A conservation-significant threatened mammal uses fire exclusions and shifts ranges in the presence of prescribed burning
Leticia F. Povh A * , Nicole Willers
A * , Nicole Willers  B , Jill M. Shephard
B , Jill M. Shephard  A and Patricia A. Fleming
A and Patricia A. Fleming  A
A
A Centre for Terrestrial Ecosystem Science and Sustainability, Harry Butler Institute, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
B Department of Biodiversity, Conservation and Attractions, Australia II Drive, Crawley, Perth, WA 6009, Australia.
International Journal of Wildland Fire 32(9) 1291-1303 https://doi.org/10.1071/WF22196
Submitted: 13 September 2022 Accepted: 30 June 2023 Published: 31 July 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of IAWF. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Understanding how animals change their use of space following prescribed burning is essential for effective conservation management, particularly a threatened species such as the quokka (Setonix brachyurus).
Aims: To determine how individual quokkas change their home ranges following burns.
Methods: The movement patterns of 20 quokkas were tracked before and after prescribed burns between 2018 and 2020. Home-range area was calculated for each individual, and behavioural change point analysis was carried out to determine whether they changed their space use after the burns.
Key results: Six quokkas that had previously resided in areas that were prescribed burned, shifted their ranges and moved into the fire exclusions, avoiding the burn areas for an average of 105 ± 65 days. After 3 months, these quokkas spent no more than 2% of their time in the burn areas. By contrast, quokkas inhabiting fire exclusion and control sites did not show any change in their space use.
Conclusions: This study highlights the importance and proximity of appropriately sized fire exclusions to ensure that populations of species dependent on dense vegetation can be retained.
Implications: Fire exclusion areas are an important part of the planning of prescribed burns to retain habitat for fauna species that rely on dense cover for refuge and food.
Keywords: conservation, fire management, home range, prescription burning, quokka, survival, threatened species, wildlife management.
Introduction
Australia has recently experienced some of the worst wildfires in its history (Teague et al. 2010; CSIRO 2020; Jalaludin and Morgan 2021). Fire has played a fundamental role in the evolution of Australia’s biota and continues to be a key driver of many of its ecosystems (Bradstock et al. 2012). However, the issue for biodiversity conservation is inappropriate fire regimes and recent increases in fire intensity in a drying climate. With changes in climate, the number of extreme weather days will keep increasing (Di Virgilio et al. 2019), and there will be greater risk of wildfires (van Oldenborgh et al. 2021) that are likely to burn with greater intensity, impacting trees and vegetation that provide important habitat for fauna species. Landscape fragmentation (e.g. due to clearing for agriculture and mining) further contributes to losses due to fire, because animals cannot readily move away from burned areas to find appropriate habitat.
Prescribed burning is the main strategy to manage flammable landscapes and reduce the intensity of wildfires (Howard et al. 2020; Radford et al. 2020). Because of their timing (usually autumn or spring), compared with wildfire, prescribed burns should have lower intensity, consume less vegetation and create a more patchy mosaic of burned and unburned areas that should allow wildlife to find unburned habitat to survive. Prescription burning can also promote biodiversity through the regeneration of vegetation seral stages and increasing habitat heterogeneity (Valkó et al. 2016; Eales et al. 2018; He et al. 2019; Radford et al. 2020) (but see Pastro et al. 2011; Berry et al. 2015; Bradshaw et al. 2018). As well as reducing risk of large-scale intense wildfire for the protection of lives and property, prescribed burning is therefore used as an important management tool to protect vegetation complexity and regenerate vegetation to provide habitat and food for fauna (Penman et al. 2011; Flanagan-Moodie et al. 2018; Howard et al. 2020).
A few studies have shown that mammals can survive fire itself. For example, Garvey et al. (2010) reported that swamp wallabies (Wallabia bicolor) survived a prescribed burn in Muogamarra Nature Reserve, New South Wales by moving into creek lines to avoid fire, and even crossing the fire front to move into burned ground. In another study, Vernes (2000) recorded comparable survival of the northern bettong (Bettongia tropica) at burned and unburned sites in north-eastern Queensland, although their mechanism of survival was not stated. In contrast, although robust studies that directly measure mortality of mammals during higher intensity fires are rare, direct mortality from fire (heat, smoke) has been inferred as the cause of death for a number of species (Ritchie et al. 2008; Penman et al. 2011; Berry et al. 2015; Flanagan-Moodie et al. 2018; Santos et al. 2022). Whether mammals burrow, hide/wait or flee is likely a result of many factors, including their typical habitat/refuge area, their size and mobility, environmental/weather cues and the scale/intensity of the fire.
There are also longer-term effects of fire on fauna. Burned areas can deprive survivors of food resources or thermal shelter, and there can be minimal protective cover from predators in these landscapes (Burbidge and McKenzie 1989; McGregor et al. 2014). Reduced survival shortly after fire due to reductions in food and shelter is the most frequently reported mechanism of fire-related decline (Santos et al. 2022). Medium-sized mammals are particularly at risk because they are less mobile and less able to find food or shelter (Gill et al. 2002). Mosaics of burned and unburned vegetation are therefore important for the maintenance of post-fire populations (Penman et al. 2007; Radford et al. 2015). For example, the long-nosed bandicoot (Perameles nasuta) is resilient to patchy burns that leave a mosaic of burned and unburned habitats (Chambers and Dickman 2002; MacGregor et al. 2015). Shaw et al. (2021) found that unburned patches of vegetation were important for the survival of the pale field rat (Rattus tunneyi) after prescribed fire in tropical savannas in the central Kimberley, Western Australia. At an even more localised scale, unburned grasstrees that escaped fire provided valuable diurnal refuge for mardo (Antechinus flavipes leucogaster) in southwest Western Australia, and these plants were used disproportionately compared with the rest of the landscape (Swinburn et al. 2007). The relative importance of unburned patches for refuge is likely to depend on the degree to which they provide resources that are otherwise unavailable within the surrounding burned area (Penman et al. 2007; Robinson et al. 2014).
Faunal use of habitat can vary in relation to temporal changes, such as seasonal differences (Couriot et al. 2018) or drought (Yospin et al. 2015). Similarly, due to substantial alterations in forest structure and the spatiotemporal distribution of key resources following fire (Clarke 2008; Wiggins et al. 2010; Styger et al. 2011), mammal assemblages often change in relative abundance and spatial distribution (Fischer et al. 1997; Morris et al. 2011). Studies in arid Australia suggest that some small mammal species avoid burned areas, moving to alternative habitat (Read 1984; Dickman et al. 1995; Anstee et al. 1997; Letnic and Dickman 2005). For example, brush-tailed bettongs (Bettongia penicillata) moved considerable distances to nest at the edge of a burn, resulting in permanent shifts in location (Christensen and Leftwich 1980). Similarly, northern bettongs relocated their home ranges from areas of grassy tussocks and logs to rocky areas and remnant patches of unburned vegetation (Vernes and Pope 2001). By contrast with these studies showing avoidance of burned areas, tracked swamp wallabies (Wallabia bicolor) selected burned over unburned habitat after a prescribed burn in a peri-urban area of north Sydney, suggesting that the impact of the low-intensity burn on habitat quality was not sufficient to warrant a shift in ranges (Garvey et al. 2010). It is possible that fire could even directly benefit herbivorous and other species by increasing availability or quality of resources, for instance higher nutrient plant growth or a post-fire pulse of grasses and herbs (Christensen and Lewis 1980; Ritchie et al. 2008; Haslem et al. 2011; Eby et al. 2014).
Prescribed burns are highly variable in severity across time and space, causing different impacts on vegetation structure and therefore the degree to which food and shelter resources of fauna species are affected. Fauna also responds to prescribed burning in different ways, according to their biology, ecology, and behaviour, as well as alternative habitat availability. The impacts of prescribed burning on immediate and long-term survival and behavioural process such as movement and resource selection of Australian fauna species therefore cannot be broadly generalised. More detailed information about the mechanisms driving fauna responses to fire is required to predict the likely impacts of prescription burning.
