Anthropogenic fire, vegetation structure and ethnobotanical uses in an alpine shrubland of Nepal’s Himalaya
Asha Paudel A B F , Scott H. Markwith B , Katie Konchar C , Mani Shrestha D E and Suresh K. Ghimire A F
D E and Suresh K. Ghimire A F
A Central Department of Botany, Tribhuvan University, Kathmandu, 44618, Nepal.
B Department of Geosciences, Florida Atlantic University, 777 Glades Road, Boca Raton, FL, 33431, USA.
C 1334 Jackson Street, Tallahassee, FL, 32301, USA.
D School of Media and Communication, RMIT University, Melbourne, Vic. 3001, Australia.
E Faculty of Information Technology, Monash University, Melbourne, Vic. 3800, Australia.
F Corresponding authors. Email: apaudel2017@fau.edu, sk.ghimire@cdbtu.edu.np
International Journal of Wildland Fire 29(3) 201-214 https://doi.org/10.1071/WF19098
Submitted: 1 July 2019 Accepted: 14 December 2019 Published: 23 January 2020
Journal Compilation © IAWF 2020 Open Access CC BY-NC-ND
Abstract
Alpine vegetation of the Himalaya is used as food, medicine or fodder, and is commonly managed with fire by agropastoralists. Prescribed fire can have positive effects on rangeland biodiversity, but studies evaluating its effects in alpine shrublands are scarce. Our objective was to examine the effects of anthropogenic fire on biophysical characteristics, species richness, abundance and composition in an alpine shrubland with socioeconomic value to local peoples in Langtang National Park in central Nepal. We surveyed biophysical variables, vascular plant species richness and composition along three transects at ascending elevations, and conducted interviews with local people and park officials on the use of fire in the region. We found 69 species of vascular plants in 89 plots; species richness was greater in burned plots and with increasing elevation, with 13 species unique to burned plots. We identified 14 indicator species in both burned and unburned plots; eight of them were Himalayan endemics. In burned plots, the indicator species were predominantly grasses and perennial forbs with ethnobotanical uses. This is the first detailed study on alpine shrubland anthropogenic fire in the Nepalese Himalaya. Burning may, at least temporarily, replace woody with more palatable herbaceous species, and weaken the elevational gradient of the shrubland.
Additional keywords: alpine pasture, endemic taxa, indicator species, species richness, transhumance.
Introduction
Fire has historically imposed intense human-induced alterations in landscapes (Thomas and McAlpine 2010), but can also increase plant species richness (Fox 1981; Peterson and Reich 2007) and support livestock grazing by initiating the regeneration of tender and palatable grasses (Mark and Holdsworth 1979; Mark 1994). Fire is also a natural disturbance process that can have a positive role in rangeland and ecosystem management and biodiversity conservation (Carlson et al. 1993; Sheuyange et al. 2005; Brandt et al. 2013; Davies et al. 2014), influence the maintenance of community structure and function, and suppress as well as foster successional processes (Chapin and Van Cleve 1981; Chapin 1983; Shang et al. 2007; Barros et al. 2017). Variable fire severities and frequencies occur across landscapes depending on several biotic and abiotic factors, such as topography, wind speed, temperature, precipitation and fuel load, as well as combustion type (smouldering v. flaming), stand composition and developmental stage of plant species (White et al. 1996; Bigler et al. 2005; Bond and Keeley 2005; Collins et al. 2007; Harris and Taylor 2015). As a function of these factors, the burn patterns vary regionally or within one landscape, resulting in patches of burned and unburned areas with different shapes, sizes and severities (White 1979; Bond and Keeley 2005). Forest gaps created by fires naturally go through a dynamic process of shifting floristic composition initiated by competition for resources, mostly light, moisture and nutrients (Huston and Smith 1987). It can alter plant species composition and richness by exposing and changing soil properties and providing space for the establishment of pioneer and r-strategist type species, with shorter life span, such as, herbs and shrubs in the case of plants (Wesche 2006; Binelli et al. 2008; de Villiers and O’Connor 2011).
Absence of fire for many years in fire-dependent ecosystem leads to change in species composition. For example, suppression of fire for many decades in dry conifer forest in California has led to a dramatic increase in shade-tolerant and fire-intolerant species, outcompeting shade-intolerant species that are also fire-tolerant woody species (Parsons and DeBenedetti 1979; Habeck 1994). Similarly, woody plant encroachment in semiarid and arid rangeland of Australia and Africa in the absence of fire is a common problem that has resulted in loss of biodiversity and affected livestock production (Watkinson and Ormerod 2001; Price and Morgan 2008; Archer 2010).
The alpine shrubland of Nepal is a region with rapidly changing climate that is likely to experience shifting vegetation structure (Gaur et al. 2003; Telwala et al. 2013; Salick et al. 2019), and is also managed with the use of anthropogenic fire to provide social and economic value to local peoples. Wildfires are common in Nepal in all physiographic zones during the dry period from March to May every year (Matin et al. 2017). Out of 30 220 fire hotspots recorded in Nepal between 2000 and 2013, 7283 (24%) occurred in alpine pastures during the hot and dry season (Parajuli et al. 2015). Approximately 50–58% of wildfires in Nepal are set deliberately by locals to enhance regeneration of grasses for pasture and hunting, clearing land for cultivation, and for firewood and non-timber forest product (NTFP) collection (Fig. 1a, b) (Karkee 1991; Bajracharya 2002; Matin et al. 2017). Along with their essential ecological roles, alpine shrublands contribute socioeconomic benefits to the local people. For example, people in the Himalaya use alpine Rhododendron and Juniperus species for incense, medicine and fuel, and for shelters for migratory pastoralists grazing their herds (Schmidt-Vogt 1990; Lama et al. 2001; Bhattacharyya 2011). The combined effects of human activities such as grazing, cutting (logging/looping) and trampling decrease the ecosystem’s natural resiliency after fire (Folke et al. 2004). Thus, the ecosystem may become more vulnerable to subsequent impacts and the previous dominant vegetation communities may not return with similar diversity or composition.

|
Beginning approximately 50 years ago, the residents of Langtang National Park observed changes in the composition of vegetation due to fire (personal interviews with local agropastoralists, July 2011). Elders recall that the south-facing forests above Chandanbari and below Lauribina Hill were dominated by Abies spectabilis (D. Don) Spach (east Himalayan fir). Intense fire and the felling of trees for fuel wood and timber has since nearly cleared the forest stands on southern slopes and allowed the spread of shrub species such as Piptanthus nepalensis (Hook.) Sweet (evergreen laburnum) and Berberis aristata DC. (Indian barberry), and herbaceous species such as Euphorbia wallichii Hook. f. (Wallich spurge) and Sambucus adnata Wall. ex DC. (east Himalayan elderberry) (Fig. 1c, d). In high-elevation areas, Rhododendron anthopogon D. Don (dwarf rhododendron) and R. setosum D. Don (bristly rhododendron) shrubs form several dominant stands with lush mosses with occasional large R. campanulatum D. Don (bell rhododendron) and Sorbus microphylla (Wall. ex Hook. f.) Wenz. (small-leaf rowan) shrubs overtopping the smaller shrub stands. However, fire can fragment the continuous vegetation starting from the tree line up to the alpine rhododendron shrubland (Fig. 1e, f).
