In vitro effects of Type I interferons (IFNτ and IFNα) on bovine hepatocytes cultured with or without Kupffer cells
Kai Josef Endriß A , Marie Margarete Meyerholz B , Teresa Fischbach A , Lutz Brimmers A , Christiane Pfarrer C , Christina Deborah Marth D and Marion Schmicke A E F
A E F
A University of Veterinary Medicine Hanover, Clinic for Cattle, Endocrinology, Bischofsholer Damm 15, 30539 Hanover, Germany.
B Clinic for Ruminants with Ambulatory and Herd Health Services, Centre for Clinical Veterinary Medicine, Ludwig-Maximilians-University Munich, Sonnenstraße 16, 85764 Oberschleißheim, Germany.
C University of Veterinary Medicine Hanover, Anatomy, Bischofsholer Damm 15, 30539 Hanover, Germany.
D Melbourne Veterinary School, The University of Melbourne, 250 Princes Highway, Werribee, Vic. 3030, Australia.
E Martin-Luther University Halle-Wittenberg, Faculty of Natural Sciences III, Institute of Agricultural and Nutritional Sciences, Animal Health Management, Theodor-Lieser-Straße 11, 06120 Halle, Germany.
F Corresponding author. Email: marion.schmicke@landw.uni-halle.de
Reproduction, Fertility and Development 33(4) 305-317 https://doi.org/10.1071/RD20278
Submitted: 16 October 2020 Accepted: 17 December 2020 Published: 12 February 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
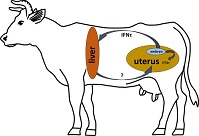
In cattle, maternal recognition of early pregnancy depends on the effects of the embryonic signal interferon (IFN)-τ. IFN-stimulated genes have been upregulated in the maternal liver during early pregnancy. In this study, primary hepatocyte cell culture models were evaluated for their suitability to test Type I IFN effects in vitro. The expression of target genes (interferon-stimulated gene 15 (ISG-15), interferon-induced GTP-binding protein (MX-1), C-X-C motif chemokine 10 (CXCL-10), CXCL-5, insulin-like growth factor 1 (IGF-1), IGF binding protein 2 (IGFBP-2)) was measured using reverse transcription–quantitative polymerase chain reaction in hepatocytes from monoculture or in indirect coculture with Kupffer cells (HKCid) on Days 1, 2, 3 and 4 of culture (n = 21 donor cows). Gene expression was also measured on Day 4 after challenging the cultures with recombinant IFNτ, IFNα, progesterone (P4), IFNτ + IFNα or IFNτ + P4 for 6 h. A significant increase in the mRNA expression of target genes in hepatocytes was shown in response to stimulation with IFNτ. The Kupffer cells in coculture did not influence the effects of IFNτ in hepatocytes. In conclusion, primary bovine hepatocyte cultures are suitable for stimulation experiments with Type I IFNs and as an extrauterine model for embryo–maternal communication. The proposed endocrine action of IFNτ in the liver may affect maternal metabolism and immune function in the liver.
Keywords: cattle, cell culture, embryo, embryo-maternal communication, endocrinology, gestation, hepatocytes, IGF binding protein, insulin-like growth factor, interferone tau
References
Antonelli, A., Martina, S., Fallahi, P., Ghiri, E., Crescioli, C., Romagnani, P., Vitti, P., Serio, M., and Ferrannini, E. (2010). Cytokine Interferon-alpha, -beta and -gamma induce CXCL9 and CXCL10 secretion by human thyrocytes: Modulation by peroxisome proliferator-activated receptor-gamma agonists. Cytokine 50, 260–267.| Cytokine Interferon-alpha, -beta and -gamma induce CXCL9 and CXCL10 secretion by human thyrocytes: Modulation by peroxisome proliferator-activated receptor-gamma agonists.Crossref | GoogleScholarGoogle Scholar | 20299237PubMed |
Arosh, J. A., Banu, S. K., Kimmins, S., Chapdelaine, P., MacLaren, L. A., and Fortier, M. A. (2004). Effect of interferon-τ on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: Evidence of polycrine actions of prostaglandin E2. Endocrinology 145, 5280–5293.
| Effect of interferon-τ on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: Evidence of polycrine actions of prostaglandin E2.Crossref | GoogleScholarGoogle Scholar | 15308607PubMed |
Bott, R. C., Ashley, R. L., Henkes, L. E., Antoniazzi, A. Q., Bruemmer, J. E., Niswender, G. D., Bazer, F. W., Spencer, T. E., Smirnova, N. P., Anthony, R. V., and Hansen, T. R. (2010). Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol. Reprod. 82, 725–735.
| Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes.Crossref | GoogleScholarGoogle Scholar | 20042537PubMed |
Capobianchi, M. R., Uleri, E., Caglioti, C., and Dolei, A. (2015). Type I IFN family members: similarity, differences and interaction. Cytokine Growth Factor Rev. 26, 103–111.
| Type I IFN family members: similarity, differences and interaction.Crossref | GoogleScholarGoogle Scholar | 25466633PubMed |
Dembic, Z. (2015). ‘The cytokines of the immune system – The role of cytokines in disease related to immune response.’ (Academic Press)
Duque, G. A., and Descoteaux, A. (2014). Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491.
| Macrophage cytokines: involvement in immunity and infectious diseases.Crossref | GoogleScholarGoogle Scholar |
Ehrhardt, S., and Schmicke, M. (2016). Isolation and cultivation of adult primary bovine hepatocytes from abattoir derived liver. EXCLI J. 15, 858–866.
| Isolation and cultivation of adult primary bovine hepatocytes from abattoir derived liver.Crossref | GoogleScholarGoogle Scholar | 28275320PubMed |
Fernandez, E. J., and Lolis, E. (2002). Structure, function, and inhibition of chemokines. Annu. Rev. Pharmacol. Toxicol. 42, 469–499.
| Structure, function, and inhibition of chemokines.Crossref | GoogleScholarGoogle Scholar | 11807180PubMed |
Forde, N., Carter, F., Fair, T., Crowe, M. A., Evans, A. C. O., Spencer, T. E., Bazer, F. W., Mcbride, R., Boland, M. P., Gaora, P. O., Lonergan, P., and Roche, J. F. (2009). Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 81, 784–794.
| Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle.Crossref | GoogleScholarGoogle Scholar | 19553605PubMed |
Gillgrass, A. E., Fernandez, S. A., Rosenthal, K. L., and Kaushic, C. (2005). Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J. Virol. 79, 3107–3116.
| Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation.Crossref | GoogleScholarGoogle Scholar | 15709030PubMed |
Gordon, S., and Martinez, F. O. (2010). Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604.
| Alternative activation of macrophages: mechanism and functions.Crossref | GoogleScholarGoogle Scholar | 20510870PubMed |
Han, H., Austin, K. J., Rempel, L. A., and Hansen, T. R. (2006). Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. J. Endocrinol. 191, 505–512.
| Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows.Crossref | GoogleScholarGoogle Scholar | 17088421PubMed |
Imakawa, K., Anthony, R. V., Kazemi, M., Marotti, K. R., Polites, H. G., and Roberts, R. M. (1987). Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature 330, 377–379.
| Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm.Crossref | GoogleScholarGoogle Scholar | 2446135PubMed |
Imakawa, K., Imai, M., Sakai, A., Suzuki, M., Nagaoka, K., Sakai, S., Lee, S. R., Chang, K.-T., Echternkamp, S. E., and Christenson, R. K. (2006). Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol. Reprod. Dev. 73, 850–585.
| Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep.Crossref | GoogleScholarGoogle Scholar | 16596627PubMed |
Kunii, H., Koyama, K., Ito, T., Suzuki, T., Balboula, A. Z., Shirozu, T., Bai, H., Nagano, M., Kawahara, M., and Takahashi, M. (2018). Hot topic: pregnancy-induced expression of interferon-stimulated genes in the cervical and vaginal mucosal membranes. J. Dairy Sci. 101, 8396–8400.
| Hot topic: pregnancy-induced expression of interferon-stimulated genes in the cervical and vaginal mucosal membranes.Crossref | GoogleScholarGoogle Scholar | 29935833PubMed |
Lassarre, C., Hardouin, S., Daffos, F., Forestier, F., Frankenne, F., and Binoux, M. (1991). Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 29, 219–225.
| Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation.Crossref | GoogleScholarGoogle Scholar | 1709729PubMed |
Li, J., and Roberts, R. M. (1994). Interferon-τ and interferon-α interact with the same receptors in bovine endometrium. Use of a readily iodinatable form of recombinant interferon-τ for binding studies. J. Biol. Chem. 269, 13544–13550.
| Interferon-τ and interferon-α interact with the same receptors in bovine endometrium. Use of a readily iodinatable form of recombinant interferon-τ for binding studies.Crossref | GoogleScholarGoogle Scholar | 8175789PubMed |
Li, Q., and Verma, I. M. (2002). NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734.
| NF-κB regulation in the immune system.Crossref | GoogleScholarGoogle Scholar | 12360211PubMed |
Matsuyama, S., Kojima, T., Kato, S., and Kimura, K. (2012). Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET. Reprod. Biol. Endocrinol. 10, 21.
