Between a rock and a hot place: do surface shelters facilitate survivable conditions for small vertebrates during prescribed fire?
Shawn Scott A B C * , Brett A Goodman D E , Joan Gibbs A C F and Sophie Petit A CA
B
C
D
E
F
Abstract
Natural and anthropogenic fires are increasing in frequency and intensity, and we know little of the conditions that animals experience during fire. Quantifying survival of skinks exposed to fire is impractical, but measuring temperatures across relevant shelter types can reveal the probable conditions they experience during fire.
To determine the thermal extremes imposed by prescribed fire, identify buffering capacity of natural shelter types, and assess whether these conditions threaten survival of skinks.
Temperatures at several shelter types, including rocks and vegetation, were measured during prescribed fires in South Australia. Peak temperatures and duration of lethal conditions were evaluated against lizard critical thermal limits.
Ambient and maximum temperatures during fire were positively associated. Logs and rocks reduced exposure of lizards to extreme temperatures, but mean temperatures were still lethal. Duration of lethal temperatures was exacerbated by increasing ambient temperature for all species.
Skinks sheltering beneath logs and rocks are afforded more protection from extreme temperatures during fire than that provided by other shelter types.
If required, prescribed burning should be undertaken when ambient conditions are mild. To prioritise biodiversity conservation, the availability of protective shelter types needs to be considered before burning.
Keywords: critical thermal maximum, fuel reduction burn, lizards, microhabitat, protective cover, refugia, reptile, shelter, skink, surface, temperatures, thermal buffering, vegetation.
Introduction
Anthropogenic climate change is increasing the frequency, intensity, and spatial extent of wildfires globally (Duane et al. 2021). The unprecedented severity of the 2019–2021 megafires across Australia, California, and South America exemplifies the destructive capacity of fires with climate warming, especially in fire-prone regions (Collins et al. 2021; Nimmo et al. 2022; Armenteras and de la Barrera 2023; Ayars et al. 2023). To mitigate the risk and severity of wildfire, authorities employ prescribed burning to reduce vegetative fuel loads (Penman et al. 2011). Although the protection of human life and assets remains the primary objective of prescribed burning in many areas, a secondary consideration is to enhance biodiversity and ecosystem heterogeneity (Parr and Andersen 2006). This pyrodiversity paradigm posits that creating a mosaic of varying post-fire ages and vegetation structures across the landscape will provide for a higher diversity of taxa with contrasting ecological requirements (Parr and Andersen 2006). However, empirical support for this concept varies considerably across taxonomic groups and biogeographic and climatic regions (Jones and Tingley 2021). In Australia, authorities typically initiate low- to mid-severity prescribed burns during mild conditions in autumn and spring to reduce flammable fuel loads (such as litter, woody debris, and vegetation) while attempting not to remove the canopy (Penman et al. 2007, 2011).
A significant increase in research concerning the effects of prescribed burning has occurred over recent decades, including studies related to post-fire population recovery, community composition, and ecosystem succession (Hiers et al. 2020; McLauchlan et al. 2020; Gordon et al. 2025). Some taxa may exhibit traits that facilitate survival during and following fire (Pausas and Parr 2018) and understanding the behavioural or trait-based mechanisms that species use is crucial to identifying taxa that may be imperilled by fire (Lazzari et al. 2022; Batista et al. 2023). For example, inappropriately timed fire may be hazardous for surface-active lizard species if it interrupts or reduces movement, foraging, and reproduction (Santos et al. 2022). Direct mortality from fire may be relatively low, and estimating the likely number of casualties from prescribed burning can be difficult depending on the species of interest (Jolly et al. 2022). Instead, inference of survivability can be made by measuring the temperatures of relevant habitat features during and after fire because they can provide an indicator of whether available refugia are likely to protect individuals.
Classically, temperatures of the soil profile during fire are measured using loggers at varying depths (e.g. Beadle 1940; Raison et al. 1986; Bradstock and Auld 1995; Williams et al. 2004). Soil and surface temperatures during prescribed burning have been measured across several ecosystem types, including forests and woodlands where studies have assessed the effects of fire temperatures on mortality and germination rates of the seed bank (e.g. Bradstock and Auld 1995; Penman and Towerton 2008; Carrington 2010). In these environments, Penman and Towerton (2008) recorded maximum temperatures of >100 and 77°C at 2-cm and 5-cm depths, respectively. Other studies, however, recorded significantly higher temperatures with increasing fuel loads, such as leaf litter and woody debris, and reduced soil moisture levels, many exceeding 600°C in dry conditions (Preisler et al. 2000; Busse et al. 2005; Perry and McDaniel 2015).
Few studies have investigated whether surface or shelter temperatures during prescribed burning exceed the physiological capacity for survival of terrestrial vertebrates. Perry and McDaniel (2015) recorded temperatures beneath leaf litter during prescribed burns and concluded that of 64 plots, only 5% were deemed survivable for bats that roost below the litter. For the hollow-denning fisher (Pekania pennanti), cavities in trees maintained stable, survivable temperatures during spring prescribed burns (Thompson and Purcell 2016). Penman et al. (2006) recorded non-lethal conditions (<30°C) for the burrowing frog (Heleioporus australiacus) when assessing temperatures at 5-cm and 10-cm depths during prescribed fire in autumn. Timing, intensity, and spatial extent of the fire, alongside the biology (physiological limits, size) and ecology (shelter choice, timing of activity, ability to flee) of taxa play integral roles in whether individuals may survive fire (Whelan et al. 2002).
For ectotherms such as reptiles, characterising the upper thermal thresholds below which operative and behavioural functioning can occur are undertaken by measuring the critical thermal maxima (CTmax) (Camacho and Rusch 2017; Taylor et al. 2021). Such studies expose individuals to increasing temperatures until they lose the ability to correct their orientation autonomously when inverted (the ‘righting reflex’). These temperatures are interpreted as the maxima that are likely to result in death if exposure continues (Heatwole and Taylor 1987; Taylor et al. 2021). Determining these limits in wild lizard populations is challenging owing to the practicalities of fitting and maintaining equipment, small body sizes of some animals, and behavioural thermoregulation where study species are unlikely to expose themselves voluntarily to deleterious conditions (Heatwole and Taylor 1987; Camacho and Rusch 2017). Instead, these studies are undertaken in laboratory settings where confounding and/or limiting factors can be mitigated (Taylor et al. 2021).
In this study, we measured surface and shelter temperatures during prescribed burning in sclerophyllous stringybark (Eucalyptus obliqua and Eucalyptus baxteri) woodlands in the Mount Lofty Ranges, South Australia. We identified the range of maximum temperatures experienced on the surface and beneath shelter types likely to be occupied by terrestrial skinks during prescribed fire. To ascertain whether these temperatures exceeded skink critical thermal limits, we compared shelter temperatures with lab-collected CTmax. Finally, we compared among skinks the relative duration of exposure to lethal temperatures during fire as a function of their natural shelters, microclimate, structural environment, and species-specific CTmax.
Materials and methods
Study region and sites
This study was undertaken in the southern Mount Lofty Ranges, South Australia (SA), approximately 15 km south-east of Adelaide (Fig. 1). The Mount Lofty Ranges form a topographically complex north-south range in southern SA, experience a Mediterranean climate, and are a biodiversity hotspot (Guerin and Lowe 2013). Stringybark woodlands (Eucalyptus obliqua and Eucalyptus baxteri) represent the dominant remnant vegetation community in the region, and the lower strata are typically dominated by dense ground cover of irongrass (Lomandra spp.) and sedges (Lepidosperma spp.) with a sparse shrub midstorey (Acacia myrtifolia, Daviesia leptophylla, and Pultenaea daphnoides) (Cochrane 1963). The region has been extensively degraded, fragmented, and cleared for human habitation, transport, and agriculture, and includes a mosaic of townships and penetrative roadways (Gill et al. 2014). Additionally, widespread seasonal prescribed burning is undertaken in spring and autumn across the region by the Department for Environment and Water (DEW) (Government of South Australia 2021). In recent years, the scope for prescribed burning has increased, and annual burn objectives target approximately 5% of remnant, ‘high risk’ vegetation across private and public lands (Department of Environment, Water and Natural Resources 2014; Government of South Australia 2021).
Study site locations in the Mount Lofty Ranges, South Australia. (a) Map of Australia (dark shading indicates South Australia). (b) South Australia (blue box, approximate study region). Map created using QGIS 3.34.14 (QGIS.org 2024).
