Thermal-treatment protocol to induce thermotolerance in bovine embryos
Clara S. Oliveira A C , Sheila C. S. Marques A , Pedro H. E. Guedes A , Viviane L. Feuchard B , Agostinho J. R. Camargo B , Celio de Freitas A and Luiz S. A. Camargo A
A C , Sheila C. S. Marques A , Pedro H. E. Guedes A , Viviane L. Feuchard B , Agostinho J. R. Camargo B , Celio de Freitas A and Luiz S. A. Camargo A
A Animal Reproduction Laboratory, Embrapa Dairy Cattle, Fazenda Santa Monica Road, Barao de Juparana, Valença, RJ, Brazil.
B Animal Biology Laboratory, Agriculture Research Company of the Rio de Janeiro State (PESAGRO RIO), Sao Boa Ventura Avenue, 770, Niterói, RJ, Brazil.
C Corresponding author. Email: clara.oliveira@embrapa.br
Reproduction, Fertility and Development 33(7) 497-501 https://doi.org/10.1071/RD20309
Submitted: 25 November 2020 Accepted: 13 March 2021 Published: 30 April 2021
Abstract
Artificial reproduction in dairy cattle is challenged by summer temperatures in tropical environments. We describe a treatment based on mild temperature increases to induce thermotolerance and improve the embryo’s performance under heat stress conditions. A protocol was established to induce upregulation of heat shock protein A (HSPA, formerly known as HSP70) but not impair embryonic development. Thermal treatment (TT) had no effect on morula/blastocyst rate or blastocyst quality (cell number and apoptosis). Heat shock given one day after TT revealed higher (P = 0.00) survival rates in TT blastocysts compared with Control. Treated embryos were transferred to recipients and no detrimental effects were observed regarding pregnancy rates, length, fetal growth or calf weight. Our results demonstrated that the established TT protocol could induce a thermal response by the embryo and is safe for further development.
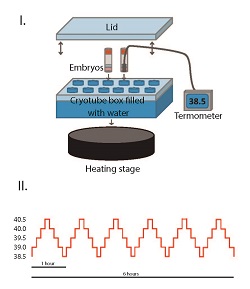
Keywords: heat shock, thermal stress, IVP, IVF, HSP, embryo survival, bovine, HSP70.
References
de Barros, F. R. O., and Paula-Lopes, F. F. (2018). Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos. Mol. Reprod. Dev. 85, 810–820.| Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos.Crossref | GoogleScholarGoogle Scholar |
Geisert, R. D., Zavy, M. T., and Biggers, B. G. (1988). Effect of heat stress on conceptus and uterine secretion in the bovine. Theriogenology 29, 1075–1082.
| Effect of heat stress on conceptus and uterine secretion in the bovine.Crossref | GoogleScholarGoogle Scholar | 16726429PubMed |
Gurbuxani, S., Schmitt, E., Cande, C., Parcellier, A., Hammann, A., Daugas, E., Kouranti, I., Spahr, C., Pance, A., Kroemer, G., and Garrido, C. (2003). Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 22, 6669–6678.
| Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor.Crossref | GoogleScholarGoogle Scholar | 14555980PubMed |
Hahnel, A. C., Gifford, D. J., Heikkila, J. J., and Schultz, G. A. (1986). Expression of the major heat shock protein (hsp 70) family during early mouse embryo development. Teratog. Carcinog. Mutagen. 6, 493–510.
| Expression of the major heat shock protein (hsp 70) family during early mouse embryo development.Crossref | GoogleScholarGoogle Scholar | 2881365PubMed |
Hansen, P. J. (2009). Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3341–3350.
| Effects of heat stress on mammalian reproduction.Crossref | GoogleScholarGoogle Scholar | 19833646PubMed |
Hansen, P. J., and Hansen, P. J. (2019). Reproductive physiology of the heat-stressed dairy cow: implications for fertility and assisted reproduction. Anim. Reprod. 16, 497–507.
