A molecular phylogeny of Boronia (Rutaceae): placement of enigmatic taxa and a revised infrageneric classification
Marco F. Duretto A * , Margaret M. Heslewood
A * , Margaret M. Heslewood  A and Michael J. Bayly
A and Michael J. Bayly  B
B
A National Herbarium of New South Wales, Australian Institute of Botanical Science, Royal Botanic Gardens & Domain Trust, Locked Bag 6002, Mount Annan, NSW 2567, Australia.
B School of BioSciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
Australian Systematic Botany 36(2) 81-106 https://doi.org/10.1071/SB22019
Submitted: 7 July 2022 Accepted: 8 March 2023 Published: 5 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
A phylogeny of Boronia (Rutaceae) is presented on the basis of maximum parsimony and Bayesian analyses of plastid (psbA–trnH, trnL–trnF, rbcL) and nuclear (ITS, ETS) markers. Analyses of either plastid or nuclear sequences recovered the same major clades, although with conflicts in resolution among them. The existing classification of Boronia is largely confirmed; sections Boronella, Pedunculatae and Valvatae are supported, and the monotypic sections Alatae and Imbricatae are isolated. Boronia corynophylla Paul G.Wilson is removed from section Algidae to the new section Corynophyllae. Boronia coriacea Paul G.Wilson is removed from section Boronia and placed, with B. inornata Turcz., in the new section Inornatae. Boronia humifusa Paul G.Wilson, B. ovata Lindl. and B. scabra Lindl. are placed in the new section Ovatae. Boronia koniambiensis is retained in section Boronella but placed in a new monotypic series. Section Boronia resolves into two clades that are confined to either south-eastern or south-western Australia, the latter containing three strongly to robustly supported subclades. An identified problem within section Boronia is the lack of morphological apomorphies to assist with formal classification. Despite this, a classification of four series, justified on the basis of the results of the molecular analysis, is proposed. Relationships among the 10 sections of Boronia remain poorly resolved apart from the sister relationships of sections Imbricatae with Pedunculatae, and, Alatae with Corynophyllae.
Keywords: Australasia, Boronia, molecular phylogenetics, moth pollination, plant systematics, Rutaceae, taxonomy.
Introduction
Boronia Sm. (Rutaceae) is an Australian and New Caledonian genus of 134 species comprising mainly shrubs but also occasionally small trees or subshrubs (Wilson 1971, 1998; Duretto 1999, 2003; Duretto et al. 2013, 2020; Bayly et al. 2015). The genus is taxonomically isolated in subfamily Zanthoxyloideae A.Juss. ex Arn. and is sister to a large clade containing genera found mainly in the Australasian–Malesian region, including those found in rainforest, for example, Acronychia J.R.Forst. & G.Forst., Euodia J.R.Forst. & G.Forst. and Melicope J.R.Forst. & G.Forst., and sclerophyllous communities, e.g. Cyanothamnus Lindl., Neobyrnesia J.A.Armstr. and Zieria Sm. (see Groppo et al. 2008; Bayly et al. 2013, 2015; Duretto et al. 2020; Appelhans et al. 2021).
There have been several infrageneric classifications proposed for Boronia over the past 150 years with most of the differences between classifications centred on taxa now placed in section Boronia or incertae sedis (i.e. uncertain placement) at the sectional level (Bayly et al. 2015; Duretto et al. 2020). Bayly et al. (2015) presented a phylogenetic analysis using molecular data and demonstrated that Boronella Baill. was nested in Boronia and, apart from section Boronia, all sections recognised for the genus Boronia in recent treatments were monophyletic or, if monotypic, then divergent from other sections, separated on relatively long terminal branches. They also showed that both section Boronia and series Boronia were polyphyletic. Bayly et al. (2015) circumscribed section Boronia in a stricter sense with no series, reduced Boronella to a section of Boronia, raised series Pedunculatae Benth. (previously placed in section Boronia) to sectional level and placed as incertae sedis (at the sectional level) four species, B. humifusa Paul G.Wilson, B. inornata Turcz., B. ovata Lindl. and B. scabra Lindl., that had been placed in series Boronia (Wilson 1998; Duretto 2003; Duretto et al. 2013). Bayly et al. (2015) also placed Boronia series Ovatae Paul G.Wilson as incertae sedis. This last series was formally described by Wilson (1971; under section Imbricatae Engl.) to accommodate B. ovata and B. scabra, although later he placed it in synonymy under series Boronia (Wilson 1998). Wilson (1998) also formally described B. humifusa and noted that the species was difficult to place although being similar to B. ovata and B. scabra in inflorescence characters.
Duretto et al. (2020) tested the monophyly of Boronia and demonstrated that Boronia (sensu Wilson 1971, 1998; Duretto 2003; Kubitzki et al. 2011; Duretto et al. 2013; Bayly et al. 2015) was polyphyletic and that section Cyanothamnus (Lindl.) F.Muell. was misclassified in Boronia and was more closely related to a large clade of genera found in rainforest, including Melicope and Acronychia. They reinstated the genus Cyanothamnus, with 23 species placed in six series.
In addition to the four incertae sedis species in Boronia, there are three other species, B. coriacea Paul G.Wilson (section Boronia), B. corynophylla Paul G.Wilson (section Algidae Duretto) and B. koniambiensis Däniker (section Boronella (Baill.) Duretto & Bayly), that are unusual morphologically, and their current taxonomic placement requires testing. The first two species have not been included in any phylogenetic analyses.
Boronia coriacea is a poorly collected and rare species from south-western Australia (SW Austr.). Wilson (1971), who described the species, and Duretto et al. (2013), in the Flora of Australia, did not discuss possible relationships of the species. Bayly et al. (2015) indicated that B. coriacea might be related to B. inornata, although critical morphological features, such as seeds, had not been described and were not available for study and, so, they retained it in section Boronia.
Boronia corynophylla was placed in section Valvatae (Benth.) Engl. when described by Wilson (1998) because it had valvate petals. Later Duretto (1999) moved it to the newly described section Algidae on the basis of it having sheathing and brown prophylls, imbricate sepals, valvate petals, and a terminal inflorescence of 1(–3) flowers. Boronia corynophylla differs from the other two species in section Algidae in having an exfoliating cuticle on its branches, simple, terete leaves and a small stigma.
Boronia koniambiensis was included in a cladistic analysis on the basis of morphological data presented by Weston et al. (1984) who resolved it as sister to the remainder of the species placed in the genus Boronella (≡Boronia section Boronella). The species is morphologically distinct from the other species of section Boronella in having glabrous branches (v. branches with tufts of simple hairs in the axils of the leaves), opposite decussate leaves (v. verticillate), an inflorescence that is a large cymose panicle (v. a pseudo-umbel), and valvate petals (v. imbricate; Hartley 1995; Bayly et al. 2015).
In Boronia, only section Valvatae has a formal infrasectional classification (Duretto and Ladiges 1998; Duretto 1999, 2008; Duretto et al. 2013), although this has not been tested using molecular data. Of the sections not discussed above, the following three do not have issues regarding monophyly: Alatae Duretto and Imbricatae are monotypic, and Pedunculatae (Benth.) Duretto & Bayly (11 spp.) is well-defined morphologically (Bayly et al. 2015). By contrast, section Boronia (23 spp.) is diverse morphologically, and the relationships of the species are unresolved even though several infrageneric taxa have been described to accommodate some of the more unusual taxa (Bayly et al. 2015). Many of the unusual morphological features found in the section are that of inflorescence and flower structures and appear to be associated with specialised host and pollination associations with day moths of the family Heliozelidae (Milla et al. 2018; Milla 2019; Wild 2022; L. Milla, A. Young, A. Mousalli, S. Wilcox, M. F. Duretto, M. F. Halsey, T. M. Jones, A. Kallies and D. J. Hilton, in prep.).
The relationships of the seven sections of Boronia and seven species of uncertain affinity are still not adequately understood. In both of the analyses presented by Bayly et al. (2015) and Duretto et al. (2020), there was strong support for sections Imbricatae and Pedunculatae being sister, and weaker support for a sister relationship between B. inornata and section Alatae, B. scabra with section Boronia, and this last clade with section Boronella.
The aims of the current study are to determine: (1) the relationships of the four species, viz. B. humifusa, B. inornata, B. ovata and B. scabra, and series Ovatae that were incertae sedis in Bayly et al. (2015), and B. coriacea (section Boronia), B. corynophylla (section Algidae) and B. koniambiensis (section Boronella); (2) the relationships of species placed in section Boronia; and (3) the relationships of the sections of Boronia. As with Duretto et al. (2020), three plastid markers (psbA–trnH, trnL–trnF and rbcL) and two nuclear ribosomal DNA markers (ITS and ETS) were used to construct robust molecular phylogenies to assess these relationships.
Materials and methods
Taxon sampling
Our dataset comprised 143 accessions of 85 species belonging to 8 genera from subfamily Zanthoxyloideae (see Appelhans et al. 2021); most are newly sequenced specimens, supplemented with samples from previously published studies (Table 1). The ingroup sits within clade D of Bayly et al. (2013, fig. 3) and the outgroups were chosen to represent other subclades within clade D, rooted with Phebalium to represent clade C of Bayly et al. (2013, fig. 3).
|
For the ingroup, 136 accessions of 78 species of Boronia were sampled from all 7 currently recognised sections (the numbers in parentheses in the following paragraph indicate: the number of samples/the number of species sampled/the number of species currently placed in that section or placed incertae sedis), namely, Alatae (1/1/1); Algidae (5/3/3); Boronella (8/4/5; note: one sample not identified to species); Boronia (78/40/43); Imbricatae (1/1/1); Pedunculatae (11/5/11); Valvatae (22/20/66); and incertae sedis (10/4/4). Sampling included representatives of most of the five subsections and nine series of section Valvatae, the only section of Boronia to have a formal infrasectional classification. Species selected for the remaining sections were chosen to cover the morphological variation in those sections. We included 10 accessions of the 4 species placed incertae sedis in Boronia, B. humifusa (1), 2 subspecies of B. inornata (6), B. ovata (1) and B. scabra (2).
