Non-invasive assessment of oocyte developmental competence
Tiffany C. Y. Tan A B C and Kylie R. Dunning
A B C and Kylie R. Dunning  A B C *
A B C *
A Robinson Research Institute, School of Biomedicine, The University of Adelaide, Adelaide, SA, Australia.
B Australian Research Council Centre of Excellence for Nanoscale Biophotonics, The University of Adelaide, Adelaide, SA, Australia.
C Institute for Photonics and Advanced Sensing, The University of Adelaide, Adelaide, SA, Australia.
Reproduction, Fertility and Development 35(2) 39-50 https://doi.org/10.1071/RD22217
Published online: 18 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Oocyte quality is a key factor influencing IVF success. The oocyte and surrounding cumulus cells, known collectively as the cumulus oocyte complex (COC), communicate bi-directionally and regulate each other’s metabolic function to support oocyte growth and maturation. Many studies have attempted to associate metabolic markers with oocyte quality, including metabolites in follicular fluid or ‘spent medium’ following maturation, gene expression of cumulus cells and measuring oxygen consumption in medium surrounding COCs. However, these methods fail to provide spatial metabolic information on the separate oocyte and cumulus cell compartments. Optical imaging of the autofluorescent cofactors – reduced nicotinamide adenine dinucleotide (phosphate) [NAD(P)H] and flavin adenine dinucleotide (FAD) – has been put forward as an approach to generate spatially resolved measurements of metabolism within individual cells of the COC. The optical redox ratio (FAD/[NAD(P)H + FAD]), calculated from these cofactors, can act as an indicator of overall metabolic activity in the oocyte and cumulus cell compartments. Confocal microscopy, fluorescence lifetime imaging microscopy (FLIM) and hyperspectral microscopy may be used for this purpose. This review provides an overview of current optical imaging techniques that capture the inner biochemistry within cells of the COC and discusses the potential for such imaging to assess oocyte developmental competence.
Keywords: autofluorescence, cellular metabolism, FAD, NAD(P)H, non-invasive, oocyte assessment, optical imaging, optical redox ratio.
Introduction
In recent years, the social trend of delayed childbearing (pregnancy occurring in women 35 years and older) is one of the growing reasons for subfertility (Johnson et al. 2012; Adashi and Gutman 2018). Advanced maternal age, along with increased incidence of lifestyle factors such as obesity and diabetes, have contributed to the statistic that one in six couples have trouble conceiving (Chambers et al. 2017). Many studies have associated maternal age with oocyte developmental competence (Cimadomo et al. 2018; Krisher 2018; Lee et al. 2021; Ntostis et al. 2022). Oocyte developmental competence is defined as the ability of an oocyte to successfully resume meiosis, undergo fertilisation and preimplantation embryo development, implant and result in the birth of a healthy offspring (Dunning et al. 2010). Importantly, oocyte quality is a key limiting factor in determining the success rate of an IVF cycle (Gilchrist et al. 2008).
The predominant method used to assess oocyte quality is morphological inspection prior to fertilisation (Lasienë et al. 2009). Several reviews discuss, and cover in detail, the morphological predictors of oocyte quality, which include assessments of cumulus expansion, zona pellucida, perivitelline space, polar body cytoplasm, and meiotic spindle analysis using polarisation microscopy (Ebner et al. 2003; Wang and Sun 2007; Lasienë et al. 2009). However, oocyte selection based on morphological criteria are highly subjective and dependent on the skill of the embryologist in predicting competency (Wang and Sun 2007; Richani et al. 2021).
There are many studies focused on understanding the metabolism of the cumulus oocyte complex (COC) (O’Brien et al. 1996; Ferguson and Leese 2006; Dunning et al. 2010; Sutton-McDowall et al. 2010; Hemmings et al. 2012) and how various metabolic pathways impact oocyte quality (for review, see Richani et al. 2021). However, current methods used to measure metabolism are potentially limited in their ability to predict oocyte developmental potential, as they mostly focus on the follicular fluid or ‘spent medium’ following oocyte maturation, which are largely reflective of the somatic follicular cells and not the oocyte (Richani et al. 2021). In this review, we will discuss how optical approaches can provide spatial metabolic information on the separate oocyte and cumulus cell compartments, as well as the potential for such imaging to assess oocyte developmental competence.
Metabolic co-dependence of the oocyte and cumulus cells
Substrates for mammalian oocyte metabolism primarily come from the surrounding environment (in vivo or in vitro culture) which include glucose, pyruvate, lactate and glutamine. Oocytes regulate their metabolism during development, with mitochondria being a vital organelle for this.
Mitochondria
Mitochondria play a multifaceted role in supporting the development of the mammalian oocyte and embryo. They are membrane-bound organelles present in almost all eukaryotic cells. The mitochondria in mammalian oocytes are different from the typical structure found in somatic cells in that they are more spherical and contain fewer, irregular cristae. Therefore, the mitochondria found in oocytes are less efficient at producing ATP compared to somatic cell mitochondria (Bentov et al. 2011). As such, mature oocytes contain the largest number of mitochondria (∼100 000) (May-Panloup et al. 2005), far more than sperm (∼50–75) (Hirata et al. 2002) or somatic cells (∼80–2000) (Cole 2016) to meet their energy requirements. Importantly, the number of mitochondria within an oocyte is one of the important factors for oocyte and subsequent embryo quality. This is because they are maternally inherited organelles, and the early stages of embryo development are entirely dependent on the starting pool of mitochondria from the oocyte until the blastocyst-stage embryo (van der Reest et al. 2021). Mitochondrial dysfunction has been associated with fertilisation failure (Santos et al. 2006), abnormal embryo development (Ge et al. 2012), implantation failure (Wai et al. 2010) and increased incidence of aneuploidy (Fragouli et al. 2015).
The primary function of mitochondria is to generate ATP through oxidative phosphorylation, which is supported by the tricarboxylic acid (TCA) cycle. The TCA cycle generates electron carriers: reduced nicotinamide adenine dinucleotide (NADH) and reduced flavin adenine dinucleotide (FADH2), mainly from catabolism of glucose and fatty acids (May-Panloup et al. 2021). Oxidative phosphorylation involves five large multi-enzymatic complexes embedded within the cristae of the inner mitochondrial membrane. The electron transport chain (ETC) functions by the transfer of electrons using dedicated electron carriers along the mitochondrial membrane, while pumping protons into the intermembrane space, giving rise to the mitochondrial membrane potential (van der Reest et al. 2021). The proton gradient generated by the transfer of electrons across the ETC is ultimately used to power the synthesis of ATP from ADP by the ATP synthase (May-Panloup et al. 2021). The ratio of reduced to oxidised electron donors (NADH/NAD+ and FADH2/FAD) in the mitochondrial matrix space forms an effective measurement of overall metabolic activity – the redox ratio (Dumollard et al. 2009; Skala and Ramanujam 2010).
Metabolism in mammalian oocytes: a requirement for oocyte developmental competence
During maturation, oocytes exhibit a high energy demand required for a series of nuclear and cytoplasmic changes. The mammalian oocyte and surrounding cumulus cells communicate bi-directionally (Eppig 2001). These two cell types are connected by gap junctions (Anderson and Albertini 1976), forming the cumulus oocyte complex (COC). Substrate utilisation, especially glucose and fatty acids, provide the oocyte with sufficient ATP to attain developmental competence (Sutton-McDowall et al. 2010; Dunning et al. 2011).
Glucose is one of the key substrates for the COC to generate ATP. The oocyte itself has a low capacity for glucose metabolism due to the lack of phosphofructokinase activity (Dumesic et al. 2015). The highly glycolytic cumulus cells therefore play an important role in metabolising glucose to pyruvate, which is then supplied via gap junctions to the oocyte for ATP production. (Biggers et al. 1967; Yeo et al. 2009; Sutton-McDowall et al. 2010; Dumesic et al. 2015). Pyruvate is converted into acetyl coenzyme A (Acetyl-CoA) in the oocyte mitochondria, which enters the TCA cycle and electron transport chain (ETC) to produce ATP. Concurrently, the oocyte ensures its own supply of pyruvate by up-regulating glycolytic genes in cumulus cells via secretion of growth differentiation factor-9 (Sugiura et al. 2007; Gilchrist et al. 2008). A small portion of glucose is metabolised by the pentose phosphate pathway in cumulus cells (Sutton-McDowall et al. 2010), which involves the conversion of glucose-6-phosphate dehydrogenase (generated from glucose) to ribose 5 phosphate, producing NADPH. The molecule NADPH is important for the biosynthesis of fatty acids and nucleotides, and for maintaining the balance between reduced and oxidised glutathione, which acts as an antioxidant against reactive oxygen species (ROS) (Gutnisky et al. 2014).