| Reproductive physiology of the heat-stressed dairy cow: implications for fertility and assisted reproduction.Crossref | GoogleScholarGoogle Scholar | 32435293PubMed |
Kumar, A., Ashraf, S., Goud, T. S., Grewal, A., Singh, S. V., Yadav, B. R., and Upadhyay, R. C. (2015). Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition. J. Therm. Biol. 51, 55–64.
| Expression profiling of major heat shock protein genes during different seasons in cattle (Bos indicus) and buffalo (Bubalus bubalis) under tropical climatic condition.Crossref | GoogleScholarGoogle Scholar | 25965018PubMed |
Lelièvre, J. M., Peynot, N., Ruffini, S., Laffont, L., Le Bourhis, D., Girard, P. M., and Duranthon, V. (2017). Regulation of heat-inducible HSPA1A gene expression during maternal-to-embryo transition and in response to heat in in vitro-produced bovine embryos. Reprod. Fertil. Dev. 29, 1868–1881.
| Regulation of heat-inducible HSPA1A gene expression during maternal-to-embryo transition and in response to heat in in vitro-produced bovine embryos.Crossref | GoogleScholarGoogle Scholar | 27851888PubMed |
Loones, M. T., Chang, Y., and Morange, M. (2000). The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation. Cell Stress Chaperones 5, 291–305.
| The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation.Crossref | GoogleScholarGoogle Scholar | 11048652PubMed |
Muller, W. U., Li, G. C., and Goldstein, L. S. (1985). Heat does not induce synthesis of heat shock proteins or thermotolerance in the earliest stage of mouse embryo development. Int. J. Hyperthermia 1, 97–102.
| Heat does not induce synthesis of heat shock proteins or thermotolerance in the earliest stage of mouse embryo development.Crossref | GoogleScholarGoogle Scholar | 3837084PubMed |
Oliveira, C. S., Saraiva, N. Z., de Lima, M. R., Oliveira, L. Z., Serapião, R. V., Garcia, J. M., Borges, C. A. V., and Camargo, L. S. A. (2016). Cell death is involved in sexual dimorphism during preimplantation development. Mech. Dev. 139, 42–50.
| Cell death is involved in sexual dimorphism during preimplantation development.Crossref | GoogleScholarGoogle Scholar | 26752320PubMed |
Oliveira, C. S., Varella Serapião, R., dos Reis Camargo, A. J., de Freitas, C., Tamy Iguma, L., Campos Carvalho, B., de Almeida Camargo, L. S., Zoccolaro Oliveira, L., and da Silva Verneque, R. (2019). Oocyte origin affects the in vitro embryo production and development of Holstein (Bos taurus taurus) - Gyr (Bos taurus indicus) reciprocal cross embryos. Anim. Reprod. Sci. 209, 106165.
| Oocyte origin affects the in vitro embryo production and development of Holstein (Bos taurus taurus) - Gyr (Bos taurus indicus) reciprocal cross embryos.Crossref | GoogleScholarGoogle Scholar | 31514927PubMed |
Sakatani, M., Alvarez, N. V., Takahashi, M., and Hansen, P. J. (2012). Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J. Dairy Sci. 95, 3080–3091.
| Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle.Crossref | GoogleScholarGoogle Scholar | 22612944PubMed |
Vasconcelos, J. L. M., Demétrio, D. G. B., Santos, R. M., Chiari, J. R., Rodrigues, C. A., and Sá Filho, O. G. (2006). Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology 65, 192–200.
| Factors potentially affecting fertility of lactating dairy cow recipients.Crossref | GoogleScholarGoogle Scholar |
Wang, F., Bonam, S. R., Schall, N., Kuhn, L., Hammann, P., Chaloin, O., Madinier, J. B., Briand, J. P., Page, N., and Muller, S. (2018). Blocking nuclear export of HSPA8 after heat shock stress severely alters cell survival. Sci. Rep. 8, 16820.
| Blocking nuclear export of HSPA8 after heat shock stress severely alters cell survival.Crossref | GoogleScholarGoogle Scholar | 30429537PubMed |