Globally, the body of research on the effects of fire in forest ecosystems of commercial value is extensive (Risser 1990; Williams et al. 1994; Bigler et al. 2005; Bond and Keeley 2005; Collins et al. 2007), but studies evaluating the effects of fire in alpine shrublands are relatively meagre (Knox and Clarke 2006) and non-existent in Nepal. Most fires above timberline in mountainous regions are severe (Wesche 2006; Williams et al. 2008), which tends to hold true in Himalayan shrublands dominated by aromatic plant species such as Rhododendron and Juniperus. Sclerophyllous shrublands are particularly susceptible to fire because they are typically dry, and may secrete flammable secondary chemicals (Christensen 1985). The low stature and single physiognomic type of shrublands commonly leads to intense crown fires (Christensen 1985). The alpine zone in the Nepal Himalaya belongs to the Western and Eastern Himalayan alpine shrub and meadow ecoregion (Olson et al. 2001). This region is known for having high species richness and supporting a large number of rare, endemic and threatened species (Shrestha and Joshi 1996; Basnet 2006), the majority of which are important from socioeconomic and cultural perspectives in addition to their conservation significance (Olsen and Larsen 2003; Ghimire et al. 2006, 2008; Salick et al. 2014). Patches of shrubs in alpine meadows are found facilitating the growth of grasses, forbs and many other important herbaceous species, either by providing suitable habitats or by protecting them from herbivores (Jacquez and Patten 1996; Körner 2003; Li et al. 2011). The mosaic – composed of sub-alpine and alpine meadows, shrublands, high-elevation agropastoral fields, forests and a large range of other habitats – is high-elevation Himalayan landscapes inscribed by human activities (Ghimire et al. 2006). Their biodiversity is shaped by the interaction among geological, climatic and topographical factors, cultural traditions and modern land-use impacts.
Studies in the Himalaya are important for understanding how traditional ecological knowledge and practices related to pastoralism are influencing and interacting with alpine shrublands, and how social and climatic developments will impact alpine zone ecology and the socioeconomic futures of the local people. The biodiverse alpine environments in the Himalaya are among the habitats experiencing the most drastic global climate change, with increasing temperature, a heavier and more unpredictable rainfall pattern, and rapidly melting permanent snows and glaciers. Research in the Himalaya suggests that the warming climate has already caused alpine plants and their habitats to shift upslope towards higher elevations (Gaur et al. 2003; Telwala et al. 2013; Salick et al. 2019). Such shifts are continually changing the communities of high-elevation regions, outcompeting threatened and endemic plants and eventually pushing the vital alpine life zones to extinction as they reach ridge tops. Climate change that threatens alpine plants also affects the traditional practices and livelihood of both indigenous peoples and massive downstream populations (Salick and Byg 2007; Salick et al. 2014).
The objective of the present study was to examine the effects of anthropogenic fire on biophysical variables, species richness, abundance and composition in an alpine shrubland with social and economic value to local peoples in Langtang National Park in the northern region of central Nepal. Specific research questions included: (1) do biophysical factors vary between burned and unburned plots; (2) do plant diversity, frequency and composition vary among burned and unburned plots; and (3) are species used by locals as NTFPs enhanced by anthropogenic burning? To address our objective and research questions, we sampled plots across a narrow elevational gradient of alpine shrubland that burned in 2009 from anthropogenic causes, and interviewed local agropastoralists with first-hand knowledge of the ethnobotanical importance of species within the alpine zone.
Methods
Study area
The study area (28°05.371′ to 28°05.660′N longitude and 85°23.337′ to 85°23.517′E latitude) is located on Lauribina Danda (danda is hill in Nepali) in the lower alpine zone of Langtang National Park (LNP) in the northern part of central Nepal (Fig. 2). LNP covers subtropical to alpine climatic conditions owing to high elevational variation. The northern aspect is cool and moist while the southern aspect is warmer and drier. LNP receives an annual precipitation of 650 mm (Langtang station, 3920 m above sea level (a.s.l.)) to 1800 mm (Dhunche station, 1950 m a.s.l.). Most of the precipitation occurs during the summer monsoon season, which lasts from June until the beginning of October. Average maximum temperature was 23°C in June and average minimum temperature was ~2°C in January.
|
The study area has 20–35° slopes with aspects ranging from 310° north-west to 30° north-east. Because sampling covered a small geographical area with plots only in the northern aspect with almost constant slope to minimise the effect of topographic variation, it is unlikely there was a difference in the amount of radiation received in burned and unburned patches. The study area was above the tree line, with a mosaic of habitats dominated by grassland and shrubland. The tree line varies within 3900–4000 m a.s.l., with Abies spectabilis and Betula utilis D. Don (Himalayan birch) in the overstorey and Rhododendron campanulatum and Sorbus microphylla in the understorey. The latter two species reach up to 4200 m a.s.l. Above forest line in lower reaches of the alpine zone (>3900–4200 m a.s.l.), the vegetation comprises vast stretches of shrubland dominated by Rhododendron anthopogon, R. lepidotum Wall. ex G. Don (pink scaly rhododendron) and R. setosum on the northern aspects. Other woody species in the lower alpine belt include Rhododendron campanulatum, Juniperus recurva Buch.-Ham. ex D. Don (Himalayan juniper), Berberis spp., Ephedra gerardiana Wall. ex Stapf (Gerard jointfir), Spiraea arcuata Hook. f. (arching spirea), Salix spp. and Potentilla fruticosa L. (shrubby cinquefoil) (Chaudhary 1998; SK Ghimire, S Thapa-Magar, MR Shrestha, B Devkota, MR Gubhaju 2008, unpubl. data).
The alpine grassland and shrubland in the study area are used by local agropastoralists for grazing livestock and collecting plants for local use. There were altogether three alpine shelters of stone construction (goth in Nepali) in the Lauribina area, which are used during the summer grazing season for shelter and rest for pastoralists, and keeping livestock temporarily. The herders traditionally managed the pastoral land through rotational grazing of livestock, and seasonal burning of vegetation (Karki and McVeigh 2000). As the study area was included under the protected area system in 1976, any unauthorised anthropogenic fire and illegal or haphazard harvesting of natural resources are subject to a certain degree of regulation. However, the national park acknowledges the traditional practice of subsistence use of natural resources. In interviews, local agropastoralists stated that the Lauribina area has received random and infrequent fire to enhance the growth of palatable species since the establishment of the national park.
Vegetation sampling and data collection
Sampling took place within the alpine rhododendron shrubland of Lauribina Danda, in June 2011, 2 years after an ~100 ha fire in 2009. Within the perimeter of the fire, a patchy mosaic was created with high-severity fire killing the majority of aboveground biomass in the burned matrix, but with numerous unburned patches completely escaping fire and absent of fire scars (mean unburned patch size 2893 m2) (see burn mosaic Fig. 1f). The timing of the fire was determined via field observations in 2009, through subsequent interviews with local agropastoralists (n = 10) from the surrounding villages, and was verified on NASA satellite imagery (NASA 2009).
Three parallel transects (each 500–700 m in length) were established paralleling the slope contour: lower summit transect (T1) at 3900 m a.s.l., mid-summit transect (T2) at 4000 m a.s.l., and upper summit transect (T3) at 4100 m a.s.l. In addition to positioning perpendicular to the elevational gradient, the transects were concomitantly perpendicular to the dominant shrub biomass gradient, with the girth (cm) of burned stumps declining with increasing elevation (girth mean ± s.d.: T1 = 25.80 ± 19.65, T2 = 6.37 ± 1.08, T3 = 3.42 ± 0.65 cm).
Each of the three transects was divided into three segments in burned matrix and three segments in unburned shrub patches. Within each segment, plots were systematically placed starting ~5 m from the matrix or patch margin and extending to ~5 m from the next matrix or patch margin, maintaining a 20–30-m distance between successive plots. A total of 89 plots were sampled, 49 burned and 40 unburned (T1 = 17 burned, 14 unburned plots; T2 = 16 burned, 14 unburned plots; T3 = 16 burned, 12 unburned plots). At each plot, we recorded geographical position (latitude and longitude) using a global positioning system, soil pH using a pH meter, and soil moisture with a moisture-reading electrode (Takemura Electric Works DM-15 soil tester).
