Could current fertility control methods be effective for landscape-scale management of populations of wild horses (Equus caballus) in Australia?
Rebecca J. Hobbs A and Lyn A. Hinds B CA Taronga Conservation Society Australia, Taronga Western Plains Zoo, Dubbo, NSW, 2830, Australia.
B CSIRO Health and Biosecurity, Canberra, ACT, 2601, Australia.
C Corresponding author. Email: lyn.hinds@csiro.au
Wildlife Research 45(3) 195-207 https://doi.org/10.1071/WR17136
Submitted: 28 September 2017 Accepted: 26 February 2018 Published: 24 May 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Context: Fertility control is seen as an attractive alternative to lethal methods for control of population size and genetic diversity in managed animal populations. Immunocontraceptive vaccines have emerged as the most promising agents for inducing long-term infertility in individual animals. However, after over 20 years of scientific testing of immunocontraceptive vaccines in the horse, the scientific consensus is that their application as a sole management approach for reducing population size is not an effective strategy.
Aims: The purpose of this review is to evaluate currently available non-lethal fertility-control methods that have been tested for their contraceptive efficacy in Equidae, and to assess their suitability for effective management of wild (feral) horses in an Australian setting.
Key results: (1) Fertility-control agents, particularly injectable immunocontraceptive vaccines based on porcine zona pellucida (PZP) or gonadotrophin-releasing hormone (GnRH), can induce multi-year infertility (up to 3 years) in the horse. Some formulations require annual or biennial booster treatments. Remote dart delivery (on foot) to horses is possible, although the efficacy of this approach when applied to large numbers of animals is yet to be determined. (2) The proportion of females that must be treated with a fertility-control agent, as well as the frequency of treatment required to achieve defined management outcomes (i.e. halting population growth in the short term and reducing population size in the long term) is likely to be >50% per annum. In national parks, treatment of a large number of wild horses over such a broad area would be challenging and impractical. (3) Fertility control for wild horses could be beneficial, but only if employed in conjunction with other broad-scale population-control practices to achieve population reduction and to minimise environmental impacts.
Conclusions: In Australia, most populations of wild horses are large, dispersed over varied and difficult-to-access terrain, are timid to approach and open to immigration and introductions. These factors make accessing and effectively managing animals logistically difficult. If application of fertility control could be achieved in more than 50% of the females, it could be used to slow the rate of increase in a population to zero (2–5 years), but it will take more than 10–20 years before population size will begin to decline without further intervention. Thus, use of fertility control as the sole technique for halting population growth is not feasible in Australia.
Additional keywords: Equidae, feral, GnRH, immunocontraception, population management, PZP.
Introduction
Wild (feral) horse (Equus caballus) management at a landscape scale in Australia (and internationally) is complex and problematic. Horses escaped domestication some 200 years ago, and since then have become an over-abundant pest in many natural environments and pastoral regions throughout mainland Australia. Their various impacts on flora and fauna, water quality and soil compaction and erosion have been documented (for reviews: Dawson et al. 2006; Nimmo and Miller 2007). The scale, location and impact of wild horses, as well as their welfare, can define their immediate management, which can include humane lethal control (shooting), trapping and mustering (herding or gathering), translocation and rehoming. However, there is often controversy surrounding management of wild horses, with community acceptance of most control methods (particularly lethal control) being low despite full engagement in the decision-making process (National Research Council 2013; Office of Environment and Heritage 2016a).
Fertility control is often considered more acceptable as an alternative to lethal methods for maintenance of population size in managed populations, whether they be captive, over-abundant native or feral species. Since the early 1990s, there has been considerable debate about the role of fertility control for wildlife management (Bomford and O’Brien 1992), with many publications now available on the development of potential fertility-control methods for application to wildlife and feral species (see Kirkpatrick and Rutberg 2001; Kirkpatrick et al. 2011; Garside et al. 2014; Massei and Cowan 2014; Massei et al. 2014; Ransom and Kaczensky 2016).
The purpose of the present review is to provide a current perspective of the most effective fertility-control methods available for use in Equidae. We also present a considered judgement of the suitability and practicality of fertility control as part of an integrated management approach for wild horses in an Australian setting.
Although many laboratory assessments of different agents have been positive, there have been few broad-scale trials of fertility control for population management of wildlife (see: Massei and Cowan 2014; Rutberg et al. 2017). The transfer to field settings is limited by the absence of cost-effective, efficient and practical field-delivery techniques. There are no proven orally deliverable formulations available for large free-ranging herbivores (Sharma and Hinds 2012); thus, all current agents require capture, or mustering for individual treatment of animals. That said, remote-dart delivery of some agents (e.g. some immunocontraceptive vaccines) is possible in the field (Kirkpatrick et al. 1990; Turner et al. 1992, 1996; Rutberg et al. 2017), and may prove the most efficacious approach in the medium to long term for relatively small isolated populations. Other issues, such as how best to remotely mark treated animals at the time of treatment or in subsequent years and learned avoidance (increasing aversion to human presence), may arise with remote delivery (e.g. Naugle and Grams 2013).
Fertility control strategies targeting females only are usually more productive in terms of efficiency, absence of adverse side effects on their behaviour or welfare, duration of infertility and overall cost effectiveness (Bomford 1990). Even in species where females predominantly mate with a single dominant territorial male (such as the horse), invoking temporary or permanent male sterility is ineffective as females have opportunities to mate with fertile extra-harem or subordinate males (Asa 1999; Scully et al. 2015). When male-oriented contraception for horses is simulated (Garrott and Siniff 1992), there are poor outcomes for overall wild population control – very high proportions of stallions needed to be sterilised to suppress population growth (Eagle et al. 1993). Moreover, no contraceptive agent (Stout and Colenbrander 2004; Janett et al. 2009) or chemical castration (Scully et al. 2015) tested thus far can completely inhibit sperm production in stallions; this leaves only surgical castration, which is highly impractical for large-scale field application. Nevertheless, Collins and Kasbohm (2017) applied both vasectomy and ovariectomy concurrently to a closed population of ~1800 horses and observed a decrease in foaling rate from >20% to <4% within 4 years.
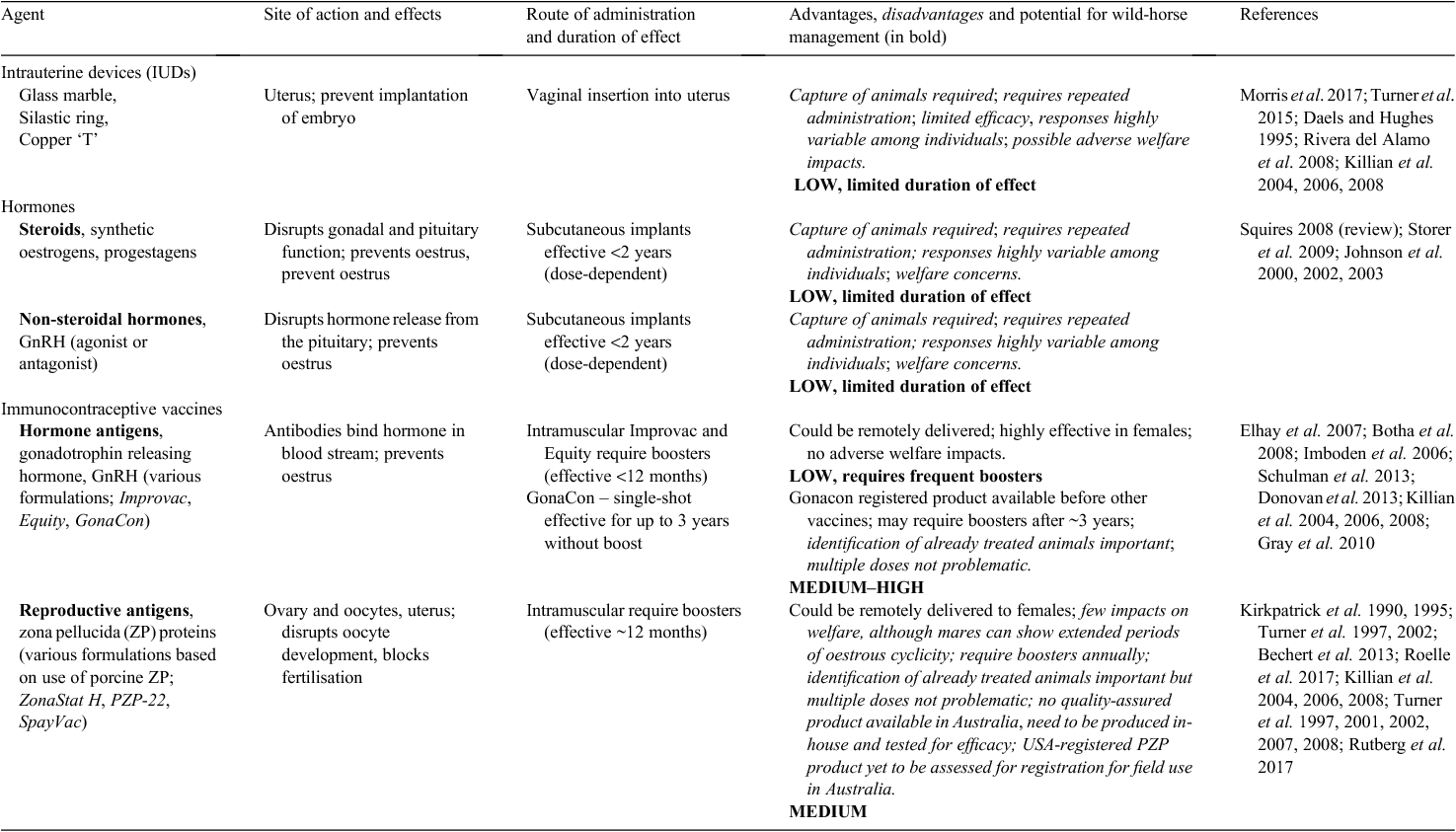
The present review, therefore, discusses only those fertility-control agents that inhibit the fertility of females and that have the potential for remote delivery. As noted by others, there are several desirable characteristics of fertility-control agents that are important if a product is to be suitable for field use (Table 1; see: Kirkpatrick and Turner 1991; Massei and Cowan 2014), particularly cost effectiveness, long-lasting efficacy and feasibility of efficient field delivery.