In recent years, the importance of lipid metabolism, specifically fatty acid- or β-oxidation, for oocyte developmental potential has been shown (Ferguson and Leese 2006; Sturmey et al. 2006; Dunning et al. 2010). Activated fatty acids (fatty acyl-CoA) are transported into mitochondria by the carnitine palmitoyl transferase 1. Fatty acyl-CoA is then metabolised via β-oxidation in the mitochondria matrix and enters the TCA cycle to generate NADH and FADH2 (Dunning et al. 2010). During ovulation, the maturing COC increases the use of energy from lipid metabolism, as metabolism of fatty acids has the capacity to produce 106 ATP, which is 3.5-fold more than glucose (Valsangkar and Downs 2013; Dunning et al. 2014). As the metabolism of fatty acids and glucose are crucial for oocyte development, it is important to maintain balanced energy homeostasis within oocytes and cumulus cells, which further supports subsequent embryo development (Dumollard et al. 2007; Blacker and Duchen 2016).
Assessment of oocyte developmental competence
To date, much of the work has focused on finding methods to select the most viable embryo for transfer, while research into developing assessments for oocyte developmental potential has received far less attention. Importantly, the quality of the oocyte cannot be improved by altering laboratory practices, as it is determined prior to oocyte pick-up from the ovary. Therefore, oocyte quality is the first checkpoint for a successful pregnancy, and is important not only for fertilisation, but also for subsequent development (Gilchrist et al. 2008).
Clinically, all morphologically normal oocytes are fertilised and selection is mainly focused on the embryo (Richani et al. 2021). As developmental competency of an embryo is highly dependent on the oocyte it is derived from, selection of an oocyte with good developmental potential is likely to improve IVF success (Richani et al. 2021). This then raises a challenge; do we have a clinically reliable and accurate diagnostic to select good quality oocytes?
Potential biomarkers in follicular fluid, culture medium and cumulus cells
There has been significant interest in finding potential biomarkers of oocyte developmental competence in follicular fluid, ‘spent’ culture medium and cumulus cells. Profiling of follicular fluid is a promising approach as the material is collected directly from the environment in which the oocyte developed and matured. Some of the potential biomarkers linked with oocyte quality include cytokines and growth factors such as granulocyte-colony stimulation factors, interleukins and growth differentiation factor-9 (Mendoza et al. 2002). Similarly, the presence and abundance of fatty acids in follicle fluid has also been shown to associate with oocyte quality (Jungheim et al. 2011; Feng et al. 2022). Additionally, proteomic (Ambekar et al. 2013; Shen et al. 2017) and metabolomic (Wu et al. 2007; O’Gorman et al. 2013) analysis of follicular fluid showed correlations between metabolites and oocyte developmental competence.
Analysis of IVM ‘spent medium’ has been proposed as a potential predictor for oocyte competence, particularly fertilisation success. In a mouse model, developmentally competent COCs that were subsequently fertilised demonstrated an increase in glucose consumption and lactate secretion, compared to those that did not fertilise (Preis et al. 2005). In a separate study, the turnover of amino acids were significantly different between bovine oocytes that failed to fertilise and those that subsequently developed to the blastocyst-stage (Hemmings et al. 2012). Specifically, oocytes that failed to fertilise utilised significantly more glutamine and produced significantly higher amounts of alanine compared to those that subsequently developed to the blastocyst-stage (Hemmings et al. 2012).
Cumulus cell gene expression, involved in cell signalling, homeostasis and metabolism was also proposed as a non-invasive viability marker (McKenzie et al. 2004; Fragouli et al. 2012). Similarly, in human cumulus cells, genes involved in metabolism and extracellular matrix formation were expressed at a significantly higher level when derived from an oocyte that resulted in a live birth (Gebhardt et al. 2011). Taking a slightly different approach, multiple studies have shown that telomere length in cumulus cells was associated with oocyte quality. (Cheng et al. 2013; Ozturk et al. 2014).
Despite the generation of a substantial body of research to develop potential predictors for oocyte developmental competence, these methods have not been routinely implemented. Perhaps this is due to the limitations of these approaches, which include (1) confounding factors caused by varying clinical practice; (2) small sample size; and (3) measurements of the entire COC that cannot detect heterogeneity between the oocyte and cumulus cell compartments. Therefore, development of a reliable, accurate and non-invasive tool to assess oocyte quality would be highly desirable.
New tools to non-invasively measure oocyte metabolism
Metabolic co-factors: NAD(P)H and FAD
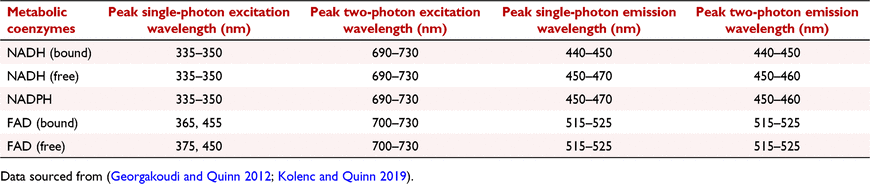
In the 1950s, Britton Chance first discovered and began to characterise the naturally fluorescent metabolic coenzymes: NADH and FAD, and associated these molecules with cellular metabolism (Chance 1952; Chance et al. 1962a). His research started in isolated cells (Chance et al. 1964; Scholz et al. 1969), and subsequently explored the potential of these molecules as reliable indicators of cell metabolism in other tissues such as the liver (Ji et al. 1979; Quistorff and Chance 1986), bladder and brain (Chance et al. 1962b). In mitochondria, NADH and FAD are directly involved in the production of ATP through oxidative phosphorylation (Fig. 1). These metabolic co-factors exist either in the oxidised (NAD+, NADP+, FAD) or reduced (NADH, NADPH and FADH2) state. Among these, only NADH, NADPH and FAD are autofluorescent. Therefore, quantifying the fluorescence intensity of these molecules has been used to identify the metabolic changes in living cells or tissues. The autofluorescence of these coenzymes (both bound and free) can be detected using specific excitation and emission bands (Table 1) (Georgakoudi and Quinn 2012; Kolenc and Quinn 2019). It is important to note that NADH and NADPH have near identical spectral properties, generally excited at 335–350 nm and emitted at 440–460 nm and are therefore collectively referred as NAD(P)H. On the other hand, FAD is excited at 450 nm and reaches peak emission at approximately 520 nm. To understand the connection between redox state and metabolism, it is important to understand the roles of NADH and FAD in oxidative phosphorylation.
|
Roles of NADH and FAD in oxidative phosphorylation
During cellular metabolism, substrates such as glucose and fatty acids are broken down to produce ATP. During glycolysis, a glucose molecule is catabolised to pyruvate, producing ATP and reducing NAD+ to NADH. Following glycolysis, pyruvate is shuttled into the mitochondria and broken down to acetyl-CoA by pyruvate dehydrogenase. Acetyl-CoA will then enter the TCA cycle, which begins to break down carbon-based molecules to CO2, producing electron carriers: NADH and FADH2. During oxidative phosphorylation, TCA cycle generated NADH and FADH2 revert back to their oxidised form in the ETC–NAD+ and FAD by the enzyme complexes I (NADH dehydrogenase) and II (succinate dehydrogenase), respectively (Fig. 1). Electrons are passed to coenzyme Q and complex III (cytochrome c reductase). The ultimate fate of these electrons is the conversion of oxygen to water at complex IV (cytochrome c oxidase). Simultaneously, proton ions are pumped into the intermembrane space of the mitochondria by complex I, III and IV. This creates a proton gradient, which is used to power ATP synthase to produce ATP (Osellame et al. 2012).