The quokka (Setonix brachyurus) is a ‘Vulnerable’ wallaby species endemic to the south-west Western Australia mainland and two offshore islands (Kitchener 1995; Burbidge and Woinarski 2020). Small, fragmented quokka populations in the Northern Jarrah Forest lie on the inland edge of the species’ geographic range, and larger connected populations are found to the southern part of their range on the mainland (Spencer et al. 2019). As a habitat specialist, in the Northern Jarrah Forest, quokkas spend most of their time refuging in dense riparian vegetation and foraging in swamp systems and adjacent open forest (Hayward 2005; Hayward et al. 2005). In the southern forests, riparian vegetation is used exclusively for movements between habitat patches, with animals spending 40% of their time in this ecotype (Bain et al. 2020). Their mean overall home range sizes are reportedly disparate between Northern Jarrah Forest (6.4 ± 0.8 ha) and the southern forest (71 ± 5.8 ha), but in both areas the largest home ranges were reported in nocturnal periods and in autumn (Hayward et al. 2004; Bain et al. 2020). The optimal post-fire age of the dense riparian vegetation is at least 5 years, and up to 25 years or longer, depending on the location/climate and vegetation type (Hayward 2002; Hayward et al. 2007). Vegetation density typically declines after 24 years and can be burned to regenerate senescing vegetation that no longer has sufficient structure and density to provide protection (Hayward 2002). Recent work carried out across the southern forests showed that quokkas are dependent on a mosaic of fuel ages that provide suitable vegetation for both food and refuge (Bain et al. 2015; Bain et al. 2016). Finding a balance of fire age mosaic is therefore an important conservation management consideration. In the fragmented Northern Jarrah Forest, managing the fire age of their habitat requires considerable planning to ensure that alternative appropriate habitat is retained within the home ranges of individuals impacted by prescribed burns. Understanding how quokkas respond to fire is required if we are to ensure habitat is appropriately managed for their conservation.
We tracked 20 mainland quokkas with VHF and GPS collars before and after three prescribed burns and compared their movement with unburned sites as treatment controls – comparing their responses at the time of the fire, immediately-post fire, and over a longer time frame. First, we examined whether quokkas were directly exposed to the fires and whether they survived prescription burning (i.e. their immediate survival). Second, we examined whether the animals immediately shifted the area that they used for diurnal rest sites or nocturnal foraging at the time of the fire. We predicted that the loss of vegetation cover would result in avoidance of the burned site for diurnal refuge, and that the loss of plant species would cause a necessary shift in their foraging activities. Third, we tested whether quokkas changed their home range area after the fires as an indication of whether they could still secure sufficient resources for their medium-term survival. We predicted that foraging resources would be more scarce post-fire, requiring greater area covered to meet their energetic needs.
Methods
This project was approved by Murdoch University Animal Ethics Committee (R3058/18) and Department of Biodiversity, Conservation and Attractions (FO25000082-2).
Study area
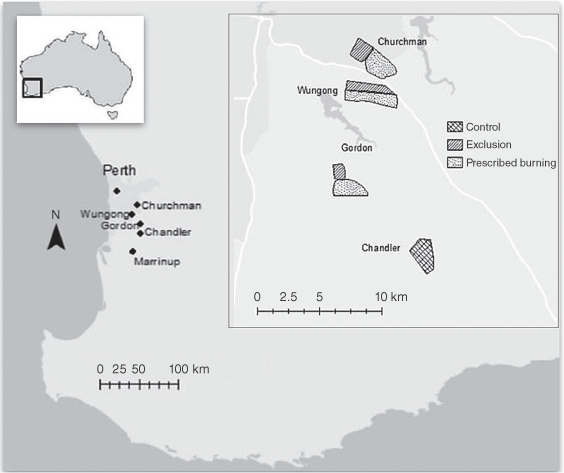
This study was undertaken in the Northern Jarrah Forest, Western Australia (Fig. 1). The research was concentrated on five forest blocks, which total 1974 ha of quokka habitat. One control site (Chandler) was not burned during this study and was monitored at the same time as three of the treatment sites were burned. One site (Marrinup) was not burned during the study period and there was no fire treatment simultaneously measured. There was also an internal control at each of the three prescription burn treatment sites, with each having both a burn area and a fire-exclusion area (adjacent unburned habitat). The area of burn at each study site was manually mapped and overlaid with the quokka location data using ArcGIS 10.8.1 (ESRI, San Diego, CA).
Study location at five sites, sites layout and treatment areas. The three prescribed burn treatment sites had both a burn and an exclusion area (adjacent unburned habitat). The prescribed burns were undertaken between October 2018 and October 2019. The area of burn at each study site is shown according to quokka habitat area, not total area of the prescribed burns. One control site (Chandler) and one site (Marrinup) were not burned during the study period.
Trapping and tracking devices
Between July 2018 and September 2019, quokkas were captured with Thomas soft-wall traps (360 × 480 × 800 mm W × H × L; Sheffield Wire Works, Welshpool, Western Australia). Quokkas were sexed and weighed (±0.01 kg; HDB 5K5N; KERN & Sohn, Balingen, Germany), microchipped (1.4 × 8.5 mm Bio-glass Parylene coating; SwissPlus ID Group, Queensland, Australia), and body measurements were recorded. All handling was undertaken by experienced ecologists. Adult quokkas weighing >2 kg were fitted with a tracking collar without the aid of anaesthesia (to reduce the possibility of attaching the collar too tightly). In the first year, quokkas were fitted with very high frequency (VHF) collars (model M1820; Advanced Telemetry Solutions, Australia). Subsequently, individuals were fitted with collars that had a store on-board global position system (GPS) system (LiteTrack30 model; Sirtrack, New Zealand) that could be located via VHF signal and have its GPS data remotely downloaded. Quokkas were then released at point of capture.
Radio-tracking
Individuals with VHF collars were tracked twice per week, using a R1000 receiver and a 6-element Yagi antenna (Sirtrack, New Zealand) from fixed telemetry stations positioned at regular intervals along access tracks running through the study sites. Upon locating each VHF collar signal, compass bearings to the strongest signal direction were taken from a minimum of three stations and less than 10 min apart. Animal location coordinates were generated from telemetry station coordinates and direction data with Locate III (Nams 2006). Location data for VHF collared individuals was collected during daylight (0600–1800 hours) and at night (1800–0600 hours).
GPS collars were programmed to take four fixes per day, with rolling intervals in multiples of 7 h. To download the data, once a GPS device was located (secondary VHF signal detected with Yagi antenna), the animal was approached quietly on foot and data were downloaded remotely with a portable PinPoint Commander (Sirtrack, New Zealand).
Data analyses
Tracking data were added to Movebank (Wikelski et al. 2020) and analysed with the ‘CTMM’ package (v0.5.5) (Calabrese et al. 2016) in R. Calibration data from two retrieved GPS collars that had fallen off and were stationary were used to estimate the user equivalent range error (UERE) for the GPS collars. Variograms were used to identify range-resident individuals, where estimated home-range area reaches an asymptote. Movement models were fitted to each individual’s spatial data separately, using maximum likelihood (Fleming and Calabrese 2017) to select the best match to each individual dataset among five possible movement models using Akaike information criterion (AIC) values (Akaike 1973). The best model fit was then applied to calculate the Kernel Density Estimation (KDE) for 95, 75, and 50% ranges (Calabrese et al. 2016) for each individual as their whole data set by month, and for nocturnal (1800–0600 hours) and diurnal (0600–1800 hours) data subsets by month.
Home-range areas were calculated separately for each individual, and the data for nocturnal and diurnal home range areas were compared by Mann–Whitney–Wilcoxon Test (Shapiro–Wilk test indicated that these data did not conform to a Gaussian distribution; P < 0.05). To test whether quokkas shifted their diurnal rest sites or nocturnal foraging activity after prescription burning, nocturnal and diurnal Log10-transformed 95% home-range areas for each month before and after the burn (predictor variable), including individual ID as a random factor to account for repeated measures, were compared by generalised linear mixed-model (GLMM) using lmer in ‘lme4’ package (Bates et al. 2014) in R. We used the ‘DHARMa’ package (Hartig 2020) in R to confirm the residual fit of the model.
To test whether quokkas shifted their home ranges at the time of the fire, movement paths were segmented into intervals corresponding with changes in the use of habitat using the ‘segclust2d’ package (v0.2.0) (Patin et al. 2020) in R. This process uses a dynamic programming algorithm to find the best segmentation that matches the data and identifies the number of segments, given a criterium based on the value of the second derivative of the penalised likelihood (Lavielle 1999). We compared the date of the change in segments with the date of the prescribed burn for all individuals at each site and compared the numbers of animals that shifted or did not shift their range at the time of the burn for burned and unburned sites using a Fisher’s exact test.