The outgroup comprised single accessions of seven species from the following six genera shown in previous studies to appropriately represent taxa closely related to Boronia: Acronychia, Cyanothamnus, Euodia, Medicosma Hook.f., Phebalium, and Zieria.
DNA extraction, PCR, sequencing, alignment
Leaf samples were taken from frozen silica-dried specimens or from herbarium sheets. The plant material was disrupted dry in a TissueLyser II (QIAGEN, Valencia, California, USA) by using tungsten beads, and total genomic DNA was extracted using the Qiagen DNeasy Plant Mini Kit, following the manufacturer’s instructions. Five DNA regions were sequenced, namely, two nuclear regions, the external (ETS) and internal (ITS) transcribed spacers of the 18S–5.8S–26S ribosomal DNA repeats; and three plastid regions, the psbA–trnH intergenic spacer (psbA–trnH), the trnL–trnF region (including the trnL intron and trnL–trnF intergenic spacer) and for a subset of 15 taxa, the rbcL gene. Sequence data for the rbcL gene were available for five outgroup taxa representing four genera, one or two taxa from five of the recognised sections in Boronia, and one taxon placed incertae sedis. We included the rbcL data, although missing from the majority of taxa, in the hope that it would test support for the major clades (see discussion on missing data and tree construction in Johnson et al. 2012). The following primers were used for PCR amplification and sequencing: ETS, myrtF (Lucas et al. 2007) and ETS–18S (Wright et al. 2001); ITS, 18SF and 26SR (Prince 2010) or ITS5 and ITS4 (White et al. 1990), with the former primer pair shown to be less likely to co-amplify fungal contaminants in extracts from herbarium material; psbA–trnH, psbAF (Sang et al. 1997) and trnH2 (Tate and Simpson 2003); trnL–trnF region, primers c and f (Taberlet et al. 1991); rbcL, RUTrbcL1F and rbcL1343R (Bayly et al. 2013).
All PCR reactions were performed in 25-μL volumes containing 200 μM of each primer, 200 μM of each dNTP, 0.004% bovine serum albumin, 2–2.5 mmol MgCl2 and 1 U of Taq DNA polymerase. ITS and trnL–trnF amplifications used Promega GoTaq DNA polymerase (Promega Corporation, Madison, WI, USA), whereas amplifications for ETS, rbcL and psbA–trnH utilised Immolase DNA polymerase (Bioline, Luckenwalde, Germany) and a hot start PCR (with an initial cycle of 10 min at 95°C). PCR reactions were subjected to 40 cycles as follows: denaturation for 30 s at 94°C; annealing for 30 s at 50–58°C; and extension for 1 min at 72°C, with a final extension for 4 min at 72°C. The annealing temperature for ETS, psbA–trnH and trnL–trnF was 53°C, ITS (Prince) 58°C or (White) 55°C and rbcL 50°C. Double-stranded PCR templates were purified, and sequencing was performed by Macrogen Inc. (Seoul, South Korea).
Consensus sequences for each sample were assembled using ABI software Sequence Navigator (ver. 1.0.1, Applied Biosystems, Inc., Foster City, CA, USA) and aligned by eye in PAUP* (ver. 4.0a build 166, see http://phylosolutions.com/paup-test; Swofford 2003). In aligning sequences, gaps were positioned to maximise conformity to known indel types such as simple and inverted duplications of adjacent sequences (Levinson and Gutman 1987; Golenberg et al. 1993). Overlapping indels of different lengths, and insertions of the same length, but bearing different relationships to surrounding sequence, were treated as having independent origins, whereas indels of the same length and position and showing minor differences in nucleotide sequence were scored as the same state (Simmons and Ochoterena 2000). Potentially informative indels were scored as additional presence or absence characters and appended to the database. Gaps were treated as missing data in the phylogenetic analyses. Coding sequences of the rbcL gene were translated in MacClade (ver. 4.08a, see http://macclade.org/; Maddison and Maddison 2000) to check for internal stop codons. The full data matrix, including indel characters, is available in the ‘Supplementary sequence’ section in the Supplementary material. A 21-bp region containing a homoplastic inversion in psbA–trnH (highly incongruent with other characters) was excluded from all analyses.
Phylogenetic analyses
Separate analyses using maximum parsimony or Bayesian inference were run using either individual loci, the concatenated chloroplast or nuclear loci and the combined chloroplast and nuclear sequences. Heuristic searches of the combined or partitioned datasets were conducted in PAUP* (ver. 4.0a build 166, in the CIPRES Science Gateway, see https://www.phylo.org; Miller et al. 2010), by using tree bisection–reconnection branch-swapping to recover all equally most-parsimonious (MP) trees. One thousand replicates of random taxon-addition searching were conducted so as to detect multiple islands of trees, with subsequent use of the ‘condense’ option to delete duplicate trees. Multistate characters were treated as polymorphisms and swapping was performed on best trees. As searching exhausted computer memory for some partitions, restricted searching was employed, saving only 100 trees per replicate. Branch supports were calculated using jackknife (JK) rather than bootstrap resampling, following the recommendations of Simmons and Freudenstein (2011). Jackknife analyses utilised faststep searching in which each replicate was performed using random-sequence addition and no branch swapping, 10 000 replicates and the percentage of characters deleted in each replicate being set at one-third. Jackknifes were interpreted as >50–74% weak support for clades; >75–89% moderate support; 90–99% strong support; and 100% was considered robust.
The MP trees generated were compared with those obtained using the Markov-chain Monte Carlo (MCMC) method implemented in MrBayes (ver. 3.2.7a, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012) in the CIPRES portal (Miller et al. 2010). Most appropriate nucleotide substitution models were determined using the Akaike’s information criterion in MrModeltest (ver. 2.3, J. A. A. Nylander, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden, see https://github.com/nylander/MrModeltest2), with data being partitioned into the five regions indicated above and excluding the appended scored indels. All regions fit general time-reversible likelihood (GTR) substitution models (nst = 6), either with gamma distribution of rate variation among sites (GTR + Γ model; trnL–trnF), or also with a proportion of invariant sites (GTR + Γ + I model; ETS, ITS, psbA–trnH, rbcL).
Bayesian posterior probabilities (PP) were estimated using two independent runs of 10 million generations by using four chains, with tree sampling every 1000 generations. All parameters were set to be unlinked and with rates variable between partitions, with all other priors for the analysis being set flat (i.e. as Dirichlet priors). Runs were assessed as sufficient when checked for convergence with Tracer (ver. 1.7.1, see https://github.com/beast-dev/tracer/releases/tag/v1.7.1; Rambaut et al. 2018) and when the standard deviation of split frequencies approached 0.001. Trees generated prior to the four Markov chains reaching stationarity (burn-in ~25%) were discarded and the remaining trees were used to construct a 50% majority-rule consensus tree, with nodes assigned posterior probabilities (PP) of 0.95–1.00 considered supported. Clades with 100% JK and PP of 1.00 were considered fully supported. Bayesian analyses were conducted, including indels from all regions combined as an extra partition. For these analyses, the indels were binary encoded and we applied a default two-state Markov model with gamma distribution of rates and coding set to variable (as there were no invariant sites). State freqpr was set to fixed (empirical) to reflect having only two states. Inclusion of indels resulted in moderate improvements in branch supports, so final analyses included them as additional characters.
Morphology
Herbarium specimens held at the National Herbarium of New South Wales were examined for all species sampled in our molecular analyses to confirm morphological information in published descriptions as well as to identify additional morphological characters previously not documented.
Results
After exclusion of 38 bp of ambiguous sequence regions, the analysed 143 accessions, 85 species dataset comprised 5268 bp, including 1200 parsimony-informative (PI; 162 being scored indels) and 472 variable but parsimony-uninformative characters. The plastid portion comprised 3773 bp, of which 549 were informative, including 103 scored indels: psbA–trnH, 313 PI (75 included indels); rbcL, 36 PI (no indels); trnL–trnF, 200 PI (28 included indels). The nuclear portion comprised 1495 bp, of which 651 were informative, including 59 scored indels: ETS, 335 PI (including 30 indels); ITS, 316 PI (including 29 indels).