The ratio of reduced to oxidised electron carriers indicates the metabolic state of cells that primarily rely on oxidative phosphorylation. As mentioned earlier, only NAD(P)H and FAD are naturally fluorescent molecules, thus, the ratio of FAD/(FAD + NAD(P)H), can be utilised to measure the cell redox state (referred to as optical redox ratio; ORR) (Chance et al. 1979). During oxidative phosphorylation, the autofluorescence of NADH decreases when oxidised to non-fluorescent NAD+ (at complex I), while the autofluorescence of FAD increases due to oxidation of FADH2 (at complex II), resulting in a higher ORR, indicative of increased ATP production (Georgakoudi and Quinn 2012). On the other hand, the ORR will decrease in the event of hypoxia or the need to increase glycolysis despite the presence of oxygen (Warburg effect), as commonly observed in cancer cells or cells undergoing high proliferation in response to disease or injury (Liberti and Locasale 2016). It is important to acknowledge that not all NADH fluorescence is associated with the bound state in oxidative phosphorylation, but can also be contributed from bound- and free-NADPH and free-NADH found in the cytoplasm (Blacker and Duchen 2016). The same is true for FAD; although the majority of FAD is found within the mitochondria and associated with oxidative phosphorylation, a small fraction of FAD fluorescence can be either attributed to electron transfer flavoproteins during the fatty acid oxidation or reduced by dithionite which is not related to cellular metabolism (Kunz and Kunz 1985).
Autofluorescence: label-free optical imaging to measure cellular metabolism
Autofluorescence within cells is commonly perceived as an interfering factor due to the impact on contrast when using a fluorescently labelled probe (Staikopoulos et al. 2016). However, a wide range of endogenous fluorophores that are integral to cellular function such as lipids, vitamins and amino acids have been identified as naturally fluorescent (Andersson et al. 1998; Croce and Bottiroli 2014). With advancements in microscopies and the ability to capture high-resolution images, autofluorescence recorded by optical imaging is able to measure the inner biochemistry of cells. Thus, this presents an opportunity for a non-invasive measurement that can directly measure the cellular metabolism of an individual oocyte, and potentially determine their developmental potential. As NADH and FAD are directly associated with mitochondrial activity (Blacker and Duchen 2016), both have been utilised to monitor the metabolic changes of oocytes and embryos in the complete absence of exogenous tags (Dumollard et al. 2004, 2007; Sutton-McDowall et al. 2012, 2015; Nohales-Córcoles et al. 2016). Importantly, we and others have shown that optical imaging has the capacity to capture the dynamic and heterogenic aspects of metabolic differences that exist between cells of the COC and the embryo (Dumollard et al. 2004, 2007; Denisenko et al. 2005; Brison et al. 2014; Sutton-McDowall et al. 2015; Tan et al. 2022a). To achieve this, various optical imaging approaches have been proposed, including standard fluorescence microscopy, Raman spectroscopy, laser scanning confocal microscopy and hyperspectral microscopy. In our recent study, we found that the ORR was an accurate reflection of metabolism in the oocyte compartment when compared with oxygen consumption measured in the whole COC (Fig. 2; Tan et al. 2022a). These findings demonstrate that label-free optical imaging of metabolic cofactors is a sensitive assay for measuring metabolic changes and has the potential to assess oocyte developmental competence.
|
|
Although optical imaging is deemed as non-invasive for living cells or tissues, this cannot be assumed to be the case for oocytes and embryos. Existing literature shows that light exposure can be detrimental to embryo development and lead to increased levels of DNA damage (Takenaka et al. 2007; Campugan et al. 2022). Therefore, the safety of any imaging modality must be assessed in detail before implementing to human IVF.
Standard fluorescence microscopy
Early autofluorescence work by Chance was not focused on providing a non-invasive assessment tool, therefore the general localisation of NAD(P)H fluorescence intensity within living cells was initially measured using a fluorimeter (Chance and Thorell 1959). This was followed by the development of the Chance Redox Scanner which allowed the capture of images (Quistorff et al. 1985). Two-photon excited fluorescence (TPEF) microscopy has emerged as another imaging tool to assess metabolic activity and cellular responses. In TPEF microscopy, molecules are excited through the simultaneous absorptions of two near-infrared photons with half the energy and double the wavelength (Quinn et al. 2013). This allows for imaging at higher penetration depths and reduces potential photodamage in the out-of-focus regions (Georgakoudi and Quinn 2012). However, longer wavelengths are not ideal for high resolution three-dimensional (3D) imaging (Swoger et al. 2014).
Laser scanning confocal microscopy
Confocal microscopy has become a standard imaging modality for biological applications when requiring 3D images. Laser scanning confocal microscopy operates by focal point illumination with a high-power laser. The ‘confocal pinhole’ is an aperture placed in front of the detector to reject any out-of-focus light and allow images to be taken at different depths (Blacker and Duchen 2016). This allows for image acquisition of high-resolution subcellular structures and provides detailed localisation of molecules of interest. This imaging approach was utilised widely to investigate a range of developmental processes in different cell types such as epithelial and endothelial cells in the cornea (Masters et al. 1993), lung tissue (Erokhina et al. 2019), plant cells (Roshchina 2012) and embryonic cells (Heppert et al. 2016). Traditionally, most of the studies that employ confocal microscopy use an exogenous tag or molecular probe to monitor cellular processes and biochemistry within the cells (Johnson 2010), such as DAPI for nucleus, Mitotracker for mitochondria and Mitosox for superoxide and antibodies. There is increasing interest in using confocal microscopy to capture metabolic cofactors, NAD(P)H and FAD, in oocytes and embryos (Dumollard et al. 2004, 2007; Sutton-McDowall et al. 2016, 2017; Santos Monteiro et al. 2021). Despite the promising results from these studies, a pitfall of this approach is the use of lasers which makes it difficult for clinical implementation due to the high probability of photodamage (Campugan et al. 2022).
Fluorescence lifetime microscopy (FLIM)
Fluorescence lifetime microscopy (FLIM) can capture autofluorescence and is able to provide discrimination between NADH and NADPH (Blacker et al. 2014). It may offer a more sensitive approach to detect metabolic cofactors by measuring the time a fluorophore remains in an excited state before being emitted, allowing the discrimination between bound- and free-NADH, and separating the NADH fluorescence from NADPH (Drozdowicz-Tomsia et al. 2014; Sanchez et al. 2019). Indeed, in reproductive medicine research, FLIM has proven successful in assessing the metabolic processes of oocytes (Sanchez et al. 2018, 2019) and provides spatial distribution of metabolic cofactors in the cytoplasm and mitochondrial regions. However, FLIM involves the use of complex laser and detection electronics for image acquisition, making it challenging for clinical translation.
Raman microscopy
Raman spectroscopy is a label-free imaging method based on inelastic scattering of monochromatic light, allowing direct evaluation of organelle structures and molecular biochemical processes inside living cells (Windom and Hahn 2013). With the well-developed knowledge that metabolism is linked to oocyte quality, Raman spectroscopy was proposed as a non-invasive method to investigate the correlation between metabolomic profile and embryo quality, as well as an indicator of developmental potential and pregnancy outcome (Seli et al. 2007; Scott et al. 2008; Perevedentseva et al. 2019). Raman spectroscopy was also employed to study lipid metabolism by quantifying lipid content of mouse oocytes (Bradley et al. 2016). However, a drawback of this approach is the use of lasers (Yakubovskaya et al. 2019), which potentially have downstream impacts on embryo development depending on the laser wavelength and dose of laser radiation. A single study by Perevedentseva et al. (2019) demonstrated that although the development rate from 2-cell to blastocyst-stage was not impacted in mouse embryos, the cell number of blastocysts was significantly reduced in the Raman-imaged group compared to control. This suggests that Raman spectroscopy may not be suitable for implementation in an IVF setting.