We used ArcGIS analysis to determine the average overlap between the animal’s space use and the planned burn areas. For those animals that shifted home range, we calculated the difference in the home range centroids of their pre- and post-burn GPS coordinates. We projected their shapefiles in R using ‘raster’ and created centroids with ‘rgeos’. Following this, we calculated distances between each individual centroid and locations of interest.
To determine how long quokkas avoided burned sites, we identified the date after the burn when the first location datapoint was recorded within the mapped burn area. To determine how much time each quokka spent within the burned areas, we calculated the percentage of nocturnal and diurnal location datapoints within the burned areas before prescribed burns, and from 3 to 7 months after burns (using ArcGIS analysis).
To test whether quokkas that had shifted their ranges used a greater area after the fires, we carried out a GLMM comparing Log10-transformed home range area data calculated each month (for 95, 75, and 50% kernel density estimates, KDE) before and after the burn (predictor variable) and including individual ID as a random factor to account for repeated measures using lmer in ‘lme4’. We used the ‘DHARMa’ package to confirm the residual fit of the model.
As a result of a 1-year delay in the prescribed burning at Marrinup site, quokkas Q19 and Q20 were not equipped with a collar during the prescribed burning at this site and were therefore not added to the segmentation analysis. Furthermore, both quokkas received a tracking device only a few days before the burns were carried out at Churchman and Wungong sites, so the Marrinup animals could not be used as formal control for comparison with the Churchman and Wungong individuals (i.e. to control for potential changes in home range area due to seasonal or weather influences).
Statistical analyses were performed in R (version 4.0.3). Significance values for all tests were set at α = 0.05, and values of response variables are reported as means ±1 standard deviation (s.d.).
Results
Were quokkas directly exposed to the fires and did they survive prescription burning?
In total, 20 male quokkas were successfully tracked: 11 in 2018 (nine using VHF collars, two with GPS collars) and nine between 2019 and 2020 (GPS collars). Two mortalities occurred before the prescribed burns: one of natural cause (in 2018) and the second was a roadkill (in 2020). Of the remaining 18 collared quokkas, no mortality occurred during or after the prescribed burns, with individuals tracked an average of 7.3 ± 2.7 months post-burns (Table 1). Two tracked individuals (Q15, Q16) were directly exposed to the prescription burns; the remaining four animals that were within burn treatment sites were known (from tracking data) to not be within the burn at the time of the prescribed burns.
| Site | Treatment | Quokka ID | Dates tracked, number of fixes, % in burned area | Tracker | Shifted home range at the time of fire | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-burn | Post-burn | |||||||||||
| Dates tracked | n fixes | % | Dates tracked | n fixes | % | |||||||
| Chandler (control) | Not-burned | Q1 | 29/08/2018 | 26/10/2018 | 31 | 0 | 31/10/2018 | 26/03/2019 | 93 | 0 | VHF | No |
| Q2 | 22/08/2018 | 23/10/2018 | 19 | 0 | 30/10/2018 | 13/03/2019 | 54 | 0 | VHF | No | ||
| Q3 | 28/08/2018 | 25/10/2018 | 40 | 0 | 30/10/2018 | 17/09/2019 | 73 | 0 | VHF | No | ||
| Q4 | 1/09/2018 | 26/10/2018 | 69 | 0 | 30/10/2018 | 26/02/2019 | 109 | 0 | VHF | No | ||
| Q5 | 23/09/2018 | 25/10/2018 | 43 | 0 | 31/10/2018 | 28/03/2019 | 99 | 0 | VHF | No | ||
| Q12 |  | 26/09/2019 | 64 | 0 | 09/10/2019 | 06/01/2020 | 291 | 0 |  /GPS /GPS | No | ||
| Q13 | 26/09/2019 | 4/10/2019 | 30 | 0 | 6/10/2019 | 3/12/2019 | 188 | 0 | GPS | No | ||
| Gordon | Not-burned (fire exclusion) | Q6 | 25/09/2018 | 25/10/2018 | 55 | 0 | 29/10/2018 | 3/03/2019 | 97 | 0 | VHF | No |
| Q7 | 1/08/2018 | 25/10/2018 | 65 | 0 | 31/10/2018 | 10/03/2019 | 100 | 0 | VHF | No | ||
| Burned (prescribed burning, manual ignition) | Q9 | 4/09/2018 | 26/10/2018 | 186 | 3 | 29/10/2018 | 4/12/2018 | 147 | 1 | GPS | No | |
| Q8 | 27/08/2018 | 24/10/2018 | 43 | 76 | 31/10/2018 | 21/01/2019 | 26 | 9 | VHF | Yes | ||
| Q10 | 30/08/2018 | 26/10/2018 | 77 | 13 | 31/10/2018 | 12/03/2019 | 108 | 0 | VHF | Yes | ||
| Q11 | 30/08/2018 | 26/10/2018 | 281 | 27 | 30/10/2018 | 22/01/2019 | 420 | 7 | GPS | Yes | ||
| Churchman | Not-burned (fire exclusion) | Q17 |  | 26/09/2019 | 118 | 0 | 28/09/2019 | 24/04/2020 | 682 | 0 |  /GPS /GPS | No |
| Q18 |  | 26/09/2019 | 178 | 0 | 28/09/2019 | 29/04/2020 | 214 | 0 |  /GPS /GPS | No | ||
| Burned (prescribed burning, manual ignition) | Q16 |  | 25/09/2019 | 101 | 100 | 28/09/2019 | 5/04/2020 | 596 | 8 |  /GPS /GPS | Yes | |
| Wungong | Burned (prescribed burning, aerial ignition) | Q14 |  | 5/10/2019 | 100 | 11 | 9/10/2019 | 9/12/2019 | 193 | 0 |  /GPS /GPS | Yes |
| Q15 | 6/09/2019 | 4/10/2019 | 97 | 37 | 9/10/2019 | 8/12/2019 | 185 | 7 | GPS | Yes | ||
| Marrinup | Not-burned (postponed) | Q19 | 13/09/2019 | 22/01/2020 | 393 | 0 | – | – | 0 | GPS | – | |
| Q20 | 04/10/2019 | 10/08/2020 | 912 | 0 | – | – | 0 | GPS | – | |||
The tracking dates for  and GPS data, the
and GPS data, the  indicates individuals tracked using VHF collars as well as GPS collars trackers. The green boxes show individuals with no overlap within the planned burn area pre-burn and post-burn. The box colours ranging from yellow (low overlap) to red (strong overlap) show the overlaps with planned burn area pre-burn and post-burn, and consequently shifts in home range after prescribed burn.
indicates individuals tracked using VHF collars as well as GPS collars trackers. The green boxes show individuals with no overlap within the planned burn area pre-burn and post-burn. The box colours ranging from yellow (low overlap) to red (strong overlap) show the overlaps with planned burn area pre-burn and post-burn, and consequently shifts in home range after prescribed burn.
Did quokkas shift their home range after prescription burning?
In total, 9905 locations were used to estimate home range areas, with an average of 453 ± 297 independent locations per quokka (Table 1). The mean 95% KDE home-range size for 20 male quokkas in the Northern Jarrah Forest was 75.2 ± 59.7 ha, the 75% KDE was 28.8 ± 24.3 ha, and the core home-range area (50% KDE) was 13.0 ± 11.8 ha. Nocturnal home ranges averaged 36% larger than diurnal ranges but there were no statistically significant differences in diurnal and nocturnal for any of the KDE isopleth areas. There was also no significant difference in the pre- and post-burn nocturnal (i.e. foraging) areas for any of the KDE isopleths (Table 2).
| KDE home ranges (%) | Diurnal (% within 25 m of the stream zone) | Nocturnal (% within 25 m of the stream zone) | Comparison diurnal and nocturnal areas |
|---|---|---|---|
| (a) Diurnal vs nocturnal home range areas | |||
| 50 | 15.6 ± 24.0 ha (74%) | 21.5 ± 31.0 ha (41%) | U23 = −2.7, P = 0.093 |
| 75 | 31.8 ± 46.3 ha (25%) | 43.4 ± 59.8 ha (56%) | U23 = −3.1, P = 0.136 |
| 95 | 72.3 ± 98.2 ha (1%) | 96.6 ± 124.0 ha (3%) | U23 = −3.2, P = 0.172 |
| (b) Pre-burn vs and post-burn overall home range areas | |||
| 50 | F1,49 = 0.173, P = 0.679 | ||
| 75 | F1,49 = 0.136, P = 0.714 | ||
| 95 | F1,49 = 0.183, P = 0.671 | ||
Numbers in brackets are the percentages of these locations within 25 m of the stream zone.