In each plot, we sampled vegetation in three 1-m2 quadrats oriented diagonally, which were further divided into four (0.5 × 0.5 m) subquadrats. In total, 267 quadrats (1 m2) and 1068 subquadrats (0.5 m2) were sampled across all 89 plots. Within each subquadrat, the team recorded presence or absence of all vascular plant species. If a species was present in all four subquadrats, it was assigned a categorical abundance value of 4. If a species was present in three out of four subquadrats, it was assigned a categorical abundance value of 3, and so on. Vegetation data from quadrats was pooled by plot for analysis purposes. We identified as many species in the field as possible following published resources (Polunin and Stainton 1984; Ghimire et al. 2008) and authors’ personal expertise on alpine plants. Botanical vouchers were deposited at Tribhuvan University Central Herbarium, Nepal, where field identifications were later confirmed. In each quadrat, we also recorded the percentage cover of each of the following biophysical variables: exposed soil, rocks, dead wood, litter, graminoids (including grasses, sedges and rushes), forbs, shrubs, trees, mosses and lichens, as well as the total number and girth of burned stumps.
We interviewed local agropastoralists (n = 10) about the use of plant species recorded in the study area and their palatability for herbivores. We specifically asked whether specific sampled plants are used by local residents, and if used, for what purpose. We also reviewed ethnobotanical literature (for example, Lama et al. 2001; Manandhar 2002; SK Ghimire, S Thapa-Magar, MR Shrestha, B Devkota, MR Gubhaju 2008, unpubl. data) to verify the use of the plant from other regions. In the case of species consumed by livestock, we asked respondents to rank the palatability. Following Daalkhaijav (2005), we categorised the palatability into preferred, desirable, consumed but less desirable, not consumable and toxic.
Both verbal and written consents were obtained for the study. Written permission for fieldwork was obtained from the authorities at LNP and Buffer Zone, and at the Department of National Park and Wildlife Conservation, Government of Nepal. Prior verbal informed consents were obtained from the local communities in Chandabari and Lauribina within LNP before establishing participation and consultation of local agropastoralists.
Data analysis
Mann–Whitney U Tests (non-parametric) were conducted using SPSS v.17 (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0) to compare biophysical data between unburned and burned plots, because the biophysical data did not meet the assumptions of parametric tests even after transformation.
Species richness, α-diversity or the number of vascular plant species per unit area, was calculated for each plot. A second measure, γ-diversity or the number of vascular plant species in unburned patches and the burned matrix, was also calculated. We used two-way ANOVA in Stata v.15 to compare vascular plant species richness at the plot scale between unburned and burned plots and among the three transects (i.e. upper, mid and lower elevations) and their interactions. Post-hoc Tukey Honest Significant Difference tests were conducted to compare pairwise differences among burned and unburned plots within and among transects.
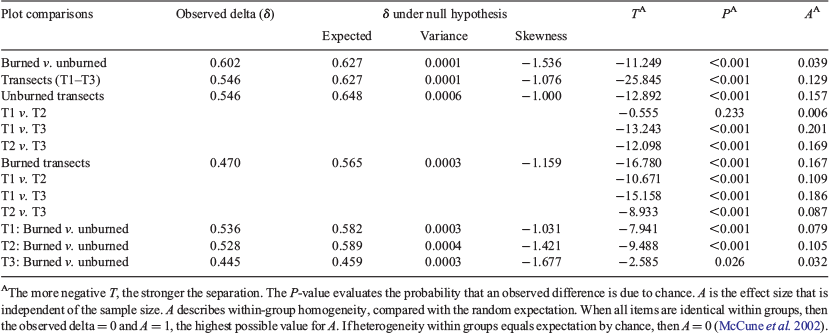
Prior to running both the multiresponse permutation procedure (MRPP) and two-way cluster analyses, species frequency in each plot was square-root-transformed and species found in less than 5% of the plots were discarded. We used MRPP (Biondini et al. 1988; McCune et al. 2002; Cai 2006) to examine species compositional differences among burned and unburned plots and transects. We performed MRPP on species frequency in each plot for combined datasets, and burned and unburned plots separately and transects separately. MRPP was performed using PC-ORD v.7 (McCune and Mefford 1999) using the Sorensen distance measure.
Two-way cluster analysis was run using PC-ORD v.7 (McCune and Mefford 1999). For the cluster analysis, species frequency was relativised by the column maximum, the distance measure was Sorensen, and a flexible β linkage method with a value of −0.25 was selected.
The presence and abundance of key indicator plant species (Dai et al. 2006) are biological characters of groups of sites representing habitat types or combinations of habitat types and are of prime interest for ecosystem conservation and management (Legendre and De Cáceres 2013). Thus, we used the indicator value method (IVM) (Dufrene and Legendre 1997) to determine the indicator species for burned and unburned plots within the three transects. Those species with high indicator values are the indicator species (McCune et al. 2002; Dai et al. 2006). We used PC-ORD v.7 (McCune and Mefford 1999) and a Monte Carlo test with 4999 permutations (McCune and Mefford 1999) to test the statistical significance of indicator values (IV).
Results
Biophysical variables
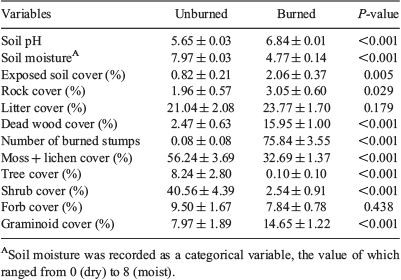
The unburned and burned plots significantly differed for 10 out of 12 variables sampled (Table 1). Unburned plots showed significantly greater cover values for tree (8.24 v. 0.10%), shrub (40.56 v. 2.54%) and moss–lichen (56.24 v. 32.69%), with Rhododendron campanulatum, R. anthopogon, R. setosum and R. lepidotum contributing the main shrub cover. Burned plots showed significantly greater cover values for graminoids (14.65 v. 7.97%), dead wood (15.95 v. 2.47%) and exposed soil (2.06 v. 0.82%) compared with unburned plots. Unburned and burned plots also differed in terms of edaphic properties. Soil in burned plots had higher pH (6.84 v. 5.65) and lower moisture (4.77 v. 7.97) compared with unburned plots (Table 1).
|
Species richness
Total vascular plant species richness (γ-diversity) varied from 55 species in unburned patches to 62 species in the burned matrix (altogether, 69 species; see Supplementary material Table S1 for the list of species recorded, their elevation range and local use). At the plot level, the two-way ANOVA resulted in an R2 = 0.585, and fire (F-value = 8.97, P = 0.004) and transect (F-value = 55.98, P < 0.001) both had significant effects on species richness, while the interaction effect was not significant (F-value = 2.49, P = 0.089). The mean vascular plant species richness was significantly greater in the burned than in the unburned plots at all three transect elevations (P < 0.05, Fig. 3). The species richness significantly increased along the elevational gradient from lower to upper transect, indicating that elevation was a statistically significant variable affecting vascular plant species richness (Fig. 3).
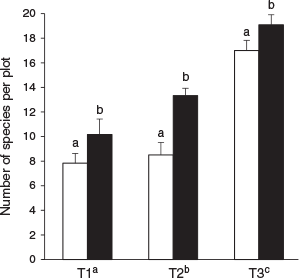
|
Species composition
The MRPP showed statistically significant compositional differences between burned and unburned plots (P < 0.001) and also between transects (P < 0.001) (Table 2). Multiple pairwise comparisons showed that burned plots in all three transects and unburned plots in Transects 1 and 3 and Transects 2 and 3 had significant compositional differences (P < 0.001 for all pairwise comparisons). In unburned plots, Transects 1 and 2 were broadly overlapping and thus the hypothesis of no difference between groups could not be rejected. In addition, within each of the three transects, burned and unburned plots had significantly differing compositions (P < 0.001 in T1 and T2, P < 0.05 in T3).
The two-way cluster analysis exhibited distinct clustering by transect (Fig. 4). There were two major groups of plots, one completely composed of plots from Transects 1 and 2 (regular and inverted triangles), and one that included all plots from Transect 3 (boxes), 10 plots from Transect 2, and 3 plots from Transect 1. Within the group composed of Transects 1 and 2, burned and unburned plots were largely separated into different subgroups, with the majority of burned plots coming from Transect 1. In addition, the 13 plots from Transects 1 and 2 that were grouped with the plots from Transect 3 were predominantly burned plots from Transect 2. These burned plots from Transect 2 continued to support Rhododendron anthopogon, as root sprouts, and had an equivalent number of burned stumps (mean 74.7) to all burned plots together (Table 1). Thus, before fire, plots in Transect 2 were dominated by large shrubs-like plots in Transect 1.
