Ovarian culture with mouse serum improves follicle development compared with fetal bovine serum, showing the importance of as yet unidentified factors in follicle growth
Nilay Kuscu A # , Sabriya Abdul Kader A # , Eleanor R. Stephens
A # , Eleanor R. Stephens  A # , Babatomisin V. Adeniran
A # , Babatomisin V. Adeniran  A , Omar F. Ammar A , Ava S. Harrison A , Belinda KM Lo
A , Omar F. Ammar A , Ava S. Harrison A , Belinda KM Lo  A and Suzannah A. Williams
A and Suzannah A. Williams  A *
A *
A
# These authors contributed equally to this paper
Handling Editor: Jennifer Juengel
Abstract
For survivors of childhood blood cancer, fertility preservation through ovarian tissue cryopreservation (OTC) and reimplantation is not recommended because of the risk of reintroducing malignant cells. Since a robust in vitro ovarian tissue culture system does not exist for humans, new approaches are needed.
To investigate new approaches to in vitro follicle growth, our aim was to determine whether mouse serum (MS) could support follicle development better in mouse ovaries in vitro compared to fetal bovine serum (FBS).
Neonatal ovaries were cultured for 14 days in either MS or FBS. Follicle development and health were assessed by histological and molecular analyses. Anti-Müllerian hormone (AMH) and laminin were analysed using immunohistochemistry.
MS supported the development of primordial follicles to preantral follicles, whereas those in FBS did not develop beyond primary. Ovaries cultured in MS had fewer atretic follicles than those in FBS. There were more AMH-positive follicles in MS-cultured than in FBS-cultured ovaries, more primary follicles that were AMH-positive and, AMH-positive primary follicles contained more AMH-positive granulosa cells than those cultured in FBS. Finally, ovaries cultured in either MS or FBS contained laminin; however, the follicle basal lamina (FBL) in MS ovaries were more defined.
MS better supported follicle development, health, and function than did BS, indicating that MS contains additional factors important for follicle development in mice.
These findings demonstrated that as-yet-unknown factors exist that are important for in vitro follicle development, and we need to define them, and explore the role of these molecules in human studies.
Keywords: culture, culture media, culture methodologies, fetal bovine serum, follicle, follicle development, histology, immunohistochemistry, mouse serum, ovarian tissue culture, ovary, serum.
Introduction
Developing methods to generate follicles and eggs in vitro is crucial as a method of fertility preservation for survivors of childhood blood cancer. For young patients who need to undertake gonadotoxic treatment regimes, ovarian tissue is cryopreserved to preserve their fertility. However, for patients with blood cancer, this tissue cannot be reimplanted because of the risk of reintroducing cancerous cells (Dolmans et al. 2013; Rosendahl et al. 2013; Moravek et al. 2023). Therefore, in vitro follicle culture techniques are being developed to produce mature oocytes from the ovarian cortex of pre-pubertal girls. This technique, although established in mice, has proven much more challenging for human ovarian tissues. At present, only two groups have generated mature human oocytes from primordial follicles in ovarian tissue, but these results have not been replicated (McLaughlin et al. 2018; Xu et al 2021). The lack of widespread success in follicle development using human tissues in culture led us to consider what might be missing in culture media because follicle development is fully supported in the human body.
The aim of tissue culture media is to mimic the in vivo environment by providing nutrients, growth factors, and hormones to support cell survival, proliferation, and function. The basic composition of culture media for ovarian tissue culture in a variety of species includes a base medium such as alpha minimal essential medium (α-MEM), McCoy bicarbonate, Waymouth’s media or Earle’s balanced salt solution (EBSS) plus pyruvate (Ghezelayagh et al. 2022) and the addition of fetal bovine serum (FBS). However, when developing a clinical treatment for humans, it is not ideal to use FBS and thus serum-free systems have been established by adding insulin–transferrin–selenium (ITS) and human serum albumin (HSA) (Bjarkadottir et al. 2021).
Although serum-free cultures are valuable for studying the effects of specific hormones or growth factors on follicle development, they may overlook synergistic interactions or factors that are active at very low concentrations, which remain unexplored or unidentified. Additionally, species-specific differences are often neglected, with FBS being commonly used in cultures without considering its compatibility with human tissues.
In mouse follicle development, follicles in cultured ovaries do not grow as well as those grown in vivo (Lo et al. 2019). In comparison to the complex array of molecules that follicles are exposed to in vivo, in vitro culture systems are simplistic and may well lack important molecules required for follicle growth. Therefore, alternatives need to be investigated to improve follicle growth in ovarian tissue culture. MS has previously been used in the culture of isolated mouse follicles and shown to support follicle growth and development, whereas FBS has been observed to inconsistently support follicle development and reduce development to the preovulatory stage (Morgan et al. 2015). However, MS has not been added to ovarian tissue culture, and comparisons have not been made with alternatives such as FBS. Therefore, the aim of this study was to investigate the effect of MS and FBS on mouse follicle development, survival, and function, as well as extracellular matrix (ECM) development in an in vitro ovarian tissue culture system. By using a Day 3 neonatal mouse model, which contains a high proportion of primordial follicles known to activate readily in culture, we were able to assess the impact of different culture conditions on early follicle development. Anti-Müllerian hormone (AMH), produced by granulosa cells (GCs) of functioning follicles, was detected to visualise GCs, count growing follicles and assess the stage of follicle development. AMH is a glycoprotein produced by GCs of preantral and early antral follicles and serves as a key regulator of follicular recruitment and growth. High AMH concentrations are typically observed in healthy, growing follicles, whereas reduced expression is associated with diminished GC activity, the onset of atresia, or cellular stress (Visser and Themmen 2005; Knight and Glister 2006; Van Houten et al. 2010). By assessing AMH expression, we gain critical insight into the functional status of early follicles and the impact of MS treatment on ovarian development.
Materials and methods
Collection of ovaries
Mouse pups were sacrificed by cervical dislocation at postnatal Day 3 (P3) and the ovaries and associated tissues were removed. The ovaries were dissected and isolated in Dulbecco’s phosphate-buffered saline (DPBS; Sigma-Aldrich, UK) containing 1 mg/mL bovine serum albumin (BSA; Fisher Scientific, UK), by using a stereomicroscope. Each ovary used in the study was collected from a different mouse.