Segmentation analysis revealed that none of the seven quokkas at the control sites, and only one of five quokkas using fire exclusions areas within burn treatment sites (i.e. unburned habitat adjacent to burn areas), shifted their home ranges during the study. By contrast, all six quokkas at burn treatment sites moved away from the burn area (Fig. 2). Segmentation analysis indicated that this shift happened on the same date as the prescribed burns. The difference in responses between control/fire exclusion and burn treatments was statistically significant (Fisher’s exact test comparing 1/12 vs 6/6, P < 0.001). The average overlap between the animal’s space use and the planned burn area averaged 44 ± 36% of location fixes pre-burn, compared with only 5 ± 4% of location fixes to the same area post-burn. All three quokkas (Q8, Q10, Q11) using sites within the planned burn area at the Gordon site (76, 13 and 27% overlap with the planned burn area) shifted their ranges post-burn (9, 0 and 7% overlap with the burn area) and their range centroids were altered (Fig. 2a–c).
Response of six mainland quokkas (a–f) to prescribed burns (red rectangles) derived from segmentation analysis showing each individual (resided or visited often burning site) home-range overlaps before (red), changes after prescribed burnings (blue), and centroid shifts (m) after burns.
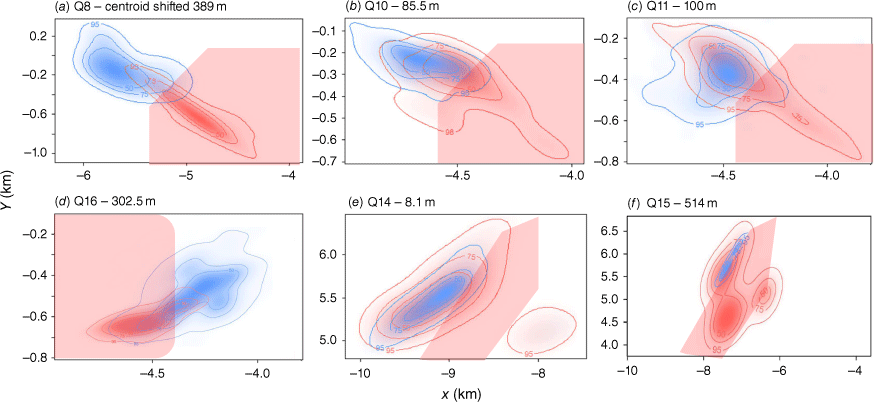
Of the six quokkas affected by the prescription burning, two were directly exposed to the prescription burns. At the Wungong site, Q15 had a 37% overlap with the planned burn area and was in the burning area during the aerial ignition (Fig. 2f). Q15 survived the fire in a small area <1 ha of unburned vegetation, moved 1.5 km to the fire exclusion site 3 days after the prescribed burn, and made minimal use of the burn area post-fire (7% overlap with the burn area). Over the next 6 months, Q15 had 4% of his diurnal and nocturnal locations in burn area. At the Churchman site, the home range of Q16 pre-burn had 96% of overlap within the planned burn area (Fig. 2d). During the burn, Q16 moved to adjoining unburned habitat, across a road, where he remained for 8 months (8% overlap with the burn area) post-burn. Three months post-burn, Q16 made irregular visits to the burned area at night (1% of locations) and to unburned borders (5% of locations). After 7 months, the percentage of diurnal and nocturnal visits to the burned area increased by 1 and 3% respectively (Table 3).
| Quokka ID | Months after PB | Fire exclusion | Burn area | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diurnal | Nocturnal | Diurnal | Nocturnal | |||||||
| Before (%) | After (%) | Before (%) | After (%) | Before (%) | After (%) | Before (%) | After (%) | Border (%) | ||
| Q8 | 6 | 11 | 55 | 2 | 45 | 52 | 0 | 34 | 0 | 0 |
| Q10 | 3 | 47 | 50 | 37 | 50 | 3 | 0 | 13 | 0 | 0 |
| Q11 | 3 | 38 | 41 | 38 | 52 | 4 | 0 | 20 | 2 | 4 |
| Q14 | 3 | 40 | 47 | 57 | 51 | 5 | 0 | 5 | 0 | 1 |
| 6 | 46 | 47 | 0 | 1 | 6 | |||||
| Q15 | 3 | 25 | 47 | 22 | 47 | 25 | 2 | 28 | 2 | 1 |
| Q16 | 3 | 38 | 44 | 20 | 50 | 11 | 0 | 31 | 1 | 5 |
| 7 | 47 | 44 | 1 | 4 | 5 | |||||
Four quokkas were not within the burn area at the time of ignition but had home ranges that overlapped the burn area and shifted their home ranges away post-burn. At the Gordon site, Q8 persisted for 6 months in the fire-exclusion area and did not spend any daytime or night-time in the burned area; 460 days after the prescribed burn, this male undertook a substantial move to an unburned site 10 km away, where it established a new range and remained for 7 months (confirmed by subsequent tracking). At this same site, two other quokkas (Q10 and Q11) shifted their range to the fire exclusion post-fire, where they remained for 7 and 6 months. Q10 did not spend any daytime or night-time in the burned area. Q11 made irregular visits to the burn area 3 months post-burn; 2% of his nocturnal locations were with the burn area and 4% within the unburned border (Table 3). At the Wungong site, pre-fire Q14 had an 11% overlap with the planned burn area. He shifted his range and did not use the burn area (0% overlap with the burn area) over the next 7 months post-burn (Fig. 2e). Over time, 1% of nocturnal locations were within unburned borders. After 6 months, he visited the burned area (1% locations) and the border visits increased by 5% (Table 3).
Did quokkas change their home range area after prescription burning?
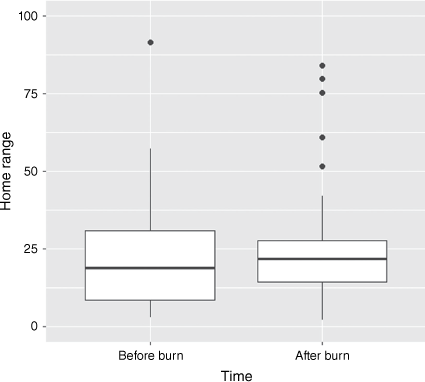
There was no significant change in the home range areas of animals that shifted their home ranges after prescribed burns (50% KDE: F1,53 = 0.159, P = 0.692; 70% KDE: F1,53 = 0.083, P = 0.774; 95% KDE: F1,53 = 0.018, P = 0.894; Fig. 3).
The comparison of monthly estimates of 95% Kernel Density Estimate (KDE) home range area for six individual quokkas (Setonix brachyurus) that shifted their ranges after three prescribed burns. Boxplots show the median (thick horizontal line) quartiles (box) and non-outlier range (whiskers). Outliers are shown as black dots.
Discussion
While previous studies have examined fire chronosequences (Hayward et al. 2005; Dundas 2013; Bain et al. 2016) or the effects of wildfire on quokkas (WWF 2016), our study is the first investigation of the impact of prescribed burning on the movement ecology of individual quokkas. Individuals with tracking devices survived fire during the prescribed burns. Post-burn, quokkas used the defined fire exclusion areas or nearby unburned habitat, resulting in home-range shifts for six individuals that had occupied sites that were burned during the prescribed burns.
Were quokkas directly exposed to the fires and did they survive prescription burning?