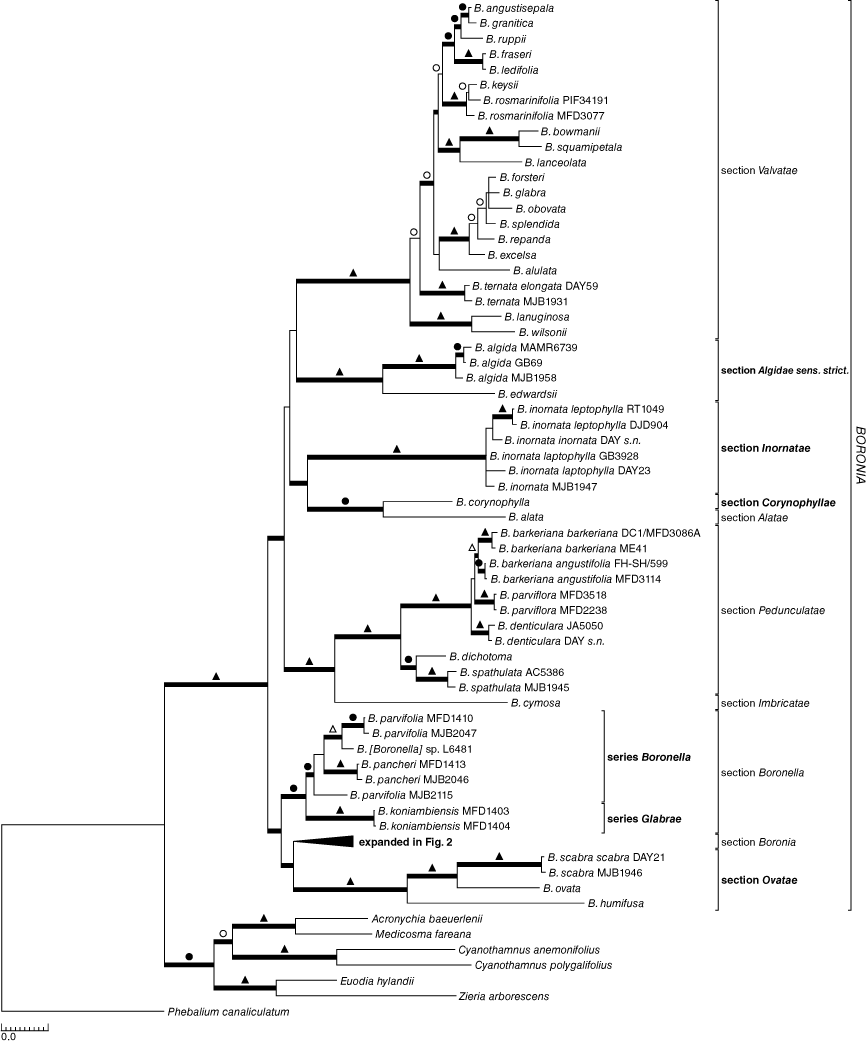
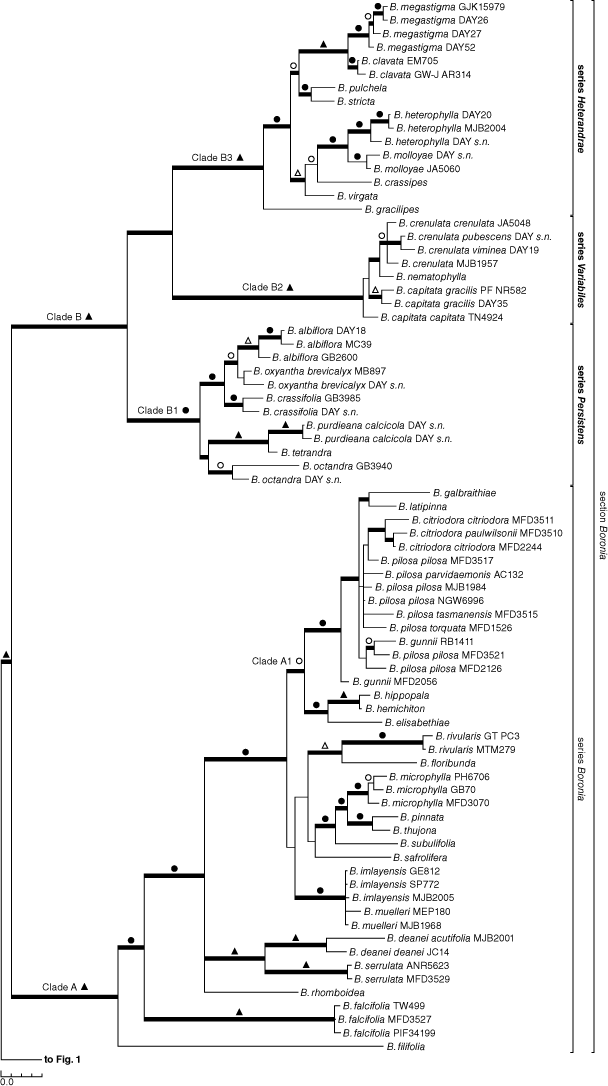
Separate analyses of the nuclear (Supplementary Fig. S1) or plastid (Supplementary Fig. S2) sequences retrieved the same major clades as did the combined analyses (Fig. 1, 2), and those clades here recognised as sections and series were also consistent throughout, excepting Boronia series Persistens Duretto & Heslewood (clade B1; formally described below), which was paraphyletic, but without jackknife support and with <0.95 posterior probability in the nuclear-only analysis. Although changes in structure were seen, the main differences were in support for clades and resolution of relationships within and between sections. In general, the jackknife supports for clades in the plastid-only analyses were weaker. On this basis our final analyses presented here focus on the combined molecular analyses, and we mention the separate analyses only where they highlight important differences. Likewise, parsimony and Bayesian analyses showed a high level of congruence, and Fig. 1 illustrates both jackknife (JK) clade support values >50% and posterior probabilities (PP) imposed on the major clades of the Bayesian majority-rule consensus tree.
|
|
The analysis of the combined dataset produced 100 000 equally most parsimonious trees of length of 4561 steps. Alignment of the nuclear loci was predominantly configured to deal with one or two base indels, whereas insertions in the plastid loci were predominantly longer repeats of adjacent sequence and at least one long deletion. A 21-bp inversion in psbA–trnH was homoplastic and that region was excluded from all analyses, with presence or absence of the inversion scored as an indel.
The genus Boronia was recovered with robust support (1.00 PP, 100% JK), and support for the four larger sections was either robust, as for Boronia, Pedunculatae and Valvatae (1.00 PP, 100% JK), or strong, as for Boronella (1.00 PP, 95% JK) (Fig. 1). The monotypic sections Alatae and Imbricatae are clearly highly divergent from other species. Section Algidae is polyphyletic in all analyses with the two south-eastern Australian species, namely, B. algida F.Muell. and B. edwardsii Benth., forming a robust clade (1.00 PP, 100% JK; section Algidae sens. strict.) and the third species, B. corynophylla, always forming a south-western Australian clade with section Alatae that has strong support (1.00 PP, 98% JK).
Three of the four species currently placed incertae sedis in Boronia (Bayly et al. 2015; Duretto et al. 2020), namely, B. humifusa, B. ovata and B. scabra, form a robustly supported clade (1.00 PP, 100% JK). The fourth species, B. inornata, which is represented by six samples that form a robust clade (1.00 PP, 100% JK), is isolated, grouping with the section Alatae + B. corynophylla clade in the combined analysis (0.98 PP) and the analysis containing nuclear data only (1.00 PP). In the analysis using plastid data only, B. inornata is part of a weakly supported clade (54% JK) that also contains section Valvatae, section Algidae sens. strict., and the section Alatae + B. corynophylla clade.
The results confirmed that section Boronella, with Boronia koniambiensis, is monophyletic with strong support (1.00 PP, 95% JK). Boronia koniambiensis is sister to a clade containing multiple accessions of both B. pancheri (Baill.) Duretto & Bayly and B. parvifolia (Baker f.) Duretto & Bayly and an unidentified specimen in both the full analysis (1.00 PP, 92% JK) and the nuclear analysis. In the analysis of plastid sequences only, B. koniambiensis forms a trichotomy with one accession of B. parvifolia and a clade containing the remaining accessions.
Section Boronia contains two robustly supported clades, clade A (1.00 PP, 100% JK) containing all species found in south-eastern Australia (SE Austr.) and Tasmania (Tas.), and clade B (1.00 PP, 100% JK) containing all species from south-western Australia (Fig. 2). Boronia citrata N.G.Walsh (Victoria, Vic.), B. coriacea (SW Austr.) and B. rozefeldsii Duretto (Tas.) were the only species of section Boronia not included in the analysis because material was not available or could not be sequenced.
In clade A, Boronia filifolia F.Muell. (South Australia, SA; W Vic.) is sister to a strongly supported clade containing the remaining taxa in the combined analysis (1.00 PP, 90% JK) and the one using plastid data only. Resolution and support within the larger clade are generally poor, except for B. falcifolia A.Cunn. ex Endl. (SE Queensland, Qld; NE New South Wales, NSW) being sister to the remainder (1.00 PP, 96% JK) and two species relationships, viz. B. imlayensis Duretto with B. muelleri (Benth.) Cheel (multiple samples of each) forming a strongly supported polytomy (1.00 PP, 95% JK; SE NSW, E Vic.), and B. deanei Maiden & Benth. sister to B. serrulata Sm. (1.00 PP, 100% JK; both simple leaved, SE NSW). By contrast, much of the backbone of clade A is reduced to an unresolved polytomy in the nuclear-only analyses. A moderately supported clade A1 (1.00 PP, 93% JK), containing all species from Tasmania (except B. rhomboidea Hook., found in Tas. and SE NSW), western Victoria and South Australia (except B. filifolia) as well as B. galbraithiae Albr. from eastern Victoria, was recovered in the combined and nuclear-only analyses.
The south-western Australian clade B contains three strongly to robustly supported clades. Clade B1 (1.00 PP, 94% JK) contains B. albiflora R.Br. ex Benth., B. oxyantha Turcz., B. crassifolia Bartl., B. octandra Paul G.Wilson (one accession of this species groups with clade B3 in the nuclear analysis with poor support, that clade being sister to the remainder of B1), B. purdieana Diels, and B. tetrandra Labill. This clade is diverse morphologically but does have an identifiable morphological apomorphy, the petioles being persistent or tardily deciduous (staying on the stems after the lamina has fallen). Elsewhere in Boronia, they are deciduous with the leaves, or the leaves are sessile (M. F. Duretto, pers. obs.). Within this clade, a close relationship is supported between B. tetrandra and B. purdieana (1.00 PP, 100% JK) and among B. albiflora, B. oxyantha and B. crassifolia (1.00 PP, 99% JK).
Clade B2 (1.00 PP, 100% JK) contains B. crenulata Sm., B. nematophylla F.Muell. and a paraphyletic B. capitata Benth., which all have simple leaves (also found in clades A, B1, B2, and other sections) and woolly staminal filaments (also found in some species in section Pedunculatae) (Duretto et al. 2013). Clade B3 (1.00 PP, 100% JK) contains the remaining 10 species of clade B (B. gracilipes F.Muell. to B. clavata Paul G.Wilson) and is morphologically diverse and a morphological apomorphy was not identified for the group. Within clade B3, three strongly supported sister species pairs were identified, namely, B. heterophylla F.Muell. with B. molloyae J.Drumm. (1.00 PP, 98% JK), and B. stricta Bartl. with B. pulchella Turcz. (1.00 PP, 93% JK) in the combined and nuclear-only analyses, and B. megastigma Nees ex Bartl. with B. clavata Paul G.Wilson (1.00 PP, 98% JK).