|
Fertility-control research
Research on the use of fertility-control agents in pest management began in the late 1970s and reflected developments in the use of steroids for human fertility and contraception (Kirkpatrick and Rutberg 2001); the FDA approved ‘the pill’ for human contraceptive use in 1960. Over the past 40 years, methods for animals have continued to evolve in parallel with studies of human contraception, whereby a range of approaches target the key physiological processes essential for successful reproduction, namely, reproductive hormones associated with the overall function of the hypothalamic–pituitary–gonadal axis, production of sperm or eggs (gametes) by the gonads (testes and ovaries), fertilisation in the oviduct, or implantation of the embryo in the uterus (Fig. 1).
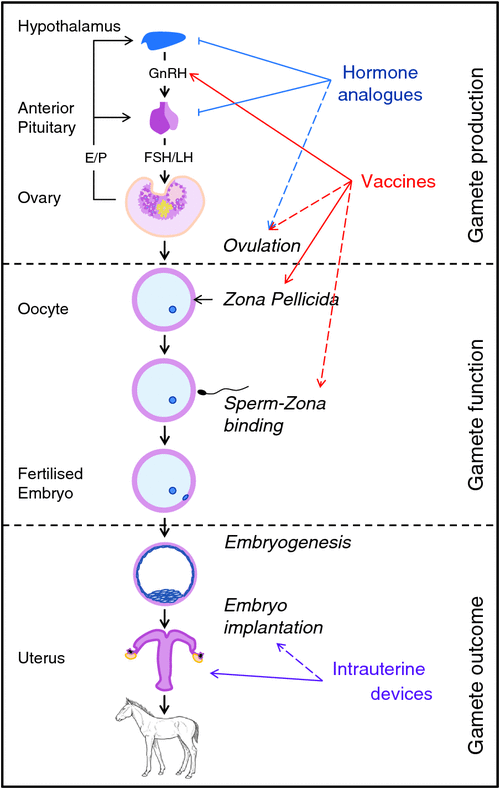
|
The physiological efficacy of various contraceptive approaches has been demonstrated in a wide range of species in captive studies, but success has been more variable in field testing (see: Massei and Cowan 2014; Massei et al. 2014). Discounting surgical castration (invasive and resource intense), the remaining contraceptive methods can be generally categorised as intrauterine devices, hormone analogues, or immunocontraceptive vaccines (see summary of studies pertinent to mares in Table 2; Stout and Colenbrander 2004; Patton et al. 2007; National Research Council 2013; Swegen and Aitken 2016; Hall et al. 2017). Although there are ongoing laboratory-based studies seeking new reproductive targets (e.g. proteins on follicles, sperm, oocytes, spermatogonial stem cells, and blastocysts), these are in the very early stages of development (see Swegen and Aitken 2016; Hall et al. 2017).
|
In 2013, in the USA, a Committee of The National Research Council undertook a major review of the Bureau of Land Management (BLM) Wild Horse and Burro Program, so as to determine ways to improve management practices. This Committee extensively reviewed methods of fertility control relevant to horses (and ungulates) and concluded that there were no fertility-control methods that were highly effective, easily delivered and affordable across all BLM herd-management areas (HMAs). Of the methods available, the Committee considered that ‘the most promising fertility-control methods for application to free ranging horses or burros are porcine zona pellucida (PZP) vaccines, GonaCon vaccine, and chemical vasectomy’ (National Research Council 2013: p.6). Although we are in general agreement with this statement, current techniques tested for chemical vasectomy in wild stallions require capture and anaesthesia (Scully et al. 2015), so will not be considered in the present review. Similarly, although IUDs and hormone implants are potential agents for wild mares, these methods are impractical for field application (see Table 2). This leaves immunocontraceptives as the most likely agents for immediate application to wild horses in Australia. ‘No other class of contraceptives has been as extensively researched in domestic and free-ranging equids as immunocontraceptives’ (National Research Council 2013: p.115). Immunocontraception involves administration of a vaccine that induces an adaptive immune response to a target tissue or molecule essential for reproduction. In equids, the two most studied immunocontraceptives are vaccines directed against gonadotrophin-releasing hormone (GnRH) and the zona pellucida proteins. Therefore, our focus below is on two immunocontraceptives (Table 2), those based either on GnRH or on PZP, both of which can be delivered remotely by dart to horses.
Immunocontraceptive vaccines
GnRH vaccines
Gonadotrophin-releasing hormone (GnRH) is a small decapeptide hormone common to both females and males. It has a major role in the endocrine feedback loop involving the hypothalamus, pituitary and gonads leading to the downstream production of sex hormones (e.g. oestrogen, progesterone and testosterone) from the gonads (Fig. 1), and is an attractive target for manipulation of reproduction. The GnRH peptide has little natural antigenicity, so it must be conjugated to a larger highly immunogenic molecule (e.g. keyhole limpet haemocyanin, diptheria toxoid) and emulsified in an adjuvant before administration. Three registered GnRH immunocontraceptive formulations are available (ImproVac, Equity, GonaCon) and have been evaluated in female horses (Table 2). GonaCon, a GnRH–haemocyanin conjugate emulsified in Adjuvac (Mycobacterium avium in mineral oil), is the only formulation to demonstrate single-shot, multi-year (long-term) efficacy (Killian et al. 2006, 2008). This immunocontraceptive vaccine was developed by the National Wildlife Research Center (USDA–APHIS Wildlife Services program). Originally formulated for non-lethal control of white-tailed deer populations in the USA, it was registered by the USA Environmental Protection Agency (EPA) in 2013 for use in wild horses and burros (GonaCon-Equine).
As with many fertility-control methods, response to GnRH vaccination varies among individuals and species (Stout and Colenbrander 2004; Miller et al. 2013). A single shot of GonaCon to mares is effective at reducing fertility for the first 12 months (up to 94% of treated females; Killian et al. 2004, 2008; Gray et al. 2010), but becomes less efficacious in subsequent years (50–70% at 3 years). A booster dose (>12 months) of GonaCon can extend the period of infertility in some other species (Miller et al. 2013, for review of GonaCon effects in other species), but has not been reported for mares.
Porcine zona pellucida (PZP) vaccines
PZP-based vaccines have a much longer history of field use and have been shown to be an effective contraceptive in ~80 ungulate species, including several equids (Kirkpatrick et al. 2009). The vaccines consist of native zona pellucida proteins (ZP1–4) isolated and purified from porcine ovaries (Dunbar et al. 1980; Naz and Saver 2016), combined with an adjuvant to increase immunogenicity. Initially, Freund’s complete adjuvant (FCA) was used, but has been replaced by modified Freund’s adjuvant (MFA), or Freund’s incomplete adjuvant (FIA), which generate smaller localised tissue reactions. Following inoculation with PZP, the mare produces antibodies that specifically recognise only the outer egg coat (the zona pellucida) of mature oocytes and bind to it; this blocks the binding of sperm to oocytes preventing fertilisation. PZP antibodies also bind to ZP proteins on developing oocytes within the ovary, altering their maturation and related hormone signals (Mask et al. 2015; Joonè et al. 2017). Three current formulations of PZP vaccines (ZonaStat-H, PZP-22 and SpayVac) are similar in their method of production, but differ in their complement of ZP antigens, additives and delivery systems, which lead to differences in duration of effect and, thus, efficacy (Table 2).
In equids, a single dose of ZonaStat-H injected by hand, jab-stick, or remote darting several months before the breeding season is an effective contraceptive in 55–70% of mares and works for ~12 months (Kirkpatrick et al. 1990). A variant of single-shot PZP vaccine using AdjuVac as the adjuvant rather than mFCA was similarly efficacious under field conditions over a 3-year period (50–63%; Gray et al. 2010). Contraceptive efficacy can be increased to 90–95% of treated mares if a booster is given 2–4 weeks after the initial priming dose; although this carries the added benefit that a primer-dose combination is effective when given at any time of year, it requires that animals are easily accessible and recognisable for this treatment regimen (Kirkpatrick and Turner 1991, 2008). In 2012, the United States EPA formally approved the PZP vaccine, ZonaStat-H (PZP plus mFCA), for use as a contraceptive in wild horses and burros.
