Comprehensive proteomic characterization and functional annotation of common carp seminal plasma
Anna Małgorzata Majewska A , Natalia Kodzik A , Mariola Aleksandra Dietrich
A , Natalia Kodzik A , Mariola Aleksandra Dietrich  A and Andrzej Ciereszko
A and Andrzej Ciereszko  A *
A *
A
Abstract
Understanding the protein composition of seminal plasma is crucial for elucidating reproductive mechanisms and improving aquaculture practices. Proteomic studies provide insights into the biological functions of seminal plasma in fish, yet comprehensive datasets for carp remain limited.
This study aimed to comprehensively characterize the proteome of carp seminal plasma, classify identified proteins on the basis of their cellular localization, and explore their functional roles in reproductive and physiological processes.
Using high-throughput liquid chromatography–tandem mass spectrometry (LC–MS/MS)-based proteomic analysis and updated Cyprinus carpio genome annotation, we identified and classified proteins on the basis of homology to human genes and fish-specific annotations. Bioinformatic tools were employed to analyze their functions and involvement in key biological pathways.
In total, 1402 proteins were identified, the highest number being reported for this species. Of the 1354 proteins homologous to human genes, 141 were secretory, 92 were both secretory and intracellular, and 1121 were intracellular. Additionally, 49 proteins were fish-specific, involved in immune response, detoxification, cold protection, and proteolytic defence. Bioinformatic analyses indicated roles in immune and stress responses, extracellular matrix organization, metabolism, and gene expression. The presence of extracellular vesicles was supported by the identification of 636 associated proteins. Reproductive-related proteins were linked to gamete generation, spermatogenesis, and sperm motility.
This dataset represents the most comprehensive proteomic profile of carp seminal plasma, offering new insights into its biological functions.
The findings enhance our understanding of carp reproductive biology and have potential applications in aquaculture, including sperm preservation, fertilization success, and disease resistance.
Keywords: Cyprinus carpio, fish, mass spectrometry, protein function, protein localization, proteome, reproductive tract, seminal plasma.
Introduction
Aquaculture of common carp
The common carp (Cyprinus carpio L.) is one of the most commercially important global aquaculture species. Historically, carp has been among the oldest freshwater species cultivated for food, playing a pivotal role in both Asian and European aquaculture. For example, in Germany and Poland, carp ranks second in production (https://www.aquaculture-welfare-standards.net/en/species/carp/, Myszkowski 2022) and is one of the key species in Asian aquaculture (Miao and Wang 2020). In aquaculture conditions, carps are raised in freshwater ponds and are often artificially reproduced using reproductive biotechniques, such as hormonal stimulation and artificial fertilization. As such, the production of high-quality gametes, including spermatozoa, is a prerequisite for successful reproduction. Sufficient knowledge regarding semen physiology and biochemistry is essential for improving reproductive biotechniques and for gaining insight into the functioning of carp reproductive system.
Seminal plasma – definition and function
The semen of vertebrates, including fish, has two components: the cellular component mainly composed of male gametes (spermatozoa), involved in fertilization, and immune cells, and the liquid component, which is the seminal plasma. In most teleost fish, seminal plasma is a secretory product of the testes and spermatic duct; some compounds may also originate from blood plasma (Ciereszko 2008; Dietrich et al. 2010a). Seminal plasma is uniquely composed of mineral and organic substances supporting spermatozoa. The main role of seminal plasma is to create an optimal environment for the storage of spermatozoa, often for prolonged periods, with special emphasis on maintaining optimal osmolality, keeping spermatozoa in the immotile state, and supporting sperm metabolism, antimicrobial and stress protection, and protection against xenobiotics (Ciereszko 2008). The composition of seminal plasma reflects species specificity of fish reproduction; for example, significant differences are observed between cold-water salmonids and warm-water cyprinids (Ciereszko et al. 2017).
Seminal proteins, their role, and proteomic approach
Proteins are an important organic composition of teleost fish seminal plasma and are involved in the protection of sperm viability (Lahnsteiner et al. 2004; Lahnsteiner 2007). Several major proteins have been purified and characterized, which allowed deeper insights into their functions. For example, protease inhibitors (Mak et al. 2004; Wojtczak et al. 2007a; Nynca et al. 2010a, 2011a) are involved in the protection of spermatozoa against proteolytic enzymes present in seminal plasma (Kowalski et al. 2003). Transferrin is a multitask protein with antioxidative and antimicrobial protection function as well as protective role against cadmium toxicity (Dietrich et al. 2010a, 2011). Parvalbumin is a calcium-binding protein that appears to be characteristic for carp semen, with the possible role in sperm motility activation (Dietrich et al. 2010b). Apolipoproteins are associated with the stabilization of sperm membranes and are characterized by antibacterial properties (Nynca et al. 2010b; Dietrich et al. 2014a), lipocalins are involved in the transportation of hydrophobic molecules (Nynca et al. 2011b), and hemopexin plays a pivotal role in antioxidative protection (Dietrich et al. 2020). Aside from major proteins that are quite easy to detect, there are hundreds of minor proteins in fish seminal plasma that can now be detected using a proteomic approach, which enables comprehensive and large-scale analysis of proteins (Ciereszko et al. 2012; Shaliutina et al. 2012; Dietrich et al. 2019a). This allows, for the first time, the acquisition of a comprehensive picture of seminal proteins and the characterization of their role by using bioinformatics tools, which opens new horizons in unraveling the new biological roles of seminal proteins. Furthermore, proteomics facilitates the selection proteins of interest for further detailed studies focusing on their structure and function.
Current knowledge and justification of the study
Earlier proteomic studies identified a limited number (22–137) of proteins in carp seminal plasma by using one-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Shaliutina-Kolešová et al. 2016; Dietrich et al. 2014b, 2019a, 2019b, 2021). These proteins were analyzed using bioinformatics tools, which significantly enhanced basic knowledge regarding seminal plasma proteins and their potential role in fish reproduction, including the regulation of sperm motility, spermatogenesis, maintenance of sperm membrane lipid stability and antioxidative protection, as well as immune and stress responses. However, because of the limited number of proteins, the results of these studies are likely to be incomplete.
Our recent comparative study focusing on bacterial Aeromonas salmonicida infection of male carps and changes in seminal plasma protein abundance in relation to infection-provided insights into the immune response of male reproductive system, including acute-phase response, complement activation response, complement activation and coagulation, and inflammation (Dietrich et al. 2022). In this study, we focused only on 44 differentiated proteins vital for infection response. This is only a fraction of all identified proteins in this study because using high-throughput liquid chromatography–tandem mass spectrometry (LC–MS/MS)-based proteomic analysis and updated Cyprinus carpio genome annotation, 1402 seminal plasma proteins were identified; this value is significantly higher than that previously reported for carp. This provides, for the first time, unique opportunity to conduct broad analysis on carp seminal plasma proteins as such a high number of proteins enhances the strength of bioinformatic analysis, potentially resulting in the accomplishment of comprehensive knowledge regarding male carp reproduction. Therefore, our study aimed to conduct comprehensive bioinformatics analysis of seminal plasma proteome of male controls from the previous experiment (Dietrich et al. 2022) by using advanced bioinformatics tools. To obtain better understanding of the protein function, we introduced a novel classification system by categorizing proteins into three groups on the basis of their cellular localization for (1) secretory proteins, (2) proteins with either secretory properties and localization to different cellular compartments, and (3) proteins without secretory properties. We assumed that secretory proteins are presumably specific seminal plasma proteins and that intracellular proteins have a multicellular origin from either the spermatozoa or diploid cells, particularly leucocytes, of the carp reproductive system. Furthermore, we provided more information on proteins involved in extracellular vesicles (EVs) owing to the emerging importance of EVs in seminal plasma function. In addition, we identified proteins specific for fish and discussed their possible role in male fish reproduction.
Materials and methods
Characteristics of common carp semen
Sperm concentration was measured using the spectrophotometric technique (Ciereszko and Dabrowski 1993) and sperm motility via computer-assisted sperm analysis, as described by Wojtczak et al. (2007b).
LC–MS analysis
For the purpose of conducting LC–MS analyses, we used seminal plasma obtained from healthy carps (n = 5) from the control group of a previously conducted experiment by Dietrich et al. (2022). LC–MS analysis and sample preparation were performed at the Mass Spectrometry Laboratory at the Institute of Biochemistry and Biophysics of Polish Academy of Sciences (PAS). We used an LC–MS system consisting of ultraperformance liquid chromatograph (M-class, Waters) coupled with a Q Exactive mass spectrometer (Thermo Scientifi, Waltham, Massachusetts, USA) using a Flex nano electrospray ionization ion source (Thermo Scientific), as previously described (Dietrich et al. 2022). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al. 2025) partner repository, with the dataset identifier PXD065911 and 10.6019/PXD065911.
Bioinformatics
We obtained information from UniProt annotations (The UniProt Consortium 2023) for each protein to divide the proteins into three groups by using subcellular location criteria. Secretory proteins were defined as proteins located outside the cell membranes. The second group of proteins were characterized as both secretory and intracellular, such as cell membrane, endoplasmic reticulum, lysosome, Golgi apparatus, cytoplasmic vesicles, and extracellular space. The third group of proteins were non-secretory and had various intracellular localizations. To evaluate the potential similarity of seminal plasma to blood protein, we searched The Human Protein Atlas (Human Protein Atlas, proteinatlas.org; Uhlén et al. 2015) to compare the results of our identified proteins with those listed in the database as secreted to blood.
We conducted separate analyses for protein groups divided according to subcellular location criteria. In addition, to gain a broader perspective, we analyzed all identified proteins and conducted a functional enrichment analysis of networks associated with EVs and reproduction.
We performed the functional profiling of the identified proteins from the selected groups into three gene ontology (GO) categories (biological processes, cellular components, and molecular functions) by using the Search Tool for the Retrieval of Interacting Genes (STRING) database (ver. 10.5, http://string-db.org/) with a medium confidence score cutoff of 0.4.
Results
Characteristics of common carp semen
The sperm concentration was 19.2 ± 0.8 × 109 mL−1, and the sperm motility was 91 ± 6%, with the following sperm kinetic parameters: curvilinear velocity (VCL) 119.7 ± 9.6 μm s−1, straight-line velocity (VSL) 66.0 ± 6.7 μm s−1, and amplitude of lateral head displacement (ALH) 6.9 ± 1.1 μm. The seminal plasma osmolality was 279.0 ± 3.8 mOsm kg−1, and the protein concentration was 1.34 ± 0.10 mg mL−1.