| Relationship between quantity of IFNT estimated by IFN-stimulated gene expression in peripheral blood mononuclear cells and bovine embryonic mortality after AI or ET.Crossref | GoogleScholarGoogle Scholar | 22439976PubMed |
McCarthy, S. D., Roche, J. F., and Forde, N. (2012). Temporal changes in endometrial gene expression and protein localization of members of the IGF family in cattle: effects of progesterone and pregnancy. Physiol. Genomics 44, 130–140.
| Temporal changes in endometrial gene expression and protein localization of members of the IGF family in cattle: effects of progesterone and pregnancy.Crossref | GoogleScholarGoogle Scholar | 22085906PubMed |
Meikle, A., Sahlin, L., Ferraris, A., Masironi, B., Blanc, J. E., Rodríguez-Irazoqui, M., Rodríguez-Piñón, M., Kindahl, H., and Forsberg, M. (2001). Endometrial mRNA expression of oestrogen receptor α, progesterone receptor and insulin-like growth factor-I (IGF-I) throughout the bovine oestrous cycle. Anim. Reprod. Sci. 68, 45–56.
| Endometrial mRNA expression of oestrogen receptor α, progesterone receptor and insulin-like growth factor-I (IGF-I) throughout the bovine oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 11600273PubMed |
Mense, K., Meyerholz, M., Gil Araujo, M., Lietzau, M., Knaack, H., Wrenzycki, C., Hoedemaker, M., and Piechotta, M. (2015). The somatotropic axis during the physiological estrus cycle in dairy heifers – effect on hepatic expression of GHR and SOCS2. J. Dairy Sci. 98, 2409–2418.
| The somatotropic axis during the physiological estrus cycle in dairy heifers – effect on hepatic expression of GHR and SOCS2.Crossref | GoogleScholarGoogle Scholar | 25704974PubMed |
Meyerholz, M. M., Mense, K., Lietzau, M., Kassens, A., Linden, M., Knaack, H., Wirthgen, E., Hoeflich, A., Raliou, M., Richard, C., Sandra, O., Schuberth, H., Hoedemaker, M., and Schmicke, M. (2015). Serum IGFBP4 concentration decreased in dairy heifers towards day 18 of pregnancy. J. Vet. Sci. 16, 413–421.
| Serum IGFBP4 concentration decreased in dairy heifers towards day 18 of pregnancy.Crossref | GoogleScholarGoogle Scholar | 26243597PubMed |
Mogensen, K. E., Lewerenz, M., Reboul, J., Lutfalla, G., and Uzé, G. (1999). The type I interferon receptor: structure, function, and evolution of a family business. J. Interferon Cytokine Res. 19, 1069–1098.
| The type I interferon receptor: structure, function, and evolution of a family business.Crossref | GoogleScholarGoogle Scholar | 10547147PubMed |
Mosser, D. M., and Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969.
| Exploring the full spectrum of macrophage activation.Crossref | GoogleScholarGoogle Scholar | 19029990PubMed |
Naito, M., Hasegawa, G., and Yamamoto, J. T. (2004). Differentiation and function of Kupffer cells. Med. Electron Microsc. 37, 16–28.
| Differentiation and function of Kupffer cells.Crossref | GoogleScholarGoogle Scholar | 15057601PubMed |
Nakamura, K., Kusama, K., Bai, R., Sakurai, T., Isuzugawa, K., Godkin, J. D., Suda, Y., and Imakawa, K. (2016). Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS One 11, e0158278.
| Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period.Crossref | GoogleScholarGoogle Scholar | 27649071PubMed |
Oeckinghaus, A., and Ghosh, S. (2009). The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 1, a000034.
| The NF-κB family of transcription factors and its regulation.Crossref | GoogleScholarGoogle Scholar | 20066092PubMed |
Ohmann, H. B., Lawman, M. J. P., and Babiuk, L. A. (1987). Bovine interferon: its biology and application in veterinary medicine. Antiviral Res. 7, 187–210.
| Bovine interferon: its biology and application in veterinary medicine.Crossref | GoogleScholarGoogle Scholar | 2441661PubMed |
Oliveira, J. F., Henkes, L. E., Ashley, R. L., Purcell, S. H., Smirnova, N. P., Veeramachaneni, D. N. R., Anthony, R. V., and Hansen, T. R. (2008). Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-τ release from the uterine vein. Endocrinology 149, 1252–1259.
| Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-τ release from the uterine vein.Crossref | GoogleScholarGoogle Scholar | 18063687PubMed |
Roberts, R. M., Liu, L., Guo, Q., Leaman, D. W., and Bixby, J. (1998). The evolution of the type I interferons. J. Interferon Cytokine Res. 18, 805–816.
