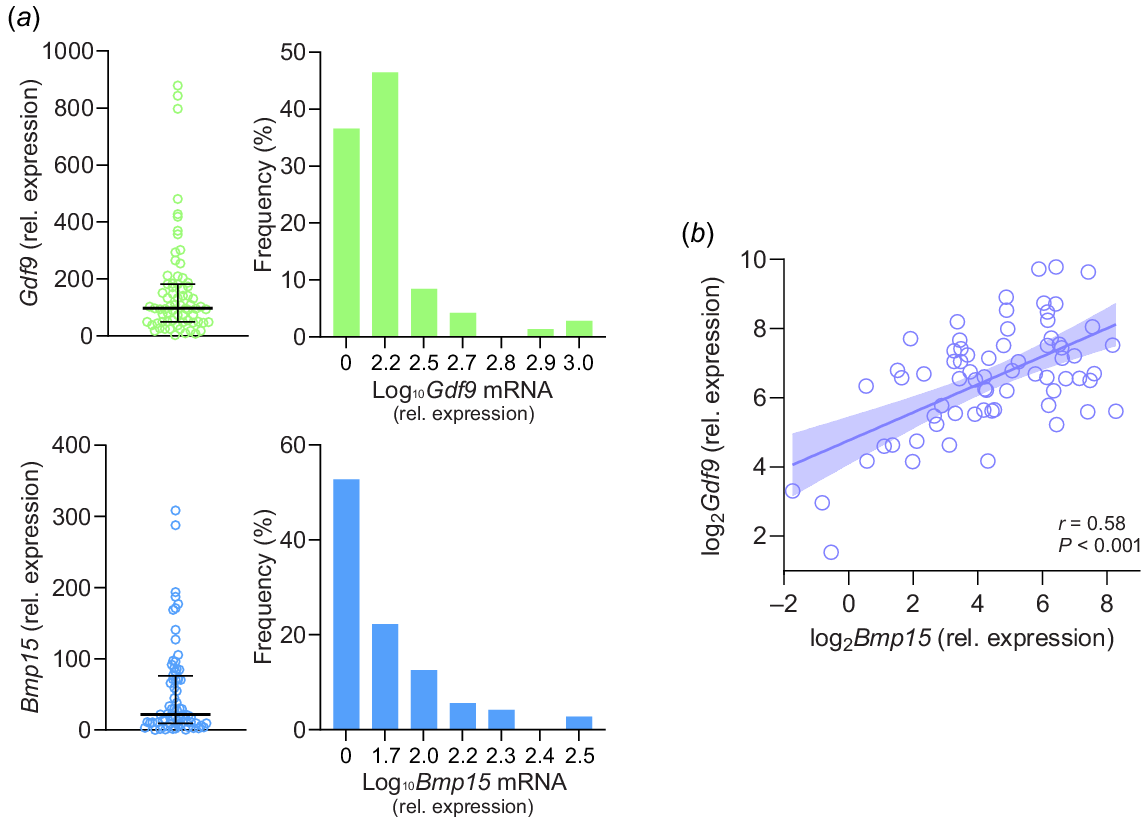
BMP15 and GDF9 both self-pair to form homodimers and also form BMP15-GDF9 heterodimers that act synergistically to increase the effects on the target cells (reviewed by Persani et al. (2014)). In mouse oocytes we found that Bmp15 and Gdf9 mRNA levels were moderately correlated (Fig. 1b). This suggests that increasing the expression of one protein dimer will generally co-occur with an increase in expression of the other. However, some oocytes with more even ratios (i.e. closer to 1:1) may be able to form heterodimers more efficiently. It is currently challenging to study oocyte-secreted factors at the protein level due to the low volume of material in single-oocyte samples, and additional complexity may occur at translational and post-translational stages. Oocytes also secrete other factors, such as BMP2, BMP6 and TGFβ2 (Paradis et al. 2009; Hao et al. 2022). Most investigation of oocyte-secreted factors has focused on BMP15 and GDF9, but the ability of an oocyte to stimulate the growth of the surrounding somatic cells, is likely to arise from the sum-total of multiple secreted signals, further complicating future research.