LC‒MS/MS analysis of seminal plasma proteins
LC–MS/MS analysis and sample preparation were performed at the Mass Spectrometry Laboratory at the Institute of Biochemistry and Biophysics of PAS. From a total of 386,204 MS/MS spectra and 5182 peptides, 2009 proteins were identified in carp seminal plasma in at least three individuals, with a minimum of two unique peptides and false discovery rate (FDR) of ≤1% (Supplementary Table S1). After removing duplicates and triplicates, the total number of proteins was 1402, among which 1354 (Table S2) were annotated to unique human gene homologs and 49 (Table 1) were fish-specific.
| Accession number | Protein name | |
|---|---|---|
| XP_018932680.1 | Natterin-3-like | |
| XP_042615378.1 | Aerolysin-like protein | |
| XP_042595238.1 | Galectin 17 | |
| XP_042628982.1 | Fucolectin-5-like | |
| XP_018978486.2 | Jeltraxin-like | |
| KTG44524.1 | Immunoglobulin kappa light chain-like | |
| BAB90985.1 | Immunoglobulin light chain, partial | |
| XP_018952365.1 | Counting factor associated protein D-like | |
| AVD68699.1 | Secreted novel AID/APOBEC-like deaminase 1 | |
| XP_018965652.2 | Secreted novel AID/APOBEC-like deaminase 1 | |
| XP_018918723.2 | Secreted novel AID/APOBEC-like deaminase 1 | |
| XP_042594085.1 | Saxitoxin and tetrodotoxin-binding protein 2-like | |
| XP_018938837.1 | Saxitoxin and tetrodotoxin-binding protein 1-like | |
| XP_018975177.1 | Type-4 ice-structuring protein LS-12 | |
| XP_042608691.1 | Prion protein, related sequence 3 isoform X2 | |
| XP_018945213.1 | Elastase 3-like | |
| XP_018948117.2 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 10a | |
| XP_042619573.1 | Probable serpin E3 | |
| XP_042617617.1 | Proprotein convertase subtilisin/kexin type-1 inhibitor-like | |
| BAF73406.1 | Vitellogenin B1 | |
| XP_042605320.1 | Vitellogenin isoform X2 | |
| XP_042621935.1 | Vitellogenin 3, phosvitinless | |
| XP_042605322.1 | Vitellogenin-like isoform X2 | |
| XP_042605323.1 | Vitellogenin-like | |
| XP_042595400.1 | Protein starmaker-like | |
| XP_042568308.1 | Cytotoxin 8-like isoform X2 | |
| XP_042632364.1 | Chromosomal protein D1-like | |
| XP_042570957.1 | Glutenin, high molecular weight subunit DX5-like | |
| XP_018936365.1 | D-threo-3-hydroxyaspartate dehydratase-like | |
| XP_042614224.1 | Sodium/potassium-transporting ATPase subunit beta-1a | |
| XP_042630804.1 | Para-nitrobenzyl esterase | |
| XP_042583538.1 | WD repeat-containing protein 76 | |
| XP_042614504.1 | Methyltransferase-like protein 7 A | |
| XP_042581821.1 | Homocysteine S-methyltransferase YbgG-like | |
| KTF74296.1 | Hypothetical protein cypCar_00046013 similar to: Immunoglobulin V-set domain | |
| XP_042604673.1 | Uncharacterized protein LOC122141410 similar to: Immunoglobulin V-set domain | |
| KTG47831.1 | Hypothetical protein cypCar_00045019 similar to: Immunoglobulin V-set domain | |
| XP_042604667.1 | Uncharacterized protein LOC109080562 isoform X1 similar to: Immunoglobulin V-set domain | |
| XP_042604929.1 | Uncharacterized protein LOC109074518 similar to: Immunoglobulin V-set domain | |
| XP_042604666.1 | Uncharacterized protein LOC122134475 similar to: Immunoglobulin V-set domain | |
| XP_042604669.1 | Uncharacterized protein LOC109080562 isoform X2 similar to: Immunoglobulin V-set domain | |
| XP_042604944.1 | Uncharacterized protein LOC122141489 isoform X3 similar to: Immunoglobulin V-set domain | |
| XP_018965146.1 | Uncharacterized protein si:ch1073-126c3.2 | |
| XP_018980481.2 | Uncharacterized protein si:rp71-15k1.1 | |
| XP_042613171.1 | Uncharacterized protein LOC109090069 | |
| XP_018926314.1 | Uncharacterized protein LOC109053368 | |
| KTF71371.1 | Hypothetical protein cypCar_00027553 | |
| XP_042632362.1 | Uncharacterized protein LOC109112387 | |
| KTG36061.1 | Hypothetical protein cypCar_00047716 |
Functional annotation of seminal plasma proteome
To gain insights into the biological roles of proteins in carp seminal plasma, we categorized the proteins by using the UniProt database into three groups on the basis of their cellular localization. Of the 1354 proteins, 233 were identified as secretory, which represents 17.13% of the total identified proteins. Among these secretory proteins, 141 were classified as exclusively secretory (Table 2), 92 as both secretory and intracellular (Table 3), and the remaining as intracellular (Fig. 1).
| Gene name | Protein name | |
|---|---|---|
| A2M | Alpha-2-macroglobulin | |
| A2ML1 | Alpha-2-macroglobulin-like 1 | |
| ACAN | Aggrecan | |
| ACE2 | Angiotensin-converting enzyme 2 | |
| ADAMTS13 | ADAM metallopeptidase with thrombospondin type-1 motif 13 | |
| AGT | Angiotensinogen | |
| AHSG | Alpha 2-HS glycoprotein | |
| AMH | Anti-Mullerian hormone | |
| AMY2A | Alpha-amylase 2 A | |
| AMY2B | Alpha-amylase 2B | |
| ANXA2 | Annexin A2 | |
| APOA1 | Apolipoprotein A1 | |
| APOA2 | 14 kDa apolipoprotein | |
| APOA4 | Apolipoprotein A-IV | |
| APOC1 | Apolipoprotein C1 | |
| APOE | Apolipoprotein E | |
| BTD | Biotinidase | |
| C1QB | Complement system-related protein C1qB | |
| C1QC | Complement system-related protein C1qC | |
| C1QL2 | Complement C1q like 2 | |
| C1QL4 | Complement C1q like 4 | |
| C1QTNF3 | C1q and TNF related 3 | |
| C2 | Complement c2 | |
| C3 | Complement C3 | |
| C5 | Complement c5 | |
| C6 | Complement c6 | |
| C6orf58 | Chromosome 6 open reading frame 58 | |
| C7 | Complement c | |
| C9 | Complement c9 | |
| C8B | Complement component C8 beta chain-like isoform X1 | |
| CBLN2 | Cerebellin 2 precursor | |
| CES5A | Carboxylesterase 5 A | |
| CFB | Complement factor B | |
| CFH | Complement factor H-like isoform X1 | |
| CFP | Complement factor properdin | |
| CFHR1 | complement factor H-related1 | |
| CFHR2 | complement factor H-related2 | |
| CFI | Complement factor I | |
| CLEC3B | C-type lectin domain family 3 member B | |
| CLU | Clusterin | |
| COL6A1 | Collagen type-VI alpha 1 chain | |
| COL6A3 | Collagen type-VI alpha 3 chain | |
| COL12A1 | Collagen type-XII alpha 1 chain | |
| COL1A1 | Collagen type-I alpha 1 chain | |
| COL1A2 | Collagen type-I alpha 2 chain | |
| CELA1 | Chymotrypsin-like elastase 1 | |
| CELA2A | Elastase 3-like | |
| CETP | Cholesteryl ester transfer protein | |
| COMP | Cartilage oligomeric matrix protein | |
| CP | Ceruloplasmin | |
| CFD | Complement factor D | |
| CPA1 | Carboxypeptidase A1 | |
| CPN2 | Carboxypeptidase N subunit 2 | |
| CST3 | Cystatin C | |
| DMBT1 | Deleted In malignant brain tumors 1 | |
| EBI3 | Epstein-Barr virus induced 3 | |
| ECM1 | extracellular matrix protein 1 | |
| EMILIN2 | Elastin microfibril interfacer 2 | |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 | |
| F5 | Coagulation factor V-like | |
| F7 | Coagulation factor VII | |
| F9 | Coagulation factor IX | |
| F10 | Coagulation factor X | |
| FBLN1 | Fibulin 1 | |
| FCGBP | Fc gamma binding protein | |
| FETUB | Fetuin B | |
| FGA | Fibrinogen alpha chain | |
| FGB | Fibrinogen beta chain | |
| FGG | Fibrinogen gamma chain | |
| FSTL1 | Follistatin-like 1 | |
| FUCA2 | Alpha-L-fucosidase 2 | |
| GAS6 | Growth arrest specific | |
| GC | Vitamin D-binding protein | |
| GPX3 | Glutathione peroxidase 3 | |
| HABP2 | Hyaluronan binding protein 2 | |
| HP | Haptoglobin | |
| HPX | Hemopexin | |
| HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein-like isoform X1 | |
| IGFALS | Insulin-like growth factor binding protein acid labile subunit | |
| IGFBP2 | Insulin-like growth factor binding protein 2 | |
| IL4R | Interleukin 4 receptor | |
| INHA | Inhibin subunit alpha | |
| INHBA | Inhibin subunit beta A | |
| ITIH2 | Inter-alpha-trypsin inhibitor heavy chain 2 | |
| ITIH3 | Inter-alpha-trypsin inhibitor heavy chain 3 | |
| ITIH4 | Inter-alpha-trypsin inhibitor heavy chain 4 | |
| KLKB1 | Kallikrein B1 | |
| KNG1 | Kininogen 1 | |
| LGALS3 | Galectin 3 | |
| LGALS3BP | Galectin 3 binding protein | |
| LRG1 | Leucine-rich alpha-2-glycoprotein 1 | |
| LTBP1 | Latent-transforming growth factor beta-binding protein 1 isoform X2 | |
| LUM | Lumican | |
| LYG2 | Lysozyme G2 | |
| MAN2B2 | Mannosidase alpha class 2B member 2 | |
| MASP2 | Mannan-binding lectin serine protease 2 | |
| MBL2 | Mannose binding lectin 2 | |
| MFAP4 | Microfibril associated protein 4 | |
| MIA | MIA SH3 domain containing | |
| MMRN2 | Multimerin 2 | |
| MSMB | Microseminoprotein beta | |
| MST1 | Macrophage stimulating 1 | |
| MUC2 | Mucin 2 | |
| MUC5B | Mucin 5B, oligomeric mucus/gel-forming | |
| MXRA5 | Matrix remodeling associated 5 | |
| NCAN | Neurocan | |
| NHLRC3 | NHL repeat containing protein | |
| PAPLN | Papilin, proteoglycan-like sulfated glycoprotein | |
| PDGFD | Platelet derived growth factor D | |
| PEBP4 | Phosphatidylethanolamine binding protein 4 | |
| PI16 | Peptidase inhibitor 16 | |
| PLG | Plasminogen | |
| PRG4 | Proteoglycan 4b | |
| PROS1 | Protein S | |
| PRSS1 | Serine protease 1 | |
| PRSS2 | Serine protease 2 | |
| PTX3 | Pentraxin 3 | |
| QPCT | Glutaminyl-peptide cyclotransferase | |
| RBP4 | Retinol binding protein 4 | |
| RNASE4 | Ribonuclease A family member 4 | |
| RNPEP | Aminopeptidase B-like isoform X1 | |
| SCG2 | Secretogranin II | |
| SELENOP | Selenoprotein P | |
| SERPINA1 | Serpin family A member 1 | |
| SERPINA6 | Serpin family A member 6 | |
| SERPINA10 | Serpin family A member 10 | |
| SERPINC1 | Antithrombin-III | |
| SERPINE3 | Serpin family E member 3 | |
| SERPINF1 | Serpin family F member 1 | |
| SERPINF2 | Serpin family F member 2 | |
| SERPING1 | Serpin family G member 1 | |
| SHBG | Sex hormone binding globulin | |
| SLPI | Secretory leukocyte peptidase inhibitor | |
| SPARC | Secreted protein acidic and cysteine rich | |
| SPINT1 | Kunitz-type protease inhibitor 1 | |
| TCN2 | Transcobalamin 2 | |
| TGFBI | Transforming growth factor beta induced | |
| TF | Transferrin | |
| THSD4 | Thrombospondin type-1 domain containing 4 | |
| TIMP2 | Tissue inhibitor of metalloproteinases 2 | |
| VMO1 | Vitelline membrane outer layer 1 homolog |
Proteins marked in red are indicated as blood proteins in humans according to The Human Protein Atlas.