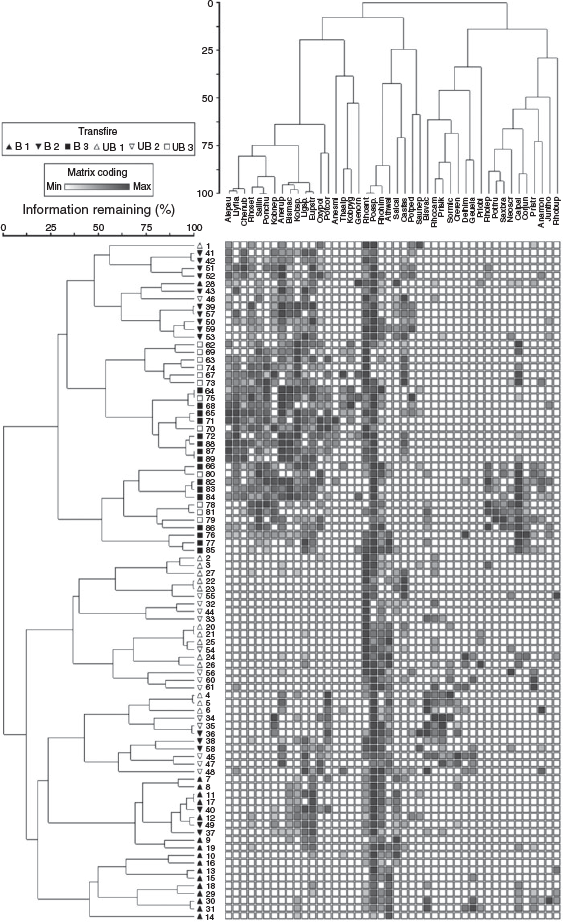
|
Indicator species
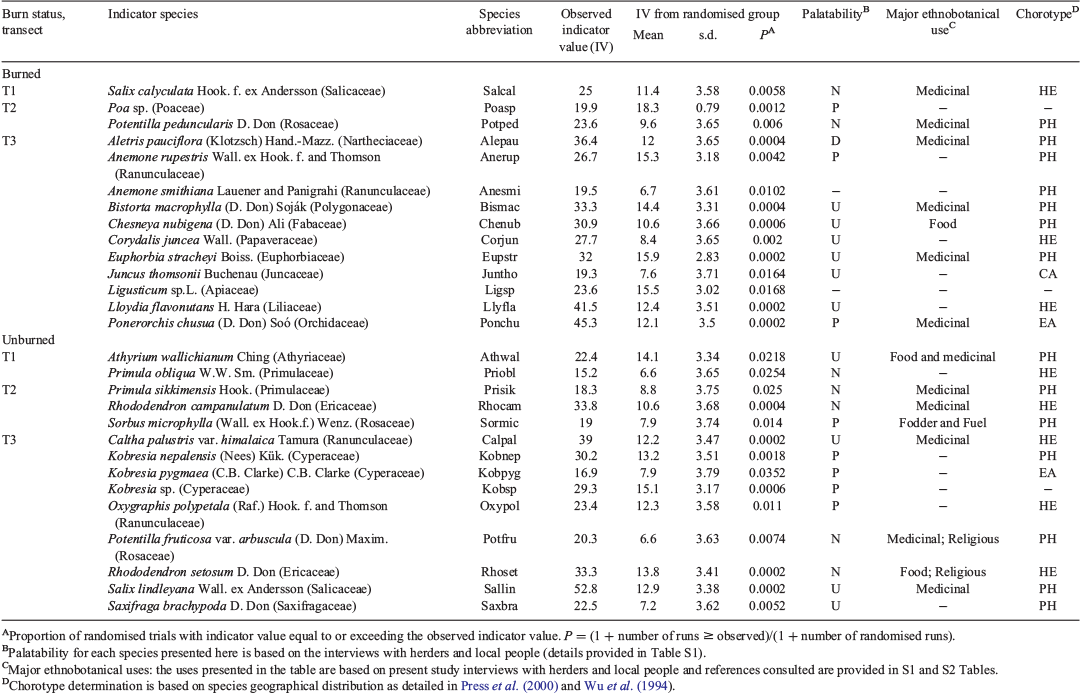
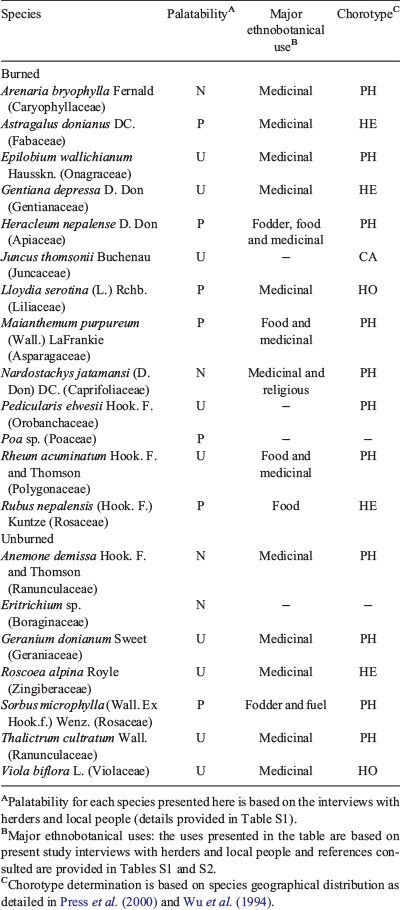
Out of 69 vascular plant species recorded in this study, 46 species were common for both burned matrix and unburned patches. The number of unique species (i.e. the species recorded only either in burned or unburned plots) was higher in burned (n = 13) than in unburned (n = 7) plots (Table 3). Several highly palatable species: Astragalus donianus DC. (dark-red milkvetch), Poa sp., Heracleum nepalense D. Don (Nepal cowparsnip), Lloydia serotina (L.) Rchb (mountain spiderwort), Maianthemum purpureum (Wall.) LaFrankie (purple mayflower), Rubus nepalensis (Hook. f.) Kuntze (Himalayan creeping bramble), and some important medicinal plant species, e.g. Gentiana depressa D. Don, Nardostachys jatamansi (D. Don) DC. (jatamansi) and Rheum acuminatum Hook. f. and Thomson (Himalayan rhubarb), were found only in the open burned matrix created by fire (Table 3). Similarly, unburned plots supported unique species like Anemone demissa Hook. f. and Thomson (floppy Himalayan anemone), Geranium donianum Sweet (Don’s geranium), and Roscoea alpina Royle (mountain roscoe lily), which were absent from the burned habitat and also had important medicinal value (Table 3). Sorbus microphylla was the only palatable species unique to unburned plots, while the other unique species were undesirable or not consumed. Himalayan and Pan-Himalayan endemics represented 15 of the 20 identified unique species (Table 3), and the majority of indicator species were also dominated by these endemics (Table 4). In total, 14 plant species were identified as indicators for burned and 14 species for unburned plots (P < 0.05, based on Monte Carlo test; Table 4).
|
The most substantial variation among burned and unburned plots was found with Transect 2. Large shrubs Rhododendron campanulatum and Sorbus microphylla were indicators of unburned plots in Transect 2, but a forb and a grass were indicators of Transect 2 in burned plots. Transects 1 and 3 were generally represented by indicators that were forbs, ferns and dwarf woody species in both burned and unburned plots. Transect 3 indicators of burned plots included several species that are recognised indicators of disturbance due to overgrazing, e.g. Anemone rupestris Wall. ex Hook. f. and Thomson (blue rock anemone), Anemone smithiana Lauener & Panigrahi, and Euphorbia stracheyi Boiss. (Himalayan prostrate spurge) (Bauer 1990; Ghimire et al. 2006).