Although there were only six tracked quokkas directly affected by the fire in the present study (i.e. residing within the boundaries of the burn area at the time of the burn), limiting our ability to extrapolate beyond the scope of the present study, we found no evidence for direct mortality caused by three prescribed burns monitored in the present study. Our results may be attributable to the presence of planned and successfully implemented fire exclusions in all monitored prescription burns. Three previous studies have similarly found no direct mortality during and immediately after burns for swamp wallabies, long-nosed bandicoots, and northern bettongs (Vernes and Pope 2001; Garvey et al. 2010; Hope 2012). A recent meta-analysis testing fire characteristics (fire type, fire severity, fire regime) and animal traits (body mass, ecological attributes, and vertebrate class) showed that only fire severity affected animal mortality, with a greater proportion of animals being killed by high- than low-severity fires (Jolly et al. 2022). Taken together, these results suggest that because prescribed fire generally burns with less intensity than wildfire (Christensen and Lewis 1980; Begg et al. 1981; Legge et al. 2008; Conner et al. 2011; Morris et al. 2011; Leahy et al. 2015), managing the intensity of burning needs to be prioritised.
Naturally unburned areas resulting from either topography, moisture differentials, or fire behaviour may act as short-term refugia for small mammals (Garvey et al. 2010; Robinson et al. 2013; Fordyce et al. 2016; Shaw et al. 2021). However, with an ongoing drying trend and less edaphic moisture barriers, larger areas of the landscape will be vulnerable to fire, making patchy burns less achievable without careful planning (Williams et al. 2009; Bain et al. 2016). In addition, fire managers should not depend on such fortuitous or occasional unburned areas, because they are unlikely to offer sufficient alternative habitat for mid- to long-term refuge and forage, especially for larger mammals and habitat specialist such as the quokka. Instead, strategically planned fire exclusion areas are likely to become a necessary conservation tool, particularly for fragmented populations, and will need to provide sufficient habitat to preserve populations over time, relative to the size of the species and population.
Despite the most careful planning, fire behaviour on the day of burning can easily increase the distance between animals and safety, and consequently their survival. In the present study, the fire exclusion area at the Gordon site was across a track from the burn area (i.e. less than 10 m away). Immediately after manual fire ignition, quokkas in the burn area could therefore move into the adjacent fire-exclusion area, which offered the same habitat in a continuous creek line. Where the distance to unburned habitat is greater, or largely dissected either by roads or fire breaks, it may inhibit or delay access to this habitat. For example, the Wungong site was aerially ignited in open forest, between exclusion and burning sites, and during the burn one of the two collared quokkas remained at the burned site in a small, unburned patch. Days after, he managed to move to the exclusion area. The operational methods of implementing fire, wind direction, and fire behaviour are also likely to influence how useful a fire-exclusion area will be as refuge for animals.
Animals may also elect to remain in known habitat rather than move to planned fire exclusions. At the Churchman site, the planned fire exclusion (containing a population of quokkas) was 2.5 km away from the burn area and was not used by the animal present at the burn area (Q16), which instead moved into closer suitable adjacent habitat that was part of the same creek system. Similarly, Hope (2012) reported that a long-nosed bandicoot slowly advanced into unburned vegetation, but when reaching the end of its known home range, avoided the unknown habitat and instead back tracked into the fire, seeking refuge in a boulder pile. Because animals may be unwilling to move to safety beyond their home range, particularly where habitat is fragmented or not continuous, it is crucial to plan proximal fire exclusions that support similar habitat to the burn area to provide appropriate resources during and post-fire.
Did quokkas shift their home range after prescription burning? And if so, how long did they avoid the area?
Quokkas affected by the prescribed burns shifted their home ranges in response to fire and did not use the burn area for at least 6 months post-fire. This result supports observations that southern forest quokkas (WWF 2016) and swamp wallabies (Ben-Ami 2005), which previously had stable home ranges, emigrated from the study site after wildfires. In contrast, Garvey et al. (2010) and Leahy et al. (2015) describe no shift in the ranges of tracked swamp wallabies and pale field rats before and after prescribed burns. Differences among species regarding whether they shift range after prescribed fires is likely to reflect their biology, behaviour, diet, and habitat specificity. For example, responses to fire are influenced by the use of burrows (Long 2009; McGregor et al. 2014; Leahy et al. 2015) and consumption of fungal fruit-bodies (Christensen and Lewis 1980; Johnson 1995; Vernes and Haydon 2001). Such differences in response highlights the importance of tracking fauna species to determine specific resources used after fire.
The new home ranges established in fire-exclusion areas by quokkas in the present study were in the same habitat, with well-developed riparian vegetation structure providing refuge and forage. This result shows the species’ high fidelity to riparian ecotype with dense understorey, as previously reported (Hayward et al. 2005; Bain et al. 2016; Dundas et al. 2018). The response of species to fire will vary with fire parameters (e.g. size, severity, timing, topography) affecting the retention of habitat, but will also be influenced by the species’ ecology. Species with broad habitat preference are likely to be more adaptable to the post-fire landscape than habitat specialists. Many grazing species will take advantage of vegetation regrowth soon after fire (Blumstein et al. 2002; Archibald and Bond 2004; Hayward et al. 2004; Hope 2012; Bain et al. 2016). However, the quokka is largely a browser (Hayward 2005), and its woody shrub diet is likely to take longer to re-grow, as suggested by the long avoidance of burned areas (average of 105 ± 65 days). In addition, the timing of understorey vegetation regrowth may differ according to the fire severity (encapsulating the joint impact that elements of a fire regime have on an ecosystem) (Burrows et al. 2008). Fire severity affects where resprouting occurs (Burrows 2013), availability and size of unburned patches (Sitters et al. 2015; Nielsen 2018), the amount and quality of retained course woody debris (Hollis et al. 2018), and stimulation of soil stored seedbanks (Ooi et al. 2006). Consequently, vegetation structure and plant responses to fire in turn influence fauna assemblages and whether a recently burned area contains habitat that is suitable for use (Densmore et al. 2023).
Did quokkas change their home range area after prescription burning?
Contrary to our predictions based on the expectation that fire would remove edible browse, therefore requiring greater movements to locate suitable food plants, we found that the prescribed burns did not affect overall home range size of the quokkas tracked in this study. We also found no significant difference in the size of diurnal (i.e. refuge locations) and nocturnal (i.e. foraging locations) home ranges before and after fire. These findings reinforce previous studies finding no significant fire-related change in the home-range sizes of brush-tailed bettong, northern bettong and long-nosed bandicoot (Christensen and Lewis 1980; Vernes and Haydon 2001; MacGregor et al. 2013). However, our result does not corroborate a previous study reporting quokkas foraged in recently burned habitat less than 3 months post-fire (Christensen and Kimber 1975). These different results could reflect differential research methods and impact of fires across specific landscapes.
Management implications of this study
This study has highlighted several important considerations to inform fire management and quokka conservation. We have demonstrated no mortalities as a direct result of prescribed fire where nearby fire exclusion areas were present, with quokkas moving into proximal unburned habitat during prescribed fire operations. Permitting natural movement into these areas will decrease the risk of mortalities where animals become confused by fire or smoke present in the direction of the exclusion areas. Fire-exclusion areas therefore need to be located close enough to facilitate quokka movement, and should be appropriately sized to cater for a predicted increase in population density over the short to medium term. Bain et al. (2016) suggested that quokkas persisted after fire in unburned habitat patches >100 ha, but not in patches smaller than this, and not in completely burned habitat. Unburned patches of small sizes were nevertheless useful as ‘stepping stones’, providing temporary shelter where quokkas could wait until the immediate threat had passed to find their way to a larger patch or fire exclusion in the longer term. Planning should consider appropriate area for the total population that is likely to use the fire-exclusion site while surrounding burned habitat regenerates.
We found that quokkas did not use burned areas for at least 6 months post-burn, so fire-exclusion areas will be critical for providing refuge, foraging resources, and protection from predators for at least this time period. Identifying when regrown vegetation becomes used again by quokkas was beyond the scope of the present project, but is imperative for future planning. Monitoring the effectiveness of planned exclusions will be critical to informing planning decisions and allowing adaptive management. Fire-exclusion size, location, quokkas density, fuel age of vegetation within exclusion, predators, and competitors can all influence how successful an exclusion area is. By monitoring or describing these parameters over time, we can determine those that maximise conservation outcomes.
