Reproduction, Fertility and Development
Volume 36 Numbers 1 & 2 2024
Special IssueProceedings of the Annual Conference of the International Embryo Technology Society, Denver, CO, USA, 9–12 January 2024
Full Papers and Abstracts for Poster Presentation
This paper deals with the 50-year history of embryo transfer technology. It covers the formation of the International Embryo Technology Society, a group of scientists and veterinarians who study or work in the field of embryos and the animals that produce them. These embryos can be frozen, moved from animal to animal, maintained outside the female, and modified by a number of procedures. Photograph by Jenson.
RD23144IETS management of the challenges associated with embryo pathogen interaction
Embryo transfer is a reproductive biotechnology that contributes to the genetic improvement of livestock for the benefit of farmers. In response to the risk of disseminating pathogens via embryo transfer, the International Embryo Technology Society commissioned its Health and Safety Advisory Committee to assess these risks and to define safe operating protocols. After 40 years of studies and reports, it ultimately led to the ‘development of the now universally accepted techniques for certification of embryo health’, subsequently validated by field experience through the safe transfer of several million embryos over the past four decades.
RD23162How I overcame problems in in vitro fertilisation of livestock animals
In vitro fertilisation (IVF) of livestock animals is socially important because it is useful for increasing livestock production, breeding improvement and gene conservation. However, there are problems that hinder the promotion of IVF, such as the necessity of co-culturing with other somatic cells for development of bovine IVF embryos. This paper presents examples of how other problems with IVF have previously been solved, in an attempt to promote joint research for the improvement of IVF. Image by Takashi Nagai.
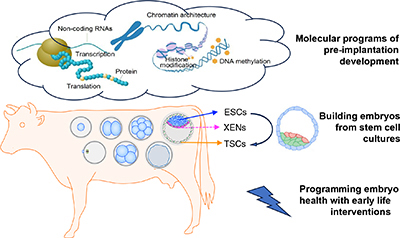
RD23146Molecular and cellular programs underlying the development of bovine pre-implantation embryos
This mini-review highlights the current status of genomic characterisations of bovine pre-implantation embryo development, programming bovine embryo health with early life interventions, as well as building bovine embryos from stem cell cultures.
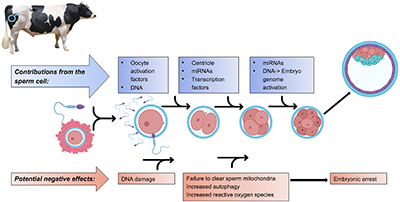
Sire characteristics can influence several events during fertilisation and preimplantation development including the binding of a spermatozoon to the oocyte, oocyte activation, paternal chromatin remodelling, centriole function, degradation of paternal mitochondria, and regulation of gene expression in the embryo through microRNAs. Defects in any of these processes can hinder embryonic development and lead to pregnancy loss. Some candidate genes have been associated with sire fertility, however, a comprehensive genetic predictor for sire fertility is yet to be fully elucidated. Image created with Biorender.
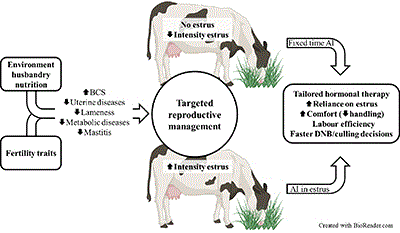
Technology and automation are ubiquitous to everyday life, including in dairy herds. Genomic testing of female dairy animals is now common in several herds, allowing for the selection for fertility traits associated with earlier resumption of oestrus cycles and more intense oestruses. Through the use of automated sensors, we are now able to identify cows that resume oestrus sooner after calving, which are more likely to conceive to spontaneous oestrus. Targeted reproductive management, based on characteristics of oestruses recorded early after calving, will result in tailored hormonal therapy, improved comfort, and reduced labour. Source: Ricardo C. Chebel. Created using Biorender.com.
The laboratory culture of mammalian embryos is of vital importance for the agricultural industry as well as clinically to help couples have a baby. Since the 1940s, embryo culture media has undergone rapid development to try and ensure that embryos are grown in a way that provides the highest possible pregnancy rates. Research continues in this area to further optimise culture media so that the embryo is provided with everything it needs to develop while minimising the need for embryo handling in the hope of providing a safer and more physiological culture environment and further improve outcomes. Photograph from Shutterstock.
RD23181Understanding conceptus–maternal interactions: what tools do we need to develop?