Relationships between sections
The relationships among the sections and other major groups are poorly resolved, with conflict between analyses and little support for these larger clades. Relationships within sections or series also show conflict between analyses, with the nuclear dataset resolving more species as monophyletic. Of note is that section Imbricatae groups with section Pedunculatae (1.00 PP, 100% JK) and B. corynophylla groups with section Alatae in all analyses with strong support (see above). In the analyses using all data or only the plastid data there are two main clades, both with full support in the Bayesian analyses (1.00 PP), but no jackknife support; the first contains sections Boronella and Boronia with the B. humifusa + B. ovata + B. scabra clade, the last two clades being sister in the combined analysis. All other sections are in the second clade, which has poor internal support, apart from the section Alatae + B. corynophylla and section Imbricatae + section Pedunculatae clades. In the analysis using nuclear data, only section Valvatae is sister to a poorly supported clade (0.77 PP) containing all other groups and for which the internal structure is poorly supported, except for those outlined above.
Discussion
The genus Boronia and sections Boronella, Boronia (less B. coriacea), Pedunculatae and Valvatae are all monophyletic, confirming the results of Bayly et al. (2015) and Duretto et al. (2020). The two monotypic sections, Alatae and Imbricatae, are highly divergent from other taxa, supporting their status as sections, and suggestive of extended periods of isolation. Section Algidae is polyphyletic, with B. corynophylla grouping with section Alatae (discussed further below). The relationships of B. humifusa, B. inornata, B. ovata, B. scabra and B. ser. Ovatae, that were placed incertae sedis by Bayly et al. (2015), and the two morphologically distinctive species, B. koniambiensis and B. corynophylla, are resolved. Boronia citrata, B. coriacea and B. rozefeldsii were the only species of section Boronia not included in this molecular study, owing to suitable material not being available or could not be sequenced. The relationships of B. citrata and B. rozefeldsii are discussed below with section Boronia and that of B. coriacea with B. inornata.
The remainder of the discussion will focus on specific sections and sectional groups.
Placement of Boronia humifusa, B. ovata, B. scabra and B. series Ovatae
Boronia humifusa, B. ovata and B. scabra (all from SW Austr.) form a robustly supported clade that is clearly separated from section Boronia, confirming the conclusions of Bayly et al. (2015). Series Ovatae was described by Wilson (1971) under section Imbricatae to accommodate B. ovata and B. scabra, although later, when reviewing the genus, Wilson (1998) included the series as a synonym of a broadly defined series Boronia. Boronia humifusa was described by Wilson (1998) who noted the species was distinct in series Boronia (equivalent to the section Boronia of Bayly et al. 2015) in having petals with only one medial vein and lacking an apiculum on the abaxial surface, as well as a unique seed type. Boronia humifusa, B. ovata and B. scabra form a strongly supported clade with B. humifusa, sister to a clade containing the other two species, and all species are separated by long branches. This clade is possibly sister to a clade comprising section Boronia (Fig. 1), although this relationship is not supported in the separate analyses, and so warrants a similar rank. All three species have simple leaves, terminal inflorescences and seeds that are smooth or minutely tuberculate with a dorsal hilum that may or may not be in a shallow groove (Wilson 1971, 1998; Choi et al. 2012; Duretto et al. 2013). These three characteristics could be morphological synapomorphies for the group, although they are homoplasious characters in Boronia. Series Ovatae is raised to the rank of a section below (see Taxonomy).
Placement of Boronia inornata and B. coriacea
Boronia inornata is found in south-western Australia and one of its two subspecies is also found in South Australia. The species has large hemispherical glands on the branches (unusual but not unique in Boronia, see B. algida (section Algidae), B. microphylla Sieber ex Rchb. (section Boronia) and B. bowmanii F.Muell. (section Valvatae), for example), imparipinnate leaves with 3(5) usually terete leaflets, terminal inflorescences of 1(–3) flowers, and the seeds are microscopically tuberculate and do not have the sunken hilum that is typical of section Boronia. In all analyses, B. inornata, which is represented by numerous samples, forms a robustly supported clade (1.00 PP, 100 JK, Fig. 1) sister to the south-western Australian B. alata Sm. + B. corynophylla clade in the combined (PP 0.98) and nuclear Bayesian analyses.
Boronia coriacea is currently placed in section Boronia (Wilson 1971; Duretto et al. 2013; Bayly et al. 2015). Bayly et al. (2015) indicated that B. coriacea may be related to B. inornata and both species have glandular verrucose stems, leaves that are 3(5)-foliolate, terminal inflorescences with few flowers, imbricate sepals and petals, petals with an obscure apiculum on the abaxial surface, and glabrous stamens. The two species differ in that B. inornata has leaflets that are usually terete and flowers that are usually solitary on short pedicels, whereas B. coriacea has flat leaflets, and an inflorescence of a few flowers that are on longer pedicels. The seed morphology of B. coriacea has not been documented, and Wilson (1971), who described the species, and Duretto et al. (2013), in the Flora of Australia, do not discuss its relationships. The features these two species share are not unique in Boronia, but the combination is. Apart from the aestivation of the petals the species are similar to B. algida (section Algidae) in appearance. Boronia inornata does not group with any species of section Algidae in our analyses.
Boronia inornata is isolated from all other species in this analysis and a new section, section Inornatae Duretto & Heslewood, is formally described below to accommodate it. As B. coriacea is morphologically similar to B. inornata, it is also placed in this newly described section.
Sections Algidae and Alatae and the placement of Boronia corynophylla
Boronia section Algidae is polyphyletic with its three species never grouping together. The south-eastern Australian clade containing B. algida (NSW, ACT, Vic.), the type species of the section, and B. edwardsii (SA) has strong support and is isolated with no clear affinity, with only the nuclear Bayesian analysis resolving it as sister to section Ovatae (0.99 PP, Supplementary Fig. S1). A narrower circumscription of the section, section Algidae sens. strict. (Fig. 1, S1, S2), is provided below in Taxonomy and the section can be defined by having imparipinnate leaves with flat leaflets, terminal inflorescences of 1(–3) flowers, imbricate sepals, valvate petals and globose stigmas that are much wider than the style.
Boronia corynophylla is restricted to a small area in inland south-western Australia and shows a strongly supported relationship with B. alata. Boronia alata is the sole member of section Alatae and is widespread in near coastal areas of south-western Australia. Branch lengths to both species are long, suggesting that they have had an extended period of isolation. This pairing is surprising, given the striking morphological differences between the two species, although they both have valvate petals. Boronia corynophylla has an exfoliating cuticle on its branches, which gives the branches a glaucous appearance and is unique in Boronia, in addition to simple, slender, terete leaves and an inflorescence of 1(–3) flowers and valvate petals. By contrast, B. alata has smooth branches, imparipinnate or bipinnate leaves with broad leaflets, inflorescences that are large, cymose panicles, and valvate and reduplicate petals. Seeds, which provide useful characters at the section and series level in Boronia (Wilson 1998; Choi et al. 2012), have not been documented for B. corynophylla. With regard to a formal taxonomy, one alternative would be to expand section Alatae to accommodate B. corynophylla but this would create a heterogenous assemblage with no clear apomorphies. Therefore, as B. corynophylla is clearly isolated taxonomically, a new monotypic section, Corynophyllae Duretto & Heslewood, is formally described below to accommodate it. This will be the fourth section of Boronia, three of which are monotypic, that is endemic to south-western Australia.
Section Boronella
Boronia section Boronella contains four described species, the three sampled here plus B. hartleyi Duretto & Bayly (Hartley 1995; Morat et al. 2011; Bayly et al. 2015; T. G. Hartley, unpubl. data) and an undescribed species (T. G. Hartley, unpubl. data; M. F. Duretto, pers. obs.; called B. sp. S’ern Grande Terre (McPherson 3961) at the National Herbarium of NSW). Boronia koniambiensis is sister to the remaining species in all but the analysis using only plastid data where one accession of B. parvifolia forms a trichotomy with B. koniambiensis and a clade containing the remaining specimens. Boronia hartleyi and B. sp. S’ern Grande Terre (McPherson 3961) are similar morphologically to B. pancheri and B. parvifolia (T. G. Hartley, unpubl. data; M. F. Duretto, pers. obs.). These four species all have verticillate leaves, branches with tufts of simple hairs in the axils of the leaves and at the base of the pedicels, inflorescences that are terminal pseudo-umbels, and narrowly imbricate petals (Hartley 1995; Bayly et al. 2015; T. G. Hartley, unpubl. data; M. F. Duretto, pers. obs.). By contrast, B. koniambiensis has opposite decussate leaves, glabrous branches, inflorescences that are terminal cymes, and petals that are valvate in bud (Hartley 1995; Bayly et al. 2015; T. G. Hartley, unpubl. data; M. F. Duretto, pers. obs.). Valvate petals have arisen several times in Boronia (see discussion above; Duretto and Ladiges 1998; Bayly et al. 2015) but in the Boronia + Boronella + Ovatae clade of the combined and plastid only analyses, they are unique to B. koniambiensis and, so, could be considered an apomorphy for that species. In no analysis was a clade retrieved of only those taxa with valvate petals. The remaining species of section Boronella are united on the basis of their verticillate leaves (not found elsewhere in Boronia, except very rarely in individual plants of a very few species, e.g. B. rosmarinifolia A.Cunn. ex Endl. (section Valvatae)), pseudo-umbellate inflorescences (seen also in sections Pedunculatae and Valvatae) and the tuft of simple hairs in the axils of the leaves and at the bases of the pedicels.
Section Boronella has a number of morphological apomorphies such as branchlets with the cortex articulated at nodes, cotyledons that are wider than the hypocotyl, and the presence of a hypodermis in the leaves (Foster 1955; Weston et al. 1984; Hartley 1995; Kubitzki et al. 2011; Bayly et al. 2015). There are two clearly defined clades in section Boronella and both are well supported by both morphological and molecular data and so warrant taxonomic recognition. Hartley (1995) did note that Boronia koniambiensis, when transferring this species from Boronia to the genus Boronella, was different from the remainder of the species in Boronella, but concluded that Boronella should be retained as one genus and that a new monotypic genus should not be erected for B. koniambiensis. We follow Hartley’s argument here, at the sectional level, and retain the one section for all New Caledonian species of Boronia but classify them into two series, namely, a new monotypic series for B. koniambiensis, series Glabrae Duretto & Heslewood, which is formally described below, as well as the typical series for the remaining species (see Taxonomy).