Hyperspectral imaging
A promising imaging modality with capacity to capture cellular autofluorescence in the oocyte and embryo is hyperspectral microscopy. This form of imaging employs multiple light-emitting diodes of varying excitation and emission wavelengths to record autofluorescent signatures. This broad-spectrum approach (excitation from 340 nm to 750 nm) allows for the detection of a range of endogenous fluorophores, not limited to NAD(P)H and FAD, thereby providing insight into the metabolism and content of a cell (Gosnell et al. 2016a). Hyperspectral imaging can detect subtle differences in metabolic signatures between disease and healthy states: pancreatic cancer cells (Gosnell et al. 2016a); olfactory cells (Gosnell et al. 2016b); and articular cartilage tissue (Mahbub et al. 2019). In the field of reproductive biology, hyperspectral microscopy demonstrated the capacity to discern between good and poor quality bovine embryos (Sutton-McDowall et al. 2017; Santos Monteiro et al. 2021) and oocytes denuded of cumulus cells from young and aged mice (Bertoldo et al. 2020). Additionally, mathematical algorithms applied to autofluorescence signals obtained from hyperspectral imaging were able to discriminate between different cell populations (Gosnell et al. 2016a; Sutton-McDowall et al. 2017; Habibalahi et al. 2019; Mahbub et al. 2021; Tan et al. 2022b). Our recent work successfully demonstrated the ability of hyperspectral imaging to separately measure metabolism in the oocyte and cumulus cell compartments of the intact COC, when compared with conventional laser scanning confocal microscopy (Fig. 3; Tan et al. 2022a). With the ability to capture metabolic heterogeneity between cells within an embryo and between compartments of a COC (cumulus cells vs the oocyte), hyperspectral microscopy may be a potential non-invasive approach to detect metabolic changes associated with oocyte and embryo quality.
|
|
Implications of non-invasive oocyte assessment for livestock breeding
Apart from human IVF, non-invasive assessment of oocyte quality could be implemented in the livestock industry. In vitro produced (IVP) embryos are widely used in the livestock industry to improve genetic gain, especially for cattle (Pontes et al. 2009). However, the efficiency of IVP is still far from optimal, with the pregnancy rate remaining below 50% (Ealy et al. 2019). To date, the industry continues to rely on the subjective analysis of oocyte and embryo quality by an embryologist based on morphological classification (Wrenzycki 2021). A plethora of basic research on new technologies to assess oocyte and embryo health exist in the clinical space. However, there are relatively few occurrences of this in livestock species. This is perhaps due to methods such as time-lapse imaging and metabolomic profiling being logistically challenging and expensive (McLennan et al. 2020). Therefore, there exists an opportunity to provide the research field with imaging modalities that are cost-effective and easy to use. This may lead to an amplification of research in this area which if successful, will result in the development of an accurate diagnostic tool for oocyte developmental competence, delivering better outcomes in breeding efficiencies and positively impacting the livestock industry.
Future directions
Existing selection methods for oocyte developmental competence are not predictive for pregnancy success. Therefore, there is a need to develop a viability assessment tool that is accurate, non-invasive, and importantly, predicts pregnancy success and live birth. Considering the importance of metabolism in determining oocyte developmental potential and the limitations of current methods in failing to detect metabolic heterogeneity between cells, label-free optical imaging of endogenous fluorophores may be a promising tool for measuring oocyte developmental competence. These advanced technologies combine non-invasive, spatially resolved, real time metabolic measurements. These metabolic measurements and their correlation with subsequent developmental outcomes will provide us with further understanding on how the dynamic metabolic changes in the COC impacts developmental competence. Importantly, metabolic imaging of COCs, following either oocyte pick up (OPU) or in vitro maturation (IVM), may facilitate a ranking of oocytes based on developmental competence leading to improved live birth rates. Although not the focus of this review, such imaging of resultant embryos may provide additional information to that acquired at the oocyte stage. This burgeoning field of research will undoubtedly benefit both clinical and agricultural IVF.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
K.R.D. is supported by a Mid-Career Fellowship from the Hospital Research Foundation (C-MCF-58-2019). K.R.D and T.C.Y.T. acknowledge support from the National Health and Medical Research Council (APP2003786).
Author contributions
T.C.Y.T. wrote the first draft of the manuscript. K.R.D. conceived the review content, provided critical feedback and edited the manuscript. All authors edited and approved the manuscript.
References
Adashi, EY, and Gutman, R (2018). Delayed childbearing as a growing, previously unrecognized contributor to the national plural birth excess. Obstetrics & Gynecology 132, 999–1006.| Delayed childbearing as a growing, previously unrecognized contributor to the national plural birth excess.Crossref | GoogleScholarGoogle Scholar |
Ambekar, AS, Nirujogi, RS, Srikanth, SM, Chavan, S, Kelkar, DS, Hinduja, I, Zaveri, K, Prasad, TS, Harsha, HC, Pandey, A, and Mukherjee, S (2013). Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. Journal of Proteomics 87, 68–77.
| Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis.Crossref | GoogleScholarGoogle Scholar |
Anderson, E, and Albertini, DF (1976). Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. Journal of Cell Biology 71, 680–686.
| Gap junctions between the oocyte and companion follicle cells in the mammalian ovary.Crossref | GoogleScholarGoogle Scholar |
Andersson, H, Baechi, T, Hoechl, M, and Richter, C (1998). Autofluorescence of living cells. Journal of Microscopy 191, 1–7.
| Autofluorescence of living cells.Crossref | GoogleScholarGoogle Scholar |
Bentov, Y, Yavorska, T, Esfandiari, N, Jurisicova, A, and Casper, RF (2011). The contribution of mitochondrial function to reproductive aging. Journal of Assisted Reproduction and Genetics 28, 773–783.
| The contribution of mitochondrial function to reproductive aging.Crossref | GoogleScholarGoogle Scholar |
Bertoldo, MJ, Listijono, DR, Ho, W-HJ, Riepsamen, AH, Goss, DM, Richani, D, Jin, XL, Mahbub, S, Campbell, JM, Habibalahi, A, Loh, W-GN, Youngson, NA, Maniam, J, Wong, ASA, Selesniemi, K, Bustamante, S, Li, C, Zhao, Y, Marinova, MB, Kim, L-J, Lau, L, Wu, RM, Mikolaizak, AS, Araki, T, Le Couteur, DG, Turner, N, Morris, MJ, Walters, KA, Goldys, E, O’Neill, C, Gilchrist, RB, Sinclair, DA, Homer, HA, and Wu, LE (2020). NAD(+) repletion rescues female fertility during reproductive aging. Cell Reports 30, 1670–1681.e7.
| NAD(+) repletion rescues female fertility during reproductive aging.Crossref | GoogleScholarGoogle Scholar |
Biggers, JD, Whittingham, DG, and Donahue, RP (1967). The pattern of energy metabolism in the mouse oöcyte and zygote. Proceedings of the National Academy of Sciences 58, 560–567.
| The pattern of energy metabolism in the mouse oöcyte and zygote.Crossref | GoogleScholarGoogle Scholar |
Blacker, TS, and Duchen, MR (2016). Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radical Biology and Medicine 100, 53–65.
| Investigating mitochondrial redox state using NADH and NADPH autofluorescence.Crossref | GoogleScholarGoogle Scholar |
Blacker, TS, Mann, ZF, Gale, JE, Ziegler, M, Bain, AJ, Szabadkai, G, and Duchen, MR (2014). Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nature Communications 5, 3936.
| Separating NADH and NADPH fluorescence in live cells and tissues using FLIM.Crossref | GoogleScholarGoogle Scholar |
Bradley, J, Pope, I, Masia, F, Sanusi, R, Langbein, W, Swann, K, and Borri, P (2016). Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 143, 2238–2247.
| Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy.Crossref | GoogleScholarGoogle Scholar |
Brison, DR, Sturmey, RG, and Leese, HJ (2014). Metabolic heterogeneity during preimplantation development: the missing link? Human Reproduction Update 20, 632–640.
| Metabolic heterogeneity during preimplantation development: the missing link?Crossref | GoogleScholarGoogle Scholar |
Campugan, CA, Lim, M, Chow, DJX, Tan, TCY, Li, T, Saini, AA, Orth, A, Reineck, P, Schartner, EP, Thompson, JG, Dholakia, K, and Dunning, KR (2022). The effect of discrete wavelengths of visible light on the developing murine embryo. Journal of Assisted Reproduction and Genetics 39, 1825–1837.