Experimental formulations of PZP aimed at increasing the duration of efficacy by encapsulating the vaccine in controlled–release pellets have been developed. Under controlled conditions, PZP emulsion plus controlled-release PZP pellets (known as PZP-22 vaccine) as a single-shot was effective up to 22 months in >85% mares (Turner et al. 2007) and SpayVac (PZP encapsulated in multi-lamellar liposomes; single-shot) maintained infertility in 50–70% of mares for up to 3 years (Killian et al. 2004, 2006, 2008; Roelle et al. 2017). In the field, application of PZP-22 to wild mares showed efficacy similar to captive trials over 12 months, with infertility prolonged if a booster dose with either PZP-22 or ZonaStat was given at 2–4 years (Rutberg et al. 2017). Due to the viscosity of these liposome formulations, hand-injection is favoured (Kirkpatrick et al. 2011), although use of large-gauge needles and improved rapid injection systems have made remote darting a possibility (Kirkpatrick et al. 2012; Rutberg et al. 2017). Quality control during the production of PZP emulsions plus controlled-release pellets has been problematic (Turner et al. 2002, 2008; Kirkpatrick et al. 2011; Rutberg et al. 2017), but follow-up boosters certainly extend the period of infertility, and, after several boosters, permanent infertility occurs (Kirkpatrick and Turner 2008). For larger species, such as the elephant, Loxodonta africana, helicopters have been used successfully to remotely dart deliver PZP vaccine (Delsink et al. 2007).
Availability
The two immunocontraceptive formulations approved for use in wild horses and burros in the United States, ZonaStat-H and GonaCon-Equine are neither produced in commercial quantities, nor are they commercially registered and available in Australia. Any fertility-control agent that has been developed and formulated overseas will require registration through the Australian Pesticides and Veterinary Medicines Authority (APVMA). Although a registration data package is substantial and generally requires Australian-based results for safety and efficacy of the product in the specific field situation, information for agents registered for wildlife use overseas can be supportive in an Australian application for registration. Currently, one of these vaccines (GonaCon) is in the process of being registered by the APVMA for use in macropods and horses in Australia. Australian quarantine regulations may prohibit the import or registration of some overseas-derived vaccines because they contain reagents that are derived directly from animals (e.g. PZP vaccines are made from pig ovaries collected at the time of slaughter). Under these circumstances, an ‘identical’ preparation may have to be produced in Australia from locally sourced porcine ovaries to prevent potential disease transfer to the Australian livestock industry.
Welfare considerations relevant to remote-field application of fertility control
When developing and assessing the potential application of any population-control strategy, humaneness, whether in the short term at the time of application or in the longer term due to post-treatment effects, is a major consideration for all stakeholders. Immunocontraceptives meet all the desired specificity, efficacy and safety (animal and environmental) requirements of a field-applicable contraceptive (Table 1), with the added potential of providing a humane and non-lethal control strategy for the management of populations of wild horses. Nevertheless, opinions differ regarding whether all the observed changes in physiology and behaviour after application of fertility-control agents in a range of species, including wild horses, constitute negative welfare impacts (Nettles 1997; Gray and Cameron 2010; Ransom et al. 2010; Hampton et al. 2015).
Stress and injury
All the contraceptive methods and agents described above require treatment to be administered either by hand or at close range (≤40 m; Massei et al. 2014); in most cases, this necessitates mustering or passive trapping. In an effort to standardise procedures and reduce negative welfare outcomes of all population-control measures in horses in Australia, a model code of practice for the humane control of wild horses (Sharp and Saunders 2012), and standard operating procedures for their trapping and mustering, have been developed for Australian conditions (Sharp 2011a, 2011b). Nevertheless, an independent technical reference group determined that both trapping and mustering cause moderate impacts on target-animal welfare (Humaneness Assessment Panel 2015).
Injection-site reactions
Minimum handling and follow-up are essential aspects of any cost-effective contraceptive treatment strategy of free-ranging animals on a large scale. Although there are no examples of death or injury of wild horses as a direct result of an injectable contraceptive (excluding necessary immobilisation for treatment), all injectable formulations of contraceptive agents have the potential to cause transient localised oedema, abscesses or tissue growth (granulomas). These responses are due to the components of the adjuvants because animals treated with the control vaccine (no reproductive antigen) also show an injection-site reaction. In studies of vaccine administration in horses, variable levels of adverse reactions have been observed, from minimal reactions with hand injection (Lyda et al. 2005; Roelle and Ransom 2009; Gray and Cameron 2010; Donovan et al. 2013), to acceptably low rates of abscess (non-aqueous adjuvant or modified Freund’s adjuvant; Roelle et al. 2017) or localised sterile non-toxic granulomas (Adjuvac; Miller et al. 2008).
Over the years, improvements in adjuvants have reduced the occurrence of adverse reactions. Granulomas are common and persist over time (Roelle et al. 2017) and abscesses occur at a rate of ~0.2% when using darts; these injection-site reactions are not observed to cause discomfort, and abscesses normally resolve without treatment after ~2 weeks (Roelle and Ransom 2009; Kirkpatrick et al. 2012; Roelle et al. 2017). In horses, immunocontraceptive treatment had no demonstrated impacts on their welfare, mobility or activity budgets (GonaCon, Baker et al. 2013; SpayVac, Roelle et al. 2017).
Behavioural changes
Depending on the type of contraceptive employed, changes in behaviour can be complex and have diverse ramifications (e.g. compensation in survival and fitness of fertile animals, emigration), although these are often not considered in study design. Contraceptives that alter sex hormone-driven behaviours (i.e. hormone analogues, GnRH vaccines), or cause a longer than normal period of reproductive receptivity (PZP vaccines, GnRH analogues), alter individual behaviour. It has been argued that behavioural changes have a negative impact on the wellbeing of PZP-contracepted females due to prolonged sexual interest from males, increased male–male aggression and increased male attention towards the remaining fertile females (Nuñez et al. 2009; Nuñez et al. 2017). However, other studies of free-living wild horses, where females were immunocontracepted, either showed no significant differences in behaviour between control and treated females (various contraceptives, Gray and Cameron 2010), or similar social behaviours by females, and changes in social behaviour in the untreated males (PZP, Ransom et al. 2010; Ransom 2012). More broadly, changes in individual behaviour have the potential to modify wild-horse ecology (Ransom et al. 2014) and, in turn, alter environmental impacts of herds and welfare of native species.
Body condition
PZP-treated females show improved body condition, which significantly increases lifespan (Kirkpatrick and Turner 2008), most likely because of decreased energetic demands in the absence of pregnancy and lactation. Although this reflects improved welfare for the individual, some authors argue that increased longevity engenders other health problems (e.g. geriatric conditions such as arthritis; Kirkpatrick et al. 1997; Kirkpatrick and Turner 2007, 2008).
Overdose and contraindications for use in pregnancy in mares
PZP-vaccines are deemed safe for use in pregnant animals (Lyda et al. 2005; Rutberg et al. 2017), and although GnRH vaccines have the potential to prevent embryo implantation if given during the first 6 weeks of pregnancy, thereafter, they are thought not to affect pregnancy (Kirkpatrick et al. 2011). From 6 weeks of gestation, pituitary luteinising hormone is no longer required (equine chorionic gonadotrophin is produced by the endometrial cups and stimulates placental progesterone production) for ongoing support of pregnancy.
Treating individuals with multiple doses of either PZP or GonaCon at a higher than necessary frequency (>annually) will not be detrimental to an animal’s wellbeing, as evidenced by ongoing use of primer-boost treatment regimes (Kirkpatrick et al. 2011). Multiple treatments may occur if treated individuals are not able to be identified accurately, if sites are visited more than annually for treatment, or if an animal is treated twice when the initial dose was thought to be unsuccessfully administered.
Field application of fertility control in free-ranging wild horses in USA
Over the past 30 years, although there has been extensive field testing of various GnRH and PZP vaccines in horses, their application has largely been in an experimental capacity to animals in the USA (Miller et al. 2013; Turner and Rutberg 2013). Results for efficacy at the individual and small-population scale are promising, with ongoing studies determining the long-term effects on population dynamics and relevance and implications for population control (Kirkpatrick et al. 2012; Rutberg et al. 2017). Research continues into development of improved formulations and delivery systems of these vaccines (Rutberg et al. 2017). The BLM also continues to support research to improve fertility-control methods (see https://www.blm.gov/programs/wild-horse-and-burro/herd-management/science-and-research [verified 10 April 2018]).
The liquid PZP vaccine, ZonaStat-H, is used at a management level on over 20 HMAs in the USA, by the National Parks Service, Rachel Carson National Estuarine Reserve, the BLM and at least five private sanctuaries (Kirkpatrick et al. 2012). The management approach using fertility control is ad hoc, with mares being treated opportunistically rather than in a targeted manner (Kirkpatrick et al. 2012; Bureau of Land Management 2016). These herds run in a variety of habitat types, from closed populations on the barrier islands of the eastern USA, to open grasslands, and mountainous and forest habitats of western ranges (Utah, Wyoming, Nevada, Colorado and Canada). The average treated herd in these HMAs contains 50 mares (Kirkpatrick et al. 2012).
The longest running (>20 years) field test of PZP (or any immunocontraceptive) on wild horses is from a closed population on Assateague Island National Seashore in Maryland, USA. By the mid-1990s, horse numbers (~175 animals) were affecting the ecology of Assateague Island and the use of PZP vaccine was implemented to reduce population size. Because of the local historical and cultural significance of these horses (introduced to the island in the 1600s), the population management objective was to reduce the population to an acceptable size and limit future population growth, rather than eradication. Under this management strategy, population health is targeted by maintaining genetic diversity; females (2–4 years old) are allowed to produce one foal, but, thereafter, are treated annually (at least 3 years) with PZP to eventually achieve permanent contraception (Kirkpatrick and Turner 2008). PZP treatment (dart delivered on foot) is 95% effective in this population, which has been treated for over 20 years without any associated health problems (Kirkpatrick and Turner 2008). Zero population growth was achieved within 2 years of commencement of the fertility-control program, and, by 8 years, population decline was apparent, such that the population had decreased by 22.8% in Year 11 (Kirkpatrick and Turner 2008). The long delay before population decline occurred was attributed to the increasing body condition scores, reduced mortality and significantly increased longevity of vaccinated females.