Section Boronia
The circumscription of Boronia section Boronia (only B. citrata and B. rozefeldsii not sampled for this molecular study) is similar to that outlined by Bayly et al. (2015), apart from the removal of B. coriacea (now placed in section Inornatae), which they had provisionally placed in section Boronia. Both unsampled species can be placed in section Boronia on the basis of morphology (see Albrecht and Walsh 1993; Duretto 2003). The section has a unique seed type: the testa is smooth and the adaxial hilum is linear and sunken in a groove that is surrounded by glossy labiose margins (Wilson 1998; Choi et al. 2012). Within the section, there are two well-supported clades, namely, clade A containing species found in south-eastern Australia and Tasmania (B. citrata and B. rozefeldsii not sampled) and including the type species of Boronia, B. pinnata Sm., and clade B with all south-western Australian species. There are no obvious morphological characters, or combinations of characters, supporting either of these clades. However, most species in clade A have pedunculate, open and multi-flowered inflorescences (exceptions are the simple leaved B. deanei, B. rhomboidea, B. serrulata and some Tasmanian species), whereas those in clade B usually have flowers that are solitary or paired, although small cymes are also present in all species of clade B2, and three-flowered cymes are also present in B. stricta and B. virgata Paul G.Wilson of clade B3.
The internal structure within clade A is not well supported and there is significant conflict between analyses. In the combined and plastid analyses, B. filifolia (SA, W Vic.) is resolved as sister to a clade containing the remainder of the species. This species is unusual in the section in being glabrous apart from the flowers and having filiform leaves or leaflets. Very narrow leaves are also found in B. deanei (NSW), which is also glabrous apart from a raised ring of tuberculae on the glands on the stems and leaves. Structure within the remainder of clade A is not well supported, although B. falcifolia (coastal NSW, SE Qld) is sometimes sister to a clade of the remaining species. In the combined (1.00 PP, 58 JK) and nuclear (1.00 PP) analyses, there is also a large polytomy (clade A1) with all Tasmanian species (excepting B. rhomboidea) as well as most species found in western Victoria and South Australia (apart from B. filifolia).
The south-western Australian clade (clade B), in contrast to the south-eastern Australian clade, has well-supported structure and contains three strongly to robustly supported clades (clades B1, B2, B3). Clade B1 is characterised by persistent or tardily caducous petioles (falling after the lamina has fallen) and the species within it show considerable morphological variation. Clade B2 is characterised by woolly staminal filaments (unique in section Boronia) and simple leaves (widespread in Boronia). The names Boronia series Variabiles Benth. (type: B. crenulata) and series Terminales Benth. (type: B. capitata) are both available for clade B2 and were published in the same publication, and the former is chosen here. Clade B3, as with clade B1, contains significant morphological variation, and like clades A and B, lacks clearly discernible morphological apomorphies. The name Boronia series Heterandrae Benth. (type: B. megastigma) is available for this clade.
Most species of Boronia have simple flowers without much modification, typical of most members of the family Rutaceae, that is, a fully open, pink or white corolla, erect stamens that are longer than the ovary, anthers that are approximately equal in size and fertile, and a cylindrical style topped with a minute stigma. Within both clades B1 and B3, there are suites of species with highly modified flowers that appear to be driven by specialised pollinator associations with day moths of the family Heliozelidae (Milla 2019; Wild 2022; L. Milla et al., in prep.). Clades B1 and B3 resolve together in the nuclear-only analyses, hinting at a possible shared origin of some of these features. For example, sterile antesepalous anthers that are significantly different in size from the antepetalous anthers occur in both clades B1 and B3. In clade B1, B. purdieana and B. tetrandra are sister species that have minute sterile antisepalous anthers. By contrast, in clade B3, B. megastigma (sister species of B. clavata), and the sister taxa B. heterophylla and B. molloyae, have very large, dark-coloured, sterile, antesepalous anthers. All these species, with B. clavata, have very large stigmas and, in both clades, some species have stigmas that have antesepalous lobes: in B1, B. purdieana and B. tetrandra, and in B3, B. megastigma (see Duretto et al. 2013, fig. 24). There are several other floral features that appear to be associated with specialised pollinator–host associations, including pendulous flowers, cup-shaped flowers, unusual petal colours (e.g. green, yellow, red, brown), contrasting petal colours (B. megastigma where the adaxial surface is yellow and abaxial surface is brown), variously shaped stamens, lobed or hairy discs, sunken ovaries, absent styles and large stigmas (see descriptions in Duretto et al. 2013). There is a diversity of floral forms that interestingly does not lend itself to a formal classification as there are many other species with more typical flowers related to these clades. Most species in south-eastern Australia and Tasmania have simple unmodified flowers. However, two species of clade A, namely, B. serrulata and B. floribunda Sieb. ex Rchb. (both confined to the Sydney region sandstones, NSW), also have modified flowers with large spherical stigmas and filaments with a dense tuft of hairs at the apex. Curiously these species are not sister taxa and the features seem to be a case of parallel evolution. This association and apparent co-evolution of certain moths of the family Heliozelidae with their host species are part of another study (Milla 2019; L. Milla et al., in prep.) and will not be dealt with further here.
A taxonomic challenge in Boronia section Boronia is that there are several clades that are strongly supported by both molecular and morphological data, including B1 (persistent or tardily caducous petioles), B2 (woolly filaments) and the species pairs in clades B1 and B3 discussed above. By contrast, clades A, B and B3 have good to strong support in the analyses presented here but do not have readily identified morphological apomorphies. This is similar to the situation seen in other Australian genera in Rutaceae, for example, Asterolasia F.Muell. and Phebalium Vent., where there is strong molecular support for clades that are confined to either eastern or south-western Australia, but no obvious morphological characters to support the clades (Duretto et al. 2023). One of the roles of a cladistic analysis is to identify well-supported groups that then can be recognised in a preferably robust formal taxonomy, which is very useful in larger taxonomic groups such as Boronia. Robust formal classification helps with the development of identification tools, identification of conservation priorities, as well as the placement of taxa new to science, which is continuing in Boronia (e.g. Barrett et al. 2015; Duretto 2019).
There are a number of options available on how to proceed with the classification of the species in section Boronia. One would be to retain the current classification with no further grouping of the species. A second would be to formally recognise those clades with morphological synapomorphies as series (clades B1, B2, B. megastigma + B. clavata etc.) and leave the remainder as incertae sedis. Unfortunately, the type species, B. pinnata, is one of the many species not part of a morphologically well-defined group. A third option is to formally recognise the four major clades with strong to robust molecular support (clades A, B1, B2, B3) as series, acknowledging that two of these are difficult to identify on morphological grounds but that all have support on the basis of molecular data. Option three does not create significant issues nomenclaturally: clade A retains the name Boronia as it contains the type species; clade B1 does not have an available name but is easily defined and thus can be formally described; clade B2 has two available names at the appropriate rank (of equal priority as they are described in the same publication and have never been considered synonymous) and is easily defined on morphological grounds; and clade B3 has a name available at the appropriate rank. The issue here is that both clades A and B3 do not have morphological apomorphies and are diverse morphologically. The result would be a complicated key to series, which is not an insurmountable issue, just not ideal. We are applying the third option (see Taxonomy).
Boronia sections Imbricatae and Pedunculatae
Boronia sections Imbricatae (1 sp., B. cymosa Endl., SW Austr.) and Pedunculatae (11 spp.; SW Austr., SE Austr., Tas.) have a well-supported relationship in all analyses (Fig. 1, S1, S2) supporting the results found in Bayly et al. (2015). This close relationship between B. cymosa and the species placed in section Pedunculatae has been postulated before. Both Bentham (1863) and Engler (1896, 1931) placed B. cymosa, along with species from other sections, with several Western Australian species of section Pedunculatae in series Pedunculatae Benth., although they both placed the known eastern Australian species of section Pedunculatae, B. parviflora Sm., in series Terminales Benth. Engler (1896, 1931) placed both these series, and four others, in a broadly defined section Imbricatae. Wilson (1971) circumscribed section Imbricatae to include only three series, series Imbricatae (Engl.) Paul G.Wilson (with only B. cymosa), series Pedunculatae (SW Austr. species only; he did not discuss B. parviflora or B. barkeriana F.Muell.) and series Ovatae (B. scabra and B. ovata). Wilson (1971) did not discuss the rationale behind this novel classification.
Species of section Imbricatae and section Pedunculatae have simple leaves and terminal inflorescences, both widespread characters in Boronia, but differ significantly in flower and seed morphology (Wilson 1971, 1998; Choi et al. 2012; Duretto et al. 2013). Section Imbricatae is characterised by imbricate and persistent sepals, and glaucous, rugulose seeds with a dorsal aril, whereas section Pedunculatae is characterised by valvate and usually deciduous sepals, and smooth, shiny seeds with a basal raphe that is a pulpy mass at the base of the seed (Wilson 1998; Duretto et al. 2013). As the sections are both well supported by both morphological characters and the molecular data presented here, we retain each section as distinct.
Boronia section Valvatae
Boronia section Valvatae is well supported by both morphological (Duretto and Ladiges 1998; Duretto 1999) and molecular data (see above; Bayly et al. 2015; Duretto et al. 2020). Morphological apomorphies that support the section are the presence of stellate hairs (unique in Boronia), valvate sepals (also found in section Pedunculatae), and valvate petals (also found in sections Algidae, Alatae, Corynophyllae and Boronella (series Glabrae)) and persistent petals (also found elsewhere in Boronia). The internal structure of the section does not agree with the classification of the section on the basis of morphological data (Duretto and Ladiges 1998; Duretto 1999, 2008; Duretto et al. 2013), but because only 20 species of the 66 species were sampled, it would be premature to propose any changes to the current classification.
Relationships between the sections of Boronia and phylogenetic diversity
The resolution of relationships between the 10 sections of Boronia is not conclusive, apart from the close relationship of section Alatae with section Corynophyllae, and that of section Imbricatae with section Pedunculatae in all analyses (Fig. 1, S1, S2). In the combined maximum-parsimony analysis, these two clades formed a polytomy with the remaining six sections. The Bayesian analysis contained more structure with sections resolving into two well-supported clades. The first contains sections Boronia and Ovatae sister with robust support (1.00 PP) and these sister to Boronella (1.00 PP). The second clade contains the remaining seven sections with little internal support except for the strongly supported Imbricatae + Pedunculatae and Alatae + Corynophyllae clades and the latter sister to section Inornatae with good support (0.98 PP).