Ovarian tissue culture
P3 ovary culture was performed as previously described (Lo et al. 2019). Briefly, whole ovaries were cultured in 300 μL of media (Waymouth-based culture media (Merck, Germany) supplemented with 1% insulin–transferrin–selenium (ITS-G; Sigma-Aldrich, UK); 100 IU/mL penicillin and 0.1 mg/mL streptomycin (Sigma-Aldrich, UK), 2.5 IU/mL recombinant follicle-stimulating hormone (FSH; Gonal-F; Merck-Serono, Feltham, UK), 10 μg/mL BSA (Factor V heat-shock treated; Fisher Scientific, UK), and 25 μg/mL ascorbic acid (Acros Organics, Belgium) in a 24-well tissue culture treated plate (Corning CoStar, Fisher Scientific, UK) on a polycarbonate Transwell membrane (6.5 mm diameter, 0.4 mm pore size; Corning Incorporated, USA). Media was supplemented with either 5% fetal bovine serum (FBS; Biosera, USA) or 5% mouse serum (MS; Biosera). All cultures used serum from a single manufacturing lot for both FBS and MS to minimise batch-to-batch variability. The culture media were replaced every 2 days during the 14-day culture period. The plates were incubated at 37°C with 5% carbon dioxide and 95% air.
Tissue processing
Whole ovaries were fixed in form-acetic (neutral buffered formalin (NBF; VWR International, UK) and 5% acetic acid (Merck, Germany); Adeniran et al. 2021) for 24 h, with gentle agitation at room temperature, then stored in 70% ethanol.
Fixed ovarian tissue samples were dehydrated in increasing concentrations of ethanol solution (70%, 80%, 95% and 3 × 100%) for 15 min each at room temperature, then cleared in three washes of xylene for 20 min each at room temperature. The whole ovary samples were then embedded in paraffin wax (Histoplast; Thermo Scientific). The embedded whole ovaries were serially sectioned at 5 μm. Every 20th section was collected and stained with PAS and haematoxylin for histological analysis, and the remaining tissue sections were used for immunohistochemistry.
Histological staining with periodic acid schiff (PAS) and haematoxylin
Every 20th section was stained with periodic acid schiff (PAS) and haematoxylin. Sections were dewaxed in three washes of xylene for 20 min and rehydrated in graded concentrations of ethanol (100% × 2, 90%, 70%, 50%), and then placed in tap water. Sections were then incubated in periodic acid stock (Sigma-Aldrich, UK) for 5 min, rinsed in dH2O, immersed in Schiff’s reagent (Merck, Germany) for 15 min, followed by washing in tap water for 5 mins, and then immersion in haematoxylin (Gills no2 Haematoxylin; Sigma-Aldrich, UK) for 90s. Sections were then dehydrated in 80%, 95% and 100% ethanol, cleared in xylene and then mounted using DPX mounting media (Sigma-Aldrich).
Immunohistochemistry
Immunohistochemistry was performed to detect AMH as a marker of follicular function and laminin to assess extracellular matrix (ECM) integrity as previously described (Adeniran et al. 2021). We define functional follicles as those with intact GC layers and detectable AMH, indicative of active growth and developmental potential. In contrast, atretic follicles show disrupted morphology with no detectable AMH. In brief, sections were dewaxed in xylene and rehydrated in graded concentrations of ethanol (100%, 90%, 70%, 50%) then washed in double-distilled water (ddH2O). Antigen retrieval was performed by placing the slides in a 1:10 dilution of 100 × Vector® antigen unmasking solution (Vector Labs, UK; according to manufacturer’s instructions) in dH2O, and microwaving for 1 min on full power and 9 min at low power. To block endogenous peroxidase activity, sections were treated with 3% hydrogen peroxide (Sigma-Aldrich, 31642) in phosphate-buffered saline (PBS; Sigma-Aldrich) for 5 min, then washed in ddH20 for 3 min, then PBS with 0.05% Tween 20 (Fisher Scientific, Loughborough, UK) (PBS-T) for 3 min. To prevent non-specific antibody binding, sections were blocked with 5% normal goat serum (NGS; Vector Laboratories, PK-6101) in PBS-T for 1 h at room temperature in a humidifying chamber. To detect AMH, sections were incubated with mouse monoclonal anti-human AMH (5 μg/mL, MCA2246, Bio-Rad UK) in a 1:50 dilution in 5% NGS in PBS-T. For laminin detection, sections were incubated with rabbit anti-laminin (1:30; Sigma-Aldrich UK, 030M4798). Negative control sections were incubated in 5% NGS in PBS-T, with the primary antibody being omitted. For both antibodies, sections were then incubated at 4°C overnight. Sections were then washed in PBS-T and all sections (including negative controls) were incubated with secondary antibodies for 1 h at room temperature: biotinylated goat anti-mouse immunoglobulin G (IgG) (1:100; Vector Laboratories, UK, BA-9200) for AMH and goat anti-rabbit IgG (1:100; Vector Laboratories, BA-1000) for laminin. Sections were washed and incubated in Vectastain Elite ABC kit (Vector Laboratories, PK-6101; according to manufacturer’s instructions) in a humidifying chamber at room temperature for 30 min. Sections were then washed in PBS-T followed by exposure to 3,3′-diaminobenzidine (DAB) peroxidase (Vector Laboratories, SK-4100; prepared according to manufacturer’s instructions). All slides in each experiment were exposed to DAB for the same amount of time. The sections were then dehydrated in graded concentrations of ethanol (50%, 70%, 90%, 2 × 100%) and cleared in xylene. Slides were mounted using DPX mounting media (Sigma-Aldrich). Slides were examined and imaged using a Leica DM2500 light microscope (Microscope Services Ltd., Woodstock, UK) and MicroPublisher 5.0 RTV camera (Qimaging; Microscope Service Ltd.). Images were captured using the Infinity analyse software (ver. 6.5.5; see https://www.teledynevisionsolutions.com/products/infinity-analyze/?model=infinityanalyze&vertical=tvs-lumenera&segment=tvs; Lumenera Corporation (now part of Teledyne Lumenera), Canada) to obtain pre-counterstained images. Following imaging, the AMH immunohistochemistry slides were counterstained with haematoxylin. First, the slides were transferred to xylene to loosen and remove the coverslips, followed by graded concentrations of ethanol before counterstaining with haematoxylin as described above.
Follicle analysis
Every 20th section stained with PAS and haematoxylin was used for follicle analyses. Images were obtained using a Leica DM2500 light microscope (Microscope Services Ltd., Woodstock, UK), and a MicroPublisher 5.0 RTV camera (Qimaging; Microscope Services Ltd.). Follicles with a visible oocyte were classified as primordial, transitional, primary, secondary, and preantral according to established criteria (Pedersen and Peters 1968; Lo et al. 2019; Adeniran et al. 2021). Follicles were classed as atretic if they had a pyknotic nucleus, shrunken oocyte or ≥10% pyknotic granulosa cells, or a combination of the above factors (Winship et al. 2019; Walker et al. 2021). Ovarian follicles as well as their components were analysed, namely, oocyte, nucleus, follicle basal lamina (FBL), granulosa cells (GCs), and theca cells (TCs). All image analysis measurements were performed blind in ImageJ with the FIJI® software (ver. 2.1.0/1.53c; National Institute of Health), with 4.39 pixels/μm as a scale. The total number of ovarian follicles at each follicle stage was calculated using the Abercrombie correction factor (Abercrombie 1946), by using the nuclear diameter (assessed using ImageJ in these ovaries). GC and TC numbers were manually counted; GCs were identified as cuboidal cells found within the FBL, whereas TCs were identified by their squamous appearance and their extension from the outer wall of the FBL to the visibly different stromal cells (Chiti et al. 2017). Follicle area (μm2) was determined by measuring the area from the outer edge of the FBL or the outer wall of the TCs by using ImageJ. Oocyte diameter (μm) was determined by averaging two measurements at the widest cross-section of follicles (Griffin et al. 2006). To analyse AMH-positive follicles, the counterstained IHC images were analysed in ImageJ to assess the number of primary, secondary and preantral follicles and cells that were AMH-positive (AMH+ve; i.e. stained brown with DAB); all other cells and follicles were considered AMH-negative. The number of AMH+ve follicles is expressed as a percentage of all follicles counted in the section, this is then broken down into follicle stages. To determine the percentage of GCs that were AMH+ve, the non-counterstained IHC images were used.