| Gene name | Protein name | |
|---|---|---|
| ADGRE5 | Adhesion G protein-coupled receptor E5 | |
| AGR2 | Anterior gradient 2, protein disulfide isomerase family member | |
| AMBP | Alpha-1-microglobulin/bikunin precursor | |
| ANXA1 | Annexin A1 | |
| APOB | Apolipoprotein B | |
| ASAH1 | Acid ceramidase | |
| B2M | Beta-2 microglobulin | |
| C4A | Complement C4A (Chido/Rodgers blood group) | |
| C4B | Complement C4B (Chido/Rodgers blood group) | |
| CALU | Calumenin | |
| CALR | Calreticulin | |
| CD48 | CD48 molecule | |
| CD59 | CD59 glycoprotein | |
| CD63 | CD63 antigen | |
| CD163 | Scavenger receptor cysteine-rich type-1 protein M130 | |
| CHIA | Chitinase acidic | |
| COPA | COPI coat complex subunit alpha | |
| CPQ | Carboxypeptidase Q | |
| CTRB1 | Chymotrypsinogen B | |
| CTSB | Cathepsin B | |
| CTSK | Cathepsin K | |
| CTSL | Procathepsin L1 | |
| CTSS | Cathepsin S | |
| DAG1 | Dystroglycan 1 | |
| DPP7 | Dipeptidyl peptidase 7 | |
| EFNA1 | Ephrin A1 | |
| EPDR1 | Ependymin related 1 | |
| FAM3C | Protein FAM3C | |
| F13A1 | Coagulation factor XIII A chain | |
| FBLN2 | Fibulin 2 | |
| FN1 | Fibronectin 1 | |
| GARS1 | Glycyl-TRNA synthetase 1 | |
| GBP1 | Guanylate binding protein 1 | |
| GGH | Gamma-glutamyl hydrolase | |
| GPC5 | Glypican 5 | |
| GPI | Glucose-6-phosphate isomerase | |
| GRN | Progranulin | |
| GSN | Gelsolin | |
| HEG1 | Protein HEG homolog 1 | |
| HLA-E | HLA class-1 histocompatibility antigen alpha chain E | |
| HMGB1 | High-mobility group protein B1 | |
| HMGB2 | High-mobility group protein B2 | |
| HPSE | Heparanase | |
| HSP90AB1 | Heat shock protein HSP 90-beta | |
| HYAL1 | Hyaluronidase-1 | |
| ICAM4 | Intercellular adhesion molecule 4 | |
| IDE | Insulin-degrading enzyme | |
| IGHM | Immunoglobulin heavy constant Mu | |
| IGKV1D-13 | Immunoglobulin kappa variable 1D-13 | |
| ITM2B | Integral membrane protein 2B | |
| ITLN1 | Intelectin 1 | |
| ITLN2 | Intelectin 2 | |
| JAM3 | Junctional adhesion molecule 3 | |
| KARS1 | lysyl-tRNA synthetase 1 | |
| LAMA1 | Laminin subunit alpha 1 | |
| LAMA4 | Laminin subunit alpha 4 | |
| LAMC1 | Laminin subunit gamma 1 | |
| LECT2 | Leukocyte cell-derived chemotaxin-2 | |
| LGALS9 | Galectin 9 | |
| MMP2 | 72 kDa type-IV collagenase | |
| NAMPT | Nicotinamide phosphoribosyltransferase | |
| NAXE | NAD(P)H-hydrate epimerase | |
| NENF | Neudesin | |
| NPC2 | NPC intracellular cholesterol transporter 2 | |
| NRCAM | Neuronal cell adhesion molecule | |
| NRP1 | Neuropilin 1 | |
| NRP2 | Neuropilin 2 | |
| NUCB1 | Nucleobindin-1 | |
| NUCB2 | Nucleobindin 2 | |
| PAM | Peptidyl-glycine alpha-amidating monooxygenase | |
| PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | |
| PLTP | Phospholipid transfer protein | |
| PLXNB1 | Plexin B1 | |
| PPIA | Peptidylprolyl isomerase A | |
| PPT1 | Palmitoyl-protein thioesterase 1 | |
| PROC | Vitamin K-dependent protein C | |
| PSAP | Prosaposin | |
| PTGDS | Prostaglandin-H2 D-isomerase | |
| QSOX1 | Sulfhydryl oxidase 1 | |
| RNASET2 | Ribonuclease T2 | |
| SCG3 | Secretogranin-3 | |
| SNCA | Alpha-synuclein | |
| SOD3 | Extracellular superoxide dismutase [Cu-Zn] | |
| SORL1 | Sortilin-related receptor | |
| SPINK2 | Serine protease inhibitor Kazal-type 2 | |
| SRGN | Serglycin | |
| TFPI | Tissue factor pathway inhibitor | |
| TGFBR3 | Transforming growth factor beta receptor type-3 | |
| TGM2 | Protein-glutamine gamma-glutamyltransferase 2 | |
| VASN | Vasorin | |
| VWC2L | Von Willebrand factor C domain containing 2-like | |
| YBX1 | Y-box-binding protein 1 |
Proteins marked in red are indicated as blood proteins in humans according to The Human Protein Atlas.
Percentage distribution of proteins unique to human gene homologs, categorized on the basis of their localization.
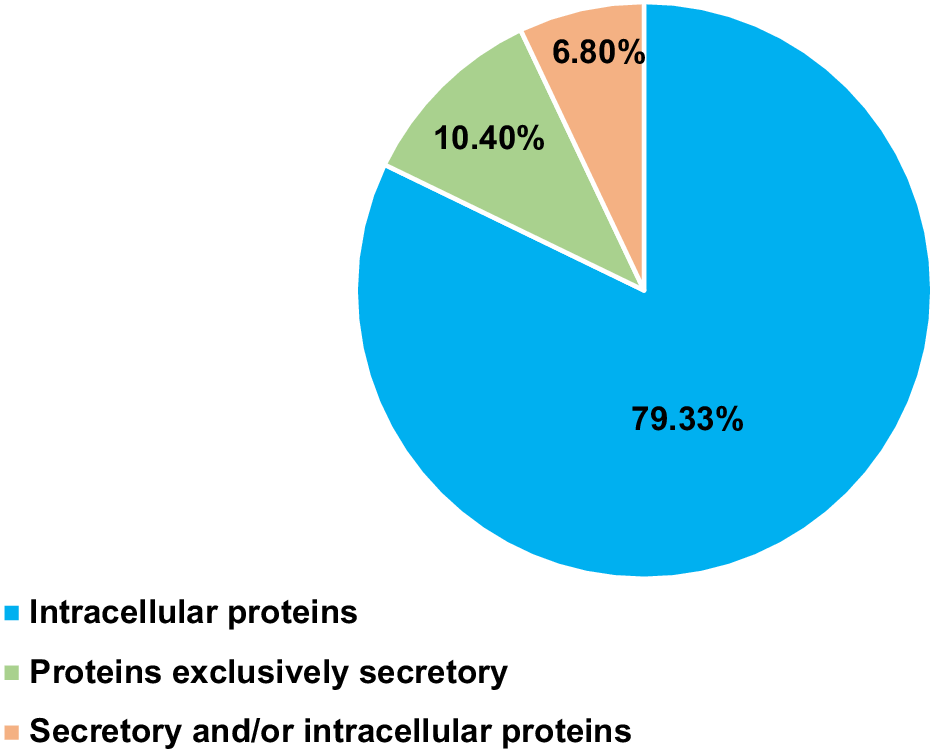
Among the 141 secretory proteins, all were found to contain signal peptides. Majority of the proteins (109) have also been indicated as blood proteins in human (Table 2).
GO analysis of biological processes showed that these proteins are involved in 222 biological processes. Major biological processes were related to immune response, response to stress, extracellular matrix organization, and lipid transport and metabolism (Fig. 2a). We also identified proteins involved in the regulation of vesicle-mediated transport and body fluid levels.
The gene ontology (GO) analysis of biological process (a), molecular function (b) and cellular component classification (c) of exclusively secretory proteins identified in carp seminal plasma.