Of the 10 sections recognised here, 6 contain 3 or fewer species. The three monotypic sections, Alatae, Corynophyllae and Imbricatae, along with section Ovatae (3 spp.), are confined to south-western Australia. Two sections contain two species, namely, Algidae sens. strict. from south-eastern Australia, and Inornatae from south-western Australia and South Australia. Sections Boronia (43 spp.) and Pedunculatae (11 spp.) are both found in south-eastern Australia (including Tas.) and south-western Australia. The largest section, Valvatae (66 spp.), is found in south-western Australia, eastern Australia (being absent from SA and Tas.) and north-western Australia, and is the only section found in tropical Australia. The final section, Boronella (4 or 5 spp.), is confined to New Caledonia.
South-western Australia contains significant phylogenetic diversity with 8 of the 10 sections present, 4 of which are endemic to the region. The two Australian sections with infrasectional classifications demonstrate contrasting patterns, with the phylogenetic diversity of section Boronia being higher in south-western Australia, whereas that for section Valvatae is higher in eastern and northern Australia.
Taxonomy
Boronia Sm., Tracts Nat. Hist. 288 (1798)
Perennial herbs, shrubs, rarely small trees; glabrous or with simple or stellate hairs. Leaves opposite, decussate, rarely in whorls of three (see series Boronella), simple or imparipinnate or rarely bipinnate (see section Alatae). Inflorescences axillary or terminal; flowers solitary or in cymes or pseudo-umbels or panicles, bisexual, 4-merous, rarely 5-merous (B. scabra subsp. attenuata Paul G.Wilson). Sepals free, open, imbricate or valvate, persistent or caducous. Petals free, imbricate or valvate, not obviously glandular; tip straight or with a subterminal apiculum on the abaxial surface; 1- or 3-veined at base; caducous or persistent. Stamens 8, rarely 4 of them caducous (B. parviflora); filaments usually inwardly curved, semiterete, glabrous or hairy, usually verrucose towards apex; anthers introrse, apiculate or not, connective usually inconspicuous or cream coloured, all or only antepetalous anthers fertile (see series Boronia and series Persistens). Disc prominent, usually entire, rarely with antepetalous (B. octandra) or antesepalous (B. tetrandra) lobes. Carpels 4; ovaries free though united at apex on adaxial margin by the solitary style. Fruit of 1–4 basally connate follicles (cocci), dehiscing explosively ventrally with separating, elastic endocarp. Seed: sclerotesta smooth or minutely tuberculate, rarely prominently rugulose (B. cymosa), glossy or dull. (Adapted from Duretto et al. 2020).
An Australian (including Tasmania) and New Caledonian genus of 134 species classified into 10 sections, including 9 confined to Australia, and 1, section Boronella, to Grande Terre, New Caledonia. Two sections, Corynophyllae and Inornatae, are newly described, and a new combination at sectional level is made for section Ovatae. Novel infrasectional classifications are provided for sections Boronella, with two series, and Boronia, with four series. Sections Alatae, Imbricatae, Pedunculatae, apart from the addition of subspecies for B. denticulata Sm. (Duretto 2019), and Valvatae, apart from the addition of five species (Barrett et al. 2015), remain as previously circumscribed by Duretto et al. (2013) and Bayly et al. (2015) and are not dealt with further here.
Key to the sections of Boronia|
1. Branches, including cortex, continuous (smooth) at nodes; leaves opposite–decussate; cotyledons linear, as wide as hypocotyl (Austr., Tas.) |
|
2. Petal aestivation unknown |
|
3. Petals imbricate in bud |
|
4. Inflorescence terminal; all hairs simple (Southern Austr., Tas.) |
|
5. Leaves imparipinnate |
|
6. Inflorescence cymose, 1(–3)-flowered; peduncle absent; leave imparipinnate (SE Austr.) |
|
7. Leaves simple |
|
8. Inflorescence terminal, sometimes also terminal on short axillary branches, 1–3-flowered; stems glandular verrucose; staminal filaments glabrous |
|
9. Seed smooth though sometimes minutely tuberculate; branches glabrous or hairy, not developing a visible cream-coloured spongy layer |
|
10. Seeds without a basal elaiosome; sepals imbricate, persistent; branches glabrous or hairy; inflorescence terminal or axillary |
|
11. Staminal filaments pilose, puberulous or glabrous |
|
12. Inflorescence in terminal and upper-axillary cymes, few to many-flowered; leaves narrowly oblong to oblong–elliptic or broadly ovate |
|
13. All hairs simple; inflorescence axillary or terminal (N and SW WA, Qld, NSW, Vic., Tas., SA) |
|
14. Leaves simple |
|
15. Inflorescence terminal, sometimes also terminal on short axillary branches |
|
16. Antepetalous anthers much larger than antesepalous anthers; branches glabrous (Kimberley Region, N WA) |
|
17. Inflorescence cymose, 1–5-flowered; staminal filaments glabrous; leaves imparipinnate |
|
18. Stigma minute, as wide as style; leaves 3(5)-foliolate; seed shiny |
|
19. Seed smooth though sometimes minutely tuberculate; branches glabrous or hairy, not developing a visible cream-coloured spongy layer |
|
20. Seeds without a basal elaiosome; sepals imbricate, persistent; branches puberulous, pilose or glabrous; inflorescence terminal or axillary |
|
21. Leaves flat or terete, if terete then flowers axillary; branches various but not exfoliating or having a grey scurfy covering |
|
22. Staminal filaments puberulous, pilose or glabrous |
|
23. Inflorescence in terminal and upper-axillary cymes, few–many-flowered; leaves narrowly oblong to oblong–elliptic or broadly ovate |
Boronia section Algidae Duretto, Muelleria 12: 16 (1999)
Hairs simple. Branches, including cortex, not articulated (continuous) at nodes, puberulous, smooth or glandular verrucose. Leaves opposite–decussate, imparipinnate. Inflorescence terminal, cymose, 1–3-flowered; peduncle absent; bracts and bracteoles persistent. Sepals imbricate in bud, persistent. Petals valvate in bud, without a subterminal apiculum on abaxial surface, multiveined from base, persistent or caducous. Staminal filaments glabrous, swollen and verrucose towards apex, with tip appearing subterminal adaxially. Stigma globular, much wider than style. Seed dull, grey to black; adaxial surface with a linear hilum; raphe basal; sclerotesta sometimes minutely verrucose; cotyledons linear, as wide as hypocotyl.
A section of two species confined to south-eastern Australia: Boronia algida (NSW, ACT, Vic.), B. edwardsii (SA).
Boronia section Boronella (Baill.) Duretto & Bayly, Austral. Syst. Bot. 28: 119 (2015)
Hairs simple. Branches, including cortex, strongly articulated at nodes, glabrous or with hairs at nodes, smooth or cuticle slightly exfoliating, not obviously glandular. Leaves verticillate in whorls of three or opposite–decussate, simple. Inflorescence a terminal cyme or pseudo-umbel, many-flowered; peduncle absent or present; bracts and bracteoles deciduous or apparently absent or so minute they are obscured by hairs at base of pedicels. Sepals imbricate or valvate in bud, persistent. Petals imbricate or valvate in bud, usually with a subterminal apiculum on abaxial surface, mostly multiveined from base or with steeply ascending basal lateral veins, persistent. Staminal filaments glabrous or hairy, swollen and verrucose towards apex, tip usually appearing subterminal adaxially. Stigma minute, not or slightly wider than style. Seed shiny, with a smooth sclerotesta, although cell walls visible; hilum usually adaxial and linear; raphe small and covered by brown outer testa; cotyledons elliptic or suborbicular, wider than hypocotyl.
A section of at least five species, of which four have been formally described, that is confined to Grande Terre, New Caledonia. A key to the described species of the genus Boronella has been provided by Hartley (1995). Here we present the first infrageneric classification for section Boronella, which includes two series.
Key to the series of Boronia section Boronella|
1. Leaves opposite–decussate; branches glabrous; petals valvate in bud |
Boronia [section Boronella] series Boronella (Baill.) Duretto & Heslewood, comb. nov.
Branches glabrous apart from a dense indumentum of simple hairs in leaf axils and at base of inflorescence. Leaves verticillate in whorls of 3, sometimes also opposite–decussate on some plants. Inflorescence a simple terminal cluster (pseudo-umbel), bracts and bracteoles absent or possibly minute and obscured by hairs at base of pedicels. Petals imbricate in bud.
A series of at least four species confined to Grande Terre, New Caledonia: Boronia hartleyi, B. pancheri, B. parvifolia, B. sp. S’ern Grande Terre (McPherson 3961) (phrase name used at the National Herbarium of NSW; T. G. Hartley, unpubl. data; M. F. Duretto, pers. obs.).
Boronia [section Boronella] series Glabrae Duretto & Heslewood, ser. nov.
Differs from series Boronella by the glabrous branches (v. pilose at nodes), opposite–decussate leave (v. verticillate in whorls of 3), and petals being valvate in bud (v. imbricate in bud).
Branches glabrous. Leaves opposite–decussate. Inflorescence a terminal cyme; bracts and bracteoles deciduous. Petals valvate in bud.
A monotypic series confined to the Koniambo Massif, north-eastern Grande Terre, New Caledonia.
Etymology
The series epithet is derived from the Latin glabrus alluding to the glabrous branches, which is one of the features that distinguishes this species from all other species in section Boronella.