The Assateague Island management program is often cited as the premier example of horse population control. However, one must keep in mind that fertility control was effective in this instance for several unique reasons, including the following: (1) 100% of mares were treated (treatment required a primary vaccination and booster in the first year and an annual booster thereafter); (2) the initial population was manageable (173 animals); (3) individual animals were easily identifiable; (4) the area was small with open habitat; (5) animals could be approached on foot for hand injection or darting; and, finally, (6) the population is isolated, so there is no possible increase in numbers as a result of immigration.
More recently, efficacy of a PZP-22 treatment with PZP boosters after 2 or 3 years was experimentally tested on two larger, free-ranging horse populations (~200–600 animals) in the USA (Rutberg et al. 2017). Although overall population outcomes were not measured, remote dart delivery of a PZP booster effectively reduced fertility in mares compared with controls for 3 years (65–72%), whether that booster was another PZP-22 dose or PZP in liquid form. What is important to note, is that contraceptive efficacy over the 3 years differed between the two HMAs; this has broader implications when considering using such data to predict efficacy of fertility control for populations in different locations.
Predicting contraceptive efficacy in free-ranging populations
Assessing the effectiveness of fertility-control practices and using this information to accurately predict future outcomes relies on the collection of quality response data. In the case of Assateague Island, each animal could be tracked individually and life history detailed from birth to death. Using 30 years of these demographic data (birth, death, removal, pedigree and contraception date), genetic data and target population numbers, Ballou and colleagues (2008) accurately modelled current population management strategies and the outcomes of the current against adapted management strategies.
With the exception of the Assateague Island National Seashore population, few studies have empirically tested the effects of fertility control on population dynamics of free-ranging horses, mostly because long-term studies are expensive to conduct. Thus, managers rely on short-term to ongoing data gathering for input into population-modelling programs that evaluate the long-term potential of management actions (Turner and Rutberg 2013).
Population dynamics (maintenance, decline or expansion of population size; changes in sex or age demographics) are governed by the following four processes: births, deaths, immigration and emigration. Although individuals can be removed (i.e. culling, trapped and removed) easily to give immediate impacts on population size, populations are fluid and numbers may again rise through compensatory density-dependent increases in birth rate, or immigration (Fowler 1981, 1987). The same is true for manipulating birth rate through fertility control; defined numbers of animals must be removed from the breeding pool through contraception to achieve efficacy as well as allowing for increased immigration or other compensatory mechanisms (Hone 1992; Barlow 1997; Barlow et al. 1997; Hobbs et al. 2000).
In the absence of empirical data, population models can be developed using a conservative set of assumptions. Early models made assumptions about birth rates, survivorship and contraceptive efficacy (see Kirkpatrick and Turner 2008). More recent models have integrated knowledge obtained from empirical studies (such as that from Assateague Island), but most lack any ability to (1) compare cost-effectiveness (essential for management decision making; de Seve and Griffin 2013), (2) undertake a staged or multi-faceted approach (Hobbs et al. 2000) or (3) consider secondary impacts of the contraceptive treatment that may affect both animal physiology and behaviour (such as band fidelity, longevity, extended breeding season, compensatory survival of offspring of fertile animals, or density-dependent increases in agonistic behaviours among males; reviewed in Gray and Cameron 2010; Ransom and Kaczensky 2016).
In the USA, BLM managers use WinEquus (Jenkins 2002) to model population effects of ongoing management practices, including fertility control. This program has been criticised for its lack of sensitivity analysis, economic-optimisation capabilities and inability to include annual environmental variations or staged interventions (de Seve and Griffin 2013), highlighting the need for further optimisation (National Research Council 2013).
Despite these shortfalls, the general consensus using population modelling is that population reduction is not achievable in large, free-ranging wild populations using fertility control as a stand-alone method (see de Seve and Griffin 2013; National Research Council 2013; Raiho et al. 2015; Ransom and Kaczensky 2016). Fertility control is appropriate for managing small, closed populations (e.g. Assateague Island), or to maintain population levels once an initial reduction effort has been applied (Bomford 1990; Barlow et al. 1997; Merrill et al. 2003, 2006). As fertility-control agents with better single-shot longevity (2–3 years) are developed, this approach may become more feasible (Rutberg et al. 2017).
Free-ranging wild horses in Australia
Australia possesses the largest population of wild horses in the world, being in excess of 300 000 animals. Wild horses occupy every mainland state and territory; yet, there is no national legislation governing the control of wild horses and pest management is the responsibility of each individual state and territory government. As a result, the status of wild horses as a declared pest differs from state to state and is reflected in the scale of implementation of population management strategies (Dawson et al. 2006). Environmentally sensitive locations (i.e. national parks and conservation reserves) often require more defined interventions for the management of pest populations for conservation purposes, but proposed management can also be associated with more vocal stakeholders (Nimmo and Miller 2007). Wild horses are regarded as a national symbol by many Australians, being subjects of bush folk lore and an integral part of our cultural heritage, particularly those populations in the highland areas of south-eastern Australia made famous by the poet Banjo Patterson (Dawson et al. 2006; Nimmo and Miller 2007).
Indeed, one of the most politically, socially and emotionally charged debates of management of wild horses in Australia surrounds the persistence of a large population of animals in ecologically sensitive alpine, subalpine and montane habitats in the Kosciuszko National Park (KNP) in south-eastern Australia. Horses are currently managed by capture and removal, with subsequent domestication where possible or transport to an abattoir or knackery for disposal, the latter of which is highly unacceptable to some members of the community (Nimmo and Miller 2007; Office of Environment and Heritage 2016a, 2016b). Despite these efforts, the population has continued to expand its range. The KNP draft horse management plan (Office of Environment and Heritage 2016a) lists population management objectives and acknowledges the cultural, economic and social values of wild horses in the park. How can these objectives be achieved to satisfy all stakeholder requirements? Can fertility control play an integral part in such management?
Case study: feasibility of horse population control using immunocontraceptives in Kosciuszko National Park (KNP)
The KNP horse population is most similar to open-ranging populations of the HMAs of the western USA, in which immunocontraceptives are not effective as a stand-alone population management tool (National Research Council 2013). The 2014, Australian Alps feral horse aerial survey estimated the population as between 3899 and 8155 horses (95% confidence interval; Cairns and Robertson 2015), with the horses occurring over almost 50% of KNP (>700 000 ha), the largest national park in the country. The park is subdivided into management zones; however, apart from a handful of isolated populations (bounded by fencing or other natural barriers), these areas have connectivity and, thus, large numbers of animals are free-ranging with the ability to move between herds and subpopulations. These constraints alone make KNP a poor candidate for a broad-scale application of fertility control as the sole management strategy. However, a multifaceted integrated management strategy that includes application of fertility management could be considered.
Mathematical modelling exercises that specifically reflect KNP horse population dynamics could predict the best combination of control methods for population reduction and stabilisation. Models have been developed for several species, including horses (Ballou et al. 2008; de Seve and Griffin 2013), and are highly informative in terms of application of fertility control alone, or in combination with other population-reduction techniques (such as culling or trapping and removal; for example, see Hone 1992, 2004; Barlow et al. 1997; Hobbs et al. 2000; Raiho et al. 2015). Pepin et al. (2017) have recently modelled the potential effects of incorporating fertility control with typical culling regimes for populations of wild pigs in the USA. Their simulations of different combinations of culling and application of fertility control were dependent on the nature of the population (closed or open to immigration), the efficacy of an applied fertility-control agent and the goal of the management program. The results of Pepin et al. (2017) clearly indicated that there would be a considerable benefit gained by applying fertility-control techniques in conjunction with other specific removal techniques to achieve a desired reduction in a population. This approach would be most efficacious if the managed population was closed to immigration.
With only sporadic demographic data from trapping and smaller horse herds under scientific study (Dawson and Hone 2012), predictions about applying fertility control to achieve the objectives of the plan across KNP is difficult, but not impossible. There are considerable life-history data available from USA studies that allow some conservative assumptions to be made; however, caution should be exercised because contraceptive efficacy has been shown to differ among HMAs (Rutberg et al. 2017). To address the social and cultural objectives (i.e. to maintain a self-sustaining horse population, irrespective of size; Office of Environment and Heritage 2016a) of the KNP plan, a level of genetic management must be incorporated into the mathematical models and ongoing management strategies (Ballou et al. 2008; Willers et al. 2014).