| The effect of discrete wavelengths of visible light on the developing murine embryo.Crossref | GoogleScholarGoogle Scholar |
Chambers, GM, Paul, RC, Harris, K, Fitzgerald, O, Boothroyd, CV, Rombauts, L, Chapman, MG, and Jorm, L (2017). Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success. Medical Journal of Australia 207, 114–118.
| Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success.Crossref | GoogleScholarGoogle Scholar |
Chance, B (1952). Spectra and reaction kinetics of respiratory pigments of homogenized and intact cells. Nature 169, 215–221.
| Spectra and reaction kinetics of respiratory pigments of homogenized and intact cells.Crossref | GoogleScholarGoogle Scholar |
Chance, B, and Thorell, B (1959). Localization and kinetics of reduced pyridine nucleotide in living cells by microfluorometry. The Journal of Biological Chemistry 234, 3044–3050.
| Localization and kinetics of reduced pyridine nucleotide in living cells by microfluorometry.Crossref | GoogleScholarGoogle Scholar |
Chance, B, Cohen, P, Jobsis, F, and Schoener, B (1962a). Intracellular oxidation-reduction states in vivo. Science 137, 499–508.
| Intracellular oxidation-reduction states in vivo.Crossref | GoogleScholarGoogle Scholar |
Chance, B, Cohen, P, Jobsis, F, and Schoener, B (1962b). Localized fluorometry of oxidation-reduction states of intracellular pyridine nucleotide in brain and kidney cortex of the anesthetized rat. Science 136, 325.
| Localized fluorometry of oxidation-reduction states of intracellular pyridine nucleotide in brain and kidney cortex of the anesthetized rat.Crossref | GoogleScholarGoogle Scholar |
Chance, B, Estabrook, RW, and Ghosh, A (1964). Damped sinusoidal oscillations of cytoplasmic reduced pyridine nucleotide in yeast cells. Proceedings of the National Academy of Sciences 51, 1244–1251.
| Damped sinusoidal oscillations of cytoplasmic reduced pyridine nucleotide in yeast cells.Crossref | GoogleScholarGoogle Scholar |
Chance, B, Schoener, B, Oshino, R, Itshak, F, and Nakase, Y (1979). Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. The Journal of Biological Chemistry 254, 4764–4771.
| Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals.Crossref | GoogleScholarGoogle Scholar |
Cheng, E-H, Chen, S-U, Lee, T-H, Pai, Y-P, Huang, L-S, Huang, C-C, and Lee, M-S (2013). Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Human Reproduction 28, 929–936.
| Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality.Crossref | GoogleScholarGoogle Scholar |
Cimadomo, D, Fabozzi, G, Vaiarelli, A, Ubaldi, N, Ubaldi, FM, and Rienzi, L (2018). Impact of maternal age on oocyte and embryo competence. Frontiers in Endocrinology 9, 327.
| Impact of maternal age on oocyte and embryo competence.Crossref | GoogleScholarGoogle Scholar |
Cole, LW (2016). The evolution of per-cell organelle number. Frontiers in Cell and Developmental Biology 4, 85.
| The evolution of per-cell organelle number.Crossref | GoogleScholarGoogle Scholar |
Croce, AC, and Bottiroli, G (2014). Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. European Journal of Histochemistry 58, 2461.
| Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis.Crossref | GoogleScholarGoogle Scholar |
Denisenko, V, Kuz’mina, TI, and Shokin, OV (2005). Dependence of Ca2+ release from intracellular stores on NADH and FAD levels in fertilized and unfertilized bovine oocytes. Tsitologiia 47, 704–708.
Drozdowicz-Tomsia, K, Anwer, AG, Cahill, MA, Madlum, KN, Maki, AM, Baker, MS, and Goldys, EM (2014). Multiphoton fluorescence lifetime imaging microscopy reveals free-to-bound NADH ratio changes associated with metabolic inhibition. Journal of Biomedical Optics 19, 086016.
| Multiphoton fluorescence lifetime imaging microscopy reveals free-to-bound NADH ratio changes associated with metabolic inhibition.Crossref | GoogleScholarGoogle Scholar |
Dumesic, DA, Meldrum, DR, Katz-Jaffe, MG, Krisher, RL, and Schoolcraft, WB (2015). Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertility and Sterility 103, 303–316.
| Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health.Crossref | GoogleScholarGoogle Scholar |
Dumollard, R, Marangos, P, Fitzharris, G, Swann, K, Duchen, M, and Carroll, J (2004). Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131, 3057–3067.
| Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production.Crossref | GoogleScholarGoogle Scholar |
Dumollard, R, Ward, Z, Carroll, J, and Duchen, MR (2007). Regulation of redox metabolism in the mouse oocyte and embryo. Development 134, 455–465.
| Regulation of redox metabolism in the mouse oocyte and embryo.Crossref | GoogleScholarGoogle Scholar |
Dumollard, R, Carroll, J, Duchen, MR, Campbell, K, and Swann, K (2009). Mitochondrial function and redox state in mammalian embryos. Seminars in Cell & Developmental Biology 20, 346–353.
| Mitochondrial function and redox state in mammalian embryos.Crossref | GoogleScholarGoogle Scholar |
Dunning, KR, Cashman, K, Russell, DL, Thompson, JG, Norman, RJ, and Robker, RL (2010). Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biology of Reproduction 83, 909–918.
| Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development.Crossref | GoogleScholarGoogle Scholar |
Dunning, KR, Akison, LK, Russell, DL, Norman, RJ, and Robker, RL (2011). Increased beta-oxidation and improved oocyte developmental competence in response to L-Carnitine during ovarian in vitro follicle development in mice. Biology of Reproduction 85, 548–555.
| Increased beta-oxidation and improved oocyte developmental competence in response to L-Carnitine during ovarian in vitro follicle development in mice.Crossref | GoogleScholarGoogle Scholar |
Dunning, KR, Anastasi, MR, Zhang, VJ, Russell, DL, and Robker, RL (2014). Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 9, e87327.
| Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists.Crossref | GoogleScholarGoogle Scholar |
Ealy, AD, Wooldridge, LK, and McCoski, SR (2019). BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. Journal of Animal Science 97, 2555–2568.
| BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle.Crossref | GoogleScholarGoogle Scholar |
Ebner, T, Moser, M, Sommergruber, M, and Tews, G (2003). Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Human Reproduction Update 9, 251–262.
| Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review.Crossref | GoogleScholarGoogle Scholar |
Eppig, JJ (2001). Oocyte control of ovarian follicular development and function in mammals. Reproduction 122, 829–838.
| Oocyte control of ovarian follicular development and function in mammals.Crossref | GoogleScholarGoogle Scholar |
Erokhina, MV, Lepekha, LN, Voronezhskaya, EE, Nezlin, LP, Avdienko, VG, and Ergeshov, AE (2019). Application of laser scanning confocal microscopy for the visualization of M. tuberculosis in lung tissue samples with weak Ziehl-Neelsen staining. Journal of Clinical Medicine 8, 1185.
| Application of laser scanning confocal microscopy for the visualization of M. tuberculosis in lung tissue samples with weak Ziehl-Neelsen staining.Crossref | GoogleScholarGoogle Scholar |
Feng, Y, Qi, J, Xue, X, Li, X, Liao, Y, Sun, Y, Tao, Y, Yin, H, Liu, W, Li, S, and Huang, R (2022). Follicular free fatty acid metabolic signatures and their effects on oocyte competence in non-obese PCOS patients. Reproduction 164, 1–8.
| Follicular free fatty acid metabolic signatures and their effects on oocyte competence in non-obese PCOS patients.Crossref | GoogleScholarGoogle Scholar |
Ferguson, EM, and Leese, HJ (2006). A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Molecular Reproduction and Development 73, 1195–1201.
| A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development.Crossref | GoogleScholarGoogle Scholar |
Fragouli, E, Wells, D, Iager, AE, Kayisli, UA, and Patrizio, P (2012). Alteration of gene expression in human cumulus cells as a potential indicator of oocyte aneuploidy. Human Reproduction 27, 2559–2568.