Boronia Sm. section Boronia
Hairs simple. Branchlets, including cortex, not articulated (continuous) at nodes, smooth or glandular–verrucose, glabrous or hairy. Leaves opposite–decussate, simple or imparipinnate. Inflorescence axillary or terminal, flowers solitary or in cymes, 1–40+-flowered; peduncle absent or present; bracts and bracteoles persistent. Sepals open or imbricate in bud, persistent. Petals imbricate in bud, with subterminal apiculum on the abaxial surface, multiveined from base or with steeply ascending basal lateral veins. Staminal filaments glabrous or hairy, swollen and usually verrucose, rarely smooth, towards apex, with tip usually appearing subterminal adaxially. Stigma minute and scarcely wider than style or massive. Seed with a smooth sclerotesta, cell walls not usually visible, glossy; hilum adaxial, linear, usually in a groove with labiose margins; raphe small and covered by brown outer testa; cotyledons linear, as wide as hypocotyl.
A section of 43 species found across southern Australia, including Tasmania, with 24 species confined to south-eastern Australia and 19 to south-western Australia. The section outlined here is equivalent to that described as section Boronia by Bayly et al. (2015) less B. coriacea, and Boronia ser. Boronia by Duretto et al. (2013), less B. coriacea, B. inornata, B. humifusa, B. ovata and B. scabra. A key to the species is provided by Duretto et al. (2013). No novel species for the section have been formally described since Duretto et al. (2013) though B. clavata has recently had novel subspecies formally described (Duretto 2019).
Here we present a novel infrageneric classification of the section that includes four series, including one newly described and two reinstated.
Key to the series of Boronia section Boronia|
1. Staminal filaments pilose, puberulous or glabrous; leaves simple or imparipinnate |
|
2. Petioles absent or deciduous with leaves; leaves simple or imparipinnate; anthers approximately equal or antisepalous anthers much larger than antipetalous anthers |
|
3. Leaves imparipinnate |
|
4. Inflorescence axillary |
|
5. Leaf margin crenulate (SW Austr.) |
|
6. Flowers axillary, solitary or in pairs, or rarely in threes; peduncle absent or minute; antisepalous approximately equal to or significantly larger than antipetalous anthers (SW Austr.) |
Boronia Sm. [section Boronia] series Boronia
Leaves imparipinnate or simple, sessile or with petiole falling with lamina. Inflorescence axillary or terminal, 1–40+-flowered; peduncle up to 30 mm long. Staminal filaments pilose, puberlous or glabrous; anthers approximately equal.
A series of 21 species restricted to south-eastern Australia, including Tasmania: Boronia citrata, B. citriodora Gunn ex Hook.f. (three subspecies), B. deanei (two subspecies), B. elisabethiae Duretto, B. falcifolia, B. filifolia, B. floribunda, B. galbraithiae, B. gunnii Hook.f., B. hemichiton Duretto, B. hippopala Duretto, B. imlayensis, B. latipinna J.H.Willis, B. microphylla, B. muelleri, B. pilosa Labill. (four subspecies), B. pinnata, B. rivularis C.T.White, B. safrolifera Cheel, B. serrulata, and B. subulifolia Cheel.
Boronia [section Boronia] series Heterandrae Benth., Fl. Austral. 1: 308, 320 (1863)
Leaves imparipinnate or simple; sessile or with petiole falling with lamina. Inflorescence axillary; 1- or 2(3)-flowered; peduncle absent or minute. Staminal filaments pilose or glabrous; anthers approximately equal or antisepalous anthers much larger than antipetalous anthers.
A series of 10 species confined to south-western Australia: Boronia clavata (2 subspecies), B. crassipes Bartl., B. gracilipes, B. heterophylla, B. megastigma, B. molloyae, B. pulchella, B. stricta, and B. virgata.
Boronia [section Boronia] series Persistens Duretto & Heslewood, ser. nov.
Differs from the other series of section Boronia by have petioles that are persistent or tardily caducous and falling after the lamina has fallen (v. absent, or deciduous with lamina).
Leaves imparipinnate; petiole persistent or tardily caducous and then after lamina has fallen. Inflorescence axillary, 1- or 2-flowered; peduncle absent or minute. Staminal filaments pilose or puberulous; anthers approximately equal or antisepalous anthers much smaller than antipetalous anthers.
A series of six species confined to south-western Australia: Boronia albiflora, B. crassifolia, B. oxyantha (two varieties), B. octandra, B. purdieana (two subspecies), and B. tetrandra.
Etymology
The series epithet is derived from the Latin persistens, alluding to the persistent petioles that remain on the branches after the leaves have fallen.
Boronia [section Boronia] series Variabiles Benth., Fl. Austral. 1: 309, 320 (1863)
Leaves simple; sessile or with petiole falling with lamina. Inflorescence axillary or terminal, flowers solitary or in small cymes; peduncle absent or minute. Staminal filaments woolly; anthers approximately equal.
A series of three species confined to south-western Australia: Boronia capitata (three subspecies), B. crenulata (four subspecies, typical subspecies with two varieties), and B. nematophylla.
Boronia section Corynophyllae Duretto & Heslewood, sect. nov.
Differs from sections Algidae and Alatae by having simple leaves (v. imparipinnate or bipinnate) and exfoliating branchlets (v. smooth).
Hairs simple. Branches, including cortex, not articulated (continuous) at nodes, smooth and not obviously glandular, scarcely puberulous, with exfoliating cuticle that gives the branches a glaucous appearance. Leaves opposite–decussate, simple. Inflorescence terminal, 1(2 or 3)-flowered; peduncle absent or minute; bracts and bracteoles persistent. Sepals imbricate in bud; persistence unknown. Petals valvate in bud, multiveined from base, without a subterminal apiculum on abaxial surface; persistence unknown. Staminal filaments pilose, swollen and verrucose towards apex, with tip appearing subterminal adaxially. Stigma minute, as wide as or slightly wider than style. Seed not seen.
A monotypic section confined to south-western Australia.
Boronia section Inornatae Duretto & Heslewood, sect. nov.
Differs from the other sections of Boronia by the having the following combination of characters: glandular, verrucose branchlets, 3(5)-foliolate leaves, a terminal inflorescence of 1(–3) flowers, imbricate sepals, imbricate petals, glabrous stamens, and seeds that are microscopically tuberculate.
Hairs simple. Branches, including cortex, not articulated (continuous) at nodes, glandular–verrucose. Leaves opposite–decussate, imparipinnate. Inflorescence terminal, 1(–3)-flowered; peduncles absent; bracts and bracteoles persistent. Sepals imbricate in bud, persistent. Petals imbricate in bud, without or with a minute subterminal apiculum on the abaxial surface, multiveined from base, caducous. Staminal filament glabrous, swollen and verrucose towards apex, with tip terminal. Stigma minute, not wider than style. Seed minutely tuberculate, glossy; hilum adaxial, linear, not in a groove; embryo unknown.
A section of two species: Boronia coriacea (SW WA), B. inornata (SW WA, SA; two subspecies).
Boronia section Ovatae (Paul G.Wilson) Duretto & Heslewood, comb. nov., stat. nov.
Hairs simple. Branches, including cortex, not articulated (continuous), obviously glandular, glabrous, puberulous or pilose. Leaves opposite–decussate, simple. Inflorescence a terminal cyme, cymes and solitary flowers sometimes also in axils of upper leaves; peduncle present, sometimes minute; bracts and bracteoles persistent. Sepals imbricate in bud, persistent. Petals imbricate in bud, with or without a subterminal apiculum on the abaxial surface, multiveined from base or with a single vein, caduous. Staminal filaments glabrous or pilose, swollen and verrucose towards apex, with tip appearing subterminal adaxially. Stigma minute, scarcely wider than style. Seed with a smooth sclerotesta or minutely tuberculate, cell walls not usually visible, glossy; adaxial surface with linear hilum in a groove, or with a glossy cover to the raphe; embryo unknown.
A section of three species confined to south-western Australia: Boronia humifusa, B. ovata, and B. scabra (three subspecies). Boronia humifusa has several unique features such as the petals having a single vein, a massive stigma and style, and a unique seed morphology (Wilson 1998).
Supplementary material
Supplementary material is available online.
Data availability
New sequence data for this study are available from GenBank https://www.ncbi.nlm.nih.gov/genbank/: ITS OP653792–OP653880, ETS OP654199–OP654291, rbcL OP654292–OP654297, psbA–trnH OP654298–OP654390, trnL–trnF OP654391–OP654466. Alignment files are available in the Supplementary material, ‘Supplementary sequence’ section. Other data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
M. Bayly is an editor for Australian Systematic Botany. Despite this relationship, he did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and has protocols that keep editors separate from the decision-making processes for their manuscripts. The authors declare that they have no other conflicts of interest.
Declaration of funding
The authors thank the Hermon Slade Foundation for funding parts of this project (Grant HSF13/6).
Acknowledgements
We thank the Directors of AD, BRI, CANB, CNS, DNA, HO, MEL, MELU, NSW, and PERTH for access to their herbaria and the loan of material; the Directors of the Royal Botanic Gardens & Domain Trust, Royal Botanic Gardens Victoria, and the Australian National Botanic Garden for permission to sample their living collections. The former Victorian Department of Sustainability and Environment, former Western Australian Department of Conservation and Land Management (later DEC), New South Wales Department of Planning, Industry and Environment, and conservation authorities of the North and South Provinces of New Caledonia (DDEE and DENV), provided permission to collect material in parks and reserves under their control. We thank our reviewers and editor for insightful comments that improved this paper.
References
Albrecht DE, Walsh NG (1993) Two new species of Boronia (Rutaceae) endemic in Victoria. Muelleria 8, 21–25.| Two new species of Boronia (Rutaceae) endemic in Victoria.Crossref | GoogleScholarGoogle Scholar |
Appelhans MS, Bayly MJ, Heslewood MM, Groppo M, Verboom GA, Forster PI, Kallunki JA, Duretto MF (2021) A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. Taxon 70, 1035–1061.
| A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers.Crossref | GoogleScholarGoogle Scholar |
Barrett RL, Barrett MD, Duretto MF (2015) Four new species of Boronia (Rutaceae) from the Kimberley region of Western Australia. Nuytsia 26, 89–109.