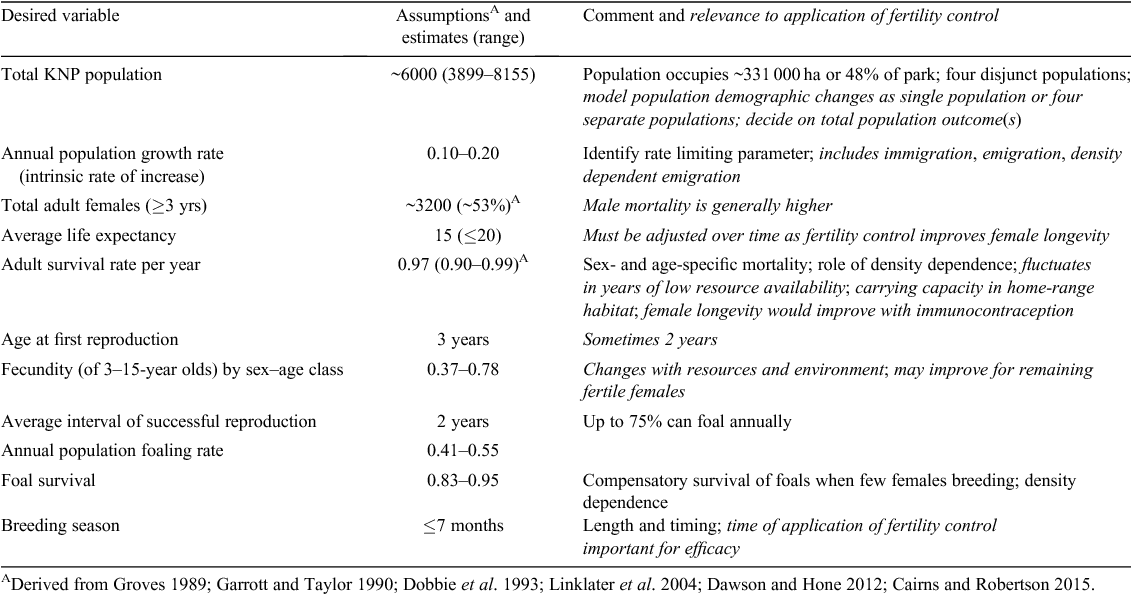
Table 3 summarises demographic data (parameters and estimations from Groves 1989; Garrott and Taylor 1990; Dobbie et al. 1993; Linklater et al. 2004; Dawson and Hone 2012), which are necessary to inform the most basic population modelling scenario. Limited demographic data are available for KNP (although sporadic over time; Independent Technical Reference Group 2016), from which informed assumptions can be made without the necessity for immediate collection of additional data. Outputs of model simulations based solely on presumptive data can be misleading compared with real on-the-ground situations (Linklater et al. 2004). Thus, it is imperative that management plans be monitored and adaptive; incorporating new data into models as they become available ensures that optimum, site-specific strategies evolve over time.
|
In simplistic terms, for a population to achieve zero growth, birth rate must equal death rate (excluding immigration, or emigration). Thus, assuming that annual mortality is between 10% and 30% (Table 3), one would need to reduce the birth rate to at least 30%, or further if population reduction is desired. For some species, population modelling has shown that there may be a broad range of levels of infertility that produces essentially no impact on population size (Hone 1992; Hobbs et al. 2000). Even the simplest model for white-tailed deer (Hobbs et al. 2000) suggests that over 50% of females must be removed from breeding each season (when using contraception alone), so as to achieve zero population growth. Similar threshold levels (30–85%) have also been shown in fertility-control trials in several other species (see table 2 in Massei and Cowan 2014). Although the deployment of improved contraceptive agents with multi-year efficacy should decrease effort in subsequent years, this hinges on treating animals before breeding and the ability to identify treated animals in subsequent years (Hobbs et al. 2000). Nonetheless, management strategies modelled for wild horses in the USA agree that a multifaceted approach combining fertility control (1–3-year efficacy) with periodic animal removals will be the most cost-effective (Bartholow 2007; de Seve and Griffin 2013) for achieving their primary objectives of maintaining specific population densities with a low inbreeding coefficient. Achieving target treatment numbers beyond the estimated 50%, to result in population reduction by fertility control alone, would involve capturing and contracepting untreated animals on an annual basis. This would be an ongoing logistical challenge for the horse population within KNP, given the complex habitat and range.
It is likely that aspects of horse ecology in Australia differ enough from those in contraceptive studies performed in USA to warrant initial smaller-scale testing of the most efficacious contraceptives. The minimum data requirements to test and monitor ongoing contraceptive efficacy at a population level are as follows: (1) accurate population counts, including an initial age–sex distribution; annual survival probabilities for each age–sex class; annual foaling rates for each age class of mares; sex ratio at birth; (2) attribution of maternity, which is essential for monitoring efficacy of a contraceptive at the individual level, which has implications for determining the frequency of treatment and percentage of mares requiring treatment (maintenance of genetic diversity); and (3) identification or marking of treated individuals to ensure no wasted effort (Hobbs et al. 2000) and to investigate band fidelity of treated females. Using these data, it would be possible to extrapolate and develop empirical models relevant to larger populations across KNP.
Until population modelling is undertaken to predict the most efficacious management scenario(s), it is not appropriate to estimate the overall cost, although this is an important consideration for long-term management strategies. More sophisticated models would incorporate the cost-effectiveness of various integrated strategies, including the use of fertility control. Moreover, costs are not linear because effort changes with terrain and population size; especially, if populations become difficult to approach, or more wary of human presence. Critically, overall KNP population numbers must be reduced by other means (shooting, trapping, mustering and removal; see Fagerstone et al. 2010) before, or in conjunction with, deployment of any fertility-control agent. Practical delivery to sufficient numbers of mares in such a large, continuous population is not feasible for KNP and will delay the achievement of conservation objectives.
Conclusions: is fertility control relevant for population management in Australian wild horses?
Throughout Australia, populations of wild horses are dispersed over large areas, in varied and difficult-to-access terrain and vegetation. Moreover, horse herds are open to immigration and introductions from neighbouring populations, making them poor choices for the successful application of currently available fertility-control agents. Applied alone, fertility control will not reduce horse numbers within 10 years and will not effectively halt population growth unless enough mares within those populations are treated concurrently and every 2–4 years in relation to the contraceptive used and to the duration of infertility induced by the specific contraceptives.
If fertility control is to be implemented in large populations, it is essential that the overall population size be reduced to a desired carrying capacity before treatment, and that an integrated management approach employing multiple methods continues over many years. This will be a logistical challenge in Australia, given that wild horses range across multiple states and territories and public and private lands.
In Australia, clear management goals must be stipulated and a pragmatic approach taken to the development of horse population control strategies; a national strategy would be beneficial. ‘The paucity of unequivocal facts makes decisions surrounding feral horse management more contentious than they otherwise would be’ (Nimmo and Miller 2007). Control of horses, particularly in areas of ecological significance, should not be any more contentious than that of other feral ungulates such as deer, camel, pig, goat, buffalo and the like.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This review was made publicly available with funding from the NSW Office of Environment and Heritage. We thank Doug Eckery (USDA) and our internal reviewers for their helpful comments on earlier versions.
References
Asa, C. S. (1999). Male reproductive success in free-ranging feral horses. Behavioral Ecology and Sociobiology 47, 89–93.| Male reproductive success in free-ranging feral horses.Crossref | GoogleScholarGoogle Scholar |
Baker, D. L., Powers, J. G., Oehler, M., Ransom, J., Gionfriddo, J. P., and Nett, T. M. (2013). Field evaluation of the immunocontraceptive, GonaCon-B, in free-ranging horses (Equus caballus) at Theodore Roosevelt National Park. Journal of Zoo and Wildlife Medicine 44, S147.
Ballou, J. D., Traylor-Holzer, K., Turner, A., Malo, A. F., Powell, D., Maldonado, J., and Eggert, L. (2008). Simulation model for contraceptive management of the Assateague Island feral horse population using individual-based data. Wildlife Research 35, 502–512.
| Simulation model for contraceptive management of the Assateague Island feral horse population using individual-based data.Crossref | GoogleScholarGoogle Scholar |
Barlow, N. (1997). Modelling immunocontraception in disseminating systems. Reproduction, Fertility and Development 9, 51–60.
| Modelling immunocontraception in disseminating systems.Crossref | GoogleScholarGoogle Scholar |
Barlow, N., Kean, J., and Briggs, C. (1997). Modelling the relative efficacy of culling and sterilisation for controlling populations. Wildlife Research 24, 129–141.
| Modelling the relative efficacy of culling and sterilisation for controlling populations.Crossref | GoogleScholarGoogle Scholar |
Bartholow, J. (2007). Economic benefit of fertility control in wild horse populations. The Journal of Wildlife Management 71, 2811–2819.
| Economic benefit of fertility control in wild horse populations.Crossref | GoogleScholarGoogle Scholar |
Bechert, U., Bartell, J., Kutzler, M., Menino, A., Bildfell, R., Anderson, M., and Fraker, M. (2013). Effects of two porcine zona pellucida immunocontraceptive vaccines on ovarian activity in horses. The Journal of Wildlife Management 77, 1386–1400.
| Effects of two porcine zona pellucida immunocontraceptive vaccines on ovarian activity in horses.Crossref | GoogleScholarGoogle Scholar |
Bomford, M. (1990). ‘A Role for Fertility Control in Wildlife Management?’ (Australian Government Publishing Service: Canberra.)
Bomford, M., and O’Brien, P. (1992). A role for fertility control wildlife management in Australia? In ‘Proceedings of the 15th Vertebrate Pest Conference’. (Ed. J. E. Borrecco and R. E. Marsh.) pp. 344–347. (University of California: Davis, CA.)
Botha, A., Schulman, M., Bertschinger, H., Guthrie, A., Annandale, C., and Hughes, S. (2008). The use of a GnRH vaccine to suppress mare ovarian activity in a large group of mares under field conditions. Wildlife Research 35, 548–554.
| The use of a GnRH vaccine to suppress mare ovarian activity in a large group of mares under field conditions.Crossref | GoogleScholarGoogle Scholar |
Bureau of Land Management (2016). ‘Wild Horse and Burro Removal and Fertility Control Treatment Schedule.’ Available at https://www.blm.gov/programs/wild-horse-and-burro/herd-management/gathers-and- removals [Accessed 5 September 2017].