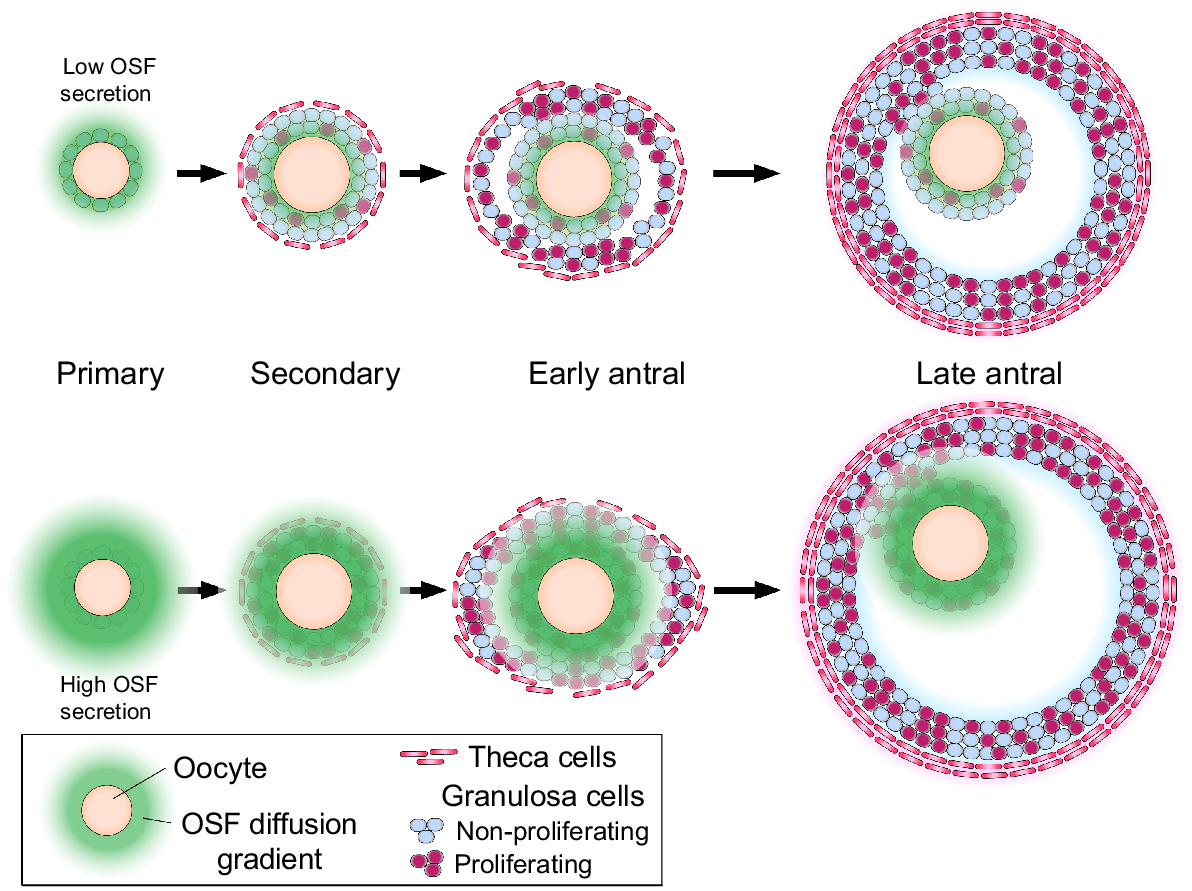
OSFs are secreted at most stages of folliculogenesis but there may be periods where OSF signalling has particular impact. OSFs help prevent apoptosis of granulosa cells and in this context, morphogenetic diffusion gradients have been shown to extend only a short range from the oocyte (Hussein et al. 2005). This may also explain some of the gene expression differences between cumulus and mural granulosa cells (Diaz et al. 2007; Shirafuta et al. 2024). Due to this short-range communication, the preantral phase likely represents the follicle stage where all somatic cells of the follicle are exposed to high levels of OSFs (Fig. 2). This phase of development is crucial, because granulosa cell proliferation is an exponential process and any early change in proliferation rates will have large consequences on final follicle size (Wilson et al. 2001; McNatty et al. 2006). At later stages of follicle development, the mural granulosa cells become distant from the oocyte and trophic support from gonadotropins becomes more important.
Morphogenetic gradients of oocyte-secreted factors. Diffusion leads to an inverse-cubic relationship between diffusion distance and concentration. At the preantral follicle stage, the oocyte has the greatest capacity to affect the granulosa cells due to (1) all cells being in proximity to the growth-promoting factors emanating from the oocyte and (2) early proliferation of somatic cells will lead to larger growth potential at later follicle stages.