Bayly MJ, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2013) Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences. PLoS One 8, e72493
| Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences.Crossref | GoogleScholarGoogle Scholar |
Bayly MJ, Duretto MF, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2015) Transfer of the New Caledonian genus Boronella to Boronia (Rutaceae) based on analyses of cpDNA and nrDNA. Australian Systematic Botany 28, 111–123.
| Transfer of the New Caledonian genus Boronella to Boronia (Rutaceae) based on analyses of cpDNA and nrDNA.Crossref | GoogleScholarGoogle Scholar |
Bentham G (1863) ‘Flora Australiensis. Vol. 1.’ (Lovell Reeve and Co: London, UK)
Choi BK, Duretto MF, Hong SP (2012) Comparative seed morphology of Boronia and related genera (Boroniinae: Rutaceae) and its systematic implications. Nordic Journal of Botany 30, 241–256.
| Comparative seed morphology of Boronia and related genera (Boroniinae: Rutaceae) and its systematic implications.Crossref | GoogleScholarGoogle Scholar |
Duretto MF (1999) Systematics of Boronia section Valvatae sensu lato (Rutaceae). Muelleria 12, 1–131.
| Systematics of Boronia section Valvatae sensu lato (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Duretto MF (2003) Notes on Boronia (Rutaceae) in eastern and northern Australia. Muelleria 17, 19–135.
| Notes on Boronia (Rutaceae) in eastern and northern Australia.Crossref | GoogleScholarGoogle Scholar |
Duretto MF (2008) A new subsection and two new subseries for Boronia Sm. section Valvatae (Benth.) Engl. (Rutaceae). Ausrobaileya 7, 665–668.
Duretto MF (2019) New subspecies for the south-western Australian species Boronia clavata and B. denticulata (Rutaceae). Telopea 22, 31–39.
| New subspecies for the south-western Australian species Boronia clavata and B. denticulata (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Duretto MF, Ladiges PY (1998) A cladistic analysis of Boronia section Valvatae (Rutaceae). Australian Systematic Botany 11, 636–665.
| A cladistic analysis of Boronia section Valvatae (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Duretto MF, Wilson PG, Ladiges PY (2013) Boronia. In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae and Zygophyllaceae’. (Ed. A Wilson) pp. 124–282. (CSIRO Publishing: Melbourne, Vic., Australia)
Duretto MF, Heslewood MM, Bayly MJ (2020) Boronia (Rutaceae) is polyphyletic: reinstating Cyanothamnus and the problems associated with inappropriately defined outgroups. Taxon 69, 481–499.
| Boronia (Rutaceae) is polyphyletic: reinstating Cyanothamnus and the problems associated with inappropriately defined outgroups.Crossref | GoogleScholarGoogle Scholar |
Duretto MF, Heslewood MM, Bayly MJ (2023) Generic and infrageneric limits of Phebalium and its allies (Rutaceae: Zanthoxyloideae). Australian Systematic Botany 36, 107–142.
| Generic and infrageneric limits of Phebalium and its allies (Rutaceae: Zanthoxyloideae).Crossref | GoogleScholarGoogle Scholar |
Engler A (1896) Rutaceae. In ‘Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen, III. Teil, Abteilung 4’ [The natural plant families together with their genera and more important species, especially the useful plants, III. Part, Section 4]. (Eds A Engler, K Prantl) pp. 95–201. (Wilhelm Engelmann: Leipzig, German Empire) [In German]
Engler HGA (1931) Rutaceae. In ‘Die natürlichen Pflanzenfamilien Teil 19a’ [The natural plant families part 19a], 2nd edn. (Eds HGA Engler, K Prantl) pp. 187–359. (Wilhelm Engelmann: Leipzig, German Republic) [In German]
Foster AS (1955) Comparative morphology of the foliar sclereids in Boronella Baill. Journal of the Arnold Arboretum 36, 189–198.
| Comparative morphology of the foliar sclereids in Boronella Baill.Crossref | GoogleScholarGoogle Scholar |
Golenberg EM, Clegg MT, Durbin ML, Doebley J, Ma DP (1993) Evolution of a noncoding region of the chloroplast genome. Molecular Phylogenetics and Evolution 2, 52–64.
| Evolution of a noncoding region of the chloroplast genome.Crossref | GoogleScholarGoogle Scholar |
Groppo M, Pirani JR, Salatino MLF, Blanco SR, Kallunki JA (2008) Phylogeny of Rutaceae based on two noncoding regions from cpDNA. American Journal of Botany 95, 985–1005.
| Phylogeny of Rutaceae based on two noncoding regions from cpDNA.Crossref | GoogleScholarGoogle Scholar |
Hartley TG (1995) A new combination in Boronella (Rutaceae) and a view on relationships of the genus. Bulletin du Muséum National d'Histoire naturelle, 4ème Série – Section B, Adansonia: Botanique, Phytochimie 17, 107–111.
Johnson KA, Holland BR, Heslewood MM, Crayn DM (2012) Supermatrices, supertrees and serendipitous scaffolding: Inferring a well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data. Molecular Phylogenetics and Evolution 62, 146–158.
| Supermatrices, supertrees and serendipitous scaffolding: Inferring a well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data.Crossref | GoogleScholarGoogle Scholar |
Kubitzki K, Kallunki JA, Duretto M, Wilson P (2011) Rutaceae. In ‘The Families and Genera of Vascular Plants Vol. X. Flowering plants Eudicots: Sapindales, Cucurbitales, Myrtaceae’. (Ed. K Kubitzki) pp. 276–356. (Springer-Verlag: Heidelberg, Germany)
| Crossref |
Levinson G, Gutman GA (1987) Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Molecular Biology and Evolution 4, 203–221.
| Slipped-strand mispairing: a major mechanism for DNA sequence evolution.Crossref | GoogleScholarGoogle Scholar |
Lucas EJ, Harris SA, Mazine FF, Belsham SR, Nic Lughadha EM, Telford A, Gasson PE, Chase MW (2007) Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales. Taxon 56, 1105–1128.
| Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales.Crossref | GoogleScholarGoogle Scholar |
Maddison WP, Maddison DR (2000) ‘MacClade 4: Analysis of phylogeny and character evolution.’ (Sinauer: Sunderland, MA, USA)
Milla E (2019) Evolution and Ecology of the Australian Heliozelidae (Adeloidea, Lepidoptera). PhD thesis, University of Melbourne, Parkville, Vic., Australia.
Milla L, van Nieukerken EJ, Vijverberg R, Doorenweerd C, Wilcox SA, Halsey M, Young DA, Jones TM, Kallies A, Hilton DJ (2018) A preliminary molecular phylogeny of shield-bearer moths (Lepidoptera: Adeloidea: Heliozelidae) highlights rich undescribed diversity. Molecular Phylogenetics and Evolution 120, 129–143.
| A preliminary molecular phylogeny of shield-bearer moths (Lepidoptera: Adeloidea: Heliozelidae) highlights rich undescribed diversity.Crossref | GoogleScholarGoogle Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA, USA. INSPEC Accession Number 11705685. (IEEE)
| Crossref |
Morat P, Jaffré T, Tronchet F, Munzinger J, Pillon Y, Veillon J-M, Chalopin M, Birnbaum P, Rigault F, Dagostini G, Tinel J, Lowry PP (2011) Le référentiel taxonomique Florical et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie. [The floral taxonomic repository and the characteristics of the native vascular flora of New Caledonia.] Adansonia 34, 179–221.
| Le référentiel taxonomique Florical et les caractéristiques de la flore vasculaire indigène de la Nouvelle-Calédonie. [The floral taxonomic repository and the characteristics of the native vascular flora of New Caledonia.]Crossref | GoogleScholarGoogle Scholar | [In French with English abstract]
Prince LM (2010) Phylogenetic relationships and species delimitation in Canna (Cannaceae). In ‘Diversity, phylogeny, and evolution in the monocotyledons: Proceedings of the Fourth International Conference on the Comparative Biology of the Monocotyledons and the Fifth International Symposium on Grass Systematics and Evolution’. (Eds O Seberg, G Petersen, AS Barfod, JI Davis) pp. 307–331. (Århus University Press: Århus, Denmark)
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901–904.
| Posterior summarization in Bayesian phylogenetics using Tracer 1.7.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542.
| MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space.Crossref | GoogleScholarGoogle Scholar |
Sang T, Crawford DJ, Stuessy TF (1997) Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). American Journal of Botany 84, 1120–1136.
| Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae).Crossref | GoogleScholarGoogle Scholar |
Simmons MP, Freudenstein JV (2011) Spurious 99% bootstrap and jackknife support for unsupported clades. Molecular Phylogenetics and Evolution 61, 177–191.
| Spurious 99% bootstrap and jackknife support for unsupported clades.Crossref | GoogleScholarGoogle Scholar |
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49, 369–381.
| Gaps as characters in sequence-based phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Swofford DL (2003) ‘PAUP*: Phylogenetic analysis using parsimony (*and other methods), Version 4.’ (Sinauer: Sunderland, MA, USA)
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17, 1105–1109.
| Universal primers for amplification of three non-coding regions of chloroplast DNA.Crossref | GoogleScholarGoogle Scholar |
Tate JA, Simpson BB (2003) Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Systematic Botany 28, 723–737.
| Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species.Crossref | GoogleScholarGoogle Scholar |
Weston P, Carolin R, Armstrong J (1984) A cladistic analysis of Boronia Sm. and Boronella Baill. (Rutaceae). Australian Journal of Botany 32, 187–203.
| A cladistic analysis of Boronia Sm. and Boronella Baill. (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In ‘PCR protocols: a guide to methods and applications’. (Eds M Innis, D Gelfand, J Sninsky, T White) pp. 315–322. (Academic Press: San Diego, CA, USA)
| Crossref |
Wild A (2022) New light on native pollinators. Ecos 294, 23 September 2022. Available at https://ecos.csiro.au/new-light-on-native-pollinators/
Wilson PG (1971) Taxonomic notes on the family Rutaceae, principally of Western Australia. Nuytsia 1, 197–207.
Wilson PG (1998) New names and new taxa in the genus Boronia (Rutaceae) from Western Australia, with notes on seed characters. Nuytsia 12, 119–154.
Wright SD, Yong CG, Wichman SR, Dawson JW, Gardner RC (2001) Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS). Journal of Biogeography 28, 769–774.
| Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS).Crossref | GoogleScholarGoogle Scholar |