Cairns, S., and Robertson, G. (2015). ‘2014 Survey of Feral Horses (Equus ferus caballus) in the Australian Alps.’ (Australian Alps Liaison Committee, Australian Alps National Parks: Canberra.)
Collins, G. H., and Kasbohm, J. W. (2017). Population dynamics and fertility control of feral horses. The Journal of Wildlife Management 81, 289–296.
| Population dynamics and fertility control of feral horses.Crossref | GoogleScholarGoogle Scholar |
Daels, P., and Hughes, J. (1995). Fertility control using intrauterine devices: an alternative for population control in wild horses. Theriogenology 44, 629–639.
| Fertility control using intrauterine devices: an alternative for population control in wild horses.Crossref | GoogleScholarGoogle Scholar |
Dawson, M., and Hone, J. (2012). Demography and dynamics of three wild horse populations in the Australian Alps. Austral Ecology 37, 97–109.
| Demography and dynamics of three wild horse populations in the Australian Alps.Crossref | GoogleScholarGoogle Scholar |
Dawson, M. J., Lane, C., and Saunders, G. (Eds) (2006). ‘Proceedings of the National Feral Horse Management Workshop. Canberra.’ (Invasive Animals Cooperative Research Centre: Canberra.)
de Seve, C. W., and Griffin, S. L. B. (2013). An economic model demonstrating the long-term cost benefits of incorporating fertility control into wild horse (Equus caballus) management programs on public lands in the United States. Journal of Zoo and Wildlife Medicine 44, S34–S37.
| An economic model demonstrating the long-term cost benefits of incorporating fertility control into wild horse (Equus caballus) management programs on public lands in the United States.Crossref | GoogleScholarGoogle Scholar |
Delsink, A. K., Van Altena, J., Grobler, D., Bertschinger, H., Kirkpatrick, J., and Slotow, R. (2007). Implementing immunocontraception in free-ranging African elephants at Makalali Conservancy. Journal of the South African Veterinary Association 78, 25–30.
| Implementing immunocontraception in free-ranging African elephants at Makalali Conservancy.Crossref | GoogleScholarGoogle Scholar |
Dobbie, W. R., Berman, D. McK., and Braysher, M. L. (Eds) (1993). ‘Managing Vertebrate Pests: Feral Horse.’ (Australian Government Publishing Service: Canberra.)
Donovan, C., Hazzard, T., Schmidt, A., LeMieux, J., Hathaway, F., and Kutzler, M. (2013). Effects of a commercial canine gonadotropin releasing hormone vaccine on estrus suppression and estrous behavior in mares. Animal Reproduction Science 142, 42–47.
| Effects of a commercial canine gonadotropin releasing hormone vaccine on estrus suppression and estrous behavior in mares.Crossref | GoogleScholarGoogle Scholar |
Dunbar, B. S., Wardrip, N. J., and Jerry, L. H. (1980). Isolation, physicochemical properties, and macromolecular composition of zona pellucida from porcine oocytes. Biochemistry 19, 356–365.
| Isolation, physicochemical properties, and macromolecular composition of zona pellucida from porcine oocytes.Crossref | GoogleScholarGoogle Scholar |
Eagle, T. C., Asa, C. S., Garrott, R. A., Plotka, E. D., Siniff, D. B., and Tester, J. R. (1993). Efficacy of dominant male sterilization to reduce reproduction in feral horses. Wildlife Society Bulletin 21, 116–121.
Elhay, M., Newbold, A., Britton, A., Turley, P., Dowsett, K., and Walker, J. (2007). Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Australian Veterinary Journal 85, 39–45.
| Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH.Crossref | GoogleScholarGoogle Scholar |
Fagerstone, K. A., Miller, L. A., Killian, G., and Yoder, C. A. (2010). Review of issues concerning the use of reproductive inhibitors, with particular emphasis on resolving human–wildlife conflicts in North America. Integrative Zoology 5, 15–30.
| Review of issues concerning the use of reproductive inhibitors, with particular emphasis on resolving human–wildlife conflicts in North America.Crossref | GoogleScholarGoogle Scholar |
Fowler, C. W. (1981). Density dependence as related to life history strategy. Ecology 62, 602–610.
| Density dependence as related to life history strategy.Crossref | GoogleScholarGoogle Scholar |
Fowler, C. W. (1987). A review of density dependence in populations of large mammals. In ‘Current Mammalogy’. (Ed. H. H. Genoways.) pp. 401–441. (Springer US: Boston, MA.)
Garrott, R., and Siniff, D. (1992). Limitations of male-oriented contraception for controlling feral horse populations. Journal of Wildlife Management 56, 456–464.
| Limitations of male-oriented contraception for controlling feral horse populations.Crossref | GoogleScholarGoogle Scholar |
Garrott, R. A., and Taylor, L. (1990). Dynamics of feral horse population in Montana. The Journal of Wildlife Management 54, 603–612.
| Dynamics of feral horse population in Montana.Crossref | GoogleScholarGoogle Scholar |
Garside, D., Gebril, A., Alsaadi, M., and Ferro, V. (2014). Fertility control in wildlife: review of current status, including novel and future technologies. In ‘Reproductive Sciences in Animal Conservation’. (Ed. W. Holt, J. Brown and P. Comizzoli.) pp. 467–488. (Springer: New York.)
Gray, M. E., and Cameron, E. Z. (2010). Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction 139, 45–55.
| Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence.Crossref | GoogleScholarGoogle Scholar |
Gray, M. E., Thain, D. S., Cameron, E. Z., and Miller, L. A. (2010). Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations. Wildlife Research 37, 475–481.
| Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations.Crossref | GoogleScholarGoogle Scholar |
Groves, C. (1989). Equidae. In ‘Fauna of Australia. Vol. IB. Mammalia’. (Ed. D. W. Walton and B. J. Richardson.) pp. 1039–1043. (Australian Government Publishing Service: Canberra.)
Hall, S., Nixon, B., and Aitken, J. (2017). Non-surgical sterilisation methods may offer a sustainable solution to feral horse (Equus caballus) overpopulation. Reproduction, Fertility and Development 29, 1655–1666.
| Non-surgical sterilisation methods may offer a sustainable solution to feral horse (Equus caballus) overpopulation.Crossref | GoogleScholarGoogle Scholar |
Hampton, J. O., Hyndman, T. H., Barnes, A., and Collins, T. (2015). Is wildlife fertility control always humane? Animals 5, 1047–1071.
| Is wildlife fertility control always humane?Crossref | GoogleScholarGoogle Scholar |
Hobbs, N. T., Bowden, D. C., and Baker, D. L. (2000). Effects of fertility control on populations of ungulates: general, stage-structured models. The Journal of Wildlife Management 64, 473–491.
| Effects of fertility control on populations of ungulates: general, stage-structured models.Crossref | GoogleScholarGoogle Scholar |
Hone, J. (1992). Rate of increase and fertility control. Journal of Applied Ecology 29, 695–698.
| Rate of increase and fertility control.Crossref | GoogleScholarGoogle Scholar |
Hone, J. (2004). Yield, compensation and fertility control: a model for vertebrate pests. Wildlife Research 31, 357–368.
| Yield, compensation and fertility control: a model for vertebrate pests.Crossref | GoogleScholarGoogle Scholar |
Humaneness Assessment Panel (2015). Assessing the humaneness of wild horse management methods: Kosciuszko National Park wild horse management plan: a report on the outcomes of a humaneness assessment panel assembled on behalf of the Independent Technical Reference Group (ITRG). NSW Office of Environment and Heritage (OEH), Sydney.
Imboden, I., Janett, F., Burger, D., Crowe, M. A., Hässig, M., and Thun, R. (2006). Influence of immunization against GnRH on reproductive cyclicity and estrous behavior in the mare. Theriogenology 66, 1866–1875.
| Influence of immunization against GnRH on reproductive cyclicity and estrous behavior in the mare.Crossref | GoogleScholarGoogle Scholar |
Independent Technical Reference Group (2016). Final report of the Independent Technical Reference Group: supplementary to the Kosciuszko National Park wild horse management plan. NSW Office of Environment and Heritage Report ID. OEH20160221. OEH, Sydney.
Janett, F., Stump, R., Burger, D., and Thun, R. (2009). Suppression of testicular function and sexual behaviour by vaccination against GnRH (Equity) in the adult stallion. Animal Reproduction Science 115, 88–102.
| Suppression of testicular function and sexual behaviour by vaccination against GnRH (Equity) in the adult stallion.Crossref | GoogleScholarGoogle Scholar |
Jenkins, S. (2002). ‘Feral Horse Population Model, WinEquus.’ Program and documentation available at https://wolfweb.unr.edu/homepage/jenkins/
Johnson, C. A. (2002). Endocrine and reproductive responses to implants of deslorein acetate in horses. PhD Disseration, Louisiana State University and Agricultural and Mechanical College, Baton Rouge Louisiana, USA.