The theory of follicle selection posits that each follicle will have a different sensitivity to gonadotropin concentrations (Fauser and van Heusden 1997; Baerwald et al. 2012) but it is not clear where in folliculogenesis this trait arises. BMP15 and GDF9 both have the capacity to influence follicle characteristics by increasing granulosa cell sensitivity to gonadotropins (Otsuka et al. 2001; Orisaka et al. 2006; Chang et al. 2013). An important question is whether early exposure to OSFs leads to persistent changes in the granulosa cells or whether constant exposure to OSFs throughout folliculogenesis is needed to maintain gonadotropin sensitivity. It should be noted that follicular fluid concentrations of GDF9 (but possibly not BMP15, (Kristensen et al. 2022)), are within the range that has been shown to elicit responses in granulosa cells in vitro (Chang et al. 2013). There is also evidence that OSF communication with corona radiata cells influences proliferation in the peripheral granulosa cells (Zhang et al. 2021). Therefore, the influence of OSFs may extend beyond the cumulus layer in antral follicles, possibly in a context-specific manner.
Currently, it is not clear if oocytes with greater OSF secretion are also higher quality. Three studies with small sample sizes provide some evidence that BMP15 and or GDF9 levels in follicular fluid may correlate with embryo quality in assisted reproduction (Wu et al. 2007; Gode et al. 2011; Huang et al. 2023). With the recent development of sensitive BMP15 and GDF9 immunoassays (Riepsamen et al. 2019), it should be possible to conduct additional studies with larger sample sizes. There have been some attempts to examine the transcription factors that activate the Bmp15 and Gdf9 gene promoters (Lan et al. 2003; Choi and Rajkovic 2006; Yan et al. 2006; Wan et al. 2015; Li et al. 2020; Ullah et al. 2023) but there is limited understanding of what drives differences in BMP15 and GDF9 secretion between individual oocytes and whether this relates to oocyte quality. One hypothesis is that oocytes suffering from defects will devote resources to correcting the issue, which may divert resources from the production of OSFs. A similar concept has been postulated for embryo quality, post-fertilisation (Leese et al. 2007), but this principle has not been widely studied during oogenesis. Ultimately, more research in this area is needed.
Conclusion
Herein, we posit the hypothesis that OSFs may be important signals that determine which of the many developing follicles are chosen to ovulate, and which undergo atresia. The hypothesis combines two concepts; (1) follicles with rapid growth rates have a higher chance of being selected for ovulation and (2) oocytes that secrete greater quantities of OSFs will increase the growth rate of the follicle’s somatic cells. Initial evidence suggests that higher levels of OSF secretion is associated with viable embryos, but more research is needed to determine if OSF secretion-rates enable follicles containing good-quality oocytes to be preferentially selected for ovulation.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
References
Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R, Hovata O, Dale L, Ritvos O (1999) Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. The Journal of Clinical Endocrinology & Metabolism 84(8), 2744-2750.
| Crossref | Google Scholar |
Adams GP, Jaiswal R, Singh J, Malhi P (2008) Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69(1), 72-80.
| Crossref | Google Scholar | PubMed |
Adriaens I, Smitz J, Jacquet P (2009) The current knowledge on radiosensitivity of ovarian follicle development stages. Human Reproduction Update 15(3), 359-377.
| Crossref | Google Scholar |
Baerwald AR, Adams GP, Pierson RA (2003) A new model for ovarian follicular development during the human menstrual cycle. Fertility and Sterility 80(1), 116-122.
| Crossref | Google Scholar | PubMed |
Baerwald AR, Adams GP, Pierson RA (2012) Ovarian antral folliculogenesis during the human menstrual cycle: a review. Human Reproduction Update 18(1), 73-91.
| Crossref | Google Scholar |
Bashir ST, Baerwald AR, Gastal MO, Pierson RA, Gastal EL (2023) Dominant follicle growth patterns and associated endocrine dynamics in anovulatory and ovulatory waves in women. Reproduction and Fertility 4(2), e220131.
| Crossref | Google Scholar |
Bodensteiner KJ, Kot K, Wiltbank MC, Ginther OJ (1996) Synchronization of emergence of follicular waves in cattle. Theriogenology 45(6), 1115-1128.