Finally, more research is needed to understand dispersal among fragmented quokka populations. We found that 20 male quokkas in the Northern Jarrah Forest had much larger home ranges (95% KDE 75.2 ± 59.7 ha) than the previously reported home-range size of males (6.92 ha) and females (5.91 ha), derived from triangulation of VHF signals (Hayward et al. 2004). With variation in the intensity of sampling between the two studies, Hayward et al. (2004) collected no more than one diurnal and one nocturnal location per day and obtained an average of 35 VHF fixes for 58 animals. In contrast, our study obtained an average of 72 VHF fixes for 14 animals and 316 GPS fixes for six quokkas pre-burn, as well as 84 VHF fixes for nine quokkas and 324 GPS fixes for nine quokkas post-burn. The use of GPS collars in the present study may partially explain the larger home ranges estimated in the present study, which captured long-range movements. Furthermore, Hayward et al. (2004) demonstrated a negative relationship between population density and home range area of individuals, which may suggest that decreasing population size over time has also contributed to larger home range areas recorded in the present study.
We reported two long-range movements of more than 9 km where tracked adult males moved between forest blocks. Hayward et al. (2005) reported no quokka dispersal between isolated populations in the Northern Jarrah Forest, as a possible response to the pressure and predation by foxes. Dundas (2019) described quokka morphological abnormalities suggesting inbreeding in two Northern Jarrah Forest populations, supported by genetic isolation between the populations (Spencer et al. 2019). On the contrary, southern quokkas routinely travel distances up to 10 km where there is optimal habitat connectivity between riparian vegetation, and where movement occurs between populations (Bain et al. 2020) and genetic mixing consequently follows (Spencer et al. 2019). Detection of two Northern Jarrah quokkas moving between distant sites from the present study warrants further long-term investigations, aiming to identify isolated and connected residents to inform fire planning.
Conclusions
Prescribed burning is a necessary but complex practice that is increasingly climatically challenging (Russell-Smith et al. 2020). The impacts of prescribed burning on many Australian fauna species are poorly researched. Most studies about fauna species in fire-prone ecosystems do not integrate animal movement and assume that species respond to fire along a post-fire successional axis (Nimmo et al. 2019). Considerations about prescribed burning in and around quokka habitat is becoming more complex in the presence of a drying climate, considering the vulnerability of the fragmented populations and uncertainties around quokka movements patterns. In Western Australia, the Northern Jarrah Forest has experienced increased temperature (Braganza and Church 2011), decreased rainfall, and reduction of soil water reservoirs causing decreases to stream flow since the 1970s (Sudmeyer et al. 2016). Consequently, the vegetation assemblage is becoming drier with less moisture differentials in riparian areas, increasing the likelihood of them being impacted by fire, and this is forecast to continue into the future. A carefully planned fire strategy for this region is therefore imperative to ensure sufficient riparian vegetation remains for quokkas to retreat to when part of their habitat has been burned. This study shows the importance of monitoring each of these fragmented populations before and after prescribed burns, both in burn and fire-exclusion areas, to define appropriately sized and located fire-exclusion areas to ensure the persistence of populations into the future.
Acknowledgements
Many thanks to Karlene Bain for assistance in the field. Thanks to Rebecca Bloomfield, Bob Huston, Michael Pasotti and all the Perth Hills fire team, particularly the Jarrahdale staff and Chris Phoebe. Thanks to the field volunteers, especially those who helped for several days during trapping and radio-tracking work, including Marcus Harris, Leah de Myer, Emma Rutter, Lenka Koporova, Cecelia Crowe, Breanna Muste, Szalina Maass, Poppy Walker, and Julia White. Leticia F. Povh was supported by the Research Training Program at Murdoch University and Department of Biodiversity, Conservation and Attractions Forest Enhancement funding.
References
Akaike H (1973) Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 60, 255-265.
| Crossref | Google Scholar |
Anstee SD, Roberts JD, O’Shea JE (1997) Social structure and patterns of movement of the western pebble-mound mouse, Pseudomys chapmani, at Marandoo, Western Australia. Wildlife Research 24, 295-305.
| Crossref | Google Scholar |
Archibald S, Bond WJ (2004) Grazer movements: spatial and temporal responses to burning in a tall-grass African savanna. International Journal of Wildland Fire 13, 377-385.
| Crossref | Google Scholar |
Bain K, Wayne A, Bencini R (2015) Risks in extrapolating habitat preferences over the geographical range of threatened taxa: a case study of the quokka (Setonix brachyurus) in the southern forests of Western Australia. Wildlife Research 42, 334-342.
| Crossref | Google Scholar |
Bain K, Wayne A, Bencini R (2016) Prescribed burning as a conservation tool for management of habitat for threatened species: the quokka, Setonix brachyurus, in the southern forests of Western Australia. International Journal of Wildland Fire 25, 608-617.
| Crossref | Google Scholar |
Bain K, Wayne AF, Bencini R (2020) Spatial ecology of the quokka (Setonix brachyurus) in the southern forests of Western Australia: implications for the maintenance, or restoration, of functional metapopulations. Australian Mammalogy 42, 38-47.
| Crossref | Google Scholar |
Begg RJ, Martin KC, Price NF (1981) The small mammals of Little Nourlangie Rock, NT. V. The effects of fire. Wildlife Research 8, 515-527.
| Crossref | Google Scholar |
Berry LE, Lindenmayer DB, Driscoll DA (2015) Large unburnt areas, not small unburnt patches, are needed to conserve avian diversity in fire-prone landscapes. Journal of Applied Ecology 52, 486-495.
| Crossref | Google Scholar |
Blumstein DT, Daniel JC, Schnell MR, Ardron JG, Evans CS (2002) Antipredator behaviour of red-necked pademelons: a factor contributing to species survival? Animal Conservation 5, 325-331.
| Crossref | Google Scholar |
Bradshaw SD, Dixon KW, Lambers H, Cross AT, Bailey J, Hopper SD (2018) Understanding the long-term impact of prescribed burning in mediterranean-climate biodiversity hotspots, with a focus on south-western Australia. International Journal of Wildland Fire 27, 643-657.
| Crossref | Google Scholar |
Burbidge AA, McKenzie NL (1989) Patterns in the modern decline of western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143-198.
| Crossref | Google Scholar |
Burbidge AA, Woinarski J (2020) Setonix brachyurus (amended version of 2019 assessment). Available at 10.2305/IUCN.UK.2020-1.RLTS.T20165A166611530.en [accessed 8 February]
Burrows GE (2013) Buds, bushfires and resprouting in the eucalypts. Australian Journal of Botany 61, 331-349.
| Crossref | Google Scholar |
Burrows ND, Wardell-Johnson G, Ward B (2008) Post-fire juvenile period of plants in south-west Australia forests and implications for fire management. Journal of the Royal Society of Western Australia 91, 163-174.
| Google Scholar |
Calabrese JM, Fleming CH, Gurarie E (2016) CTMM: an r package for analyzing animal relocation data as a continuous-time stochastic process. Methods in Ecology and Evolution 7, 1124-1132.
| Crossref | Google Scholar |
Chambers LK, Dickman CR (2002) Habitat selection of the long-nosed bandicoot, Perameles nasuta (Mammalia, Peramelidae), in a patchy urban environment. Austral Ecology 27, 334-342.
| Crossref | Google Scholar |
Christensen PES, Kimber PC (1975) Effect of prescribed burning on the flora and fauna of south-western Australian forests. Proceedings of the Ecological Society of Australia 85-106.
| Google Scholar |
Christensen P, Leftwich T (1980) Observations on the nest-building habits of the brush-tailed rat-kangaroo or woylie (Bettongia penicillata). Journal of the Royal Society of Western Australia 63, 33-38.
| Google Scholar |
Clarke MF (2008) Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research 35, 385-394.
| Crossref | Google Scholar |
Conner LM, Castleberry SB, Derrick AM (2011) Effects of mesopredators and prescribed fire on hispid cotton rat survival and cause-specific mortality. The Journal of Wildlife Management 75, 938-944.
| Crossref | Google Scholar |
Couriot O, Hewison AJM, Saïd S, Cagnacci F, Chamaillé-Jammes S, Linnell JDC, Mysterud A, Peters W, Urbano F, Heurich M, Kjellander P, Nicoloso S, Berger A, Sustr P, Kroeschel M, Soennichsen L, Sandfort R, Gehr B, Morellet N (2018) Truly sedentary? The multi-range tactic as a response to resource heterogeneity and unpredictability in a large herbivore. Oecologia 187, 47-60.
| Crossref | Google Scholar |
CSIRO (2020) State of the climate 2020. (Bureau of Meteorology and Commonwealth Scientific and Industrial Research Organisation) Available at https://www.csiro.au/en/Showcase/state-of-the-climate [accessed 4 June 2021]
Densmore VS, van Dongen RJ, Ong R, Harris BG (2023) OzCBI: the composite burn index adapted to assess fire severity and key fauna habitat features in Australian ecosystems. Australian Forestry 86, 1-21.
| Crossref | Google Scholar |
Dickman CR, Predavec M, Downey FJ (1995) Long-range movements of small mammals in arid Australia: implications for land management. Journal of Arid Environments 31, 441-452.
| Crossref | Google Scholar |
Di Virgilio G, Evans JP, Blake SAP, Armstrong M, Dowdy AJ, Sharples J, McRae R (2019) Climate change increases the potential for extreme wildfires. Geophysical Research Letters 46, 8517-8526.
| Crossref | Google Scholar |
Dundas SJ (2019) Tell-tale testicles: observations of morphological abnormalities in small, spatially restricted mainland quokka (Setonix brachyurus) populations. Australian Mammalogy 41, 150.
| Crossref | Google Scholar |
Dundas SJ, Adams PJ, Fleming PA (2018) Population monitoring of an endemic macropod, the quokka (Setonix brachyurus), in the northern jarrah forest, Western Australia. Australian Mammalogy 40, 26-35.
| Crossref | Google Scholar |
Eales J, Haddaway NR, Bernes C, Cooke SJ, Jonsson BG, Kouki J, Petrokofsky G, Taylor JJ (2018) What is the effect of prescribed burning in temperate and boreal forest on biodiversity, beyond pyrophilous and saproxylic species? A systematic review. Environmental Evidence 7, 19.
| Crossref | Google Scholar |
Eby SL, Anderson TM, Mayemba EP, Ritchie ME (2014) The effect of fire on habitat selection of mammalian herbivores: the role of body size and vegetation characteristics. Journal of Animal Ecology 83, 1196-1205.
| Crossref | Google Scholar |
Fischer RA, Wakkinen WL, Reese KP, Connelly JW (1997) Effects of prescribed fire on movements of female sage grouse from breeding to summer ranges. The Wilson Bulletin 109, 82-91.
| Google Scholar |
Flanagan-Moodie AK, Holland GJ, Clarke MF, Bennett AF (2018) Prescribed burning reduces the abundance of den sites for a hollow-using mammal in a dry forest ecosystem. Forest Ecology and Management 429, 233-243.
| Crossref | Google Scholar |
Fleming CH, Calabrese JM (2017) A new kernel density estimator for accurate home-range and species-range area estimation. Methods in Ecology and Evolution 8, 571-579.
| Crossref | Google Scholar |
Fordyce A, Hradsky BA, Ritchie EG, Di Stefano J (2016) Fire affects microhabitat selection, movement patterns, and body condition of an Australian rodent (Rattus fuscipes). Journal of Mammalogy 97, 102-111.
| Crossref | Google Scholar |
Garvey N, Ben-Ami D, Ramp D, Croft DB (2010) Survival behaviour of swamp wallabies during prescribed burning and wildfire. Wildlife Research 37, 1-12.
| Crossref | Google Scholar |
Haslem A, Kelly LT, Nimmo DG, Watson SJ, Kenny SA, Taylor RS, Avitabile SC, Callister KE, Spence-Bailey LM, Clarke MF, Bennett AF (2011) Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire. Journal of Applied Ecology 48, 247-256.
| Crossref | Google Scholar |
Hayward MW (2005) Diet of the quokka (Setonix brachyurus) (Macropodidae: Marsupialia) in the northern jarrah forest of Western Australia. Wildlife Research 32, 15-22.
| Crossref | Google Scholar |
Hayward MW, de Tores PJ, Augee ML, Fox BJ, Banks PB (2004) Home range and movements of the quokka Setonix brachyurus (Macropodidae: Marsupialia), and its impact on the viability of the metapopulation on the Australian mainland. Journal of Zoology 263, 219-228.
| Crossref | Google Scholar |
Hayward MW, de Tores PJ, Banks PB (2005) Habitat use of the quokka, Setonix brachyurus (Macropodidae: Marsupialia) in the northern jarrah forest of Australia. Journal of Mammalogy 86, 683-688.
| Crossref | Google Scholar |
Hayward MW, de Tores PJ, Dillon MJ, Banks PB (2007) Predicting the occurrence of the quokka, Setonix brachyurus (Macropodidae: Marsupialia), in Western Australia’s northern jarrah forest. Wildlife Research 34, 194-199.
| Crossref | Google Scholar |
He T, Lamont BB, Pausas JG (2019) Fire as a key driver of Earth’s biodiversity. Biological Reviews 94, 1983-2010.
| Crossref | Google Scholar |
Hollis JJ, McCaw WL, Cruz MG (2018) The effect of woody fuel characteristics on fuel ignition and consumption: a case study from a eucalypt forest in south-west Western Australia. International Journal of Wildland Fire 27, 363-375.
| Crossref | Google Scholar |
Hope B (2012) Short-term response of the long-nosed bandicoot, Perameles nasuta, and the southern brown bandicoot, Isoodon obesulus obesulus, to low-intensity prescribed fire in heathland vegetation. Wildlife Research 39, 731-744.
| Crossref | Google Scholar |
Howard T, Burrows N, Smith T, Daniel G, McCaw L (2020) A framework for prioritising prescribed burning on public land in Western Australia. International journal of Wildland Fire 29, 314-325.
| Crossref | Google Scholar |
Jalaludin B, Morgan GG (2021) What does climate change have to do with bushfires? Australian Health Review 45, 4-6.
| Crossref | Google Scholar |
Johnson CN (1995) Interactions between Fire, Mycophagous Mammals, and Dispersal of Ectromycorrhizal Fungi in Eucalyptus Forests. Oecologia 104, 467-475.
| Crossref | Google Scholar |
Jolly CJ, Dickman CR, Doherty TS, van Eeden LM, Geary WL, Legge SM, Woinarski JCZ, Nimmo DG (2022) Animal mortality during fire. Global Change Biology 28, 2053-2065.
| Crossref | Google Scholar |
Lavielle M (1999) Detection of multiple changes in a sequence of dependent variables. Stochastic Processes and their Applications 83, 79-102.
| Crossref | Google Scholar |
Leahy L, Legge SM, Tuft K, McGregor HW, Barmuta LA, Jones ME, Johnson CN (2015) Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42, 705-716.
| Crossref | Google Scholar |
Legge S, Murphy S, Heathcote J, Flaxman E, Augusteyn J, Crossman M (2008) The short-term effects of an extensive and high-intensity fire on vertebrates in the tropical savannas of the central Kimberley, northern Australia. Wildlife Research 35, 33-43.
| Crossref | Google Scholar |
Letnic M, Dickman CR (2005) The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia: effects of fire and rainfall on small mammals. Austral Ecology 30, 24-39.
| Crossref | Google Scholar |
Long K (2009) Burrowing bandicoots – an adaptation to life in a fire-prone environment? Australian Mammalogy 31, 57-59.
| Crossref | Google Scholar |
MacGregor CI, Wood JT, Dexter N, Lindenmayer DB (2013) Home range size and use by the long-nosed bandicoot (Perameles nasuta) following fire. Australian Mammalogy 35, 206-216.
| Crossref | Google Scholar |
MacGregor CI, Cunningham RB, Lindenmayer DB (2015) Nest-site selection of the long-nosed bandicoot (Perameles nasuta) in a postfire environment. Australian Journal of Zoology 63, 324-330.
| Crossref | Google Scholar |
McGregor HW, Legge S, Jones ME, Johnson CN (2014) Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS One 9, e109097.
| Crossref | Google Scholar |
Morris G, Hostetler JA, Oli MK, Conner LM (2011) Effects of predation, fire, and supplemental feeding on populations of two species of Peromyscus mice. Journal of Mammalogy 92, 934-944.
