Sealed culture system for supporting mouse preimplantation embryo development in vitro
Jie Liu A B * , Zhao Wang A B * , Zhen Gao A B , Hui Zhang A B , Jianfeng Gu A B , Xiaoe Zhao A B , Qiang Wei A B and Baohua Ma A B C
A B C
A College of Veterinary Medicine, Northwest A&F University, Yangling, Shaanxi, 712100, China.
B Key Laboratory of Animal Biotechnology, Ministry of Agriculture, Yangling, Shaanxi, 712100, China.
C Corresponding author: Email: malab@nwafu.edu.cn
Reproduction, Fertility and Development 32(9) 879-884 https://doi.org/10.1071/RD19086
Submitted: 7 March 2019 Accepted: 24 December 2019 Published: 25 May 2020
Abstract
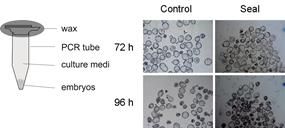
This study investigated the possibility of a sealed culture system in polymerase chain reaction (PCR) tubes to maintain embryo development. The embryo density that could support the development of 2-cell stage mouse embryos to the hatching stage was determined. At an embryo density of 1 : 2 (100 embryos cultured in 200 μL CZB medium that had been pretreated with a reference gas containing 5% O2), the developmental rate was higher and fewer embryos exhibited reactive oxygen species- or hypoxia-induced injury compared with other sealed culture groups. Expression of growth factors (insulin-like growth factor (IGF) 1, IGF2, epidermal growth factor and transforming growth factor-α) and their receptors was evaluated, with similar expression patterns seen for embryos in sealed culture (5% O2, embryo density of 1 : 2) compared with the control group (embryos cultured in microdrops and placed in a 37°C, 5% CO2 water-jacketed incubator; P > 0.05). After transfer of blastocysts generated by the sealed culture into recipients, there were no obvious differences in the rate of normal live pups births between the sealed culture and control groups (P > 0.05). Thus, the sealed embryo culture system in PCR tubes is feasible for use in situations which cannot use a traditional incubator, such as in space and during the transport of embryos.
Additional keywords: apoptosis, hypoxia-inducible factor (HIF), reactive oxygen species (ROS).
References
Azad, M. B., Chen, Y., Henson, E. S., Cizeau, J., Mcmillanward, E., Israels, S. J., and Gibson, S. B. (2008). Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4, 195–204.| Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3.Crossref | GoogleScholarGoogle Scholar | 18059169PubMed |
Chatot, C. L., Ziomek, C. A., Bavister, B. D., Lewis, J. L., and Torres, I. (1989). An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 86, 679–688.
| An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro.Crossref | GoogleScholarGoogle Scholar | 2760894PubMed |
Itoi, F., Tokoro, M., Terashita, Y., Yamagata, K., Fukunaga, N., Asada, Y., and Wakayama, T. (2012). Offspring from mouse embryos developed using a simple incubator-free culture system with a deoxidizing agent. PLoS One 7, e47512.
| Offspring from mouse embryos developed using a simple incubator-free culture system with a deoxidizing agent.Crossref | GoogleScholarGoogle Scholar | 23056643PubMed |
Kawamura, K., Chen, Y., Shu, Y., Cheng, Y., Qiao, J., Behr, B., Pera, R. A. R., and Hsueh, A. J. W. (2012). Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One 7, e49328.
| Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors.Crossref | GoogleScholarGoogle Scholar | 23152897PubMed |
Khurana, N. K., and Niemann, H. (2000). Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 54, 741–756.
| Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos.Crossref | GoogleScholarGoogle Scholar | 11101035PubMed |
Nowacki, D., Klinger, F. G., Mazur, G., and De Felici, M. (2015). Effect of culture in simulated microgravity on the development of mouse embryonic testes. Adv. Clin. Exp. Med. 24, 769–774.
| Effect of culture in simulated microgravity on the development of mouse embryonic testes.Crossref | GoogleScholarGoogle Scholar | 26768626PubMed |
Peng, Z. F., Shi, S. L., Jin, H. X., Yao, G. D., Wang, E. Y., Yang, H. Y., Song, W. Y., and Sun, Y. P. (2015). Impact of oxygen concentrations on fertilization, cleavage, implantation, and pregnancy rates of in vitro generated human embryos. Int. J. Clin. Exp. Med. 8, 6179–6185.
| 26131222PubMed |
Romek, M., Gajda, B., Krzysztofowicz, E., Kucia, M., Uzarowska, A., and Smorag, Z. (2017). Improved quality of porcine embryos cultured with hyaluronan due to the modification of the mitochondrial membrane potential and reactive oxygen species level. Theriogenology 102, 1–9.
| Improved quality of porcine embryos cultured with hyaluronan due to the modification of the mitochondrial membrane potential and reactive oxygen species level.Crossref | GoogleScholarGoogle Scholar | 28708486PubMed |
Tokoro, M., Fukunaga, N., Yamanaka, K., Itoi, F., Terashita, Y., Kamada, Y., Wakayama, S., Asada, Y., and Wakayama, T. (2015). A simple method for transportation of mouse embryos using microtubes and a warm box. PLoS One 10, e0138854.
| A simple method for transportation of mouse embryos using microtubes and a warm box.Crossref | GoogleScholarGoogle Scholar | 26393931PubMed |
Umaoka, Y., Noda, Y., Narimoto, K., and Mori, T. (1992). Effects of oxygen toxicity on early development of mouse embryos. Mol. Reprod. Dev. 31, 28–33.
| Effects of oxygen toxicity on early development of mouse embryos.Crossref | GoogleScholarGoogle Scholar | 1562324PubMed |
Vutyavanich, T., Saeng-anan, U., Sirisukkasem, S., and Piromlertamorn, W. (2011). Effect of embryo density and microdrop volume on the blastocyst development of mouse two-cell embryos. Fertil. Steril. 95, 1435–1439.
| Effect of embryo density and microdrop volume on the blastocyst development of mouse two-cell embryos.Crossref | GoogleScholarGoogle Scholar | 20561617PubMed |
Wu, C., Guo, X., Wang, F., Li, X., Tian, X. C., Li, L., Wu, Z., and Zhang, S. (2011). Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing. PLoS One 6, e22214.
| Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing.Crossref | GoogleScholarGoogle Scholar | 22195028PubMed |
Zhang, S., Zheng, D., Wu, Y., Lin, W., Chen, Z., Meng, L., Liu, J., and Zhou, Y. (2016). Simulated microgravity using a rotary culture system compromises the in vitro development of mouse preantral follicles. PLoS One 11, e0151062.
| Simulated microgravity using a rotary culture system compromises the in vitro development of mouse preantral follicles.Crossref | GoogleScholarGoogle Scholar | 28030618PubMed |