| Crossref | Google Scholar | PubMed |
Braw-Tal R, McNatty KP, Smith P, Heath DA, Hudson NL, Phillips DJ, McLeod BJ, Davis GH (1993) Ovaries of ewes homozygous for the X-linked Inverdale gene (FecXI) are devoid of secondary and tertiary follicles but contain many abnormal structures. Biology of Reproduction 49(5), 895-907.
| Crossref | Google Scholar |
Chang H-M, Cheng J-C, Klausen C, Leung PCK (2013) BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Molecular Endocrinology 27(12), 2093-2104.
| Crossref | Google Scholar |
Choi Y, Rajkovic A (2006) Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. Journal of Biological Chemistry 281(47), 35747-35756.
| Crossref | Google Scholar |
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L (2018) Impact of maternal age on oocyte and embryo competence. Frontiers in Endocrinology 9, 327.
| Crossref | Google Scholar |
de Resende LOT, Vireque AA, Santana LF, Moreno DA, de Sa Rosa e Silva ACJ, Ferriani RA, Scrideli CA, Reis RM (2012) Single-cell expression analysis of BMP15 and GDF9 in mature oocytes and BMPR2 in cumulus cells of women with polycystic ovary syndrome undergoing controlled ovarian hyperstimulation. Journal of Assisted Reproduction and Genetics 29(10), 1057-1065.
| Crossref | Google Scholar |
Diaz FJ, Wigglesworth K, Eppig JJ (2007) Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Developmental Biology 305(1), 300-311.
| Crossref | Google Scholar |
Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P (1998) Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proceedings of the National Academy of Sciences of the United States of America 95(23), 13612-13617.
| Crossref | Google Scholar |
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383(6600), 531-535.
| Crossref | Google Scholar |
Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM (1998) The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Molecular Endocrinology 12(12), 1809-1817.
| Crossref | Google Scholar |
Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM (1999) Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Molecular Endocrinology 13(6), 1035-1048.
| Crossref | Google Scholar |
Etches RJ, Petitte JN (1990) Reptilian and avian follicular hierarchies: models for the study of ovarian development. Journal of Experimental Zoology 256, 112-122.
| Crossref | Google Scholar |
Fauser BCJM, van Heusden AM (1997) Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocrine Reviews 18(1), 71-106.
| Crossref | Google Scholar |
Fenwick MA, Mora JM, Mansour YT, Baithun C, Franks S, Hardy K (2013) Investigations of TGF-β signaling in preantral follicles of female mice reveal differential roles for bone morphogenetic protein 15. Endocrinology 154(9), 3423-3436.
| Crossref | Google Scholar |
Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O (2000) Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nature Genetics 25(3), 279-283.
| Crossref | Google Scholar |
Gastal EL, Gastal MO, Bergfelt DR, Ginther OJ (1997) Role of diameter differences among follicles in selection of a future dominant follicle in mares. Biology of Reproduction 57(6), 1320-1327.
| Crossref | Google Scholar |
Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, Myllymaa S, Kaivo-Oja N, Lankinen H, Mottershead DG, Groome NP, Ritvos O (2004) Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biology of Reproduction 71(3), 732-739.
| Crossref | Google Scholar |
Gilchrist RB, Lane M, Thompson JG (2008) Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Human Reproduction Update 14(2), 159-177.
| Crossref | Google Scholar |
Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G (2003) Time to pregnancy: results of the German prospective study and impact on the management of infertility. Human Reproduction 18(9), 1959-1966.
| Crossref | Google Scholar |
Gode F, Gulekli B, Dogan E, Korhan P, Dogan S, Bige O, Cimrin D, Atabey N (2011) Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertility and Sterility 95(7), 2274-2278.
| Crossref | Google Scholar | PubMed |
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Reviews 17(2), 121-155.
| Crossref | Google Scholar |
Grier HJ, Uribe MC, Lo Nostro FL, Mims SD, Parenti LR (2016) Conserved form and function of the germinal epithelium through 500 million years of vertebrate evolution. Journal of Morphology 277(8), 1014-1044.
| Crossref | Google Scholar |
Haadsma ML, Bukman A, Groen H, Roeloffzen EMA, Groenewoud ER, Heineman MJ, Hoek A (2007) The number of small antral follicles (2-6 mm) determines the outcome of endocrine ovarian reserve tests in a subfertile population. Human Reproduction 22(7), 1925-1931.