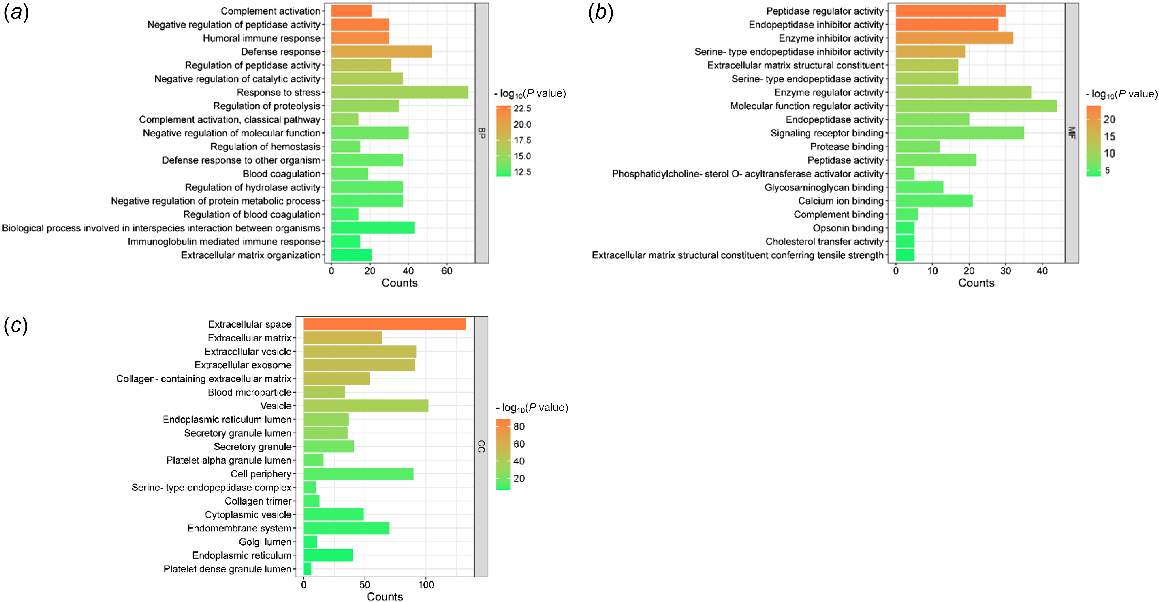
Molecular function analysis showed mostly the activities of proteolytic enzymes and their regulators. Binding activities were also identified for protease, signaling receptor, glycosaminoglycan, calcium ions, and complement. Other molecular functions include extracellular matrix structural constituent and phosphatidylcholine-sterol O-acyltransferase activator activity (Fig. 2b).
According to the cellular component criteria, secretory proteins were mainly located in different extracellular regions, secretory granules, and lumens of several cell structure (Fig. 2c). Moreover, secretory proteins were associated with the endoplasmic reticulum, Golgi apparatus, vesicles, and membranes. They were also associated with various extracellular protein complexes, such as serine-type endopeptidase complex, high-density lipoprotein particle, very-low-density lipoprotein particle, and chylomicrons.
The complete results of the GO analysis of exclusively secretory proteins are presented in Table S3.
A subset of 92 proteins with both secretory and intracellular localizations included 40 proteins also classified as blood proteins in humans (Table 3).
GO analysis showed 244 biological processes associated with these proteins. Major biological processes (Fig. 3a) were related to various immune responses and responses to stress. Several processes were also associated with response to stimulus, which is defined as any process that results in a change in state or activity of a cell or an organism (in terms of movement, secretion, enzyme production, gene expression, etc.). Other processes were related to organismal process and development; cell differentiation, migration, and adhesion; and blood vessel morphogenesis.
The gene ontology (GO) analysis of (a) biological process, (b) molecular function and (c) cellular component classifications of proteins characterized by both secretory and intracellular localizations, and proteins exclusively localized intracellularly, identified in carp seminal plasma.
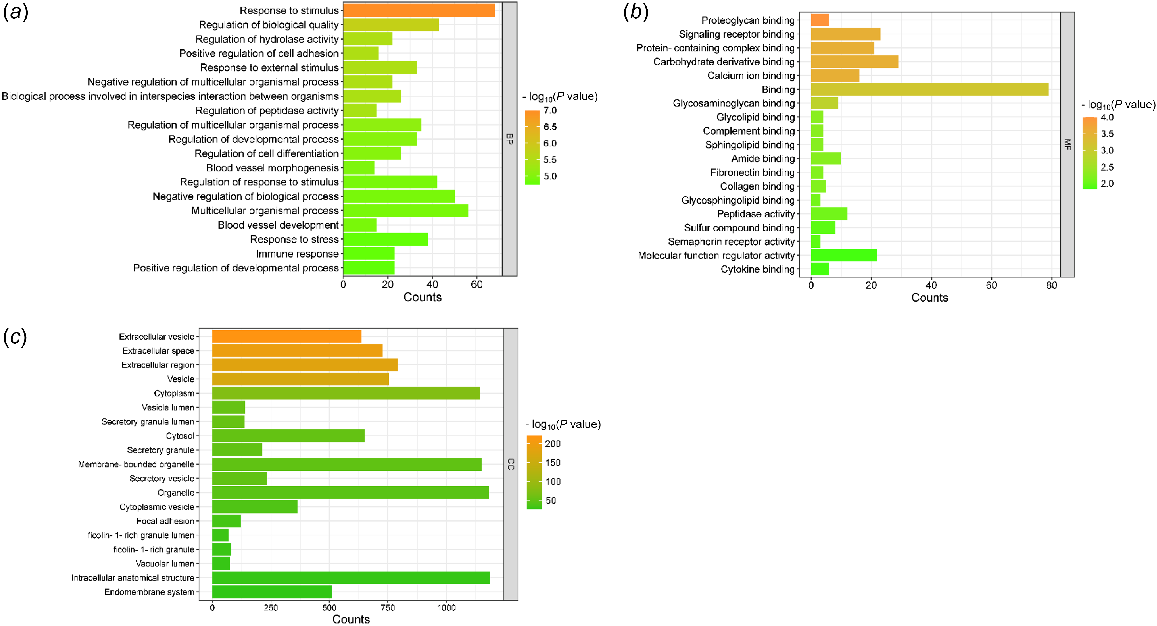
Molecular function analysis uncovered various binding activities, including those involving proteins and protein-containing complexes, signaling receptors, proteoglycans, and calcium ions (Fig. 3b). According to the cellular component criteria, proteins were mainly located in different extracellular regions, membranes, exosomes, and vesicles as well as in the endoplasmic reticulum, lysosome, and Golgi apparatus (Fig. 3c). The complete results of the GO analysis are presented in Table S4.
The majority of proteins (1121) lacked the secretory properties and were predominantly intracellular. A K-means clustering analysis using the STRING database categorized these proteins into four functional clusters.
Cluster 1 consisted of 570 proteins mainly involved in metabolic processes with molecular functions related to binding and enzyme activities. Cellular component analyses linked these proteins to majority of cellular structures (Table S5).
Cluster 2 consisted of 463 proteins associated with numerous cellular processes, such as organization of structures and proteins, regulation, transport, motility, and response to stimulus. Moreover, we found processes related to immune cells, cell development and migration, as well as protein quality control. Molecular functions were mainly related to binding. Cellular component analysis showed that among others, several processes related to exosomes, vesicles, extracellular spaces, Golgi apparatus, lysosomes, and endoplasmic reticulum (Table S6).
Cluster 3 consisted of 55 proteins related to the maintenance and regulation of the sperm motility apparatus with molecular functions being associated with microtubule binding and activity. These proteins were mainly localized to the axonemal structure (Table S7).
Cluster 4 consisted only two proteins, ABHD14B and tetratricopeptide repeat domain 38; however, no significant functional enrichment was detected.
Proteins related to extracellular vesicles
According to the cellular component criteria, we analyzed the entire protein set and performed functional enrichment of the cellular component GO:1903561 (EV; PPI enrichment P-value: <1.0 × 10−16), which comprised 636 molecules (Supplementary Fig. S1).
Functional enrichment of the selected GO term identified 713 biological processes, including those involving more than 400 proteins, such as cellular process, biological process regulation, metabolic process and biological regulation. In addition, the biological functions involving over 200 proteins include, response to stimulus, cellular metabolic process, nitrogen compound metabolic process, organic substance metabolic process, primary metabolic process, protein metabolic process, developmental process, and response to stress. Other important activities comprised response to organic substance, cellular response to chemical stimulus, regulation of protein metabolic activity, negative and positive regulations of the biological process, regulation of metabolic function, anatomical structure development, cellular metabolic process, cell differentiation, multicellular organismal process, and multicellular organism development (Table S8).
We also identified 155 significant molecular functions, including those with participation of more than 200 proteins, such as binding, protein binding, as well as hydrolase and catalytic activities (Table S8).
Table S8 presents 185 cellular component categories with involvement of more than 100 proteins, such as extracellular exosome, EV secretory granule, secretory vesicle, cytoplasmic vesicle, cytosol, endomembrane system, cell junction, intracellular anatomical structure, intracellular organelle, intracellular membrane-bound organelle, cell periphery, and protein-containing complex.
Proteins related to reproduction
To conduct a detailed analysis of proteins related to reproduction, we analyzed the entire protein set and performed functional enrichment of the networks for reproductive processes (GO:0022414; PPI enrichment P-value: <1.0 × 10−16) and sexual reproduction (GO:0019953; PPI enrichment P-value: <1.0 × 10−16), which showed 340 and 106 significant biological processes respectively.
We found that seminal plasma proteins were associated with gamete generation, spermatogenesis, and spermatozoa motility apparatus. The biological processes of both networks are presented in Table S9.
Fish-specific proteins
We identified 49 fish-specific proteins (Table 1). Among the identified proteins, the majority were molecules associated with immune responses. In addition, we noted the presence of vitellogenin group proteins, proteins characterized by other functions, and seven uncharacterized proteins.
Discussion
Most abundant carp seminal plasma proteins are secretory and also present in blood plasma
The most abundant proteins of carp seminal plasma were established in our previous studies (Dietrich et al. 2014b; Ciereszko et al. 2017), and the results of the present study clearly indicated that these proteins are of secretory origin, including transferrin, apolipoprotein (APO) E, 14-kDa APOA2, APOA1, complement C3, retinol-binding protein, serpin 1 (alpha-1 antitrypsin), fetuin, and hemopexin. Heat shock protein 90-alpha has been classified as secreted and at different cellular localizations. These proteins are likely to be mainly produced by spermatic duct and/or originate from blood because majority of carp seminal proteins are also present in blood plasma (Lahnsteiner et al. 1993, 1995; Lahnsteiner 2003; Dietrich et al. 2014b); see also Table 2. This suggestion is supported by our recent results that found the expression of major proteins, such as APOA1, APOA2, and CR, both in carp testis and liver, kidney, and spleen (Dietrich et al. 2022).
Immune response
The results of our study clearly suggest that participation in immune response, both innate and humoral, is a major role of fish seminal plasma. The introduction of proteomic approach to studies on fish reproduction clearly indicated that acute-phase proteins are major proteins of fish semen, which highlights the importance of immune response in male fish reproduction (Dietrich et al. 2022). The acute-phase response is the first rapid mechanism of protection against microbes and pathogens, tissue damage repair, and inactivation of proteases in fish (Bayne and Gerwick 2001). The immunological processes are precisely regulated, often by proteolysis, which is clearly highlighted by the presence of several proteases and their inhibitors, such as inhibitors of serine proteases (serpins) and metalloproteases (TIMP).
