Phylogenomics of Australian sundews (Drosera: Droseraceae)
Luis T. Williamson A * , Ed Biffin
A * , Ed Biffin  B , Timothy A. Hammer
B , Timothy A. Hammer  A B , Kor-jent van Dijk
A B , Kor-jent van Dijk  B , John G. Conran
B , John G. Conran  A and Michelle Waycott
A and Michelle Waycott  A B
A B
A
B
Abstract
Drosera (Droseraceae) is one of the largest carnivorous plant genera globally, with Australia considered a nexus for the evolution of the genus. We present the most densely sampled phylogenomic analyses for Australian Drosera to date. As part of the Genomics for Australian Plants Initiative Stage II, 92 Drosera samples representing all major clades within Australia and key extra-Australian taxa were newly sequenced using Angiosperm353 and OzBaits nuclear bait sets, and the OzBaits plastid bait set. In total, 380 nuclear and 57 plastid loci were included in our final analyses. Our findings are broadly in congruence with conclusions of previous morphological studies that were informed by molecular data regarding the major lineages within Drosera. Incongruencies between the results from plastid and nuclear sequence data sets were primarily restricted to within-clade relationships, with high discordance noted in two closely affiliated species groups with centres of diversity across northern Australia and south-west Western Australia. Potential drivers of this phylogenetic discordance are investigated using Quartet Sampling and are discussed. An important outcome of these data is to highlight the diversity of novel evolutionary lineages within Australia for this group of plants that exhibit highly modified traits to survive in arid Australian environments.
Keywords: Angiosperms353, Australia, Caryophyllales, carnivorous plant, Drosera, Droseraceae, Genomics for Australian Plants, OzBaits, phylogenomics, phylogeny, sundew.
Introduction
Droseraceae is a family of three extant carnivorous genera of annual or perennial herbs; two are monotypic (Aldrovanda vesiculosa L. and Dionaea muscipula J.Ellis), whereas Drosera L. is speciose (~260 spp.) and cosmopolitan. Outcomes of recent angiosperm-wide analysis confirm Droseraceae resolving as a member of a clade in the eudicot order Caryophyllales along with Nepenthaceae, Drosophyllaceae, Dioncophyllaceae and Ancistrocladaceae (Baker et al. 2022; Zuntini et al. 2024). Drosera is morphologically diverse (Fig. 1), with a range of growth habits, leaf lamina forms, reproductive strategies and dormancy systems (Fleischmann et al. 2017a). Centres of diversity for the genus occur in Southern Hemisphere Mediterranean climates (Yesson and Culham 2006), particularly within Australia, South Africa and South America (Lowrie et al. 2017a, 2017b; Robinson et al. 2017). Australia is the global centre of diversity for the genus with 3 of 4 subgenera, 10 of 13 sections (Fleischmann et al. 2017a) and ~170 spp. occurring there, representing ~65% of globally recognised species.
Drosera clades showing variation in habit and floral morphological characteristics. (a) Drosera regia. Photograph: Z. Poulsen (see https://www.inaturalist.org/photos/15164989, CC BY-NC). (b) Drosera arcturi. Photograph: J. G. Conran. (c) Drosera burmanni. Photograph: J. G. Conran. (d) Drosera uniflora. Photograph: J. J. Bruhl. (e) Drosera spatulata. Photograph: J. G. Conran. (f) Drosera hamiltonii. Photograph: J. G. Conran. (g) Drosera finlaysoniana. Photograph: J. G. Conran. (h) Drosera adelae flower. Photograph: J. G. Conran. (i) Drosera glanduligera. Photograph: T. A. Hammer. (j) Drosera petiolaris. Photograph: J. G. Conran. (k) Drosera pygmaea. Photograph: J. G. Conran. (l) Drosera barbigera. Photograph: J. G. Conran. (m) Drosera × sidjamesii. Photograph: J. G. Conran. (n) Drosera binata habit. Photograph: J. G. Conran. (o) Drosera binata flower. Photograph: J. G. Conran. (p) Drosera squamosa. Photograph: J. G. Conran. (q) Drosera ramellosa. Photograph: J. G. Conran. (r) Drosera hookeri. Photograph: J. G. Conran. (s) Drosera menziesii. Photograph: J. G. Conran. (t) Drosera macrantha subsp. planchonii tubers. Photograph: J. G. Conran.

Drosera has been the subject of taxonomic interest since the type species D. rotundifolia L. was described by Linnaeus (1753). Numerous taxonomic revisions have applied infrageneric ranks at the subgeneric, sectional and series ranks (e.g. Planchon 1848; DeBuhr 1977; Seine and Barthlott 1994; Lowrie 2005, 2013a, 2013b, 2013c; Fleischmann et al. 2017a, 2018; Lowrie et al. 2017a, 2017b; Robinson et al. 2017). Ongoing taxonomic descriptions by A. Lowrie (1948–2021), N. G. Marchant, J. G. Conran, R. Gibson, A. Fleischmann, T. A. Krueger and several other authors have led to additional new Drosera species being recognised in Australia (Krueger 2022). These works range across individual species descriptions (de Salas 2018; Baleeiro et al. 2020; Mathieson and Thompson 2020; Krueger and Fleischmann 2021, 2023b), species complex revisions (Krueger et al. 2023a), revisions raising subspecies to species rank (Lowrie 2013a, 2013b, 2013c), works synonymising taxa (Krueger and Fleischmann 2020) and major revisions of the higher taxonomy within Drosera (Lowrie 2013a, 2013b, 2013c; Fleischmann et al. 2017a).
The earliest phylogenetic study of carnivorous plants (Albert et al. 1992) established that carnivory occurs among distantly related plant lineages, with diverse methods having evolved independently for trapping and digesting prey. This life history strategy is now documented in at least 19 angiosperm genera (Fleischmann et al. 2017b). Carnivorous flypaper traps (i.e. extended glandular trichomes) have evolved independently in the Caryophyllales (Drosera L., Drosophyllum Link and Triphyophyllum Airy Shaw.) and Lamiales (Byblis Salisb., Philcoxia P.Taylor & V.C.Souza and Pinguicula L.) (Fleischmann et al. 2017b; Fleck and Jobson 2023). Similar flypaper traps are known in the Ericales (Roridula Burm. ex L.); however, unlike the previously mentioned genera, these do not produce digestive enzymes and are considered protocarnivorous (Anderson and Midgley 2003). Recent phylogenomic publications using nuclear DNA data (Baker et al. 2022; Zuntini et al. 2024) explored the origins of different modes of carnivory in flowering plants (Fleck and Jobson 2023). These identified that within the Droseraceae, the Drosera flypaper strategy was associated with sister taxa having modified bilobed laminae that, when triggered, rapidly close to form a digestive chamber (Fleischmann et al. 2017a) referred to as a leaf steel-trap and concluded that the deeper time phylogenetic origin of carnivory for this lineage remains uncertain (Fleck and Jobson 2023).
There are currently two major classification systems for Drosera concurrent in the literature, proposing subgenus and section groups. One is a morphological classification proposed by Lowrie (2013a, 2013b, 2013c) that has been refined more recently in a series of publications (Lowrie et al. 2017a, 2017b; Robinson et al. 2017; Nunn and Lowrie 2021; referred to hereafter as the Lowrie classification). This has been informally applied widely as a classification system grouping for Drosera in Australia. The other classification, phylogenetically informed and based on the analysis of rbcL and ITS sequence data (Rivadavia et al. 2003), included some Australian Drosera species representing the major groups; however, the within-group diversity of Australian taxa was limited. Significant incongruence between the morphologically informed Lowrie classification and the molecular based Rivadavia et al. (2003) system were apparent and Fleischmann et al. (2017a) proposed an updated infrageneric classification rationalising morphological groups but informed by molecular sequence data. Most significantly, Fleischmann et al. (2017a, 2018) proposed that compared to the Lowrie classification, Drosera subgenus Ergaleium should be expanded to include taxa previously placed within subgenus Drosera. In addition, the revised classification raises section Arcturia Planch. to subgenus Arcturia (Planch.) Schlauer, along with additional changes within subgenus Drosera (Fleischmann et al. 2017a, 2018).
As in previous studies utilising molecular data (see above), the most recent phylogenetic study of Drosera (Mohn et al. 2023) was based on sanger sequencing data, with chromosome characters mapped to a phylogeny that encompasses the broad Drosera clades with only a few Australian taxa represented. The molecular studies to date do not provide unequivocal resolution among the different and somewhat conflicting infrageneric taxonomies of Lowrie (2013a, 2013b, 2013c) and Fleischmann et al. (2017a). For example, Lowrie (2013a, 2013b, 2013c) recognise a narrow definition for subgenus Ergaleium based on morphological characters that includes five sections (Ergaleium Planch., Erythrorhiza (Planch.) Diels, Luniferae (Planch.) Lowrie, Macrantha Lowrie and Stolonifera DeBuhr) of tuber forming, summer aestivating taxa. Using phylogenetic datasets, Fleischmann et al. (2017a) recognises a broader subgenus Ergaleium, synonymising all tuberous taxa into an expanded section Ergaleium and placing other species groups previously placed within subgenus Drosera (sections Bryastrum Planch., Coelophylla Planch., Lasiocephala Planch. and Phycopsis Planch.) in subgenus Ergaleium. Furthermore, two unusual erect annual taxa that were variously placed within sections Ergaleium (Marchant and George 1982) and Annuerecta (Lowrie 2013a, 2013b, 2013c) were also synonymised with section Lasiocephala by Fleischmann et al. (2017a), changes that were accepted in subsequent publications (Nunn and Lowrie 2021). The relationships between lineages are particularly poorly resolved with the methods previously used to generate phylogenies; this is most evident for the species depauperate subgenera Arcturia (D. arcturi and D. murfetii; Australia and New Zealand) and Regiae (D. regia; South Africa) (Rivadavia et al. 2003; Mohn et al. 2023) that share several morphological similarities (Gibson 1999).
The Australian Drosera diversity includes numerous and diverse adaptations to persist in the variable and often arid environments that dominate a large proportion of the Australian continent. Prolific plantlet production arising from moisture-exposed leaves or light-exposed roots are common within sections Drosera, Phycopsis and Prolifera (Lowrie 2013a, 2013b, 2013c). Alternate survival and vegetative reproductive strategies have also become specialised within section Ergaleium; taxa within this section seasonally aestivate as tubers over summer, with many species also producing additional daughter tubers from lateral stolons or more rarely, from dropper roots arising from leaves (Conran 2008; Lowrie 2013a, 2013b, 2013c; Krueger et al. 2023a). A differing and rare (among angiosperms) clonal reproductive strategy is utilised within section Bryastrum; taxa within this clade reproduce by fragmentation, utilising highly modified leaves (gemmae) that, when struck by winter rains, dislodge from the parent plants and readily form daughter plantlets (Fig. 1m) (Karlsson and Pate 1992). As in many fire-adapted Australian taxa (Gill and Ingwersen 1976; Lamont and Downes 2011), some species within section Ergaleium, e.g. D. erythrorhiza and D. zonaria are also known to flower en masse following bushfire events (Dixon and Pate 1978; Lowrie 2013a, 2013b, 2013c). Short and predominantly annual life cycles are also common for taxa in arid, tropical (sections Arachnopus, Thelocalyx and two species within section Lasiocephala) and temperate (section Coelophylla) environments (Lowrie 2013a, 2013b, 2013c). Australian Drosera also have considerably higher variation in ploidy levels than taxa in other species diverse regions (e.g. South Africa and South America) (Mohn et al. 2023), potentially due to the long evolutionary history of the genus in the region.
