Generation and characterisation of a COV434 cell clone carrying a monoallelic FecBB mutation introduced by CRISPR/Cas9
Kai Zhang A , Peiqing Cong A , Delin Mo A , Yaosheng Chen A and Zuyong He A B
A B
A State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, No.3 Road of Higher Education Mega Centre North, Guangzhou 510006, P. R. China.
B Corresponding author. Email: zuyonghe@foxmail.com
Reproduction, Fertility and Development 32(13) 1145-1155 https://doi.org/10.1071/RD20068
Submitted: 12 March 2020 Accepted: 25 July 2020 Published: 14 August 2020
Abstract
The FecBB mutation, which increases the ovulation quota, was identified in a hyperprolific sheep breed, and is associated with a single point mutation (c.A746G; p.Q249R) in bone morphogenetic protein receptor-type 1B (BMPR1B). However, the mechanism of action of the FecBB mutation remains unclear. Here, we describe the application of clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 (CRISPR/Cas9) to introduce the FecBB mutation into the human granulosa cell line COV434 via homology-directed repair (HDR), using single-stranded oligodeoxynucleotides (ssODNs) as template. Upon screening single cell-derived clones, we found one clone containing the FecBB mutation on one allele and knockout of BMPR1B on the other allele, and another clone harbouring an in-frame deletion on one allele and knockout of BMPR1B on the other. These two clones were subjected to functional analysis. We found that the FecBB mutation and knockout of BMPR1B could upregulate the expression of cumulus expansion-related genes, potentially mediated by enhanced activation of the Sma- and Mad-related 1/5 (SMAD1/5) pathway. The FecBB mutation, an in-frame mutation, and knockout of BMPR1B could impair the synthesis of oestradiol in COV434 cells, possibly through inhibition of the SMAD2/3 pathway. Our study provides a useful cell model for further investigation into the mechanism of action of the FecBB mutation.
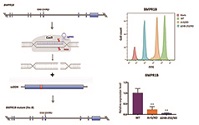
Additional keywords: BMP15, BMPR1B, fertility, GDF9.
References
Abdoli, R., Zamani, P., Mirhoseini, S. Z., Ghavi Hossein-Zadeh, N., and Nadri, S. (2016). A review on prolificacy genes in sheep. Reprod. Domest. Anim. 51, 631–637.| A review on prolificacy genes in sheep.Crossref | GoogleScholarGoogle Scholar | 27491513PubMed |
Al-Musawi, S. L., Walton, K. L., Heath, D., Simpson, C. M., and Harrison, C. A. (2013). Species differences in the expression and activity of bone morphogenetic protein 15. Endocrinology 154, 888–899.
| Species differences in the expression and activity of bone morphogenetic protein 15.Crossref | GoogleScholarGoogle Scholar | 23284103PubMed |
Anzalone, A. V., Randolph, P. B., Davis, J. R., Sousa, A. A., Koblan, L. W., Levy, J. M., Chen, P. J., Wilson, C., Newby, G. A., Raguram, A., and Liu, D. R. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157.
| Search-and-replace genome editing without double-strand breaks or donor DNA.Crossref | GoogleScholarGoogle Scholar | 31634902PubMed |
Brabender, J., Danenberg, K. D., Metzger, R., Schneider, P. M., Park, J., Salonga, D., Holscher, A. H., and Danenberg, P. V. (2001). Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer is correlated with survival. Clin. Cancer Res. 7, 1850–1855.
| 11448895PubMed |
Chang, H. M., Cheng, J. C., Fang, L., Qiu, X., Klausen, C., Taylor, E. L., and Leung, P. C. (2015). Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells. J. Clin. Endocrinol. Metab. 100, E365–E374.
| Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 25514099PubMed |
Chen, L., Russell, P. T., and Larsen, W. J. (1993). Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol. Reprod. Dev. 34, 87–93.
| Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass.Crossref | GoogleScholarGoogle Scholar | 8418823PubMed |
Conley, A. J., Howard, H. J., Slanger, W. D., and Ford, J. J. (1994). Steroidogenesis in the preovulatory porcine follicle1. Biol. Reprod. 51, 655–661.
| Steroidogenesis in the preovulatory porcine follicle1.Crossref | GoogleScholarGoogle Scholar | 7819446PubMed |
Crawford, J. L., and McNatty, K. P. (2012). The ratio of growth differentiation factor 9: bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates. Mol. Cell. Endocrinol. 348, 339–343.