Statistical analysis
GraphPad Prism (ver. 9.2.0; GraphPad Software Inc., USA) was used for statistical analysis and creating graphs. For all results, a Shapiro–Wilk test and the associated Q–Q plot was used to determine whether data were normally or abnormally distributed. Normally distributed data were compared using two-sample Student’s t-tests; this was performed for atretic counts and AMH+ve follicle counts. Abnormally distributed data were analysed using the non-parametric Mann–Whitney test; this was performed for total follicle counts, somatic cell counts, and proportions including follicles without a defined FBL and AMH+ve GCs. Linear regressions were also performed and plotted to correlate follicle area and oocyte diameter to GC number. Results are presented as mean ± standard deviation (STDEV), with P < 0.05 being considered statistically significant.
Ethical approval
All animal studies and experimental procedures using mice were conducted with approval from the Local Ethical Review Panel at the University of Oxford, in accordance with the UK Animals (Scientific Procedures) Act 1986, under a project licence held by Professor Suzannah Williams (Project Licence number: PP1638762). Male C57BL/6J mice, purchased from the Biomedical Services at the University of Oxford, UK, were mated in-house with female CD-1 mice purchased from Charles River, UK.
Results
Follicle health and development is improved in mouse ovarian tissue cultured in MS versus FBS
Follicles were analysed in PAS and haematoxylin-stained sections (FBS, n = 3; MS, n = 3). Follicles at different stages of development were found in ovarian tissue cultured in both FBS-supplemented media and MS-supplemented media (Fig. 1a); however, there was no difference in the total number of follicles between the two groups (Fig. 1b; P = 0.700).
Analysis of follicle number, follicular somatic cells, and atretic follicles in neonatal mouse ovaries cultured in fetal bovine serum (FBS)- and mouse serum (MS)-supplemented culture media. (a) P3 mice ovaries were cultured for 14 days in Waymouth’s media supplemented with either FBS (n = 3) or MS (n = 3), and sections were stained in PAS and haematoxylin. Scale bar = 100 μm. (b) The total number of follicles in FBS- and MS-cultured ovaries was not significantly (P > 0.05) different; n.s., non-significant. (c) Representative images of each follicle type. No secondary or preantral follicles were found in the FBS group. Scale bar = 50 μm. (d) The number of follicles at each developmental stage did not differ although secondary and preantral follicles were only in the MS group. (e) The proportion of atretic follicles was lower in the MS-supplemented culture group (*P < 0.05); however, the stage of follicle development when atresia occurred did not differ (f). Data are means ± std.
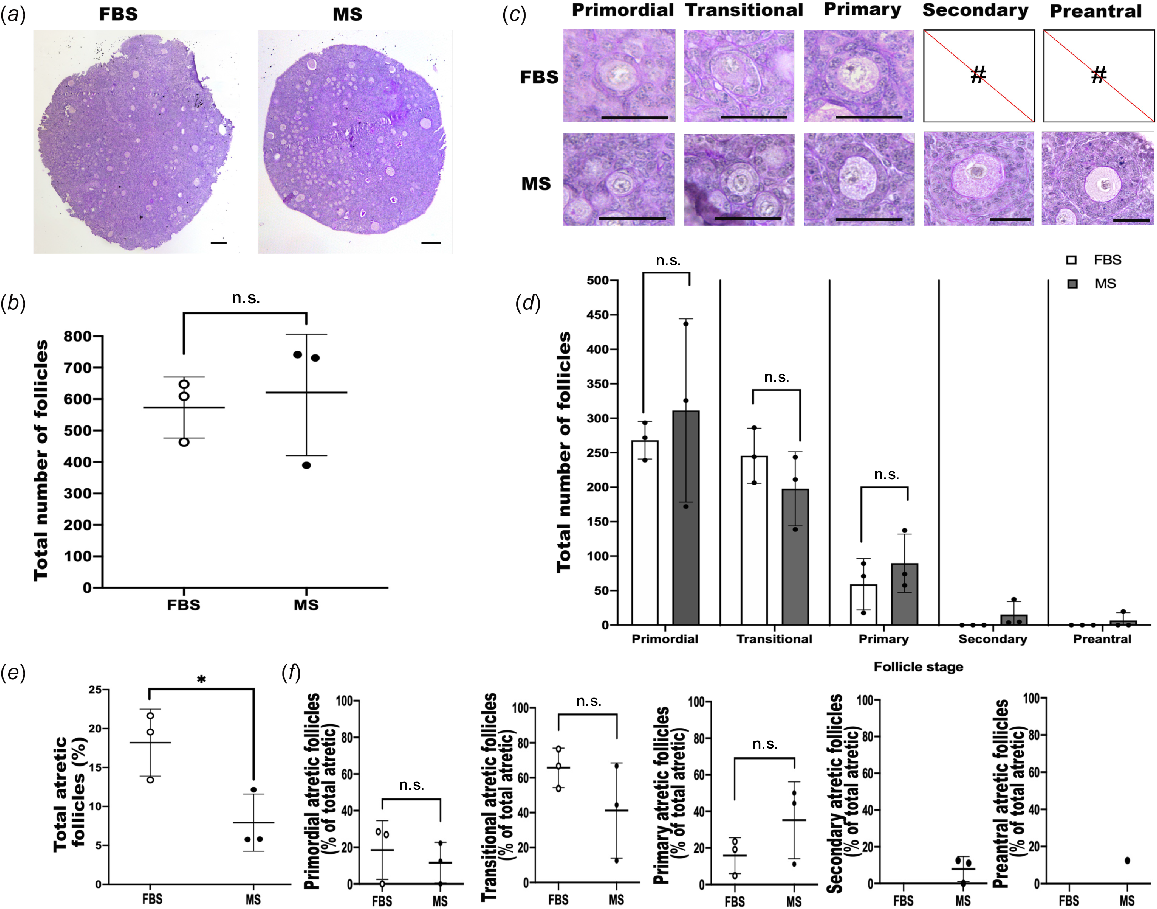
To evaluate the development and growth of follicles in FBS- versus MS-supplemented media, the number of follicles at each stage of development was determined. Ovaries cultured with FBS-supplemented media were present only at the early stages of development, with no secondary or preantral follicles present, whereas ovaries cultured with MS-supplemented media contained follicles at all stages of development; primordial to preantral (Fig. 1c). Therefore, although there was a clear difference in the more developed follicles, there were no differences in earlier-stage follicles (P > 0.05) (Fig. 1d). Focusing on later follicle development, all three MS-treated ovaries contained secondary follicles, whereas none of the FBS-treated ovaries contained secondary follicles. Moreover, although preantral follicles were found only in the MS-treated group, these were restricted to one individual.