Discussion
Anthropogenic burning in alpine shrublands may be sustainable as a temporally and spatially dynamic process, providing fodder, food, fuel, medicine and religiously significant resources to practitioners of burning. Agropastoralists of the Nepalese Himalaya have used fire in alpine pastures purposefully for social and economic reasons, i.e. for the regeneration of grasses and for the promotion of grazing, for an unknown number of generations. Although prescribed fire used as a management tool has been studied in Nepal’s subtropical grasslands, where grazing is prohibited (e.g. Peet et al. 1999), this is the first detailed study of anthropogenic fire in Himalayan alpine shrublands, where burning and grazing are common disturbances linked to the livelihood of the local people.
The number of unique species was greater in burned than in unburned plots, and most of the unique species were Himalayan or Pan-Himalayan endemics. Some of the species unique to burned plots were rare and threatened forbs, such as Nardostachys jatamansi (International Union for Conservation of Nature, IUCN Redlist critically endangered) and Rheum acuminatum, which have substantial local use and medicinal values (Manandhar 2002; SK Ghimire, S Thapa-Magar, MR Shrestha, B Devkota, MR Gubhaju 2008, unpubl. data). Some wider-ranging species, such as Juncus thomsonii found across central Asia, were also present only in burned habitat. Earlier studies have shown that rare and threatened plant species may be favoured, to some extent, by intermediate burning (Dudley and Lajtha 1993; Van Lear et al. 2005). A study evaluating the detailed demographic effects of fire on rare and threatened plant species should be completed in the Himalayan alpine ecosystem to inform best management practices for conservation of species and continued socioeconomic benefit to local agropastoralists.
Many studies have shown that fire positively influences species diversity by maximising the coexistence of numerous species owing to the removal of competing woody biomass and making habitat suitable for forbs, grasses and fire-adapted perennial species (Reilly et al. 2006; Twidwell et al. 2012; Bowles and Jones 2013). Fire is one of the dominant ecological factors in ericaceous shrublands (Allen et al. 1996), and in Rhododendron shrublands, fire is favoured by its flammable secondary metabolites, the presence of dense woolly indumentum on leaves (Ng and Corlett 2003; Innocenti et al. 2010; Paul et al. 2010; Guleria et al. 2011), a lush thicket of mature stumps and good-quality fuels. The results of the MRPP and two-way cluster analysis demonstrate that the effects of burning can, at least temporarily, foster a change in habitat composition from one dominated by shrubland species to one where grassland species may thrive. The Lauribina Danda fire created a mosaic of high-severity matrix and unburned patches, converting a homogeneous shrubland to a more heterogeneous community of mixed grassland and shrubland.
There appears to be an interaction between elevation and fire in changes in community composition. Stronger variation in composition and species richness was observed between burned and unburned plots in the mid-elevation Transect 2 compared with the lowest-elevation Transect 1 and highest-elevation Transect 3. The IV analysis demonstrated a change at mid-elevation, at least temporarily, in vegetation composition from shrubland to a grassland similar to higher-elevation alpine grasslands, i.e. large R. campanulatum and Sorbus microphylla shrub dominance in unburned areas compared with grass, forb and dwarf shrub dominance in burned areas of Transect 2. In addition, in the cluster analysis, unburned plots from Transect 2 grouped nearly uniformly with plots from Transect 1 (also unburned plots did not differ between Transects 1 and 2 with the MRPP analysis), but burned plots from Transect 2 were much more likely to be grouped with plots from Transect 3. The pattern in Transect 2 is similar to results reported from other regions (Walker 2001; Sheuyange et al. 2005), where herbaceous and graminoid species are favoured by burning and shrub cover is temporarily reduced (Sheuyange et al. 2005).
The dominant shrubland species were not extirpated, and a longer monitoring period is required, but our data indicate that the community likely possesses the capacity to respond resiliently to disturbance and may not permanently shift away from the pre-burn vegetation structure. Although their aboveground biomass was either mineralised or harvested, the shrubs readily sprouted from the remaining rootstock and will likely regain canopy dominance in a cyclical pattern shifting across the alpine shrubland zone as mature patches are burned, utilised and left fallow. Because Rododendron shrubs are highly valued as medicinal and aromatic plants, their resprouting and regrowth is an important step in the process that maintains their future availability for ethnobotanical use.
Changes in species range sizes due to climate change are especially likely in montane regions (Myers et al. 2000). Climate change is not only a threat to the plant species and ecology of montane regions, but to the social and economic sustainability of local people who utilise vegetation on the slopes of mountain ranges like the Himalaya. Annual temperature is projected to increase in the Himalaya by 4–5°C by the end of the 21st century (Kumar et al. 2006). Feeley and Silman (2010) calculated that similar temperature changes are expected on the eastern slopes of the Andes, and that plants will need to migrate upslope >900 m by the end of the century to remain at climate equilibrium. Thus, on some ridges, such as Lauribina Hill, alpine plants will be squeezed between the ridge top and the upward-migrating shrubs and trees. The only remaining habitat options for persistence of herbaceous alpine species may become burned patches where woody cover is consumed. Our results suggest that local agropastoralist’s use of fire opens gaps in the shrub canopy and favours herbaceous species at least temporarily. Their use of fire in the future may be the only mechanism for maintaining adequate grazing fodder and other species of ethnobotanical significance in proximity to their current settlements. However, the interaction of increasing temperature with fire may create more dangerous burning conditions over time.
In accordance with the results of earlier studies (Xiang et al. 2014), we found that burned and unburned shrub habitat patches differed considerably in edaphic properties; burned soils had higher, approximately neutral, pH and lower soil moisture compared with unburned soils. Generally, fire increases soil pH and nutrient availability, but severe wildfire can have deleterious and lasting effects on soil nutrients, structure, porosity and microbial activity (Certini 2005; Xiang et al. 2014), and hydrophobicity of burned soil reduces water absorption capacity and makes soil more prone to erosion (Certini 2005). The present study was completed 2 years after fire, and the observed differences in vegetation composition may have been influenced to an unknown extent by changes in soils. Further, a detailed account of soil property changes due to fire is beyond the scope of this research and requires separate and detailed study. As observed in LNP, colonisation and growth of grasses can benefit from burning with exposed soils, both of which were significantly greater in burned areas on Lauribina Danda, and reduced dominance of competitive woody species (Walker 2001), which covered a significantly smaller area in the burned matrix in our study area.
Conclusions
Fire at LNP in central Nepal was found to open the shrub canopy of Rhododendron species and increase species richness of herbaceous plants, including grasses and forbs of ethnobotanical value 2 years after fire. Our study area showed early signs of secondary succession in fire-affected shrublands of the subalpine zone. We also found that the IV analysis demonstrated a change at mid-elevation, at least temporarily, in vegetation composition from shrubland to a grassland similar to higher-elevation alpine grasslands. Burning to some extent also favoured Himalaya and Pan-Himalaya endemics and some rare and threatened species. Climate change and the potential for increasing anthropogenic impacts from alteration of historic fire regimes, medicinal plant harvesting, logging and grazing may place alpine shrublands at greater risk. Reduction and potential elimination of those shrublands in central Nepal not only threatens biodiversity but the local livelihoods of the people of LNP. Additional data may expose relationships and responses not apparent in the earliest years after high-elevation fires (DellaSala et al. 2015). We recommend a landscape-scale analysis of the alpine shrubland and pasture zone to examine the potential existence of a shifting mosaic steady state (Bormann and Likens 1979) created by anthropogenic fire and local management. We also suggest the establishment of long-term monitoring on the effects of anthropogenic fire on alpine shrublands and a plan that includes locals for sustainable management of this valuable habitat.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was completed in conjunction with botanical research supported by the Missouri Botanical Garden in collaboration with Central Department of Botany, Tribhuvan University. We are thankful to authorities at LNP and the Department of National Park and Wildlife Conservation (DNPWC), Government of Nepal, for providing the permit to work inside the park. We are thankful to Binod Koirala for assisting in the fieldwork and to Dr Sheetal Vaidya for her critical suggestions on the manuscript. We are very grateful to the locals who supported the team during fieldwork. MS is thankful to Associate Professors Adrian G. Dyer and Alan Dorin for support, and also acknowledges a research award from the School of Media and Communication to facilitate part of this research.