| The ratio of growth differentiation factor 9: bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates.Crossref | GoogleScholarGoogle Scholar | 21970812PubMed |
Elvin, J. A., Clark, A. T., Wang, P., Wolfman, N. M., and Matzuk, M. M. (1999). Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol. 13, 1035–1048.
| Paracrine actions of growth differentiation factor-9 in the mammalian ovary.Crossref | GoogleScholarGoogle Scholar | 10379900PubMed |
Elvin, J. A., Yan, C., and Matzuk, M. M. (2000). Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc. Natl. Acad. Sci. USA 97, 10288–10293.
| Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway.Crossref | GoogleScholarGoogle Scholar | 10944203PubMed |
Eppig, J. J. (1979). Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro. J. Exp. Zool. 208, 111–120.
| Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: general conditions for expansion in vitro.Crossref | GoogleScholarGoogle Scholar | 224135PubMed |
Fabre, S., Pierre, A., Pisselet, C., Mulsant, P., Lecerf, F., Pohl, J., Monget, P., and Monniaux, D. (2003). The Booroola mutation in sheep is associated with an alteration of the bone morphogenetic protein receptor-IB functionality. J. Endocrinol. 177, 435–444.
| The Booroola mutation in sheep is associated with an alteration of the bone morphogenetic protein receptor-IB functionality.Crossref | GoogleScholarGoogle Scholar | 12773124PubMed |
Fogarty, N. M. (2009). A review of the effects of the Booroola gene (FecB) on sheep production. Small Rumin. Res. 85, 75–84.
| A review of the effects of the Booroola gene (FecB) on sheep production.Crossref | GoogleScholarGoogle Scholar |
Gasperin, B. G., Ferreira, R., Rovani, M. T., Bordignon, V., Duggavathi, R., Buratini, J., Oliveira, J. F., and Goncalves, P. B. (2014). Expression of receptors for BMP15 is differentially regulated in dominant and subordinate follicles during follicle deviation in cattle. Anim. Reprod. Sci. 144, 72–78.
| Expression of receptors for BMP15 is differentially regulated in dominant and subordinate follicles during follicle deviation in cattle.Crossref | GoogleScholarGoogle Scholar | 24388700PubMed |
Gaudelli, N. M., Komor, A. C., Rees, H. A., Packer, M. S., Badran, A. H., Bryson, D. I., and Liu, D. R. (2017). Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471.
| Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage.Crossref | GoogleScholarGoogle Scholar | 29160308PubMed |
Gilchrist, R. B., and Ritter, L. J. (2011). Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction 142, 647–657.
| Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion.Crossref | GoogleScholarGoogle Scholar | 21896635PubMed |
Gootwine, E. (2020). Invited review: opportunities for genetic improvement toward higher prolificacy in sheep. Small Rumin. Res. 186, 106090.
| Invited review: opportunities for genetic improvement toward higher prolificacy in sheep.Crossref | GoogleScholarGoogle Scholar |
Goyal, S., Aggarwal, J., Dubey, P. K., Mishra, B. P., Ghalsasi, P., Nimbkar, C., Joshi, B. K., and Kataria, R. S. (2017). Expression analysis of genes associated with prolificacy in FecB carrier and noncarrier Indian sheep. Anim. Biotechnol. 28, 220–227.
| Expression analysis of genes associated with prolificacy in FecB carrier and noncarrier Indian sheep.Crossref | GoogleScholarGoogle Scholar | 28075701PubMed |
He, Z., Shi, X., Du, B., Qin, Y., Cong, P., and Chen, Y. (2015). Highly efficient enrichment of porcine cells with deletions induced by CRISPR/Cas9 using dual fluorescence selection. J. Biotechnol. 214, 69–74.
| Highly efficient enrichment of porcine cells with deletions induced by CRISPR/Cas9 using dual fluorescence selection.Crossref | GoogleScholarGoogle Scholar | 26200831PubMed |
He, Z., Proudfoot, C., Whitelaw, C. B., and Lillico, S. G. (2016). Comparison of CRISPR/Cas9 and TALENs on editing an integrated EGFP gene in the genome of HEK293FT cells. Springerplus 5, 814.
| Comparison of CRISPR/Cas9 and TALENs on editing an integrated EGFP gene in the genome of HEK293FT cells.Crossref | GoogleScholarGoogle Scholar | 27390654PubMed |
Hobeika, E., Armouti, M., Kala, H., Fierro, M. A., Winston, N. J., Scoccia, B., Zamah, A. M., and Stocco, C. (2019). Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. J. Clin. Endocrinol. Metab. 104, 1667–1676.
| Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells.Crossref | GoogleScholarGoogle Scholar | 30541132PubMed |
Holbrook, J. A., Neu-Yilik, G., Hentze, M. W., and Kulozik, A. E. (2004). Nonsense-mediated decay approaches the clinic. Nat. Genet. 36, 801–808.
| Nonsense-mediated decay approaches the clinic.Crossref | GoogleScholarGoogle Scholar | 15284851PubMed |
Jin, S., Zong, Y., Gao, Q., Zhu, Z., Wang, Y., Qin, P., Liang, C., Wang, D., Qiu, J. L., Zhang, F., and Gao, C. (2019). Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295.
| Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice.Crossref | GoogleScholarGoogle Scholar | 30819931PubMed |
Karimian, A., Azizian, K., Parsian, H., Rafieian, S., Shafiei-Irannejad, V., Kheyrollah, M., Yousefi, M., Majidinia, M., and Yousefi, B. (2019). CRISPR/Cas9 technology as a potent molecular tool for gene therapy. J. Cell. Physiol. 234, 12267–12277.
| CRISPR/Cas9 technology as a potent molecular tool for gene therapy.Crossref | GoogleScholarGoogle Scholar | 30697727PubMed |
Kim, H., Um, E., Cho, S. R., Jung, C., Kim, H., and Kim, J. S. (2011). Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nat. Methods 8, 941–943.
| Surrogate reporters for enrichment of cells with nuclease-induced mutations.Crossref | GoogleScholarGoogle Scholar | 21983922PubMed |
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A., and Liu, D. R. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424.
| Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage.Crossref | GoogleScholarGoogle Scholar | 27096365PubMed |
Lim, H., Paria, B. C., Das, S. K., Dinchuk, J. E., Langenbach, R., Trzaskos, J. M., and Dey, S. K. (1997). Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208.
| Multiple female reproductive failures in cyclooxygenase 2-deficient mice.Crossref | GoogleScholarGoogle Scholar | 9346237PubMed |
Liu, X., Liu, H., Wang, M., Li, R., Zeng, J., Mo, D., Cong, P., Liu, X., Chen, Y., and He, Z. (2019). Disruption of the ZBED6 binding site in intron 3 of IGF2 by CRISPR/Cas9 leads to enhanced muscle development in Liang Guang Small Spotted pigs. Transgenic Res. 28, 141–150.
| Disruption of the ZBED6 binding site in intron 3 of IGF2 by CRISPR/Cas9 leads to enhanced muscle development in Liang Guang Small Spotted pigs.Crossref | GoogleScholarGoogle Scholar | 30488155PubMed |
Luong, H. T., Chaplin, J., McRae, A. F., Medland, S. E., Willemsen, G., Nyholt, D. R., Henders, A. K., Hoekstra, C., Duffy, D. L., Martin, N. G., Boomsma, D. I., Montgomery, G. W., and Painter, J. N. (2011). Variation in BMPR1B, TGFRB1 and BMPR2 and control of dizygotic twinning. Twin Res. Hum. Genet. 14, 408–416.
| Variation in BMPR1B, TGFRB1 and BMPR2 and control of dizygotic twinning.Crossref | GoogleScholarGoogle Scholar | 21962132PubMed |
Matzuk, M. M., and Burns, K. H. (2012). Genetics of mammalian reproduction: modeling the end of the germline. Annu. Rev. Physiol. 74, 503–528.
| Genetics of mammalian reproduction: modeling the end of the germline.Crossref | GoogleScholarGoogle Scholar | 22335799PubMed |
Matzuk, M. M., Burns, K. H., Viveiros, M. M., and Eppig, J. J. (2002). Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296, 2178–2180.
| Intercellular communication in the mammalian ovary: oocytes carry the conversation.Crossref | GoogleScholarGoogle Scholar | 12077402PubMed |
McNatty, K. P., and Henderson, K. M. (1987). Gonadotrophins, fecundity genes and ovarian follicular function. J. Steroid Biochem. 27, 365–373.
| Gonadotrophins, fecundity genes and ovarian follicular function.Crossref | GoogleScholarGoogle Scholar | 2826892PubMed |
Montgomery, G. W., McNatty, K. P., and Davis, G. H. (1992). Physiology and molecular genetics of mutations that increase ovulation rate in sheep. Endocr. Rev. 13, 309–328.