| Crossref | Google Scholar |
Hao X, Yuan F, Cui Y, Zhang M (2022) Oocyte-secreted factor TGFB2 enables mouse cumulus cell expansion in vitro. Molecular Reproduction and Development 89(11), 554-562.
| Crossref | Google Scholar |
Hayashi M, McGee EA, Min G, Klein C, Rose UM, Duin Mv, Hsueh AJW (1999) Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 140(3), 1236-1244.
| Crossref | Google Scholar |
Hobeika E, Armouti M, Kala H, Fierro MA, Winston NJ, Scoccia B, Zamah AM, Stocco C (2019) Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. The Journal of Clinical Endocrinology & Metabolism 104(5), 1667-1676.
| Crossref | Google Scholar |
Huang T-H, Chen F-R, Zhang Y-N, Chen S-Q, Long F-Y, Wei J-J, Zhang K, Zeng J-Z, Zhu Q-Y, Li-Ling J, Gong Y (2023) Decreased GDF9 and BMP15 in follicle fluid and granulosa cells and outcomes of IVF-ET among young patients with low prognosis. Journal of Assisted Reproduction and Genetics 40(3), 567-576.
| Crossref | Google Scholar |
Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB (2005) Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. Journal of Cell Science 118(22), 5257-5268.
| Crossref | Google Scholar |
Jaatinen R, Laitinen MP, Vuojolainen K, Aaltonen J, Louhio H, Heikinheimo K, Lehtonen E, Ritvos O (1999) Localization of growth differentiation factor-9 (GDF-9) mRNA and protein in rat ovaries and cDNA cloning of rat GDF-9 and its novel homolog GDF-9B. Molecular and Cellular Endocrinology 156(1-2), 189-193.
| Crossref | Google Scholar |
Jaiswal RS, Singh J, Adams GP (2004) Developmental pattern of small antral follicles in the bovine ovary. Biology of Reproduction 71(4), 1244-1251.
| Crossref | Google Scholar |
Johnson AL (2014) The avian ovary and follicle development: some comparative and practical insights. Turkish Journal of Veterinary and Animal Sciences 38(6), 660-669.
| Crossref | Google Scholar |
Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, O’Connell AR, Laitinen MPE, Cranfield M, Groome NP, Ritvos O, McNatty KP (2002) Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biology of Reproduction 67(6), 1777-1789.
| Crossref | Google Scholar |
Karsch FJ, Weick RF, Butler WR, Dierschke DJ, Krey LC, Weiss G, Hotchkiss J, Yamaji T, Knobil E (1973) Induced LH surges in the rhesus monkey: strength-duration characteristics of the estrogen stimulus. Endocrinology 92(6), 1740-1747.
| Crossref | Google Scholar |
Kristensen SG, Kumar A, Mamsen LS, Kalra B, Pors SE, Bøtkjær JA, Macklon KT, Fedder J, Ernst E, Hardy K, Franks S, Andersen CY (2022) Intrafollicular concentrations of the oocyte-secreted factors GDF9 and BMP15 vary inversely in polycystic ovaries. The Journal of Clinical Endocrinology & Metabolism 107(8), e3374-e3383.
| Crossref | Google Scholar |
Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O (1998) A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mechanisms of Development 78(1-2), 135-140.
| Crossref | Google Scholar | PubMed |
Lan Z-J, Gu P, Xu X, Jackson KJ, DeMayo FJ, O’Malley BW, Cooney AJ (2003) GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility. The EMBO Journal 22(16), 4070-4081.
| Crossref | Google Scholar |
Leese HJ, Sturmey RG, Baumann CG, McEvoy TG (2007) Embryo viability and metabolism: obeying the quiet rules. Human Reproduction 22(12), 3047-3050.
| Crossref | Google Scholar |
Li Y, Jin W, Wang Y, Zhang J, Meng C, Wang H, Qian Y, Li Q, Cao S (2020) Three complete linkage SNPs of GDF9 gene affect the litter size probably mediated by OCT1 in Hu sheep. DNA and Cell Biology 39(4), 563-571.
| Crossref | Google Scholar |
Maeda K-I, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H (2010) Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Research 1364, 103-115.