References
Allen RB, Basher LR, Comrie J (1996) The use of fire for conservation management in New Zealand. Science for Conservation 23, 1173–2946. Department of Conservation. (Wellington, New Zealand)Archer SR (2010) Rangeland conservation and shrub encroachment: new perspectives on an old problem. In ‘Wild rangelands: conserving wildlife while maintaining livestock in semi-arid ecosystems’. (Eds JT du Toit, R Kock, JC Deutsch) pp. 53–97. (Wiley–Blackwell: Oxford, UK)
Bajracharya KM (2002) Forest fire situation in Nepal. International Forest Fire News 26, 84–86.
Barros AMG, Ager AA, Day MA, Preisler HK, Spies TA, White E, Pabst RJ, Olsen KA, Platt E, Bailey JD, Bolte JP (2017) Spatiotemporal dynamics of simulated wildfire, forest management, and forest succession in central Oregon, USA. Ecology and Society 22, 24
| Spatiotemporal dynamics of simulated wildfire, forest management, and forest succession in central Oregon, USA.Crossref | GoogleScholarGoogle Scholar |
Basnet K (2006) Effects of anthropogenic disturbances on biodiversity: a major issue of protected-area management in Nepal. In ‘Land use change and mountain biodiversity’. (Eds EM Spehn, M Liberman, C Körner) pp. 293–306. (CRC Press, Taylor and Francis Group: Boca Raton, FL, USA)
Bauer JJ (1990) The analysis of plant–herbivore interactions between ungulates and vegetation on alpine grasslands in the Himalayan region of Nepal. Vegetatio 90, 15–34.
| The analysis of plant–herbivore interactions between ungulates and vegetation on alpine grasslands in the Himalayan region of Nepal.Crossref | GoogleScholarGoogle Scholar |
Bhattacharyya D (2011) Rhododendron species and their uses with special reference to Himalayas – A review. Assam University Journal of Science and Technology 7, 161–167.
Bigler C, Kulakowski D, Veblen TT (2005) Multiple disturbance interactions and drought influence fire severity in Rocky Mountain subalpine forests. Ecology 86, 3018–3029.
| Multiple disturbance interactions and drought influence fire severity in Rocky Mountain subalpine forests.Crossref | GoogleScholarGoogle Scholar |
Binelli EK, Gholz HL, Duryea ML (2008) Plant succession and disturbances in the urban forest ecosystem. In ‘Restoring the urban forest ecosystem”. (Eds ML Duryea, E Kampf Binelli, LV Korhnak) pp. 1–23. (School of Forest Resources and Conservation, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL).
Biondini ME, Mielke PW, Berry KJ (1988) Data-dependent permutation techniques for the analysis of ecological data. Vegetatio 75, 161–168.
| Data-dependent permutation techniques for the analysis of ecological data.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20, 387–394.
| Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bormann FH, Likens GE (1979) ‘Pattern and process in a forested ecosystem: disturbance, development and the steady state based on the Hubbard Brook ecosystem study.’ (Springer-Verlag: New York, NY, USA)
Bowles ML, Jones MD (2013) Repeated burning of eastern tallgrass prairie increases richness and diversity, stabilizing late successional vegetation. Ecological Applications 23, 464–478.
| Repeated burning of eastern tallgrass prairie increases richness and diversity, stabilizing late successional vegetation.Crossref | GoogleScholarGoogle Scholar | 23634595PubMed |
Brandt JS, Haynes MA, Kuemmerle T, Waller DM, Radeloff VC (2013) Regime shift on the roof of the world: alpine meadows converting to shrublands in the southern Himalayas. Biological Conservation 158, 116–127.
| Regime shift on the roof of the world: alpine meadows converting to shrublands in the southern Himalayas.Crossref | GoogleScholarGoogle Scholar |
Cai L (2006) Multiresponse permutation procedure as an alternative to the analysis of variance: an SPSS implementation. Behavior Research Methods 38, 51–59.
| Multiresponse permutation procedure as an alternative to the analysis of variance: an SPSS implementation.Crossref | GoogleScholarGoogle Scholar | 16817513PubMed |
Carlson PC, Tanner GW, Wood JM, Humphrey SR (1993) Fire in key deer habitat improves browse, prevents succession, and preserves endemic herbs. The Journal of Wildlife Management 57, 914–928.
| Fire in key deer habitat improves browse, prevents succession, and preserves endemic herbs.Crossref | GoogleScholarGoogle Scholar |
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10.
| Effects of fire on properties of forest soils: a review.Crossref | GoogleScholarGoogle Scholar | 15688212PubMed |
Chapin FS (1983) Patterns of nutrient absorption and use by plants from natural and man-modified environments. In ‘Disturbance and ecosystems. Ecological studies (analysis and synthesis), Vol. 44’. (Eds HA Mooney, M Godron) pp. 175–187. (Springer: Berlin, Heidelberg)
Chapin FS, III, Van Cleve K (1981) Plant nutrient absorption and retention under differing fire regimes. In ‘Fire regimes and ecosystem properties’. (Eds HA Mooney, TM Bonnickson, NL Christensen, JE Lotan, WA Reiners). USDA Forest Service, General Technical Report WO 26, pp. 301–321.
Chaudhary RP (1998) ‘Biodiversity in Nepal: status and conservation’. (S Devi, Saharanpur)
Christensen NL (1985) Shrubland fire regimes and their evolutionary consequences. In ‘The ecology of natural disturbance and patch dynamics’. (Eds STA Pickett, PS White) pp. 86–100. (Academic Press Inc.: Orlando, FL, USA)
Collins BM, Kelly M, Van Wagtendonk JW, Stephens SL (2007) Spatial patterns of large natural fires in Sierra Nevada wilderness areas. Landscape Ecology 22, 545–557.
| Spatial patterns of large natural fires in Sierra Nevada wilderness areas.Crossref | GoogleScholarGoogle Scholar |
Daalkhaijav D (2005) ‘Palatability of Mongolian rangeland plants.’ (Eastern Oregon Agricultural Research Center/Union Station: Corvallis, OR, USA)
Dai X, Page B, Duffy KJ (2006) Indicator value analysis as a group prediction technique in community classification. South African Journal of Botany 72, 589–596.
| Indicator value analysis as a group prediction technique in community classification.Crossref | GoogleScholarGoogle Scholar |
Davies KW, Bates JD, Boyd CS, Nafus AM (2014) Is fire exclusion in mountain big sagebrush communities prudent? Soil nutrient, plant diversity and arthropod response to burning. International Journal of Wildland Fire 23, 417–424.
| Is fire exclusion in mountain big sagebrush communities prudent? Soil nutrient, plant diversity and arthropod response to burning.Crossref | GoogleScholarGoogle Scholar |
de Villiers AD, O’Connor T (2011) Effect of a single fire on woody vegetation in Catchment IX, Cathedral Peak, KwaZulu–Natal Drakensberg, following extended partial exclusion of fire. African Journal of Range & Forage Science 28, 111–120.
| Effect of a single fire on woody vegetation in Catchment IX, Cathedral Peak, KwaZulu–Natal Drakensberg, following extended partial exclusion of fire.Crossref | GoogleScholarGoogle Scholar |
DellaSala DA, Lindenmayer DB, Hanson CT, Furnish J (2015) In the aftermath of fire: logging and related actions degrade mixed- and high-severity burn areas. In ‘The ecological importance of mixed-severity fires: nature’s phoenix’. (Eds DA DellaSala, CT Hanson) pp. 313–347. (Elsevier Inc, Amsterdam, the Netherlands)
Dudley JL, Lajtha K (1993) The effects of prescribed burning on nutrient availability and primary production in sandplain grasslands. American Midland Naturalist 130, 286–298.