| The evolution of the type I interferons.Crossref | GoogleScholarGoogle Scholar | 9809615PubMed |
Robinson, R. S., Hammond, A. J., Wathes, D. C., Hunter, M. G., and Mann, G. E. (2008). Corpus luteum–endometrium–embryo interactions in the dairy cow: underlying mechanisms and clinical relevance. Reprod. Domest. Anim. 43, 104–112.
| Corpus luteum–endometrium–embryo interactions in the dairy cow: underlying mechanisms and clinical relevance.Crossref | GoogleScholarGoogle Scholar | 18638111PubMed |
Romero, J. J., Antoniazzi, A. Q., Nett, T. M., Ashley, R. L., Webb, B. T., Smirnova, N. P., Bott, R. C., Bruemmer, J. E., Bazer, F. W., Anthony, R. V., and Hansen, T. R. (2015). Temporal release, paracrine and endocrine actions of ovine conceptus-derived interferon-tau during early pregnancy. Biol. Reprod. 93, 146.
| Temporal release, paracrine and endocrine actions of ovine conceptus-derived interferon-tau during early pregnancy.Crossref | GoogleScholarGoogle Scholar | 26559679PubMed |
Ruhmann, B., Giller, K., Hankele, A. K., Ulbrich, S. E., and Schmicke, M. (2017). Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy. Theriogenology 104, 198–204.
| Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy.Crossref | GoogleScholarGoogle Scholar | 28888122PubMed |
Sen, G. C., and Sarkar, S. N. (2007). The Interferon-Stimulated Genes: Targets of Direct Signaling by Interferons, Double-Stranded RNA, and Viruses. In ‘Interferon: The 50th Anniversary’. (Ed. P. M. Pitha.) pp. 233–250. (Springer.)
Smedsrød, B., Pertoft, H., Eggertsen, G., and Sundström, C. (1985). Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 241, 639–649.
| Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence.Crossref | GoogleScholarGoogle Scholar | 2992796PubMed |
Spencer, T. E., Forde, N., and Lonergan, P. (2016). The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants1. J. Dairy Sci. 99, 5941–5950.
| The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants1.Crossref | GoogleScholarGoogle Scholar | 26387021PubMed |
Stein, M., Keshav, S., Harris, N., and Gordon, S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292.
| Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation.Crossref | GoogleScholarGoogle Scholar | 1613462PubMed |
Teixeira, M. G., Austin, K. J., Perry, D. J., Dooley, V. D., Johnson, G. A., Francis, B. R., and Hansen, T. R. (1997). Bovine granulocyte chemotactic protein-2 is secreted by the endometrium in response to interferon-tau (IFN-τ). Endocrine 6, 31–37.
| Bovine granulocyte chemotactic protein-2 is secreted by the endometrium in response to interferon-tau (IFN-τ).Crossref | GoogleScholarGoogle Scholar | 9225113PubMed |
Uzé, G., Schreiber, G., Piehler, J., and Pellegrini, S. (2007). The Receptor of the Type I Interferon Family. In ‘Interferon: The 50th Anniversary’. (Ed. P. M. Pitha.) pp. 71–90. (Springer.)
Vinken, M., Maes, M., Oliveira, A. G., Cogliati, B., Marques, P. E., Menezes, G. B., Dagli, M. L. Z., Vanhaecke, T., and Rogiers, V. (2014). Primary hepatocytes and their cultures in liver apoptosis research. Arch. Toxicol. 88, 199–212.
| Primary hepatocytes and their cultures in liver apoptosis research.Crossref | GoogleScholarGoogle Scholar | 24013573PubMed |
Witte, S., Brockelmann, Y., Haeger, J. D., and Schmicke, M. (2019). Establishing a model of primary bovine hepatocytes with responsive growth hormone receptor expression. J. Dairy Sci. 102, 7522–7535.
| Establishing a model of primary bovine hepatocytes with responsive growth hormone receptor expression.Crossref | GoogleScholarGoogle Scholar | 31155243PubMed |
Wynn, T. A., Chawla, A., and Pollard, J. W. (2013). Origins and hallmarks of macrophages: development, homeostasis, and disease. Nature 496, 445–455.
| Origins and hallmarks of macrophages: development, homeostasis, and disease.Crossref | GoogleScholarGoogle Scholar | 23619691PubMed |
Yankey, S. J., Hicks, B. A., Carnahan, K. G., Assiri, A. M., Sinor, S. J., Kodali, K., Stellflug, J. N., and Ott, T. L. (2001). Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. J. Endocrinol. 170, R7–R11.
| Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes.Crossref | GoogleScholarGoogle Scholar | 11479146PubMed |