To compare follicle health of mouse ovaries cultured in FBS- and MS-supplemented media, the number of atretic follicles was determined; follicle atresia was defined as follicles exhibiting a pyknotic nucleus, a notably shrunken oocyte, or ≥10% pyknotic granulosa cells. The proportion of atretic follicles in the MS group was significantly lower than in the FBS group (P < 0.02; Fig. 1e). However, of the atretic follicles, there was no difference in the number of atretic follicles at each stage of development between the two culture media supplement groups (Fig. 1f). Because no secondary or preantral follicles were observed in the FBS group, the percentage of atresia for these stages could not be compared with the MS group.
Analysis of follicular cells and follicle size in mouse ovaries cultured in FBS- versus MS-supplemented culture media
The number of GCs and TCs were determined to assess how MS affected somatic cells (Fig. 2a). However, since ovaries cultured in FBS did not contain any secondary (FBS, n = 0; MS, n = 11) or preantral (FBS, n = 0; MS, n = 5) follicles, only primary follicles were compared (FBS, n = 39; MS, n = 58). Despite MS clearly supporting later stages of development evidenced by the presence of secondary and preantral follicles, there was no difference in the number of GCs and TCs in primary follicles, although this is unsurprising because primary follicles are categorised on the basis of the number of GCs (Fig. 2b, c).
Evaluation of follicular somatic cells and follicle development in mouse ovaries cultured in FBS- versus MS-supplemented culture media. (a) Representative images of the granulosa and theca cells in a late-stage follicle. Sections were stained in PAS and haematoxylin. The black arrow indicates GCs, and the yellow arrow indicates TCs. Scale bar = 50 μm. The number of (b) granulosa and (c) theca cells around follicles from the primary stage onwards were counted. n.s., non-significant. Primary follicles: FBS, n = 39; MS, n = 58. Secondary follicles: MS, n = 11. Preantral follicles: MS, n = 5. Data are means ± std. (d) Follicle area and (e) oocyte diameter were plotted against GC number for primary follicles and linear regressions were performed: FBS, n = 39 follicles, and MS, n = 58 follicles. No significant difference was found between the gradient of the slopes for either analysis.
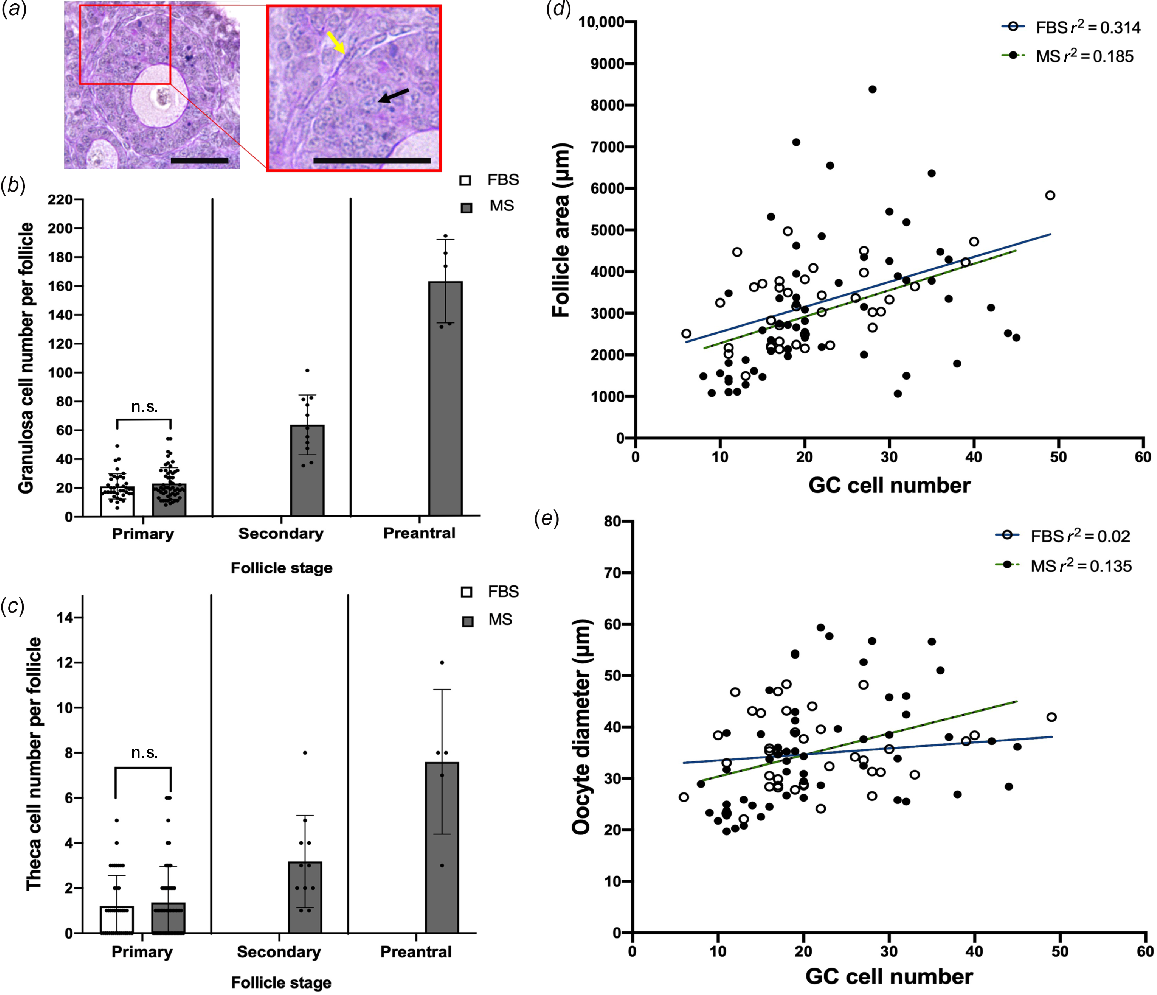
Another parameter measured to compare follicle development between ovaries cultured in FBS and those cultured in MS-supplemented media was the growth rate of follicles and oocytes. As the number of GCs increases, it is expected that the GC area, and thus follicle area, will also increase (Lo et al. 2019). Therefore, the rate of area increase per GC can reflect follicle growth rate. To investigate this, follicle area and oocyte diameter were assessed relative to GC number for primary follicles in both groups. Primary follicles were chosen because they represent the initial stage of follicle development where a rapid increase in follicle area, GC count, and oocyte size occurs; secondary and preantral follicles were not assessed as none were observed in the FBS group. Linear regression lines were plotted (Fig. 2d, e) to analyse the relationships between follicle area and GC count, as well as oocyte diameter and GC count in both groups. The linear regressions showed weak but positive correlations between follicle area and GC count, as well as oocyte diameter and GC count (follicle area: MS vs FBS, r2 = 0.314 and r2 = 0.185; Oocyte diameter: MS vs FBS, r2 = 0.02 and r2 = 0.135). No significant difference was found in the slope gradient, and thus growth trajectories, between the MS and FBS groups for either follicle area or oocyte diameter.