| The effects of prescribed burning on nutrient availability and primary production in sandplain grasslands.Crossref | GoogleScholarGoogle Scholar |
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67, 345–366.
| Species assemblages and indicator species: the need for a flexible asymmetrical approach.Crossref | GoogleScholarGoogle Scholar |
Feeley KJ, Silman MR (2010) Land-use and climate change effects on population size and extinction risk of Andean plants. Global Change Biology 16, 3215–3222.
| Land-use and climate change effects on population size and extinction risk of Andean plants.Crossref | GoogleScholarGoogle Scholar |
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS (2004) Regime shifts, resilience, and biodiversity in ecosystem management. Annual Review of Ecology, Evolution, and Systematics 35, 557–581.
| Regime shifts, resilience, and biodiversity in ecosystem management.Crossref | GoogleScholarGoogle Scholar |
Fox JF (1981) Intermediate levels of soil disturbance maximise alpine plant diversity. Nature 293, 564–565.
| Intermediate levels of soil disturbance maximise alpine plant diversity.Crossref | GoogleScholarGoogle Scholar |
Gaur UN, Raturi GP, Bhatt AB (2003) Quantitative response of vegetation in glacial moraine of central Himalaya. The Environmentalist 23, 237–247.
| Quantitative response of vegetation in glacial moraine of central Himalaya.Crossref | GoogleScholarGoogle Scholar |
Ghimire SK, McKey D, Aumeeruddy-Thomas Y (2006) Himalayan medicinal plant diversity in an ecologically complex high-altitude anthropogenic landscape, Dolpo, Nepal. Environmental Conservation 33, 128–140.
| Himalayan medicinal plant diversity in an ecologically complex high-altitude anthropogenic landscape, Dolpo, Nepal.Crossref | GoogleScholarGoogle Scholar |
Ghimire SK, Sapkota IB, Oli BR, Parajuli RR (2008) ‘Non-timber forest products of Nepal Himalaya: database of some important species found in the mountain protected areas and surrounding regions.’ (WWF Nepal: Kathmandu, Nepal)
Guleria S, Jaitak V, Saini R, Kaul VK, Lal B, Babu GDK, Singh B, Singh RD (2011) Comparative studies of volatile oil composition of Rhododendron anthopogon by hydrodistillation, supercritical carbon dioxide extraction and head space analysis. Natural Product Research 25, 1271–1277.
| Comparative studies of volatile oil composition of Rhododendron anthopogon by hydrodistillation, supercritical carbon dioxide extraction and head space analysis.Crossref | GoogleScholarGoogle Scholar | 21854174PubMed |
Habeck JR (1994) Using general land office records to assess forest succession in ponderosa pine/Douglas-fir forests in western Montana. Northwest Science 68, 69–78.
Harris L, Taylor AH (2015) Topography, fuels, and fire exclusion drive fire severity of the Rim Fire in an old-growth mixed-conifer forest, Yosemite National Park, USA. Ecosystems 18, 1192–1208.
| Topography, fuels, and fire exclusion drive fire severity of the Rim Fire in an old-growth mixed-conifer forest, Yosemite National Park, USA.Crossref | GoogleScholarGoogle Scholar |
Huston M, Smith T (1987) Plant succession: life history and competition. American Naturalist 130, 168–198.
| Plant succession: life history and competition.Crossref | GoogleScholarGoogle Scholar |
Innocenti G, Dall’Acqua S, Scialino G, Banfi E, Sosa S, Gurung K, Barbera M, Carrara M (2010) Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules 15, 2326–2338.
| Chemical composition and biological properties of Rhododendron anthopogon essential oil.Crossref | GoogleScholarGoogle Scholar | 20428045PubMed |
Jacquez GM, Patten DT (1996) Chesneya nubigena on a Himalayan glacial moraine: a case of facilitation in primary succession? Mountain Research and Development 16, 265–273.
| Chesneya nubigena on a Himalayan glacial moraine: a case of facilitation in primary succession?Crossref | GoogleScholarGoogle Scholar |
Karkee TB (1991) Forest fire – causes and its relationship with economic variables. Nepal. Journal of Forestry 6, 75–80.
Karki J, McVeigh C (2000) Status paper of Langtang National Park. In ‘Grassland ecology and management in protected areas of Nepal’. (Eds C Richard, K Basnet, JP Sah, Y Raut) pp. 121–132. (International Center for Integrated Mountain Development (ICIMOD): Kathmandu, Nepal)
Knox KJE, Clarke PJ (2006) Response of resprouting shrubs to repeated fires in the dry sclerophyll forest of Gibraltar Range National Park. Proceedings of the Linnean Society of New South Wales 127, 49–56.
Körner C (2003) ‘Alpine plant life: functional plant ecology of high mountain ecosystems.’ (Springer: New York, NY, USA)
Kumar KR, Sahai AK, Krishna Kumar K, Patwardhan SK, Mishra PK, Revadekar JV, Kamala K, Pant GB (2006) High-resolution climate change scenarios for India for the 21st century. Current Science 90, 334–345.
Lama YC, Ghimire SK, Aumeeruddy-Thomas Y (2001) ‘Medicinal plants of Dolpo: Amchis’ knowledge and conservation.’ (WWF Nepal Program: Kathmandu, Nepal).
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16, 951–963.
| Beta diversity as the variance of community data: dissimilarity coefficients and partitioning.Crossref | GoogleScholarGoogle Scholar | 23809147PubMed |
Li PX, Krüsi BO, Li SL, Cai XH, Yu FH (2011) Can Potentilla fruticosa Linn. shrubs facilitate the herb layer of heavily grazed pasture on the eastern Tibetan Plateau? Polish Journal of Ecology 59, 129–140.
Manandhar NP (2002) ‘Plants and people of Nepal.’ (Timber Press: Portland, OR, USA).
Mark AF (1994) Effects of burning and grazing on sustainable utilization of upland snow tussock (Chionochloa spp.) rangelands for pastoralism in South Island, New Zealand. Australian Journal of Botany 42, 149–161.
| Effects of burning and grazing on sustainable utilization of upland snow tussock (Chionochloa spp.) rangelands for pastoralism in South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Mark AF, Holdsworth DK (1979) Yield and macronutrient content of water in relation to plant cover from the snow tussock grassland zone of eastern and central Otago, New Zealand. Progress in Water Technology 11, 449–462.
Matin MA, Chitale VS, Murthy MSR, Uddin K, Bajracharya B, Pradhan S (2017) Understanding forest fire patterns and risk in Nepal using remote sensing, geographic information system and historical fire data. International Journal of Wildland Fire 26, 276–286.
| Understanding forest fire patterns and risk in Nepal using remote sensing, geographic information system and historical fire data.Crossref | GoogleScholarGoogle Scholar |
McCune B, Mefford MJ (1999) ‘PC-ORD: Multivariate analysis of ecological data, version 4 for Windows.’ (MjM Software Design: Glenden Beach, OR, USA)
McCune B, Grace JB, Urban DL (2002) ‘Analysis of ecological communities.’ (MjM Software Design: Glenden Beach, OR, USA)
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
NASA (2009) Forest fires in Nepal. (NASA Earth Observatory) Available at https://earthobservatory.nasa.gov/images/37518/forest-fires-in-nepal [Verified 24 December 2019]
Ng SC, Corlett RT (2003) The ecology of six rhododendron species (Ericaceae) with contrasting local abundance and distribution patterns in Hong Kong, China. Plant Ecology 164, 225–233.
| The ecology of six rhododendron species (Ericaceae) with contrasting local abundance and distribution patterns in Hong Kong, China.Crossref | GoogleScholarGoogle Scholar |
Olsen CS, Larsen HO (2003) Alpine medicinal plant trade and Himalayan mountain livelihood strategies. The Geographical Journal 169, 243–254.
| Alpine medicinal plant trade and Himalayan mountain livelihood strategies.Crossref | GoogleScholarGoogle Scholar |
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR (2001) Terrestrial ecoregions of the world: a map of life on Earth. Bioscience 51, 933–938.
| Terrestrial ecoregions of the world: a map of life on Earth.Crossref | GoogleScholarGoogle Scholar |
Parajuli A, Chand DB, Rayamajhi B, Khanal R, Baral S, Malla Y, Poudel S (2015) Spatial and temporal distribution of forest fires in Nepal. Paper presented at XIV World Forestry Congress, Durban, South Africa, pp. 7–11 September 2015. Available at http://www.forestrynepal.org/images/publications/wfc2015_forestfirenepal.pdf [Verified 28 February 2017].