| Alteration of gene expression in human cumulus cells as a potential indicator of oocyte aneuploidy.Crossref | GoogleScholarGoogle Scholar |
Fragouli, E, Spath, K, Alfarawati, S, Kaper, F, Craig, A, Michel, C-E, Kokocinski, F, Cohen, J, Munne, S, and Wells, D (2015). Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genetics 11, e1005241.
| Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential.Crossref | GoogleScholarGoogle Scholar |
Ge, H, Tollner, TL, Hu, Z, Dai, M, Li, X, Guan, H, Shan, D, Zhang, X, Lv, J, Huang, C, and Dong, Q (2012). The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Molecular Reproduction and Development 79, 392–401.
| The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence.Crossref | GoogleScholarGoogle Scholar |
Gebhardt, KM, Feil, DK, Dunning, KR, Lane, M, and Russell, DL (2011). Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertility and Sterility 96, 47–52.e2.
| Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer.Crossref | GoogleScholarGoogle Scholar |
Georgakoudi, I, and Quinn, KP (2012). Optical imaging using endogenous contrast to assess metabolic state. Annual Review of Biomedical Engineering 14, 351–367.
| Optical imaging using endogenous contrast to assess metabolic state.Crossref | GoogleScholarGoogle Scholar |
Gilchrist, RB, Lane, M, and Thompson, JG (2008). Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Human Reproduction Update 14, 159–177.
| Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality.Crossref | GoogleScholarGoogle Scholar |
Gosnell, ME, Anwer, AG, Mahbub, SB, Menon Perinchery, S, Inglis, DW, Adhikary, PP, Jazayeri, JA, Cahill, MA, Saad, S, Pollock, CA, Sutton-McDowall, ML, Thompson, JG, and Goldys, EM (2016a). Quantitative non-invasive cell characterisation and discrimination based on multispectral autofluorescence features. Scientific Reports 6, 23453.
| Quantitative non-invasive cell characterisation and discrimination based on multispectral autofluorescence features.Crossref | GoogleScholarGoogle Scholar |
Gosnell, ME, Anwer, AG, Cassano, JC, Sue, CM, and Goldys, EM (2016b). Functional hyperspectral imaging captures subtle details of cell metabolism in olfactory neurosphere cells, disease-specific models of neurodegenerative disorders. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863, 56–63.
| Functional hyperspectral imaging captures subtle details of cell metabolism in olfactory neurosphere cells, disease-specific models of neurodegenerative disorders.Crossref | GoogleScholarGoogle Scholar |
Gutnisky, C, Dalvit, GC, Thompson, JG, and Cetica, PD (2014). Pentose phosphate pathway activity: effect on in vitro maturation and oxidative status of bovine oocytes. Reproduction, Fertility and Development 26, 931–942.
| Pentose phosphate pathway activity: effect on in vitro maturation and oxidative status of bovine oocytes.Crossref | GoogleScholarGoogle Scholar |
Habibalahi, A, Bala, C, Allende, A, Anwer, AG, and Goldys, EM (2019). Novel automated non invasive detection of ocular surface squamous neoplasia using multispectral autofluorescence imaging. The Ocular Surface 17, 540–550.
| Novel automated non invasive detection of ocular surface squamous neoplasia using multispectral autofluorescence imaging.Crossref | GoogleScholarGoogle Scholar |
Hemmings, KE, Leese, HJ, and Picton, HM (2012). Amino acid turnover by bovine oocytes provides an index of oocyte developmental competence in vitro. Biology of Reproduction 165, 1–12.
| Amino acid turnover by bovine oocytes provides an index of oocyte developmental competence in vitro.Crossref | GoogleScholarGoogle Scholar |
Heppert, JK, Dickinson, DJ, Pani, AM, Higgins, CD, Steward, A, Ahringer, J, Kuhn, JR, and Goldstein, B (2016). Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system. Molecular Biology of the Cell 27, 3385–3394.
| Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system.Crossref | GoogleScholarGoogle Scholar |
Hirata, S, Hoshi, K, Shoda, T, and Mabuchi, T (2002). Spermatozoon and mitochondrial DNA. Reproductive Medicine and Biology 1, 41–47.
| Spermatozoon and mitochondrial DNA.Crossref | GoogleScholarGoogle Scholar |
Ji, S, Chance, B, Nishiki, K, Smith, T, and Rich, T (1979). Micro-light guides: a new method for measuring tissue fluorescence and reflectance. American Journal of Physiology - Cell Physiology 236, C144–C156.
| Micro-light guides: a new method for measuring tissue fluorescence and reflectance.Crossref | GoogleScholarGoogle Scholar |
Johnson ID (2010) ‘Molecular probes handbook: a guide to fluorescent probes and labeling technologies.’ (Life Technologies Corporation)
Johnson, JA, Tough, S, and Sogc Genetics, C (2012). Delayed child-bearing. Journal of Obstetrics and Gynaecology Canada 34, 80–93.
| Delayed child-bearing.Crossref | GoogleScholarGoogle Scholar |
Jungheim, ES, Macones, GA, Odem, RR, Patterson, BW, Lanzendorf, SE, Ratts, VS, and Moley, KH (2011). Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertility and Sterility 95, 1970–1974.
| Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization.Crossref | GoogleScholarGoogle Scholar |
Kolenc, OI, and Quinn, KP (2019). Evaluating cell metabolism through autofluorescence imaging of NAD(P)H and FAD. Antioxidants & Redox Signaling 30, 875–889.
| Evaluating cell metabolism through autofluorescence imaging of NAD(P)H and FAD.Crossref | GoogleScholarGoogle Scholar |
Krisher, RL (2018). Maternal age affects oocyte developmental potential at both ends of the age spectrum. Reproduction, Fertility and Development 31, 1–9.
| Maternal age affects oocyte developmental potential at both ends of the age spectrum.Crossref | GoogleScholarGoogle Scholar |
Kunz, WS, and Kunz, W (1985). Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria. Biochimica et Biophysica Acta (BBA) - General Subjects 841, 237–246.
| Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria.Crossref | GoogleScholarGoogle Scholar |
Lasienë, K, Vitkus, A, Valanèiûtë, A, and Lasys, V (2009). Morphological criteria of oocyte quality. Medicina 45, 509.
| Morphological criteria of oocyte quality.Crossref | GoogleScholarGoogle Scholar |
Lee, JH, Park, JK, Yoon, SY, Park, EA, Jun, JH, Lim, HJ, Kim, J, and Song, H (2021). Advanced maternal age deteriorates the developmental competence of vitrified oocytes in mice. Cells 10, 1563.
| Advanced maternal age deteriorates the developmental competence of vitrified oocytes in mice.Crossref | GoogleScholarGoogle Scholar |
Liberti, MV, and Locasale, JW (2016). The Warburg effect: how does it benefit cancer cells? Trends in Biochemical Sciences 41, 211–218.
| The Warburg effect: how does it benefit cancer cells?Crossref | GoogleScholarGoogle Scholar |
Mahbub, SB, Guller, A, Campbell, JM, Anwer, AG, Gosnell, ME, Vesey, G, and Goldys, EM (2019). Non-invasive monitoring of functional state of articular cartilage tissue with label-free unsupervised hyperspectral imaging. Scientific Reports 9, 4398.
| Non-invasive monitoring of functional state of articular cartilage tissue with label-free unsupervised hyperspectral imaging.Crossref | GoogleScholarGoogle Scholar |
Mahbub, SB, Nguyen, LT, Habibalahi, A, Campbell, JM, Anwer, AG, Qadri, UM, Gill, A, Chou, A, Wong, MG, Gosnell, ME, Pollock, CA, Saad, S, and Goldys, EM (2021). Non-invasive assessment of exfoliated kidney cells extracted from urine using multispectral autofluorescence features. Scientific Reports 11, 10655.
| Non-invasive assessment of exfoliated kidney cells extracted from urine using multispectral autofluorescence features.Crossref | GoogleScholarGoogle Scholar |
Masters, BR, Kriete, A, and Kukulies, J (1993). Ultraviolet confocal fluorescence microscopy of the in vitro cornea: redox metabolic imaging. Applied Optics 32, 592–596.