| Physiology and molecular genetics of mutations that increase ovulation rate in sheep.Crossref | GoogleScholarGoogle Scholar | 1618165PubMed |
Mulsant, P., Lecerf, F., Fabre, S., Schibler, L., Monget, P., Lanneluc, I., Pisselet, C., Riquet, J., Monniaux, D., Callebaut, I., Cribiu, E., Thimonier, J., Teyssier, J., Bodin, L., Cognie, Y., Chitour, N., and Elsen, J. M. (2001). Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes. Proc. Natl. Acad. Sci. USA 98, 5104–5109.
| Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes.Crossref | GoogleScholarGoogle Scholar | 11320249PubMed |
Nonis, D., McTavish, K. J., and Shimasaki, S. (2013). Essential but differential role of FOXL2wt and FOXL2C134W in GDF-9 stimulation of follistatin transcription in co-operation with Smad3 in the human granulosa cell line COV434. Mol. Cell. Endocrinol. 372, 42–48.
| Essential but differential role of FOXL2wt and FOXL2C134W in GDF-9 stimulation of follistatin transcription in co-operation with Smad3 in the human granulosa cell line COV434.Crossref | GoogleScholarGoogle Scholar | 23523567PubMed |
Otsuka, F., McTavish, K. J., and Shimasaki, S. (2011). Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 78, 9–21.
| Integral role of GDF-9 and BMP-15 in ovarian function.Crossref | GoogleScholarGoogle Scholar | 21226076PubMed |
Peng, J., Li, Q., Wigglesworth, K., Rangarajan, A., Kattamuri, C., Peterson, R. T., Eppig, J. J., Thompson, T. B., and Matzuk, M. M. (2013). Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl Acad. Sci. U. S. A. 110, E776–E785.
| Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions.Crossref | GoogleScholarGoogle Scholar | 23382188PubMed |
Qi, X., Salem, M., Zhou, W., Sato-Shimizu, M., Ye, G., Smitz, J., and Peng, C. (2016). Neurokinin B exerts direct effects on the ovary to stimulate estradiol production. Endocrinology 157, 3355–3365.
| Neurokinin B exerts direct effects on the ovary to stimulate estradiol production.Crossref | GoogleScholarGoogle Scholar | 27580802PubMed |
Ran, F. A., Hsu, P. D., Wright, J., Agarwala, V., Scott, D. A., and Zhang, F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308.
| Genome engineering using the CRISPR-Cas9 system.Crossref | GoogleScholarGoogle Scholar | 24157548PubMed |
Reader, K. L., Heath, D. A., Lun, S., McIntosh, C. J., Western, A. H., Littlejohn, R. P., McNatty, K. P., and Juengel, J. L. (2011). Signalling pathways involved in the cooperative effects of ovine and murine GDF9+BMP15-stimulated thymidine uptake by rat granulosa cells. Reproduction 142, 123–131.
| Signalling pathways involved in the cooperative effects of ovine and murine GDF9+BMP15-stimulated thymidine uptake by rat granulosa cells.Crossref | GoogleScholarGoogle Scholar | 21474603PubMed |
Renault, L., Patiño, L. C., Magnin, F., Delemer, B., Young, J., Laissue, P., Binart, N., and Beau, I. (2020). BMPR1A and BMPR1B missense mutations cause primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 105, e1449–e1457.
| BMPR1A and BMPR1B missense mutations cause primary ovarian insufficiency.Crossref | GoogleScholarGoogle Scholar |
Rivera-Torres, N., Banas, K., Bialk, P., Bloh, K. M., and Kmiec, E. B. (2017). Insertional mutagenesis by CRISPR/Cas9 ribonucleoprotein gene editing in cells targeted for point mutation repair directed by short single-stranded DNA oligonucleotides. PLoS One 12, e0169350.
| Insertional mutagenesis by CRISPR/Cas9 ribonucleoprotein gene editing in cells targeted for point mutation repair directed by short single-stranded DNA oligonucleotides.Crossref | GoogleScholarGoogle Scholar | 28052104PubMed |
Roberts, A. B., and Derynck, R. (2001). Meeting report: signaling schemes for TGF-beta. Sci. STKE 2001, pe43.
| 11752631PubMed |
Souza, C. J., MacDougall, C., MacDougall, C., Campbell, B. K., McNeilly, A. S., and Baird, D. T. (2001). The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J. Endocrinol. 169, R1–R6.
| The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene.Crossref | GoogleScholarGoogle Scholar | 11312159PubMed |
Su, Y. Q., Sugiura, K., Wigglesworth, K., O’Brien, M. J., Affourtit, J. P., Pangas, S. A., Matzuk, M. M., and Eppig, J. J. (2008). Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135, 111–121.
| Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells.Crossref | GoogleScholarGoogle Scholar | 18045843PubMed |
Tang, J., Hu, W., Di, R., Liu, Q., Wang, X., Zhang, X., Zhang, J., and Chu, M. (2018). Expression analysis of the prolific candidate genes, BMPR1B, BMP15, and GDF9 in Small Tail Han ewes with three fecundity (FecB gene) genotypes. Animals (Basel) 8, 166.
| Expression analysis of the prolific candidate genes, BMPR1B, BMP15, and GDF9 in Small Tail Han ewes with three fecundity (FecB gene) genotypes.Crossref | GoogleScholarGoogle Scholar |
Varani, S., Elvin, J. A., Yan, C., DeMayo, J., DeMayo, F. J., Horton, H. F., Byrne, M. C., and Matzuk, M. M. (2002). Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 16, 1154–1167.
| Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility.Crossref | GoogleScholarGoogle Scholar | 12040004PubMed |
Wilson, T., Wu, X. Y., Juengel, J. L., Ross, I. K., Lumsden, J. M., Lord, E. A., Dodds, K. G., Walling, G. A., McEwan, J. C., O’Connell, A. R., McNatty, K. P., and Montgomery, G. W. (2001). Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol. Reprod. 64, 1225–1235.
| Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells.Crossref | GoogleScholarGoogle Scholar | 11259271PubMed |
Xu, Y., Li, E., Han, Y., Chen, L., and Xie, Z. (2010). Differential expression of mRNAs encoding BMP/Smad pathway molecules in antral follicles of high- and low-fecundity Hu sheep. Anim. Reprod. Sci. 120, 47–55.
| Differential expression of mRNAs encoding BMP/Smad pathway molecules in antral follicles of high- and low-fecundity Hu sheep.Crossref | GoogleScholarGoogle Scholar | 20303683PubMed |
Yao, Y., Reheman, A., Xu, Y., and Li, Q. (2019). miR-125b Contributes to ovarian granulosa cell apoptosis through targeting BMPR1B, a major gene for sheep prolificacy. Reprod. Sci. 26, 295–305.
| miR-125b Contributes to ovarian granulosa cell apoptosis through targeting BMPR1B, a major gene for sheep prolificacy.Crossref | GoogleScholarGoogle Scholar | 29661099PubMed |
Yee, J. K. (2016). Off-target effects of engineered nucleases. FEBS J. 283, 3239–3248.
| Off-target effects of engineered nucleases.Crossref | GoogleScholarGoogle Scholar | 27208701PubMed |
Yi, S. E., LaPolt, P. S., Yoon, B. S., Chen, J. Y., Lu, J. K., and Lyons, K. M. (2001). The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA 98, 7994–7999.
| The type I BMP receptor BmprIB is essential for female reproductive function.Crossref | GoogleScholarGoogle Scholar | 11416163PubMed |
Zhang, H., Tian, S., Klausen, C., Zhu, H., Liu, R., and Leung, P. C. (2016). Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol. Cell. Endocrinol. 428, 17–27.
| Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 26992562PubMed |
Zhang, X., Li, W., Wu, Y., Peng, X., Lou, B., Wang, L., and Liu, M. (2017). Disruption of the sheep BMPR-IB gene by CRISPR/Cas9 in in vitro-produced embryos. Theriogenology 91, 163–172.e2.
| Disruption of the sheep BMPR-IB gene by CRISPR/Cas9 in in vitro-produced embryos.Crossref | GoogleScholarGoogle Scholar | 28108032PubMed |
Zhou, S., Yu, H., Zhao, X., Cai, B., Ding, Q., Huang, Y., Li, Y., Li, Y., Niu, Y., Lei, A., Kou, Q., Huang, X., Petersen, B., Ma, B., Chen, Y., and Wang, X. (2018). Generation of gene-edited sheep with a defined Booroola fecundity gene (FecB(B)) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9. Reprod. Fertil. Dev. 30, 1616–1621.
| Generation of gene-edited sheep with a defined Booroola fecundity gene (FecB(B)) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9.Crossref | GoogleScholarGoogle Scholar | 31039970PubMed |
Zuo, E., Sun, Y., Wei, W., Yuan, T., Ying, W., Sun, H., Yuan, L., Steinmetz, L. M., Li, Y., and Yang, H. (2019). Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 364, 289–292.
| Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos.Crossref | GoogleScholarGoogle Scholar | 30819928PubMed |