Parsons DJ, DeBenedetti SH (1979) Impact of fire suppression on a mixed-conifer forest. Forest Ecology and Management 2, 21–33.
| Impact of fire suppression on a mixed-conifer forest.Crossref | GoogleScholarGoogle Scholar |
Paul A, Khan ML, Das AK (2010) Utilization of rhododendrons by Monpas in western Arunachal Pradesh, India. Journal - American Rhododendron Society 64, 81–84.
Peet NB, Watkinson AR, Bell DJ, Kattel BJ (1999) Plant diversity in the threatened subtropical grasslands of Nepal. Biological Conservation 88, 193–206.
| Plant diversity in the threatened subtropical grasslands of Nepal.Crossref | GoogleScholarGoogle Scholar |
Peterson DW, Reich PB (2007) Fire frequency and tree canopy structure influence plant species diversity in a forest–grassland ecotone. Plant Ecology 194, 5–16.
| Fire frequency and tree canopy structure influence plant species diversity in a forest–grassland ecotone.Crossref | GoogleScholarGoogle Scholar |
Polunin O, Stainton A (1984) ‘Flowers of the Himalaya.’ (Oxford University Press: New Delhi, India)
Press JR, Shrestha KK, Sutton DA (2000) ‘Annotated checklist of the flowering plants of Nepal.’ (The Natural History Museum: London, UK)
Price JN, Morgan JW (2008) Woody plant encroachment reduces species richness of herb‐rich woodlands in southern Australia. Austral Ecology 33, 278–289.
| Woody plant encroachment reduces species richness of herb‐rich woodlands in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Reilly MJ, Wimberly MC, Newell CL (2006) Wildfire effects on plant species richness at multiple spatial scales in forest communities of the southern Appalachians. Journal of Ecology 94, 118–130.
| Wildfire effects on plant species richness at multiple spatial scales in forest communities of the southern Appalachians.Crossref | GoogleScholarGoogle Scholar |
Risser PG (1990) Landscape processes and the vegetation of the North American grassland. In ‘Fire in North American tallgrass prairies’. (Eds SL Collins, LL Wallace) pp. 133–146. (University of Oklahoma Press: Oklahoma)
Salick J, Byg A (2007) ‘Indigenous peoples and climate change.’ (Tyndall Centre for Climate Change Research: Oxford, UK)
Salick J, Ghimire SK, Fang Z, Dema S, Konchar KM (2014) Himalayan alpine vegetation, climate change and mitigation. Journal of Ethnobiology 34, 276–294.
| Himalayan alpine vegetation, climate change and mitigation.Crossref | GoogleScholarGoogle Scholar |
Salick J, Fang Z, Hart R (2019) Rapid changes in eastern Himalayan alpine flora with climate change. American Journal of Botany 106, 520–530.
| Rapid changes in eastern Himalayan alpine flora with climate change.Crossref | GoogleScholarGoogle Scholar | 30934119PubMed |
Schmidt-Vogt D (1990) Fire in high-altitude forests of the Nepal Himalaya. In ‘Fire in ecosystem dynamics: Mediterranean and northern perspectives’. (Eds JG Goldammer, MJ Jenkins) pp. 191–199. (SPB Academic Publishing: The Hague, the Netherlands)
Shang ZB, He HS, Lytle DE, Shifley SR, Crow TR (2007) Modeling the long-term effects of fire suppression on central hardwood forests in Missouri Ozarks, using LANDIS. Forest Ecology and Management 242, 776–790.
| Modeling the long-term effects of fire suppression on central hardwood forests in Missouri Ozarks, using LANDIS.Crossref | GoogleScholarGoogle Scholar |
Sheuyange A, Oba G, Management RW (2005) Effects of anthropogenic fire history on savanna vegetation. Journal of Environmental Management 75, 189–198.
| Effects of anthropogenic fire history on savanna vegetation.Crossref | GoogleScholarGoogle Scholar | 15829362PubMed |
Shrestha TB, Joshi RM (1996) ‘Rare, endemic and endangered plants of Nepal.’ (WWF Nepal Program: Kathmandu, Nepal)
Telwala Y, Brook BW, Manish K, Pandit MK (2013) Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS One 8, e57103
| Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre.Crossref | GoogleScholarGoogle Scholar | 23437322PubMed |
Thomas PA, McAlpine RS (2010) ‘Fire in the forest.’ (Cambridge University Press: New York, NY, USA)
Twidwell D, Rogers WE, McMahon EA, Thomas BR, Kreuter UP, Blankenship TL (2012) Prescribed extreme fire effects on richness and invasion in Coastal Prairie. Invasive Plant Science and Management 5, 330–340.
| Prescribed extreme fire effects on richness and invasion in Coastal Prairie.Crossref | GoogleScholarGoogle Scholar |
Van Lear DH, Carroll WD, Kapeluck PR, Johnson R (2005) History and restoration of the longleaf pine–grassland ecosystem: implications for species at risk. Forest Ecology and Management 211, 150–165.
| History and restoration of the longleaf pine–grassland ecosystem: implications for species at risk.Crossref | GoogleScholarGoogle Scholar |
Walker B (2001) Tropical savanna. In ‘Global biodiversity in a changing environment: scenarios for the 21st century’. (Eds FS Chapin, OE Sala, E Huber-Sannwald) pp. 139–156. (Springer: New York, NY, USA)
Watkinson AR, Ormerod SJ (2001) Grasslands, grazing and biodiversity: editors’ introduction. Journal of Applied Ecology 38, 233–237.
| Grasslands, grazing and biodiversity: editors’ introduction.Crossref | GoogleScholarGoogle Scholar |
Wesche K (2006) Is Afroalpine plant biodiversity negatively affected by high-altitude fires? In ‘Land use change and mountain biodiversity’. (Eds EM Spehn, M Liberman, C Körner) pp. 39–49. (CRC Press, Taylor and Francis Group: Boca Raton, FL, USA)
White JD, Ryan KC, Key CC, Running SW (1996) Remote sensing of forest fire severity and vegetation recovery. International Journal of Wildland Fire 6, 125–136.
| Remote sensing of forest fire severity and vegetation recovery.Crossref | GoogleScholarGoogle Scholar |
White PS (1979) Pattern, process, and natural disturbance in vegetation. Botanical Review 45, 229–299.
| Pattern, process, and natural disturbance in vegetation.Crossref | GoogleScholarGoogle Scholar |
Williams JE, Whelan RJ, Gill AM (1994) Fire and environmental heterogeneity in southern temperate forest ecosystems: implications for management. Australian Journal of Botany 42, 125–137.
| Fire and environmental heterogeneity in southern temperate forest ecosystems: implications for management.Crossref | GoogleScholarGoogle Scholar |
Williams RJ, Wahren C-H, Tolsma AD, Sanecki GM, Papst WA, Myers BA, McDougall KL, Heinze DA, Green K (2008) Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity. International Journal of Wildland Fire 17, 793–808.
| Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity.Crossref | GoogleScholarGoogle Scholar |
Wu ZY, Raven PH, Hong DY (1994) ‘Flora of China’. (Science Press: Beijing, China, and Missouri Botanical Garden Press: St Louis, MO, USA). Available online at efloras.org [Verified 16 January 2020]
Xiang X, Shi Y, Yang J, Kong J, Lin X, Zhang H, Zeng J, Chu H (2014) Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Scientific Reports 4, 3829
| Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest.Crossref | GoogleScholarGoogle Scholar | 24452061PubMed |