Ours is the first broad-scale phylogenomic study to investigate the evolutionary relationships between these morphologically diverse Australian Drosera clades. This study was completed as part of the Bioplatforms Australia (see https://bioplatforms.com/) Genomics for Australian Plants (GAP) Phylogenomics Stage II initiative (see https://www.genomicsforaustralianplants.com/) and was also partially supported by an Australian Research Council (ARC) Discovery Project grant. Large genomic libraries, consisting of high copy plastid and low copy nuclear loci were recovered using the Angiosperms353 (A353; Johnson et al. 2018) and OzBaits (Waycott et al. 2021) probe kits. As part of this study, we aimed to (1) assess nuclear and plastid gene recovery for the Genomics for Australian Plants (GAP) protocol (see https://www.genomicsforaustralianplants.com/protocols/) using the A353 and OzBaits probe kits; (2) use these large genomic libraries to detect potential incongruence between plastid and nuclear phylogenies; and (3) compare phylogenomic tree topologies against previous infrageneric and species concepts. We intended to provide a framework phylogeny for Drosera in Australia through this study and this is the first in a series of detailed studies investigating the evolution of the genus in Australia.
Materials and methods
Taxon sampling
We included sequence data from 92 Drosera samples (Table 1) in collections held at the State Herbarium of South Australia (AD), the Queensland Herbarium (BRI) and the Beadle Herbarium (NE), supplemented with publicly available sequence data at GenBank (see https://www.ncbi.nlm.nih.gov/genbank/) and the Kew Tree of Life (see https://treeoflife.kew.org/tree-of-life) (Baker et al. 2022; Zuntini et al. 2024). Taxon sampling included representatives of all subgenera and sections occurring in Australia and selected taxa from other continents, following the classification system of Fleischmann et al. (2017a) and Mohn et al. (2023). These included type species acting as representatives for subgenus Arcturia (2 spp.), subgenus Drosera (~110 spp.), subgenus Ergaleium (~150 spp.) and subgenus Regiae Seine & Barthlott (1 sp.). Type species of the recently synonymised (Fleischmann et al. 2017a) sections Annuerecta Lowrie, Ergaleium, Erythrorhiza (Planch.) Diels, Stolonifera DeBuhr, Macrantha Lowrie and Luniferae Lowrie, as had been circumscribed by Lowrie (2013a, 2013b, 2013c), were included.
| Taxon | Subgenus | Section | Sample ID; A353 Library ID; OzBaits Library ID (GAP), GenBank (GB) or European Nucleotide Archive (ENA) Accession number | Collector code (herbarium Accession number); locality | |
|---|---|---|---|---|---|
| Aldrovanda vesiculosa L. | – | – | ERR7599885 (ENA) | ||
| Aldrovanda vesiculosa L. | – | – | NC_035416.1 (GB) | ||
| Dionaea muscipula J.Ellis | – | – | SRR2807634 (ENA) | ||
| Dionaea muscipula J.Ellis | – | – | KY679201.1 (GB) | ||
| Nepenthes madagascariensis Poir. | – | – | ERR3672062 (ENA) | ||
| Drosera aberrans (Lowrie & Carlquist) Lowrie & Conran | Ergaleium | Ergaleium | 378115; 382924; 382972 (GAP) | L.T. Williamson 142 (AD 289673) AU:SA | |
| Drosera adelae F.Muell. | Drosera | Prolifera | 376555; 380873; 380921 (GAP) | A. Ford 3588 & J. Holmes (AQ 559665) AU:Qld | |
| Drosera andersoniana W.Fitzg. ex Ewart & Jean White | Ergaleium | Ergaleium | 378103; 382912; 382960 (GAP) | A.P. Brown 116 (AD 224688) AU:WA | |
| Drosera arcturi Hook. | Arcturia | – | 376515; 380833; 380881 (GAP) | J.G. Conran 3971A (AD 289649) NZ | |
| Drosera auriculata Backh. ex Planch. | Ergaleium | Ergaleium | 378129; 382938; 382986 (GAP) | L.T. Williamson 53 (AD 286692) AU:SA | |
| Drosera banksii R.Br. ex DC. | Ergaleium | Lasiocephala | 376542; 380860; 380908 (GAP) | D.E. Murfet 7211 (AD 246630) AU:NT | |
| Drosera barbigera Planch. | Ergaleium | Bryastrum | 376517; 380835; 380883 (GAP) | J.G. Conran 4077 & L.T. Williamson (AD 286722) AU:WA | |
| Drosera binata Labill. | Ergaleium | Phycopsis | 376553; 380871; 380919 (GAP) | F.J. Nge 1402 & R. Khan (AD 286718) AU:Tas. | |
| Drosera brevicornis Lowrie | Ergaleium | Lasiocephala | 376543; 380861; 380909 (GAP) | D.E. Murfet 5583 & A. Lowrie (AD 206677) AU:NT | |
| Drosera bulbosa Hook. | Ergaleium | Ergaleium | 378116; 382925; 382973 (GAP) | J.G. Conran 4187 & L.T. Williamson (AD 286705) AU:WA | |
| Drosera burmanni Vahl | Drosera | Thelocalyx | 378101; 382910; 382958 (GAP) | D.E. Murfet 6868 (AD 238728) AU:NT | |
| Drosera buubugujin M.T.Mathieson | Drosera | Prolifera | 376556; 380874; 380922 (GAP) | S.L. Thompson 15127 & G. Luscombe & L. Brown (AQ 856868) AU:Qld | |
| Drosera collina (N.G.Marchant & Lowrie) Lowrie | Ergaleium | Ergaleium | 378117; 382926; 382974 (GAP) | L.T. Williamson 191 (AD 289672) AU:WA | |
| Drosera coomallo Lowrie & Conran | Ergaleium | Bryastrum | 376518; 380836; 380884 (GAP) | J.G. Conran 4078 & L.T. Williamson (AD 289653) AU:WA | |
| Drosera darwinensis Lowrie | Ergaleium | Lasiocephala | 376544; 380862; 380910 (GAP) | D.E. Murfet 6052 (AD 224091) AU:NT | |
| Drosera derbyensis Lowrie | Ergaleium | Lasiocephala | 376541; 380859; 380907 (GAP) | W.K. Harris 47 (AD 98223503Y) AU:WA | |
| Drosera dilatatopetiolaris K.Kondo | Ergaleium | Lasiocephala | 376546; 380864; 380912 (GAP) | D.E. Murfet 5601 & A. Lowrie (AD 206633) AU:NT | |
| Drosera drummondii Planch. | Ergaleium | Ergaleium | 378104; 382913; 382961 (GAP) | L.T. Williamson 154 (AD 289667) AU:WA | |
| Drosera eneabba N.G.Marchant & Lowrie | Ergaleium | Bryastrum | 376519; 380837; 380885 (GAP) | J.G. Conran 4084 & L.T. Williamson (AD 289652) AU:WA | |
| Drosera enodes N.G.Marchant & Lowrie | Ergaleium | Bryastrum | 376520; 380838; 380886 (GAP) | G. Cassis L17 H23 & B. Buirchell (AD 289661) AU:WA | |
| Drosera erythrorhiza Lindl. | Ergaleium | Ergaleium | 378114; 382923; 382971 (GAP) | J.G. Conran 4227 & L.T. Williamson (AD 286700) AU:WA | |
| Drosera finlaysoniana Wall. ex Arn. | Drosera | Arachnopus | 376512; 380830; 380878 (GAP) | D.E. Murfet 7316 (AD 249712) AU:SA | |
| Drosera fragrans Lowrie | Drosera | Arachnopus | 376513; 380831; 380879 (GAP) | D.E. Murfet 6511 & A. Lowrie (AD 228763) AU:NT | |
| Drosera geniculata (N.G.Marchant & Lowrie) Lowrie | Ergaleium | Ergaleium | 378105; 382914; 382962 (GAP) | T.A. Halliday 257 (AD 97611051) AU:WA | |
| Drosera gibsonii P.Mann | Ergaleium | Bryastrum | 376521; 380839; 380887 (GAP) | L.T. Williamson 183 (AD 289651) AU:WA | |
| Drosera gigantea Lindl. | Ergaleium | Ergaleium | 378106; 382915; 382963 (GAP) | L.T. Williamson 197 (AD 286695) AU:WA | |
| Drosera glanduligera Lehm. | Ergaleium | Coelophylla | 376536; 380854; 380902 (GAP) | L.T. Williamson 186 (AD 286691) AU:WA | |
| Drosera gracilis Hook.f. ex Planch. | Ergaleium | Ergaleium | 378128; 382937; 382985 (GAP) | D.E. Murfet 7630 (AD 265695) AU:SA | |
| Drosera gunniana (Planch.) Mig.F.Salas | Ergaleium | Ergaleium | 378130; 382939; 382987 (GAP) | L.T. Williamson 135 (AD 286693) AU:SA | |
| Drosera hamiltonii C.R.P.Andrews | Drosera | Stelogyne | 378100; 382909; 382957 (GAP) | J.G. Conran 3292 (AD 286706) AU:WA | |
| Drosera heterophylla Lindl. | Ergaleium | Ergaleium | 378107; 382916; 382964 (GAP) | J.G. Conran 4122 & L.T. Williamson (AD 286696) AU:WA | |
| Drosera hookeri R.P.Gibson, B.J.Conn & Conran | Ergaleium | Ergaleium | 378131; 382940; 382988 (GAP) | L.T. Williamson 147 (AD 286694) AU:SA | |
| Drosera huegelii var. phillmanniana Y.-A.Utz & R.P.Gibson | Ergaleium | Ergaleium | 378109; 382918; 382966 (GAP) | L.T. Williamson 188 (AD 286699) AU:WA | |
| Drosera humilis Planch. | Ergaleium | Ergaleium | 378144; 382953; 383001 (GAP) | J.G. Conran 4103 & L.T. Williamson (AD 286714) AU:WA | |
| Drosera hyperostigma N.G.Marchant & Lowrie | Ergaleium | Bryastrum | 376522; 380840; 380888 (GAP) | J.G. Conran 4178 & L.T. Williamson (AD 289654) AU:WA | |
| Drosera intricata Planch. | Ergaleium | Ergaleium | 378136; 382945; 382993 (GAP) | J.G. Conran 4186 & L.T. Williamson (AD 286716) AU:WA | |
| Drosera kenneallyi Lowrie | Ergaleium | Lasiocephala | 376548; 380866; 380914 (GAP) | D.E. Murfet 5633 & A. Lowrie (AD 206657) AU:NT | |
| Drosera lanata K.Kondo | Ergaleium | Lasiocephala | 376549; 380867; 380915 (GAP) | D.E. Murfet 4960 (AD 184805) AU:NT | |
| Drosera lasiantha Lowrie & Carlquist | Ergaleium | Bryastrum | 376523; 380841; 380889 (GAP) | G. Cassis L3 H7 & B. Buirchell (AD 289662) AU:WA | |
| Drosera macrantha Endl. | Ergaleium | Ergaleium | 378134; 382943; 382991 (GAP) | J.G. Conran 4074 & L.T. Williamson (AD 286707) AU:WA | |
| Drosera macrantha Endl. | Ergaleium | Ergaleium | 378139; 382948; 382996 (GAP) | J.G. Conran 4144 & L.T. Williamson (AD 289678) AU:WA | |
| Drosera macrantha subsp. planchonii (Hook.f. ex Planch.) N.G.Marchant | Ergaleium | Ergaleium | 378135; 382944; 382992 (GAP) | L.T. Williamson 116 (AD 286708) AU:SA | |
| Drosera macrophylla Lindl. | Ergaleium | Ergaleium | 378118; 382927; 382975 (GAP) | J.G. Conran 4147 & L.T. Williamson (AD 286704) AU:WA | |
| Drosera magna (N.G.Marchant & Lowrie) Lowrie | Ergaleium | Ergaleium | 378119; 382928; 382976 (GAP) | J.G. Conran 4082 & L.T. Williamson (AD 289676) AU:WA | |
| Drosera marchantii Endl. | Ergaleium | Ergaleium | 378108; 382917; 382965 (GAP) | L.T. Williamson 167 (AD 289666) AU:WA | |
| Drosera menziesii R.Br. ex DC. | Ergaleium | Ergaleium | 378102; 382911; 382959 (GAP) | J.G. Conran 4161 & L.T. Williamson (AD 286697) AU:WA | |
| Drosera miniata Diels | Ergaleium | Bryastrum | 376524; 380842; 380890 (GAP) | L.T. Williamson 153 (AD 289650) AU:WA | |
| Drosera modesta Diels | Ergaleium | Ergaleium | 378137; 382946; 382994 (GAP) | L.T. Williamson 185 (AD 286717) AU:WA | |
| Drosera monantha (Lowrie & Carlquist) Lowrie | Ergaleium | Ergaleium | 378120; 382929; 382977 (GAP) | A.A. Munir 5249 (AD 97342275) AU:WA | |
| Drosera nana Lowrie | Drosera | Arachnopus | 376514; 380832; 380880 (GAP) | D.E. Murfet 6798 & A. Lowrie (AD 236575) AU:NT | |
| Drosera neesii Lehm. | Ergaleium | Ergaleium | 378110; 382919; 382967 (GAP) | J.G. Conran 4102 & L.T. Williamson (AD 289669) AU:WA | |
| Drosera nitidula Planch. | Ergaleium | Bryastrum | 376525; 380843; 380891 (GAP) | J.G. Conran 4175 & L.T. Williamson (AD 286725) AU:WA | |
| Drosera ordensis Lowrie | Ergaleium | Lasiocephala | 376550; 380868; 380916 (GAP) | D.E. Murfet 5556 & A. Lowrie (AD 206614) AU:WA | |
| Drosera paleacea DC. | Ergaleium | Bryastrum | 376526; 380844; 380892 (GAP) | J.G. Conran 4198 & L.T. Williamson (AD 289655) AU:WA | |
| Drosera pallida Lindl. | Ergaleium | Ergaleium | 378138; 382947; 382995 (GAP) | L.T. Williamson 171 (AD 286709) AU:WA | |
| Drosera paradoxa Lowrie | Ergaleium | Lasiocephala | 376551; 380869; 380917 (GAP) | D.E. Murfet 5621 & A. Lowrie (AD 206651) AU:NT | |
| Drosera patens Lowrie & Conran | Ergaleium | Bryastrum | 376527; 380845; 380893 (GAP) | J.G. Conran 4231 & L.T. Williamson (AD 289656) AU:WA | |
| Drosera peltata Thunb. | Ergaleium | Ergaleium | 378127; 382936; 382984 (GAP) | J. Whinray 13272 (AD 246594) AU:Tas. | |
| Drosera petiolaris DC. | Ergaleium | Lasiocephala | 376540; 380858; 380906 (GAP) | D.E. Murfet 6115 & A. Lowrie (AD 225027) AU:Qld | |
| Drosera platypoda Turcz. | Ergaleium | Ergaleium | 378143; 382952; 383000 (GAP) | L.T. Williamson 179 (AD 286712) AU:WA | |
| Drosera platystigma Lehm. | Ergaleium | Bryastrum | 376528; 380846; 380894 (GAP) | J.G. Conran 4210 & L.T. Williamson (AD 289657) AU:WA | |
| Drosera praefolia Tepper | Ergaleium | Ergaleium | 378121; 382930; 382978 (GAP) | L.T. Williamson 129 (AD 289674) AU:SA | |
| Drosera prolifera C.T.White | Drosera | Prolifera | 376554; 380872; 380920 (GAP) | B. Gray 2066 (AQ 569880) AU:Qld | |
| Drosera pulchella Lehm. | Ergaleium | Bryastrum | 376529; 380847; 380895 (GAP) | J.G. Conran 4219 & L.T. Williamson (AD 286724) AU:WA | |
| Drosera purpurascens Schlotth. | Ergaleium | Ergaleium | 378145; 382954; 383002 (GAP) | L.T. Williamson 181 (AD 286710) AU:WA | |
| Drosera pycnoblasta Diels | Ergaleium | Bryastrum | 376530; 380848; 380896 (GAP) | J.G. Conran 4139 & L.T. Williamson (AD 289658) AU:WA | |
| Drosera pygmaea DC. | Ergaleium | Bryastrum | 376516; 380834; 380882 (GAP) | L.T. Williamson 68 & F.J. Nge & A. Thornhill (AD 286721) AU:SA | |
| Drosera radicans N.G.Marchant | Ergaleium | Ergaleium | 378111; 382920; 382968 (GAP) | J.G. Conran 4100 & L.T. Williamson (AD 289668) AU:WA | |
| Drosera ramellosa Lehm. | Ergaleium | Ergaleium | 378146; 382955; 383003 (GAP) | J.G. Conran 4134 & L.T. Williamson (AD 286713) AU:WA | |
| Drosera regia Stephens | Regiae | – | 378147; 382956; 383004 (GAP) | L.T. Williamson 201 (AD 289680) STH AFR | |
| Drosera rosulata Lehm. | Ergaleium | Ergaleium | 378122; 382931; 382979 (GAP) | L.T. Williamson 166 (AD 286702) AU:WA | |
| Drosera rotundifolia L. | Drosera | Drosera | 376537; 380855; 380903 (GAP) | R.D. Worthington 16787 (AD 99301134) USA:ME | |
| Drosera schizandra Diels | Drosera | Prolifera | 376557; 380875; 380923 (GAP) | R. Booth 2770 & R. Jensen (AQ 555995) AU:Qld | |
| Drosera schmutzii Lowrie & Conran | Ergaleium | Ergaleium | 378123; 382932; 382980 (GAP) | L.T. Williamson 84 & F.J. Nge & A. Thornhill (AD 289675) AU:SA | |
| Drosera scorpioides Planch. | Ergaleium | Bryastrum | 376531; 380849; 380897 (GAP) | J.G. Conran 4159 & L.T. Williamson (AD 286723) AU:WA | |
| Drosera spatulata Labill. | Drosera | Drosera | 376539; 380857; 380905 (GAP) | C. Schultz D2 & G. McGregor & J. Marshall (AD 286719) AU:Qld | |
| Drosera spatulata Labill. | Drosera | Drosera | 376559; 380877; 380925 (GAP) | J.G. Conran 3972A (AD 289665) NZ | |
| Drosera spilos N.G.Marchant & Lowrie | Ergaleium | Bryastrum | 376532; 380850; 380898 (GAP) | J.G. Conran 4119 & L.T. Williamson (AD 286720) AU:WA | |
| Drosera squamosa Benth. | Ergaleium | Ergaleium | 378124; 382933; 382981 (GAP) | L.T. Williamson 199 (AD 289671) AU:WA | |
| Drosera stelliflora Lowrie & Carlquist | Ergaleium | Bryastrum | 376533; 380851; 380899 (GAP) | J.G. Conran 4224 & L.T. Williamson (AD 289659) AU:WA | |
| Drosera stolonifera Endl. | Ergaleium | Ergaleium | 378141; 382950; 382998 (GAP) | J.G. Conran 4226 & L.T. Williamson (AD 286715) AU:WA | |
| Drosera stricticaulis (Diels) O.H.Sarg. | Ergaleium | Ergaleium | 378112; 382921; 382969 (GAP) | J.G. Conran 4135 & L.T. Williamson (AD 286698) AU:WA | |
| Drosera subtilis N.G.Marchant | Ergaleium | Lasiocephala | 376552; 380870; 380918 (GAP) | D.E. Murfet 7593 (AD 262960) AU:NT | |
| Drosera sulphurea Lehm. | Ergaleium | Ergaleium | 378113; 382922; 382970 (GAP) | G. Cassis L15 H19 & B. Buirchell (AD 289670) AU:WA | |
| Drosera thysanosepala Diels | Ergaleium | Ergaleium | 378140; 382949; 382997 (GAP) | J.G. Conran 4098 & L.T. Williamson (AD 289679) AU:WA | |
| Drosera trichocaulis (Diels) Lowrie & Conran | Ergaleium | Bryastrum | 376534; 380852; 380900 (GAP) | J.G. Conran 4211 & L.T. Williamson (AD 289660) AU:WA | |
| Drosera uniflora Willd. | Drosera | Psychophila | 376558; 380876; 380924 (GAP) | J.J. Bruhl 2244 & A. Plos (NE 86465) ARG | |
| Drosera verrucata Lowrie & Conran | Ergaleium | Bryastrum | 376535; 380853; 380901 (GAP) | G. Cassis L9 H13 & B. Buirchell (AD 289663) AU:WA | |
| Drosera whittakeri Planch. | Ergaleium | Ergaleium | 378125; 382934; 382982 (GAP) | L.T. Williamson 139 (AD 286701) AU:SA | |
| Drosera yilgarnensis R.P.Gibson & B.J.Conn | Ergaleium | Ergaleium | 378132; 382941; 382989 (GAP) | L. Haegi 951 (AD 97704672) AU:WA | |
| Drosera zigzagia Lowrie | Ergaleium | Ergaleium | 378133; 382942; 382990 (GAP) | D.E. Murfet 6315 & A. Lowrie (AD 222841) AU:WA | |
| Drosera zonaria Planch. | Ergaleium | Ergaleium | 378126; 382935; 382983 (GAP) | J.G. Conran 4152 & L.T. Williamson (AD 286703) AU:WA |
The classification system follows Fleischmann et al. (2017a). Collection locality abbreviations are: AUS, Australia (NT, Northern Territory; Qld, Queensland; SA, South Australia; Tas., Tasmania; WA, Western Australia); ARG, Argentina; NZ, New Zealand; STH AFR, South Africa; USA, United States of America (ME, Maine).
To fill sampling gaps, herbarium vouchers were made from cultivated D. regia Stephens and D. hamiltonii C.R.P.Andrews held in living collections at The University of Adelaide. Dionaea muscipula (SRR2807634), Aldrovanda vesiculosa (ERR7599885) and Nepenthes madagascariensis Poir. (ERR3672062) nuclear sequence data from the Kew Tree of Life (Baker et al. 2022; Zuntini et al. 2024) were included in our nuclear analyses as outgroups. Plastid sequence data for D. muscipula (NC_035417) and A. vesiculosa (NC_035416) (Nevill et al. 2019) from GenBank were included in our plastid analyses as outgroups.
All described taxa within Drosera subgenus Regiae (1 sp.), D. subgenus Drosera sections Prolifera (4 spp.), Stelogyne (1 sp.), D. subgenus Ergaleium sections Phycopsis Planch. (1 sp.) and Coelophylla Planch. (1 sp.) were sampled. Other species groups, i.e. D. subgenus Arcturia, D. subgenus Drosera sections Arachnopus and Psychophila Planch., D. subgenus Ergaleium sections Bryastrum, Ergaleium and Lasiocephala were not sampled exhaustively. Taxon sampling for this study represents ~50% of the species diversity in Australia; for a comprehensive sample list, see Table 1.
DNA extraction, library preparation and sequencing
Material was removed from herbarium specimens using forceps sterilised by immersing in a 6% sodium hypochlorite solution, followed by a RO water and 70% ethanol rinse. The forceps were physically dried with a Kimwipe (Kimtech Science) before progressing to the next sample. DNA extraction, library preparation and sequencing were performed at the Australian Genome Research Facility (AGRF, Melbourne, Vic., Australia) as part of the GAP initiative supported by Bioplatforms Australia (Sydney, NSW, Australia). Dried plant tissue (~20–30 mg) was ground using a TissueLyser II (Qiagen) with tungsten carbide beads for simultaneous disruption and homogenisation of the sample, as per the manufacturer’s instructions. Genomic DNA was extracted using the DNeasy Plant mini kit (Qiagen) as per the manufacturer’s instructions on a QIAcube Connect (Qiagen). DNA quality was assessed using 1% E-gel with Sybr Safe dye (ThermoFisher) to visualise fragment size range and concentrations were measured with a Quantifluor dsDNA assay (Promega). Libraries were prepared using the NEBNext Ultra II FS Library Prep Kit (New England Biolabs, Ipswich, MA, USA), following the manufacturer’s instructions with inserts on average having a length of ~350 bp. As part of this workflow DNA was enzymatically fragmented to the required size. Libraries prepared by AGRF were enriched using both the Angiosperms353 (A353; Johnson et al. 2018) and OzBaits (Waycott et al. 2021) probe kits. Pooled libraries (12–16 plex) were enriched using the A353 probe kit by hybridising at 65°C with the Arbor Biosciences MyBaits Expert Plant A353 v1 bait set with v5 chemistry (catalogue number 308108.v5) and Arbor Biosciences MyBaits custom OzBaits_NR set (catalogue number 300496R.V5) set with V5 chemistry and 64°C hybridisation following the manufacturer’s instructions.
Remaining genomic DNA was sent to the Advanced DNA, Identification and Forensic Facility at The University of Adelaide and additional libraries were prepared based on similar conditions to the GAP libraries but following the two-step library preparation protocol from Waycott et al. (2021). This was done to enrich ~60 chloroplast loci using the OzBaits_CP bait set. Libraries were pooled 16 plex (6 pools) and enriched with the OzBaits_CP v2.0 (Waycott et al. 2021) chloroplast probe kit (catalogue number 300196R.v5) at 65°C using V5 chemistry. Post-capture libraries were amplified with indexed primers and purified with 1× AMPure XP beads (Beckman Coulter).
To obtain additional plastid and ribosomal sequence coverage, a subsample of the 6 un-enriched pooled libraries was used for a genome skim (i.e. Zeng et al. 2018); 1 µL of library was amplified (8 cycles, 65°C annealing temperature) in a 25-µL reaction with indexed primers using the KAPA HiFi Hotstart ReadyMix (Roche). All libraries were purified with AMPure XP beads (Beckman Coulter) and quantified on a 4150 TapeStation using a High Sensitivity DNA screen tape (Agilent). Both plastid and genome skim libraries were pooled to equimolar concentrations and size-selected (300–700 bp) on a Pippin Prep (Sage Sciences) 1.5% agarose 250–1500-bp gel cassette. The resulting cleaned up product was subsequently sequenced on a NovaSeq. 6000 (Illumina Inc., San Diego, CA, USA) with v1.5 chemistry and 150-bp paired-end reads.
Data processing
Paired-end Illumina reads (150 bp in length) were imported into CLC Genomics Workbench (ver. 20.0.2, see https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-clc-genomics-workbench/) for demultiplexing and adapter trimming. Reads with a Phred score (Ewing et al. 1998) >13 (corresponding to a quality score cutoff of 0.05) were retained. To reduce computational processing time, individuals were randomly sampled to 2.5 million paired-end reads. Sampled reads were then de novo assembled with MEGAHIT (ver. 1.2.9, see https://github.com/voutcn/megahit; Li et al. 2015), using the ‘assemble’ command in CAPTUS bioinformatics pipeline (ver. 1.0.0, see https://github.com/edgardomortiz/Captus; Raza et al. 2023) with settings left as default with the recommended ‘–min_count 3’ and ‘–prune_level 3’ arguments.
We used the CAPTUS ‘extract’ function to extract target gene regions using custom nuclear and plastid references. A set of reference sequences of OzBaits gene targets was derived from sequences of 43 genera within the Caryophyllales that were sourced from the 1KP data set (Carpenter et al. 2019), Genbank (Drosera capensis genome: GCA_001925005; Butts et al. 2016) and the transcript sequences of Aldrovanda vesiculosa and Dionaea muscipula available at https://www.biozentrum.uni-wuerzburg.de/carnivorom/resources (Palfalvi et al. 2020). References for the A353 gene targets were sourced from the Kew Tree of Life Explorer (Baker et al. 2022), consisting of Aldrovanda vesiculosa (ERR3639225), Dionaea muscipula (SRR2807634), Drosera dichrosepala Turcz. (ERR3672058) and Nepenthes madagascariensis (ERR3672062). Plastid references were sourced from GenBank and included coding regions extracted from the plastid genome sequences of Aldrovanda vesiculosa (MK397911, NC_035416), Dionaea muscipula (MK397918, NC_035417), Drosera erythrorhiza (KY651214, NC_035241), Drosera indica L. (MK397919), Drosera regia (KY679199, NC_035415) and Drosera rotundifolia (KU168830, NC_029770). As parameters for the CAPTUS ‘extract’ function, we used a minimum score of ‘0.2’ and minimum identity of ‘80’ for the nuclear data, and a minimum score of ‘0.3’ and minimum identity of ‘90’ for the plastid data.
The CAPTUS ‘align’ command was used to generate multiple sequence alignments for the extracted nuclear and plastid loci using the ‘genes’ format. Contigs were aligned with MAFFT (ver. 7.520, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013) with the recommended ‘mafft_auto’ argument and the following paralog filtering settings: ‘–max_paralogs 3’, ‘–filter_method informed’ and ‘–tolerance 1’ (refer to the CAPTUS user manual for a full description of the settings for details, see https://edgardomortiz.github.io/captus.docs/). A maximum of three paralogs was included in the pre-filtered alignments based upon initial runs that indicated values above three led to the retention of short and highly divergent copies indicative of contamination. Alignments were trimmed in CAPTUS using the default ‘gappy’ trimming mode in ClipKIT (ver. 2.1.1, see https://github.com/JLSteenwyk/ClipKIT; Steenwyk et al. 2020). Subsequently, the Paragone (ver. 1.0.0, see https://github.com/chrisjackson-pellicle/ParaGone; Yang and Smith 2014; Morales-Briones et al. 2021) pipeline was used to refine alignment quality. Paragone removes sequencing and alignment errors, and rogue taxa from alignments using HmmCleaner (ver. 0.243280, see https://metacpan.org/dist/Bio-MUST-Apps-HmmCleaner; Di Franco et al. 2019), TreeShrink (ver. 1.3.9, see https://github.com/uym2/TreeShrink; Mai and Mirarab 2018) and TrimAl (ver. 1.5.0, see http://trimal.cgenomics.org/; Capella-Gutiérrez et al. 2009). Aligned loci represented by fewer than 20 samples were removed from downstream analyses. Nuclear OzBaits and A353 targeted loci were combined to create the nuclear dataset for use in the subsequent analyses. Any duplicate loci sequenced by both nuclear bait sets were represented only once (i.e. the longest aligned locus was retained) in the final data set.
Phylogenetic analyses
Concatenated nuclear and plastid datasets were each analysed separately with a maximum likelihood approach in IQ-TREE (ver. 2.2.0, see http://www.iqtree.org/; Nguyen et al. 2015; Chernomor et al. 2016), with partitioning for individual loci. The best nucleotide substitution model for each partition was found using ModelFinder (see http://www.iqtree.org/ModelFinder/), as implemented in IQ-TREE (MFP + MERGE) and like partitions were subsequently merged (Kalyaanamoorthy et al. 2017). Only the top 10% of partition merging schemes were examined by using the relaxed hierarchical clustering algorithm to save computational time (Lanfear et al. 2014). Topological support was assessed using 1000 ultrafast bootstrap replicates (UFBS; Minh et al. 2013) and the approximate likelihood ratio test (ALRT; Guindon et al. 2010), as implemented in IQ-TREE. We interpreted support values following Minh et al. (2013) and recommendations in the IQ-TREE manual (see https://iqtree.github.io/doc/iqtree-doc.pdf). Additionally, we used IQ-TREE to produce individual locus trees of the nuclear dataset as input for a coalescent-based analysis using wASTRAL–hybrid (ver. 1.4.2.3, see https://github.com/smirarab/ASTRAL) in Accurate Species Tree Estimator (ASTER, hereafter referred to as ASTRAL) using the ‘likelihood’ preset (i.e. the ALRT value is used to weight branch support using default values). This method reimplements ASTRAL-III and accounts for phylogenetic uncertainty by considering signals derived from branch length and branch support in gene trees (Zhang et al. 2018; Zhang and Mirarab 2022). While a general concern for phylogenomic analyses is the presence of undetected gene copies, species tree inference using ASTRAL is considered robust to the presence of paralogs (Yan et al. 2022). Nevertheless, we conducted a further analysis using ASTRAL-Pro (ASTRAL for paralogs and orthologs, see https://github.com/chaoszhang/A-pro; Zhang et al. 2020) that attempts to identify the most likely species tree given a set of paralog gene trees. Here, we used the ‘unfiltered’ alignments from the CAPTUS align output to infer paralog gene trees using IQ-TREE (analysis settings as above) as input to ASTRAL-Pro and analyses were conducted using default settings.
The concatenated and coalescent trees of the nuclear dataset were both analysed using Quartet Sampling (QS, see https://github.com/fephyfofum/quartetsampling; Pease et al. 2018). QS is a quartet-based method that provides a wealth of information on how well the data support the topology and when support is poor, can indicate which underlying biological factor may be contributing to support values. Branch support for the QS analyses was assessed by the associated Quartet Concordance (QC), Quartet Differential (QD) and Quartet Informativeness (QI) values that were described in Pease et al. (2018). Branches with a QC value ≥0.5 were considered concordant, indicating that half or more of all quartet trees are concordant with the focal branch. QD values indicate whether neither of the discordant topologies are favoured (high QD) or if there is a skew to one discordant topology (low QD). QD can therefore provide evidence as to whether discordance is due to incomplete lineage sorting (ILS) or introgression, with the former suggested by high QD and the latter by low QD (Pease et al. 2018; Paetzold et al. 2019). QI reports the proportion of the replicates that are informative for the quartet and can be used to distinguish lack of support (low QI) from conflict (high QI). Additionally, terminal nodes were assessed by the associated Quartet Fidelity (QF) values. QF reports the proportion of replicates that included the taxon and resulted in a concordant topology and therefore can identify ‘rogue taxa’ (Pease et al. 2018) that may otherwise decay support values.
Results
Dataset creation
Following assembly, 431 nuclear gene region alignments were recovered, including 343 A353 loci and 88 OzBaits loci. Prior to tree building, 39 loci were excluded from further analyses; these included alignments with fewer than 20 sequences (12 A353 loci) and loci overlapping between OzBaits and A353 (27 loci). The final nuclear dataset included 392 loci (331 A353 and 61 OzBaits loci) for Drosera and outgroups, with a concatenated length of ~259,000 bp, for a total of 95 terminals. Gene recovery for nuclear alignments was high, with a mean of ~229 loci represented for each sample. Across all samples, 50–380 nuclear loci (Supplementary Table S1) were represented with an ungapped sequence length of ~17,000–189,000 bp, with 62/92 samples having an ungapped length of ≥50% of the total alignment length (≥94,697 bp), excluding outgroups.
The final plastid dataset included 59 loci, with a concatenated length of ~41,000 bp across 80 terminals. Between 2 and 57 plastid loci were retained in the final dataset (Supplementary Table S1), with an average of ~35.7 loci represented for each sample. Samples with an ungapped length ≥50% of the total plastid alignment length (≥20,551 bp) were represented by 45/78 samples, excluding outgroups. Full recovery statistics for the nuclear and plastid datasets are available as Supplementary Table S1.
Phylogenetic analyses
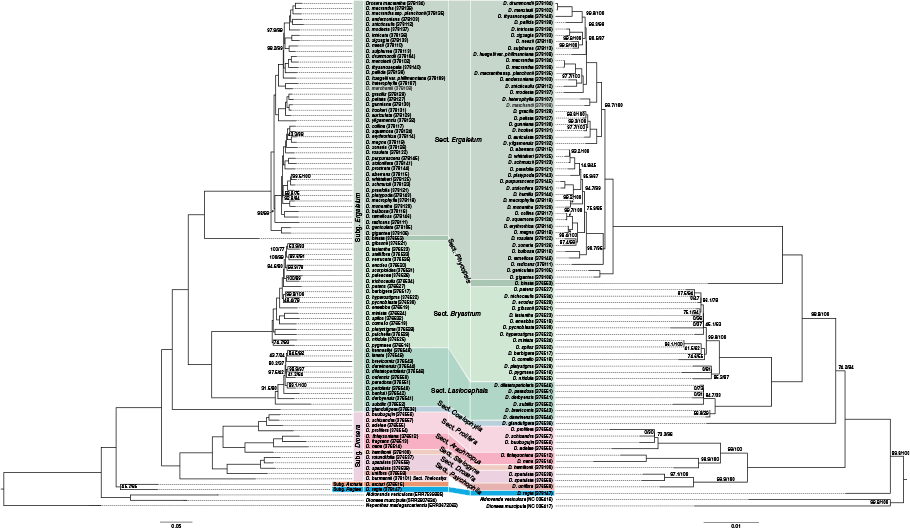
IQ-TREE analyses for each of the concatenated nuclear and plastid datasets recovered trees with largely congruent topologies and with well-supported relationships between the major clades (Fig. 2). The ASTRAL tree of the nuclear dataset (Supplementary Fig. S1) and the ASTRAL-Pro nuclear species tree topology generated from the paralog gene alignments (Supplementary Fig. S2) were also found to have well-supported relationships between major clades that are consistent with those found by the IQ-TREE analyses. Differences between the trees are confined to relationships that are poorly supported amongst analyses. We confine our discussion to the IQ-TREE and ASTRAL results. Tree topologies that are mostly consistent with the infrageneric classification of Fleischmann et al. (2017a) were recovered by our analyses, with all subgenera and sections resolved as monophyletic in both nuclear and plastid datasets (Fig. 2). Unlike previous studies by Rivadavia et al. (2003) and Fleischmann et al. (2017a), subgenera Arcturia (D. arcturi) and Regiae (D. regia) are resolved as sister in IQ-TREE and ASTRAL analyses of the nuclear dataset (Fig. 3), with weak support for this relationship from the IQ-TREE analysis (Fig. 2; ALRT 45.7 and UFBS 84) but high support in the ASTRAL tree (Supplementary Fig. S1; Local Posterior Probability (LPP) 0.99). Drosera arcturi was not included in the plastid dataset due to poor locus recovery (Supplementary Table S1). Subgenus Ergaleium was well supported in the nuclear tree but was unsupported in the plastid tree, with low support (ALRT 74.2 and UFBS 84, Fig. 2) for the node subtending section Coelophylla from the remaining subgenus Ergaleium. Some internal nodes within sections Lasiocephala and Bryastrum were moderately to poorly supported in the nuclear trees (Fig. 2, 3). In the plastid tree (Fig. 2), many relationships within these sections were very poorly supported with low branch lengths, including clades encompassing D. patens Lowrie & Conran to D. trichocaulis (Diels) Lowrie & Conran (section Bryastrum), D. platystigma Lehm. to D. nitidula Planch. (section Bryastrum), and D. dilatatopetiolaris K.Kondo to D. subtilis N.G.Marchant (section Lasiocephala). In all analyses, section Phycopsis (D. binata) was well resolved as sister to section Ergaleium and, apart from a few nodes, there was high support for most relationships within section Ergaleium in both the nuclear and plastid IQ-TREE analyses (Fig. 2).
Comparative nuclear (left) and plastid (right) phylogenies using IQ-TREE analysis. Ultrafast bootstrap support (UFBS) and approximate likelihood ratio test values (ALRT) shown adjacent to nodes where values are <100. Subgeneric and sectional classifications follow Fleischmann et al. (2017a).
Comparative IQ-TREE (left) and ASTRAL (right) phylogenies from recovered nuclear sequence data using Quartet Sampling. Phylogenies are presented as cladograms for readability. Internal nodes are coloured based on the associated Quartet Concordance (QC) and Quartet Differential (QD) values; nodes with <0.5 QC have the associated QC, QD and Quartet Informativeness (QI) values reported, respectively. Terminal nodes are coloured based on the associated Quartet Fidelity (QF) values.
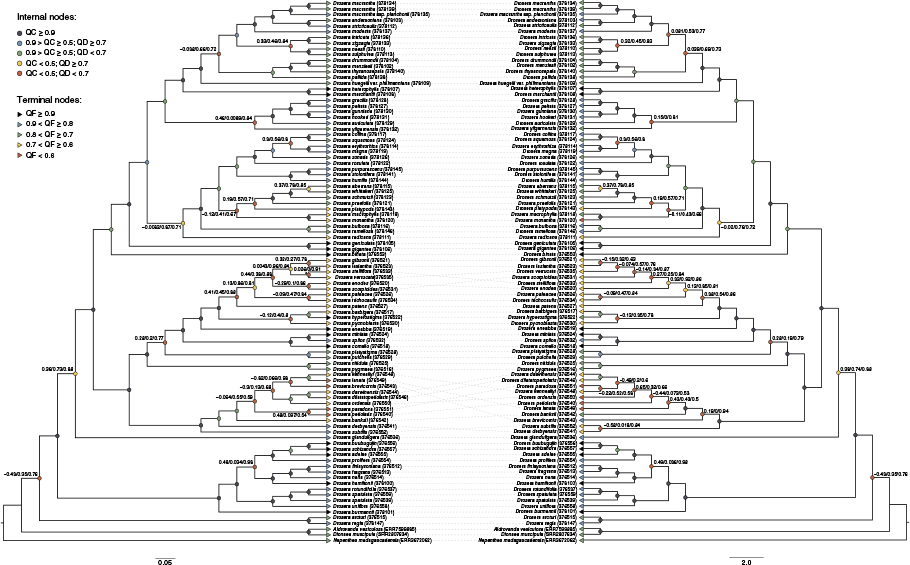
Concordance and discordance on the nuclear phylogenies analysed with QS analyses show a large degree of congruence between the IQ-TREE and ASTRAL analyses (Fig. 3). In some cases, discordant nodes in the QS analyses correspond to those highly supported by ALRT, UFBS or LPP (Fig. 2, Supplementary Fig. S1). In particular, the Drosera crown node has a very low QC value (−0.43) and a low QD value (0.35) (Fig. 3) that indicates that an alternate topology is favoured for this node. The node segregating section Coelophylla from the remainder of subgenus Ergaleium is also discordant (QC 0.36 and 0.38 on the IQ-TREE and ASTRAL trees respectively; Fig. 3). Interestingly, the sister relationship between subgenera Arcturia and Regiae was highly concordant in the QS analyses (Fig. 3), in contrast to the low support found by ALRT and UFBS (Fig. 2).
Both QS analyses showed low QC values for internal nodes within sections Bryastrum (10 IQ-TREE and ASTRAL nodes) and Lasiocephala (4 IQ-TREE and 7 ASTRAL nodes), and subgenus Drosera at the internal node bifurcating sections Arachnopus and Thelocalyx from section Prolifera. Comparatively few nodes with low QC values were found within section Ergaleium (8 IQ-TREE and 9 ASTRAL nodes). High discordance within clades corresponds to low QF scores found at terminal nodes, with lowest being in section Lasiocephala.
Discussion
Ours is the first study to successfully generate genomic based data sets from both nuclear and plastid genomes including taxa from all major Drosera subgenera and sections occurring in Australia (Supplementary Table S1), and most lineages globally. Phylogenetic hypotheses inferred from these data represent a significant milestone in our ability to understand the systematics and evolution of the major lineages and species present in Australia. As has been recovered in previous molecular studies, we identify that most of the evolutionary diversity within Drosera occurs in lineages referred to by Fleischmann et al. (2017a) as subgenera. We provide a rich data set that successfully creates an evolutionary framework to identify natural groupings, informing subgeneric classification and in future studies, trait associations. The phylogenetic hypotheses recovered with the data rich nuclear and plastid based analyses do not support the adoption of sections summarised by Lowrie (2013a, 2013b, 2013c) (i.e. Fig. 4) and are more aligned with those proposed by Fleischmann et al. (2017a) (Fig. 2). The outcomes of our analyses broadly align with previous molecular studies (Rivadavia et al. 2003; Brittnacher 2011; Fleischmann et al. 2017a; Mohn et al. 2023) and some morphological concepts (Lowrie 2013a, 2013b, 2013c; Lowrie et al. 2017a, 2017b) but with notable exceptions for some groups.
Comparative IQ-TREE (left) phylogeny recovered from nuclear sequence data with subgeneric and sectional classifications sensu Lowrie (2013a, 2013b, 2013c) (right). Ultrafast bootstrap support (UFBS) and approximate likelihood ratio test values (ALRT) are shown adjacent to nodes where values are <100.
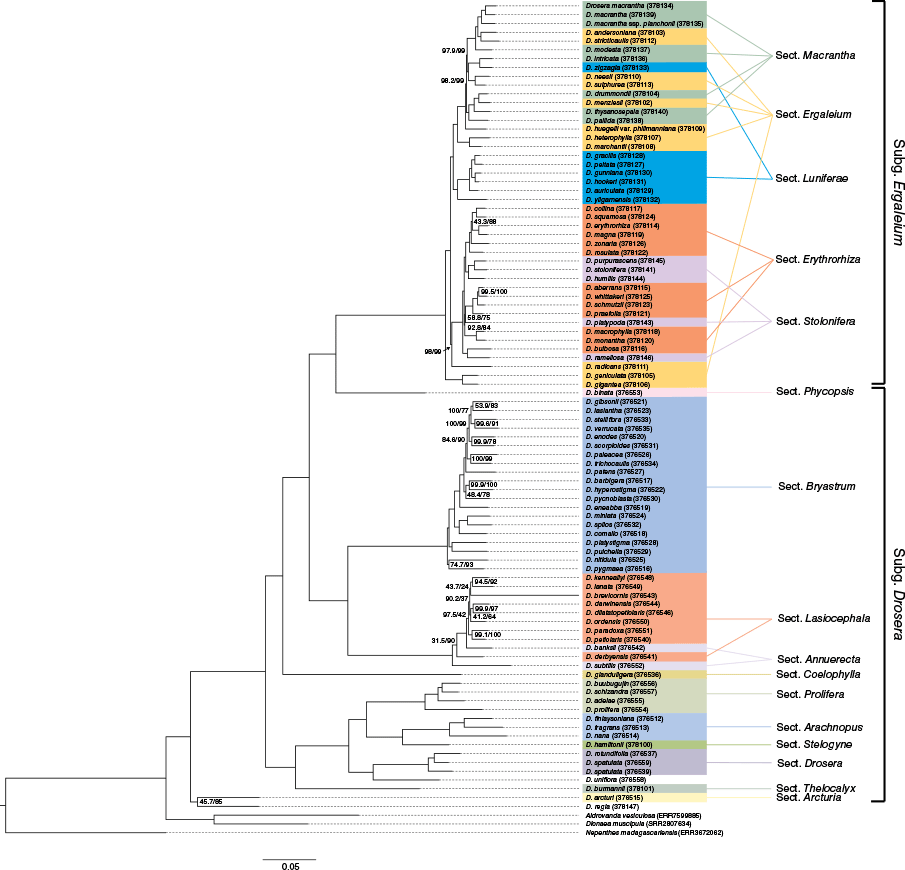
The subgenera Drosera and Ergaleium were resolved as two distinct, well supported clades, with six sections in subgenus Drosera and five sections in subgenus Ergaleium, with the caveat that subgenus Drosera sections Brasilianae and Ptycnostigma were not included in this analysis. Using subgeneric concepts outlined in Fleischmann et al. (2017a), the more detailed relationships within subgenus Ergaleium are resolved into five clades by our analyses, with section Coelophylla (D. glanduligera) as sister to the remaining four. We resolved section Phycopsis, comprising the unusual species D. binata, and section Ergaleium as sister, within a clade sister to sections Lasiocephala and Bryastrum (Fig. 2).
Within the northern Australian tropical taxa of section Lasiocephala, relatively poor plastid and nuclear tree support values (Fig. 2) and discordance between IQ-TREE and ASTRAL analyses (Fig. 3) limited interpretation of internal evolutionary relationships. Nevertheless, in contrast to previous morphological assignments placing D. banksii and D. subtilis variously within sections Annuerecta or Ergaleium (Marchant and George 1982; Lowrie 2013a, 2013b, 2013c), the results of our analyses show a clear affinity with section Lasiocephala for these taxa (Fig. 2–4, Supplementary Fig. S1). In both IQ-TREE and ASTRAL analyses of the nuclear dataset (Fig. 3), D. darwinensis and D. dilatatopetiolaris are consistently resolved as sister taxa; however, due the clearly discordant tree topologies within this clade (Fig. 3), the evolutionary relationships within section Lasiocephala are largely not discussed. Section Bryastrum was resolved as a monophyletic sister clade to section Lasiocephala within subgenus Ergaleium (Fig. 2); however, as in section Lasiocephala, nuclear tree topologies within this clade are discordant depending on the analysis method used (Fig. 3) and are not discussed in detail. This group will benefit from more widespread and detailed sampling to resolve relationships.
Higher classification systems applied to the summer aestivating, tuber-forming Drosera differ significantly between Lowrie (2013a, 2013b, 2013c) and Fleischmann et al. (2017a). Lowrie (2013a, 2013b, 2013c) segregate these taxa into sections Ergaleium, Erythrorhiza, Stolonifera, Luniferae and Macrantha based on morphological characters that are synonymised into an expanded section Ergaleium by Fleischmann et al. (2017a). These differing higher taxonomic concepts are mapped to phylogenetic trees in Fig. 2 (sensu Fleischmann et al. 2017a) and Fig. 4 (sensu Lowrie 2013a, 2013b, 2013c). Broadly, the climbing and tall tuber-forming taxa (Fig. 4; clade from D. macrantha to D. yilgarnensis) form a sister clade to the rosetted and erect, fan leaved taxa (Fig. 4; clade from D. collina to D. radicans). Two major subclades are nested within this tall and climbing tuberous Drosera clade. The D. peltata complex, excluding D. zigzagia, is recovered as a subclade that occurs in eastern Australia, New Zealand, South-east Asia, Japan, India (the clade extending from D. gracilis to D. auriculata) and Western Australia (D. yilgarnensis). The other subclade (extending from D. macrantha to D. marchantii) includes most tall and climbing taxa endemic to western Australia, except for D. gigantea, D. geniculata and D. radicans (Fig. 4). These latter taxa were resolved in unexpected phylogenetic positions and do not cluster with the other tall and climbing taxa in the upper clades of Fig. 4.
The clade including D. gigantea and D. geniculata is consistently resolved as sister to the remainder of tuberous Drosera (Fig. 2–4). This relationship is similar to the phylogeny resolved by Mohn et al. (2023) but differs from Rivadavia et al. (2003), who resolved D. peltata and D. gigantea as sister taxa. Similarly, the erect stemmed and self-supporting D. radicans is not affiliated with other tall or climbing taxa and is affiliated with the rosetted and fan-leaved taxa instead (Fig. 4; former sections Erythrorhiza and Stolonifera). This broad former section Erythrorhiza and Stolonifera clade is highly paraphyletic when applying previous taxonomic concepts (Fig. 4) and includes three major subclades, with each subclade including novel relationships between paraphyletic taxa formerly assigned to sections Erythrorhiza and Stolonifera. Our analyses clearly do not support the former segregation of tuberous Drosera into five morphologically defined sections proposed by Lowrie (2013a, 2013b, 2013c) and Lowrie et al. (2017a). These novel paraphyletic relationships within the tuber-forming clade are currently under review (L. T. Williamson et al., unpubl. data) and will be discussed in a future publication with denser sampling.
As previously noted, the classification schemes of Lowrie (2013a, 2013b, 2013c) and Lowrie et al. (2017a) identified several monophyletic species groups; however, many others are not supported by nuclear or plastid DNA based phylogenetic outputs (Fig. 4). Incongruence with our tree topologies and concepts by Lowrie (2013a, 2013b, 2013c) are mainly restricted to within-group species relationships among the tuberous Drosera and the recently synonymised section Annuerecta (Fig. 4). By contrast, the updated higher classification system of Fleischmann et al. (2017a) is most consistent with our phylogenies.
The determination that subgenera Arcturia (D. arcturi) and Regiae (D. regia) were resolved as sisters in the nuclear dataset is of particular interest (Fig. 2); however, there was an unfortunate lack of plastid data available for equivalent comparison. Support for alternate relationships between subgenus Regiae and the remainder of currently circumscribed Drosera (Fig. 3) is not a new finding. Chrtek and Slavíková (1996) proposed that D. regia should be recognised in a new genus, Freatulina (Stephens) Chrtek & Slavíková, based on strongly differentiated pollen characters, whereas Gibson (1999) has noted a series of morphological similarities between D. regia and the robust form of D. arcturi from Tasmania. Given the additional data availability in this study, the potentially novel sister relationship between subgenera Arcturia and Regiae, the lack of sequence data for D. murfetii within section Arcturia (Lowrie and Conran 2014) and improved morphological and biological data sets, relationships between Drosera s.l. and this species depauperate sister clade clearly require revision.
Biogeographic patterns within Australian Drosera
Patterns of disjunct sister clades occurring in South Africa (D. regia), south-eastern Australia (D. arcturi and D. murfetii) and New Zealand (D. arcturi) appear similar to other prominent Gondwanan Southern Hemisphere angiosperm families, e.g. Proteaceae (tribes Petrophileae and Persoonieae) (Barker et al. 2007) and Myrtaceae (tribes Myrteae and Syzygieae) (Thornhill et al. 2015). However recent molecular phylogenies (Thornhill et al. 2015; Lamont et al. 2024) highlight complex biogeographic histories, including both vicariance and intercontinental long distance dispersal events for these families. As support values are low for the sister relationship between D. arcturi and D. regia in the nuclear tree (Fig. 2), plastid sequence data were not recovered for D. arcturi (Fig. 2), taxon sampling is incomplete for subgenus Arcturia and molecular clock methods are not applied to our analyses, we interpret these results only as moderate support for an ancient Gondwanan relationship between subgenera Arcturia and Regiae, with a great deal of additional work remaining.
The highest levels of diversity and endemism at both sectional and species levels of Australian Drosera occur in the Southwest Australian Floristic Region (SWAFR), in part due to the combination of long-term, stable but heterogeneous habitats occurring on ancient, highly infertile soils (Groom and Lamont 2015; Brundrett 2021), with sections Bryastrum and Ergaleium representing most of the species diversity in this region. The single representative of section Bryastrum from eastern Australia and New Zealand represented in our analyses (D. pygmaea from South Australia) is sister to D. nitidula in the SWAFR in our nuclear phylogenies (Fig. 2, 3), and in a polytomy with D. nitidula and D. platystigma in the plastid phylogeny (Fig. 2). There is a second region of Drosera diversity for sections Lasiocephala and Arachnopus across the seasonally dry monsoonal tropics of northern Australia, particularly in the Kimberley regions, with section Prolifera confined to wetter regions of the wet tropics of far north Queensland (Lowrie 2013a, 2013b, 2013c; Nunn and Bourke 2017). The close molecular affinity between sections Arachnopus, Stelogyne and Prolifera first reported by Rivadavia et al. (2003) is supported in our analyses (Fig. 2); these taxa occur respectively in northern and central Australia, the SWAFR and in the wet tropics of Queensland but are poorly sampled in our analyses (section Arachnopus) or species depauperate (sections Prolifera and Stelogyne). Within section Prolifera, a close affinity between D. adelae and D. schizandra has been implied by phytochemical analyses (Schlauer et al. 2019), aligning with Fig. 2 and 3 that show a close affiliation between D. adelae, D. buubugujin and D. schizandra, with D. prolifera more distantly related. There is no overlap in the modern distribution of each species (Nunn and Bourke 2017; Mathieson and Thompson 2020) and despite D. schizandra and D. prolifera hybrids (D. × ‘Andromeda’) existing in cultivation, our QS analyses find no evidence of ILS or introgression between section Prolifera taxa (Fig. 3). These factors support a hypothesis of extended geographic isolation between section Prolifera taxa within the wet tropical region, with limited opportunity for introgression between species.
The dryland growing members of section Ergaleium are most diverse in the Mediterranean climatic regions of southern Australia, especially in the SWAFR, they (and Drosera species generally) are largely absent from chenopod-dominated vegetation types across this region (Specht and Specht 1999), although some taxa in the section extend to New Zealand and Asia (Lowrie et al. 2017a, 2017b). Our analyses support three major east–west disjunctions across the Nullarbor Plain within section Erglaeium. A close sister relationship is resolved by our analyses between D. macrantha ssp. planchoni in South Australia and D. macrantha in the SWAFR (Fig. 3). As D. macrantha ssp. planchonii occurs in both south-eastern Australia and the arid eastern extent of the SWAFR (Lowrie 2013a, 2013b, 2013c), this close trans-Nullarbor affiliation is possibly due to a comparatively recent dispersal event from the SWAFR to south-eastern Australia; however, denser sampling is required to further resolve the D. macrantha complex. The D. whittakeri complex (Fig. 3; clade extending from D. whittakeri to D. praefolia) represents a second radiation of section Ergaleium in south-east Australia; the closest relatives to that complex in our analyses are D. macrophylla, D. monantha and D. platypoda from the SWAFR but with a comparatively large molecular divergence, low support values and discordance for internal nodes (Fig. 2, 3). A third, well-supported radiation within the predominantly eastern Australian D. peltata complex (Fig. 3; clade extending from D. gracilis to D. auriculata) is resolved as a distant sister clade to D. yilgarnensis (Fig. 2, 3), a species endemic to the SWAFR correctly considered by Lowrie (2013a, 2013b, 2013c) and Lowrie et al. (2017a) to have a close affiliation with the D. peltata complex. This relationship potentially supports a model of ancient dispersal or vicariance for the broad D. peltata clade; however, additional sampling of D. bicolor in the SWAFR and D. lunata from eastern Australia and overseas is required to further resolve relationships within this complex. Most subgenus Ergaleium species are confined to either the eastern or western sides of the Nullarbor Plain, however, D. binata Labill., D. glanduligera Lehm., D. pygmaea DC. and D. stricticaulis (Diels) O.H.Sarg. (Conran and Lowrie 2007; Lowrie 2013a, 2013b, 2013c) also extend across the east–west continental divide. The degree of molecular divergence between populations across this significant biogeographic barrier (Crisp and Cook 2007) remains uncharacterised here for these taxa; however, duplicate sampling across the Nullarbor Plain has been conducted for many of these species (L. T. Williamson et al., unpubl. data) and will be discussed in a future publication.
Potential drivers of phylogenetic discordance
Despite differences in support between datasets, presumably reflecting differences in alignment length and evolutionary rate between these genomic compartments (Rokas and Carroll 2005; Asar et al. 2024), topologies for the nuclear and plastid trees are broadly congruent. Support values within sections Bryastrum and Lasiocephala are, however, consistently low in both nuclear and plastid trees (Fig. 2, 3). Similar patterns of poor within-clade support and discordance have been documented in other angiosperm groups; these are typically driven by ancient (Lin et al. 2019; Zhou et al. 2022) or modern (Scharmann et al. 2021) introgression events, ILS (Cai et al. 2021; McLay et al. 2023) and gene tree estimation errors (Cai et al. 2021).
The comparatively poor support values and inconsistent topologies resolved by IQ-TREE and ASTRAL analyses within subgenus Ergaleium sections Bryastrum and Lasiocephala may be driven by ILS (section Bryastrum) and introgression (sections Bryastrum and Lasiocephala) (Fig. 3). The pattern of introgression implied by low QC and QD values (Fig. 3) within section Lasiocephala is supported by common field observations of hybridisation between natural populations (Lowrie 1991). Introgression zones where D. stipularis Baleeiro, R.W.Jobson & R.L.Barrett and D. petiolaris R.Br. ex DC. hybridise are known in the wild (B. Ng, pers. comm.), and numerous other section Lasiocephala hybrids have been documented where preferred habitats and soil types co-occur (Lowrie 1991). Widespread hybridisation between section Lasiocephala taxa is also reported by carnivorous plant cultivators, with 30 known hybrids listed by International Carnivorous Plant Society (2024) in private collections. Although further study is needed, this potentially represents an example of introgression playing a major role in the evolution of a carnivorous plant genus, similar to Nepenthes (Nauheimer et al. 2019; Scharmann et al. 2021) and Sarracenia (Baldwin et al. 2023). Unlike the widespread introgression within Nepenthes, potential introgression appears to be more common in two sections in our analyses and within section Drosera, as reported by other authors (Wood 1955; Hoshi et al. 1994; Schnell 1995; Nakano et al. 2004; Pearman 2004; Schlauer and Fleischmann 2016).
Taxa within section Bryastrum also hybridise in both natural conditions and cultivation (Lowrie 2013a, 2013b, 2013c; International Carnivorous Plant Society 2024), with hybrid populations potentially able to persist indefinitely by clonal gemmae reproduction. Interpreting relationships among section Bryastrum taxa is limited by the same issues present for section Lasiocephala, namely poorly supported relationships (Fig. 2) reflecting extensive gene tree conflict underlying the species tree and the plastid topology (Fig. 3). These observed patterns of poor support and discordance within sections Bryastrum and Lasiocephala are confounding factors that require additional analyses (e.g. networks) and denser (population-level) sampling for resolution.
Conclusions
Our study was conducted as a part of the Bioplatforms Australia Genomics for Australian Plants Stage II initiative and successfully recovered large nuclear and plastid genomic libraries that were used to reconstruct evolutionary relationships between Drosera species in Australia.
Strong support for subgeneric and sectional classifications within Drosera was recovered with the exception of D. regia and D. arcturi, in which species were resolved as sister to the remaining Drosera species in some analyses. Incongruence, poor support values and discordance were mostly restricted to two sister sections that are known to hybridise with closely affiliated species in wild populations, whereas other clades were resolved with higher support values and less discordance. Current and future work will include more detailed taxonomic and geographic sampling of Drosera in Australia to better reflect the distributions of wide-ranging species and enabling additional analyses.
Our study is the first to apply phylogenomic methods to Drosera and utilises widely adopted target capture probe sets used by other authors to resolve relationships between all angiosperms (Zuntini et al. 2024), multiple genera (Nge et al. 2021) and within species (van Dijk et al. 2023). Data generated as part of this study provide a robust foundation for further phylogenomic studies, particularly for Australian clades that were under-sampled in this study (e.g. section Arachnopus) and extra-Australian clades (e.g. sections Brasilianae, Drosera and Ptycnostigma) not included in our dataset.
Data availability
GAP, GenBank and PAFTOL Accession numbers for the data used in this study are listed in Table 1. The final datasets generated and analysed trees produced in the current study are available on FigShare repository (see https://figshare.com/s/00e0fda166d6064360e1).
Conflicts of interest
M. Waycott is an Associate Editor for Australian Systematic Botany. Despite this relationship, she took no part in the review and acceptance of this manuscript, in line with the publishing policy. The authors declare that they have no further conflicts.
Declaration of funding
Cofunding for generation of the nuclear data was provided by Bioplatforms Australia (see https://bioplatforms.com/) as part of Genomics for Australian Plants Framework Initiative (GAP) Stage II (see https://www.genomicsforaustralianplants.com/). Additional cofunding for nuclear and plastid data was provided by an Australian Research Council (ARC) Discovery Project (DP190101676) awarded in 2019, of which M. Waycott and J. G. Conran are chief investigators and E. Biffin is a partner investigator.
Acknowledgements
We are thankful to Andrew McDougall, Andrew Thornhill, Bevan Buirchell, Cameron Schulz, Francis Nge, Gerry Cassis, Glenn McGregor, Jeremy Bruhl, Jonathan Marshall and Mike Mathieson for assistance in securing material for sequencing. Ainsley Calladine provided support in data management. We thank directors, collection managers and staff at AD, BRI and NE for granting access and permission for destructive sampling of herbarium Accession numbers.
References
Albert VA, Williams SE, Chase MW (1992) Carnivorous plants: phylogeny and structural evolution. Science 257, 1491-1495.
| Crossref | Google Scholar | PubMed |
Anderson B, Midgley JJ (2003) Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102, 221-224.
| Crossref | Google Scholar |
Asar Y, Sauquet H, Ho SYW (2024) Evolutionary rates of nuclear and organellar genomes are linked in land plants. BioRxiv 2024, 2024.08.05.606707 [Preprint, published 21 October 2024].
| Crossref | Google Scholar |
Baker WJ, Bailey P, Barber V, Barker A, Bellot S, Bishop D, Botigué LR, Brewer G, Carruthers T, Clarkson JJ, Cook J, Cowan RS, Dodsworth S, Epitawalage N, Françoso E, Gallego B, Johnson MG, Kim JT, Leempoel K, Maurin O, McGinnie C, Pokorny L, Roy S, Stone M, Toledo E, Wickett NJ, Zuntini AR, Eiserhardt WL, Kersey PJ, Leitch IJ, Forest F (2022) A comprehensive phylogenomic platform for exploring the Angiosperm Tree of Life. Systematic Biology 71, 301-319.
| Crossref | Google Scholar | PubMed |
Baldwin E, McNair M, Leebens-Mack J (2023) Rampant chloroplast capture in Sarracenia revealed by plastome phylogeny. Frontiers in Plant Science 14, 1237749.
| Crossref | Google Scholar | PubMed |
Baleeiro P, Jobson R, Barrett R (2020) Drosera stipularis, a new species for the D. petiolaris complex from Cape York Peninsula, Queensland. Telopea 23, 35-40.
| Crossref | Google Scholar |
Barker N, Weston P, Rutschmann F, Sauquet H (2007) Molecular dating of the ‘Gondwanan’ plant family Proteaceae is only partly congruent with the timing of the break-up of Gondwana. Journal of Biogeography 34, 2012-2027.
| Crossref | Google Scholar |
Brittnacher J (2011) Drosera phylogeny. In ‘International Carnivorous Plant Society’. (ICPS) Available at https://www.carnivorousplants.org/cp/evolution/DroseraPhylogeny [Verified 2 July 2025]
Brundrett M (2021) One biodiversity hotspot to rule them all: southwestern Australia – an extraordinary evolutionary centre for plant functional and taxonomic diversity. Journal of the Royal Society of Western Australia 104, 91-122.
| Crossref | Google Scholar |
Butts CT, Bierma JC, Martin RW (2016) Novel proteases from the genome of the carnivorous plant Drosera capensis: structural prediction and comparative analysis. Proteins 84, 1517-1533.
| Crossref | Google Scholar | PubMed |
Cai L, Xi Z, Lemmon EM, Lemmon AR, Mast A, Buddenhagen CE, Liu L, Davis CC (2021) The perfect storm: gene tree estimation error, incomplete lineage sorting, and ancient gene flow explain the most recalcitrant ancient angiosperm clade, Malpighiales. Systematic Biology 70, 491-507.
| Crossref | Google Scholar | PubMed |
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972-1973.
| Crossref | Google Scholar | PubMed |
Carpenter EJ, Matasci N, Ayyampalayam S, Wu S, Sun J, Yu J, Vieira FRJ, Bowler C, Dorrell RG, Gitzendanner MA, Li L, Du W, Ullrich KK, Wickett NJ, Barkmann TJ, Barker MS, Leebens-Mack JH, Wong GK-S (2019) Access to RNA-sequencing data from 1,173 plant species: the 1000 Plant transcriptomes initiative (1KP). GigaScience 8, giz126.
| Crossref | Google Scholar | PubMed |
Chernomor O, von Haeseler A, Minh BQ (2016) Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65, 997-1008.
| Crossref | Google Scholar | PubMed |
Chrtek JA, Slavíková Z (1996) Comments on the families Drosophyllaceae and Droseraceae. Journal of the National Museum (Prague), Natural History Series 165, 139-141.
| Google Scholar |
Conran JG (2008) Aestivation organ structure in Drosera subgen. Ergaleium (Droseraceae): corms or tubers; roots or shoots? Australian Journal of Botany 56, 144-152.
| Crossref | Google Scholar |
Conran JG, Lowrie A (2007) The biogeography of Drosera stricticaulis (Droseraceae) in Australia: a disjunct ‘island’ refugee? Transactions of the Royal Society of South Australia 132, 142-151.
| Crossref | Google Scholar |
Crisp MD, Cook LG (2007) A congruent molecular signature of vicariance across multiple plant lineages. Molecular Phylogenetics and Evolution 43, 1106-1117.
| Crossref | Google Scholar | PubMed |
DeBuhr L (1977) Sectional reclassification of Drosera subgenus Ergaleium (Droseraceae). Australian Journal of Botany 25, 209-218.
| Crossref | Google Scholar |
de Salas MF (2018) Drosera gunniana comb. et stat. nov., a species in the Drosera peltata (Droseraceae) complex. Muelleria 36, 97-106.
| Crossref | Google Scholar |
Di Franco A, Poujol R, Baurain D, Philippe H (2019) Evaluating the usefulness of alignment filtering methods to reduce the impact of errors on evolutionary inferences. BMC Evolutionary Biology 19, 21.
| Crossref | Google Scholar | PubMed |
Dixon KW, Pate JS (1978) Phenology, morphology and reproductive biology of the tuberous sundew, Drosera erythrorhiza Lindl. Australian Journal of Botany 26, 441-454.
| Crossref | Google Scholar |
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Research 8, 175-185.
| Crossref | Google Scholar | PubMed |
Fleck SJ, Jobson RW (2023) Molecular phylogenomics reveals the deep evolutionary history of carnivory across land plants. Plants 12, 3356.
| Crossref | Google Scholar | PubMed |
Fleischmann A, Gonella PM, Rivadavia F (2018) A new sectional name for the Brazilian tetraploid clade of Drosera subgenus Drosera. Carnivorous Plant Newsletter 47, 4-9.
| Crossref | Google Scholar |
Gibson R (1999) Drosera arcturi in Tasmania and a comparison with Drosera regia. Carnivorous Plant Newsletter 28, 76-80.
| Crossref | Google Scholar |
Gill AM, Ingwersen F (1976) Growth of Xanthorrhoea australis R.Br. in relation to fire. Journal of Applied Ecology 13, 195-203.
| Crossref | Google Scholar |
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307-321.
| Crossref | Google Scholar |
Hoshi Y, Hizume M, Kondo K (1994) Genomic in situ hybridization to improve a hypothesis on natural-hybrid origin of the hexaploid Drosera spathulata ‘Kansai type’. La Kromosomo II 75, 2619-2623.
| Google Scholar |
International Carnivorous Plant Society (2024) Drosera hybrids. In ‘International Carnivorous Plant Society’. (ICPS) Available at https://www.carnivorousplants.org/cp/evolution/DroseraHybrids [Verified 2 July 2025]
Johnson MG, Pokorny L, Dodsworth S, Botigué LR, Cowan RS, Devault A, Eiserhardt WL, Epitawalage N, Forest F, Kim JT, Leebens-Mack JH, Leitch IJ, Maurin O, Soltis DE, Soltis PS, Wong GK-s, Baker WJ, Wickett NJ (2018) A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68, 594-606.
| Crossref | Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Karlsson PS, Pate JS (1992) Resource allocation to asexual gemma production and sexual reproduction in south-western Australian pygmy and micro stilt-form species of sundew (Drosera spp. Droseraceae). Australian Journal of Botany 40, 353-364.
| Crossref | Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Krueger T (2022) New Australian Drosera taxa published since Allen Lowrie’s magnum opus. Carnivorous Plant Newsletter 50, 41-47.
| Crossref | Google Scholar |
Krueger T, Fleischmann A (2020) When three become two: Drosera coalara links Drosera citrina with Drosera nivea. Carnivorous Plant Newsletter 49, 6-16.
| Crossref | Google Scholar |
Krueger T, Fleischmann A (2021) A new species of Drosera section Arachnopus (Droseraceae) from the western Kimberley, Australia, and amendments to the range and circumscription of Drosera finlaysoniana. Phytotaxa 501, 56-84.
| Crossref | Google Scholar |
Krueger T, Robinson A, Bourke G, Fleischmann A (2023a) Small leaves, big diversity: citizen science and taxonomic revision triples species number in the carnivorous Drosera microphylla complex (D. section Ergaleium, Droseraceae). Biology 12, 141.
| Crossref | Google Scholar |
Krueger T, Cross A, Rangers D, Fleischmann A (2023b) Drosera maanyaa-gooljoo, a new species of Drosera section Arachnopus (Droseraceae) from the Buccaneer Archipelago and Yampi Peninsula, northwest Kimberley region, Western Australia. Phytotaxa 618, 31-46.
| Crossref | Google Scholar |
Lamont BB, Downes KS (2011) Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecology 212, 2111-2125.
| Crossref | Google Scholar |
Lamont BB, He T, Milne LA, Cowling RM (2024) Out of Africa: linked continents, overland migration and differential survival explain abundance of Proteaceae in Australia. Perspectives in Plant Ecology, Evolution and Systematics 62, 125778.
| Crossref | Google Scholar |
Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Ecology and Evolution 14, 82.
| Crossref | Google Scholar | PubMed |
Li D, Liu C-M, Luo R, Sadakane K, Lam T-W (2015) MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674-1676.
| Crossref | Google Scholar | PubMed |
Lin H-Y, Hao Y-J, Li J-H, Fu C-X, Soltis PS, Soltis DE, Zhao Y-P (2019) Phylogenomic conflict resulting from ancient introgression following species diversification in Stewartia s.l. (Theaceae). Molecular Phylogenetics and Evolution 135, 1-11.
| Crossref | Google Scholar | PubMed |
Lowrie A (1991) A field trip to Darwin. Carnivorous Plant Newsletter 20, 114-123.
| Crossref | Google Scholar |
Lowrie A (2005) A taxonomic revision of Drosera section Stolonifera. Nuytsia 15, 355-393.
| Crossref | Google Scholar |
Lowrie A, Conran JG (2014) Drosera murfetii (Droseraceae): a new species from Tasmania, Australia. Journal of the Adelaide Botanic Gardens 27, 7-21.
| Google Scholar |
Mai U, Mirarab S (2018) TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19, 272.
| Crossref | Google Scholar | PubMed |
Mathieson MT, Thompson SL (2020) Drosera buubugujin M.T.Mathieson (Droseraceae, Drosera section Prolifera C.T.White), a spectacular new species of sundew from the Cape York Peninsula bioregion. Austrobaileya 10, 549-557.
| Crossref | Google Scholar |
McLay TGB, Fowler RM, Fahey PS, Murphy DJ, Udovicic F, Cantrill DJ, Bayly MJ (2023) Phylogenomics reveals extreme gene tree discordance in a lineage of dominant trees: hybridization, introgression, and incomplete lineage sorting blur deep evolutionary relationships despite clear species groupings in Eucalyptus subgenus Eudesmia. Molecular Phylogenetics and Evolution 187, 107869.
| Crossref | Google Scholar | PubMed |
Minh BQ, Nguyen MAT, von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30, 1188-1195.
| Crossref | Google Scholar | PubMed |
Mohn RA, Zenil-Ferguson R, Krueger TA, Fleischmann AS, Cross AT, Yang Y (2023) Dramatic difference in rate of chromosome number evolution among sundew (Drosera L., Droseraceae) lineages. Evolution 77, 2314-2325.
| Crossref | Google Scholar | PubMed |
Morales-Briones DF, Gehrke B, Huang C-H, Liston A, Ma H, Marx HE, Tank DC, Yang Y (2021) Analysis of paralogs in target enrichment data pinpoints multiple ancient polyploidy events in Alchemilla s.l. (Rosaceae). Systematic Biology 71, 190-207.
| Crossref | Google Scholar |
Nakano M, Kinoshita E, Ueda K (2004) Life history traits and coexistence of an amphidiploid, Drosera tokaiensis, and its parental species, D. rotundifolia and D. spatulata (Droseraceae). Plant Species Biology 19, 59-72.
| Crossref | Google Scholar |
Nauheimer L, Cui L, Clarke C, Crayn DM, Bourke G, Nargar K (2019) Genome skimming provides well resolved plastid and nuclear phylogenies, showing patterns of deep reticulate evolution in the tropical carnivorous plant genus Nepenthes (Caryophyllales). Australian Systematic Botany 32, 243-254.
| Crossref | Google Scholar |
Nevill PG, Howell KA, Cross AT, Williams AV, Zhong X, Tonti-Filippini J, Boykin LM, Dixon KW, Small I (2019) Plastome-wide rearrangements and gene losses in carnivorous Droseraceae. Genome Biology and Evolution 11, 472-485.
| Crossref | Google Scholar | PubMed |
Nge F, Biffin E, Waycott M, Thiele K (2021) Phylogenomics and continental biogeographic disjunctions:insight from the Australian starflowers (Calytrix). American Journal of Botany 109, 291-308.
| Crossref | Google Scholar |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar | PubMed |
Nunn R, Bourke G (2017) An account of Drosera section Prolifera. Carnivorous Plant Newsletter 46, 92-100.
| Crossref | Google Scholar |
Nunn R, Lowrie A (2021) An account of Drosera section Lasiocephala. Carnivorous Plant Newsletter 50, 118-132.
| Crossref | Google Scholar |
Paetzold C, Wood KR, Eaton DAR, Wagner WL, Appelhans MS (2019) Phylogeny of Hawaiian Melicope (Rutaceae): RAD-seq resolves species relationships and reveals ancient introgression. Frontiers in Plant Science 10, 1074.
| Crossref | Google Scholar | PubMed |
Palfalvi G, Hackl T, Terhoeven N, Shibata TF, Nishiyama T, Ankenbrand M, Becker D, Förster F, Freund M, Iosip A, Kreuzer I, Saul F, Kamida C, Fukushima K, Shigenobu S, Tamada Y, Adamec L, Hoshi Y, Ueda K, Winkelmann T, Fuchs J, Schubert I, Schwacke R, Al-Rasheid K, Schultz J, Hasebe M, Hedrich R (2020) Genomes of the venus flytrap and close relatives unveil the roots of plant carnivory. Current Biology 30, 2312-2320.
| Crossref | Google Scholar | PubMed |
Pearman DA (2004) Drosera×belezeana Camus confirmed for the British Isles. Watsonia 25, 115-119.
| Google Scholar |
Pease JB, Brown JW, Walker JF, Hinchliff CE, Smith SA (2018) Quartet Sampling distinguishes lack of support from conflicting support in the green plant tree of life. American Journal of Botany 105, 385-403.
| Crossref | Google Scholar | PubMed |
Planchon JÉ (1848) Sur la famille des Droséracées. Annales des Sciences Naturelles Botanique (Paris) Séries 3 9, 285-309 [In French].
| Google Scholar |
Raza M, Ortiz EM, Schwung L, Shigita G, Schaefer H (2023) Resolving the phylogeny of Thladiantha (Cucurbitaceae) with three different target capture pipelines. BMC Ecology and Evolution 23, 75.
| Crossref | Google Scholar |
Rivadavia F, Kondo K, Kato M, Hasebe M (2003) Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. American Journal of Botany 90, 123-130.
| Crossref | Google Scholar | PubMed |
Rokas A, Carroll SB (2005) More genes or more taxa? The relative contribution of gene number and taxon number to phylogenetic accuracy. Molecular Biology and Evolution 22, 1337-1344.
| Crossref | Google Scholar | PubMed |
Scharmann M, Wistuba A, Widmer A (2021) Introgression is widespread in the radiation of carnivorous Nepenthes pitcher plants. Molecular Phylogenetics and Evolution 163, 107214.
| Crossref | Google Scholar | PubMed |
Schlauer J, Fleischmann A (2016) Chemical evidence for hybridity in Drosera (Droseraceae). Biochemical Systematics and Ecology 66, 33-36.
| Crossref | Google Scholar |
Schlauer J, Hartmeyer S, Hartmeyer I (2019) Chemistry and surface micromorphology of the Queensland sundews (Drosera section Prolifera). Carnivorous Plant Newsletter 48, 111-116.
| Crossref | Google Scholar |
Schnell DE (1995) A natural hybrid of Drosera anglica Huds. and Drosera linearis Goldie in Michigan. Rhodora 97, 164-170.
| Google Scholar |
Seine R, Barthlott W (1994) Some proposals on the infrageneric classification of Drosera L. Taxon 43, 583-589.
| Crossref | Google Scholar |
Steenwyk JL, Buida TJ, III, Li Y, Shen X-X, Rokas A (2020) ClipKIT: a multiple sequence alignment trimming software for accurate phylogenomic inference. PLoS Biology 18, e3001007.
| Crossref | Google Scholar | PubMed |
Thornhill AH, Ho SYW, Külheim C, Crisp MD (2015) Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution 93, 29-43.
| Crossref | Google Scholar | PubMed |
van Dijk K, Waycott M, Biffin E, Creed JC, Albertazzi FJ, Samper-Villarreal J (2023) Phylogenomic Insights into the Phylogeography of Halophila baillonii Asch. Diversity 15, 111.
| Crossref | Google Scholar |
Waycott M, van Dijk K, Biffin E (2021) A hybrid capture RNA bait set for resolving genetic and evolutionary relationships in angiosperms from deep phylogeny to intraspecific lineage hybridization. BioRxiv 2021, 2021.09.06.456727 [Preprint, published 7 September 2021].
| Crossref | Google Scholar |
Wood CE (1955) Evidence for the hybrid origin of Drosera anglica. Rhodora 57, 105-130.
| Google Scholar |
Yan Z, Smith ML, Du P, Hahn MW, Nakhleh L (2022) Species tree inference methods intended to deal with incomplete lineage sorting are robust to the presence of paralogs. Systematic Biology 71, 367-381.
| Crossref | Google Scholar | PubMed |
Yang Y, Smith SA (2014) Orthology inference in nonmodel organisms using transcriptomes and low-coverage genomes: improving accuracy and matrix occupancy for phylogenomics. Molecular Biology and Evolution 31(11), 3081-3092.
| Crossref | Google Scholar |
Yesson C, Culham A (2006) Phyloclimatic modeling: combining phylogenetics and bioclimatic modeling. Systematic Biology 55, 785-802.
| Crossref | Google Scholar | PubMed |
Zeng C-X, Hollingsworth PM, Yang J, He Z-S, Zhang Z-R, Li D-Z, Yang J-B (2018) Genome skimming herbarium specimens for DNA barcoding and phylogenomics. Plant Methods 14, 43.
| Crossref | Google Scholar | PubMed |
Zhang C, Mirarab S (2022) Weighting by gene tree uncertainty improves accuracy of quartet-based species trees. Molecular Biology and Evolution 39, msac215.
| Crossref | Google Scholar | PubMed |
Zhang C, Rabiee M, Sayyari E, Mirarab S (2018) ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19, 153.
| Crossref | Google Scholar | PubMed |
Zhang C, Scornavacca C, Molloy EK, Mirarab S (2020) ASTRAL-Pro: quartet-based species-tree inference despite paralogy. Molecular Biology and Evolution 37, 3292-3307.
| Crossref | Google Scholar | PubMed |
Zhou BF, Yuan S, Crowl AA, Liang Y-Y, Shi Y, Chen X-Y, An Q-Q, Kang M, Manos PS (2022) Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the Northern Hemisphere. Nature Communications 13, 1320.
| Crossref | Google Scholar | PubMed |
Zuntini AR, Carruthers T, Maurin O, et al. (2024) Phylogenomics and the rise of the angiosperms. Nature 629, 843-850.
| Crossref | Google Scholar | PubMed |