Analysis of follicle basal lamina in mouse ovaries cultured in FBS- versus MS-supplemented culture media
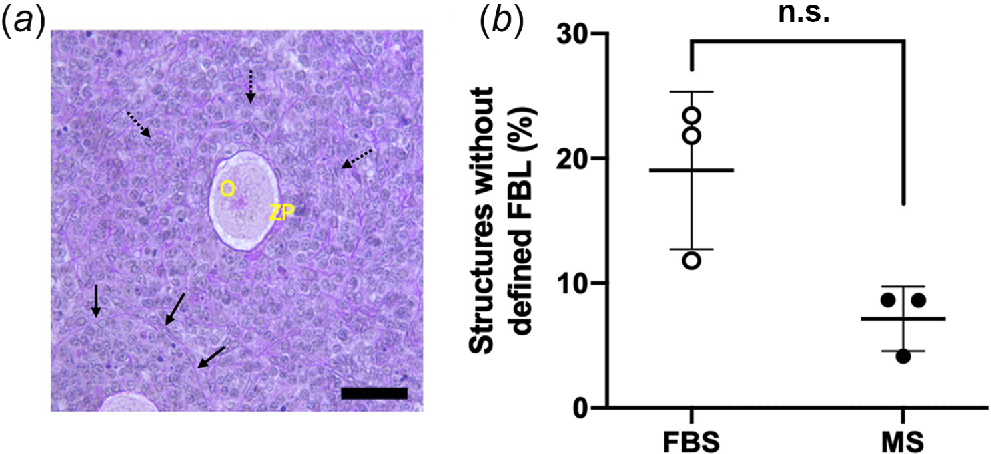
During follicle analysis, some follicles were found that lacked a well-defined FBL and thus the stage of development could not be classified according to established follicle classification criteria (Fig. 3a). To investigate whether the occurrence of these was improved by the addition of MS, we analysed the proportion of these in the two conditions. Although there was no significant difference in the incidence of follicles with an undefined FBL (FBS vs MS, P = 0.100), the mean proportion in MS was half the proportion found in FBS-supplemented media (Fig. 3b).
Analysis of follicle basal lamina in mouse ovaries cultured for 14 days in FBS- and MS-supplemented culture media. (a) A representative image of a follicle-like structure with a defined FBL and an indistinguishable FBL; black solid arrows indicate a clear FBL surrounding the follicle, and broken black arrows indicate follicles without a defined FBL. O, oocyte; ZP, zona pellucida. Sections were stained in PAS and haematoxylin. (b) The percentage of follicles without a defined FBL (P = 0.1). n.s., non-significant. Data are means ± std.
Assessment of follicle function in ovaries cultured in FBS-supplemented media compared with MS-supplemented media
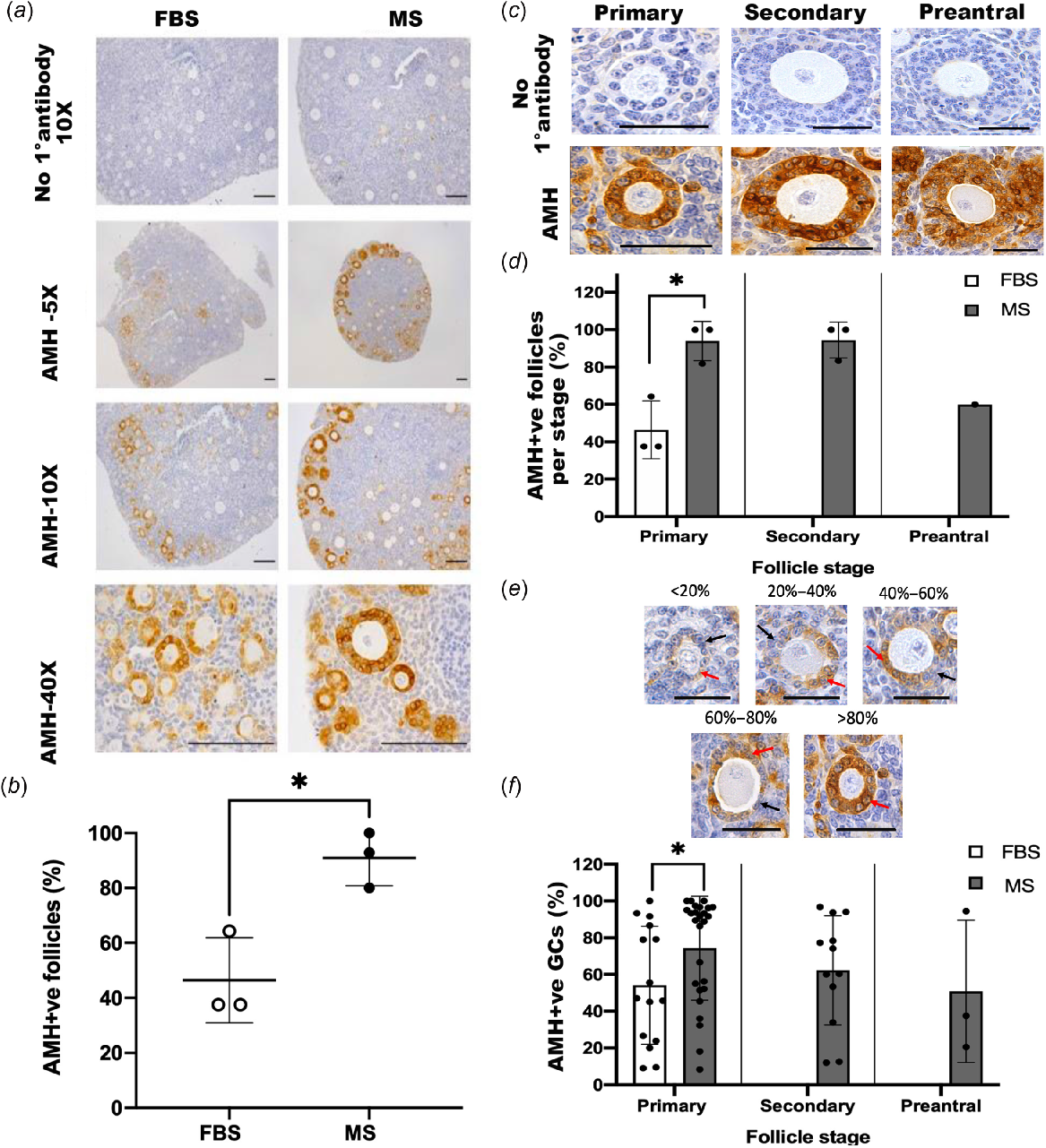
To assess follicle function, we detected AMH by using immunohistochemistry. Ovaries cultured in either FBS or MS both contained follicles that were clearly positive for AMH (AMH+ve; Fig. 4a). However, ovaries cultured in media supplemented with MS had more AMH+ve follicles than did those in FBS-supplemented media (P < 0.05; Fig. 4b). To determine whether this reflected the increased number of later-stage follicles in MS or a difference in AMH at a specific stage of development, the percentage of AMH+ve follicles per stage was analysed (Fig. 4c, d). Interestingly, the proportion of AMH+ve primary follicles was higher in the MS-supplemented group compared with the FBS-supplemented group (P < 0.05). Since no secondary or preantral follicles were detected in ovaries cultured with FBS-supplemented media, no comparison could be made at the later stages of development. All GCs within AMH-positive (AMH+ve) follicles were not equally stained (Fig. 4e). With the aim of distinguishing between them, the percentage of GCs that were AMH+ve was calculated for each AMH+ve follicle to determine whether ovaries cultured in MS-supplemented media had a higher proportion of AMH+ve GCs than those in FBS-supplemented media (Fig. 4f). This analysis showed that a higher percentage of GCs was AMH+ve in the primary follicles of ovaries cultured in MS-supplemented media compared to those in FBS-supplemented media (P < 0.05).
Analysis of anti-Müllerian hormone in mouse ovaries cultured in fetal bovine serum (FBS)- or mouse serum (MS)-supplemented media for 14 days. (a) Representative images of ovarian sections using immunohistochemistry to detect anti-Müllerian hormone (AMH). Images are from the same experiment with the same DAB exposure to allow for direct comparison. Scale bar = 100 μm. (b) The percentage of AMH+ve follicles in the MS group was higher than in the FBS group (P < 0.05). (c) Representative images of AMH+ve primary, secondary and preantral follicles detected using immunohistochemistry with a DAB chromogen. Scale bar = 50 μm. (d) The percentage of AMH+ve follicles per stage was analysed and the proportion of primary AMH+ve follicles was higher in the MS group at the primary stage than in the FBS group (P < 0.05). There were no secondary and preantral follicles in the FBS group and thus no comparisons could be made. (e) Representative sections of AMH+ve follicles with varying proportions of AMH+ve GCs. The red arrows point to an example of AMH+ve GCs, whereas the black arrows point to AMH-negative GCs. Scale bar = 50 μm. (f) The percentage of AMH+ve per follicle at each stage of development. (Primary follicles: FBS, n = 15; MS, n = 26; secondary follicles: FBS, n = 0; MS, n = 12; preantral follicles: FBS, n = 0; MS, n = 3). There was a higher proportion of AMH+ve in primary follicles in the MS group than in the FBS group (*P < 0.05). There were no secondary and preantral follicles in the FBS group and thus no comparison could be made. Data are means ± std.
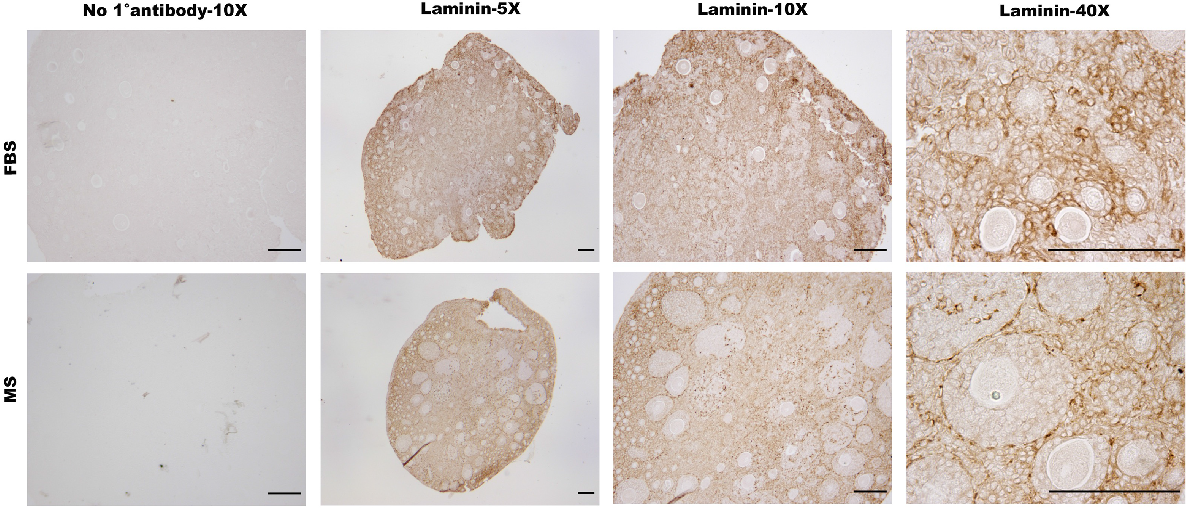
ECM evaluation via laminin in MS- and FBS-cultured mouse ovarian tissue
With the aim of identifying the FBL, TCs and exploring the composition of the ECM, laminin was detected in ovarian sections by immunohistochemistry (Fig. 5). In ovarian tissue cultured within both FBS- and MS-supplemented media, the majority of the section was positive for laminin, with the exception of the GCs within follicles. Visible laminin staining suggests the presence of the FBL around follicles in both conditions, appearing more prominent in later-stage follicles that were cultured in MS. Thus, both MS- and FBS-supplemented culture media supported the expression of laminin in ovarian tissue, indicating their potential to maintain essential ECM integrity for follicle development.
Detection of laminin using immunohistochemistry in mouse ovarian tissue cultured in FBS- versus MS-supplemented culture media. P3 mice ovaries were cultured for 14 days in Waymouth’s media supplemented with either FBS or MS. Control sections were not exposed to the primary antibody. Images are representative of the same experiment with the same DAB exposure to allow for direct comparison. Scale bar = 100 μm.
Discussion
Optimising the culture of ovarian tissue to develop mature follicles in vitro is a crucial technique with significant clinical implications, such as providing new fertility preservation options for prepubertal girls undergoing gonadotoxic therapies and advancing reproductive biology research. Because there is no clinical method to develop mature eggs from primordial follicles by using human ovarian tissue, we hypothesised that by not adding serum from the species in question, crucial components that support follicle development are lacking. Therefore, using a mouse model, we investigated whether mouse serum (MS) improved early in vitro follicle development in mouse ovaries compared with the standard fetal bovine serum (FBS). Our study yielded the following key findings: (1) media supplemented with mouse serum resulted in follicles at later stages of development (secondary and preantral) (2) follicles in MS media had better survival and fewer atretic follicles compared to those in FBS; (3) primary follicles developed in MS media contained more AMH-positive GCs, indicating better follicle function than in FBS; and (4) although both MS and FBS media maintained similar laminin staining patterns throughout the ovary, the FBL was also clearly visible around the more developed follicles in MS-cultured ovaries.
Follicle development in cultured ovaries was assessed using histological analysis. Both FBS and MS supported follicle development in neonatal ovaries during the 2-week culture period, as evidenced by the development of growing follicles. However, although the number of primordial, transitional, and primary follicles did not differ in the different culture conditions, FBS-supplemented media failed to support the development of secondary and preantral follicles, because these were observed exclusively in the MS group. The presence of secondary follicles in all three MS-treated ovaries clearly demonstrated consistent enhanced support of follicle development by MS as opposed to FBS, because no secondary follicles were observed in any of the FBS-treated ovaries. However, although preantral follicles were found in the MS-treated group, they were observed in only one ovary. Such variability in follicle development in different individuals could result from a mild difference in ovary age. Although ovaries were all collected on P3, a difference in time of birth (for example, 6 am vs 6 pm Day 0) would mean that some ovaries were from older animals, thus containing slightly more developed follicles (Pedersen 1969; Peters 1969), which could result in the observed variability in follicle development after culture. While the current study has provided preliminary evidence that MS better supports later follicle development, larger studies are needed to fully elucidate the underlying mechanisms and also to determine the broader applicability of these observations.
Follicle health was also improved by the addition of MS compared with FBS, with approximately half the number of atretic follicles in the MS group. Although there is currently no literature specifically investigating the effects of MS-supplemented media on mouse follicle health and comparing it to the commonly used FBS (Eppig and O’Brien 1996), MS obtained from whole mouse blood has been shown to support isolated mouse follicle growth and development (Morgan et al. 2015). Even though FBS is standard for various mouse ovarian culture systems, using serum from the same species as the ovarian tissue might be more beneficial.
The impact of varying MS concentrations on mouse embryo viability suggested potential benefits of higher MS concentrations by improving overall embryo health (Saito et al. 1984). Future research directions could explore the use of elevated MS concentrations to further enhance follicle health during culture and also determine the molecular mechanisms of the apoptotic pathways involved.
FBS is widely used in cell culture studies and is routinely used in the culture of mouse ovaries and mouse follicles (Kaune et al 2017; Lo et al. 2019). However, FBS is not used when culturing human ovarian tissues because relying on a reagent to develop follicles and eggs in vitro that would not be suitable for a clinical treatment seems counterintuitive. Crucially, this stripped-back approach may be the reason why a robust technique for culturing ovarian human tissues to develop mature eggs from primordial follicles has proved elusive, because perhaps there are other molecules that are also required.
Since primary follicles appear to develop similarly in both conditions but did not progress further in the FBS, we sought to examine follicle development in more depth. Interestingly, the number of GCs and TCs in primary follicles did not differ in FBS- versus MS-supplemented media, indicating that MS did not affect the development of somatic cells in these very early follicles compared with FBS supplementation. However, analysis of AMH, as a marker of follicle function, showed that ovaries cultured in MS were more likely to be AMH+ve. Moreover, the proportion of GCs that were AMH+ve was also higher in primary follicles and, thus, despite the fact that the number of primary follicles did not differ between the groups, their functionality did. These results are likely to suggest improved granulosa cell functionality and overall follicle function in MS media. However, the assessment was limited to granulosa cells, and future studies could include markers such as c-Kit receptor expression to assess the functionality of TCs (Thomas and Vanderhyden 2006) or GDF-9 for oocytes (Hreinsson et al. 2002) to obtain more insight into the molecular modifications of follicle development.
The FBL plays a critical role in regulating molecular exchange within the follicle, creating an optimal environment for oocyte development, and supporting the proliferation and differentiation of GCs (Irving-Rodgers and Rodgers 2000). GCs also produce factors that recruit TCs, which reciprocally support GCs, influencing overall follicle quality (Young and McNeilly 2010). An underdeveloped FBL may disrupt these interactions between GCs and TCs, potentially resulting in follicles of poorer quality. Follicles lacking a distinct FBL occurred twice as often in the FBS group as in the MS group; however, this was not statistically significant likely because of the small sample size. In similar culture conditions, the absence of a distinct FBL was routinely observed in ovaries cultured in vitro compared with their in vivo counterparts (Lo et al 2019), and, thus, if the inclusion of mouse serum can improve FBL development, this indicates that something is missing precluding robust FBL development when culturing in FBS alone. There is a close relationship between the number of GCs and the formation of the FBL; however, whether this is a direct effect owing to missing factor(s) in the media, or indirect owing to a lack of factor(s) usually produced by other ovarian or follicular cells, is unknown. For example, GDF-9, a member of the TGF-β superfamily generated by mammalian oocytes, could potentially improve TC development and subsequently enhance GC count and FBL formation (Elvin et al. 1999).
Considering that the FBL was improved in MS, we explored the presence of laminin as a crucial ECM molecule (Irving-Rodgers and Rodgers 2000). Both MS and FBS media preserved laminin distribution within the ECM of cultured ovarian tissue, indicating preserved ECM integrity, a crucial component for follicle development and function (Berkholtz et al. 2006; Grosbois et al. 2023). However, detection of laminin in the MS ovaries clearly outlined the follicles readily defining the granulosa–FBL border. The role of other ECM components such as collagen and elastin should be explored in future studies to fully understand ECM dynamics under different culture conditions.
Conclusions
In conclusion, we have provided preliminary evidence that MS supports the growth of mouse primordial follicles to preantral follicles in vitro more effectively than does standard FBS, offering new insights into follicle development. These findings suggest potential advantages of MS over FBS in supporting follicle development, health, and function, as well as maintaining crucial ECM components in mice. Further research is required to identify the specific components in MS responsible for these benefits and to explore their applicability to human systems. Although further studies need to be performed in humans, these findings may ultimately aid in the establishment of the in vitro ovarian tissue culture system and provide female childhood cancer survivors, particularly those with blood cancers, a safe fertility preservation option.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
Declaration of funding
This work was supported by a Clarendon Fund Scholarship to B. K. M. Lo, Nuffield Department of Women’s and Reproductive Health funding to B. K. M. Lo, S. A. Kader, E. R. Stephens and John Fell OUP to S. A. Williams to fund O. F. Ammar. This project has also been made possible in part by Grant number 2021-238038 from the Chan Zuckerberg Initiative DAF, an advised fund of Silicon Valley Community Foundation, to S. A. Williams to fund N. Kuscu.