Johnson, C. A., Thompson, D. L., Kulinski, K. M., and Guitreau, A. M. (2000). Prolonged interovulatory interval and hormonal changes in mares following the use of Ovuplant™ to hasten ovulation. Journal of Equine Veterinary Science 20, 331–336.
| Prolonged interovulatory interval and hormonal changes in mares following the use of Ovuplant™ to hasten ovulation.Crossref | GoogleScholarGoogle Scholar |
Johnson, C., Thompson, D., and Cartmill, J. (2003). Effects of deslorelin acetate implants in horses: single implants in stallions and steroid-treated geldings and multiple implants in mares. Journal of Animal Science 81, 1300–1307.
| Effects of deslorelin acetate implants in horses: single implants in stallions and steroid-treated geldings and multiple implants in mares.Crossref | GoogleScholarGoogle Scholar |
Joonè, C. J., Schulman, M. L., and Bertschinger, H. J. (2017). Ovarian dysfunction associated with zona pellucida-based immunocontraceptive vaccines. Theriogenology 89, 329–337.
| Ovarian dysfunction associated with zona pellucida-based immunocontraceptive vaccines.Crossref | GoogleScholarGoogle Scholar |
Killian, G., Miller, L. A., Diehl, N. K., Rhyan, J., and Thain, D. (2004). Evaluation of three contraceptive approaches for population control in wild horses. In ‘Proceedings of the 21st Vertebrate Pest Conference’. (Ed. R M. Timm and W. P. Gorenzel.) pp. 263–268. (University of California: Davis, CA.)
Killian, G., Diehl, N. K., Miller, L., Rhyan, J., and Thain, D. (2006). Long-term efficacy of three contraceptive approaches for population control of wild horses. In ‘Proceedings of the 22nd Vertebrate Pest Conference’. (Ed. R. M. Timm and J. M. O’Brien.) pp. 67–71. (University of California: Davis, CA.)
Killian, G., Thain, D., Diehl, N. K., Rhyan, J., and Miller, L. (2008). Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildlife Research 35, 531–539.
| Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J. F., and Rutberg, A. T. (2001). Fertility control in animals. In ‘The State of the Animals 2001’. (Eds D. J. Salem snd A. N. Rowan.) pp. 183–198. (Humane Society Press: Washington, DC.)
Kirkpatrick, J. F., and Turner, J. W. (1991). Reversible contraception in nondomestic animals. Journal of Zoo and Wildlife Medicine 22, 392–408.
Kirkpatrick, J. F., and Turner, A. (2007). Immunocontraception and increased longevity in equids. Zoo Biology 26, 237–244.
| Immunocontraception and increased longevity in equids.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J. F., and Turner, A. (2008). Achieving population goals in a long-lived wildlife species (Equus caballus) with contraception. Wildlife Research 35, 513–519.
| Achieving population goals in a long-lived wildlife species (Equus caballus) with contraception.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J. F., Liu, I., and Turner, J. W. (1990). Remotely-delivered immunocontraception in feral horses. Wildlife Society Bulletin 18, 326–330.
Kirkpatrick, J. F., Zimmermann, W., Kolter, L., Liu, I., and Turner, J. (1995). Immunocontraception of captive exotic species. I. Przewalski’s horses (Equus przewalskii) and banteng (Bos javanicus). Zoo Biology 14, 403–416.
| Immunocontraception of captive exotic species. I. Przewalski’s horses (Equus przewalskii) and banteng (Bos javanicus).Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J., Turner, J., Liu, I., Fayrer-Hosken, R., and Rutberg, A. (1997). Case studies in wildlife immunocontraception: wild and feral equids and white-tailed deer. Reproduction, Fertility and Development 9, 105–110.
| Case studies in wildlife immunocontraception: wild and feral equids and white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J., Rowan, A., Lamberski, N., Wallace, R., Frank, K., and Lyda, R. (2009). The practical side of immunocontraception: zona proteins and wildlife. Journal of Reproductive Immunology 83, 151–157.
| The practical side of immunocontraception: zona proteins and wildlife.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J., Robin, L., and Kimberly, F. (2011). Contraceptive vaccines for wildlife: a review. American Journal of Reproductive Immunology 66, 40–50.
| Contraceptive vaccines for wildlife: a review.Crossref | GoogleScholarGoogle Scholar |
Kirkpatrick, J. F., Rutberg, A. T., and Coates-Markle, L. (2012). ‘Immunocontraceptive Reproductive Control Utilizing Porcine Zona Pellucida (PZP) in Federal Wild Horse Populations.’ 4th edn. (Ed. P. M. Fazio.) (Science and Conservation Center: Billings, MT.) Available at http://www.sccpzp.org/wp-content/uploads/PZP-QA-February-2012.pdf [verified 10 April 2018]
Linklater, W. L., Cameron, E. Z., Minot, E. O., and Stafford, K. J. (2004). Feral horse demography and population growth in the Kaimanawa Ranges, New Zealand. Wildlife Research 31, 119–128.
| Feral horse demography and population growth in the Kaimanawa Ranges, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Lyda, R. O., Hall, J. R., and Kirkpatrick, J. F. (2005). A comparison of Freund’s complete and Freund’s modified adjuvants used with a contraceptive vaccine in wild horses (Equus caballus). Journal of Zoo and Wildlife Medicine 36, 610–616.
| A comparison of Freund’s complete and Freund’s modified adjuvants used with a contraceptive vaccine in wild horses (Equus caballus).Crossref | GoogleScholarGoogle Scholar |
Mask, T. A., Schoenecker, K. A., Kane, A. J., Ransom, J. I., and Bruemmer, J. E. (2015). Serum antibody immunoreactivity to equine zona protein after SpayVac vaccination. Theriogenology 84, 261–267.
| Serum antibody immunoreactivity to equine zona protein after SpayVac vaccination.Crossref | GoogleScholarGoogle Scholar |
Massei, G., and Cowan, D. (2014). Fertility control to mitigate human–wildlife conflicts: a review. Wildlife Research 41, 1–21.
| Fertility control to mitigate human–wildlife conflicts: a review.Crossref | GoogleScholarGoogle Scholar |
Massei, G., Cowan, D., and Eckery, D. (2014). Novel management methods: immunocontraception and other fertility control tools. In ‘Behaviour and Management of European Ungulates’. (Ed. R. Putman and A. Marco.) pp. 209–235. (Whittles Publishing: Dunbeath, UK.) Available at http://www.sccpzp.org/publications/
Merrill, J. A., Cooch, E. G., and Curtis, P. D. (2003). Time to reduction: factors influencing management efficacy in sterilizing overabundant white-tailed deer. The Journal of Wildlife Management 67, 267–279.
| Time to reduction: factors influencing management efficacy in sterilizing overabundant white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Merrill, J. A., Cooch, E. G., and Curtis, P. D. (2006). Managing an overabundant deer population by sterilization: effects of immigration, stochasticity and the capture process. The Journal of Wildlife Management 70, 268–277.
| Managing an overabundant deer population by sterilization: effects of immigration, stochasticity and the capture process.Crossref | GoogleScholarGoogle Scholar |
Miller, L., Fagerstone, K., Kemp, J., Killian, G., and Rhyan, J. (2008). Immune mechanisms and characterization of injection site reactions involved in the multi-year contraceptive effect of the GonaCon™ vaccine.In ‘Proceedings of the 23rd Vertebrate Pest Conference’. (Eds R. M. Timm and M. B. Madon.) pp. 244–249. (University of California: Davis, CA.)
Miller, L. A., Fagerstone, K. A., and Eckery, D. C. (2013). Twenty years of immunocontraceptive research: lessons learned. Journal of Zoo and Wildlife Medicine 44, S84–S96.
| Twenty years of immunocontraceptive research: lessons learned.Crossref | GoogleScholarGoogle Scholar |
Morris, L. H. A., Fraser, B. S. L., Cantley, C., and Wilsher, S. (2017). The hazards associated with the use of intrauterine glass balls to suppress oestrus in mares. Equine Veterinary Education 29, 125–130.
| The hazards associated with the use of intrauterine glass balls to suppress oestrus in mares.Crossref | GoogleScholarGoogle Scholar |
National Research Council (2013). ‘Using Science to Improve the BLM Wild Horse and Burro Program: a Way Forward.’ (National Academies Press: Washington, DC.)
Naugle, R., and Grams, K. (2013). Long-term methods and effects of remotely treating wildlife with immunocontraception. Journal of Zoo and Wildlife Medicine 44, S138–S140.
| Long-term methods and effects of remotely treating wildlife with immunocontraception.Crossref | GoogleScholarGoogle Scholar |
Naz, R. K., and Saver, A. E. (2016). Immunocontraception for animals: current status and future perspective. American Journal of Reproductive Immunology 75, 426–439.
| Immunocontraception for animals: current status and future perspective.Crossref | GoogleScholarGoogle Scholar |
Nettles, V. F. (1997). Potential consequences and problems with wildlife contraceptives. Reproduction, Fertility and Development 9, 137–144.
| Potential consequences and problems with wildlife contraceptives.Crossref | GoogleScholarGoogle Scholar |
Nimmo, D. G., and Miller, K. K. (2007). Ecological and human dimensions of management of feral horses in Australia: a review. Wildlife Research 34, 408–417.
| Ecological and human dimensions of management of feral horses in Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Nuñez, C. M. V., Adelman, J. S., Mason, C., and Rubenstein, D. I. (2009). Immunocontraception decreases group fidelity in a feral horse population during the non-breeding season. Applied Animal Behaviour Science 117, 74–83.
| Immunocontraception decreases group fidelity in a feral horse population during the non-breeding season.Crossref | GoogleScholarGoogle Scholar |
Nuñez, C. M. V., Adelman, J. S., Carr, H. A., Alvarez, C. M., and Rubenstein, D. I. (2017). Lingering effects of contraception management on feral mare (Equus caballus) fertility and social behavior. Conservation Physiology 5, cox018.
| Lingering effects of contraception management on feral mare (Equus caballus) fertility and social behavior.Crossref | GoogleScholarGoogle Scholar |
Office of Environment and Heritage (2016a). Draft wild horse management plan: Kosciuszko National Park. NSW Office of Environment and Heritage Report ID. OEH20160271. OEH, Sydney.