| Crossref | Google Scholar |
Malizia BA, Hacker MR, Penzias AS (2009) Cumulative live-birth rates after in vitro fertilization. New England Journal of Medicine 360(3), 236-243.
| Crossref | Google Scholar |
Matova N, Cooley L (2001) Comparative aspects of animal oogenesis. Developmental Biology 231(2), 291-320.
| Crossref | Google Scholar |
McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL (2008) The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biology of Reproduction 79(5), 889-896.
| Crossref | Google Scholar |
McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP (2005) Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction 129(4), 473-480.
| Crossref | Google Scholar | PubMed |
McNatty KP, Lawrence S, Groome NP, Meerasahib MF, Hudson NL, Whiting L, Heath DA, Juengel JL (2006) Meat and Livestock Association Plenary Lecture 2005. Oocyte signalling molecules and their effects on reproduction in ruminants. Reproduction, Fertility and Development 18(4), 403-412.
| Crossref | Google Scholar | PubMed |
McNatty KP, Hudson NL, Whiting L, Reader KL, Lun S, Western A, Heath DA, Smith P, Moore LG, Juengel JL (2007) The effects of immunizing sheep with different BMP15 or GDF9 peptide sequences on ovarian follicular activity and ovulation rate. Biology of Reproduction 76(4), 552-560.
| Crossref | Google Scholar |
McNatty KP, Heath DA, Hudson NL, Lun S, Juengel JL, Moore LG (2009) Gonadotrophin-responsiveness of granulosa cells from bone morphogenetic protein 15 heterozygous mutant sheep. Reproduction 138(3), 545-551.
| Crossref | Google Scholar | PubMed |
McNatty KP, Heath DA, Clark Z, Reader K, Juengel JL, Pitman JL (2017) Ovarian characteristics in sheep with multiple fecundity genes. Reproduction 153(2), 233-240.
| Crossref | Google Scholar | PubMed |
Mihm M, Austin EJ (2002) The final stages of dominant follicle selection in cattle. Domestic Animal Endocrinology 23(1-2), 155-166.
| Crossref | Google Scholar | PubMed |
Monniaux D, Clément F, Dalbiès-Tran R, Estienne A, Fabre S, Mansanet C, Monget P (2014) The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biology of Reproduction 90(4), 85.
| Crossref | Google Scholar |
Munné S, Bahçe M, Sandalinas M, Escudero T, Márquez C, Velilla E, Colls P, Oter M, Alikani M, Cohen J (2004) Differences in chromosome susceptibility to aneuploidy and survival to first trimester. Reproductive BioMedicine Online 8(1), 81-90.
| Crossref | Google Scholar | PubMed |
Ogielska M, Rozenblut B, Augustyńska R, Kotusz A (2010) Degeneration of germ line cells in amphibian ovary. Acta Zoologica 91(3), 319-327.
| Crossref | Google Scholar |
Orisaka M, Orisaka S, Jiang J-Y, Craig J, Wang Y, Kotsuji F, Tsang BK (2006) Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Molecular Endocrinology 20(10), 2456-2468.
| Crossref | Google Scholar |
Otsuka F, Moore RK, Iemura S-I, Ueno N, Shimasaki S (2001) Follistatin inhibits the function of the oocyte-derived factor BMP-15. Biochemical and Biophysical Research Communications 289(5), 961-966.
| Crossref | Google Scholar |
Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR (2009) Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 138(1), 115-129.
| Crossref | Google Scholar | PubMed |
Pepling ME, de Cuevas M, Spradling AC (1999) Germline cysts: a conserved phase of germ cell development? Trends in Cell Biology 9(7), 257-262.
| Crossref | Google Scholar |
Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S (2014) The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Human Reproduction Update 20(6), 869-883.
| Crossref | Google Scholar |
Richard S, Zhou Y, Jasoni CL, Pankhurst MW (2024) Ovarian follicle size or growth rate can both be determinants of ovulatory follicle selection in mice. Biology of Reproduction 110(1), 130-139.
| Crossref | Google Scholar |
Riepsamen AH, Chan K, Lien S, Sweeten P, Donoghoe MW, Walker G, Fraison EHJ, Stocker WA, Walton KL, Harrison CA, Ledger WL, Robertson DM, Gilchrist RB (2019) Serum concentrations of oocyte-secreted factors BMP15 and GDF9 during IVF and in women with reproductive pathologies. Endocrinology 160(10), 2298-2313.