| Crossref | Google Scholar |
Nimmo DG, Avitabile S, Banks SC, Bliege Bird R, Callister K, Clarke MF, Dickman CR, Doherty TS, Driscoll DA, Greenville AC, Haslem A, Kelly LT, Kenny SA, Lahoz-Monfort JJ, Lee C, Leonard S, Moore H, Newsome TM, Parr CL, Ritchie EG, Schneider K, Turner JM, Watson S, Westbrooke M, Wouters M, White M, Bennett AF (2019) Animal movements in fire-prone landscapes. Biological Reviews 94, 981-998.
| Crossref | Google Scholar |
Ooi MKJ, Whelan RJ, Auld TD (2006) Persistence of obligate-seeding species at the population scale: effects of fire intensity, fire patchiness and long fire-free intervals. International Journal of Wildland Fire 15, 261-269.
| Crossref | Google Scholar |
Pastro LA, Dickman CR, Letnic M (2011) Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals. Ecological Applications 21, 3238-3253.
| Crossref | Google Scholar |
Patin R, Etienne MP, Lebarbier E, Chamaillé-Jammes S, Benhamou S (2020) Identifying stationary phases in multivariate time series for highlighting behavioural modes and home range settlements. Journal of Animal Ecology 89, 44-56.
| Crossref | Google Scholar |
Penman TD, Kavanagh RP, Binns DL, Melick DR (2007) Patchiness of prescribed burns in dry sclerophyll eucalypt forests in south-eastern Australia. Forest Ecology and Management 252, 24-32.
| Crossref | Google Scholar |
Penman TD, Christie FJ, Andersen AN, Bradstock RA, Cary GJ, Henderson MK, Price O, Tran C, Wardle GM, Williams RJ, York A (2011) Prescribed burning: how can it work to conserve the things we value? International Journal of Wildland Fire 20, 721-733.
| Crossref | Google Scholar |
Radford IJ, Gibson LA, Corey B, Carnes K, Fairman R (2015) Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley. PLoS One 10, e0130721.
| Crossref | Google Scholar |
Radford IJ, Woolley L-A, Corey B, Vigilante T, Wunambal Gaambera Aboriginal Corporation, , Hatherley E, Fairman R, Carnes K, Start AN (2020) Prescribed burning benefits threatened mammals in northern Australia. Biodiversity and Conservation 29, 2985-3007.
| Crossref | Google Scholar |
Read D (1984) Movements and home ranges of three sympatric dasyruids, Sminthopsis crassicaudata, Planigale gilesi and P. tenuirostris (Marsupialaia), in semiarid western New South Wales. Wildlife Research 11, 223-234.
| Crossref | Google Scholar |
Ritchie EG, Martin JK, Krockenberger AK, Garnett S, Johnson CN (2008) Large-herbivore distribution and abundance: intra-and interspecific niche variation in the tropics. Ecological Monographs 78, 105-122.
| Crossref | Google Scholar |
Robinson NM, Leonard SWJ, Ritchie EG, Bassett M, Chia EK, Buckingham S, Gibb H, Bennett AF, Clarke MF (2013) REVIEW: Refuges for fauna in fire-prone landscapes: their ecological function and importance. Journal of Applied Ecology 50, 1321-1329.
| Crossref | Google Scholar |
Robinson NM, Leonard SWJ, Bennett AF, Clarke MF (2014) Refuges for birds in fire-prone landscapes: the influence of fire severity and fire history on the distribution of forest birds. Forest Ecology and Management 318, 110-121.
| Crossref | Google Scholar |
Russell-Smith J, McCaw L, Leavesley A (2020) Adaptive prescribed burning in Australia for the early 21st Century – context, status, challenges. International Journal of Wildland Fire 29, 305-313.
| Crossref | Google Scholar |
Santos JL, Hradsky BA, Keith DA, Rowe KC, Senior KL, Sitters H, Kelly LT (2022) Beyond inappropriate fire regimes: a synthesis of fire-driven declines of threatened mammals in Australia. Conservation Letters 15, e12905.
| Crossref | Google Scholar |
Shaw RE, James AI, Tuft K, Legge S, Cary GJ, Peakall R, Banks SC (2021) Unburnt habitat patches are critical for survival and in situ population recovery in a small mammal after fire. Journal of Applied Ecology 58, 1325-1335.
| Crossref | Google Scholar |
Sitters H, Di Stefano J, Christie FJ, Sunnucks P, York A (2015) Bird diversity increases after patchy prescribed fire: implications from a before–after control–impact study. International Journal of Wildland Fire 24, 690-701.
| Crossref | Google Scholar |
Spencer PBS, Bain K, Hayward MW, Hillyer M, Friend JAT (2019) Persistence of remnant patches and genetic loss at the distribution periphery in island and mainland populations of the quokka. Australian Journal of Zoology 67, 38-50.
| Crossref | Google Scholar |
Styger JK, Kirkpatrick JB, Marsden-Smedley JON, Leonard SWJ (2011) Fire incidence, but not fire size, affects macropod densities. Austral Ecology 36, 679-686.
| Crossref | Google Scholar |
Swinburn ML, Fleming PA, Craig MD, Grigg AH, Garkaklis MJ, Hobbs RJ, Hardy GESJ (2007) The importance of grasstrees (Xanthorrhoea preissii) as habitat for mardo (Antechinus flavipes leucogaster) during post-fire recovery. Wildlife Research 34, 640-651.
| Crossref | Google Scholar |
Teague B, McLeod R, Pascoe S (2010) The 2009 Victoria Bushfires Royal Commission final report. (Government Printer for the State of Victoria: Melbourne, Vic., Australia) Available at http://royalcommission.vic.gov.au/Commission-Reports/Final-Report/Volume-1/Print-Friendly-Version.html [accessed 9 June 2021]
Valkó O, Deák B, Magura T, Török P, Kelemen A, Tóth K, Horváth R, Nagy DD, Debnár Z, Zsigrai G, Kapocsi I, Tóthmérész B (2016) Supporting biodiversity by prescribed burning in grasslands — A multi-taxa approach. Science of the Total Environment 572, 1377-1384.
| Crossref | Google Scholar |
van Oldenborgh GJ, Krikken F, Lewis S, Leach NJ, Lehner F, Saunders KR, van Weele M, Haustein K, Li S, Wallom D, Sparrow S, Arrighi J, Singh RK, van Aalst MK, Philip SY, Vautard R, Otto FEL (2021) Attribution of the Australian bushfire risk to anthropogenic climate change. Natural Hazards and Earth System Sciences 21, 941-960.
| Crossref | Google Scholar |
Vernes K (2000) Immediate effects of fire on survivorship of the northern bettong (Bettongia tropica): an endangered Australian marsupial. Biological Conservation 96, 305-309.
| Crossref | Google Scholar |
Vernes K, Haydon DT (2001) Effect of fire on northern bettong (Bettongia tropica) foraging behaviour. Austral Ecology 26, 649-659.
| Crossref | Google Scholar |
Vernes K, Pope LC (2001) Stability of nest range, home range and movement of the northern bettong (Bettongia tropica) following moderate-intensity fire in a tropical woodland, north-eastern Queensland. Wildlife Research 28, 141-150.
| Crossref | Google Scholar |
Wiggins NL, Williamson GJ, McCallum HI, McMahon CR, Bowman DMJS (2010) Shifts in macropod home ranges in response to wildlife management interventions. Wildlife Research 37, 379-391.
| Crossref | Google Scholar |
Wikelski M, Davidson S, Kays R (2020) Movebank: archive, analysis and sharing of animal movement data. (Hosted by the Max Planck Institute of Animal Behavior) Available at www.movebank.org [accessed 10 October]
Williams, RJ, Bradstock, RA, Cary, GJ, Enright, NJ, Gill, AM, Leidloff, AC, Lucas C, Whelan RJ, Andersen AN, Bowman DJMS (2009) Interactions between climate change, fire regimes and biodiversity in Australia: A preliminary assessment. (Department of Climate Change and Department of the Environment, Water, Heritage and the Arts)
WWF (2016) Survival of quokkas in the 2015 Northcliffe bushfire. Available at https://www.wwf.org.au/what-we-do/species/quokka#gs.538yi5 [accessed 6 July 2021]
Yospin GI, Wood SW, Holz A, Bowman DMJS, Keane RE, Whitlock C (2015) Modeling vegetation mosaics in sub-alpine Tasmania under various fire regimes. Modeling Earth Systems and Environment 1, 16.
| Crossref | Google Scholar |