Notably, response to wounding and wound healing were also indicated as significant biological processes. As such, these results suggest that the male reproductive system is equipped with a protective mechanism against injuries that can occur during spawning runs and male fight during spawning.
Lipid transport and metabolism
We detected several processes related to lipid transport and metabolism, including remodeling of lipoprotein, phospholipid efflux, and cholesterol transport, with apolipoproteins being the major proteins involved in these processes (Dietrich et al. 2014a). These results agree with our previous finding indicating that the presence of lipid-/cholesterol-binding proteins is a specific feature of carp seminal plasma because the abundance of these proteins is higher in seminal plasma than in blood (Dietrich et al. 2014b). Interestingly, molecular function analysis highlighted phosphatidylcholine-sterol O-acyltransferase activator activity. This enzyme is a major source of cholesterol ester in human plasma and can be activated by apolipoprotein E (Steinmetz et al. 1985). The latter is a secretory protein in carp seminal plasma identified in our study. The main role of lipids is likely to stabilize sperm membranes and provide energy source to spermatozoa. Our recent data also suggest that lipoproteins play a role in the reproductive tract of carp during bacterial infection (Dietrich et al. 2022).
Regulation of body fluid levels
For the first time, we identified a biological process involved in the regulation of body fluid levels. This is particularly important for better understanding of the mechanism of hormonal stimulation of male carps raised under aquaculture conditions to enhance sperm production and quality (Dietrich et al. 2019a). Hydration of the testis and spermatic duct is a pre-requisite to enhancing spermiation by increasing seminal fluid production and sperm maturation (Mylonas et al. 2017). The mechanism of semen hydration is not well understood. Our results indicated the presence of 23 proteins that can regulate seminal fluid level (Table 4). Interestingly, among these proteins, we have identified plasma kallikrein (KLKB1), which can release bradykinin from kininogen (KNG1). Furthermore, we detected an angiotensin-I-converting enzyme (detected in proteins that were both secretory and intracellular), which is involved in bradykinin hydrolysis. Bradykinin is reportedly affected by sodium and water movements in tissues (Crocker and Willavoys 1975; Zaika et al. 2011). We also identified an angiotensin-like protein. These results suggest the involvement of the kallikrein–kinin system in the regulation of carp seminal plasma volume, presumably in semen hydration. This suggestion needs to be tested in further studies.
| F9 | Coagulation factor IXa heavy chain | |
| COMP | Cartilage oligomeric matrix protein | |
| APOE | Apolipoprotein E | |
| EMILIN2 | EMILIN-2 | |
| SERPINA10 | Protein Z-dependent protease inhibitor | |
| KLKB1 | Plasma kallikrein heavy chain | |
| SERPING1 | Plasma protease C1 inhibitor | |
| FGB | Fibrinogen beta chain | |
| PLG | Plasmin heavy chain A | |
| SERPINF2 | Alpha-2-antiplasmin | |
| FBLN1 | Fibulin-1 | |
| GAS6 | Growth arrest-specific protein 6 | |
| FGG | Fibrinogen gamma chain | |
| ANXA2 | Annexin A2 | |
| SERPINC1 | Antithrombin-III | |
| F5 | Coagulation factor V heavy chain | |
| ADAMTS13 | A disintegrin and metalloproteinase with thrombospondin motifs 13 | |
| F10 | Activated factor Xa heavy chain | |
| F7 | Coagulation factor VII | |
| PROS1 | Vitamin K-dependent protein S | |
| SERPINA1 | Short peptide from AAT | |
| KNG1 | Low molecular weight growth-promoting factor | |
| FGA | Fibrinogen alpha chain |
Secretory proteins with intracellular localizations
Analyses of proteins that were both secretory and intracellular showed that they had overlapping and extended biological functions, particularly secretory proteins, as observed in immune response and EV-related processes. This group is further characterized by several processes associated with development, such as organismal processes, blood vessel morphogenesis, as well as cell differentiation, migration, and adhesion. These findings suggest the involvement of these processes in spermatogenesis and sperm maturation. The latter may be supported by processes related to EVs, which play a pivotal role in sperm maturation (see below).
Proteins without secretory properties
Cluster 1 is characterized by proteins involved in a wide range of metabolic processes (both anabolic and catabolic) and gene expression activities, including post-transcriptional regulation, translation regulation, as well as ribonucleoprotein complex and ribosome biogenesis.
Interestingly, only in this cluster did we identify proteins related to proteasomes, such as proteasome complex and proteasome-mediated ubiquitin-dependent protein catabolic process. Our results strongly indicated the importance of protein ubiquitination in seminal plasma. The ubiquitin–proteasome system together with ubiquitin-like protein conjugation pathways are integral to cellular protein homeostasis (Bedford et al. 2011). Notably, the FAT10 signaling pathway links ubiquitination with the immune system as the FAT10 protein is part of the immune system and exhibits both adaptive and innate immune responses (Aichem and Groettrup 2020). The biological role of the FAT10 protein has been associated with antiviral functions (Bedford et al. 2011), male fertility traits, and immune-related functions of seminal plasma (Kosova et al. 2012).
Cluster 2 includes proteins associated with immune cell structures, developmental processes, and protein quality control mechanisms, enhancing the understanding of immune functions within the reproductive system of carp.
Our analysis uncovered structures of immune cells with cytotoxic activities. Phagocytic vesicles, which contain acid hydrolases, are formed in macrophages and are involved in the phagocytosis of microbes (Stossel et al. 1972). Azurophilic storage granules are part of neutrophilic leukocytes that are used for the digestion of phagocytized microorganisms and distinguished by the presence of myeloperoxidases, elastases, and defensins (Cieutat et al. 1998). Highly exocytosable gelatinase-poor granules are found in neutrophils and are rich in ficolin-1. Ficolin-1 is present in neutrophil granules and bind to carbohydrates present on the surface of microorganisms and function as recognition molecules in the lectin complement pathway (Rørvig et al. 2009).
We identified proteins linked to lamellipodia; ruffles and filopodia are actin-based protrusions particularly relevant in the context of mesenchymal (fibroblast-like) cell motility (Innocenti 2018). Filopodia are finger-like cellular protrusions found throughout the metazoan kingdom and have fundamental cellular functions during cell development and cell migration. They are also involved in numerous cellular processes, such as cell migration, wound healing, adhesion to the extracellular matrix, guidance toward chemoattractants, neuronal growth-cone pathfinding, and embryonic development (Mattila and Lappalainen 2008). Membrane ruffling (also known as cell ruffling) refers to the formation of actin-rich membrane protrusions. This occurs in cellular zones undergoing rapid re-organization of the plasma membrane and often precedes the formation of a lamellipodium. Lamellipodia and ruffles are veil-shaped cell protrusions that are composed of a highly branched actin filament meshwork assembled by the Arp2/3 complex. These structures hallmark the leading edge of cells adopting the adhesion-based mesenchymal mode of migration; they are also thought to drive cell movement.
Our results indicated the presence of podosomes, which are F-actin-rich structures at the basal sides of monocytes, macrophages, dendritic cells (DCs), and osteoclasts that are involved in cell adhesion and motility, matrix degradation, and bone resorption. Podosomes and invadopodia are actin-based dynamic protrusions of the plasma membrane. They act as sites of attachment to and degradation of the extracellular matrix (Murphy and Courtneidge 2011). It is noteworthy that cell development and migration were also attributed to proteins that are both secretory and intracellular (see above).
Protein misfolding is a serious threat to cell viability. It can occur de novo during imprecise protein folding or can be induced by disturbance to protein structure owing to oxidative and thermal stresses. There are two mechanisms of protein quality control aimed at coping with protein misfolding; the first is aimed at protecting native protein structure and repairing its defects, and the second is aimed at removing proteins toxic to cells and whose structures are irreversibly damaged (Dai et al. 2005). The first mechanism is based on the action of molecular chaperones and is indicated in Cluster 2, whereas the second is based on the action of the ubiquitin–proteasome system and is indicated in Cluster 1 (see above).
The proteins in cluster three are predominantly related to spermatozoa (some sperm proteins were also indicated in Cluster 2). This is an important finding that enables the discrimination of several sperm-associated proteins in carp seminal plasma. Although the exact reason for their presence is currently unknown, it is likely that they originate from damaged spermatozoa. The second possible origin is the remnants of spermatogenesis and sperm maturation. Therefore, it can be inferred that these sperm-specific proteins can potentially be used as markers for the evaluation of spermatogenesis and/or damage to spermatozoa in the carp reproductive system.
Proteins related to extracellular vesicles
Our results clearly indicated the presence of EVs in carp seminal plasma, because we identified 636 proteins associated with these structures. Owing to the tight DNA packaging in male haploid gametes, support for sperm function cannot be exerted through transcription and translation (with the possible exception of translation of pre-existing nuclear transcripts by using mitochondrial translational machinery) (Gur and Breitbart 2008; Boguenet et al. 2021). Instead, post-translational modifications (PTMs), secretory proteins, and EVs are used (Samanta et al. 2018; Belleannée et al. 2022; Castillo et al. 2023; Parra et al. 2023).
In addition, we found molecules involved in their formation, secretion, as well as cargo uptake and sorting processes. Furthermore, we identified 19 GPI-anchored proteins associated with EVs, such as CD48, CD59, LYPD6B, and NT5E (Özhan et al. 2013; Klein-Scory et al. 2014; Karasu et al. 2018; Zhao et al. 2023). Proteins involved in EV formation mechanisms, driven by the endosomal sorting complexes required for transport (ESCRT), were also detected. These include ALG-2-interacting protein X, implicated in miRNA packaging, and vacuolar protein sorting-associated protein 4, essential for membrane remodeling during vesicle biogenesis (Alonso Y Adell et al. 2016; Iavello et al. 2016). Moreover, we demonstrated the presence of tetraspanin CD63, a widely recognized EV marker. CD63 regulates the ESCRT-independent pathway of endosomal sorting, is required for exosome formation, and acts as a crucial regulator of endosomal compartment fate as well as an inducer of the secretory pathway (Toribio and Yáñez-Mó 2022).
Interestingly, we found many members of Ras-associated binding (Rab) proteins regulating the intracellular signaling of EVs (e.g. Rab2A, Rab8a, Rab35, Rab 15, CDC42) and soluble N-ethylmaleimide-sensitive factor attachment protein receptors, membrane proteins that function in a complex and are essential for regulating the docking of granules and vesicles to target membranes, although they participate in the final step of exosome secretion (e.g. Vamp3, Vamp7, STX2, STXBP2). Moreover, we found that co-operation between members of these families is necessary for the proper regulation of membrane fusion and EV trafficking (Margiotta 2022).