| Crossref | Google Scholar |
Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt M-A, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, Monget P, Monniaux D, Viñoles C, Webb R (2011) Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reproduction, Fertility and Development 23(3), 444-467.
| Crossref | Google Scholar | PubMed |
Schipper I, Hop WCJ, Fauser BCJM (1998) The follicle-stimulating hormone (FSH) threshold/window concept examined by different interventions with exogenous FSH during the follicular phase of the normal menstrual cycle: duration, rather than magnitude, of FSH increase affects follicle development. The Journal of Clinical Endocrinology & Metabolism 83(4), 1292-1298.
| Crossref | Google Scholar |
Shirafuta Y, Tamura I, Shiroshita A, Fujimura T, Maekawa R, Taketani T, Sugino N (2024) Analysis of cell-cell interaction between mural granulosa cells and cumulus granulosa cells during ovulation using single-cell RNA sequencing data of mouse ovary. Reproductive Medicine and Biology 23(1), e12564.
| Crossref | Google Scholar |
Smith P, O W-S, Corrigan KA, Smith T, Lundy T, Davis GH, McNatty KP (1997) Ovarian morphology and endocrine characteristics of female sheep fetuses that are heterozygous or homozygous for the inverdale prolificacy gene (fecX’). Biology of Reproduction 57(5), 1183-1192.
| Crossref | Google Scholar |
Stolwijk AM, Zielhuis GA, Sauer MV, Hamilton CJCM, Paulson RJ (1997) The impact of the woman’s age on the success of standard and donor in vitro fertilization. Fertility and Sterility 67(4), 702-710.
| Crossref | Google Scholar | PubMed |
Tarin JJ, García-Pérez MA, Cano A (2014) Assisted reproductive technology results: why are live-birth percentages so low? Molecular Reproduction and Development 81(7), 568-583.
| Crossref | Google Scholar |
Tingen C, Kim A, Woodruff TK (2009) The primordial pool of follicles and nest breakdown in mammalian ovaries. Molecular Human Reproduction 15(12), 795-803.
| Crossref | Google Scholar |
Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K (2013) Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Science Translational Medicine 5(172), 172ra121.
| Crossref | Google Scholar |
Ullah A, Khan R, Suhail SM, Ahmad I, Anwar Khan F, Subhan Qureshi M, Khan NA, Ayari-Akkari A, Ahmed DAEM (2023) Bioinformatics analysis and the association of polymorphisms within the caprine GDF9 gene promoter with economically useful traits in Damani goats. Animal Biotechnology 34(8), 3449-3460.
| Crossref | Google Scholar |
Wallace WHB, Kelsey TW (2010) Human ovarian reserve from conception to the menopause. PLoS ONE 5(1), e8772.
| Crossref | Google Scholar |
Wan Q, Wang Y, Wang H (2015) Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter. International Journal of Molecular Sciences 16(10), 25759-25772.
| Crossref | Google Scholar |
Wilson T, Wu X-Y, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan JC, O’Connell AR, McNatty KP, Montgomery GW (2001) Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biology of Reproduction 64(4), 1225-1235.
| Crossref | Google Scholar |
Wu Y-T, Tang L, Cai J, Lu X-E, Xu J, Zhu X-M, Luo Q, Huang H-F (2007) High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Human Reproduction 22(6), 1526-1531.
| Crossref | Google Scholar |
Xu Z, Allen Garverick H, Smith GW, Smith MF, Hamilton SA, Youngquist RS (1995) Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biology of Reproduction 53(4), 951-957.
| Crossref | Google Scholar |
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM (2001) Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Molecular Endocrinology 15(6), 854-866.
| Crossref | Google Scholar |
Yan C, Elvin JA, Lin Y-N, Hadsell LA, Wang J, DeMayo FJ, Matzuk MM (2006) Regulation of growth differentiation factor 9 expression in oocytes in vivo: a key role of the E-box. Biology of Reproduction 74(6), 999-1006.
| Crossref | Google Scholar |
Zhang Y, Wang Y, Feng X, Zhang S, Xu X, Li L, Niu S, Bo Y, Wang C, Li Z, Xia G, Zhang H (2021) Oocyte-derived microvilli control female fertility by optimizing ovarian follicle selection in mice. Nature Communications 12(1), 2523.
| Crossref | Google Scholar |