| Ultraviolet confocal fluorescence microscopy of the in vitro cornea: redox metabolic imaging.Crossref | GoogleScholarGoogle Scholar |
May-Panloup, P, Chrétien, MF, Jacques, C, Vasseur, C, Malthièry, Y, and Reynier, P (2005). Low oocyte mitochondrial DNA content in ovarian insufficiency. Human Reproduction 20, 593–597.
| Low oocyte mitochondrial DNA content in ovarian insufficiency.Crossref | GoogleScholarGoogle Scholar |
May-Panloup, P, Boguenet, M, El Hachem, H, Bouet, P-E, and Reynier, P (2021). Embryo and its mitochondria. Antioxidants 10, 139.
| Embryo and its mitochondria.Crossref | GoogleScholarGoogle Scholar |
McKenzie, LJ, Pangas, SA, Carson, SA, Kovanci, E, Cisneros, P, Buster, JE, Amato, P, and Matzuk, MM (2004). Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Human Reproduction 19, 2869–2874.
| Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF.Crossref | GoogleScholarGoogle Scholar |
McLennan, HJ, Saini, A, Dunning, KR, and Thompson, JG (2020). Oocyte and embryo evaluation by AI and multi-spectral auto-fluorescence imaging: livestock embryology needs to catch-up to clinical practice. Theriogenology 150, 255–262.
| Oocyte and embryo evaluation by AI and multi-spectral auto-fluorescence imaging: livestock embryology needs to catch-up to clinical practice.Crossref | GoogleScholarGoogle Scholar |
Mendoza, C, Ruiz-Requena, E, Ortega, E, Cremades, N, Martinez, F, Bernabeu, R, Greco, E, and Tesarik, J (2002). Follicular fluid markers of oocyte developmental potential. Human Reproduction 17, 1017–1022.
| Follicular fluid markers of oocyte developmental potential.Crossref | GoogleScholarGoogle Scholar |
Nohales-Córcoles, M, Sevillano-Almerich, G, Di Emidio, G, Tatone, C, Cobo, AC, Dumollard, R, and De los Santos Molina, MJ (2016). Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Human Reproduction 31, 1850–1858.
| Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte.Crossref | GoogleScholarGoogle Scholar |
Ntostis, P, Iles, D, Kokkali, G, Vaxevanoglou, T, Kanavakis, E, Pantou, A, Huntriss, J, Pantos, K, and Picton, HM (2022). The impact of maternal age on gene expression during the GV to MII transition in euploid human oocytes. Human Reproduction 37, 80–92.
| The impact of maternal age on gene expression during the GV to MII transition in euploid human oocytes.Crossref | GoogleScholarGoogle Scholar |
O’Brien, JK, Dwarte, D, Ryan, JP, Maxwell, WM, and Evans, G (1996). Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reproduction, Fertility and Development 8, 1029–1037.
| Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep.Crossref | GoogleScholarGoogle Scholar |
O’Gorman, A, Wallace, M, Cottell, E, Gibney, MJ, McAuliffe, FM, Wingfield, M, and Brennan, L (2013). Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 146, 389–395.
| Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar |
Osellame, LD, Blacker, TS, and Duchen, MR (2012). Cellular and molecular mechanisms of mitochondrial function. Best Practice & Research Clinical Endocrinology & Metabolism 26, 711–723.
| Cellular and molecular mechanisms of mitochondrial function.Crossref | GoogleScholarGoogle Scholar |
Ozturk, S, Sozen, B, and Demir, N (2014). Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Molecular Human Reproduction 20, 15–30.
| Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species.Crossref | GoogleScholarGoogle Scholar |
Perevedentseva, E, Krivokharchenko, A, Karmenyan, AV, Chang, H-H, and Cheng, C-L (2019). Raman spectroscopy on live mouse early embryo while it continues to develop into blastocyst in vitro. Scientific Reports 9, 6636.
| Raman spectroscopy on live mouse early embryo while it continues to develop into blastocyst in vitro.Crossref | GoogleScholarGoogle Scholar |
Pontes, JHF, Nonato-Junior, I, Sanches, BV, Ereno-Junior, JC, Uvo, S, Barreiros, TRR, Oliveira, JA, Hasler, JF, and Seneda, MM (2009). Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 71, 690–697.
| Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows.Crossref | GoogleScholarGoogle Scholar |
Preis, KA, Seidel, G, and Gardner, DK (2005). Metabolic markers of developmental competence for in vitro-matured mouse oocytes. Reproduction 130, 475–483.
| Metabolic markers of developmental competence for in vitro-matured mouse oocytes.Crossref | GoogleScholarGoogle Scholar |
Quinn, KP, Sridharan, GV, Hayden, RS, Kaplan, DL, Lee, K, and Georgakoudi, I (2013). Quantitative metabolic imaging using endogenous fluorescence to detect stem cell differentiation. Scientific Reports 3, 3432.
| Quantitative metabolic imaging using endogenous fluorescence to detect stem cell differentiation.Crossref | GoogleScholarGoogle Scholar |
Quistorff B, Chance B (1986) Redox scanning in the study of metabolic zonation of liver. In ‘Regulation of hepatic metabolism: intra- and intercellular compartmentation’. (Eds RG Thurman, FC Kauffman, K Jungermann) pp. 185–207. (Springer US: Boston, MA)
Quistorff, B, Haselgrove, JC, and Chance, B (1985). High spatial resolution readout of 3-D metabolic organ structure: an automated, low-temperature redox ratio-scanning instrument. Analytical Biochemistry 148, 389–400.
| High spatial resolution readout of 3-D metabolic organ structure: an automated, low-temperature redox ratio-scanning instrument.Crossref | GoogleScholarGoogle Scholar |
Richani, D, Dunning, KR, Thompson, JG, and Gilchrist, RB (2021). Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Human Reproduction Update 27, 27–47.
| Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar |
Roshchina, VV (2012). Vital autofluorescence: application to the study of plant living cells. International Journal of Spectroscopy 2012, 124672.
| Vital autofluorescence: application to the study of plant living cells.Crossref | GoogleScholarGoogle Scholar |
Sanchez, T, Wang, T, Pedro, MV, Zhang, M, Esencan, E, Sakkas, D, Needleman, D, and Seli, E (2018). Metabolic imaging with the use of fluorescence lifetime imaging microscopy (FLIM) accurately detects mitochondrial dysfunction in mouse oocytes. Fertility and Sterility 110, 1387–1397.
| Metabolic imaging with the use of fluorescence lifetime imaging microscopy (FLIM) accurately detects mitochondrial dysfunction in mouse oocytes.Crossref | GoogleScholarGoogle Scholar |
Sanchez, T, Venturas, M, Aghvami, SA, Yang, X, Fraden, S, Sakkas, D, and Needleman, DJ (2019). Combined noninvasive metabolic and spindle imaging as potential tools for embryo and oocyte assessment. Human Reproduction 34, 2349–2361.
| Combined noninvasive metabolic and spindle imaging as potential tools for embryo and oocyte assessment.Crossref | GoogleScholarGoogle Scholar |
Santos, TA, El Shourbagy, S, and St. John, JC (2006). Mitochondrial content reflects oocyte variability and fertilization outcome. Fertility and Sterility 85, 584–591.
| Mitochondrial content reflects oocyte variability and fertilization outcome.Crossref | GoogleScholarGoogle Scholar |
Santos Monteiro, CA, Chow, DJX, Leal, GR, Tan, TCY, Reis Ferreira, AM, Thompson, JG, and Dunning, KR (2021). Optical imaging of cleavage stage bovine embryos using hyperspectral and confocal approaches reveals metabolic differences between on-time and fast-developing embryos. Theriogenology 159, 60–68.
| Optical imaging of cleavage stage bovine embryos using hyperspectral and confocal approaches reveals metabolic differences between on-time and fast-developing embryos.Crossref | GoogleScholarGoogle Scholar |
Scholz, R, Thurman, RG, Williamson, JR, Chance, B, and Bücher, T (1969). Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. The Journal of Biological Chemistry 244, 2317–2324.
| Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins.Crossref | GoogleScholarGoogle Scholar |
Scott, R, Seli, E, Miller, K, Sakkas, D, Scott, K, and Burns, DH (2008). Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertility and Sterility 90, 77–83.
| Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study.Crossref | GoogleScholarGoogle Scholar |
Seli, E, Sakkas, D, Scott, R, Kwok, SC, Rosendahl, SM, and Burns, DH (2007). Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertility and Sterility 88, 1350–1357.
| Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization.Crossref | GoogleScholarGoogle Scholar |
Shen, X, Liu, X, Zhu, P, Zhang, Y, Wang, J, Wang, Y, Wang, W, Liu, J, Li, N, and Liu, F (2017). Proteomic analysis of human follicular fluid associated with successful in vitro fertilization. Reproductive Biology and Endocrinology 15, 58.
| Proteomic analysis of human follicular fluid associated with successful in vitro fertilization.Crossref | GoogleScholarGoogle Scholar |
Skala M, Ramanujam N (2010) Multiphoton redox ratio imaging for metabolic monitoring in vivo. In ‘Advanced protocols in oxidative stress II. Vol. 594’. (Ed. D Armstrong) pp. 155–162. Methods in molecular biology. (Humana Press: Totowa, NJ). 10.1007/978-1-60761-411-1_11
Staikopoulos V, Gosnell ME, Anwer AG, Mustafa S, Hutchinson MR, Goldys EM (2016) Hyperspectral imaging of endogenous fluorescent metabolic molecules to identify pain states in central nervous system tissue. In ‘SPIE BioPhotonics Australasia’. p. 8. (SPIE)
Sturmey, RG, O’Toole, PJ, and Leese, HJ (2006). Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte. Reproduction 132, 829–837.
| Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte.Crossref | GoogleScholarGoogle Scholar |
Sugiura, K, Su, Y-Q, Diaz, FJ, Pangas, SA, Sharma, S, Wigglesworth, K, O’Brien, MJ, Matzuk, MM, Shimasaki, S, and Eppig, JJ (2007). Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134, 2593–2603.
| Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells.Crossref | GoogleScholarGoogle Scholar |
Sutton-McDowall, ML, Gilchrist, RB, and Thompson, JG (2010). The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139, 685–695.
| The pivotal role of glucose metabolism in determining oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar |
Sutton-McDowall, ML, Mottershead, DG, Gardner, DK, Gilchrist, RB, and Thompson, JG (2012). Metabolic differences in bovine cumulus-oocyte complexes matured in vitro in the presence or absence of follicle-stimulating hormone and bone morphogenetic protein 15. Biology of Reproduction 87, 1–8.
| Metabolic differences in bovine cumulus-oocyte complexes matured in vitro in the presence or absence of follicle-stimulating hormone and bone morphogenetic protein 15.Crossref | GoogleScholarGoogle Scholar |
Sutton-McDowall, ML, Purdey, M, Brown, HM, Abell, AD, Mottershead, DG, Cetica, PD, Dalvit, GC, Goldys, EM, Gilchrist, RB, Gardner, DK, and Thompson, JG (2015). Redox and anti-oxidant state within cattle oocytes following in vitro maturation with bone morphogenetic protein 15 and follicle stimulating hormone. Molecular Reproduction and Development 82, 281–294.
| Redox and anti-oxidant state within cattle oocytes following in vitro maturation with bone morphogenetic protein 15 and follicle stimulating hormone.Crossref | GoogleScholarGoogle Scholar |
Sutton-McDowall, ML, Wu, LLY, Purdey, M, Abell, AD, Goldys, EM, MacMillan, KL, Thompson, JG, and Robker, RL (2016). Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biology of Reproduction 94, 23.
| Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence.Crossref | GoogleScholarGoogle Scholar |
Sutton-McDowall, ML, Gosnell, M, Anwer, AG, White, M, Purdey, M, Abell, AD, Goldys, EM, and Thompson, JG (2017). Hyperspectral microscopy can detect metabolic heterogeneity within bovine post-compaction embryos incubated under two oxygen concentrations (7% versus 20%). Human Reproduction 32, 2016–2025.
| Hyperspectral microscopy can detect metabolic heterogeneity within bovine post-compaction embryos incubated under two oxygen concentrations (7% versus 20%).Crossref | GoogleScholarGoogle Scholar |
Swoger, J, Pampaloni, F, and Stelzer, EHK (2014). Light-sheet-based fluorescence microscopy for three-dimensional imaging of biological samples. Cold Spring Harbor Protocols 2014, 1–8.
| Light-sheet-based fluorescence microscopy for three-dimensional imaging of biological samples.Crossref | GoogleScholarGoogle Scholar |
Takenaka, M, Horiuchi, T, and Yanagimachi, R (2007). Effects of light on development of mammalian zygotes. Proceedings of the National Academy of Sciences 104, 14289–14293.
| Effects of light on development of mammalian zygotes.Crossref | GoogleScholarGoogle Scholar |
Tan, TCY, Brown, HM, Thompson, JG, Mustafa, S, and Dunning, KR (2022a). Optical imaging detects metabolic signatures associated with oocyte quality. Biology of Reproduction , ioac145.
| Optical imaging detects metabolic signatures associated with oocyte quality.Crossref | GoogleScholarGoogle Scholar |
Tan, TCY, Mahbub, SB, Campbell, JM, Habibalahi, A, Campugan, CA, Rose, RD, Chow, DJX, Mustafa, S, Goldys, EM, and Dunning, KR (2022b). Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo. Human Reproduction 37, 14–29.
| Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo.Crossref | GoogleScholarGoogle Scholar |
Valsangkar, D, and Downs, SM (2013). A requirement for fatty acid oxidation in the hormone-induced meiotic maturation of mouse oocytes. Biology of Reproduction 89, 43.
| A requirement for fatty acid oxidation in the hormone-induced meiotic maturation of mouse oocytes.Crossref | GoogleScholarGoogle Scholar |
van der Reest, J, Nardini Cecchino, G, Haigis, MC, and Kordowitzki, P (2021). Mitochondria: their relevance during oocyte ageing. Ageing Research Reviews 70, 101378.
| Mitochondria: their relevance during oocyte ageing.Crossref | GoogleScholarGoogle Scholar |
Wai, T, Ao, A, Zhang, X, Cyr, D, Dufort, D, and Shoubridge, EA (2010). The role of mitochondrial DNA copy number in mammalian fertility. Biology of Reproduction 83, 52–62.
| The role of mitochondrial DNA copy number in mammalian fertility.Crossref | GoogleScholarGoogle Scholar |
Wang, Q, and Sun, Q-Y (2007). Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reproduction, Fertility and Development 19, 1–12.
| Evaluation of oocyte quality: morphological, cellular and molecular predictors.Crossref | GoogleScholarGoogle Scholar |
Windom BC, Hahn DW (2013) Raman spectroscopy. In ‘Encyclopedia of tribology’. (Eds QJ Wang, Y-W Chung) pp. 2742–2747. (Springer US: Boston, MA)
Wrenzycki, C (2021). Parameters to identify good quality oocytes and embryos in cattle. Reproduction, Fertility and Development 34, 190–202.
| Parameters to identify good quality oocytes and embryos in cattle.Crossref | GoogleScholarGoogle Scholar |
Wu, Y-T, Tang, L, Cai, J, Lu, X-E, Xu, J, Zhu, X-M, Luo, Q, and Huang, H-F (2007). High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Human Reproduction 22, 1526–1531.
| High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development.Crossref | GoogleScholarGoogle Scholar |
Yakubovskaya, E, Zaliznyak, T, Martínez Martínez, J, and Taylor, GT (2019). Tear down the fluorescent curtain: a new fluorescence suppression method for raman microspectroscopic analyses. Scientific Reports 9, 15785.
| Tear down the fluorescent curtain: a new fluorescence suppression method for raman microspectroscopic analyses.Crossref | GoogleScholarGoogle Scholar |
Yeo, CX, Gilchrist, RB, and Lane, M (2009). Disruption of bidirectional oocyte-cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence. Biology of Reproduction 80, 1072–1080.
| Disruption of bidirectional oocyte-cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar |