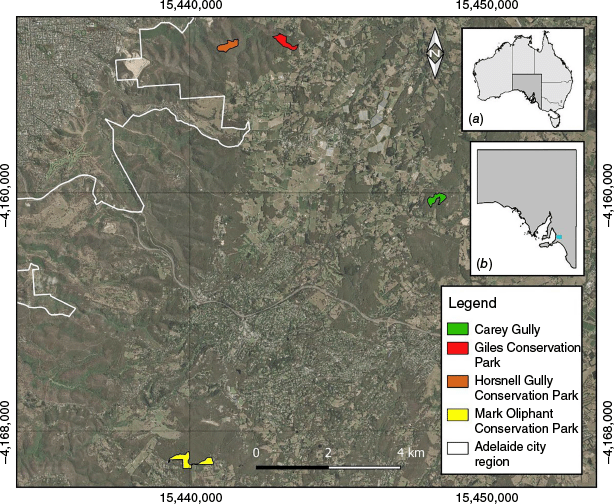
We selected four planned burns to establish our treatment sites (Table 1). Sites were selected using the following four criteria: (1) remnant stringybark woodland; (2) minimum interval of 15 years since previous fire; (3) reasonable access for pre- and post-fire sampling; and (4) no known history of ground cover modification (e.g. grazing). In view of climatic and timing constraints during our study period, only four planned burns were undertaken that met our requirements (three in spring 2023, and one in autumn 2024). Burning occurred in accordance with standard practice, whereby ground crews used drip torches to ignite surface and mid-storey fires within the pre-established fire boundary.
| Site | Season | Fire date | Burn area (ha) | Previous fire | Logging success | Distance to ambient logger (m) | Mean ambient temperature (°C) | Mean fuel consumed (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Carey Gully (private property) | Spring | 15 September 2023 | 14.6 | Unknown | 12/14 | 423 | 17.4 | 80.44 | |
| Horsnell Gully Conservation Park | Spring | 08 October 2023 | 11.0 | 16/02/1983 | 14/14 | 535 | 19.1 | 76.73 | |
| Giles Conservation Park | Spring | 27 October 2023 | 16.8 | 16/11/2005 | 14/14 | 595 | 21.4 | 77.86 | |
| Mark Oliphant Conservation Park | Autumn | 28 April 2024 | 12.1 | 08/12/2005 | 14/14 | 916 | 20.0 | 87.76 |
Burn area=total area burnt by prescribed fire; logging success = number of data loggers that successfully logged temperatures during prescribed fire; distance to ambient logger = distance between burnt and unburnt sites where ambient temperatures were recorded; mean ambient temp = mean ambient temperature during each prescribed fire; mean fuel consumed = mean percentage difference between pre- and post-fire fuel assessments from seven quadrats at each site.
Study species and critical thermal maxima
Terrestrial skinks constitute the most abundant and speciose reptile group in the region, with Hemiergis decresiensis (Cuvier, 1829; three-toed earless skink), Lampropholis guichenoti (Duméril and Bibron, 1839; garden skink), and Lerista bougainvillii (Gray, 1839; Bougainville’s skink) being among the most common (Armstrong et al. 2003). They average 45–70 mm in snout-to-vent length and are typically active and reproductive from spring through autumn (Greer 1989; Wilson and Swan 2021). All are associated with habitats that provide vegetation and/or rocky ground cover, often with leaf litter (Cogger 2014; Wilson and Swan 2021). Localised population densities may be high and diurnal basking behaviours are common in sunny conditions, particularly for L. guichenoti (Torre and Shine 1996; Armstrong et al. 2003).
For two of these species, H. decresiensis and L. bougainvillii, critical thermal data were determined for individuals used in a separate study (B. Goodman, unpubl. data; see Supplementary material S1). As intraspecific CTmax may change across climatic and distributional gradients (Camacho and Rusch 2017), we used the CTmax values for H. decresiensis and L. bougainvillii from individuals collected within 100 km of the study region and applied values for L. guichenoti as per Anderson et al. (2023).
Groundcover and thermal measurements
At each site, we established a 60-m transect where we visually estimated percentage groundcover pre- and post-fire, and installed loggers for use before, during, and immediately after fire. We placed seven 1-m2 quadrats at 10-m intervals along the transects to assess percentage cover of leaf litter, fine woody debris (FWD: ≤30-mm diameter) and coarse woody debris (CWD: >31-mm diameter), rock, surface, duff, bark, and live vegetation, including small plants, shrubs, graminoids, midstorey, and trees. Owing to layering of ground cover, total percentage cover often exceeded 100%. For analyses of vegetation shelter types, we grouped Lepidosperma semiteres and Lomandra fibrata as ‘graminoids’ and Acrotriche fasciculiflora, Hibbertia exutiacies, and H. sericea as ‘shrubs’ because of their structural similarities. We calculated fuel consumed as the cumulative percentage difference of leaf litter, FWD, CWD, bark, small plants, shrubs, graminoids, midstorey, and trees between the pre- and post-fire measurements (similarly to Penman and Towerton 2008). The mean fuel depth was estimated using five measurements of litter or plant cover depth from the corners and centre of each quadrat. Soil moisture (%) was measured by inserting a soil moisture meter 100 mm into the soil (PMS-714, Lutron Electronic Enterprise, Taiwan) centrally per quadrat. These surveys occurred within five days pre- and post-fire.
At each quadrat, we set data probes at two locations to measure temperatures before, during, and after fire. Locations were established under potential lizard shelters such as: rocks, logs, litter, graminoids, shrubs, bark, or duff. Loggers were buried (150 mm) next to each quadrat and the probes were placed beneath rocks, logs, and leaf litter, or at the base of graminoids and shrubs. If two shelters were not present, we included one shelter and the bare surface. Probes were 300 mm long, aluminium, flexible, and were wholly buried, excepting the recording point at the final 10 mm, which was positioned appropriately at the shelter. The depth of the probe beneath each shelter (range: 2–260 mm) and the shelter size (mm) were measured. Aside from minor changes to soil when positioning probes, we did not alter shelters or their respective cover values prior to the fire. At four quadrats, temperatures were recorded using HOBO UX120-014M thermocouple loggers (Onset, Bourne, MA, USA) equipped with two K-type probes. At each of the remaining three quadrats, two HOBO U12-014 thermocouple loggers (Onset, Bourne, MA, USA), each equipped with a K-type probe, were used. Loggers recorded temperatures every second from the morning of the fire and with a 2–4-h lead time before ignition. Ambient temperatures (°C) during fire were recorded every 20 min using EL-USB-2 data loggers (Lascar Electronics, Hong Kong) positioned 1.5 m above the surface at unburnt sites <1000 m away (Table 1). For analyses, we applied the mean ambient temperature between ignition and the cessation of fire at a particular shelter to represent the specific ambient conditions during which a shelter reached its maximum temperature.
Statistical analyses
We implemented a multimodel inference approach (Burnham and Anderson 2002) to identify variables that may influence shelter temperatures and the duration of high temperatures that may threaten lizard survival during fire. We used generalised linear mixed-models (GLMMs) with a gamma distribution and log-link function undertaken with the glmmTMB package (Brooks et al. 2017) in R (R Core Team 2023). Our first model set included maximum temperature (°C) during fire as the response variable. In the second model set, duration of temperatures above CTmax was fitted as the response variable and this model set was run for each of the three species separately. As intraspecific CTmax values ranged over 3–4°C (Table 2), we allocated the mean of those values as the singular value per species. To determine whether the duration of temperatures above the CTmax differed among species, we ran a simplified model fitted with duration as the response variable and species as the predictor. We assessed correlations and multicollinearity among predictor variables using Spearman’s correlation and generalised variance inflation factors, respectively. Owing to high multicollinearity (GVIF >3; Zuur et al. 2009, 2010), aspect, cumulative rainfall over the preceding 5 days, and season were removed from further analyses. All continuous predictor variables were centred and scaled prior to analyses.
| Taxon | Min CTmax | Max CTmax | Mean CTmax | S | Source | |
|---|---|---|---|---|---|---|
| Hemiergis decresiensis | 35.6 | 39.4 | 37.5 | 14 | B. Goodman, unpubl. data | |
| Lampropholis guichenoti | 37.9 | 41.5 | 39.4 | 15 | Anderson et al. (2023) | |
| Lerista bougainvillii | 40.9 | 44.4 | 43.0 | 13 | B. Goodman, unpubl. data |
CTmax = critical thermal maxima (°C); S = sample size. For H. decresiensis and L. bougainvillii, data from individuals collected within 100 km of the study region were used in analyses.
In both model sets, we included predictor variables such as ambient temperature, soil moisture, fuel consumed, fuel depth, probe depth, shelter type, and shelter size (size categories assigned based on range of shelter sizes measured). In model set 2, we also included maximum temperature as a predictor. Size or depth categories were assigned per shelter type (Table S1) and this predictor variable was fitted to models only as an interaction effect with shelter type. Considering the small sample sizes, we excluded bark (n = 1) and surface (n = 2) from analyses. Quadrat and site were included in all models as nested random effects to account for intra-site repeated measures and spatial autocorrelation (Zuur et al. 2009). Models were built and run using an additive approach, whereby additional covariates and interactions were included or removed based on relative contributions, significance to model convergence and/or variance explained, and realistic likelihood of important interactions (Burnham and Anderson 2002; Harrison et al. 2018). We fitted and compared 30 candidate models in each model set and those in the second set were run per species. Model fit was assessed visually using histograms and QQ plots and residual dispersion, heteroscedasticity, and normality were checked using the DHARMa package (Hartig 2022).
To compare and select models among our candidate model sets, we used an information-theoretic approach with Akaike’s information criterion (AIC) (Burnham and Anderson 2002). As the ratio of our sample size to the number of parameters in the most parameterised model did not exceed 40, we ranked model performance using AIC corrected for small sample sizes (AICc) (Burnham and Anderson 2002). Top-ranking models with <2 ΔAICc in model set 1 were interpreted as being equally plausible. Model coefficients were similar between the two top-performing models (Table 3), and the results from the highest performing model are detailed here. However, model set 2 contained a greater number of high-performing models based on AICc. To account for this model uncertainty, we conducted model averaging in the MuMIn package (Burnham and Anderson 2002; Zuur et al. 2009; Dormann et al. 2018; Barton 2024). The top candidate models that summed to AICc weights of >0.95 were fitted during model averaging to improve predictions and ensure inclusion of all potentially informative predictors and interactions from models that may have been penalised by AICc (Burnham and Anderson 2002; Symonds and Moussalli 2011). Where significant differences were detected in the maximum temperatures among shelter types and the duration of lethal temperatures across the three species, we used the ‘joint_tests’ and ‘pairs’ functions in the emmeans package (Lenth 2024) to determine the overall significance of the predictor and undertake pairwise comparisons of the levels therein (with Tukey-adjusted P-values for multiple comparisons), respectively. All plots were created using the package, ggplot2 (Wickham 2016).
| No. | Model | Log likelihood | K | AICc | ΔAICc | AICc weight | |
|---|---|---|---|---|---|---|---|
| 1 | Ambient + Shelter type | −318.0 | 9 | 660.379 | 0.000 | 0.582 |
| Model term | Estimate | s.e. | Z-value | P-value | |
|---|---|---|---|---|---|
| Intercept | 6.283 | 0.307 | 20.487 | <0.001 | |
| Ambient | 0.588 | 0.125 | 4.722 | <0.001 | |
| Shelter type: litter | −0.538 | 0.370 | −1.453 | 0.146 | |
| Shelter type: log | −1.597 | 0.422 | −3.781 | <0.001 | |
| Shelter type: rock | −2.320 | 0.391 | −5.940 | <0.001 | |
| Shelter type: graminoid | −0.648 | 0.367 | −1.767 | 0.077 | |
| Shelter type: shrub | −0.437 | 0.474 | −0.922 | 0.356 |
| No. | Model | Log likelihood | K | AICc | ΔAICc | AICc weight | |
|---|---|---|---|---|---|---|---|
| 2 | Ambient + Fuel depth + Shelter type | −316.8 | 10 | 661.052 | 0.673 | 0.416 |
| Model term | Estimate | s.e. | Z-value | P-value | |
|---|---|---|---|---|---|
| Intercept | 6.346 | 0.307 | 20.695 | <0.001 | |
| Ambient | 0.661 | 0.129 | 5.125 | <0.001 | |
| Shelter type: litter | −0.700 | 0.381 | −1.835 | 0.067 | |
| Shelter type: log | −1.692 | 0.418 | −4.051 | <0.001 | |
| Shelter type: rock | −2.393 | 0.388 | −6.173 | <0.001 | |
| Shelter type: graminoid | −0.666 | 0.361 | −1.844 | 0.065 | |
| Shelter type: shrub | −0.502 | 0.471 | −1.066 | 0.287 | |
| Fuel depth | −0.178 | 0.112 | −1.592 | 0.111 |
Significant values are highlighted in bold. K = number of parameters; ambient = ambient temperature (°C) during prescribed fire; fuel depth = mean of five measurements (mm) of fuel depth from quadrat pre-fire; shelter type = shelter where temperatures were recorded.
Results
Across the four burns, all 28 quadrats were burnt by prescribed fire. One logger (two thermocouples) failed during a burn in spring 2023, resulting in 54 successful logging sessions during fire. Maximum temperatures attained during prescribed fires ranged from 14.53–727.56°C (median = 286.52, IQR = 51.85–433.44). Of the 54 successful logging sessions, 11 loggers (20.8%) recorded maximum temperatures that did not exceed the mean CTmax for any species. Maximum temperatures from the remaining 43 loggers were considered lethal for the three skink species, ranging from 45.71 to 727.56°C (median = 383.45; IQR = 202.29–487.90). Across the measured shelter types during fire, maximum temperatures recorded varied considerably (Fig. S1). Median duration of lethal temperatures was 1663 s (IQR = 908–2530, range = 388–6710) for H. decresiensis, 1519 s for L. guichenoti (IQR = 827–2079, range = 342–5854), and 1218 s for L. bougainvillii (IQR = 723–1636, range = 272–4660).
Maximum temperatures during fire
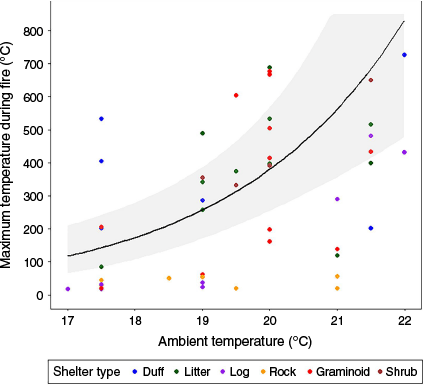
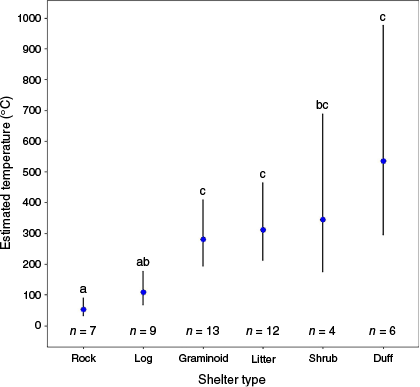
Two candidate models with <2 ΔAICc strongly outcompeted other models (all others >12 ΔAICc; Table S2). In each top-ranked model, ambient temperature and shelter type (log and rock) were significant predictors of maximum temperature during fire. The random effects site and quadrat did not contribute significantly to the observed effects (variances <0.001). Increasing ambient temperature was positively associated with maximum temperature during fire (estimate = 0.588, Z = 4.722, P < 0.001; Fig. 2). Overall, shelter type significantly influenced maximum temperatures during prescribed fire (F(5, Inf) = 10.806, χ² = 54.030, P < 0.001; Table S3). The maximum temperatures beneath rocks (emmean = 52.6, s.e. = 14.6, 95% CI = 31–91) were significantly lower than were maximum temperatures under all other shelter types except logs (Fig. 3). Maximum temperatures under logs (emmean = 108.4, s.e. = 27.3, 95% CI = 66–178) were significantly lower than those beneath most shelter types other than rocks (estimate = 0.723, Z = 1.829, P = 0.447) and shrubs (estimate = −1.160, Z = −2.726, P = 0.070), which showed a non-significant trend.
Relationship between ambient temperature and maximum temperature during prescribed fire. The line represents the predicted increase in maximum temperature with increasing ambient temperature during this study (~17–22°C). The shading around the line represents 95% confidence intervals. Coloured points represent the temperatures measured for each shelter type.
Estimated means with 95% confidence intervals for maximum temperatures during prescribed fire at each of the shelter types included in analyses. Different letters indicate significant differences. Rocks, and to a lesser extent, logs maintained the coolest temperatures during fire. Numbers below each error bar represent the sample size for each shelter type.
Duration of lethal temperatures during fire
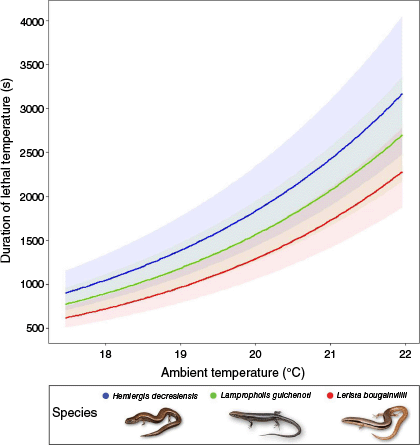
For each species, the three top-ranked models concerning duration of lethal temperatures included ambient temperature, fuel consumed, and soil moisture. The number of candidate models included during model-averaging differed among taxa owing to relative differences in AICc values and weights (Table 4; Table S4). Ambient temperature was significantly and positively related to the duration of lethal temperatures during fire for all species (Fig. 4): H. decresiensis (estimate = 0.385, Z = 2.965, P = 0.003, Fig. S2a), L. guichenoti (estimate = 0.384, Z = 3.338, P = 0.001, Fig. S2b), and L. bougainvilii (estimate = 0.401, Z = 3.871, P < 0.001, Fig. S2c). Pairwise comparisons indicated no overall significant difference in duration of lethal temperatures among the different shelter types for L. bougainvillii (lowest P = 0.097; Table S5).
| No. | Model | Log likelihood | K | AICc | ΔAICc | AICc weight | |
|---|---|---|---|---|---|---|---|
| Hemiergis decresiensis | |||||||
| 1 | Ambient | −333.501 | 5 | 678.77 | 0.00 | 0.44 | |
| 2 | Ambient + Fuel consumed | −332.589 | 6 | 679.72 | 0.96 | 0.27 | |
| 3 | Ambient + Fuel consumed + Soil moisture | −332.241 | 7 | 681.98 | 3.22 | 0.09 | |
| 4 | Fuel consumed | −335.520 | 5 | 682.80 | 4.04 | 0.06 | |
| 5 | Temperature | −335.792 | 5 | 683.35 | 4.58 | 0.04 | |
| 6 | Ambient + Probe depth + Soil moisture | −332.928 | 7 | 683.36 | 4.59 | 0.04 | |
| 7 | Fuel depth | −336.155 | 5 | 684.07 | 5.31 | 0.03 | |
| 8 | Probe depth | −336.212 | 5 | 684.19 | 5.42 | 0.03 | |
| Lampropholis guichenoti | |||||||
| 1 | Ambient + Fuel consumed | −322.60 | 6 | 659.75 | 0.00 | 0.30 | |
| 2 | Ambient | −324.00 | 5 | 659.77 | 0.01 | 0.30 | |
| 3 | Ambient + Fuel consumed + Soil moisture | −321.58 | 7 | 660.66 | 0.91 | 0.19 | |
| 4 | Ambient + Probe depth + Soil moisture | −322.68 | 7 | 662.86 | 3.11 | 0.06 | |
| 5 | Fuel consumed | −325.73 | 5 | 663.23 | 3.48 | 0.05 | |
| 6 | Temperature | −326.32 | 5 | 664.41 | 4.65 | 0.03 | |
| 7 | Soil moisture | −326.56 | 5 | 664.89 | 5.13 | 0.02 | |
| 8 | Fuel depth | −326.72 | 5 | 665.20 | 5.44 | 0.02 | |
| 9 | Probe depth | −326.77 | 5 | 665.31 | 5.56 | 0.02 | |
| Lerista bougainvillii | |||||||
| 1 | Ambient + Fuel consumed | −310.99 | 6 | 636.52 | 0.00 | 0.33 | |
| 2 | Ambient + Fuel consumed + Soil moisture | −309.77 | 7 | 637.04 | 0.52 | 0.26 | |
| 3 | Ambient | −312.68 | 5 | 637.12 | 0.60 | 0.24 | |
| 4 | Ambient + Probe depth + Soil moisture | −311.16 | 7 | 639.83 | 3.31 | 0.06 | |
| 5 | Ambient + Fuel consumed + Shelter type | −304.59 | 11 | 640.60 | 4.08 | 0.04 | |
| 6 | Fuel consumed | −314.72 | 5 | 641.21 | 4.69 | 0.03 | |
| 7 | Temperature | −315.40 | 5 | 642.56 | 6.04 | 0.02 | |
| 8 | Soil moisture | −315.47 | 5 | 642.71 | 6.19 | 0.01 | |
| Model term | Estimate | Adjusted s.e. | Z-value | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hd | Lg | Lb | Hd | Lg | Lb | Hd | Lg | Lb | Hd | Lg | Lb | ||
| Intercept | 7.46 | 7.30 | 7.11 | 0.15 | 0.13 | 0.12 | 49.30 | 54.84 | 57.68 | <0.01 | <0.01 | <0.01 | |
| Ambient | 0.39 | 0.38 | 0.40 | 0.13 | 0.12 | 0.10 | 2.97 | 3.34 | 3.87 | <0.01 | <0.01 | <0.01 | |
| Fuel depth | 0.16 | 0.14 | – | 0.17 | 0.15 | – | 0.92 | 0.91 | – | 0.36 | 0.36 | – | |
| Shelter type: graminoid | – | – | −0.50 | – | – | 0.21 | – | – | 2.31 | – | – | 0.02 | |
| Fuel consumed | 0.18 | 0.18 | 0.18 | 0.14 | 0.12 | 0.10 | 1.29 | 1.53 | 1.80 | 0.20 | 0.13 | 0.07 | |
| Probe depth | 0.05 | 0.04 | 0.02 | 0.08 | 0.08 | 0.08 | 0.60 | 0.46 | 0.31 | 0.55 | 0.64 | 0.76 | |
| Soil moisture | 0.12 | 0.17 | 0.16 | 0.15 | 0.12 | 0.11 | 0.82 | 1.36 | 1.52 | 0.41 | 0.17 | 0.13 | |
| Temperature | 0.12 | 0.12 | 0.11 | 0.09 | 0.09 | 0.09 | 1.30 | 1.30 | 1.30 | 0.19 | 0.19 | 0.20 | |
Reported models are those with AICc weights that sum to >0.95. Hd = Hemiergis decresiensis; Lg = Lampropholis guichenoti; Lb = Lerista bougainvillii. Significant values are highlighted in bold. K = number of parameters; ambient = ambient temperature (°C) during prescribed fire; fuel consumed = percentage difference of fuel cover between pre- and post-fire fuel measurements; fuel depth = mean of five measurements (mm) of fuel depth from quadrat pre-fire; probe depth = depth of probe when positioned beneath a shelter during fire; shelter type = shelter where temperatures were recorded; soil moisture = single measure (%) taken from the centre of each quadrat prior to fire; temperature = maximum temperature achieved during fire.
Positive association between duration of lethal temperatures and increasing ambient temperature for each of the three skink species. Shading around each line represents 95% confidence intervals. Image credit: Hemiergi decresiensis and Lerista bougainvsillii (S. Scott); Lampropholis guichenoti (S. Mahony, with permission).
Pairwise contrasts among the log-means for each species demonstrated significant differences in duration of exposure to lethal temperatures (Fig. 5): H. decresiensis vs L. guichenoti (estimate = 0.171, s.e. = 0.072; P = 0.046), H. decresiensis vs L. bougainvillii (estimate = 0.372, s.e. = 0.072, P < 0.001), and L. guichenoti vs L. bougainvillii (estimate = 0.202, s.e. = 0.072, P = 0.014). Specifically, H. decresiensis was exposed to lethal temperatures during fire for longer times (emmean = 1570, s.e. = 1.230, 95% CI = 1047–2354) than were L. guichenoti (emmean = 1324, s.e. = 1.230, 95% CI = 883–1984) and L. bougainvillii (emmean = 1082, s.e. = 1.230, 95% CI = 722–1622).
Significant differences in duration of exposure to lethal temperatures across the three skink species. Points show the estimated marginal mean duration of exposure to lethal temperatures and the error bars represent 95% confidence intervals. Hemiergis decresiensis was exposed to lethal conditions for the longest; 1.19× longer than Lampropholis guichenoti and 1.45× longer than Lerista bougainvilii. L. bougainvilii experienced the shortest period of exposure to lethal temperatures during fire, being 1.22× shorter than the exposure time of L. guichenoti. Values above the bars denote the relative pairwise P-values (with Tukey adjustment for multiple comparisons) associated with differences in duration of lethal temperatures among species.

Discussion
Prescribed burning in the Mount Lofty Ranges exposes skinks to extreme thermal conditions that far exceed their physiological thresholds. Individual survival is mediated by many species-specific behavioural, ecological, morphological, and physiological traits and components of the fire regime, and the capacity for an individual to flee or seek shelter plays a significant role in whether it survives fire (Pausas and Parr 2018; Nimmo et al. 2021; Jolly et al. 2022). In considering our focal species, fleeing across large distances to escape fire is unlikely (Ensbey et al. 2023). For lizards occupying areas on or near the fire boundary, fleeing the oncoming fire may be rapid and enhance survival, as observed for skinks during these fires (DEW, unpubl. data). However, when individuals inhabit the fire interior and escape is not feasible because of multiple fire fronts or rapid fire spread, seeking shelter becomes the primary means of survival (Batista et al. 2023).
Quantifying mortality during fire is an inherently challenging process and requires the tracking of individuals exposed to fire (Whelan et al. 2002), which is impractical for small vertebrates such as skinks. Assessing the capacity of natural shelter types to buffer extreme conditions facilitates inference on the implications of fire on animal survival. Among the many models tested, maximum temperatures during fire were reduced by logs and rocks, shelters typically suggested as suitable protective cover (Batista et al. 2023; Ensbey et al. 2023). Rocks were clearly the most effective shelter type for buffering high temperatures during fire, which agrees with evidence from other studies (Stoof et al. 2011). Despite maintaining temperatures significantly cooler than those beneath other shelter types, the mean estimated maximum temperatures under logs and rocks were ~108°C and ~53°C, respectively, which exceed the respective CTmax of our three skink species (37.5–43.0°C). Furthermore, we identified no difference in the duration of lethal temperatures among shelter types. Despite comparatively low temperatures afforded by logs and rocks, their mean temperatures and ineffectiveness to reduce the duration of these temperatures indicate that not all are adequate shelters for small vertebrates during fire.
Our results indicated that maximum temperature during fire increased with ambient temperature, which is unsurprising considering that microclimate, including high daytime temperatures, low humidity, and high wind speed, are associated with increasing fire temperature and severity (Flannigan et al. 2009; Bradstock 2010; Lindenmayer et al. 2023). We observed a marked increase in maximum temperatures during fire (predicted difference of up to ~700°C) with increasing ambient temperatures over a ~5°C gradient (~17–22°C). This relationship between ambient temperature and fire intensity has been described elsewhere (see Knapp et al. 2009 and references therein) and prevailing air temperatures affect the decision to undertake prescribed burning. Our analyses did not highlight any significant effect of soil moisture on maximum temperatures during prescribed fire, but the role of increasing ambient temperatures in drying and warming surface fuels that subsequently promote higher burn intensities has received considerable support (Baeza et al. 2002; Liu et al. 2014; Hiers et al. 2020).
Duration of lethal temperatures was primarily mediated by ambient temperatures for all species, with shortest periods of exposure at cooler ambient temperatures. A similar relationship between increasing ambient temperature and lethal heat dosages (the combined effects of maximum temperature and duration) during prescribed fire was reported by Hill et al. (2017) for grassland butterfly larvae. In our study, duration of lethal temperatures increased concomitantly with ambient temperature, ranging from ~500–1000 s at ~17°C to 2000–3000 s at ~22°C depending on the species and CTmax. The positive relationship of increasing ambient temperature with increasing maximum temperatures and duration of these temperatures, also reported by Hill et al. (2017) for lethal heat dosages, has substantial implications for prescribed fire planning. To mitigate the risk of high severity and maintain control of prescribed fire, burning typically occurs during mild temperatures outside of the primary wildfire season (Hiers et al. 2020). Our results suggest that even during relatively mild conditions (~17–22°C) in our study region, the maximum temperatures and duration of lethal temperatures achieved during prescribed burning are likely to threaten small vertebrates that rely on these shelters for protection. With the demonstrated interaction of ambient temperatures with fire intensity, limiting prescribed burning to cooler weather conditions is crucial for survival of small vertebrates occupying these surface-level shelters and control of fire spread, extreme temperatures, and duration (Knapp et al. 2009; Penman et al. 2011).
Duration of exposure to lethal temperatures was shorter for species with higher CTmax. H. decresiensis exhibited the lowest CTmax, resulting in increased periods of vulnerability to high temperature during fire compared to L. guichenoti and L. bougainvillii. Our range of CTmax values for H. decresiensis (Table 2) largely agrees with published data (32.0–33.5°C, Brattstrom 1971; 39.2–39.4°C, Spellerberg 1972; 38.6°C, Bennett and John-Alder 1986). High rates of water loss and occupancy of mesic, well-vegetated environments are suggested to be implicit in their sensitivity to increasing temperatures (Warburg 1966; Greer 1989). Pairwise estimates indicated a higher similarity among the durations of lethal temperatures experienced by H. decresiensis and L. guichenoti, which may be attributed to the closer CTmax values and their largely similar occupancy of forested environments with abundant ground cover (Cogger 2014; Wilson and Swan 2021). Lerista bougainvillii demonstrated the highest CTmax and lowest duration values of the three skinks. Greer (1980) reported that among skinks measured, L. bougainvillii exhibited one of the highest mean CTmax values (43.8°C) and suggested that this comparatively higher thermal tolerance may result from its evolution within, and occupation of, warmer and drier conditions across parts of its distribution.
We did not detect any effect of shelter size on maximum temperature, probably because of low sample sizes across our shelter size categories, and it would be valuable for future studies to focus on the role of different shelter sizes in more detail. It is likely that large logs and rocks, and tree hollows with insulating bark or moist substrate underneath would be valuable shelters during fire (Whelan et al. 2002; Batista et al. 2023). Logs and rocks of varying sizes are likely to differ in their efficacy in protecting animals during fire. Increased sampling across a greater range of shelter sizes and ambient temperatures would clarify the interactions between shelter form and ambient temperature, and their consequences for maximum temperature and its duration. Such information would contribute to informed prescribed fire management and the development of burning regimes with objectives that aim to enhance the survival of fauna. Logs and rocks are frequently used as shelters by many lizard species, and their value as protective cover during fire has been suggested elsewhere (Lindenmayer et al. 2002; Cogger 2014; Santos et al. 2016; Michael et al. 2021). Although logs and rocks may protect animals during fire, the retention of these shelters must be accompanied by the consideration of ambient temperature when planning burns to reduce fatalities.
Our study has highlighted the roles of ambient temperature and shelter type in mediating maximum temperatures and duration of these temperatures during prescribed fire, with consideration for the implications of these conditions on lizard survival. In other studies, seasonal and environmental characteristics, including wind speed, fuel loads, and surface moisture, affected significantly the maximum temperatures attained and the duration of these temperatures during prescribed fire (Molina and Llinares 2001; Wanthongchai et al. 2011; Wotton et al. 2012; Perry and McDaniel 2015; Menges et al. 2021). Penman and Towerton (2008) reported that higher temperatures during fire were associated with increased consumption of fuel, which we did not detect as part of our analyses. Our limited sample size may explain why some of these drivers of fire temperature and duration were not confirmed here.
To complement our findings, future research could target alternative shelter types used by small vertebrates (e.g. deep soil, burrows, beneath tree bark), environmental conditions, and species with contrasting traits that may affect their chances of survival during fire (Whelan et al. 2002; Penman et al. 2006). Increased seasonal replication during spring and autumn, particularly with higher ambient conditions, would allow the assessment of season-based differences in shelter temperatures and duration of lethal conditions during fire at these times. As surface and plant moisture conditions in spring are typically higher than in autumn, maximum temperatures during autumn prescribed fires are expected to be higher because of drier fuel loads (Knapp and Keeley 2006; Knapp et al. 2009). Identifying whether season exacerbates deleterious thermal conditions for biodiversity during fire, while considering surface activity and reproductive behaviour, will inform on appropriate timing for prescribed burning. High temperatures during fire may also threaten the survival of lizard nests positioned beneath surface shelters, such as logs and rocks, if they are not adequately protected by these shelters or buried to a depth unaffected by fire temperatures (e.g. Penman and Towerton 2008; Shine et al. 2016). Prescribed burning in spring may correspond with increased lizard reproductive activity and evaluating whether prescribed burning typically exposes these nests to lethal conditions should be considered when developing plans for prescribed fire, especially in areas with species that exhibit narrow reproductive phenologies.
Inappropriate fire regimes are identified as significant threats to global biodiversity, including reptiles (Kelly et al. 2020; Geyle et al. 2021; Santos et al. 2022). Despite some evidence suggesting that mortality during fire is low (Jolly et al. 2022), our understanding of the conditions during prescribed burning and their implications for reptiles is poor (Whelan et al. 2002; Doherty et al. 2024). Although our study was limited to three skink species and their respective CTmax, temperatures beneath most shelters during fire would threaten survival of other animal groups, as these conditions dramatically exceed the ~60°C threshold for most terrestrial vertebrates (Tattersall et al. 2012). Logs and rocks were the most effective shelters for buffering extreme temperatures during prescribed fire in our study. However, the maximum temperatures and duration of these conditions may still prove lethal for small vertebrates if prescribed burning is undertaken during conditions that exacerbate fire severity. With dramatically reduced availability of shelters following combustion of woody or vegetative fuels during fire (e.g. Petit and Frazer 2023), the importance of rocks and large intact logs as crucial cover for lizards during and after fire is emphasised when considering that individuals must contend with the simplified and potentially dangerous conditions of the post-fire landscape (Whelan et al. 2002; Batista et al. 2023).
As frequency and severity of global wildfire regimes are expected to intensify with climate change, prescribed burning practices may increase (Clarke et al. 2019). Although symptomatic of many fire-prone regions, frequency and spatial extent of prescribed burning in the study region have precipitously increased in recent decades and we know little of its interactive effects on biodiversity with processes such as landscape fragmentation (Willson and Bignall 2009; Gill et al. 2014; Driscoll et al. 2021). When considering the combined effects of increasing ambient temperature for higher shelter temperatures and duration of lethal conditions during prescribed fire, we recommend that practitioners of prescribed burning consider carefully the conditions in which it is undertaken and select cooler temperatures if animal survival is to be prioritised. By evaluating and accommodating at the taxonomic and geographic scales, the crucial resource requirements, physiological limits, and reproductive phenology of affected species, recommendations for informed prescribed fire regimes can be made. With the expected increase of prescribed burning in response to elevated wildfire risk induced by climatic change, fauna of fire-prone biomes, such as those of Mediterranean-climatic regions, will require additional consideration as part of the prescribed burning process if effective biodiversity conservation is to be considered a condition of prescribed burning (Driscoll et al. 2010; Penman et al. 2011; Bradshaw et al. 2018).
Declaration of funding
Some field equipment used during this study was purchased using funding provided to SS by the Holsworth Wildlife Research Endowment through the Ecological Society of Australia.
Acknowledgements
Our work was conducted on the traditional lands of the Kaurna and Peramangk peoples, to whom we pay our respect. For support with fieldwork, planning, and use of equipment, we are grateful to Andrew Sheath and Tim Groves at the Department for Environment and Water. For helpful discussions during this project, we thank Jas Armstrong, Miguel de Barros Lopes, Gunnar Keppel, Peter Matejcic, Jack Bilby, Tom Prosser, Flynn Balshaw, and Max Tibby. We also thank Rodolfo Anderson for providing additional information related to the critical thermal limits of L. guichenoti. For support with producing figures, we thank Jas Armstrong and Paul Corcoran. Thanks to Stephen Mahony for providing the image of L. guichenoti used in Fig. 4 and to Phil Ainsley and Jason van Weenen for their support. Access and use of protected lands for the purpose of this research was authorised under DEW Scientific Research Permit E27131-1–6. We thank the anonymous reviewers for their insightful and constructive feedback on an earlier version of this manuscript.
References
Anderson RO, Tingley R, Hoskin CJ, White CR, Chapple DG (2023) Linking physiology and climate to infer species distributions in Australian skinks. Journal of Animal Ecology 92, 2094-2108.
| Crossref | Google Scholar | PubMed |
Armenteras D, de la Barrera F (2023) Landscape management is urgently needed to address the rise of megafires in South America. Communications Earth & Environment 4, 305.
| Crossref | Google Scholar |
Ayars J, Kramer HA, Jones GM (2023) The 2020 to 2021 California megafires and their impacts on wildlife habitat. Proceedings of the National Academy of Sciences of the United States of America 120, e2312909120.
| Crossref | Google Scholar | PubMed |
Baeza MJ, De Luís M, Raventós J, Escarré A (2002) Factors influencing fire behaviour in shrublands of different stand ages and the implications for using prescribed burning to reduce wildfire risk. Journal of Environmental Management 65, 199-208.
| Crossref | Google Scholar | PubMed |
Barton K (2024) MuMIn: Multi-model inference. R package version 1.48.4. Available at https://cran.r-project=MuMIn [verified 16 October 2024]
Batista EKL, Figueira JEC, Solar RRC, de Azevedo CS, Beirão MV, Berlinck CN, Brandão RA, de Castro FS, Costa HC, Costa LM, Feitosa RM, Freitas AVL, Freitas GHS, Galdino CAB, Júnior JES, Leite FS, Lopes L, Ludwig S, do Nascimento MC, Negreiros D, Oki Y, Paprocki H, Perillo LN, Perini FA, Resende FM, Rosa AHB, Salvador LF, Jr, Silva LM, Silveira LF, DeSouza O, Vieira EM, Fernandes GW (2023) In case of fire, escape or die: a trait-based approach for identifying animal species threatened by fire. Fire 6, 242.
| Crossref | Google Scholar |
Beadle NCW (1940) Soil temperatures during forest fires and their effect on the survival of vegetation. Journal of Ecology 28, 180-192.
| Crossref | Google Scholar |
Bennett AF, John-Alder H (1986) Thermal relations of some Australian skinks (Sauria: Scincidae). Copeia 1986, 57-64.
| Crossref | Google Scholar |
Bradshaw SD, Dixon KW, Lambers H, Cross AT, Bailey J, Hopper S (2018) Understanding the long-term impact of prescribed burning in mediterranean-climate biodiversity hotspots, with a focus on south-western Australia. International Journal of Wildland Fire 27, 643-657.
| Crossref | Google Scholar |
Bradstock RA (2010) A biogeographic model of fire regimes in Australia: current and future implication. Global Ecology and Biogeography 19, 145-158.
| Crossref | Google Scholar |
Bradstock RA, Auld TD (1995) Soil temperatures during experimental bushfires in relation to fire intensity: consequences for legume germination and fire management in south-eastern Australia. Journal of Applied Ecology 32, 76-84.
| Crossref | Google Scholar |
Brattstrom BH (1971) Critical thermal maxima of some Australian skinks. Copeia 1971, 554-557.
| Crossref | Google Scholar |
Brooks ME, Kristensen K, van , Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378-400.
| Crossref | Google Scholar |
Busse MD, Hubbert KR, Fiddler GO, Shestak CJ, Powers RF (2005) Lethal soil temperatures during burning of masticated forest residues. International Journal of Wildland Fire 14, 267-276.
| Crossref | Google Scholar |
Camacho A, Rusch TW (2017) Methods and pitfalls of measuring thermal preference and tolerance in lizards. Journal of Thermal Biology 68, 63-72.
| Crossref | Google Scholar | PubMed |
Carrington ME (2010) Effects of soil temperature during fire on seed survival in Florida sand pine scrub. International Journal of Forestry Research 2010, 402346.
| Crossref | Google Scholar |
Clarke H, Tran B, Boer MM, Price O, Kenny B, Bradstock R (2019) Climate change effects on the frequency, seasonality and interannual variability of suitable prescribed burning weather conditions in south-eastern Australia. Agricultural and Forest Meteorology 271, 148-157.
| Crossref | Google Scholar |
Cochrane GR (1963) Vegetation studies in forest-fire areas of the Mount Lofty Ranges, South Australia. Ecology 44, 41-52.
| Crossref | Google Scholar |
Collins L, Bradstock RA, Clarke H, Clarke MF, Nolan RH, Penman TD (2021) The 2019/2020 mega-fires exposed Australian ecosystems to an unprecedented extent of high-severity fire. Environmental Research Letters 16, 044029.
| Crossref | Google Scholar |
Doherty TS, Johnson B, Friend GR, Wayne AF (2024) Multi-year responses of reptiles to prescribed burning in a eucalypt forest ecosystem. Austral Ecology 49, e13572.
| Crossref | Google Scholar |
Dormann CF, Calabrese JM, Guillera-Arroita G, Matechou E, Bahn V, Bartoń K, Beale CM, Ciuti S, Elith J, Gerstner K, Guelat J, Keil P, Lahoz-Monfort JJ, Pollock LJ, Reineking B, Roberts DR, Schröder B, Thuiller W, Warton DI, Wintle BA, Wood SN, Wüest RO, Hartig F (2018) Model averaging in ecology: a review of Bayesian, information-theoretic, and tactical approaches for predictive inference. Ecological Monographs 88, 485-504.
| Crossref | Google Scholar |
Driscoll DA, Lindenmayer DB, Bennett AF, Bode M, Bradstock RA, Cary GJ, Clarke MF, Dexter N, Fensham R, Friend G, Gill M, James S, Kay G, Keith DA, MacGregor C, Russell-Smith J, Salt D, Watson JEM, Williams RJ, York A (2010) Fire management for biodiversity conservation: key research questions and our capacity to answer them. Biological Conservation 143, 1928-1939.
| Crossref | Google Scholar |
Driscoll DA, Armenteras D, Bennett AF, Brotons L, Clarke MF, Doherty TS, Haslem A, Kelly LT, Sato CF, Sitters H, Aquilué N, Bell K, Chadid M, Duane A, Meza-Elizalde MC, Giljohann KM, González TM, Jambhekar R, Lazzari J, Morán-Ordóñez A, Wevill T (2021) How fire interacts with habitat loss and fragmentation. Biological Reviews 96, 976-998.
| Crossref | Google Scholar | PubMed |
Duane A, Castellnou M, Brotons L (2021) Towards a comprehensive look at global drivers of novel extreme wildfire events. Climatic Change 165, 43.
| Crossref | Google Scholar |
Ensbey M, Legge S, Jolly CJ, Garnett ST, Gallagher RV, Lintermans M, Nimmo DG, Rumpff L, Scheele BC, Whiterod NS, Woinarski JCZ, Ahyong ST, Blackmore CJ, Bower DS, Burbidge AH, Burns PA, Butler G, Catullo R, Chapple DG, Dickman CR, Doyle KE, Ferris J, Fisher DO, Geyle HM, Gillespie GR, Greenlees MJ, Hohnen R, Hoskin CJ, Kennard M, King AJ, Kuchinke D, Law B, Lawler I, Lawler S, Loyn R, Lunney D, Lyon J, MacHunter J, Mahony M, Mahony S, McCormack R, Melville J, Menkhorst P, Michael D, Mitchell N, Mulder E, Newell D, Pearce L, Raadik TA, Rowley JJL, Sitters H, Southwell DG, Spencer R, West M, Zukowski S (2023) Animal population decline and recovery after severe fire: relating ecological and life history traits with expert estimates of population impacts from the Australian 2019-20 megafires. Biological Conservation 283, 110021.
| Crossref | Google Scholar |
Flannigan MD, Krawchuk MA, de Groot WJ, Wotton BM, Gowman LM (2009) Implications of changing climate for global wildland fire. International Journal of Wildland Fire 18, 483-507.
| Crossref | Google Scholar |
Geyle HM, Tingley R, Amey AP, Cogger H, Couper PJ, Cowan M, Craig MD, Doughty P, Driscoll DA, Ellis RJ, Emery J-P, Fenner A, Gardner MG, Garnett ST, Gillespie GR, Greenlees MJ, Hoskin CJ, Keogh JS, Lloyd R, Melville J, McDonald PJ, Michael DR, Mitchell NJ, Sanderson C, Shea GM, Sumner J, Wapstra E, Woinarski JCZ, Chapple DG (2021) Reptiles on the brink: identifying the Australian terrestrial snake and lizard species most at risk of extinction. Pacific Conservation Biology 27, 3-12.
| Crossref | Google Scholar |
Gill AM, McKenna DJ, Wouters MA (2014) Landscape fire, biodiversity decline and a rapidly changing milieu: a microcosm of global issues in an Australian biodiversity hotspot. Land 3, 1091-1136.
| Crossref | Google Scholar |
Gordon L, Evans MJ, Zylstra P, Lindenmayer DB (2025) Trends and gaps in prescribed burning research. Environmental Management 75, 746-760.
| Crossref | Google Scholar | PubMed |
Greer AE (1980) Critical thermal maximum temperatures in Australian scincid lizards: their ecological and evolutionary significance. Australian Journal of Zoology 28, 91-102.
| Crossref | Google Scholar |
Guerin GR, Lowe AJ (2013) Multi-species distribution modelling highlights the Adelaide Geosyncline, South Australia, as an important continental-scale arid-zone refugium. Austral Ecology 38, 427-435.
| Crossref | Google Scholar |
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6, e4794.
| Crossref | Google Scholar | PubMed |
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. Available at https://cran.r-project.org/package=DHARMa [verified 16 October 2024]
Hiers JK, O’Brien JJ, Varner JM, Butler BW, Dickinson M, Furman J, Gallagher M, Godwin D, Goodrick SL, Hood SM, Hudak A, Kobziar LN, Linn R, Loudermilk EL, McCaffery S, Robertson K, Rowell EM, Skowronski N, Watts AN, Yedinak KM (2020) Prescribed fire science: the case for a refined research agenda. Fire Ecology 16, 11.
| Crossref | Google Scholar |
Hill KC, Bakker JD, Dunwiddie PW (2017) Prescribed fire in grassland butterfly habitat: targeting weather and fuel conditions to reduce soil temperatures and burn severity. Fire Ecology 13, 24-41.
| Crossref | Google Scholar |
Jolly CJ, Dickman CR, Doherty TS, van Eeden LM, Geary WL, Legge SM, Woinarski JCZ, Nimmo DG (2022) Animal mortality during fire. Global Change Biology 28, 2053-2065.
| Crossref | Google Scholar | PubMed |
Jones GM, Tingley MW (2021) Pyrodiversity and biodiversity: a history, synthesis, and outlook. Diversity and Distributions 28, 386-403.
| Crossref | Google Scholar |
Kelly LT, Giljohann KM, Duane A, Aquilué N, Archibald S, Batllori E, Bennett AF, Buckland ST, Canelles Q, Clarke MF, Fortin M-J, Hermoso V, Herrando S, Keane RE, Lake FK, McCarthy MA, Morán-Ordóñez A, Parr CL, Pausas JG, Penman TD, Regos A, Rumpff L, Santos JL, Smith AL, Syphard AD, Tingley MW, Brotons L (2020) Fire and biodiversity in the Anthropocene. Science 370, 929.
| Crossref | Google Scholar | PubMed |
Knapp EE, Keeley JE (2006) Heterogeneity in fire severity within early season and late season prescribed burns in a mixed-conifer forest. International Journal of Wildland Fire 15, 37-45.
| Crossref | Google Scholar |
Lazzari J, Sato CF, Driscoll DA (2022) Traits influence reptile responses to fire in a fragmented agricultural landscape. Landscape Ecology 37, 2363-2382.
| Crossref | Google Scholar |
Lenth, R (2024) _emmeans: Estimates Marginal Means, aka Least-Squares Means_. R package version 1.10.3. Available at http://cran.r-project.org/package=emmeans [verified 11 February 2025]
Lindenmayer DB, Claridge AW, Gilmore AM, Michael D, Lindenmayer BD (2002) The ecological roles of logs in Australian forests and the potential impacts of harvesting intensification on log-using biota. Pacific Conservation Biology 8, 121-140.
| Crossref | Google Scholar |
Lindenmayer D, Taylor C, Blanchard W, Zylstra P, Evans MJ (2023) What environmental and climatic factors influence multidecadal fire frequency? Ecosphere 14, e4610.
| Crossref | Google Scholar |
Liu Y, Goodrick S, Heilman W (2014) Wildland fire emissions, carbon, and climate: wildfire-climate interactions. Forest Ecology and Management 317, 80-96.
| Crossref | Google Scholar |
McLauchlan KK, Higuera PE, Miesel J, Rogers BM, Schweitzer J, Shuman JK, Tepley AJ, Varner JM, Veblen TT, Adalsteinsson SA, Balch JK, Baker P, Batllori E, Bigio E, Brando P, Cattau M, Chipman ML, Coen J, Crandall R, Daniels L, Enright N, Gross WS, Harvey BJ, Hatten JA, Hermann S, Hewitt RE, Kobziar LN, Landesmann JB, Loranty MM, Maezumi SY, Mearns L, Moritz M, Myers JA, Pausas JG, Pellegrini AFA, Platt WJ, Roozeboom J, Safford H, Santos F, Scheller RM, Sherriff RL, Smith KG, Smith MD, Watts AC (2020) Fire as a fundamental ecological process: research advances and frontiers. Journal of Ecology 108, 2047-2069.
| Crossref | Google Scholar |
Menges ES, Smith SA, Clarke GL, Koontz SM (2021) Are fire temperatures and residence times good predictors of survival and regrowth for resprouters in Florida, USA, scrub? Fire Ecology 17, 16.
| Crossref | Google Scholar |
Michael DR, Moore H, Wassens S, Craig MD, Tingley R, Chapple DG, O’Sullivan J, Hobbs RJ, Nimmo DG (2021) Rock removal associated with agricultural intensification will exacerbate the loss of reptile diversity. Journal of Applied Ecology 58, 1557-1565.
| Crossref | Google Scholar |
Molina MJ, Llinares JB (2001) Temperature-time curves at the soil surface in maquis summer fires. International Journal of Wildland Fire 10, 45-52.
| Crossref | Google Scholar |
Nimmo DG, Carthey AJR, Jolly CJ, Blumstein DT (2021) Welcome to the Pyrocene: animal survival in the age of megafire. Global Change Biology 27, 5684-5693.
| Crossref | Google Scholar | PubMed |
Nimmo DG, Andersen AN, Archibald S, Boer MM, Brotons L, Parr CL, Tingley MW (2022) Fire ecology for the 21st century: conserving biodiversity in the age of megafire. Diversity and Distributions 28, 350-356.
| Crossref | Google Scholar |
Parr CL, Andersen AN (2006) Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20, 1610-1619.
| Crossref | Google Scholar | PubMed |
Pausas JG, Parr CL (2018) Towards an understanding of the evolutionary role of fire in animals. Evolutionary Ecology 32, 113-125.
| Crossref | Google Scholar |
Penman TD, Towerton AL (2008) Soil temperatures during autumn prescribed burning: implications for the germination of fire responsive species? International Journal of Wildland Fire 17, 572-578.
| Crossref | Google Scholar |
Penman TD, Lemckert FL, Mahony MJ (2006) A preliminary investigation in the potential impacts of fire on a forest dependent burrowing frog species. Pacific Conservation Biology 12, 78-83.
| Crossref | Google Scholar |
Penman TD, Kavanagh RP, Binns DL, Melick DR (2007) Patchiness of prescribed burns in dry sclerophyll eucalypt forests in south-eastern Australia. Forest Ecology and Management 252, 24-32.
| Crossref | Google Scholar |
Penman TD, Christie FJ, Andersen AN, Bradstock RA, Cary GJ, Henderson MK, Price O, Tran C, Wardle GM, Williams RJ, York A (2011) Prescribed burning: how can it work to conserve the things we value? International Journal of Wildland Fire 20, 721-733.
| Crossref | Google Scholar |
Perry RW, McDaniel VL (2015) Temperatures below leaf litter during winter prescribed burns: implications for litter-roosting bats. International Journal of Wildland Fire 24, 544-549.
| Crossref | Google Scholar |
Petit S, Frazer DS (2023) The role of grass-tree Xanthorrhoea semiplana (Asphodelaceae) canopies in temperature regulation and waterproofing for ground-dwelling wildlife. Pacific Conservation Biology 29, 445-455.
| Crossref | Google Scholar |
Preisler HK, Haase SM, Sackett SS (2000) Modeling and risk assessment for soil temperatures beneath prescribed forest fires. Environmental and Ecological Statistics 7, 239-254.
| Crossref | Google Scholar |
QGIS.org (2024) ‘QGIS Geographic Information System.’ (QGIS Association: Switzerland) Available at https://www.qgis.org
Raison RJ, Woods PV, Jakobsen BF, Bary GAV (1986) Soil temperatures during and following low-intensity prescribed burning in a Eucalyptus pauciflora forest. Australian Journal of Soil Research 24, 33-47.
| Crossref | Google Scholar |
R Core Team (2023) R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org [verified 16 October 2024]
Santos X, Badiane A, Matos C (2016) Contrasts in short- and long-term responses of Mediterranean reptile species to fire and habitat structure. Oecologia 180, 205-216.
| Crossref | Google Scholar | PubMed |
Santos JL, Sitters H, Keith DA, Geary WL, Tingley R, Kelly LT (2022) A demographic framework for understanding fire-driven reptile declines in the ‘land of the lizards. Global Ecology and Biogeography 31, 2105-2119.
| Crossref | Google Scholar |
Shine R, Brown GP, Elphick MJ (2016) Effects of intense wildfires on the nesting ecology of oviparous montane lizards. Austral Ecology 41, 756-767.
| Crossref | Google Scholar |
Spellerberg IF (1972) Temperature tolerances of southeast Australian reptiles examined in relation to reptile thermoregulatory behaviour and distribution. Oecologia 9, 23-46.
| Crossref | Google Scholar | PubMed |
Stoof CR, De Kort A, Bishop TFA, Moore D, Wesseling JG, Ritsema CJ (2011) How rock fragments and moisture affect soil temperatures during fire. Soil Science Society of America Journal 75, 1133-1143.
| Crossref | Google Scholar |
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology 65, 13-21.
| Crossref | Google Scholar |
Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK (2012) Coping with thermal challenges: physiological adaptations to environmental temperatures. Comprehensive Physiology 2, 2151-2202.
| Crossref | Google Scholar | PubMed |
Taylor EN, Diele-Viegas LM, Gangloff EJ, Hall JM, Halpern B, Massey MD, Rödder D, Rollinson N, Spears S, Sun B, Telemeco RS (2021) The thermal ecology and physiology of reptiles and amphibians: a user’s guide. Journal of Experimental Zoology 335, 13-44.
| Crossref | Google Scholar | PubMed |
Thompson CM, Purcell KL (2016) Conditions inside fisher dens during prescribed fires; what is the risk posed by spring underburns? Forest Ecology and Management 359, 156-161.
| Crossref | Google Scholar |
Torre GA, Shine R (1996) Patterns of dominance in the small scincid lizard Lampropholis guichenoti. Journal of Herpetology 30, 230-237.
| Crossref | Google Scholar |
Wanthongchai K, Goldammer JG, Bauhus J (2011) Effects of fire frequency on prescribed fire behaviour and soil temperatures in dry dipterocarp forests. International Journal of Wildland Fire 20, 35-45.
| Crossref | Google Scholar |
Warburg MR (1966) On the water economy of several Australian geckos, agamids, and skinks. Copeia 1966(2), 230-235.
| Crossref | Google Scholar |
Whelan RJ, Rodgerson L, Dickman CR, Sutherland EF (2002) Critical life cycles of plants and animals: developing a process-based understanding of population changes in fire-prone landscapes. In ‘Flammable Australia: The Fire Regimes and Biodiversity of a Continent’. (Eds RA Bradstock, JE Williams, AM Gill) pp. 94–124. (Cambridge University Press: Cambridge, UK)
Wickham H (2016) ggplot2: elegant graphics for data analysis. Available at https://ggplot2.tidyverse.org [verified 16 October 2024]
Williams PR, Congdon RA, Grice AC, Clarke PJ (2004) Soil temperature and depth of legume germination during early and late dry season fires in a tropical eucalypt savanna of north-eastern Australia. Austral Ecology 29, 258-263.
| Crossref | Google Scholar |
Wotton BM, Gould JS, McCaw WL, Cheney NP, Taylor SW (2012) Flame temperature and residence time of fires in dry eucalypt forest. International Journal of Wildland Fire 21, 270-281.
| Crossref | Google Scholar |
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3-14.
| Crossref | Google Scholar |