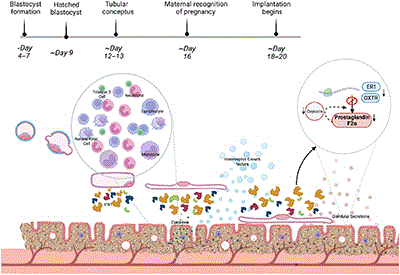
The majority of pregnancy loss in cattle (and other mammals) occurs in the peri-implantation period. The contribution of endometrial dysfunction to this loss remains elusive. Furthering our understanding the function/dysfunction of the endometrium is difficult given the lack of in vitro models that appropriately recapitulate the in vivo scenario or allow high-throughput testing of conceptus–maternal interactions. Here we review the current state of the art of in vitro and in silico model development, which will allow us to test the molecular interactions required for pregnancy success in cattle and other mammalian species. Created with BioRender.com.
RD23181 Abstract | RD23181 Full Text | RD23181PDF (1.5 MB) Open Access Article
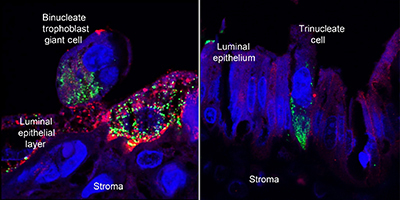
RD23119Understanding placentation in ruminants: a review focusing on cows and sheep
 , Fuller W. Bazer, Heewon Seo, Robert C. Burghardt, Guoyao Wu, Ky G. Pohler
, Fuller W. Bazer, Heewon Seo, Robert C. Burghardt, Guoyao Wu, Ky G. Pohler  and Joe W. Cain
and Joe W. Cain
The United States cattle industry is burdened by losses of pregnancies of up to 45% of cows bred. Many of these losses in ruminant species are the result of errors in the development of the placenta. This review describes and discusses what is currently known about some of the complex molecular and physiological events that underlie successful placentation in cows and sheep. Increased understanding of placentation is critical for developing advances to improve reproductive efficiency in animal agriculture enterprises that provide high-quality animal protein for human consumption. Photograph by Dr Robert C. Burghardt.
RD23163Production of light-coloured, low heat-absorbing Holstein Friesian cattle by precise embryo-mediated genome editing
 , Brigid Brophy, Sally-Ann Cole, Shane Leath, Björn Oback, Jens Boch, David N. Wells and Götz Laible
, Brigid Brophy, Sally-Ann Cole, Shane Leath, Björn Oback, Jens Boch, David N. Wells and Götz Laible
With warming climates, grazing cattle are becoming increasingly heat stressed. Embryo-mediated genome editing provides a strategy to rapidly adapt cattle. It was successfully applied to introduce a mutation in the PMEL gene, known to lighten coat colour, which reduced thermal energy absorption and might help to reduce heat stress impacts. Photograph by G. Laible.
RD23163 Abstract | RD23163 Full Text | RD23163PDF (1.9 MB) | RD23163Supplementary Material (1.7 MB) Open Access Article
RD23169From fertilised oocyte to cultivated meat – harnessing bovine embryonic stem cells in the cultivated meat industry
Global demand for sustainable, nutritious, and animal welfare–conscious protein sources encourages research dedicated to the production of cultivated meat. In this paper, we outline the approach that we took to produce bovine embryonic stem cell lines and present our strategy to expand and grow cells in quantities suitable for mass production. Our work demonstrates the potential benefits of harnessing bESCs in the production of cultivated meat. Photograph by Dr. Irena Vertkin (researcher, R&D department, Aleph Farms).
RD23169 Abstract | RD23169 Full Text | RD23169PDF (2.2 MB) Open Access Article
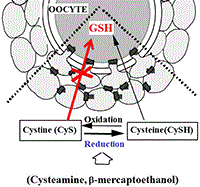
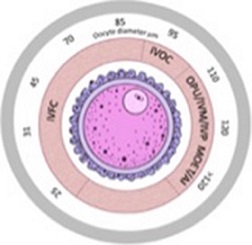
The development of the bovine oocyte from its differentiation in the ovary to ovulation is described from the perspective of each stage of oocyte and follicle development. The characteristic processes and pathways and the regulatory factors critical to each stage are detailed, along with their relevance to key aspects of individual assisted reproductive technologies. The historical timeline is also highlighted. Graphic designed by Trudee Fair, incorporating artwork by Iseult Lonergan.