Fish-specific proteins
Natterins, proteins that are of very ancient origin (about 400 million years), are recognized as important constituents of the fish innate immune defence system (Lima et al. 2021). Natterin and aerolysin (also identified in our study and recently in sturgeon seminal plasma; Kodzik et al. 2023) are pore-forming proteins acting on the membranes of pathogens (Lima et al. 2021). Natterin-3, produced by hepatic tissues, was found to be a part of biochemical immune responses in carp (Yu et al. 2018). We have recently detected natterin in pikeperch seminal plasma (Dietrich et al. 2021) and ovarian fluid (Nynca et al. 2022), suggesting that this protein is universally involved in the protection of male teleost fish and female gametes against microbial attack.
Lectins are carbohydrate-binding proteins that play an important role in the innate immune system of fish with antimicrobial action against viruses, bacteria, and fungi (for a recent review, see Elumalai et al. 2019). These authors summarized major tissue distribution of fish lectins, including gills, skin mucus, roe, kidney, serum and liver; however, information on male reproductive system is unavailable. Our results indicated that lectins belong to different families, such as galectins (galectin 17), pentraxin (jeltraxin), and F-type lectin (fucolectin 5). The latter was found to be involved in immune response to the hypoxic challenge of fish (Mu et al. 2020). In our previous study, we also identified another lectin family, namely, intelectin (Dietrich et al. 2019b). Notably, lectins are also an important component of fish ovarian fluid (Nynca et al. 2022). As such, it can be concluded that lectins in fluids surrounding both male and female fish gametes are important for their antimicrobial defence.
Aside from proteins involved in innate immune response, we identified immunoglobins (IGs), which are part of the adaptive immune system. Three IG isotypes are produced in teleosts, namely, IgD, IgM, and IgT/Z (Mokhtar et al. 2023). The presence of IGs in seminal plasma corresponds to the presence of white blood cells (Ciereszko et al. 2004) and other immune cells (see above); thus, it is highly likely that IGs can be produced within the male reproductive system.
To our knowledge, the counting factor-associated protein D (CfaD) identified in our study has not yet been described for fish. CfaD has been described for a simple eukaryote, Dictyostelium discoideum (social amoeba), as a secreted factor (chalone) that controls cell proliferation (Bakthavatsalam et al. 2008). The role of CfaD in vertebrates is unknown; however, a recent study has suggested an analogy between the functions of Dictyostelium and mammalian macrophages has been suggested (Bajgar and Krejčová 2023). Thus, there is a possibility that CfaD has a role in the regulation of macrophage proliferation in male carp reproductive system.
In our previous study (Dietrich et al. 2018), we identified a novel ‘uncharacterized protein’ named Cap31 in carp blood plasma, which showed an important response to cold acclimation. Recently, we identified Cap31 as Secreted Novel AID/APOBEC-like deaminase (SNAD1) which likely catalyze the deamination of bases in nucleic acids, resulting in cytidine–uridine transition, which leads to changes in the information coded by nucleic acids. As a consequence, the direct action of SNAD1 on nucleic acids of pathogens may occur, or such an action can be directed against the nucleic acids of the immune cells of carp, thereby increasing the variability of IG (Majewska et al. 2024). We suggest that SNAD1 has zinc-dependent cytidine deaminase activity involved in various immunological processes, including innate and/or adaptive immune response owing to bacterial or virial infection. We identified 13 SNAD1 genes in carp. It is likely that different SNAD1 forms can have specific immunological functions as they responded differently to bacterial and virial challenges as well as to acclimation. We have also recorded a high level of SNAD1 expression in carp testis, indicating its role in male reproduction for this species and possible presence in seminal plasma.
In this study, we provided evidence of the presence of three SNAD1 proteins in carp seminal plasma. Interestingly, three SNAD1 proteins were previously found to be upregulated in carp liver in response to exposure to cold conditions; however, the expression of the fourth SNAD1 protein was rather related to viral and bacterial infections (Majewska et al. 2024). This suggests the specific protective role of SNAD1 proteins for the male reproductive system, particularly under cold conditions. However, it is unclear why three SNAD1 proteins are necessary for cold acclimation. In summary, our data indicated that the male reproductive system of carp is equipped in previously unknown defense system based on the action of several forms of SNAD1. Deciphering the specific functions of particular SNADs seems to be an exciting challenge for future research.
Saxitoxin and tetrodotoxin-binding proteins are known to bind to neurotoxins produced by bacteria, namely, saxitoxin and tetrodotoxin, which are well known in pufferfish owing to human intoxications and fatalities. These protein were suggested to neutralize tributyltin, a potent pollutant with a strong endocrine disruptor activity (Takai et al. 2020). We previously identified saxitoxin- and tetrodotoxin-binding proteins in pikeperch seminal plasma (Dietrich et al. 2021) and recently in seminal plasma of carp infected with Aeromonas salmonicida (Dietrich et al. 2022). In the latter, we observed an enrichment of this protein following bacterial infection, demonstrating its involvement in detoxification mechanisms in the protection of spermatozoa and reproductive tract tissue against tetrodotoxin produced by A. salmonicida. In our opinion, the presence of saxitoxin and tetrodotoxin-binding protein in carp seminal plasma may be a part of defence of carp reproductive system against A. salmonicida and numerous other bacteria-producing tetrodotoxins (Magarlamov et al. 2017).
Ice-structuring proteins can survive in temperatures below the freezing point of water by binding to small ice crystals and inhibiting both freezing and melting. Their presence in male fish reproductive system has recently been demonstrated. We recently identified type-4 ice-structuring protein as a major protein in pikeperch seminal and sturgeon plasma (Dietrich et al. 2021; Kodzik et al. 2023). The results of our study also confirmed the presence of type-4 ice-structuring protein in carp seminal plasma. As such, it can be suggested that ice-structuring proteins are universally present on the seminal plasma of freshwater fish, with a possible role in the preservation of male gametes against injuries caused by cold temperature.
Prion proteins are well known for their role in neurodegenerative disorders; however, their biological role is poorly understood. Protein genes are present in fish. A recent transcriptomic study conducted by Pollock et al. (2021) that involved the use of prion protein mutants in zebrafish reported their potential role in cell adhesion, proteolysis, oxidation–reduction process, and apoptotic regulation. Moreover, the latter suggests the involvement of prion proteins in the innate immune system. Our results indicated the presence of prion proteins in the fish reproductive system. Interestingly, fish prion molecules contain the GPI domain (Málaga-Trillo et al. 2011), indicating their association with EVs (Fevrier et al. 2004). Meanwhile, the prion molecules in mammals are thought to be involved in several reproductive processes, such as spermatogenesis, sperm motility, zona binding, and fertilization (Pimenta and Pereira 2014). They have also been proposed as epigenetic factors important for male non-genetic transgenerational inheritance (Immler 2018). Their role in fish reproduction remains to be established.
Vitellogenin is an egg protein produced in female liver and is a precursor of egg yolk. However, the presence of vitellogenin in males has also been reported, including seminal plasma of rainbow trout (Nynca et al. 2014), pikeperch (Dietrich et al. 2021), and recently carp (Dietrich et al. 2022). The presence of vitellogenin in male fish is mostly explained by the activation of the gene for vitellogenin after exposure to estrogens or estrogen mimics, leading to disturbance to male fertility (Hennies et al. 2003). However, this effect seems to be controversial because no clear relationship between reproductive dysfunction and plasma vitellogenin concentration has been established (Mills et al. 2003). Moreover, it is possible that vitellogenin is involved in fish maturation, reflecting the participation of estradiol in maturation (Ma et al. 2005). This suggestion is supported by our recent results indicating the presence of vitellogenin in immature male pikeperch (Dietrich et al. 2021). Our recent results also indicated that vitellogenin can play a role in defence responses and acute-phase responses in fish (Dietrich et al. 2022). This strongly suggests that the production of vitellogenin in male fish is not only a negative result of pollution by environmental estrogens, but can also be important for fish biology, including maturation and defence responses. This suggestion is further supported by the identification of another egg protein, vitelline membrane outer layer protein 1 homolog (VMO1), in our study. VMO1 is a secretory protein (see Table 2) and has been suggested to participate in innate defense birds (Da Silva et al. 2019). This protein has also been identified in chicken semen (Labas et al. 2015), which suggests its presence in vertebrates.
Conclusions
This study has substantially enhanced our knowledge on the protein composition of fish seminal plasma. Seminal plasma appeared to be equipped in complex mechanism for immunological defence, which secures the safety of male gametes. Moreover, through the seminal EV system, seminal plasma can potentially influence the protein composition of spermatozoa, leading to their maturation. We believe that this new knowledge will stimulate further research into many processes vital for the functioning of male fish reproductive system.
CRediT authorship contribution
Anna M. Majewska: conceptualization, methodology, data curation; writing – original draft; writing – review and editing. Natalia Kodzik: data curation. Mariola A. Dietrich: data curation; writing – review and editing. Andrzej Ciereszko: conceptualization; supervision; funding acquisition; writing – original draft; writing – review and editing.
Declaration of funding
This research was support by the project 2021/43/B/NZ9/02869 (PI Andrzej Ciereszko) from National Science Centre, Poland.
Acknowledgements
We are grateful to Agata Malinowska and Bianka Świderska from Institute of Biochemistry and Biophysics, Mass Spectrometry Laboratory, Polish Academy of Sciences, for the LC–MS/MS analysis.
References
Aichem A, Groettrup M (2020) The ubiquitin-like modifier FAT10 – much more than a proteasome-targeting signal. Journal of Cell Science 133(14), jcs246041.
| Crossref | Google Scholar |
Alonso Y Adell M, Migliano SM, Teis D (2016) ESCRT-III and Vps4: a dynamic multipurpose tool for membrane budding and scission. The FEBS Journal 283(18), 3288-3302.
| Crossref | Google Scholar |
Bajgar A, Krejčová G (2023) On the origin of the functional versatility of macrophages. Frontiers in Physiology 14, 1128984.
| Crossref | Google Scholar | PubMed |
Bakthavatsalam D, Brock DA, Nikravan NN, Houston KD, Hatton RD, Gomer RH (2008) The secreted Dictyostelium protein CfaD is a chalone. Journal of Cell Science 121(15), 2473-2480.
| Crossref | Google Scholar |
Bayne CJ, Gerwick L (2001) The acute phase response and innate immunity of fish. Developmental and Comparative Immunology 25(8–9), 725-743.
| Crossref | Google Scholar | PubMed |
Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE (2011) Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nature Reviews Drug Discovery 10(1), 29-46.
| Crossref | Google Scholar | PubMed |
Belleannée C, Viana AGA, Lavoie-Ouellet C (2022) Intra and intercellular signals governing sperm maturation. Reproduction, Fertility and Development 35(2), 27-38.
| Crossref | Google Scholar | PubMed |
Boguenet M, Bouet PE, Spiers A, Reynier P, May-Panloup P (2021) Mitochondria: their role in spermatozoa and in male infertility. Human Reproduction Update 27(4), 697-719.
| Crossref | Google Scholar | PubMed |
Castillo J, de la Iglesia A, Leiva M, Jodar M, Oliva R (2023) Proteomics of human spermatozoa. Human Reproduction 38(12), 2312-2320.
| Crossref | Google Scholar | PubMed |
Ciereszko A, Dabrowski K (1993) Estimation of sperm concentration of rainbow trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture 109, 367-373.
| Crossref | Google Scholar |
Ciereszko A, Wlasow T, Dobosz S, Goryczko K, Glogowski J (2004) Blood cells in rainbow trout Oncorhynchus mykiss milt: relation to milt collection method and sampling period. Theriogenology 62(7), 1353-1364.
| Crossref | Google Scholar | PubMed |
Ciereszko A, Dietrich MA, Nynca J (2012) The identification of seminal proteins in fish: from a traditional approach to proteomics. Journal of Applied Ichthyology 28(6), 865-872.
| Crossref | Google Scholar |
Ciereszko A, Dietrich MA, Nynca J (2017) Fish semen proteomics – new opportunities in fish reproductive research. Aquaculture 472, 81-92.
| Crossref | Google Scholar |
Cieutat A-M, Lobel P, August JT, Kjeldsen L, Sengeløv H, Borregaard N, Bainton DF (1998) Azurophilic granules of human neutrophilic leukocytes are deficient in lysosome-associated membrane proteins but retain the mannose 6-phosphate recognition marker. Blood 91(3), 1044-1058.
| Crossref | Google Scholar | PubMed |
Crocker AD, Willavoys SP (1975) Effect of bradykinin on transepithelial transfer of sodium and water in vitro. The Journal of Physiology 253(2), 401-410.
| Crossref | Google Scholar | PubMed |
Da Silva M, Dombre C, Brionne A, Monget P, Chessé M, De Pauw M, Mills M, Combes-Soia L, Labas V, Guyot N, Nys Y, Réhault-Godbert S (2019) The unique features of proteins depicting the chicken amniotic fluid. Molecular and Cellular Proteomics 18, S174-S190.
| Crossref | Google Scholar | PubMed |
Dai Q, Qian S-B, Li H-H, McDonough H, Borchers C, Huang D, Takayama S, Younger JM, Ren HY, Cyr DM, Patterson C (2005) Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. Journal of Biological Chemistry 280(46), 38673-38681.
| Crossref | Google Scholar | PubMed |
Dietrich MA, Żmijewski D, Karol H, Hejmej A, Bilińska B, Jurecka P, Irnazarow I, Słowińska M, Hliwa P, Ciereszko A (2010a) Isolation and characterization of transferrin from common carp (Cyprinus carpio L) seminal plasma. Fish and Shellfish Immunology 29, 66-74.
| Crossref | Google Scholar |
Dietrich MA, Nynca J, Bilińska B, Kuba J, Kotula-Balak M, Karol H, Ciereszko A (2010b) Identification of parvalbumin-like protein as a major protein of common carp (Cyprinus carpio L.) spermatozoa which appears during final stage of spermatogenesis. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 157(2), 220-227.
| Crossref | Google Scholar |
Dietrich MA, Dietrich GJ, Hliwa P, Ciereszko A (2011) Carp transferrin can protect spermatozoa against toxic effects of cadmium ions. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 153(4), 422-429.
| Crossref | Google Scholar | PubMed |
Dietrich MA, Adamek M, Bilińska B, Hejmej A, Steinhagen D, Ciereszko A (2014a) Characterization, expression and antibacterial properties of apolipoproteins A from carp (Cyprinus carpio L.) seminal plasma. Fish and Shellfish Immunology 41(2), 389-401.
| Crossref | Google Scholar |
Dietrich MA, Arnold GJ, Nynca J, Fröhlich T, Otte K, Ciereszko A (2014b) Characterization of carp seminal plasma proteome in relation to blood plasma. Journal of Proteomics 98, 218-232.
| Crossref | Google Scholar |
Dietrich MA, Hliwa P, Adamek M, Steinhagen D, Karol H, Ciereszko A (2018) Acclimation to cold and warm temperatures is associated with differential expression of male carp blood proteins involved in acute phase and stress responses, and lipid metabolism. Fish and Shellfish Immunology 76, 305-315.
| Crossref | Google Scholar | PubMed |
Dietrich MA, Irnazarow I, Inglot M, Adamek M, Jurecka P, Steinhagen D, Ciereszko A (2019a) Hormonal stimulation of carp is accompanied by changes in seminal plasma proteins associated with the immune and stress responses. Journal of Proteomics 202, 103369.
| Crossref | Google Scholar |
Dietrich MA, Nynca J, Ciereszko A (2019b) Proteomic and metabolomic insights into the functions of the male reproductive system in fishes. Theriogenology 132, 182-200.
| Crossref | Google Scholar |
Dietrich MA, Adamek M, Jung-Schroers V, Rakus K, Chadzińska M, Hejmej A, Hliwa P, Bilińska B, Karol H, Ciereszko A (2020) Characterization of carp seminal plasma Wap65-2 and its participation in the testicular immune response and temperature acclimation. Veterinary Research 51(1), 142.
| Crossref | Google Scholar | PubMed |
Dietrich MA, Judycka S, Słowińska M, Kodzik N, Ciereszko A (2021) Short-term storage-induced changes in the proteome of carp (Cyprinus carpio L.) spermatozoa. Aquaculture 530, 735784.
| Crossref | Google Scholar |
Dietrich MA, Adamek M, Teitge F, Teich L, Jung-Schroers V, Malinowska A, Świderska B, Rakus K, Kodzik N, Chadzińska M, Karol H, Liszewska E, Ciereszko A (2022) Proteomic analysis of carp seminal plasma provides insights into the immune response to bacterial infection of the male reproductive system. Fish and Shellfish Immunology 127, 822-835.
| Crossref | Google Scholar | PubMed |
Elumalai P, Rubeena AS, Arockiaraj J, Wongpanya R, Cammarata M, Ringø E, Vaseeharan B (2019) The role of lectins in finfish: a review. Reviews in Fisheries Science and Aquaculture 27(2), 152-169.
| Crossref | Google Scholar |
Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G (2004) Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America 101(26), 9683-9688.
| Crossref | Google Scholar |
Gur Y, Breitbart H (2008) Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Molecular and Cellular Endocrinology 282(1–2), 45-55.
| Crossref | Google Scholar | PubMed |
Hennies M, Wiesmann M, Allner B, Sauerwein H (2003) Vitellogenin in carp (Cyprinus carpio) and perch (Perca fluviatilis): purification, characterization and development of an ELISA for the detection of estrogenic effects. Science of The Total Environment 309(1–3), 93-103.
| Crossref | Google Scholar | PubMed |
Iavello A, Frech VSL, Gai C, Deregibus MC, Quesenberry PJ, Camussi G (2016) Role of Alix in miRNA packaging during extracellular vesicle biogenesis. International Journal of Molecular Medicine 37(4), 958-966.
| Crossref | Google Scholar | PubMed |
Immler S (2018) The sperm factor: paternal impact beyond genes. Heredity 121(3), 239-247.
| Crossref | Google Scholar | PubMed |
Innocenti M (2018) New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration. Cell Adhesion and Migration 12(5), 401-416.
| Crossref | Google Scholar | PubMed |
Karasu E, Eisenhardt SU, Harant J, Huber-Lang M (2018) Extracellular vesicles: packages sent with complement. Frontiers in Immunology 9, 721.
| Crossref | Google Scholar | PubMed |
Klein-Scory S, Tehrani MM, Eilert-Micus C, Adamczyk KA, Wojtalewicz N, Schnölzer M, Hahn SA, Schmiegel W, Schwarte-Waldhoff I (2014) New insights in the composition of extracellular vesicles from pancreatic cancer cells: implications for biomarkers and functions. Proteome Science 12(1), 50.
| Crossref | Google Scholar | PubMed |
Kodzik N, Ciereszko A, Szczepkowski M, Karol H, Judycka S, Malinowska A, Świderska B, Dietrich MA (2023) Comprehensive proteomic characterization and functional annotation of Siberian sturgeon seminal plasma proteins. Aquaculture 568, 739326.
| Crossref | Google Scholar |
Kosova G, Scott NM, Niederberger C, Prins GS, Ober C (2012) Genome-wide association study identifies candidate genes for male fertility traits in humans. American Journal of Human Genetics 90(6), 950-961.
| Crossref | Google Scholar | PubMed |
Kowalski R, Glogowski J, Kucharczyk D, Goryczko K, Dobosz S, Ciereszko A (2003) Proteolytic activity and electrophoretic profiles of proteases from seminal plasma of teleosts. Journal of Fish Biology 63, 1008-1019.
| Crossref | Google Scholar |
Labas V, Grasseau I, Cahier K, Gargaros A, Harichaux G, Teixeira-Gomes AP, Alves S, Bourin M, Gérard N, Blesbois E (2015) Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. Journal of Proteomics 112, 313-335.
| Crossref | Google Scholar | PubMed |
Lahnsteiner F (2003) Morphology, fine structure, biochemistry, and function of the spermatic ducts in marine fish. Tissue and Cell 35(5), 363-373.
| Crossref | Google Scholar | PubMed |
Lahnsteiner F (2007) Characterization of seminal plasma proteins stabilizing the sperm viability in rainbow trout (Oncorhynchus mykiss). Animal Reproduction Science 97(1–2), 151-164.
| Crossref | Google Scholar | PubMed |
Lahnsteiner F, Patzner RA, Welsmann T (1993) The spermatic ducts of salmonid fishes (Salmonidae, Teleostei). Morphology, histochemistry and composition of the secretion. Journal of Fish Biology 42, 79-93.
| Crossref | Google Scholar |
Lahnsteiner F, Berger B, Weismann T, Patzner R (1995) Fine structure and motility of spermatozoa and composition of the seminal plasma in the perch. Journal of Fish Biology 47, 492-508.
| Crossref | Google Scholar |
Lahnsteiner F, Mansour N, Berger B (2004) Seminal plasma proteins prolong the viability of rainbow trout (Oncorynchus mykiss) spermatozoa. Theriogenology 62(5), 801-808.
| Crossref | Google Scholar | PubMed |
Lima C, Disner GR, Falcão MAP, Seni-Silva AC, Maleski ALA, Souza MM, Reis Tonello MC, Lopes-Ferreira M (2021) The natterin proteins diversity: a review on phylogeny, structure, and immune function. Toxins 13(8), 538.
| Crossref | Google Scholar | PubMed |
Ma YX, Matsuda K, Uchiyama M (2005) Seasonal variations in plasma concentrations of sex steroid hormones and vitellogenin in wild male Japanese dace (Tribolodon hakonensis) collected from different sites of the Jinzu river basin. Zoological Science 22(8), 861-868.
| Crossref | Google Scholar | PubMed |
Magarlamov TY, Melnikova DI, Chernyshev AV (2017) Tetrodotoxin-producing bacteria: detection, distribution and migration of the toxin in aquatic systems. Toxins 9(5), 166.
| Crossref | Google Scholar | PubMed |
Majewska AM, Dietrich MA, Budzko L, Adamek M, Figlerowicz M, Ciereszko A (2024) Secreted novel AID/APOBEC-like deaminase 1 (SNAD1) – a new important player in fish immunology. Frontiers in Immunology 15, 1340273.
| Crossref | Google Scholar | PubMed |
Mak M, Mak P, Olczak M, Szalewicz A, Glogowski J, Dubin A, Watorek W, Ciereszko A (2004) Isolation, characterization, and cDNA sequencing of alpha-1-antiproteinase-like protein from rainbow trout seminal plasma. Biochimica et Biophysica Acta 1671(1–3), 93-105.
| Crossref | Google Scholar | PubMed |
Málaga-Trillo E, Salta E, Figueras A, Panagiotidis C, Sklaviadis T (2011) Fish models in prion biology: underwater issues. Biochimica et Biophysica Acta 1812(3), 402-414.
| Crossref | Google Scholar | PubMed |
Margiotta A (2022) Membrane fusion and SNAREs: interaction with Ras proteins. International Journal of Molecular Sciences 23(15), 8067.
| Crossref | Google Scholar | PubMed |
Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nature Reviews Molecular Cell Biology 9(6), 446-454.
| Crossref | Google Scholar | PubMed |
Miao W, Wang W (2020) Trends of aquaculture production and trade: carp, tilapia, and shrimp. Asian Fisheries Science 33(S1), 1-10.
| Crossref | Google Scholar |
Mills LJ, Gutjahr-Gobell RE, Horowitz DB, Denslow ND, Chow MC, Zaroogian GE (2003) Relationship between reproductive success and male plasma vitellogenin concentrations in cunner, Tautogolabrus adspersus. Environmental Health Perspectives 111(1), 93-100.
| Crossref | Google Scholar | PubMed |
Mokhtar DM, Zaccone G, Alesci A, Kuciel M, Hussein MT, Sayed RKA (2023) Main components of fish immunity: an overview of the fish immune system. Fishes 8(2), 93.
| Crossref | Google Scholar |
Mu Y, Li W, Wu B, Chen J, Chen X (2020) Transcriptome analysis reveals new insights into immune response to hypoxia challenge of large yellow croaker (Larimichthys crocea). Fish and Shellfish Immunology 98, 738-747.
| Crossref | Google Scholar | PubMed |
Murphy DA, Courtneidge SA (2011) The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature Reviews Molecular Cell Biology 12(7), 413-426.
| Crossref | Google Scholar | PubMed |
Mylonas CC, Duncan NJ, Asturiano JF (2017) Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 472, 21-44.
| Crossref | Google Scholar |
Myszkowski L (2022) Obraz polskiej akwakultury w 2021 roku na podstawie badań statystycznych przy zastosowaniu kwestionariusza RRW-22. In ‘Materiały konferencyjnych z XLVII Szkolenia - Konferencji Hodowców Ryb Łososiowatych, 13–14 Października 2022, Gdynia’. pp. 7–13. Available at http://sprl.pl/konferencje/konferencja-2022/materialy-konferencyjne. [In Polish]
Nynca J, Hórvath A, Dietrich MA, Müller T, Karol H, Urbányi B, Kotrik L, Ciereszko A (2010a) Serine proteinase inhibitors in the seminal plasma of percid fish. Journal of Applied Ichthyology 26, 742-745.
| Crossref | Google Scholar |
Nynca J, Dietrich MA, Karol H, Ciereszko A (2010b) Identification of apolipoprotein C-I in rainbow trout seminal plasma. Reproduction, Fertility and Development 22(8), 1183-1187.
| Crossref | Google Scholar |
Nynca J, Słowińska M, Dietrich MA, Bilińska B, Kotula-Balak M, Ciereszko A (2011a) Isolation and identification of fetuin-B-like protein from rainbow trout seminal plasma and its localization in the reproductive system. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 158, 106-116.
| Crossref | Google Scholar |
Nynca J, Dietrich MA, Bilińska B, Kotula-Balak M, Kiełbasa T, Karol H, Ciereszko A (2011b) Isolation of lipocalin-type protein from rainbow trout seminal plasma and its localisation in the reproductive system. Reproduction, Fertililty and Development 23, 381-389.
| Crossref | Google Scholar |
Nynca J, Arnold GJ, Fröhlich T, Otte K, Flenkenthaler F, Ciereszko A (2014) Proteomic identification of rainbow trout seminal plasma proteins. Proteomics 14(1), 133-140.
| Crossref | Google Scholar | PubMed |
Nynca J, Żarski D, Fröhlich T, Köster M, Bobe J, Ciereszko A (2022) Comparative proteomic analysis reveals the importance of the protective role of ovarian fluid over eggs during the reproduction of pikeperch. Aquaculture 548, 737656.
| Crossref | Google Scholar |
Özhan G, Sezgin E, Wehner D, Pfister AS, Kühl SJ, Kagermeier-Schenk B, Kühl M, Schwille P, Weidinger G (2013) Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Developmental Cell 26(4), 331-345.
| Crossref | Google Scholar | PubMed |
Parra A, Padilla L, Lucas X, Rodriguez-Martinez H, Barranco I, Roca J (2023) Seminal extracellular vesicles and their involvement in male (in)fertility: a systematic review. International Journal of Molecular Science 24(5), 4818.
| Crossref | Google Scholar |
Perez-Riverol Y, Bandla C, Kundu DJ, Kamatchinathan S, Bai J, Hewapathirana S, John NS, Prakash A, Walzer M, Wang S, Vizcaíno JA (2025) The PRIDE database at 20 years: 2025 update. Nucleic Acids Research 53(D1), D543-D553.
| Crossref | Google Scholar |
Pimenta J, Pereira R (2014) New perspectives on the study of prion and prion like proteins: key roles in the physiology of sperm and male fertility. In ‘Spermatozoa: biology, motility and function and chromosomal abnormalities’. (Ed. BT Erickson) pp. 123–138. (Nova Science Publishers: New York, NY, USA)
Pollock NM, Leighton P, Neil G, Allison WT (2021) Transcriptomic analysis of zebrafish prion protein mutants supports conserved cross-species function of the cellular prion protein. Prion 15(1), 70-81.
| Crossref | Google Scholar | PubMed |
Rørvig S, Honore C, Larsson LI, Ohlsson S, Pedersen CC, Jacobsen LC, Cowland JB, Garred P, Borregaard N (2009) Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. Journal of Leukocyte Biology 86(6), 1439-1449.
| Crossref | Google Scholar | PubMed |
Samanta L, Parida R, Dias TR, Agarwal A (2018) The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reproductive Biology and Endocrinology 16(1), 41.
| Crossref | Google Scholar | PubMed |
Shaliutina A, Hulak M, Dzuyba B, Linhart O (2012) Spermatozoa motility and variation in the seminal plasma proteome of Eurasian perch (Perca fluviatilis) during the reproductive season. Molecular Reproduction and Development 79(12), 879-887.
| Crossref | Google Scholar | PubMed |
Shaliutina-Kolešová A, Kotas P, Štěrba J, Rodina M, Dzyuba B, Cosson J, Linhart O (2016) Protein profile of seminal plasma and functionality of spermatozoa during the reproductive season in the common carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss). Molecular Reproduction and Development 83(11), 968-982.
| Crossref | Google Scholar | PubMed |
Steinmetz A, Kaffarnik H, Utermann G (1985) Activation of phosphatidylcholine-sterol acyltransferase by human apolipoprotein E isoforms. European Journal of Biochemistry 152(3), 747-751.
| Crossref | Google Scholar | PubMed |
Stossel TP, Mason RJ, Pollard TD, Vaughan M (1972) Isolation and properties of phagocytic vesicles. II. Alveolar macrophages. Journal of Clinical Investigation 51(3), 604-614.
| Crossref | Google Scholar | PubMed |
Takai Y, Mizoguchi N, Kinoshita M, Qiu X, Shimasaki Y, Oshima Y (2020) Establishment of a Japanese medaka (Oryzias latipes) transgenic line expressing Takifugu rubripes pufferfish saxitoxin and tetrodotoxin binding protein 1, and evaluation of tributyltin toxicity via in ovo nanoinjection. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 234, 108785.
| Crossref | Google Scholar |
The UniProt Consortium (2023) UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Research 51(D1), D523-D531.
| Crossref | Google Scholar |
Toribio V, Yáñez-Mó M (2022) Tetraspanins interweave EV secretion, endosomal network dynamics and cellular metabolism. European Journal of Cell Biology 101(3), 151229.
| Crossref | Google Scholar | PubMed |
Uhlén M, Fagerberg L, Hallström BM, Lindsko C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson IM, Edlund K, Lundberg E, Navani S, Szigyarto CA-K, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Proteomics. Tissue-based map of the human proteome. Science 347(6220), 1260419.
| Crossref | Google Scholar | PubMed |
Wojtczak M, Całka J, Glogowski J, Ciereszko A (2007a) Isolation and characterization of alpha1-proteinase inhibitor from common carp (Cyprinus carpio) seminal plasma. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 148(3), 264-276.
| Crossref | Google Scholar |
Wojtczak M, Dietrich GJ, Irnazarow I, Jurecka P, Słowińska M, Ciereszko A (2007b) Polymorphism of transferrin of carp seminal plasma: relationship to blood transferrin and sperm motility characteristics. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 148(4), 426-431.
| Crossref | Google Scholar |
Yu F, Li J, Li H, Tang Y, Wang M, Hu W, Yu J (2018) Effects of glucose on biochemical immune responses and hepatic gene expression in common carp, Cyprinus carpio L. Biotechnology and Biotechnological Equipment 32(6), 1440-1446.
| Crossref | Google Scholar |
Zaika O, Mamenko M, O’Neil RG, Pochynyuk O (2011) Bradykinin acutely inhibits activity of the epithelial Na+ channel in mammalian aldosterone-sensitive distal nephron. American Journal of Physiology. Renal Physiology 300(5), F1105-F1115.
| Crossref | Google Scholar | PubMed |
Zhao W, Zhang H, Liu R, Cui R (2023) Advances in immunomodulatory mechanisms of mesenchymal stem cells-derived exosome on immune cells in scar formation. International Journal of Nanomedicine 18, 3643-3662.
| Crossref | Google Scholar |