Office of Environment and Heritage. (2016b). Review of the 2008 horse management plan and wild horse management program, Kosciuszko National Park. NSW Office of Environment and Heritage Report ID. OEH20160272. OEH, Sydney.
Patton, M., Jöchle, W., and Penfold, L. (2007). Review of contraception in ungulate species. Zoo Biology 26, 311–326.
| Review of contraception in ungulate species.Crossref | GoogleScholarGoogle Scholar |
Pepin, K. M., Davis, A. J., Cunningham, F. L., VerCauteren, K. C., and Eckery, D. C. (2017). Potential effects of incorporating fertility control into typical culling regimes in wild pig populations. PLoS One 12, e0183441.
| Potential effects of incorporating fertility control into typical culling regimes in wild pig populations.Crossref | GoogleScholarGoogle Scholar |
Raiho, A. M., Hooten, M. B., Bates, S., and Hobbs, N. T. (2015). Forecasting the effects of fertility control on overabundant ungulates: white-tailed deer in the national capital region. PLoS One 10, e0143122.
| Forecasting the effects of fertility control on overabundant ungulates: white-tailed deer in the national capital region.Crossref | GoogleScholarGoogle Scholar |
Ransom, J. I. (2012). Population ecology of feral horses in an era of fertility control management. Ph.D. Disseration, Colorado State University Fort Collins, Colorado USA.
Ransom, J., and Kaczensky, P. (Eds) (2016). ‘Wild Equids: Ecology, Management, and Conservation.’ (JHU Press: Baltimore, MD.)
Ransom, J. I., Cade, B. S., and Hobbs, N. T. (2010). Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses. Applied Animal Behaviour Science 124, 51–60.
| Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses.Crossref | GoogleScholarGoogle Scholar |
Ransom, J. I., Powers, J. G., Hobbs, N. T., and Baker, D. L. (2014). Ecological feedbacks can reduce population‐level efficacy of wildlife fertility control. Journal of Applied Ecology 51, 259–269.
| Ecological feedbacks can reduce population‐level efficacy of wildlife fertility control.Crossref | GoogleScholarGoogle Scholar |
Rivera del Alamo, M. R., Reilas, T., Kindahl, H., and Katila, T. (2008). Mechanisms behind intrauterine device-induced luteal persistence in mares. Animal Reproduction Science 107, 94–106.
| Mechanisms behind intrauterine device-induced luteal persistence in mares.Crossref | GoogleScholarGoogle Scholar |
Roelle, J. E., and Ransom, J. I. (2009). Injection-site reactions in wild horses (Equus caballus) receiving an immunocontraceptive vaccine. US Geological Survey Scientific Investigations Report 2009-5038. US Geological Survey, Reston, VA.
Roelle, J. E., Germaine, S. S., Kane, A. J., and Cade, B. S. (2017). Efficacy of SpayVac® as a contraceptive in feral horses. Wildlife Society Bulletin 41, 107–115.
| Efficacy of SpayVac® as a contraceptive in feral horses.Crossref | GoogleScholarGoogle Scholar |
Rutberg, A., Grams, K., Turner, J. W., and Hopkins, H. (2017). Contraceptive efficacy of priming and boosting doses of controlled-release PZP in wild horses. Wildlife Research 44, 174–181.
| Contraceptive efficacy of priming and boosting doses of controlled-release PZP in wild horses.Crossref | GoogleScholarGoogle Scholar |
Schulman, M., Botha, A., Muenscher, S., Annandale, C., Guthrie, A., and Bertschinger, H. (2013). Reversibility of the effects of GnRH‐vaccination used to suppress reproductive function in mares. Equine Veterinary Journal 45, 111–113.
| Reversibility of the effects of GnRH‐vaccination used to suppress reproductive function in mares.Crossref | GoogleScholarGoogle Scholar |
Scully, C. M., Lee, R. L., Pielstick, L., Medlock, J., Patton, K. M., Collins, G. H., and Kutzler, M. A. (2015). Comparison of chemical and surgical vasetomy on testicular activity in free-roaming horses (Equus caballus). Journal of Zoo and Wildlife Medicine 46, 815–824.
Sharma, S., and Hinds, L. A. (2012). Formulation and delivery of vaccines: ongoing challenges for animal management. Journal of Pharmacy & Bioallied Sciences 4, 258–266.
| Formulation and delivery of vaccines: ongoing challenges for animal management.Crossref | GoogleScholarGoogle Scholar |
Sharp, T. (2011a). ‘Standard Operating Procedure HOR003: Mustering of Feral Horses.’ (Invasive Animals Cooperative Research Centre: Canberra.) Available at https://www.pestsmart.org.au/pest-animal-species/horse/ [verified 10 April 2018]
Sharp, T. (2011b). ‘Standard Operating Procedure HOR004: Trapping of Feral Horses.’ (Invasive Animals Cooperative Research Centre: Canberra.) Available at https://www.pestsmart.org.au/pest-animal-species/horse/ [verified 10 April 2018]
Sharp, T., and Saunders, G. (2012). ‘Model Code of Practice for the Humane Control Feral Horses.’ Invasive Animals Cooperative Research Centre. (Department of Sustainability Environment, Water, Population and Communities: Canberra.) Available at https://www.pestsmart.org.au/pest-animal-species/horse/ [verified 10 April 2018]
Squires, E. L. (2008). Hormonal manipulation of the mare: a review. Journal of Equine Veterinary Science 28, 627–634.
| Hormonal manipulation of the mare: a review.Crossref | GoogleScholarGoogle Scholar |
Storer, W. A., Thompson, D. L., Gilley, R. M., and Burns, P. J. (2009). Evaluation of injectable sustained release progestin formulations for suppression of estrus and ovulation in mares. Journal of Equine Veterinary Science 29, 33–36.
| Evaluation of injectable sustained release progestin formulations for suppression of estrus and ovulation in mares.Crossref | GoogleScholarGoogle Scholar |
Stout, T., and Colenbrander, B. (2004). Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Animal Reproduction Science 82-83, 633–643.
| Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists.Crossref | GoogleScholarGoogle Scholar |
Swegen, A., and Aitken, J. (2016). Prospects for immunocontraception in feral horse population control: exploring novel targets for an equine fertility vaccine. Reproduction, Fertility and Development 28, 853–863.
| Prospects for immunocontraception in feral horse population control: exploring novel targets for an equine fertility vaccine.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., and Rutberg, A. T. (2013). From the pens to the field: real-world wildlife contraception. Journal of Zoo and Wildlife Medicine 44, S102–S110.
| From the pens to the field: real-world wildlife contraception.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Liu, I., and Kirkpatrick, J. (1992). Remotely delivered immunocontraception in captive white-tailed deer. The Journal of Wildlife Management 56, 154–157.
| Remotely delivered immunocontraception in captive white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Liu, I., and Kirkpatrick, J. (1996). Remotely delivered immunocontraception in free-roaming feral burros (Equus asinus). Journal of Reproduction and Fertility 107, 31–35.
| Remotely delivered immunocontraception in free-roaming feral burros (Equus asinus).Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Liu, I., Rutberg, A., and Kirkpatrick, J. (1997). Immunocontraception limits foal production in free-roaming feral horses in Nevada. The Journal of Wildlife Management 61, 873–880.
| Immunocontraception limits foal production in free-roaming feral horses in Nevada.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Liu, I. K., Flanagan, D. R., Rutberg, A. T., and Kirkpatrick, J. F. (2001). Immunocontraception in feral horses: one inoculation provides one year of infertility. The Journal of Wildlife Management 65, 235–241.
| Immunocontraception in feral horses: one inoculation provides one year of infertility.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Liu, I., Flanagan, D., Bynum, K., and Rutberg, A. (2002). Porcine zona pellucida (PZP) immunocontraception of wild horses (Equus caballus) in Nevada: a 10 year study. Reproduction 60, 177–186.
Turner, J. W., Liu, I. K., Flanagan, D. R., Rutberg, A. T., and Kirkpatrick, J. F. (2007). Immunocontraception in wild horses: one inoculation provides two years of infertility. The Journal of Wildlife Management 71, 662–667.
| Immunocontraception in wild horses: one inoculation provides two years of infertility.Crossref | GoogleScholarGoogle Scholar |
Turner, J. W., Rutberg, A. T., Naugle, R. E., Kaur, M. A., Flanagan, D. R., Bertschinger, H. J., and Liu, I. K. (2008). Controlled-release components of PZP contraceptive vaccine extend duration of infertility. Wildlife Research 35, 555–562.
| Controlled-release components of PZP contraceptive vaccine extend duration of infertility.Crossref | GoogleScholarGoogle Scholar |
Turner, R. M., Vanderwall, D. K., and Stawicki, R. (2015). Complications associated with the presence of two intrauterine glass balls used for oestrus suppression in a mare. Equine Veterinary Education 27, 340–343.
| Complications associated with the presence of two intrauterine glass balls used for oestrus suppression in a mare.Crossref | GoogleScholarGoogle Scholar |
Willers, N., Berry, O., and Roberts, J. D. (2014). Fine-scale genetic structure and the design of optimal fertility control for an overabundant mammal. Conservation Genetics 15, 1053–1062.
| Fine-scale genetic structure and the design of optimal fertility control for an overabundant mammal.Crossref | GoogleScholarGoogle Scholar |