Author contributions
S. A. Williams conceived the study, S. A. Williams, B. K. M. Lo, B. V. Adeniran designed the study, samples were generated and analysed by B. V. Adeniran, S. A. Kader, E. R. Stephens, O. F. Ammar, A. S. Harrison, and N. Kuscu. The manuscript draft and figures were generated by N. Kuscu on the basis of MSc dissertations generated by S. A. Kader and E. R. Stephens. All authors edited and approved the final draft.
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. The Anatomical Record 94(2), 239-247.
| Crossref | Google Scholar | PubMed |
Adeniran BV, Bjarkadottir BD, Appeltant R, Lane S, Williams SA (2021) Improved preservation of ovarian tissue morphology that is compatible with antigen detection using a fixative mixture of formalin and acetic acid. Human Reproduction 36(7), 1871-1890.
| Crossref | Google Scholar | PubMed |
Berkholtz CB, Shea LD, Woodruff TK (2006) Extracellular matrix functions in follicle maturation. Seminars in Reproductive Medicine 24(4), 262-269.
| Crossref | Google Scholar | PubMed |
Bjarkadottir BD, Walker CA, Fatum M, Lane S, Williams SA (2021) Analysing culture methods of frozen human ovarian tissue to improve follicle survival. Reproduction and Fertility 2(1), 59-68.
| Crossref | Google Scholar | PubMed |
Chiti MC, Dolmans MM, Lucci CM, Paulini F, Donnez J, Amorim CA (2017) Further insights into the impact of mouse follicle stage on graft outcome in an artificial ovary environment. Molecular Human Reproduction 23(6), 381-392.
| Crossref | Google Scholar | PubMed |
Dolmans M-M, Luyckx V, Donnez J, Andersen CY, Greve T (2013) Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertility and Sterility 99(6), 1514-1522.
| Crossref | Google Scholar | PubMed |
Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM (1999) Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Molecular Endocrinology 13(6), 1018-1034.
| Crossref | Google Scholar | PubMed |
Eppig JJ, O’Brien MJ (1996) Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction 54(1), 197-207.
| Crossref | Google Scholar | PubMed |
Ghezelayagh Z, Khoshdel-Rad N, Ebrahimi B (2022) Human ovarian tissue in-vitro culture: primordial follicle activation as a new strategy for female fertility preservation. Cytotechnology 74, 1-15.
| Crossref | Google Scholar | PubMed |
Griffin J, Emery BR, Huang I, Peterson CM, Carrell DT (2006) Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). Journal of Experimental & Clinical Assisted Reproduction 3, 2.
| Crossref | Google Scholar |
Grosbois J, Bailie EC, Kelsey TW, Anderson RA, Telfer EE (2023) Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture. Human Reproduction 38(3), 444-458.
| Crossref | Google Scholar | PubMed |
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O (2002) Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. The Journal of Clinical Endocrinology & Metabolism 87(1), 316-321.
| Crossref | Google Scholar | PubMed |
Irving-Rodgers HF, Rodgers RJ (2000) Ultrastructure of the basal lamina of bovine ovarian follicles and its relationship to the membrana granulosa. Reproduction 118(2), 221-228.
| Crossref | Google Scholar |
Kaune H, Sheikh S, Williams SA (2017) Analysis of in vitro follicle development during the onset of premature ovarian insufficiency in a mouse model. Reproduction, Fertility and Development 29(8), 1538-1544.
| Crossref | Google Scholar | PubMed |
Knight PG, Glister C (2006) TGF-β superfamily members and ovarian follicle development. Reproduction 132(2), 191-206.
| Crossref | Google Scholar | PubMed |
Lo BKM, Sheikh S, Williams SA (2019) In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries. Reproduction 157(2), 135-148.
| Crossref | Google Scholar | PubMed |
McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE (2018) Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Molecular Human Reproduction 24(3), 135-142.
| Crossref | Google Scholar | PubMed |
Moravek MB, Pavone ME, Burns K, Kashanian JA, Anderson RA, Klosky JL, Rotz SJ, Stern CJ, Rodriguez-Wallberg KA, Levine JM, Meacham LR (2023) Fertility assessment and treatment in adolescent and young adult cancer survivors. Pediatric Blood & Cancer 70, e28854.
| Crossref | Google Scholar |
Morgan S, Campbell L, Allison V, Murray A, Spears N (2015) Culture and co-culture of mouse ovaries and ovarian follicles. Journal of Visualized Experiments 97, e52458.
| Crossref | Google Scholar |
Pedersen T (1969) Follicle growth in the immature mouse ovary. Acta Endocrinologica 62(1), 117-132.
| Crossref | Google Scholar | PubMed |
Pedersen T, Peters H (1968) Proposal for a classification of oocytes and follicles in the mouse ovary. Reproduction 17(3), 555-557.
| Crossref | Google Scholar |
Peters H (1969) The development of the mouse ovary from birth to maturity. Acta Endocrinologica 62(1), 98-116.
| Google Scholar | PubMed |
Rosendahl M, Greve T, Andersen CY (2013) The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. Journal of Assisted Reproduction and Genetics 30, 11-24.
| Crossref | Google Scholar | PubMed |
Saito H, Berger T, Mishell D, Marrs RP (1984) Effect of variable concentration of serum on mouse embryo development. Fertility and Sterility 41(3), 460-464.
| Crossref | Google Scholar | PubMed |
Thomas FH, Vanderhyden BC (2006) Oocyte–granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reproductive Biology and Endocrinology 4, 19.
| Crossref | Google Scholar |
Van Houten ELAF, Themmen AP, Visser JA (2010) Anti-Müllerian hormone (AMH): regulator and marker of ovarian function. Annales d’Endocrinologie 71(3), 191-197.
| Crossref | Google Scholar | PubMed |
Visser JA, Themmen APN (2005) Anti-Müllerian hormone and folliculogenesis. Molecular and Cellular Endocrinology 234(1–2), 81-86.
| Crossref | Google Scholar | PubMed |
Walker CA, Bjarkadottir BD, Fatum M, Lane S, Williams S (2021) Variation in follicle health and development in cultured cryopreserved ovarian cortical tissue: a study of ovarian tissue from patients undergoing fertility preservation. Human Fertility 24(3), 188-198.
| Crossref | Google Scholar | PubMed |
Winship AL, Carpenter M, Griffiths M, Hutt KJ (2019) Vincristine chemotherapy induces atresia of growing ovarian follicles in mice. Toxicological Sciences 169(1), 43-53.
| Crossref | Google Scholar | PubMed |
Xu F, Lawson MS, Bean Y, Ting AY, Pejovic T, De Geest K, Moffitt M, Mitalipov SM, Xu J (2021) Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Human Reproduction 36, 1326-1338.
| Crossref | Google Scholar | PubMed |
Young JM, McNeilly AS (2010) Theca: the forgotten cell of the ovarian follicle. Reproduction 140(4), 489-504.
| Crossref | Google Scholar | PubMed |


